User login
Generalized Pustular Psoriasis: A Review of the Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
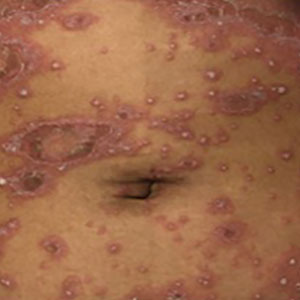
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4
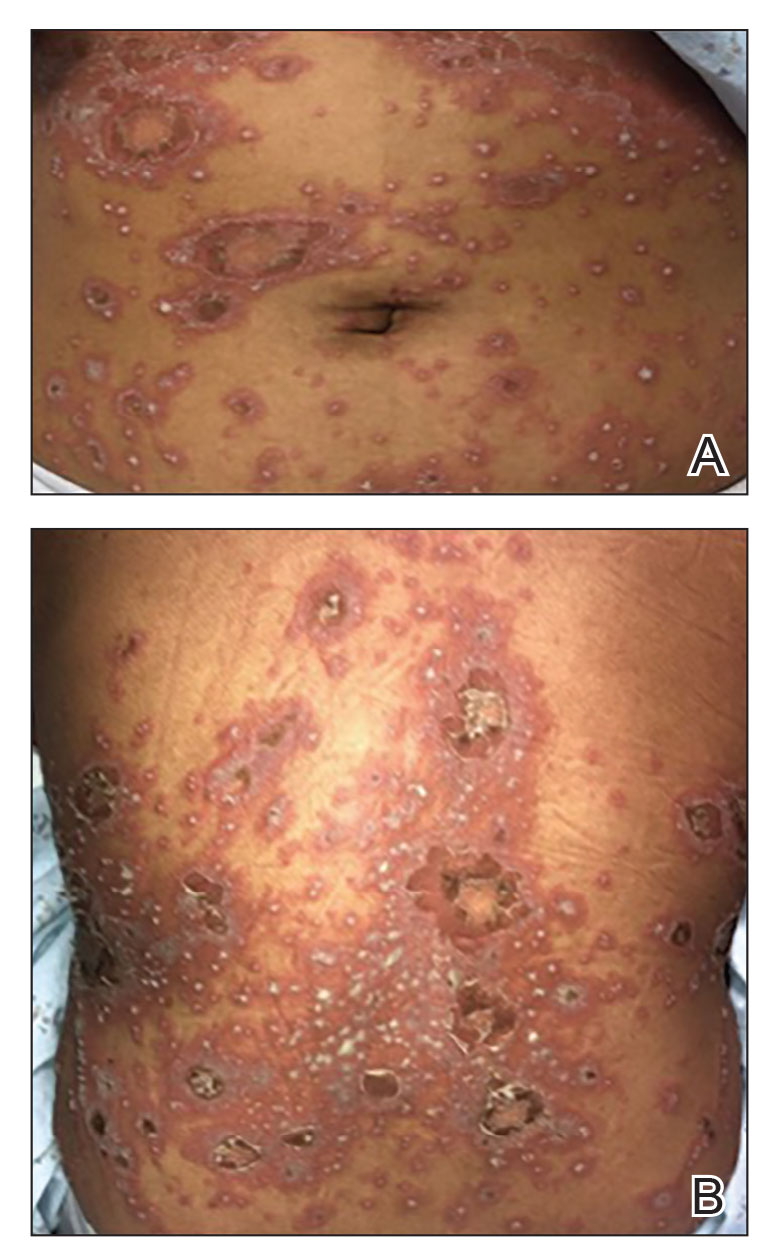
The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
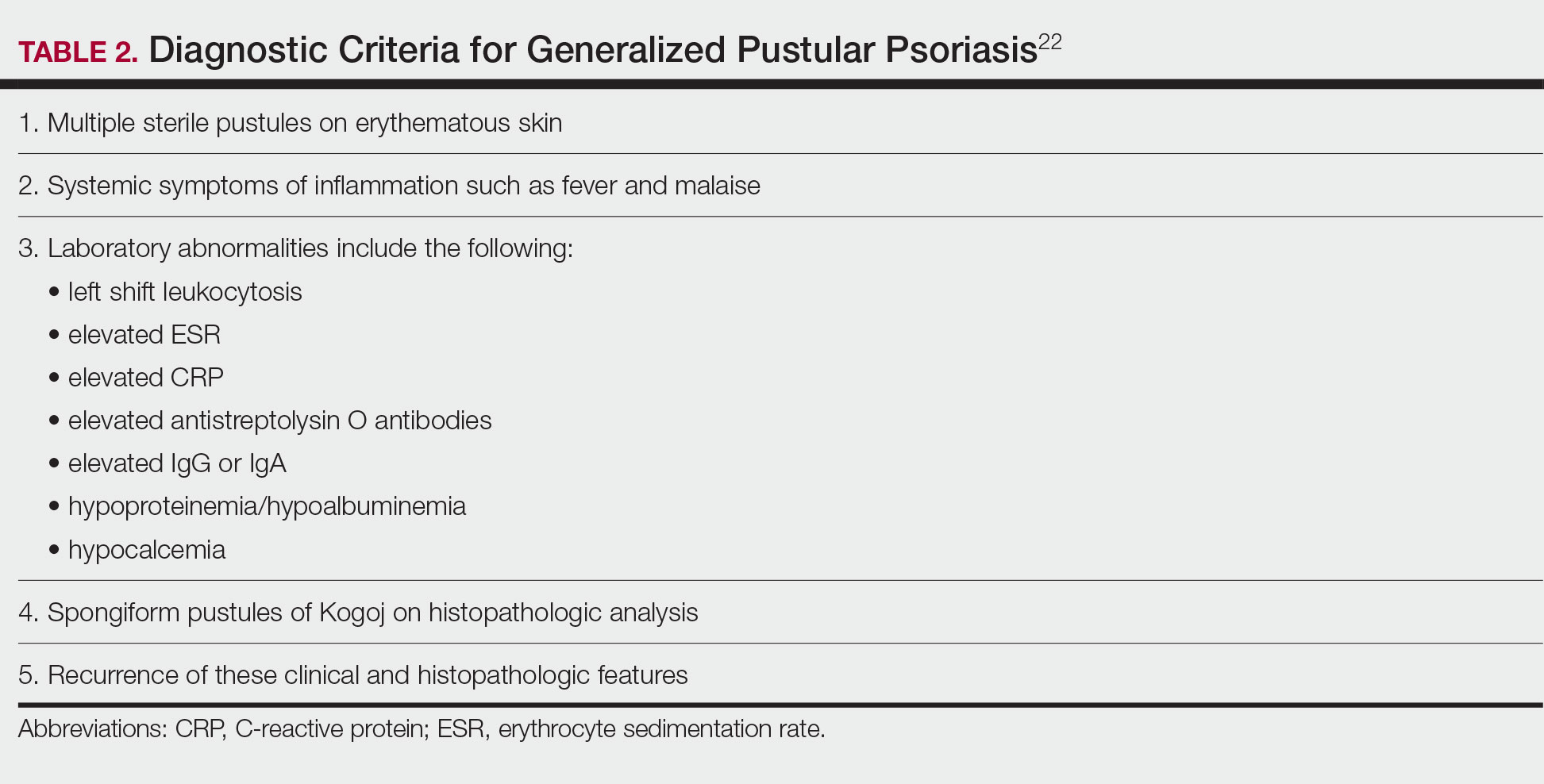
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25
Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4
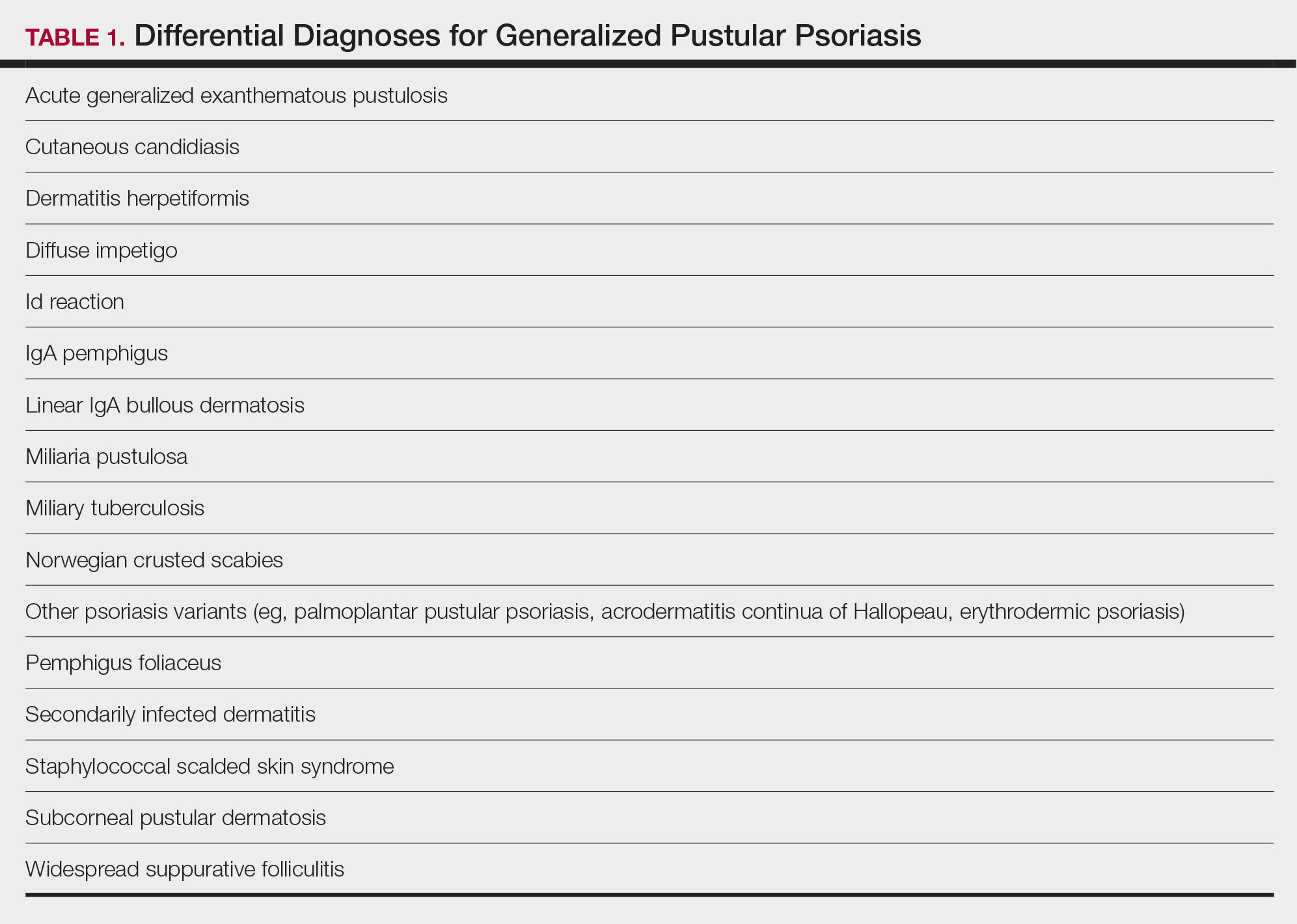
Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
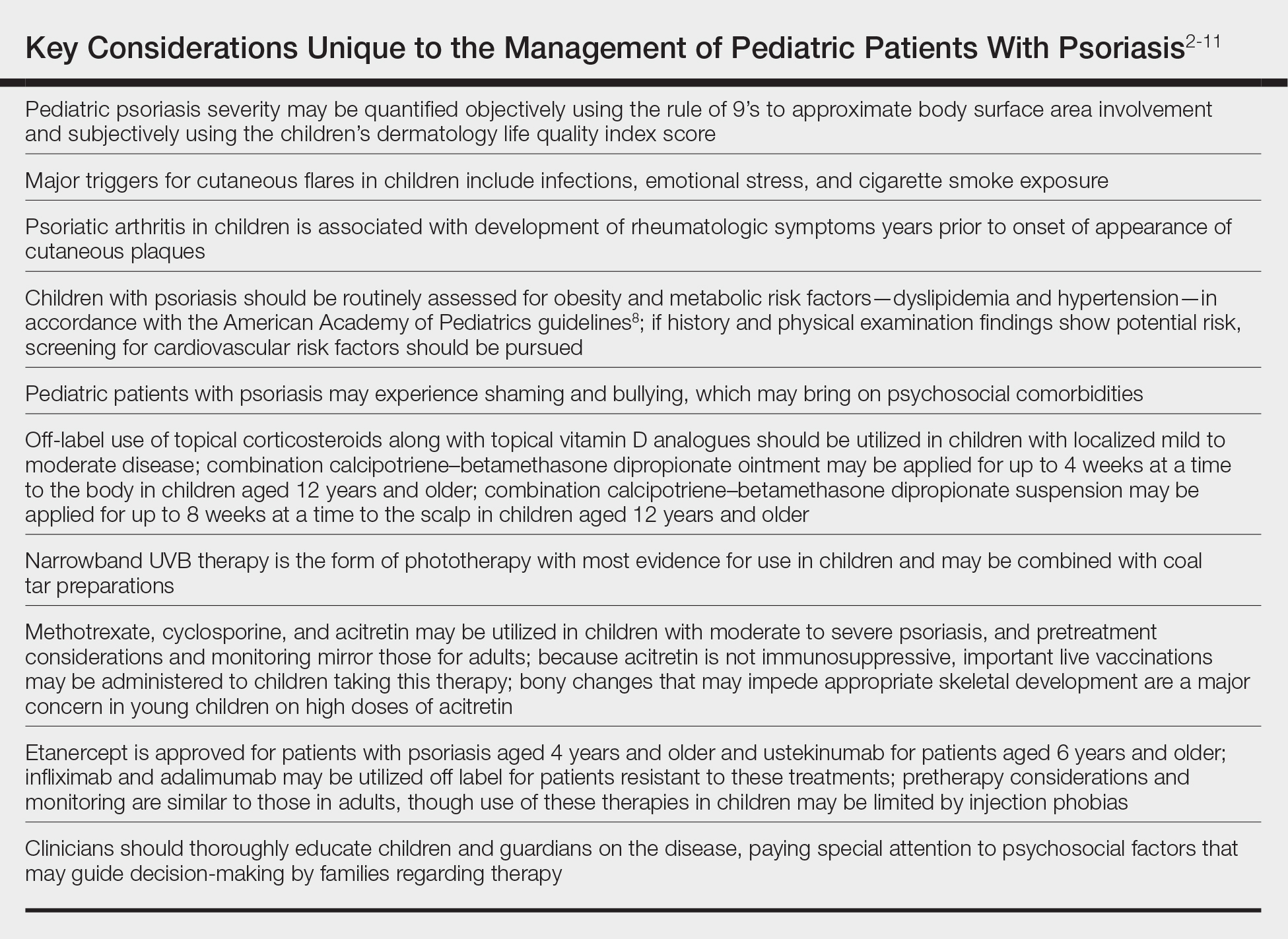
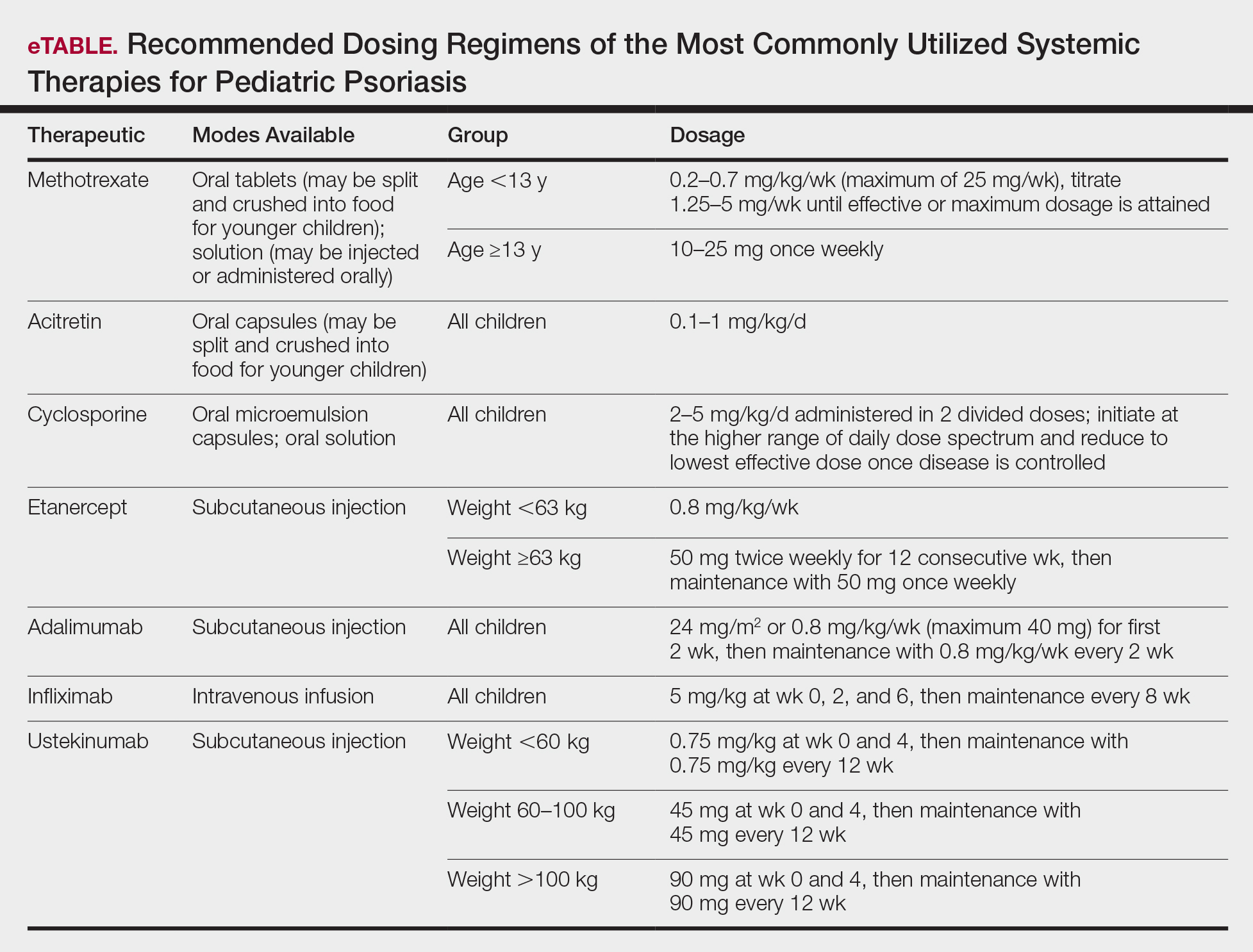
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Practice Points
- Generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis that is characterized by the abrupt widespread onset of small pustules.
- Although no treatments have specifically been approved for GPP, various biologics, especially infliximab, may be effective in achieving rapid clearance in patients with GPP. Other oral systemic agents including acitretin, cyclosporine, and methotrexate also have been shown to be effective.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis in Pediatric Patients
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
Practice Points
- For children, several environmental factors may prompt psoriasis flares, and it is critical to identify and eliminate these triggers.
- Although the use of biologics may be limited by cost and injection phobias in children, they may be an appropriate option for children with moderate to severe psoriasis when other therapies have failed. A growing body of literature is establishing the safety and effectiveness of biologics in children.
- Clinicians should thoroughly educate parents/ guardians on the course of psoriasis and treatment options as well as pay special attention to treatment goals and psychosocial factors that may guide decision-making regarding therapy.
Risk for Deep Fungal Infections During IL-17 and IL-23 Inhibitor Therapy for Psoriasis
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.
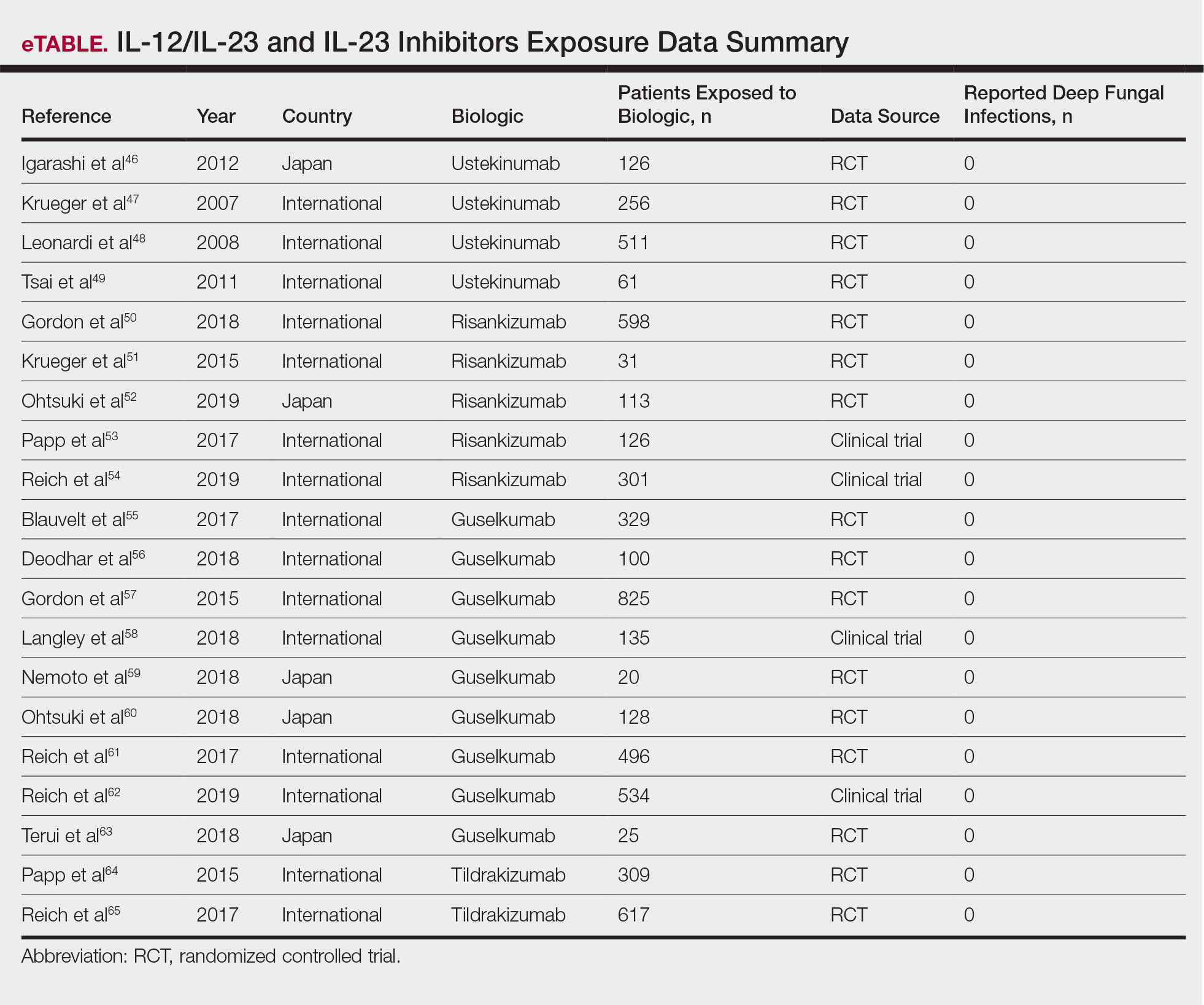
IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Practice Points
- The use of IL-17, IL-12/IL-23, and IL-23 inhibitors for psoriasis and other inflammatory conditions does not appear to increase the risk for deep fungal infections.
- Physicians should still be cautiously optimistic in prescribing these medications, as IL-17 and IL-23 play a central role in immunologic defenses, particularly against fungi.
- A high index of suspicion should be maintained for patients from endemic areas who are being treated with biologics.
Tumor Necrosis Factor Inhibitors May Reduce Cardiovascular Morbidity in Patients With Psoriasis
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to Clinical Practice
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.
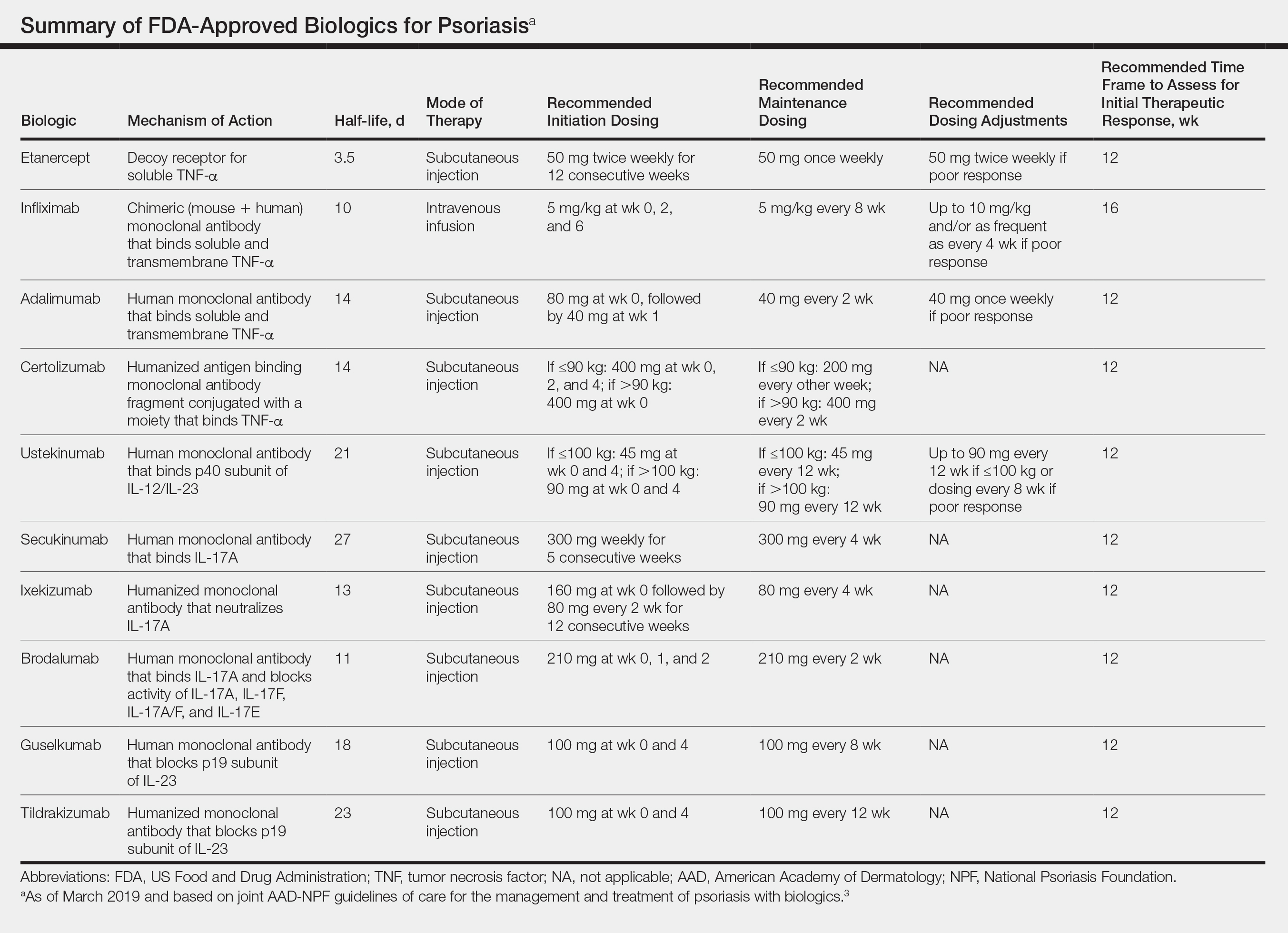
In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.

In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.

In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
Practice Points
- There are currently 11 biologics approved for psoriasis, but there is no first-line or optimalbiologic. The choice must be made using clinical judgment based on a variety of medical and social factors.
- Frequent assessment for efficacy of and adverse events due to biologic therapy is warranted, as lack of response, loss of response, or severe side effects may warrant addition of concurrent therapies or switching to a different biologic.
- There are important considerations to make when immunizing and planning for surgery in patients on biologics.