User login
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 3: Oral Therapies
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
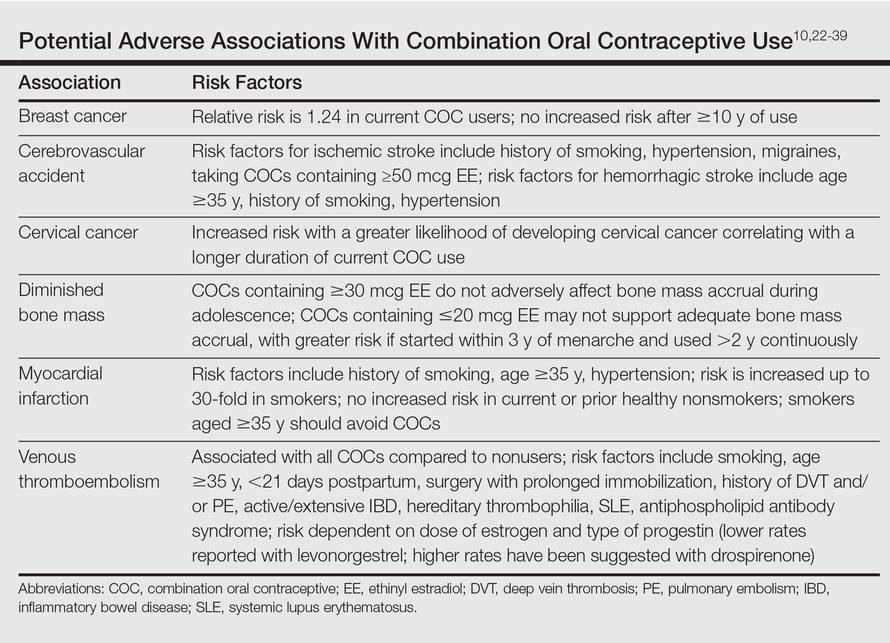
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33
The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33
The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33
The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Practice Points
- Use of combination oral contraceptives to treat acne vulgaris (AV) in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception.
- Spironolactone is widely accepted as an oral agent that can be effective in treating adult women with AV and may be used in combination with other therapies.
- Monotherapy with oral antibiotics should be avoided in the treatment of adult women with AV, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.
- Oral isotretinoin use in adult women with AV warrants strict adherence to pregnancy prevention measures and requirements set forth by the federally mandated iPLEDGE™ risk management program.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 1: Overview, Clinical Characteristics, and Laboratory Evaluation
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
Practice Points
- Acne in adult women is common and may persist beyond the adolescent years or may be late in onset with emergence usually during the early to mid-20s.
- Adult women with acne often are frustrated, as they perceive it as a disorder of teenagers and are perplexed by its presence later in life. They often are distressed by unpredictable flares as well as difficulty with covering lesions and associated dyschromia and scarring.
- Clinical patterns of acne in adult women are mixed inflammatory and comedonal facial acne or a U-shaped pattern of inflammatory lesions involving the lower face and neck.
- Laboratory testing is not considered mandatory in all cases. The clinician is encouraged to carefully evaluate each case and determine if further evaluation to detect a cause of androgen excess is warranted.
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 5: A Guide on the Management of Rosacea
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 4: A Status Report on Physical Modalities and Devices
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 3: A Status Report on Systemic Therapies
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 2: A Status Report on Topical Agents
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 1: A Status Report on the Disease State, General Measures, and Adjunctive Skin Care
Test your knowledge on consensus recommendations for rosacea with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.
Standard Management Options for Rosacea, Part 2: Options According to Subtype
Management of Rosacea by Subtype
The management of rosacea should be tailored to address the individual signs and symptoms of each patient and often may be keyed to subtypes and levels of severity while noting that patients often experience more than one subtype concurrently.1 Using the standard grading system, primary features of each subtype are graded as mild, moderate, or severe (grades 1–3, respectively), and most secondary signs and symptoms are graded as simply present or absent (Tables 1–4, PLEASE REFER TO THE PDF TO VIEW THE TABLES). All patients should be advised of proper skin care procedures, including the use of sunscreen as well as avoidance of environmental and lifestyle factors that may affect their individual cases.
Subtype 1: Erythematotelangiectatic Rosacea
Erythematotelangiectatic rosacea is primarily characterized by flushing and persistent erythema of the central face. The appearance of telangiectases is common but not essential to the diagnosis, while burning and stinging sensations, edema, and roughness or scaling are common secondary features. Patients with this subtype often have a history of flushing alone.
Because this subtype of rosacea may be difficult to treat, identification and avoidance of environmental and lifestyle triggers to minimize flushing and irritation of the skin may be especially important.1
Although no drugs to reduce flushing have been approved by the US Food and Drug Administration (FDA), off-label use of certain medications may potentially have a moderating effect for grades 2 and 3 flushing. It should be noted, however, that there are no broad-spectrumantiflushing medications, and those specific to the cause of the flushing should be chosen.
Flushing is a phenomenon of vasodilation that can be considered an abnormality of cutaneous vascular smooth muscle control. Vascular smooth muscle is controlled by circulating vasoactive agents or by autonomic nerves.
Circulating vasoactive agents are associated with dry flushing and may be exogenous (eg, alcohol, calcium channel-blocking agents, and nicotinic acid [niacin]) or endogenous (eg, histamine and prostaglandins). Management options to mediate flushing caused by endogenous agents may include aspirin, indomethacin, or other nonsteroidal anti-inflammatory drugs that also may reduce erythema. Antihistamines may be prescribed to reduce flushing related to histamines produced endogenously or exogenously by certain foods.2
When vasodilation is controlled by autonomic nerves, it is accompanied by sweating.2 This flushing usually results from heat in the ambient surroundings or from exercise or hot drinks, for example. In this case, flushing may be reduced by cooling the neck and face with a cold wet towel or a fan. Ice chips held in the mouth and ingestion of ice water may be effective.2 Flushing also may be diminished, of course, by avoidance of other potential environmental and lifestyle factors.1
In severe cases, the alpha 2 agonist clonidine or a beta-blocker such as nadolol may sometimes reduce neurally mediated flushing.2 For women with menopausal flushing, hormone replacement therapy prescribed by a gynecologist or primary care physician could be considered but should be used with caution.
Flushing also may have emotional origins for some patients, and these individuals may additionally benefit from psychological counseling or biofeedback.
Telangiectases and background erythema are commonly treated with laser therapy,3-8 including long-pulsed dye, potassium-titanyl-phosphate, and diode lasers, which have been associated with little or no purpura.4 They also may be reduced by intense pulsed light therapy,7,9 and electrocautery is an additional option for telangiectasia. Although published clinical data are limited, lasers and intense pulsed light also may be used to reduce flushing.6
The appearance of flushing, erythema, and telangiectases also may be concealed with cosmetics.1 In addition, burning, stinging, roughness, and/or scaling may be minimized by selecting appropriate over-the-counter products, including nonirritating cosmetics, nonsoap cleansers, moisturizers, and appropriate cleansing techniques. Sunblocks or sunscreens may be particularly important.
Subtype 2: Papulopustular Rosacea
This subtype is characterized by persistent central facial erythema with transient central facial papules or pustules, or both (Figure, PLEASE REFER TO THE PDF TO VIEW THE FiGURE). In these patients, topical therapies and oral antibiotics are prescribed, though the modes of action have not been definitively established.
Topical therapies FDA approved for the treatment of rosacea including metronidazole and azelaic acid as well as topical sodium sulfacetamide–sulfur may be used alone or in conjunction with oral therapy administered initially or at any point during treatment. A controlled-release formulation of oral doxycycline is FDA approved for rosacea with low plasma levels that do not exert antimicrobial effects while retaining anti-inflammatory activity.10 Topical therapy and/or a controlled-release oral therapy for rosacea may be used for grades 1 and 2 disease. For grade 3, an oral antibiotic may be used initially with a topical therapy to bring the disorder under immediate control. Once remission has been achieved, it often may be maintained on a long-term basis with a topical or controlled-release agent alone for an indefinite period.11
In some cases, oral drug therapy for grades 2 and 3and/or in patients with ocular involvement may consist of off-label systemic tetracycline (or other members of the tetracycline family) administered as 1 g/d in divided doses for 2 to 3 weeks, followed by 0.5 g/d for 2 to 3 weeks.12 Some physicians may prescribe higher doses, longer courses, or other tetracyclines such as doxycycline or minocycline.
In refractory cases, off label oral trimethoprim-sulfamethoxazole, trimethoprim alone, metronidazole, erythromycin, ampicillin, clindamycin, or dapsone may be prescribed. Off-label isotretinoin reportedly may be effective, especially in otherwise refractory cases or when the patulous follicles of incipient rhinophyma are present. Use of isotretinoin requires careful monitoring, and long-lasting remission is not common.
Additional off-label alternatives for refractory rosacea may include other antibacterial agents, mild topical retinoids, or adapalene.13,14 It also has been suggested in isolated reports that drugs eradicating Demodex folliculorum may play a role in treating certain cases of papulopustular rosacea, including topical permethrin; systemic ivermectin; and topical crotamiton, sulfur, and lindane.15 Use on the face for patients with rosacea is off label, and if prescribed, patients should be cautioned about the irritation potential of these agents.
While there was historical speculation on the treatment of Helicobacter pylori to manage rosacea, studies found no substantial difference in the abatement of rosacea following H pylori treatment compared with patients in placebo control groups.16,17
In certain exceptional cases, short-term use of a low-strength topical steroid may be considered for rapid resolution of inflammation. However, long-term use of these agents often produces rosacealike manifestations, commonly called steroid-induced rosacea, and therefore should be avoided. Topical calcineurin inhibitors such as tacrolimus and pimecrolimus also may be of value in treating erythema from active inflammation,12 though they have been reported to induce a rosacealike eruption.18-21 Topical retinoids have been recommended by some to repair the dermis by decreasing abnormal elastin, increasing collagen, increasing glycosaminoglycan, and decreasing telangiectases.22
Possible burning and stinging sensations may be dealt with as described for erythematotelangiectatic rosacea.
Subtype 3: Phymatous Rosacea
In addition to a primary feature of rosacea (flushing, erythema, telangiectases, papules or pustules), phymatous rosacea may include skin thickening, irregular surface nodularities, and patulous follicles. Enlargement commonly occurs on the nose (rhinophyma), though other affected locations may include the chin, forehead, cheeks, and ears.
Management options for grade 1 phymatous rosacea, with patulous follicles but no contour changes, include topical and systemic antibiotics if inflammatory lesions are present. Isotretinoin has been demonstrated to decrease nasal volume in rhinophyma, especially in younger patients with less advanced disease, though volume may increase again after therapy is stopped.22,23 During isotretinoin therapy, numerous large sebaceous glands were reported to be diminished in size and number.22 There also is evidence that topical retinoids may decrease fibrosis, elastosis, and sebaceous gland hypertrophy.24-26
Grades 2—change in contour without a nodular component—and 3—change in contour with a nodular component—phymatous rosacea may require surgical therapy, such as cryosurgery, radiofrequency ablation, electrosurgery, heated scalpel, electrocautery, tangential excision combined with scissor sculpturing, skin grafting, and dermabrasion. CO2 or erbium:YAG lasers may be used as a bloodless scalpel to remove excess tissue and recontour the nose. Fractional resurfacing can be of value in mild cases.
Subtype 4: Ocular Rosacea
The common presentations of ocular rosacea are a watery or bloodshot appearance, foreign body sensation, burning or stinging, dryness, itching, light sensitivity, and blurred vision. A history of styes (chalazion, hordeolum) is a strong indication, as well as dry eye, recurrent conjunctivitis, or blepharitis. Telangiectases of the eyelid margins or lid and periocular erythema also may be present.
The meibomian glands typically are obstructed and often may be blocked. The formation of collarettes, narrow rims of loosened keratin around the base of the eyelashes, is common. A gritty granular symptom indicates damage to the ocular surface. Undiagnosed ocular rosacea may present with recurring inflammation such as episcleritis, iritis,
and keratitis.
Ocular rosacea may appear in advance of the cutaneous form, and more than 60% of patients with cutaneous rosacea also may have ocular involvement. Treatment of cutaneous rosacea alone may be inadequate in lessening the risk for vision loss resulting from ocular rosacea, and referral to an ophthalmologist may be needed.
Treatment of grades 1 and 2 ocular rosacea may initially include artificial tears, and on a long-term basis, the patient should apply a warm compress and cleanse the eyelashes twice daily with baby shampoo on a wet washcloth rubbed onto the upper and lower eyelashes of the closed eyes. Antibiotic ointment may be appropriate to decrease the presence of Propionibacterium acnes, Staphylococcus epidermidis, and Staphylococcus aureus, and to soften any collarettes, allowing easy removal by the patient during eyelash hygiene. An oral tetracycline such as low-dose doxycycline may be necessary, and for grade 3 ocular rosacea, a topical steroid, cyclosporine ophthalmic emulsion, or alternative oral medications may be prescribed by the ophthalmologist. Any corneal ulceration requires immediate attention by an ophthalmologist, as it may involve loss of visual acuity.
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively known and the etiology and pathogenesis of rosacea are more completely understood. Meanwhile, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments—The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
- Odom R, Dahl M, Dover J, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 1: overview and broad spectrum of care. Cutis. 2009;84:43-47.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Mark KA, Sparacio RM, Voigt A, et al. Objective and quantitative improvement of rosacea-associated erythema after intense pulsed light treatment. Dermatol Surg. 2003;29:600-604.
- Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Bikowski JB. The pharmacologic therapy of rosacea: a paradigm shift in progress. Cutis. 2005;75(suppl 3):27-32.
- Altinyazar HC, Koca R, Tekin NS, et al. Adapalene vs. metronidazole gel for the treatment of rosacea. Int J Dermatol. 2005;44:252-255.
- Scheinfeld NS. Rosacea. Skinmed. 2006;5:191-194.
- Scheinfeld N. When rosacea resists standard therapies. Skin & Aging. 2006;8:46-48.
- Bamford JT, Tilden RL, Blankush JL, et al. Effect of treatment of Helicobacter pylori infection on rosacea. Arch Dermatol. 1999;135:659-663.
- Herr H, You CH. Relationship between Helicobacter pylori and rosacea: it may be a myth. J Korean Med Sci. 2000;15:551-554.
- Gorman CR, White SW. Rosaceiform dermatitis as a complication of treatment of facial seborrheic dermatitis with 1% pimecrolimus cream [letter]. Arch Dermatol. 2005;141:1168.
- El Sayed F, Ammoury A, Dhaybi R, et al. Rosaceiform eruption to pimecrolimus [letter]. J Am Acad Dermatol. 2005;54:548-550.
- Antille C, Saurat JH, Lübbe J. Induction of rosaceiform dermatitis during treatment of facial inflammatory dermatoses with tacrolimus ointment. Arch Dermatol. 2004;140:457-460.
- Bernard LA, Cunningham BB, Al-Suwaidan S, et al. A rosacea-like granulomatous eruption in a patient using tacrolimus ointment for atopic dermatitis. Arch Dermatol. 2003;139:229-231.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- Daly TJ, Weston WL. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. J Am Acad Dermatol. 1986;15(4, pt 2):900-902.
- Schmidt JB, Gebhart W, Raff M, et al. 13-cis-retinoic acid in rosacea. clinical and laboratory findings. Acta Derm Venereol. 1984;64:15-21.
- Yamamoto O, Bhawan J, Solares G, et al. Ultrastructural effects of topical tretinoin on dermo-epidermal junction and papillary dermis in photodamaged skin. a controlled study. Exp Dermatol. 1995;4:146-154.
Management of Rosacea by Subtype
The management of rosacea should be tailored to address the individual signs and symptoms of each patient and often may be keyed to subtypes and levels of severity while noting that patients often experience more than one subtype concurrently.1 Using the standard grading system, primary features of each subtype are graded as mild, moderate, or severe (grades 1–3, respectively), and most secondary signs and symptoms are graded as simply present or absent (Tables 1–4, PLEASE REFER TO THE PDF TO VIEW THE TABLES). All patients should be advised of proper skin care procedures, including the use of sunscreen as well as avoidance of environmental and lifestyle factors that may affect their individual cases.
Subtype 1: Erythematotelangiectatic Rosacea
Erythematotelangiectatic rosacea is primarily characterized by flushing and persistent erythema of the central face. The appearance of telangiectases is common but not essential to the diagnosis, while burning and stinging sensations, edema, and roughness or scaling are common secondary features. Patients with this subtype often have a history of flushing alone.
Because this subtype of rosacea may be difficult to treat, identification and avoidance of environmental and lifestyle triggers to minimize flushing and irritation of the skin may be especially important.1
Although no drugs to reduce flushing have been approved by the US Food and Drug Administration (FDA), off-label use of certain medications may potentially have a moderating effect for grades 2 and 3 flushing. It should be noted, however, that there are no broad-spectrumantiflushing medications, and those specific to the cause of the flushing should be chosen.
Flushing is a phenomenon of vasodilation that can be considered an abnormality of cutaneous vascular smooth muscle control. Vascular smooth muscle is controlled by circulating vasoactive agents or by autonomic nerves.
Circulating vasoactive agents are associated with dry flushing and may be exogenous (eg, alcohol, calcium channel-blocking agents, and nicotinic acid [niacin]) or endogenous (eg, histamine and prostaglandins). Management options to mediate flushing caused by endogenous agents may include aspirin, indomethacin, or other nonsteroidal anti-inflammatory drugs that also may reduce erythema. Antihistamines may be prescribed to reduce flushing related to histamines produced endogenously or exogenously by certain foods.2
When vasodilation is controlled by autonomic nerves, it is accompanied by sweating.2 This flushing usually results from heat in the ambient surroundings or from exercise or hot drinks, for example. In this case, flushing may be reduced by cooling the neck and face with a cold wet towel or a fan. Ice chips held in the mouth and ingestion of ice water may be effective.2 Flushing also may be diminished, of course, by avoidance of other potential environmental and lifestyle factors.1
In severe cases, the alpha 2 agonist clonidine or a beta-blocker such as nadolol may sometimes reduce neurally mediated flushing.2 For women with menopausal flushing, hormone replacement therapy prescribed by a gynecologist or primary care physician could be considered but should be used with caution.
Flushing also may have emotional origins for some patients, and these individuals may additionally benefit from psychological counseling or biofeedback.
Telangiectases and background erythema are commonly treated with laser therapy,3-8 including long-pulsed dye, potassium-titanyl-phosphate, and diode lasers, which have been associated with little or no purpura.4 They also may be reduced by intense pulsed light therapy,7,9 and electrocautery is an additional option for telangiectasia. Although published clinical data are limited, lasers and intense pulsed light also may be used to reduce flushing.6
The appearance of flushing, erythema, and telangiectases also may be concealed with cosmetics.1 In addition, burning, stinging, roughness, and/or scaling may be minimized by selecting appropriate over-the-counter products, including nonirritating cosmetics, nonsoap cleansers, moisturizers, and appropriate cleansing techniques. Sunblocks or sunscreens may be particularly important.
Subtype 2: Papulopustular Rosacea
This subtype is characterized by persistent central facial erythema with transient central facial papules or pustules, or both (Figure, PLEASE REFER TO THE PDF TO VIEW THE FiGURE). In these patients, topical therapies and oral antibiotics are prescribed, though the modes of action have not been definitively established.
Topical therapies FDA approved for the treatment of rosacea including metronidazole and azelaic acid as well as topical sodium sulfacetamide–sulfur may be used alone or in conjunction with oral therapy administered initially or at any point during treatment. A controlled-release formulation of oral doxycycline is FDA approved for rosacea with low plasma levels that do not exert antimicrobial effects while retaining anti-inflammatory activity.10 Topical therapy and/or a controlled-release oral therapy for rosacea may be used for grades 1 and 2 disease. For grade 3, an oral antibiotic may be used initially with a topical therapy to bring the disorder under immediate control. Once remission has been achieved, it often may be maintained on a long-term basis with a topical or controlled-release agent alone for an indefinite period.11
In some cases, oral drug therapy for grades 2 and 3and/or in patients with ocular involvement may consist of off-label systemic tetracycline (or other members of the tetracycline family) administered as 1 g/d in divided doses for 2 to 3 weeks, followed by 0.5 g/d for 2 to 3 weeks.12 Some physicians may prescribe higher doses, longer courses, or other tetracyclines such as doxycycline or minocycline.
In refractory cases, off label oral trimethoprim-sulfamethoxazole, trimethoprim alone, metronidazole, erythromycin, ampicillin, clindamycin, or dapsone may be prescribed. Off-label isotretinoin reportedly may be effective, especially in otherwise refractory cases or when the patulous follicles of incipient rhinophyma are present. Use of isotretinoin requires careful monitoring, and long-lasting remission is not common.
Additional off-label alternatives for refractory rosacea may include other antibacterial agents, mild topical retinoids, or adapalene.13,14 It also has been suggested in isolated reports that drugs eradicating Demodex folliculorum may play a role in treating certain cases of papulopustular rosacea, including topical permethrin; systemic ivermectin; and topical crotamiton, sulfur, and lindane.15 Use on the face for patients with rosacea is off label, and if prescribed, patients should be cautioned about the irritation potential of these agents.
While there was historical speculation on the treatment of Helicobacter pylori to manage rosacea, studies found no substantial difference in the abatement of rosacea following H pylori treatment compared with patients in placebo control groups.16,17
In certain exceptional cases, short-term use of a low-strength topical steroid may be considered for rapid resolution of inflammation. However, long-term use of these agents often produces rosacealike manifestations, commonly called steroid-induced rosacea, and therefore should be avoided. Topical calcineurin inhibitors such as tacrolimus and pimecrolimus also may be of value in treating erythema from active inflammation,12 though they have been reported to induce a rosacealike eruption.18-21 Topical retinoids have been recommended by some to repair the dermis by decreasing abnormal elastin, increasing collagen, increasing glycosaminoglycan, and decreasing telangiectases.22
Possible burning and stinging sensations may be dealt with as described for erythematotelangiectatic rosacea.
Subtype 3: Phymatous Rosacea
In addition to a primary feature of rosacea (flushing, erythema, telangiectases, papules or pustules), phymatous rosacea may include skin thickening, irregular surface nodularities, and patulous follicles. Enlargement commonly occurs on the nose (rhinophyma), though other affected locations may include the chin, forehead, cheeks, and ears.
Management options for grade 1 phymatous rosacea, with patulous follicles but no contour changes, include topical and systemic antibiotics if inflammatory lesions are present. Isotretinoin has been demonstrated to decrease nasal volume in rhinophyma, especially in younger patients with less advanced disease, though volume may increase again after therapy is stopped.22,23 During isotretinoin therapy, numerous large sebaceous glands were reported to be diminished in size and number.22 There also is evidence that topical retinoids may decrease fibrosis, elastosis, and sebaceous gland hypertrophy.24-26
Grades 2—change in contour without a nodular component—and 3—change in contour with a nodular component—phymatous rosacea may require surgical therapy, such as cryosurgery, radiofrequency ablation, electrosurgery, heated scalpel, electrocautery, tangential excision combined with scissor sculpturing, skin grafting, and dermabrasion. CO2 or erbium:YAG lasers may be used as a bloodless scalpel to remove excess tissue and recontour the nose. Fractional resurfacing can be of value in mild cases.
Subtype 4: Ocular Rosacea
The common presentations of ocular rosacea are a watery or bloodshot appearance, foreign body sensation, burning or stinging, dryness, itching, light sensitivity, and blurred vision. A history of styes (chalazion, hordeolum) is a strong indication, as well as dry eye, recurrent conjunctivitis, or blepharitis. Telangiectases of the eyelid margins or lid and periocular erythema also may be present.
The meibomian glands typically are obstructed and often may be blocked. The formation of collarettes, narrow rims of loosened keratin around the base of the eyelashes, is common. A gritty granular symptom indicates damage to the ocular surface. Undiagnosed ocular rosacea may present with recurring inflammation such as episcleritis, iritis,
and keratitis.
Ocular rosacea may appear in advance of the cutaneous form, and more than 60% of patients with cutaneous rosacea also may have ocular involvement. Treatment of cutaneous rosacea alone may be inadequate in lessening the risk for vision loss resulting from ocular rosacea, and referral to an ophthalmologist may be needed.
Treatment of grades 1 and 2 ocular rosacea may initially include artificial tears, and on a long-term basis, the patient should apply a warm compress and cleanse the eyelashes twice daily with baby shampoo on a wet washcloth rubbed onto the upper and lower eyelashes of the closed eyes. Antibiotic ointment may be appropriate to decrease the presence of Propionibacterium acnes, Staphylococcus epidermidis, and Staphylococcus aureus, and to soften any collarettes, allowing easy removal by the patient during eyelash hygiene. An oral tetracycline such as low-dose doxycycline may be necessary, and for grade 3 ocular rosacea, a topical steroid, cyclosporine ophthalmic emulsion, or alternative oral medications may be prescribed by the ophthalmologist. Any corneal ulceration requires immediate attention by an ophthalmologist, as it may involve loss of visual acuity.
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively known and the etiology and pathogenesis of rosacea are more completely understood. Meanwhile, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments—The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
Management of Rosacea by Subtype
The management of rosacea should be tailored to address the individual signs and symptoms of each patient and often may be keyed to subtypes and levels of severity while noting that patients often experience more than one subtype concurrently.1 Using the standard grading system, primary features of each subtype are graded as mild, moderate, or severe (grades 1–3, respectively), and most secondary signs and symptoms are graded as simply present or absent (Tables 1–4, PLEASE REFER TO THE PDF TO VIEW THE TABLES). All patients should be advised of proper skin care procedures, including the use of sunscreen as well as avoidance of environmental and lifestyle factors that may affect their individual cases.
Subtype 1: Erythematotelangiectatic Rosacea
Erythematotelangiectatic rosacea is primarily characterized by flushing and persistent erythema of the central face. The appearance of telangiectases is common but not essential to the diagnosis, while burning and stinging sensations, edema, and roughness or scaling are common secondary features. Patients with this subtype often have a history of flushing alone.
Because this subtype of rosacea may be difficult to treat, identification and avoidance of environmental and lifestyle triggers to minimize flushing and irritation of the skin may be especially important.1
Although no drugs to reduce flushing have been approved by the US Food and Drug Administration (FDA), off-label use of certain medications may potentially have a moderating effect for grades 2 and 3 flushing. It should be noted, however, that there are no broad-spectrumantiflushing medications, and those specific to the cause of the flushing should be chosen.
Flushing is a phenomenon of vasodilation that can be considered an abnormality of cutaneous vascular smooth muscle control. Vascular smooth muscle is controlled by circulating vasoactive agents or by autonomic nerves.
Circulating vasoactive agents are associated with dry flushing and may be exogenous (eg, alcohol, calcium channel-blocking agents, and nicotinic acid [niacin]) or endogenous (eg, histamine and prostaglandins). Management options to mediate flushing caused by endogenous agents may include aspirin, indomethacin, or other nonsteroidal anti-inflammatory drugs that also may reduce erythema. Antihistamines may be prescribed to reduce flushing related to histamines produced endogenously or exogenously by certain foods.2
When vasodilation is controlled by autonomic nerves, it is accompanied by sweating.2 This flushing usually results from heat in the ambient surroundings or from exercise or hot drinks, for example. In this case, flushing may be reduced by cooling the neck and face with a cold wet towel or a fan. Ice chips held in the mouth and ingestion of ice water may be effective.2 Flushing also may be diminished, of course, by avoidance of other potential environmental and lifestyle factors.1
In severe cases, the alpha 2 agonist clonidine or a beta-blocker such as nadolol may sometimes reduce neurally mediated flushing.2 For women with menopausal flushing, hormone replacement therapy prescribed by a gynecologist or primary care physician could be considered but should be used with caution.
Flushing also may have emotional origins for some patients, and these individuals may additionally benefit from psychological counseling or biofeedback.
Telangiectases and background erythema are commonly treated with laser therapy,3-8 including long-pulsed dye, potassium-titanyl-phosphate, and diode lasers, which have been associated with little or no purpura.4 They also may be reduced by intense pulsed light therapy,7,9 and electrocautery is an additional option for telangiectasia. Although published clinical data are limited, lasers and intense pulsed light also may be used to reduce flushing.6
The appearance of flushing, erythema, and telangiectases also may be concealed with cosmetics.1 In addition, burning, stinging, roughness, and/or scaling may be minimized by selecting appropriate over-the-counter products, including nonirritating cosmetics, nonsoap cleansers, moisturizers, and appropriate cleansing techniques. Sunblocks or sunscreens may be particularly important.
Subtype 2: Papulopustular Rosacea
This subtype is characterized by persistent central facial erythema with transient central facial papules or pustules, or both (Figure, PLEASE REFER TO THE PDF TO VIEW THE FiGURE). In these patients, topical therapies and oral antibiotics are prescribed, though the modes of action have not been definitively established.
Topical therapies FDA approved for the treatment of rosacea including metronidazole and azelaic acid as well as topical sodium sulfacetamide–sulfur may be used alone or in conjunction with oral therapy administered initially or at any point during treatment. A controlled-release formulation of oral doxycycline is FDA approved for rosacea with low plasma levels that do not exert antimicrobial effects while retaining anti-inflammatory activity.10 Topical therapy and/or a controlled-release oral therapy for rosacea may be used for grades 1 and 2 disease. For grade 3, an oral antibiotic may be used initially with a topical therapy to bring the disorder under immediate control. Once remission has been achieved, it often may be maintained on a long-term basis with a topical or controlled-release agent alone for an indefinite period.11
In some cases, oral drug therapy for grades 2 and 3and/or in patients with ocular involvement may consist of off-label systemic tetracycline (or other members of the tetracycline family) administered as 1 g/d in divided doses for 2 to 3 weeks, followed by 0.5 g/d for 2 to 3 weeks.12 Some physicians may prescribe higher doses, longer courses, or other tetracyclines such as doxycycline or minocycline.
In refractory cases, off label oral trimethoprim-sulfamethoxazole, trimethoprim alone, metronidazole, erythromycin, ampicillin, clindamycin, or dapsone may be prescribed. Off-label isotretinoin reportedly may be effective, especially in otherwise refractory cases or when the patulous follicles of incipient rhinophyma are present. Use of isotretinoin requires careful monitoring, and long-lasting remission is not common.
Additional off-label alternatives for refractory rosacea may include other antibacterial agents, mild topical retinoids, or adapalene.13,14 It also has been suggested in isolated reports that drugs eradicating Demodex folliculorum may play a role in treating certain cases of papulopustular rosacea, including topical permethrin; systemic ivermectin; and topical crotamiton, sulfur, and lindane.15 Use on the face for patients with rosacea is off label, and if prescribed, patients should be cautioned about the irritation potential of these agents.
While there was historical speculation on the treatment of Helicobacter pylori to manage rosacea, studies found no substantial difference in the abatement of rosacea following H pylori treatment compared with patients in placebo control groups.16,17
In certain exceptional cases, short-term use of a low-strength topical steroid may be considered for rapid resolution of inflammation. However, long-term use of these agents often produces rosacealike manifestations, commonly called steroid-induced rosacea, and therefore should be avoided. Topical calcineurin inhibitors such as tacrolimus and pimecrolimus also may be of value in treating erythema from active inflammation,12 though they have been reported to induce a rosacealike eruption.18-21 Topical retinoids have been recommended by some to repair the dermis by decreasing abnormal elastin, increasing collagen, increasing glycosaminoglycan, and decreasing telangiectases.22
Possible burning and stinging sensations may be dealt with as described for erythematotelangiectatic rosacea.
Subtype 3: Phymatous Rosacea
In addition to a primary feature of rosacea (flushing, erythema, telangiectases, papules or pustules), phymatous rosacea may include skin thickening, irregular surface nodularities, and patulous follicles. Enlargement commonly occurs on the nose (rhinophyma), though other affected locations may include the chin, forehead, cheeks, and ears.
Management options for grade 1 phymatous rosacea, with patulous follicles but no contour changes, include topical and systemic antibiotics if inflammatory lesions are present. Isotretinoin has been demonstrated to decrease nasal volume in rhinophyma, especially in younger patients with less advanced disease, though volume may increase again after therapy is stopped.22,23 During isotretinoin therapy, numerous large sebaceous glands were reported to be diminished in size and number.22 There also is evidence that topical retinoids may decrease fibrosis, elastosis, and sebaceous gland hypertrophy.24-26
Grades 2—change in contour without a nodular component—and 3—change in contour with a nodular component—phymatous rosacea may require surgical therapy, such as cryosurgery, radiofrequency ablation, electrosurgery, heated scalpel, electrocautery, tangential excision combined with scissor sculpturing, skin grafting, and dermabrasion. CO2 or erbium:YAG lasers may be used as a bloodless scalpel to remove excess tissue and recontour the nose. Fractional resurfacing can be of value in mild cases.
Subtype 4: Ocular Rosacea
The common presentations of ocular rosacea are a watery or bloodshot appearance, foreign body sensation, burning or stinging, dryness, itching, light sensitivity, and blurred vision. A history of styes (chalazion, hordeolum) is a strong indication, as well as dry eye, recurrent conjunctivitis, or blepharitis. Telangiectases of the eyelid margins or lid and periocular erythema also may be present.
The meibomian glands typically are obstructed and often may be blocked. The formation of collarettes, narrow rims of loosened keratin around the base of the eyelashes, is common. A gritty granular symptom indicates damage to the ocular surface. Undiagnosed ocular rosacea may present with recurring inflammation such as episcleritis, iritis,
and keratitis.
Ocular rosacea may appear in advance of the cutaneous form, and more than 60% of patients with cutaneous rosacea also may have ocular involvement. Treatment of cutaneous rosacea alone may be inadequate in lessening the risk for vision loss resulting from ocular rosacea, and referral to an ophthalmologist may be needed.
Treatment of grades 1 and 2 ocular rosacea may initially include artificial tears, and on a long-term basis, the patient should apply a warm compress and cleanse the eyelashes twice daily with baby shampoo on a wet washcloth rubbed onto the upper and lower eyelashes of the closed eyes. Antibiotic ointment may be appropriate to decrease the presence of Propionibacterium acnes, Staphylococcus epidermidis, and Staphylococcus aureus, and to soften any collarettes, allowing easy removal by the patient during eyelash hygiene. An oral tetracycline such as low-dose doxycycline may be necessary, and for grade 3 ocular rosacea, a topical steroid, cyclosporine ophthalmic emulsion, or alternative oral medications may be prescribed by the ophthalmologist. Any corneal ulceration requires immediate attention by an ophthalmologist, as it may involve loss of visual acuity.
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively known and the etiology and pathogenesis of rosacea are more completely understood. Meanwhile, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments—The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
- Odom R, Dahl M, Dover J, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 1: overview and broad spectrum of care. Cutis. 2009;84:43-47.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Mark KA, Sparacio RM, Voigt A, et al. Objective and quantitative improvement of rosacea-associated erythema after intense pulsed light treatment. Dermatol Surg. 2003;29:600-604.
- Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Bikowski JB. The pharmacologic therapy of rosacea: a paradigm shift in progress. Cutis. 2005;75(suppl 3):27-32.
- Altinyazar HC, Koca R, Tekin NS, et al. Adapalene vs. metronidazole gel for the treatment of rosacea. Int J Dermatol. 2005;44:252-255.
- Scheinfeld NS. Rosacea. Skinmed. 2006;5:191-194.
- Scheinfeld N. When rosacea resists standard therapies. Skin & Aging. 2006;8:46-48.
- Bamford JT, Tilden RL, Blankush JL, et al. Effect of treatment of Helicobacter pylori infection on rosacea. Arch Dermatol. 1999;135:659-663.
- Herr H, You CH. Relationship between Helicobacter pylori and rosacea: it may be a myth. J Korean Med Sci. 2000;15:551-554.
- Gorman CR, White SW. Rosaceiform dermatitis as a complication of treatment of facial seborrheic dermatitis with 1% pimecrolimus cream [letter]. Arch Dermatol. 2005;141:1168.
- El Sayed F, Ammoury A, Dhaybi R, et al. Rosaceiform eruption to pimecrolimus [letter]. J Am Acad Dermatol. 2005;54:548-550.
- Antille C, Saurat JH, Lübbe J. Induction of rosaceiform dermatitis during treatment of facial inflammatory dermatoses with tacrolimus ointment. Arch Dermatol. 2004;140:457-460.
- Bernard LA, Cunningham BB, Al-Suwaidan S, et al. A rosacea-like granulomatous eruption in a patient using tacrolimus ointment for atopic dermatitis. Arch Dermatol. 2003;139:229-231.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- Daly TJ, Weston WL. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. J Am Acad Dermatol. 1986;15(4, pt 2):900-902.
- Schmidt JB, Gebhart W, Raff M, et al. 13-cis-retinoic acid in rosacea. clinical and laboratory findings. Acta Derm Venereol. 1984;64:15-21.
- Yamamoto O, Bhawan J, Solares G, et al. Ultrastructural effects of topical tretinoin on dermo-epidermal junction and papillary dermis in photodamaged skin. a controlled study. Exp Dermatol. 1995;4:146-154.
- Odom R, Dahl M, Dover J, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 1: overview and broad spectrum of care. Cutis. 2009;84:43-47.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Mark KA, Sparacio RM, Voigt A, et al. Objective and quantitative improvement of rosacea-associated erythema after intense pulsed light treatment. Dermatol Surg. 2003;29:600-604.
- Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Bikowski JB. The pharmacologic therapy of rosacea: a paradigm shift in progress. Cutis. 2005;75(suppl 3):27-32.
- Altinyazar HC, Koca R, Tekin NS, et al. Adapalene vs. metronidazole gel for the treatment of rosacea. Int J Dermatol. 2005;44:252-255.
- Scheinfeld NS. Rosacea. Skinmed. 2006;5:191-194.
- Scheinfeld N. When rosacea resists standard therapies. Skin & Aging. 2006;8:46-48.
- Bamford JT, Tilden RL, Blankush JL, et al. Effect of treatment of Helicobacter pylori infection on rosacea. Arch Dermatol. 1999;135:659-663.
- Herr H, You CH. Relationship between Helicobacter pylori and rosacea: it may be a myth. J Korean Med Sci. 2000;15:551-554.
- Gorman CR, White SW. Rosaceiform dermatitis as a complication of treatment of facial seborrheic dermatitis with 1% pimecrolimus cream [letter]. Arch Dermatol. 2005;141:1168.
- El Sayed F, Ammoury A, Dhaybi R, et al. Rosaceiform eruption to pimecrolimus [letter]. J Am Acad Dermatol. 2005;54:548-550.
- Antille C, Saurat JH, Lübbe J. Induction of rosaceiform dermatitis during treatment of facial inflammatory dermatoses with tacrolimus ointment. Arch Dermatol. 2004;140:457-460.
- Bernard LA, Cunningham BB, Al-Suwaidan S, et al. A rosacea-like granulomatous eruption in a patient using tacrolimus ointment for atopic dermatitis. Arch Dermatol. 2003;139:229-231.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- Daly TJ, Weston WL. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. J Am Acad Dermatol. 1986;15(4, pt 2):900-902.
- Schmidt JB, Gebhart W, Raff M, et al. 13-cis-retinoic acid in rosacea. clinical and laboratory findings. Acta Derm Venereol. 1984;64:15-21.
- Yamamoto O, Bhawan J, Solares G, et al. Ultrastructural effects of topical tretinoin on dermo-epidermal junction and papillary dermis in photodamaged skin. a controlled study. Exp Dermatol. 1995;4:146-154.
Standard Management Options for Rosacea, Part 1: Overview and Broad Spectrum of Care
Rosacea is well established as a chronic typology or syndrome, primarily affecting the convexities of the central face (ie, cheeks, nose, chin, forehead) and often affecting the eyes. In 2002, the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea reported on a standard classification system that identified primary and secondary features of rosacea and described 4 common patterns of signs and symptoms designated as subtypes.1 In 2004, the committee published a standard grading system for assessing the relative severity of rosacea to enhance the utility of the classification system for researchers and clinicians.2
Developed and reviewed by 21 experts worldwide, these standard systems are essential to perform research; analyze results and compare data from different sources; and provide a common terminology and reference for the diagnosis, treatment, and assessment of results in clinical practice. Because present scientific knowledge of the etiology of rosacea is limited, these systems are considered provisional and are based on morphologic characteristics alone to avoid assumptions about pathogenesis and progression. They are intended to facilitate communication and ultimately the development of a research-based understanding of the disorder.
As a final step, the committee has developed standard management options based on these standard criteria to assist in providing optimal patient care. Because it is fundamental in the management of rosacea to consider the broad spectrum of potential therapies, the consensus committee and review panel have been expanded to include leading experts in dermatology, laser therapy, skin care, and ophthalmology. As with the standard classification and grading systems, the standard management options are considered provisional and may be expanded and updated as scientific knowledge increases and additional therapies become available.
Although rosacea encompasses various combinations of signs and symptoms, in most cases, some rather than all of these features appear in any given patient and often are characterized by remissions and exacerbations. Therefore, it is important to define the roles of respective treatment modalities as well as lifestyle management and skin care within the context of specific potential manifestations. In this way, an optimal management approach may be tailored for each individual patient.3,4
The standard management options are intended to serve as a menu of options rather than a treatment protocol. Although there is no cure for rosacea, its various signs and symptoms may be reduced or controlled with a range of therapeutic modalities, even though their actions may not be fully defined by clinical data.5 It should be noted that clinical trials are rarely a reflection of clinical practice because they are typically intended to discern only the contribution of a specific treatment.6 In practice, clinicians rarely rely on a single mode of care alone, and in the case of rosacea, factors such as proper skin care and avoidance of exacerbating factors may substantially improve results. Thus, patients often may experience better outcomes than might be suggested by clinical studies designed to isolate the effect of a single therapy.
Part 1 of this 2-part series will review the patient evaluation process and respective modalities of care.
Medical History
In addition to clinical observation of potential primary and secondary features of rosacea (Table 1, PLEASE REFER TO THE PDF TO VIEW THE TABLE), a medical history is needed to identify features that may not be visually evident or present at the time of the patient visit, to rule out alternative diagnoses, and to help identify potential environmental and lifestyle triggers. There is no laboratory test for rosacea and a biopsy is warranted only to rule out alternative diagnoses.
It may be difficult to clinically distinguish between the effects of chronic actinic damage on sun-sensitive skin (heliodermatitis) and subtype 1 (erythematotelangiectactic) rosacea. In some individuals, there may be overlapping features. A medical history may be especially useful in differentiating between erythematotelangiectactic rosacea and isolated photodamage. For example, any patient whose occupation or lifestyle has involved extensive sun exposure may experience chronic actinic damage, whereas patients with a history of flushing alone may be more likely to have rosacea. In addition, in the case of rosacea, erythema and telangiectasia tend to present with a central facial distribution.7 Other differential diagnoses include seborrheic dermatitis, lupus erythematosus, polycythemia vera, and carcinoid syndrome, with flushing mimicking rosacea.
A medical history also may be relevant for treatment purposes in distinguishing between dry flushing, which often is caused by exogenous or endogenous vasoactive agents, and wet flushing, which is accompanied by sweating that is regulated by the autonomic nervous system. Flushing can be further divided according to causes such as physical exertion, heat, or emotional reaction.8
Importantly, a medical history can uncover ocular involvement that may not be currently present or readily apparent from clinical observation as well as identify physical discomfort such as burning or stinging that may substantially affect quality of life for many patients.
Because rosacea affects facial appearance, its presence also may have considerable impact on an individual patient’s psychologic well-being and ability to interact socially or professionally. An assessment can help guide the physician toward providing an appropriate level of care.
Drug Therapy
The papules and pustules of rosacea, as well as nodules, plaques, or perilesional erythema, can be effectively treated in most patients with drugs that have been extensively studied in clinical trials and approved by the US Food and Drug Administration for rosacea, such as topical metronidazole, topical azelaic acid, and oral controlled-release doxycycline 40 mg, all approved for the treatment of inflammatory lesions of rosacea. In addition, the efficacy of topical sodium
sulfacetamide–sulfur is supported by many years of clinical experience in treating rosacea, though it was allowed to be marketed for rosacea prior to more stringent modern requirements for clinical studies and US Food and Drug Administration review. Options for the use of approved medications as well as off-label use of other medications such as oral tetracycline are reviewed in detail in part 2 of this series.9
Several oral antibiotics commonly are prescribed on an off-label basis for subtype 4 (ocular) rosacea. Moreover, when appropriate, the off-label use of other medical therapies may be administered to treat flushing and background erythema, which will be discussed in detail in part 2 of this series.9 The committee encourages further drug research aimed to improve the treatment of background erythema, which represents a great unmet clinical need in rosacea therapy.10
In all cases, physicians should review the package insert for prescribing information. This document is not intended to suggest the monitoring and actual dosing practices for drugs.
Laser and Light Therapy
The efficacy of laser therapy for the treatment of telangiectasia has been well established in clinical practice,11-16 and limited studies also have suggested that it may reduce erythema and flushing.11,15,17 Most lasers used to treat vascular components of rosacea have wavelengths in the 500 to 600 nm range and are known as nonablative (they do not destroy tissue). Recent developments using long-pulsed pulsed dye lasers,13 a technique of stacking pulses,18 or 532-nm potassium-titanyl-phosphate lasers19 may produce excellent improvement in erythema and telangiectasia without purpura.13
Polychromatic light-emitting devices such as intense pulsed light devices (515–1200 nm) also have been found to be effective in reducing erythema and telangiectasia.15,20
Ablative lasers, such as the 2.94-nm erbium:YAG or 10,600-nm CO2 lasers, destroy tissue and may be used to treat subtype 3 (phymatous) rosacea.3,12,21
Lifestyle Management
Signs and symptoms of rosacea often appear to be triggered by environmental or lifestyle factors, most related to flushing. Some of the most common rosacea triggers include sun exposure, emotional stress, hot or cold weather, wind, heavy exercise, alcohol consumption, hot baths, spicy foods, humidity, indoor heat, certain skin care products, heated beverages, certain cosmetics, medications, medical conditions, and certain foods (Table 2, PLEASE REFER TO THE PDF TO VIEW THE TABLE).22 However, triggers that may affect one patient may not affect another, and avoidance of every potential factor may be unnecessary as well as impractical.
An appropriate management strategy identifies and avoids only those lifestyle factors that trigger or exacerbate rosacea symptoms in each individual patient. To help identify a patient’s individual rosacea triggers, the patient can record daily contact with the most common rosacea triggers and other possible factors and then match them to flare-ups of signs and symptoms. In unscientific surveys of patients with rosacea who identified and avoided their personal rosacea triggers, more than 90% reported that their condition had improved in varying degrees.23
Adjunctive Care
Skin Care Products
Because patients with rosacea often have skin that is sensitive and easily irritated, causing redness, inflammation, and stinging, skin care is an important component of rosacea management.21,24 The goal of everyday skin care for patients with rosacea is to maintain the integrity of the skin barrier while avoiding agents that cause inflammation or flushing.
Complicating skin care is the typical heightened neurosensory response in many patients with rosacea who may experience stinging and burning from minor irritants more frequently than the general population. Patients may therefore be advised to select cleansers and moisturizers that do not irritate their skin.
Sunscreens or sunblocks effective against the full spectrum of UVA and UVB radiation can be especially important for patients with rosacea whose facial skin may be particularly susceptible to actinic damage and consequent rosacea flare-ups. A sun protection factor of 15 or higher is recommended, and physical blocks utilizing zinc or titanium dioxide may be effective if chemical sunscreens cause irritation.
A useful rule of thumb may be to select products for patients with rosacea that contain no sensory provoking ingredients, no volatile substances, no minor irritants or allergens, minimal botanical agents, and no unnecessary ingredients.
Cleansing Regimen
Patients should be informed that compliance with instructions on facial cleansing and topical medication application may be critical to avoiding irritation, burning, and stinging. They may be advised to wash the face gently with a nonirritating cleanser, avoiding the use of abrasive materials such as washcloths and loofahs. They also may be advised to blot, not rub, the face dry with a soft towel and wait up to 30 minutes for the face to completely dry before applying topical medication or other products, as stinging most often occurs when the skin is wet.6,8
After this routine is established and the face is not irritated, the patient can reduce the amount of time waiting to dry by 5 minutes every day to determine the shortest waiting time necessary for the individual patient.
Cosmetics
Cosmetics, especially those with a green or yellow tint, may be effective in reducing the appearance of redness. However, as with skin cleansers and moisturizers, care should be taken to minimize irritation.
Patients should be advised to avoid any products that cause burning, stinging, itching, or other discomfort. They also may be advised that waterproof cosmetics may be difficult to remove, requiring the use of harsh agents that may induce irritation.
New cosmetics should be regularly purchased to minimize microbial contamination and degradation. Brushes are preferred over sponges to avoid abrasion and because brushes can be easily cleaned to decrease bacterial contamination.24
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively established. Until the etiology and pathogenesis are more completely understood, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments
The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Powell FC. Rosacea. N Engl J Med. 2005;352:793-803.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.
- Wilkin JK. Use of topical products for maintaining remission in rosacea. Arch Dermatol. 1999;135:79-80.
- Odom R. Rosacea, acne rosacea, and actinic telangiectasia: in reply. J Am Acad Dermatol. 2005;53:1103-1104.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Odom R, Dahl M, Dover K, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 2: options according to subtype. Cutis. In press.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective a1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Goldberg DJ. Lasers and light sources for rosacea. Cutis. 2005;75(suppl 3):22-26, 33-36.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Tan SR, Tope WD. Pulsed dye laser treatment of rosacea improves erythema, symptomatology, and quality of life. J Am Acad Dermatol. 2004;51:592-599.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Railan D, Parlette EC, Uebelhoer NS, et al. Laser treatment of vascular lesions. Clin Dermatol. 2006;24:8-15.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Drake L, ed; National Rosacea Society. New survey pinpoints leading factors that trigger symptoms. Rosacea Review. Summer 2002. http://www.rosacea.org/rr/2002/summer/article_3.php. Accessed June 15, 2009.
- Drake L, ed; National Rosacea Society. Survey shows lifestyle changes help control rosacea flare-ups. Rosacea Review. Winter 1998. http://www.rosacea.org/rr/1998/winter/article_3.php. Accessed June 15, 2009.
- Draelos ZD. Cosmetics in acne and rosacea. Semin Cutan Med Surg. 2001;20:209-214.
Rosacea is well established as a chronic typology or syndrome, primarily affecting the convexities of the central face (ie, cheeks, nose, chin, forehead) and often affecting the eyes. In 2002, the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea reported on a standard classification system that identified primary and secondary features of rosacea and described 4 common patterns of signs and symptoms designated as subtypes.1 In 2004, the committee published a standard grading system for assessing the relative severity of rosacea to enhance the utility of the classification system for researchers and clinicians.2
Developed and reviewed by 21 experts worldwide, these standard systems are essential to perform research; analyze results and compare data from different sources; and provide a common terminology and reference for the diagnosis, treatment, and assessment of results in clinical practice. Because present scientific knowledge of the etiology of rosacea is limited, these systems are considered provisional and are based on morphologic characteristics alone to avoid assumptions about pathogenesis and progression. They are intended to facilitate communication and ultimately the development of a research-based understanding of the disorder.
As a final step, the committee has developed standard management options based on these standard criteria to assist in providing optimal patient care. Because it is fundamental in the management of rosacea to consider the broad spectrum of potential therapies, the consensus committee and review panel have been expanded to include leading experts in dermatology, laser therapy, skin care, and ophthalmology. As with the standard classification and grading systems, the standard management options are considered provisional and may be expanded and updated as scientific knowledge increases and additional therapies become available.
Although rosacea encompasses various combinations of signs and symptoms, in most cases, some rather than all of these features appear in any given patient and often are characterized by remissions and exacerbations. Therefore, it is important to define the roles of respective treatment modalities as well as lifestyle management and skin care within the context of specific potential manifestations. In this way, an optimal management approach may be tailored for each individual patient.3,4
The standard management options are intended to serve as a menu of options rather than a treatment protocol. Although there is no cure for rosacea, its various signs and symptoms may be reduced or controlled with a range of therapeutic modalities, even though their actions may not be fully defined by clinical data.5 It should be noted that clinical trials are rarely a reflection of clinical practice because they are typically intended to discern only the contribution of a specific treatment.6 In practice, clinicians rarely rely on a single mode of care alone, and in the case of rosacea, factors such as proper skin care and avoidance of exacerbating factors may substantially improve results. Thus, patients often may experience better outcomes than might be suggested by clinical studies designed to isolate the effect of a single therapy.
Part 1 of this 2-part series will review the patient evaluation process and respective modalities of care.
Medical History
In addition to clinical observation of potential primary and secondary features of rosacea (Table 1, PLEASE REFER TO THE PDF TO VIEW THE TABLE), a medical history is needed to identify features that may not be visually evident or present at the time of the patient visit, to rule out alternative diagnoses, and to help identify potential environmental and lifestyle triggers. There is no laboratory test for rosacea and a biopsy is warranted only to rule out alternative diagnoses.
It may be difficult to clinically distinguish between the effects of chronic actinic damage on sun-sensitive skin (heliodermatitis) and subtype 1 (erythematotelangiectactic) rosacea. In some individuals, there may be overlapping features. A medical history may be especially useful in differentiating between erythematotelangiectactic rosacea and isolated photodamage. For example, any patient whose occupation or lifestyle has involved extensive sun exposure may experience chronic actinic damage, whereas patients with a history of flushing alone may be more likely to have rosacea. In addition, in the case of rosacea, erythema and telangiectasia tend to present with a central facial distribution.7 Other differential diagnoses include seborrheic dermatitis, lupus erythematosus, polycythemia vera, and carcinoid syndrome, with flushing mimicking rosacea.
A medical history also may be relevant for treatment purposes in distinguishing between dry flushing, which often is caused by exogenous or endogenous vasoactive agents, and wet flushing, which is accompanied by sweating that is regulated by the autonomic nervous system. Flushing can be further divided according to causes such as physical exertion, heat, or emotional reaction.8
Importantly, a medical history can uncover ocular involvement that may not be currently present or readily apparent from clinical observation as well as identify physical discomfort such as burning or stinging that may substantially affect quality of life for many patients.
Because rosacea affects facial appearance, its presence also may have considerable impact on an individual patient’s psychologic well-being and ability to interact socially or professionally. An assessment can help guide the physician toward providing an appropriate level of care.
Drug Therapy
The papules and pustules of rosacea, as well as nodules, plaques, or perilesional erythema, can be effectively treated in most patients with drugs that have been extensively studied in clinical trials and approved by the US Food and Drug Administration for rosacea, such as topical metronidazole, topical azelaic acid, and oral controlled-release doxycycline 40 mg, all approved for the treatment of inflammatory lesions of rosacea. In addition, the efficacy of topical sodium
sulfacetamide–sulfur is supported by many years of clinical experience in treating rosacea, though it was allowed to be marketed for rosacea prior to more stringent modern requirements for clinical studies and US Food and Drug Administration review. Options for the use of approved medications as well as off-label use of other medications such as oral tetracycline are reviewed in detail in part 2 of this series.9
Several oral antibiotics commonly are prescribed on an off-label basis for subtype 4 (ocular) rosacea. Moreover, when appropriate, the off-label use of other medical therapies may be administered to treat flushing and background erythema, which will be discussed in detail in part 2 of this series.9 The committee encourages further drug research aimed to improve the treatment of background erythema, which represents a great unmet clinical need in rosacea therapy.10
In all cases, physicians should review the package insert for prescribing information. This document is not intended to suggest the monitoring and actual dosing practices for drugs.
Laser and Light Therapy
The efficacy of laser therapy for the treatment of telangiectasia has been well established in clinical practice,11-16 and limited studies also have suggested that it may reduce erythema and flushing.11,15,17 Most lasers used to treat vascular components of rosacea have wavelengths in the 500 to 600 nm range and are known as nonablative (they do not destroy tissue). Recent developments using long-pulsed pulsed dye lasers,13 a technique of stacking pulses,18 or 532-nm potassium-titanyl-phosphate lasers19 may produce excellent improvement in erythema and telangiectasia without purpura.13
Polychromatic light-emitting devices such as intense pulsed light devices (515–1200 nm) also have been found to be effective in reducing erythema and telangiectasia.15,20
Ablative lasers, such as the 2.94-nm erbium:YAG or 10,600-nm CO2 lasers, destroy tissue and may be used to treat subtype 3 (phymatous) rosacea.3,12,21
Lifestyle Management
Signs and symptoms of rosacea often appear to be triggered by environmental or lifestyle factors, most related to flushing. Some of the most common rosacea triggers include sun exposure, emotional stress, hot or cold weather, wind, heavy exercise, alcohol consumption, hot baths, spicy foods, humidity, indoor heat, certain skin care products, heated beverages, certain cosmetics, medications, medical conditions, and certain foods (Table 2, PLEASE REFER TO THE PDF TO VIEW THE TABLE).22 However, triggers that may affect one patient may not affect another, and avoidance of every potential factor may be unnecessary as well as impractical.
An appropriate management strategy identifies and avoids only those lifestyle factors that trigger or exacerbate rosacea symptoms in each individual patient. To help identify a patient’s individual rosacea triggers, the patient can record daily contact with the most common rosacea triggers and other possible factors and then match them to flare-ups of signs and symptoms. In unscientific surveys of patients with rosacea who identified and avoided their personal rosacea triggers, more than 90% reported that their condition had improved in varying degrees.23
Adjunctive Care
Skin Care Products
Because patients with rosacea often have skin that is sensitive and easily irritated, causing redness, inflammation, and stinging, skin care is an important component of rosacea management.21,24 The goal of everyday skin care for patients with rosacea is to maintain the integrity of the skin barrier while avoiding agents that cause inflammation or flushing.
Complicating skin care is the typical heightened neurosensory response in many patients with rosacea who may experience stinging and burning from minor irritants more frequently than the general population. Patients may therefore be advised to select cleansers and moisturizers that do not irritate their skin.
Sunscreens or sunblocks effective against the full spectrum of UVA and UVB radiation can be especially important for patients with rosacea whose facial skin may be particularly susceptible to actinic damage and consequent rosacea flare-ups. A sun protection factor of 15 or higher is recommended, and physical blocks utilizing zinc or titanium dioxide may be effective if chemical sunscreens cause irritation.
A useful rule of thumb may be to select products for patients with rosacea that contain no sensory provoking ingredients, no volatile substances, no minor irritants or allergens, minimal botanical agents, and no unnecessary ingredients.
Cleansing Regimen
Patients should be informed that compliance with instructions on facial cleansing and topical medication application may be critical to avoiding irritation, burning, and stinging. They may be advised to wash the face gently with a nonirritating cleanser, avoiding the use of abrasive materials such as washcloths and loofahs. They also may be advised to blot, not rub, the face dry with a soft towel and wait up to 30 minutes for the face to completely dry before applying topical medication or other products, as stinging most often occurs when the skin is wet.6,8
After this routine is established and the face is not irritated, the patient can reduce the amount of time waiting to dry by 5 minutes every day to determine the shortest waiting time necessary for the individual patient.
Cosmetics
Cosmetics, especially those with a green or yellow tint, may be effective in reducing the appearance of redness. However, as with skin cleansers and moisturizers, care should be taken to minimize irritation.
Patients should be advised to avoid any products that cause burning, stinging, itching, or other discomfort. They also may be advised that waterproof cosmetics may be difficult to remove, requiring the use of harsh agents that may induce irritation.
New cosmetics should be regularly purchased to minimize microbial contamination and degradation. Brushes are preferred over sponges to avoid abrasion and because brushes can be easily cleaned to decrease bacterial contamination.24
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively established. Until the etiology and pathogenesis are more completely understood, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments
The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
Rosacea is well established as a chronic typology or syndrome, primarily affecting the convexities of the central face (ie, cheeks, nose, chin, forehead) and often affecting the eyes. In 2002, the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea reported on a standard classification system that identified primary and secondary features of rosacea and described 4 common patterns of signs and symptoms designated as subtypes.1 In 2004, the committee published a standard grading system for assessing the relative severity of rosacea to enhance the utility of the classification system for researchers and clinicians.2
Developed and reviewed by 21 experts worldwide, these standard systems are essential to perform research; analyze results and compare data from different sources; and provide a common terminology and reference for the diagnosis, treatment, and assessment of results in clinical practice. Because present scientific knowledge of the etiology of rosacea is limited, these systems are considered provisional and are based on morphologic characteristics alone to avoid assumptions about pathogenesis and progression. They are intended to facilitate communication and ultimately the development of a research-based understanding of the disorder.
As a final step, the committee has developed standard management options based on these standard criteria to assist in providing optimal patient care. Because it is fundamental in the management of rosacea to consider the broad spectrum of potential therapies, the consensus committee and review panel have been expanded to include leading experts in dermatology, laser therapy, skin care, and ophthalmology. As with the standard classification and grading systems, the standard management options are considered provisional and may be expanded and updated as scientific knowledge increases and additional therapies become available.
Although rosacea encompasses various combinations of signs and symptoms, in most cases, some rather than all of these features appear in any given patient and often are characterized by remissions and exacerbations. Therefore, it is important to define the roles of respective treatment modalities as well as lifestyle management and skin care within the context of specific potential manifestations. In this way, an optimal management approach may be tailored for each individual patient.3,4
The standard management options are intended to serve as a menu of options rather than a treatment protocol. Although there is no cure for rosacea, its various signs and symptoms may be reduced or controlled with a range of therapeutic modalities, even though their actions may not be fully defined by clinical data.5 It should be noted that clinical trials are rarely a reflection of clinical practice because they are typically intended to discern only the contribution of a specific treatment.6 In practice, clinicians rarely rely on a single mode of care alone, and in the case of rosacea, factors such as proper skin care and avoidance of exacerbating factors may substantially improve results. Thus, patients often may experience better outcomes than might be suggested by clinical studies designed to isolate the effect of a single therapy.
Part 1 of this 2-part series will review the patient evaluation process and respective modalities of care.
Medical History
In addition to clinical observation of potential primary and secondary features of rosacea (Table 1, PLEASE REFER TO THE PDF TO VIEW THE TABLE), a medical history is needed to identify features that may not be visually evident or present at the time of the patient visit, to rule out alternative diagnoses, and to help identify potential environmental and lifestyle triggers. There is no laboratory test for rosacea and a biopsy is warranted only to rule out alternative diagnoses.
It may be difficult to clinically distinguish between the effects of chronic actinic damage on sun-sensitive skin (heliodermatitis) and subtype 1 (erythematotelangiectactic) rosacea. In some individuals, there may be overlapping features. A medical history may be especially useful in differentiating between erythematotelangiectactic rosacea and isolated photodamage. For example, any patient whose occupation or lifestyle has involved extensive sun exposure may experience chronic actinic damage, whereas patients with a history of flushing alone may be more likely to have rosacea. In addition, in the case of rosacea, erythema and telangiectasia tend to present with a central facial distribution.7 Other differential diagnoses include seborrheic dermatitis, lupus erythematosus, polycythemia vera, and carcinoid syndrome, with flushing mimicking rosacea.
A medical history also may be relevant for treatment purposes in distinguishing between dry flushing, which often is caused by exogenous or endogenous vasoactive agents, and wet flushing, which is accompanied by sweating that is regulated by the autonomic nervous system. Flushing can be further divided according to causes such as physical exertion, heat, or emotional reaction.8
Importantly, a medical history can uncover ocular involvement that may not be currently present or readily apparent from clinical observation as well as identify physical discomfort such as burning or stinging that may substantially affect quality of life for many patients.
Because rosacea affects facial appearance, its presence also may have considerable impact on an individual patient’s psychologic well-being and ability to interact socially or professionally. An assessment can help guide the physician toward providing an appropriate level of care.
Drug Therapy
The papules and pustules of rosacea, as well as nodules, plaques, or perilesional erythema, can be effectively treated in most patients with drugs that have been extensively studied in clinical trials and approved by the US Food and Drug Administration for rosacea, such as topical metronidazole, topical azelaic acid, and oral controlled-release doxycycline 40 mg, all approved for the treatment of inflammatory lesions of rosacea. In addition, the efficacy of topical sodium
sulfacetamide–sulfur is supported by many years of clinical experience in treating rosacea, though it was allowed to be marketed for rosacea prior to more stringent modern requirements for clinical studies and US Food and Drug Administration review. Options for the use of approved medications as well as off-label use of other medications such as oral tetracycline are reviewed in detail in part 2 of this series.9
Several oral antibiotics commonly are prescribed on an off-label basis for subtype 4 (ocular) rosacea. Moreover, when appropriate, the off-label use of other medical therapies may be administered to treat flushing and background erythema, which will be discussed in detail in part 2 of this series.9 The committee encourages further drug research aimed to improve the treatment of background erythema, which represents a great unmet clinical need in rosacea therapy.10
In all cases, physicians should review the package insert for prescribing information. This document is not intended to suggest the monitoring and actual dosing practices for drugs.
Laser and Light Therapy
The efficacy of laser therapy for the treatment of telangiectasia has been well established in clinical practice,11-16 and limited studies also have suggested that it may reduce erythema and flushing.11,15,17 Most lasers used to treat vascular components of rosacea have wavelengths in the 500 to 600 nm range and are known as nonablative (they do not destroy tissue). Recent developments using long-pulsed pulsed dye lasers,13 a technique of stacking pulses,18 or 532-nm potassium-titanyl-phosphate lasers19 may produce excellent improvement in erythema and telangiectasia without purpura.13
Polychromatic light-emitting devices such as intense pulsed light devices (515–1200 nm) also have been found to be effective in reducing erythema and telangiectasia.15,20
Ablative lasers, such as the 2.94-nm erbium:YAG or 10,600-nm CO2 lasers, destroy tissue and may be used to treat subtype 3 (phymatous) rosacea.3,12,21
Lifestyle Management
Signs and symptoms of rosacea often appear to be triggered by environmental or lifestyle factors, most related to flushing. Some of the most common rosacea triggers include sun exposure, emotional stress, hot or cold weather, wind, heavy exercise, alcohol consumption, hot baths, spicy foods, humidity, indoor heat, certain skin care products, heated beverages, certain cosmetics, medications, medical conditions, and certain foods (Table 2, PLEASE REFER TO THE PDF TO VIEW THE TABLE).22 However, triggers that may affect one patient may not affect another, and avoidance of every potential factor may be unnecessary as well as impractical.
An appropriate management strategy identifies and avoids only those lifestyle factors that trigger or exacerbate rosacea symptoms in each individual patient. To help identify a patient’s individual rosacea triggers, the patient can record daily contact with the most common rosacea triggers and other possible factors and then match them to flare-ups of signs and symptoms. In unscientific surveys of patients with rosacea who identified and avoided their personal rosacea triggers, more than 90% reported that their condition had improved in varying degrees.23
Adjunctive Care
Skin Care Products
Because patients with rosacea often have skin that is sensitive and easily irritated, causing redness, inflammation, and stinging, skin care is an important component of rosacea management.21,24 The goal of everyday skin care for patients with rosacea is to maintain the integrity of the skin barrier while avoiding agents that cause inflammation or flushing.
Complicating skin care is the typical heightened neurosensory response in many patients with rosacea who may experience stinging and burning from minor irritants more frequently than the general population. Patients may therefore be advised to select cleansers and moisturizers that do not irritate their skin.
Sunscreens or sunblocks effective against the full spectrum of UVA and UVB radiation can be especially important for patients with rosacea whose facial skin may be particularly susceptible to actinic damage and consequent rosacea flare-ups. A sun protection factor of 15 or higher is recommended, and physical blocks utilizing zinc or titanium dioxide may be effective if chemical sunscreens cause irritation.
A useful rule of thumb may be to select products for patients with rosacea that contain no sensory provoking ingredients, no volatile substances, no minor irritants or allergens, minimal botanical agents, and no unnecessary ingredients.
Cleansing Regimen
Patients should be informed that compliance with instructions on facial cleansing and topical medication application may be critical to avoiding irritation, burning, and stinging. They may be advised to wash the face gently with a nonirritating cleanser, avoiding the use of abrasive materials such as washcloths and loofahs. They also may be advised to blot, not rub, the face dry with a soft towel and wait up to 30 minutes for the face to completely dry before applying topical medication or other products, as stinging most often occurs when the skin is wet.6,8
After this routine is established and the face is not irritated, the patient can reduce the amount of time waiting to dry by 5 minutes every day to determine the shortest waiting time necessary for the individual patient.
Cosmetics
Cosmetics, especially those with a green or yellow tint, may be effective in reducing the appearance of redness. However, as with skin cleansers and moisturizers, care should be taken to minimize irritation.
Patients should be advised to avoid any products that cause burning, stinging, itching, or other discomfort. They also may be advised that waterproof cosmetics may be difficult to remove, requiring the use of harsh agents that may induce irritation.
New cosmetics should be regularly purchased to minimize microbial contamination and degradation. Brushes are preferred over sponges to avoid abrasion and because brushes can be easily cleaned to decrease bacterial contamination.24
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively established. Until the etiology and pathogenesis are more completely understood, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments
The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Powell FC. Rosacea. N Engl J Med. 2005;352:793-803.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.
- Wilkin JK. Use of topical products for maintaining remission in rosacea. Arch Dermatol. 1999;135:79-80.
- Odom R. Rosacea, acne rosacea, and actinic telangiectasia: in reply. J Am Acad Dermatol. 2005;53:1103-1104.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Odom R, Dahl M, Dover K, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 2: options according to subtype. Cutis. In press.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective a1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Goldberg DJ. Lasers and light sources for rosacea. Cutis. 2005;75(suppl 3):22-26, 33-36.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Tan SR, Tope WD. Pulsed dye laser treatment of rosacea improves erythema, symptomatology, and quality of life. J Am Acad Dermatol. 2004;51:592-599.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Railan D, Parlette EC, Uebelhoer NS, et al. Laser treatment of vascular lesions. Clin Dermatol. 2006;24:8-15.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Drake L, ed; National Rosacea Society. New survey pinpoints leading factors that trigger symptoms. Rosacea Review. Summer 2002. http://www.rosacea.org/rr/2002/summer/article_3.php. Accessed June 15, 2009.
- Drake L, ed; National Rosacea Society. Survey shows lifestyle changes help control rosacea flare-ups. Rosacea Review. Winter 1998. http://www.rosacea.org/rr/1998/winter/article_3.php. Accessed June 15, 2009.
- Draelos ZD. Cosmetics in acne and rosacea. Semin Cutan Med Surg. 2001;20:209-214.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Powell FC. Rosacea. N Engl J Med. 2005;352:793-803.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.
- Wilkin JK. Use of topical products for maintaining remission in rosacea. Arch Dermatol. 1999;135:79-80.
- Odom R. Rosacea, acne rosacea, and actinic telangiectasia: in reply. J Am Acad Dermatol. 2005;53:1103-1104.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Odom R, Dahl M, Dover K, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 2: options according to subtype. Cutis. In press.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective a1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Goldberg DJ. Lasers and light sources for rosacea. Cutis. 2005;75(suppl 3):22-26, 33-36.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Tan SR, Tope WD. Pulsed dye laser treatment of rosacea improves erythema, symptomatology, and quality of life. J Am Acad Dermatol. 2004;51:592-599.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Railan D, Parlette EC, Uebelhoer NS, et al. Laser treatment of vascular lesions. Clin Dermatol. 2006;24:8-15.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Drake L, ed; National Rosacea Society. New survey pinpoints leading factors that trigger symptoms. Rosacea Review. Summer 2002. http://www.rosacea.org/rr/2002/summer/article_3.php. Accessed June 15, 2009.
- Drake L, ed; National Rosacea Society. Survey shows lifestyle changes help control rosacea flare-ups. Rosacea Review. Winter 1998. http://www.rosacea.org/rr/1998/winter/article_3.php. Accessed June 15, 2009.
- Draelos ZD. Cosmetics in acne and rosacea. Semin Cutan Med Surg. 2001;20:209-214.