User login
Acute Coronary Syndrome Update
Acute coronary syndrome (ACS) remains a major healthcare challenge. Currently, the majority of patients with ACS have nonST‐segment (ST, part of an electrocardiogram between the QRS complex and the T wave) elevation myocardial infarction (MI) and unstable angina.1 Nevertheless, ST‐segment elevation MI is also an important cause of morbidity and mortality. In recent years, our understanding of ACS has improved as a result of several major advances based on results from multiple randomized clinical trials and registry analyses. The results of these analyses have influenced guidelines issued by professional societies and in some cases have become performance metrics. Therefore, it is particularly important for physicians involved in the care of patients with ACS to be aware of evolving treatment patterns (Table 1).
|
| Hospitalists should demonstrate a knowledge of: |
| ACS without enzyme leak, NSTEMI, and STEMI |
| Variable presentations of unstable angina, acute MI |
| Conditions that mimic ACS |
| Cardiac biomarkers |
| Role of noninvasive cardiac testing |
| Risks; indications for cardiac catheterization |
| Risk factors for CAD |
| Validated risk stratification tools |
| Indications for hospitalization of patients with chest pain |
| Indications, contraindications for thrombolytic therapy |
| Indications, contraindications, and pharmacology of drugs for ACS |
| Indications for early invasive interventions |
| Angiography, stenting and/or CABG |
| Laboratory studies or imaging indicative of disease severity |
| Safe hospital discharge |
| Hospitalists should demonstrate skill in: |
| History and physical exam relative to cardiac disease |
| Recognizing signs and severity of ACS |
| Diagnosing ACS through appropriate testing |
| History and physical, ECG, x‐rays, biomarkers |
| Risk stratification using validated tools |
| Formulating an evidence‐based treatment plan |
| Identifying patients for thrombolytics and/or early revascularization |
| Recognizing and treating patient discomfort |
| Recognizing decompensation, initiating immediate therapy |
| Managing complicating factors |
| Bleeding, inadequate response, cardiopulmonary compromise |
| Timely patient assessment, co‐management with other providers |
| Hospitalists should demonstrate attitudes that facilitate: |
| Communication with patients and families relative to cardiac disease and all aspects of care plan |
| Obtain informed consent |
| Early specialty consultation |
| Initiation of secondary prevention measures before discharge |
| Multidisciplinary care throughout the hospital stay |
| Safe discharge and transition back into primary care |
Case Study
A 64‐year‐old man presents to the emergency department with the chief complaint of chest pressure for the past 2 hours. His chest pressure began after he moved furniture in his home. He initially believed that a pulled muscle was the cause of the pain, but when the discomfort did not improve with rest and continued to worsen, he thought it best that his wife drive him to the emergency department, where he continues to have chest pressure. He has never had this symptom before. His past medical history is notable only for mild hypertension for which he takes hydrochlorothiazide 25 mg daily. Otherwise, he has been healthy.
Clinical Presentation and Risk Assessment
The clinical presentation of ACS is not always straightforward. Although physicians frequently inquire about chest pain, the pain often manifests as chest heaviness or chest pressure. Additionally, some patients have a more atypical presentation, where the predominant symptom of acute coronary ischemia is dyspnea or extreme fatigue. These atypical presentations are believed to be somewhat more common in women and in the elderly, but it is important to realize that they can occur in any patient. Nausea, vomiting, or diaphoresis may accompany these symptoms or occur in isolation. Chest discomfort radiating to the jaw, neck, or left arm may be present, but is not necessary to the diagnosis. Thus, we see a variety of symptoms presenting in a patient with ACS.
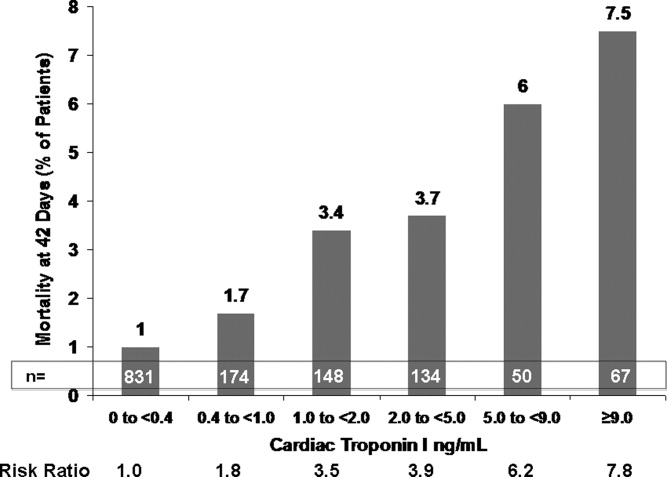
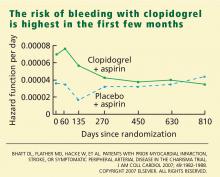
This varied presentation makes objective assessment of ACS particularly important. To inform assessment, biomarkers have emerged as a quick and effective tool to help with the diagnosis of ACS. In particular, troponin measurement is important and serial troponin measurement is useful to exclude myonecrosis. It should be noted that the initial troponin level may be normal during the early stages of ACS. A bedside troponin measurement can be useful for rapid identification of myocardial damage. Quantitative troponin measurement also adds value, as higher levels of troponin are associated with progressively worse outcomes, including mortality (Figure 1). Although a number of biomarkers are available, the most important commonly used at present is troponin.0, 0, 0, 0
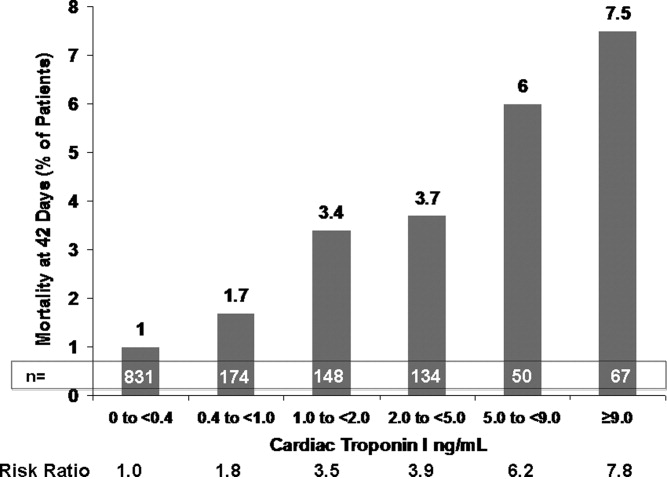
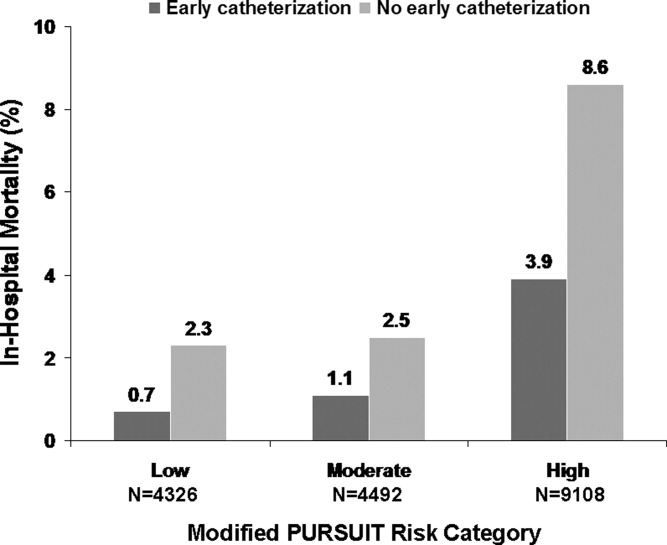
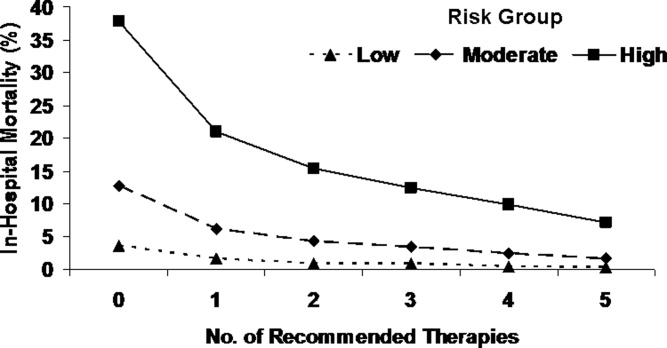
In addition to biomarkers, the electrocardiogram (ECG) remains extremely important in diagnosis and risk stratification. In a moderate‐level emergency department, the triage nurse should obtain a 12‐lead ECG for a patient with a history that is suspicious for coronary ischemia, and ask the attending physician to review the ECG immediately. If there is ST‐segment elevation or any new or presumed new left bundle‐branch block, the patient should be triaged to the ST‐segment elevation MI pathway of care. If there is ST‐segment depression or marked T‐wave inversion, this greatly raises the suspicion for nonST‐segment elevation MI or unstable angina. The presence of any of these features on the ECG places the patient at markedly elevated risk of short‐term ischemic complications.
A protocol should be in place for rapid treatment of patients with ST‐segment elevation MI.2 If the hospital has 24/7 percutaneous coronary intervention (PCI) capability, the catheterization lab should be immediately activated and the patient should proceed to primary PCI. The goal door‐to‐balloon time is 90 minutes or less. A patient who presents to a hospital without primary PCI capability should receive either fibrinolysis or be transferred to a center that can perform primary PCI. If fibrinolytic therapy is planned, it is essential that the patient not have any absolute contraindications to fibrinolytic therapy. Fibrinolysis should be administered within 30 minutes of patient contact. If transfer for primary PCI is planned, it is important that systems to support the transfer are in place so that the time from first medical contact to PCI does not exceed 90 minutes. As a practical point, it can be difficult to achieve these short transfer times in many geographic regions of the United States. However, with organized systems of care, it is certainly possible to have effective transfer systems and to achieve a short door‐to‐balloon time.3
If the patient does not have ST‐segment elevation MI, the next step depends on the patient's level of risk, where risk stratification is particularly important. As mentioned above, troponin measurement and the ECG are both essential aspects of risk stratification, but they alone are not sufficient to establish risk. It is recommended that an objective risk tool also be used. This is especially important because the patient can be initially troponin‐negative and have a normal ECG but still be at high risk for ischemic complications. The TIMI risk score (Table 2) is 1 of a number of resources that can help determine whether patients are at high risk for short‐term ischemic complications using means more objective than the eyeball test (Table 3).
|
| Historical |
| Age 65 year |
| 3 CAD risk factors |
| Family history, hypertension, hypercholesterolemia, diabetes, active smoker |
| Known CAD (stenosis 50%) |
| ASA use in past 7 days |
| At presentation |
| Recent (24 hours) severe angina |
| Elevated cardiac markers |
| ST deviation 0.5 mm |
| Risk score = total points, range: 07 |
| Risk Score | Death or MI (%) | Death, MI, or Urgent Revascularization (%) |
|---|---|---|
| ||
| 0/1 | 3 | 5 |
| 2 | 3 | 8 |
| 3 | 5 | 13 |
| 4 | 7 | 20 |
| 5 | 12 | 26 |
| 6/7 | 19 | 41 |
Delineation of the coronary anatomy in the catheterization lab is warranted for patients judged to be at high risk on the basis of the TIMI risk score, which would include most patients with elevated troponin or ST‐segment deviation. Many patients will undergo PCI on the basis of those test results and a smaller percentage might undergo coronary artery bypass grafting (CABG). Additionally, a sizeable minority of patients will be managed medically. This latter group is challenging because it consists of patients who have either trivial coronary artery disease or extensive coronary artery disease not amenable to revascularization and who have either a very low or very high risk of ischemic complications.
Even though catheterization may not be necessary, further evaluation is warranted in patients with ACS deemed to be at low risk. Typically, some form of functional assessment is indicated. In patients who are able to exercise, this would consist of exercise stress testing, often with an imaging modality. If the stress test is abnormal, cardiac catheterization is often the next step.
Case Study (cont)
An ECG is rapidly obtained on this patient and there is ST‐segment depression in leads II, III, and aVF. A bedside troponin is positive. The patient is at high risk of ischemic complications. He is diagnosed with nonST‐segment elevation MI. The next step is to initiate medical therapy. Presumably, the patient would have already (or at least should have already) received aspirin. Chewing or swallowing a dose of 325 mg nonenteric coated aspirin should provide a prompt aspirin effect. It would be reasonable to initiate anticoagulation as well, and the guidelines support a number of choices such as unfractionated heparin or low molecular weight heparin. Consideration should also be given to starting additional antiplatelet therapy, such as a loading dose of clopidogrel. Although aspirin provides some degree of antiplatelet effect, in a patient with activated platelets who presents with an ACS, additional antiplatelet therapy is necessary, although the exact timing of it is a matter of debate. Finally, consideration needs to be given to the need for catheterization. This patient, on the basis of his high ischemic risk and lack of obvious contraindications, should go to the catheterization laboratory, and the timing of catheterization requires further thought.
Guideline Update
The American College of Cardiology/American Heart Association 2009 Focused Guideline Update provides new information and recommendations pertinent to the care of patients with ACS2 and incorporates new data relevant to the initial emergency care and subsequent inpatient care of patients with ACS. Guideline highlights are presented in Table 4.
| Intervention | Recommendation |
|---|---|
| |
| GP IIb/IIIa receptor antagonists | |
| Class IIa | Start abciximab, tirofiban, or eptifibatide at primary PCI (with/without stenting) in selected patients with STEMI. |
| Class IIb | Uncertain value in STEMI when given before arrival at catheterization lab. |
| Thienopyridines | |
| Class I | Use loading dose for planned PCI in STEMI. Regimens for primary and nonprimary PCI are detailed within the guideline. |
| Duration of therapy after stent placement of at least 12 months. Stop early if bleeding risk outweighs benefit. | |
| Discontinue before planned, delayed CABG (5 d clopidogrel; 7 d prasugrel) unless the need for CABG outweighs bleeding risk. | |
| Class IIb | After DES placement, consider continuing clopidogrel or prasugrel beyond the first 15 months of therapy. |
| Class III | Prasugrel is not recommended for primary PCI in patients with STEMI who have a history of stroke or TIA. |
| Parenteral anticoagulants | |
| Class I | In primary PCI, supportive anticoagulants include unfractionated heparin, enoxaparin, fondaparinux, or bivalirudin, following ASA and a thienopyridine. |
| Class IIa | Bivalirudin is reasonable in patients at high risk of bleeding undergoing PCI for STEMI. |
| Triage and transfer for PCI | |
| Class I | STEMI system of care is supported by dedicated teams and protocols required for all communities. |
| Class IIa | Transfer patients who received fibrinolytic therapy at a nonPCI‐capable facility to a PCI‐capable facility. Consider preparatory antithrombotic regimen before or during transfer. |
| Class IIb | Consider expeditious transfer of nonhigh‐risk patients from a nonPCI‐capable facility to a PCI‐capable facility after administration of fibrinolytic. Consider preparatory antithrombotic regimen before or during transfer. |
| Intensive glucose control in STEMI | |
| Class IIa | Insulin is reasonable to maintain glucose <180 mg/dL (avoid hypoglycemia) for any patient with STEMI. |
| Thrombus aspiration during PCI of STEMI | |
| Class IIa | Aspiration thombectomy is reasonable. |
| Use of stents in STEMI | |
| Class IIa | DES is a reasonable alternative to BMS for primary PCI in STEMI. |
| Class IIb | Consider DES when clinical or anatomical factors suggest favorable safety and efficacy for DES. |
| Angiography in CKD | |
| Class I | Isomolar contrast or low molecular weight contrast (not ioxaglate or iohexol) is indicated for CKD patients not on dialysis. |
| Fractional flow reserve | |
| Class IIa | FFR is useful to assess a specific coronary lesion or as an alternative to noninvasive functional testing to justify PCI. Reasonable for intermediate coronary stenosis in patients with angina. |
| Class III | Routine use of FFR is not recommended to assess severity of CAD in patients with angina who have had a positive, unequivocal, noninvasive functional study. |
| PCI for unprotected left main CAD | |
| Class IIb | PCI of left main coronary artery with stents is an alternative to CABG for anatomy associated with low risk of PCI complications and a clinical scenario with higher risk of adverse surgical outcomes. |
| Timing of angiography and antiplatelet therapy in UA/NSTEMI | |
| Class I | Initiate dual‐antiplatelet therapy for UA/NSTEMI and an invasive approach. Start ASA on presentation. Clopidogrel (before or at PCI) or prasugrel (at PCI) as a second antiplatelet agent. |
| Class IIa | Early invasive strategy within 12 to 24 hours of admission is reasonable for stabilized high‐risk UA/NSTEMI; an early approach is also reasonable for UA/STEMI not at high‐risk. |
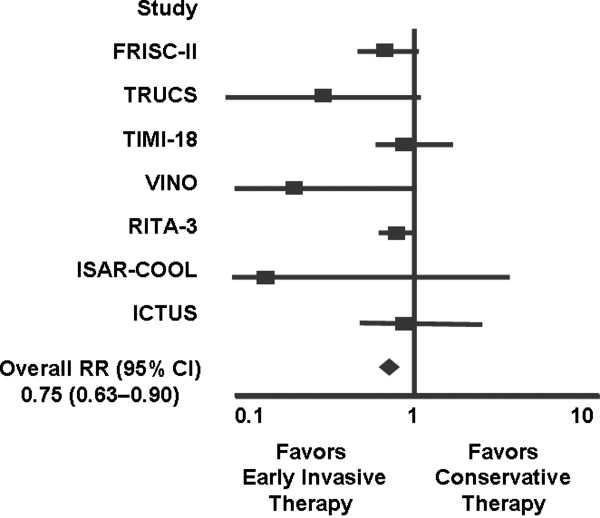
Several studies support an invasive strategy to assess high‐risk ACS patients. Randomized clinical trials and meta‐analyses of these trials have confirmed a significant reduction in subsequent ischemic events, including mortality, in patients who undergo an invasive vs. a more conservative strategy.4 Registry data have confirmed that these randomized clinical trial data reflect patients in the real‐world setting of clinical practice.5
The timing of angiography has recently been examined in detail.6, 7 It appears that for patients with nonST‐segment elevation ACS, unlike those with ST‐segment elevation MI, there is no need for emergent transfer to the catheterization laboratory, assuming patients are electrically and hemodynamically stable. Emergency transfer is warranted for unstable patients and those with ongoing chest discomfort. Otherwise, it appears sufficient to send the patient with nonST‐segment elevation ACS for catheterization within the subsequent 48 hours, or, alternatively, to adopt a more expectant approach in which catheterization is deferred until either recurrent symptoms develop or risk stratification suggests that there is substantial myocardium in jeopardy.
PCI is performed in the catheterization laboratory most often in the setting of ACS.5 When PCI is performed, an important consideration is whether to use a bare metal stent or a drug‐eluting stent.8 Drug‐eluting stents have been shown to have a significant benefit in reducing restenosis and the need for repeat revascularization. However, in aggregate, they have not been shown to either increase or decrease mortality.9 A key issue for the referring physician is to ascertain whether patients who go to the catheterization laboratory are likely to tolerate and be compliant with prolonged dual antiplatelet therapy. If it appears that the patient can or will not be compliant, a bare metal stent is preferable to a drug‐eluting stent; a bare metal stent requires dual antiplatelet therapy of shorter duration.
Additional considerations when sending patients to the catheterization laboratory are related to renal function. In patients with renal dysfunction, the most important way to prevent contrast nephropathy is adequate hydration prior to the procedure. In patients with left ventricular dysfunction, hydration must be done judiciously. Other strategies for preventing contrast nephropathy are being studied, although it is not entirely clear which strategies beyond hydration are truly effective.
Use of upstream glycoprotein IIb/IIIa inhibitors has become more common in patients with nonST‐segment elevation ACS. However, the most recent trial to examine this issue, the Early Glycoprotein IIb/IIIa Inhibition in NonST‐Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial, did not find a clear benefit for routine administration of upstream glycoprotein IIb/IIIa inhibitors when studying all patients with ACS.10 There did appear to be a signal of benefit in troponin‐positive patients, but as an overall strategy no significant benefit and even some detriment associated with an increase in bleeding were shown. Similarly, the results of the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial did not support the benefit of upstream glycoprotein IIb/IIIa inhibitors in patients with ACS.11
New data have also been released with respect to the thienopyridines. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with PrasugrelThrombolysis in Myocardial Infarction (TRITONTIMI) 38 study found that the more potent thienopyridine prasugrel significantly reduced ischemic events when compared with clopidogrel in patients with ACS undergoing PCI.1214 A significant reduction in stent thrombosis was reported regardless of the type of stent.15 However, the study reported a significant increase in major bleeding and a small, but statistically significant, excess of fatal bleeding. A subgroup analysis of patients with diabetes or with ST‐segment elevation MI from the TRITON‐TIMI 38 study showed a particularly large benefit associated with the use of prasugrel vs. clopidogrel and, interestingly, bleeding hazards were attenuated in these subgroups.16, 17 In the small subgroup of patients with prior stroke or transient ischemic attack (TIA), there was an excessive rate of intracranial hemorrhage with prasugrel vs. clopidogrel, indicating that prasugrel should not be used in these patients. Patients age 75 years or older or who weighed less than 60 kg also appeared to have a higher bleeding risk with prasugrel compared to clopidogrel. Careful thought is needed before using prasugrel in those patients identified as having a higher risk of bleeding.
Recently, a higher clopidogrel loading dose of 600 mg vs. the standard 300 mg dose was tested in patients who presented with ACS in the Clopidogrel optimal loading dose Usage to Reduce Recurrent EveNTs‐Organization to Assess Strategies in Ischemic Syndromes (CURRENT‐OASIS) 7 trial.18 Patients also received 150 mg of clopidogrel daily for the ensuing 6 days vs. the standard 75 mg daily dose. All patients then received 75 mg clopidogrel for 1 month of follow‐up. In the overall population, there was no benefit to using the higher clopidogrel loading dose. In contrast, there was a significant reduction in stent thrombosis in patients who received stents. The higher loading dose of clopidogrel was associated with a higher rate of bleeding.
Ticagrelor is a novel adenosine diphosphate receptor antagonist that was compared with clopidogrel in patients with ACS.19, 20 Compared to clopidogrel, ticagrelor significantly reduced ischemic events and there was also a significant reduction in cardiovascular mortality and in all‐cause mortality. Surprisingly, overall major bleeding did not increase with ticagrelor, but nonCABG‐related major bleeding increased.
The use of proton pump inhibitors (PPIs) in patients receiving dual antiplatelet therapy has also been a matter of vigorous recent debate.21 Evidence to date suggests there is no significant clinical interaction between PPIs and prasugrel. The data with clopidogrel and PPIs are mixed, although data are limited because much were derived from observational studies. Randomized clinical trial data are needed to assess whether there is an interaction between clopidogrel and PPIs that warrants clinical action, although preliminary data suggest there is no adverse cardiovascular interaction.22
New data regarding the intravenous anticoagulant bivalirudin have become available and have been incorporated into the Focused Guideline Update. Although bivalirudin is to be used primarily in the catheterization laboratory during PCI, it does appear to be associated with significantly less bleeding than heparin plus glycoprotein IIb/IIIa inhibitors.11, 2326
Case Study (cont)
The patient undergoes cardiac catheterization. An occluded dominant left circumflex artery is noted and is opened up with balloon angioplasty after aspiration thrombectomy. The patient receives 60 mg of prasugrel as a loading dose and bivalirudin as the anticoagulant during the procedure. A drug‐eluting stent is implanted with excellent results. The patient is transferred to the cardiac care unit for further care. It appears that this is a patient who functionally has an ST‐segment elevation MI with an occluded artery, although it manifested on the ECG as ST depression. Because of the patient's ongoing chest discomfort, it was fortunate that prompt angiography was performed.
Discussion
Patients with ACS present several challenges in management. Risk stratification is particularly important for nonST‐segment elevation ACS and requires thoughtful evaluation by the physician. Additionally, the large amount of new data and guideline updates create a rapidly evolving field, making it difficult to keep abreast of new developments. Physicians of patients with ACS need to be aware of these key developments so that they can provide optimal care to their patients with potentially life‐threatening ACS.
Acknowledgements
Denise M. Erkkila, RPh of DIME, provided editorial assistance consisting of help with tables, figures, and reference formatting for this manuscript.
- ,,, et al.Heart disease and stroke statistics‐‐2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,, et al.2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol.2009;54:2205–2241.
- ,,, et al.Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes.JAMA.2009;302:2207–2213.
- ,,,,.Benefit of early invasive therapy in acute coronary syndromes: a meta‐analysis of contemporary randomized clinical trials.J Am Coll Cardiol.2006;48:1319–1325.
- ,,, et al.Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative.JAMA.2004;292:2096–2104.
- ,,, et al.Early versus delayed invasive intervention in acute coronary syndromes.N Engl J Med.2009;360:2165–2175.
- ,,, et al.Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial.JAMA.2009;302:947–954.
- ,.Drug‐eluting stents: dual antiplatelet therapy for every survivor?Circulation.2007;116:696–699.
- ,.Appropriate use of drug‐eluting stents: balancing the reduction in restenosis with the concern of late thrombosis.Lancet.2008;371:2134–2143.
- ,,, et al.Early versus delayed, provisional eptifibatide in acute coronary syndromes.N Engl J Med.2009;360:2176–2190.
- ,,, et al.Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one‐year results from the ACUITY trial.JAMA.2007;298:2497–2506.
- .Intensifying platelet inhibition‐‐navigating between Scylla and Charybdis.N Engl J Med.2007;357:2078–2081.
- .Prasugrel in clinical practice.N Engl J Med.2009;361:940–942.
- ,,, et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2007;357:2001–2015.
- ,,, et al.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON‐TIMI 38 trial: a subanalysis of a randomised trial.Lancet.2008;371:1353–1363.
- ,,, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial.Lancet.2009;373:723–731.
- ,,, et al.Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel—Thrombolysis in Myocardial Infarction 38.Circulation.2008;118:1626–1636.
- , CURRENT Investigators. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Available at: http://www.escardio.org/congresses/esc‐2009/congress‐reports/Pages/706003‐706004‐mehta‐vandewerf.aspx#discussant.2009. Accessed July 2010.
- .Antiplatelet therapy: ticagrelor in ACS‐what does PLATO teach us?Nat Rev Cardiol.2009;6:737–738.
- ,,, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2009;361:1045–1057.
- ,.Omeprazole and clopidogrel: Should clinicians be worried?Cleve Clin J Med.2010;77:113–116.
- .COGENT: a prospective, randomized, placebo‐controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Transcatheter Cardiovascular Therapeutics (TCT) 2009; September 24,2009;San Francisco, CA.
- ,,, et al.The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) Trial: study design and rationale.Am Heart J.2008;156:44–56.
- ,,, et al.Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial.Lancet.2009;374:1149–1159.
- ,,, et al.Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale.Am Heart J.2004;148:764–775.
- ,,, et al.Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial.Lancet.2007;369:907–919.
- Society of Hospital Medicine.Acute coronary syndrome.J Hosp Med.2006;1 (suppl 1):2–3.
- ,,, et al.The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making.JAMA.2000;284:835–842.
Acute coronary syndrome (ACS) remains a major healthcare challenge. Currently, the majority of patients with ACS have nonST‐segment (ST, part of an electrocardiogram between the QRS complex and the T wave) elevation myocardial infarction (MI) and unstable angina.1 Nevertheless, ST‐segment elevation MI is also an important cause of morbidity and mortality. In recent years, our understanding of ACS has improved as a result of several major advances based on results from multiple randomized clinical trials and registry analyses. The results of these analyses have influenced guidelines issued by professional societies and in some cases have become performance metrics. Therefore, it is particularly important for physicians involved in the care of patients with ACS to be aware of evolving treatment patterns (Table 1).
|
| Hospitalists should demonstrate a knowledge of: |
| ACS without enzyme leak, NSTEMI, and STEMI |
| Variable presentations of unstable angina, acute MI |
| Conditions that mimic ACS |
| Cardiac biomarkers |
| Role of noninvasive cardiac testing |
| Risks; indications for cardiac catheterization |
| Risk factors for CAD |
| Validated risk stratification tools |
| Indications for hospitalization of patients with chest pain |
| Indications, contraindications for thrombolytic therapy |
| Indications, contraindications, and pharmacology of drugs for ACS |
| Indications for early invasive interventions |
| Angiography, stenting and/or CABG |
| Laboratory studies or imaging indicative of disease severity |
| Safe hospital discharge |
| Hospitalists should demonstrate skill in: |
| History and physical exam relative to cardiac disease |
| Recognizing signs and severity of ACS |
| Diagnosing ACS through appropriate testing |
| History and physical, ECG, x‐rays, biomarkers |
| Risk stratification using validated tools |
| Formulating an evidence‐based treatment plan |
| Identifying patients for thrombolytics and/or early revascularization |
| Recognizing and treating patient discomfort |
| Recognizing decompensation, initiating immediate therapy |
| Managing complicating factors |
| Bleeding, inadequate response, cardiopulmonary compromise |
| Timely patient assessment, co‐management with other providers |
| Hospitalists should demonstrate attitudes that facilitate: |
| Communication with patients and families relative to cardiac disease and all aspects of care plan |
| Obtain informed consent |
| Early specialty consultation |
| Initiation of secondary prevention measures before discharge |
| Multidisciplinary care throughout the hospital stay |
| Safe discharge and transition back into primary care |
Case Study
A 64‐year‐old man presents to the emergency department with the chief complaint of chest pressure for the past 2 hours. His chest pressure began after he moved furniture in his home. He initially believed that a pulled muscle was the cause of the pain, but when the discomfort did not improve with rest and continued to worsen, he thought it best that his wife drive him to the emergency department, where he continues to have chest pressure. He has never had this symptom before. His past medical history is notable only for mild hypertension for which he takes hydrochlorothiazide 25 mg daily. Otherwise, he has been healthy.
Clinical Presentation and Risk Assessment
The clinical presentation of ACS is not always straightforward. Although physicians frequently inquire about chest pain, the pain often manifests as chest heaviness or chest pressure. Additionally, some patients have a more atypical presentation, where the predominant symptom of acute coronary ischemia is dyspnea or extreme fatigue. These atypical presentations are believed to be somewhat more common in women and in the elderly, but it is important to realize that they can occur in any patient. Nausea, vomiting, or diaphoresis may accompany these symptoms or occur in isolation. Chest discomfort radiating to the jaw, neck, or left arm may be present, but is not necessary to the diagnosis. Thus, we see a variety of symptoms presenting in a patient with ACS.
This varied presentation makes objective assessment of ACS particularly important. To inform assessment, biomarkers have emerged as a quick and effective tool to help with the diagnosis of ACS. In particular, troponin measurement is important and serial troponin measurement is useful to exclude myonecrosis. It should be noted that the initial troponin level may be normal during the early stages of ACS. A bedside troponin measurement can be useful for rapid identification of myocardial damage. Quantitative troponin measurement also adds value, as higher levels of troponin are associated with progressively worse outcomes, including mortality (Figure 1). Although a number of biomarkers are available, the most important commonly used at present is troponin.0, 0, 0, 0





In addition to biomarkers, the electrocardiogram (ECG) remains extremely important in diagnosis and risk stratification. In a moderate‐level emergency department, the triage nurse should obtain a 12‐lead ECG for a patient with a history that is suspicious for coronary ischemia, and ask the attending physician to review the ECG immediately. If there is ST‐segment elevation or any new or presumed new left bundle‐branch block, the patient should be triaged to the ST‐segment elevation MI pathway of care. If there is ST‐segment depression or marked T‐wave inversion, this greatly raises the suspicion for nonST‐segment elevation MI or unstable angina. The presence of any of these features on the ECG places the patient at markedly elevated risk of short‐term ischemic complications.
A protocol should be in place for rapid treatment of patients with ST‐segment elevation MI.2 If the hospital has 24/7 percutaneous coronary intervention (PCI) capability, the catheterization lab should be immediately activated and the patient should proceed to primary PCI. The goal door‐to‐balloon time is 90 minutes or less. A patient who presents to a hospital without primary PCI capability should receive either fibrinolysis or be transferred to a center that can perform primary PCI. If fibrinolytic therapy is planned, it is essential that the patient not have any absolute contraindications to fibrinolytic therapy. Fibrinolysis should be administered within 30 minutes of patient contact. If transfer for primary PCI is planned, it is important that systems to support the transfer are in place so that the time from first medical contact to PCI does not exceed 90 minutes. As a practical point, it can be difficult to achieve these short transfer times in many geographic regions of the United States. However, with organized systems of care, it is certainly possible to have effective transfer systems and to achieve a short door‐to‐balloon time.3
If the patient does not have ST‐segment elevation MI, the next step depends on the patient's level of risk, where risk stratification is particularly important. As mentioned above, troponin measurement and the ECG are both essential aspects of risk stratification, but they alone are not sufficient to establish risk. It is recommended that an objective risk tool also be used. This is especially important because the patient can be initially troponin‐negative and have a normal ECG but still be at high risk for ischemic complications. The TIMI risk score (Table 2) is 1 of a number of resources that can help determine whether patients are at high risk for short‐term ischemic complications using means more objective than the eyeball test (Table 3).
|
| Historical |
| Age 65 year |
| 3 CAD risk factors |
| Family history, hypertension, hypercholesterolemia, diabetes, active smoker |
| Known CAD (stenosis 50%) |
| ASA use in past 7 days |
| At presentation |
| Recent (24 hours) severe angina |
| Elevated cardiac markers |
| ST deviation 0.5 mm |
| Risk score = total points, range: 07 |
| Risk Score | Death or MI (%) | Death, MI, or Urgent Revascularization (%) |
|---|---|---|
| ||
| 0/1 | 3 | 5 |
| 2 | 3 | 8 |
| 3 | 5 | 13 |
| 4 | 7 | 20 |
| 5 | 12 | 26 |
| 6/7 | 19 | 41 |
Delineation of the coronary anatomy in the catheterization lab is warranted for patients judged to be at high risk on the basis of the TIMI risk score, which would include most patients with elevated troponin or ST‐segment deviation. Many patients will undergo PCI on the basis of those test results and a smaller percentage might undergo coronary artery bypass grafting (CABG). Additionally, a sizeable minority of patients will be managed medically. This latter group is challenging because it consists of patients who have either trivial coronary artery disease or extensive coronary artery disease not amenable to revascularization and who have either a very low or very high risk of ischemic complications.
Even though catheterization may not be necessary, further evaluation is warranted in patients with ACS deemed to be at low risk. Typically, some form of functional assessment is indicated. In patients who are able to exercise, this would consist of exercise stress testing, often with an imaging modality. If the stress test is abnormal, cardiac catheterization is often the next step.
Case Study (cont)
An ECG is rapidly obtained on this patient and there is ST‐segment depression in leads II, III, and aVF. A bedside troponin is positive. The patient is at high risk of ischemic complications. He is diagnosed with nonST‐segment elevation MI. The next step is to initiate medical therapy. Presumably, the patient would have already (or at least should have already) received aspirin. Chewing or swallowing a dose of 325 mg nonenteric coated aspirin should provide a prompt aspirin effect. It would be reasonable to initiate anticoagulation as well, and the guidelines support a number of choices such as unfractionated heparin or low molecular weight heparin. Consideration should also be given to starting additional antiplatelet therapy, such as a loading dose of clopidogrel. Although aspirin provides some degree of antiplatelet effect, in a patient with activated platelets who presents with an ACS, additional antiplatelet therapy is necessary, although the exact timing of it is a matter of debate. Finally, consideration needs to be given to the need for catheterization. This patient, on the basis of his high ischemic risk and lack of obvious contraindications, should go to the catheterization laboratory, and the timing of catheterization requires further thought.
Guideline Update
The American College of Cardiology/American Heart Association 2009 Focused Guideline Update provides new information and recommendations pertinent to the care of patients with ACS2 and incorporates new data relevant to the initial emergency care and subsequent inpatient care of patients with ACS. Guideline highlights are presented in Table 4.
| Intervention | Recommendation |
|---|---|
| |
| GP IIb/IIIa receptor antagonists | |
| Class IIa | Start abciximab, tirofiban, or eptifibatide at primary PCI (with/without stenting) in selected patients with STEMI. |
| Class IIb | Uncertain value in STEMI when given before arrival at catheterization lab. |
| Thienopyridines | |
| Class I | Use loading dose for planned PCI in STEMI. Regimens for primary and nonprimary PCI are detailed within the guideline. |
| Duration of therapy after stent placement of at least 12 months. Stop early if bleeding risk outweighs benefit. | |
| Discontinue before planned, delayed CABG (5 d clopidogrel; 7 d prasugrel) unless the need for CABG outweighs bleeding risk. | |
| Class IIb | After DES placement, consider continuing clopidogrel or prasugrel beyond the first 15 months of therapy. |
| Class III | Prasugrel is not recommended for primary PCI in patients with STEMI who have a history of stroke or TIA. |
| Parenteral anticoagulants | |
| Class I | In primary PCI, supportive anticoagulants include unfractionated heparin, enoxaparin, fondaparinux, or bivalirudin, following ASA and a thienopyridine. |
| Class IIa | Bivalirudin is reasonable in patients at high risk of bleeding undergoing PCI for STEMI. |
| Triage and transfer for PCI | |
| Class I | STEMI system of care is supported by dedicated teams and protocols required for all communities. |
| Class IIa | Transfer patients who received fibrinolytic therapy at a nonPCI‐capable facility to a PCI‐capable facility. Consider preparatory antithrombotic regimen before or during transfer. |
| Class IIb | Consider expeditious transfer of nonhigh‐risk patients from a nonPCI‐capable facility to a PCI‐capable facility after administration of fibrinolytic. Consider preparatory antithrombotic regimen before or during transfer. |
| Intensive glucose control in STEMI | |
| Class IIa | Insulin is reasonable to maintain glucose <180 mg/dL (avoid hypoglycemia) for any patient with STEMI. |
| Thrombus aspiration during PCI of STEMI | |
| Class IIa | Aspiration thombectomy is reasonable. |
| Use of stents in STEMI | |
| Class IIa | DES is a reasonable alternative to BMS for primary PCI in STEMI. |
| Class IIb | Consider DES when clinical or anatomical factors suggest favorable safety and efficacy for DES. |
| Angiography in CKD | |
| Class I | Isomolar contrast or low molecular weight contrast (not ioxaglate or iohexol) is indicated for CKD patients not on dialysis. |
| Fractional flow reserve | |
| Class IIa | FFR is useful to assess a specific coronary lesion or as an alternative to noninvasive functional testing to justify PCI. Reasonable for intermediate coronary stenosis in patients with angina. |
| Class III | Routine use of FFR is not recommended to assess severity of CAD in patients with angina who have had a positive, unequivocal, noninvasive functional study. |
| PCI for unprotected left main CAD | |
| Class IIb | PCI of left main coronary artery with stents is an alternative to CABG for anatomy associated with low risk of PCI complications and a clinical scenario with higher risk of adverse surgical outcomes. |
| Timing of angiography and antiplatelet therapy in UA/NSTEMI | |
| Class I | Initiate dual‐antiplatelet therapy for UA/NSTEMI and an invasive approach. Start ASA on presentation. Clopidogrel (before or at PCI) or prasugrel (at PCI) as a second antiplatelet agent. |
| Class IIa | Early invasive strategy within 12 to 24 hours of admission is reasonable for stabilized high‐risk UA/NSTEMI; an early approach is also reasonable for UA/STEMI not at high‐risk. |
Several studies support an invasive strategy to assess high‐risk ACS patients. Randomized clinical trials and meta‐analyses of these trials have confirmed a significant reduction in subsequent ischemic events, including mortality, in patients who undergo an invasive vs. a more conservative strategy.4 Registry data have confirmed that these randomized clinical trial data reflect patients in the real‐world setting of clinical practice.5
The timing of angiography has recently been examined in detail.6, 7 It appears that for patients with nonST‐segment elevation ACS, unlike those with ST‐segment elevation MI, there is no need for emergent transfer to the catheterization laboratory, assuming patients are electrically and hemodynamically stable. Emergency transfer is warranted for unstable patients and those with ongoing chest discomfort. Otherwise, it appears sufficient to send the patient with nonST‐segment elevation ACS for catheterization within the subsequent 48 hours, or, alternatively, to adopt a more expectant approach in which catheterization is deferred until either recurrent symptoms develop or risk stratification suggests that there is substantial myocardium in jeopardy.
PCI is performed in the catheterization laboratory most often in the setting of ACS.5 When PCI is performed, an important consideration is whether to use a bare metal stent or a drug‐eluting stent.8 Drug‐eluting stents have been shown to have a significant benefit in reducing restenosis and the need for repeat revascularization. However, in aggregate, they have not been shown to either increase or decrease mortality.9 A key issue for the referring physician is to ascertain whether patients who go to the catheterization laboratory are likely to tolerate and be compliant with prolonged dual antiplatelet therapy. If it appears that the patient can or will not be compliant, a bare metal stent is preferable to a drug‐eluting stent; a bare metal stent requires dual antiplatelet therapy of shorter duration.
Additional considerations when sending patients to the catheterization laboratory are related to renal function. In patients with renal dysfunction, the most important way to prevent contrast nephropathy is adequate hydration prior to the procedure. In patients with left ventricular dysfunction, hydration must be done judiciously. Other strategies for preventing contrast nephropathy are being studied, although it is not entirely clear which strategies beyond hydration are truly effective.
Use of upstream glycoprotein IIb/IIIa inhibitors has become more common in patients with nonST‐segment elevation ACS. However, the most recent trial to examine this issue, the Early Glycoprotein IIb/IIIa Inhibition in NonST‐Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial, did not find a clear benefit for routine administration of upstream glycoprotein IIb/IIIa inhibitors when studying all patients with ACS.10 There did appear to be a signal of benefit in troponin‐positive patients, but as an overall strategy no significant benefit and even some detriment associated with an increase in bleeding were shown. Similarly, the results of the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial did not support the benefit of upstream glycoprotein IIb/IIIa inhibitors in patients with ACS.11
New data have also been released with respect to the thienopyridines. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with PrasugrelThrombolysis in Myocardial Infarction (TRITONTIMI) 38 study found that the more potent thienopyridine prasugrel significantly reduced ischemic events when compared with clopidogrel in patients with ACS undergoing PCI.1214 A significant reduction in stent thrombosis was reported regardless of the type of stent.15 However, the study reported a significant increase in major bleeding and a small, but statistically significant, excess of fatal bleeding. A subgroup analysis of patients with diabetes or with ST‐segment elevation MI from the TRITON‐TIMI 38 study showed a particularly large benefit associated with the use of prasugrel vs. clopidogrel and, interestingly, bleeding hazards were attenuated in these subgroups.16, 17 In the small subgroup of patients with prior stroke or transient ischemic attack (TIA), there was an excessive rate of intracranial hemorrhage with prasugrel vs. clopidogrel, indicating that prasugrel should not be used in these patients. Patients age 75 years or older or who weighed less than 60 kg also appeared to have a higher bleeding risk with prasugrel compared to clopidogrel. Careful thought is needed before using prasugrel in those patients identified as having a higher risk of bleeding.
Recently, a higher clopidogrel loading dose of 600 mg vs. the standard 300 mg dose was tested in patients who presented with ACS in the Clopidogrel optimal loading dose Usage to Reduce Recurrent EveNTs‐Organization to Assess Strategies in Ischemic Syndromes (CURRENT‐OASIS) 7 trial.18 Patients also received 150 mg of clopidogrel daily for the ensuing 6 days vs. the standard 75 mg daily dose. All patients then received 75 mg clopidogrel for 1 month of follow‐up. In the overall population, there was no benefit to using the higher clopidogrel loading dose. In contrast, there was a significant reduction in stent thrombosis in patients who received stents. The higher loading dose of clopidogrel was associated with a higher rate of bleeding.
Ticagrelor is a novel adenosine diphosphate receptor antagonist that was compared with clopidogrel in patients with ACS.19, 20 Compared to clopidogrel, ticagrelor significantly reduced ischemic events and there was also a significant reduction in cardiovascular mortality and in all‐cause mortality. Surprisingly, overall major bleeding did not increase with ticagrelor, but nonCABG‐related major bleeding increased.
The use of proton pump inhibitors (PPIs) in patients receiving dual antiplatelet therapy has also been a matter of vigorous recent debate.21 Evidence to date suggests there is no significant clinical interaction between PPIs and prasugrel. The data with clopidogrel and PPIs are mixed, although data are limited because much were derived from observational studies. Randomized clinical trial data are needed to assess whether there is an interaction between clopidogrel and PPIs that warrants clinical action, although preliminary data suggest there is no adverse cardiovascular interaction.22
New data regarding the intravenous anticoagulant bivalirudin have become available and have been incorporated into the Focused Guideline Update. Although bivalirudin is to be used primarily in the catheterization laboratory during PCI, it does appear to be associated with significantly less bleeding than heparin plus glycoprotein IIb/IIIa inhibitors.11, 2326
Case Study (cont)
The patient undergoes cardiac catheterization. An occluded dominant left circumflex artery is noted and is opened up with balloon angioplasty after aspiration thrombectomy. The patient receives 60 mg of prasugrel as a loading dose and bivalirudin as the anticoagulant during the procedure. A drug‐eluting stent is implanted with excellent results. The patient is transferred to the cardiac care unit for further care. It appears that this is a patient who functionally has an ST‐segment elevation MI with an occluded artery, although it manifested on the ECG as ST depression. Because of the patient's ongoing chest discomfort, it was fortunate that prompt angiography was performed.
Discussion
Patients with ACS present several challenges in management. Risk stratification is particularly important for nonST‐segment elevation ACS and requires thoughtful evaluation by the physician. Additionally, the large amount of new data and guideline updates create a rapidly evolving field, making it difficult to keep abreast of new developments. Physicians of patients with ACS need to be aware of these key developments so that they can provide optimal care to their patients with potentially life‐threatening ACS.
Acknowledgements
Denise M. Erkkila, RPh of DIME, provided editorial assistance consisting of help with tables, figures, and reference formatting for this manuscript.
Acute coronary syndrome (ACS) remains a major healthcare challenge. Currently, the majority of patients with ACS have nonST‐segment (ST, part of an electrocardiogram between the QRS complex and the T wave) elevation myocardial infarction (MI) and unstable angina.1 Nevertheless, ST‐segment elevation MI is also an important cause of morbidity and mortality. In recent years, our understanding of ACS has improved as a result of several major advances based on results from multiple randomized clinical trials and registry analyses. The results of these analyses have influenced guidelines issued by professional societies and in some cases have become performance metrics. Therefore, it is particularly important for physicians involved in the care of patients with ACS to be aware of evolving treatment patterns (Table 1).
|
| Hospitalists should demonstrate a knowledge of: |
| ACS without enzyme leak, NSTEMI, and STEMI |
| Variable presentations of unstable angina, acute MI |
| Conditions that mimic ACS |
| Cardiac biomarkers |
| Role of noninvasive cardiac testing |
| Risks; indications for cardiac catheterization |
| Risk factors for CAD |
| Validated risk stratification tools |
| Indications for hospitalization of patients with chest pain |
| Indications, contraindications for thrombolytic therapy |
| Indications, contraindications, and pharmacology of drugs for ACS |
| Indications for early invasive interventions |
| Angiography, stenting and/or CABG |
| Laboratory studies or imaging indicative of disease severity |
| Safe hospital discharge |
| Hospitalists should demonstrate skill in: |
| History and physical exam relative to cardiac disease |
| Recognizing signs and severity of ACS |
| Diagnosing ACS through appropriate testing |
| History and physical, ECG, x‐rays, biomarkers |
| Risk stratification using validated tools |
| Formulating an evidence‐based treatment plan |
| Identifying patients for thrombolytics and/or early revascularization |
| Recognizing and treating patient discomfort |
| Recognizing decompensation, initiating immediate therapy |
| Managing complicating factors |
| Bleeding, inadequate response, cardiopulmonary compromise |
| Timely patient assessment, co‐management with other providers |
| Hospitalists should demonstrate attitudes that facilitate: |
| Communication with patients and families relative to cardiac disease and all aspects of care plan |
| Obtain informed consent |
| Early specialty consultation |
| Initiation of secondary prevention measures before discharge |
| Multidisciplinary care throughout the hospital stay |
| Safe discharge and transition back into primary care |
Case Study
A 64‐year‐old man presents to the emergency department with the chief complaint of chest pressure for the past 2 hours. His chest pressure began after he moved furniture in his home. He initially believed that a pulled muscle was the cause of the pain, but when the discomfort did not improve with rest and continued to worsen, he thought it best that his wife drive him to the emergency department, where he continues to have chest pressure. He has never had this symptom before. His past medical history is notable only for mild hypertension for which he takes hydrochlorothiazide 25 mg daily. Otherwise, he has been healthy.
Clinical Presentation and Risk Assessment
The clinical presentation of ACS is not always straightforward. Although physicians frequently inquire about chest pain, the pain often manifests as chest heaviness or chest pressure. Additionally, some patients have a more atypical presentation, where the predominant symptom of acute coronary ischemia is dyspnea or extreme fatigue. These atypical presentations are believed to be somewhat more common in women and in the elderly, but it is important to realize that they can occur in any patient. Nausea, vomiting, or diaphoresis may accompany these symptoms or occur in isolation. Chest discomfort radiating to the jaw, neck, or left arm may be present, but is not necessary to the diagnosis. Thus, we see a variety of symptoms presenting in a patient with ACS.
This varied presentation makes objective assessment of ACS particularly important. To inform assessment, biomarkers have emerged as a quick and effective tool to help with the diagnosis of ACS. In particular, troponin measurement is important and serial troponin measurement is useful to exclude myonecrosis. It should be noted that the initial troponin level may be normal during the early stages of ACS. A bedside troponin measurement can be useful for rapid identification of myocardial damage. Quantitative troponin measurement also adds value, as higher levels of troponin are associated with progressively worse outcomes, including mortality (Figure 1). Although a number of biomarkers are available, the most important commonly used at present is troponin.0, 0, 0, 0





In addition to biomarkers, the electrocardiogram (ECG) remains extremely important in diagnosis and risk stratification. In a moderate‐level emergency department, the triage nurse should obtain a 12‐lead ECG for a patient with a history that is suspicious for coronary ischemia, and ask the attending physician to review the ECG immediately. If there is ST‐segment elevation or any new or presumed new left bundle‐branch block, the patient should be triaged to the ST‐segment elevation MI pathway of care. If there is ST‐segment depression or marked T‐wave inversion, this greatly raises the suspicion for nonST‐segment elevation MI or unstable angina. The presence of any of these features on the ECG places the patient at markedly elevated risk of short‐term ischemic complications.
A protocol should be in place for rapid treatment of patients with ST‐segment elevation MI.2 If the hospital has 24/7 percutaneous coronary intervention (PCI) capability, the catheterization lab should be immediately activated and the patient should proceed to primary PCI. The goal door‐to‐balloon time is 90 minutes or less. A patient who presents to a hospital without primary PCI capability should receive either fibrinolysis or be transferred to a center that can perform primary PCI. If fibrinolytic therapy is planned, it is essential that the patient not have any absolute contraindications to fibrinolytic therapy. Fibrinolysis should be administered within 30 minutes of patient contact. If transfer for primary PCI is planned, it is important that systems to support the transfer are in place so that the time from first medical contact to PCI does not exceed 90 minutes. As a practical point, it can be difficult to achieve these short transfer times in many geographic regions of the United States. However, with organized systems of care, it is certainly possible to have effective transfer systems and to achieve a short door‐to‐balloon time.3
If the patient does not have ST‐segment elevation MI, the next step depends on the patient's level of risk, where risk stratification is particularly important. As mentioned above, troponin measurement and the ECG are both essential aspects of risk stratification, but they alone are not sufficient to establish risk. It is recommended that an objective risk tool also be used. This is especially important because the patient can be initially troponin‐negative and have a normal ECG but still be at high risk for ischemic complications. The TIMI risk score (Table 2) is 1 of a number of resources that can help determine whether patients are at high risk for short‐term ischemic complications using means more objective than the eyeball test (Table 3).
|
| Historical |
| Age 65 year |
| 3 CAD risk factors |
| Family history, hypertension, hypercholesterolemia, diabetes, active smoker |
| Known CAD (stenosis 50%) |
| ASA use in past 7 days |
| At presentation |
| Recent (24 hours) severe angina |
| Elevated cardiac markers |
| ST deviation 0.5 mm |
| Risk score = total points, range: 07 |
| Risk Score | Death or MI (%) | Death, MI, or Urgent Revascularization (%) |
|---|---|---|
| ||
| 0/1 | 3 | 5 |
| 2 | 3 | 8 |
| 3 | 5 | 13 |
| 4 | 7 | 20 |
| 5 | 12 | 26 |
| 6/7 | 19 | 41 |
Delineation of the coronary anatomy in the catheterization lab is warranted for patients judged to be at high risk on the basis of the TIMI risk score, which would include most patients with elevated troponin or ST‐segment deviation. Many patients will undergo PCI on the basis of those test results and a smaller percentage might undergo coronary artery bypass grafting (CABG). Additionally, a sizeable minority of patients will be managed medically. This latter group is challenging because it consists of patients who have either trivial coronary artery disease or extensive coronary artery disease not amenable to revascularization and who have either a very low or very high risk of ischemic complications.
Even though catheterization may not be necessary, further evaluation is warranted in patients with ACS deemed to be at low risk. Typically, some form of functional assessment is indicated. In patients who are able to exercise, this would consist of exercise stress testing, often with an imaging modality. If the stress test is abnormal, cardiac catheterization is often the next step.
Case Study (cont)
An ECG is rapidly obtained on this patient and there is ST‐segment depression in leads II, III, and aVF. A bedside troponin is positive. The patient is at high risk of ischemic complications. He is diagnosed with nonST‐segment elevation MI. The next step is to initiate medical therapy. Presumably, the patient would have already (or at least should have already) received aspirin. Chewing or swallowing a dose of 325 mg nonenteric coated aspirin should provide a prompt aspirin effect. It would be reasonable to initiate anticoagulation as well, and the guidelines support a number of choices such as unfractionated heparin or low molecular weight heparin. Consideration should also be given to starting additional antiplatelet therapy, such as a loading dose of clopidogrel. Although aspirin provides some degree of antiplatelet effect, in a patient with activated platelets who presents with an ACS, additional antiplatelet therapy is necessary, although the exact timing of it is a matter of debate. Finally, consideration needs to be given to the need for catheterization. This patient, on the basis of his high ischemic risk and lack of obvious contraindications, should go to the catheterization laboratory, and the timing of catheterization requires further thought.
Guideline Update
The American College of Cardiology/American Heart Association 2009 Focused Guideline Update provides new information and recommendations pertinent to the care of patients with ACS2 and incorporates new data relevant to the initial emergency care and subsequent inpatient care of patients with ACS. Guideline highlights are presented in Table 4.
| Intervention | Recommendation |
|---|---|
| |
| GP IIb/IIIa receptor antagonists | |
| Class IIa | Start abciximab, tirofiban, or eptifibatide at primary PCI (with/without stenting) in selected patients with STEMI. |
| Class IIb | Uncertain value in STEMI when given before arrival at catheterization lab. |
| Thienopyridines | |
| Class I | Use loading dose for planned PCI in STEMI. Regimens for primary and nonprimary PCI are detailed within the guideline. |
| Duration of therapy after stent placement of at least 12 months. Stop early if bleeding risk outweighs benefit. | |
| Discontinue before planned, delayed CABG (5 d clopidogrel; 7 d prasugrel) unless the need for CABG outweighs bleeding risk. | |
| Class IIb | After DES placement, consider continuing clopidogrel or prasugrel beyond the first 15 months of therapy. |
| Class III | Prasugrel is not recommended for primary PCI in patients with STEMI who have a history of stroke or TIA. |
| Parenteral anticoagulants | |
| Class I | In primary PCI, supportive anticoagulants include unfractionated heparin, enoxaparin, fondaparinux, or bivalirudin, following ASA and a thienopyridine. |
| Class IIa | Bivalirudin is reasonable in patients at high risk of bleeding undergoing PCI for STEMI. |
| Triage and transfer for PCI | |
| Class I | STEMI system of care is supported by dedicated teams and protocols required for all communities. |
| Class IIa | Transfer patients who received fibrinolytic therapy at a nonPCI‐capable facility to a PCI‐capable facility. Consider preparatory antithrombotic regimen before or during transfer. |
| Class IIb | Consider expeditious transfer of nonhigh‐risk patients from a nonPCI‐capable facility to a PCI‐capable facility after administration of fibrinolytic. Consider preparatory antithrombotic regimen before or during transfer. |
| Intensive glucose control in STEMI | |
| Class IIa | Insulin is reasonable to maintain glucose <180 mg/dL (avoid hypoglycemia) for any patient with STEMI. |
| Thrombus aspiration during PCI of STEMI | |
| Class IIa | Aspiration thombectomy is reasonable. |
| Use of stents in STEMI | |
| Class IIa | DES is a reasonable alternative to BMS for primary PCI in STEMI. |
| Class IIb | Consider DES when clinical or anatomical factors suggest favorable safety and efficacy for DES. |
| Angiography in CKD | |
| Class I | Isomolar contrast or low molecular weight contrast (not ioxaglate or iohexol) is indicated for CKD patients not on dialysis. |
| Fractional flow reserve | |
| Class IIa | FFR is useful to assess a specific coronary lesion or as an alternative to noninvasive functional testing to justify PCI. Reasonable for intermediate coronary stenosis in patients with angina. |
| Class III | Routine use of FFR is not recommended to assess severity of CAD in patients with angina who have had a positive, unequivocal, noninvasive functional study. |
| PCI for unprotected left main CAD | |
| Class IIb | PCI of left main coronary artery with stents is an alternative to CABG for anatomy associated with low risk of PCI complications and a clinical scenario with higher risk of adverse surgical outcomes. |
| Timing of angiography and antiplatelet therapy in UA/NSTEMI | |
| Class I | Initiate dual‐antiplatelet therapy for UA/NSTEMI and an invasive approach. Start ASA on presentation. Clopidogrel (before or at PCI) or prasugrel (at PCI) as a second antiplatelet agent. |
| Class IIa | Early invasive strategy within 12 to 24 hours of admission is reasonable for stabilized high‐risk UA/NSTEMI; an early approach is also reasonable for UA/STEMI not at high‐risk. |
Several studies support an invasive strategy to assess high‐risk ACS patients. Randomized clinical trials and meta‐analyses of these trials have confirmed a significant reduction in subsequent ischemic events, including mortality, in patients who undergo an invasive vs. a more conservative strategy.4 Registry data have confirmed that these randomized clinical trial data reflect patients in the real‐world setting of clinical practice.5
The timing of angiography has recently been examined in detail.6, 7 It appears that for patients with nonST‐segment elevation ACS, unlike those with ST‐segment elevation MI, there is no need for emergent transfer to the catheterization laboratory, assuming patients are electrically and hemodynamically stable. Emergency transfer is warranted for unstable patients and those with ongoing chest discomfort. Otherwise, it appears sufficient to send the patient with nonST‐segment elevation ACS for catheterization within the subsequent 48 hours, or, alternatively, to adopt a more expectant approach in which catheterization is deferred until either recurrent symptoms develop or risk stratification suggests that there is substantial myocardium in jeopardy.
PCI is performed in the catheterization laboratory most often in the setting of ACS.5 When PCI is performed, an important consideration is whether to use a bare metal stent or a drug‐eluting stent.8 Drug‐eluting stents have been shown to have a significant benefit in reducing restenosis and the need for repeat revascularization. However, in aggregate, they have not been shown to either increase or decrease mortality.9 A key issue for the referring physician is to ascertain whether patients who go to the catheterization laboratory are likely to tolerate and be compliant with prolonged dual antiplatelet therapy. If it appears that the patient can or will not be compliant, a bare metal stent is preferable to a drug‐eluting stent; a bare metal stent requires dual antiplatelet therapy of shorter duration.
Additional considerations when sending patients to the catheterization laboratory are related to renal function. In patients with renal dysfunction, the most important way to prevent contrast nephropathy is adequate hydration prior to the procedure. In patients with left ventricular dysfunction, hydration must be done judiciously. Other strategies for preventing contrast nephropathy are being studied, although it is not entirely clear which strategies beyond hydration are truly effective.
Use of upstream glycoprotein IIb/IIIa inhibitors has become more common in patients with nonST‐segment elevation ACS. However, the most recent trial to examine this issue, the Early Glycoprotein IIb/IIIa Inhibition in NonST‐Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial, did not find a clear benefit for routine administration of upstream glycoprotein IIb/IIIa inhibitors when studying all patients with ACS.10 There did appear to be a signal of benefit in troponin‐positive patients, but as an overall strategy no significant benefit and even some detriment associated with an increase in bleeding were shown. Similarly, the results of the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial did not support the benefit of upstream glycoprotein IIb/IIIa inhibitors in patients with ACS.11
New data have also been released with respect to the thienopyridines. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with PrasugrelThrombolysis in Myocardial Infarction (TRITONTIMI) 38 study found that the more potent thienopyridine prasugrel significantly reduced ischemic events when compared with clopidogrel in patients with ACS undergoing PCI.1214 A significant reduction in stent thrombosis was reported regardless of the type of stent.15 However, the study reported a significant increase in major bleeding and a small, but statistically significant, excess of fatal bleeding. A subgroup analysis of patients with diabetes or with ST‐segment elevation MI from the TRITON‐TIMI 38 study showed a particularly large benefit associated with the use of prasugrel vs. clopidogrel and, interestingly, bleeding hazards were attenuated in these subgroups.16, 17 In the small subgroup of patients with prior stroke or transient ischemic attack (TIA), there was an excessive rate of intracranial hemorrhage with prasugrel vs. clopidogrel, indicating that prasugrel should not be used in these patients. Patients age 75 years or older or who weighed less than 60 kg also appeared to have a higher bleeding risk with prasugrel compared to clopidogrel. Careful thought is needed before using prasugrel in those patients identified as having a higher risk of bleeding.
Recently, a higher clopidogrel loading dose of 600 mg vs. the standard 300 mg dose was tested in patients who presented with ACS in the Clopidogrel optimal loading dose Usage to Reduce Recurrent EveNTs‐Organization to Assess Strategies in Ischemic Syndromes (CURRENT‐OASIS) 7 trial.18 Patients also received 150 mg of clopidogrel daily for the ensuing 6 days vs. the standard 75 mg daily dose. All patients then received 75 mg clopidogrel for 1 month of follow‐up. In the overall population, there was no benefit to using the higher clopidogrel loading dose. In contrast, there was a significant reduction in stent thrombosis in patients who received stents. The higher loading dose of clopidogrel was associated with a higher rate of bleeding.
Ticagrelor is a novel adenosine diphosphate receptor antagonist that was compared with clopidogrel in patients with ACS.19, 20 Compared to clopidogrel, ticagrelor significantly reduced ischemic events and there was also a significant reduction in cardiovascular mortality and in all‐cause mortality. Surprisingly, overall major bleeding did not increase with ticagrelor, but nonCABG‐related major bleeding increased.
The use of proton pump inhibitors (PPIs) in patients receiving dual antiplatelet therapy has also been a matter of vigorous recent debate.21 Evidence to date suggests there is no significant clinical interaction between PPIs and prasugrel. The data with clopidogrel and PPIs are mixed, although data are limited because much were derived from observational studies. Randomized clinical trial data are needed to assess whether there is an interaction between clopidogrel and PPIs that warrants clinical action, although preliminary data suggest there is no adverse cardiovascular interaction.22
New data regarding the intravenous anticoagulant bivalirudin have become available and have been incorporated into the Focused Guideline Update. Although bivalirudin is to be used primarily in the catheterization laboratory during PCI, it does appear to be associated with significantly less bleeding than heparin plus glycoprotein IIb/IIIa inhibitors.11, 2326
Case Study (cont)
The patient undergoes cardiac catheterization. An occluded dominant left circumflex artery is noted and is opened up with balloon angioplasty after aspiration thrombectomy. The patient receives 60 mg of prasugrel as a loading dose and bivalirudin as the anticoagulant during the procedure. A drug‐eluting stent is implanted with excellent results. The patient is transferred to the cardiac care unit for further care. It appears that this is a patient who functionally has an ST‐segment elevation MI with an occluded artery, although it manifested on the ECG as ST depression. Because of the patient's ongoing chest discomfort, it was fortunate that prompt angiography was performed.
Discussion
Patients with ACS present several challenges in management. Risk stratification is particularly important for nonST‐segment elevation ACS and requires thoughtful evaluation by the physician. Additionally, the large amount of new data and guideline updates create a rapidly evolving field, making it difficult to keep abreast of new developments. Physicians of patients with ACS need to be aware of these key developments so that they can provide optimal care to their patients with potentially life‐threatening ACS.
Acknowledgements
Denise M. Erkkila, RPh of DIME, provided editorial assistance consisting of help with tables, figures, and reference formatting for this manuscript.
- ,,, et al.Heart disease and stroke statistics‐‐2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,, et al.2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol.2009;54:2205–2241.
- ,,, et al.Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes.JAMA.2009;302:2207–2213.
- ,,,,.Benefit of early invasive therapy in acute coronary syndromes: a meta‐analysis of contemporary randomized clinical trials.J Am Coll Cardiol.2006;48:1319–1325.
- ,,, et al.Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative.JAMA.2004;292:2096–2104.
- ,,, et al.Early versus delayed invasive intervention in acute coronary syndromes.N Engl J Med.2009;360:2165–2175.
- ,,, et al.Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial.JAMA.2009;302:947–954.
- ,.Drug‐eluting stents: dual antiplatelet therapy for every survivor?Circulation.2007;116:696–699.
- ,.Appropriate use of drug‐eluting stents: balancing the reduction in restenosis with the concern of late thrombosis.Lancet.2008;371:2134–2143.
- ,,, et al.Early versus delayed, provisional eptifibatide in acute coronary syndromes.N Engl J Med.2009;360:2176–2190.
- ,,, et al.Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one‐year results from the ACUITY trial.JAMA.2007;298:2497–2506.
- .Intensifying platelet inhibition‐‐navigating between Scylla and Charybdis.N Engl J Med.2007;357:2078–2081.
- .Prasugrel in clinical practice.N Engl J Med.2009;361:940–942.
- ,,, et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2007;357:2001–2015.
- ,,, et al.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON‐TIMI 38 trial: a subanalysis of a randomised trial.Lancet.2008;371:1353–1363.
- ,,, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial.Lancet.2009;373:723–731.
- ,,, et al.Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel—Thrombolysis in Myocardial Infarction 38.Circulation.2008;118:1626–1636.
- , CURRENT Investigators. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Available at: http://www.escardio.org/congresses/esc‐2009/congress‐reports/Pages/706003‐706004‐mehta‐vandewerf.aspx#discussant.2009. Accessed July 2010.
- .Antiplatelet therapy: ticagrelor in ACS‐what does PLATO teach us?Nat Rev Cardiol.2009;6:737–738.
- ,,, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2009;361:1045–1057.
- ,.Omeprazole and clopidogrel: Should clinicians be worried?Cleve Clin J Med.2010;77:113–116.
- .COGENT: a prospective, randomized, placebo‐controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Transcatheter Cardiovascular Therapeutics (TCT) 2009; September 24,2009;San Francisco, CA.
- ,,, et al.The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) Trial: study design and rationale.Am Heart J.2008;156:44–56.
- ,,, et al.Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial.Lancet.2009;374:1149–1159.
- ,,, et al.Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale.Am Heart J.2004;148:764–775.
- ,,, et al.Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial.Lancet.2007;369:907–919.
- Society of Hospital Medicine.Acute coronary syndrome.J Hosp Med.2006;1 (suppl 1):2–3.
- ,,, et al.The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making.JAMA.2000;284:835–842.
- ,,, et al.Heart disease and stroke statistics‐‐2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,, et al.2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol.2009;54:2205–2241.
- ,,, et al.Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes.JAMA.2009;302:2207–2213.
- ,,,,.Benefit of early invasive therapy in acute coronary syndromes: a meta‐analysis of contemporary randomized clinical trials.J Am Coll Cardiol.2006;48:1319–1325.
- ,,, et al.Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative.JAMA.2004;292:2096–2104.
- ,,, et al.Early versus delayed invasive intervention in acute coronary syndromes.N Engl J Med.2009;360:2165–2175.
- ,,, et al.Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial.JAMA.2009;302:947–954.
- ,.Drug‐eluting stents: dual antiplatelet therapy for every survivor?Circulation.2007;116:696–699.
- ,.Appropriate use of drug‐eluting stents: balancing the reduction in restenosis with the concern of late thrombosis.Lancet.2008;371:2134–2143.
- ,,, et al.Early versus delayed, provisional eptifibatide in acute coronary syndromes.N Engl J Med.2009;360:2176–2190.
- ,,, et al.Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one‐year results from the ACUITY trial.JAMA.2007;298:2497–2506.
- .Intensifying platelet inhibition‐‐navigating between Scylla and Charybdis.N Engl J Med.2007;357:2078–2081.
- .Prasugrel in clinical practice.N Engl J Med.2009;361:940–942.
- ,,, et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2007;357:2001–2015.
- ,,, et al.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON‐TIMI 38 trial: a subanalysis of a randomised trial.Lancet.2008;371:1353–1363.
- ,,, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial.Lancet.2009;373:723–731.
- ,,, et al.Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel—Thrombolysis in Myocardial Infarction 38.Circulation.2008;118:1626–1636.
- , CURRENT Investigators. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Available at: http://www.escardio.org/congresses/esc‐2009/congress‐reports/Pages/706003‐706004‐mehta‐vandewerf.aspx#discussant.2009. Accessed July 2010.
- .Antiplatelet therapy: ticagrelor in ACS‐what does PLATO teach us?Nat Rev Cardiol.2009;6:737–738.
- ,,, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2009;361:1045–1057.
- ,.Omeprazole and clopidogrel: Should clinicians be worried?Cleve Clin J Med.2010;77:113–116.
- .COGENT: a prospective, randomized, placebo‐controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Transcatheter Cardiovascular Therapeutics (TCT) 2009; September 24,2009;San Francisco, CA.
- ,,, et al.The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) Trial: study design and rationale.Am Heart J.2008;156:44–56.
- ,,, et al.Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial.Lancet.2009;374:1149–1159.
- ,,, et al.Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale.Am Heart J.2004;148:764–775.
- ,,, et al.Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial.Lancet.2007;369:907–919.
- Society of Hospital Medicine.Acute coronary syndrome.J Hosp Med.2006;1 (suppl 1):2–3.
- ,,, et al.The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making.JAMA.2000;284:835–842.
Omeprazole and clopidogrel: Should clinicians be worried?
Many clinicians are concerned about a possible interaction between the proton pump inhibitor omeprazole (Prilosec) and the antiplatelet drug clopidogrel (Plavix), which is often given to patients as part of dual antiplatelet therapy after an acute coronary syndrome or a percutaneous coronary intervention. Indeed, the US Food and Drug Administration (FDA) has warned that omeprazole reduces the antiplatelet effect of clopidogrel.
Although we should not take such warnings lightly, we also should not be alarmed. The data on which the FDA warning was based came mostly from laboratory assays of platelet function. Preliminary results from a randomized, controlled clinical trial with hard end points show that, for the time being, we should not change the way we manage patients.
PROTON PUMP INHIBITORS DECREASE GASTROINTESTINAL BLEEDING
Dual antiplatelet therapy with aspirin and clopidogrel decreases the risk of major adverse cardiac events after an acute coronary syndrome or a percutaneous coronary intervention compared with aspirin alone.1 However, it also increases the risk of gastrointestinal bleeding. A recent analysis determined that dual antiplatelet therapy was the most significant risk factor associated with serious or fatal gastrointestinal bleeding in high-risk survivors of myocardial infarction.2
Given the risks of significant morbidity and death in patients on dual antiplatelet therapy who develop gastrointestinal bleeding, an expert consensus panel recommended the use of proton pump inhibitors in patients on dual antiplatelet therapy who have risk factors for gastrointestinal bleeding.3 Accordingly, these drugs are commonly used for gastrointestinal protection in patients requiring dual antiplatelet therapy.
A POSSIBLE CYP450 INTERACTION
Clopidogrel is metabolized from a prodrug to its active metabolite by the cytochrome P450 (CYP450) system. Proton pump inhibitors also are metabolized by the CYP450 system.4 Proton pump inhibitors are thought to diminish the activity of clopidogrel via inhibition of the CYP2C19 isoenzyme. However, the clinical significance of this inhibition is not clear. Different drugs of this class inhibit the CYP450 system to varying degrees.
The potential interaction between proton pump inhibitors and clopidogrel is worrisome for many physicians, since adverse cardiovascular outcomes are more common in patients in whom the antiplatelet response to clopidogrel is impaired.1 This interaction led to the publication of numerous articles, and prompted the FDA to carefully analyze the potential clinical implications.
In several randomized trials, omeprazole diminished the response to clopidogrel (measured via platelet function assays).5,6 It is unclear if this is a class effect, as proton pump inhibitors other than omeprazole have not consistently been shown to have this effect.6,7 Observational studies of the effect of co-administration of a proton pump inhibitor and clopidogrel on cardiovascular outcomes following acute coronary syndromes have had conflicting findings.8–11
THE FDA ISSUES AN ADVISORY
Given the reports of an impaired platelet response to clopidogrel with omeprazole, the FDA asked the manufacturer for data on this potential interaction. The data showed diminished platelet inhibition when clopidogrel was co-administered with omeprazole or when the two were taken 12 hours apart.
On November 17, 2009, the FDA issued a patient advisory and updated the patient safety information on the package insert for clopidogrel about this drug interaction.12 Specifically, the FDA warns that omeprazole reduces the antiplatelet effect of clopidogrel by about 50%. The FDA warning sparked debate in the medical community, as the decision was based in part on ex vivo data.
POST HOC ANALYSES FROM RANDOMIZED CONTROLLED TRIALS
Several post hoc analyses of large randomized clinical trials have studied the potential interaction between proton pump inhibitors and clopidogrel.
In the Clopidogrel for the Reduction of Events During Observation (CREDO) trial, clopidogrel reduced the incidence of death, myocardial infarction, or stroke to a similar extent regardless of baseline use of a proton pump inhibitor.13
In patients undergoing percutaneous coronary intervention, the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation—Thrombolysis in Myocardial Infarction 44 (PRINCIPLE-TIMI 44) trial found that those taking a proton pump inhibitor had significantly less platelet inhibition with clopidogrel compared with those not on one.14 However, patients taking prasugrel (Effient) and a proton pump inhibitor only had a slight trend towards diminished platelet inhibition.14
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) found that proton pump inhibitors did not influence the long-term outcome of cardiovascular death, myocardial infarction, or stroke for patients on clopidogrel or prasugrel after an acute coronary syndrome.14 A subanalysis did not reveal any differences between omeprazole or other drugs of this class as to an effect on the primary outcome.
Though informative, the results of these post hoc analyses need to be validated with data from randomized clinical trials.
‘COGENT’ TRIAL HALTED EARLY, BUT PRELIMINARY RESULTS AVAILABLE
The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) trial was the first randomized clinical study of the effect of the interaction between clopidogrel and omeprazole on cardiovascular and gastrointestinal outcomes.15 In a double-blind fashion, patients with acute coronary syndromes or undergoing percutaneous coronary interventions were randomized to receive a fixed-dose combination pill containing either clopidogrel and delayed-release omeprazole or clopidogrel alone. All patients also received aspirin.
Unfortunately, the trial was stopped early because the sponsor declared bankruptcy. However, preliminary results revealed no significant difference in cardiovascular outcomes for patients on clopidogrel and omeprazole compared with clopidogrel alone.15 Furthermore, adverse gastrointestinal events were significantly fewer in patients on clopidogrel and omeprazole.
Thus, omeprazole appears to be safe and may offer gastrointestinal protection to patients on dual antiplatelet therapy, though we need to await publication of the full results.
‘SPICE ’ TRIAL TO EVALUATE POSSIBLE MECHANISMS OF INTERACTION
The Evaluation of the Influence of Statins and Proton Pump Inhibitors on Clopidogrel Antiplatelet Effects (SPICE) trial is a mechanistic study that will evaluate platelet function and genetic polymorphisms in patients on clopidogrel and aspirin after a percutaneous coronary intervention. They will be randomized to statin therapy plus different proton pump inhibitors.16 Prior concerns about an ex vivo interaction between clopidogrel and certain statins were not validated by clinical data.17
OUR RECOMMENDATIONS
Based on the current evidence, patients on aspirin and clopidogrel who have an indication for a proton pump inhibitor or who are at risk of gastrointestinal bleeding can continue or start taking a proton pump inhibitor, including omeprazole.
Switching to another proton pump inhibitor is not currently supported by any randomized clinical trial, nor is changing to a histamine H2-receptor antagonist. The effect of proton pump inhibitors other than omeprazole on clopidogrel is unclear, and it is not known if the interaction with clopidogrel is a class effect or specific to certain drugs of this class.18 On the other hand, we still have no compelling evidence of any major clinical interaction between alternative proton pump inhibitors and clopidogrel.18
Also, separating the dosing times of clopidogrel and omeprazole by 12 hours is not supported by any randomized clinical trial, and runs contrary to at least some ex vivo data.
It is important that all physicians assess the need for a proton pump inhibitor in their patients, as overuse of these drugs has been documented in certain settings.19
Clopidogrel and omeprazole share a common metabolic link via CYP2C19. Omeprazole, along with some other proton pump inhibitors, interacts with clopidogrel at the level of the CYP450 system. Platelet function studies show that platelet inhibition by clopidogrel is impaired, though the astute clinician should be aware of the wide variability associated with platelet function assays and clopidogrel.1,20 However, what may appear to be an interaction at the enzymatic level does not necessarily translate into worse clinical outcomes. Additionally, reliance on nonrandomized studies rather than on randomized clinical trials can be misleading.
- Depta JP, Bhatt DL. Aspirin and platelet adenosine diphosphate receptor antagonists in acute coronary syndromes and percutaneous coronary intervention: role in therapy and strategies to overcome resistance. Am J Cardiovasc Drugs 2008; 8:91–112.
- Moukarbel GV, Signorovitch JE, Pfeffer MA, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT trial. Eur Heart J 2009; 30:2226–2232.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2008; 52:1502–1517.
- Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 2004; 32:821–827.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Cuisset T, Frere C, Quilici J, et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose. The PACA (Proton Pump Inhibitors and Clopidogrel Association) prospective randomized study. J Am Coll Cardiol 2009; 54:1149–1153.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–148.e5.
- Aubert RE, Epstein RS, Teagarden JR, et al. Proton pump inhibitors effect on clopidogrel effectiveness: the Clopidogrel Medco Outcomes Study [abstract]. Circulation 2008; 118( suppl):S28–10–2008.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Rassen JA, Choudhry NK, Avorn J, Schneeweiss S. Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome. Circulation 2009; 120:2322–2329.
- US Food and Drug Administration. Public health advisory: updated safety information about a drug interaction between clopidogrel bisulfate (marketed as Plavix) and omeprazole (marketed as Prilosec and Prilosec OTC). www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm190825.htm. Accessed 1/6/2010.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial. Circulation 2008; 118:S_815. Abstract 3999.
- O‘Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Bhatt DL. COGENT: A prospective, randomized, placebo-controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Presented at Transcatheter Cardiovascular Therapeutics; September 24, 2009; San Francisco, Calif.
- National Institutes of Health. Evaluation of the Influence of Statins and Proton Pump Inhibitors on Clopidogrel Antiplatelet Effects (SPICE). http://clinicaltrials.gov/ct2/show/NCT00930670. Accessed 1/6/2010.
- Saw J, Brennan DM, Steinhubl SR, et al. Lack of evidence of clopidogrel-statin interaction in the CHARISMA trial. J Am Coll Cardiol 2007; 50:291–295.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2009 Nov 10. [Epub ahead of print].
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
Many clinicians are concerned about a possible interaction between the proton pump inhibitor omeprazole (Prilosec) and the antiplatelet drug clopidogrel (Plavix), which is often given to patients as part of dual antiplatelet therapy after an acute coronary syndrome or a percutaneous coronary intervention. Indeed, the US Food and Drug Administration (FDA) has warned that omeprazole reduces the antiplatelet effect of clopidogrel.
Although we should not take such warnings lightly, we also should not be alarmed. The data on which the FDA warning was based came mostly from laboratory assays of platelet function. Preliminary results from a randomized, controlled clinical trial with hard end points show that, for the time being, we should not change the way we manage patients.
PROTON PUMP INHIBITORS DECREASE GASTROINTESTINAL BLEEDING
Dual antiplatelet therapy with aspirin and clopidogrel decreases the risk of major adverse cardiac events after an acute coronary syndrome or a percutaneous coronary intervention compared with aspirin alone.1 However, it also increases the risk of gastrointestinal bleeding. A recent analysis determined that dual antiplatelet therapy was the most significant risk factor associated with serious or fatal gastrointestinal bleeding in high-risk survivors of myocardial infarction.2
Given the risks of significant morbidity and death in patients on dual antiplatelet therapy who develop gastrointestinal bleeding, an expert consensus panel recommended the use of proton pump inhibitors in patients on dual antiplatelet therapy who have risk factors for gastrointestinal bleeding.3 Accordingly, these drugs are commonly used for gastrointestinal protection in patients requiring dual antiplatelet therapy.
A POSSIBLE CYP450 INTERACTION
Clopidogrel is metabolized from a prodrug to its active metabolite by the cytochrome P450 (CYP450) system. Proton pump inhibitors also are metabolized by the CYP450 system.4 Proton pump inhibitors are thought to diminish the activity of clopidogrel via inhibition of the CYP2C19 isoenzyme. However, the clinical significance of this inhibition is not clear. Different drugs of this class inhibit the CYP450 system to varying degrees.
The potential interaction between proton pump inhibitors and clopidogrel is worrisome for many physicians, since adverse cardiovascular outcomes are more common in patients in whom the antiplatelet response to clopidogrel is impaired.1 This interaction led to the publication of numerous articles, and prompted the FDA to carefully analyze the potential clinical implications.
In several randomized trials, omeprazole diminished the response to clopidogrel (measured via platelet function assays).5,6 It is unclear if this is a class effect, as proton pump inhibitors other than omeprazole have not consistently been shown to have this effect.6,7 Observational studies of the effect of co-administration of a proton pump inhibitor and clopidogrel on cardiovascular outcomes following acute coronary syndromes have had conflicting findings.8–11
THE FDA ISSUES AN ADVISORY
Given the reports of an impaired platelet response to clopidogrel with omeprazole, the FDA asked the manufacturer for data on this potential interaction. The data showed diminished platelet inhibition when clopidogrel was co-administered with omeprazole or when the two were taken 12 hours apart.
On November 17, 2009, the FDA issued a patient advisory and updated the patient safety information on the package insert for clopidogrel about this drug interaction.12 Specifically, the FDA warns that omeprazole reduces the antiplatelet effect of clopidogrel by about 50%. The FDA warning sparked debate in the medical community, as the decision was based in part on ex vivo data.
POST HOC ANALYSES FROM RANDOMIZED CONTROLLED TRIALS
Several post hoc analyses of large randomized clinical trials have studied the potential interaction between proton pump inhibitors and clopidogrel.
In the Clopidogrel for the Reduction of Events During Observation (CREDO) trial, clopidogrel reduced the incidence of death, myocardial infarction, or stroke to a similar extent regardless of baseline use of a proton pump inhibitor.13
In patients undergoing percutaneous coronary intervention, the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation—Thrombolysis in Myocardial Infarction 44 (PRINCIPLE-TIMI 44) trial found that those taking a proton pump inhibitor had significantly less platelet inhibition with clopidogrel compared with those not on one.14 However, patients taking prasugrel (Effient) and a proton pump inhibitor only had a slight trend towards diminished platelet inhibition.14
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) found that proton pump inhibitors did not influence the long-term outcome of cardiovascular death, myocardial infarction, or stroke for patients on clopidogrel or prasugrel after an acute coronary syndrome.14 A subanalysis did not reveal any differences between omeprazole or other drugs of this class as to an effect on the primary outcome.
Though informative, the results of these post hoc analyses need to be validated with data from randomized clinical trials.
‘COGENT’ TRIAL HALTED EARLY, BUT PRELIMINARY RESULTS AVAILABLE
The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) trial was the first randomized clinical study of the effect of the interaction between clopidogrel and omeprazole on cardiovascular and gastrointestinal outcomes.15 In a double-blind fashion, patients with acute coronary syndromes or undergoing percutaneous coronary interventions were randomized to receive a fixed-dose combination pill containing either clopidogrel and delayed-release omeprazole or clopidogrel alone. All patients also received aspirin.
Unfortunately, the trial was stopped early because the sponsor declared bankruptcy. However, preliminary results revealed no significant difference in cardiovascular outcomes for patients on clopidogrel and omeprazole compared with clopidogrel alone.15 Furthermore, adverse gastrointestinal events were significantly fewer in patients on clopidogrel and omeprazole.
Thus, omeprazole appears to be safe and may offer gastrointestinal protection to patients on dual antiplatelet therapy, though we need to await publication of the full results.
‘SPICE ’ TRIAL TO EVALUATE POSSIBLE MECHANISMS OF INTERACTION
The Evaluation of the Influence of Statins and Proton Pump Inhibitors on Clopidogrel Antiplatelet Effects (SPICE) trial is a mechanistic study that will evaluate platelet function and genetic polymorphisms in patients on clopidogrel and aspirin after a percutaneous coronary intervention. They will be randomized to statin therapy plus different proton pump inhibitors.16 Prior concerns about an ex vivo interaction between clopidogrel and certain statins were not validated by clinical data.17
OUR RECOMMENDATIONS
Based on the current evidence, patients on aspirin and clopidogrel who have an indication for a proton pump inhibitor or who are at risk of gastrointestinal bleeding can continue or start taking a proton pump inhibitor, including omeprazole.
Switching to another proton pump inhibitor is not currently supported by any randomized clinical trial, nor is changing to a histamine H2-receptor antagonist. The effect of proton pump inhibitors other than omeprazole on clopidogrel is unclear, and it is not known if the interaction with clopidogrel is a class effect or specific to certain drugs of this class.18 On the other hand, we still have no compelling evidence of any major clinical interaction between alternative proton pump inhibitors and clopidogrel.18
Also, separating the dosing times of clopidogrel and omeprazole by 12 hours is not supported by any randomized clinical trial, and runs contrary to at least some ex vivo data.
It is important that all physicians assess the need for a proton pump inhibitor in their patients, as overuse of these drugs has been documented in certain settings.19
Clopidogrel and omeprazole share a common metabolic link via CYP2C19. Omeprazole, along with some other proton pump inhibitors, interacts with clopidogrel at the level of the CYP450 system. Platelet function studies show that platelet inhibition by clopidogrel is impaired, though the astute clinician should be aware of the wide variability associated with platelet function assays and clopidogrel.1,20 However, what may appear to be an interaction at the enzymatic level does not necessarily translate into worse clinical outcomes. Additionally, reliance on nonrandomized studies rather than on randomized clinical trials can be misleading.
Many clinicians are concerned about a possible interaction between the proton pump inhibitor omeprazole (Prilosec) and the antiplatelet drug clopidogrel (Plavix), which is often given to patients as part of dual antiplatelet therapy after an acute coronary syndrome or a percutaneous coronary intervention. Indeed, the US Food and Drug Administration (FDA) has warned that omeprazole reduces the antiplatelet effect of clopidogrel.
Although we should not take such warnings lightly, we also should not be alarmed. The data on which the FDA warning was based came mostly from laboratory assays of platelet function. Preliminary results from a randomized, controlled clinical trial with hard end points show that, for the time being, we should not change the way we manage patients.
PROTON PUMP INHIBITORS DECREASE GASTROINTESTINAL BLEEDING
Dual antiplatelet therapy with aspirin and clopidogrel decreases the risk of major adverse cardiac events after an acute coronary syndrome or a percutaneous coronary intervention compared with aspirin alone.1 However, it also increases the risk of gastrointestinal bleeding. A recent analysis determined that dual antiplatelet therapy was the most significant risk factor associated with serious or fatal gastrointestinal bleeding in high-risk survivors of myocardial infarction.2
Given the risks of significant morbidity and death in patients on dual antiplatelet therapy who develop gastrointestinal bleeding, an expert consensus panel recommended the use of proton pump inhibitors in patients on dual antiplatelet therapy who have risk factors for gastrointestinal bleeding.3 Accordingly, these drugs are commonly used for gastrointestinal protection in patients requiring dual antiplatelet therapy.
A POSSIBLE CYP450 INTERACTION
Clopidogrel is metabolized from a prodrug to its active metabolite by the cytochrome P450 (CYP450) system. Proton pump inhibitors also are metabolized by the CYP450 system.4 Proton pump inhibitors are thought to diminish the activity of clopidogrel via inhibition of the CYP2C19 isoenzyme. However, the clinical significance of this inhibition is not clear. Different drugs of this class inhibit the CYP450 system to varying degrees.
The potential interaction between proton pump inhibitors and clopidogrel is worrisome for many physicians, since adverse cardiovascular outcomes are more common in patients in whom the antiplatelet response to clopidogrel is impaired.1 This interaction led to the publication of numerous articles, and prompted the FDA to carefully analyze the potential clinical implications.
In several randomized trials, omeprazole diminished the response to clopidogrel (measured via platelet function assays).5,6 It is unclear if this is a class effect, as proton pump inhibitors other than omeprazole have not consistently been shown to have this effect.6,7 Observational studies of the effect of co-administration of a proton pump inhibitor and clopidogrel on cardiovascular outcomes following acute coronary syndromes have had conflicting findings.8–11
THE FDA ISSUES AN ADVISORY
Given the reports of an impaired platelet response to clopidogrel with omeprazole, the FDA asked the manufacturer for data on this potential interaction. The data showed diminished platelet inhibition when clopidogrel was co-administered with omeprazole or when the two were taken 12 hours apart.
On November 17, 2009, the FDA issued a patient advisory and updated the patient safety information on the package insert for clopidogrel about this drug interaction.12 Specifically, the FDA warns that omeprazole reduces the antiplatelet effect of clopidogrel by about 50%. The FDA warning sparked debate in the medical community, as the decision was based in part on ex vivo data.
POST HOC ANALYSES FROM RANDOMIZED CONTROLLED TRIALS
Several post hoc analyses of large randomized clinical trials have studied the potential interaction between proton pump inhibitors and clopidogrel.
In the Clopidogrel for the Reduction of Events During Observation (CREDO) trial, clopidogrel reduced the incidence of death, myocardial infarction, or stroke to a similar extent regardless of baseline use of a proton pump inhibitor.13
In patients undergoing percutaneous coronary intervention, the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation—Thrombolysis in Myocardial Infarction 44 (PRINCIPLE-TIMI 44) trial found that those taking a proton pump inhibitor had significantly less platelet inhibition with clopidogrel compared with those not on one.14 However, patients taking prasugrel (Effient) and a proton pump inhibitor only had a slight trend towards diminished platelet inhibition.14
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) found that proton pump inhibitors did not influence the long-term outcome of cardiovascular death, myocardial infarction, or stroke for patients on clopidogrel or prasugrel after an acute coronary syndrome.14 A subanalysis did not reveal any differences between omeprazole or other drugs of this class as to an effect on the primary outcome.
Though informative, the results of these post hoc analyses need to be validated with data from randomized clinical trials.
‘COGENT’ TRIAL HALTED EARLY, BUT PRELIMINARY RESULTS AVAILABLE
The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) trial was the first randomized clinical study of the effect of the interaction between clopidogrel and omeprazole on cardiovascular and gastrointestinal outcomes.15 In a double-blind fashion, patients with acute coronary syndromes or undergoing percutaneous coronary interventions were randomized to receive a fixed-dose combination pill containing either clopidogrel and delayed-release omeprazole or clopidogrel alone. All patients also received aspirin.
Unfortunately, the trial was stopped early because the sponsor declared bankruptcy. However, preliminary results revealed no significant difference in cardiovascular outcomes for patients on clopidogrel and omeprazole compared with clopidogrel alone.15 Furthermore, adverse gastrointestinal events were significantly fewer in patients on clopidogrel and omeprazole.
Thus, omeprazole appears to be safe and may offer gastrointestinal protection to patients on dual antiplatelet therapy, though we need to await publication of the full results.
‘SPICE ’ TRIAL TO EVALUATE POSSIBLE MECHANISMS OF INTERACTION
The Evaluation of the Influence of Statins and Proton Pump Inhibitors on Clopidogrel Antiplatelet Effects (SPICE) trial is a mechanistic study that will evaluate platelet function and genetic polymorphisms in patients on clopidogrel and aspirin after a percutaneous coronary intervention. They will be randomized to statin therapy plus different proton pump inhibitors.16 Prior concerns about an ex vivo interaction between clopidogrel and certain statins were not validated by clinical data.17
OUR RECOMMENDATIONS
Based on the current evidence, patients on aspirin and clopidogrel who have an indication for a proton pump inhibitor or who are at risk of gastrointestinal bleeding can continue or start taking a proton pump inhibitor, including omeprazole.
Switching to another proton pump inhibitor is not currently supported by any randomized clinical trial, nor is changing to a histamine H2-receptor antagonist. The effect of proton pump inhibitors other than omeprazole on clopidogrel is unclear, and it is not known if the interaction with clopidogrel is a class effect or specific to certain drugs of this class.18 On the other hand, we still have no compelling evidence of any major clinical interaction between alternative proton pump inhibitors and clopidogrel.18
Also, separating the dosing times of clopidogrel and omeprazole by 12 hours is not supported by any randomized clinical trial, and runs contrary to at least some ex vivo data.
It is important that all physicians assess the need for a proton pump inhibitor in their patients, as overuse of these drugs has been documented in certain settings.19
Clopidogrel and omeprazole share a common metabolic link via CYP2C19. Omeprazole, along with some other proton pump inhibitors, interacts with clopidogrel at the level of the CYP450 system. Platelet function studies show that platelet inhibition by clopidogrel is impaired, though the astute clinician should be aware of the wide variability associated with platelet function assays and clopidogrel.1,20 However, what may appear to be an interaction at the enzymatic level does not necessarily translate into worse clinical outcomes. Additionally, reliance on nonrandomized studies rather than on randomized clinical trials can be misleading.
- Depta JP, Bhatt DL. Aspirin and platelet adenosine diphosphate receptor antagonists in acute coronary syndromes and percutaneous coronary intervention: role in therapy and strategies to overcome resistance. Am J Cardiovasc Drugs 2008; 8:91–112.
- Moukarbel GV, Signorovitch JE, Pfeffer MA, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT trial. Eur Heart J 2009; 30:2226–2232.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2008; 52:1502–1517.
- Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 2004; 32:821–827.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Cuisset T, Frere C, Quilici J, et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose. The PACA (Proton Pump Inhibitors and Clopidogrel Association) prospective randomized study. J Am Coll Cardiol 2009; 54:1149–1153.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–148.e5.
- Aubert RE, Epstein RS, Teagarden JR, et al. Proton pump inhibitors effect on clopidogrel effectiveness: the Clopidogrel Medco Outcomes Study [abstract]. Circulation 2008; 118( suppl):S28–10–2008.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Rassen JA, Choudhry NK, Avorn J, Schneeweiss S. Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome. Circulation 2009; 120:2322–2329.
- US Food and Drug Administration. Public health advisory: updated safety information about a drug interaction between clopidogrel bisulfate (marketed as Plavix) and omeprazole (marketed as Prilosec and Prilosec OTC). www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm190825.htm. Accessed 1/6/2010.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial. Circulation 2008; 118:S_815. Abstract 3999.
- O‘Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Bhatt DL. COGENT: A prospective, randomized, placebo-controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Presented at Transcatheter Cardiovascular Therapeutics; September 24, 2009; San Francisco, Calif.
- National Institutes of Health. Evaluation of the Influence of Statins and Proton Pump Inhibitors on Clopidogrel Antiplatelet Effects (SPICE). http://clinicaltrials.gov/ct2/show/NCT00930670. Accessed 1/6/2010.
- Saw J, Brennan DM, Steinhubl SR, et al. Lack of evidence of clopidogrel-statin interaction in the CHARISMA trial. J Am Coll Cardiol 2007; 50:291–295.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2009 Nov 10. [Epub ahead of print].
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Depta JP, Bhatt DL. Aspirin and platelet adenosine diphosphate receptor antagonists in acute coronary syndromes and percutaneous coronary intervention: role in therapy and strategies to overcome resistance. Am J Cardiovasc Drugs 2008; 8:91–112.
- Moukarbel GV, Signorovitch JE, Pfeffer MA, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT trial. Eur Heart J 2009; 30:2226–2232.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2008; 52:1502–1517.
- Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 2004; 32:821–827.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Cuisset T, Frere C, Quilici J, et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose. The PACA (Proton Pump Inhibitors and Clopidogrel Association) prospective randomized study. J Am Coll Cardiol 2009; 54:1149–1153.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–148.e5.
- Aubert RE, Epstein RS, Teagarden JR, et al. Proton pump inhibitors effect on clopidogrel effectiveness: the Clopidogrel Medco Outcomes Study [abstract]. Circulation 2008; 118( suppl):S28–10–2008.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Rassen JA, Choudhry NK, Avorn J, Schneeweiss S. Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome. Circulation 2009; 120:2322–2329.
- US Food and Drug Administration. Public health advisory: updated safety information about a drug interaction between clopidogrel bisulfate (marketed as Plavix) and omeprazole (marketed as Prilosec and Prilosec OTC). www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm190825.htm. Accessed 1/6/2010.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial. Circulation 2008; 118:S_815. Abstract 3999.
- O‘Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Bhatt DL. COGENT: A prospective, randomized, placebo-controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Presented at Transcatheter Cardiovascular Therapeutics; September 24, 2009; San Francisco, Calif.
- National Institutes of Health. Evaluation of the Influence of Statins and Proton Pump Inhibitors on Clopidogrel Antiplatelet Effects (SPICE). http://clinicaltrials.gov/ct2/show/NCT00930670. Accessed 1/6/2010.
- Saw J, Brennan DM, Steinhubl SR, et al. Lack of evidence of clopidogrel-statin interaction in the CHARISMA trial. J Am Coll Cardiol 2007; 50:291–295.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2009 Nov 10. [Epub ahead of print].
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
KEY POINTS
- Proton pump inhibitors such as omeprazole reduce the risk of gastrointestinal bleeding in patients on antiplatelet therapy after an acute coronary syndrome or percutaneous coronary intervention.
- Omeprazole diminishes the antiplatelet activity of clopidogrel by inhibiting the CYP2C19 isoenzyme.
- Although the interaction between omeprazole and clopidogrel can be demonstrated on platelet function studies, the clinical significance of this interaction is not clear.
Controversies in non-ST-elevation acute coronary syndromes and percutaneous coronary interventions
Despite all the attention paid to ST-segment-elevation myocardial infarction (MI), in terms of sheer numbers, non-ST-elevation MI and unstable angina are where the action is. Acute coronary syndromes account for 2.43 million hospital discharges per year. Of these, 0.46 million are for ST-elevation MI and 1.97 million are for non-ST-elevation MI and unstable angina.1,2
A number of recent studies have begun to answer some of the pressing questions about treating these types of acute coronary syndromes. In this article, I update the reader on these studies, along with recent findings regarding stenting and antiplatelet agents. As you will see, they are all interconnected.
TO CATHETERIZE IS BETTER THAN NOT TO CATHETERIZE
In the 1990s, a topic of debate was whether patients presenting with unstable angina or non-ST-elevation MI should routinely undergo catheterization or whether they would do just as well with a conservative approach, ie, undergoing catheterization only if they developed recurrent, spontaneous, or stress-induced ischemia. Now, the data are reasonably clear and favor an aggressive strategy.3
Mehta et al4 performed a meta-analysis of seven randomized controlled trials (N = 9,212 patients) of aggressive vs conservative angiography and revascularization for non-ST-elevation MI or unstable angina. The results favored the aggressive strategy. At 17 months of follow-up, death or MI had occurred in 7.4% of patients who received the aggressive therapy compared with 11.0% of those who received the conservative therapy, for an odds ratio of 0.82 (P = .001).
The CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implemention of the ACC/AHA Guidelines?) Quality Improvement Initiative5 analyzed data from a registry of 17,926 patients with non-ST-elevation acute coronary syndrome who were at high risk because of positive cardiac markers or ischemic electrocardiographic changes. Overall, 2.0% of patients who received early invasive care (catheterization within the first 48 hours) died in the hospital compared with 6.2% of those who got no early invasive care, for an adjusted odds ratio of 0.63 (95% confidence interval [CI] 0.52–0.77).
The investigators also stratified the patients into those at low, medium, and high risk, using the criteria of the PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin [eptifibatide] Therapy) risk score. There were fewer deaths with early invasive therapy in each risk group, and the risk reduction was greatest in the high-risk group.5
Bavry et al6 performed an updated meta-analysis of randomized trials. At a mean follow-up of 24 months, the relative risk of death from any cause was 0.75 in patients who received early invasive therapy.
In another meta-analysis, O’Donoghue et al7 found that the odds ratio of death, MI, or rehospitalization with acute coronary syndromes was 0.73 (95% CI 0.55–0.98) in men who received invasive vs conservative therapy; in women it was 0.81 (95% CI 0.65–1.01). In women, the benefit was statistically significant in those who had elevations of creatine kinase MB or troponin but not in those who did not, though the benefit in men appeared to be less dependent on the presence of biomarker abnormalities.
MUST ANGIOGRAPHY BE DONE IN THE FIRST 24 HOURS?
Although a number of trials showed that a routine invasive strategy leads to better outcomes than a conservative strategy, until recently we had no information as to whether the catheterization needed to be done early (eg, within the first 24 hours) or if it could be delayed a day or two while the patient received medical therapy.
Mehta et al8 conducted a trial to find out: the Timing of Intervention in Acute Coronary Syndrome (TIMACS) trial. Patients were included if they had unstable angina or non-ST-elevation MI, presented to a hospital within 24 hours of the onset of symptoms, and had two of three high-risk features: age 60 years or older, elevated cardiac biomarkers, or electrocardiographic findings compatible with ischemia. All received standard medical therapy, and 3,031 were randomly assigned to undergo angiography either within 24 hours after randomization or 36 or more hours after randomization.
At 6 months, the primary outcome of death, new MI, or stroke had occurred in 9.6% of the patients in the early-intervention group and in 11.3% of those in the delayed-intervention group, but the difference was not statistically significant. However, the difference in the rate of a secondary end point, death, MI, or refractory ischemia, was statistically significant: 9.5% vs 12.9%, P = .003, owing mainly to less refractory ischemia with early intervention.
The patients were also stratified into two groups by baseline risk. The rate of the primary outcome was significantly lower with early intervention in high-risk patients, but not in those at intermediate or low risk. Thus, early intervention may be beneficial in patients at high risk, such as those with ongoing chest pain, but not necessarily in those at low risk.
LEAVE NO LESION BEHIND?
Coronary artery disease often affects more than one segment. Until recently, it was not known whether we should stent all stenotic segments in patients presenting with non-ST-elevation MI or unstable angina, or only the “culprit lesion.”
Shishehbor et al9 examined data from a Cleveland Clinic registry of 1,240 patients with acute coronary syndrome and multivessel coronary artery disease who underwent bare-metal stenting. The median follow-up was 2.3 years. Using a propensity model to match patients in the two groups with similar baseline characteristics, they found that the rate of repeat revascularization was less with multivessel intervention than with culprit-only stenting, as was the rate of the combined end point of death, MI, or revascularization, but not that of all-cause mortality or the composite of death or MI.
BARE-METAL VS DRUG-ELUTING STENTS: BALANCING THE RISKS AND BENEFITS
After a patient receives a stent, two bad things can happen: the artery can close up again either gradually, in a process called restenosis, or suddenly, via thrombosis.
Drug-eluting stents were invented to solve the problem of restenosis, and they work very well. Stone et al10 pooled the data from four double-blind trials of sirolimus (Rapamune) stents and five double-blind trials of paclitaxel (Taxol) stents and found that, at 4 years, the rates of target-lesion revascularization (for restenosis) were 7.8% with sirolimus stents vs 23.6% with bare-metal stents (P < .001), and 10.1% with paclitaxel stents vs 20.0% with bare-metal stents (P < .001).
Thrombosis was much less common in these studies, occurring in 1.2% of the sirolimus stent groups vs 0.6% of the bare-metal stent groups (P = .20), and in 1.3% of the paclitaxel stent groups vs 0.9% of the bare-metal stent groups (P = .30).10
However, drug-eluting stents appear to increase the risk of thrombosis later on, ie, after 1 year. Bavry et al,11 in a meta-analysis, calculated that when stent thrombosis occurred, the median time after implantation was 15.5 months with sirolimus stents vs 4 months with bare-metal stents (P = .0052), and 18 months with paclitaxel stents vs 3.5 months with bare-metal stents (P = .04). The absolute risk of very late stent thrombosis after 1 year was very low, with five events per 1,000 patients with drug-eluting stents vs no events with bare-metal stents (P = .02). Nevertheless, this finding has practical implications. How long must patients continue dual antiplatelet therapy? And what if a patient needs surgery a year later?
Restenosis is not always so gradual
Although stent thrombosis is serious and often fatal, bare-metal stent restenosis is not always benign either, despite the classic view that stent restenosis is a gradual process that results in exertional angina. Reviewing 1,186 cases of bare-metal stent restenosis in 984 patients at Cleveland Clinic, Chen et al12 reported that 9.5% of cases presented as acute MI (2.2% as ST-elevation MI and 7.3% as non-ST-elevation MI), and 26.4% as unstable angina requiring hospitalization.
A Mayo Clinic study13 corroborated these findings. The 10-year incidence of clinical bare-metal stent restenosis was 18.1%, and the incidence of MI was 2.1%. The 10-year rate of bare-metal stent thrombosis was 2%. Off-label use, primarily in saphenous vein grafts, increased the incidence; other correlates were prior MI, peripheral arterial disease, and ulcerated lesions.
Furthermore, bare-metal stent thrombosis can also occur later. We saw a case that occurred 13 years after the procedure, 3 days after the patient stopped taking aspirin because he was experiencing flu-like symptoms, ran out of aspirin, and felt too sick to go out and buy more. The presentation was with ST-elevation MI. The patient recovered after treatment with intracoronary abciximab (ReoPro), percutaneous thrombectomy, balloon angioplasty, and, eventually, bypass surgery.14
No difference in risk of death with drug-eluting vs bare-metal stents
Even though drug-eluting stents pose a slightly higher risk of thrombosis than bare-metal stents, the risk of death is no higher.15
I believe the reason is that there are competing risks, and that the higher risk of thrombosis with first-generation drug-eluting stents and the higher risk of restenosis with bare-metal stents essentially cancel each other out. For most patients, there is an absolute benefit with drug-eluting stents, which reduce the need for revascularization with no effect in terms of either increasing or decreasing the risk of MI or death. Second-generation drug-eluting stents may have advantages in reducing rates of death or MI compared with first-generation drug-eluting stents, though this remains to be proven conclusively.
The right revascularization for the right patient
Bavry and I16 developed an algorithm for deciding on revascularization, posing a series of questions:
- Does the patient need any form of revascularization?
- Is he or she at higher risk of both stent thrombosis and restenosis, as in patients with diabetes, diffuse multivessel disease with bifurcation lesions, or chronic total occlusions? If so, coronary artery bypass grafting remains an excellent option.
- Does he or she have a low risk of restenosis, as in patients without diabetes with focal lesions in large vessels? If so, one could consider a bare-metal stent, which would probably be more cost-effective than a drug-eluting stent in this situation.
- Does the patient have relative contraindications to drug-eluting stents? Examples are a history of noncompliance with medical therapy, financial issues such as lack of insurance that would make buying clopidogrel (Plavix) a problem, long-term anticoagulation, or anticipated need for surgery in the next few years.
If a drug-eluting stent is used, certain measures can help ensure that it is used optimally. It should often be placed under high pressure with a noncompliant balloon so that it achieves contact with the artery wall all around. One should consider intravascular ultrasonographic guidance to make sure the stent is well opposed if it is in a very calcified lesion. Dual antiplatelet therapy with clopidogrel and aspirin should be given for at least 1 year, and if there is no bleeding, perhaps longer, pending further data.16
LEAVE NO PLATELET ACTIVATED?
Platelets have several types of receptors that, when bound by their respective ligands, lead to platelet activation and aggregation and, ultimately, thrombus formation. Antagonists to some of these receptors are available or are being developed.17
For long-term therapy, blocking the process “upstream,” ie, preventing platelet activation, is better than blocking it “downstream,” ie, preventing aggregation. For example, clopidogrel, ticlopipine (Ticlid), and prasugrel (Effient) have active metabolites that bind to a subtype of the adenosine diphosphate receptor and prevent platelet activation, whereas the glycoprotein IIb/IIIa inhibitors such as abciximab work downstream, binding to a different receptor and preventing aggregation.18
Dual therapy for 1 year is the standard of care after acute coronary syndromes
The evidence for using dual antiplatelet therapy (ie, aspirin plus clopidogrel) in patients with acute coronary syndromes without ST-elevation is very well established.
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial,19 published in 2001, found a 20% relative risk reduction and a 2% absolute risk reduction in the incidence of MI, stroke, or cardiovascular death in patients randomly assigned to receive clopidogrel plus aspirin for 1 year vs aspirin alone for 1 year (P < .001). In the subgroup of patients who underwent percutaneous coronary intervention, the relative risk reduction in the incidence of MI or cardiovascular death at 1 year of follow-up was 31% (P = .002).20
As a result of these findings, the cardiology society guidelines21 recommend a year of dual antiplatelet therapy after acute coronary syndromes, regardless of whether the patient is treated medically, percutaneously, or surgically.
But what happens after clopidogrel is withdrawn? Ho et al22 retrospectively analyzed data from Veterans Affairs hospitals and found a spike in the incidence of death or MI in the first 90 days after stopping clopidogrel treatment. This was true in medically treated patients as well as in those treated with percutaneous coronary interventions, in those with or without diabetes mellitus, in those who received a drug-eluting stent or a bare-metal stent, and in those treated longer than 9 months.
The investigators concluded that there might be a “clopidogrel rebound effect.” However, I believe that a true rebound effect, such as after withdrawal of heparin or warfarin, is biologically unlikely with clopidogrel, since clopidogrel irreversibly binds to its receptor for the 7- to 10-day life span of the platelet. Rather, I believe the phenomenon must be due to withdrawal of protection in patients at risk.
In stable patients, dual therapy is not as beneficial
Would dual antiplatelet therapy with clopidogrel and aspirin also benefit patients at risk of atherothrombotic events but without acute coronary syndromes?
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial23 included 15,603 patients with either clinically evident but stable cardiovascular disease or multiple risk factors for athero-thrombosis. They were randomly assigned to receive either clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin. At a median of 28 months, the groups did not differ significantly in the rate of MI, stroke, or death from cardiovascular causes.
However, the subgroup of patients who had documented prior MI, ischemic stroke, or symptomatic peripheral arterial disease did appear to derive significant benefit from dual therapy.24 In this subgroup, the rate of MI, stroke, or cardiovascular death at a median follow-up of 27.6 months was 8.8% with placebo plus aspirin compared with 7.3% with clopidogrel plus aspirin, for a hazard ratio of 0.83 (95% CI 0.72–0.96, P = .01). Unstented patients with stable coronary artery disease but without prior MI derived no benefit.
Bleeding and thrombosis: The Scylla and Charybdis of antiplatelet therapy
However, with dual antiplatelet therapy, we steer between the Scylla of bleeding and the Charybdis of thrombosis.25
In the CHARISMA subgroup who had prior MI, ischemic stroke, or symptomatic peripheral arterial disease, the incidence of moderate or severe bleeding was higher with dual therapy than with aspirin alone, but the rates converged after about 1 year of treatment.24 Further, there was no difference in fatal bleeding or intracranial bleeding, although the rate of moderate bleeding (defined as the need for transfusion) was higher with dual therapy (2.0% vs 1.3%, P = .004).
I believe the data indicate that if a patient can tolerate dual antiplatelet therapy for 9 to 12 months without any bleeding issues, he or she is unlikely to have a major bleeding episode if dual therapy is continued beyond this time.
About half of bleeding events in patients on chronic antiplatelet therapy are gastrointestinal. To address this risk, in 2008 an expert committee from the American College of Cardiology, American College of Gastroenterology, and American Heart Association issued a consensus document26 in which they recommended assessing gastrointestinal risk factors in patients on antiplatelet therapy, such as history of ulcers (and testing for and treating Helicobacter pylori infection if present), history of gastrointestinal bleeding, concomitant anticoagulant therapy, and dual antiplatelet therapy. If any of these were present, the committee recommended considering a proton pump inhibitor. The committee also recommended a proton pump inhibitor for patients on antiplatelet therapy who have more than one of the following: age 60 years or more, corticosteroid use, or dyspepsia or gastroesophageal reflux symptoms.
Some ex vivo platelet studies and observational analyses have suggested that there might be an adverse interaction between clopidogrel and proton pump inhibitors due to a blunting of clopidogrel’s antiplatelet effect. A large randomized clinical trial was designed and launched to determine if a single-pill combination of the proton pump inhibitor omeprazole (Prilosec) and clopidogrel would be safer than clopidogrel alone when added to aspirin. Called COGENT-1 (Clopidogrel and the Optimization of GI Events Trial), it was halted early in 2009 when it lost its funding. However, preliminary data did not show an adverse interaction between clopidogrel and omeprazole.
What is the right dose of aspirin?
Steinhubl et al27 performed a post hoc observational analysis of data from the CHARISMA trial. Their findings suggested that higher doses of aspirin are not more effective than lower doses for chronic therapy. Furthermore, in the group receiving clopidogrel plus aspirin, the incidence of severe or life-threatening bleeding was significantly greater with aspirin doses higher than 100 mg than with doses lower than 100 mg, 2.6% vs 1.7%, P = .040.
A randomized, controlled trial called Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions (CURRENT/OASIS 7)28 recently reported that higher-dose aspirin (ie, 325 mg) may be better than lower dose aspirin (ie, 81 mg) in patients with acute coronary syndromes undergoing percutaneous coronary intervention and receiving clopidogrel. During this 30-day study, there was no increase in overall bleeding with the higher dose of aspirin, though gastrointestinal bleeding was slightly increased.29 In a factorial design, the second part of this trial found that a higher-dose clopidogrel regimen reduced stent thrombosis.29
Should nonresponders get higher doses of clopidogrel?
In vitro, response to clopidogrel shows a normal bell-shaped distribution.30 In theory, therefore, patients who are hyperresponders may be at higher risk of bleeding, and those who are hyporesponders may be at risk of ischemic events.
A clinical trial is under way to examine whether hyporesponders should get higher doses. Called GRAVITAS (Gauging Responsiveness With a VerifyNow Assay Impact on Thrombosis and Safety), it will use a point-of-care platelet assay and then allocate patients to receive either standard therapy or double the dose of clopidogrel. The primary end point will be the rate of cardiovascular death, nonfatal MI, or stent thrombosis at 6 months.
Is prasugrel better than clopidogrel?
Prasugrel (Effient) is a new drug of the same class as clopidogrel, ie, a thienopyridine, with its active metabolite binding to the same platelet receptor as clopidogrel and inhibiting platelet aggregation more rapidly, more consistently, and to a greater extent than clopidogrel. Prasugrel was recently approved by the Food and Drug Administration. But is it better?31
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI 38) compared prasugrel and clopidogrel in 13,608 patients with moderate- to high-risk acute coronary syndromes who were scheduled to undergo percutaneous coronary intervention.32
Overall, prasugrel was better. At 15 months, the incidence of the primary end point (death from cardiovascular causes, nonfatal MI, or nonfatal stroke) was significantly lower with prasugrel therapy than with clopidogrel in the entire cohort (9.9% vs 12.1%, hazard ratio 0.81, 95% CI 0.73–0.90, P < .001), in the subgroup with ST-segment elevation MI, and in the subgroup with unstable angina or non-ST-elevation MI.
However, there was a price to pay. The rate of major bleeding was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% CI 1.03–1.68, P = .03). Assessing the balance between the risk and the benefit, the investigators identified three subgroups who did not derive a net clinical benefit from prasugrel: patients who had had a previous stroke or transient ischemic attack (this group actually had a net harm from prasugrel), patients 75 years of age or older, and patients weighing less than 60 kg (132 pounds).
More work is needed to determine which patients are best served by standard-dose clopidogrel, higher doses of clopidogrel, platelet-assay-guided dosing of clopidogrel, or prasugrel.24
Short-acting, potent intravenous platelet blockade with an agent such as cangrelor is theoretically appealing, but further research is necessary.33,34 Ticagrelor, a reversible adenosine diphosphate receptor antagonist, provides yet another potential option in antiplatelet therapy for acute coronary syndromes. In the recent PLATO trial (Study of Platelet Inhibition and Patient Outcomes), compared with clopidogrel, ticagrelor reduced the risk of ischemic events, including death.35,36 Here, too, there was more major bleeding (unrelated to coronary artery bypass grafting) with ticagrelor.
Thus, clinical assessment of an individual patient’s ischemic and bleeding risks will continue to be critical as therapeutic strategies evolve.
- Wiviott SD, Morrow DA, Giugliano RP, et al. Performance of the Thrombolysis In Myocardial Infarction risk index for early acute coronary syndrome in the National Registry of Myocardial Infarction: a simple risk index predicts mortality in both ST and non-ST elevation myocardial infarction [abstract]. J Am Coll Cardiol 2003; 43( suppl 2):365A–366A.
- Thom T, Haase N, Rosamond W, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006; 113:e85–e151. Errata in Circulation 2006; 113:e696 and Circulation 2006 114:e630.
- Bhatt DL. To cath or not to cath. That is no longer the question. JAMA 2005; 293:2935–2937.
- Mehta SR, Cannon CP, Fox KA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA 2005; 293:2908–2917.
- Bhatt DL, Roe MT, Peterson ED, et al; for the CRUSADE Investigators. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004; 292:2096–2104.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- O’Donoghue MO, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST segment elevation myocardial infarction: a meta-analysis. JAMA 2008; 300:71–80.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Shishehbor MH, Lauer MS, Singh IM, et al. In unstable angina or non-ST-segment acute coronary syndrome, should patients with multivessel coronary artery disease undergo multivessel or culpritonly stenting? J Am Coll Cardiol 2007; 49:849–854.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J 2006; 151:1260–1264.
- Doyle B, Rihal CS, O’Sullivan CJ, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation 2007; 116:2391–2398.
- Sarkees ML, Bavry AA, Galla JM, Bhatt DL. Bare metal stent thrombosis 13 years after implantation. Cardiovasc Revasc Med 2009; 10:58–91.
- Bavry AA, Bhatt DL. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet 2008; 371:2134–2143.
- Bavry AA, Bhatt DL. Drug-eluting stents: dual antiplatelet therapy for every survivor? Circulation 2007; 116:696–699.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502. Errata in N Engl J Med 2001; 345:1506 and N Engl J Med 2001; 345:1716.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction); american College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- Ho PM, Peterson ED, Wang L, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA 2008; 299:532–539. Erratum in JAMA 2008; 299:2390.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL. Intensifying platelet inhibition—navigating between Scylla and Charybdis. N Engl J Med 2007; 357:2078–2081.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med 2009; 150:379–386.
- Mehta SR, Bassand JP, Chrolavicius S, et al; CURRENT-OASIS 7 Steering Committee. Design and rationale of CURRENT-OASIS 7: a randomized, 2 x 2 factorial trial evaluating optimal dosing strategies for clopidogrel and aspirin in patients with ST and non-ST-elevation acute coronary syndromes managed with an early invasive strategy. Am Heart J 2008; 156:1080–1088.
- Mehta SR, Van de Werf F. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Paper presented at the European Society of Cardiology Congress; August 30, 2009; Barcelona, Spain. Also available online at www.Escardio.org/congresses/esc-2009/congress-reports. Accessed December 12, 2009.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AT, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Bhatt DL. Prasugrel in clinical practice [perspective]. N Engl J Med 2009; 361:940–942.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Bhatt DL, Lincoff AM, Gibson CM, et al; for the CHAMPION PLATFORM Investigators. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med 2009 Nov 15(epub ahead of print).
- Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patient sundergoing PCI. N Engl J Med 2009 Nov 17(epub ahead of print).
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Bhatt DL. Ticagrelor in ACS—what does PLATO teach us? Nat Rev Cardiol 2009; 6:737–738.
Despite all the attention paid to ST-segment-elevation myocardial infarction (MI), in terms of sheer numbers, non-ST-elevation MI and unstable angina are where the action is. Acute coronary syndromes account for 2.43 million hospital discharges per year. Of these, 0.46 million are for ST-elevation MI and 1.97 million are for non-ST-elevation MI and unstable angina.1,2
A number of recent studies have begun to answer some of the pressing questions about treating these types of acute coronary syndromes. In this article, I update the reader on these studies, along with recent findings regarding stenting and antiplatelet agents. As you will see, they are all interconnected.
TO CATHETERIZE IS BETTER THAN NOT TO CATHETERIZE
In the 1990s, a topic of debate was whether patients presenting with unstable angina or non-ST-elevation MI should routinely undergo catheterization or whether they would do just as well with a conservative approach, ie, undergoing catheterization only if they developed recurrent, spontaneous, or stress-induced ischemia. Now, the data are reasonably clear and favor an aggressive strategy.3
Mehta et al4 performed a meta-analysis of seven randomized controlled trials (N = 9,212 patients) of aggressive vs conservative angiography and revascularization for non-ST-elevation MI or unstable angina. The results favored the aggressive strategy. At 17 months of follow-up, death or MI had occurred in 7.4% of patients who received the aggressive therapy compared with 11.0% of those who received the conservative therapy, for an odds ratio of 0.82 (P = .001).
The CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implemention of the ACC/AHA Guidelines?) Quality Improvement Initiative5 analyzed data from a registry of 17,926 patients with non-ST-elevation acute coronary syndrome who were at high risk because of positive cardiac markers or ischemic electrocardiographic changes. Overall, 2.0% of patients who received early invasive care (catheterization within the first 48 hours) died in the hospital compared with 6.2% of those who got no early invasive care, for an adjusted odds ratio of 0.63 (95% confidence interval [CI] 0.52–0.77).
The investigators also stratified the patients into those at low, medium, and high risk, using the criteria of the PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin [eptifibatide] Therapy) risk score. There were fewer deaths with early invasive therapy in each risk group, and the risk reduction was greatest in the high-risk group.5
Bavry et al6 performed an updated meta-analysis of randomized trials. At a mean follow-up of 24 months, the relative risk of death from any cause was 0.75 in patients who received early invasive therapy.
In another meta-analysis, O’Donoghue et al7 found that the odds ratio of death, MI, or rehospitalization with acute coronary syndromes was 0.73 (95% CI 0.55–0.98) in men who received invasive vs conservative therapy; in women it was 0.81 (95% CI 0.65–1.01). In women, the benefit was statistically significant in those who had elevations of creatine kinase MB or troponin but not in those who did not, though the benefit in men appeared to be less dependent on the presence of biomarker abnormalities.
MUST ANGIOGRAPHY BE DONE IN THE FIRST 24 HOURS?
Although a number of trials showed that a routine invasive strategy leads to better outcomes than a conservative strategy, until recently we had no information as to whether the catheterization needed to be done early (eg, within the first 24 hours) or if it could be delayed a day or two while the patient received medical therapy.
Mehta et al8 conducted a trial to find out: the Timing of Intervention in Acute Coronary Syndrome (TIMACS) trial. Patients were included if they had unstable angina or non-ST-elevation MI, presented to a hospital within 24 hours of the onset of symptoms, and had two of three high-risk features: age 60 years or older, elevated cardiac biomarkers, or electrocardiographic findings compatible with ischemia. All received standard medical therapy, and 3,031 were randomly assigned to undergo angiography either within 24 hours after randomization or 36 or more hours after randomization.
At 6 months, the primary outcome of death, new MI, or stroke had occurred in 9.6% of the patients in the early-intervention group and in 11.3% of those in the delayed-intervention group, but the difference was not statistically significant. However, the difference in the rate of a secondary end point, death, MI, or refractory ischemia, was statistically significant: 9.5% vs 12.9%, P = .003, owing mainly to less refractory ischemia with early intervention.
The patients were also stratified into two groups by baseline risk. The rate of the primary outcome was significantly lower with early intervention in high-risk patients, but not in those at intermediate or low risk. Thus, early intervention may be beneficial in patients at high risk, such as those with ongoing chest pain, but not necessarily in those at low risk.
LEAVE NO LESION BEHIND?
Coronary artery disease often affects more than one segment. Until recently, it was not known whether we should stent all stenotic segments in patients presenting with non-ST-elevation MI or unstable angina, or only the “culprit lesion.”
Shishehbor et al9 examined data from a Cleveland Clinic registry of 1,240 patients with acute coronary syndrome and multivessel coronary artery disease who underwent bare-metal stenting. The median follow-up was 2.3 years. Using a propensity model to match patients in the two groups with similar baseline characteristics, they found that the rate of repeat revascularization was less with multivessel intervention than with culprit-only stenting, as was the rate of the combined end point of death, MI, or revascularization, but not that of all-cause mortality or the composite of death or MI.
BARE-METAL VS DRUG-ELUTING STENTS: BALANCING THE RISKS AND BENEFITS
After a patient receives a stent, two bad things can happen: the artery can close up again either gradually, in a process called restenosis, or suddenly, via thrombosis.
Drug-eluting stents were invented to solve the problem of restenosis, and they work very well. Stone et al10 pooled the data from four double-blind trials of sirolimus (Rapamune) stents and five double-blind trials of paclitaxel (Taxol) stents and found that, at 4 years, the rates of target-lesion revascularization (for restenosis) were 7.8% with sirolimus stents vs 23.6% with bare-metal stents (P < .001), and 10.1% with paclitaxel stents vs 20.0% with bare-metal stents (P < .001).
Thrombosis was much less common in these studies, occurring in 1.2% of the sirolimus stent groups vs 0.6% of the bare-metal stent groups (P = .20), and in 1.3% of the paclitaxel stent groups vs 0.9% of the bare-metal stent groups (P = .30).10
However, drug-eluting stents appear to increase the risk of thrombosis later on, ie, after 1 year. Bavry et al,11 in a meta-analysis, calculated that when stent thrombosis occurred, the median time after implantation was 15.5 months with sirolimus stents vs 4 months with bare-metal stents (P = .0052), and 18 months with paclitaxel stents vs 3.5 months with bare-metal stents (P = .04). The absolute risk of very late stent thrombosis after 1 year was very low, with five events per 1,000 patients with drug-eluting stents vs no events with bare-metal stents (P = .02). Nevertheless, this finding has practical implications. How long must patients continue dual antiplatelet therapy? And what if a patient needs surgery a year later?
Restenosis is not always so gradual
Although stent thrombosis is serious and often fatal, bare-metal stent restenosis is not always benign either, despite the classic view that stent restenosis is a gradual process that results in exertional angina. Reviewing 1,186 cases of bare-metal stent restenosis in 984 patients at Cleveland Clinic, Chen et al12 reported that 9.5% of cases presented as acute MI (2.2% as ST-elevation MI and 7.3% as non-ST-elevation MI), and 26.4% as unstable angina requiring hospitalization.
A Mayo Clinic study13 corroborated these findings. The 10-year incidence of clinical bare-metal stent restenosis was 18.1%, and the incidence of MI was 2.1%. The 10-year rate of bare-metal stent thrombosis was 2%. Off-label use, primarily in saphenous vein grafts, increased the incidence; other correlates were prior MI, peripheral arterial disease, and ulcerated lesions.
Furthermore, bare-metal stent thrombosis can also occur later. We saw a case that occurred 13 years after the procedure, 3 days after the patient stopped taking aspirin because he was experiencing flu-like symptoms, ran out of aspirin, and felt too sick to go out and buy more. The presentation was with ST-elevation MI. The patient recovered after treatment with intracoronary abciximab (ReoPro), percutaneous thrombectomy, balloon angioplasty, and, eventually, bypass surgery.14
No difference in risk of death with drug-eluting vs bare-metal stents
Even though drug-eluting stents pose a slightly higher risk of thrombosis than bare-metal stents, the risk of death is no higher.15
I believe the reason is that there are competing risks, and that the higher risk of thrombosis with first-generation drug-eluting stents and the higher risk of restenosis with bare-metal stents essentially cancel each other out. For most patients, there is an absolute benefit with drug-eluting stents, which reduce the need for revascularization with no effect in terms of either increasing or decreasing the risk of MI or death. Second-generation drug-eluting stents may have advantages in reducing rates of death or MI compared with first-generation drug-eluting stents, though this remains to be proven conclusively.
The right revascularization for the right patient
Bavry and I16 developed an algorithm for deciding on revascularization, posing a series of questions:
- Does the patient need any form of revascularization?
- Is he or she at higher risk of both stent thrombosis and restenosis, as in patients with diabetes, diffuse multivessel disease with bifurcation lesions, or chronic total occlusions? If so, coronary artery bypass grafting remains an excellent option.
- Does he or she have a low risk of restenosis, as in patients without diabetes with focal lesions in large vessels? If so, one could consider a bare-metal stent, which would probably be more cost-effective than a drug-eluting stent in this situation.
- Does the patient have relative contraindications to drug-eluting stents? Examples are a history of noncompliance with medical therapy, financial issues such as lack of insurance that would make buying clopidogrel (Plavix) a problem, long-term anticoagulation, or anticipated need for surgery in the next few years.
If a drug-eluting stent is used, certain measures can help ensure that it is used optimally. It should often be placed under high pressure with a noncompliant balloon so that it achieves contact with the artery wall all around. One should consider intravascular ultrasonographic guidance to make sure the stent is well opposed if it is in a very calcified lesion. Dual antiplatelet therapy with clopidogrel and aspirin should be given for at least 1 year, and if there is no bleeding, perhaps longer, pending further data.16
LEAVE NO PLATELET ACTIVATED?
Platelets have several types of receptors that, when bound by their respective ligands, lead to platelet activation and aggregation and, ultimately, thrombus formation. Antagonists to some of these receptors are available or are being developed.17
For long-term therapy, blocking the process “upstream,” ie, preventing platelet activation, is better than blocking it “downstream,” ie, preventing aggregation. For example, clopidogrel, ticlopipine (Ticlid), and prasugrel (Effient) have active metabolites that bind to a subtype of the adenosine diphosphate receptor and prevent platelet activation, whereas the glycoprotein IIb/IIIa inhibitors such as abciximab work downstream, binding to a different receptor and preventing aggregation.18
Dual therapy for 1 year is the standard of care after acute coronary syndromes
The evidence for using dual antiplatelet therapy (ie, aspirin plus clopidogrel) in patients with acute coronary syndromes without ST-elevation is very well established.
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial,19 published in 2001, found a 20% relative risk reduction and a 2% absolute risk reduction in the incidence of MI, stroke, or cardiovascular death in patients randomly assigned to receive clopidogrel plus aspirin for 1 year vs aspirin alone for 1 year (P < .001). In the subgroup of patients who underwent percutaneous coronary intervention, the relative risk reduction in the incidence of MI or cardiovascular death at 1 year of follow-up was 31% (P = .002).20
As a result of these findings, the cardiology society guidelines21 recommend a year of dual antiplatelet therapy after acute coronary syndromes, regardless of whether the patient is treated medically, percutaneously, or surgically.
But what happens after clopidogrel is withdrawn? Ho et al22 retrospectively analyzed data from Veterans Affairs hospitals and found a spike in the incidence of death or MI in the first 90 days after stopping clopidogrel treatment. This was true in medically treated patients as well as in those treated with percutaneous coronary interventions, in those with or without diabetes mellitus, in those who received a drug-eluting stent or a bare-metal stent, and in those treated longer than 9 months.
The investigators concluded that there might be a “clopidogrel rebound effect.” However, I believe that a true rebound effect, such as after withdrawal of heparin or warfarin, is biologically unlikely with clopidogrel, since clopidogrel irreversibly binds to its receptor for the 7- to 10-day life span of the platelet. Rather, I believe the phenomenon must be due to withdrawal of protection in patients at risk.
In stable patients, dual therapy is not as beneficial
Would dual antiplatelet therapy with clopidogrel and aspirin also benefit patients at risk of atherothrombotic events but without acute coronary syndromes?
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial23 included 15,603 patients with either clinically evident but stable cardiovascular disease or multiple risk factors for athero-thrombosis. They were randomly assigned to receive either clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin. At a median of 28 months, the groups did not differ significantly in the rate of MI, stroke, or death from cardiovascular causes.
However, the subgroup of patients who had documented prior MI, ischemic stroke, or symptomatic peripheral arterial disease did appear to derive significant benefit from dual therapy.24 In this subgroup, the rate of MI, stroke, or cardiovascular death at a median follow-up of 27.6 months was 8.8% with placebo plus aspirin compared with 7.3% with clopidogrel plus aspirin, for a hazard ratio of 0.83 (95% CI 0.72–0.96, P = .01). Unstented patients with stable coronary artery disease but without prior MI derived no benefit.
Bleeding and thrombosis: The Scylla and Charybdis of antiplatelet therapy
However, with dual antiplatelet therapy, we steer between the Scylla of bleeding and the Charybdis of thrombosis.25
In the CHARISMA subgroup who had prior MI, ischemic stroke, or symptomatic peripheral arterial disease, the incidence of moderate or severe bleeding was higher with dual therapy than with aspirin alone, but the rates converged after about 1 year of treatment.24 Further, there was no difference in fatal bleeding or intracranial bleeding, although the rate of moderate bleeding (defined as the need for transfusion) was higher with dual therapy (2.0% vs 1.3%, P = .004).
I believe the data indicate that if a patient can tolerate dual antiplatelet therapy for 9 to 12 months without any bleeding issues, he or she is unlikely to have a major bleeding episode if dual therapy is continued beyond this time.
About half of bleeding events in patients on chronic antiplatelet therapy are gastrointestinal. To address this risk, in 2008 an expert committee from the American College of Cardiology, American College of Gastroenterology, and American Heart Association issued a consensus document26 in which they recommended assessing gastrointestinal risk factors in patients on antiplatelet therapy, such as history of ulcers (and testing for and treating Helicobacter pylori infection if present), history of gastrointestinal bleeding, concomitant anticoagulant therapy, and dual antiplatelet therapy. If any of these were present, the committee recommended considering a proton pump inhibitor. The committee also recommended a proton pump inhibitor for patients on antiplatelet therapy who have more than one of the following: age 60 years or more, corticosteroid use, or dyspepsia or gastroesophageal reflux symptoms.
Some ex vivo platelet studies and observational analyses have suggested that there might be an adverse interaction between clopidogrel and proton pump inhibitors due to a blunting of clopidogrel’s antiplatelet effect. A large randomized clinical trial was designed and launched to determine if a single-pill combination of the proton pump inhibitor omeprazole (Prilosec) and clopidogrel would be safer than clopidogrel alone when added to aspirin. Called COGENT-1 (Clopidogrel and the Optimization of GI Events Trial), it was halted early in 2009 when it lost its funding. However, preliminary data did not show an adverse interaction between clopidogrel and omeprazole.
What is the right dose of aspirin?
Steinhubl et al27 performed a post hoc observational analysis of data from the CHARISMA trial. Their findings suggested that higher doses of aspirin are not more effective than lower doses for chronic therapy. Furthermore, in the group receiving clopidogrel plus aspirin, the incidence of severe or life-threatening bleeding was significantly greater with aspirin doses higher than 100 mg than with doses lower than 100 mg, 2.6% vs 1.7%, P = .040.
A randomized, controlled trial called Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions (CURRENT/OASIS 7)28 recently reported that higher-dose aspirin (ie, 325 mg) may be better than lower dose aspirin (ie, 81 mg) in patients with acute coronary syndromes undergoing percutaneous coronary intervention and receiving clopidogrel. During this 30-day study, there was no increase in overall bleeding with the higher dose of aspirin, though gastrointestinal bleeding was slightly increased.29 In a factorial design, the second part of this trial found that a higher-dose clopidogrel regimen reduced stent thrombosis.29
Should nonresponders get higher doses of clopidogrel?
In vitro, response to clopidogrel shows a normal bell-shaped distribution.30 In theory, therefore, patients who are hyperresponders may be at higher risk of bleeding, and those who are hyporesponders may be at risk of ischemic events.
A clinical trial is under way to examine whether hyporesponders should get higher doses. Called GRAVITAS (Gauging Responsiveness With a VerifyNow Assay Impact on Thrombosis and Safety), it will use a point-of-care platelet assay and then allocate patients to receive either standard therapy or double the dose of clopidogrel. The primary end point will be the rate of cardiovascular death, nonfatal MI, or stent thrombosis at 6 months.
Is prasugrel better than clopidogrel?
Prasugrel (Effient) is a new drug of the same class as clopidogrel, ie, a thienopyridine, with its active metabolite binding to the same platelet receptor as clopidogrel and inhibiting platelet aggregation more rapidly, more consistently, and to a greater extent than clopidogrel. Prasugrel was recently approved by the Food and Drug Administration. But is it better?31
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI 38) compared prasugrel and clopidogrel in 13,608 patients with moderate- to high-risk acute coronary syndromes who were scheduled to undergo percutaneous coronary intervention.32
Overall, prasugrel was better. At 15 months, the incidence of the primary end point (death from cardiovascular causes, nonfatal MI, or nonfatal stroke) was significantly lower with prasugrel therapy than with clopidogrel in the entire cohort (9.9% vs 12.1%, hazard ratio 0.81, 95% CI 0.73–0.90, P < .001), in the subgroup with ST-segment elevation MI, and in the subgroup with unstable angina or non-ST-elevation MI.
However, there was a price to pay. The rate of major bleeding was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% CI 1.03–1.68, P = .03). Assessing the balance between the risk and the benefit, the investigators identified three subgroups who did not derive a net clinical benefit from prasugrel: patients who had had a previous stroke or transient ischemic attack (this group actually had a net harm from prasugrel), patients 75 years of age or older, and patients weighing less than 60 kg (132 pounds).
More work is needed to determine which patients are best served by standard-dose clopidogrel, higher doses of clopidogrel, platelet-assay-guided dosing of clopidogrel, or prasugrel.24
Short-acting, potent intravenous platelet blockade with an agent such as cangrelor is theoretically appealing, but further research is necessary.33,34 Ticagrelor, a reversible adenosine diphosphate receptor antagonist, provides yet another potential option in antiplatelet therapy for acute coronary syndromes. In the recent PLATO trial (Study of Platelet Inhibition and Patient Outcomes), compared with clopidogrel, ticagrelor reduced the risk of ischemic events, including death.35,36 Here, too, there was more major bleeding (unrelated to coronary artery bypass grafting) with ticagrelor.
Thus, clinical assessment of an individual patient’s ischemic and bleeding risks will continue to be critical as therapeutic strategies evolve.
Despite all the attention paid to ST-segment-elevation myocardial infarction (MI), in terms of sheer numbers, non-ST-elevation MI and unstable angina are where the action is. Acute coronary syndromes account for 2.43 million hospital discharges per year. Of these, 0.46 million are for ST-elevation MI and 1.97 million are for non-ST-elevation MI and unstable angina.1,2
A number of recent studies have begun to answer some of the pressing questions about treating these types of acute coronary syndromes. In this article, I update the reader on these studies, along with recent findings regarding stenting and antiplatelet agents. As you will see, they are all interconnected.
TO CATHETERIZE IS BETTER THAN NOT TO CATHETERIZE
In the 1990s, a topic of debate was whether patients presenting with unstable angina or non-ST-elevation MI should routinely undergo catheterization or whether they would do just as well with a conservative approach, ie, undergoing catheterization only if they developed recurrent, spontaneous, or stress-induced ischemia. Now, the data are reasonably clear and favor an aggressive strategy.3
Mehta et al4 performed a meta-analysis of seven randomized controlled trials (N = 9,212 patients) of aggressive vs conservative angiography and revascularization for non-ST-elevation MI or unstable angina. The results favored the aggressive strategy. At 17 months of follow-up, death or MI had occurred in 7.4% of patients who received the aggressive therapy compared with 11.0% of those who received the conservative therapy, for an odds ratio of 0.82 (P = .001).
The CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implemention of the ACC/AHA Guidelines?) Quality Improvement Initiative5 analyzed data from a registry of 17,926 patients with non-ST-elevation acute coronary syndrome who were at high risk because of positive cardiac markers or ischemic electrocardiographic changes. Overall, 2.0% of patients who received early invasive care (catheterization within the first 48 hours) died in the hospital compared with 6.2% of those who got no early invasive care, for an adjusted odds ratio of 0.63 (95% confidence interval [CI] 0.52–0.77).
The investigators also stratified the patients into those at low, medium, and high risk, using the criteria of the PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin [eptifibatide] Therapy) risk score. There were fewer deaths with early invasive therapy in each risk group, and the risk reduction was greatest in the high-risk group.5
Bavry et al6 performed an updated meta-analysis of randomized trials. At a mean follow-up of 24 months, the relative risk of death from any cause was 0.75 in patients who received early invasive therapy.
In another meta-analysis, O’Donoghue et al7 found that the odds ratio of death, MI, or rehospitalization with acute coronary syndromes was 0.73 (95% CI 0.55–0.98) in men who received invasive vs conservative therapy; in women it was 0.81 (95% CI 0.65–1.01). In women, the benefit was statistically significant in those who had elevations of creatine kinase MB or troponin but not in those who did not, though the benefit in men appeared to be less dependent on the presence of biomarker abnormalities.
MUST ANGIOGRAPHY BE DONE IN THE FIRST 24 HOURS?
Although a number of trials showed that a routine invasive strategy leads to better outcomes than a conservative strategy, until recently we had no information as to whether the catheterization needed to be done early (eg, within the first 24 hours) or if it could be delayed a day or two while the patient received medical therapy.
Mehta et al8 conducted a trial to find out: the Timing of Intervention in Acute Coronary Syndrome (TIMACS) trial. Patients were included if they had unstable angina or non-ST-elevation MI, presented to a hospital within 24 hours of the onset of symptoms, and had two of three high-risk features: age 60 years or older, elevated cardiac biomarkers, or electrocardiographic findings compatible with ischemia. All received standard medical therapy, and 3,031 were randomly assigned to undergo angiography either within 24 hours after randomization or 36 or more hours after randomization.
At 6 months, the primary outcome of death, new MI, or stroke had occurred in 9.6% of the patients in the early-intervention group and in 11.3% of those in the delayed-intervention group, but the difference was not statistically significant. However, the difference in the rate of a secondary end point, death, MI, or refractory ischemia, was statistically significant: 9.5% vs 12.9%, P = .003, owing mainly to less refractory ischemia with early intervention.
The patients were also stratified into two groups by baseline risk. The rate of the primary outcome was significantly lower with early intervention in high-risk patients, but not in those at intermediate or low risk. Thus, early intervention may be beneficial in patients at high risk, such as those with ongoing chest pain, but not necessarily in those at low risk.
LEAVE NO LESION BEHIND?
Coronary artery disease often affects more than one segment. Until recently, it was not known whether we should stent all stenotic segments in patients presenting with non-ST-elevation MI or unstable angina, or only the “culprit lesion.”
Shishehbor et al9 examined data from a Cleveland Clinic registry of 1,240 patients with acute coronary syndrome and multivessel coronary artery disease who underwent bare-metal stenting. The median follow-up was 2.3 years. Using a propensity model to match patients in the two groups with similar baseline characteristics, they found that the rate of repeat revascularization was less with multivessel intervention than with culprit-only stenting, as was the rate of the combined end point of death, MI, or revascularization, but not that of all-cause mortality or the composite of death or MI.
BARE-METAL VS DRUG-ELUTING STENTS: BALANCING THE RISKS AND BENEFITS
After a patient receives a stent, two bad things can happen: the artery can close up again either gradually, in a process called restenosis, or suddenly, via thrombosis.
Drug-eluting stents were invented to solve the problem of restenosis, and they work very well. Stone et al10 pooled the data from four double-blind trials of sirolimus (Rapamune) stents and five double-blind trials of paclitaxel (Taxol) stents and found that, at 4 years, the rates of target-lesion revascularization (for restenosis) were 7.8% with sirolimus stents vs 23.6% with bare-metal stents (P < .001), and 10.1% with paclitaxel stents vs 20.0% with bare-metal stents (P < .001).
Thrombosis was much less common in these studies, occurring in 1.2% of the sirolimus stent groups vs 0.6% of the bare-metal stent groups (P = .20), and in 1.3% of the paclitaxel stent groups vs 0.9% of the bare-metal stent groups (P = .30).10
However, drug-eluting stents appear to increase the risk of thrombosis later on, ie, after 1 year. Bavry et al,11 in a meta-analysis, calculated that when stent thrombosis occurred, the median time after implantation was 15.5 months with sirolimus stents vs 4 months with bare-metal stents (P = .0052), and 18 months with paclitaxel stents vs 3.5 months with bare-metal stents (P = .04). The absolute risk of very late stent thrombosis after 1 year was very low, with five events per 1,000 patients with drug-eluting stents vs no events with bare-metal stents (P = .02). Nevertheless, this finding has practical implications. How long must patients continue dual antiplatelet therapy? And what if a patient needs surgery a year later?
Restenosis is not always so gradual
Although stent thrombosis is serious and often fatal, bare-metal stent restenosis is not always benign either, despite the classic view that stent restenosis is a gradual process that results in exertional angina. Reviewing 1,186 cases of bare-metal stent restenosis in 984 patients at Cleveland Clinic, Chen et al12 reported that 9.5% of cases presented as acute MI (2.2% as ST-elevation MI and 7.3% as non-ST-elevation MI), and 26.4% as unstable angina requiring hospitalization.
A Mayo Clinic study13 corroborated these findings. The 10-year incidence of clinical bare-metal stent restenosis was 18.1%, and the incidence of MI was 2.1%. The 10-year rate of bare-metal stent thrombosis was 2%. Off-label use, primarily in saphenous vein grafts, increased the incidence; other correlates were prior MI, peripheral arterial disease, and ulcerated lesions.
Furthermore, bare-metal stent thrombosis can also occur later. We saw a case that occurred 13 years after the procedure, 3 days after the patient stopped taking aspirin because he was experiencing flu-like symptoms, ran out of aspirin, and felt too sick to go out and buy more. The presentation was with ST-elevation MI. The patient recovered after treatment with intracoronary abciximab (ReoPro), percutaneous thrombectomy, balloon angioplasty, and, eventually, bypass surgery.14
No difference in risk of death with drug-eluting vs bare-metal stents
Even though drug-eluting stents pose a slightly higher risk of thrombosis than bare-metal stents, the risk of death is no higher.15
I believe the reason is that there are competing risks, and that the higher risk of thrombosis with first-generation drug-eluting stents and the higher risk of restenosis with bare-metal stents essentially cancel each other out. For most patients, there is an absolute benefit with drug-eluting stents, which reduce the need for revascularization with no effect in terms of either increasing or decreasing the risk of MI or death. Second-generation drug-eluting stents may have advantages in reducing rates of death or MI compared with first-generation drug-eluting stents, though this remains to be proven conclusively.
The right revascularization for the right patient
Bavry and I16 developed an algorithm for deciding on revascularization, posing a series of questions:
- Does the patient need any form of revascularization?
- Is he or she at higher risk of both stent thrombosis and restenosis, as in patients with diabetes, diffuse multivessel disease with bifurcation lesions, or chronic total occlusions? If so, coronary artery bypass grafting remains an excellent option.
- Does he or she have a low risk of restenosis, as in patients without diabetes with focal lesions in large vessels? If so, one could consider a bare-metal stent, which would probably be more cost-effective than a drug-eluting stent in this situation.
- Does the patient have relative contraindications to drug-eluting stents? Examples are a history of noncompliance with medical therapy, financial issues such as lack of insurance that would make buying clopidogrel (Plavix) a problem, long-term anticoagulation, or anticipated need for surgery in the next few years.
If a drug-eluting stent is used, certain measures can help ensure that it is used optimally. It should often be placed under high pressure with a noncompliant balloon so that it achieves contact with the artery wall all around. One should consider intravascular ultrasonographic guidance to make sure the stent is well opposed if it is in a very calcified lesion. Dual antiplatelet therapy with clopidogrel and aspirin should be given for at least 1 year, and if there is no bleeding, perhaps longer, pending further data.16
LEAVE NO PLATELET ACTIVATED?
Platelets have several types of receptors that, when bound by their respective ligands, lead to platelet activation and aggregation and, ultimately, thrombus formation. Antagonists to some of these receptors are available or are being developed.17
For long-term therapy, blocking the process “upstream,” ie, preventing platelet activation, is better than blocking it “downstream,” ie, preventing aggregation. For example, clopidogrel, ticlopipine (Ticlid), and prasugrel (Effient) have active metabolites that bind to a subtype of the adenosine diphosphate receptor and prevent platelet activation, whereas the glycoprotein IIb/IIIa inhibitors such as abciximab work downstream, binding to a different receptor and preventing aggregation.18
Dual therapy for 1 year is the standard of care after acute coronary syndromes
The evidence for using dual antiplatelet therapy (ie, aspirin plus clopidogrel) in patients with acute coronary syndromes without ST-elevation is very well established.
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial,19 published in 2001, found a 20% relative risk reduction and a 2% absolute risk reduction in the incidence of MI, stroke, or cardiovascular death in patients randomly assigned to receive clopidogrel plus aspirin for 1 year vs aspirin alone for 1 year (P < .001). In the subgroup of patients who underwent percutaneous coronary intervention, the relative risk reduction in the incidence of MI or cardiovascular death at 1 year of follow-up was 31% (P = .002).20
As a result of these findings, the cardiology society guidelines21 recommend a year of dual antiplatelet therapy after acute coronary syndromes, regardless of whether the patient is treated medically, percutaneously, or surgically.
But what happens after clopidogrel is withdrawn? Ho et al22 retrospectively analyzed data from Veterans Affairs hospitals and found a spike in the incidence of death or MI in the first 90 days after stopping clopidogrel treatment. This was true in medically treated patients as well as in those treated with percutaneous coronary interventions, in those with or without diabetes mellitus, in those who received a drug-eluting stent or a bare-metal stent, and in those treated longer than 9 months.
The investigators concluded that there might be a “clopidogrel rebound effect.” However, I believe that a true rebound effect, such as after withdrawal of heparin or warfarin, is biologically unlikely with clopidogrel, since clopidogrel irreversibly binds to its receptor for the 7- to 10-day life span of the platelet. Rather, I believe the phenomenon must be due to withdrawal of protection in patients at risk.
In stable patients, dual therapy is not as beneficial
Would dual antiplatelet therapy with clopidogrel and aspirin also benefit patients at risk of atherothrombotic events but without acute coronary syndromes?
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial23 included 15,603 patients with either clinically evident but stable cardiovascular disease or multiple risk factors for athero-thrombosis. They were randomly assigned to receive either clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin. At a median of 28 months, the groups did not differ significantly in the rate of MI, stroke, or death from cardiovascular causes.
However, the subgroup of patients who had documented prior MI, ischemic stroke, or symptomatic peripheral arterial disease did appear to derive significant benefit from dual therapy.24 In this subgroup, the rate of MI, stroke, or cardiovascular death at a median follow-up of 27.6 months was 8.8% with placebo plus aspirin compared with 7.3% with clopidogrel plus aspirin, for a hazard ratio of 0.83 (95% CI 0.72–0.96, P = .01). Unstented patients with stable coronary artery disease but without prior MI derived no benefit.
Bleeding and thrombosis: The Scylla and Charybdis of antiplatelet therapy
However, with dual antiplatelet therapy, we steer between the Scylla of bleeding and the Charybdis of thrombosis.25
In the CHARISMA subgroup who had prior MI, ischemic stroke, or symptomatic peripheral arterial disease, the incidence of moderate or severe bleeding was higher with dual therapy than with aspirin alone, but the rates converged after about 1 year of treatment.24 Further, there was no difference in fatal bleeding or intracranial bleeding, although the rate of moderate bleeding (defined as the need for transfusion) was higher with dual therapy (2.0% vs 1.3%, P = .004).
I believe the data indicate that if a patient can tolerate dual antiplatelet therapy for 9 to 12 months without any bleeding issues, he or she is unlikely to have a major bleeding episode if dual therapy is continued beyond this time.
About half of bleeding events in patients on chronic antiplatelet therapy are gastrointestinal. To address this risk, in 2008 an expert committee from the American College of Cardiology, American College of Gastroenterology, and American Heart Association issued a consensus document26 in which they recommended assessing gastrointestinal risk factors in patients on antiplatelet therapy, such as history of ulcers (and testing for and treating Helicobacter pylori infection if present), history of gastrointestinal bleeding, concomitant anticoagulant therapy, and dual antiplatelet therapy. If any of these were present, the committee recommended considering a proton pump inhibitor. The committee also recommended a proton pump inhibitor for patients on antiplatelet therapy who have more than one of the following: age 60 years or more, corticosteroid use, or dyspepsia or gastroesophageal reflux symptoms.
Some ex vivo platelet studies and observational analyses have suggested that there might be an adverse interaction between clopidogrel and proton pump inhibitors due to a blunting of clopidogrel’s antiplatelet effect. A large randomized clinical trial was designed and launched to determine if a single-pill combination of the proton pump inhibitor omeprazole (Prilosec) and clopidogrel would be safer than clopidogrel alone when added to aspirin. Called COGENT-1 (Clopidogrel and the Optimization of GI Events Trial), it was halted early in 2009 when it lost its funding. However, preliminary data did not show an adverse interaction between clopidogrel and omeprazole.
What is the right dose of aspirin?
Steinhubl et al27 performed a post hoc observational analysis of data from the CHARISMA trial. Their findings suggested that higher doses of aspirin are not more effective than lower doses for chronic therapy. Furthermore, in the group receiving clopidogrel plus aspirin, the incidence of severe or life-threatening bleeding was significantly greater with aspirin doses higher than 100 mg than with doses lower than 100 mg, 2.6% vs 1.7%, P = .040.
A randomized, controlled trial called Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions (CURRENT/OASIS 7)28 recently reported that higher-dose aspirin (ie, 325 mg) may be better than lower dose aspirin (ie, 81 mg) in patients with acute coronary syndromes undergoing percutaneous coronary intervention and receiving clopidogrel. During this 30-day study, there was no increase in overall bleeding with the higher dose of aspirin, though gastrointestinal bleeding was slightly increased.29 In a factorial design, the second part of this trial found that a higher-dose clopidogrel regimen reduced stent thrombosis.29
Should nonresponders get higher doses of clopidogrel?
In vitro, response to clopidogrel shows a normal bell-shaped distribution.30 In theory, therefore, patients who are hyperresponders may be at higher risk of bleeding, and those who are hyporesponders may be at risk of ischemic events.
A clinical trial is under way to examine whether hyporesponders should get higher doses. Called GRAVITAS (Gauging Responsiveness With a VerifyNow Assay Impact on Thrombosis and Safety), it will use a point-of-care platelet assay and then allocate patients to receive either standard therapy or double the dose of clopidogrel. The primary end point will be the rate of cardiovascular death, nonfatal MI, or stent thrombosis at 6 months.
Is prasugrel better than clopidogrel?
Prasugrel (Effient) is a new drug of the same class as clopidogrel, ie, a thienopyridine, with its active metabolite binding to the same platelet receptor as clopidogrel and inhibiting platelet aggregation more rapidly, more consistently, and to a greater extent than clopidogrel. Prasugrel was recently approved by the Food and Drug Administration. But is it better?31
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI 38) compared prasugrel and clopidogrel in 13,608 patients with moderate- to high-risk acute coronary syndromes who were scheduled to undergo percutaneous coronary intervention.32
Overall, prasugrel was better. At 15 months, the incidence of the primary end point (death from cardiovascular causes, nonfatal MI, or nonfatal stroke) was significantly lower with prasugrel therapy than with clopidogrel in the entire cohort (9.9% vs 12.1%, hazard ratio 0.81, 95% CI 0.73–0.90, P < .001), in the subgroup with ST-segment elevation MI, and in the subgroup with unstable angina or non-ST-elevation MI.
However, there was a price to pay. The rate of major bleeding was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% CI 1.03–1.68, P = .03). Assessing the balance between the risk and the benefit, the investigators identified three subgroups who did not derive a net clinical benefit from prasugrel: patients who had had a previous stroke or transient ischemic attack (this group actually had a net harm from prasugrel), patients 75 years of age or older, and patients weighing less than 60 kg (132 pounds).
More work is needed to determine which patients are best served by standard-dose clopidogrel, higher doses of clopidogrel, platelet-assay-guided dosing of clopidogrel, or prasugrel.24
Short-acting, potent intravenous platelet blockade with an agent such as cangrelor is theoretically appealing, but further research is necessary.33,34 Ticagrelor, a reversible adenosine diphosphate receptor antagonist, provides yet another potential option in antiplatelet therapy for acute coronary syndromes. In the recent PLATO trial (Study of Platelet Inhibition and Patient Outcomes), compared with clopidogrel, ticagrelor reduced the risk of ischemic events, including death.35,36 Here, too, there was more major bleeding (unrelated to coronary artery bypass grafting) with ticagrelor.
Thus, clinical assessment of an individual patient’s ischemic and bleeding risks will continue to be critical as therapeutic strategies evolve.
- Wiviott SD, Morrow DA, Giugliano RP, et al. Performance of the Thrombolysis In Myocardial Infarction risk index for early acute coronary syndrome in the National Registry of Myocardial Infarction: a simple risk index predicts mortality in both ST and non-ST elevation myocardial infarction [abstract]. J Am Coll Cardiol 2003; 43( suppl 2):365A–366A.
- Thom T, Haase N, Rosamond W, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006; 113:e85–e151. Errata in Circulation 2006; 113:e696 and Circulation 2006 114:e630.
- Bhatt DL. To cath or not to cath. That is no longer the question. JAMA 2005; 293:2935–2937.
- Mehta SR, Cannon CP, Fox KA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA 2005; 293:2908–2917.
- Bhatt DL, Roe MT, Peterson ED, et al; for the CRUSADE Investigators. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004; 292:2096–2104.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- O’Donoghue MO, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST segment elevation myocardial infarction: a meta-analysis. JAMA 2008; 300:71–80.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Shishehbor MH, Lauer MS, Singh IM, et al. In unstable angina or non-ST-segment acute coronary syndrome, should patients with multivessel coronary artery disease undergo multivessel or culpritonly stenting? J Am Coll Cardiol 2007; 49:849–854.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J 2006; 151:1260–1264.
- Doyle B, Rihal CS, O’Sullivan CJ, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation 2007; 116:2391–2398.
- Sarkees ML, Bavry AA, Galla JM, Bhatt DL. Bare metal stent thrombosis 13 years after implantation. Cardiovasc Revasc Med 2009; 10:58–91.
- Bavry AA, Bhatt DL. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet 2008; 371:2134–2143.
- Bavry AA, Bhatt DL. Drug-eluting stents: dual antiplatelet therapy for every survivor? Circulation 2007; 116:696–699.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502. Errata in N Engl J Med 2001; 345:1506 and N Engl J Med 2001; 345:1716.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction); american College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- Ho PM, Peterson ED, Wang L, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA 2008; 299:532–539. Erratum in JAMA 2008; 299:2390.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL. Intensifying platelet inhibition—navigating between Scylla and Charybdis. N Engl J Med 2007; 357:2078–2081.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med 2009; 150:379–386.
- Mehta SR, Bassand JP, Chrolavicius S, et al; CURRENT-OASIS 7 Steering Committee. Design and rationale of CURRENT-OASIS 7: a randomized, 2 x 2 factorial trial evaluating optimal dosing strategies for clopidogrel and aspirin in patients with ST and non-ST-elevation acute coronary syndromes managed with an early invasive strategy. Am Heart J 2008; 156:1080–1088.
- Mehta SR, Van de Werf F. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Paper presented at the European Society of Cardiology Congress; August 30, 2009; Barcelona, Spain. Also available online at www.Escardio.org/congresses/esc-2009/congress-reports. Accessed December 12, 2009.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AT, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Bhatt DL. Prasugrel in clinical practice [perspective]. N Engl J Med 2009; 361:940–942.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Bhatt DL, Lincoff AM, Gibson CM, et al; for the CHAMPION PLATFORM Investigators. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med 2009 Nov 15(epub ahead of print).
- Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patient sundergoing PCI. N Engl J Med 2009 Nov 17(epub ahead of print).
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Bhatt DL. Ticagrelor in ACS—what does PLATO teach us? Nat Rev Cardiol 2009; 6:737–738.
- Wiviott SD, Morrow DA, Giugliano RP, et al. Performance of the Thrombolysis In Myocardial Infarction risk index for early acute coronary syndrome in the National Registry of Myocardial Infarction: a simple risk index predicts mortality in both ST and non-ST elevation myocardial infarction [abstract]. J Am Coll Cardiol 2003; 43( suppl 2):365A–366A.
- Thom T, Haase N, Rosamond W, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006; 113:e85–e151. Errata in Circulation 2006; 113:e696 and Circulation 2006 114:e630.
- Bhatt DL. To cath or not to cath. That is no longer the question. JAMA 2005; 293:2935–2937.
- Mehta SR, Cannon CP, Fox KA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA 2005; 293:2908–2917.
- Bhatt DL, Roe MT, Peterson ED, et al; for the CRUSADE Investigators. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004; 292:2096–2104.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- O’Donoghue MO, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST segment elevation myocardial infarction: a meta-analysis. JAMA 2008; 300:71–80.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Shishehbor MH, Lauer MS, Singh IM, et al. In unstable angina or non-ST-segment acute coronary syndrome, should patients with multivessel coronary artery disease undergo multivessel or culpritonly stenting? J Am Coll Cardiol 2007; 49:849–854.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J 2006; 151:1260–1264.
- Doyle B, Rihal CS, O’Sullivan CJ, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation 2007; 116:2391–2398.
- Sarkees ML, Bavry AA, Galla JM, Bhatt DL. Bare metal stent thrombosis 13 years after implantation. Cardiovasc Revasc Med 2009; 10:58–91.
- Bavry AA, Bhatt DL. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet 2008; 371:2134–2143.
- Bavry AA, Bhatt DL. Drug-eluting stents: dual antiplatelet therapy for every survivor? Circulation 2007; 116:696–699.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502. Errata in N Engl J Med 2001; 345:1506 and N Engl J Med 2001; 345:1716.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction); american College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- Ho PM, Peterson ED, Wang L, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA 2008; 299:532–539. Erratum in JAMA 2008; 299:2390.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL. Intensifying platelet inhibition—navigating between Scylla and Charybdis. N Engl J Med 2007; 357:2078–2081.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med 2009; 150:379–386.
- Mehta SR, Bassand JP, Chrolavicius S, et al; CURRENT-OASIS 7 Steering Committee. Design and rationale of CURRENT-OASIS 7: a randomized, 2 x 2 factorial trial evaluating optimal dosing strategies for clopidogrel and aspirin in patients with ST and non-ST-elevation acute coronary syndromes managed with an early invasive strategy. Am Heart J 2008; 156:1080–1088.
- Mehta SR, Van de Werf F. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Paper presented at the European Society of Cardiology Congress; August 30, 2009; Barcelona, Spain. Also available online at www.Escardio.org/congresses/esc-2009/congress-reports. Accessed December 12, 2009.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AT, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Bhatt DL. Prasugrel in clinical practice [perspective]. N Engl J Med 2009; 361:940–942.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Bhatt DL, Lincoff AM, Gibson CM, et al; for the CHAMPION PLATFORM Investigators. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med 2009 Nov 15(epub ahead of print).
- Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patient sundergoing PCI. N Engl J Med 2009 Nov 17(epub ahead of print).
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Bhatt DL. Ticagrelor in ACS—what does PLATO teach us? Nat Rev Cardiol 2009; 6:737–738.
KEY POINTS
- The data favor an aggressive strategy of routine catheterization, rather than a conservative strategy of catheterization only if a patient develops recurrent, spontaneous, or stress-induced ischemia.
- Early percutaneous intervention (within 24 hours) may be beneficial in patients at higher risk, but not necessarily in those at lower risk.
- Drug-eluting stents appear safe, assuming dual antiplatelet therapy is used. It is unclear how long this therapy needs to be continued.
- The choice of revascularization strategy—bypass surgery, bare-metal stent, or drug-eluting stent—should be individualized based on the risk of restenosis, thrombosis, and other factors.
Platelet response in practice: Applying new insights and tools for testing and treatment
CASE STUDY: THROMBOSIS AFTER STENTING DESPITE ANTIPLATELET THERAPY
Dr. Deepak Bhatt: We have taken in a wealth of terrific information from the three preceding talks in this symposium. Let’s now share some questions from the audience and explore some of the points raised in the preceding talks in a bit more practical detail for clinicians. Our three prior speakers are joined in this panel discussion by Cleveland Clinic’s Dr. Frank Peacock, who brings an emergency medicine perspective.
Let’s begin with a case-based question supplied from the audience. The patient is a 42-year-old morbidly obese man without diabetes who had a non-ST-elevation myocardial infarction (MI) less than 1 year ago. A drug-eluting stent was placed at the time of his MI, and now restenosis has occurred. He is on aspirin and clopidogrel 75 mg/day. Do you recommend running a vasodilator-stimulated phosphoprotein (VASP) test and possibly increasing the clopidogrel dose to 150 mg/ day, or should the patient just be switched to prasugrel (assuming it is commercially available) without running the VASP test?
I’ll take a quick initial stab at this question. Studies of antiplatelet therapies to prevent instent restenosis have been a mixed bag. Some of the trials with glycoprotein IIb/IIIa inhibitors have shown an effect on restenosis, but most have not. Similarly, some of the analyses of the thienopyridines ticlopodine and clopidogrel have shown an effect on restenosis, but most have not.
For the most part, restenosis does not appear to be heavily mediated by platelets, at least not in a way that we can influence by therapy. On the other hand, stent thrombosis is highly platelet mediated, so I would alter the case to one in which stent thrombosis is the clinical problem. Assuming that the patient has been adherent to his antiplatelet regimen, which tests would you perform, and how would you act on the information from those tests?
Dr. Kandice Kottke-Marchant: The 2007 guidelines on acute coronary syndrome (ACS) management from the American College of Cardiology and American Heart Association (ACC/AHA)1 do not address platelet function testing, and almost none of the clinical trials of antiplatelet agents had an arm that included testing and dose adjustment based on platelet function studies. Platelet testing is available at some centers; at Cleveland Clinic, we use platelet aggregation testing. One can do platelet aggregation testing on a patient-by-patient basis; if inhibition appears to be suboptimal, a treatment decision should be made, but there is little guidance from the literature to steer that decision. I have seen clinicians increase the dose of clopidogrel or aspirin in response to platelet function tests, which occasionally triggers a confirmatory call from the pharmacy department.
Dr. Bhatt: When I was still at Cleveland Clinic, our chief medical resident did an analysis of platelet function testing, and it was remarkable how much testing was performed and how often it changed management, largely in the absence of any outcomes data, as Dr. Kottke-Marchant pointed out. Dr. Alexander, what are your recommendations with respect to platelet function testing today?
Dr. John Alexander: The case you describe is one in which applying evidence is not easy. There are no trials to supply any evidence to change therapy in this patient, a morbidly obese man receiving 75 mg/day of clopidogrel. There is certainly a rationale, however, to believe that a standard “one size fits all” 75-mg daily dose of clopidogrel may not be enough for him. The trade-off with a higher dosage is a higher risk of bleeding, however, so I would first be sure that he has been adherent to his current regimen of clopidogrel and aspirin.
Dr. Bhatt: Is there a role for point-of-care testing to determine whether he is adherent to the medicines?
Dr. Kottke-Marchant: Several of the point-of-care tests, such as the VerifyNow rapid platelet function analyzer, have specific cartridges for aspirin and for clopidogrel. If platelets were not being inhibited, it would suggest that the doses were too low, given the patient’s weight, but you probably would not be able to determine whether he was resistant to clopidogrel.
Dr. W. Frank Peacock: One way to verify that patients are taking their aspirin is to take a small urine sample and squirt in 2 mL of ferric chloride. If the sample turns purple, it means they are taking their aspirin. Once that is established, you can try to determine whether the drug is working on their platelets.
Dr. Alexander: Another potential explanation for stent thrombosis is faulty stent placement. In this case I would consider asking an interventional colleague to perform intravascular ultrasonography to make sure the stent was implanted properly before I changed the patient’s antithrombotic therapy.
Dr. Bhatt: That’s a great technical point. We always want to make sure that a case of stent thrombosis is not due to a mechanical problem. We should be asking: Is the stent properly sized and well opposed? Is there a distal dissection or any other issue that could predispose to stent thrombosis?
Dr. Alexander: This case illustrates a host of other challenges that underscore how much more work we need to do to define optimal antiplatelet therapy. Suppose we perform platelet function testing and find a low level of platelet inhibition in this patient with stent thrombosis, and we change his antiplatelet regimen. When should we test him again? If we retest in 3 months and find that he has a higher than expected level of platelet inhibition on the new antiplatelet regimen, do we dial down the intensity? Once again, there is no evidence to guide these decisions, and levels of platelet inhibition are driven not just by the medications but also by what is going on in the patient’s platelets—it is quite multifactorial.
POINT-OF-CARE PLATELET FUNCTION TESTING: CURRENT LIMITS, FUTURE ROLES
Dr. Bhatt: While we’re discussing platelet function testing, I found it interesting, Dr. Kottke-Marchant, that you said the use of bleeding time as a platelet test is finally going away. Testing of bleeding time has been around forever, but I agree that it doesn’t have much value in clinical practice. Do you think bleeding time will continue to have any role in drug development? Most phase 2 trials, and certainly phase 1 trials, still capture bleeding time to assess whether or not a drug is working. Should that, too, be jettisoned, or does bleeding time still have some merit in this context?
Dr. Kottke-Marchant: I would jettison it in drug development as well because of the considerable variability in bleeding time. It is not a test that can be standardized, and no quality control can be done. The results depend on skin turgor, age, and many other variables.
We need a global assay that will pick up multiple aspects of platelet function, such as flow-based adhesion, aggregation, and granule release. The bleeding time is a shear-dependent test, whereas the platelet aggregation test that is used in most drug trials is an artificial assay that measures only aggregation, but not under shear. The VerifyNow rapid platelet function analyzer does not measure platelets under shear and is not a global assay.
Dr. Marc Sabatine: I would underscore the need for a reliable point-of-care test of platelet function. When we prescribe a statin or an antihypertensive drug, we don’t just send the patient out the door and hope that everything will be okay. We measure the response, knowing that genotype, environmental factors, or medication factors can affect the response. When we prescribe an antiplatelet drug, we need a reliable point-of-care device to make certain that the patient is getting appropriate platelet inhibition.
I am reminded of a recent study of point-of-care measurement of platelet inhibition in patients receiving clopidogrel prior to nonemergent percutaneous coronary intervention (PCI).2 Rather than just treating patients with PCI and sending them out the door, the investigators kept giving patients clopidogrel and measuring their platelet inhibition until they achieved an appropriate degree of inhibition, after which PCI was performed. Event rates were significantly reduced in the patient group treated this way, which suggests a need to individualize therapy and move away from the “one size fits all” mindset.
Dr. Bhatt: Dr. Peacock, you’ve led a study of point-of-care assays in the emergency department. What might ultimately be the role of point-of-care testing in emergency medicine, and might it influence drug selection?
Dr. Peacock: My short answer is that I think there will be a role for point-of-care testing, with all the caveats that have been discussed. There may even be a day when we do genetic testing and look for DNA. Honestly, though, I’m somewhat of a skeptic because I’m not looking at the genetics. I see many patients who do crack cocaine who come to the emergency room with chest pain and have risk factors, but I send these patients home because they are not having an event. The real question is, “Is it an event?” If a patient is having an event and he or she has platelet resistance or hyperreactivity—whatever we term it—then you have to decide the next step.
As you mentioned, we just completed a study that evaluated a couple hundred patients for platelet inhibition resistance to aspirin, and one finding was that the incidence of platelet resistance to aspirin was much lower than we had anticipated. Studies from the literature suggest that the prevalence of resistance is around 30%, but in our study it was 6.5%.3
Dr. Kottke-Marchant: It depends on how and in whom you measure resistance. Different tests will give you different numbers. Even among studies using the same measurement techniques, the results depend on the patient population. If it’s a fairly stable cardiac population, you may see aspirin resistance rates of 4% or 5%. If it’s a population of patients who have had multiple MIs, the rate may be higher.
Dr. Peacock: That’s exactly my point. In the emergency department we see a mixed bag. We see many people who have had no prior events and have no acute event occurring. So in that setting you are going to get results that suggest that no intervention is required, whereas in that small percentage of patients in whom something is happening, your drug choice may be different.
Dr. Alexander: We are still talking about resistance to antiplatelet drugs as though it were a patient-level variable, but it’s my impression that it changes over time and within a patient.
Dr. Kottke-Marchant: It can change over time. There aren’t many good longitudinal studies. Most of the studies of “aspirin resistance” are really snapshot studies with measurements taken at one point in time. A term I prefer is “platelet reactivity.” To really assess treatment efficacy, we are going to have to look at the basal level of platelet reactivity.
WHAT ROLE FOR GENOTYPING IN GUIDING ANTIPLATELET THERAPY?
Dr. Bhatt: Dr. Peacock alluded to a potential role for genetic testing. Dr. Sabatine, you have done a lot of interesting work with genotyping in the TRITON-TIMI 38 study of prasugrel and clopidogrel. What is the future role of genotyping in determining which antiplatelet therapy is best for which patient?
Dr. Sabatine: As I mentioned, cytochrome P450 enzymes play a critical role in the metabolism of clopidogrel. These enzymes are fairly polymorphic—mutations in their encoding genes are responsible for subtle changes in effect, unlike the traditional mutations that we think about for sickle cell disease, for example. A wealth of data has been published showing that genetic variants are associated with decreased functional activity of cytochrome P450 enzymes, demonstrating the pharmacologic importance of these variants.
Individuals who carry variant alleles appear to respond differently to clopidogrel. A variety of small studies show that those who carry specific variants—particularly in the CYP2C19 enzyme, but in other enzymes as well—appear to have a diminished response to clopidogrel. There are also data showing that individuals with a diminished response to clopidogrel have worse outcomes.4 Our group is studying the impact of genetic variants that decrease the functional activity of cytochrome P450 enzymes on clinical outcomes. (Editor’s note: This study has since been published by Mega et al.5)
The practical implication may lie in point-of-care genotyping, which appears possible and will be clinically useful if a strong link can be demonstrated between genotype and outcomes. If point-of-care genotyping becomes practical, it will raise the question of whether both genotyping and platelet aggregation testing are needed. I think they might indeed be complementary in risk prediction, as is the case with genetic variants that affect low-density lipoprotein cholesterol (LDL-C) levels. In the lipid arena, we have seen that genetic effects and lipid levels provide independent incremental information about risk. That’s because of the high degree of variation in LDL-C levels: an LDL-C measurement is a snapshot in time, yet a variety of factors can influence LDL-C levels. In contrast, genotype is an invariant factor. Similarly, in the platelet arena, platelet aggregation studies and genotyping may be synergistic in predicting an individual’s predisposition to events and response to medications.
Dr. Bhatt: While we’re discussing pathways of metabolism, the literature, though scant, suggests a potential interaction between proton pump inhibitors and clopidogrel. I was co-chair of a recent American College of Cardiology/ American Heart Association/American College of Gastro-enterology consensus document that endorsed liberal use of proton pump inhibitors in patients who are at gastrointestinal risk, including those on antiplatelet therapy.6 The gastroenterologists believed strongly that proton pump inhibitors were safe and in fact underused in these patients. What do you think about the clopidogrel–proton pump inhibitor interaction? Should we be concerned?
Dr. Sabatine: Proton pump inhibitors are not only substrates for, but also inhibitors of, CYP2C19, a key enzyme that helps transform clopidogrel into an active metabolite. For this reason, there has been interest in whether concomitant use of proton pump inhibitors would blunt the efficacy of clopidogrel. The same concern was raised about giving clopidogrel with certain statin drugs that are also metabolized by the cytochrome P450 system, and several studies have shown an effect of these statins on clopidogrel’s platelet inhibition. However, there is no evidence that coadministration of these statins has affected clinical outcomes with clopidogrel in clinical trials. So it may be that while competition for the cytochrome P450 system is one factor, it’s not enough of a factor to tip the scale and result in a clinical event. The same may be true of coadministration of proton pump inhibitors; meanwhile, we await definitive data that concomitant use with clopidogrel leads to higher rates of ischemic events.
DIAGNOSTIC UNCERTAINTY IN THE EMERGENCY SETTING
Dr. Bhatt: We heard about quite a few new antiplatelet drugs in Dr. Sabatine’s presentation, some of which will likely be taken up in clinical practice. Dr. Peacock, from an emergency department perspective, how will you integrate all these new agents with the numerous therapies already available? What should emergency departments do to come to grips with and ultimately take advantage of these different forms of therapy as well as emerging platelet function tests?
Dr. Peacock: The piece that’s unique or especially pertinent to the emergency department is diagnostic uncertainty. Diagnosis and management are easy when a patient has an ST-elevation MI because we all know what that looks like and we know what to do in response. To some extent non-ST-elevation MI is fairly simple too. ACS is a lot more difficult because we don’t have a good definition for unstable angina, and that’s where diagnosis and management become problematic. And with high-sensitivity troponins coming out now, the question of non-ST-elevation MI is going to get more and more confusing because we will have a lot more patients who meet criteria without having an acute coronary artery event.
So it is going to be important that studies be designed correctly. A lot of the studies reviewed today were efficacy studies, showing that a particular drug works well in a carefully defined population, but they were not efficiency studies: they did not take into account the real-world diagnostic uncertainty—and inevitable misdiagnoses—that emergency departments encounter before starting therapy.
Take the CURE trial, for example. It was a great study, showing that clopidogrel reduced the hazard ratio for major coronary events by 20% in patients with unstable angina,7 and the message was that everybody should be using clopidogrel. A close look at the study, however, reveals that about half the patients did not receive clopidogrel in the emergency department. When patients did receive it early, it was driven by the cardiologist, who was absolutely certain of the diagnosis. But if the study was not designed to test early use, then it is a big leap to extrapolate its findings to this circumstance.
Many of the patients in the CURE trial were enrolled the day after presentation, when their diagnosis was certain—ie, they had a rise in troponin after their symptoms. But when a patient first arrives in the emergency department, we are not certain of the diagnosis. And if we use a drug such as clopidogrel, with a duration of action as long as 5 days, we have committed the entire medical system to a certain course of management for that period of time. If we get the diagnosis wrong, this commitment could restrict management options for up to 5 days.
The question for emergency physicians becomes, “How long is long enough to know whether I can pull the trigger on a therapy and be correct?” With all the new drugs coming along, the way to answer this is to do efficiency studies in a real-world environment in addition to efficacy studies.
Dr. Alexander: Yes, one of the biggest limitations of antiplatelet drug studies to date is that they usually haven’t really addressed the timing of drug initiation. We often assume that if a drug is shown to be beneficial, then it should be started as soon as possible. As we just heard, that may have been an unfounded extrapolation from the CURE trial. The same sort of thing happened with the ISIS trial of aspirin in patients with ST-elevation MI.8 In response to the ISIS results, clinicians rushed to give patients aspirin right away even though many of the patients in the trial may have received their aspirin the day after presentation. For these reasons, the EARLY-ACS study,9 which is addressing a very simple question—whether early upstream use of glycoprotein IIb/IIIa inhibitors is beneficial—has been a challenging trial to complete.
WHAT ROLE FOR THIENOPYRIDINE PRETREATMENT?
Dr. Bhatt: Dr. Sabatine, you presented data from the large TRITON-TIMI 38 trial comparing prasugrel with clopidogrel. I’m interested in how you would use prasugrel in practice, assuming it receives marketing approval, especially in light of its bleeding risk, particularly in patients in whom coronary artery bypass graft surgery (CABG) is planned. Many hospitals pretreat patients with clopidogrel in the emergency department. How would you manage a patient who shows up in the emergency room with ACS? Would you give clopidogrel, would you wait and give prasugrel, or would you do something else? If you gave clopidogrel, what loading dose would you use—300 mg, 600 mg, or, as some have suggested, 900 or 1,200 mg?
Dr. Sabatine: I am a strong proponent of pretreatment. Data from multiple studies show a benefit to this strategy, and even the original CURE trial showed a roughly 30% reduction in ischemic events within the first 24 hours of clopidogrel initiation.7
I think the dosing strategy depends on how the patient is going to be managed. If management is going to be conservative, then I would start the patient on 300 mg of clopidogrel when he or she came in. If the patient is going to the cardiac catheterization laboratory in a few hours, I would pretreat with 600 mg of clopidogrel. For prasugrel, the need for pretreatment is less clear, given the drug’s faster onset of action and greater degree of platelet inhibition. In the TRITON-TIMI 38 study,10 prasugrel was given, by and large, after diagnostic angiography, and thus one could use that approach in practice.
In terms of clopidogrel versus prasugrel, I would embrace prasugrel for the large majority of my patients, being mindful of the risk of bleeding. I would not hesitate to give the medication to diabetics or to younger, more robust patients. The 50% reduction in stent thrombosis with prasugrel versus clopidogrel in TRITON-TIMI 38 is huge,11 given that the risk of death with stent thrombosis is probably 25% or greater. So I would want to have prasugrel on board to reduce the risk of stent thrombosis, especially if a drug-eluting stent were being implanted.
Dr. Bhatt: Dr. Alexander, let’s get your take on a similar scenario. Assuming that prasugrel gains marketing approval, how would you manage patients with non-ST-elevation MI who present to the emergency department? Would you pretreat with clopidogrel? Would you wait until angiography and then, depending on the anatomy, treat with prasugrel? Or would you potentially pretreat with prasugrel, which has not been studied and would not be a labeled indication? How would you reconcile the data?
Dr. Alexander: At Duke, I expect that prasugrel will not be used prior to the catheterization laboratory in patients with non-ST-elevation ACS due to concerns about whether the patients will undergo PCI or be managed medically or with CABG.
Dr. Bhatt: That makes sense, since there was a fair amount of bleeding with prasugrel in those patients in TRITON-TIMI 38.
Dr. Alexander: Correct. Moreover, at Duke we don’t use as much upstream clopidogrel as we would, based on the evidence, if I were managing all the patients. There is still a lot of pushback about upstream clopidogrel from our surgeons because patients are going to surgery quickly these days, sometimes just a day after catheterization, and that’s when a loading dose of clopidogrel can be problematic. We are also still fairly heavy users of glycoprotein IIb/IIIa inhibitors.
Where I can see prasugrel being used prior to the cath lab at Duke is in ST-elevation MI, where the rate of PCI is very high. In primary angioplasty for ST-elevation MI, it would likely be given upstream. The bigger issue for us will be that we serve as a referral base for a lot of regional hospitals, and thus have some influence on their practices.
Dr. Bhatt: In that case, what would you advise those regional hospitals to do for non-ST-elevation MI?
Dr. Alexander: For the time being, we would advise continuing with our current practice, which is to load clopidogrel in patients in whom there is a reasonable certainty that CABG will not be performed, and to use glycoprotein IIb/IIIa inhibitors in high-risk patients. As we get more experience with prasugrel or with additional trial results, however, that practice could easily change.
Dr. Bhatt: So you would still use glycoprotein IIb/IIIa inhibitors?
Dr. Alexander: Yes, I advocate upstream clopidogrel use, but not all my colleagues do. Based on the guidelines, I’d use one or the other—either clopidogrel or a glycoprotein IIb/IIIa inhibitor. As I mentioned in my talk, if a patient is at high risk for bleeding, I am more inclined to use clopidogrel, although patients at higher risk of bleeding are often at higher risk for ischemic events as well.
WHAT’S DRIVEN THE DROPOFF IN GLYCOPROTEIN IIb/IIIa INHIBITOR USE?
Dr. Bhatt: While we’re on the topic of glycoprotein IIb/IIIa inhibitors, a question card from the audience asks why there has been a decrease in glycoprotein IIb/ IIIa inhibitor use and whether this decline is appropriate or inappropriate. Have clopidogrel pretreatment, higher loading doses of clopidogrel, and use of the direct thrombin inhibitor bivalirudin contributed to the decrease in glycoprotein IIb/IIIa inhibitor use?
Dr. Alexander: I do think that the decline has been driven by the changing environment, with greater use of other antithrombotic strategies that include clopidogrel and bivalirudin, as you suggest, as well as an increased attention to bleeding. From an evidence-based standpoint, we don’t know whether the decrease in glycoprotein IIb/IIIa use is appropriate or not because the studies of these agents were conducted before the widespread upstream use of clopidogrel and bivalirudin. Clopidogrel is attractive because it’s a pill given as one dose in the emergency department, the wards, or the catheterization laboratory, rather than a much more complicated infusion with weight-based dosing and dosage adjustments based on creatinine clearance. It is possible that we should perhaps be dosing clopidogrel the same way, but we don’t know that yet.
PRASUGREL IN PRACTICE: HOW LOW CAN THE DOSE GO, AND IS THERE A GENDER EFFECT?
Dr. Bhatt: Let’s stick with this focus on dosing but turn back to discussion of prasugrel. In your presentation of the TRITON-TIMI 38 data, Dr. Sabatine, you proposed a potential prasugrel dosage modification, down to a 5-mg loading dose, in subgroups that were identified as being at high bleeding risk—namely, elderly patients and patients with low body weight. However, no outcomes data with 5 mg of prasugrel came out of TRITON-TIMI 38.10 Is this proposed modification based on pharmacokinetic extrapolation? Could clinicians be comfortable using 5 mg of prasugrel, assuming the drug receives regulatory approval and a 5-mg tablet would be available?
Dr. Sabatine: Of course, evidence at the grade A level would consist of a trial showing that patients who received a lower dose enjoyed the same benefit as those who got standard dosing in TRITON-TIMI 38—a 60-mg loading dose followed by 10 mg/day—with an acceptable risk profile. However, such a trial would be difficult and costly to conduct, and would take roughly half a decade to pull off. It is only through large trials like TRITON-TIMI 38 that you identify subgroups that respond differently, and then to go back and do a separate trial for those subgroups takes a great deal of time. It may not be practical.
I think the Food and Drug Administration is moving toward embracing careful pharmacokinetic/pharmacodynamic substudies within trials, with these substudies having adequate numbers of subjects to provide a sense for the ideal target dose and what an acceptable dose range would be, without limiting approval to a single dose. The analogy would be warfarin dosing, with the aim being to figure out an acceptable dose range, discover which patients fall outside that range, and then model the effect of a lower dose in those patients. Thus, approving a 5-mg dose of prasugrel based on TRITON-TIMI 38 would be a reasonable approach if this dose passed muster under pharmacokinetic/pharmacodynamic modeling. If this approach were taken, there would clearly be a need for postmarketing surveillance to confirm whether the modeling on the effects of the lower dose was borne out by actual outcomes.
Dr. Bhatt: The audience has posed another interesting question raised by TRITON-TIMI 38: Can you comment on the lesser effect of prasugrel in women?
Dr. Sabatine: It is true that there was not a statistically significant effect of prasugrel among women in TRITON-TIMI 38, but statistical tests among subgroups found no significant heterogeneity for the effect between men and women, and that is the relevant measure to determine any gender effect. Keep in mind that not all subgroups represent a univariate slice of the population. For example, women generally have lower body weight than men, and since prasugrel’s net clinical benefit was reduced in patients with lower body weight, that may explain some of the differing extent of effect between men and women.
Dr. Bhatt: That’s a good point about the lack of heterogeneity between men and women. In fact, a meta-analysis of clopidogrel data conducted by one of the fellows I work with revealed that men and women appear to benefit similarly from clopidogrel.12 There was a slight signal of excess bleeding in women, but there were more elderly women in the pooled population, which may have been a confounding factor. As best as anyone can tell, antiplate-let therapy works well in both men and women.
NAVIGATING MANAGEMENT ACROSS THE SPECTRUM OF CARE
Dr. Bhatt: I would like to explore a bit further how all of these issues translate across the spectrum of care, beginning in the emergency department, which we know is a key component of the entire ACS management strategy for a health care system. What should emergency medicine doctors do, given all of the potential options—clopidogrel, different loading doses of clopidogrel, prasugrel, glycoprotein IIb/IIIa inhibitors, even bivalirudin?
Dr. Peacock: It depends on the practice setting. Some emergency physicians work at community hospitals with no backup. They must have relationships with the larger centers to which they’ll be transferring patients, because ACS patients should not be staying at community hospitals. These emergency physicians must have close relationships with the physicians who will be receiving their patients, and they know the potential head-butting with surgeons surrounding early clopidogrel use better than anybody does. If they treat with clopidogrel in the emergency room, and it turns out that the patient needs to go to the catheterization laboratory, can the receiving hospital use platelet testing to shorten the standard 5-day interval from treatment to catheterization?
Dr. Bhatt: Yes, that’s a rather useful, although not completely validated, way of using point-of-care platelet testing—to potentially reduce the time to surgery.
Dr. Peacock: Right. So if the policies for handling these types of transfer-related issues are worked out in advance, all players have a pathway to follow, which can allow quick action when necessary. If you don’t have these issues worked out in advance, you either lose many opportunities to act quickly in the emergency room or you risk taking actions that will cause problems later in the course of management.
Dr. Alexander: I totally agree. The key is to sit down with all the players involved—the surgeons, the interventional cardiologists, the intensivists, the emergency room personnel—and come up with strategies for different populations of patients. Write down the collective strategy and hang it on the wall so that everybody can be comfortable with it. The strategy can be reevaluated when prasugrel or other new antithrombotic drugs come on the market.
Dr. Peacock: The other environment is the academic center, which is even more challenging, but for different reasons. At a large academic center like the Cleveland Clinic, any of 25 different cardiologists may be taking call and receiving patients from the emergency department on a particular night. A lot of phone interaction is required to elicit the planned management strategy, including if and when the patient will be going to the cath lab. Individualizing care to a particular cardiologist then becomes a time-consuming challenge, especially in clinical situations where outcomes are time-dependent.
Dr. Alexander: Agreed. Management needs to be integrated across the entire spectrum of care. The anticoagulants that we plan to use in the cath lab will affect the antithrombotic regimen used upstream.
Dr. Kottke-Marchant: One circumstance where platelet function testing has been helpful is in determining the washout of the clopidogrel effect before surgery. At Cleveland Clinic, we have implemented platelet function testing in this circumstance instead of waiting a blanket 5 days after clopidogrel administration to go to surgery. A return to normal platelet function on platelet aggregation testing, depending on the cutoff value used, is an indicator that the patient can proceed to surgery.
Dr. Bhatt: That’s a logical approach. How should we be using antiplatelet therapy in the medically managed patient, Dr. Alexander?
Dr. Alexander: When I think of medical management, I include patients who don’t go to the cath lab, but also those who do, with regards to their management prior to and following their time in the cath lab.
In patients who don’t go to the cath lab for angiography, the ACC/AHA guidelines recommend aspirin and either clopidogrel, a glycoprotein IIb/IIIa inhibitor, or both.1 In making this choice, I consider the patient’s risk of bleeding and the dosing complexity of the regimen, especially with the use of glycoprotein IIb/IIIa inhibitors in a patient with renal insufficiency. In a patient at relatively low risk for bleeding, I often use both clopidogrel and a glycoprotein IIb/IIIa inhibitor, although this strategy does not have a lot of data to support it.
The more challenging population consists of patients who go to the cath lab but do not undergo PCI; this population is managed medically too. We often drop the ball with clopidogrel in this population. Many patients in whom PCI is not performed do not receive clopidogrel upstream, for all of the reasons we’ve discussed, and there is pretty good evidence that if clopidogrel is not instituted before hospital discharge, the patient is not likely to be receiving it at 30 days either. We have an obligation to treat these patients.
Treatment following bypass surgery is much murkier, and I don’t really know what we should be doing. The ACC/AHA guidelines suggest that clopidogrel be started in a patient with non-ST-elevation ACS after bypass surgery,1 but I believe the evidence to support that recommendation is pretty weak.
Dr. Bhatt: Well, the CURE trial did contain a sizeable group that underwent bypass surgery,7 and although this group was underpowered in some respects, it was still a very large group, so I personally favor treatment in those patients. We should mention that an ongoing trial called TRILOGY ACS is comparing clopidogrel and prasugrel specifically in patients who are being managed medically,13 so more data on this strategy will be emerging.
ARE GUIDELINES DESTINED TO BECOME EVER MORE COMPLEX?
Dr. Bhatt: Here’s a comment and question from the audience that pulls together a lot of what we’ve discussed while also looking forward: The antiplatelet therapy guidelines are already complicated. If the ongoing studies of emerging antiplatelet drugs all have results that are qualitatively similar to those of the TRITON-TIMI 38 study of prasugrel—ie, better efficacy with more potent therapy but more bleeding—how do you foresee these antiplatelet drugs being used in clinical practice?
Dr. Sabatine: The contrast between the US guidelines and the European guidelines for ACS management is stark. The US guidelines—from the ACC and AHA1—are essentially an encyclopedia that includes nearly every trial of anti-platelet therapy in ACS along with complicated algorithms; they do a wonderful job of being complete. The European guidelines14 are probably one tenth the size of their US counterpart document, and they suggest treatments for various patient types; they are very simple.
In a sense, the US guidelines lay out the data and force practitioners to evaluate the trials and consider how our patients fit into the study populations. In this way they are analogous to current guidelines for anticoagulant therapy. Several anticoagulants have been compared with heparin in clinical trials. These newer anticoagulants appear to reduce the risk of ischemic events compared with heparin; some have lower rates of bleeding, while others have higher rates of bleeding. There have been few head-to-head studies of these agents, however, so we wind up with guidelines that are not definitive but rather suggest agents to “consider” in various settings.
It’s unlikely that a head-to-head trial will be conducted comparing prasugrel with the reversible P2Y12 antagonist AZD6140, assuming that both are approved for marketing. If the drugs appear equally efficacious in placebo-controlled trials, it will take consensus to determine the appropriate choice at your hospital, factoring in your patient profile, the cost of the drugs, and other variables. It’s more complicated when one agent is slightly more efficacious but causes more bleeding or, conversely, a little less efficacious but less apt to cause bleeding. In such cases, you may need to tailor therapy to the patient, trying to gauge bleeding risk. All of the emerging data appear to point to the importance of bleeding on outcomes: patients who bleed fare poorly, in part due to the bleeding itself and in part perhaps because they have a proclivity for bleeding.
THE FUTURE: MONITORING-BASED DOSING AND NICHE ANTIPLATELETS?
Dr. Bhatt: That’s a good observation. Let’s wrap up by having the other panelists share any final thoughts you may have.
Dr. Alexander: I’d like to return to the issue of measuring antiplatelet response and using it to guide therapy. Earlier we cited the examples of antihypertensive therapy and lipid-lowering therapy to support this model of monitoring-based treatment. Guidelines for dyslipidemia treatment recommend using LDL-C levels to guide therapy, but this practice is difficult to study in a randomized trial. In fact, none of the randomized trials of statins used LDL-C levels to guide therapy. They all studied fixed doses of statins versus placebo or fixed doses of another statin. Higher doses of statins were always beneficial compared with lower doses, and this finding was extrapolated into the guidelines as a justification to treat to target LDL-C levels.
Dr. Bhatt: It’s not even necessarily clear that LDL-C level is the best target, if you consider the JUPITER trial, in which patients received statin therapy based on their baseline level of high-sensitivity C-reactive protein, not their LDL-C level.15 It goes to show how incomplete our knowledge of a class of drugs may be, even decades after the drugs are introduced.
Dr. Kottke-Marchant: To speak to Dr. Alexander’s point, dose adjustment guided by platelet monitoring is a bit more problematic for antiplatelet drugs that are irreversible inhibitors, such as clopidogrel and aspirin, than for those that are reversible inhibitors, which are being developed and may eventually make more sense to use. From a drug development standpoint, a drug that requires monitoring and dose adjustment will not gain wide acceptance because it will increase medical costs and morbidity.
Dr. Bhatt: Yes, we know from experience with warfarin that doctors and patients don’t like the ongoing need for monitoring and testing.
Dr. Peacock: The drugs that are going to be adopted by the emergency department are those with the shortest half-lives, for several reasons: (1) using a drug with a short half-life won’t commit us to a particular course of action; (2) the potential for drug interactions is lower; and (3) in the event of an erroneous diagnosis, the consequence of misapplication may be mitigated by early recognition and termination of the drug. If we later decide that we’ve gone down the wrong therapeutic road or reached a wrong diagnosis, or if a complication occurs, we can turn off the therapy quickly. That level of flexibility is needed.
Dr. Kottke-Marchant: I think we are moving into an era of niche antiplatelet drugs. One might be used in a patient going to surgery, for example, and another for long-term therapy.
Dr. Peacock: One thing that I don’t have a feel for is how to transition from one drug to another. When you change drugs for a patient, it so often seems like it goes badly. If we’re eventually going to use drugs with ultra-short half-lives in the in the emergency department for the first day or two, and then switch patients to a pill for a week, a lot more platelet function testing may be needed.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:e1–e157.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Glauser J, Emerman CL, Bhatt DL, Peacock WF. Platelet aspirin resistance in emergency department patients with suspected acute coronary syndrome. Am J Emerg Med. In press
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2008; 52:1128–1133.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. J Am Coll Cardiol 2008; 52:1502–1517.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- EARLY-ACS: Glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome. Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00089895. Updated December 17, 2008. Accessed December 18, 2008.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral anti-platelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary dyndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Berger JS, Bhatt DL, Chen Z, et al. The relationship between sex, mortality and cardiovascular events among patients with established cardiovascular disease: a meta-analysis [ACC abstract 1012-149]. J Am Coll Cardiol 2008; 51 10 suppl A:A247.
- TRILOGY ACS: A comparison of prasugrel and clopidogrel in acute coronary syndrome subjects. ClinicalTrials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00699998. Updated December 15, 2008. Accessed January 2, 2009.
- Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
CASE STUDY: THROMBOSIS AFTER STENTING DESPITE ANTIPLATELET THERAPY
Dr. Deepak Bhatt: We have taken in a wealth of terrific information from the three preceding talks in this symposium. Let’s now share some questions from the audience and explore some of the points raised in the preceding talks in a bit more practical detail for clinicians. Our three prior speakers are joined in this panel discussion by Cleveland Clinic’s Dr. Frank Peacock, who brings an emergency medicine perspective.
Let’s begin with a case-based question supplied from the audience. The patient is a 42-year-old morbidly obese man without diabetes who had a non-ST-elevation myocardial infarction (MI) less than 1 year ago. A drug-eluting stent was placed at the time of his MI, and now restenosis has occurred. He is on aspirin and clopidogrel 75 mg/day. Do you recommend running a vasodilator-stimulated phosphoprotein (VASP) test and possibly increasing the clopidogrel dose to 150 mg/ day, or should the patient just be switched to prasugrel (assuming it is commercially available) without running the VASP test?
I’ll take a quick initial stab at this question. Studies of antiplatelet therapies to prevent instent restenosis have been a mixed bag. Some of the trials with glycoprotein IIb/IIIa inhibitors have shown an effect on restenosis, but most have not. Similarly, some of the analyses of the thienopyridines ticlopodine and clopidogrel have shown an effect on restenosis, but most have not.
For the most part, restenosis does not appear to be heavily mediated by platelets, at least not in a way that we can influence by therapy. On the other hand, stent thrombosis is highly platelet mediated, so I would alter the case to one in which stent thrombosis is the clinical problem. Assuming that the patient has been adherent to his antiplatelet regimen, which tests would you perform, and how would you act on the information from those tests?
Dr. Kandice Kottke-Marchant: The 2007 guidelines on acute coronary syndrome (ACS) management from the American College of Cardiology and American Heart Association (ACC/AHA)1 do not address platelet function testing, and almost none of the clinical trials of antiplatelet agents had an arm that included testing and dose adjustment based on platelet function studies. Platelet testing is available at some centers; at Cleveland Clinic, we use platelet aggregation testing. One can do platelet aggregation testing on a patient-by-patient basis; if inhibition appears to be suboptimal, a treatment decision should be made, but there is little guidance from the literature to steer that decision. I have seen clinicians increase the dose of clopidogrel or aspirin in response to platelet function tests, which occasionally triggers a confirmatory call from the pharmacy department.
Dr. Bhatt: When I was still at Cleveland Clinic, our chief medical resident did an analysis of platelet function testing, and it was remarkable how much testing was performed and how often it changed management, largely in the absence of any outcomes data, as Dr. Kottke-Marchant pointed out. Dr. Alexander, what are your recommendations with respect to platelet function testing today?
Dr. John Alexander: The case you describe is one in which applying evidence is not easy. There are no trials to supply any evidence to change therapy in this patient, a morbidly obese man receiving 75 mg/day of clopidogrel. There is certainly a rationale, however, to believe that a standard “one size fits all” 75-mg daily dose of clopidogrel may not be enough for him. The trade-off with a higher dosage is a higher risk of bleeding, however, so I would first be sure that he has been adherent to his current regimen of clopidogrel and aspirin.
Dr. Bhatt: Is there a role for point-of-care testing to determine whether he is adherent to the medicines?
Dr. Kottke-Marchant: Several of the point-of-care tests, such as the VerifyNow rapid platelet function analyzer, have specific cartridges for aspirin and for clopidogrel. If platelets were not being inhibited, it would suggest that the doses were too low, given the patient’s weight, but you probably would not be able to determine whether he was resistant to clopidogrel.
Dr. W. Frank Peacock: One way to verify that patients are taking their aspirin is to take a small urine sample and squirt in 2 mL of ferric chloride. If the sample turns purple, it means they are taking their aspirin. Once that is established, you can try to determine whether the drug is working on their platelets.
Dr. Alexander: Another potential explanation for stent thrombosis is faulty stent placement. In this case I would consider asking an interventional colleague to perform intravascular ultrasonography to make sure the stent was implanted properly before I changed the patient’s antithrombotic therapy.
Dr. Bhatt: That’s a great technical point. We always want to make sure that a case of stent thrombosis is not due to a mechanical problem. We should be asking: Is the stent properly sized and well opposed? Is there a distal dissection or any other issue that could predispose to stent thrombosis?
Dr. Alexander: This case illustrates a host of other challenges that underscore how much more work we need to do to define optimal antiplatelet therapy. Suppose we perform platelet function testing and find a low level of platelet inhibition in this patient with stent thrombosis, and we change his antiplatelet regimen. When should we test him again? If we retest in 3 months and find that he has a higher than expected level of platelet inhibition on the new antiplatelet regimen, do we dial down the intensity? Once again, there is no evidence to guide these decisions, and levels of platelet inhibition are driven not just by the medications but also by what is going on in the patient’s platelets—it is quite multifactorial.
POINT-OF-CARE PLATELET FUNCTION TESTING: CURRENT LIMITS, FUTURE ROLES
Dr. Bhatt: While we’re discussing platelet function testing, I found it interesting, Dr. Kottke-Marchant, that you said the use of bleeding time as a platelet test is finally going away. Testing of bleeding time has been around forever, but I agree that it doesn’t have much value in clinical practice. Do you think bleeding time will continue to have any role in drug development? Most phase 2 trials, and certainly phase 1 trials, still capture bleeding time to assess whether or not a drug is working. Should that, too, be jettisoned, or does bleeding time still have some merit in this context?
Dr. Kottke-Marchant: I would jettison it in drug development as well because of the considerable variability in bleeding time. It is not a test that can be standardized, and no quality control can be done. The results depend on skin turgor, age, and many other variables.
We need a global assay that will pick up multiple aspects of platelet function, such as flow-based adhesion, aggregation, and granule release. The bleeding time is a shear-dependent test, whereas the platelet aggregation test that is used in most drug trials is an artificial assay that measures only aggregation, but not under shear. The VerifyNow rapid platelet function analyzer does not measure platelets under shear and is not a global assay.
Dr. Marc Sabatine: I would underscore the need for a reliable point-of-care test of platelet function. When we prescribe a statin or an antihypertensive drug, we don’t just send the patient out the door and hope that everything will be okay. We measure the response, knowing that genotype, environmental factors, or medication factors can affect the response. When we prescribe an antiplatelet drug, we need a reliable point-of-care device to make certain that the patient is getting appropriate platelet inhibition.
I am reminded of a recent study of point-of-care measurement of platelet inhibition in patients receiving clopidogrel prior to nonemergent percutaneous coronary intervention (PCI).2 Rather than just treating patients with PCI and sending them out the door, the investigators kept giving patients clopidogrel and measuring their platelet inhibition until they achieved an appropriate degree of inhibition, after which PCI was performed. Event rates were significantly reduced in the patient group treated this way, which suggests a need to individualize therapy and move away from the “one size fits all” mindset.
Dr. Bhatt: Dr. Peacock, you’ve led a study of point-of-care assays in the emergency department. What might ultimately be the role of point-of-care testing in emergency medicine, and might it influence drug selection?
Dr. Peacock: My short answer is that I think there will be a role for point-of-care testing, with all the caveats that have been discussed. There may even be a day when we do genetic testing and look for DNA. Honestly, though, I’m somewhat of a skeptic because I’m not looking at the genetics. I see many patients who do crack cocaine who come to the emergency room with chest pain and have risk factors, but I send these patients home because they are not having an event. The real question is, “Is it an event?” If a patient is having an event and he or she has platelet resistance or hyperreactivity—whatever we term it—then you have to decide the next step.
As you mentioned, we just completed a study that evaluated a couple hundred patients for platelet inhibition resistance to aspirin, and one finding was that the incidence of platelet resistance to aspirin was much lower than we had anticipated. Studies from the literature suggest that the prevalence of resistance is around 30%, but in our study it was 6.5%.3
Dr. Kottke-Marchant: It depends on how and in whom you measure resistance. Different tests will give you different numbers. Even among studies using the same measurement techniques, the results depend on the patient population. If it’s a fairly stable cardiac population, you may see aspirin resistance rates of 4% or 5%. If it’s a population of patients who have had multiple MIs, the rate may be higher.
Dr. Peacock: That’s exactly my point. In the emergency department we see a mixed bag. We see many people who have had no prior events and have no acute event occurring. So in that setting you are going to get results that suggest that no intervention is required, whereas in that small percentage of patients in whom something is happening, your drug choice may be different.
Dr. Alexander: We are still talking about resistance to antiplatelet drugs as though it were a patient-level variable, but it’s my impression that it changes over time and within a patient.
Dr. Kottke-Marchant: It can change over time. There aren’t many good longitudinal studies. Most of the studies of “aspirin resistance” are really snapshot studies with measurements taken at one point in time. A term I prefer is “platelet reactivity.” To really assess treatment efficacy, we are going to have to look at the basal level of platelet reactivity.
WHAT ROLE FOR GENOTYPING IN GUIDING ANTIPLATELET THERAPY?
Dr. Bhatt: Dr. Peacock alluded to a potential role for genetic testing. Dr. Sabatine, you have done a lot of interesting work with genotyping in the TRITON-TIMI 38 study of prasugrel and clopidogrel. What is the future role of genotyping in determining which antiplatelet therapy is best for which patient?
Dr. Sabatine: As I mentioned, cytochrome P450 enzymes play a critical role in the metabolism of clopidogrel. These enzymes are fairly polymorphic—mutations in their encoding genes are responsible for subtle changes in effect, unlike the traditional mutations that we think about for sickle cell disease, for example. A wealth of data has been published showing that genetic variants are associated with decreased functional activity of cytochrome P450 enzymes, demonstrating the pharmacologic importance of these variants.
Individuals who carry variant alleles appear to respond differently to clopidogrel. A variety of small studies show that those who carry specific variants—particularly in the CYP2C19 enzyme, but in other enzymes as well—appear to have a diminished response to clopidogrel. There are also data showing that individuals with a diminished response to clopidogrel have worse outcomes.4 Our group is studying the impact of genetic variants that decrease the functional activity of cytochrome P450 enzymes on clinical outcomes. (Editor’s note: This study has since been published by Mega et al.5)
The practical implication may lie in point-of-care genotyping, which appears possible and will be clinically useful if a strong link can be demonstrated between genotype and outcomes. If point-of-care genotyping becomes practical, it will raise the question of whether both genotyping and platelet aggregation testing are needed. I think they might indeed be complementary in risk prediction, as is the case with genetic variants that affect low-density lipoprotein cholesterol (LDL-C) levels. In the lipid arena, we have seen that genetic effects and lipid levels provide independent incremental information about risk. That’s because of the high degree of variation in LDL-C levels: an LDL-C measurement is a snapshot in time, yet a variety of factors can influence LDL-C levels. In contrast, genotype is an invariant factor. Similarly, in the platelet arena, platelet aggregation studies and genotyping may be synergistic in predicting an individual’s predisposition to events and response to medications.
Dr. Bhatt: While we’re discussing pathways of metabolism, the literature, though scant, suggests a potential interaction between proton pump inhibitors and clopidogrel. I was co-chair of a recent American College of Cardiology/ American Heart Association/American College of Gastro-enterology consensus document that endorsed liberal use of proton pump inhibitors in patients who are at gastrointestinal risk, including those on antiplatelet therapy.6 The gastroenterologists believed strongly that proton pump inhibitors were safe and in fact underused in these patients. What do you think about the clopidogrel–proton pump inhibitor interaction? Should we be concerned?
Dr. Sabatine: Proton pump inhibitors are not only substrates for, but also inhibitors of, CYP2C19, a key enzyme that helps transform clopidogrel into an active metabolite. For this reason, there has been interest in whether concomitant use of proton pump inhibitors would blunt the efficacy of clopidogrel. The same concern was raised about giving clopidogrel with certain statin drugs that are also metabolized by the cytochrome P450 system, and several studies have shown an effect of these statins on clopidogrel’s platelet inhibition. However, there is no evidence that coadministration of these statins has affected clinical outcomes with clopidogrel in clinical trials. So it may be that while competition for the cytochrome P450 system is one factor, it’s not enough of a factor to tip the scale and result in a clinical event. The same may be true of coadministration of proton pump inhibitors; meanwhile, we await definitive data that concomitant use with clopidogrel leads to higher rates of ischemic events.
DIAGNOSTIC UNCERTAINTY IN THE EMERGENCY SETTING
Dr. Bhatt: We heard about quite a few new antiplatelet drugs in Dr. Sabatine’s presentation, some of which will likely be taken up in clinical practice. Dr. Peacock, from an emergency department perspective, how will you integrate all these new agents with the numerous therapies already available? What should emergency departments do to come to grips with and ultimately take advantage of these different forms of therapy as well as emerging platelet function tests?
Dr. Peacock: The piece that’s unique or especially pertinent to the emergency department is diagnostic uncertainty. Diagnosis and management are easy when a patient has an ST-elevation MI because we all know what that looks like and we know what to do in response. To some extent non-ST-elevation MI is fairly simple too. ACS is a lot more difficult because we don’t have a good definition for unstable angina, and that’s where diagnosis and management become problematic. And with high-sensitivity troponins coming out now, the question of non-ST-elevation MI is going to get more and more confusing because we will have a lot more patients who meet criteria without having an acute coronary artery event.
So it is going to be important that studies be designed correctly. A lot of the studies reviewed today were efficacy studies, showing that a particular drug works well in a carefully defined population, but they were not efficiency studies: they did not take into account the real-world diagnostic uncertainty—and inevitable misdiagnoses—that emergency departments encounter before starting therapy.
Take the CURE trial, for example. It was a great study, showing that clopidogrel reduced the hazard ratio for major coronary events by 20% in patients with unstable angina,7 and the message was that everybody should be using clopidogrel. A close look at the study, however, reveals that about half the patients did not receive clopidogrel in the emergency department. When patients did receive it early, it was driven by the cardiologist, who was absolutely certain of the diagnosis. But if the study was not designed to test early use, then it is a big leap to extrapolate its findings to this circumstance.
Many of the patients in the CURE trial were enrolled the day after presentation, when their diagnosis was certain—ie, they had a rise in troponin after their symptoms. But when a patient first arrives in the emergency department, we are not certain of the diagnosis. And if we use a drug such as clopidogrel, with a duration of action as long as 5 days, we have committed the entire medical system to a certain course of management for that period of time. If we get the diagnosis wrong, this commitment could restrict management options for up to 5 days.
The question for emergency physicians becomes, “How long is long enough to know whether I can pull the trigger on a therapy and be correct?” With all the new drugs coming along, the way to answer this is to do efficiency studies in a real-world environment in addition to efficacy studies.
Dr. Alexander: Yes, one of the biggest limitations of antiplatelet drug studies to date is that they usually haven’t really addressed the timing of drug initiation. We often assume that if a drug is shown to be beneficial, then it should be started as soon as possible. As we just heard, that may have been an unfounded extrapolation from the CURE trial. The same sort of thing happened with the ISIS trial of aspirin in patients with ST-elevation MI.8 In response to the ISIS results, clinicians rushed to give patients aspirin right away even though many of the patients in the trial may have received their aspirin the day after presentation. For these reasons, the EARLY-ACS study,9 which is addressing a very simple question—whether early upstream use of glycoprotein IIb/IIIa inhibitors is beneficial—has been a challenging trial to complete.
WHAT ROLE FOR THIENOPYRIDINE PRETREATMENT?
Dr. Bhatt: Dr. Sabatine, you presented data from the large TRITON-TIMI 38 trial comparing prasugrel with clopidogrel. I’m interested in how you would use prasugrel in practice, assuming it receives marketing approval, especially in light of its bleeding risk, particularly in patients in whom coronary artery bypass graft surgery (CABG) is planned. Many hospitals pretreat patients with clopidogrel in the emergency department. How would you manage a patient who shows up in the emergency room with ACS? Would you give clopidogrel, would you wait and give prasugrel, or would you do something else? If you gave clopidogrel, what loading dose would you use—300 mg, 600 mg, or, as some have suggested, 900 or 1,200 mg?
Dr. Sabatine: I am a strong proponent of pretreatment. Data from multiple studies show a benefit to this strategy, and even the original CURE trial showed a roughly 30% reduction in ischemic events within the first 24 hours of clopidogrel initiation.7
I think the dosing strategy depends on how the patient is going to be managed. If management is going to be conservative, then I would start the patient on 300 mg of clopidogrel when he or she came in. If the patient is going to the cardiac catheterization laboratory in a few hours, I would pretreat with 600 mg of clopidogrel. For prasugrel, the need for pretreatment is less clear, given the drug’s faster onset of action and greater degree of platelet inhibition. In the TRITON-TIMI 38 study,10 prasugrel was given, by and large, after diagnostic angiography, and thus one could use that approach in practice.
In terms of clopidogrel versus prasugrel, I would embrace prasugrel for the large majority of my patients, being mindful of the risk of bleeding. I would not hesitate to give the medication to diabetics or to younger, more robust patients. The 50% reduction in stent thrombosis with prasugrel versus clopidogrel in TRITON-TIMI 38 is huge,11 given that the risk of death with stent thrombosis is probably 25% or greater. So I would want to have prasugrel on board to reduce the risk of stent thrombosis, especially if a drug-eluting stent were being implanted.
Dr. Bhatt: Dr. Alexander, let’s get your take on a similar scenario. Assuming that prasugrel gains marketing approval, how would you manage patients with non-ST-elevation MI who present to the emergency department? Would you pretreat with clopidogrel? Would you wait until angiography and then, depending on the anatomy, treat with prasugrel? Or would you potentially pretreat with prasugrel, which has not been studied and would not be a labeled indication? How would you reconcile the data?
Dr. Alexander: At Duke, I expect that prasugrel will not be used prior to the catheterization laboratory in patients with non-ST-elevation ACS due to concerns about whether the patients will undergo PCI or be managed medically or with CABG.
Dr. Bhatt: That makes sense, since there was a fair amount of bleeding with prasugrel in those patients in TRITON-TIMI 38.
Dr. Alexander: Correct. Moreover, at Duke we don’t use as much upstream clopidogrel as we would, based on the evidence, if I were managing all the patients. There is still a lot of pushback about upstream clopidogrel from our surgeons because patients are going to surgery quickly these days, sometimes just a day after catheterization, and that’s when a loading dose of clopidogrel can be problematic. We are also still fairly heavy users of glycoprotein IIb/IIIa inhibitors.
Where I can see prasugrel being used prior to the cath lab at Duke is in ST-elevation MI, where the rate of PCI is very high. In primary angioplasty for ST-elevation MI, it would likely be given upstream. The bigger issue for us will be that we serve as a referral base for a lot of regional hospitals, and thus have some influence on their practices.
Dr. Bhatt: In that case, what would you advise those regional hospitals to do for non-ST-elevation MI?
Dr. Alexander: For the time being, we would advise continuing with our current practice, which is to load clopidogrel in patients in whom there is a reasonable certainty that CABG will not be performed, and to use glycoprotein IIb/IIIa inhibitors in high-risk patients. As we get more experience with prasugrel or with additional trial results, however, that practice could easily change.
Dr. Bhatt: So you would still use glycoprotein IIb/IIIa inhibitors?
Dr. Alexander: Yes, I advocate upstream clopidogrel use, but not all my colleagues do. Based on the guidelines, I’d use one or the other—either clopidogrel or a glycoprotein IIb/IIIa inhibitor. As I mentioned in my talk, if a patient is at high risk for bleeding, I am more inclined to use clopidogrel, although patients at higher risk of bleeding are often at higher risk for ischemic events as well.
WHAT’S DRIVEN THE DROPOFF IN GLYCOPROTEIN IIb/IIIa INHIBITOR USE?
Dr. Bhatt: While we’re on the topic of glycoprotein IIb/IIIa inhibitors, a question card from the audience asks why there has been a decrease in glycoprotein IIb/ IIIa inhibitor use and whether this decline is appropriate or inappropriate. Have clopidogrel pretreatment, higher loading doses of clopidogrel, and use of the direct thrombin inhibitor bivalirudin contributed to the decrease in glycoprotein IIb/IIIa inhibitor use?
Dr. Alexander: I do think that the decline has been driven by the changing environment, with greater use of other antithrombotic strategies that include clopidogrel and bivalirudin, as you suggest, as well as an increased attention to bleeding. From an evidence-based standpoint, we don’t know whether the decrease in glycoprotein IIb/IIIa use is appropriate or not because the studies of these agents were conducted before the widespread upstream use of clopidogrel and bivalirudin. Clopidogrel is attractive because it’s a pill given as one dose in the emergency department, the wards, or the catheterization laboratory, rather than a much more complicated infusion with weight-based dosing and dosage adjustments based on creatinine clearance. It is possible that we should perhaps be dosing clopidogrel the same way, but we don’t know that yet.
PRASUGREL IN PRACTICE: HOW LOW CAN THE DOSE GO, AND IS THERE A GENDER EFFECT?
Dr. Bhatt: Let’s stick with this focus on dosing but turn back to discussion of prasugrel. In your presentation of the TRITON-TIMI 38 data, Dr. Sabatine, you proposed a potential prasugrel dosage modification, down to a 5-mg loading dose, in subgroups that were identified as being at high bleeding risk—namely, elderly patients and patients with low body weight. However, no outcomes data with 5 mg of prasugrel came out of TRITON-TIMI 38.10 Is this proposed modification based on pharmacokinetic extrapolation? Could clinicians be comfortable using 5 mg of prasugrel, assuming the drug receives regulatory approval and a 5-mg tablet would be available?
Dr. Sabatine: Of course, evidence at the grade A level would consist of a trial showing that patients who received a lower dose enjoyed the same benefit as those who got standard dosing in TRITON-TIMI 38—a 60-mg loading dose followed by 10 mg/day—with an acceptable risk profile. However, such a trial would be difficult and costly to conduct, and would take roughly half a decade to pull off. It is only through large trials like TRITON-TIMI 38 that you identify subgroups that respond differently, and then to go back and do a separate trial for those subgroups takes a great deal of time. It may not be practical.
I think the Food and Drug Administration is moving toward embracing careful pharmacokinetic/pharmacodynamic substudies within trials, with these substudies having adequate numbers of subjects to provide a sense for the ideal target dose and what an acceptable dose range would be, without limiting approval to a single dose. The analogy would be warfarin dosing, with the aim being to figure out an acceptable dose range, discover which patients fall outside that range, and then model the effect of a lower dose in those patients. Thus, approving a 5-mg dose of prasugrel based on TRITON-TIMI 38 would be a reasonable approach if this dose passed muster under pharmacokinetic/pharmacodynamic modeling. If this approach were taken, there would clearly be a need for postmarketing surveillance to confirm whether the modeling on the effects of the lower dose was borne out by actual outcomes.
Dr. Bhatt: The audience has posed another interesting question raised by TRITON-TIMI 38: Can you comment on the lesser effect of prasugrel in women?
Dr. Sabatine: It is true that there was not a statistically significant effect of prasugrel among women in TRITON-TIMI 38, but statistical tests among subgroups found no significant heterogeneity for the effect between men and women, and that is the relevant measure to determine any gender effect. Keep in mind that not all subgroups represent a univariate slice of the population. For example, women generally have lower body weight than men, and since prasugrel’s net clinical benefit was reduced in patients with lower body weight, that may explain some of the differing extent of effect between men and women.
Dr. Bhatt: That’s a good point about the lack of heterogeneity between men and women. In fact, a meta-analysis of clopidogrel data conducted by one of the fellows I work with revealed that men and women appear to benefit similarly from clopidogrel.12 There was a slight signal of excess bleeding in women, but there were more elderly women in the pooled population, which may have been a confounding factor. As best as anyone can tell, antiplate-let therapy works well in both men and women.
NAVIGATING MANAGEMENT ACROSS THE SPECTRUM OF CARE
Dr. Bhatt: I would like to explore a bit further how all of these issues translate across the spectrum of care, beginning in the emergency department, which we know is a key component of the entire ACS management strategy for a health care system. What should emergency medicine doctors do, given all of the potential options—clopidogrel, different loading doses of clopidogrel, prasugrel, glycoprotein IIb/IIIa inhibitors, even bivalirudin?
Dr. Peacock: It depends on the practice setting. Some emergency physicians work at community hospitals with no backup. They must have relationships with the larger centers to which they’ll be transferring patients, because ACS patients should not be staying at community hospitals. These emergency physicians must have close relationships with the physicians who will be receiving their patients, and they know the potential head-butting with surgeons surrounding early clopidogrel use better than anybody does. If they treat with clopidogrel in the emergency room, and it turns out that the patient needs to go to the catheterization laboratory, can the receiving hospital use platelet testing to shorten the standard 5-day interval from treatment to catheterization?
Dr. Bhatt: Yes, that’s a rather useful, although not completely validated, way of using point-of-care platelet testing—to potentially reduce the time to surgery.
Dr. Peacock: Right. So if the policies for handling these types of transfer-related issues are worked out in advance, all players have a pathway to follow, which can allow quick action when necessary. If you don’t have these issues worked out in advance, you either lose many opportunities to act quickly in the emergency room or you risk taking actions that will cause problems later in the course of management.
Dr. Alexander: I totally agree. The key is to sit down with all the players involved—the surgeons, the interventional cardiologists, the intensivists, the emergency room personnel—and come up with strategies for different populations of patients. Write down the collective strategy and hang it on the wall so that everybody can be comfortable with it. The strategy can be reevaluated when prasugrel or other new antithrombotic drugs come on the market.
Dr. Peacock: The other environment is the academic center, which is even more challenging, but for different reasons. At a large academic center like the Cleveland Clinic, any of 25 different cardiologists may be taking call and receiving patients from the emergency department on a particular night. A lot of phone interaction is required to elicit the planned management strategy, including if and when the patient will be going to the cath lab. Individualizing care to a particular cardiologist then becomes a time-consuming challenge, especially in clinical situations where outcomes are time-dependent.
Dr. Alexander: Agreed. Management needs to be integrated across the entire spectrum of care. The anticoagulants that we plan to use in the cath lab will affect the antithrombotic regimen used upstream.
Dr. Kottke-Marchant: One circumstance where platelet function testing has been helpful is in determining the washout of the clopidogrel effect before surgery. At Cleveland Clinic, we have implemented platelet function testing in this circumstance instead of waiting a blanket 5 days after clopidogrel administration to go to surgery. A return to normal platelet function on platelet aggregation testing, depending on the cutoff value used, is an indicator that the patient can proceed to surgery.
Dr. Bhatt: That’s a logical approach. How should we be using antiplatelet therapy in the medically managed patient, Dr. Alexander?
Dr. Alexander: When I think of medical management, I include patients who don’t go to the cath lab, but also those who do, with regards to their management prior to and following their time in the cath lab.
In patients who don’t go to the cath lab for angiography, the ACC/AHA guidelines recommend aspirin and either clopidogrel, a glycoprotein IIb/IIIa inhibitor, or both.1 In making this choice, I consider the patient’s risk of bleeding and the dosing complexity of the regimen, especially with the use of glycoprotein IIb/IIIa inhibitors in a patient with renal insufficiency. In a patient at relatively low risk for bleeding, I often use both clopidogrel and a glycoprotein IIb/IIIa inhibitor, although this strategy does not have a lot of data to support it.
The more challenging population consists of patients who go to the cath lab but do not undergo PCI; this population is managed medically too. We often drop the ball with clopidogrel in this population. Many patients in whom PCI is not performed do not receive clopidogrel upstream, for all of the reasons we’ve discussed, and there is pretty good evidence that if clopidogrel is not instituted before hospital discharge, the patient is not likely to be receiving it at 30 days either. We have an obligation to treat these patients.
Treatment following bypass surgery is much murkier, and I don’t really know what we should be doing. The ACC/AHA guidelines suggest that clopidogrel be started in a patient with non-ST-elevation ACS after bypass surgery,1 but I believe the evidence to support that recommendation is pretty weak.
Dr. Bhatt: Well, the CURE trial did contain a sizeable group that underwent bypass surgery,7 and although this group was underpowered in some respects, it was still a very large group, so I personally favor treatment in those patients. We should mention that an ongoing trial called TRILOGY ACS is comparing clopidogrel and prasugrel specifically in patients who are being managed medically,13 so more data on this strategy will be emerging.
ARE GUIDELINES DESTINED TO BECOME EVER MORE COMPLEX?
Dr. Bhatt: Here’s a comment and question from the audience that pulls together a lot of what we’ve discussed while also looking forward: The antiplatelet therapy guidelines are already complicated. If the ongoing studies of emerging antiplatelet drugs all have results that are qualitatively similar to those of the TRITON-TIMI 38 study of prasugrel—ie, better efficacy with more potent therapy but more bleeding—how do you foresee these antiplatelet drugs being used in clinical practice?
Dr. Sabatine: The contrast between the US guidelines and the European guidelines for ACS management is stark. The US guidelines—from the ACC and AHA1—are essentially an encyclopedia that includes nearly every trial of anti-platelet therapy in ACS along with complicated algorithms; they do a wonderful job of being complete. The European guidelines14 are probably one tenth the size of their US counterpart document, and they suggest treatments for various patient types; they are very simple.
In a sense, the US guidelines lay out the data and force practitioners to evaluate the trials and consider how our patients fit into the study populations. In this way they are analogous to current guidelines for anticoagulant therapy. Several anticoagulants have been compared with heparin in clinical trials. These newer anticoagulants appear to reduce the risk of ischemic events compared with heparin; some have lower rates of bleeding, while others have higher rates of bleeding. There have been few head-to-head studies of these agents, however, so we wind up with guidelines that are not definitive but rather suggest agents to “consider” in various settings.
It’s unlikely that a head-to-head trial will be conducted comparing prasugrel with the reversible P2Y12 antagonist AZD6140, assuming that both are approved for marketing. If the drugs appear equally efficacious in placebo-controlled trials, it will take consensus to determine the appropriate choice at your hospital, factoring in your patient profile, the cost of the drugs, and other variables. It’s more complicated when one agent is slightly more efficacious but causes more bleeding or, conversely, a little less efficacious but less apt to cause bleeding. In such cases, you may need to tailor therapy to the patient, trying to gauge bleeding risk. All of the emerging data appear to point to the importance of bleeding on outcomes: patients who bleed fare poorly, in part due to the bleeding itself and in part perhaps because they have a proclivity for bleeding.
THE FUTURE: MONITORING-BASED DOSING AND NICHE ANTIPLATELETS?
Dr. Bhatt: That’s a good observation. Let’s wrap up by having the other panelists share any final thoughts you may have.
Dr. Alexander: I’d like to return to the issue of measuring antiplatelet response and using it to guide therapy. Earlier we cited the examples of antihypertensive therapy and lipid-lowering therapy to support this model of monitoring-based treatment. Guidelines for dyslipidemia treatment recommend using LDL-C levels to guide therapy, but this practice is difficult to study in a randomized trial. In fact, none of the randomized trials of statins used LDL-C levels to guide therapy. They all studied fixed doses of statins versus placebo or fixed doses of another statin. Higher doses of statins were always beneficial compared with lower doses, and this finding was extrapolated into the guidelines as a justification to treat to target LDL-C levels.
Dr. Bhatt: It’s not even necessarily clear that LDL-C level is the best target, if you consider the JUPITER trial, in which patients received statin therapy based on their baseline level of high-sensitivity C-reactive protein, not their LDL-C level.15 It goes to show how incomplete our knowledge of a class of drugs may be, even decades after the drugs are introduced.
Dr. Kottke-Marchant: To speak to Dr. Alexander’s point, dose adjustment guided by platelet monitoring is a bit more problematic for antiplatelet drugs that are irreversible inhibitors, such as clopidogrel and aspirin, than for those that are reversible inhibitors, which are being developed and may eventually make more sense to use. From a drug development standpoint, a drug that requires monitoring and dose adjustment will not gain wide acceptance because it will increase medical costs and morbidity.
Dr. Bhatt: Yes, we know from experience with warfarin that doctors and patients don’t like the ongoing need for monitoring and testing.
Dr. Peacock: The drugs that are going to be adopted by the emergency department are those with the shortest half-lives, for several reasons: (1) using a drug with a short half-life won’t commit us to a particular course of action; (2) the potential for drug interactions is lower; and (3) in the event of an erroneous diagnosis, the consequence of misapplication may be mitigated by early recognition and termination of the drug. If we later decide that we’ve gone down the wrong therapeutic road or reached a wrong diagnosis, or if a complication occurs, we can turn off the therapy quickly. That level of flexibility is needed.
Dr. Kottke-Marchant: I think we are moving into an era of niche antiplatelet drugs. One might be used in a patient going to surgery, for example, and another for long-term therapy.
Dr. Peacock: One thing that I don’t have a feel for is how to transition from one drug to another. When you change drugs for a patient, it so often seems like it goes badly. If we’re eventually going to use drugs with ultra-short half-lives in the in the emergency department for the first day or two, and then switch patients to a pill for a week, a lot more platelet function testing may be needed.
CASE STUDY: THROMBOSIS AFTER STENTING DESPITE ANTIPLATELET THERAPY
Dr. Deepak Bhatt: We have taken in a wealth of terrific information from the three preceding talks in this symposium. Let’s now share some questions from the audience and explore some of the points raised in the preceding talks in a bit more practical detail for clinicians. Our three prior speakers are joined in this panel discussion by Cleveland Clinic’s Dr. Frank Peacock, who brings an emergency medicine perspective.
Let’s begin with a case-based question supplied from the audience. The patient is a 42-year-old morbidly obese man without diabetes who had a non-ST-elevation myocardial infarction (MI) less than 1 year ago. A drug-eluting stent was placed at the time of his MI, and now restenosis has occurred. He is on aspirin and clopidogrel 75 mg/day. Do you recommend running a vasodilator-stimulated phosphoprotein (VASP) test and possibly increasing the clopidogrel dose to 150 mg/ day, or should the patient just be switched to prasugrel (assuming it is commercially available) without running the VASP test?
I’ll take a quick initial stab at this question. Studies of antiplatelet therapies to prevent instent restenosis have been a mixed bag. Some of the trials with glycoprotein IIb/IIIa inhibitors have shown an effect on restenosis, but most have not. Similarly, some of the analyses of the thienopyridines ticlopodine and clopidogrel have shown an effect on restenosis, but most have not.
For the most part, restenosis does not appear to be heavily mediated by platelets, at least not in a way that we can influence by therapy. On the other hand, stent thrombosis is highly platelet mediated, so I would alter the case to one in which stent thrombosis is the clinical problem. Assuming that the patient has been adherent to his antiplatelet regimen, which tests would you perform, and how would you act on the information from those tests?
Dr. Kandice Kottke-Marchant: The 2007 guidelines on acute coronary syndrome (ACS) management from the American College of Cardiology and American Heart Association (ACC/AHA)1 do not address platelet function testing, and almost none of the clinical trials of antiplatelet agents had an arm that included testing and dose adjustment based on platelet function studies. Platelet testing is available at some centers; at Cleveland Clinic, we use platelet aggregation testing. One can do platelet aggregation testing on a patient-by-patient basis; if inhibition appears to be suboptimal, a treatment decision should be made, but there is little guidance from the literature to steer that decision. I have seen clinicians increase the dose of clopidogrel or aspirin in response to platelet function tests, which occasionally triggers a confirmatory call from the pharmacy department.
Dr. Bhatt: When I was still at Cleveland Clinic, our chief medical resident did an analysis of platelet function testing, and it was remarkable how much testing was performed and how often it changed management, largely in the absence of any outcomes data, as Dr. Kottke-Marchant pointed out. Dr. Alexander, what are your recommendations with respect to platelet function testing today?
Dr. John Alexander: The case you describe is one in which applying evidence is not easy. There are no trials to supply any evidence to change therapy in this patient, a morbidly obese man receiving 75 mg/day of clopidogrel. There is certainly a rationale, however, to believe that a standard “one size fits all” 75-mg daily dose of clopidogrel may not be enough for him. The trade-off with a higher dosage is a higher risk of bleeding, however, so I would first be sure that he has been adherent to his current regimen of clopidogrel and aspirin.
Dr. Bhatt: Is there a role for point-of-care testing to determine whether he is adherent to the medicines?
Dr. Kottke-Marchant: Several of the point-of-care tests, such as the VerifyNow rapid platelet function analyzer, have specific cartridges for aspirin and for clopidogrel. If platelets were not being inhibited, it would suggest that the doses were too low, given the patient’s weight, but you probably would not be able to determine whether he was resistant to clopidogrel.
Dr. W. Frank Peacock: One way to verify that patients are taking their aspirin is to take a small urine sample and squirt in 2 mL of ferric chloride. If the sample turns purple, it means they are taking their aspirin. Once that is established, you can try to determine whether the drug is working on their platelets.
Dr. Alexander: Another potential explanation for stent thrombosis is faulty stent placement. In this case I would consider asking an interventional colleague to perform intravascular ultrasonography to make sure the stent was implanted properly before I changed the patient’s antithrombotic therapy.
Dr. Bhatt: That’s a great technical point. We always want to make sure that a case of stent thrombosis is not due to a mechanical problem. We should be asking: Is the stent properly sized and well opposed? Is there a distal dissection or any other issue that could predispose to stent thrombosis?
Dr. Alexander: This case illustrates a host of other challenges that underscore how much more work we need to do to define optimal antiplatelet therapy. Suppose we perform platelet function testing and find a low level of platelet inhibition in this patient with stent thrombosis, and we change his antiplatelet regimen. When should we test him again? If we retest in 3 months and find that he has a higher than expected level of platelet inhibition on the new antiplatelet regimen, do we dial down the intensity? Once again, there is no evidence to guide these decisions, and levels of platelet inhibition are driven not just by the medications but also by what is going on in the patient’s platelets—it is quite multifactorial.
POINT-OF-CARE PLATELET FUNCTION TESTING: CURRENT LIMITS, FUTURE ROLES
Dr. Bhatt: While we’re discussing platelet function testing, I found it interesting, Dr. Kottke-Marchant, that you said the use of bleeding time as a platelet test is finally going away. Testing of bleeding time has been around forever, but I agree that it doesn’t have much value in clinical practice. Do you think bleeding time will continue to have any role in drug development? Most phase 2 trials, and certainly phase 1 trials, still capture bleeding time to assess whether or not a drug is working. Should that, too, be jettisoned, or does bleeding time still have some merit in this context?
Dr. Kottke-Marchant: I would jettison it in drug development as well because of the considerable variability in bleeding time. It is not a test that can be standardized, and no quality control can be done. The results depend on skin turgor, age, and many other variables.
We need a global assay that will pick up multiple aspects of platelet function, such as flow-based adhesion, aggregation, and granule release. The bleeding time is a shear-dependent test, whereas the platelet aggregation test that is used in most drug trials is an artificial assay that measures only aggregation, but not under shear. The VerifyNow rapid platelet function analyzer does not measure platelets under shear and is not a global assay.
Dr. Marc Sabatine: I would underscore the need for a reliable point-of-care test of platelet function. When we prescribe a statin or an antihypertensive drug, we don’t just send the patient out the door and hope that everything will be okay. We measure the response, knowing that genotype, environmental factors, or medication factors can affect the response. When we prescribe an antiplatelet drug, we need a reliable point-of-care device to make certain that the patient is getting appropriate platelet inhibition.
I am reminded of a recent study of point-of-care measurement of platelet inhibition in patients receiving clopidogrel prior to nonemergent percutaneous coronary intervention (PCI).2 Rather than just treating patients with PCI and sending them out the door, the investigators kept giving patients clopidogrel and measuring their platelet inhibition until they achieved an appropriate degree of inhibition, after which PCI was performed. Event rates were significantly reduced in the patient group treated this way, which suggests a need to individualize therapy and move away from the “one size fits all” mindset.
Dr. Bhatt: Dr. Peacock, you’ve led a study of point-of-care assays in the emergency department. What might ultimately be the role of point-of-care testing in emergency medicine, and might it influence drug selection?
Dr. Peacock: My short answer is that I think there will be a role for point-of-care testing, with all the caveats that have been discussed. There may even be a day when we do genetic testing and look for DNA. Honestly, though, I’m somewhat of a skeptic because I’m not looking at the genetics. I see many patients who do crack cocaine who come to the emergency room with chest pain and have risk factors, but I send these patients home because they are not having an event. The real question is, “Is it an event?” If a patient is having an event and he or she has platelet resistance or hyperreactivity—whatever we term it—then you have to decide the next step.
As you mentioned, we just completed a study that evaluated a couple hundred patients for platelet inhibition resistance to aspirin, and one finding was that the incidence of platelet resistance to aspirin was much lower than we had anticipated. Studies from the literature suggest that the prevalence of resistance is around 30%, but in our study it was 6.5%.3
Dr. Kottke-Marchant: It depends on how and in whom you measure resistance. Different tests will give you different numbers. Even among studies using the same measurement techniques, the results depend on the patient population. If it’s a fairly stable cardiac population, you may see aspirin resistance rates of 4% or 5%. If it’s a population of patients who have had multiple MIs, the rate may be higher.
Dr. Peacock: That’s exactly my point. In the emergency department we see a mixed bag. We see many people who have had no prior events and have no acute event occurring. So in that setting you are going to get results that suggest that no intervention is required, whereas in that small percentage of patients in whom something is happening, your drug choice may be different.
Dr. Alexander: We are still talking about resistance to antiplatelet drugs as though it were a patient-level variable, but it’s my impression that it changes over time and within a patient.
Dr. Kottke-Marchant: It can change over time. There aren’t many good longitudinal studies. Most of the studies of “aspirin resistance” are really snapshot studies with measurements taken at one point in time. A term I prefer is “platelet reactivity.” To really assess treatment efficacy, we are going to have to look at the basal level of platelet reactivity.
WHAT ROLE FOR GENOTYPING IN GUIDING ANTIPLATELET THERAPY?
Dr. Bhatt: Dr. Peacock alluded to a potential role for genetic testing. Dr. Sabatine, you have done a lot of interesting work with genotyping in the TRITON-TIMI 38 study of prasugrel and clopidogrel. What is the future role of genotyping in determining which antiplatelet therapy is best for which patient?
Dr. Sabatine: As I mentioned, cytochrome P450 enzymes play a critical role in the metabolism of clopidogrel. These enzymes are fairly polymorphic—mutations in their encoding genes are responsible for subtle changes in effect, unlike the traditional mutations that we think about for sickle cell disease, for example. A wealth of data has been published showing that genetic variants are associated with decreased functional activity of cytochrome P450 enzymes, demonstrating the pharmacologic importance of these variants.
Individuals who carry variant alleles appear to respond differently to clopidogrel. A variety of small studies show that those who carry specific variants—particularly in the CYP2C19 enzyme, but in other enzymes as well—appear to have a diminished response to clopidogrel. There are also data showing that individuals with a diminished response to clopidogrel have worse outcomes.4 Our group is studying the impact of genetic variants that decrease the functional activity of cytochrome P450 enzymes on clinical outcomes. (Editor’s note: This study has since been published by Mega et al.5)
The practical implication may lie in point-of-care genotyping, which appears possible and will be clinically useful if a strong link can be demonstrated between genotype and outcomes. If point-of-care genotyping becomes practical, it will raise the question of whether both genotyping and platelet aggregation testing are needed. I think they might indeed be complementary in risk prediction, as is the case with genetic variants that affect low-density lipoprotein cholesterol (LDL-C) levels. In the lipid arena, we have seen that genetic effects and lipid levels provide independent incremental information about risk. That’s because of the high degree of variation in LDL-C levels: an LDL-C measurement is a snapshot in time, yet a variety of factors can influence LDL-C levels. In contrast, genotype is an invariant factor. Similarly, in the platelet arena, platelet aggregation studies and genotyping may be synergistic in predicting an individual’s predisposition to events and response to medications.
Dr. Bhatt: While we’re discussing pathways of metabolism, the literature, though scant, suggests a potential interaction between proton pump inhibitors and clopidogrel. I was co-chair of a recent American College of Cardiology/ American Heart Association/American College of Gastro-enterology consensus document that endorsed liberal use of proton pump inhibitors in patients who are at gastrointestinal risk, including those on antiplatelet therapy.6 The gastroenterologists believed strongly that proton pump inhibitors were safe and in fact underused in these patients. What do you think about the clopidogrel–proton pump inhibitor interaction? Should we be concerned?
Dr. Sabatine: Proton pump inhibitors are not only substrates for, but also inhibitors of, CYP2C19, a key enzyme that helps transform clopidogrel into an active metabolite. For this reason, there has been interest in whether concomitant use of proton pump inhibitors would blunt the efficacy of clopidogrel. The same concern was raised about giving clopidogrel with certain statin drugs that are also metabolized by the cytochrome P450 system, and several studies have shown an effect of these statins on clopidogrel’s platelet inhibition. However, there is no evidence that coadministration of these statins has affected clinical outcomes with clopidogrel in clinical trials. So it may be that while competition for the cytochrome P450 system is one factor, it’s not enough of a factor to tip the scale and result in a clinical event. The same may be true of coadministration of proton pump inhibitors; meanwhile, we await definitive data that concomitant use with clopidogrel leads to higher rates of ischemic events.
DIAGNOSTIC UNCERTAINTY IN THE EMERGENCY SETTING
Dr. Bhatt: We heard about quite a few new antiplatelet drugs in Dr. Sabatine’s presentation, some of which will likely be taken up in clinical practice. Dr. Peacock, from an emergency department perspective, how will you integrate all these new agents with the numerous therapies already available? What should emergency departments do to come to grips with and ultimately take advantage of these different forms of therapy as well as emerging platelet function tests?
Dr. Peacock: The piece that’s unique or especially pertinent to the emergency department is diagnostic uncertainty. Diagnosis and management are easy when a patient has an ST-elevation MI because we all know what that looks like and we know what to do in response. To some extent non-ST-elevation MI is fairly simple too. ACS is a lot more difficult because we don’t have a good definition for unstable angina, and that’s where diagnosis and management become problematic. And with high-sensitivity troponins coming out now, the question of non-ST-elevation MI is going to get more and more confusing because we will have a lot more patients who meet criteria without having an acute coronary artery event.
So it is going to be important that studies be designed correctly. A lot of the studies reviewed today were efficacy studies, showing that a particular drug works well in a carefully defined population, but they were not efficiency studies: they did not take into account the real-world diagnostic uncertainty—and inevitable misdiagnoses—that emergency departments encounter before starting therapy.
Take the CURE trial, for example. It was a great study, showing that clopidogrel reduced the hazard ratio for major coronary events by 20% in patients with unstable angina,7 and the message was that everybody should be using clopidogrel. A close look at the study, however, reveals that about half the patients did not receive clopidogrel in the emergency department. When patients did receive it early, it was driven by the cardiologist, who was absolutely certain of the diagnosis. But if the study was not designed to test early use, then it is a big leap to extrapolate its findings to this circumstance.
Many of the patients in the CURE trial were enrolled the day after presentation, when their diagnosis was certain—ie, they had a rise in troponin after their symptoms. But when a patient first arrives in the emergency department, we are not certain of the diagnosis. And if we use a drug such as clopidogrel, with a duration of action as long as 5 days, we have committed the entire medical system to a certain course of management for that period of time. If we get the diagnosis wrong, this commitment could restrict management options for up to 5 days.
The question for emergency physicians becomes, “How long is long enough to know whether I can pull the trigger on a therapy and be correct?” With all the new drugs coming along, the way to answer this is to do efficiency studies in a real-world environment in addition to efficacy studies.
Dr. Alexander: Yes, one of the biggest limitations of antiplatelet drug studies to date is that they usually haven’t really addressed the timing of drug initiation. We often assume that if a drug is shown to be beneficial, then it should be started as soon as possible. As we just heard, that may have been an unfounded extrapolation from the CURE trial. The same sort of thing happened with the ISIS trial of aspirin in patients with ST-elevation MI.8 In response to the ISIS results, clinicians rushed to give patients aspirin right away even though many of the patients in the trial may have received their aspirin the day after presentation. For these reasons, the EARLY-ACS study,9 which is addressing a very simple question—whether early upstream use of glycoprotein IIb/IIIa inhibitors is beneficial—has been a challenging trial to complete.
WHAT ROLE FOR THIENOPYRIDINE PRETREATMENT?
Dr. Bhatt: Dr. Sabatine, you presented data from the large TRITON-TIMI 38 trial comparing prasugrel with clopidogrel. I’m interested in how you would use prasugrel in practice, assuming it receives marketing approval, especially in light of its bleeding risk, particularly in patients in whom coronary artery bypass graft surgery (CABG) is planned. Many hospitals pretreat patients with clopidogrel in the emergency department. How would you manage a patient who shows up in the emergency room with ACS? Would you give clopidogrel, would you wait and give prasugrel, or would you do something else? If you gave clopidogrel, what loading dose would you use—300 mg, 600 mg, or, as some have suggested, 900 or 1,200 mg?
Dr. Sabatine: I am a strong proponent of pretreatment. Data from multiple studies show a benefit to this strategy, and even the original CURE trial showed a roughly 30% reduction in ischemic events within the first 24 hours of clopidogrel initiation.7
I think the dosing strategy depends on how the patient is going to be managed. If management is going to be conservative, then I would start the patient on 300 mg of clopidogrel when he or she came in. If the patient is going to the cardiac catheterization laboratory in a few hours, I would pretreat with 600 mg of clopidogrel. For prasugrel, the need for pretreatment is less clear, given the drug’s faster onset of action and greater degree of platelet inhibition. In the TRITON-TIMI 38 study,10 prasugrel was given, by and large, after diagnostic angiography, and thus one could use that approach in practice.
In terms of clopidogrel versus prasugrel, I would embrace prasugrel for the large majority of my patients, being mindful of the risk of bleeding. I would not hesitate to give the medication to diabetics or to younger, more robust patients. The 50% reduction in stent thrombosis with prasugrel versus clopidogrel in TRITON-TIMI 38 is huge,11 given that the risk of death with stent thrombosis is probably 25% or greater. So I would want to have prasugrel on board to reduce the risk of stent thrombosis, especially if a drug-eluting stent were being implanted.
Dr. Bhatt: Dr. Alexander, let’s get your take on a similar scenario. Assuming that prasugrel gains marketing approval, how would you manage patients with non-ST-elevation MI who present to the emergency department? Would you pretreat with clopidogrel? Would you wait until angiography and then, depending on the anatomy, treat with prasugrel? Or would you potentially pretreat with prasugrel, which has not been studied and would not be a labeled indication? How would you reconcile the data?
Dr. Alexander: At Duke, I expect that prasugrel will not be used prior to the catheterization laboratory in patients with non-ST-elevation ACS due to concerns about whether the patients will undergo PCI or be managed medically or with CABG.
Dr. Bhatt: That makes sense, since there was a fair amount of bleeding with prasugrel in those patients in TRITON-TIMI 38.
Dr. Alexander: Correct. Moreover, at Duke we don’t use as much upstream clopidogrel as we would, based on the evidence, if I were managing all the patients. There is still a lot of pushback about upstream clopidogrel from our surgeons because patients are going to surgery quickly these days, sometimes just a day after catheterization, and that’s when a loading dose of clopidogrel can be problematic. We are also still fairly heavy users of glycoprotein IIb/IIIa inhibitors.
Where I can see prasugrel being used prior to the cath lab at Duke is in ST-elevation MI, where the rate of PCI is very high. In primary angioplasty for ST-elevation MI, it would likely be given upstream. The bigger issue for us will be that we serve as a referral base for a lot of regional hospitals, and thus have some influence on their practices.
Dr. Bhatt: In that case, what would you advise those regional hospitals to do for non-ST-elevation MI?
Dr. Alexander: For the time being, we would advise continuing with our current practice, which is to load clopidogrel in patients in whom there is a reasonable certainty that CABG will not be performed, and to use glycoprotein IIb/IIIa inhibitors in high-risk patients. As we get more experience with prasugrel or with additional trial results, however, that practice could easily change.
Dr. Bhatt: So you would still use glycoprotein IIb/IIIa inhibitors?
Dr. Alexander: Yes, I advocate upstream clopidogrel use, but not all my colleagues do. Based on the guidelines, I’d use one or the other—either clopidogrel or a glycoprotein IIb/IIIa inhibitor. As I mentioned in my talk, if a patient is at high risk for bleeding, I am more inclined to use clopidogrel, although patients at higher risk of bleeding are often at higher risk for ischemic events as well.
WHAT’S DRIVEN THE DROPOFF IN GLYCOPROTEIN IIb/IIIa INHIBITOR USE?
Dr. Bhatt: While we’re on the topic of glycoprotein IIb/IIIa inhibitors, a question card from the audience asks why there has been a decrease in glycoprotein IIb/ IIIa inhibitor use and whether this decline is appropriate or inappropriate. Have clopidogrel pretreatment, higher loading doses of clopidogrel, and use of the direct thrombin inhibitor bivalirudin contributed to the decrease in glycoprotein IIb/IIIa inhibitor use?
Dr. Alexander: I do think that the decline has been driven by the changing environment, with greater use of other antithrombotic strategies that include clopidogrel and bivalirudin, as you suggest, as well as an increased attention to bleeding. From an evidence-based standpoint, we don’t know whether the decrease in glycoprotein IIb/IIIa use is appropriate or not because the studies of these agents were conducted before the widespread upstream use of clopidogrel and bivalirudin. Clopidogrel is attractive because it’s a pill given as one dose in the emergency department, the wards, or the catheterization laboratory, rather than a much more complicated infusion with weight-based dosing and dosage adjustments based on creatinine clearance. It is possible that we should perhaps be dosing clopidogrel the same way, but we don’t know that yet.
PRASUGREL IN PRACTICE: HOW LOW CAN THE DOSE GO, AND IS THERE A GENDER EFFECT?
Dr. Bhatt: Let’s stick with this focus on dosing but turn back to discussion of prasugrel. In your presentation of the TRITON-TIMI 38 data, Dr. Sabatine, you proposed a potential prasugrel dosage modification, down to a 5-mg loading dose, in subgroups that were identified as being at high bleeding risk—namely, elderly patients and patients with low body weight. However, no outcomes data with 5 mg of prasugrel came out of TRITON-TIMI 38.10 Is this proposed modification based on pharmacokinetic extrapolation? Could clinicians be comfortable using 5 mg of prasugrel, assuming the drug receives regulatory approval and a 5-mg tablet would be available?
Dr. Sabatine: Of course, evidence at the grade A level would consist of a trial showing that patients who received a lower dose enjoyed the same benefit as those who got standard dosing in TRITON-TIMI 38—a 60-mg loading dose followed by 10 mg/day—with an acceptable risk profile. However, such a trial would be difficult and costly to conduct, and would take roughly half a decade to pull off. It is only through large trials like TRITON-TIMI 38 that you identify subgroups that respond differently, and then to go back and do a separate trial for those subgroups takes a great deal of time. It may not be practical.
I think the Food and Drug Administration is moving toward embracing careful pharmacokinetic/pharmacodynamic substudies within trials, with these substudies having adequate numbers of subjects to provide a sense for the ideal target dose and what an acceptable dose range would be, without limiting approval to a single dose. The analogy would be warfarin dosing, with the aim being to figure out an acceptable dose range, discover which patients fall outside that range, and then model the effect of a lower dose in those patients. Thus, approving a 5-mg dose of prasugrel based on TRITON-TIMI 38 would be a reasonable approach if this dose passed muster under pharmacokinetic/pharmacodynamic modeling. If this approach were taken, there would clearly be a need for postmarketing surveillance to confirm whether the modeling on the effects of the lower dose was borne out by actual outcomes.
Dr. Bhatt: The audience has posed another interesting question raised by TRITON-TIMI 38: Can you comment on the lesser effect of prasugrel in women?
Dr. Sabatine: It is true that there was not a statistically significant effect of prasugrel among women in TRITON-TIMI 38, but statistical tests among subgroups found no significant heterogeneity for the effect between men and women, and that is the relevant measure to determine any gender effect. Keep in mind that not all subgroups represent a univariate slice of the population. For example, women generally have lower body weight than men, and since prasugrel’s net clinical benefit was reduced in patients with lower body weight, that may explain some of the differing extent of effect between men and women.
Dr. Bhatt: That’s a good point about the lack of heterogeneity between men and women. In fact, a meta-analysis of clopidogrel data conducted by one of the fellows I work with revealed that men and women appear to benefit similarly from clopidogrel.12 There was a slight signal of excess bleeding in women, but there were more elderly women in the pooled population, which may have been a confounding factor. As best as anyone can tell, antiplate-let therapy works well in both men and women.
NAVIGATING MANAGEMENT ACROSS THE SPECTRUM OF CARE
Dr. Bhatt: I would like to explore a bit further how all of these issues translate across the spectrum of care, beginning in the emergency department, which we know is a key component of the entire ACS management strategy for a health care system. What should emergency medicine doctors do, given all of the potential options—clopidogrel, different loading doses of clopidogrel, prasugrel, glycoprotein IIb/IIIa inhibitors, even bivalirudin?
Dr. Peacock: It depends on the practice setting. Some emergency physicians work at community hospitals with no backup. They must have relationships with the larger centers to which they’ll be transferring patients, because ACS patients should not be staying at community hospitals. These emergency physicians must have close relationships with the physicians who will be receiving their patients, and they know the potential head-butting with surgeons surrounding early clopidogrel use better than anybody does. If they treat with clopidogrel in the emergency room, and it turns out that the patient needs to go to the catheterization laboratory, can the receiving hospital use platelet testing to shorten the standard 5-day interval from treatment to catheterization?
Dr. Bhatt: Yes, that’s a rather useful, although not completely validated, way of using point-of-care platelet testing—to potentially reduce the time to surgery.
Dr. Peacock: Right. So if the policies for handling these types of transfer-related issues are worked out in advance, all players have a pathway to follow, which can allow quick action when necessary. If you don’t have these issues worked out in advance, you either lose many opportunities to act quickly in the emergency room or you risk taking actions that will cause problems later in the course of management.
Dr. Alexander: I totally agree. The key is to sit down with all the players involved—the surgeons, the interventional cardiologists, the intensivists, the emergency room personnel—and come up with strategies for different populations of patients. Write down the collective strategy and hang it on the wall so that everybody can be comfortable with it. The strategy can be reevaluated when prasugrel or other new antithrombotic drugs come on the market.
Dr. Peacock: The other environment is the academic center, which is even more challenging, but for different reasons. At a large academic center like the Cleveland Clinic, any of 25 different cardiologists may be taking call and receiving patients from the emergency department on a particular night. A lot of phone interaction is required to elicit the planned management strategy, including if and when the patient will be going to the cath lab. Individualizing care to a particular cardiologist then becomes a time-consuming challenge, especially in clinical situations where outcomes are time-dependent.
Dr. Alexander: Agreed. Management needs to be integrated across the entire spectrum of care. The anticoagulants that we plan to use in the cath lab will affect the antithrombotic regimen used upstream.
Dr. Kottke-Marchant: One circumstance where platelet function testing has been helpful is in determining the washout of the clopidogrel effect before surgery. At Cleveland Clinic, we have implemented platelet function testing in this circumstance instead of waiting a blanket 5 days after clopidogrel administration to go to surgery. A return to normal platelet function on platelet aggregation testing, depending on the cutoff value used, is an indicator that the patient can proceed to surgery.
Dr. Bhatt: That’s a logical approach. How should we be using antiplatelet therapy in the medically managed patient, Dr. Alexander?
Dr. Alexander: When I think of medical management, I include patients who don’t go to the cath lab, but also those who do, with regards to their management prior to and following their time in the cath lab.
In patients who don’t go to the cath lab for angiography, the ACC/AHA guidelines recommend aspirin and either clopidogrel, a glycoprotein IIb/IIIa inhibitor, or both.1 In making this choice, I consider the patient’s risk of bleeding and the dosing complexity of the regimen, especially with the use of glycoprotein IIb/IIIa inhibitors in a patient with renal insufficiency. In a patient at relatively low risk for bleeding, I often use both clopidogrel and a glycoprotein IIb/IIIa inhibitor, although this strategy does not have a lot of data to support it.
The more challenging population consists of patients who go to the cath lab but do not undergo PCI; this population is managed medically too. We often drop the ball with clopidogrel in this population. Many patients in whom PCI is not performed do not receive clopidogrel upstream, for all of the reasons we’ve discussed, and there is pretty good evidence that if clopidogrel is not instituted before hospital discharge, the patient is not likely to be receiving it at 30 days either. We have an obligation to treat these patients.
Treatment following bypass surgery is much murkier, and I don’t really know what we should be doing. The ACC/AHA guidelines suggest that clopidogrel be started in a patient with non-ST-elevation ACS after bypass surgery,1 but I believe the evidence to support that recommendation is pretty weak.
Dr. Bhatt: Well, the CURE trial did contain a sizeable group that underwent bypass surgery,7 and although this group was underpowered in some respects, it was still a very large group, so I personally favor treatment in those patients. We should mention that an ongoing trial called TRILOGY ACS is comparing clopidogrel and prasugrel specifically in patients who are being managed medically,13 so more data on this strategy will be emerging.
ARE GUIDELINES DESTINED TO BECOME EVER MORE COMPLEX?
Dr. Bhatt: Here’s a comment and question from the audience that pulls together a lot of what we’ve discussed while also looking forward: The antiplatelet therapy guidelines are already complicated. If the ongoing studies of emerging antiplatelet drugs all have results that are qualitatively similar to those of the TRITON-TIMI 38 study of prasugrel—ie, better efficacy with more potent therapy but more bleeding—how do you foresee these antiplatelet drugs being used in clinical practice?
Dr. Sabatine: The contrast between the US guidelines and the European guidelines for ACS management is stark. The US guidelines—from the ACC and AHA1—are essentially an encyclopedia that includes nearly every trial of anti-platelet therapy in ACS along with complicated algorithms; they do a wonderful job of being complete. The European guidelines14 are probably one tenth the size of their US counterpart document, and they suggest treatments for various patient types; they are very simple.
In a sense, the US guidelines lay out the data and force practitioners to evaluate the trials and consider how our patients fit into the study populations. In this way they are analogous to current guidelines for anticoagulant therapy. Several anticoagulants have been compared with heparin in clinical trials. These newer anticoagulants appear to reduce the risk of ischemic events compared with heparin; some have lower rates of bleeding, while others have higher rates of bleeding. There have been few head-to-head studies of these agents, however, so we wind up with guidelines that are not definitive but rather suggest agents to “consider” in various settings.
It’s unlikely that a head-to-head trial will be conducted comparing prasugrel with the reversible P2Y12 antagonist AZD6140, assuming that both are approved for marketing. If the drugs appear equally efficacious in placebo-controlled trials, it will take consensus to determine the appropriate choice at your hospital, factoring in your patient profile, the cost of the drugs, and other variables. It’s more complicated when one agent is slightly more efficacious but causes more bleeding or, conversely, a little less efficacious but less apt to cause bleeding. In such cases, you may need to tailor therapy to the patient, trying to gauge bleeding risk. All of the emerging data appear to point to the importance of bleeding on outcomes: patients who bleed fare poorly, in part due to the bleeding itself and in part perhaps because they have a proclivity for bleeding.
THE FUTURE: MONITORING-BASED DOSING AND NICHE ANTIPLATELETS?
Dr. Bhatt: That’s a good observation. Let’s wrap up by having the other panelists share any final thoughts you may have.
Dr. Alexander: I’d like to return to the issue of measuring antiplatelet response and using it to guide therapy. Earlier we cited the examples of antihypertensive therapy and lipid-lowering therapy to support this model of monitoring-based treatment. Guidelines for dyslipidemia treatment recommend using LDL-C levels to guide therapy, but this practice is difficult to study in a randomized trial. In fact, none of the randomized trials of statins used LDL-C levels to guide therapy. They all studied fixed doses of statins versus placebo or fixed doses of another statin. Higher doses of statins were always beneficial compared with lower doses, and this finding was extrapolated into the guidelines as a justification to treat to target LDL-C levels.
Dr. Bhatt: It’s not even necessarily clear that LDL-C level is the best target, if you consider the JUPITER trial, in which patients received statin therapy based on their baseline level of high-sensitivity C-reactive protein, not their LDL-C level.15 It goes to show how incomplete our knowledge of a class of drugs may be, even decades after the drugs are introduced.
Dr. Kottke-Marchant: To speak to Dr. Alexander’s point, dose adjustment guided by platelet monitoring is a bit more problematic for antiplatelet drugs that are irreversible inhibitors, such as clopidogrel and aspirin, than for those that are reversible inhibitors, which are being developed and may eventually make more sense to use. From a drug development standpoint, a drug that requires monitoring and dose adjustment will not gain wide acceptance because it will increase medical costs and morbidity.
Dr. Bhatt: Yes, we know from experience with warfarin that doctors and patients don’t like the ongoing need for monitoring and testing.
Dr. Peacock: The drugs that are going to be adopted by the emergency department are those with the shortest half-lives, for several reasons: (1) using a drug with a short half-life won’t commit us to a particular course of action; (2) the potential for drug interactions is lower; and (3) in the event of an erroneous diagnosis, the consequence of misapplication may be mitigated by early recognition and termination of the drug. If we later decide that we’ve gone down the wrong therapeutic road or reached a wrong diagnosis, or if a complication occurs, we can turn off the therapy quickly. That level of flexibility is needed.
Dr. Kottke-Marchant: I think we are moving into an era of niche antiplatelet drugs. One might be used in a patient going to surgery, for example, and another for long-term therapy.
Dr. Peacock: One thing that I don’t have a feel for is how to transition from one drug to another. When you change drugs for a patient, it so often seems like it goes badly. If we’re eventually going to use drugs with ultra-short half-lives in the in the emergency department for the first day or two, and then switch patients to a pill for a week, a lot more platelet function testing may be needed.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:e1–e157.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Glauser J, Emerman CL, Bhatt DL, Peacock WF. Platelet aspirin resistance in emergency department patients with suspected acute coronary syndrome. Am J Emerg Med. In press
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2008; 52:1128–1133.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. J Am Coll Cardiol 2008; 52:1502–1517.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- EARLY-ACS: Glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome. Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00089895. Updated December 17, 2008. Accessed December 18, 2008.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral anti-platelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary dyndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Berger JS, Bhatt DL, Chen Z, et al. The relationship between sex, mortality and cardiovascular events among patients with established cardiovascular disease: a meta-analysis [ACC abstract 1012-149]. J Am Coll Cardiol 2008; 51 10 suppl A:A247.
- TRILOGY ACS: A comparison of prasugrel and clopidogrel in acute coronary syndrome subjects. ClinicalTrials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00699998. Updated December 15, 2008. Accessed January 2, 2009.
- Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:e1–e157.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Glauser J, Emerman CL, Bhatt DL, Peacock WF. Platelet aspirin resistance in emergency department patients with suspected acute coronary syndrome. Am J Emerg Med. In press
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2008; 52:1128–1133.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. J Am Coll Cardiol 2008; 52:1502–1517.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- EARLY-ACS: Glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome. Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00089895. Updated December 17, 2008. Accessed December 18, 2008.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral anti-platelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary dyndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Berger JS, Bhatt DL, Chen Z, et al. The relationship between sex, mortality and cardiovascular events among patients with established cardiovascular disease: a meta-analysis [ACC abstract 1012-149]. J Am Coll Cardiol 2008; 51 10 suppl A:A247.
- TRILOGY ACS: A comparison of prasugrel and clopidogrel in acute coronary syndrome subjects. ClinicalTrials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00699998. Updated December 15, 2008. Accessed January 2, 2009.
- Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
What is the role of dual antiplatelet therapy with clopidogrel and aspirin?
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
KEY POINTS
- Platelets are key players in atherothrombosis, and antiplatelet drugs such as aspirin and clopidogrel prevent events in patients at risk.
- In studies leading up to CHARISMA, the combination of clopidogrel and aspirin was found to be beneficial in patients with acute coronary syndromes and in those undergoing percutaneous coronary interventions.
- Clopidogrel should not be combined with aspirin as a primary preventive therapy (ie, for people without established vascular disease). How dual antiplatelet therapy should be used as secondary prevention in stable patients needs further study.