User login
JUPITER to Earth: A statin helps people with normal LDL-C and high hs-CRP, but what does it mean?
The medical community has struggled with two important questions for the past 10 years: When it comes to the low-density lipoprotein cholesterol (LDL-C) level, how low should one go and at what cost? And are there other markers of risk that can identify a higher-risk subpopulation in relatively healthy people? The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) provided partial answers for these questions by finding that a highly potent statin lowered the risk of cardiovascular events in patients with “normal” LDL-C but elevated levels of high-sensitivity C-reactive protein (hs-CRP).1
In this article, we will critically evaluate the methods, results, and conclusions of the JUPITER trial. Additionally, we will discuss its limitations and areas of uncertainty.
BEFORE JUPITER
The LDL-C-lowering drugs called statins have revolutionized cardiovascular medicine.2 They are beneficial in both the primary prevention setting and in acute coronary syndromes, stable angina, and unstable angina and can halt the progression of coronary artery disease—in some cases even resulting in modest regression of plaque.3–6
Many experts have credited the reduction in LDL-C as being the sole factor responsible for the decrease in major adverse events seen with statin therapy.7 However, statins have other, non-lipid-lowering properties, including anti-inflammatory and antioxidant effects, that may also contribute to their benefits.8–15
One of the anti-inflammatory actions of statins is evidenced by lower levels of the acute-phase reactant CRP.10,11,15,16 Measuring systemic CRP levels with a highly sensitive assay (yielding the so-called high-sensitivity or hs-CRP level) provides significant clinical prognostic value across a spectrum of clinical situations, ranging from risk screening in apparently healthy people to stable and unstable angina.17–22 People with higher hs-CRP levels are, on average, at higher risk of adverse cardiovascular events. However, controversy remains as to whether hs-CRP plays a mechanistic role in plaque formation and acute complications. Indeed, recent genetic studies argue strongly that hs-CRP lies outside the mechanistic path of atherosclerosis.23 Nonetheless, an overwhelming amount of data indicates that hs-CRP serves as a marker of disease.17–21
Nissen et al10 showed that the rate of progression of atherosclerosis is lower when the levels of atherogenic lipoproteins and hs-CRP are both lowered with statin therapy. Simultaneously, Ridker et al11 showed that patients who have lower hs-CRP levels after statin therapy have better clinical outcomes than those with higher hs-CRP levels, regardless of their achieved level of LDL-C.
Collectively, these studies and others have led some to believe that, in people with relatively low LDL-C but persistently elevated hs-CRP, statin therapy may reduce the rate of events.15,24 The JUPITER trial was undertaken to test this hypothesis.
JUPITER STUDY DESIGN
JUPITER was designed to see whether highly potent statin therapy is beneficial in people with elevated hs-CRP who otherwise do not meet the criteria for lipid-lowering therapy. The study was conducted at 1,315 sites in 26 countries. It was sponsored by AstraZeneca, the maker of rosuvastatin (Crestor).
Inclusion and exclusion criteria
All participants had to be free of known cardiovascular disease, have an LDL-C level lower than 130 mg/dL, and have an hs-CRP level of 2.0 mg/L or greater. Patients were excluded if they were previous or current users of lipid-lowering drugs; had severe arthritis, lupus, or inflammatory bowel disease; or were taking immune-modulating drugs such as cyclosporine (Sandimmune, others), tacrolimus (Prograf), azathioprine (Azasan, Imuran), or long-term oral corticosteroids.
Rosuvastatin therapy
Participants were randomly assigned in a 1:1 ratio to receive rosuvastatin 20 mg daily or a matching placebo in a double-blind fashion.
End points
The primary end point was the composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes. Secondary end points were the individual components of the primary end point.
Statistical analysis
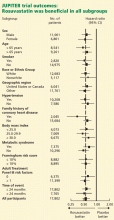
The study was powered to detect a 25% reduction in the primary end point among those treated with rosuvastatin. The trial was designed to run until 520 end point events had occurred. However, on March 29, 2008, after the first prespecified interim analysis, the Data and Safety Monitoring Board stopped the trial due to a significant reduction in the primary end point in the rosuvastatin group. As in most randomized clinical trials, all analyses were done on an intention-to-treat basis. Prespecified subgroup analyses were also performed.
STUDY RESULTS
Patient recruitment and eligibility
Between February 4, 2003, and December 15, 2006, a total of 89,890 people were screened. Of these, 17,802 met the inclusion and exclusion criteria and were included in the study. Of the 72,088 people who were excluded, 25,993 (36.1%) had an hs-CRP level below 2 mg/L and 37,611 (52.2%) had an LDL-C level of 130 mg/dL or higher.
A not-so-healthy population
The aim of the investigators was to include relatively healthy people. The median age was 66 years, about 16% of participants were current smokers, about 11% had a family history of heart disease, and about 41% met the criteria for metabolic syndrome, all conditions that are associated with elevated hs-CRP.25 Of note, the median hs-CRP level was 4.2 mg/L, a level indicating higher global risk according to the American College of Cardiology/American Heart Association consensus statement.26
Reduction in lipid levels and hs-CRP
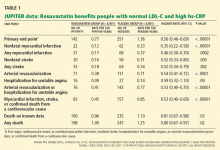
By 12 months, in the rosuvastatin group, the median LDL-C level had fallen by 50% (from 108 to 55 mg/dL), and the median hs-CRP level had fallen by 37% (from 4.2 to 2.2 mg/L). Additionally, the triglyceride level had fallen by 17%. The high-density lipoprotein cholesterol levels did not change significantly.
Impact on end points
Adverse events
WHAT DOES THIS MEAN?
Is lower LDL-C better?
The JUPITER trial is the latest of several statin trials that have shown significant reductions in major adverse cardiovascular events when LDL-C was lowered below what has been recommended by the current guidelines.27,28
In 2002, the Heart Protection Study29 showed a significant reduction in major adverse cardiovascular events in patients at high risk of coronary artery disease if they received simvastatin (Zocor), even if they had LDL-C levels lower than 100 mg/dL at baseline. Similarly, the Pravastatin or Atorvastatin Evaluation and Infection-Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial30 showed a 16% relative risk reduction in a composite end point in patients presenting with acute coronary syndrome if they received intensive statin therapy.
These two studies led to an update by the National Cholesterol Education Program (Adult Treatment Panel III), suggesting an optimal LDL-C goal of less than 70 mg/dL in those with coronary artery disease or its risk equivalent (ie, diabetes mellitus, peripheral vascular disease). Furthermore, in support of the “lower is better” theory, a number of studies that used intravascular ultrasonography have shown regression of coronary plaque with aggressive LDL-C lowering. Notably, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (the ASTEROID trial),5 rosuvastatin 40 mg daily caused significant plaque regression while lowering LDL-C to 61 mg/dL over a 24-month period.
A number of high-dose statin trials have shown that lowering LDL-C to less than 70 mg/dL significantly reduces major adverse cardiovascular events.31–39 The JUPITER trial was unique in that it extended these findings to people without known coronary disease (ie, primary prevention) or elevated cholesterol but with elevated levels of a marker of inflammation—hs-CRP. In view of the JUPITER results and of studies using intravascular ultrasonography in the primary prevention setting, it seems clear that lowering LDL-C to levels less than 70 mg/dL also reduces both atherosclerotic plaque progression and the rate of first major adverse cardiovascular events in primary prevention in patients at higher global risk.
Did the study prove that reducing hs-CRP lowers risk?
Measuring hs-CRP levels has been extensively studied in apparently healthy populations, stable angina, unstable angina, and other cardiovascular settings.18,21,40–43 It has been shown to have significant prognostic implications in a number of primary and secondary trials.44 Additionally, those with elevated LDL-C and hs-CRP levels benefit the most from statin therapy.16,45,46 Animal studies have also provided some evidence that CRP may play a role in atherogenesis.47,48 However, recent clinical and genetic studies have raised doubt about the direct causal relationship between CRP and coronary artery disease,23,49,50 and epidemiologic studies have questioned its usefulness as a marker of risk.51,52
The JUPITER study adds little to clear up the controversy about whether hs-CRP is a mechanistic participant in atherosclerotic disease. However, it also shows that this issue is somewhat irrelevant, in that selection of patients for high-potency statin therapy solely on the basis of high hs-CRP without other indications for lipid-lowering therapy clearly reduces risk and improves survival.
JUPITER did not examine whether people with higher hs-CRP levels benefited more from statin therapy than those with lower levels. The hypothesis-generating data for JUPITER came from an analysis of changes in hs-CRP and LDL-C in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).16 Thus, JUPITER did not include people with both low LDL-C and low hs-CRP because, in the AFCAPS/TexCAPS analysis, those with low LDL-C and low hs-CRP had extremely low event rates and no clinical efficacy of statin therapy, despite good LDL-C reduction. In marked contrast, those with low LDL-C but elevated hs-CRP had high event rates and large relative risk reductions— hence the need for JUPITER to prospectively test this hypothesis. Nevertheless, the initial results of JUPITER as presented do not yet make it clear that there is a dose-response relationship between higher levels of hs-CRP and a greater reduction in events, even in a cohort with elevated hs-CRP at baseline. This analysis will no doubt be forthcoming in another manuscript from Ridker and colleagues. Specifically, it will be of interest to examine whether those with the highest hs-CRP levels benefited the most from rosuvastatin on both an absolute and relative scale, and whether those with the greatest hs-CRP reduction also benefited more. With the present data available from JUPITER, a reasonable interpretation is that an elevated hs-CRP simply widens the inclusion criterion for those for whom high-potency statin therapy improves clinical outcomes.53
Better markers are needed
Even with a nonspecific marker such as hs-CRP, patients at higher global risk and with LDL-C below the recommended levels could be identified and treated aggressively. This benefit, however, required that approximately 100 people be treated with rosuvastatin for 2 years to prevent one event. Additionally, only 20% of all patients screened were eligible for the trial. Therefore, one could argue that its generalizability is limited.
Markers of risk that are more specific and sensitive are needed to identify people at higher global risk who would otherwise be considered to be at low risk with the current risk assessment tools. A number of such inflammatory and oxidative markers are under development.54–60
Absolute vs relative risk reduction and the public health burden
The 44% reduction in the number of primary end point events in the rosuvastatin group was considerable in relative terms. However, in absolute terms, 95 people had to be treated for up to 2 years in order to prevent one event.53 In making recommendations, the United States Department of Health and Human Services has to consider the clinical benefit of a test or a drug in light of its cost. With health care costs increasing, many agencies are refusing to pay for therapies on the basis of cost or small absolute benefit.
While we do not have the answer as to whether treating 95 people for 2 years to see one benefit is cost-effective, one thing is clear: the field of medicine is in desperate need of a better way to identify individuals who may benefit from a test or therapy.61 Additionally, we think it is important to note that the “numbers-needed-to-treat” (95 at 2 years and 25 at 5 years) derived from JUPITER are actually smaller than the values observed in the AFCAPS/TexCAPS and the West of Scotland Coronary Prevention Study.62,63 This suggests that statin therapy is at least as cost-effective in those with elevated hs-CRP as in those with elevated LDL-C. Even our most robust therapies are effective in only a minority of patients treated.61
Should ‘healthy’ people be tested for hs-CRP?
In 2003, we wrote in this journal21 that measuring hs-CRP may add to the current risk-prediction models by identifying people at increased risk who would otherwise not be considered as such by current risk models. The US Centers for Disease Control and Prevention and the American Heart Association have also stated that measuring hs-CRP in those at intermediate risk may be reasonable.26
The JUPITER investigators intended to study a relatively healthy population, but, as we mentioned, a close look at the cohort’s baseline characteristics indicates a substantial proportion met the criteria for metabolic syndrome. Therefore, one could challenge whether we really need hs-CRP in such a population to identify who will benefit from statin therapy.
We agree with the recommendation from the Centers for Disease Control and Prevention and the American Heart Association that measuring hs-CRP in people at intermediate risk is a reasonable option.26 We also believe that hs-CRP should be tested as a secondary risk factor, in combination with blood pressure, lipids, diabetes, smoking, serum creatinine, and fasting blood glucose. Factors such as obesity, sedentary lifestyle, family history of heart disease, and emotional and physical stress should also be considered.
Safety of high-dose statin therapy
High-dose statin therapy has been well tolerated in clinical trials, but rates of discontinuation have been higher (7%–10%) than with moderate-dose therapy (4%–5%).64 Fortunately, the rates of serious adverse events have in general been low. For example, with simvastatin 80 mg, the rates of myopathy and rhabdomyolysis were quite low.31
Rates of elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with high-dose statin therapy have been reported to be below 1.3%. Studies have shown that reducing LDL-C to below 100 mg/dL is associated with a higher incidence of ALT and AST elevations. However, these elevations have usually been benign and often return to normal when the drug is reduced in dose or withdrawn.
In previous studies of rosuvastatin,65 the incidence of myopathy and liver function abnormalities was less than 0.1%. Rates of proteinuria were similarly low, and in many patients renal function actually improved on rosuvastatin.66,67 Furthermore, rosuvastatin may have different pharmacokinetic properties than atorvastatin (Lipitor) and simvastatin, which may result in a lower incidence of musculoskeletal toxicity.68,69
In general, the incidence of cancer has been similar in those treated with high-dose statins and those treated with placebo. The Treating to New Targets trial70 suggested that the incidence of cancer was higher with atorvastatin 80 mg daily than with 20 mg daily. However, a meta-analysis of 14 trials of moderate-dose statin therapy did not show any evidence of increased cancer rates with these agents.70 Indeed, in JUPITER, there was a reduction in cancer-related mortality rates, which could have been due to chance.
The JUPITER trial also showed an increase in the physician-reported incidence of diabetes mellitus with rosuvastatin. This is an important finding, and it may be a class effect because modest increases have similarly been reported with other statins in other major trials, eg, with pravastatin (Pravachol) in PROSPER, simvastatin in the Heart Protection Study, and atorvastatin in PROVE-IT. However, even in those with diabetes or impaired fasting glucose, the reduction in the rate of major adverse events is significant. For example, in JUPITER, almost all of the cases of “incident diabetes” were in those with impaired fasting glucose at baseline, and this group had nearly a 50% reduction in rates of myocardial infarction, stroke, and cardiovascular death. Therefore, on balance, the modest risk of earlier diagnosis of diabetes with statin therapy seems substantially offset by the marked reduction in rates of major adverse cardiovascular events in people with diabetes and impaired fasting glucose on statin therapy.
TAKE-HOME POINTS
The JUPITER trial, like previous high-dose statin trials, calls into question whether current LDL-C guidelines are appropriate for people at higher global risk with otherwise “normal” LDL-C levels.27,28 This trial heralds a new era in preventive therapy because it extends beyond LDL-C as an indication for statin therapy within the primary prevention setting. Statins have revolutionized the therapy of cardiovascular disease, and they continue to show benefit even in the “healthy.”
Clearly, hs-CRP serves as a nonlipid marker to identify those who may benefit from statin therapy. Nonetheless, more specific and sensitive markers (or panels) of cardiovascular risk are necessary. In the future, we will need markers that not only identify people at higher global risk, but that also tell us who would benefit from certain medical or surgical therapies. Elevated hs-CRP in a patient who otherwise would not be a candidate for statin therapy should trigger a reassessment of the risks vs benefits of statin therapy—JUPITER teaches us that statin therapy will benefit these patients.
Aggressive lifestyle modification that encompasses a balanced diet, routine exercise, and smoking cessation should be applied in both primary and secondary prevention. Additionally, risk factors such as elevated blood pressure and hyperlipidemia should be aggressively treated with appropriate medications.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Topol EJ. Intensive statin therapy—a sea change in cardiovascular prevention. N Engl J Med 2004; 350:1562–1564.
- Cannon CP, Murphy SA, Braunwald E. Intensive lipid lowering with atorvastatin in coronary disease. N Engl J Med 2005; 353:93–96.
- Cohen DJ, Carrozza JP, Baim DS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999; 341:1853–1854.
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005; 46:1855–1862.
- Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001; 103:276–283.
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol 2005; 96:24F–33F.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003; 289:1675–1680.
- Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003; 108:426–431.
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–1719.
- Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med 2006; 73:760–766.
- Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344:1959–1965.
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998; 98:731–733.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001; 285:2481–2485.
- Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 2001; 47:403–411.
- Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med 2003; 70:634–640.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–979.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Ridker PM. Are statins anti-inflammatory? Issues in the design and conduct of the pravastatin inflammation C-reactive protein evaluation. Curr Cardiol Rep 2000; 2:269–273.
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107:391–397.
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107:499–511.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002; 56:53–56.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Liem AH, van Boven AJ, Veeger NJ, et al. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 2002; 23:1931–1937.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999; 341:70–76.
- Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of highdose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005; 46:1405–1410.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007; 28:664–672.
- Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med 2000; 45:391–418.
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103:1813–1818.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97:2007–2011.
- Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 2005; 112:25–31.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007; 49:2129–2138.
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286:64–70.
- Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100:230–235.
- Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res 2005; 96:714–716.
- Schwedler SB, Amann K, Wernicke K, et al. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation 2005; 112:1016–1023.
- Pfister R, Hellmich M. Multiple biomarkers and cardiovascular risk. N Engl J Med 2008; 359:760.
- Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis— chicken or egg? N Engl J Med 2008; 359:1953–1955.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–1397.
- Kushner I, Sehgal AR. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch Intern Med 2002; 162:867–869.
- Hlatky MA. Expanding the orbit of primary prevention—moving beyond JUPITER. N Engl J Med 2008; 359:2280–2282.
- Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep 2004; 6:243–250.
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005; 25:1102–1111.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299:1265–1276.
- Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol 2008; 52:24–32.
- Hakonarson H, Thorvaldsson S, Helgadottir A, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA 2005; 293:2245–2256.
- Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol 2008; 51:1653–1662.
- Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2007; 27:134–140.
- Mukherjee D, Topol EJ. Pharmacogenomics in cardiovascular diseases. Curr Probl Cardiol 2003; 28:317–347
- West of Scotland Coronary Prevention Study: identification of highrisk groups and comparison with other cardiovascular intervention trials. Lancet 1996; 348:1339–1342.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol 2007; 49:1753–1762.
- Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf 2004; 3:547–557.
- Kasiske BL, Wanner C, O’Neill WC. An assessment of statin safety by nephrologists. Am J Cardiol 2006; 97:82C–85C.
- McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001; 87:28B–32B.
- Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol 2006; 97:44C–51C.
- Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol 2004; 94:1140–1146.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
The medical community has struggled with two important questions for the past 10 years: When it comes to the low-density lipoprotein cholesterol (LDL-C) level, how low should one go and at what cost? And are there other markers of risk that can identify a higher-risk subpopulation in relatively healthy people? The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) provided partial answers for these questions by finding that a highly potent statin lowered the risk of cardiovascular events in patients with “normal” LDL-C but elevated levels of high-sensitivity C-reactive protein (hs-CRP).1
In this article, we will critically evaluate the methods, results, and conclusions of the JUPITER trial. Additionally, we will discuss its limitations and areas of uncertainty.
BEFORE JUPITER
The LDL-C-lowering drugs called statins have revolutionized cardiovascular medicine.2 They are beneficial in both the primary prevention setting and in acute coronary syndromes, stable angina, and unstable angina and can halt the progression of coronary artery disease—in some cases even resulting in modest regression of plaque.3–6
Many experts have credited the reduction in LDL-C as being the sole factor responsible for the decrease in major adverse events seen with statin therapy.7 However, statins have other, non-lipid-lowering properties, including anti-inflammatory and antioxidant effects, that may also contribute to their benefits.8–15
One of the anti-inflammatory actions of statins is evidenced by lower levels of the acute-phase reactant CRP.10,11,15,16 Measuring systemic CRP levels with a highly sensitive assay (yielding the so-called high-sensitivity or hs-CRP level) provides significant clinical prognostic value across a spectrum of clinical situations, ranging from risk screening in apparently healthy people to stable and unstable angina.17–22 People with higher hs-CRP levels are, on average, at higher risk of adverse cardiovascular events. However, controversy remains as to whether hs-CRP plays a mechanistic role in plaque formation and acute complications. Indeed, recent genetic studies argue strongly that hs-CRP lies outside the mechanistic path of atherosclerosis.23 Nonetheless, an overwhelming amount of data indicates that hs-CRP serves as a marker of disease.17–21
Nissen et al10 showed that the rate of progression of atherosclerosis is lower when the levels of atherogenic lipoproteins and hs-CRP are both lowered with statin therapy. Simultaneously, Ridker et al11 showed that patients who have lower hs-CRP levels after statin therapy have better clinical outcomes than those with higher hs-CRP levels, regardless of their achieved level of LDL-C.
Collectively, these studies and others have led some to believe that, in people with relatively low LDL-C but persistently elevated hs-CRP, statin therapy may reduce the rate of events.15,24 The JUPITER trial was undertaken to test this hypothesis.
JUPITER STUDY DESIGN
JUPITER was designed to see whether highly potent statin therapy is beneficial in people with elevated hs-CRP who otherwise do not meet the criteria for lipid-lowering therapy. The study was conducted at 1,315 sites in 26 countries. It was sponsored by AstraZeneca, the maker of rosuvastatin (Crestor).
Inclusion and exclusion criteria
All participants had to be free of known cardiovascular disease, have an LDL-C level lower than 130 mg/dL, and have an hs-CRP level of 2.0 mg/L or greater. Patients were excluded if they were previous or current users of lipid-lowering drugs; had severe arthritis, lupus, or inflammatory bowel disease; or were taking immune-modulating drugs such as cyclosporine (Sandimmune, others), tacrolimus (Prograf), azathioprine (Azasan, Imuran), or long-term oral corticosteroids.
Rosuvastatin therapy
Participants were randomly assigned in a 1:1 ratio to receive rosuvastatin 20 mg daily or a matching placebo in a double-blind fashion.
End points
The primary end point was the composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes. Secondary end points were the individual components of the primary end point.
Statistical analysis
The study was powered to detect a 25% reduction in the primary end point among those treated with rosuvastatin. The trial was designed to run until 520 end point events had occurred. However, on March 29, 2008, after the first prespecified interim analysis, the Data and Safety Monitoring Board stopped the trial due to a significant reduction in the primary end point in the rosuvastatin group. As in most randomized clinical trials, all analyses were done on an intention-to-treat basis. Prespecified subgroup analyses were also performed.
STUDY RESULTS
Patient recruitment and eligibility
Between February 4, 2003, and December 15, 2006, a total of 89,890 people were screened. Of these, 17,802 met the inclusion and exclusion criteria and were included in the study. Of the 72,088 people who were excluded, 25,993 (36.1%) had an hs-CRP level below 2 mg/L and 37,611 (52.2%) had an LDL-C level of 130 mg/dL or higher.
A not-so-healthy population
The aim of the investigators was to include relatively healthy people. The median age was 66 years, about 16% of participants were current smokers, about 11% had a family history of heart disease, and about 41% met the criteria for metabolic syndrome, all conditions that are associated with elevated hs-CRP.25 Of note, the median hs-CRP level was 4.2 mg/L, a level indicating higher global risk according to the American College of Cardiology/American Heart Association consensus statement.26
Reduction in lipid levels and hs-CRP
By 12 months, in the rosuvastatin group, the median LDL-C level had fallen by 50% (from 108 to 55 mg/dL), and the median hs-CRP level had fallen by 37% (from 4.2 to 2.2 mg/L). Additionally, the triglyceride level had fallen by 17%. The high-density lipoprotein cholesterol levels did not change significantly.
Impact on end points
Adverse events
WHAT DOES THIS MEAN?
Is lower LDL-C better?
The JUPITER trial is the latest of several statin trials that have shown significant reductions in major adverse cardiovascular events when LDL-C was lowered below what has been recommended by the current guidelines.27,28
In 2002, the Heart Protection Study29 showed a significant reduction in major adverse cardiovascular events in patients at high risk of coronary artery disease if they received simvastatin (Zocor), even if they had LDL-C levels lower than 100 mg/dL at baseline. Similarly, the Pravastatin or Atorvastatin Evaluation and Infection-Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial30 showed a 16% relative risk reduction in a composite end point in patients presenting with acute coronary syndrome if they received intensive statin therapy.
These two studies led to an update by the National Cholesterol Education Program (Adult Treatment Panel III), suggesting an optimal LDL-C goal of less than 70 mg/dL in those with coronary artery disease or its risk equivalent (ie, diabetes mellitus, peripheral vascular disease). Furthermore, in support of the “lower is better” theory, a number of studies that used intravascular ultrasonography have shown regression of coronary plaque with aggressive LDL-C lowering. Notably, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (the ASTEROID trial),5 rosuvastatin 40 mg daily caused significant plaque regression while lowering LDL-C to 61 mg/dL over a 24-month period.
A number of high-dose statin trials have shown that lowering LDL-C to less than 70 mg/dL significantly reduces major adverse cardiovascular events.31–39 The JUPITER trial was unique in that it extended these findings to people without known coronary disease (ie, primary prevention) or elevated cholesterol but with elevated levels of a marker of inflammation—hs-CRP. In view of the JUPITER results and of studies using intravascular ultrasonography in the primary prevention setting, it seems clear that lowering LDL-C to levels less than 70 mg/dL also reduces both atherosclerotic plaque progression and the rate of first major adverse cardiovascular events in primary prevention in patients at higher global risk.
Did the study prove that reducing hs-CRP lowers risk?
Measuring hs-CRP levels has been extensively studied in apparently healthy populations, stable angina, unstable angina, and other cardiovascular settings.18,21,40–43 It has been shown to have significant prognostic implications in a number of primary and secondary trials.44 Additionally, those with elevated LDL-C and hs-CRP levels benefit the most from statin therapy.16,45,46 Animal studies have also provided some evidence that CRP may play a role in atherogenesis.47,48 However, recent clinical and genetic studies have raised doubt about the direct causal relationship between CRP and coronary artery disease,23,49,50 and epidemiologic studies have questioned its usefulness as a marker of risk.51,52
The JUPITER study adds little to clear up the controversy about whether hs-CRP is a mechanistic participant in atherosclerotic disease. However, it also shows that this issue is somewhat irrelevant, in that selection of patients for high-potency statin therapy solely on the basis of high hs-CRP without other indications for lipid-lowering therapy clearly reduces risk and improves survival.
JUPITER did not examine whether people with higher hs-CRP levels benefited more from statin therapy than those with lower levels. The hypothesis-generating data for JUPITER came from an analysis of changes in hs-CRP and LDL-C in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).16 Thus, JUPITER did not include people with both low LDL-C and low hs-CRP because, in the AFCAPS/TexCAPS analysis, those with low LDL-C and low hs-CRP had extremely low event rates and no clinical efficacy of statin therapy, despite good LDL-C reduction. In marked contrast, those with low LDL-C but elevated hs-CRP had high event rates and large relative risk reductions— hence the need for JUPITER to prospectively test this hypothesis. Nevertheless, the initial results of JUPITER as presented do not yet make it clear that there is a dose-response relationship between higher levels of hs-CRP and a greater reduction in events, even in a cohort with elevated hs-CRP at baseline. This analysis will no doubt be forthcoming in another manuscript from Ridker and colleagues. Specifically, it will be of interest to examine whether those with the highest hs-CRP levels benefited the most from rosuvastatin on both an absolute and relative scale, and whether those with the greatest hs-CRP reduction also benefited more. With the present data available from JUPITER, a reasonable interpretation is that an elevated hs-CRP simply widens the inclusion criterion for those for whom high-potency statin therapy improves clinical outcomes.53
Better markers are needed
Even with a nonspecific marker such as hs-CRP, patients at higher global risk and with LDL-C below the recommended levels could be identified and treated aggressively. This benefit, however, required that approximately 100 people be treated with rosuvastatin for 2 years to prevent one event. Additionally, only 20% of all patients screened were eligible for the trial. Therefore, one could argue that its generalizability is limited.
Markers of risk that are more specific and sensitive are needed to identify people at higher global risk who would otherwise be considered to be at low risk with the current risk assessment tools. A number of such inflammatory and oxidative markers are under development.54–60
Absolute vs relative risk reduction and the public health burden
The 44% reduction in the number of primary end point events in the rosuvastatin group was considerable in relative terms. However, in absolute terms, 95 people had to be treated for up to 2 years in order to prevent one event.53 In making recommendations, the United States Department of Health and Human Services has to consider the clinical benefit of a test or a drug in light of its cost. With health care costs increasing, many agencies are refusing to pay for therapies on the basis of cost or small absolute benefit.
While we do not have the answer as to whether treating 95 people for 2 years to see one benefit is cost-effective, one thing is clear: the field of medicine is in desperate need of a better way to identify individuals who may benefit from a test or therapy.61 Additionally, we think it is important to note that the “numbers-needed-to-treat” (95 at 2 years and 25 at 5 years) derived from JUPITER are actually smaller than the values observed in the AFCAPS/TexCAPS and the West of Scotland Coronary Prevention Study.62,63 This suggests that statin therapy is at least as cost-effective in those with elevated hs-CRP as in those with elevated LDL-C. Even our most robust therapies are effective in only a minority of patients treated.61
Should ‘healthy’ people be tested for hs-CRP?
In 2003, we wrote in this journal21 that measuring hs-CRP may add to the current risk-prediction models by identifying people at increased risk who would otherwise not be considered as such by current risk models. The US Centers for Disease Control and Prevention and the American Heart Association have also stated that measuring hs-CRP in those at intermediate risk may be reasonable.26
The JUPITER investigators intended to study a relatively healthy population, but, as we mentioned, a close look at the cohort’s baseline characteristics indicates a substantial proportion met the criteria for metabolic syndrome. Therefore, one could challenge whether we really need hs-CRP in such a population to identify who will benefit from statin therapy.
We agree with the recommendation from the Centers for Disease Control and Prevention and the American Heart Association that measuring hs-CRP in people at intermediate risk is a reasonable option.26 We also believe that hs-CRP should be tested as a secondary risk factor, in combination with blood pressure, lipids, diabetes, smoking, serum creatinine, and fasting blood glucose. Factors such as obesity, sedentary lifestyle, family history of heart disease, and emotional and physical stress should also be considered.
Safety of high-dose statin therapy
High-dose statin therapy has been well tolerated in clinical trials, but rates of discontinuation have been higher (7%–10%) than with moderate-dose therapy (4%–5%).64 Fortunately, the rates of serious adverse events have in general been low. For example, with simvastatin 80 mg, the rates of myopathy and rhabdomyolysis were quite low.31
Rates of elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with high-dose statin therapy have been reported to be below 1.3%. Studies have shown that reducing LDL-C to below 100 mg/dL is associated with a higher incidence of ALT and AST elevations. However, these elevations have usually been benign and often return to normal when the drug is reduced in dose or withdrawn.
In previous studies of rosuvastatin,65 the incidence of myopathy and liver function abnormalities was less than 0.1%. Rates of proteinuria were similarly low, and in many patients renal function actually improved on rosuvastatin.66,67 Furthermore, rosuvastatin may have different pharmacokinetic properties than atorvastatin (Lipitor) and simvastatin, which may result in a lower incidence of musculoskeletal toxicity.68,69
In general, the incidence of cancer has been similar in those treated with high-dose statins and those treated with placebo. The Treating to New Targets trial70 suggested that the incidence of cancer was higher with atorvastatin 80 mg daily than with 20 mg daily. However, a meta-analysis of 14 trials of moderate-dose statin therapy did not show any evidence of increased cancer rates with these agents.70 Indeed, in JUPITER, there was a reduction in cancer-related mortality rates, which could have been due to chance.
The JUPITER trial also showed an increase in the physician-reported incidence of diabetes mellitus with rosuvastatin. This is an important finding, and it may be a class effect because modest increases have similarly been reported with other statins in other major trials, eg, with pravastatin (Pravachol) in PROSPER, simvastatin in the Heart Protection Study, and atorvastatin in PROVE-IT. However, even in those with diabetes or impaired fasting glucose, the reduction in the rate of major adverse events is significant. For example, in JUPITER, almost all of the cases of “incident diabetes” were in those with impaired fasting glucose at baseline, and this group had nearly a 50% reduction in rates of myocardial infarction, stroke, and cardiovascular death. Therefore, on balance, the modest risk of earlier diagnosis of diabetes with statin therapy seems substantially offset by the marked reduction in rates of major adverse cardiovascular events in people with diabetes and impaired fasting glucose on statin therapy.
TAKE-HOME POINTS
The JUPITER trial, like previous high-dose statin trials, calls into question whether current LDL-C guidelines are appropriate for people at higher global risk with otherwise “normal” LDL-C levels.27,28 This trial heralds a new era in preventive therapy because it extends beyond LDL-C as an indication for statin therapy within the primary prevention setting. Statins have revolutionized the therapy of cardiovascular disease, and they continue to show benefit even in the “healthy.”
Clearly, hs-CRP serves as a nonlipid marker to identify those who may benefit from statin therapy. Nonetheless, more specific and sensitive markers (or panels) of cardiovascular risk are necessary. In the future, we will need markers that not only identify people at higher global risk, but that also tell us who would benefit from certain medical or surgical therapies. Elevated hs-CRP in a patient who otherwise would not be a candidate for statin therapy should trigger a reassessment of the risks vs benefits of statin therapy—JUPITER teaches us that statin therapy will benefit these patients.
Aggressive lifestyle modification that encompasses a balanced diet, routine exercise, and smoking cessation should be applied in both primary and secondary prevention. Additionally, risk factors such as elevated blood pressure and hyperlipidemia should be aggressively treated with appropriate medications.
The medical community has struggled with two important questions for the past 10 years: When it comes to the low-density lipoprotein cholesterol (LDL-C) level, how low should one go and at what cost? And are there other markers of risk that can identify a higher-risk subpopulation in relatively healthy people? The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) provided partial answers for these questions by finding that a highly potent statin lowered the risk of cardiovascular events in patients with “normal” LDL-C but elevated levels of high-sensitivity C-reactive protein (hs-CRP).1
In this article, we will critically evaluate the methods, results, and conclusions of the JUPITER trial. Additionally, we will discuss its limitations and areas of uncertainty.
BEFORE JUPITER
The LDL-C-lowering drugs called statins have revolutionized cardiovascular medicine.2 They are beneficial in both the primary prevention setting and in acute coronary syndromes, stable angina, and unstable angina and can halt the progression of coronary artery disease—in some cases even resulting in modest regression of plaque.3–6
Many experts have credited the reduction in LDL-C as being the sole factor responsible for the decrease in major adverse events seen with statin therapy.7 However, statins have other, non-lipid-lowering properties, including anti-inflammatory and antioxidant effects, that may also contribute to their benefits.8–15
One of the anti-inflammatory actions of statins is evidenced by lower levels of the acute-phase reactant CRP.10,11,15,16 Measuring systemic CRP levels with a highly sensitive assay (yielding the so-called high-sensitivity or hs-CRP level) provides significant clinical prognostic value across a spectrum of clinical situations, ranging from risk screening in apparently healthy people to stable and unstable angina.17–22 People with higher hs-CRP levels are, on average, at higher risk of adverse cardiovascular events. However, controversy remains as to whether hs-CRP plays a mechanistic role in plaque formation and acute complications. Indeed, recent genetic studies argue strongly that hs-CRP lies outside the mechanistic path of atherosclerosis.23 Nonetheless, an overwhelming amount of data indicates that hs-CRP serves as a marker of disease.17–21
Nissen et al10 showed that the rate of progression of atherosclerosis is lower when the levels of atherogenic lipoproteins and hs-CRP are both lowered with statin therapy. Simultaneously, Ridker et al11 showed that patients who have lower hs-CRP levels after statin therapy have better clinical outcomes than those with higher hs-CRP levels, regardless of their achieved level of LDL-C.
Collectively, these studies and others have led some to believe that, in people with relatively low LDL-C but persistently elevated hs-CRP, statin therapy may reduce the rate of events.15,24 The JUPITER trial was undertaken to test this hypothesis.
JUPITER STUDY DESIGN
JUPITER was designed to see whether highly potent statin therapy is beneficial in people with elevated hs-CRP who otherwise do not meet the criteria for lipid-lowering therapy. The study was conducted at 1,315 sites in 26 countries. It was sponsored by AstraZeneca, the maker of rosuvastatin (Crestor).
Inclusion and exclusion criteria
All participants had to be free of known cardiovascular disease, have an LDL-C level lower than 130 mg/dL, and have an hs-CRP level of 2.0 mg/L or greater. Patients were excluded if they were previous or current users of lipid-lowering drugs; had severe arthritis, lupus, or inflammatory bowel disease; or were taking immune-modulating drugs such as cyclosporine (Sandimmune, others), tacrolimus (Prograf), azathioprine (Azasan, Imuran), or long-term oral corticosteroids.
Rosuvastatin therapy
Participants were randomly assigned in a 1:1 ratio to receive rosuvastatin 20 mg daily or a matching placebo in a double-blind fashion.
End points
The primary end point was the composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes. Secondary end points were the individual components of the primary end point.
Statistical analysis
The study was powered to detect a 25% reduction in the primary end point among those treated with rosuvastatin. The trial was designed to run until 520 end point events had occurred. However, on March 29, 2008, after the first prespecified interim analysis, the Data and Safety Monitoring Board stopped the trial due to a significant reduction in the primary end point in the rosuvastatin group. As in most randomized clinical trials, all analyses were done on an intention-to-treat basis. Prespecified subgroup analyses were also performed.
STUDY RESULTS
Patient recruitment and eligibility
Between February 4, 2003, and December 15, 2006, a total of 89,890 people were screened. Of these, 17,802 met the inclusion and exclusion criteria and were included in the study. Of the 72,088 people who were excluded, 25,993 (36.1%) had an hs-CRP level below 2 mg/L and 37,611 (52.2%) had an LDL-C level of 130 mg/dL or higher.
A not-so-healthy population
The aim of the investigators was to include relatively healthy people. The median age was 66 years, about 16% of participants were current smokers, about 11% had a family history of heart disease, and about 41% met the criteria for metabolic syndrome, all conditions that are associated with elevated hs-CRP.25 Of note, the median hs-CRP level was 4.2 mg/L, a level indicating higher global risk according to the American College of Cardiology/American Heart Association consensus statement.26
Reduction in lipid levels and hs-CRP
By 12 months, in the rosuvastatin group, the median LDL-C level had fallen by 50% (from 108 to 55 mg/dL), and the median hs-CRP level had fallen by 37% (from 4.2 to 2.2 mg/L). Additionally, the triglyceride level had fallen by 17%. The high-density lipoprotein cholesterol levels did not change significantly.
Impact on end points
Adverse events
WHAT DOES THIS MEAN?
Is lower LDL-C better?
The JUPITER trial is the latest of several statin trials that have shown significant reductions in major adverse cardiovascular events when LDL-C was lowered below what has been recommended by the current guidelines.27,28
In 2002, the Heart Protection Study29 showed a significant reduction in major adverse cardiovascular events in patients at high risk of coronary artery disease if they received simvastatin (Zocor), even if they had LDL-C levels lower than 100 mg/dL at baseline. Similarly, the Pravastatin or Atorvastatin Evaluation and Infection-Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial30 showed a 16% relative risk reduction in a composite end point in patients presenting with acute coronary syndrome if they received intensive statin therapy.
These two studies led to an update by the National Cholesterol Education Program (Adult Treatment Panel III), suggesting an optimal LDL-C goal of less than 70 mg/dL in those with coronary artery disease or its risk equivalent (ie, diabetes mellitus, peripheral vascular disease). Furthermore, in support of the “lower is better” theory, a number of studies that used intravascular ultrasonography have shown regression of coronary plaque with aggressive LDL-C lowering. Notably, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (the ASTEROID trial),5 rosuvastatin 40 mg daily caused significant plaque regression while lowering LDL-C to 61 mg/dL over a 24-month period.
A number of high-dose statin trials have shown that lowering LDL-C to less than 70 mg/dL significantly reduces major adverse cardiovascular events.31–39 The JUPITER trial was unique in that it extended these findings to people without known coronary disease (ie, primary prevention) or elevated cholesterol but with elevated levels of a marker of inflammation—hs-CRP. In view of the JUPITER results and of studies using intravascular ultrasonography in the primary prevention setting, it seems clear that lowering LDL-C to levels less than 70 mg/dL also reduces both atherosclerotic plaque progression and the rate of first major adverse cardiovascular events in primary prevention in patients at higher global risk.
Did the study prove that reducing hs-CRP lowers risk?
Measuring hs-CRP levels has been extensively studied in apparently healthy populations, stable angina, unstable angina, and other cardiovascular settings.18,21,40–43 It has been shown to have significant prognostic implications in a number of primary and secondary trials.44 Additionally, those with elevated LDL-C and hs-CRP levels benefit the most from statin therapy.16,45,46 Animal studies have also provided some evidence that CRP may play a role in atherogenesis.47,48 However, recent clinical and genetic studies have raised doubt about the direct causal relationship between CRP and coronary artery disease,23,49,50 and epidemiologic studies have questioned its usefulness as a marker of risk.51,52
The JUPITER study adds little to clear up the controversy about whether hs-CRP is a mechanistic participant in atherosclerotic disease. However, it also shows that this issue is somewhat irrelevant, in that selection of patients for high-potency statin therapy solely on the basis of high hs-CRP without other indications for lipid-lowering therapy clearly reduces risk and improves survival.
JUPITER did not examine whether people with higher hs-CRP levels benefited more from statin therapy than those with lower levels. The hypothesis-generating data for JUPITER came from an analysis of changes in hs-CRP and LDL-C in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).16 Thus, JUPITER did not include people with both low LDL-C and low hs-CRP because, in the AFCAPS/TexCAPS analysis, those with low LDL-C and low hs-CRP had extremely low event rates and no clinical efficacy of statin therapy, despite good LDL-C reduction. In marked contrast, those with low LDL-C but elevated hs-CRP had high event rates and large relative risk reductions— hence the need for JUPITER to prospectively test this hypothesis. Nevertheless, the initial results of JUPITER as presented do not yet make it clear that there is a dose-response relationship between higher levels of hs-CRP and a greater reduction in events, even in a cohort with elevated hs-CRP at baseline. This analysis will no doubt be forthcoming in another manuscript from Ridker and colleagues. Specifically, it will be of interest to examine whether those with the highest hs-CRP levels benefited the most from rosuvastatin on both an absolute and relative scale, and whether those with the greatest hs-CRP reduction also benefited more. With the present data available from JUPITER, a reasonable interpretation is that an elevated hs-CRP simply widens the inclusion criterion for those for whom high-potency statin therapy improves clinical outcomes.53
Better markers are needed
Even with a nonspecific marker such as hs-CRP, patients at higher global risk and with LDL-C below the recommended levels could be identified and treated aggressively. This benefit, however, required that approximately 100 people be treated with rosuvastatin for 2 years to prevent one event. Additionally, only 20% of all patients screened were eligible for the trial. Therefore, one could argue that its generalizability is limited.
Markers of risk that are more specific and sensitive are needed to identify people at higher global risk who would otherwise be considered to be at low risk with the current risk assessment tools. A number of such inflammatory and oxidative markers are under development.54–60
Absolute vs relative risk reduction and the public health burden
The 44% reduction in the number of primary end point events in the rosuvastatin group was considerable in relative terms. However, in absolute terms, 95 people had to be treated for up to 2 years in order to prevent one event.53 In making recommendations, the United States Department of Health and Human Services has to consider the clinical benefit of a test or a drug in light of its cost. With health care costs increasing, many agencies are refusing to pay for therapies on the basis of cost or small absolute benefit.
While we do not have the answer as to whether treating 95 people for 2 years to see one benefit is cost-effective, one thing is clear: the field of medicine is in desperate need of a better way to identify individuals who may benefit from a test or therapy.61 Additionally, we think it is important to note that the “numbers-needed-to-treat” (95 at 2 years and 25 at 5 years) derived from JUPITER are actually smaller than the values observed in the AFCAPS/TexCAPS and the West of Scotland Coronary Prevention Study.62,63 This suggests that statin therapy is at least as cost-effective in those with elevated hs-CRP as in those with elevated LDL-C. Even our most robust therapies are effective in only a minority of patients treated.61
Should ‘healthy’ people be tested for hs-CRP?
In 2003, we wrote in this journal21 that measuring hs-CRP may add to the current risk-prediction models by identifying people at increased risk who would otherwise not be considered as such by current risk models. The US Centers for Disease Control and Prevention and the American Heart Association have also stated that measuring hs-CRP in those at intermediate risk may be reasonable.26
The JUPITER investigators intended to study a relatively healthy population, but, as we mentioned, a close look at the cohort’s baseline characteristics indicates a substantial proportion met the criteria for metabolic syndrome. Therefore, one could challenge whether we really need hs-CRP in such a population to identify who will benefit from statin therapy.
We agree with the recommendation from the Centers for Disease Control and Prevention and the American Heart Association that measuring hs-CRP in people at intermediate risk is a reasonable option.26 We also believe that hs-CRP should be tested as a secondary risk factor, in combination with blood pressure, lipids, diabetes, smoking, serum creatinine, and fasting blood glucose. Factors such as obesity, sedentary lifestyle, family history of heart disease, and emotional and physical stress should also be considered.
Safety of high-dose statin therapy
High-dose statin therapy has been well tolerated in clinical trials, but rates of discontinuation have been higher (7%–10%) than with moderate-dose therapy (4%–5%).64 Fortunately, the rates of serious adverse events have in general been low. For example, with simvastatin 80 mg, the rates of myopathy and rhabdomyolysis were quite low.31
Rates of elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with high-dose statin therapy have been reported to be below 1.3%. Studies have shown that reducing LDL-C to below 100 mg/dL is associated with a higher incidence of ALT and AST elevations. However, these elevations have usually been benign and often return to normal when the drug is reduced in dose or withdrawn.
In previous studies of rosuvastatin,65 the incidence of myopathy and liver function abnormalities was less than 0.1%. Rates of proteinuria were similarly low, and in many patients renal function actually improved on rosuvastatin.66,67 Furthermore, rosuvastatin may have different pharmacokinetic properties than atorvastatin (Lipitor) and simvastatin, which may result in a lower incidence of musculoskeletal toxicity.68,69
In general, the incidence of cancer has been similar in those treated with high-dose statins and those treated with placebo. The Treating to New Targets trial70 suggested that the incidence of cancer was higher with atorvastatin 80 mg daily than with 20 mg daily. However, a meta-analysis of 14 trials of moderate-dose statin therapy did not show any evidence of increased cancer rates with these agents.70 Indeed, in JUPITER, there was a reduction in cancer-related mortality rates, which could have been due to chance.
The JUPITER trial also showed an increase in the physician-reported incidence of diabetes mellitus with rosuvastatin. This is an important finding, and it may be a class effect because modest increases have similarly been reported with other statins in other major trials, eg, with pravastatin (Pravachol) in PROSPER, simvastatin in the Heart Protection Study, and atorvastatin in PROVE-IT. However, even in those with diabetes or impaired fasting glucose, the reduction in the rate of major adverse events is significant. For example, in JUPITER, almost all of the cases of “incident diabetes” were in those with impaired fasting glucose at baseline, and this group had nearly a 50% reduction in rates of myocardial infarction, stroke, and cardiovascular death. Therefore, on balance, the modest risk of earlier diagnosis of diabetes with statin therapy seems substantially offset by the marked reduction in rates of major adverse cardiovascular events in people with diabetes and impaired fasting glucose on statin therapy.
TAKE-HOME POINTS
The JUPITER trial, like previous high-dose statin trials, calls into question whether current LDL-C guidelines are appropriate for people at higher global risk with otherwise “normal” LDL-C levels.27,28 This trial heralds a new era in preventive therapy because it extends beyond LDL-C as an indication for statin therapy within the primary prevention setting. Statins have revolutionized the therapy of cardiovascular disease, and they continue to show benefit even in the “healthy.”
Clearly, hs-CRP serves as a nonlipid marker to identify those who may benefit from statin therapy. Nonetheless, more specific and sensitive markers (or panels) of cardiovascular risk are necessary. In the future, we will need markers that not only identify people at higher global risk, but that also tell us who would benefit from certain medical or surgical therapies. Elevated hs-CRP in a patient who otherwise would not be a candidate for statin therapy should trigger a reassessment of the risks vs benefits of statin therapy—JUPITER teaches us that statin therapy will benefit these patients.
Aggressive lifestyle modification that encompasses a balanced diet, routine exercise, and smoking cessation should be applied in both primary and secondary prevention. Additionally, risk factors such as elevated blood pressure and hyperlipidemia should be aggressively treated with appropriate medications.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Topol EJ. Intensive statin therapy—a sea change in cardiovascular prevention. N Engl J Med 2004; 350:1562–1564.
- Cannon CP, Murphy SA, Braunwald E. Intensive lipid lowering with atorvastatin in coronary disease. N Engl J Med 2005; 353:93–96.
- Cohen DJ, Carrozza JP, Baim DS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999; 341:1853–1854.
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005; 46:1855–1862.
- Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001; 103:276–283.
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol 2005; 96:24F–33F.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003; 289:1675–1680.
- Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003; 108:426–431.
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–1719.
- Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med 2006; 73:760–766.
- Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344:1959–1965.
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998; 98:731–733.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001; 285:2481–2485.
- Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 2001; 47:403–411.
- Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med 2003; 70:634–640.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–979.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Ridker PM. Are statins anti-inflammatory? Issues in the design and conduct of the pravastatin inflammation C-reactive protein evaluation. Curr Cardiol Rep 2000; 2:269–273.
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107:391–397.
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107:499–511.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002; 56:53–56.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Liem AH, van Boven AJ, Veeger NJ, et al. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 2002; 23:1931–1937.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999; 341:70–76.
- Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of highdose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005; 46:1405–1410.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007; 28:664–672.
- Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med 2000; 45:391–418.
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103:1813–1818.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97:2007–2011.
- Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 2005; 112:25–31.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007; 49:2129–2138.
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286:64–70.
- Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100:230–235.
- Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res 2005; 96:714–716.
- Schwedler SB, Amann K, Wernicke K, et al. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation 2005; 112:1016–1023.
- Pfister R, Hellmich M. Multiple biomarkers and cardiovascular risk. N Engl J Med 2008; 359:760.
- Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis— chicken or egg? N Engl J Med 2008; 359:1953–1955.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–1397.
- Kushner I, Sehgal AR. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch Intern Med 2002; 162:867–869.
- Hlatky MA. Expanding the orbit of primary prevention—moving beyond JUPITER. N Engl J Med 2008; 359:2280–2282.
- Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep 2004; 6:243–250.
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005; 25:1102–1111.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299:1265–1276.
- Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol 2008; 52:24–32.
- Hakonarson H, Thorvaldsson S, Helgadottir A, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA 2005; 293:2245–2256.
- Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol 2008; 51:1653–1662.
- Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2007; 27:134–140.
- Mukherjee D, Topol EJ. Pharmacogenomics in cardiovascular diseases. Curr Probl Cardiol 2003; 28:317–347
- West of Scotland Coronary Prevention Study: identification of highrisk groups and comparison with other cardiovascular intervention trials. Lancet 1996; 348:1339–1342.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol 2007; 49:1753–1762.
- Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf 2004; 3:547–557.
- Kasiske BL, Wanner C, O’Neill WC. An assessment of statin safety by nephrologists. Am J Cardiol 2006; 97:82C–85C.
- McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001; 87:28B–32B.
- Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol 2006; 97:44C–51C.
- Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol 2004; 94:1140–1146.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Topol EJ. Intensive statin therapy—a sea change in cardiovascular prevention. N Engl J Med 2004; 350:1562–1564.
- Cannon CP, Murphy SA, Braunwald E. Intensive lipid lowering with atorvastatin in coronary disease. N Engl J Med 2005; 353:93–96.
- Cohen DJ, Carrozza JP, Baim DS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999; 341:1853–1854.
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005; 46:1855–1862.
- Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001; 103:276–283.
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol 2005; 96:24F–33F.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003; 289:1675–1680.
- Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003; 108:426–431.
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–1719.
- Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med 2006; 73:760–766.
- Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344:1959–1965.
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998; 98:731–733.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001; 285:2481–2485.
- Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 2001; 47:403–411.
- Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med 2003; 70:634–640.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–979.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Ridker PM. Are statins anti-inflammatory? Issues in the design and conduct of the pravastatin inflammation C-reactive protein evaluation. Curr Cardiol Rep 2000; 2:269–273.
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107:391–397.
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107:499–511.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002; 56:53–56.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Liem AH, van Boven AJ, Veeger NJ, et al. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 2002; 23:1931–1937.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999; 341:70–76.
- Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of highdose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005; 46:1405–1410.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007; 28:664–672.
- Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med 2000; 45:391–418.
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103:1813–1818.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97:2007–2011.
- Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 2005; 112:25–31.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007; 49:2129–2138.
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286:64–70.
- Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100:230–235.
- Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res 2005; 96:714–716.
- Schwedler SB, Amann K, Wernicke K, et al. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation 2005; 112:1016–1023.
- Pfister R, Hellmich M. Multiple biomarkers and cardiovascular risk. N Engl J Med 2008; 359:760.
- Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis— chicken or egg? N Engl J Med 2008; 359:1953–1955.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–1397.
- Kushner I, Sehgal AR. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch Intern Med 2002; 162:867–869.
- Hlatky MA. Expanding the orbit of primary prevention—moving beyond JUPITER. N Engl J Med 2008; 359:2280–2282.
- Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep 2004; 6:243–250.
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005; 25:1102–1111.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299:1265–1276.
- Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol 2008; 52:24–32.
- Hakonarson H, Thorvaldsson S, Helgadottir A, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA 2005; 293:2245–2256.
- Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol 2008; 51:1653–1662.
- Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2007; 27:134–140.
- Mukherjee D, Topol EJ. Pharmacogenomics in cardiovascular diseases. Curr Probl Cardiol 2003; 28:317–347
- West of Scotland Coronary Prevention Study: identification of highrisk groups and comparison with other cardiovascular intervention trials. Lancet 1996; 348:1339–1342.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol 2007; 49:1753–1762.
- Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf 2004; 3:547–557.
- Kasiske BL, Wanner C, O’Neill WC. An assessment of statin safety by nephrologists. Am J Cardiol 2006; 97:82C–85C.
- McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001; 87:28B–32B.
- Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol 2006; 97:44C–51C.
- Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol 2004; 94:1140–1146.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
KEY POINTS
- LDL-C is the current gold standard diagnostic marker of risk, and elevated values should be aggressively treated in both primary and secondary prevention.
- The optional LDL-C goal of 70 mg/dL for patients at high risk may need to be extended to others at higher global risk, such as those with elevated hs-CRP.
- Although elevated hs-CRP may identify some people with low LDL-C who are nevertheless at higher global risk, more sensitive and specific markers of risk are needed.
Percutaneous treatment of aortic valve stenosis
Stenosis of the aortic valve has a long, latent, asymptomatic phase, but when symptoms finally occur, clinical deterioration can be rapid. For patients with severe stenosis, the standard treatment has long been replacement of the aortic valve via open heart surgery. But many patients with severe stenosis are considered too high-risk for this procedure.
Until about 5 years ago, these patients had no other option but medical therapy or percutaneous aortic balloon valvuloplasty as a palliative measure or as a bridge to open heart surgery. But 5 years of experience with percutaneous techniques to implant prosthetic aortic valves show that this less-invasive approach may become a viable option for patients with severe symptomatic aortic valve stenosis.
In this review, we discuss current prosthetic valves and percutaneous techniques and their relative advantages and limitations and the potential future role of this new treatment option.
THE NEED FOR A LESS-INVASIVE APPROACH
Calcific aortic stenosis is the most common valvular heart disease, affecting 2% to 4% of adults over age 65 in the United States alone.1,2 The aging of our population and the lack of drug therapies to prevent, halt, or effectively slow aortic valve stenosis are leading to a greater burden of this condition.1,3,4 Already in the United States more than 50,000 surgical aortic valve replacements are performed every year for severe aortic stenosis.1,2 The associated in-hospital death rate is 8.8% in patients over age 65 years, and as high as 13% in low-volume centers.1,5
The steady increase in the number of patients requiring aortic valve replacement, the high surgical risk in patients with multiple comorbidities, the reluctance of some patients to undergo the trauma and pain associated with open heart surgery via sternotomy, and the fact that percutaneous procedures are less traumatic and offer faster recovery and fewer hospital days—all these are forces that have been driving the development of percutaneous techniques for the treatment of aortic stenosis.6–11 In addition, a recent study12 showed that 33% of patients over age 75 were deemed too high-risk for open heart surgery and thus were left untreated.12
The evolution of percutaneous aortic valve replacement
The idea of percutaneous treatment of aortic stenosis was first put into clinical practice in 1985, when Cribier performed an aortic balloon valvuloplasty.6 This was followed in 200013 by the first successful implantation of a catheter-based stent valve in a human, and in 2002 by the first successful percutaneous aortic valve replacement in a human.13–15 In the following sections, we discuss the percutaneous approaches in current use for the treatment of degenerative aortic stenosis.
AORTIC BALLOON VALVULOPLASTY
Percutaneous aortic balloon valvuloplasty, partial dilation of the stenotic aortic valve with a balloon inserted via a catheter,1,16–19 improves symptoms but has failed to show a sustained benefit on rates of mortality or morbidity.1,16–18 The restenosis rate is high, and symptoms recur in most patients within months to a year.1,16–18 Procedural complication rates are about 10%, and complication rates at the catheter access site are even higher.1,16–18 The 30-day death rate in the National Heart, Lung, and Blood Institute’s Balloon Valvuloplasty Registry, which included more than 600 patients, was 14%.18 In a retrospective study of 212 patients who underwent single or repeat percutaneous aortic balloon valvuloplasty,20 the 1-year mortality rate was 36% for the entire cohort, with a median survival of 3 years. Patients who underwent a repeat procedure (33%) had 1-year mortality rate of 42%, compared with 16% in patients who did not undergo a repeat procedure.20
Percutaneous aortic balloon valvuloplasty serves best as palliative therapy in severely symptomatic patients, and as a bridge to surgery in hemodynamically unstable adult patients.21,22 Percutaneous aortic balloon valvuloplasty is not an option in patients who are good candidates for surgical valve replacement.1
PERCUTANEOUS AORTIC VALVE REPLACEMENT: THREE TECHNIQUES
The antegrade technique
The retrograde technique
In the retrograde (ie, transfemoral) technique, access to the femoral artery is gained and the catheter with the prosthetic aortic valve is advanced to the stenotic aortic valve.8,11,26,28–30 This approach is faster and technically easier than the antegrade approach, but it can be associated with injury to the aortofemoral vessels and with failure of the prosthesis to cross the aortic arch or the stenotic aortic valve.11,23,30
The transapical technique
In the transapical technique, the valve delivery system is inserted via a small incision made between the ribs. The apex of the left ventricle is punctured with a needle, and the prosthetic valve is positioned within the stenotic aortic valve.27,31–33 The main advantage of this approach is that it allows more direct access to the aortic valve and eliminates the need for a large peripheral vascular access site in patients with peripheral vascular disease, small tortuous vasculature, or a history of major vascular complications or vascular repairs.31–33 Potential disadvantages are related to the left ventricular apical puncture and include adverse ventricular remodeling, left ventricular aneurysm or pseudoaneurysm, pericardial complications, pneumothorax, malignant ventricular arrhythmias, coronary artery injury, and the need for general anesthesia and chest tubes.27,31–35
Common features of the three approaches
The three percutaneous approaches have certain final steps in common.11,23,30,33 The position of final deployment of the prosthetic valve is determined by the patient’s native valvular structure and anatomy and is optimized by using fluoroscopic imaging of the native aortic valve calcification as an anatomical marker, along with guidance from supra-aortic angiography and transesophageal echocardiography.11,23,30,33 Ideally, the aortic valve prosthesis is placed at mid-position in the patient’s aortic valve, taking care to not to impinge on the coronary ostia or to impede the motion of the anterior mitral leaflet.11,23,30,33 In all three procedures, the prosthesis is then deployed by maximally inflating, rapidly deflating, and immediately withdrawing the delivery balloon. This final step is carried out during temporary high-rate right ventricular apical pacing, which produces ventricular tachycardia at 180 to 220 beats/min for up to 10 seconds.11,23,30,33 This leads to an immediate decrease in stroke volume, resulting in minimal forward flow through the aortic valve, which in turn facilitates precise positioning of the prosthetic valve.
So far, only the Cribier-Edwards valve has been deployed via all three techniques. The CoreValve has been deployed only via the retrograde technique. The Edwards SAPIEN valve has been deployed with retrograde and transapical approaches (see www.edwards.com/Products/TranscatheterValves/SapienTHV.htm and www.corevalve.com for animations depicting these techniques).
EXPERIENCE WITH THE CRIBIER-EDWARDS VALVE
The Cribier-Edwards valve has three leaflets made from equine pericardial tissue sutured inside a balloon-expandable stainless steel 14-mm stent (Table 1).11,23,33 With the use of a specially designed mechanical crimping device, the aortic valve prosthesis is mounted over a 3-cm-long balloon catheter, expandable to a diameter of 22 to 26 mm (NuMed Inc, Hopkinton, NY).11,23,30,33
After this prosthesis was tested in animal models,14,15 a trial for compassionate use in humans was begun, called the Initial Registry of Endovascular Implantation of Valves in Europe (I-REVIVE) trial. This trial was later continued as the Registry of Endovascular Critical Aortic Stenosis Treatment (RECAST) trial.23 All patients were formally evaluated by two cardio-thoracic surgeons and were deemed inappropriate for surgical aortic valve replacement.23
The success rate with the antegrade percutaneous approach was 85% (23 of 27 patients) and 57% for the retrograde approach (4 of 7 patients).11,23,30–33 Procedural limitations were migration or embolization of the prosthetic valve, failure to cross the stenotic aortic valve, and paravalvular aortic regurgitation.23 Anatomic and functional success was evidenced by improvement in aortic valve area, increase in left ventricular ejection fraction, and improved New York Heart Association functional class, all of which were sustained at up to 24 months.23
Webb et al11 reported similar results with retrograde implantation of the Cribier-Edwards valve in a cohort of 50 patients.11 The main difference between the two studies was the expected occurrence of aortofemoral complications with the retrograde approach.11,26 Procedural success increased from 76% in the first 25 patients to 96% in the second 25, and the 30-day mortality rate fell from 16% to 8%, which reflected the learning curve. Importantly, no patients needed conversion to open surgery during the first 30 days, and at a median follow-up of 359 days 35 (81%) of 43 patients who underwent successful transcatheter aortic valve replacement were still alive.11 Additionally, significant improvement was noted in left ventricular ejection fraction, mitral regurgitation, and New York Heart Association functional class, and these improvements persisted at 1 year.11
Lichtenstein et al31 and Walther et al32 successfully implanted the Cribier-Edwards valve using the transapical approach in a very high-risk elderly population with poor functional class. All patients were deemed unsuitable for standard surgical valve replacement and also for percutaneous transfemoral aortic valve implantation because of severe aorto-iliac disease. In both studies, the short-term and mid-term results were encouraging.
These experiences with the Cribier-Edwards valve showed that device- and technique-related shortcomings could be addressed. To date, more than 500 percutaneous aortic valve replacement procedures have been done with the Cribier-Edwards valve worldwide, with a greater than 95% technical success rate in the latest cohorts.36 Importantly, use of a larger (26-mm) prosthetic valve has been associated with a lower rate of prosthetic valve migration or embolization, and with a significantly lower rate of paravalvular aortic regurgitation.11,23
EXPERIENCE WITH THE COREVALVE SYSTEM
The CoreValve ReValving system is based on retrograde implantation of the CoreValve prosthesis—a self-expanding aortic valve prosthesis composed of three bovine pericardial leaflets mounted and sutured within a self-expanding 50-mm-long nitinol stent (Table 1).28–30 The inner diameter is 21 to 22 mm.28–30 This prosthesis has three distinct structural segments.28–30 The bottom portion exerts a high radial force that expands and pushes aside the calcified leaflets and avoids recoil; the central portion carries the valve, and it tapers to avoid the coronary artery ostia; and the upper portion flares to fixate and stabilize the deployed aortic valve prosthesis in the ascending aorta, thus preventing migration or embolization of the device.28–30 The main difference between the CoreValve and the Cribier-Edwards valve is that the Core-Valve is self-expanding, which theoretically permits it to conform to different aortic sizes and to anchor well in the aortic annulus.28–30 This feature allows the CoreValve to be used in patients with severe aortic insufficiency and other noncalcific aortic valvular conditions. The CoreValve has not yet been deployed via antegrade or transapical technique.
The first-generation CoreValve prosthesis was first implanted in a human recipient in 2005.29 Since then, improvements have been made, leading to the development of second- and third-generation devices. A pilot study of implantation of the first-generation CoreValve28 via the retrograde approach in elderly patients with poor functional class and severe aortic stenosis had a short-term procedural success rate of 84% (21 of 25 patients), with a significant reduction in the mean aortic valve gradient and improved functional class at 30-day follow-up.28 At 30 days, 17 (94%) of 18 patients had no or only mild aortic regurgitation.28 Procedural limitations and complications were similar to those with the Cribier-Edwards valve.
In a study of second- and third-generation devices (50 patients received a second-generation device, and 36 received a third-generation device),30 again in elderly patients with poor functional class and severe aortic stenosis, the short-term success rate of the device was 88% (76 of 86) in each group. After the procedure, the mean aortic valve gradient decreased significantly and functional class improved significantly.30 Immediate after implantation, no patient had more than moderate aortic regurgitation, and in 51 patients (66%) the aortic regurgitation remained unchanged or improved after CoreValve implantation.30 These results were maintained at 30-day follow-up.
CoreValve was approved in May 2007 for clinical use in Europe.36 Of note, CoreValve has also been used to treat severe aortic regurgitation of a degenerated bioprosthetic aortic valve in an 80-year-old man with multiple comorbidities.37
EXPERIENCE WITH THE EDWARDS SAPIEN VALVE
The Edwards SAPIEN valve is a modification of the initial Cribier-Edwards valve and is the latest percutaneous aortic valve prosthesis to enter clinical trials (Table 1). It is a trileaflet balloon-expandable stainless steel valve made from bovine pericardial tissue, available in two sizes (23 mm and 26 mm). In September 2007, it was approved for use in Europe with the RetroFlex transfemoral delivery system. The Ascendra transapical delivery system for the Edward SAPIEN valve has received approval in Europe.
The multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial in North America is continuing to enroll patients, with enrollment projected to be complete by the end of 2008. The aim of this prospective randomized clinical trial is to enroll 1,040 patients in two separate treatment arms. The surgical arm of the trial is comparing the Edwards SAPIEN valve with standard surgical aortic valve replacement, with the objective of demonstrating non-inferiority. The medical management arm of the trial is comparing percutaneous valve replacement against medical therapy or balloon valvuloplasty in patients considered too high-risk for conventional surgical valve replacement.
The primary end point in both arms is death at 1 year; secondary end points focus on long-term (1-year) composite cardiovascular events, valve performance, and quality-of-life indicators. Preliminary data on the first 100 patients (74 via the transfemoral [ie, retrograde] and 26 via the transapical approach) who underwent percutaneous Edwards SAPIEN valve implantation for compassionate use showed device durability and symptom relief at up to 2 years.38 Overall procedural success was 91%, but, as with other trials, there was a steep learning curve, so that excluding the first 25 patients increased the procedural success rate to 96%.38 Aortic valve size and hemodynamics, left ventricular systolic function, mitral regurgitation, and functional class were all significantly improved. Mild aortic regurgitation was common, but none of the patients had severe aortic regurgitation. Importantly, the 15% 30-day death rate was significantly lower than the expected rate of 33%. The 6-month survival rate was 78%, but the 2-year rate was 60% in this high-risk elderly cohort.
Walther et al39 recently reported outcomes on their first 50 patients who underwent transapical implantation of the Edwards SAPIEN valve. The operators were able to implant the prosthesis in all 50 patients, but 3 required early conversion to open surgery with sternotomy. The overall survival at 30 days was 92%, but in the last 25 patients the 30-day survival rate was 96%, with a 1-year survival rate of 80%.
PUTTING THE DATA IN PERSPECTIVE
As noted in this review, a number of factors make a strong case for timely aortic valve replacement: the aging population, the increase in incidence and prevalence of aortic stenosis,1,3,4,27,40 the multiple comorbidities in older patients, and the eventually aggressive natural course of aortic stenosis.1,3,4,27,40–43 Yet current standards dictate not to proceed with standard surgical aortic valve replacement in patients who are truly asymptomatic and who have normal left ventricular systolic function,1,40 mainly because the risks of surgical valve replacement outweigh the benefits in this population.1,40 Aortic valve surgery carries a risk of early death of 15% for patients ages 80 to 84 and of 18% for patients age 85.3,9,10,12,43–45
These figures seem high when compared with death rates of 12% in recent studies of percutaneous valve replacement in similar patients.11,23,30,33 The rates become lower as the learning curve improves.11,21,23,27,30,33 Thus, as the design of aortic valve prostheses and the techniques to implant them are refined and tested for safety, the risk-benefit balance may change in favor of earlier intervention in aortic stenosis with a percutaneous approach.11,21,27,46 Some experts believe that in 10 years 10% to 30% of patients undergoing conventional valve replacement will be candidates for a percutaneous approach.
Of the techniques used to date, the retrograde approach seems most amenable to widespread acceptance, given its inherent advantage of being faster and easier.11,21,30 Limitations with the retrograde approach seen in earlier trials—challenges and complications associated with large-bore arterial vascular access, difficulty traversing the aortic arch with bulky devices, and the inability to cross the stenotic aortic valve to deploy the prosthesis even after balloon valvuloplasty11,21,30—are correctable with refinements in the devices and in technique.
New types of prosthetic aortic valves entering early human studies are improving on current devices, for example, by using collapsible, inflatable valve frames for retrievability before final deployment.
Surgical aortic valve replacement remains the gold standard treatment for patients with symptomatic aortic stenosis. And while studies of percutaneous aortic valve replacement show great promise for this less-invasive treat-men, enthusiasm about percutaneous aortic valve replacement should be tempered by an awareness of persistent limitations of this approach, such as vascular and mechanical complications and operator inexperience, which still need attention.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–231.
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005; 111:3316–3326.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg 2003; 76:1131–1137.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Vahanian A, Palacios IF. Percutaneous approaches to valvular disease. Circulation 2004; 109:1572–1579.
- Webb JG, Munt B, Makkar RR, Naqvi TZ, Dang N. Percutaneous stent-mounted valve for treatment of aortic or pulmonary valve disease. Catheter Cardiovasc Interv 2004; 63:89–93.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients =80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Mittermair RP, Muller LC. Quality of life after cardiac surgery in the elderly. J Cardiovasc Surg (Torino) 2002; 43:43–47.
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000; 356:1403–1405.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704–708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med 1988; 319:125–130.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Safian RD, Mandell VS, Thurer RE, et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J Am Coll Cardiol 1987; 9:655–660.
- Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol 2005; 95:43–47.
- Kapadia SR, Wazni OM, Tan WA, et al. Aortic valvuloplasty in 1990's: experience from a single center in United States. Circulation 1999; 100 18 suppl 1:1–448.
- Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol 1995; 26:1522–1528.
- Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006; 47:1214–1223.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698–703.
- Rajagopal V, Kapadia SR, Tuzcu EM. Advances in the percutaneous treatment of aortic and mitral valve disease. Minerva Cardioangiol 2007; 55:83–94.
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006; 113:842–850.
- Salemi A. Percutaneous valve interventions. Curr Opin Anaesthesiol 2007; 20:70–74.
- Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006; 114:1616–1624.
- Grube E, Laborde JC, Zickmann B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv 2005; 66:465–469.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation—proof of concept. Eur J Cardiothorac Surg 2007; 31:9–15.
- Ye J, Cheung A, Lichtenstein SV, et al. Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur J Cardiothorac Surg 2007; 31:16–21.
- Turgut T, Deeb M, Moscucci M. Left ventricular apical puncture: a procedure surviving well into the new millennium. Catheter Cardiovasc Interv 2000; 49:68–73.
- Zuguchi M, Shindoh C, Chida K, et al. Safety and clinical benefits of transsubxiphoidal left ventricular puncture. Catheter Cardiovasc Interv 2002; 55:58–65.
- Sinha AK, Kini AS, Sharma SK. Percutaneous valve replacement: a paradigm shift. Curr Opin Cardiol 2007; 22:471–477.
- Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the CoreValve ReValving system. Catheter Cardiovasc Interv 2007; 70:760–764.
- Pasupati S, Humphries K, AlAli A, et al. Balloon expandable aortic valve (BEAV) implantation. The first 100 Canadian patients. Circulation 2007; 116 suppl:357.
- Walther T, Falk V, Kempfert J, et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 2008; 33:983–988. Epub 2008 February 21.
- Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002; 346:677–682.
- Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15:1012–1017.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38:61–67.
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000; 35:747–756.
- Society of Thoracic Surgeons National Cardiac Surgery Database. Available at www.sts.org/documents/pdf/Spring2005STS-ExecutiveSummary.pdf. Accessed 9/11/2008.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346:1128–1137.
- Wenger NK, Weber MA, Scheidt S. Valvular heart disease at elderly age: new vistas. Am J Geriatr Cardiol 2006; 15:273–274.
Stenosis of the aortic valve has a long, latent, asymptomatic phase, but when symptoms finally occur, clinical deterioration can be rapid. For patients with severe stenosis, the standard treatment has long been replacement of the aortic valve via open heart surgery. But many patients with severe stenosis are considered too high-risk for this procedure.
Until about 5 years ago, these patients had no other option but medical therapy or percutaneous aortic balloon valvuloplasty as a palliative measure or as a bridge to open heart surgery. But 5 years of experience with percutaneous techniques to implant prosthetic aortic valves show that this less-invasive approach may become a viable option for patients with severe symptomatic aortic valve stenosis.
In this review, we discuss current prosthetic valves and percutaneous techniques and their relative advantages and limitations and the potential future role of this new treatment option.
THE NEED FOR A LESS-INVASIVE APPROACH
Calcific aortic stenosis is the most common valvular heart disease, affecting 2% to 4% of adults over age 65 in the United States alone.1,2 The aging of our population and the lack of drug therapies to prevent, halt, or effectively slow aortic valve stenosis are leading to a greater burden of this condition.1,3,4 Already in the United States more than 50,000 surgical aortic valve replacements are performed every year for severe aortic stenosis.1,2 The associated in-hospital death rate is 8.8% in patients over age 65 years, and as high as 13% in low-volume centers.1,5
The steady increase in the number of patients requiring aortic valve replacement, the high surgical risk in patients with multiple comorbidities, the reluctance of some patients to undergo the trauma and pain associated with open heart surgery via sternotomy, and the fact that percutaneous procedures are less traumatic and offer faster recovery and fewer hospital days—all these are forces that have been driving the development of percutaneous techniques for the treatment of aortic stenosis.6–11 In addition, a recent study12 showed that 33% of patients over age 75 were deemed too high-risk for open heart surgery and thus were left untreated.12
The evolution of percutaneous aortic valve replacement
The idea of percutaneous treatment of aortic stenosis was first put into clinical practice in 1985, when Cribier performed an aortic balloon valvuloplasty.6 This was followed in 200013 by the first successful implantation of a catheter-based stent valve in a human, and in 2002 by the first successful percutaneous aortic valve replacement in a human.13–15 In the following sections, we discuss the percutaneous approaches in current use for the treatment of degenerative aortic stenosis.
AORTIC BALLOON VALVULOPLASTY
Percutaneous aortic balloon valvuloplasty, partial dilation of the stenotic aortic valve with a balloon inserted via a catheter,1,16–19 improves symptoms but has failed to show a sustained benefit on rates of mortality or morbidity.1,16–18 The restenosis rate is high, and symptoms recur in most patients within months to a year.1,16–18 Procedural complication rates are about 10%, and complication rates at the catheter access site are even higher.1,16–18 The 30-day death rate in the National Heart, Lung, and Blood Institute’s Balloon Valvuloplasty Registry, which included more than 600 patients, was 14%.18 In a retrospective study of 212 patients who underwent single or repeat percutaneous aortic balloon valvuloplasty,20 the 1-year mortality rate was 36% for the entire cohort, with a median survival of 3 years. Patients who underwent a repeat procedure (33%) had 1-year mortality rate of 42%, compared with 16% in patients who did not undergo a repeat procedure.20
Percutaneous aortic balloon valvuloplasty serves best as palliative therapy in severely symptomatic patients, and as a bridge to surgery in hemodynamically unstable adult patients.21,22 Percutaneous aortic balloon valvuloplasty is not an option in patients who are good candidates for surgical valve replacement.1
PERCUTANEOUS AORTIC VALVE REPLACEMENT: THREE TECHNIQUES
The antegrade technique
The retrograde technique
In the retrograde (ie, transfemoral) technique, access to the femoral artery is gained and the catheter with the prosthetic aortic valve is advanced to the stenotic aortic valve.8,11,26,28–30 This approach is faster and technically easier than the antegrade approach, but it can be associated with injury to the aortofemoral vessels and with failure of the prosthesis to cross the aortic arch or the stenotic aortic valve.11,23,30
The transapical technique
In the transapical technique, the valve delivery system is inserted via a small incision made between the ribs. The apex of the left ventricle is punctured with a needle, and the prosthetic valve is positioned within the stenotic aortic valve.27,31–33 The main advantage of this approach is that it allows more direct access to the aortic valve and eliminates the need for a large peripheral vascular access site in patients with peripheral vascular disease, small tortuous vasculature, or a history of major vascular complications or vascular repairs.31–33 Potential disadvantages are related to the left ventricular apical puncture and include adverse ventricular remodeling, left ventricular aneurysm or pseudoaneurysm, pericardial complications, pneumothorax, malignant ventricular arrhythmias, coronary artery injury, and the need for general anesthesia and chest tubes.27,31–35
Common features of the three approaches
The three percutaneous approaches have certain final steps in common.11,23,30,33 The position of final deployment of the prosthetic valve is determined by the patient’s native valvular structure and anatomy and is optimized by using fluoroscopic imaging of the native aortic valve calcification as an anatomical marker, along with guidance from supra-aortic angiography and transesophageal echocardiography.11,23,30,33 Ideally, the aortic valve prosthesis is placed at mid-position in the patient’s aortic valve, taking care to not to impinge on the coronary ostia or to impede the motion of the anterior mitral leaflet.11,23,30,33 In all three procedures, the prosthesis is then deployed by maximally inflating, rapidly deflating, and immediately withdrawing the delivery balloon. This final step is carried out during temporary high-rate right ventricular apical pacing, which produces ventricular tachycardia at 180 to 220 beats/min for up to 10 seconds.11,23,30,33 This leads to an immediate decrease in stroke volume, resulting in minimal forward flow through the aortic valve, which in turn facilitates precise positioning of the prosthetic valve.
So far, only the Cribier-Edwards valve has been deployed via all three techniques. The CoreValve has been deployed only via the retrograde technique. The Edwards SAPIEN valve has been deployed with retrograde and transapical approaches (see www.edwards.com/Products/TranscatheterValves/SapienTHV.htm and www.corevalve.com for animations depicting these techniques).
EXPERIENCE WITH THE CRIBIER-EDWARDS VALVE
The Cribier-Edwards valve has three leaflets made from equine pericardial tissue sutured inside a balloon-expandable stainless steel 14-mm stent (Table 1).11,23,33 With the use of a specially designed mechanical crimping device, the aortic valve prosthesis is mounted over a 3-cm-long balloon catheter, expandable to a diameter of 22 to 26 mm (NuMed Inc, Hopkinton, NY).11,23,30,33
After this prosthesis was tested in animal models,14,15 a trial for compassionate use in humans was begun, called the Initial Registry of Endovascular Implantation of Valves in Europe (I-REVIVE) trial. This trial was later continued as the Registry of Endovascular Critical Aortic Stenosis Treatment (RECAST) trial.23 All patients were formally evaluated by two cardio-thoracic surgeons and were deemed inappropriate for surgical aortic valve replacement.23
The success rate with the antegrade percutaneous approach was 85% (23 of 27 patients) and 57% for the retrograde approach (4 of 7 patients).11,23,30–33 Procedural limitations were migration or embolization of the prosthetic valve, failure to cross the stenotic aortic valve, and paravalvular aortic regurgitation.23 Anatomic and functional success was evidenced by improvement in aortic valve area, increase in left ventricular ejection fraction, and improved New York Heart Association functional class, all of which were sustained at up to 24 months.23
Webb et al11 reported similar results with retrograde implantation of the Cribier-Edwards valve in a cohort of 50 patients.11 The main difference between the two studies was the expected occurrence of aortofemoral complications with the retrograde approach.11,26 Procedural success increased from 76% in the first 25 patients to 96% in the second 25, and the 30-day mortality rate fell from 16% to 8%, which reflected the learning curve. Importantly, no patients needed conversion to open surgery during the first 30 days, and at a median follow-up of 359 days 35 (81%) of 43 patients who underwent successful transcatheter aortic valve replacement were still alive.11 Additionally, significant improvement was noted in left ventricular ejection fraction, mitral regurgitation, and New York Heart Association functional class, and these improvements persisted at 1 year.11
Lichtenstein et al31 and Walther et al32 successfully implanted the Cribier-Edwards valve using the transapical approach in a very high-risk elderly population with poor functional class. All patients were deemed unsuitable for standard surgical valve replacement and also for percutaneous transfemoral aortic valve implantation because of severe aorto-iliac disease. In both studies, the short-term and mid-term results were encouraging.
These experiences with the Cribier-Edwards valve showed that device- and technique-related shortcomings could be addressed. To date, more than 500 percutaneous aortic valve replacement procedures have been done with the Cribier-Edwards valve worldwide, with a greater than 95% technical success rate in the latest cohorts.36 Importantly, use of a larger (26-mm) prosthetic valve has been associated with a lower rate of prosthetic valve migration or embolization, and with a significantly lower rate of paravalvular aortic regurgitation.11,23
EXPERIENCE WITH THE COREVALVE SYSTEM
The CoreValve ReValving system is based on retrograde implantation of the CoreValve prosthesis—a self-expanding aortic valve prosthesis composed of three bovine pericardial leaflets mounted and sutured within a self-expanding 50-mm-long nitinol stent (Table 1).28–30 The inner diameter is 21 to 22 mm.28–30 This prosthesis has three distinct structural segments.28–30 The bottom portion exerts a high radial force that expands and pushes aside the calcified leaflets and avoids recoil; the central portion carries the valve, and it tapers to avoid the coronary artery ostia; and the upper portion flares to fixate and stabilize the deployed aortic valve prosthesis in the ascending aorta, thus preventing migration or embolization of the device.28–30 The main difference between the CoreValve and the Cribier-Edwards valve is that the Core-Valve is self-expanding, which theoretically permits it to conform to different aortic sizes and to anchor well in the aortic annulus.28–30 This feature allows the CoreValve to be used in patients with severe aortic insufficiency and other noncalcific aortic valvular conditions. The CoreValve has not yet been deployed via antegrade or transapical technique.
The first-generation CoreValve prosthesis was first implanted in a human recipient in 2005.29 Since then, improvements have been made, leading to the development of second- and third-generation devices. A pilot study of implantation of the first-generation CoreValve28 via the retrograde approach in elderly patients with poor functional class and severe aortic stenosis had a short-term procedural success rate of 84% (21 of 25 patients), with a significant reduction in the mean aortic valve gradient and improved functional class at 30-day follow-up.28 At 30 days, 17 (94%) of 18 patients had no or only mild aortic regurgitation.28 Procedural limitations and complications were similar to those with the Cribier-Edwards valve.
In a study of second- and third-generation devices (50 patients received a second-generation device, and 36 received a third-generation device),30 again in elderly patients with poor functional class and severe aortic stenosis, the short-term success rate of the device was 88% (76 of 86) in each group. After the procedure, the mean aortic valve gradient decreased significantly and functional class improved significantly.30 Immediate after implantation, no patient had more than moderate aortic regurgitation, and in 51 patients (66%) the aortic regurgitation remained unchanged or improved after CoreValve implantation.30 These results were maintained at 30-day follow-up.
CoreValve was approved in May 2007 for clinical use in Europe.36 Of note, CoreValve has also been used to treat severe aortic regurgitation of a degenerated bioprosthetic aortic valve in an 80-year-old man with multiple comorbidities.37
EXPERIENCE WITH THE EDWARDS SAPIEN VALVE
The Edwards SAPIEN valve is a modification of the initial Cribier-Edwards valve and is the latest percutaneous aortic valve prosthesis to enter clinical trials (Table 1). It is a trileaflet balloon-expandable stainless steel valve made from bovine pericardial tissue, available in two sizes (23 mm and 26 mm). In September 2007, it was approved for use in Europe with the RetroFlex transfemoral delivery system. The Ascendra transapical delivery system for the Edward SAPIEN valve has received approval in Europe.
The multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial in North America is continuing to enroll patients, with enrollment projected to be complete by the end of 2008. The aim of this prospective randomized clinical trial is to enroll 1,040 patients in two separate treatment arms. The surgical arm of the trial is comparing the Edwards SAPIEN valve with standard surgical aortic valve replacement, with the objective of demonstrating non-inferiority. The medical management arm of the trial is comparing percutaneous valve replacement against medical therapy or balloon valvuloplasty in patients considered too high-risk for conventional surgical valve replacement.
The primary end point in both arms is death at 1 year; secondary end points focus on long-term (1-year) composite cardiovascular events, valve performance, and quality-of-life indicators. Preliminary data on the first 100 patients (74 via the transfemoral [ie, retrograde] and 26 via the transapical approach) who underwent percutaneous Edwards SAPIEN valve implantation for compassionate use showed device durability and symptom relief at up to 2 years.38 Overall procedural success was 91%, but, as with other trials, there was a steep learning curve, so that excluding the first 25 patients increased the procedural success rate to 96%.38 Aortic valve size and hemodynamics, left ventricular systolic function, mitral regurgitation, and functional class were all significantly improved. Mild aortic regurgitation was common, but none of the patients had severe aortic regurgitation. Importantly, the 15% 30-day death rate was significantly lower than the expected rate of 33%. The 6-month survival rate was 78%, but the 2-year rate was 60% in this high-risk elderly cohort.
Walther et al39 recently reported outcomes on their first 50 patients who underwent transapical implantation of the Edwards SAPIEN valve. The operators were able to implant the prosthesis in all 50 patients, but 3 required early conversion to open surgery with sternotomy. The overall survival at 30 days was 92%, but in the last 25 patients the 30-day survival rate was 96%, with a 1-year survival rate of 80%.
PUTTING THE DATA IN PERSPECTIVE
As noted in this review, a number of factors make a strong case for timely aortic valve replacement: the aging population, the increase in incidence and prevalence of aortic stenosis,1,3,4,27,40 the multiple comorbidities in older patients, and the eventually aggressive natural course of aortic stenosis.1,3,4,27,40–43 Yet current standards dictate not to proceed with standard surgical aortic valve replacement in patients who are truly asymptomatic and who have normal left ventricular systolic function,1,40 mainly because the risks of surgical valve replacement outweigh the benefits in this population.1,40 Aortic valve surgery carries a risk of early death of 15% for patients ages 80 to 84 and of 18% for patients age 85.3,9,10,12,43–45
These figures seem high when compared with death rates of 12% in recent studies of percutaneous valve replacement in similar patients.11,23,30,33 The rates become lower as the learning curve improves.11,21,23,27,30,33 Thus, as the design of aortic valve prostheses and the techniques to implant them are refined and tested for safety, the risk-benefit balance may change in favor of earlier intervention in aortic stenosis with a percutaneous approach.11,21,27,46 Some experts believe that in 10 years 10% to 30% of patients undergoing conventional valve replacement will be candidates for a percutaneous approach.
Of the techniques used to date, the retrograde approach seems most amenable to widespread acceptance, given its inherent advantage of being faster and easier.11,21,30 Limitations with the retrograde approach seen in earlier trials—challenges and complications associated with large-bore arterial vascular access, difficulty traversing the aortic arch with bulky devices, and the inability to cross the stenotic aortic valve to deploy the prosthesis even after balloon valvuloplasty11,21,30—are correctable with refinements in the devices and in technique.
New types of prosthetic aortic valves entering early human studies are improving on current devices, for example, by using collapsible, inflatable valve frames for retrievability before final deployment.
Surgical aortic valve replacement remains the gold standard treatment for patients with symptomatic aortic stenosis. And while studies of percutaneous aortic valve replacement show great promise for this less-invasive treat-men, enthusiasm about percutaneous aortic valve replacement should be tempered by an awareness of persistent limitations of this approach, such as vascular and mechanical complications and operator inexperience, which still need attention.
Stenosis of the aortic valve has a long, latent, asymptomatic phase, but when symptoms finally occur, clinical deterioration can be rapid. For patients with severe stenosis, the standard treatment has long been replacement of the aortic valve via open heart surgery. But many patients with severe stenosis are considered too high-risk for this procedure.
Until about 5 years ago, these patients had no other option but medical therapy or percutaneous aortic balloon valvuloplasty as a palliative measure or as a bridge to open heart surgery. But 5 years of experience with percutaneous techniques to implant prosthetic aortic valves show that this less-invasive approach may become a viable option for patients with severe symptomatic aortic valve stenosis.
In this review, we discuss current prosthetic valves and percutaneous techniques and their relative advantages and limitations and the potential future role of this new treatment option.
THE NEED FOR A LESS-INVASIVE APPROACH
Calcific aortic stenosis is the most common valvular heart disease, affecting 2% to 4% of adults over age 65 in the United States alone.1,2 The aging of our population and the lack of drug therapies to prevent, halt, or effectively slow aortic valve stenosis are leading to a greater burden of this condition.1,3,4 Already in the United States more than 50,000 surgical aortic valve replacements are performed every year for severe aortic stenosis.1,2 The associated in-hospital death rate is 8.8% in patients over age 65 years, and as high as 13% in low-volume centers.1,5
The steady increase in the number of patients requiring aortic valve replacement, the high surgical risk in patients with multiple comorbidities, the reluctance of some patients to undergo the trauma and pain associated with open heart surgery via sternotomy, and the fact that percutaneous procedures are less traumatic and offer faster recovery and fewer hospital days—all these are forces that have been driving the development of percutaneous techniques for the treatment of aortic stenosis.6–11 In addition, a recent study12 showed that 33% of patients over age 75 were deemed too high-risk for open heart surgery and thus were left untreated.12
The evolution of percutaneous aortic valve replacement
The idea of percutaneous treatment of aortic stenosis was first put into clinical practice in 1985, when Cribier performed an aortic balloon valvuloplasty.6 This was followed in 200013 by the first successful implantation of a catheter-based stent valve in a human, and in 2002 by the first successful percutaneous aortic valve replacement in a human.13–15 In the following sections, we discuss the percutaneous approaches in current use for the treatment of degenerative aortic stenosis.
AORTIC BALLOON VALVULOPLASTY
Percutaneous aortic balloon valvuloplasty, partial dilation of the stenotic aortic valve with a balloon inserted via a catheter,1,16–19 improves symptoms but has failed to show a sustained benefit on rates of mortality or morbidity.1,16–18 The restenosis rate is high, and symptoms recur in most patients within months to a year.1,16–18 Procedural complication rates are about 10%, and complication rates at the catheter access site are even higher.1,16–18 The 30-day death rate in the National Heart, Lung, and Blood Institute’s Balloon Valvuloplasty Registry, which included more than 600 patients, was 14%.18 In a retrospective study of 212 patients who underwent single or repeat percutaneous aortic balloon valvuloplasty,20 the 1-year mortality rate was 36% for the entire cohort, with a median survival of 3 years. Patients who underwent a repeat procedure (33%) had 1-year mortality rate of 42%, compared with 16% in patients who did not undergo a repeat procedure.20
Percutaneous aortic balloon valvuloplasty serves best as palliative therapy in severely symptomatic patients, and as a bridge to surgery in hemodynamically unstable adult patients.21,22 Percutaneous aortic balloon valvuloplasty is not an option in patients who are good candidates for surgical valve replacement.1
PERCUTANEOUS AORTIC VALVE REPLACEMENT: THREE TECHNIQUES
The antegrade technique
The retrograde technique
In the retrograde (ie, transfemoral) technique, access to the femoral artery is gained and the catheter with the prosthetic aortic valve is advanced to the stenotic aortic valve.8,11,26,28–30 This approach is faster and technically easier than the antegrade approach, but it can be associated with injury to the aortofemoral vessels and with failure of the prosthesis to cross the aortic arch or the stenotic aortic valve.11,23,30
The transapical technique
In the transapical technique, the valve delivery system is inserted via a small incision made between the ribs. The apex of the left ventricle is punctured with a needle, and the prosthetic valve is positioned within the stenotic aortic valve.27,31–33 The main advantage of this approach is that it allows more direct access to the aortic valve and eliminates the need for a large peripheral vascular access site in patients with peripheral vascular disease, small tortuous vasculature, or a history of major vascular complications or vascular repairs.31–33 Potential disadvantages are related to the left ventricular apical puncture and include adverse ventricular remodeling, left ventricular aneurysm or pseudoaneurysm, pericardial complications, pneumothorax, malignant ventricular arrhythmias, coronary artery injury, and the need for general anesthesia and chest tubes.27,31–35
Common features of the three approaches
The three percutaneous approaches have certain final steps in common.11,23,30,33 The position of final deployment of the prosthetic valve is determined by the patient’s native valvular structure and anatomy and is optimized by using fluoroscopic imaging of the native aortic valve calcification as an anatomical marker, along with guidance from supra-aortic angiography and transesophageal echocardiography.11,23,30,33 Ideally, the aortic valve prosthesis is placed at mid-position in the patient’s aortic valve, taking care to not to impinge on the coronary ostia or to impede the motion of the anterior mitral leaflet.11,23,30,33 In all three procedures, the prosthesis is then deployed by maximally inflating, rapidly deflating, and immediately withdrawing the delivery balloon. This final step is carried out during temporary high-rate right ventricular apical pacing, which produces ventricular tachycardia at 180 to 220 beats/min for up to 10 seconds.11,23,30,33 This leads to an immediate decrease in stroke volume, resulting in minimal forward flow through the aortic valve, which in turn facilitates precise positioning of the prosthetic valve.
So far, only the Cribier-Edwards valve has been deployed via all three techniques. The CoreValve has been deployed only via the retrograde technique. The Edwards SAPIEN valve has been deployed with retrograde and transapical approaches (see www.edwards.com/Products/TranscatheterValves/SapienTHV.htm and www.corevalve.com for animations depicting these techniques).
EXPERIENCE WITH THE CRIBIER-EDWARDS VALVE
The Cribier-Edwards valve has three leaflets made from equine pericardial tissue sutured inside a balloon-expandable stainless steel 14-mm stent (Table 1).11,23,33 With the use of a specially designed mechanical crimping device, the aortic valve prosthesis is mounted over a 3-cm-long balloon catheter, expandable to a diameter of 22 to 26 mm (NuMed Inc, Hopkinton, NY).11,23,30,33
After this prosthesis was tested in animal models,14,15 a trial for compassionate use in humans was begun, called the Initial Registry of Endovascular Implantation of Valves in Europe (I-REVIVE) trial. This trial was later continued as the Registry of Endovascular Critical Aortic Stenosis Treatment (RECAST) trial.23 All patients were formally evaluated by two cardio-thoracic surgeons and were deemed inappropriate for surgical aortic valve replacement.23
The success rate with the antegrade percutaneous approach was 85% (23 of 27 patients) and 57% for the retrograde approach (4 of 7 patients).11,23,30–33 Procedural limitations were migration or embolization of the prosthetic valve, failure to cross the stenotic aortic valve, and paravalvular aortic regurgitation.23 Anatomic and functional success was evidenced by improvement in aortic valve area, increase in left ventricular ejection fraction, and improved New York Heart Association functional class, all of which were sustained at up to 24 months.23
Webb et al11 reported similar results with retrograde implantation of the Cribier-Edwards valve in a cohort of 50 patients.11 The main difference between the two studies was the expected occurrence of aortofemoral complications with the retrograde approach.11,26 Procedural success increased from 76% in the first 25 patients to 96% in the second 25, and the 30-day mortality rate fell from 16% to 8%, which reflected the learning curve. Importantly, no patients needed conversion to open surgery during the first 30 days, and at a median follow-up of 359 days 35 (81%) of 43 patients who underwent successful transcatheter aortic valve replacement were still alive.11 Additionally, significant improvement was noted in left ventricular ejection fraction, mitral regurgitation, and New York Heart Association functional class, and these improvements persisted at 1 year.11
Lichtenstein et al31 and Walther et al32 successfully implanted the Cribier-Edwards valve using the transapical approach in a very high-risk elderly population with poor functional class. All patients were deemed unsuitable for standard surgical valve replacement and also for percutaneous transfemoral aortic valve implantation because of severe aorto-iliac disease. In both studies, the short-term and mid-term results were encouraging.
These experiences with the Cribier-Edwards valve showed that device- and technique-related shortcomings could be addressed. To date, more than 500 percutaneous aortic valve replacement procedures have been done with the Cribier-Edwards valve worldwide, with a greater than 95% technical success rate in the latest cohorts.36 Importantly, use of a larger (26-mm) prosthetic valve has been associated with a lower rate of prosthetic valve migration or embolization, and with a significantly lower rate of paravalvular aortic regurgitation.11,23
EXPERIENCE WITH THE COREVALVE SYSTEM
The CoreValve ReValving system is based on retrograde implantation of the CoreValve prosthesis—a self-expanding aortic valve prosthesis composed of three bovine pericardial leaflets mounted and sutured within a self-expanding 50-mm-long nitinol stent (Table 1).28–30 The inner diameter is 21 to 22 mm.28–30 This prosthesis has three distinct structural segments.28–30 The bottom portion exerts a high radial force that expands and pushes aside the calcified leaflets and avoids recoil; the central portion carries the valve, and it tapers to avoid the coronary artery ostia; and the upper portion flares to fixate and stabilize the deployed aortic valve prosthesis in the ascending aorta, thus preventing migration or embolization of the device.28–30 The main difference between the CoreValve and the Cribier-Edwards valve is that the Core-Valve is self-expanding, which theoretically permits it to conform to different aortic sizes and to anchor well in the aortic annulus.28–30 This feature allows the CoreValve to be used in patients with severe aortic insufficiency and other noncalcific aortic valvular conditions. The CoreValve has not yet been deployed via antegrade or transapical technique.
The first-generation CoreValve prosthesis was first implanted in a human recipient in 2005.29 Since then, improvements have been made, leading to the development of second- and third-generation devices. A pilot study of implantation of the first-generation CoreValve28 via the retrograde approach in elderly patients with poor functional class and severe aortic stenosis had a short-term procedural success rate of 84% (21 of 25 patients), with a significant reduction in the mean aortic valve gradient and improved functional class at 30-day follow-up.28 At 30 days, 17 (94%) of 18 patients had no or only mild aortic regurgitation.28 Procedural limitations and complications were similar to those with the Cribier-Edwards valve.
In a study of second- and third-generation devices (50 patients received a second-generation device, and 36 received a third-generation device),30 again in elderly patients with poor functional class and severe aortic stenosis, the short-term success rate of the device was 88% (76 of 86) in each group. After the procedure, the mean aortic valve gradient decreased significantly and functional class improved significantly.30 Immediate after implantation, no patient had more than moderate aortic regurgitation, and in 51 patients (66%) the aortic regurgitation remained unchanged or improved after CoreValve implantation.30 These results were maintained at 30-day follow-up.
CoreValve was approved in May 2007 for clinical use in Europe.36 Of note, CoreValve has also been used to treat severe aortic regurgitation of a degenerated bioprosthetic aortic valve in an 80-year-old man with multiple comorbidities.37
EXPERIENCE WITH THE EDWARDS SAPIEN VALVE
The Edwards SAPIEN valve is a modification of the initial Cribier-Edwards valve and is the latest percutaneous aortic valve prosthesis to enter clinical trials (Table 1). It is a trileaflet balloon-expandable stainless steel valve made from bovine pericardial tissue, available in two sizes (23 mm and 26 mm). In September 2007, it was approved for use in Europe with the RetroFlex transfemoral delivery system. The Ascendra transapical delivery system for the Edward SAPIEN valve has received approval in Europe.
The multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial in North America is continuing to enroll patients, with enrollment projected to be complete by the end of 2008. The aim of this prospective randomized clinical trial is to enroll 1,040 patients in two separate treatment arms. The surgical arm of the trial is comparing the Edwards SAPIEN valve with standard surgical aortic valve replacement, with the objective of demonstrating non-inferiority. The medical management arm of the trial is comparing percutaneous valve replacement against medical therapy or balloon valvuloplasty in patients considered too high-risk for conventional surgical valve replacement.
The primary end point in both arms is death at 1 year; secondary end points focus on long-term (1-year) composite cardiovascular events, valve performance, and quality-of-life indicators. Preliminary data on the first 100 patients (74 via the transfemoral [ie, retrograde] and 26 via the transapical approach) who underwent percutaneous Edwards SAPIEN valve implantation for compassionate use showed device durability and symptom relief at up to 2 years.38 Overall procedural success was 91%, but, as with other trials, there was a steep learning curve, so that excluding the first 25 patients increased the procedural success rate to 96%.38 Aortic valve size and hemodynamics, left ventricular systolic function, mitral regurgitation, and functional class were all significantly improved. Mild aortic regurgitation was common, but none of the patients had severe aortic regurgitation. Importantly, the 15% 30-day death rate was significantly lower than the expected rate of 33%. The 6-month survival rate was 78%, but the 2-year rate was 60% in this high-risk elderly cohort.
Walther et al39 recently reported outcomes on their first 50 patients who underwent transapical implantation of the Edwards SAPIEN valve. The operators were able to implant the prosthesis in all 50 patients, but 3 required early conversion to open surgery with sternotomy. The overall survival at 30 days was 92%, but in the last 25 patients the 30-day survival rate was 96%, with a 1-year survival rate of 80%.
PUTTING THE DATA IN PERSPECTIVE
As noted in this review, a number of factors make a strong case for timely aortic valve replacement: the aging population, the increase in incidence and prevalence of aortic stenosis,1,3,4,27,40 the multiple comorbidities in older patients, and the eventually aggressive natural course of aortic stenosis.1,3,4,27,40–43 Yet current standards dictate not to proceed with standard surgical aortic valve replacement in patients who are truly asymptomatic and who have normal left ventricular systolic function,1,40 mainly because the risks of surgical valve replacement outweigh the benefits in this population.1,40 Aortic valve surgery carries a risk of early death of 15% for patients ages 80 to 84 and of 18% for patients age 85.3,9,10,12,43–45
These figures seem high when compared with death rates of 12% in recent studies of percutaneous valve replacement in similar patients.11,23,30,33 The rates become lower as the learning curve improves.11,21,23,27,30,33 Thus, as the design of aortic valve prostheses and the techniques to implant them are refined and tested for safety, the risk-benefit balance may change in favor of earlier intervention in aortic stenosis with a percutaneous approach.11,21,27,46 Some experts believe that in 10 years 10% to 30% of patients undergoing conventional valve replacement will be candidates for a percutaneous approach.
Of the techniques used to date, the retrograde approach seems most amenable to widespread acceptance, given its inherent advantage of being faster and easier.11,21,30 Limitations with the retrograde approach seen in earlier trials—challenges and complications associated with large-bore arterial vascular access, difficulty traversing the aortic arch with bulky devices, and the inability to cross the stenotic aortic valve to deploy the prosthesis even after balloon valvuloplasty11,21,30—are correctable with refinements in the devices and in technique.
New types of prosthetic aortic valves entering early human studies are improving on current devices, for example, by using collapsible, inflatable valve frames for retrievability before final deployment.
Surgical aortic valve replacement remains the gold standard treatment for patients with symptomatic aortic stenosis. And while studies of percutaneous aortic valve replacement show great promise for this less-invasive treat-men, enthusiasm about percutaneous aortic valve replacement should be tempered by an awareness of persistent limitations of this approach, such as vascular and mechanical complications and operator inexperience, which still need attention.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–231.
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005; 111:3316–3326.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg 2003; 76:1131–1137.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Vahanian A, Palacios IF. Percutaneous approaches to valvular disease. Circulation 2004; 109:1572–1579.
- Webb JG, Munt B, Makkar RR, Naqvi TZ, Dang N. Percutaneous stent-mounted valve for treatment of aortic or pulmonary valve disease. Catheter Cardiovasc Interv 2004; 63:89–93.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients =80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Mittermair RP, Muller LC. Quality of life after cardiac surgery in the elderly. J Cardiovasc Surg (Torino) 2002; 43:43–47.
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000; 356:1403–1405.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704–708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med 1988; 319:125–130.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Safian RD, Mandell VS, Thurer RE, et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J Am Coll Cardiol 1987; 9:655–660.
- Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol 2005; 95:43–47.
- Kapadia SR, Wazni OM, Tan WA, et al. Aortic valvuloplasty in 1990's: experience from a single center in United States. Circulation 1999; 100 18 suppl 1:1–448.
- Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol 1995; 26:1522–1528.
- Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006; 47:1214–1223.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698–703.
- Rajagopal V, Kapadia SR, Tuzcu EM. Advances in the percutaneous treatment of aortic and mitral valve disease. Minerva Cardioangiol 2007; 55:83–94.
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006; 113:842–850.
- Salemi A. Percutaneous valve interventions. Curr Opin Anaesthesiol 2007; 20:70–74.
- Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006; 114:1616–1624.
- Grube E, Laborde JC, Zickmann B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv 2005; 66:465–469.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation—proof of concept. Eur J Cardiothorac Surg 2007; 31:9–15.
- Ye J, Cheung A, Lichtenstein SV, et al. Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur J Cardiothorac Surg 2007; 31:16–21.
- Turgut T, Deeb M, Moscucci M. Left ventricular apical puncture: a procedure surviving well into the new millennium. Catheter Cardiovasc Interv 2000; 49:68–73.
- Zuguchi M, Shindoh C, Chida K, et al. Safety and clinical benefits of transsubxiphoidal left ventricular puncture. Catheter Cardiovasc Interv 2002; 55:58–65.
- Sinha AK, Kini AS, Sharma SK. Percutaneous valve replacement: a paradigm shift. Curr Opin Cardiol 2007; 22:471–477.
- Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the CoreValve ReValving system. Catheter Cardiovasc Interv 2007; 70:760–764.
- Pasupati S, Humphries K, AlAli A, et al. Balloon expandable aortic valve (BEAV) implantation. The first 100 Canadian patients. Circulation 2007; 116 suppl:357.
- Walther T, Falk V, Kempfert J, et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 2008; 33:983–988. Epub 2008 February 21.
- Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002; 346:677–682.
- Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15:1012–1017.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38:61–67.
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000; 35:747–756.
- Society of Thoracic Surgeons National Cardiac Surgery Database. Available at www.sts.org/documents/pdf/Spring2005STS-ExecutiveSummary.pdf. Accessed 9/11/2008.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346:1128–1137.
- Wenger NK, Weber MA, Scheidt S. Valvular heart disease at elderly age: new vistas. Am J Geriatr Cardiol 2006; 15:273–274.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–231.
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005; 111:3316–3326.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg 2003; 76:1131–1137.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Vahanian A, Palacios IF. Percutaneous approaches to valvular disease. Circulation 2004; 109:1572–1579.
- Webb JG, Munt B, Makkar RR, Naqvi TZ, Dang N. Percutaneous stent-mounted valve for treatment of aortic or pulmonary valve disease. Catheter Cardiovasc Interv 2004; 63:89–93.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients =80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Mittermair RP, Muller LC. Quality of life after cardiac surgery in the elderly. J Cardiovasc Surg (Torino) 2002; 43:43–47.
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000; 356:1403–1405.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704–708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med 1988; 319:125–130.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Safian RD, Mandell VS, Thurer RE, et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J Am Coll Cardiol 1987; 9:655–660.
- Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol 2005; 95:43–47.
- Kapadia SR, Wazni OM, Tan WA, et al. Aortic valvuloplasty in 1990's: experience from a single center in United States. Circulation 1999; 100 18 suppl 1:1–448.
- Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol 1995; 26:1522–1528.
- Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006; 47:1214–1223.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698–703.
- Rajagopal V, Kapadia SR, Tuzcu EM. Advances in the percutaneous treatment of aortic and mitral valve disease. Minerva Cardioangiol 2007; 55:83–94.
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006; 113:842–850.
- Salemi A. Percutaneous valve interventions. Curr Opin Anaesthesiol 2007; 20:70–74.
- Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006; 114:1616–1624.
- Grube E, Laborde JC, Zickmann B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv 2005; 66:465–469.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation—proof of concept. Eur J Cardiothorac Surg 2007; 31:9–15.
- Ye J, Cheung A, Lichtenstein SV, et al. Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur J Cardiothorac Surg 2007; 31:16–21.
- Turgut T, Deeb M, Moscucci M. Left ventricular apical puncture: a procedure surviving well into the new millennium. Catheter Cardiovasc Interv 2000; 49:68–73.
- Zuguchi M, Shindoh C, Chida K, et al. Safety and clinical benefits of transsubxiphoidal left ventricular puncture. Catheter Cardiovasc Interv 2002; 55:58–65.
- Sinha AK, Kini AS, Sharma SK. Percutaneous valve replacement: a paradigm shift. Curr Opin Cardiol 2007; 22:471–477.
- Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the CoreValve ReValving system. Catheter Cardiovasc Interv 2007; 70:760–764.
- Pasupati S, Humphries K, AlAli A, et al. Balloon expandable aortic valve (BEAV) implantation. The first 100 Canadian patients. Circulation 2007; 116 suppl:357.
- Walther T, Falk V, Kempfert J, et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 2008; 33:983–988. Epub 2008 February 21.
- Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002; 346:677–682.
- Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15:1012–1017.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38:61–67.
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000; 35:747–756.
- Society of Thoracic Surgeons National Cardiac Surgery Database. Available at www.sts.org/documents/pdf/Spring2005STS-ExecutiveSummary.pdf. Accessed 9/11/2008.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346:1128–1137.
- Wenger NK, Weber MA, Scheidt S. Valvular heart disease at elderly age: new vistas. Am J Geriatr Cardiol 2006; 15:273–274.
KEY POINTS
- Aortic stenosis is the most common valvular condition, affecting 3% of the general population; its incidence and prevalence are increasing as the population ages.
- Many patients with severe aortic valve stenosis are considered too high-risk for standard surgical valve replacement but may be candidates for percutaneous valve replacement.
- Of the approaches now undergoing refinement, the most promising is retrograde (ie, femoral arterial) placement of the Edwards SAPIEN valve or the CoreValve.
- The technology is still evolving, and the learning curve is substantial, yet cautious enthusiasm about percutaneous aortic valve replacement is justified.