User login
Acute coronary syndrome (ACS) remains a major healthcare challenge. Currently, the majority of patients with ACS have nonST‐segment (ST, part of an electrocardiogram between the QRS complex and the T wave) elevation myocardial infarction (MI) and unstable angina.1 Nevertheless, ST‐segment elevation MI is also an important cause of morbidity and mortality. In recent years, our understanding of ACS has improved as a result of several major advances based on results from multiple randomized clinical trials and registry analyses. The results of these analyses have influenced guidelines issued by professional societies and in some cases have become performance metrics. Therefore, it is particularly important for physicians involved in the care of patients with ACS to be aware of evolving treatment patterns (Table 1).
|
| Hospitalists should demonstrate a knowledge of: |
| ACS without enzyme leak, NSTEMI, and STEMI |
| Variable presentations of unstable angina, acute MI |
| Conditions that mimic ACS |
| Cardiac biomarkers |
| Role of noninvasive cardiac testing |
| Risks; indications for cardiac catheterization |
| Risk factors for CAD |
| Validated risk stratification tools |
| Indications for hospitalization of patients with chest pain |
| Indications, contraindications for thrombolytic therapy |
| Indications, contraindications, and pharmacology of drugs for ACS |
| Indications for early invasive interventions |
| Angiography, stenting and/or CABG |
| Laboratory studies or imaging indicative of disease severity |
| Safe hospital discharge |
| Hospitalists should demonstrate skill in: |
| History and physical exam relative to cardiac disease |
| Recognizing signs and severity of ACS |
| Diagnosing ACS through appropriate testing |
| History and physical, ECG, x‐rays, biomarkers |
| Risk stratification using validated tools |
| Formulating an evidence‐based treatment plan |
| Identifying patients for thrombolytics and/or early revascularization |
| Recognizing and treating patient discomfort |
| Recognizing decompensation, initiating immediate therapy |
| Managing complicating factors |
| Bleeding, inadequate response, cardiopulmonary compromise |
| Timely patient assessment, co‐management with other providers |
| Hospitalists should demonstrate attitudes that facilitate: |
| Communication with patients and families relative to cardiac disease and all aspects of care plan |
| Obtain informed consent |
| Early specialty consultation |
| Initiation of secondary prevention measures before discharge |
| Multidisciplinary care throughout the hospital stay |
| Safe discharge and transition back into primary care |
Case Study
A 64‐year‐old man presents to the emergency department with the chief complaint of chest pressure for the past 2 hours. His chest pressure began after he moved furniture in his home. He initially believed that a pulled muscle was the cause of the pain, but when the discomfort did not improve with rest and continued to worsen, he thought it best that his wife drive him to the emergency department, where he continues to have chest pressure. He has never had this symptom before. His past medical history is notable only for mild hypertension for which he takes hydrochlorothiazide 25 mg daily. Otherwise, he has been healthy.
Clinical Presentation and Risk Assessment
The clinical presentation of ACS is not always straightforward. Although physicians frequently inquire about chest pain, the pain often manifests as chest heaviness or chest pressure. Additionally, some patients have a more atypical presentation, where the predominant symptom of acute coronary ischemia is dyspnea or extreme fatigue. These atypical presentations are believed to be somewhat more common in women and in the elderly, but it is important to realize that they can occur in any patient. Nausea, vomiting, or diaphoresis may accompany these symptoms or occur in isolation. Chest discomfort radiating to the jaw, neck, or left arm may be present, but is not necessary to the diagnosis. Thus, we see a variety of symptoms presenting in a patient with ACS.
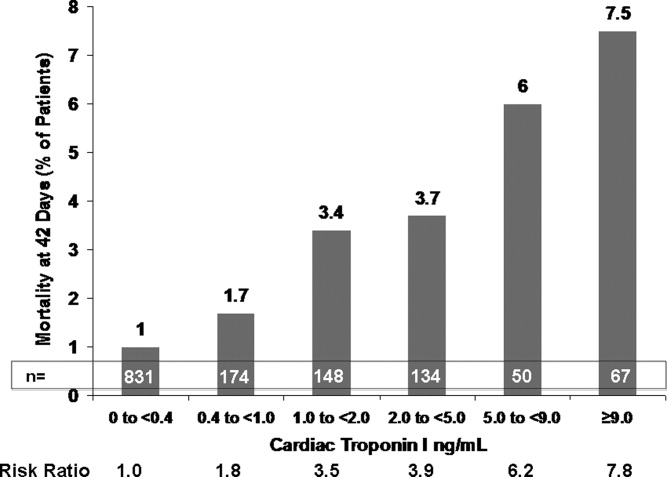
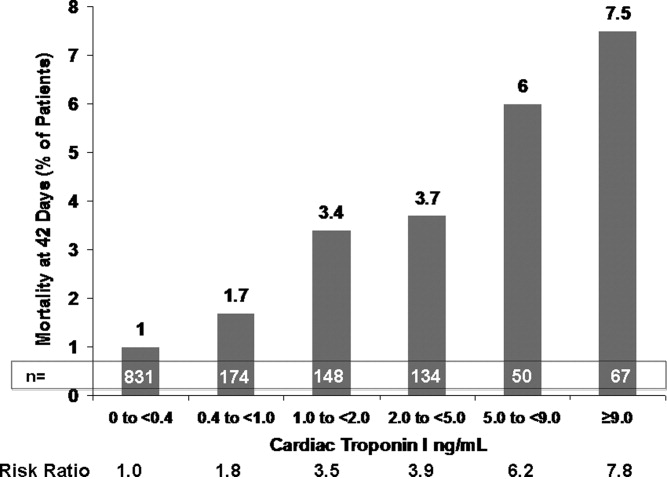
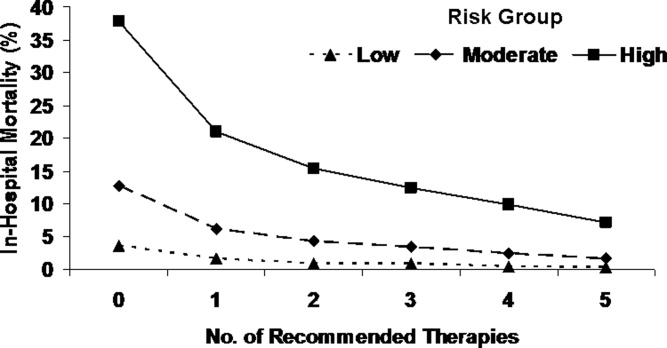
This varied presentation makes objective assessment of ACS particularly important. To inform assessment, biomarkers have emerged as a quick and effective tool to help with the diagnosis of ACS. In particular, troponin measurement is important and serial troponin measurement is useful to exclude myonecrosis. It should be noted that the initial troponin level may be normal during the early stages of ACS. A bedside troponin measurement can be useful for rapid identification of myocardial damage. Quantitative troponin measurement also adds value, as higher levels of troponin are associated with progressively worse outcomes, including mortality (Figure 1). Although a number of biomarkers are available, the most important commonly used at present is troponin.0, 0, 0, 0
In addition to biomarkers, the electrocardiogram (ECG) remains extremely important in diagnosis and risk stratification. In a moderate‐level emergency department, the triage nurse should obtain a 12‐lead ECG for a patient with a history that is suspicious for coronary ischemia, and ask the attending physician to review the ECG immediately. If there is ST‐segment elevation or any new or presumed new left bundle‐branch block, the patient should be triaged to the ST‐segment elevation MI pathway of care. If there is ST‐segment depression or marked T‐wave inversion, this greatly raises the suspicion for nonST‐segment elevation MI or unstable angina. The presence of any of these features on the ECG places the patient at markedly elevated risk of short‐term ischemic complications.
A protocol should be in place for rapid treatment of patients with ST‐segment elevation MI.2 If the hospital has 24/7 percutaneous coronary intervention (PCI) capability, the catheterization lab should be immediately activated and the patient should proceed to primary PCI. The goal door‐to‐balloon time is 90 minutes or less. A patient who presents to a hospital without primary PCI capability should receive either fibrinolysis or be transferred to a center that can perform primary PCI. If fibrinolytic therapy is planned, it is essential that the patient not have any absolute contraindications to fibrinolytic therapy. Fibrinolysis should be administered within 30 minutes of patient contact. If transfer for primary PCI is planned, it is important that systems to support the transfer are in place so that the time from first medical contact to PCI does not exceed 90 minutes. As a practical point, it can be difficult to achieve these short transfer times in many geographic regions of the United States. However, with organized systems of care, it is certainly possible to have effective transfer systems and to achieve a short door‐to‐balloon time.3
If the patient does not have ST‐segment elevation MI, the next step depends on the patient's level of risk, where risk stratification is particularly important. As mentioned above, troponin measurement and the ECG are both essential aspects of risk stratification, but they alone are not sufficient to establish risk. It is recommended that an objective risk tool also be used. This is especially important because the patient can be initially troponin‐negative and have a normal ECG but still be at high risk for ischemic complications. The TIMI risk score (Table 2) is 1 of a number of resources that can help determine whether patients are at high risk for short‐term ischemic complications using means more objective than the eyeball test (Table 3).
|
| Historical |
| Age 65 year |
| 3 CAD risk factors |
| Family history, hypertension, hypercholesterolemia, diabetes, active smoker |
| Known CAD (stenosis 50%) |
| ASA use in past 7 days |
| At presentation |
| Recent (24 hours) severe angina |
| Elevated cardiac markers |
| ST deviation 0.5 mm |
| Risk score = total points, range: 07 |
| Risk Score | Death or MI (%) | Death, MI, or Urgent Revascularization (%) |
|---|---|---|
| ||
| 0/1 | 3 | 5 |
| 2 | 3 | 8 |
| 3 | 5 | 13 |
| 4 | 7 | 20 |
| 5 | 12 | 26 |
| 6/7 | 19 | 41 |
Delineation of the coronary anatomy in the catheterization lab is warranted for patients judged to be at high risk on the basis of the TIMI risk score, which would include most patients with elevated troponin or ST‐segment deviation. Many patients will undergo PCI on the basis of those test results and a smaller percentage might undergo coronary artery bypass grafting (CABG). Additionally, a sizeable minority of patients will be managed medically. This latter group is challenging because it consists of patients who have either trivial coronary artery disease or extensive coronary artery disease not amenable to revascularization and who have either a very low or very high risk of ischemic complications.
Even though catheterization may not be necessary, further evaluation is warranted in patients with ACS deemed to be at low risk. Typically, some form of functional assessment is indicated. In patients who are able to exercise, this would consist of exercise stress testing, often with an imaging modality. If the stress test is abnormal, cardiac catheterization is often the next step.
Case Study (cont)
An ECG is rapidly obtained on this patient and there is ST‐segment depression in leads II, III, and aVF. A bedside troponin is positive. The patient is at high risk of ischemic complications. He is diagnosed with nonST‐segment elevation MI. The next step is to initiate medical therapy. Presumably, the patient would have already (or at least should have already) received aspirin. Chewing or swallowing a dose of 325 mg nonenteric coated aspirin should provide a prompt aspirin effect. It would be reasonable to initiate anticoagulation as well, and the guidelines support a number of choices such as unfractionated heparin or low molecular weight heparin. Consideration should also be given to starting additional antiplatelet therapy, such as a loading dose of clopidogrel. Although aspirin provides some degree of antiplatelet effect, in a patient with activated platelets who presents with an ACS, additional antiplatelet therapy is necessary, although the exact timing of it is a matter of debate. Finally, consideration needs to be given to the need for catheterization. This patient, on the basis of his high ischemic risk and lack of obvious contraindications, should go to the catheterization laboratory, and the timing of catheterization requires further thought.
Guideline Update
The American College of Cardiology/American Heart Association 2009 Focused Guideline Update provides new information and recommendations pertinent to the care of patients with ACS2 and incorporates new data relevant to the initial emergency care and subsequent inpatient care of patients with ACS. Guideline highlights are presented in Table 4.
| Intervention | Recommendation |
|---|---|
| |
| GP IIb/IIIa receptor antagonists | |
| Class IIa | Start abciximab, tirofiban, or eptifibatide at primary PCI (with/without stenting) in selected patients with STEMI. |
| Class IIb | Uncertain value in STEMI when given before arrival at catheterization lab. |
| Thienopyridines | |
| Class I | Use loading dose for planned PCI in STEMI. Regimens for primary and nonprimary PCI are detailed within the guideline. |
| Duration of therapy after stent placement of at least 12 months. Stop early if bleeding risk outweighs benefit. | |
| Discontinue before planned, delayed CABG (5 d clopidogrel; 7 d prasugrel) unless the need for CABG outweighs bleeding risk. | |
| Class IIb | After DES placement, consider continuing clopidogrel or prasugrel beyond the first 15 months of therapy. |
| Class III | Prasugrel is not recommended for primary PCI in patients with STEMI who have a history of stroke or TIA. |
| Parenteral anticoagulants | |
| Class I | In primary PCI, supportive anticoagulants include unfractionated heparin, enoxaparin, fondaparinux, or bivalirudin, following ASA and a thienopyridine. |
| Class IIa | Bivalirudin is reasonable in patients at high risk of bleeding undergoing PCI for STEMI. |
| Triage and transfer for PCI | |
| Class I | STEMI system of care is supported by dedicated teams and protocols required for all communities. |
| Class IIa | Transfer patients who received fibrinolytic therapy at a nonPCI‐capable facility to a PCI‐capable facility. Consider preparatory antithrombotic regimen before or during transfer. |
| Class IIb | Consider expeditious transfer of nonhigh‐risk patients from a nonPCI‐capable facility to a PCI‐capable facility after administration of fibrinolytic. Consider preparatory antithrombotic regimen before or during transfer. |
| Intensive glucose control in STEMI | |
| Class IIa | Insulin is reasonable to maintain glucose <180 mg/dL (avoid hypoglycemia) for any patient with STEMI. |
| Thrombus aspiration during PCI of STEMI | |
| Class IIa | Aspiration thombectomy is reasonable. |
| Use of stents in STEMI | |
| Class IIa | DES is a reasonable alternative to BMS for primary PCI in STEMI. |
| Class IIb | Consider DES when clinical or anatomical factors suggest favorable safety and efficacy for DES. |
| Angiography in CKD | |
| Class I | Isomolar contrast or low molecular weight contrast (not ioxaglate or iohexol) is indicated for CKD patients not on dialysis. |
| Fractional flow reserve | |
| Class IIa | FFR is useful to assess a specific coronary lesion or as an alternative to noninvasive functional testing to justify PCI. Reasonable for intermediate coronary stenosis in patients with angina. |
| Class III | Routine use of FFR is not recommended to assess severity of CAD in patients with angina who have had a positive, unequivocal, noninvasive functional study. |
| PCI for unprotected left main CAD | |
| Class IIb | PCI of left main coronary artery with stents is an alternative to CABG for anatomy associated with low risk of PCI complications and a clinical scenario with higher risk of adverse surgical outcomes. |
| Timing of angiography and antiplatelet therapy in UA/NSTEMI | |
| Class I | Initiate dual‐antiplatelet therapy for UA/NSTEMI and an invasive approach. Start ASA on presentation. Clopidogrel (before or at PCI) or prasugrel (at PCI) as a second antiplatelet agent. |
| Class IIa | Early invasive strategy within 12 to 24 hours of admission is reasonable for stabilized high‐risk UA/NSTEMI; an early approach is also reasonable for UA/STEMI not at high‐risk. |
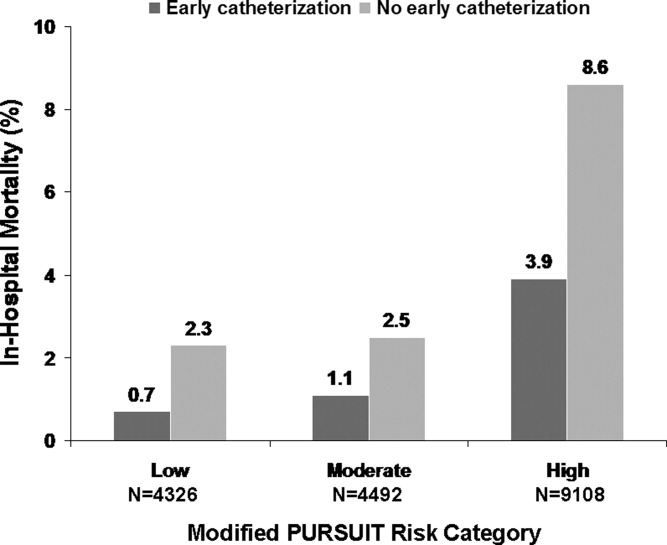
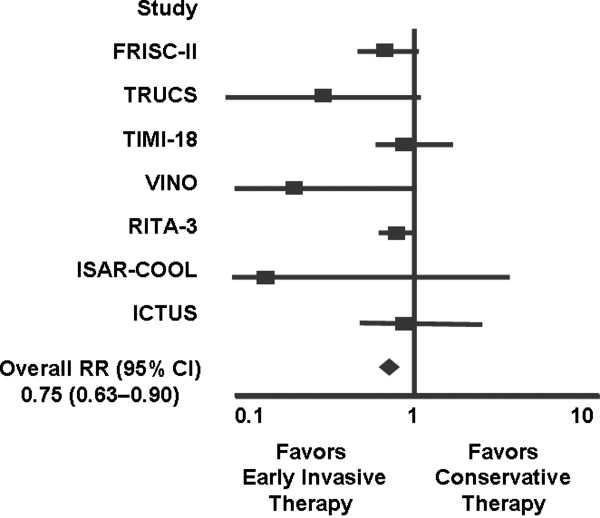
Several studies support an invasive strategy to assess high‐risk ACS patients. Randomized clinical trials and meta‐analyses of these trials have confirmed a significant reduction in subsequent ischemic events, including mortality, in patients who undergo an invasive vs. a more conservative strategy.4 Registry data have confirmed that these randomized clinical trial data reflect patients in the real‐world setting of clinical practice.5
The timing of angiography has recently been examined in detail.6, 7 It appears that for patients with nonST‐segment elevation ACS, unlike those with ST‐segment elevation MI, there is no need for emergent transfer to the catheterization laboratory, assuming patients are electrically and hemodynamically stable. Emergency transfer is warranted for unstable patients and those with ongoing chest discomfort. Otherwise, it appears sufficient to send the patient with nonST‐segment elevation ACS for catheterization within the subsequent 48 hours, or, alternatively, to adopt a more expectant approach in which catheterization is deferred until either recurrent symptoms develop or risk stratification suggests that there is substantial myocardium in jeopardy.
PCI is performed in the catheterization laboratory most often in the setting of ACS.5 When PCI is performed, an important consideration is whether to use a bare metal stent or a drug‐eluting stent.8 Drug‐eluting stents have been shown to have a significant benefit in reducing restenosis and the need for repeat revascularization. However, in aggregate, they have not been shown to either increase or decrease mortality.9 A key issue for the referring physician is to ascertain whether patients who go to the catheterization laboratory are likely to tolerate and be compliant with prolonged dual antiplatelet therapy. If it appears that the patient can or will not be compliant, a bare metal stent is preferable to a drug‐eluting stent; a bare metal stent requires dual antiplatelet therapy of shorter duration.
Additional considerations when sending patients to the catheterization laboratory are related to renal function. In patients with renal dysfunction, the most important way to prevent contrast nephropathy is adequate hydration prior to the procedure. In patients with left ventricular dysfunction, hydration must be done judiciously. Other strategies for preventing contrast nephropathy are being studied, although it is not entirely clear which strategies beyond hydration are truly effective.
Use of upstream glycoprotein IIb/IIIa inhibitors has become more common in patients with nonST‐segment elevation ACS. However, the most recent trial to examine this issue, the Early Glycoprotein IIb/IIIa Inhibition in NonST‐Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial, did not find a clear benefit for routine administration of upstream glycoprotein IIb/IIIa inhibitors when studying all patients with ACS.10 There did appear to be a signal of benefit in troponin‐positive patients, but as an overall strategy no significant benefit and even some detriment associated with an increase in bleeding were shown. Similarly, the results of the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial did not support the benefit of upstream glycoprotein IIb/IIIa inhibitors in patients with ACS.11
New data have also been released with respect to the thienopyridines. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with PrasugrelThrombolysis in Myocardial Infarction (TRITONTIMI) 38 study found that the more potent thienopyridine prasugrel significantly reduced ischemic events when compared with clopidogrel in patients with ACS undergoing PCI.1214 A significant reduction in stent thrombosis was reported regardless of the type of stent.15 However, the study reported a significant increase in major bleeding and a small, but statistically significant, excess of fatal bleeding. A subgroup analysis of patients with diabetes or with ST‐segment elevation MI from the TRITON‐TIMI 38 study showed a particularly large benefit associated with the use of prasugrel vs. clopidogrel and, interestingly, bleeding hazards were attenuated in these subgroups.16, 17 In the small subgroup of patients with prior stroke or transient ischemic attack (TIA), there was an excessive rate of intracranial hemorrhage with prasugrel vs. clopidogrel, indicating that prasugrel should not be used in these patients. Patients age 75 years or older or who weighed less than 60 kg also appeared to have a higher bleeding risk with prasugrel compared to clopidogrel. Careful thought is needed before using prasugrel in those patients identified as having a higher risk of bleeding.
Recently, a higher clopidogrel loading dose of 600 mg vs. the standard 300 mg dose was tested in patients who presented with ACS in the Clopidogrel optimal loading dose Usage to Reduce Recurrent EveNTs‐Organization to Assess Strategies in Ischemic Syndromes (CURRENT‐OASIS) 7 trial.18 Patients also received 150 mg of clopidogrel daily for the ensuing 6 days vs. the standard 75 mg daily dose. All patients then received 75 mg clopidogrel for 1 month of follow‐up. In the overall population, there was no benefit to using the higher clopidogrel loading dose. In contrast, there was a significant reduction in stent thrombosis in patients who received stents. The higher loading dose of clopidogrel was associated with a higher rate of bleeding.
Ticagrelor is a novel adenosine diphosphate receptor antagonist that was compared with clopidogrel in patients with ACS.19, 20 Compared to clopidogrel, ticagrelor significantly reduced ischemic events and there was also a significant reduction in cardiovascular mortality and in all‐cause mortality. Surprisingly, overall major bleeding did not increase with ticagrelor, but nonCABG‐related major bleeding increased.
The use of proton pump inhibitors (PPIs) in patients receiving dual antiplatelet therapy has also been a matter of vigorous recent debate.21 Evidence to date suggests there is no significant clinical interaction between PPIs and prasugrel. The data with clopidogrel and PPIs are mixed, although data are limited because much were derived from observational studies. Randomized clinical trial data are needed to assess whether there is an interaction between clopidogrel and PPIs that warrants clinical action, although preliminary data suggest there is no adverse cardiovascular interaction.22
New data regarding the intravenous anticoagulant bivalirudin have become available and have been incorporated into the Focused Guideline Update. Although bivalirudin is to be used primarily in the catheterization laboratory during PCI, it does appear to be associated with significantly less bleeding than heparin plus glycoprotein IIb/IIIa inhibitors.11, 2326
Case Study (cont)
The patient undergoes cardiac catheterization. An occluded dominant left circumflex artery is noted and is opened up with balloon angioplasty after aspiration thrombectomy. The patient receives 60 mg of prasugrel as a loading dose and bivalirudin as the anticoagulant during the procedure. A drug‐eluting stent is implanted with excellent results. The patient is transferred to the cardiac care unit for further care. It appears that this is a patient who functionally has an ST‐segment elevation MI with an occluded artery, although it manifested on the ECG as ST depression. Because of the patient's ongoing chest discomfort, it was fortunate that prompt angiography was performed.
Discussion
Patients with ACS present several challenges in management. Risk stratification is particularly important for nonST‐segment elevation ACS and requires thoughtful evaluation by the physician. Additionally, the large amount of new data and guideline updates create a rapidly evolving field, making it difficult to keep abreast of new developments. Physicians of patients with ACS need to be aware of these key developments so that they can provide optimal care to their patients with potentially life‐threatening ACS.
Acknowledgements
Denise M. Erkkila, RPh of DIME, provided editorial assistance consisting of help with tables, figures, and reference formatting for this manuscript.
- ,,, et al.Heart disease and stroke statistics‐‐2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,, et al.2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol.2009;54:2205–2241.
- ,,, et al.Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes.JAMA.2009;302:2207–2213.
- ,,,,.Benefit of early invasive therapy in acute coronary syndromes: a meta‐analysis of contemporary randomized clinical trials.J Am Coll Cardiol.2006;48:1319–1325.
- ,,, et al.Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative.JAMA.2004;292:2096–2104.
- ,,, et al.Early versus delayed invasive intervention in acute coronary syndromes.N Engl J Med.2009;360:2165–2175.
- ,,, et al.Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial.JAMA.2009;302:947–954.
- ,.Drug‐eluting stents: dual antiplatelet therapy for every survivor?Circulation.2007;116:696–699.
- ,.Appropriate use of drug‐eluting stents: balancing the reduction in restenosis with the concern of late thrombosis.Lancet.2008;371:2134–2143.
- ,,, et al.Early versus delayed, provisional eptifibatide in acute coronary syndromes.N Engl J Med.2009;360:2176–2190.
- ,,, et al.Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one‐year results from the ACUITY trial.JAMA.2007;298:2497–2506.
- .Intensifying platelet inhibition‐‐navigating between Scylla and Charybdis.N Engl J Med.2007;357:2078–2081.
- .Prasugrel in clinical practice.N Engl J Med.2009;361:940–942.
- ,,, et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2007;357:2001–2015.
- ,,, et al.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON‐TIMI 38 trial: a subanalysis of a randomised trial.Lancet.2008;371:1353–1363.
- ,,, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial.Lancet.2009;373:723–731.
- ,,, et al.Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel—Thrombolysis in Myocardial Infarction 38.Circulation.2008;118:1626–1636.
- , CURRENT Investigators. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Available at: http://www.escardio.org/congresses/esc‐2009/congress‐reports/Pages/706003‐706004‐mehta‐vandewerf.aspx#discussant.2009. Accessed July 2010.
- .Antiplatelet therapy: ticagrelor in ACS‐what does PLATO teach us?Nat Rev Cardiol.2009;6:737–738.
- ,,, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2009;361:1045–1057.
- ,.Omeprazole and clopidogrel: Should clinicians be worried?Cleve Clin J Med.2010;77:113–116.
- .COGENT: a prospective, randomized, placebo‐controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Transcatheter Cardiovascular Therapeutics (TCT) 2009; September 24,2009;San Francisco, CA.
- ,,, et al.The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) Trial: study design and rationale.Am Heart J.2008;156:44–56.
- ,,, et al.Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial.Lancet.2009;374:1149–1159.
- ,,, et al.Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale.Am Heart J.2004;148:764–775.
- ,,, et al.Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial.Lancet.2007;369:907–919.
- Society of Hospital Medicine.Acute coronary syndrome.J Hosp Med.2006;1 (suppl 1):2–3.
- ,,, et al.The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making.JAMA.2000;284:835–842.
Acute coronary syndrome (ACS) remains a major healthcare challenge. Currently, the majority of patients with ACS have nonST‐segment (ST, part of an electrocardiogram between the QRS complex and the T wave) elevation myocardial infarction (MI) and unstable angina.1 Nevertheless, ST‐segment elevation MI is also an important cause of morbidity and mortality. In recent years, our understanding of ACS has improved as a result of several major advances based on results from multiple randomized clinical trials and registry analyses. The results of these analyses have influenced guidelines issued by professional societies and in some cases have become performance metrics. Therefore, it is particularly important for physicians involved in the care of patients with ACS to be aware of evolving treatment patterns (Table 1).
|
| Hospitalists should demonstrate a knowledge of: |
| ACS without enzyme leak, NSTEMI, and STEMI |
| Variable presentations of unstable angina, acute MI |
| Conditions that mimic ACS |
| Cardiac biomarkers |
| Role of noninvasive cardiac testing |
| Risks; indications for cardiac catheterization |
| Risk factors for CAD |
| Validated risk stratification tools |
| Indications for hospitalization of patients with chest pain |
| Indications, contraindications for thrombolytic therapy |
| Indications, contraindications, and pharmacology of drugs for ACS |
| Indications for early invasive interventions |
| Angiography, stenting and/or CABG |
| Laboratory studies or imaging indicative of disease severity |
| Safe hospital discharge |
| Hospitalists should demonstrate skill in: |
| History and physical exam relative to cardiac disease |
| Recognizing signs and severity of ACS |
| Diagnosing ACS through appropriate testing |
| History and physical, ECG, x‐rays, biomarkers |
| Risk stratification using validated tools |
| Formulating an evidence‐based treatment plan |
| Identifying patients for thrombolytics and/or early revascularization |
| Recognizing and treating patient discomfort |
| Recognizing decompensation, initiating immediate therapy |
| Managing complicating factors |
| Bleeding, inadequate response, cardiopulmonary compromise |
| Timely patient assessment, co‐management with other providers |
| Hospitalists should demonstrate attitudes that facilitate: |
| Communication with patients and families relative to cardiac disease and all aspects of care plan |
| Obtain informed consent |
| Early specialty consultation |
| Initiation of secondary prevention measures before discharge |
| Multidisciplinary care throughout the hospital stay |
| Safe discharge and transition back into primary care |
Case Study
A 64‐year‐old man presents to the emergency department with the chief complaint of chest pressure for the past 2 hours. His chest pressure began after he moved furniture in his home. He initially believed that a pulled muscle was the cause of the pain, but when the discomfort did not improve with rest and continued to worsen, he thought it best that his wife drive him to the emergency department, where he continues to have chest pressure. He has never had this symptom before. His past medical history is notable only for mild hypertension for which he takes hydrochlorothiazide 25 mg daily. Otherwise, he has been healthy.
Clinical Presentation and Risk Assessment
The clinical presentation of ACS is not always straightforward. Although physicians frequently inquire about chest pain, the pain often manifests as chest heaviness or chest pressure. Additionally, some patients have a more atypical presentation, where the predominant symptom of acute coronary ischemia is dyspnea or extreme fatigue. These atypical presentations are believed to be somewhat more common in women and in the elderly, but it is important to realize that they can occur in any patient. Nausea, vomiting, or diaphoresis may accompany these symptoms or occur in isolation. Chest discomfort radiating to the jaw, neck, or left arm may be present, but is not necessary to the diagnosis. Thus, we see a variety of symptoms presenting in a patient with ACS.
This varied presentation makes objective assessment of ACS particularly important. To inform assessment, biomarkers have emerged as a quick and effective tool to help with the diagnosis of ACS. In particular, troponin measurement is important and serial troponin measurement is useful to exclude myonecrosis. It should be noted that the initial troponin level may be normal during the early stages of ACS. A bedside troponin measurement can be useful for rapid identification of myocardial damage. Quantitative troponin measurement also adds value, as higher levels of troponin are associated with progressively worse outcomes, including mortality (Figure 1). Although a number of biomarkers are available, the most important commonly used at present is troponin.0, 0, 0, 0





In addition to biomarkers, the electrocardiogram (ECG) remains extremely important in diagnosis and risk stratification. In a moderate‐level emergency department, the triage nurse should obtain a 12‐lead ECG for a patient with a history that is suspicious for coronary ischemia, and ask the attending physician to review the ECG immediately. If there is ST‐segment elevation or any new or presumed new left bundle‐branch block, the patient should be triaged to the ST‐segment elevation MI pathway of care. If there is ST‐segment depression or marked T‐wave inversion, this greatly raises the suspicion for nonST‐segment elevation MI or unstable angina. The presence of any of these features on the ECG places the patient at markedly elevated risk of short‐term ischemic complications.
A protocol should be in place for rapid treatment of patients with ST‐segment elevation MI.2 If the hospital has 24/7 percutaneous coronary intervention (PCI) capability, the catheterization lab should be immediately activated and the patient should proceed to primary PCI. The goal door‐to‐balloon time is 90 minutes or less. A patient who presents to a hospital without primary PCI capability should receive either fibrinolysis or be transferred to a center that can perform primary PCI. If fibrinolytic therapy is planned, it is essential that the patient not have any absolute contraindications to fibrinolytic therapy. Fibrinolysis should be administered within 30 minutes of patient contact. If transfer for primary PCI is planned, it is important that systems to support the transfer are in place so that the time from first medical contact to PCI does not exceed 90 minutes. As a practical point, it can be difficult to achieve these short transfer times in many geographic regions of the United States. However, with organized systems of care, it is certainly possible to have effective transfer systems and to achieve a short door‐to‐balloon time.3
If the patient does not have ST‐segment elevation MI, the next step depends on the patient's level of risk, where risk stratification is particularly important. As mentioned above, troponin measurement and the ECG are both essential aspects of risk stratification, but they alone are not sufficient to establish risk. It is recommended that an objective risk tool also be used. This is especially important because the patient can be initially troponin‐negative and have a normal ECG but still be at high risk for ischemic complications. The TIMI risk score (Table 2) is 1 of a number of resources that can help determine whether patients are at high risk for short‐term ischemic complications using means more objective than the eyeball test (Table 3).
|
| Historical |
| Age 65 year |
| 3 CAD risk factors |
| Family history, hypertension, hypercholesterolemia, diabetes, active smoker |
| Known CAD (stenosis 50%) |
| ASA use in past 7 days |
| At presentation |
| Recent (24 hours) severe angina |
| Elevated cardiac markers |
| ST deviation 0.5 mm |
| Risk score = total points, range: 07 |
| Risk Score | Death or MI (%) | Death, MI, or Urgent Revascularization (%) |
|---|---|---|
| ||
| 0/1 | 3 | 5 |
| 2 | 3 | 8 |
| 3 | 5 | 13 |
| 4 | 7 | 20 |
| 5 | 12 | 26 |
| 6/7 | 19 | 41 |
Delineation of the coronary anatomy in the catheterization lab is warranted for patients judged to be at high risk on the basis of the TIMI risk score, which would include most patients with elevated troponin or ST‐segment deviation. Many patients will undergo PCI on the basis of those test results and a smaller percentage might undergo coronary artery bypass grafting (CABG). Additionally, a sizeable minority of patients will be managed medically. This latter group is challenging because it consists of patients who have either trivial coronary artery disease or extensive coronary artery disease not amenable to revascularization and who have either a very low or very high risk of ischemic complications.
Even though catheterization may not be necessary, further evaluation is warranted in patients with ACS deemed to be at low risk. Typically, some form of functional assessment is indicated. In patients who are able to exercise, this would consist of exercise stress testing, often with an imaging modality. If the stress test is abnormal, cardiac catheterization is often the next step.
Case Study (cont)
An ECG is rapidly obtained on this patient and there is ST‐segment depression in leads II, III, and aVF. A bedside troponin is positive. The patient is at high risk of ischemic complications. He is diagnosed with nonST‐segment elevation MI. The next step is to initiate medical therapy. Presumably, the patient would have already (or at least should have already) received aspirin. Chewing or swallowing a dose of 325 mg nonenteric coated aspirin should provide a prompt aspirin effect. It would be reasonable to initiate anticoagulation as well, and the guidelines support a number of choices such as unfractionated heparin or low molecular weight heparin. Consideration should also be given to starting additional antiplatelet therapy, such as a loading dose of clopidogrel. Although aspirin provides some degree of antiplatelet effect, in a patient with activated platelets who presents with an ACS, additional antiplatelet therapy is necessary, although the exact timing of it is a matter of debate. Finally, consideration needs to be given to the need for catheterization. This patient, on the basis of his high ischemic risk and lack of obvious contraindications, should go to the catheterization laboratory, and the timing of catheterization requires further thought.
Guideline Update
The American College of Cardiology/American Heart Association 2009 Focused Guideline Update provides new information and recommendations pertinent to the care of patients with ACS2 and incorporates new data relevant to the initial emergency care and subsequent inpatient care of patients with ACS. Guideline highlights are presented in Table 4.
| Intervention | Recommendation |
|---|---|
| |
| GP IIb/IIIa receptor antagonists | |
| Class IIa | Start abciximab, tirofiban, or eptifibatide at primary PCI (with/without stenting) in selected patients with STEMI. |
| Class IIb | Uncertain value in STEMI when given before arrival at catheterization lab. |
| Thienopyridines | |
| Class I | Use loading dose for planned PCI in STEMI. Regimens for primary and nonprimary PCI are detailed within the guideline. |
| Duration of therapy after stent placement of at least 12 months. Stop early if bleeding risk outweighs benefit. | |
| Discontinue before planned, delayed CABG (5 d clopidogrel; 7 d prasugrel) unless the need for CABG outweighs bleeding risk. | |
| Class IIb | After DES placement, consider continuing clopidogrel or prasugrel beyond the first 15 months of therapy. |
| Class III | Prasugrel is not recommended for primary PCI in patients with STEMI who have a history of stroke or TIA. |
| Parenteral anticoagulants | |
| Class I | In primary PCI, supportive anticoagulants include unfractionated heparin, enoxaparin, fondaparinux, or bivalirudin, following ASA and a thienopyridine. |
| Class IIa | Bivalirudin is reasonable in patients at high risk of bleeding undergoing PCI for STEMI. |
| Triage and transfer for PCI | |
| Class I | STEMI system of care is supported by dedicated teams and protocols required for all communities. |
| Class IIa | Transfer patients who received fibrinolytic therapy at a nonPCI‐capable facility to a PCI‐capable facility. Consider preparatory antithrombotic regimen before or during transfer. |
| Class IIb | Consider expeditious transfer of nonhigh‐risk patients from a nonPCI‐capable facility to a PCI‐capable facility after administration of fibrinolytic. Consider preparatory antithrombotic regimen before or during transfer. |
| Intensive glucose control in STEMI | |
| Class IIa | Insulin is reasonable to maintain glucose <180 mg/dL (avoid hypoglycemia) for any patient with STEMI. |
| Thrombus aspiration during PCI of STEMI | |
| Class IIa | Aspiration thombectomy is reasonable. |
| Use of stents in STEMI | |
| Class IIa | DES is a reasonable alternative to BMS for primary PCI in STEMI. |
| Class IIb | Consider DES when clinical or anatomical factors suggest favorable safety and efficacy for DES. |
| Angiography in CKD | |
| Class I | Isomolar contrast or low molecular weight contrast (not ioxaglate or iohexol) is indicated for CKD patients not on dialysis. |
| Fractional flow reserve | |
| Class IIa | FFR is useful to assess a specific coronary lesion or as an alternative to noninvasive functional testing to justify PCI. Reasonable for intermediate coronary stenosis in patients with angina. |
| Class III | Routine use of FFR is not recommended to assess severity of CAD in patients with angina who have had a positive, unequivocal, noninvasive functional study. |
| PCI for unprotected left main CAD | |
| Class IIb | PCI of left main coronary artery with stents is an alternative to CABG for anatomy associated with low risk of PCI complications and a clinical scenario with higher risk of adverse surgical outcomes. |
| Timing of angiography and antiplatelet therapy in UA/NSTEMI | |
| Class I | Initiate dual‐antiplatelet therapy for UA/NSTEMI and an invasive approach. Start ASA on presentation. Clopidogrel (before or at PCI) or prasugrel (at PCI) as a second antiplatelet agent. |
| Class IIa | Early invasive strategy within 12 to 24 hours of admission is reasonable for stabilized high‐risk UA/NSTEMI; an early approach is also reasonable for UA/STEMI not at high‐risk. |
Several studies support an invasive strategy to assess high‐risk ACS patients. Randomized clinical trials and meta‐analyses of these trials have confirmed a significant reduction in subsequent ischemic events, including mortality, in patients who undergo an invasive vs. a more conservative strategy.4 Registry data have confirmed that these randomized clinical trial data reflect patients in the real‐world setting of clinical practice.5
The timing of angiography has recently been examined in detail.6, 7 It appears that for patients with nonST‐segment elevation ACS, unlike those with ST‐segment elevation MI, there is no need for emergent transfer to the catheterization laboratory, assuming patients are electrically and hemodynamically stable. Emergency transfer is warranted for unstable patients and those with ongoing chest discomfort. Otherwise, it appears sufficient to send the patient with nonST‐segment elevation ACS for catheterization within the subsequent 48 hours, or, alternatively, to adopt a more expectant approach in which catheterization is deferred until either recurrent symptoms develop or risk stratification suggests that there is substantial myocardium in jeopardy.
PCI is performed in the catheterization laboratory most often in the setting of ACS.5 When PCI is performed, an important consideration is whether to use a bare metal stent or a drug‐eluting stent.8 Drug‐eluting stents have been shown to have a significant benefit in reducing restenosis and the need for repeat revascularization. However, in aggregate, they have not been shown to either increase or decrease mortality.9 A key issue for the referring physician is to ascertain whether patients who go to the catheterization laboratory are likely to tolerate and be compliant with prolonged dual antiplatelet therapy. If it appears that the patient can or will not be compliant, a bare metal stent is preferable to a drug‐eluting stent; a bare metal stent requires dual antiplatelet therapy of shorter duration.
Additional considerations when sending patients to the catheterization laboratory are related to renal function. In patients with renal dysfunction, the most important way to prevent contrast nephropathy is adequate hydration prior to the procedure. In patients with left ventricular dysfunction, hydration must be done judiciously. Other strategies for preventing contrast nephropathy are being studied, although it is not entirely clear which strategies beyond hydration are truly effective.
Use of upstream glycoprotein IIb/IIIa inhibitors has become more common in patients with nonST‐segment elevation ACS. However, the most recent trial to examine this issue, the Early Glycoprotein IIb/IIIa Inhibition in NonST‐Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial, did not find a clear benefit for routine administration of upstream glycoprotein IIb/IIIa inhibitors when studying all patients with ACS.10 There did appear to be a signal of benefit in troponin‐positive patients, but as an overall strategy no significant benefit and even some detriment associated with an increase in bleeding were shown. Similarly, the results of the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial did not support the benefit of upstream glycoprotein IIb/IIIa inhibitors in patients with ACS.11
New data have also been released with respect to the thienopyridines. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with PrasugrelThrombolysis in Myocardial Infarction (TRITONTIMI) 38 study found that the more potent thienopyridine prasugrel significantly reduced ischemic events when compared with clopidogrel in patients with ACS undergoing PCI.1214 A significant reduction in stent thrombosis was reported regardless of the type of stent.15 However, the study reported a significant increase in major bleeding and a small, but statistically significant, excess of fatal bleeding. A subgroup analysis of patients with diabetes or with ST‐segment elevation MI from the TRITON‐TIMI 38 study showed a particularly large benefit associated with the use of prasugrel vs. clopidogrel and, interestingly, bleeding hazards were attenuated in these subgroups.16, 17 In the small subgroup of patients with prior stroke or transient ischemic attack (TIA), there was an excessive rate of intracranial hemorrhage with prasugrel vs. clopidogrel, indicating that prasugrel should not be used in these patients. Patients age 75 years or older or who weighed less than 60 kg also appeared to have a higher bleeding risk with prasugrel compared to clopidogrel. Careful thought is needed before using prasugrel in those patients identified as having a higher risk of bleeding.
Recently, a higher clopidogrel loading dose of 600 mg vs. the standard 300 mg dose was tested in patients who presented with ACS in the Clopidogrel optimal loading dose Usage to Reduce Recurrent EveNTs‐Organization to Assess Strategies in Ischemic Syndromes (CURRENT‐OASIS) 7 trial.18 Patients also received 150 mg of clopidogrel daily for the ensuing 6 days vs. the standard 75 mg daily dose. All patients then received 75 mg clopidogrel for 1 month of follow‐up. In the overall population, there was no benefit to using the higher clopidogrel loading dose. In contrast, there was a significant reduction in stent thrombosis in patients who received stents. The higher loading dose of clopidogrel was associated with a higher rate of bleeding.
Ticagrelor is a novel adenosine diphosphate receptor antagonist that was compared with clopidogrel in patients with ACS.19, 20 Compared to clopidogrel, ticagrelor significantly reduced ischemic events and there was also a significant reduction in cardiovascular mortality and in all‐cause mortality. Surprisingly, overall major bleeding did not increase with ticagrelor, but nonCABG‐related major bleeding increased.
The use of proton pump inhibitors (PPIs) in patients receiving dual antiplatelet therapy has also been a matter of vigorous recent debate.21 Evidence to date suggests there is no significant clinical interaction between PPIs and prasugrel. The data with clopidogrel and PPIs are mixed, although data are limited because much were derived from observational studies. Randomized clinical trial data are needed to assess whether there is an interaction between clopidogrel and PPIs that warrants clinical action, although preliminary data suggest there is no adverse cardiovascular interaction.22
New data regarding the intravenous anticoagulant bivalirudin have become available and have been incorporated into the Focused Guideline Update. Although bivalirudin is to be used primarily in the catheterization laboratory during PCI, it does appear to be associated with significantly less bleeding than heparin plus glycoprotein IIb/IIIa inhibitors.11, 2326
Case Study (cont)
The patient undergoes cardiac catheterization. An occluded dominant left circumflex artery is noted and is opened up with balloon angioplasty after aspiration thrombectomy. The patient receives 60 mg of prasugrel as a loading dose and bivalirudin as the anticoagulant during the procedure. A drug‐eluting stent is implanted with excellent results. The patient is transferred to the cardiac care unit for further care. It appears that this is a patient who functionally has an ST‐segment elevation MI with an occluded artery, although it manifested on the ECG as ST depression. Because of the patient's ongoing chest discomfort, it was fortunate that prompt angiography was performed.
Discussion
Patients with ACS present several challenges in management. Risk stratification is particularly important for nonST‐segment elevation ACS and requires thoughtful evaluation by the physician. Additionally, the large amount of new data and guideline updates create a rapidly evolving field, making it difficult to keep abreast of new developments. Physicians of patients with ACS need to be aware of these key developments so that they can provide optimal care to their patients with potentially life‐threatening ACS.
Acknowledgements
Denise M. Erkkila, RPh of DIME, provided editorial assistance consisting of help with tables, figures, and reference formatting for this manuscript.
Acute coronary syndrome (ACS) remains a major healthcare challenge. Currently, the majority of patients with ACS have nonST‐segment (ST, part of an electrocardiogram between the QRS complex and the T wave) elevation myocardial infarction (MI) and unstable angina.1 Nevertheless, ST‐segment elevation MI is also an important cause of morbidity and mortality. In recent years, our understanding of ACS has improved as a result of several major advances based on results from multiple randomized clinical trials and registry analyses. The results of these analyses have influenced guidelines issued by professional societies and in some cases have become performance metrics. Therefore, it is particularly important for physicians involved in the care of patients with ACS to be aware of evolving treatment patterns (Table 1).
|
| Hospitalists should demonstrate a knowledge of: |
| ACS without enzyme leak, NSTEMI, and STEMI |
| Variable presentations of unstable angina, acute MI |
| Conditions that mimic ACS |
| Cardiac biomarkers |
| Role of noninvasive cardiac testing |
| Risks; indications for cardiac catheterization |
| Risk factors for CAD |
| Validated risk stratification tools |
| Indications for hospitalization of patients with chest pain |
| Indications, contraindications for thrombolytic therapy |
| Indications, contraindications, and pharmacology of drugs for ACS |
| Indications for early invasive interventions |
| Angiography, stenting and/or CABG |
| Laboratory studies or imaging indicative of disease severity |
| Safe hospital discharge |
| Hospitalists should demonstrate skill in: |
| History and physical exam relative to cardiac disease |
| Recognizing signs and severity of ACS |
| Diagnosing ACS through appropriate testing |
| History and physical, ECG, x‐rays, biomarkers |
| Risk stratification using validated tools |
| Formulating an evidence‐based treatment plan |
| Identifying patients for thrombolytics and/or early revascularization |
| Recognizing and treating patient discomfort |
| Recognizing decompensation, initiating immediate therapy |
| Managing complicating factors |
| Bleeding, inadequate response, cardiopulmonary compromise |
| Timely patient assessment, co‐management with other providers |
| Hospitalists should demonstrate attitudes that facilitate: |
| Communication with patients and families relative to cardiac disease and all aspects of care plan |
| Obtain informed consent |
| Early specialty consultation |
| Initiation of secondary prevention measures before discharge |
| Multidisciplinary care throughout the hospital stay |
| Safe discharge and transition back into primary care |
Case Study
A 64‐year‐old man presents to the emergency department with the chief complaint of chest pressure for the past 2 hours. His chest pressure began after he moved furniture in his home. He initially believed that a pulled muscle was the cause of the pain, but when the discomfort did not improve with rest and continued to worsen, he thought it best that his wife drive him to the emergency department, where he continues to have chest pressure. He has never had this symptom before. His past medical history is notable only for mild hypertension for which he takes hydrochlorothiazide 25 mg daily. Otherwise, he has been healthy.
Clinical Presentation and Risk Assessment
The clinical presentation of ACS is not always straightforward. Although physicians frequently inquire about chest pain, the pain often manifests as chest heaviness or chest pressure. Additionally, some patients have a more atypical presentation, where the predominant symptom of acute coronary ischemia is dyspnea or extreme fatigue. These atypical presentations are believed to be somewhat more common in women and in the elderly, but it is important to realize that they can occur in any patient. Nausea, vomiting, or diaphoresis may accompany these symptoms or occur in isolation. Chest discomfort radiating to the jaw, neck, or left arm may be present, but is not necessary to the diagnosis. Thus, we see a variety of symptoms presenting in a patient with ACS.
This varied presentation makes objective assessment of ACS particularly important. To inform assessment, biomarkers have emerged as a quick and effective tool to help with the diagnosis of ACS. In particular, troponin measurement is important and serial troponin measurement is useful to exclude myonecrosis. It should be noted that the initial troponin level may be normal during the early stages of ACS. A bedside troponin measurement can be useful for rapid identification of myocardial damage. Quantitative troponin measurement also adds value, as higher levels of troponin are associated with progressively worse outcomes, including mortality (Figure 1). Although a number of biomarkers are available, the most important commonly used at present is troponin.0, 0, 0, 0





In addition to biomarkers, the electrocardiogram (ECG) remains extremely important in diagnosis and risk stratification. In a moderate‐level emergency department, the triage nurse should obtain a 12‐lead ECG for a patient with a history that is suspicious for coronary ischemia, and ask the attending physician to review the ECG immediately. If there is ST‐segment elevation or any new or presumed new left bundle‐branch block, the patient should be triaged to the ST‐segment elevation MI pathway of care. If there is ST‐segment depression or marked T‐wave inversion, this greatly raises the suspicion for nonST‐segment elevation MI or unstable angina. The presence of any of these features on the ECG places the patient at markedly elevated risk of short‐term ischemic complications.
A protocol should be in place for rapid treatment of patients with ST‐segment elevation MI.2 If the hospital has 24/7 percutaneous coronary intervention (PCI) capability, the catheterization lab should be immediately activated and the patient should proceed to primary PCI. The goal door‐to‐balloon time is 90 minutes or less. A patient who presents to a hospital without primary PCI capability should receive either fibrinolysis or be transferred to a center that can perform primary PCI. If fibrinolytic therapy is planned, it is essential that the patient not have any absolute contraindications to fibrinolytic therapy. Fibrinolysis should be administered within 30 minutes of patient contact. If transfer for primary PCI is planned, it is important that systems to support the transfer are in place so that the time from first medical contact to PCI does not exceed 90 minutes. As a practical point, it can be difficult to achieve these short transfer times in many geographic regions of the United States. However, with organized systems of care, it is certainly possible to have effective transfer systems and to achieve a short door‐to‐balloon time.3
If the patient does not have ST‐segment elevation MI, the next step depends on the patient's level of risk, where risk stratification is particularly important. As mentioned above, troponin measurement and the ECG are both essential aspects of risk stratification, but they alone are not sufficient to establish risk. It is recommended that an objective risk tool also be used. This is especially important because the patient can be initially troponin‐negative and have a normal ECG but still be at high risk for ischemic complications. The TIMI risk score (Table 2) is 1 of a number of resources that can help determine whether patients are at high risk for short‐term ischemic complications using means more objective than the eyeball test (Table 3).
|
| Historical |
| Age 65 year |
| 3 CAD risk factors |
| Family history, hypertension, hypercholesterolemia, diabetes, active smoker |
| Known CAD (stenosis 50%) |
| ASA use in past 7 days |
| At presentation |
| Recent (24 hours) severe angina |
| Elevated cardiac markers |
| ST deviation 0.5 mm |
| Risk score = total points, range: 07 |
| Risk Score | Death or MI (%) | Death, MI, or Urgent Revascularization (%) |
|---|---|---|
| ||
| 0/1 | 3 | 5 |
| 2 | 3 | 8 |
| 3 | 5 | 13 |
| 4 | 7 | 20 |
| 5 | 12 | 26 |
| 6/7 | 19 | 41 |
Delineation of the coronary anatomy in the catheterization lab is warranted for patients judged to be at high risk on the basis of the TIMI risk score, which would include most patients with elevated troponin or ST‐segment deviation. Many patients will undergo PCI on the basis of those test results and a smaller percentage might undergo coronary artery bypass grafting (CABG). Additionally, a sizeable minority of patients will be managed medically. This latter group is challenging because it consists of patients who have either trivial coronary artery disease or extensive coronary artery disease not amenable to revascularization and who have either a very low or very high risk of ischemic complications.
Even though catheterization may not be necessary, further evaluation is warranted in patients with ACS deemed to be at low risk. Typically, some form of functional assessment is indicated. In patients who are able to exercise, this would consist of exercise stress testing, often with an imaging modality. If the stress test is abnormal, cardiac catheterization is often the next step.
Case Study (cont)
An ECG is rapidly obtained on this patient and there is ST‐segment depression in leads II, III, and aVF. A bedside troponin is positive. The patient is at high risk of ischemic complications. He is diagnosed with nonST‐segment elevation MI. The next step is to initiate medical therapy. Presumably, the patient would have already (or at least should have already) received aspirin. Chewing or swallowing a dose of 325 mg nonenteric coated aspirin should provide a prompt aspirin effect. It would be reasonable to initiate anticoagulation as well, and the guidelines support a number of choices such as unfractionated heparin or low molecular weight heparin. Consideration should also be given to starting additional antiplatelet therapy, such as a loading dose of clopidogrel. Although aspirin provides some degree of antiplatelet effect, in a patient with activated platelets who presents with an ACS, additional antiplatelet therapy is necessary, although the exact timing of it is a matter of debate. Finally, consideration needs to be given to the need for catheterization. This patient, on the basis of his high ischemic risk and lack of obvious contraindications, should go to the catheterization laboratory, and the timing of catheterization requires further thought.
Guideline Update
The American College of Cardiology/American Heart Association 2009 Focused Guideline Update provides new information and recommendations pertinent to the care of patients with ACS2 and incorporates new data relevant to the initial emergency care and subsequent inpatient care of patients with ACS. Guideline highlights are presented in Table 4.
| Intervention | Recommendation |
|---|---|
| |
| GP IIb/IIIa receptor antagonists | |
| Class IIa | Start abciximab, tirofiban, or eptifibatide at primary PCI (with/without stenting) in selected patients with STEMI. |
| Class IIb | Uncertain value in STEMI when given before arrival at catheterization lab. |
| Thienopyridines | |
| Class I | Use loading dose for planned PCI in STEMI. Regimens for primary and nonprimary PCI are detailed within the guideline. |
| Duration of therapy after stent placement of at least 12 months. Stop early if bleeding risk outweighs benefit. | |
| Discontinue before planned, delayed CABG (5 d clopidogrel; 7 d prasugrel) unless the need for CABG outweighs bleeding risk. | |
| Class IIb | After DES placement, consider continuing clopidogrel or prasugrel beyond the first 15 months of therapy. |
| Class III | Prasugrel is not recommended for primary PCI in patients with STEMI who have a history of stroke or TIA. |
| Parenteral anticoagulants | |
| Class I | In primary PCI, supportive anticoagulants include unfractionated heparin, enoxaparin, fondaparinux, or bivalirudin, following ASA and a thienopyridine. |
| Class IIa | Bivalirudin is reasonable in patients at high risk of bleeding undergoing PCI for STEMI. |
| Triage and transfer for PCI | |
| Class I | STEMI system of care is supported by dedicated teams and protocols required for all communities. |
| Class IIa | Transfer patients who received fibrinolytic therapy at a nonPCI‐capable facility to a PCI‐capable facility. Consider preparatory antithrombotic regimen before or during transfer. |
| Class IIb | Consider expeditious transfer of nonhigh‐risk patients from a nonPCI‐capable facility to a PCI‐capable facility after administration of fibrinolytic. Consider preparatory antithrombotic regimen before or during transfer. |
| Intensive glucose control in STEMI | |
| Class IIa | Insulin is reasonable to maintain glucose <180 mg/dL (avoid hypoglycemia) for any patient with STEMI. |
| Thrombus aspiration during PCI of STEMI | |
| Class IIa | Aspiration thombectomy is reasonable. |
| Use of stents in STEMI | |
| Class IIa | DES is a reasonable alternative to BMS for primary PCI in STEMI. |
| Class IIb | Consider DES when clinical or anatomical factors suggest favorable safety and efficacy for DES. |
| Angiography in CKD | |
| Class I | Isomolar contrast or low molecular weight contrast (not ioxaglate or iohexol) is indicated for CKD patients not on dialysis. |
| Fractional flow reserve | |
| Class IIa | FFR is useful to assess a specific coronary lesion or as an alternative to noninvasive functional testing to justify PCI. Reasonable for intermediate coronary stenosis in patients with angina. |
| Class III | Routine use of FFR is not recommended to assess severity of CAD in patients with angina who have had a positive, unequivocal, noninvasive functional study. |
| PCI for unprotected left main CAD | |
| Class IIb | PCI of left main coronary artery with stents is an alternative to CABG for anatomy associated with low risk of PCI complications and a clinical scenario with higher risk of adverse surgical outcomes. |
| Timing of angiography and antiplatelet therapy in UA/NSTEMI | |
| Class I | Initiate dual‐antiplatelet therapy for UA/NSTEMI and an invasive approach. Start ASA on presentation. Clopidogrel (before or at PCI) or prasugrel (at PCI) as a second antiplatelet agent. |
| Class IIa | Early invasive strategy within 12 to 24 hours of admission is reasonable for stabilized high‐risk UA/NSTEMI; an early approach is also reasonable for UA/STEMI not at high‐risk. |
Several studies support an invasive strategy to assess high‐risk ACS patients. Randomized clinical trials and meta‐analyses of these trials have confirmed a significant reduction in subsequent ischemic events, including mortality, in patients who undergo an invasive vs. a more conservative strategy.4 Registry data have confirmed that these randomized clinical trial data reflect patients in the real‐world setting of clinical practice.5
The timing of angiography has recently been examined in detail.6, 7 It appears that for patients with nonST‐segment elevation ACS, unlike those with ST‐segment elevation MI, there is no need for emergent transfer to the catheterization laboratory, assuming patients are electrically and hemodynamically stable. Emergency transfer is warranted for unstable patients and those with ongoing chest discomfort. Otherwise, it appears sufficient to send the patient with nonST‐segment elevation ACS for catheterization within the subsequent 48 hours, or, alternatively, to adopt a more expectant approach in which catheterization is deferred until either recurrent symptoms develop or risk stratification suggests that there is substantial myocardium in jeopardy.
PCI is performed in the catheterization laboratory most often in the setting of ACS.5 When PCI is performed, an important consideration is whether to use a bare metal stent or a drug‐eluting stent.8 Drug‐eluting stents have been shown to have a significant benefit in reducing restenosis and the need for repeat revascularization. However, in aggregate, they have not been shown to either increase or decrease mortality.9 A key issue for the referring physician is to ascertain whether patients who go to the catheterization laboratory are likely to tolerate and be compliant with prolonged dual antiplatelet therapy. If it appears that the patient can or will not be compliant, a bare metal stent is preferable to a drug‐eluting stent; a bare metal stent requires dual antiplatelet therapy of shorter duration.
Additional considerations when sending patients to the catheterization laboratory are related to renal function. In patients with renal dysfunction, the most important way to prevent contrast nephropathy is adequate hydration prior to the procedure. In patients with left ventricular dysfunction, hydration must be done judiciously. Other strategies for preventing contrast nephropathy are being studied, although it is not entirely clear which strategies beyond hydration are truly effective.
Use of upstream glycoprotein IIb/IIIa inhibitors has become more common in patients with nonST‐segment elevation ACS. However, the most recent trial to examine this issue, the Early Glycoprotein IIb/IIIa Inhibition in NonST‐Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial, did not find a clear benefit for routine administration of upstream glycoprotein IIb/IIIa inhibitors when studying all patients with ACS.10 There did appear to be a signal of benefit in troponin‐positive patients, but as an overall strategy no significant benefit and even some detriment associated with an increase in bleeding were shown. Similarly, the results of the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial did not support the benefit of upstream glycoprotein IIb/IIIa inhibitors in patients with ACS.11
New data have also been released with respect to the thienopyridines. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with PrasugrelThrombolysis in Myocardial Infarction (TRITONTIMI) 38 study found that the more potent thienopyridine prasugrel significantly reduced ischemic events when compared with clopidogrel in patients with ACS undergoing PCI.1214 A significant reduction in stent thrombosis was reported regardless of the type of stent.15 However, the study reported a significant increase in major bleeding and a small, but statistically significant, excess of fatal bleeding. A subgroup analysis of patients with diabetes or with ST‐segment elevation MI from the TRITON‐TIMI 38 study showed a particularly large benefit associated with the use of prasugrel vs. clopidogrel and, interestingly, bleeding hazards were attenuated in these subgroups.16, 17 In the small subgroup of patients with prior stroke or transient ischemic attack (TIA), there was an excessive rate of intracranial hemorrhage with prasugrel vs. clopidogrel, indicating that prasugrel should not be used in these patients. Patients age 75 years or older or who weighed less than 60 kg also appeared to have a higher bleeding risk with prasugrel compared to clopidogrel. Careful thought is needed before using prasugrel in those patients identified as having a higher risk of bleeding.
Recently, a higher clopidogrel loading dose of 600 mg vs. the standard 300 mg dose was tested in patients who presented with ACS in the Clopidogrel optimal loading dose Usage to Reduce Recurrent EveNTs‐Organization to Assess Strategies in Ischemic Syndromes (CURRENT‐OASIS) 7 trial.18 Patients also received 150 mg of clopidogrel daily for the ensuing 6 days vs. the standard 75 mg daily dose. All patients then received 75 mg clopidogrel for 1 month of follow‐up. In the overall population, there was no benefit to using the higher clopidogrel loading dose. In contrast, there was a significant reduction in stent thrombosis in patients who received stents. The higher loading dose of clopidogrel was associated with a higher rate of bleeding.
Ticagrelor is a novel adenosine diphosphate receptor antagonist that was compared with clopidogrel in patients with ACS.19, 20 Compared to clopidogrel, ticagrelor significantly reduced ischemic events and there was also a significant reduction in cardiovascular mortality and in all‐cause mortality. Surprisingly, overall major bleeding did not increase with ticagrelor, but nonCABG‐related major bleeding increased.
The use of proton pump inhibitors (PPIs) in patients receiving dual antiplatelet therapy has also been a matter of vigorous recent debate.21 Evidence to date suggests there is no significant clinical interaction between PPIs and prasugrel. The data with clopidogrel and PPIs are mixed, although data are limited because much were derived from observational studies. Randomized clinical trial data are needed to assess whether there is an interaction between clopidogrel and PPIs that warrants clinical action, although preliminary data suggest there is no adverse cardiovascular interaction.22
New data regarding the intravenous anticoagulant bivalirudin have become available and have been incorporated into the Focused Guideline Update. Although bivalirudin is to be used primarily in the catheterization laboratory during PCI, it does appear to be associated with significantly less bleeding than heparin plus glycoprotein IIb/IIIa inhibitors.11, 2326
Case Study (cont)
The patient undergoes cardiac catheterization. An occluded dominant left circumflex artery is noted and is opened up with balloon angioplasty after aspiration thrombectomy. The patient receives 60 mg of prasugrel as a loading dose and bivalirudin as the anticoagulant during the procedure. A drug‐eluting stent is implanted with excellent results. The patient is transferred to the cardiac care unit for further care. It appears that this is a patient who functionally has an ST‐segment elevation MI with an occluded artery, although it manifested on the ECG as ST depression. Because of the patient's ongoing chest discomfort, it was fortunate that prompt angiography was performed.
Discussion
Patients with ACS present several challenges in management. Risk stratification is particularly important for nonST‐segment elevation ACS and requires thoughtful evaluation by the physician. Additionally, the large amount of new data and guideline updates create a rapidly evolving field, making it difficult to keep abreast of new developments. Physicians of patients with ACS need to be aware of these key developments so that they can provide optimal care to their patients with potentially life‐threatening ACS.
Acknowledgements
Denise M. Erkkila, RPh of DIME, provided editorial assistance consisting of help with tables, figures, and reference formatting for this manuscript.
- ,,, et al.Heart disease and stroke statistics‐‐2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,, et al.2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol.2009;54:2205–2241.
- ,,, et al.Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes.JAMA.2009;302:2207–2213.
- ,,,,.Benefit of early invasive therapy in acute coronary syndromes: a meta‐analysis of contemporary randomized clinical trials.J Am Coll Cardiol.2006;48:1319–1325.
- ,,, et al.Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative.JAMA.2004;292:2096–2104.
- ,,, et al.Early versus delayed invasive intervention in acute coronary syndromes.N Engl J Med.2009;360:2165–2175.
- ,,, et al.Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial.JAMA.2009;302:947–954.
- ,.Drug‐eluting stents: dual antiplatelet therapy for every survivor?Circulation.2007;116:696–699.
- ,.Appropriate use of drug‐eluting stents: balancing the reduction in restenosis with the concern of late thrombosis.Lancet.2008;371:2134–2143.
- ,,, et al.Early versus delayed, provisional eptifibatide in acute coronary syndromes.N Engl J Med.2009;360:2176–2190.
- ,,, et al.Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one‐year results from the ACUITY trial.JAMA.2007;298:2497–2506.
- .Intensifying platelet inhibition‐‐navigating between Scylla and Charybdis.N Engl J Med.2007;357:2078–2081.
- .Prasugrel in clinical practice.N Engl J Med.2009;361:940–942.
- ,,, et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2007;357:2001–2015.
- ,,, et al.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON‐TIMI 38 trial: a subanalysis of a randomised trial.Lancet.2008;371:1353–1363.
- ,,, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial.Lancet.2009;373:723–731.
- ,,, et al.Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel—Thrombolysis in Myocardial Infarction 38.Circulation.2008;118:1626–1636.
- , CURRENT Investigators. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Available at: http://www.escardio.org/congresses/esc‐2009/congress‐reports/Pages/706003‐706004‐mehta‐vandewerf.aspx#discussant.2009. Accessed July 2010.
- .Antiplatelet therapy: ticagrelor in ACS‐what does PLATO teach us?Nat Rev Cardiol.2009;6:737–738.
- ,,, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2009;361:1045–1057.
- ,.Omeprazole and clopidogrel: Should clinicians be worried?Cleve Clin J Med.2010;77:113–116.
- .COGENT: a prospective, randomized, placebo‐controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Transcatheter Cardiovascular Therapeutics (TCT) 2009; September 24,2009;San Francisco, CA.
- ,,, et al.The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) Trial: study design and rationale.Am Heart J.2008;156:44–56.
- ,,, et al.Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial.Lancet.2009;374:1149–1159.
- ,,, et al.Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale.Am Heart J.2004;148:764–775.
- ,,, et al.Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial.Lancet.2007;369:907–919.
- Society of Hospital Medicine.Acute coronary syndrome.J Hosp Med.2006;1 (suppl 1):2–3.
- ,,, et al.The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making.JAMA.2000;284:835–842.
- ,,, et al.Heart disease and stroke statistics‐‐2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,, et al.2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol.2009;54:2205–2241.
- ,,, et al.Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes.JAMA.2009;302:2207–2213.
- ,,,,.Benefit of early invasive therapy in acute coronary syndromes: a meta‐analysis of contemporary randomized clinical trials.J Am Coll Cardiol.2006;48:1319–1325.
- ,,, et al.Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative.JAMA.2004;292:2096–2104.
- ,,, et al.Early versus delayed invasive intervention in acute coronary syndromes.N Engl J Med.2009;360:2165–2175.
- ,,, et al.Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial.JAMA.2009;302:947–954.
- ,.Drug‐eluting stents: dual antiplatelet therapy for every survivor?Circulation.2007;116:696–699.
- ,.Appropriate use of drug‐eluting stents: balancing the reduction in restenosis with the concern of late thrombosis.Lancet.2008;371:2134–2143.
- ,,, et al.Early versus delayed, provisional eptifibatide in acute coronary syndromes.N Engl J Med.2009;360:2176–2190.
- ,,, et al.Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one‐year results from the ACUITY trial.JAMA.2007;298:2497–2506.
- .Intensifying platelet inhibition‐‐navigating between Scylla and Charybdis.N Engl J Med.2007;357:2078–2081.
- .Prasugrel in clinical practice.N Engl J Med.2009;361:940–942.
- ,,, et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2007;357:2001–2015.
- ,,, et al.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON‐TIMI 38 trial: a subanalysis of a randomised trial.Lancet.2008;371:1353–1363.
- ,,, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial.Lancet.2009;373:723–731.
- ,,, et al.Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel—Thrombolysis in Myocardial Infarction 38.Circulation.2008;118:1626–1636.
- , CURRENT Investigators. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: results of the CURRENT OASIS 7 trial. Available at: http://www.escardio.org/congresses/esc‐2009/congress‐reports/Pages/706003‐706004‐mehta‐vandewerf.aspx#discussant.2009. Accessed July 2010.
- .Antiplatelet therapy: ticagrelor in ACS‐what does PLATO teach us?Nat Rev Cardiol.2009;6:737–738.
- ,,, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes.N Engl J Med.2009;361:1045–1057.
- ,.Omeprazole and clopidogrel: Should clinicians be worried?Cleve Clin J Med.2010;77:113–116.
- .COGENT: a prospective, randomized, placebo‐controlled trial of omeprazole in patients receiving aspirin and clopidogrel. Transcatheter Cardiovascular Therapeutics (TCT) 2009; September 24,2009;San Francisco, CA.
- ,,, et al.The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) Trial: study design and rationale.Am Heart J.2008;156:44–56.
- ,,, et al.Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial.Lancet.2009;374:1149–1159.
- ,,, et al.Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale.Am Heart J.2004;148:764–775.
- ,,, et al.Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial.Lancet.2007;369:907–919.
- Society of Hospital Medicine.Acute coronary syndrome.J Hosp Med.2006;1 (suppl 1):2–3.
- ,,, et al.The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making.JAMA.2000;284:835–842.