User login
Botulinum Toxin and Glycopyrrolate Combination Therapy for Hailey-Hailey Disease
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
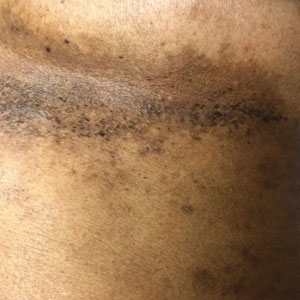
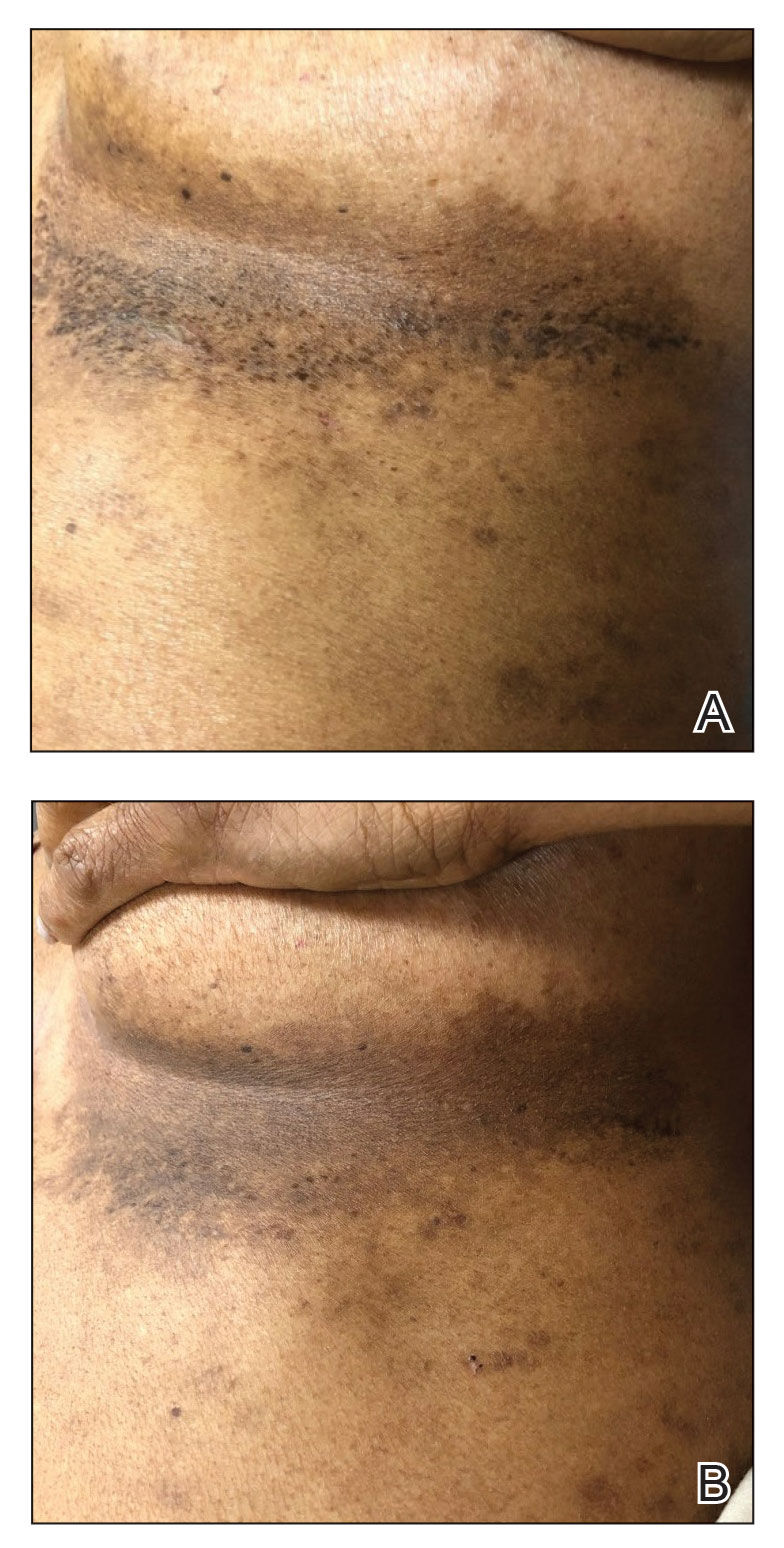
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.
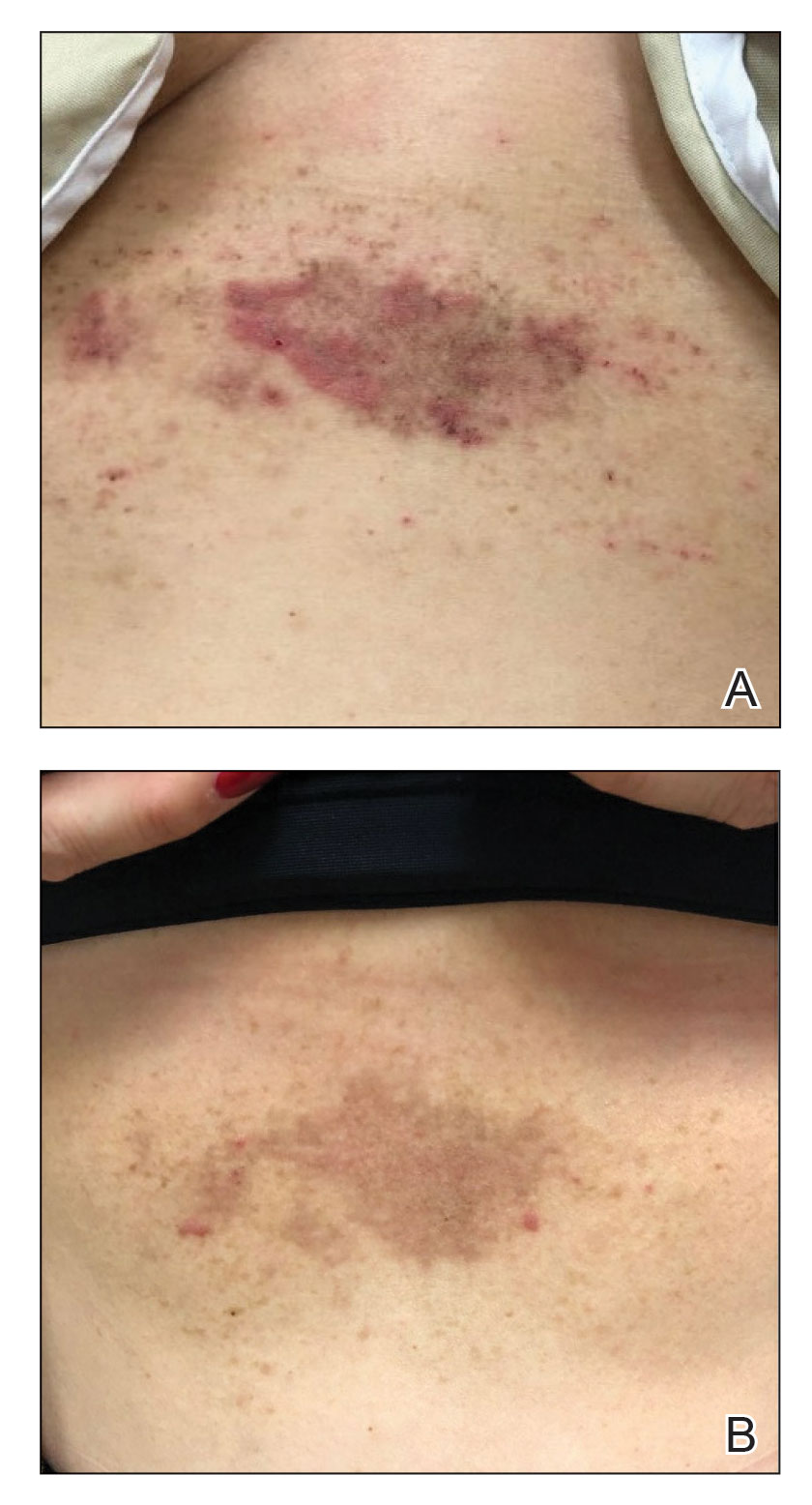
A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.
Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.

A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.

Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.

A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.

Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
Practice Points
- Hailey-Hailey disease is associated with decreased quality of life for patients, and current treatment options are limited.
- A combination of local neuromodulator injections and systemic oral anticholinergic therapy may provide sustained disease remission compared to neuromodulator therapy alone.
Methacrylate Polymer Powder Dressing for a Lower Leg Surgical Defect
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
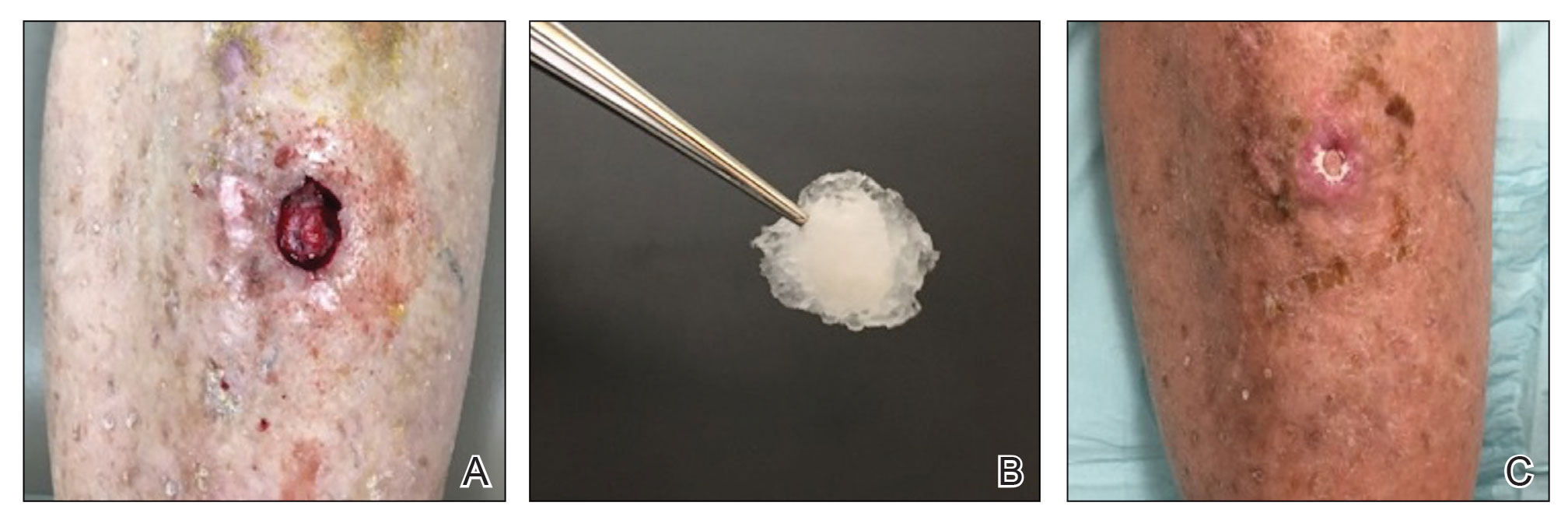
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
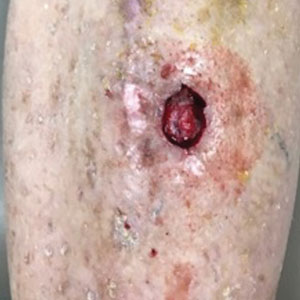
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.
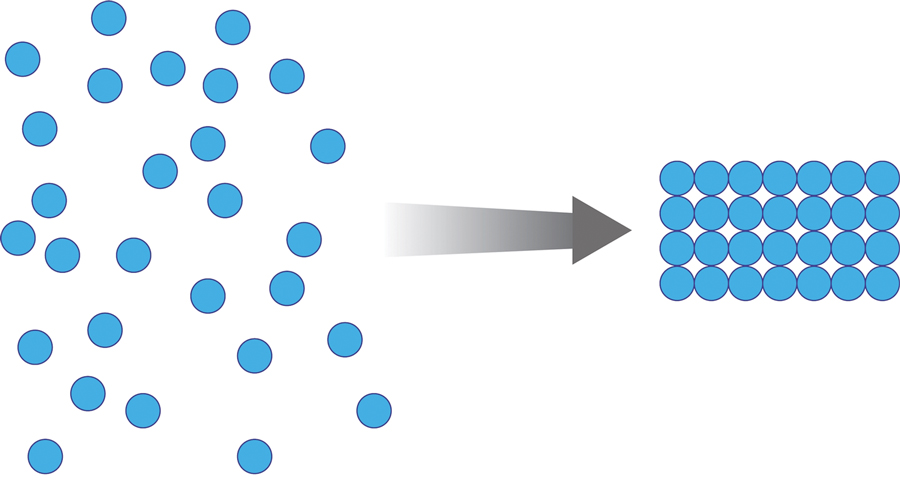
Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1
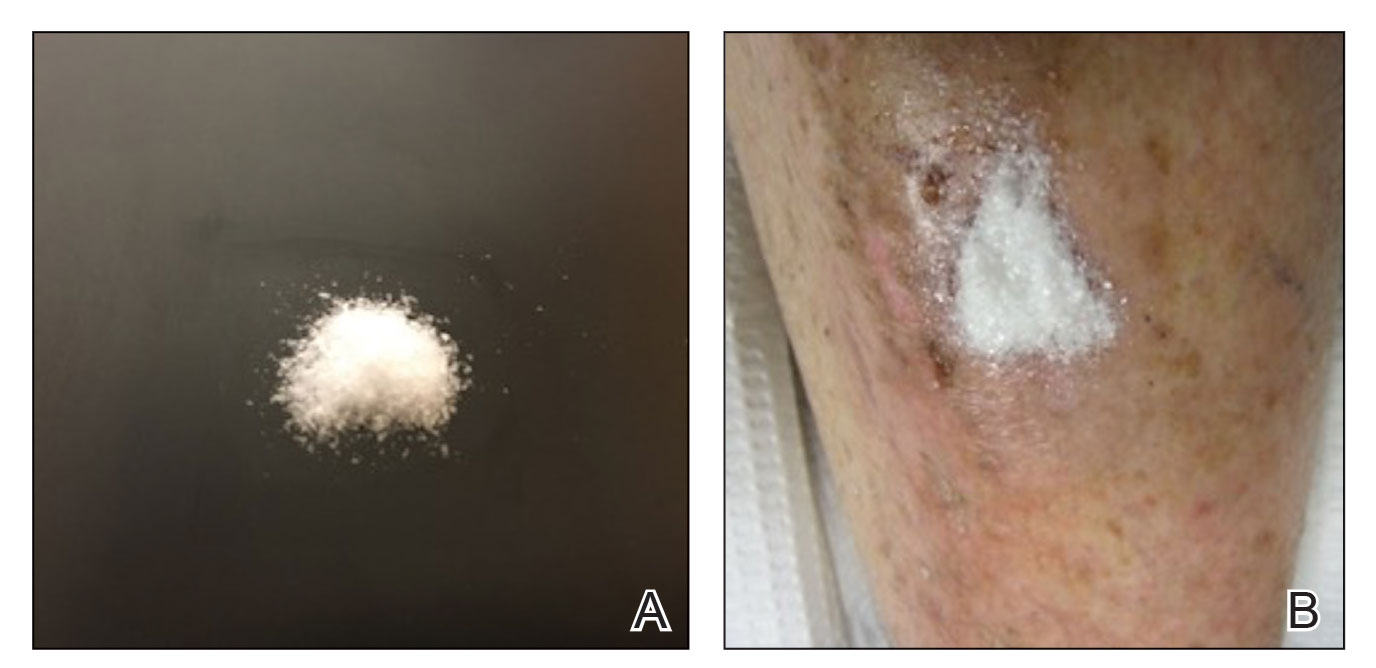
Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1
This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
PRACTICE POINTS
- Lower leg surgical wounds are difficult to manage, as venous stasis, bacterial colonization, and high tension may contribute to protracted healing.
- A methacrylate polymer powder dressing is user friendly and facilitates granulation and reduction in size of difficult lower leg wounds.
Buccal Fat Pad Reduction With Intraoperative Fat Transfer to the Temple
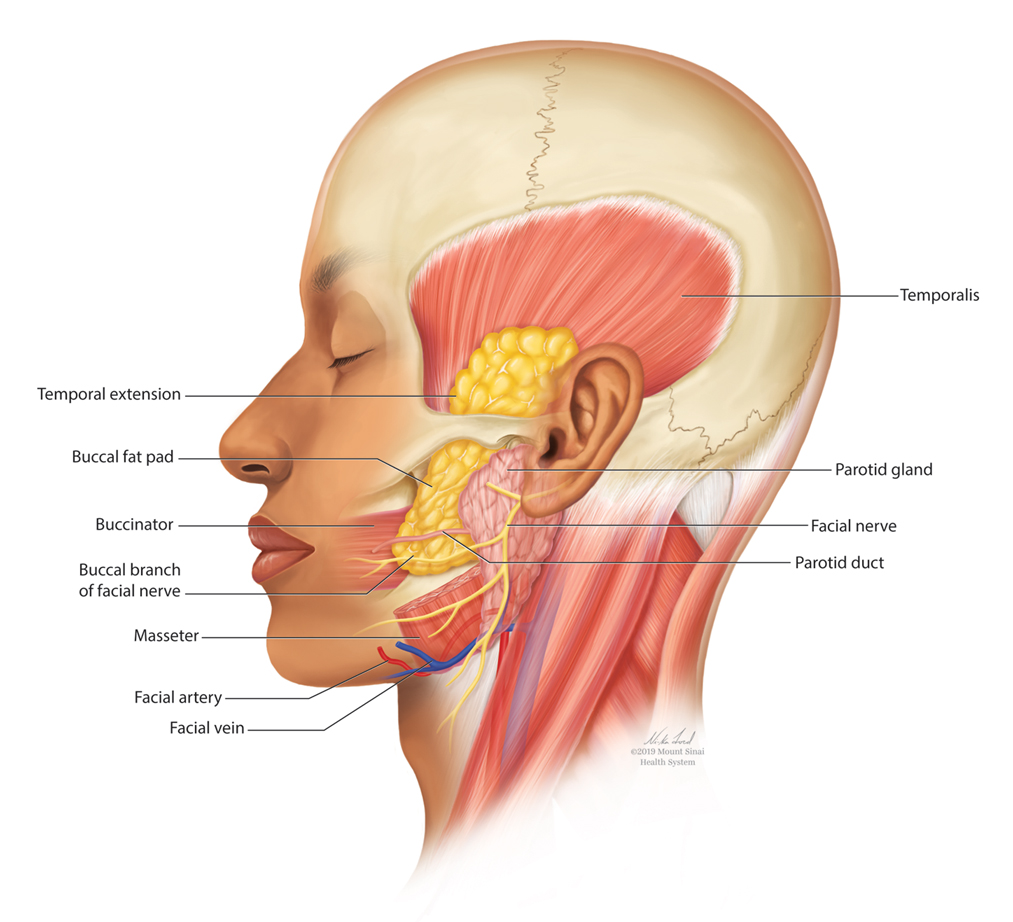
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
Practice Points
- Buccal fat pad reduction is an increasingly popular procedure for facial shaping.
- Buccal fat pad reduction in addition to natural aging can result in volume depletion of the temporal fossae.
- Removed buccal fat can be transferred to the temples for increased volume.