User login
Coding the “Spot Check”: Part 2
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
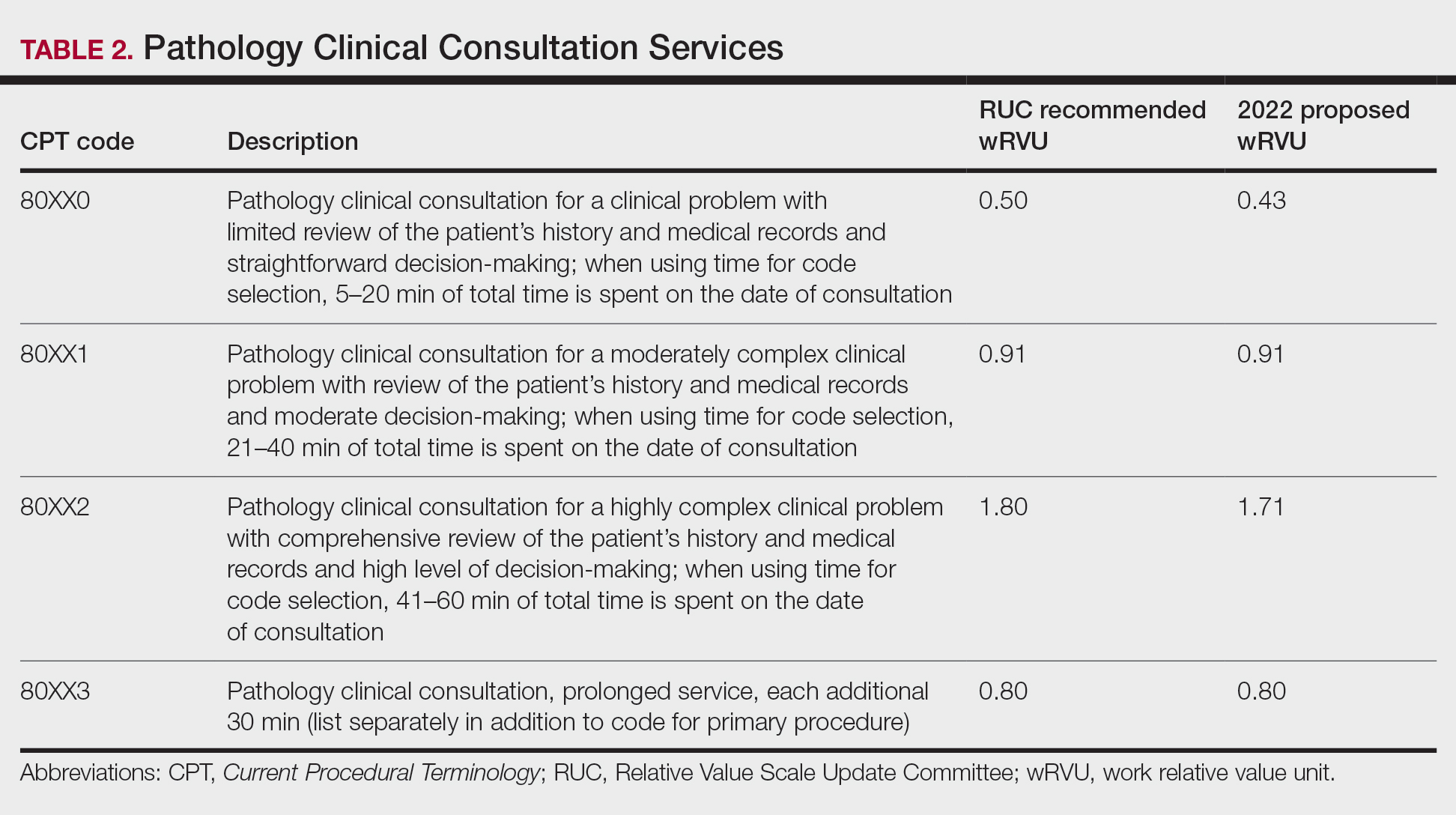
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
Coding the “Spot Check”: Part 1
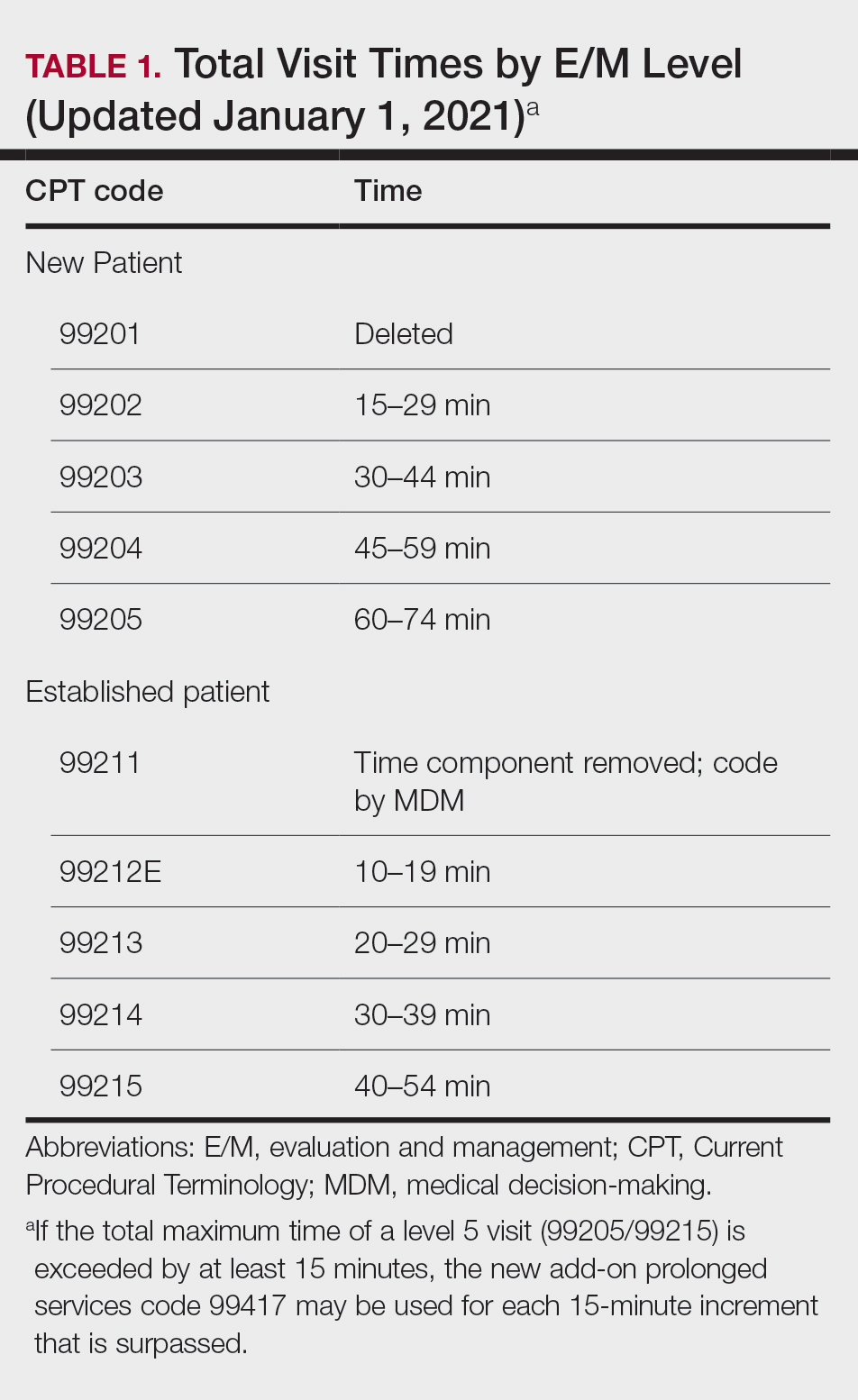
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
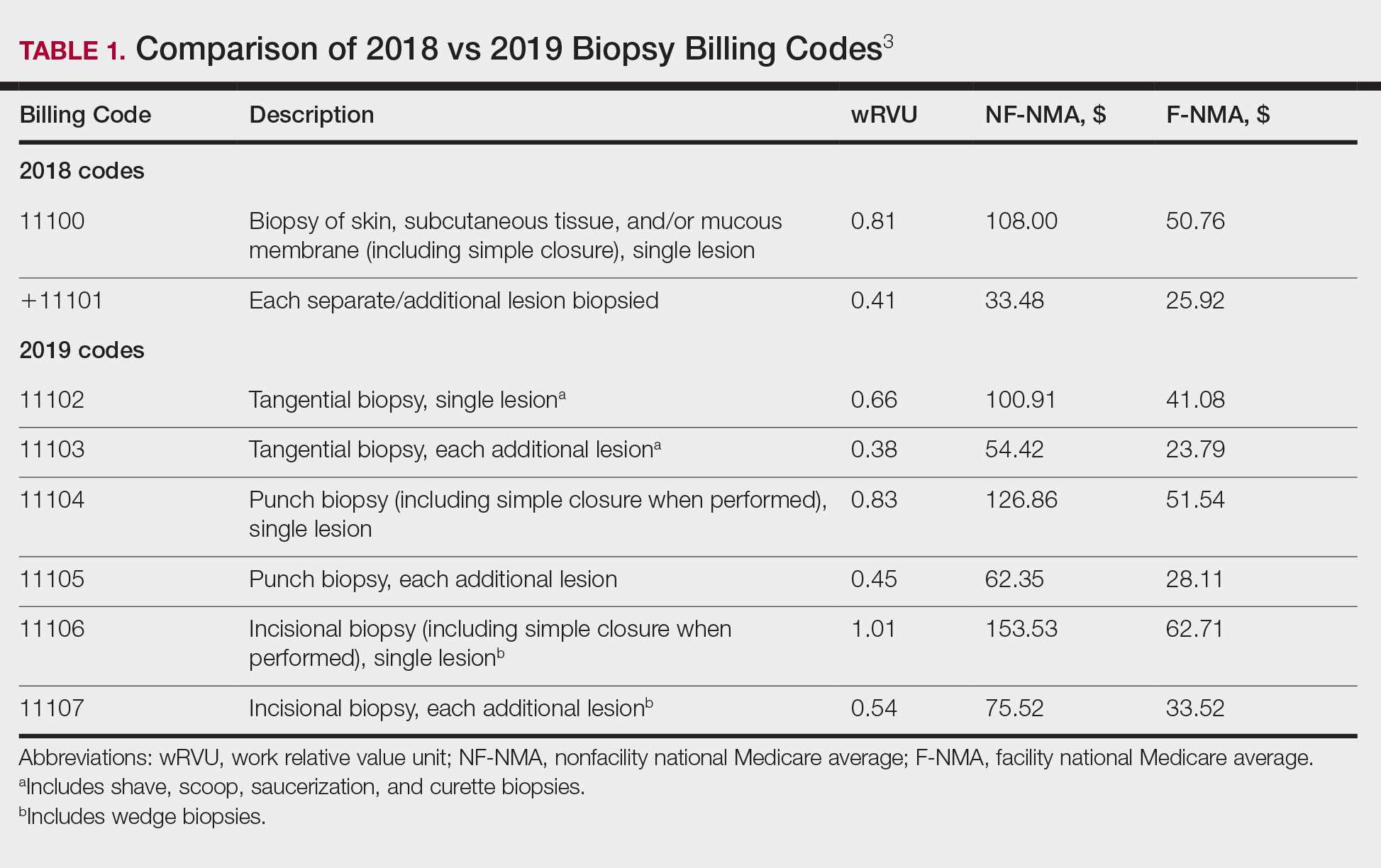
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
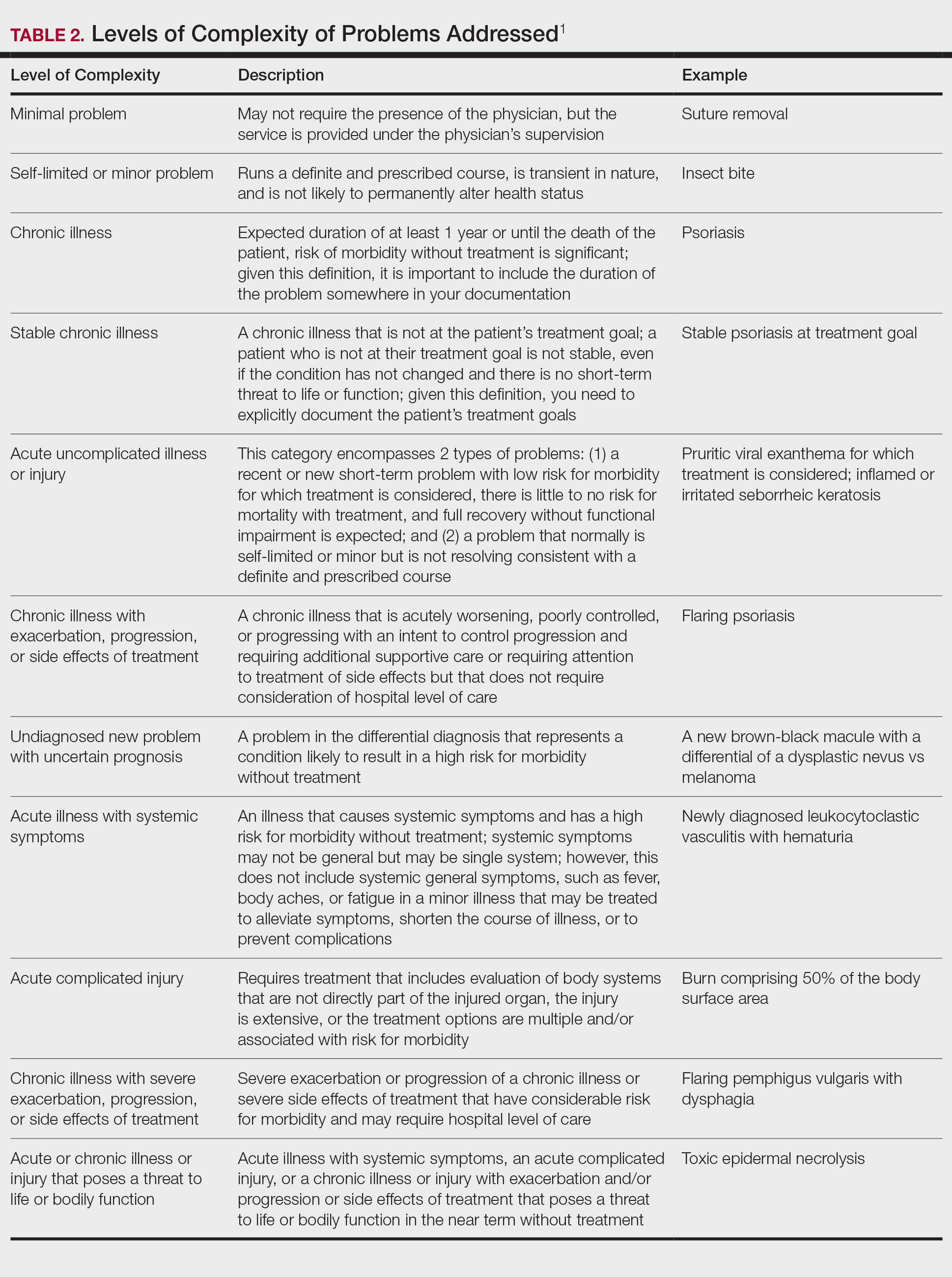
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
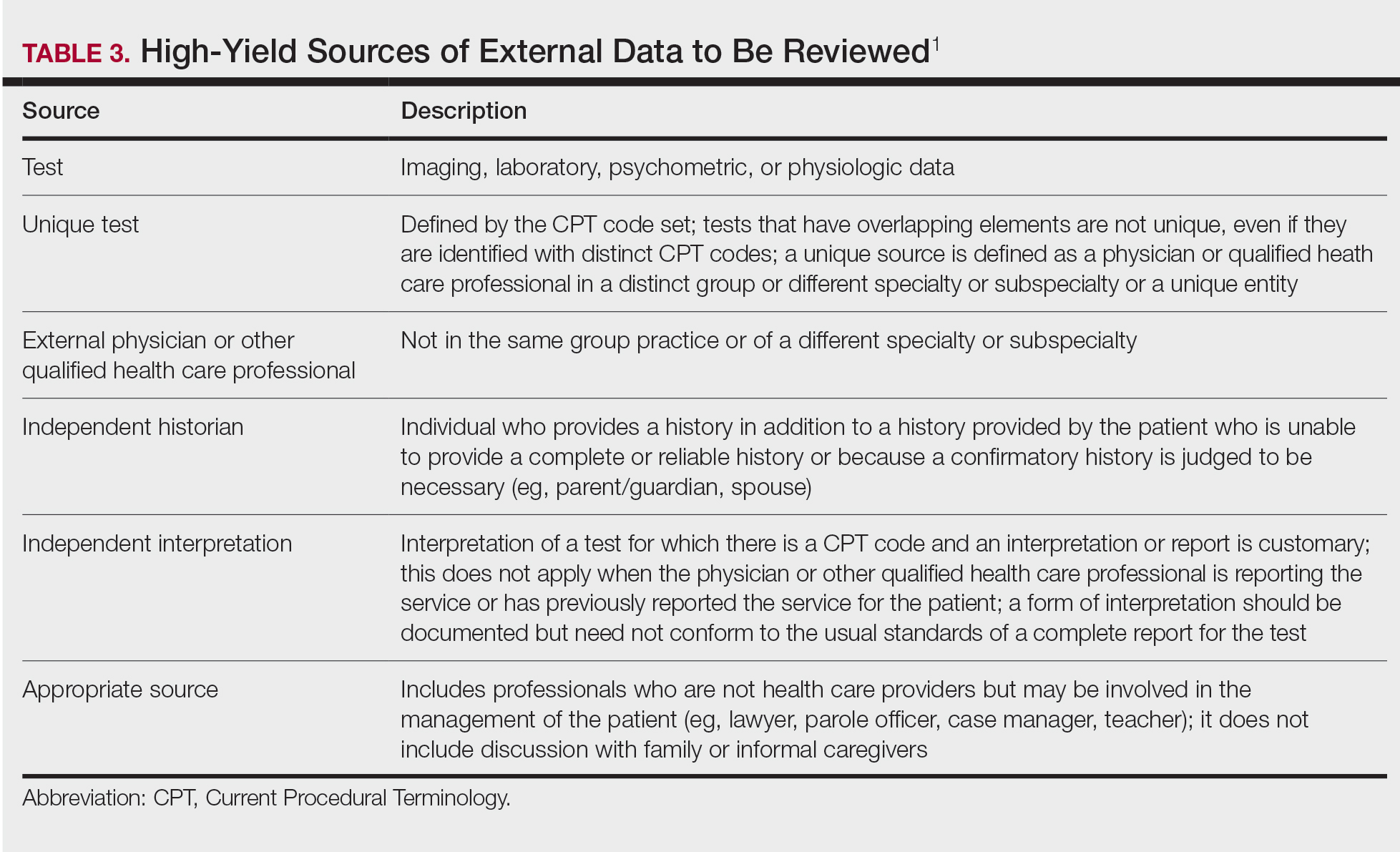
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
Proper Use and Compliance of Facial Masks During the COVID-19 Pandemic: An Observational Study of Hospitals in New York City
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
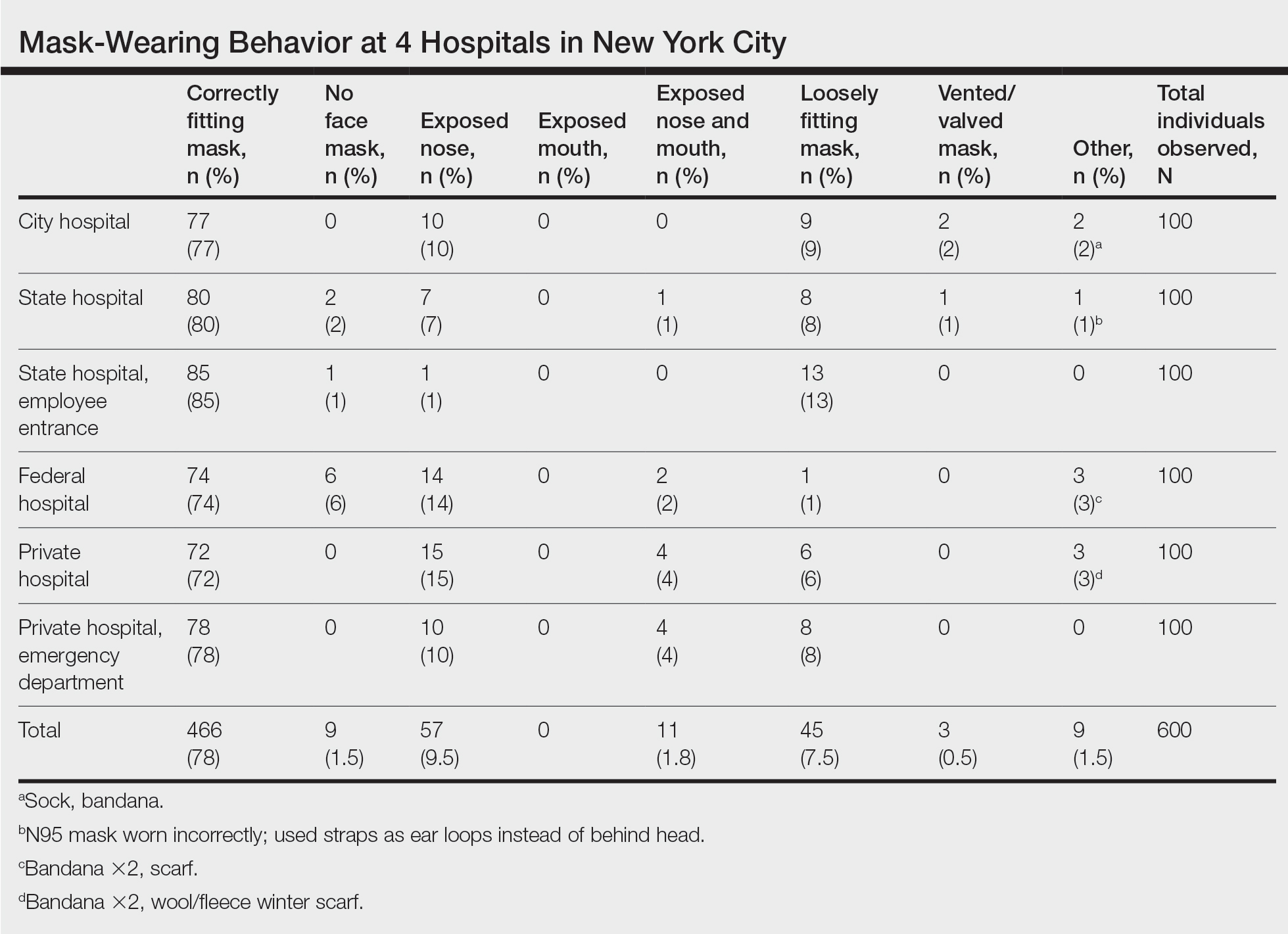
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Practice Points
- Enormous financial and human resources have been utilized by health care systems to prevent the spread of COVID-19 in health care settings, including universal temperature checks, clinical symptom triage, and masking policies. Despite these mitigation practices, mask noncompliance continues to be a major problem in hospitals.
- Mask compliance among 600 individuals entering 4 New York City hospitals was observed to be 78%, despite months of policies for universal masking and the city’s high mortality rates during the first COVID-19 wave.
- Masks have been shown to reduce the spread of COVID-19, and proper mask compliance is an important issue that must be addressed by health care administrations and governmental agencies.
Advocacy Update: Is Your Practice Equipped to Handle Looming Changes in Dermatopathology?
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
Practice Points
- A proposed 2022 fee schedule negatively impacting dermatopathology practices has been published by the Centers for Medicare & Medicaid Services (CMS) in July 2021.
- New pathology consultation codes with new payment rates proposed by CMS can be used starting January 1, 2022.
- The 21st Century Cures Act Final Rule has information blocking provisions.
Mobile App Usage Among Dermatology Residents in America
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Practice Points
- Virtual resources, including mobile apps, have become critical tools for learning and patient care during dermatology resident training for reasons that should be elucidated.
- Dermatology residents of different years and sexes utilize mobile apps in different amounts and for different purposes.
E/M Coding in 2021: The Times (and More) Are A-Changin’
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Practice Points
- The outpatient evaluation and management (E/M) codes have undergone substantial changes that took effect January 1, 2021.
- Outpatient E/M visits are now coded based on time or level of medical decision-making (MDM).
- Time now includes all preservice, intraservice, and postservice time the physician spends with the patient on the date of the encounter.
- Many of the key definitions used in order to determine level of MDM have been streamlined and updated.
Virtual Dermatology: A COVID-19 Update
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.
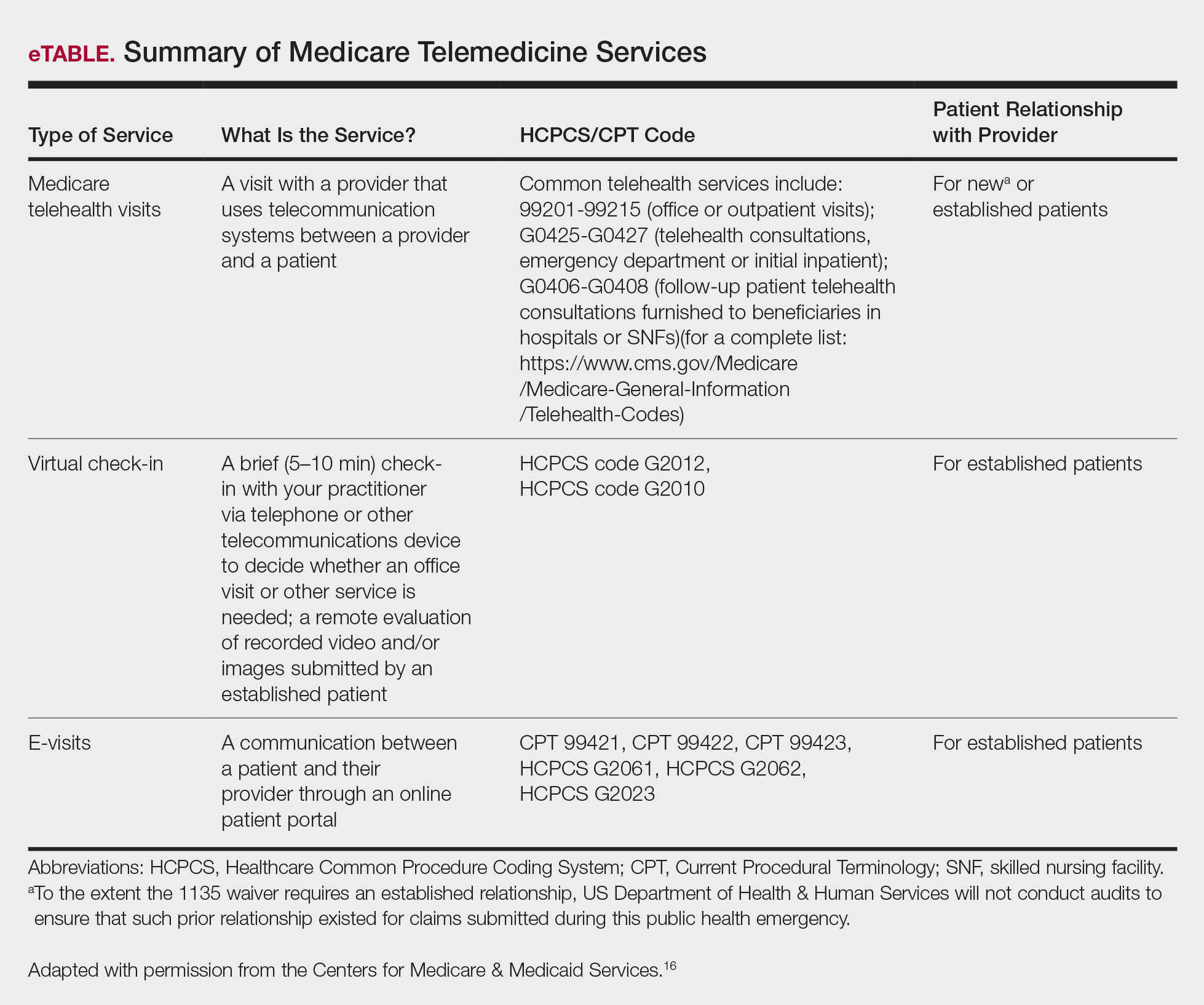
Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
New Diagnostic Procedure Codes and Reimbursement
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.
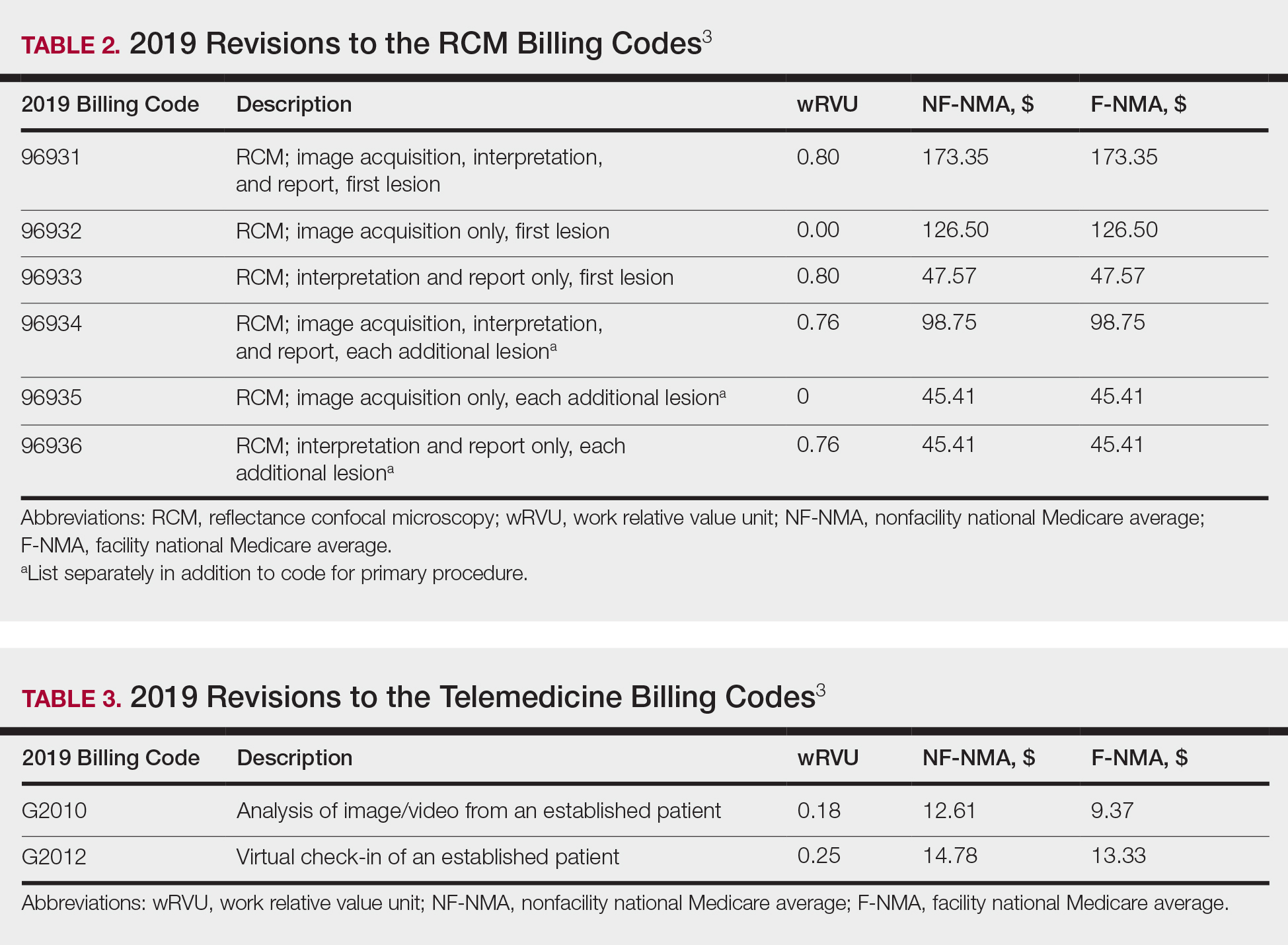
Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.

Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.

Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
PRACTICE POINTS
- Reimbursement typically is proportional to the relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.
- The total RVU consists of the work RVU, practice expense RVU, and malpractice expense RVU.
- The new 2019 biopsy codes reflect the complexity of the sampling technique (ie, whether the biopsy is tangential, punch, or incisional).
- Accurate completion of Relative Value Scale Update Committee surveys sent to practitioners will allow RVUs to be valued appropriately.
Topical Natural Products in Managing Dermatologic Conditions: Observations and Recommendations
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Practice Points
- Patients are increasingly interested in and asking for nature-based products and formulations to manage dermatologic conditions.
- Physicians can satisfy patient interests with nature-based formulations that are as beneficial or more so than synthetic formulations because of the physiologic activity of the ingredients within these formulations.
- Physicians should have resources available to them that adequately educate on nature-based ingredients and how to recommend them.
Strategies to Reduce Youth Indoor Tanning Injuries
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.