User login
Cutting Edge Technology in Dermatology: Virtual Reality and Artificial Intelligence
The clinical practice of dermatology is changing at a rapid pace. Advances in technology and new inventions in rapid diagnostics are revolutionizing how physicians approach medical care.
Given these advances, how does today’s dermatologist integrate into the future of the specialty? If leveraged properly, current technologies such as teledermatology and patient portals integrated with electronic medical records can be beneficial to dermatology practices by improving access to care, facilitating triage of patients, and improving communication between patients and health care team members. Herein, we discuss some of the emerging technologies that have the potential to shape clinical dermatology practice and remove barriers to care.
Virtual Reality
Teledermatology can be practiced through live video or, more commonly, via a store-and-forward method in which dermatologists review clinical photographs and the patient's history asynchronously with the in-office visit.6 Virtual reality has the potential to augment teledermatology services by enabling a live, interactive visit that more closely models the traditional face-to-face visit. Virtual reality already is available for patients at home with the use of a commercially marketed headset and a smartphone, and the marriage of virtual reailty and telemedicine has the potential to transform health care.
Virtual reality also can be used to deliver an essential component of the physical examination of a patient: sensory information from palpation. Haptic feedback, also known as haptics, is used to relay force and tactile information to the user of a device (eg, a haptic glove).7 In dermatology, this information pertains to the skin texture, skin profile, and physical properties (eg, stiffness, temperature).8 Assessing the texture of the skin surface can help when distinguishing epidermal processes such as psoriasis versus atopic dermatitis or when evaluating edema, induration, and depth of a leg ulcer.9
One model for conducting a teledermatology encounter that captures sensory information would consist of a haptic probe located at a referring medical provider’s office for examining patients and a master robot that controls the probe located at the consulting dermatologist’s facility.8 Another model converts 2-dimensional images taken from traditional full-body optical imaging systems into virtual 3-dimensional (3D) images that can be felt using a haptic device.10,11 In this method, the user is able to both visualize and touch the skin surface at the same time. Currently, 3D imaging of skin lesions is available in the form of a specialized handheld imager that allows the dermatologist to appreciate the texture and elevation of single lesions when viewing clinical photographs. Additionally, full-body 3D mapping of the skin surface is available for monitoring pigmented lesions or other diseases of the skin.12,13
Artificial Intelligence and Machine Learning
Computer algorithms can be helpful in assisting physicians with disease diagnosis. Machine learning is a subfield of artificial intelligence (AI) in which computer programs learn automatically from experience without explicit programming instructions. A machine learning algorithm uses a labeled data set known as a training set to create a function that can make predictions about new inputs in the future.14 The algorithm can successively compare its predicted outputs with the correct outputs and modify its function as errors are found; for example, a database of images of healthy skin as well as the skin of psoriasis patients can be fed into a machine learning algorithm that picks up features such as color and skin texture from the labeled photographs, allowing it to learn how to diagnose psoriasis.15
In one instance, researchers at Stanford University (Stanford, California) used approximately 130,000 images representing over 2000 different skin diseases in order to train their machine learning algorithm to recognize benign and malignant skin lesions.4 Although the algorithm was able to match the performance of experienced dermatologists in many diagnostic categories, further testing in a real-world clinical setting still needs to be done. In the future, nondermatologists may have the option to consult with decision-support systems that include image analysis software, such as the one developed at Stanford University, for making decisions in triage or diagnosis, which may be critical in areas where access to a dermatologist is limited.4 Future AI systems also may provide supplemental assistance in managing patients to dermatology trainees until they have the experience of a more established dermatologist.
Currently, AI cannot match general human intelligence and life experience; therefore, physicians will continue to make the final decisions when it comes to diagnosis and treatment. In the future, AI algorithms may integrate into clinical dermatology practice, leading to more accurate triage of lesions, potentially streamlined referral to dermatologists for skin conditions that require prompt consultation, and improved quality of care.
Summary
In conclusion, emerging technologies have the power to augment and revolutionize dermatology practice. Savvy dermatologists may incorporate new tools in a way that works for their practice, leading to increased efficiency and improved patient outcomes. Eventually, the technology that is most beneficial to clinical practice will likely be adopted by and integrated into mainstream dermatologic care, making it available for the majority of clinicians to use.
- Health Information Technology for Economic and Clinical Health (HITECH) Act, Pub L No. 111-5, 123 Stat 226 (2009).
- Holmgren AJ, Adler-Milstein J. Health information exchange in US hospitals: the current landscape and a path to improved information sharing. J Hosp Med. 2017;12:193-198.
- The IMLC. Interstate Medical Licensure Compact website. http://www.imlcc.org. Accessed March 21, 2018.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The Associated Press. Microsoft eyes buffer zone in TV airwaves for rural internet. ABC News website. http://abcnews.go.com/amp/Technology/wireStory/microsoft-announces-rural-broadband-initiative-48562282. Published July 11, 2017. Accessed March 21, 2018.
- Practice guidelines for dermatology. American Telemedicine Association website. https://higherlogicdownload.s3.amazonaws.com/AMERICANTELEMED/3c09839a-fffd-46f7-916c-692c11d78933/UploadedImages/SIGs/Teledermatology.Final.pdf. Accessed March 27, 2018. Published April 28, 2016.
- Lee O, Lee K, Oh C, et al. Prototype tactile feedback system for examination by skin touch. Skin Res Technol. 2014;20:307-314.
- Waldron KJ, Enedah C, Gladstone H. Stiffness and texture perception for teledermatology. Stud Health Technol Inform. 2005;111:579-585.
- Cox NH. A literally blinded trial of palpation in dermatologic diagnosis. J Am Acad Dermatol. 2007;56:949-951.
- Kim K. Roughness based perceptual analysis towards digital skin imaging system with haptic feedback. Skin Res Technol. 2016;22:334-340.
- Kim K, Lee S. Perception-based 3D tactile rendering from a single image for human skin examinations by dynamic touch. Skin Res Technol. 2015;21:164-174.
- How it works. 3Derm website. https://www.3derm.com. Accessed March 21, 2018.
- Vectra 3D. Canfield Scientific website. http://www.canfieldsci.com/imaging-systems/vectra-wb360-imaging-system. Accessed March 21, 2018.
- Alpaydin E. Introduction to Machine Learning. Cambridge, MA: MIT Press; 2014.
- Shrivastava VK, Londhe ND, Sonawane RS, et al. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: a first comparative study of its kind. Comput Methods Programs Biomed. 2016;126:98-109.
The clinical practice of dermatology is changing at a rapid pace. Advances in technology and new inventions in rapid diagnostics are revolutionizing how physicians approach medical care.
Given these advances, how does today’s dermatologist integrate into the future of the specialty? If leveraged properly, current technologies such as teledermatology and patient portals integrated with electronic medical records can be beneficial to dermatology practices by improving access to care, facilitating triage of patients, and improving communication between patients and health care team members. Herein, we discuss some of the emerging technologies that have the potential to shape clinical dermatology practice and remove barriers to care.
Virtual Reality
Teledermatology can be practiced through live video or, more commonly, via a store-and-forward method in which dermatologists review clinical photographs and the patient's history asynchronously with the in-office visit.6 Virtual reality has the potential to augment teledermatology services by enabling a live, interactive visit that more closely models the traditional face-to-face visit. Virtual reality already is available for patients at home with the use of a commercially marketed headset and a smartphone, and the marriage of virtual reailty and telemedicine has the potential to transform health care.
Virtual reality also can be used to deliver an essential component of the physical examination of a patient: sensory information from palpation. Haptic feedback, also known as haptics, is used to relay force and tactile information to the user of a device (eg, a haptic glove).7 In dermatology, this information pertains to the skin texture, skin profile, and physical properties (eg, stiffness, temperature).8 Assessing the texture of the skin surface can help when distinguishing epidermal processes such as psoriasis versus atopic dermatitis or when evaluating edema, induration, and depth of a leg ulcer.9
One model for conducting a teledermatology encounter that captures sensory information would consist of a haptic probe located at a referring medical provider’s office for examining patients and a master robot that controls the probe located at the consulting dermatologist’s facility.8 Another model converts 2-dimensional images taken from traditional full-body optical imaging systems into virtual 3-dimensional (3D) images that can be felt using a haptic device.10,11 In this method, the user is able to both visualize and touch the skin surface at the same time. Currently, 3D imaging of skin lesions is available in the form of a specialized handheld imager that allows the dermatologist to appreciate the texture and elevation of single lesions when viewing clinical photographs. Additionally, full-body 3D mapping of the skin surface is available for monitoring pigmented lesions or other diseases of the skin.12,13
Artificial Intelligence and Machine Learning
Computer algorithms can be helpful in assisting physicians with disease diagnosis. Machine learning is a subfield of artificial intelligence (AI) in which computer programs learn automatically from experience without explicit programming instructions. A machine learning algorithm uses a labeled data set known as a training set to create a function that can make predictions about new inputs in the future.14 The algorithm can successively compare its predicted outputs with the correct outputs and modify its function as errors are found; for example, a database of images of healthy skin as well as the skin of psoriasis patients can be fed into a machine learning algorithm that picks up features such as color and skin texture from the labeled photographs, allowing it to learn how to diagnose psoriasis.15
In one instance, researchers at Stanford University (Stanford, California) used approximately 130,000 images representing over 2000 different skin diseases in order to train their machine learning algorithm to recognize benign and malignant skin lesions.4 Although the algorithm was able to match the performance of experienced dermatologists in many diagnostic categories, further testing in a real-world clinical setting still needs to be done. In the future, nondermatologists may have the option to consult with decision-support systems that include image analysis software, such as the one developed at Stanford University, for making decisions in triage or diagnosis, which may be critical in areas where access to a dermatologist is limited.4 Future AI systems also may provide supplemental assistance in managing patients to dermatology trainees until they have the experience of a more established dermatologist.
Currently, AI cannot match general human intelligence and life experience; therefore, physicians will continue to make the final decisions when it comes to diagnosis and treatment. In the future, AI algorithms may integrate into clinical dermatology practice, leading to more accurate triage of lesions, potentially streamlined referral to dermatologists for skin conditions that require prompt consultation, and improved quality of care.
Summary
In conclusion, emerging technologies have the power to augment and revolutionize dermatology practice. Savvy dermatologists may incorporate new tools in a way that works for their practice, leading to increased efficiency and improved patient outcomes. Eventually, the technology that is most beneficial to clinical practice will likely be adopted by and integrated into mainstream dermatologic care, making it available for the majority of clinicians to use.
The clinical practice of dermatology is changing at a rapid pace. Advances in technology and new inventions in rapid diagnostics are revolutionizing how physicians approach medical care.
Given these advances, how does today’s dermatologist integrate into the future of the specialty? If leveraged properly, current technologies such as teledermatology and patient portals integrated with electronic medical records can be beneficial to dermatology practices by improving access to care, facilitating triage of patients, and improving communication between patients and health care team members. Herein, we discuss some of the emerging technologies that have the potential to shape clinical dermatology practice and remove barriers to care.
Virtual Reality
Teledermatology can be practiced through live video or, more commonly, via a store-and-forward method in which dermatologists review clinical photographs and the patient's history asynchronously with the in-office visit.6 Virtual reality has the potential to augment teledermatology services by enabling a live, interactive visit that more closely models the traditional face-to-face visit. Virtual reality already is available for patients at home with the use of a commercially marketed headset and a smartphone, and the marriage of virtual reailty and telemedicine has the potential to transform health care.
Virtual reality also can be used to deliver an essential component of the physical examination of a patient: sensory information from palpation. Haptic feedback, also known as haptics, is used to relay force and tactile information to the user of a device (eg, a haptic glove).7 In dermatology, this information pertains to the skin texture, skin profile, and physical properties (eg, stiffness, temperature).8 Assessing the texture of the skin surface can help when distinguishing epidermal processes such as psoriasis versus atopic dermatitis or when evaluating edema, induration, and depth of a leg ulcer.9
One model for conducting a teledermatology encounter that captures sensory information would consist of a haptic probe located at a referring medical provider’s office for examining patients and a master robot that controls the probe located at the consulting dermatologist’s facility.8 Another model converts 2-dimensional images taken from traditional full-body optical imaging systems into virtual 3-dimensional (3D) images that can be felt using a haptic device.10,11 In this method, the user is able to both visualize and touch the skin surface at the same time. Currently, 3D imaging of skin lesions is available in the form of a specialized handheld imager that allows the dermatologist to appreciate the texture and elevation of single lesions when viewing clinical photographs. Additionally, full-body 3D mapping of the skin surface is available for monitoring pigmented lesions or other diseases of the skin.12,13
Artificial Intelligence and Machine Learning
Computer algorithms can be helpful in assisting physicians with disease diagnosis. Machine learning is a subfield of artificial intelligence (AI) in which computer programs learn automatically from experience without explicit programming instructions. A machine learning algorithm uses a labeled data set known as a training set to create a function that can make predictions about new inputs in the future.14 The algorithm can successively compare its predicted outputs with the correct outputs and modify its function as errors are found; for example, a database of images of healthy skin as well as the skin of psoriasis patients can be fed into a machine learning algorithm that picks up features such as color and skin texture from the labeled photographs, allowing it to learn how to diagnose psoriasis.15
In one instance, researchers at Stanford University (Stanford, California) used approximately 130,000 images representing over 2000 different skin diseases in order to train their machine learning algorithm to recognize benign and malignant skin lesions.4 Although the algorithm was able to match the performance of experienced dermatologists in many diagnostic categories, further testing in a real-world clinical setting still needs to be done. In the future, nondermatologists may have the option to consult with decision-support systems that include image analysis software, such as the one developed at Stanford University, for making decisions in triage or diagnosis, which may be critical in areas where access to a dermatologist is limited.4 Future AI systems also may provide supplemental assistance in managing patients to dermatology trainees until they have the experience of a more established dermatologist.
Currently, AI cannot match general human intelligence and life experience; therefore, physicians will continue to make the final decisions when it comes to diagnosis and treatment. In the future, AI algorithms may integrate into clinical dermatology practice, leading to more accurate triage of lesions, potentially streamlined referral to dermatologists for skin conditions that require prompt consultation, and improved quality of care.
Summary
In conclusion, emerging technologies have the power to augment and revolutionize dermatology practice. Savvy dermatologists may incorporate new tools in a way that works for their practice, leading to increased efficiency and improved patient outcomes. Eventually, the technology that is most beneficial to clinical practice will likely be adopted by and integrated into mainstream dermatologic care, making it available for the majority of clinicians to use.
- Health Information Technology for Economic and Clinical Health (HITECH) Act, Pub L No. 111-5, 123 Stat 226 (2009).
- Holmgren AJ, Adler-Milstein J. Health information exchange in US hospitals: the current landscape and a path to improved information sharing. J Hosp Med. 2017;12:193-198.
- The IMLC. Interstate Medical Licensure Compact website. http://www.imlcc.org. Accessed March 21, 2018.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The Associated Press. Microsoft eyes buffer zone in TV airwaves for rural internet. ABC News website. http://abcnews.go.com/amp/Technology/wireStory/microsoft-announces-rural-broadband-initiative-48562282. Published July 11, 2017. Accessed March 21, 2018.
- Practice guidelines for dermatology. American Telemedicine Association website. https://higherlogicdownload.s3.amazonaws.com/AMERICANTELEMED/3c09839a-fffd-46f7-916c-692c11d78933/UploadedImages/SIGs/Teledermatology.Final.pdf. Accessed March 27, 2018. Published April 28, 2016.
- Lee O, Lee K, Oh C, et al. Prototype tactile feedback system for examination by skin touch. Skin Res Technol. 2014;20:307-314.
- Waldron KJ, Enedah C, Gladstone H. Stiffness and texture perception for teledermatology. Stud Health Technol Inform. 2005;111:579-585.
- Cox NH. A literally blinded trial of palpation in dermatologic diagnosis. J Am Acad Dermatol. 2007;56:949-951.
- Kim K. Roughness based perceptual analysis towards digital skin imaging system with haptic feedback. Skin Res Technol. 2016;22:334-340.
- Kim K, Lee S. Perception-based 3D tactile rendering from a single image for human skin examinations by dynamic touch. Skin Res Technol. 2015;21:164-174.
- How it works. 3Derm website. https://www.3derm.com. Accessed March 21, 2018.
- Vectra 3D. Canfield Scientific website. http://www.canfieldsci.com/imaging-systems/vectra-wb360-imaging-system. Accessed March 21, 2018.
- Alpaydin E. Introduction to Machine Learning. Cambridge, MA: MIT Press; 2014.
- Shrivastava VK, Londhe ND, Sonawane RS, et al. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: a first comparative study of its kind. Comput Methods Programs Biomed. 2016;126:98-109.
- Health Information Technology for Economic and Clinical Health (HITECH) Act, Pub L No. 111-5, 123 Stat 226 (2009).
- Holmgren AJ, Adler-Milstein J. Health information exchange in US hospitals: the current landscape and a path to improved information sharing. J Hosp Med. 2017;12:193-198.
- The IMLC. Interstate Medical Licensure Compact website. http://www.imlcc.org. Accessed March 21, 2018.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The Associated Press. Microsoft eyes buffer zone in TV airwaves for rural internet. ABC News website. http://abcnews.go.com/amp/Technology/wireStory/microsoft-announces-rural-broadband-initiative-48562282. Published July 11, 2017. Accessed March 21, 2018.
- Practice guidelines for dermatology. American Telemedicine Association website. https://higherlogicdownload.s3.amazonaws.com/AMERICANTELEMED/3c09839a-fffd-46f7-916c-692c11d78933/UploadedImages/SIGs/Teledermatology.Final.pdf. Accessed March 27, 2018. Published April 28, 2016.
- Lee O, Lee K, Oh C, et al. Prototype tactile feedback system for examination by skin touch. Skin Res Technol. 2014;20:307-314.
- Waldron KJ, Enedah C, Gladstone H. Stiffness and texture perception for teledermatology. Stud Health Technol Inform. 2005;111:579-585.
- Cox NH. A literally blinded trial of palpation in dermatologic diagnosis. J Am Acad Dermatol. 2007;56:949-951.
- Kim K. Roughness based perceptual analysis towards digital skin imaging system with haptic feedback. Skin Res Technol. 2016;22:334-340.
- Kim K, Lee S. Perception-based 3D tactile rendering from a single image for human skin examinations by dynamic touch. Skin Res Technol. 2015;21:164-174.
- How it works. 3Derm website. https://www.3derm.com. Accessed March 21, 2018.
- Vectra 3D. Canfield Scientific website. http://www.canfieldsci.com/imaging-systems/vectra-wb360-imaging-system. Accessed March 21, 2018.
- Alpaydin E. Introduction to Machine Learning. Cambridge, MA: MIT Press; 2014.
- Shrivastava VK, Londhe ND, Sonawane RS, et al. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: a first comparative study of its kind. Comput Methods Programs Biomed. 2016;126:98-109.
Genital Wart Treatment
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
Erythema and Induration on the Right Ear and Maxilla
Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
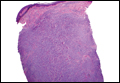
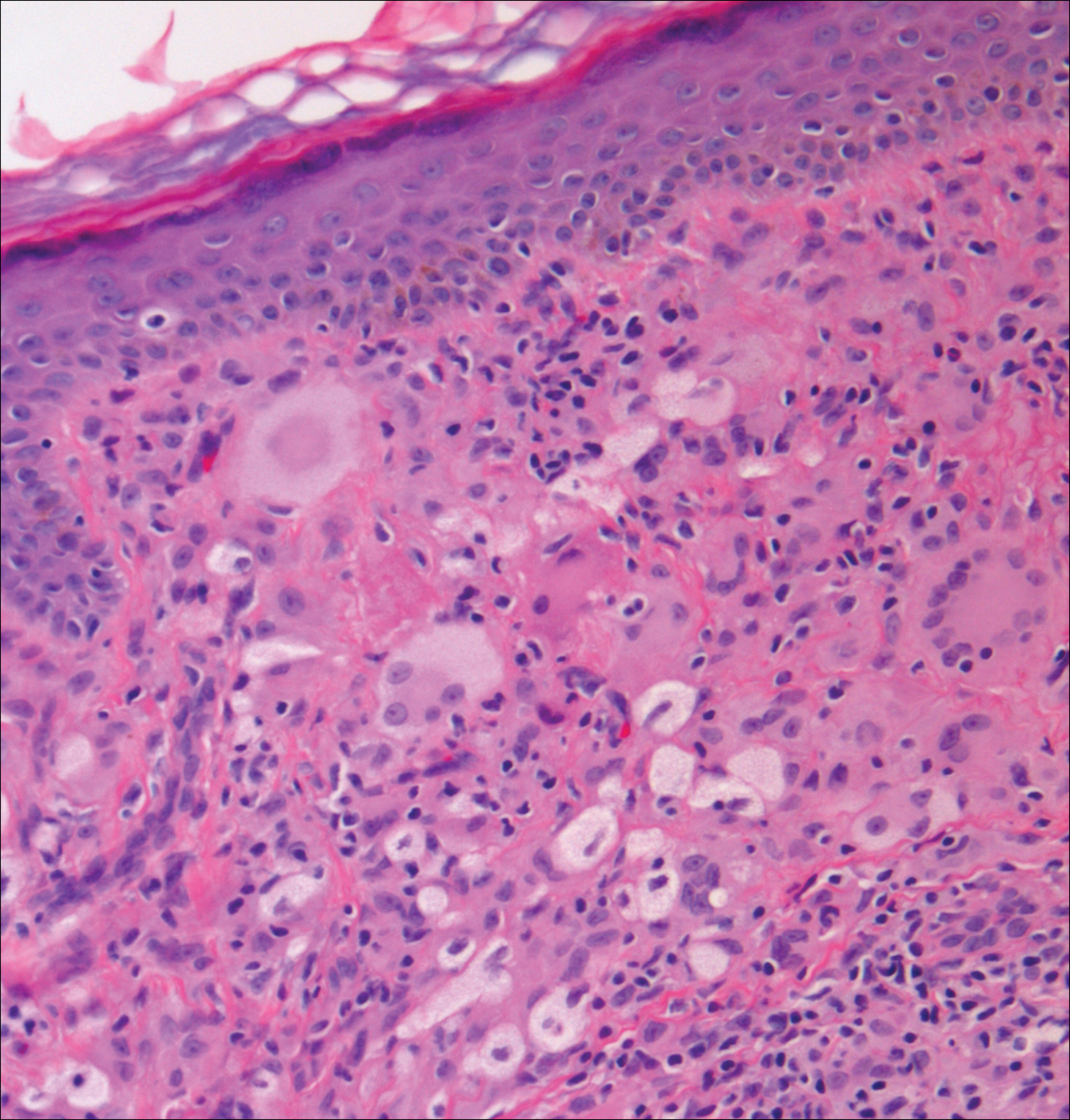
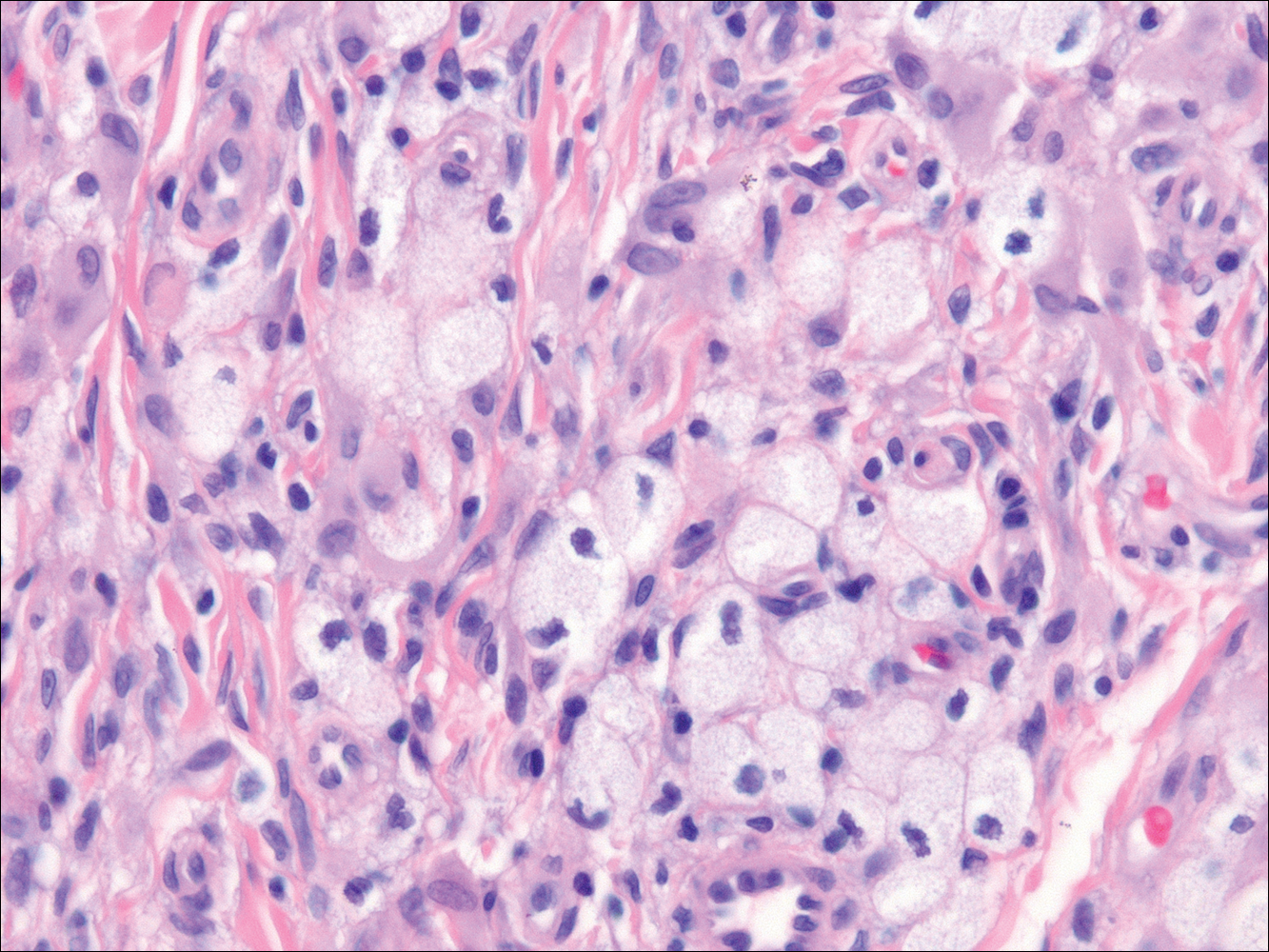
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
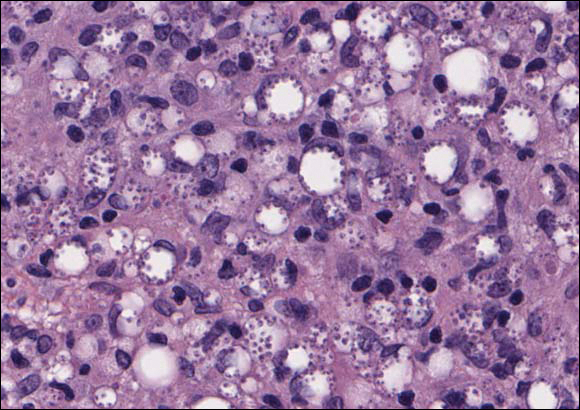
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9
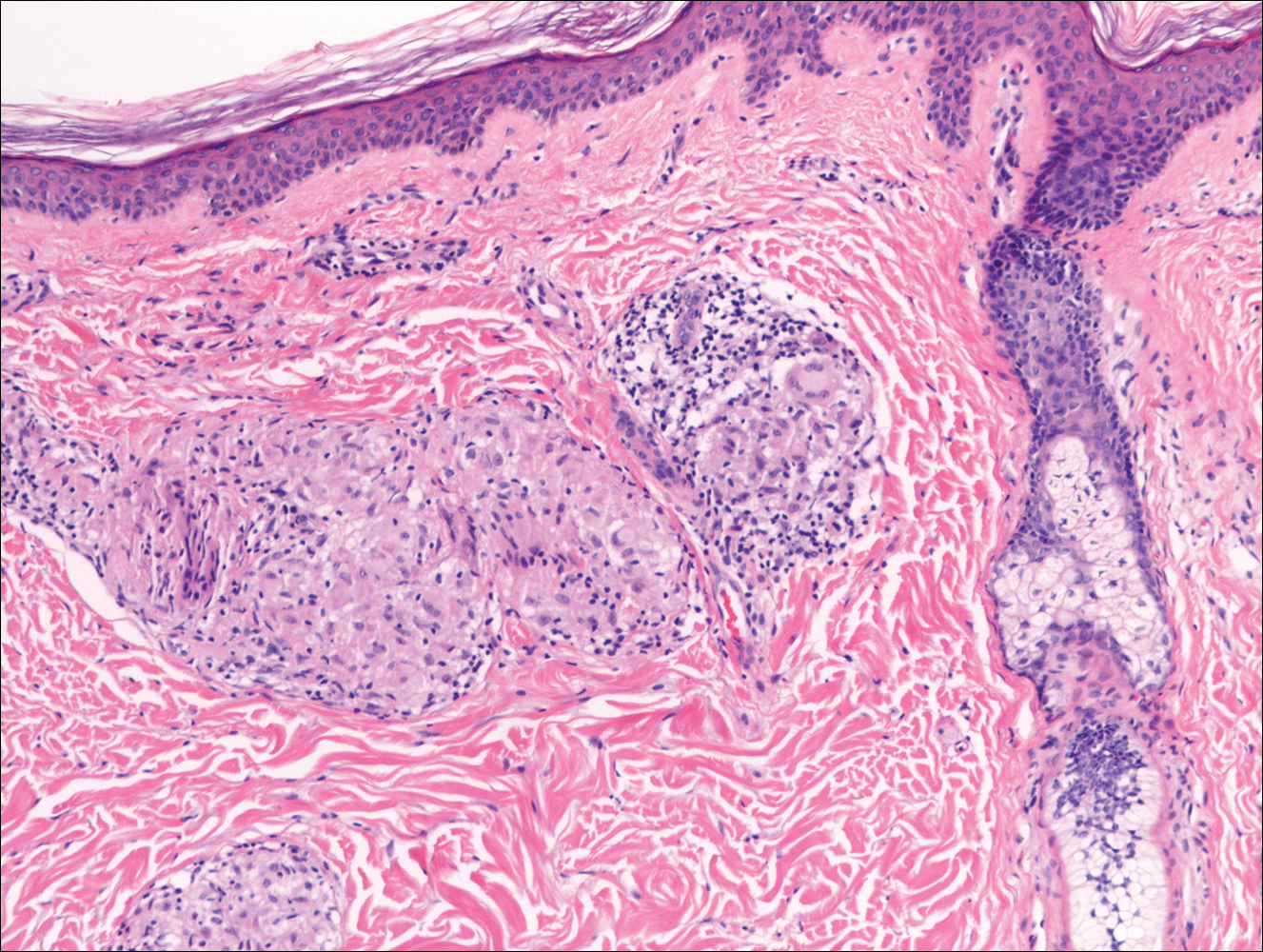
In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.
Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).
- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9

In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.

Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).


Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9

In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.

Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).


- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
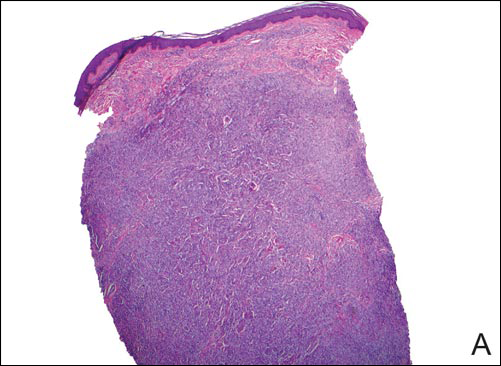
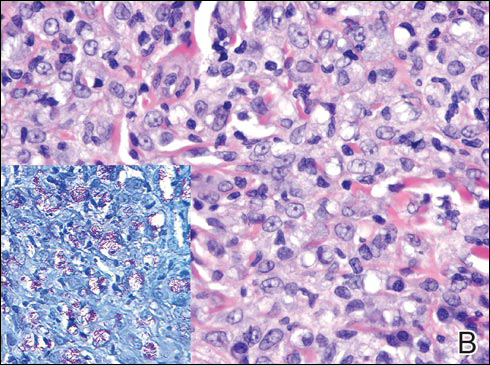
A 43-year-old man from Ghana presented with erythema and induration on the skin of the right maxillary region and right ear of several weeks’ duration.
The best diagnosis is:
a. cutaneous leishmaniasis
b. lepromatous leprosy
c. sarcoidosis
d. xanthogranuloma
e. xanthoma