User login
Lupus Erythematosus Tumidus Clinical Characteristics and Treatment: A Retrospective Review of 25 Patients
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
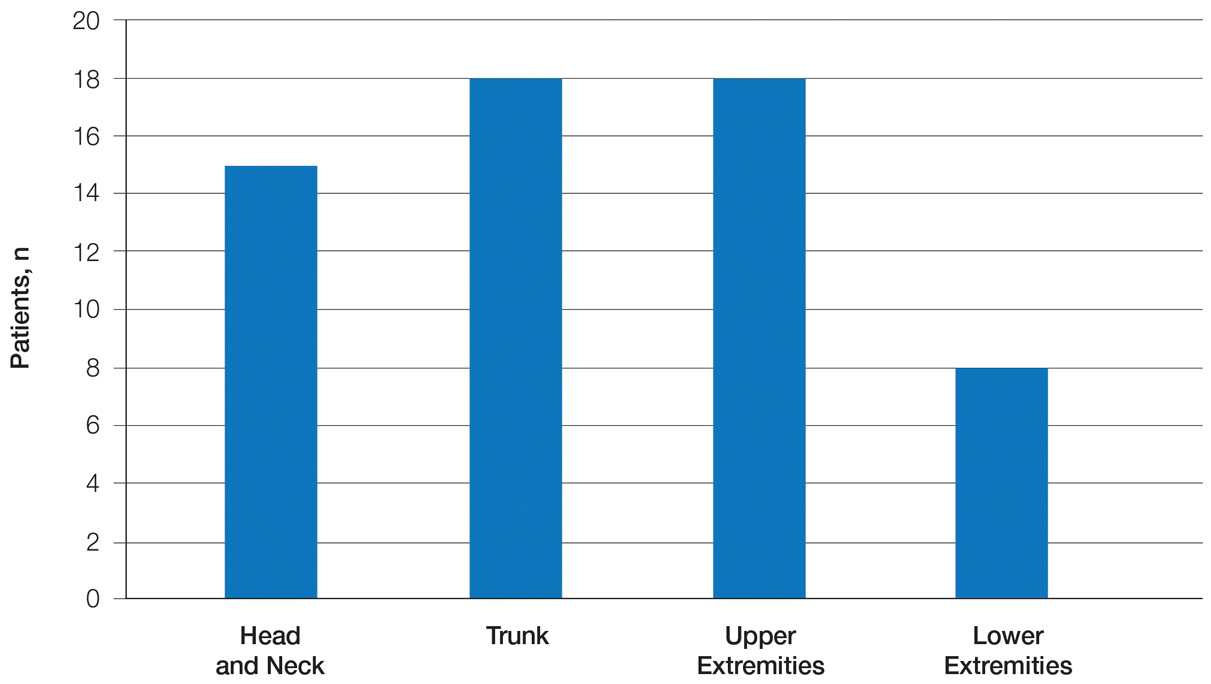
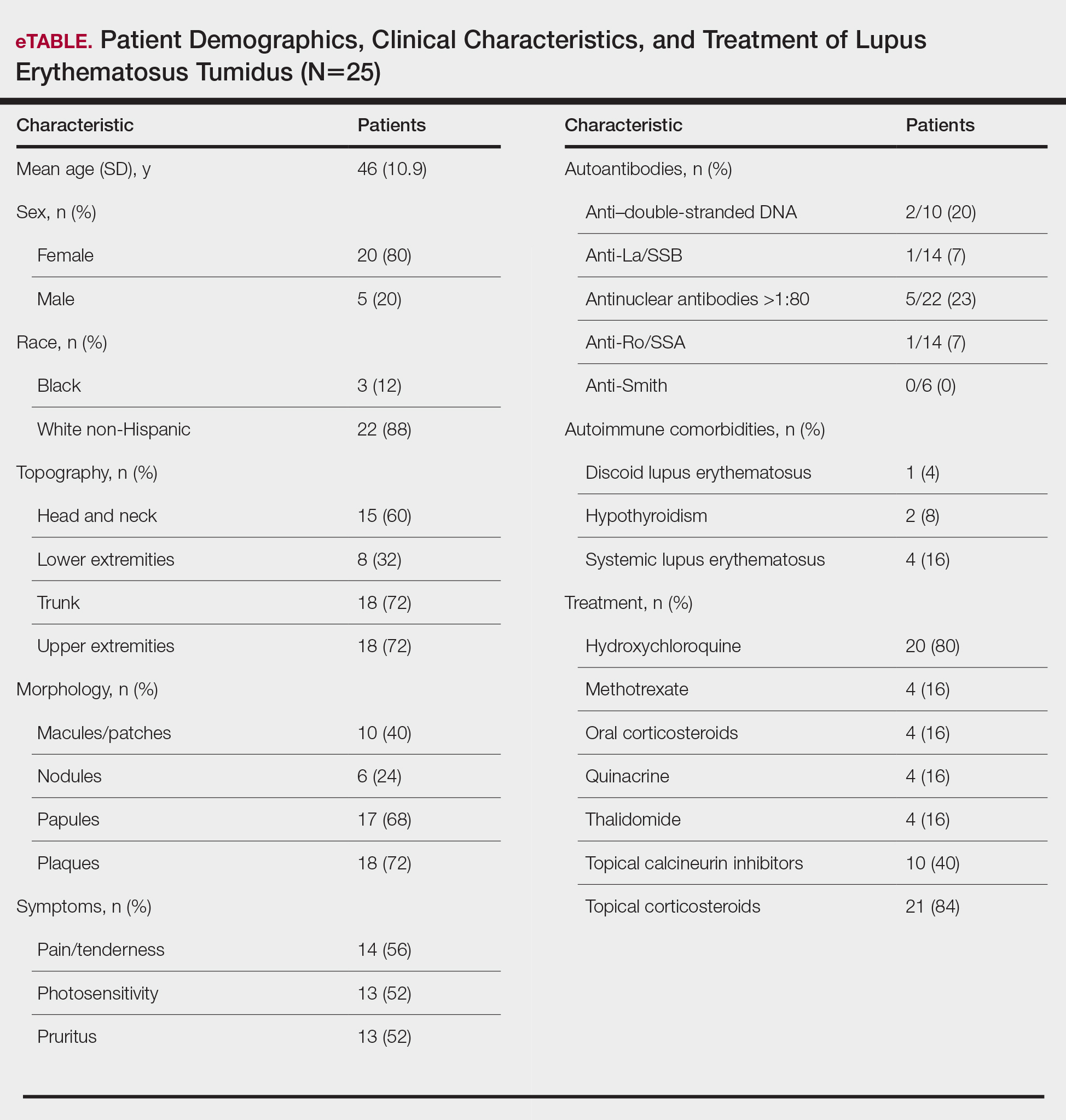
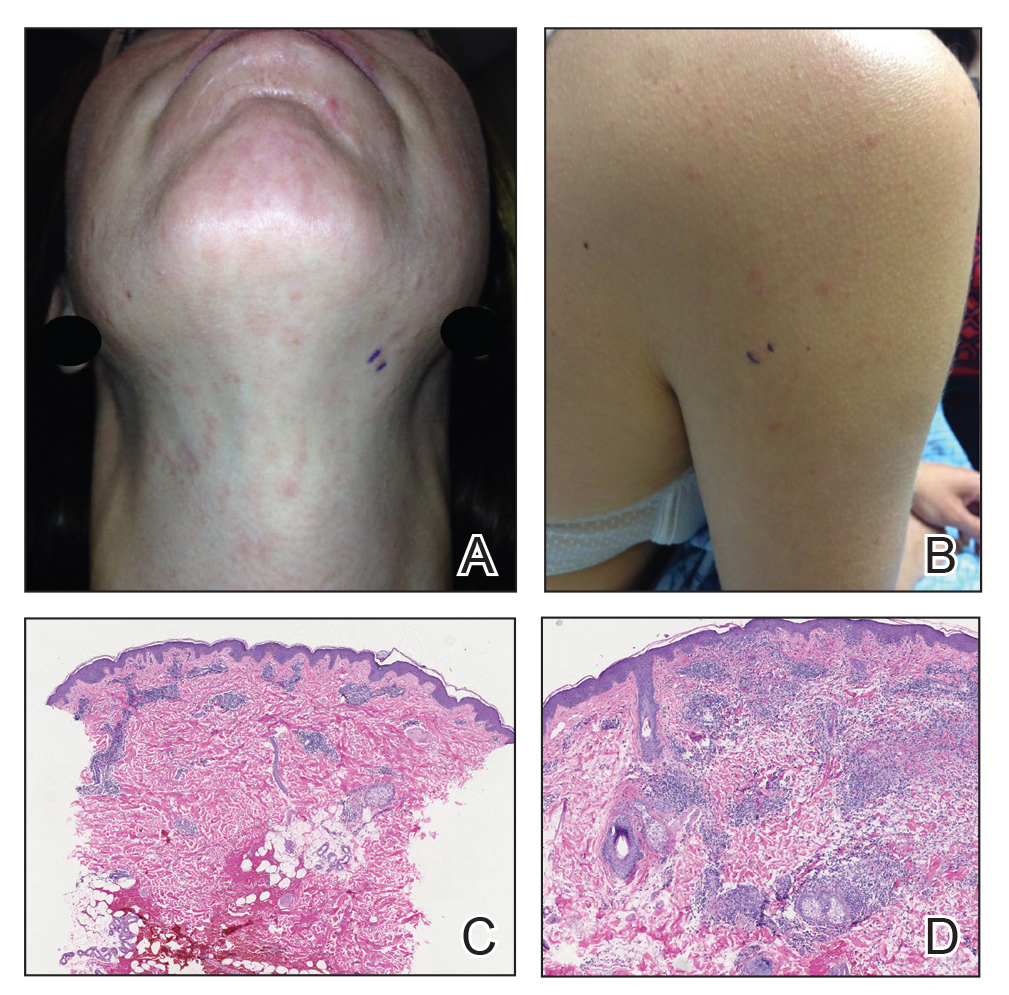
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Practice Points
- Approximately 20% of patients with lupus erythematosus tumidus (LET) will have positive antinuclear antibody titers.
- Along with cutaneous manifestations, approximately 50% of patients with LET also will have pruritus, tenderness, and photosensitivity.
- If LET is resistant to hydroxychloroquine, consider using quinacrine, methotrexate, or thalidomide.
Psychosocial Impact of Psoriasis: A Review for Dermatology Residents
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
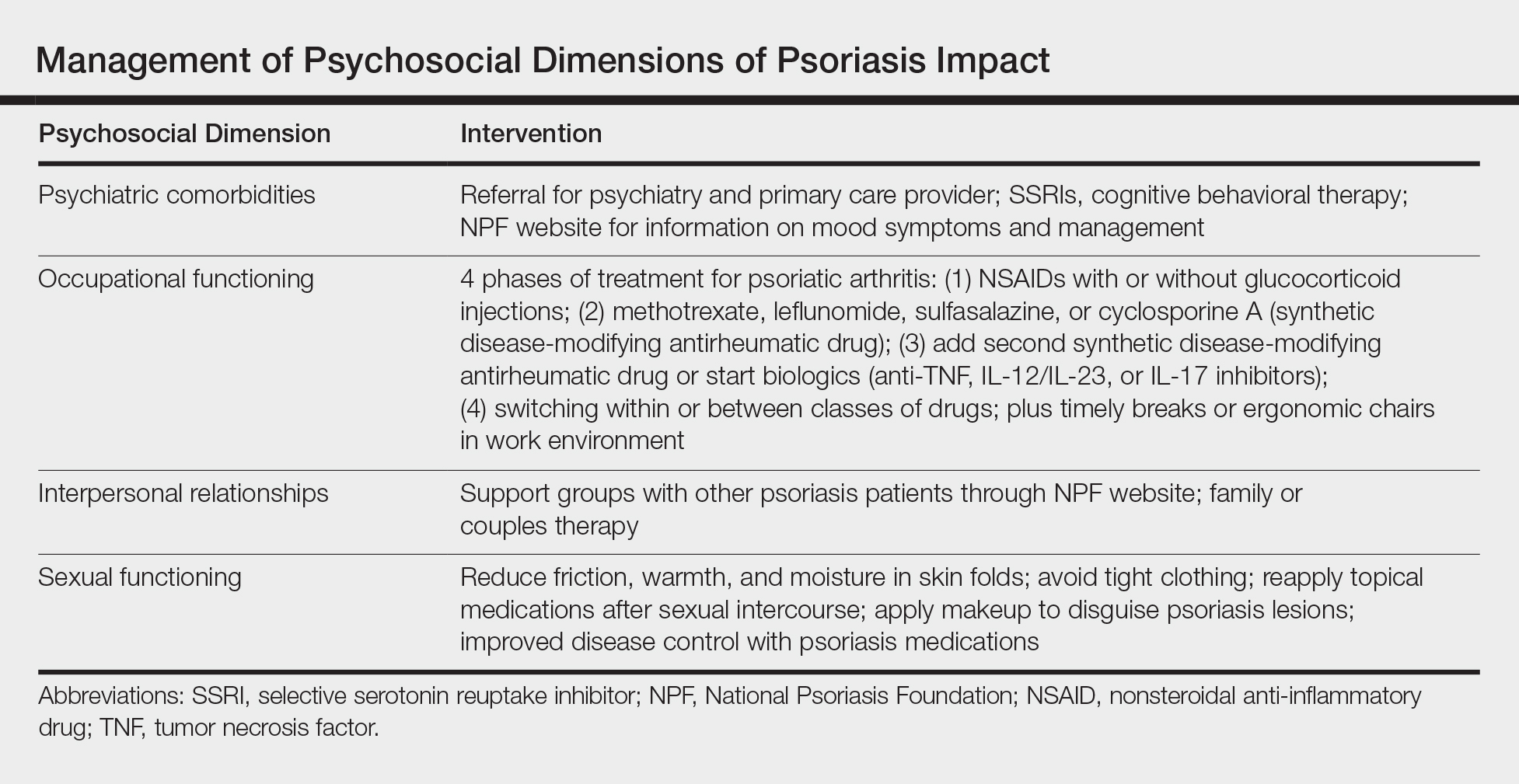
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
Practice Points
- The psychosocial impact of psoriasis is an important component of the disease burden leading to reduced quality of life.
- Assessment of psychosocial dysfunction can be done through short questionnaires, asking patients directly about these issues and anticipating these problems in patients who are most vulnerable.
- Management of psychosocial impact ranges from pharmacological interventions to helpful resources such as the National Psoriasis Foundation website.