User login
Lupus Erythematosus Tumidus Clinical Characteristics and Treatment: A Retrospective Review of 25 Patients
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
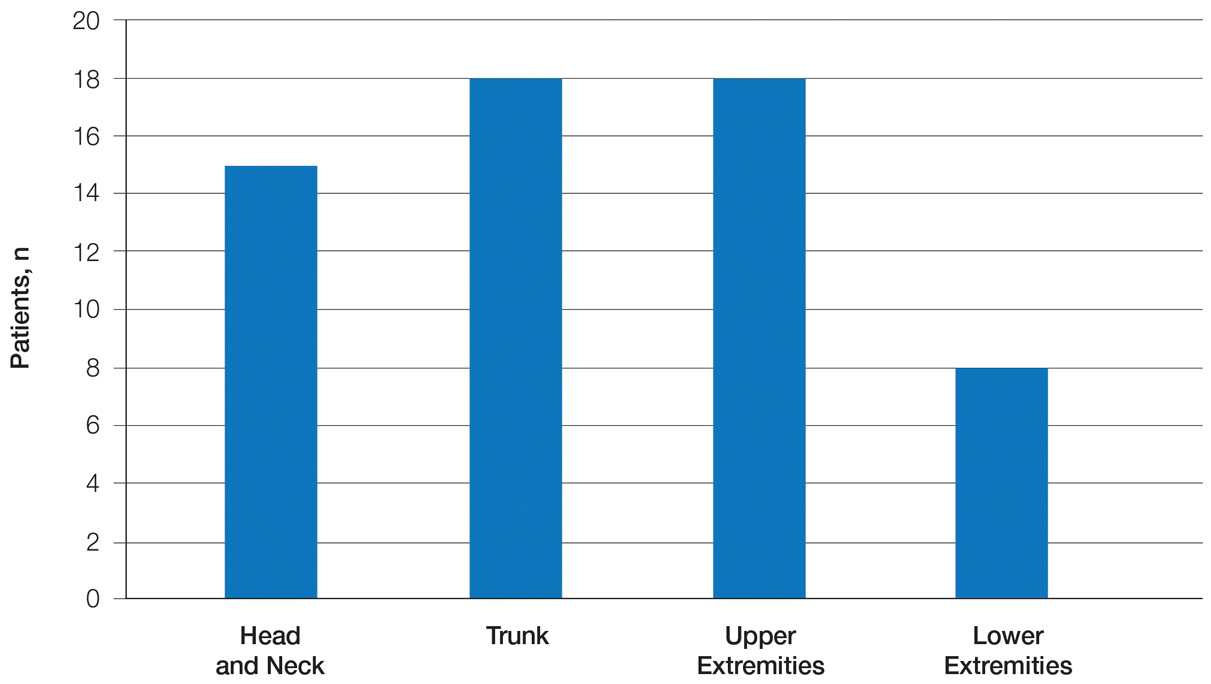
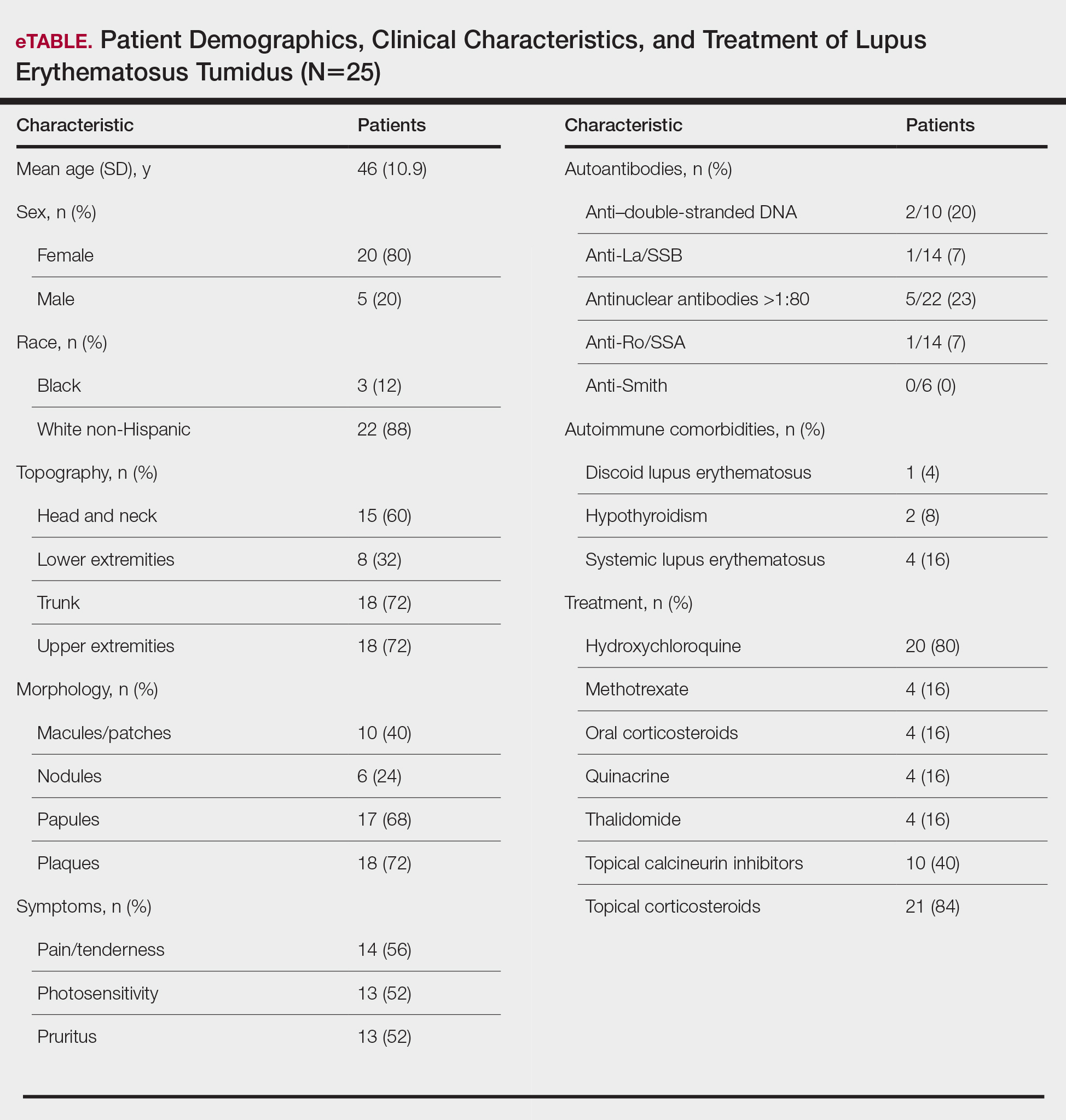
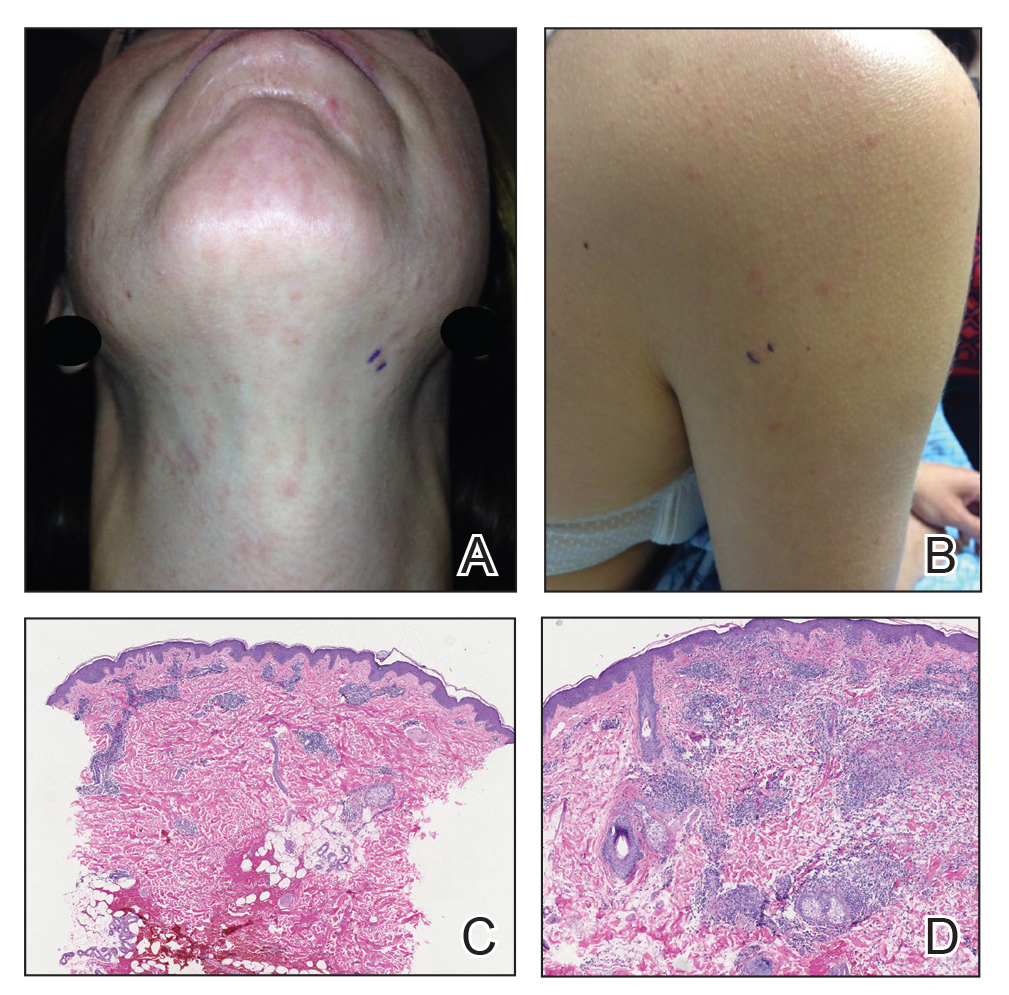
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Practice Points
- Approximately 20% of patients with lupus erythematosus tumidus (LET) will have positive antinuclear antibody titers.
- Along with cutaneous manifestations, approximately 50% of patients with LET also will have pruritus, tenderness, and photosensitivity.
- If LET is resistant to hydroxychloroquine, consider using quinacrine, methotrexate, or thalidomide.
Paronychia and Target Lesions After Hematopoietic Cell Transplant
The Diagnosis: Fusariosis
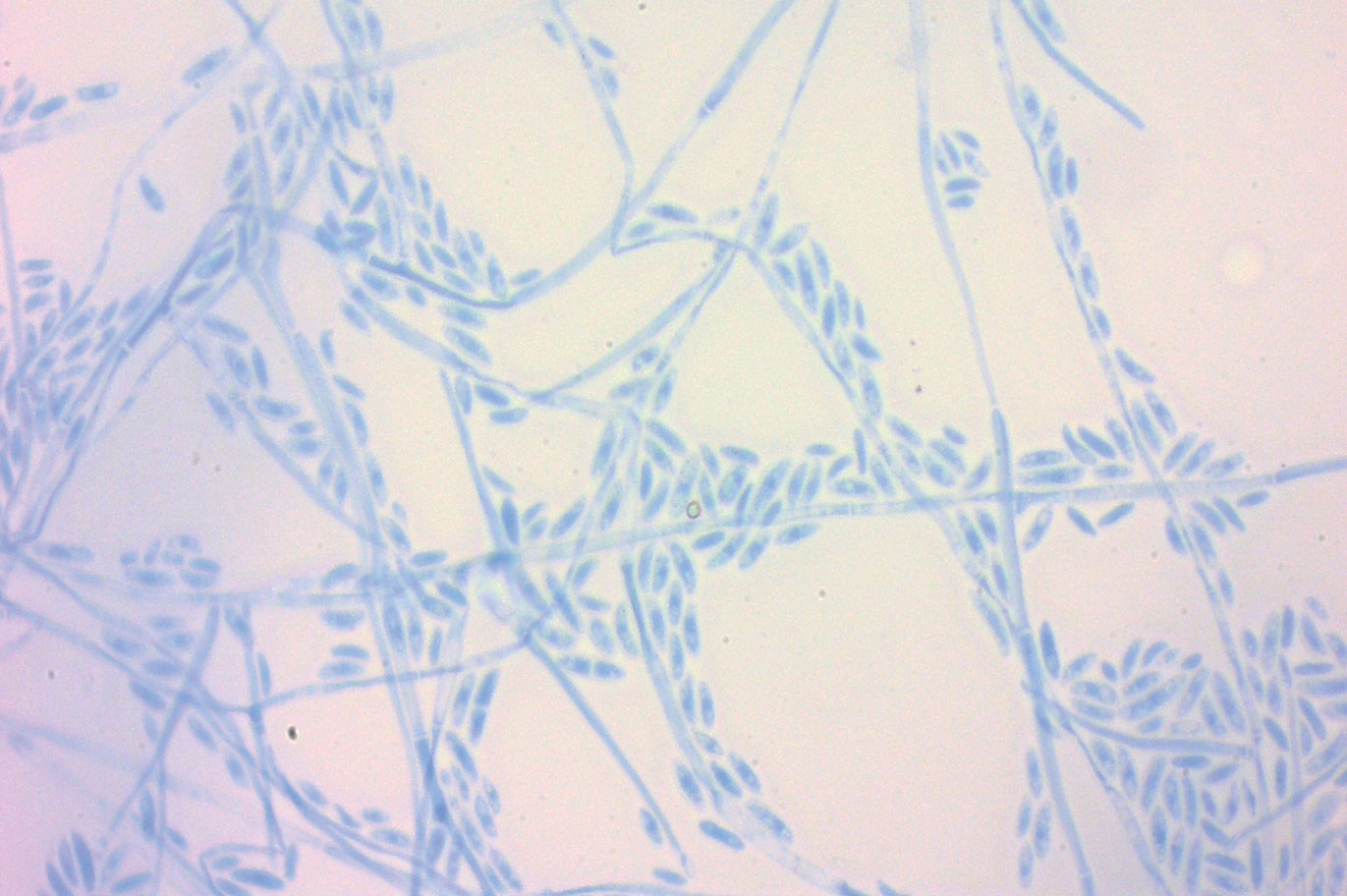
A periodic acid-Schiff stain of the seropurulent drainage from a skin nodule revealed neutrophils and scarce branching hyaline hyphae. Skin and blood cultures grew a white cottony colony. Microscopic examination showed sickle-shaped macroconidia and septate hyaline hyphae with branching acute angles (Figure). Molecular analysis by polymerase chain reaction yielded Fusarium solani species complex. Histopathology as well as culture and molecular findings were consistent with a diagnosis of disseminated fusariosis. Amphotericin B was started with rapid clinical improvement. The patient was asymptomatic upon discharge with voriconazole 200 mg twice daily.
Fusariosis is an emerging, opportunistic, and life-threatening mycosis. In immunocompetent patients it may cause onychomycosis and keratitis.1 Invasive fusariosis predominantly is caused by the F solani species complex and affects immunocompromised patients, especially those with neutropenia or acute leukemia or hematopoietic stem cell transplant recipients.2
Before invasion, the infection frequently may begin by affecting the nail apparatus as onychomycosis or paronychia of the skin. As in our case, trauma or manipulation of the nail favors dissemination.3 Skin manifestations include erythematous to violaceous papules, macules, and nodules with central necrosis or crust; some may exhibit target morphology. Other organs may be affected, including the sinuses, lungs, liver, spleen, and kidneys. A comprehensive clinical examination before hematopoietic cell transplant and during fever and neutropenia may opportunely identify these potential infective foci.3,4
The differential diagnosis of disseminated fusariosis includes bacterial infections, especially Staphylococcus aureus and Pseudomonas aeruginosa, and other invasive fungal infections, particularly aspergillosis, mucormycosis, and candidiasis.5 Symptom persistence after broad-spectrum antibiotic initiation should raise diagnostic suspicion of systemic mycosis or mycobacterial infection. Mucormycosis and candidiasis have histopathologic profiles that differ from fusariosis, presenting with broad ribbonlike hyphae with 90° angulation and pseudohyphae with budding yeast cells, respectively. Differentiation of disseminated fusariosis and aspergillosis in neutropenic patients is difficult. Hyphae cannot be differentiated from those of Aspergillus species on histology.6 Furthermore, serologic assays, such as galactomannan and (1,3)-β-D-glucan, cross-react with both genera. Clinically, Fusarium species exhibit metastatic skin lesions, cellulitis, and positive blood cultures due to adventitious sporulation more frequently than Aspergillus species. Patients with aspergillosis more commonly present with sinusitis, pneumonia, and pulmonary macronodules with the halo sign.6 Although nocardiosis presents with disseminated subcutaneous nodules with pulmonary affection in immunocompromised patients, its morphology is very different from fusariosis. Nocardia presents with a gram-positive bacillus with the microscopic appearance of branching filaments. Yeastlike microorganisms with morphology ranging from oval to sausagelike are found in talaromycosis, an uncommon fungal infection predominantly caused by Talaromyces marneffei. Fusarium species culture reveals white cottony colonies with characteristic hyaline, canoe-shaped or sickle-shaped (banana-shaped), multicellular macroconidia, and microconidia. Precise species identification requires molecular analyses such as polymerase chain reaction.
Mortality is high, ranging from 50% to 70% of cases.5 Voriconazole or lipid-based amphotericin B are considered first-line treatments. Posaconazole may be employed as a second-line alternative. Surgical debridement of infected tissues and removal of colonized venous catheters is recommended. Secondary prophylaxis should be considered with agents such as voriconazole, posaconazole, or amphotericin B.5 Resolution of immunosuppression and neutropenia is an important factor to reduce the mortality rate.
- Ranawaka RR, Nagahawatte A, Gunasekara TA. Fusarium onychomycosis: prevalence, clinical presentations, response toitraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int J Dermatol. 2015;54:1275-1282.
- Nucci F, Nouer SA, Capone D, et al. Fusariosis. Semin Respir Crit Care Med. 2015;36:706-714.
- Varon AG, Nouer SA, Barreiros G, et al. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J Infect. 2014;68:85-89.
- Hay RJ. Fusarium infections of the skin. Curr Opin Infect Dis. 2007;20:115-117.
- Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20:27-46.
- Nucci F, Nouer SA, Capone D, et al. Invasive mould disease in haematologic patients: comparison between fusariosis and aspergillosis. Clin Microbiol Infect. 2018;24:1105.e1-1105.e4.
The Diagnosis: Fusariosis
A periodic acid-Schiff stain of the seropurulent drainage from a skin nodule revealed neutrophils and scarce branching hyaline hyphae. Skin and blood cultures grew a white cottony colony. Microscopic examination showed sickle-shaped macroconidia and septate hyaline hyphae with branching acute angles (Figure). Molecular analysis by polymerase chain reaction yielded Fusarium solani species complex. Histopathology as well as culture and molecular findings were consistent with a diagnosis of disseminated fusariosis. Amphotericin B was started with rapid clinical improvement. The patient was asymptomatic upon discharge with voriconazole 200 mg twice daily.

Fusariosis is an emerging, opportunistic, and life-threatening mycosis. In immunocompetent patients it may cause onychomycosis and keratitis.1 Invasive fusariosis predominantly is caused by the F solani species complex and affects immunocompromised patients, especially those with neutropenia or acute leukemia or hematopoietic stem cell transplant recipients.2
Before invasion, the infection frequently may begin by affecting the nail apparatus as onychomycosis or paronychia of the skin. As in our case, trauma or manipulation of the nail favors dissemination.3 Skin manifestations include erythematous to violaceous papules, macules, and nodules with central necrosis or crust; some may exhibit target morphology. Other organs may be affected, including the sinuses, lungs, liver, spleen, and kidneys. A comprehensive clinical examination before hematopoietic cell transplant and during fever and neutropenia may opportunely identify these potential infective foci.3,4
The differential diagnosis of disseminated fusariosis includes bacterial infections, especially Staphylococcus aureus and Pseudomonas aeruginosa, and other invasive fungal infections, particularly aspergillosis, mucormycosis, and candidiasis.5 Symptom persistence after broad-spectrum antibiotic initiation should raise diagnostic suspicion of systemic mycosis or mycobacterial infection. Mucormycosis and candidiasis have histopathologic profiles that differ from fusariosis, presenting with broad ribbonlike hyphae with 90° angulation and pseudohyphae with budding yeast cells, respectively. Differentiation of disseminated fusariosis and aspergillosis in neutropenic patients is difficult. Hyphae cannot be differentiated from those of Aspergillus species on histology.6 Furthermore, serologic assays, such as galactomannan and (1,3)-β-D-glucan, cross-react with both genera. Clinically, Fusarium species exhibit metastatic skin lesions, cellulitis, and positive blood cultures due to adventitious sporulation more frequently than Aspergillus species. Patients with aspergillosis more commonly present with sinusitis, pneumonia, and pulmonary macronodules with the halo sign.6 Although nocardiosis presents with disseminated subcutaneous nodules with pulmonary affection in immunocompromised patients, its morphology is very different from fusariosis. Nocardia presents with a gram-positive bacillus with the microscopic appearance of branching filaments. Yeastlike microorganisms with morphology ranging from oval to sausagelike are found in talaromycosis, an uncommon fungal infection predominantly caused by Talaromyces marneffei. Fusarium species culture reveals white cottony colonies with characteristic hyaline, canoe-shaped or sickle-shaped (banana-shaped), multicellular macroconidia, and microconidia. Precise species identification requires molecular analyses such as polymerase chain reaction.
Mortality is high, ranging from 50% to 70% of cases.5 Voriconazole or lipid-based amphotericin B are considered first-line treatments. Posaconazole may be employed as a second-line alternative. Surgical debridement of infected tissues and removal of colonized venous catheters is recommended. Secondary prophylaxis should be considered with agents such as voriconazole, posaconazole, or amphotericin B.5 Resolution of immunosuppression and neutropenia is an important factor to reduce the mortality rate.
The Diagnosis: Fusariosis
A periodic acid-Schiff stain of the seropurulent drainage from a skin nodule revealed neutrophils and scarce branching hyaline hyphae. Skin and blood cultures grew a white cottony colony. Microscopic examination showed sickle-shaped macroconidia and septate hyaline hyphae with branching acute angles (Figure). Molecular analysis by polymerase chain reaction yielded Fusarium solani species complex. Histopathology as well as culture and molecular findings were consistent with a diagnosis of disseminated fusariosis. Amphotericin B was started with rapid clinical improvement. The patient was asymptomatic upon discharge with voriconazole 200 mg twice daily.

Fusariosis is an emerging, opportunistic, and life-threatening mycosis. In immunocompetent patients it may cause onychomycosis and keratitis.1 Invasive fusariosis predominantly is caused by the F solani species complex and affects immunocompromised patients, especially those with neutropenia or acute leukemia or hematopoietic stem cell transplant recipients.2
Before invasion, the infection frequently may begin by affecting the nail apparatus as onychomycosis or paronychia of the skin. As in our case, trauma or manipulation of the nail favors dissemination.3 Skin manifestations include erythematous to violaceous papules, macules, and nodules with central necrosis or crust; some may exhibit target morphology. Other organs may be affected, including the sinuses, lungs, liver, spleen, and kidneys. A comprehensive clinical examination before hematopoietic cell transplant and during fever and neutropenia may opportunely identify these potential infective foci.3,4
The differential diagnosis of disseminated fusariosis includes bacterial infections, especially Staphylococcus aureus and Pseudomonas aeruginosa, and other invasive fungal infections, particularly aspergillosis, mucormycosis, and candidiasis.5 Symptom persistence after broad-spectrum antibiotic initiation should raise diagnostic suspicion of systemic mycosis or mycobacterial infection. Mucormycosis and candidiasis have histopathologic profiles that differ from fusariosis, presenting with broad ribbonlike hyphae with 90° angulation and pseudohyphae with budding yeast cells, respectively. Differentiation of disseminated fusariosis and aspergillosis in neutropenic patients is difficult. Hyphae cannot be differentiated from those of Aspergillus species on histology.6 Furthermore, serologic assays, such as galactomannan and (1,3)-β-D-glucan, cross-react with both genera. Clinically, Fusarium species exhibit metastatic skin lesions, cellulitis, and positive blood cultures due to adventitious sporulation more frequently than Aspergillus species. Patients with aspergillosis more commonly present with sinusitis, pneumonia, and pulmonary macronodules with the halo sign.6 Although nocardiosis presents with disseminated subcutaneous nodules with pulmonary affection in immunocompromised patients, its morphology is very different from fusariosis. Nocardia presents with a gram-positive bacillus with the microscopic appearance of branching filaments. Yeastlike microorganisms with morphology ranging from oval to sausagelike are found in talaromycosis, an uncommon fungal infection predominantly caused by Talaromyces marneffei. Fusarium species culture reveals white cottony colonies with characteristic hyaline, canoe-shaped or sickle-shaped (banana-shaped), multicellular macroconidia, and microconidia. Precise species identification requires molecular analyses such as polymerase chain reaction.
Mortality is high, ranging from 50% to 70% of cases.5 Voriconazole or lipid-based amphotericin B are considered first-line treatments. Posaconazole may be employed as a second-line alternative. Surgical debridement of infected tissues and removal of colonized venous catheters is recommended. Secondary prophylaxis should be considered with agents such as voriconazole, posaconazole, or amphotericin B.5 Resolution of immunosuppression and neutropenia is an important factor to reduce the mortality rate.
- Ranawaka RR, Nagahawatte A, Gunasekara TA. Fusarium onychomycosis: prevalence, clinical presentations, response toitraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int J Dermatol. 2015;54:1275-1282.
- Nucci F, Nouer SA, Capone D, et al. Fusariosis. Semin Respir Crit Care Med. 2015;36:706-714.
- Varon AG, Nouer SA, Barreiros G, et al. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J Infect. 2014;68:85-89.
- Hay RJ. Fusarium infections of the skin. Curr Opin Infect Dis. 2007;20:115-117.
- Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20:27-46.
- Nucci F, Nouer SA, Capone D, et al. Invasive mould disease in haematologic patients: comparison between fusariosis and aspergillosis. Clin Microbiol Infect. 2018;24:1105.e1-1105.e4.
- Ranawaka RR, Nagahawatte A, Gunasekara TA. Fusarium onychomycosis: prevalence, clinical presentations, response toitraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int J Dermatol. 2015;54:1275-1282.
- Nucci F, Nouer SA, Capone D, et al. Fusariosis. Semin Respir Crit Care Med. 2015;36:706-714.
- Varon AG, Nouer SA, Barreiros G, et al. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J Infect. 2014;68:85-89.
- Hay RJ. Fusarium infections of the skin. Curr Opin Infect Dis. 2007;20:115-117.
- Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20:27-46.
- Nucci F, Nouer SA, Capone D, et al. Invasive mould disease in haematologic patients: comparison between fusariosis and aspergillosis. Clin Microbiol Infect. 2018;24:1105.e1-1105.e4.
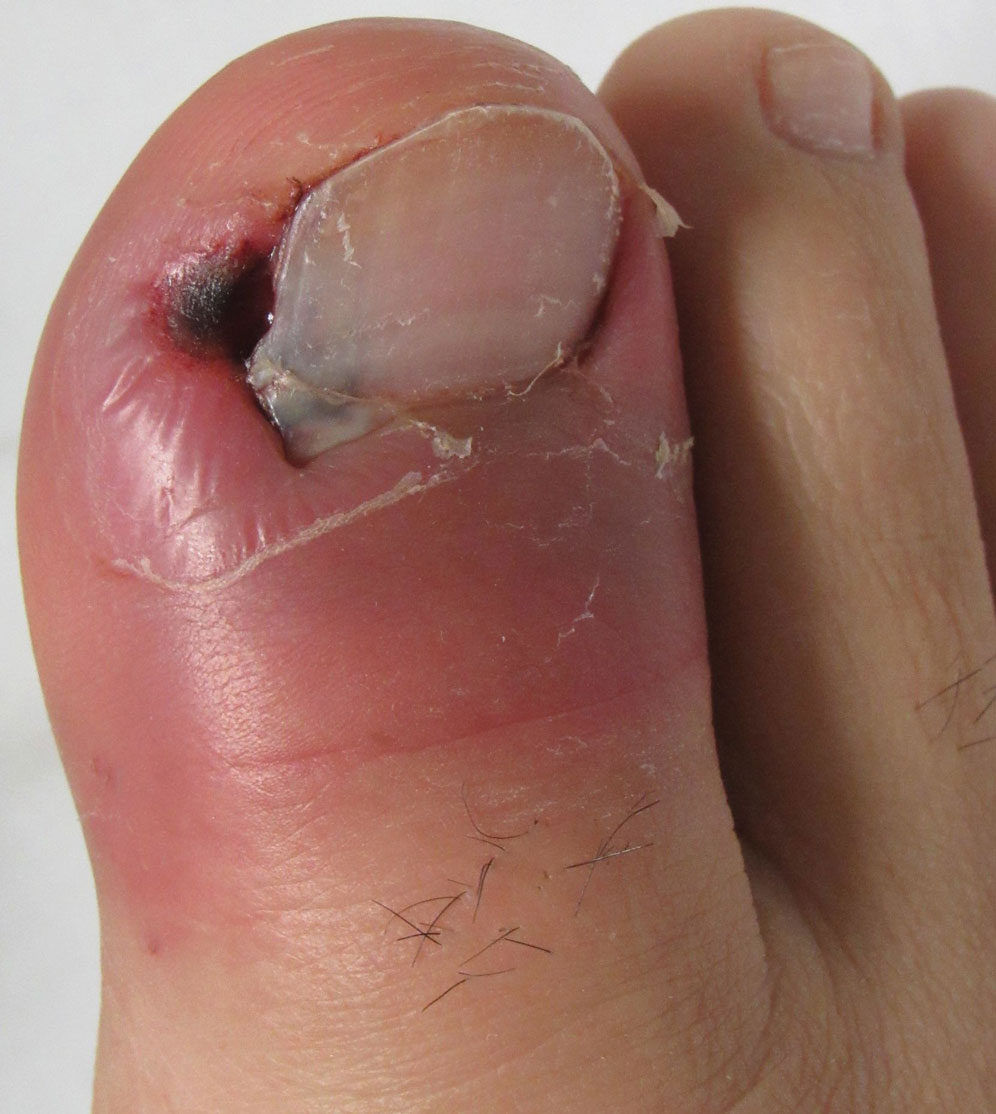
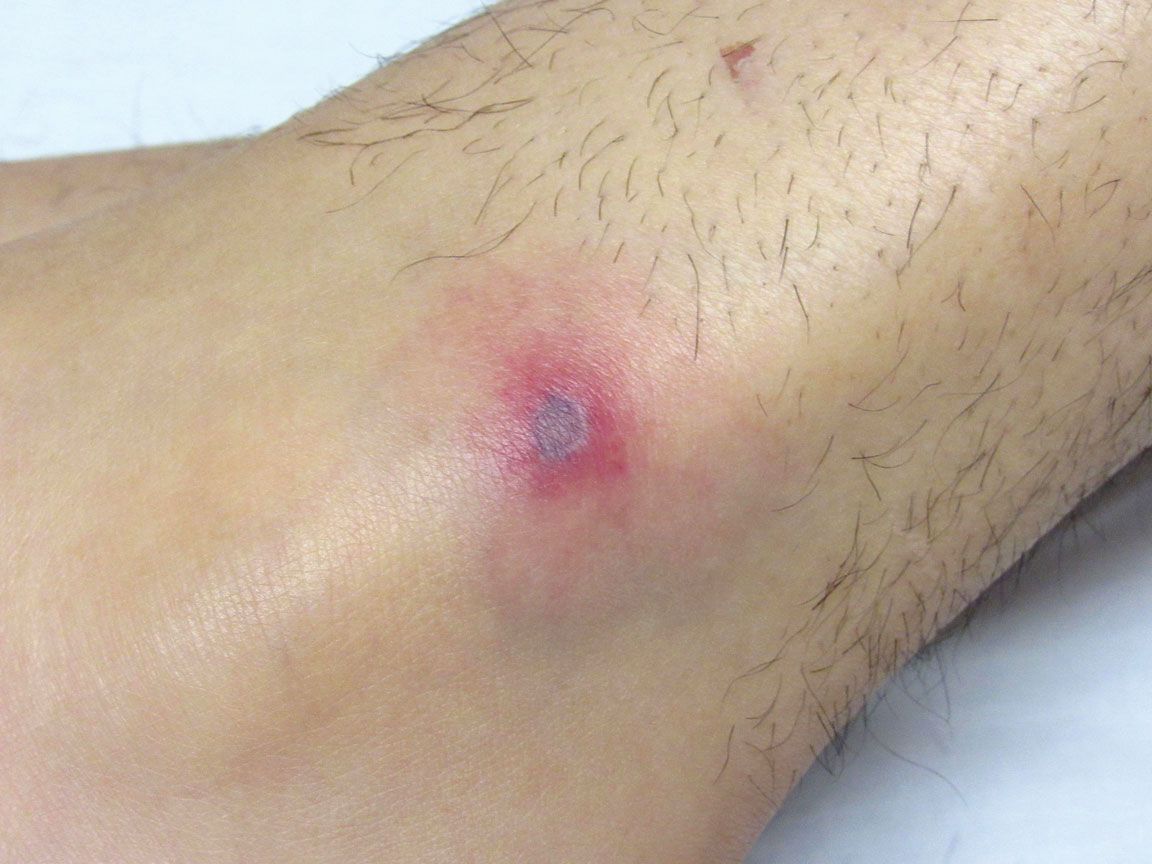
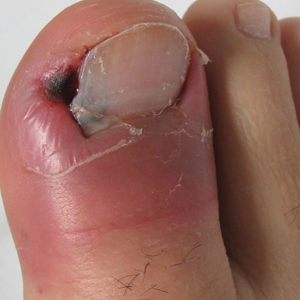
A 19-year-old man with acute lymphoblastic leukemia was admitted for an allogeneic hematopoietic cell transplant. On the 11th day of hospitalization, he experienced a right toe trauma in his hospital room and subsequently developed edema, erythema, and pain on the right hallux (top). The next day, a general surgeon performed a minor incision and drainage of the affected area. After 2 days, the patient developed a fever and a disseminated dermatosis located on the arms and legs characterized by target lesions with a necrotic center and erythematous papules and macules (bottom). On day 3, he developed severe neutropenia (0.042×109 cells/L [reference range, 2.0–6.9×109 cells/L]). Broad-spectrum antibiotics were initiated without clinical improvement. The patient developed dyspnea on day 5. Skin, nail, and blood cultures were obtained. High-resolution computed tomography of the chest displayed multiple small pulmonary nodules, ground-glass opacities, and the tree-in-bud sign.