User login
FDA attacks antibiotic resistance with new strategy
WASHINGTON – A strategy combining stewardship and science is needed to help combat antimicrobial resistance, and updated plans from the U.S. Food and Drug Administration include four key components to address all aspects of product development and use, FDA commissioner Scott Gottlieb, MD, said in a press briefing in Washington on Sept. 14.
“The FDA plays a unique role in advancing human and animal health” that provides a unique vantage point for coordinating all aspects of product development and application, he said.
The FDA’s comprehensive approach to the challenge of antimicrobial resistance (AMR) includes:
- Facilitating product development.
- Promoting antimicrobial stewardship.
- Supporting the development of new tools for surveillance.
- Advancing scientific initiatives, including research for the development of alternative treatments.
The FDA’s product development plan to combat AMR includes the creation of incentives for companies to develop new antibiotic products and create a robust pipeline, which is a challenge because of the lack of immediate economic gain, Dr. Gottlieb said.
“It necessary to change the perception that the costs and risks of antibiotic innovation are too high relative to their expected gains,” he emphasized.
Strategies to incentivize companies include fast track designation, priority review, and breakthrough therapy designation. In addition, the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) is designed to promote development of antimicrobial drugs for limited and underserved populations, Dr. Gottlieb said. The FDA plan also calls for pursuing reimbursement options with the Centers for Medicare & Medicaid Services.
Promoting antimicrobial stewardship remains an ongoing element of the FDA’s plan to reduce AMR. In conjunction with the release of the FDA’s updated approach to AMR, the FDA’s Center for Veterinary Medicine CVM released a 5-year action plan to promote and support antimicrobial stewardship in not only the agricultural arena, but in companion animals as well.
The FDA plans to bring all antimicrobials of medical importance that are approved for use in animals under the oversight of CVM, which will pursue the improve labeling on antimicrobial drugs used in the feed and water of food-producing animals, including defining durations of use, Dr. Gottlieb noted.
Supporting the development and improvement of surveillance tools is “essential to understanding the drivers of resistance in human and veterinary settings and formulating appropriate responses” to outbreaks, Dr. Gottlieb said.
To help meet this goal, the FDA will expand sampling via the National Antimicrobial Resistance Monitoring System (NARMS) database, he said. Other surveillance goals include supporting genomics research and expanding AMR monitoring to include pathogens associated with animal feed and companion animals, he added.
As part of the final component of the FDA’s AMR strategy to advance scientific initiatives, the FDA has released a new Request for Information “to obtain additional, external input on how best to develop an annual list of regulatory science initiatives specific for antimicrobial products,” Dr. Gottlieb announced. The FDA intends to use the information gained from clinicians and others in its creation of guidance documents and recommendations to streamline the antibiotic development process. He also cited the FDA’s ongoing support of partnerships with public and private organizations such as the Clinical Trials Transformation Initiative, which focuses on drug development for severe bacterial infections with current unmet medical need.
“We need to harness science and policy to help our public health systems and researchers become nimbler in the battle against drug-resistant pathogens,” Dr. Gottlieb concluded.
In a panel discussion following the briefing, several experts offered perspective on the FDA’s goals and on the challenges of AMR.
William Flynn, DVM, deputy director of science policy for the Center of Veterinary Medicine, noted some goals for reducing the use of antibiotics in the veterinary arena.
“We are trying to focus on the driver: What are the disease conditions that drive use of the product,” he said. Ideally, better management of disease conditions can reduce reliance on antibiotics, he added.
Also in the panel discussion, Steven Gitterman, MD, deputy director of the division of microbiology devices at the Center for Devices and Radiological Health, emphasized the value of sustainable trial databases so AMR research can continue on an ongoing basis. Finally, Carolyn Wilson, PhD, associate director of research at the Center for Biologics Evaluation and Research, noted that the FDA’s research and development efforts include antibiotic alternatives, including live biotherapeutic products, fecal microbiota transplantation, and bacteriophage therapy.
Visit www.fda.gov for a transcript of Dr. Gottlieb’s talk, and for the updated FDA website page with more details on the agency’s plans to combat antimicrobial resistance.
Dr. Gottlieb and the panelists had no financial conflicts to disclose.
WASHINGTON – A strategy combining stewardship and science is needed to help combat antimicrobial resistance, and updated plans from the U.S. Food and Drug Administration include four key components to address all aspects of product development and use, FDA commissioner Scott Gottlieb, MD, said in a press briefing in Washington on Sept. 14.
“The FDA plays a unique role in advancing human and animal health” that provides a unique vantage point for coordinating all aspects of product development and application, he said.
The FDA’s comprehensive approach to the challenge of antimicrobial resistance (AMR) includes:
- Facilitating product development.
- Promoting antimicrobial stewardship.
- Supporting the development of new tools for surveillance.
- Advancing scientific initiatives, including research for the development of alternative treatments.
The FDA’s product development plan to combat AMR includes the creation of incentives for companies to develop new antibiotic products and create a robust pipeline, which is a challenge because of the lack of immediate economic gain, Dr. Gottlieb said.
“It necessary to change the perception that the costs and risks of antibiotic innovation are too high relative to their expected gains,” he emphasized.
Strategies to incentivize companies include fast track designation, priority review, and breakthrough therapy designation. In addition, the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) is designed to promote development of antimicrobial drugs for limited and underserved populations, Dr. Gottlieb said. The FDA plan also calls for pursuing reimbursement options with the Centers for Medicare & Medicaid Services.
Promoting antimicrobial stewardship remains an ongoing element of the FDA’s plan to reduce AMR. In conjunction with the release of the FDA’s updated approach to AMR, the FDA’s Center for Veterinary Medicine CVM released a 5-year action plan to promote and support antimicrobial stewardship in not only the agricultural arena, but in companion animals as well.
The FDA plans to bring all antimicrobials of medical importance that are approved for use in animals under the oversight of CVM, which will pursue the improve labeling on antimicrobial drugs used in the feed and water of food-producing animals, including defining durations of use, Dr. Gottlieb noted.
Supporting the development and improvement of surveillance tools is “essential to understanding the drivers of resistance in human and veterinary settings and formulating appropriate responses” to outbreaks, Dr. Gottlieb said.
To help meet this goal, the FDA will expand sampling via the National Antimicrobial Resistance Monitoring System (NARMS) database, he said. Other surveillance goals include supporting genomics research and expanding AMR monitoring to include pathogens associated with animal feed and companion animals, he added.
As part of the final component of the FDA’s AMR strategy to advance scientific initiatives, the FDA has released a new Request for Information “to obtain additional, external input on how best to develop an annual list of regulatory science initiatives specific for antimicrobial products,” Dr. Gottlieb announced. The FDA intends to use the information gained from clinicians and others in its creation of guidance documents and recommendations to streamline the antibiotic development process. He also cited the FDA’s ongoing support of partnerships with public and private organizations such as the Clinical Trials Transformation Initiative, which focuses on drug development for severe bacterial infections with current unmet medical need.
“We need to harness science and policy to help our public health systems and researchers become nimbler in the battle against drug-resistant pathogens,” Dr. Gottlieb concluded.
In a panel discussion following the briefing, several experts offered perspective on the FDA’s goals and on the challenges of AMR.
William Flynn, DVM, deputy director of science policy for the Center of Veterinary Medicine, noted some goals for reducing the use of antibiotics in the veterinary arena.
“We are trying to focus on the driver: What are the disease conditions that drive use of the product,” he said. Ideally, better management of disease conditions can reduce reliance on antibiotics, he added.
Also in the panel discussion, Steven Gitterman, MD, deputy director of the division of microbiology devices at the Center for Devices and Radiological Health, emphasized the value of sustainable trial databases so AMR research can continue on an ongoing basis. Finally, Carolyn Wilson, PhD, associate director of research at the Center for Biologics Evaluation and Research, noted that the FDA’s research and development efforts include antibiotic alternatives, including live biotherapeutic products, fecal microbiota transplantation, and bacteriophage therapy.
Visit www.fda.gov for a transcript of Dr. Gottlieb’s talk, and for the updated FDA website page with more details on the agency’s plans to combat antimicrobial resistance.
Dr. Gottlieb and the panelists had no financial conflicts to disclose.
WASHINGTON – A strategy combining stewardship and science is needed to help combat antimicrobial resistance, and updated plans from the U.S. Food and Drug Administration include four key components to address all aspects of product development and use, FDA commissioner Scott Gottlieb, MD, said in a press briefing in Washington on Sept. 14.
“The FDA plays a unique role in advancing human and animal health” that provides a unique vantage point for coordinating all aspects of product development and application, he said.
The FDA’s comprehensive approach to the challenge of antimicrobial resistance (AMR) includes:
- Facilitating product development.
- Promoting antimicrobial stewardship.
- Supporting the development of new tools for surveillance.
- Advancing scientific initiatives, including research for the development of alternative treatments.
The FDA’s product development plan to combat AMR includes the creation of incentives for companies to develop new antibiotic products and create a robust pipeline, which is a challenge because of the lack of immediate economic gain, Dr. Gottlieb said.
“It necessary to change the perception that the costs and risks of antibiotic innovation are too high relative to their expected gains,” he emphasized.
Strategies to incentivize companies include fast track designation, priority review, and breakthrough therapy designation. In addition, the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) is designed to promote development of antimicrobial drugs for limited and underserved populations, Dr. Gottlieb said. The FDA plan also calls for pursuing reimbursement options with the Centers for Medicare & Medicaid Services.
Promoting antimicrobial stewardship remains an ongoing element of the FDA’s plan to reduce AMR. In conjunction with the release of the FDA’s updated approach to AMR, the FDA’s Center for Veterinary Medicine CVM released a 5-year action plan to promote and support antimicrobial stewardship in not only the agricultural arena, but in companion animals as well.
The FDA plans to bring all antimicrobials of medical importance that are approved for use in animals under the oversight of CVM, which will pursue the improve labeling on antimicrobial drugs used in the feed and water of food-producing animals, including defining durations of use, Dr. Gottlieb noted.
Supporting the development and improvement of surveillance tools is “essential to understanding the drivers of resistance in human and veterinary settings and formulating appropriate responses” to outbreaks, Dr. Gottlieb said.
To help meet this goal, the FDA will expand sampling via the National Antimicrobial Resistance Monitoring System (NARMS) database, he said. Other surveillance goals include supporting genomics research and expanding AMR monitoring to include pathogens associated with animal feed and companion animals, he added.
As part of the final component of the FDA’s AMR strategy to advance scientific initiatives, the FDA has released a new Request for Information “to obtain additional, external input on how best to develop an annual list of regulatory science initiatives specific for antimicrobial products,” Dr. Gottlieb announced. The FDA intends to use the information gained from clinicians and others in its creation of guidance documents and recommendations to streamline the antibiotic development process. He also cited the FDA’s ongoing support of partnerships with public and private organizations such as the Clinical Trials Transformation Initiative, which focuses on drug development for severe bacterial infections with current unmet medical need.
“We need to harness science and policy to help our public health systems and researchers become nimbler in the battle against drug-resistant pathogens,” Dr. Gottlieb concluded.
In a panel discussion following the briefing, several experts offered perspective on the FDA’s goals and on the challenges of AMR.
William Flynn, DVM, deputy director of science policy for the Center of Veterinary Medicine, noted some goals for reducing the use of antibiotics in the veterinary arena.
“We are trying to focus on the driver: What are the disease conditions that drive use of the product,” he said. Ideally, better management of disease conditions can reduce reliance on antibiotics, he added.
Also in the panel discussion, Steven Gitterman, MD, deputy director of the division of microbiology devices at the Center for Devices and Radiological Health, emphasized the value of sustainable trial databases so AMR research can continue on an ongoing basis. Finally, Carolyn Wilson, PhD, associate director of research at the Center for Biologics Evaluation and Research, noted that the FDA’s research and development efforts include antibiotic alternatives, including live biotherapeutic products, fecal microbiota transplantation, and bacteriophage therapy.
Visit www.fda.gov for a transcript of Dr. Gottlieb’s talk, and for the updated FDA website page with more details on the agency’s plans to combat antimicrobial resistance.
Dr. Gottlieb and the panelists had no financial conflicts to disclose.
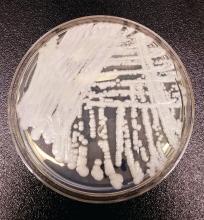
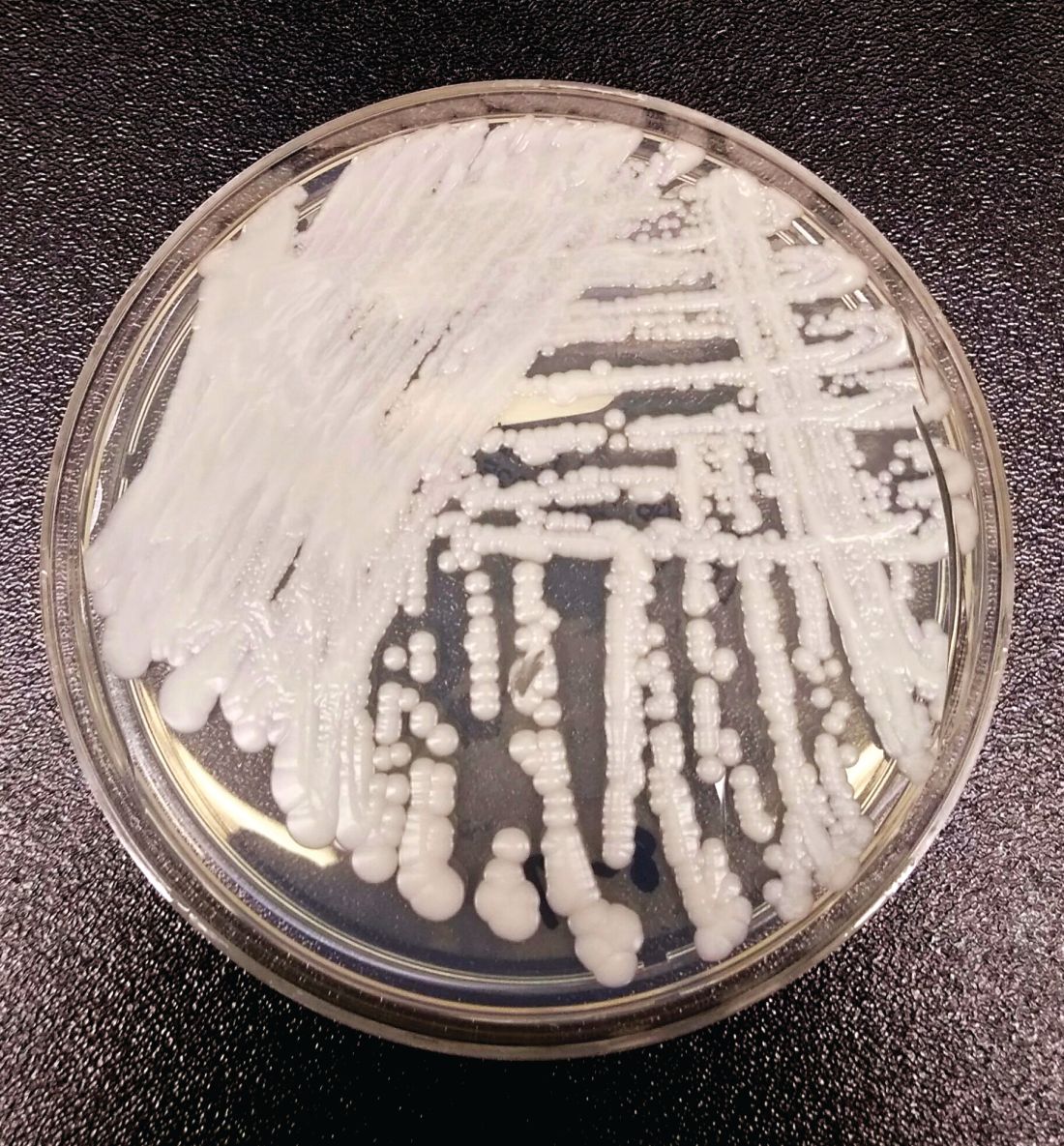
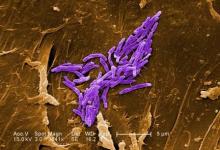
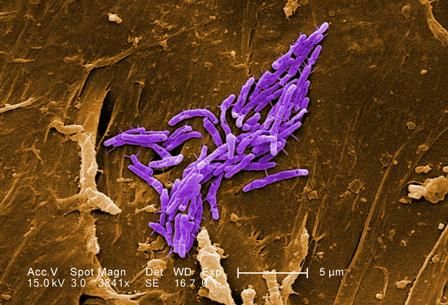
NYC outbreak of Candida auris linked to 45% mortality
Mortality within 90 days of infection was 45% among 51 patients diagnosed with antibiotic-resistant Candida auris infections in a multihospital outbreak in New York City from 2012 to 2017.
Transmission is ongoing in health care facilities, primarily among patients with extensive health care exposures, according to a report published in Emerging Infectious Diseases.
“Intensive infection prevention and control efforts continue; the goals are delaying endemicity, preventing outbreaks within facilities, reducing transmission and geographic spread, and blunting the effect of C. auris in New York and the rest of the United States,” Eleanor Adams, MD, of the New York Health Department, and her colleagues wrote. “Among medically fragile patients in NYC who had a history of extensive contact with health care facilities, clinicians should include C. auris in the differential diagnosis for patients with symptoms compatible with bloodstream infection.”
In the intensive case-patient analysis conducted by the New York State Health Department, 21 cases were from seven hospitals in Brooklyn, 16 were from three hospitals and one private medical office in Queens, 12 were from five hospitals and one long-term acute care hospital in Manhattan, and 1 was from a hospital in the Bronx. The remaining clinical case was identified in a western New York hospital in a patient who had recently been admitted to an involved Brooklyn hospital.
Among these patients, 31 (61%) had resided in long-term care facilities immediately before being admitted to the hospital in which their infection was diagnosed, and 19 of these 31 resided in skilled nursing facilities with ventilator beds; 1 (2%) resided in a long-term acute care hospital; 5 (10%) had been transferred from another hospital; and 4 (8%) had traveled internationally within 5 years before diagnosis, according to the investigators.
Isolates from 50 patients (98%) were resistant to fluconazole and 13 (25%) were resistant to fluconazole and amphotericin B. No initial isolates were resistant to echinocandins, although subsequent isolates obtained from 3 persons who had received an echinocandin acquired resistance to it, according to the researchers. Whole-genome sequencing performed at The Centers for Disease Control and Prevention indicated that 50 of 51 isolates belonged to a South Asia clade; the remaining isolate was the only one susceptible to fluconazole.
The work was supported by the CDC. No disclosures were reported.
SOURCE: Adams E et al. Emerg Infect Dis. 2018 Sep 12; 24(10); ID: 18-0649.
Mortality within 90 days of infection was 45% among 51 patients diagnosed with antibiotic-resistant Candida auris infections in a multihospital outbreak in New York City from 2012 to 2017.
Transmission is ongoing in health care facilities, primarily among patients with extensive health care exposures, according to a report published in Emerging Infectious Diseases.
“Intensive infection prevention and control efforts continue; the goals are delaying endemicity, preventing outbreaks within facilities, reducing transmission and geographic spread, and blunting the effect of C. auris in New York and the rest of the United States,” Eleanor Adams, MD, of the New York Health Department, and her colleagues wrote. “Among medically fragile patients in NYC who had a history of extensive contact with health care facilities, clinicians should include C. auris in the differential diagnosis for patients with symptoms compatible with bloodstream infection.”
In the intensive case-patient analysis conducted by the New York State Health Department, 21 cases were from seven hospitals in Brooklyn, 16 were from three hospitals and one private medical office in Queens, 12 were from five hospitals and one long-term acute care hospital in Manhattan, and 1 was from a hospital in the Bronx. The remaining clinical case was identified in a western New York hospital in a patient who had recently been admitted to an involved Brooklyn hospital.
Among these patients, 31 (61%) had resided in long-term care facilities immediately before being admitted to the hospital in which their infection was diagnosed, and 19 of these 31 resided in skilled nursing facilities with ventilator beds; 1 (2%) resided in a long-term acute care hospital; 5 (10%) had been transferred from another hospital; and 4 (8%) had traveled internationally within 5 years before diagnosis, according to the investigators.
Isolates from 50 patients (98%) were resistant to fluconazole and 13 (25%) were resistant to fluconazole and amphotericin B. No initial isolates were resistant to echinocandins, although subsequent isolates obtained from 3 persons who had received an echinocandin acquired resistance to it, according to the researchers. Whole-genome sequencing performed at The Centers for Disease Control and Prevention indicated that 50 of 51 isolates belonged to a South Asia clade; the remaining isolate was the only one susceptible to fluconazole.
The work was supported by the CDC. No disclosures were reported.
SOURCE: Adams E et al. Emerg Infect Dis. 2018 Sep 12; 24(10); ID: 18-0649.
Mortality within 90 days of infection was 45% among 51 patients diagnosed with antibiotic-resistant Candida auris infections in a multihospital outbreak in New York City from 2012 to 2017.
Transmission is ongoing in health care facilities, primarily among patients with extensive health care exposures, according to a report published in Emerging Infectious Diseases.
“Intensive infection prevention and control efforts continue; the goals are delaying endemicity, preventing outbreaks within facilities, reducing transmission and geographic spread, and blunting the effect of C. auris in New York and the rest of the United States,” Eleanor Adams, MD, of the New York Health Department, and her colleagues wrote. “Among medically fragile patients in NYC who had a history of extensive contact with health care facilities, clinicians should include C. auris in the differential diagnosis for patients with symptoms compatible with bloodstream infection.”
In the intensive case-patient analysis conducted by the New York State Health Department, 21 cases were from seven hospitals in Brooklyn, 16 were from three hospitals and one private medical office in Queens, 12 were from five hospitals and one long-term acute care hospital in Manhattan, and 1 was from a hospital in the Bronx. The remaining clinical case was identified in a western New York hospital in a patient who had recently been admitted to an involved Brooklyn hospital.
Among these patients, 31 (61%) had resided in long-term care facilities immediately before being admitted to the hospital in which their infection was diagnosed, and 19 of these 31 resided in skilled nursing facilities with ventilator beds; 1 (2%) resided in a long-term acute care hospital; 5 (10%) had been transferred from another hospital; and 4 (8%) had traveled internationally within 5 years before diagnosis, according to the investigators.
Isolates from 50 patients (98%) were resistant to fluconazole and 13 (25%) were resistant to fluconazole and amphotericin B. No initial isolates were resistant to echinocandins, although subsequent isolates obtained from 3 persons who had received an echinocandin acquired resistance to it, according to the researchers. Whole-genome sequencing performed at The Centers for Disease Control and Prevention indicated that 50 of 51 isolates belonged to a South Asia clade; the remaining isolate was the only one susceptible to fluconazole.
The work was supported by the CDC. No disclosures were reported.
SOURCE: Adams E et al. Emerg Infect Dis. 2018 Sep 12; 24(10); ID: 18-0649.
FROM EMERGING INFECTIOUS DISEASES
Review protocols, follow reprocessing guidelines to cut device-related HAIs
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
REPORTING FROM ICEID 2018
Key clinical point: Medical devices can be reservoirs and transmission vectors for health care–associated infections.
Major finding: Of 285 consultations, 48 involved medical devices or device reprocessing.
Study details: A review of records from 285 consultations
Disclosures: Dr. Benowitz reported having no disclosures
Source: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
United Kingdom experience provides important lessons for controlling C. auris outbreaks
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at candidaauris@cdc.gov.”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at candidaauris@cdc.gov.”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at candidaauris@cdc.gov.”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Ten additional cases occurred in the 7 months after the axillary probes were removed from the environment.
Study details: A review of the epidemiology and control of a C. auris outbreak affecting 76 patients.
Disclosures: Dr. Eyre reported having no disclosures.
Source: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
Hand hygiene linked to reduced ICU health care–associated infections
A hospital-wide infection control program (ICP) was found to be associated with reduced health care-associated severe sepsis/septic shock or death in the ICU, but it was not clear whether this decrease was a consequence of the ICP or because of a concomitant improvement in HAI case management, according to a the results of a prospective analysis.
In addition, there was no significant decrease in overall HAIs seen despite implementation of the program, according to the report published online in Clinical Microbiology and Infection (doi: 10.1016/j.cmi.2018.07.010), according to Stefan Hagel, MD, of the Institute for Infectious Diseases and Infection Control, Jena (Germany) University Hospital, and his colleagues.
They assessed two surveillance periods (September 2011 to August 2012 and May 2013 to August 2014). The ICP started in October 2012, and included hand-hygiene promotion and bundle implementation for common HAIs.
The data were analyzed by segmented mixed-effects Poisson regression and multi-state models and reported as adjusted incidence rate ratios (aIRR) and 50 adjusted hazard ratios (aHR) with 95% confidence intervals (CI).
In the first period, 62,154 patients were under surveillance, with 1,568 HAIs identified in 1,170 patients (4.3/100 admissions) and 2,336 HAIs identified in 1,711 patients (4.9/100 admissions) in the second surveillance period. No differences were found in the overall HAI incidence rates between the periods in the general wards and ICUs. There was only a slight decline in the incidence rate of HAIs in the ICUs (aIRR 0.98 [0.97, 1.00] per 1-week increment), compared with the general wards (aIRR 1.01 [1.00, 1.02]).
However, a reduction in severe HAIs (aIRR 0.13 [0.05, 0.32]) and a lower probability of HAI-associated in-hospital deaths (aHR 0.56 [0.31, 0.99]) were observed in the second period in the ICUs.
In attempting to explain the variance seen between the results for general wards and the ICU, an analysis of alcohol-based handrub solution consumption as a marker of hand-hygiene behavior indicated that a remarkable increase in consumption occurred in the ICUs while a less pronounced increase occurred in the general wards. “This finding might explain the observed decline in the HAI incidence after starting the campaign in the ICUs, which was not observed on the general wards.” Dr. Hagel and his colleagues suggested.
The authors discussed how several confounding factors that influenced the incidence of HAIs needed to be considered. As a consequence of the improvement in HAI management, the number of collected blood culture sets nearly doubled hospital-wide from 13,126 to 25,805 per year between 2011 and 2014, which likely undermined the study objective, they stated. The increase in cultures may have impacted the number of overall HAIs found.
“Although the primary aim of the study of reducing the overall incidence of HAIs was not achieved, the study demonstrated a decline of severe HAIs in patients in ICUs in the second surveillance period. Whether this result was a consequence of the ICP or a general improvement in HAI management remains unclear,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Hagel S et al. Clin Microbiol Infect. doi: 10.1016/j.cmi.2018.07.010].
A hospital-wide infection control program (ICP) was found to be associated with reduced health care-associated severe sepsis/septic shock or death in the ICU, but it was not clear whether this decrease was a consequence of the ICP or because of a concomitant improvement in HAI case management, according to a the results of a prospective analysis.
In addition, there was no significant decrease in overall HAIs seen despite implementation of the program, according to the report published online in Clinical Microbiology and Infection (doi: 10.1016/j.cmi.2018.07.010), according to Stefan Hagel, MD, of the Institute for Infectious Diseases and Infection Control, Jena (Germany) University Hospital, and his colleagues.
They assessed two surveillance periods (September 2011 to August 2012 and May 2013 to August 2014). The ICP started in October 2012, and included hand-hygiene promotion and bundle implementation for common HAIs.
The data were analyzed by segmented mixed-effects Poisson regression and multi-state models and reported as adjusted incidence rate ratios (aIRR) and 50 adjusted hazard ratios (aHR) with 95% confidence intervals (CI).
In the first period, 62,154 patients were under surveillance, with 1,568 HAIs identified in 1,170 patients (4.3/100 admissions) and 2,336 HAIs identified in 1,711 patients (4.9/100 admissions) in the second surveillance period. No differences were found in the overall HAI incidence rates between the periods in the general wards and ICUs. There was only a slight decline in the incidence rate of HAIs in the ICUs (aIRR 0.98 [0.97, 1.00] per 1-week increment), compared with the general wards (aIRR 1.01 [1.00, 1.02]).
However, a reduction in severe HAIs (aIRR 0.13 [0.05, 0.32]) and a lower probability of HAI-associated in-hospital deaths (aHR 0.56 [0.31, 0.99]) were observed in the second period in the ICUs.
In attempting to explain the variance seen between the results for general wards and the ICU, an analysis of alcohol-based handrub solution consumption as a marker of hand-hygiene behavior indicated that a remarkable increase in consumption occurred in the ICUs while a less pronounced increase occurred in the general wards. “This finding might explain the observed decline in the HAI incidence after starting the campaign in the ICUs, which was not observed on the general wards.” Dr. Hagel and his colleagues suggested.
The authors discussed how several confounding factors that influenced the incidence of HAIs needed to be considered. As a consequence of the improvement in HAI management, the number of collected blood culture sets nearly doubled hospital-wide from 13,126 to 25,805 per year between 2011 and 2014, which likely undermined the study objective, they stated. The increase in cultures may have impacted the number of overall HAIs found.
“Although the primary aim of the study of reducing the overall incidence of HAIs was not achieved, the study demonstrated a decline of severe HAIs in patients in ICUs in the second surveillance period. Whether this result was a consequence of the ICP or a general improvement in HAI management remains unclear,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Hagel S et al. Clin Microbiol Infect. doi: 10.1016/j.cmi.2018.07.010].
A hospital-wide infection control program (ICP) was found to be associated with reduced health care-associated severe sepsis/septic shock or death in the ICU, but it was not clear whether this decrease was a consequence of the ICP or because of a concomitant improvement in HAI case management, according to a the results of a prospective analysis.
In addition, there was no significant decrease in overall HAIs seen despite implementation of the program, according to the report published online in Clinical Microbiology and Infection (doi: 10.1016/j.cmi.2018.07.010), according to Stefan Hagel, MD, of the Institute for Infectious Diseases and Infection Control, Jena (Germany) University Hospital, and his colleagues.
They assessed two surveillance periods (September 2011 to August 2012 and May 2013 to August 2014). The ICP started in October 2012, and included hand-hygiene promotion and bundle implementation for common HAIs.
The data were analyzed by segmented mixed-effects Poisson regression and multi-state models and reported as adjusted incidence rate ratios (aIRR) and 50 adjusted hazard ratios (aHR) with 95% confidence intervals (CI).
In the first period, 62,154 patients were under surveillance, with 1,568 HAIs identified in 1,170 patients (4.3/100 admissions) and 2,336 HAIs identified in 1,711 patients (4.9/100 admissions) in the second surveillance period. No differences were found in the overall HAI incidence rates between the periods in the general wards and ICUs. There was only a slight decline in the incidence rate of HAIs in the ICUs (aIRR 0.98 [0.97, 1.00] per 1-week increment), compared with the general wards (aIRR 1.01 [1.00, 1.02]).
However, a reduction in severe HAIs (aIRR 0.13 [0.05, 0.32]) and a lower probability of HAI-associated in-hospital deaths (aHR 0.56 [0.31, 0.99]) were observed in the second period in the ICUs.
In attempting to explain the variance seen between the results for general wards and the ICU, an analysis of alcohol-based handrub solution consumption as a marker of hand-hygiene behavior indicated that a remarkable increase in consumption occurred in the ICUs while a less pronounced increase occurred in the general wards. “This finding might explain the observed decline in the HAI incidence after starting the campaign in the ICUs, which was not observed on the general wards.” Dr. Hagel and his colleagues suggested.
The authors discussed how several confounding factors that influenced the incidence of HAIs needed to be considered. As a consequence of the improvement in HAI management, the number of collected blood culture sets nearly doubled hospital-wide from 13,126 to 25,805 per year between 2011 and 2014, which likely undermined the study objective, they stated. The increase in cultures may have impacted the number of overall HAIs found.
“Although the primary aim of the study of reducing the overall incidence of HAIs was not achieved, the study demonstrated a decline of severe HAIs in patients in ICUs in the second surveillance period. Whether this result was a consequence of the ICP or a general improvement in HAI management remains unclear,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Hagel S et al. Clin Microbiol Infect. doi: 10.1016/j.cmi.2018.07.010].
FROM CLINICAL MICROBIOLOGY AND INFECTION
Key clinical point: Hand hygiene was the key factor associated with a decrease in severe HAIs.
Major finding: A reduction in severe HAIs (aIRR 0.13) and a lower probability of HAI-associated in-hospital deaths (aHR 0.56]) were observed.
Study details: A prospective database analysis of more nearly 65,000 hospitalized patients.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Hagel S et al. Clin Microbiol Infect. doi: 10.1016/j.cmi.2018.07.010.
Fewer groin infections with closed incision negative pressure therapy after vascular surgery
Closed incision negative pressure therapy (ciNPT) was found to reduce surgical site infections (SSI) in vascular surgery, according to the results of a prospective, randomized, industry-sponsored trial of patients who underwent vascular surgery for peripheral artery disease (PAD) published online in the European Journal of Vascular and Endovascular Surgery.
The investigator-initiated Reduction of Groin Wound Infections After Vascular Surgery by Using an Incision Management System trial (NCT02395159) included 204 patients who underwent vascular surgery involving longitudinal groin incision to treat the lower extremity or the iliac arteries between July 2015 and May 2017 at two study centers.
The primary endpoint was the occurrence of SSI assessed by the Szilagyi classification (grades I-III). The mean patient age was nearly 67 years and 70% were men. In terms of PAD staging, 52% were stage 2B, 28% were stage 3, and 19% were stage 4. Among the patients, 45% had a previous groin incision and 42% had diabetes.
All patients underwent similar preoperative treatment: hair shaving and preparation with Poly Alcohol (Antiseptica, Pulheim, Germany) and Braunoderm (Braun, Melsungen, Germany). At 30 minutes preincision, patients received intravenous antibiotic treatment (1.5 g cefuroxime or 600 mg clindamycin, if allergic to penicillin). After closure, the incision and surrounding skin area was cleaned and dried using sterile gauze. In the control group, a sterile adhesive wound dressing was applied to the wound, which was changed daily. In the treatment group, ciNPT was applied under sterile conditions in the operating room using the Prevena device, which exerts a continuous negative pressure of 125 mm Hg on the closed incision during the time of application. The device was removed at 5-7 days postoperatively, and no further wound dressings were used in the treatment group unless an SSI occurred.
The control group experienced more frequent SSIs (33.3%) than the intervention group (13.2%) (P =.0015). This difference was based on an increased rate of Szilagyi grade I SSI in the control group (24.6% vs. 8.1%, P = .0012), according to Alexander Gombert, MD, of the University Hospital Aachen (Germany), and his colleagues. The absolute risk difference based on the Szilagyi classification was –20.1 per 100 (95% confidence interval, –31.9 to –8.2).
In addition, there was a statistically significantly lower rate of SSI when using ciNPT within the subgroups at greater risk of infection, compared with controls: PAD stage greater than or equal to 3 (P less than .001), body mass index greater than 25 kg/m2 (P less than .001), and previous groin incision (P = .016).
There were no statistical differences between the two groups in Szilagyi grade II and III SSIs (which occurred in 5.8% of all procedures).
No potentially device-related complications were observed in the trial and there were no failures of the device seen.
“The use of ciNPT rather than standard wound dressing after groin incision as access for vascular surgery was associated with a reduced rate of superficial SSI classified by Szilagyi, suggesting that ciNPT may be useful for reducing the SSI rate among high-risk patients,” the researchers concluded.
The trial was funded by Acelity. Dr. Gombert received travel grants from Acelity.
SOURCE: Gombert A et al. Eur J Vasc Surg. 2018 Jul 2. doi: 10.1016/j.ejvs.2018.05.018.
Closed incision negative pressure therapy (ciNPT) was found to reduce surgical site infections (SSI) in vascular surgery, according to the results of a prospective, randomized, industry-sponsored trial of patients who underwent vascular surgery for peripheral artery disease (PAD) published online in the European Journal of Vascular and Endovascular Surgery.
The investigator-initiated Reduction of Groin Wound Infections After Vascular Surgery by Using an Incision Management System trial (NCT02395159) included 204 patients who underwent vascular surgery involving longitudinal groin incision to treat the lower extremity or the iliac arteries between July 2015 and May 2017 at two study centers.
The primary endpoint was the occurrence of SSI assessed by the Szilagyi classification (grades I-III). The mean patient age was nearly 67 years and 70% were men. In terms of PAD staging, 52% were stage 2B, 28% were stage 3, and 19% were stage 4. Among the patients, 45% had a previous groin incision and 42% had diabetes.
All patients underwent similar preoperative treatment: hair shaving and preparation with Poly Alcohol (Antiseptica, Pulheim, Germany) and Braunoderm (Braun, Melsungen, Germany). At 30 minutes preincision, patients received intravenous antibiotic treatment (1.5 g cefuroxime or 600 mg clindamycin, if allergic to penicillin). After closure, the incision and surrounding skin area was cleaned and dried using sterile gauze. In the control group, a sterile adhesive wound dressing was applied to the wound, which was changed daily. In the treatment group, ciNPT was applied under sterile conditions in the operating room using the Prevena device, which exerts a continuous negative pressure of 125 mm Hg on the closed incision during the time of application. The device was removed at 5-7 days postoperatively, and no further wound dressings were used in the treatment group unless an SSI occurred.
The control group experienced more frequent SSIs (33.3%) than the intervention group (13.2%) (P =.0015). This difference was based on an increased rate of Szilagyi grade I SSI in the control group (24.6% vs. 8.1%, P = .0012), according to Alexander Gombert, MD, of the University Hospital Aachen (Germany), and his colleagues. The absolute risk difference based on the Szilagyi classification was –20.1 per 100 (95% confidence interval, –31.9 to –8.2).
In addition, there was a statistically significantly lower rate of SSI when using ciNPT within the subgroups at greater risk of infection, compared with controls: PAD stage greater than or equal to 3 (P less than .001), body mass index greater than 25 kg/m2 (P less than .001), and previous groin incision (P = .016).
There were no statistical differences between the two groups in Szilagyi grade II and III SSIs (which occurred in 5.8% of all procedures).
No potentially device-related complications were observed in the trial and there were no failures of the device seen.
“The use of ciNPT rather than standard wound dressing after groin incision as access for vascular surgery was associated with a reduced rate of superficial SSI classified by Szilagyi, suggesting that ciNPT may be useful for reducing the SSI rate among high-risk patients,” the researchers concluded.
The trial was funded by Acelity. Dr. Gombert received travel grants from Acelity.
SOURCE: Gombert A et al. Eur J Vasc Surg. 2018 Jul 2. doi: 10.1016/j.ejvs.2018.05.018.
Closed incision negative pressure therapy (ciNPT) was found to reduce surgical site infections (SSI) in vascular surgery, according to the results of a prospective, randomized, industry-sponsored trial of patients who underwent vascular surgery for peripheral artery disease (PAD) published online in the European Journal of Vascular and Endovascular Surgery.
The investigator-initiated Reduction of Groin Wound Infections After Vascular Surgery by Using an Incision Management System trial (NCT02395159) included 204 patients who underwent vascular surgery involving longitudinal groin incision to treat the lower extremity or the iliac arteries between July 2015 and May 2017 at two study centers.
The primary endpoint was the occurrence of SSI assessed by the Szilagyi classification (grades I-III). The mean patient age was nearly 67 years and 70% were men. In terms of PAD staging, 52% were stage 2B, 28% were stage 3, and 19% were stage 4. Among the patients, 45% had a previous groin incision and 42% had diabetes.
All patients underwent similar preoperative treatment: hair shaving and preparation with Poly Alcohol (Antiseptica, Pulheim, Germany) and Braunoderm (Braun, Melsungen, Germany). At 30 minutes preincision, patients received intravenous antibiotic treatment (1.5 g cefuroxime or 600 mg clindamycin, if allergic to penicillin). After closure, the incision and surrounding skin area was cleaned and dried using sterile gauze. In the control group, a sterile adhesive wound dressing was applied to the wound, which was changed daily. In the treatment group, ciNPT was applied under sterile conditions in the operating room using the Prevena device, which exerts a continuous negative pressure of 125 mm Hg on the closed incision during the time of application. The device was removed at 5-7 days postoperatively, and no further wound dressings were used in the treatment group unless an SSI occurred.
The control group experienced more frequent SSIs (33.3%) than the intervention group (13.2%) (P =.0015). This difference was based on an increased rate of Szilagyi grade I SSI in the control group (24.6% vs. 8.1%, P = .0012), according to Alexander Gombert, MD, of the University Hospital Aachen (Germany), and his colleagues. The absolute risk difference based on the Szilagyi classification was –20.1 per 100 (95% confidence interval, –31.9 to –8.2).
In addition, there was a statistically significantly lower rate of SSI when using ciNPT within the subgroups at greater risk of infection, compared with controls: PAD stage greater than or equal to 3 (P less than .001), body mass index greater than 25 kg/m2 (P less than .001), and previous groin incision (P = .016).
There were no statistical differences between the two groups in Szilagyi grade II and III SSIs (which occurred in 5.8% of all procedures).
No potentially device-related complications were observed in the trial and there were no failures of the device seen.
“The use of ciNPT rather than standard wound dressing after groin incision as access for vascular surgery was associated with a reduced rate of superficial SSI classified by Szilagyi, suggesting that ciNPT may be useful for reducing the SSI rate among high-risk patients,” the researchers concluded.
The trial was funded by Acelity. Dr. Gombert received travel grants from Acelity.
SOURCE: Gombert A et al. Eur J Vasc Surg. 2018 Jul 2. doi: 10.1016/j.ejvs.2018.05.018.
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point: Closed incision negative pressure therapy lessened the incidence of groin infection after vascular surgery.
Major finding: The control group experienced more frequent surgical site infections (33.3%) than the intervention group (13.2%) (P =.0015).
Study details: A randomized, controlled trial of 204 patients with peripheral artery disease who underwent vascular surgery.
Disclosures: The trial was funded by Acelity. Dr. Gombert received travel grants from Acelity.
Source: Gombert A et al. Eur J Vasc Surg. 2018 Jul 2. doi: 10.1016/j.ejvs.2018.05.018.
Glucocorticoids linked with surgical infections in RA patients
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point:
Major finding: RA patients on more than 10 mg/day prednisone had a more than twofold higher rate of postsurgical hospitalized infections.
Study details: Review of Medicare and MarketScan administrative claims data for 11,021 patients with rheumatoid arthritis who underwent joint surgery.
Disclosures: The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept (Orencia). Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
Source: George MD et al. EULAR 2018. Abstract OP0228.
Hospital-acquired conditions drop 8% since 2014, saving 8,000 lives and $3 billion
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
Severity of sepsis-associated coagulopathy predicts hospital mortality
Patients with appear to be at heightened risk of death, according to results of a large retrospective cohort study.
The risk of death in the study increased with the severity of the sepsis-associated coagulopathy, which was defined using international normalized ratio (INR) and platelet counts.
Those findings suggest that the severity of coagulation abnormalities might be used to quantify mortality risk, according to investigator Patrick G. Lyons, MD, of the division of pulmonary and critical care medicine, Washington University, St. Louis, and his coinvestigators.
“Future trials of sepsis therapies targeting the coagulation cascade should take into account the presence or absence of sepsis-associated coagulopathy, as well as the severity of sepsis-associated coagulopathy, when formulating potential trial designs,” the investigators wrote in the journal Critical Care Medicine.
Their retrospective cohort study included 6,148 consecutive patients with sepsis or septic shock hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015. Of that group, 26% had sepsis-associated coagulopathy, defined as having both an INR of 1.2 or higher and a platelet count less than 150,000/mcL. Sepsis-associated coagulopathy was classified as mild for 4%, moderate for 16%, and severe for 6% of the cohort.
Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, the research team found, increasing progressively from 27.0% for mild, 40.7% for moderate, and 56.1% for patients in the most severe category of sepsis-associated coagulopathy (P less than .001).
Hospital and ICU days also increased progressively according to the severity of coagulopathy, they reported.
Both presence and severity of sepsis-associated coagulopathy remained independently associated with hospital mortality even after adjustments were made for patient characteristics, hospitalization variables, and interactions between sepsis-associated coagulopathy and cancer, investigators said. Odds ratios ranged from 1.33 to 2.14 for presence of sepsis-associated coagulopathy, and from 1.18 to 1.51 for severity, they reported in the journal.
These data have potential implications for managing patients with sepsis, according to Dr. Lyons and coinvestigators. In particular, severity of sepsis-associated coagulopathy might be used as “another relatively simple way” to compare sepsis patient populations, similar to other markers of severity such as the Sequential Organ Failure Assessment score.
“This could have important implications for comparing the outcomes of patients with sepsis from different hospitals, especially with increasing requirements for public reporting of such data through systems such as the Severe Sepsis/Septic Shock Early Management Bundle-1 and New York State’s Rory’s Regulations,” the investigators wrote.
Reported disclosures for the study included institutional funding from Asahi Kasei Pharma America by one coauthor, and support from Barnes-Jewish Hospital Foundation by another. No other potential conflicts of interest were reported.
SOURCE: Lyons PG et al. Crit Care Med. 2018 May;46(5):736-42.
This study outlines a simplified classification scheme for coagulopathy with implications that are potentially “profound,” according to authors of an editorial accompanying the journal article.
“Despite the frequency with which hemostatic derangements occur in sepsis, there has not been a widely accepted system for stratification of coagulopathies,” said editorialists Garrett W. Britton, DO, Cody Babcock, PharmD, and Christopher J. Colombo, MD. “Of the most cited criteria, all have varying concordance, and one does not seem to have an advantage over another.”
In the present study, patients with sepsis-associated coagulopathy were stratified into mild, moderate, and severe categories based on international normalized ratio (INR) levels and platelet counts.
While the study has limitations including a sicker patient cohort and arbitrarily chosen severity thresholds, the investigators did find progressively increasing mortality rates that correlated with severity and were independent of confounding variables.
“Overall, this stratification system will prove useful in identifying target populations in future interventional studies,” the editorial authors wrote.
Since sepsis-related mortality remains high, the ultimate goal of research should be identifying varying phenotypes of the disease and targeting them with specific therapies, they added.
“Lyons et al. have aided the first steps in that process with their straightforward classification scheme for sepsis-associated coagulopathy,” they wrote. “Intelligently designed therapeutic trials ‘evaluating’ the response of these phenotypes to new (or old) pharmacotherapy should be the ultimate goal.”
Garrett W. Britton, DO, is with the department of medicine, critical care section, Walter Reed National Military Medical Center, Bethesda, Md. Cody Babcock, PharmD, and Christopher J. Colombo, MD, are with the department of medicine, critical care section, Dwight David Eisenhower Army Medical Center, Fort Gordon, Ga. These comments are derived from their editorial in Critical Care Medicine . The authors had no disclosures beyond reporting government work.
This study outlines a simplified classification scheme for coagulopathy with implications that are potentially “profound,” according to authors of an editorial accompanying the journal article.
“Despite the frequency with which hemostatic derangements occur in sepsis, there has not been a widely accepted system for stratification of coagulopathies,” said editorialists Garrett W. Britton, DO, Cody Babcock, PharmD, and Christopher J. Colombo, MD. “Of the most cited criteria, all have varying concordance, and one does not seem to have an advantage over another.”
In the present study, patients with sepsis-associated coagulopathy were stratified into mild, moderate, and severe categories based on international normalized ratio (INR) levels and platelet counts.
While the study has limitations including a sicker patient cohort and arbitrarily chosen severity thresholds, the investigators did find progressively increasing mortality rates that correlated with severity and were independent of confounding variables.
“Overall, this stratification system will prove useful in identifying target populations in future interventional studies,” the editorial authors wrote.
Since sepsis-related mortality remains high, the ultimate goal of research should be identifying varying phenotypes of the disease and targeting them with specific therapies, they added.
“Lyons et al. have aided the first steps in that process with their straightforward classification scheme for sepsis-associated coagulopathy,” they wrote. “Intelligently designed therapeutic trials ‘evaluating’ the response of these phenotypes to new (or old) pharmacotherapy should be the ultimate goal.”
Garrett W. Britton, DO, is with the department of medicine, critical care section, Walter Reed National Military Medical Center, Bethesda, Md. Cody Babcock, PharmD, and Christopher J. Colombo, MD, are with the department of medicine, critical care section, Dwight David Eisenhower Army Medical Center, Fort Gordon, Ga. These comments are derived from their editorial in Critical Care Medicine . The authors had no disclosures beyond reporting government work.
This study outlines a simplified classification scheme for coagulopathy with implications that are potentially “profound,” according to authors of an editorial accompanying the journal article.
“Despite the frequency with which hemostatic derangements occur in sepsis, there has not been a widely accepted system for stratification of coagulopathies,” said editorialists Garrett W. Britton, DO, Cody Babcock, PharmD, and Christopher J. Colombo, MD. “Of the most cited criteria, all have varying concordance, and one does not seem to have an advantage over another.”
In the present study, patients with sepsis-associated coagulopathy were stratified into mild, moderate, and severe categories based on international normalized ratio (INR) levels and platelet counts.
While the study has limitations including a sicker patient cohort and arbitrarily chosen severity thresholds, the investigators did find progressively increasing mortality rates that correlated with severity and were independent of confounding variables.
“Overall, this stratification system will prove useful in identifying target populations in future interventional studies,” the editorial authors wrote.
Since sepsis-related mortality remains high, the ultimate goal of research should be identifying varying phenotypes of the disease and targeting them with specific therapies, they added.
“Lyons et al. have aided the first steps in that process with their straightforward classification scheme for sepsis-associated coagulopathy,” they wrote. “Intelligently designed therapeutic trials ‘evaluating’ the response of these phenotypes to new (or old) pharmacotherapy should be the ultimate goal.”
Garrett W. Britton, DO, is with the department of medicine, critical care section, Walter Reed National Military Medical Center, Bethesda, Md. Cody Babcock, PharmD, and Christopher J. Colombo, MD, are with the department of medicine, critical care section, Dwight David Eisenhower Army Medical Center, Fort Gordon, Ga. These comments are derived from their editorial in Critical Care Medicine . The authors had no disclosures beyond reporting government work.
Patients with appear to be at heightened risk of death, according to results of a large retrospective cohort study.
The risk of death in the study increased with the severity of the sepsis-associated coagulopathy, which was defined using international normalized ratio (INR) and platelet counts.
Those findings suggest that the severity of coagulation abnormalities might be used to quantify mortality risk, according to investigator Patrick G. Lyons, MD, of the division of pulmonary and critical care medicine, Washington University, St. Louis, and his coinvestigators.
“Future trials of sepsis therapies targeting the coagulation cascade should take into account the presence or absence of sepsis-associated coagulopathy, as well as the severity of sepsis-associated coagulopathy, when formulating potential trial designs,” the investigators wrote in the journal Critical Care Medicine.
Their retrospective cohort study included 6,148 consecutive patients with sepsis or septic shock hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015. Of that group, 26% had sepsis-associated coagulopathy, defined as having both an INR of 1.2 or higher and a platelet count less than 150,000/mcL. Sepsis-associated coagulopathy was classified as mild for 4%, moderate for 16%, and severe for 6% of the cohort.
Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, the research team found, increasing progressively from 27.0% for mild, 40.7% for moderate, and 56.1% for patients in the most severe category of sepsis-associated coagulopathy (P less than .001).
Hospital and ICU days also increased progressively according to the severity of coagulopathy, they reported.
Both presence and severity of sepsis-associated coagulopathy remained independently associated with hospital mortality even after adjustments were made for patient characteristics, hospitalization variables, and interactions between sepsis-associated coagulopathy and cancer, investigators said. Odds ratios ranged from 1.33 to 2.14 for presence of sepsis-associated coagulopathy, and from 1.18 to 1.51 for severity, they reported in the journal.
These data have potential implications for managing patients with sepsis, according to Dr. Lyons and coinvestigators. In particular, severity of sepsis-associated coagulopathy might be used as “another relatively simple way” to compare sepsis patient populations, similar to other markers of severity such as the Sequential Organ Failure Assessment score.
“This could have important implications for comparing the outcomes of patients with sepsis from different hospitals, especially with increasing requirements for public reporting of such data through systems such as the Severe Sepsis/Septic Shock Early Management Bundle-1 and New York State’s Rory’s Regulations,” the investigators wrote.
Reported disclosures for the study included institutional funding from Asahi Kasei Pharma America by one coauthor, and support from Barnes-Jewish Hospital Foundation by another. No other potential conflicts of interest were reported.
SOURCE: Lyons PG et al. Crit Care Med. 2018 May;46(5):736-42.
Patients with appear to be at heightened risk of death, according to results of a large retrospective cohort study.
The risk of death in the study increased with the severity of the sepsis-associated coagulopathy, which was defined using international normalized ratio (INR) and platelet counts.
Those findings suggest that the severity of coagulation abnormalities might be used to quantify mortality risk, according to investigator Patrick G. Lyons, MD, of the division of pulmonary and critical care medicine, Washington University, St. Louis, and his coinvestigators.
“Future trials of sepsis therapies targeting the coagulation cascade should take into account the presence or absence of sepsis-associated coagulopathy, as well as the severity of sepsis-associated coagulopathy, when formulating potential trial designs,” the investigators wrote in the journal Critical Care Medicine.
Their retrospective cohort study included 6,148 consecutive patients with sepsis or septic shock hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015. Of that group, 26% had sepsis-associated coagulopathy, defined as having both an INR of 1.2 or higher and a platelet count less than 150,000/mcL. Sepsis-associated coagulopathy was classified as mild for 4%, moderate for 16%, and severe for 6% of the cohort.
Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, the research team found, increasing progressively from 27.0% for mild, 40.7% for moderate, and 56.1% for patients in the most severe category of sepsis-associated coagulopathy (P less than .001).
Hospital and ICU days also increased progressively according to the severity of coagulopathy, they reported.
Both presence and severity of sepsis-associated coagulopathy remained independently associated with hospital mortality even after adjustments were made for patient characteristics, hospitalization variables, and interactions between sepsis-associated coagulopathy and cancer, investigators said. Odds ratios ranged from 1.33 to 2.14 for presence of sepsis-associated coagulopathy, and from 1.18 to 1.51 for severity, they reported in the journal.
These data have potential implications for managing patients with sepsis, according to Dr. Lyons and coinvestigators. In particular, severity of sepsis-associated coagulopathy might be used as “another relatively simple way” to compare sepsis patient populations, similar to other markers of severity such as the Sequential Organ Failure Assessment score.
“This could have important implications for comparing the outcomes of patients with sepsis from different hospitals, especially with increasing requirements for public reporting of such data through systems such as the Severe Sepsis/Septic Shock Early Management Bundle-1 and New York State’s Rory’s Regulations,” the investigators wrote.
Reported disclosures for the study included institutional funding from Asahi Kasei Pharma America by one coauthor, and support from Barnes-Jewish Hospital Foundation by another. No other potential conflicts of interest were reported.
SOURCE: Lyons PG et al. Crit Care Med. 2018 May;46(5):736-42.
FROM CRITICAL CARE MEDICINE
Key clinical point: Risk of hospital mortality increased incrementally with the severity of sepsis-related coagulopathy.
Major finding: Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, increasing progressively up to 56.1% for patients in the most severe category.
Study details: A retrospective cohort study including 6,148 consecutive patients hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015.
Disclosures: One author reported institutional funding from Asahi Kasei Pharma America and another noted support from Barnes-Jewish Hospital Foundation. No other potential conflicts of interest were reported.
Source: Lyons PG et al. Crit Care Med. 2018 May;46(5):73642.
Hospital safety program curbs surgical site infections
The Agency for Healthcare Research and Quality (AHRQ) designed the program to reduce surgical site infections (SSIs), which are harmful to patients and expensive for the health care system, wrote Della M. Lin, MD, of Johns Hopkins University, Baltimore, and the department of surgery at the University of Hawaii, Honolulu, and her colleagues.
In a study published in the Journal of the American College of Surgeons, the researchers reviewed data from a statewide intervention conducted at 15 hospitals in Hawaii from January 2013 to June 2015. The intervention included the Comprehensive Unit-based Safety Program and individualized interventions for each hospital to help reduce SSIs. The primary outcome was the number of colorectal SSIs. A secondary outcome of hospital safety culture was assessed using the AHRQ Hospital Survey on Patient Safety Culture. The participating hospitals ranged from a 25-bed critical-access hospital to a 533-bed academic medical center.
Overall, the colorectal SSI rate decreased significantly (from 12% to 5%) from the first quarter of 2013 to the second quarter of 2015, with a significant linear decrease over the study period. The rate of superficial SSIs decreased significantly, falling from 8% to 3%. However, the rate of deep SSIs was not significantly different before and after the intervention program (2% vs. 0%), nor was the organ space SSI rate (3% vs. 2%). The standardized infection ratio decreased from 1.83 to 0.92.
The culture of safety in the hospitals improved, but more modestly, in 10 of 12 areas that were measured over the study period.
The overall perception of patient safety improved from 49% to 53%, teamwork across different units improved from 49% to 54%, management and support for patient safety improved from 53% to 60%, and nonpunitive response to errors improved from 36% to 40%.
In addition, communication and openness improved from 50% to 53%, frequency of reported events improved from 51% to 60%, feedback and communication about errors improved from 52% to 59%, organizational learning and continuous improvement increased from 59% to 70%, teamwork within units improved from 68% to 75%, and expectations and actions by supervisors and managers to promote safety improved from 58% to 64%. Staff responses reflect agreement on improvement in the areas of issues of communication, feedback mechanisms, and teamwork, but the change in culture was not on the order of the SSI change.
The most common interventions to reduce SSIs were the use of reliable chlorhexidine wash or wipe before surgery/surgical prep; appropriate use of antibiotics with respect to selection, dosage, and timing; standardized postsurgical debriefing; and differentiating clean/dirty/clean in the use of anastomosis trays and closing trays.
One bundle component, the implementation of the standard operating room debrief, was found to be of particular value to participants. The investigators noted that debrief questions such as “What went well?” and “What needs to be improved?” had “encouraged new processes of thinking beyond first-order problem solving. The debrief challenge embraced by the teams emphasized that ‘bundles’ did not consist of only technical interventions [e.g. clean/dirty trays, chlorhexidine gluconate wipes in preop], but embedded culture interventions—new processes for problem solving.”
The study findings were limited by several factors, such as the use of public SSI data that were not audited for accuracy and the inability to monitor the reliability of the implementation of the various interventions, the researchers said. In addition, “In this current study, there was a change in SSI rates and a change in safety culture, but correlations between the two were negligible or weak for most domains of safety culture,” they noted. The question of sustainability of the SSI improvement without the concomitant staff support of culture change was not addressed by the investigators.
However, the results suggest that a 62% decrease is robust, and that for some hospitals with a low volume of colorectal cases, “teams could attend to iteratively reduce surgical harm beyond SSI,” the researchers wrote.
The study was supported in part by the AHRQ. Dr. Lin disclosed serving as a board member and as a paid independent contractor to the Hawaii Medical Service Association. Her coauthors had no financial conflicts to disclose.
SOURCE: Lin DM et al. J Am Coll Surg. 2018 May 18. doi: 10.1016/j.jamcollsurg.2018.04.031.
The Agency for Healthcare Research and Quality (AHRQ) designed the program to reduce surgical site infections (SSIs), which are harmful to patients and expensive for the health care system, wrote Della M. Lin, MD, of Johns Hopkins University, Baltimore, and the department of surgery at the University of Hawaii, Honolulu, and her colleagues.
In a study published in the Journal of the American College of Surgeons, the researchers reviewed data from a statewide intervention conducted at 15 hospitals in Hawaii from January 2013 to June 2015. The intervention included the Comprehensive Unit-based Safety Program and individualized interventions for each hospital to help reduce SSIs. The primary outcome was the number of colorectal SSIs. A secondary outcome of hospital safety culture was assessed using the AHRQ Hospital Survey on Patient Safety Culture. The participating hospitals ranged from a 25-bed critical-access hospital to a 533-bed academic medical center.
Overall, the colorectal SSI rate decreased significantly (from 12% to 5%) from the first quarter of 2013 to the second quarter of 2015, with a significant linear decrease over the study period. The rate of superficial SSIs decreased significantly, falling from 8% to 3%. However, the rate of deep SSIs was not significantly different before and after the intervention program (2% vs. 0%), nor was the organ space SSI rate (3% vs. 2%). The standardized infection ratio decreased from 1.83 to 0.92.
The culture of safety in the hospitals improved, but more modestly, in 10 of 12 areas that were measured over the study period.
The overall perception of patient safety improved from 49% to 53%, teamwork across different units improved from 49% to 54%, management and support for patient safety improved from 53% to 60%, and nonpunitive response to errors improved from 36% to 40%.
In addition, communication and openness improved from 50% to 53%, frequency of reported events improved from 51% to 60%, feedback and communication about errors improved from 52% to 59%, organizational learning and continuous improvement increased from 59% to 70%, teamwork within units improved from 68% to 75%, and expectations and actions by supervisors and managers to promote safety improved from 58% to 64%. Staff responses reflect agreement on improvement in the areas of issues of communication, feedback mechanisms, and teamwork, but the change in culture was not on the order of the SSI change.
The most common interventions to reduce SSIs were the use of reliable chlorhexidine wash or wipe before surgery/surgical prep; appropriate use of antibiotics with respect to selection, dosage, and timing; standardized postsurgical debriefing; and differentiating clean/dirty/clean in the use of anastomosis trays and closing trays.
One bundle component, the implementation of the standard operating room debrief, was found to be of particular value to participants. The investigators noted that debrief questions such as “What went well?” and “What needs to be improved?” had “encouraged new processes of thinking beyond first-order problem solving. The debrief challenge embraced by the teams emphasized that ‘bundles’ did not consist of only technical interventions [e.g. clean/dirty trays, chlorhexidine gluconate wipes in preop], but embedded culture interventions—new processes for problem solving.”
The study findings were limited by several factors, such as the use of public SSI data that were not audited for accuracy and the inability to monitor the reliability of the implementation of the various interventions, the researchers said. In addition, “In this current study, there was a change in SSI rates and a change in safety culture, but correlations between the two were negligible or weak for most domains of safety culture,” they noted. The question of sustainability of the SSI improvement without the concomitant staff support of culture change was not addressed by the investigators.
However, the results suggest that a 62% decrease is robust, and that for some hospitals with a low volume of colorectal cases, “teams could attend to iteratively reduce surgical harm beyond SSI,” the researchers wrote.
The study was supported in part by the AHRQ. Dr. Lin disclosed serving as a board member and as a paid independent contractor to the Hawaii Medical Service Association. Her coauthors had no financial conflicts to disclose.
SOURCE: Lin DM et al. J Am Coll Surg. 2018 May 18. doi: 10.1016/j.jamcollsurg.2018.04.031.
The Agency for Healthcare Research and Quality (AHRQ) designed the program to reduce surgical site infections (SSIs), which are harmful to patients and expensive for the health care system, wrote Della M. Lin, MD, of Johns Hopkins University, Baltimore, and the department of surgery at the University of Hawaii, Honolulu, and her colleagues.
In a study published in the Journal of the American College of Surgeons, the researchers reviewed data from a statewide intervention conducted at 15 hospitals in Hawaii from January 2013 to June 2015. The intervention included the Comprehensive Unit-based Safety Program and individualized interventions for each hospital to help reduce SSIs. The primary outcome was the number of colorectal SSIs. A secondary outcome of hospital safety culture was assessed using the AHRQ Hospital Survey on Patient Safety Culture. The participating hospitals ranged from a 25-bed critical-access hospital to a 533-bed academic medical center.
Overall, the colorectal SSI rate decreased significantly (from 12% to 5%) from the first quarter of 2013 to the second quarter of 2015, with a significant linear decrease over the study period. The rate of superficial SSIs decreased significantly, falling from 8% to 3%. However, the rate of deep SSIs was not significantly different before and after the intervention program (2% vs. 0%), nor was the organ space SSI rate (3% vs. 2%). The standardized infection ratio decreased from 1.83 to 0.92.
The culture of safety in the hospitals improved, but more modestly, in 10 of 12 areas that were measured over the study period.
The overall perception of patient safety improved from 49% to 53%, teamwork across different units improved from 49% to 54%, management and support for patient safety improved from 53% to 60%, and nonpunitive response to errors improved from 36% to 40%.
In addition, communication and openness improved from 50% to 53%, frequency of reported events improved from 51% to 60%, feedback and communication about errors improved from 52% to 59%, organizational learning and continuous improvement increased from 59% to 70%, teamwork within units improved from 68% to 75%, and expectations and actions by supervisors and managers to promote safety improved from 58% to 64%. Staff responses reflect agreement on improvement in the areas of issues of communication, feedback mechanisms, and teamwork, but the change in culture was not on the order of the SSI change.
The most common interventions to reduce SSIs were the use of reliable chlorhexidine wash or wipe before surgery/surgical prep; appropriate use of antibiotics with respect to selection, dosage, and timing; standardized postsurgical debriefing; and differentiating clean/dirty/clean in the use of anastomosis trays and closing trays.
One bundle component, the implementation of the standard operating room debrief, was found to be of particular value to participants. The investigators noted that debrief questions such as “What went well?” and “What needs to be improved?” had “encouraged new processes of thinking beyond first-order problem solving. The debrief challenge embraced by the teams emphasized that ‘bundles’ did not consist of only technical interventions [e.g. clean/dirty trays, chlorhexidine gluconate wipes in preop], but embedded culture interventions—new processes for problem solving.”
The study findings were limited by several factors, such as the use of public SSI data that were not audited for accuracy and the inability to monitor the reliability of the implementation of the various interventions, the researchers said. In addition, “In this current study, there was a change in SSI rates and a change in safety culture, but correlations between the two were negligible or weak for most domains of safety culture,” they noted. The question of sustainability of the SSI improvement without the concomitant staff support of culture change was not addressed by the investigators.
However, the results suggest that a 62% decrease is robust, and that for some hospitals with a low volume of colorectal cases, “teams could attend to iteratively reduce surgical harm beyond SSI,” the researchers wrote.
The study was supported in part by the AHRQ. Dr. Lin disclosed serving as a board member and as a paid independent contractor to the Hawaii Medical Service Association. Her coauthors had no financial conflicts to disclose.
SOURCE: Lin DM et al. J Am Coll Surg. 2018 May 18. doi: 10.1016/j.jamcollsurg.2018.04.031.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Hospital participation in an Agency for Healthcare Research and Quality safety program improved safety culture and reduced surgical site infections.
Major finding: Surgical site infections among colorectal surgery patients decreased by 61.7% after the intervention.
Study details: The data come from a cohort study of 15 hospitals in Hawaii from January 2013 to June 2015.
Disclosures: The study was supported in part by the AHRQ. Dr. Lin disclosed serving as a board member and as a paid independent contractor to the Hawaii Medical Service Association. Her coauthors had no financial conflicts to disclose.
Source: Lin DM et al. J Am Coll Surg. 2018 May 18. doi: 10.1016/j.jamcollsurg.2018.04.031.