User login
Study highlights diagnostic challenges of differentiating lichen sclerosus from vitiligo
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
FROM SPD 2023
Enlarging Pigmented Lesion on the Thigh
The Diagnosis: Localized Cutaneous Argyria
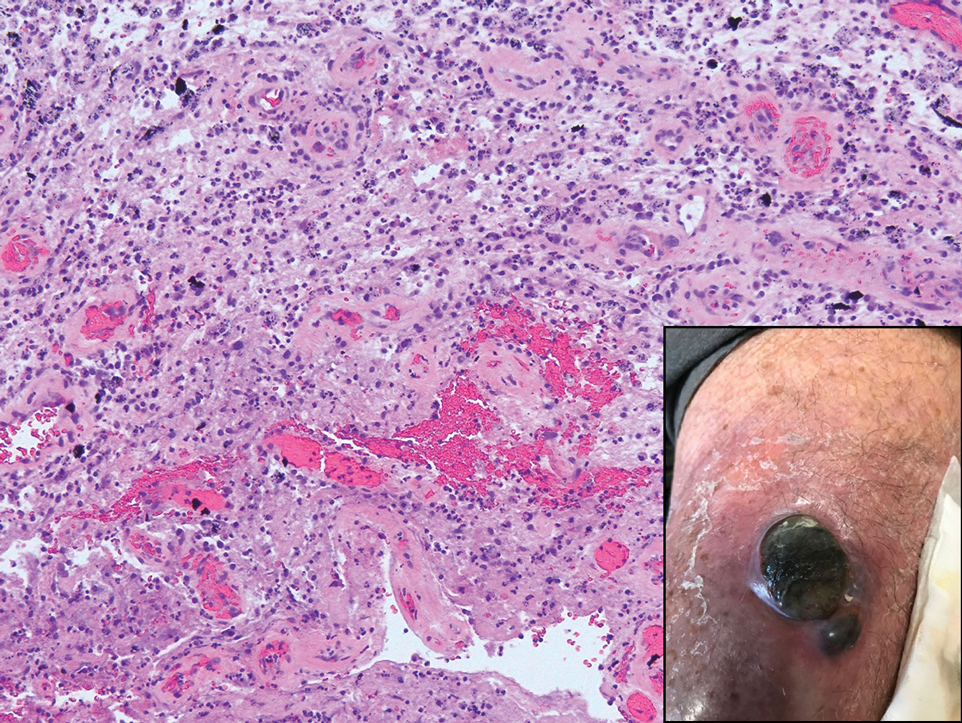
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
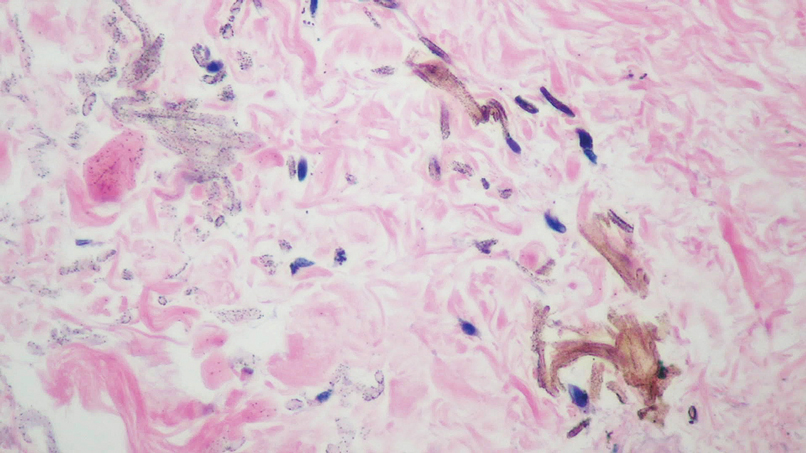
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
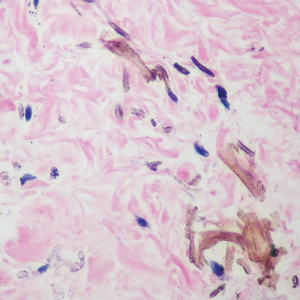
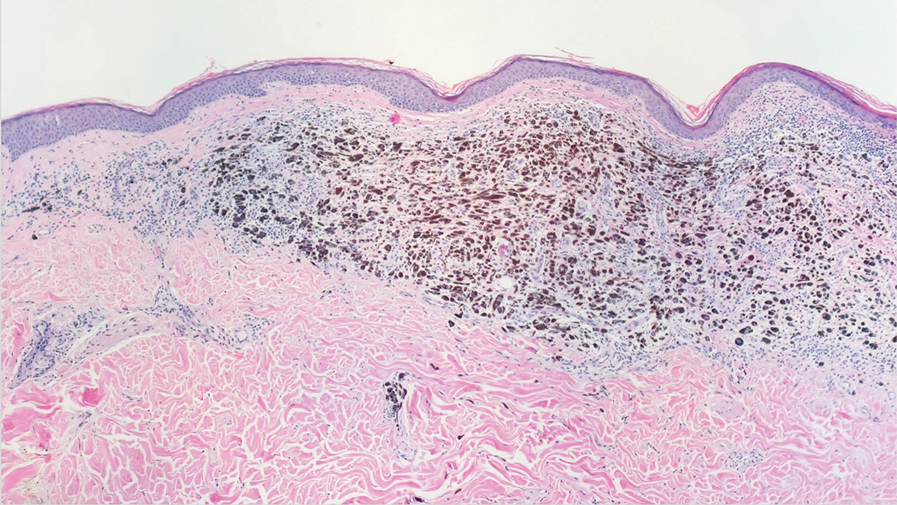
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6
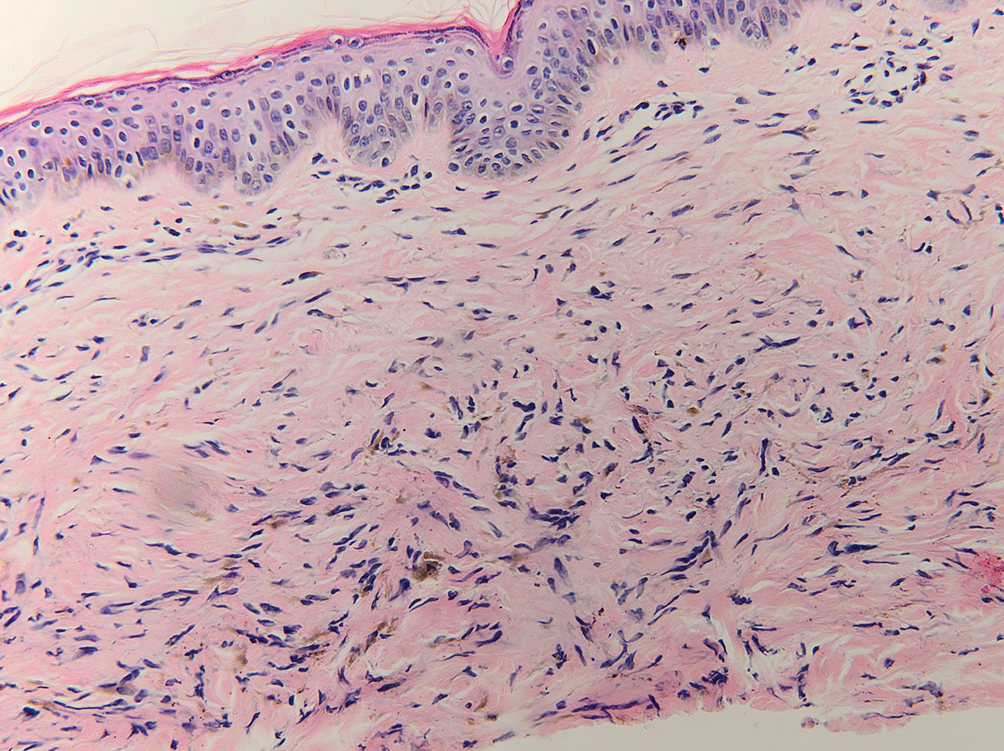
Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7
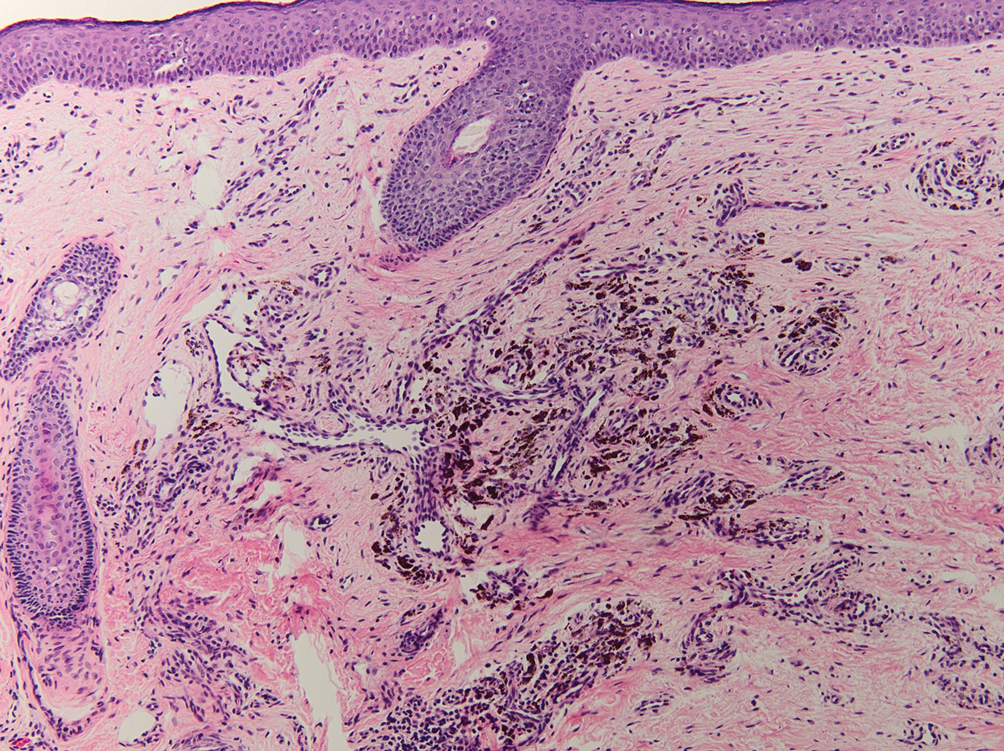
Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8
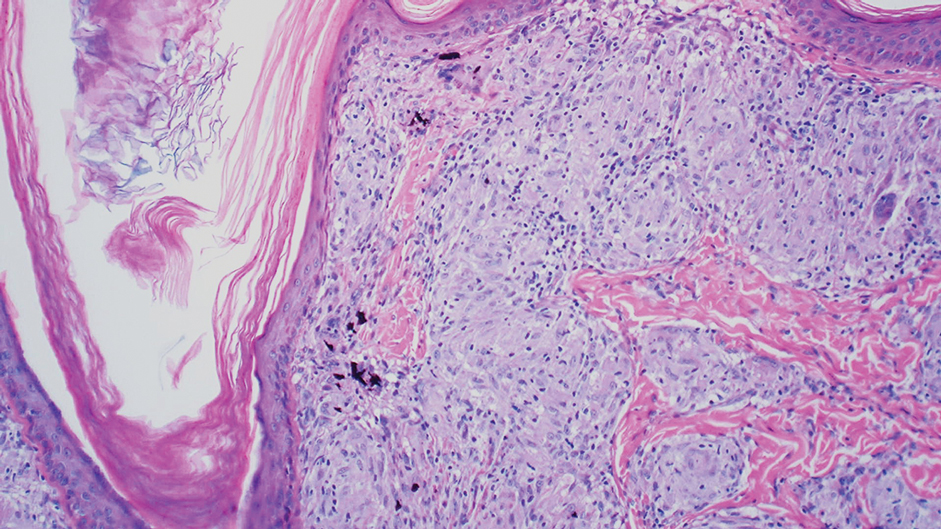
Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3
Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
An 80-year-old man presented with a pigmented lesion on the left lateral thigh near the knee that had been gradually enlarging over several weeks (top [inset]). He underwent a left knee replacement surgery for advanced osteoarthritis many months prior that was complicated by postoperative Staphylococcus aureus infection with sinus tract formation that was persistent for 6 months and treated with a topical medication. A pigmented lesion developed near the opening of the sinus tract. His medical history was remarkable for extensive actinic damage as well as multiple actinic keratoses treated with cryotherapy but no history of melanoma. An excisional biopsy was performed (top and bottom).
Many users of skin-lightening product unaware of risks
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Study examines burden of vitiligo in the U.S.
To investigate the incidence and prevalence of diagnosed vitiligo in the United States, researchers used a 15% random sample of electronic medical records from the IBM Explorys database. Two cohorts were included: 2,980,778 patients diagnosed with vitiligo between Jan. 1, 2015, and Dec. 31, 2019 (incidence analysis), and 1,057,534 patients diagnosed with vitiligo between Jan. 1 and Dec. 31, 2019 (prevalence analysis).The main outcomes were incidence (per 100,000 person-years) and prevalence of diagnosed vitiligo overall and by age, race/ethnicity, and sex. Amit Garg, MD, a dermatologist with Northwell Health, New Hyde Park, N.Y., led the study, which was published in JAMA Dermatology.
The age- and sex-adjusted overall incidence rate of diagnosed vitiligo was 22.6 per 100,000 person-years, and the prevalence was 0.16%, the authors reported. The sex-adjusted IR was highest among patients aged 60-69 years (25.3 per 100,000 person-years); prevalence was highest among patients aged 70 years or older (0.21%).
The highest age-adjusted IR was among Asian American patients (41.2 per 100,000 person-years), followed by Hispanic/Latino patients (37.3 per 100,000 PY), those reporting other or multiple races (31.1 per 100,000), Black patients (29.6 per 100,000 person-years), and White patients (18.7 per 100,000 person-years). The highest age-adjusted prevalence was among Hispanic/Latino patients (0.29%), followed by Asian American patients (0.27%), those reporting other or multiple races (0.24%), Black patients (0.22%), and White patients (0.13%).
The burden of vitiligo in the United States is poorly understood, and the findings “may support improving awareness of vitiligo disease burden in medical and public sectors, informing research agendas, improving enrollment of racial and ethnic minority populations in trials, and developing health policies,” the authors wrote.
Limitations of the study included that the analysis only captured patients who sought care in health systems included in the database, and there was the potential for underreporting, “since not all patients with vitiligo seek care,” the authors noted.
Dr. Garg reported being an adviser for and receiving honoraria from many pharmaceutical companies. He has also received research grants from AbbVie, UCB, the National Psoriasis Foundation, and the CHORD COUSIN Collaboration. No other disclosures were reported.
A version of this article first appeared on Medscape.com .
To investigate the incidence and prevalence of diagnosed vitiligo in the United States, researchers used a 15% random sample of electronic medical records from the IBM Explorys database. Two cohorts were included: 2,980,778 patients diagnosed with vitiligo between Jan. 1, 2015, and Dec. 31, 2019 (incidence analysis), and 1,057,534 patients diagnosed with vitiligo between Jan. 1 and Dec. 31, 2019 (prevalence analysis).The main outcomes were incidence (per 100,000 person-years) and prevalence of diagnosed vitiligo overall and by age, race/ethnicity, and sex. Amit Garg, MD, a dermatologist with Northwell Health, New Hyde Park, N.Y., led the study, which was published in JAMA Dermatology.
The age- and sex-adjusted overall incidence rate of diagnosed vitiligo was 22.6 per 100,000 person-years, and the prevalence was 0.16%, the authors reported. The sex-adjusted IR was highest among patients aged 60-69 years (25.3 per 100,000 person-years); prevalence was highest among patients aged 70 years or older (0.21%).
The highest age-adjusted IR was among Asian American patients (41.2 per 100,000 person-years), followed by Hispanic/Latino patients (37.3 per 100,000 PY), those reporting other or multiple races (31.1 per 100,000), Black patients (29.6 per 100,000 person-years), and White patients (18.7 per 100,000 person-years). The highest age-adjusted prevalence was among Hispanic/Latino patients (0.29%), followed by Asian American patients (0.27%), those reporting other or multiple races (0.24%), Black patients (0.22%), and White patients (0.13%).
The burden of vitiligo in the United States is poorly understood, and the findings “may support improving awareness of vitiligo disease burden in medical and public sectors, informing research agendas, improving enrollment of racial and ethnic minority populations in trials, and developing health policies,” the authors wrote.
Limitations of the study included that the analysis only captured patients who sought care in health systems included in the database, and there was the potential for underreporting, “since not all patients with vitiligo seek care,” the authors noted.
Dr. Garg reported being an adviser for and receiving honoraria from many pharmaceutical companies. He has also received research grants from AbbVie, UCB, the National Psoriasis Foundation, and the CHORD COUSIN Collaboration. No other disclosures were reported.
A version of this article first appeared on Medscape.com .
To investigate the incidence and prevalence of diagnosed vitiligo in the United States, researchers used a 15% random sample of electronic medical records from the IBM Explorys database. Two cohorts were included: 2,980,778 patients diagnosed with vitiligo between Jan. 1, 2015, and Dec. 31, 2019 (incidence analysis), and 1,057,534 patients diagnosed with vitiligo between Jan. 1 and Dec. 31, 2019 (prevalence analysis).The main outcomes were incidence (per 100,000 person-years) and prevalence of diagnosed vitiligo overall and by age, race/ethnicity, and sex. Amit Garg, MD, a dermatologist with Northwell Health, New Hyde Park, N.Y., led the study, which was published in JAMA Dermatology.
The age- and sex-adjusted overall incidence rate of diagnosed vitiligo was 22.6 per 100,000 person-years, and the prevalence was 0.16%, the authors reported. The sex-adjusted IR was highest among patients aged 60-69 years (25.3 per 100,000 person-years); prevalence was highest among patients aged 70 years or older (0.21%).
The highest age-adjusted IR was among Asian American patients (41.2 per 100,000 person-years), followed by Hispanic/Latino patients (37.3 per 100,000 PY), those reporting other or multiple races (31.1 per 100,000), Black patients (29.6 per 100,000 person-years), and White patients (18.7 per 100,000 person-years). The highest age-adjusted prevalence was among Hispanic/Latino patients (0.29%), followed by Asian American patients (0.27%), those reporting other or multiple races (0.24%), Black patients (0.22%), and White patients (0.13%).
The burden of vitiligo in the United States is poorly understood, and the findings “may support improving awareness of vitiligo disease burden in medical and public sectors, informing research agendas, improving enrollment of racial and ethnic minority populations in trials, and developing health policies,” the authors wrote.
Limitations of the study included that the analysis only captured patients who sought care in health systems included in the database, and there was the potential for underreporting, “since not all patients with vitiligo seek care,” the authors noted.
Dr. Garg reported being an adviser for and receiving honoraria from many pharmaceutical companies. He has also received research grants from AbbVie, UCB, the National Psoriasis Foundation, and the CHORD COUSIN Collaboration. No other disclosures were reported.
A version of this article first appeared on Medscape.com .
FROM JAMA DERMATOLOGY
Pigmenting Purpuric Dermatoses: Striking But Not a Manifestation of COVID-19 Infection
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6
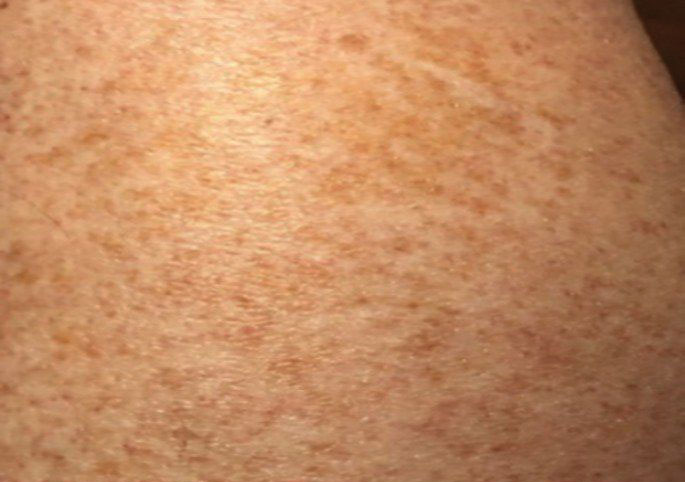
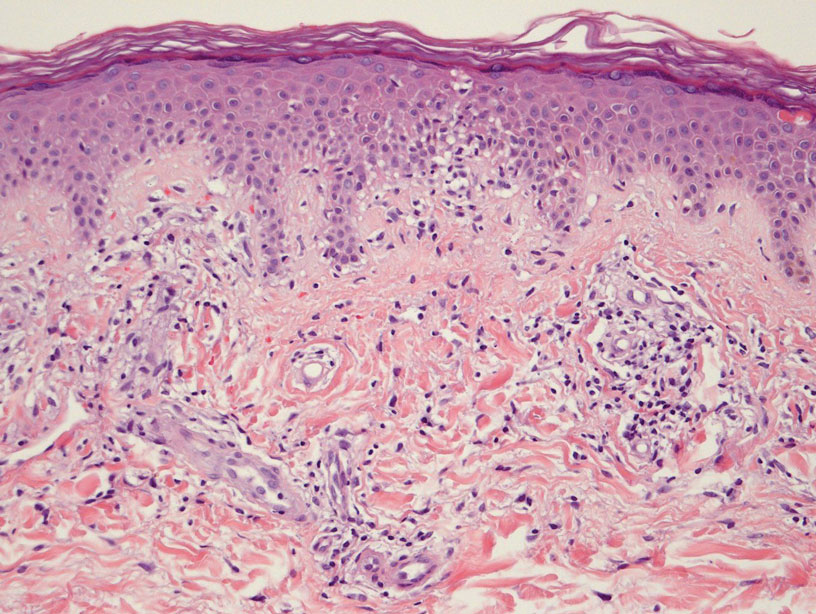
Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1
Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24
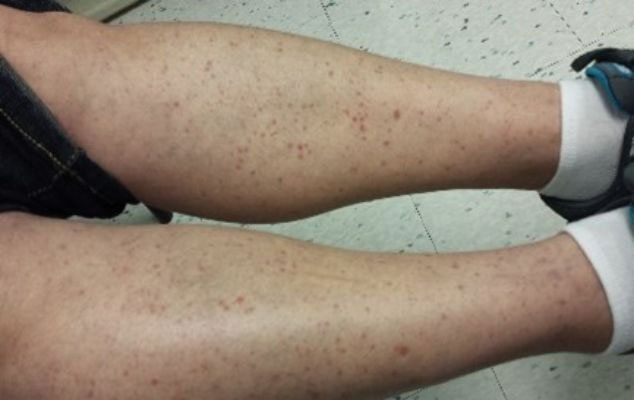
Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.
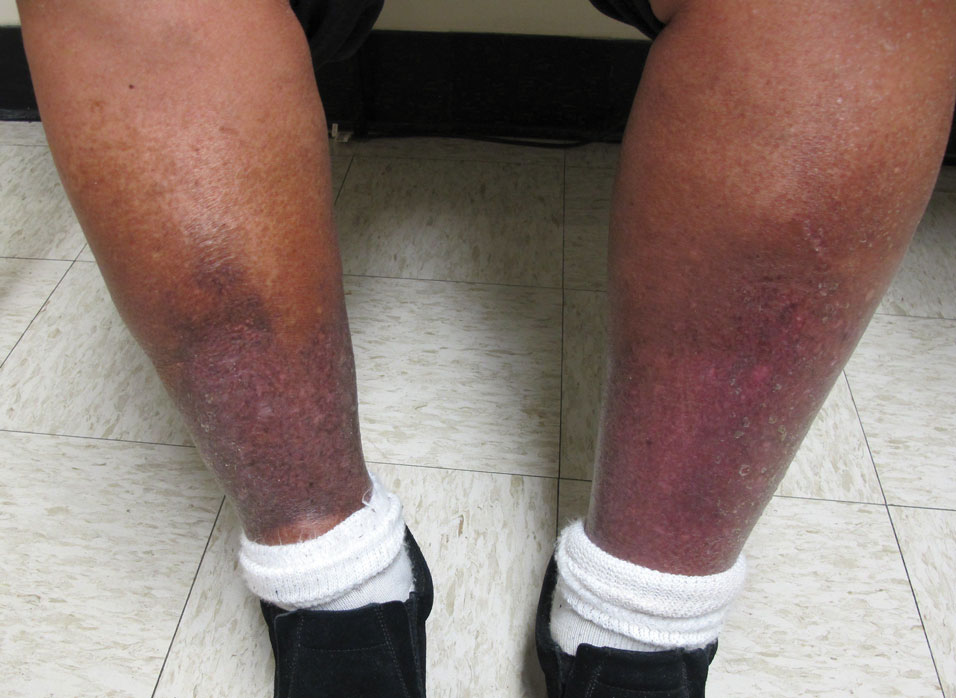
Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Practice Points
- Dermatologists should be aware of the clinical presentations of pigmenting purpuric dermatoses (PPDs).
- Certain PPDs may resemble the thromboembolic events seen in COVID-19. Clinicians should especially be aware of how to differentiate these benign pigmentary disorders from other serious conditions.
- Teledermatology is widely utilized, but caution may be prudent when evaluating erythematous or purpuric dermatoses, especially those of the lower extremities.
- Pigmenting purpuric dermatoses generally are benign and do not require immediate treatment.
Camp Discovery: A place for children to be comfortable in their own skin
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.
Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.
Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.
Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
White Spots on the Extremities
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
A 52-year-old Black woman presented with self-described whitened spots on the arms and legs of 2 years’ duration. She experienced no improvement with ketoconazole cream and topical calcineurin inhibitors prescribed during a prior dermatology visit at an outside institution. She denied pain or pruritus. A review of systems as well as the patient’s medical history were noncontributory. A prior biopsy at an outside institution revealed an interface dermatitis suggestive of cutaneous lupus erythematosus. The patient noted social drinking and denied tobacco use. She had no known allergies to medications and currently was on tamoxifen for breast cancer following a right mastectomy. Physical examination showed hypopigmented macules and patches on the left upper arm and right proximal leg. The center of the lesions was not erythematous or scaly. Palpation did not reveal enlarged lymph nodes, and laboratory analyses ruled out low levels of red blood cells, white blood cells, or platelets. Punch biopsies from the left arm and right thigh were performed.
Halting active inflammation key in treating PIH
CHICAGO –
Dr. Desai, clinical assistant professor in the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, spoke at the Pigmentary Disorders Exchange Symposium, provided by MedscapeLive!
Like all dermatologists, he said at the meeting, he sees lots of acne cases. However, PIH is often the presenting reason for the visit in his practice, which focuses predominantly on skin of color.
“Most of my patients come in not even worried about the acne,” he said. “They come in wanting me to fix the dark spots.”
Inflammation persists
Dermatologists, Dr. Desai said, should educate patients with active PIH resulting from acne or other diseases that even though the condition has been labeled post- inflammatory hyperpigmentation, the inflammation continues to be a problem.
He said, while patients may think PIH is “just scars,” the inflammation is still active and the condition needs to be treated from a skin-lightening perspective but, more importantly, with a focus on halting the inflammation. “If you were to biopsy the areas of hyperpigmentation, you would find a high density of active inflammatory behaviors still present in the skin,” he said.
When treating patients, it’s critical to first treat the underlying skin condition aggressively, he said. “Things like topical retinoids and azelaic acid mechanistically actually make a lot more sense for PIH than even hydroquinone, in some cases, because these therapies are actually anti-inflammatory for many of the diseases we treat.”
Dr. Desai noted that, in patients with darker skin tones, even diseases like seborrheic dermatitis and plaque psoriasis can result in PIH, while in patients with lighter skin tones, the same diseases may leave some residual postinflammatory erythema.
“I think it’s very important, particularly when you’re treating a darker skin–toned patient, to arrest the erythema early on to prevent that further worsening of hyperpigmentation,” he said.
Biopsies important
In cases of PIH, determining the best treatment requires finding out where the pigment is and how deep it is, Dr. Desai said.
He noted dermatologists are often worried about doing biopsies, particularly in patients with darker skin, because of the risk of scarring and keloid formation for those more prone to keloids. The preference is also for a therapeutic effect without using invasive procedures.
“But particularly with PIH, in patients who have been therapeutically challenging, I don’t hesitate to do very small biopsies – 2- and 3-mm punch biopsies – particularly if they are from the head and neck area.”
He suggests doing biopsies on part of the ear, lower jaw line, or the neck area, as these areas tend to heal nicely. “You don’t have to be so concerned about the scarring if you counsel appropriately,” he said.
The biopsy can be valuable in determining whether a very expensive treatment will reach the intended target.
Topical retinoids play an important role as anti-inflammatories for PIH, Dr. Desai said.
He gave an example of a patient with Fitzpatrick skin type IV or V with chronic acne and extensive PIH. “Are you going to effectively tell that patient to apply 4% hydroquinone triple-combination compound across 30 different areas of PIH on their face? The answer is that’s really not very efficient or effective.”
That’s why therapies, such as retinoids, that target the pathogenesis of PIH, particularly the inflammatory component, are important, he added.
Psychological burden
PIH comes with significant stigma and loss of quality of life loss that can last many years.
During another presentation at the meeting, Susan C. Taylor, MD, professor and vice chair of diversity, equity and inclusion in the department of dermatology, at the University of Pennsylvania, Philadelphia, pointed out that in a 2016 study of 324 patients in seven Asian countries, acne-related PIH lasted longer than 1 year in 65.2% of patients and 5 years or longer in 22.3%, significantly affecting their quality of life.
Dr. Desai added that, in a paper recently published in the British Journal of Dermatology, on the impact of postacne hyperpigmentation in patients, the authors pointed out that the reported prevalence of PIH in patients with acne ranges between 45.5% and 87.2%, depending on skin phototype, and that in most cases, PIH takes more than a year to fade.
“Studies have demonstrated that patients with acne and resulting scarring often face stigmatization, leading to quality of life impairment, social withdrawal and body image disorders, which can further contribute to higher risk for depression and social anxiety,” the paper’s authors wrote.
Dr. Desai reported no financial disclosures relevant to his talk.
CHICAGO –
Dr. Desai, clinical assistant professor in the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, spoke at the Pigmentary Disorders Exchange Symposium, provided by MedscapeLive!
Like all dermatologists, he said at the meeting, he sees lots of acne cases. However, PIH is often the presenting reason for the visit in his practice, which focuses predominantly on skin of color.
“Most of my patients come in not even worried about the acne,” he said. “They come in wanting me to fix the dark spots.”
Inflammation persists
Dermatologists, Dr. Desai said, should educate patients with active PIH resulting from acne or other diseases that even though the condition has been labeled post- inflammatory hyperpigmentation, the inflammation continues to be a problem.
He said, while patients may think PIH is “just scars,” the inflammation is still active and the condition needs to be treated from a skin-lightening perspective but, more importantly, with a focus on halting the inflammation. “If you were to biopsy the areas of hyperpigmentation, you would find a high density of active inflammatory behaviors still present in the skin,” he said.
When treating patients, it’s critical to first treat the underlying skin condition aggressively, he said. “Things like topical retinoids and azelaic acid mechanistically actually make a lot more sense for PIH than even hydroquinone, in some cases, because these therapies are actually anti-inflammatory for many of the diseases we treat.”
Dr. Desai noted that, in patients with darker skin tones, even diseases like seborrheic dermatitis and plaque psoriasis can result in PIH, while in patients with lighter skin tones, the same diseases may leave some residual postinflammatory erythema.
“I think it’s very important, particularly when you’re treating a darker skin–toned patient, to arrest the erythema early on to prevent that further worsening of hyperpigmentation,” he said.
Biopsies important
In cases of PIH, determining the best treatment requires finding out where the pigment is and how deep it is, Dr. Desai said.
He noted dermatologists are often worried about doing biopsies, particularly in patients with darker skin, because of the risk of scarring and keloid formation for those more prone to keloids. The preference is also for a therapeutic effect without using invasive procedures.
“But particularly with PIH, in patients who have been therapeutically challenging, I don’t hesitate to do very small biopsies – 2- and 3-mm punch biopsies – particularly if they are from the head and neck area.”
He suggests doing biopsies on part of the ear, lower jaw line, or the neck area, as these areas tend to heal nicely. “You don’t have to be so concerned about the scarring if you counsel appropriately,” he said.
The biopsy can be valuable in determining whether a very expensive treatment will reach the intended target.
Topical retinoids play an important role as anti-inflammatories for PIH, Dr. Desai said.
He gave an example of a patient with Fitzpatrick skin type IV or V with chronic acne and extensive PIH. “Are you going to effectively tell that patient to apply 4% hydroquinone triple-combination compound across 30 different areas of PIH on their face? The answer is that’s really not very efficient or effective.”
That’s why therapies, such as retinoids, that target the pathogenesis of PIH, particularly the inflammatory component, are important, he added.
Psychological burden
PIH comes with significant stigma and loss of quality of life loss that can last many years.
During another presentation at the meeting, Susan C. Taylor, MD, professor and vice chair of diversity, equity and inclusion in the department of dermatology, at the University of Pennsylvania, Philadelphia, pointed out that in a 2016 study of 324 patients in seven Asian countries, acne-related PIH lasted longer than 1 year in 65.2% of patients and 5 years or longer in 22.3%, significantly affecting their quality of life.
Dr. Desai added that, in a paper recently published in the British Journal of Dermatology, on the impact of postacne hyperpigmentation in patients, the authors pointed out that the reported prevalence of PIH in patients with acne ranges between 45.5% and 87.2%, depending on skin phototype, and that in most cases, PIH takes more than a year to fade.
“Studies have demonstrated that patients with acne and resulting scarring often face stigmatization, leading to quality of life impairment, social withdrawal and body image disorders, which can further contribute to higher risk for depression and social anxiety,” the paper’s authors wrote.
Dr. Desai reported no financial disclosures relevant to his talk.
CHICAGO –
Dr. Desai, clinical assistant professor in the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, spoke at the Pigmentary Disorders Exchange Symposium, provided by MedscapeLive!
Like all dermatologists, he said at the meeting, he sees lots of acne cases. However, PIH is often the presenting reason for the visit in his practice, which focuses predominantly on skin of color.
“Most of my patients come in not even worried about the acne,” he said. “They come in wanting me to fix the dark spots.”
Inflammation persists
Dermatologists, Dr. Desai said, should educate patients with active PIH resulting from acne or other diseases that even though the condition has been labeled post- inflammatory hyperpigmentation, the inflammation continues to be a problem.
He said, while patients may think PIH is “just scars,” the inflammation is still active and the condition needs to be treated from a skin-lightening perspective but, more importantly, with a focus on halting the inflammation. “If you were to biopsy the areas of hyperpigmentation, you would find a high density of active inflammatory behaviors still present in the skin,” he said.
When treating patients, it’s critical to first treat the underlying skin condition aggressively, he said. “Things like topical retinoids and azelaic acid mechanistically actually make a lot more sense for PIH than even hydroquinone, in some cases, because these therapies are actually anti-inflammatory for many of the diseases we treat.”
Dr. Desai noted that, in patients with darker skin tones, even diseases like seborrheic dermatitis and plaque psoriasis can result in PIH, while in patients with lighter skin tones, the same diseases may leave some residual postinflammatory erythema.
“I think it’s very important, particularly when you’re treating a darker skin–toned patient, to arrest the erythema early on to prevent that further worsening of hyperpigmentation,” he said.
Biopsies important
In cases of PIH, determining the best treatment requires finding out where the pigment is and how deep it is, Dr. Desai said.
He noted dermatologists are often worried about doing biopsies, particularly in patients with darker skin, because of the risk of scarring and keloid formation for those more prone to keloids. The preference is also for a therapeutic effect without using invasive procedures.
“But particularly with PIH, in patients who have been therapeutically challenging, I don’t hesitate to do very small biopsies – 2- and 3-mm punch biopsies – particularly if they are from the head and neck area.”
He suggests doing biopsies on part of the ear, lower jaw line, or the neck area, as these areas tend to heal nicely. “You don’t have to be so concerned about the scarring if you counsel appropriately,” he said.
The biopsy can be valuable in determining whether a very expensive treatment will reach the intended target.
Topical retinoids play an important role as anti-inflammatories for PIH, Dr. Desai said.
He gave an example of a patient with Fitzpatrick skin type IV or V with chronic acne and extensive PIH. “Are you going to effectively tell that patient to apply 4% hydroquinone triple-combination compound across 30 different areas of PIH on their face? The answer is that’s really not very efficient or effective.”
That’s why therapies, such as retinoids, that target the pathogenesis of PIH, particularly the inflammatory component, are important, he added.
Psychological burden
PIH comes with significant stigma and loss of quality of life loss that can last many years.
During another presentation at the meeting, Susan C. Taylor, MD, professor and vice chair of diversity, equity and inclusion in the department of dermatology, at the University of Pennsylvania, Philadelphia, pointed out that in a 2016 study of 324 patients in seven Asian countries, acne-related PIH lasted longer than 1 year in 65.2% of patients and 5 years or longer in 22.3%, significantly affecting their quality of life.
Dr. Desai added that, in a paper recently published in the British Journal of Dermatology, on the impact of postacne hyperpigmentation in patients, the authors pointed out that the reported prevalence of PIH in patients with acne ranges between 45.5% and 87.2%, depending on skin phototype, and that in most cases, PIH takes more than a year to fade.
“Studies have demonstrated that patients with acne and resulting scarring often face stigmatization, leading to quality of life impairment, social withdrawal and body image disorders, which can further contribute to higher risk for depression and social anxiety,” the paper’s authors wrote.
Dr. Desai reported no financial disclosures relevant to his talk.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM
Macular dermal hyperpigmentation: Treatment tips from an expert
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM
Tips, contraindications for superficial chemical peels reviewed
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
AT THE MEDSCAPE LIVE! PIGMENTARY DISORDERS SYMPOSIUM