User login
Pigmenting Purpuric Dermatoses: Striking But Not a Manifestation of COVID-19 Infection
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
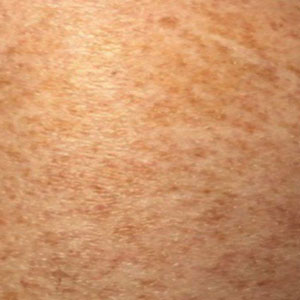
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6
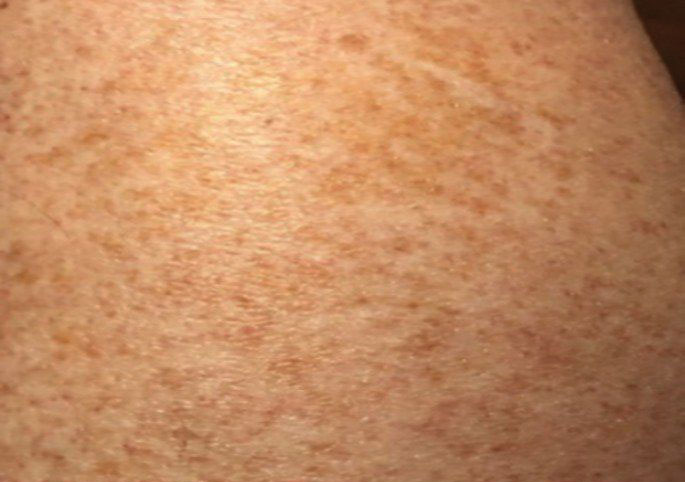
Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1
Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
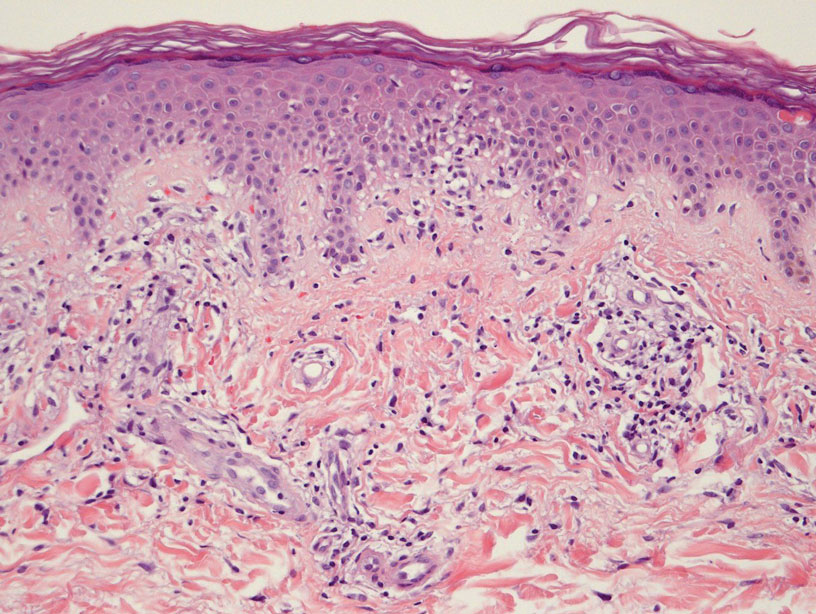
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24
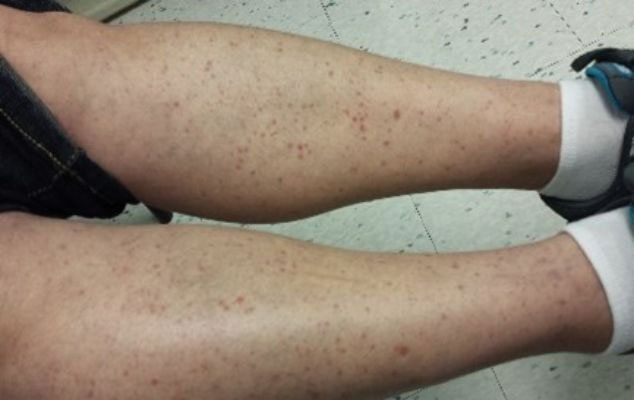
Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.
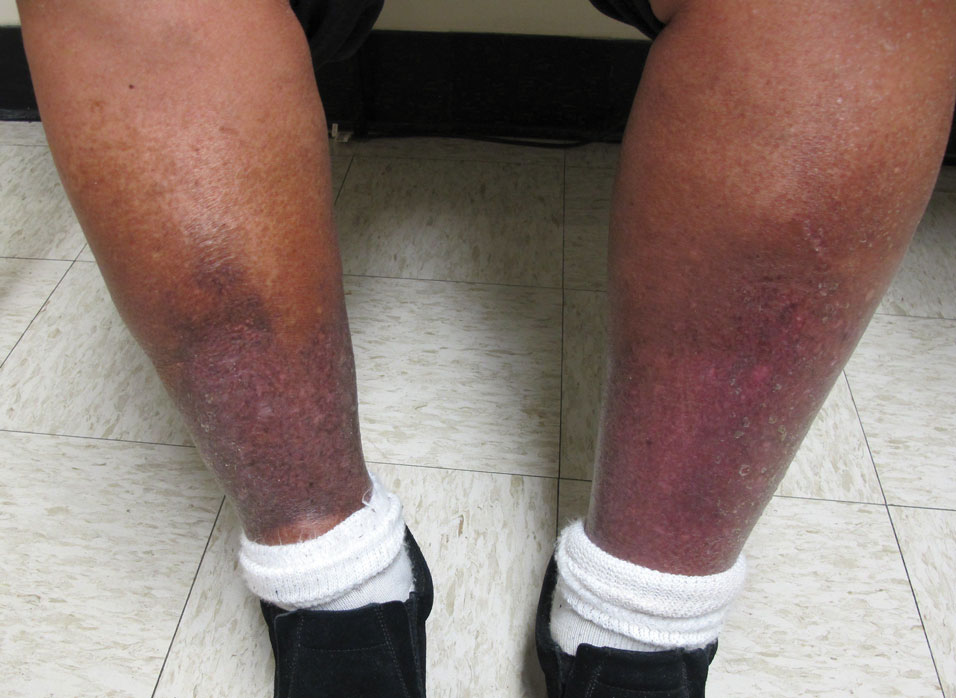
Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Practice Points
- Dermatologists should be aware of the clinical presentations of pigmenting purpuric dermatoses (PPDs).
- Certain PPDs may resemble the thromboembolic events seen in COVID-19. Clinicians should especially be aware of how to differentiate these benign pigmentary disorders from other serious conditions.
- Teledermatology is widely utilized, but caution may be prudent when evaluating erythematous or purpuric dermatoses, especially those of the lower extremities.
- Pigmenting purpuric dermatoses generally are benign and do not require immediate treatment.
Cross-sectional Analysis of Matched Dermatology Residency Applicants Without US Home Programs
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.
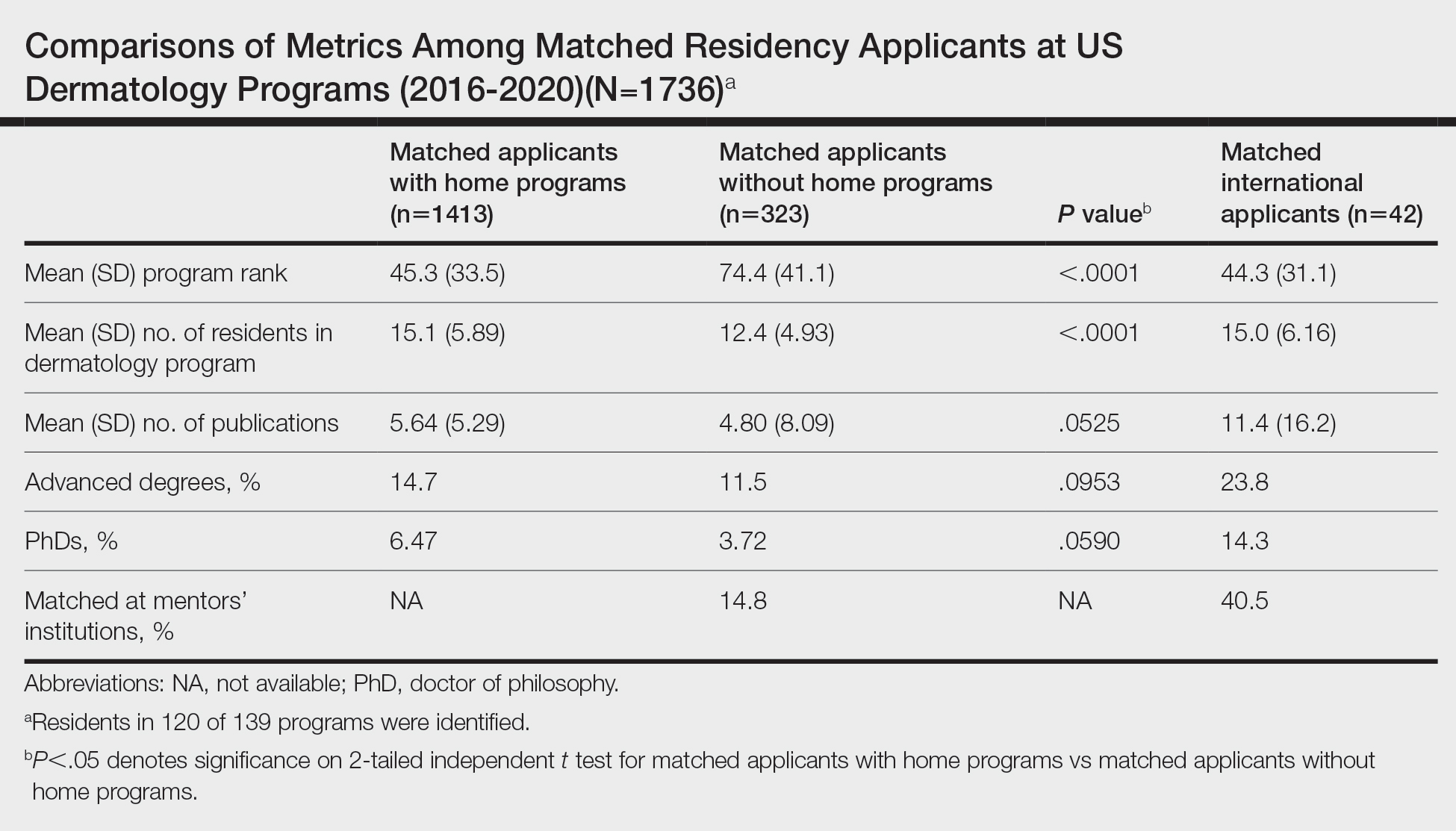
Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
Practice Points
- Our study suggests that matched dermatology applicants with and without home programs (HPs) had similar achievements, on average, for number of publications and holding advanced degrees.
- Because of the potential benefits of having program connections for matching in dermatology, applicants without HPs should seek dermatology research mentors.