User login
Dental Health: What It Means in Kidney Disease
Q) I teach nephrology at a local PA program, and they want us to integrate dental care into each module. What’s the connection between the two?
Dental health is frequently overlooked in the medical realm, as many clinicians feel that dental issues are out of our purview. Hematuria worries us, but bleeding gums and other signs of periodontal disease are often ignored. Surprisingly, many patients don’t seem to mind when their gums bleed every time they brush; they believe that this is normal, when really, it’s not.
Growing evidence supports associations between dental health and multiple medical issues—chronic kidney disease (CKD) among them. Periodontal disease is one of several inflammatory diseases caused by an interaction between gram-negative periodontal bacterial species and the immune system. It manifests with sore, red, bleeding gums and can lead to tooth loss if left untreated.
Chronic inflammation in the gums is a good indicator of inflammation elsewhere in the body. In and of itself, periodontitis can set off an inflammatory cascade in the body. Poor dentition can also lead to poor nutrition, which then causes a feedback loop, leading to even more inflammation.
Patients with periodontal disease have higher levels of C-reactive protein and a higher erythrocyte sedimentation rate than those without the disease.1 And a recent study by Zhang et al showed that periodontal disease increased risk for all-cause mortality in patients with CKD.2
The high cost of CKD from both a financial and personal view makes any intervention worth exploring, as the risk factors are difficult to modify and the CKD population is growing worldwide. We, as medical providers, should reiterate what our dental colleagues have been saying for years: Encourage patients with CKD to practice good dental hygiene by brushing twice a day and flossing daily, in an attempt to improve their overall outcomes.
LCDR Julie Taylor, PA-C
United States Public Health Service, Boston
1. Zhang J, Jiang H, Sun M, Chen J. Association between periodontal disease and mortality in people with CKD: a meta-analysis of cohort studies. BMC Nephrol. 2017;18(1):269.
2. Chen YT, Shin CJ, Ou SM, et al; Taiwan Geriatric Kidney Disease (TGKD) Research Group. Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis. 2015; 66(2):223-230.
Q) I teach nephrology at a local PA program, and they want us to integrate dental care into each module. What’s the connection between the two?
Dental health is frequently overlooked in the medical realm, as many clinicians feel that dental issues are out of our purview. Hematuria worries us, but bleeding gums and other signs of periodontal disease are often ignored. Surprisingly, many patients don’t seem to mind when their gums bleed every time they brush; they believe that this is normal, when really, it’s not.
Growing evidence supports associations between dental health and multiple medical issues—chronic kidney disease (CKD) among them. Periodontal disease is one of several inflammatory diseases caused by an interaction between gram-negative periodontal bacterial species and the immune system. It manifests with sore, red, bleeding gums and can lead to tooth loss if left untreated.
Chronic inflammation in the gums is a good indicator of inflammation elsewhere in the body. In and of itself, periodontitis can set off an inflammatory cascade in the body. Poor dentition can also lead to poor nutrition, which then causes a feedback loop, leading to even more inflammation.
Patients with periodontal disease have higher levels of C-reactive protein and a higher erythrocyte sedimentation rate than those without the disease.1 And a recent study by Zhang et al showed that periodontal disease increased risk for all-cause mortality in patients with CKD.2
The high cost of CKD from both a financial and personal view makes any intervention worth exploring, as the risk factors are difficult to modify and the CKD population is growing worldwide. We, as medical providers, should reiterate what our dental colleagues have been saying for years: Encourage patients with CKD to practice good dental hygiene by brushing twice a day and flossing daily, in an attempt to improve their overall outcomes.
LCDR Julie Taylor, PA-C
United States Public Health Service, Boston
Q) I teach nephrology at a local PA program, and they want us to integrate dental care into each module. What’s the connection between the two?
Dental health is frequently overlooked in the medical realm, as many clinicians feel that dental issues are out of our purview. Hematuria worries us, but bleeding gums and other signs of periodontal disease are often ignored. Surprisingly, many patients don’t seem to mind when their gums bleed every time they brush; they believe that this is normal, when really, it’s not.
Growing evidence supports associations between dental health and multiple medical issues—chronic kidney disease (CKD) among them. Periodontal disease is one of several inflammatory diseases caused by an interaction between gram-negative periodontal bacterial species and the immune system. It manifests with sore, red, bleeding gums and can lead to tooth loss if left untreated.
Chronic inflammation in the gums is a good indicator of inflammation elsewhere in the body. In and of itself, periodontitis can set off an inflammatory cascade in the body. Poor dentition can also lead to poor nutrition, which then causes a feedback loop, leading to even more inflammation.
Patients with periodontal disease have higher levels of C-reactive protein and a higher erythrocyte sedimentation rate than those without the disease.1 And a recent study by Zhang et al showed that periodontal disease increased risk for all-cause mortality in patients with CKD.2
The high cost of CKD from both a financial and personal view makes any intervention worth exploring, as the risk factors are difficult to modify and the CKD population is growing worldwide. We, as medical providers, should reiterate what our dental colleagues have been saying for years: Encourage patients with CKD to practice good dental hygiene by brushing twice a day and flossing daily, in an attempt to improve their overall outcomes.
LCDR Julie Taylor, PA-C
United States Public Health Service, Boston
1. Zhang J, Jiang H, Sun M, Chen J. Association between periodontal disease and mortality in people with CKD: a meta-analysis of cohort studies. BMC Nephrol. 2017;18(1):269.
2. Chen YT, Shin CJ, Ou SM, et al; Taiwan Geriatric Kidney Disease (TGKD) Research Group. Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis. 2015; 66(2):223-230.
1. Zhang J, Jiang H, Sun M, Chen J. Association between periodontal disease and mortality in people with CKD: a meta-analysis of cohort studies. BMC Nephrol. 2017;18(1):269.
2. Chen YT, Shin CJ, Ou SM, et al; Taiwan Geriatric Kidney Disease (TGKD) Research Group. Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis. 2015; 66(2):223-230.
For Patients With CKD, Don’t Wait—Vaccinate!
Q) What can I tell my kidney patients to increase acceptance of the influenza and pneumonia vaccines during cold and flu season?
The CDC recommends that everyone ages 6 months and older receive an annual flu vaccination, unless contraindicated.1 Additionally, administration of either the 13-valent pneumococcal conjugate vaccine (PCV13) or the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for all adults ages 65 and older and for younger adults (ages 19 to 64) with diabetes, chronic kidney disease (CKD), chronic heart disease, and/or solid organ transplant.1 Despite these recommendations, patients often decline vaccination. What they may not realize is that CKD increases their risk for infection.
In a cohort of more than 1 million Swedish patients, researchers found that any stage of CKD increased risk for community-acquired infection and that the risk for lower respiratory tract infection increased as glomerular filtration rate declined.2 Patients on hemodialysis have an increased risk for pneumonia and an incidence of pneumonia-related mortality that is up to 16 times higher than that of the general population.3 Pneumonia also increases the risk for cardiovascular events among all patients with CKD, regardless of stage.4
So, can vaccines reduce these risks in our kidney patients? McGrath and colleagues found that patients with end-stage renal disease (ESRD) who were vaccinated against the flu had lower mortality rates than those who were not vaccinated—even when the vaccine was poorly matched to the circulating virus strain.5 Additional research has demonstrated that for patients with any stage of CKD, including those on dialysis, the flu vaccine is safe and effective, and its protection may be durable over time.6
For pneumonia vaccines, antibody response in patients with CKD may be suboptimal; however, Medicare data have demonstrated that patients with ESRD who are vaccinated against pneumonia have lower rates of all-cause and cardiovascular mortality than unvaccinated patients do.5 Given their increased vulnerability to vaccine-preventable respiratory illnesses, it is imperative that our kidney patients receive both the flu and pneumonia vaccines.
Nicole DeFeo McCormick, DNP, MBA, NP-C, CCTC
Assistant Professor
School of Medicine at the University of Colorado
1. CDC. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. www.cdc.gov/vaccines/schedules/hcp/index.html. Accessed November 22, 2017.
2. Xu H, Gasparini A, Ishigami J, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017; 12(9):1399-1408.
3. Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6): 1883-1887.
4. Mathew R, Mason D, Kennedy JS. Vaccination issues in patients with chronic kidney disease. Expert Rev Vaccines. 2014;13(2):285-298.
5. McGrath LJ, Kshirsagar AV, Cole SR, et al. Evaluating influenza vaccine effectiveness among hemodialysis patients using a natural experiment. Arch Intern Med. 2012;172(7): 548-554.
6. Janus N, Vacher L, Karie S, et al. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):800-807.
Q) What can I tell my kidney patients to increase acceptance of the influenza and pneumonia vaccines during cold and flu season?
The CDC recommends that everyone ages 6 months and older receive an annual flu vaccination, unless contraindicated.1 Additionally, administration of either the 13-valent pneumococcal conjugate vaccine (PCV13) or the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for all adults ages 65 and older and for younger adults (ages 19 to 64) with diabetes, chronic kidney disease (CKD), chronic heart disease, and/or solid organ transplant.1 Despite these recommendations, patients often decline vaccination. What they may not realize is that CKD increases their risk for infection.
In a cohort of more than 1 million Swedish patients, researchers found that any stage of CKD increased risk for community-acquired infection and that the risk for lower respiratory tract infection increased as glomerular filtration rate declined.2 Patients on hemodialysis have an increased risk for pneumonia and an incidence of pneumonia-related mortality that is up to 16 times higher than that of the general population.3 Pneumonia also increases the risk for cardiovascular events among all patients with CKD, regardless of stage.4
So, can vaccines reduce these risks in our kidney patients? McGrath and colleagues found that patients with end-stage renal disease (ESRD) who were vaccinated against the flu had lower mortality rates than those who were not vaccinated—even when the vaccine was poorly matched to the circulating virus strain.5 Additional research has demonstrated that for patients with any stage of CKD, including those on dialysis, the flu vaccine is safe and effective, and its protection may be durable over time.6
For pneumonia vaccines, antibody response in patients with CKD may be suboptimal; however, Medicare data have demonstrated that patients with ESRD who are vaccinated against pneumonia have lower rates of all-cause and cardiovascular mortality than unvaccinated patients do.5 Given their increased vulnerability to vaccine-preventable respiratory illnesses, it is imperative that our kidney patients receive both the flu and pneumonia vaccines.
Nicole DeFeo McCormick, DNP, MBA, NP-C, CCTC
Assistant Professor
School of Medicine at the University of Colorado
Q) What can I tell my kidney patients to increase acceptance of the influenza and pneumonia vaccines during cold and flu season?
The CDC recommends that everyone ages 6 months and older receive an annual flu vaccination, unless contraindicated.1 Additionally, administration of either the 13-valent pneumococcal conjugate vaccine (PCV13) or the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for all adults ages 65 and older and for younger adults (ages 19 to 64) with diabetes, chronic kidney disease (CKD), chronic heart disease, and/or solid organ transplant.1 Despite these recommendations, patients often decline vaccination. What they may not realize is that CKD increases their risk for infection.
In a cohort of more than 1 million Swedish patients, researchers found that any stage of CKD increased risk for community-acquired infection and that the risk for lower respiratory tract infection increased as glomerular filtration rate declined.2 Patients on hemodialysis have an increased risk for pneumonia and an incidence of pneumonia-related mortality that is up to 16 times higher than that of the general population.3 Pneumonia also increases the risk for cardiovascular events among all patients with CKD, regardless of stage.4
So, can vaccines reduce these risks in our kidney patients? McGrath and colleagues found that patients with end-stage renal disease (ESRD) who were vaccinated against the flu had lower mortality rates than those who were not vaccinated—even when the vaccine was poorly matched to the circulating virus strain.5 Additional research has demonstrated that for patients with any stage of CKD, including those on dialysis, the flu vaccine is safe and effective, and its protection may be durable over time.6
For pneumonia vaccines, antibody response in patients with CKD may be suboptimal; however, Medicare data have demonstrated that patients with ESRD who are vaccinated against pneumonia have lower rates of all-cause and cardiovascular mortality than unvaccinated patients do.5 Given their increased vulnerability to vaccine-preventable respiratory illnesses, it is imperative that our kidney patients receive both the flu and pneumonia vaccines.
Nicole DeFeo McCormick, DNP, MBA, NP-C, CCTC
Assistant Professor
School of Medicine at the University of Colorado
1. CDC. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. www.cdc.gov/vaccines/schedules/hcp/index.html. Accessed November 22, 2017.
2. Xu H, Gasparini A, Ishigami J, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017; 12(9):1399-1408.
3. Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6): 1883-1887.
4. Mathew R, Mason D, Kennedy JS. Vaccination issues in patients with chronic kidney disease. Expert Rev Vaccines. 2014;13(2):285-298.
5. McGrath LJ, Kshirsagar AV, Cole SR, et al. Evaluating influenza vaccine effectiveness among hemodialysis patients using a natural experiment. Arch Intern Med. 2012;172(7): 548-554.
6. Janus N, Vacher L, Karie S, et al. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):800-807.
1. CDC. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. www.cdc.gov/vaccines/schedules/hcp/index.html. Accessed November 22, 2017.
2. Xu H, Gasparini A, Ishigami J, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017; 12(9):1399-1408.
3. Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6): 1883-1887.
4. Mathew R, Mason D, Kennedy JS. Vaccination issues in patients with chronic kidney disease. Expert Rev Vaccines. 2014;13(2):285-298.
5. McGrath LJ, Kshirsagar AV, Cole SR, et al. Evaluating influenza vaccine effectiveness among hemodialysis patients using a natural experiment. Arch Intern Med. 2012;172(7): 548-554.
6. Janus N, Vacher L, Karie S, et al. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):800-807.
Diagnosing & Treating Neuromyelitis Optica Spectrum Disorder
Q) How do you know if a neurologic symptom is due to a relapse of neuromyelitis optica spectrum disorder? And how should a confirmed relapse be treated?
Neuromyelitis optica spectrum disorder (NMOSD) is a severe, relapsing autoimmune disease of the central nervous system (CNS) that targets the optic nerves and spinal cord, leading to blindness and paralysis.1,2 Whereas multiple sclerosis (MS) is characterized by demyelination, NMOSD is associated with astrocytic damage and tissue necrosis.3 Because longitudinally extensive inflammatory lesions are typical with NMOSD, permanent CNS damage is common with each relapse.4
Health care providers first need to determine whether a patient with NMOSD who presents with new or worsening symptoms is having a relapse. A relapse is caused by a breach of the blood-brain barrier by the peripheral immune system, which leads to inflammation and damage to the CNS.5 This causes neurologic symptoms that depend on the anatomic location. Once damage has occurred, symptoms may result either from a new relapse in the same location as a previous inflammatory event or from a pseudorelapse.6
Pseudorelapses are triggered by a systemic metabolic imbalance; they exacerbate symptoms from previous CNS damage. Differentiating between a true relapse and a pseudorelapse can be a diagnostic challenge for even the most seasoned of health care providers. Kessler et al retrospectively examined which clinical factors can distinguish relapses from pseudorelapses.6 Their findings suggest that while clinical examination alone may be effective in events involving vision loss, MRI may be necessary when signs and symptoms are attributable to a spinal cord lesion.
In fact, they found that the degree of clinical worsening in patients with spinal cord symptoms caused by a pseudorelapse was similar to that of a true relapse. The most common causes of pseudorelapse included infection, dysautonomia, metabolic abnormalities, and changes to medication regimens. Interestingly, the presence of infection did not rule out a relapse, as patients experiencing relapses were equally likely as those with pseudorelapse to have a urinary tract infection. The authors concluded, based on their data, that an MRI is warranted to verify a relapse in patients who experience worsening of symptoms localized to the spinal cord but is not necessary to rule out a pseudorelapse of optic neu
In contrast to MS, a progressive phase is not believed to be associated with NMOSD.7 Instead, accrual of disability occurs with each relapse. The majority of patients with NMOSD do not return to baseline following an untreated relapse, making it especially important that patients receive adequate acute treatment to mitigate the damage.8
Currently, there are no medications approved by the FDA for the acute or preventive treatment of NMOSD. However, off-label use of immunotherapies, including rituximab, mycophenolate mofetil, azathioprine, prednisone, methotrexate, tocilizumab, and mitoxantrone, have been studied for relapse prevention.2 In addition, there are three ongoing phase III trials investigating eculizumab (C5 complement inhibitor), inebilizumab (CD19 monoclonal antibody), and SA237 (IL6R blocker); results from these studies could potentially widen the landscape of immunotherapy use in NMOSD.2
Less investigation into appropriate acute treatment of new relapses has been conducted, however, leaving clinicians and patients uncertain about how to manage a new inflammatory event. Traditionally, firstline treatment for acute NMOSD relapses has been the same as for MS relapses—high-dose methylprednisolone. However, due to the severity of NMOSD relapses and the relative lack of response to steroids alone, methylprednisolone is commonly followed by plasma exchange (PLEX).2
Most data to guide clinical decision-making suggest that patients with NMOSD relapses recover better when PLEX is added to steroid treatment. Abboud et al found that 65% of patients who received both PLEX and methylprednisolone recovered to their prerelapse baseline, compared to 35% of those who received methylprednisolone alone.9 These findings were supported by a larger retrospective investigation by Kleiter et al, which found improved recovery with treatment escalation in their cohort.8 These data support the recommendation to use PLEX as an adjunct therapy in acute relapses—particularly in relapses with severe presentations.
Because diagnosis and treatment of relapses involve many factors, ranging from accrual of disability, long-term immunotherapy decisions, and medical costs, diligence in provider decision-making is essential when caring for patients with NMOSD. -MAM
Maureen A. Mealy, BSN, MSCN
Neuromyelitis Optica Research Program Manager, Senior Research Nurse of the Transverse Myelitis & Multiple Sclerosis Centers, PhD candidate at Johns Hopkins School of Nursing in Baltimore
1. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107-1114.
2. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
3. Popescu BF, Lucchinetti CF. Immunopathology: autoimmune glial diseases and differentiation from multiple sclerosis. Handb Clin Neurol. 2016;133:95-106.
4. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14.
5. Orman G, Wang KY, Pekcevik Y, et al. Enhancing brain lesions during acute optic neuritis and/or longitudinally extensive transverse myelitis may portend a higher relapse rate in neuromyelitis optica spectrum disorders. Am J Neuroradiol. 2017;38(5):949-953.
6. Kessler RA, Mealy MA, Levy M. Early indicators of relapses vs pseudorelapses in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e269.
7. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603-605.
8. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216.
9. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185-192.
Q) How do you know if a neurologic symptom is due to a relapse of neuromyelitis optica spectrum disorder? And how should a confirmed relapse be treated?
Neuromyelitis optica spectrum disorder (NMOSD) is a severe, relapsing autoimmune disease of the central nervous system (CNS) that targets the optic nerves and spinal cord, leading to blindness and paralysis.1,2 Whereas multiple sclerosis (MS) is characterized by demyelination, NMOSD is associated with astrocytic damage and tissue necrosis.3 Because longitudinally extensive inflammatory lesions are typical with NMOSD, permanent CNS damage is common with each relapse.4
Health care providers first need to determine whether a patient with NMOSD who presents with new or worsening symptoms is having a relapse. A relapse is caused by a breach of the blood-brain barrier by the peripheral immune system, which leads to inflammation and damage to the CNS.5 This causes neurologic symptoms that depend on the anatomic location. Once damage has occurred, symptoms may result either from a new relapse in the same location as a previous inflammatory event or from a pseudorelapse.6
Pseudorelapses are triggered by a systemic metabolic imbalance; they exacerbate symptoms from previous CNS damage. Differentiating between a true relapse and a pseudorelapse can be a diagnostic challenge for even the most seasoned of health care providers. Kessler et al retrospectively examined which clinical factors can distinguish relapses from pseudorelapses.6 Their findings suggest that while clinical examination alone may be effective in events involving vision loss, MRI may be necessary when signs and symptoms are attributable to a spinal cord lesion.
In fact, they found that the degree of clinical worsening in patients with spinal cord symptoms caused by a pseudorelapse was similar to that of a true relapse. The most common causes of pseudorelapse included infection, dysautonomia, metabolic abnormalities, and changes to medication regimens. Interestingly, the presence of infection did not rule out a relapse, as patients experiencing relapses were equally likely as those with pseudorelapse to have a urinary tract infection. The authors concluded, based on their data, that an MRI is warranted to verify a relapse in patients who experience worsening of symptoms localized to the spinal cord but is not necessary to rule out a pseudorelapse of optic neu
In contrast to MS, a progressive phase is not believed to be associated with NMOSD.7 Instead, accrual of disability occurs with each relapse. The majority of patients with NMOSD do not return to baseline following an untreated relapse, making it especially important that patients receive adequate acute treatment to mitigate the damage.8
Currently, there are no medications approved by the FDA for the acute or preventive treatment of NMOSD. However, off-label use of immunotherapies, including rituximab, mycophenolate mofetil, azathioprine, prednisone, methotrexate, tocilizumab, and mitoxantrone, have been studied for relapse prevention.2 In addition, there are three ongoing phase III trials investigating eculizumab (C5 complement inhibitor), inebilizumab (CD19 monoclonal antibody), and SA237 (IL6R blocker); results from these studies could potentially widen the landscape of immunotherapy use in NMOSD.2
Less investigation into appropriate acute treatment of new relapses has been conducted, however, leaving clinicians and patients uncertain about how to manage a new inflammatory event. Traditionally, firstline treatment for acute NMOSD relapses has been the same as for MS relapses—high-dose methylprednisolone. However, due to the severity of NMOSD relapses and the relative lack of response to steroids alone, methylprednisolone is commonly followed by plasma exchange (PLEX).2
Most data to guide clinical decision-making suggest that patients with NMOSD relapses recover better when PLEX is added to steroid treatment. Abboud et al found that 65% of patients who received both PLEX and methylprednisolone recovered to their prerelapse baseline, compared to 35% of those who received methylprednisolone alone.9 These findings were supported by a larger retrospective investigation by Kleiter et al, which found improved recovery with treatment escalation in their cohort.8 These data support the recommendation to use PLEX as an adjunct therapy in acute relapses—particularly in relapses with severe presentations.
Because diagnosis and treatment of relapses involve many factors, ranging from accrual of disability, long-term immunotherapy decisions, and medical costs, diligence in provider decision-making is essential when caring for patients with NMOSD. -MAM
Maureen A. Mealy, BSN, MSCN
Neuromyelitis Optica Research Program Manager, Senior Research Nurse of the Transverse Myelitis & Multiple Sclerosis Centers, PhD candidate at Johns Hopkins School of Nursing in Baltimore
Q) How do you know if a neurologic symptom is due to a relapse of neuromyelitis optica spectrum disorder? And how should a confirmed relapse be treated?
Neuromyelitis optica spectrum disorder (NMOSD) is a severe, relapsing autoimmune disease of the central nervous system (CNS) that targets the optic nerves and spinal cord, leading to blindness and paralysis.1,2 Whereas multiple sclerosis (MS) is characterized by demyelination, NMOSD is associated with astrocytic damage and tissue necrosis.3 Because longitudinally extensive inflammatory lesions are typical with NMOSD, permanent CNS damage is common with each relapse.4
Health care providers first need to determine whether a patient with NMOSD who presents with new or worsening symptoms is having a relapse. A relapse is caused by a breach of the blood-brain barrier by the peripheral immune system, which leads to inflammation and damage to the CNS.5 This causes neurologic symptoms that depend on the anatomic location. Once damage has occurred, symptoms may result either from a new relapse in the same location as a previous inflammatory event or from a pseudorelapse.6
Pseudorelapses are triggered by a systemic metabolic imbalance; they exacerbate symptoms from previous CNS damage. Differentiating between a true relapse and a pseudorelapse can be a diagnostic challenge for even the most seasoned of health care providers. Kessler et al retrospectively examined which clinical factors can distinguish relapses from pseudorelapses.6 Their findings suggest that while clinical examination alone may be effective in events involving vision loss, MRI may be necessary when signs and symptoms are attributable to a spinal cord lesion.
In fact, they found that the degree of clinical worsening in patients with spinal cord symptoms caused by a pseudorelapse was similar to that of a true relapse. The most common causes of pseudorelapse included infection, dysautonomia, metabolic abnormalities, and changes to medication regimens. Interestingly, the presence of infection did not rule out a relapse, as patients experiencing relapses were equally likely as those with pseudorelapse to have a urinary tract infection. The authors concluded, based on their data, that an MRI is warranted to verify a relapse in patients who experience worsening of symptoms localized to the spinal cord but is not necessary to rule out a pseudorelapse of optic neu
In contrast to MS, a progressive phase is not believed to be associated with NMOSD.7 Instead, accrual of disability occurs with each relapse. The majority of patients with NMOSD do not return to baseline following an untreated relapse, making it especially important that patients receive adequate acute treatment to mitigate the damage.8
Currently, there are no medications approved by the FDA for the acute or preventive treatment of NMOSD. However, off-label use of immunotherapies, including rituximab, mycophenolate mofetil, azathioprine, prednisone, methotrexate, tocilizumab, and mitoxantrone, have been studied for relapse prevention.2 In addition, there are three ongoing phase III trials investigating eculizumab (C5 complement inhibitor), inebilizumab (CD19 monoclonal antibody), and SA237 (IL6R blocker); results from these studies could potentially widen the landscape of immunotherapy use in NMOSD.2
Less investigation into appropriate acute treatment of new relapses has been conducted, however, leaving clinicians and patients uncertain about how to manage a new inflammatory event. Traditionally, firstline treatment for acute NMOSD relapses has been the same as for MS relapses—high-dose methylprednisolone. However, due to the severity of NMOSD relapses and the relative lack of response to steroids alone, methylprednisolone is commonly followed by plasma exchange (PLEX).2
Most data to guide clinical decision-making suggest that patients with NMOSD relapses recover better when PLEX is added to steroid treatment. Abboud et al found that 65% of patients who received both PLEX and methylprednisolone recovered to their prerelapse baseline, compared to 35% of those who received methylprednisolone alone.9 These findings were supported by a larger retrospective investigation by Kleiter et al, which found improved recovery with treatment escalation in their cohort.8 These data support the recommendation to use PLEX as an adjunct therapy in acute relapses—particularly in relapses with severe presentations.
Because diagnosis and treatment of relapses involve many factors, ranging from accrual of disability, long-term immunotherapy decisions, and medical costs, diligence in provider decision-making is essential when caring for patients with NMOSD. -MAM
Maureen A. Mealy, BSN, MSCN
Neuromyelitis Optica Research Program Manager, Senior Research Nurse of the Transverse Myelitis & Multiple Sclerosis Centers, PhD candidate at Johns Hopkins School of Nursing in Baltimore
1. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107-1114.
2. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
3. Popescu BF, Lucchinetti CF. Immunopathology: autoimmune glial diseases and differentiation from multiple sclerosis. Handb Clin Neurol. 2016;133:95-106.
4. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14.
5. Orman G, Wang KY, Pekcevik Y, et al. Enhancing brain lesions during acute optic neuritis and/or longitudinally extensive transverse myelitis may portend a higher relapse rate in neuromyelitis optica spectrum disorders. Am J Neuroradiol. 2017;38(5):949-953.
6. Kessler RA, Mealy MA, Levy M. Early indicators of relapses vs pseudorelapses in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e269.
7. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603-605.
8. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216.
9. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185-192.
1. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107-1114.
2. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
3. Popescu BF, Lucchinetti CF. Immunopathology: autoimmune glial diseases and differentiation from multiple sclerosis. Handb Clin Neurol. 2016;133:95-106.
4. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14.
5. Orman G, Wang KY, Pekcevik Y, et al. Enhancing brain lesions during acute optic neuritis and/or longitudinally extensive transverse myelitis may portend a higher relapse rate in neuromyelitis optica spectrum disorders. Am J Neuroradiol. 2017;38(5):949-953.
6. Kessler RA, Mealy MA, Levy M. Early indicators of relapses vs pseudorelapses in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e269.
7. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603-605.
8. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216.
9. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185-192.
Bladder Complications in MS
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
Insulin Pump Therapy: Who, Why, and How
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
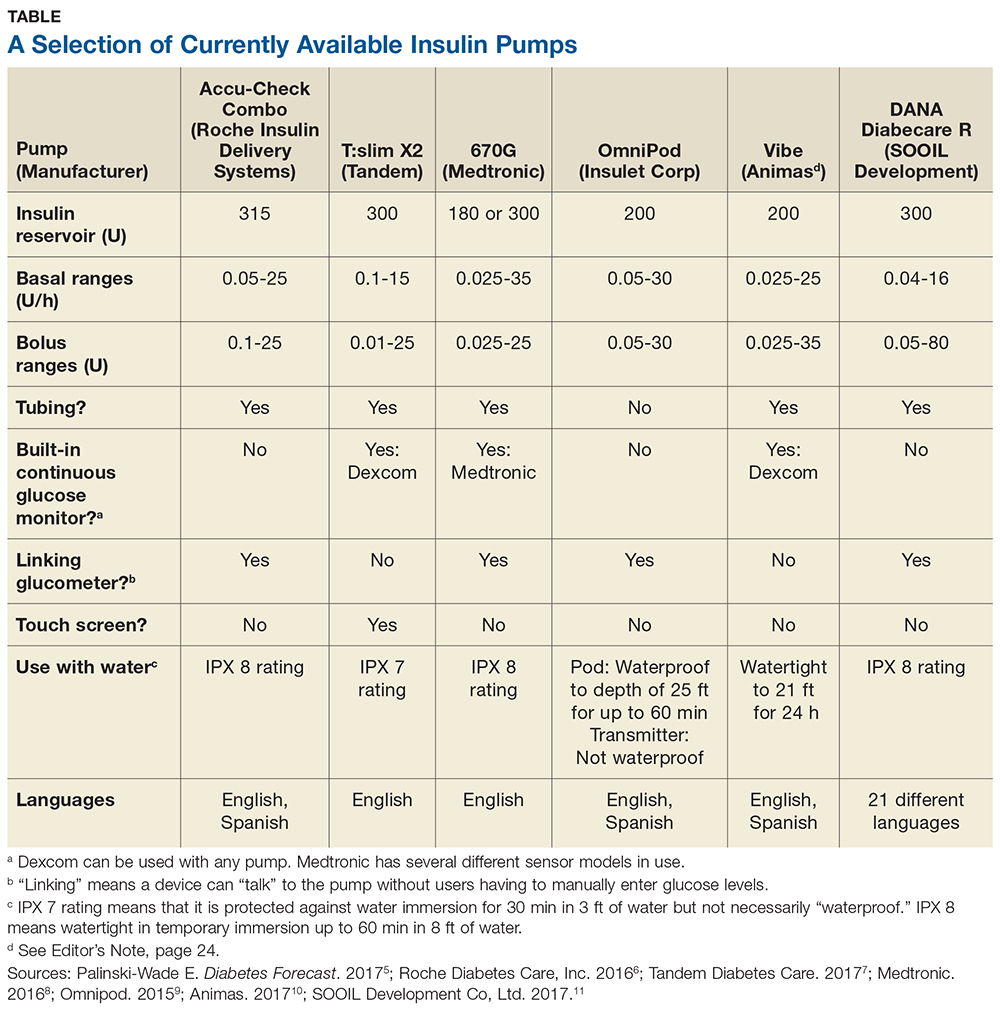
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
How to Interpret Positive Troponin Tests in CKD
Q) Recently, when I have sent my patients with chronic kidney disease (CKD) to the emergency department (ED) for complaints of chest pain or shortness of breath, their troponin levels are high. I know CKD increases risk for cardiovascular disease, but I find it hard to believe that every CKD patient is having an MI. What gives?
Cardiovascular disease remains the most common cause of death in patients with CKD, accounting for 45% to 50% of all deaths. Therefore, accurate diagnosis of acute myocardial infarction (AMI) in this patient population is vital to assure prompt identification and treatment.1,2
Cardiac troponins are the gold standard for detecting myocardial injury in patients presenting to the ED with suggestive symptoms.1 But the chronic baseline elevation in serum troponin levels among patients with CKD often results in a false-positive reading, making the detection of AMI difficult.1
With the recent introduction of high-sensitivity troponin assays, as many as 97% of patients on hemodialysis exhibit elevated troponin levels; this is also true for patients with CKD, on a sliding scale (lower kidney function = higher baseline troponins).2 The use of high-sensitivity testing has increased substantially in the past 15 years, and it is expected to become the benchmark for troponin evaluation. While older troponin tests had a false-positive rate of 30% to 85% in patients with stage 5 CKD, the newer troponin tests display elevated troponins in almost 100% of these patients.1,2
Numerous studies have been conducted to determine the best way to interpret positive troponin tests in patients with CKD to ensure an accurate diagnosis of AMI.2 One study determined that a 20% increase in troponin levels was a more accurate determinant of AMI in patients with CKD than one isolated positive level.3 Another study demonstrated that serial troponin measurements conducted over time yielded higher diagnostic accuracy than one measurement above the 99th percentile.4
The American College of Cardiology Foundation task force found that monitoring changes in troponin concentration over time (3-6 h) is more accurate than a single elevated troponin when diagnosing AMI in symptomatic patients.3 Correlation between elevated troponin levels and clinical suspicion proved helpful in determining the significance of troponin results and the probability of AMI in patients with CKD.2
The significance and interpretation of elevated troponin levels in patients with CKD remains an important topic for further study, as cardiovascular disease continues to be the leading cause of mortality in patients with kidney dysfunction.1,2 More definitive studies need to be conducted on patients with CKD as high-sensitivity troponin assay testing becomes standard for diagnosing AMI.
So, the reason you see more positive troponin results in your CKD population is due to both the increased accuracy of the newer tests and the fact that CKD often causes a false-positive result. Monitoring your patients with serial troponins for at least three hours is essential to confirm or rule out an AMI. —MS-G
Marlene Shaw-Gallagher, MS, PA-C
University of Detroit Mercy, Michigan
Division of Nephrology, University of Michigan, Ann Arbor
1. Robitaille R, Lafrance JP, Leblanc M. Altered laboratory findings associated with end-stage renal disease. Semin Dial. 2006;19(5):373.
2. Howard CE, McCullough PA. Decoding acute myocardial infarction among patients on dialysis. J Am Soc Nephrol. 2017;28(5):1337-1339.
3. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012; 60(23):2427-2463.
4. Mahajan VS, Petr Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350-2354.
Q) Recently, when I have sent my patients with chronic kidney disease (CKD) to the emergency department (ED) for complaints of chest pain or shortness of breath, their troponin levels are high. I know CKD increases risk for cardiovascular disease, but I find it hard to believe that every CKD patient is having an MI. What gives?
Cardiovascular disease remains the most common cause of death in patients with CKD, accounting for 45% to 50% of all deaths. Therefore, accurate diagnosis of acute myocardial infarction (AMI) in this patient population is vital to assure prompt identification and treatment.1,2
Cardiac troponins are the gold standard for detecting myocardial injury in patients presenting to the ED with suggestive symptoms.1 But the chronic baseline elevation in serum troponin levels among patients with CKD often results in a false-positive reading, making the detection of AMI difficult.1
With the recent introduction of high-sensitivity troponin assays, as many as 97% of patients on hemodialysis exhibit elevated troponin levels; this is also true for patients with CKD, on a sliding scale (lower kidney function = higher baseline troponins).2 The use of high-sensitivity testing has increased substantially in the past 15 years, and it is expected to become the benchmark for troponin evaluation. While older troponin tests had a false-positive rate of 30% to 85% in patients with stage 5 CKD, the newer troponin tests display elevated troponins in almost 100% of these patients.1,2
Numerous studies have been conducted to determine the best way to interpret positive troponin tests in patients with CKD to ensure an accurate diagnosis of AMI.2 One study determined that a 20% increase in troponin levels was a more accurate determinant of AMI in patients with CKD than one isolated positive level.3 Another study demonstrated that serial troponin measurements conducted over time yielded higher diagnostic accuracy than one measurement above the 99th percentile.4
The American College of Cardiology Foundation task force found that monitoring changes in troponin concentration over time (3-6 h) is more accurate than a single elevated troponin when diagnosing AMI in symptomatic patients.3 Correlation between elevated troponin levels and clinical suspicion proved helpful in determining the significance of troponin results and the probability of AMI in patients with CKD.2
The significance and interpretation of elevated troponin levels in patients with CKD remains an important topic for further study, as cardiovascular disease continues to be the leading cause of mortality in patients with kidney dysfunction.1,2 More definitive studies need to be conducted on patients with CKD as high-sensitivity troponin assay testing becomes standard for diagnosing AMI.
So, the reason you see more positive troponin results in your CKD population is due to both the increased accuracy of the newer tests and the fact that CKD often causes a false-positive result. Monitoring your patients with serial troponins for at least three hours is essential to confirm or rule out an AMI. —MS-G
Marlene Shaw-Gallagher, MS, PA-C
University of Detroit Mercy, Michigan
Division of Nephrology, University of Michigan, Ann Arbor
Q) Recently, when I have sent my patients with chronic kidney disease (CKD) to the emergency department (ED) for complaints of chest pain or shortness of breath, their troponin levels are high. I know CKD increases risk for cardiovascular disease, but I find it hard to believe that every CKD patient is having an MI. What gives?
Cardiovascular disease remains the most common cause of death in patients with CKD, accounting for 45% to 50% of all deaths. Therefore, accurate diagnosis of acute myocardial infarction (AMI) in this patient population is vital to assure prompt identification and treatment.1,2
Cardiac troponins are the gold standard for detecting myocardial injury in patients presenting to the ED with suggestive symptoms.1 But the chronic baseline elevation in serum troponin levels among patients with CKD often results in a false-positive reading, making the detection of AMI difficult.1
With the recent introduction of high-sensitivity troponin assays, as many as 97% of patients on hemodialysis exhibit elevated troponin levels; this is also true for patients with CKD, on a sliding scale (lower kidney function = higher baseline troponins).2 The use of high-sensitivity testing has increased substantially in the past 15 years, and it is expected to become the benchmark for troponin evaluation. While older troponin tests had a false-positive rate of 30% to 85% in patients with stage 5 CKD, the newer troponin tests display elevated troponins in almost 100% of these patients.1,2
Numerous studies have been conducted to determine the best way to interpret positive troponin tests in patients with CKD to ensure an accurate diagnosis of AMI.2 One study determined that a 20% increase in troponin levels was a more accurate determinant of AMI in patients with CKD than one isolated positive level.3 Another study demonstrated that serial troponin measurements conducted over time yielded higher diagnostic accuracy than one measurement above the 99th percentile.4
The American College of Cardiology Foundation task force found that monitoring changes in troponin concentration over time (3-6 h) is more accurate than a single elevated troponin when diagnosing AMI in symptomatic patients.3 Correlation between elevated troponin levels and clinical suspicion proved helpful in determining the significance of troponin results and the probability of AMI in patients with CKD.2
The significance and interpretation of elevated troponin levels in patients with CKD remains an important topic for further study, as cardiovascular disease continues to be the leading cause of mortality in patients with kidney dysfunction.1,2 More definitive studies need to be conducted on patients with CKD as high-sensitivity troponin assay testing becomes standard for diagnosing AMI.
So, the reason you see more positive troponin results in your CKD population is due to both the increased accuracy of the newer tests and the fact that CKD often causes a false-positive result. Monitoring your patients with serial troponins for at least three hours is essential to confirm or rule out an AMI. —MS-G
Marlene Shaw-Gallagher, MS, PA-C
University of Detroit Mercy, Michigan
Division of Nephrology, University of Michigan, Ann Arbor
1. Robitaille R, Lafrance JP, Leblanc M. Altered laboratory findings associated with end-stage renal disease. Semin Dial. 2006;19(5):373.
2. Howard CE, McCullough PA. Decoding acute myocardial infarction among patients on dialysis. J Am Soc Nephrol. 2017;28(5):1337-1339.
3. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012; 60(23):2427-2463.
4. Mahajan VS, Petr Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350-2354.
1. Robitaille R, Lafrance JP, Leblanc M. Altered laboratory findings associated with end-stage renal disease. Semin Dial. 2006;19(5):373.
2. Howard CE, McCullough PA. Decoding acute myocardial infarction among patients on dialysis. J Am Soc Nephrol. 2017;28(5):1337-1339.
3. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012; 60(23):2427-2463.
4. Mahajan VS, Petr Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350-2354.
Do PPIs Pose a Danger to Kidneys?
Q) Is it true that PPI use can cause kidney disease?
Proton pump inhibitors (PPIs) have been available in the United States since 1990, with OTC options available since 2009. While these medications play a vital role in the treatment of gastrointestinal (GI) conditions, observational studies have linked PPI use to serious adverse events, including dementia, community-acquired pneumonia, hip fracture, and Clostridium difficile infection.1-4
Studies have also found an association between PPI use and kidney problems such as acute kidney injury (AKI), acute interstitial nephritis, and incident chronic kidney disease (CKD).5-7 One observational study used the Department of Veterans Affairs (VA) national databases to track the renal outcomes of 173,321 new PPI users and 20,270 new histamine H2 receptor antagonist (H2RA) users over the course of five years. Those who used PPIs demonstrated a significant risk for decreased renal function, lower estimated glomerular filtration rate (eGFR), doubled serum creatinine levels, and progression to end-stage renal disease (ESRD).8
Another study of 10,482 patients (322 PPI; 956 H2RA; 9,204 nonusers) and a replicate study of 248,751 patients (16,900 PPI; 6,640 H2RA; 225,211 nonusers) with an initial eGFR ≥ 60 mL/min/1.73m2 also found an association between PPI use and incident CKD, which persisted when compared to the other groups. Additionally, twice-daily PPI use was associated with a higher CKD risk than once-daily use.9
The pathophysiology of PPI use and kidney deterioration is poorly understood at this point. It is known that AKI can increase the risk for CKD, and AKI has been an assumed precursor to PPI-associated CKD. However, a study by Xie and colleagues reported an association between PPI use and increased risk for CKD, progression of CKD, and ESRD in the absence of preceding AKI. Using the VA databases, the researchers identified 144,032 new users of acid-suppressing medications (125,596 PPI; 18,436 H2RA) who had no history of kidney disease and followed them for five years. PPI users were found to be at increased risk for CKD, and a graded association was discovered between length of PPI use and risk for CKD.10
While these studies are observational and therefore do not prove causation, they do suggest a need for attentive monitoring of kidney function in patients using PPIs. Evaluating the need for PPIs and inquiring about OTC use of these medications is highly recommended, as research has found 25% to 70% of PPI prescriptions are not prescribed for an appropriate indication.11 Considerations regarding PPI use should include dosage, length of use, and whether alternate use of an H2RA is appropriate. —CAS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants, PLLC, South Charleston, West Virginia
1. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
2. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PloS One. 2015;10(6):e0128004.
3. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296(24):2947-2953.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
5. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
6. Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837-844.
7. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-171.
8. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153-3163.
9. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
10. Xie Y, Bowe B, Li T, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91(6):1482-1494.
11. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
Q) Is it true that PPI use can cause kidney disease?
Proton pump inhibitors (PPIs) have been available in the United States since 1990, with OTC options available since 2009. While these medications play a vital role in the treatment of gastrointestinal (GI) conditions, observational studies have linked PPI use to serious adverse events, including dementia, community-acquired pneumonia, hip fracture, and Clostridium difficile infection.1-4
Studies have also found an association between PPI use and kidney problems such as acute kidney injury (AKI), acute interstitial nephritis, and incident chronic kidney disease (CKD).5-7 One observational study used the Department of Veterans Affairs (VA) national databases to track the renal outcomes of 173,321 new PPI users and 20,270 new histamine H2 receptor antagonist (H2RA) users over the course of five years. Those who used PPIs demonstrated a significant risk for decreased renal function, lower estimated glomerular filtration rate (eGFR), doubled serum creatinine levels, and progression to end-stage renal disease (ESRD).8
Another study of 10,482 patients (322 PPI; 956 H2RA; 9,204 nonusers) and a replicate study of 248,751 patients (16,900 PPI; 6,640 H2RA; 225,211 nonusers) with an initial eGFR ≥ 60 mL/min/1.73m2 also found an association between PPI use and incident CKD, which persisted when compared to the other groups. Additionally, twice-daily PPI use was associated with a higher CKD risk than once-daily use.9
The pathophysiology of PPI use and kidney deterioration is poorly understood at this point. It is known that AKI can increase the risk for CKD, and AKI has been an assumed precursor to PPI-associated CKD. However, a study by Xie and colleagues reported an association between PPI use and increased risk for CKD, progression of CKD, and ESRD in the absence of preceding AKI. Using the VA databases, the researchers identified 144,032 new users of acid-suppressing medications (125,596 PPI; 18,436 H2RA) who had no history of kidney disease and followed them for five years. PPI users were found to be at increased risk for CKD, and a graded association was discovered between length of PPI use and risk for CKD.10
While these studies are observational and therefore do not prove causation, they do suggest a need for attentive monitoring of kidney function in patients using PPIs. Evaluating the need for PPIs and inquiring about OTC use of these medications is highly recommended, as research has found 25% to 70% of PPI prescriptions are not prescribed for an appropriate indication.11 Considerations regarding PPI use should include dosage, length of use, and whether alternate use of an H2RA is appropriate. —CAS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants, PLLC, South Charleston, West Virginia
Q) Is it true that PPI use can cause kidney disease?
Proton pump inhibitors (PPIs) have been available in the United States since 1990, with OTC options available since 2009. While these medications play a vital role in the treatment of gastrointestinal (GI) conditions, observational studies have linked PPI use to serious adverse events, including dementia, community-acquired pneumonia, hip fracture, and Clostridium difficile infection.1-4
Studies have also found an association between PPI use and kidney problems such as acute kidney injury (AKI), acute interstitial nephritis, and incident chronic kidney disease (CKD).5-7 One observational study used the Department of Veterans Affairs (VA) national databases to track the renal outcomes of 173,321 new PPI users and 20,270 new histamine H2 receptor antagonist (H2RA) users over the course of five years. Those who used PPIs demonstrated a significant risk for decreased renal function, lower estimated glomerular filtration rate (eGFR), doubled serum creatinine levels, and progression to end-stage renal disease (ESRD).8
Another study of 10,482 patients (322 PPI; 956 H2RA; 9,204 nonusers) and a replicate study of 248,751 patients (16,900 PPI; 6,640 H2RA; 225,211 nonusers) with an initial eGFR ≥ 60 mL/min/1.73m2 also found an association between PPI use and incident CKD, which persisted when compared to the other groups. Additionally, twice-daily PPI use was associated with a higher CKD risk than once-daily use.9
The pathophysiology of PPI use and kidney deterioration is poorly understood at this point. It is known that AKI can increase the risk for CKD, and AKI has been an assumed precursor to PPI-associated CKD. However, a study by Xie and colleagues reported an association between PPI use and increased risk for CKD, progression of CKD, and ESRD in the absence of preceding AKI. Using the VA databases, the researchers identified 144,032 new users of acid-suppressing medications (125,596 PPI; 18,436 H2RA) who had no history of kidney disease and followed them for five years. PPI users were found to be at increased risk for CKD, and a graded association was discovered between length of PPI use and risk for CKD.10
While these studies are observational and therefore do not prove causation, they do suggest a need for attentive monitoring of kidney function in patients using PPIs. Evaluating the need for PPIs and inquiring about OTC use of these medications is highly recommended, as research has found 25% to 70% of PPI prescriptions are not prescribed for an appropriate indication.11 Considerations regarding PPI use should include dosage, length of use, and whether alternate use of an H2RA is appropriate. —CAS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants, PLLC, South Charleston, West Virginia
1. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
2. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PloS One. 2015;10(6):e0128004.
3. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296(24):2947-2953.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
5. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
6. Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837-844.
7. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-171.
8. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153-3163.
9. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
10. Xie Y, Bowe B, Li T, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91(6):1482-1494.
11. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
1. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
2. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PloS One. 2015;10(6):e0128004.
3. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296(24):2947-2953.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
5. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
6. Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837-844.
7. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-171.
8. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153-3163.
9. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
10. Xie Y, Bowe B, Li T, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91(6):1482-1494.
11. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
The Benefits of Exercise for Patients With Multiple Sclerosis
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
Does Diet Matter in Multiple Sclerosis?
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
Thyroid Storm: Early Management and Prevention
A 73-year-old man is transported to the emergency department (ED) by ambulance for nausea, vomiting, diarrhea, and weakness of three days’ duration. Earlier today, he presented to his primary care provider with these symptoms and was found to be hypotensive; he was advised to go to the ED but instead went home against medical advice.
The patient’s medical history is significant for type 2 diabetes, stage 3b chronic kidney disease, dyslipidemia, hypertension, coronary artery disease, and benign prostatic hyperplasia. He has undergone stent placement and triple coronary artery bypass graft surgery. His medication list includes insulin glargine, glimepiride, liraglutide, atorvastatin, benazepril, carvedilol, amlodipine, clopidogrel, and tamsulosin.
Upon admission, the patient has a pulse of 98 beats/min; temperature, 98.2°F; respiratory rate, 18 breaths/min-1; and PO2, 98 mm Hg. An ECG, chest radiograph, and CT (without contrast) of the head, chest, and abdomen are all within normal limits. Lab evaluation is significant for severe thyrotoxicosis (see Table 1).
Endocrinology consult is requested. Further testing yields the following findings
- Thyroid-stimulating immunoglobulin: 309% (reference range, < 30%)
- Nuclear medicine thyroid scan with uptake: 6-hour uptake of 70.3% (10%-25%) and 24-hour uptake, 81.8% (15%-35%)
- Homogeneous radiotracer uptake within the thyroid gland: no evidence of hot or cold nodules
- Thyroid ultrasound: bilateral enlarged heterogeneous gland and multiple subcentimeter nodules (largest measuring 6 × 7 mm)
These results confirm a diagnosis of Graves’ disease. Treatment options, including antithyroid medications, radioactive iodine ablation (RAI), and surgery, are discussed. The patient is treated with RAI therapy (10 mCi) and discharged from the hospital.
Six days later, however, he returns to the ED with severe intermittent dizziness and lightheadedness of two hours’ duration, new-onset atrial fibrillation (A-fib), and mild shortness of breath. His vital signs include a pulse of 116 beats/min; temperature, 98.1°F; respiratory rate, 18 breaths/min-1, blood pressure, 154/88 mm Hg; and PO2, 100 mm Hg.
His lab values include
- TSH < 0.005 uIU/mL
- Free T4, 8.01 ng/dL
- Free T3, 3,701 pg/dL
- eGFR, 60 mL/min/1.73 m2
Cardiology consult is requested. A pacemaker is placed for bradycardia-tachycardia syndrome, and the patient is put on rivaroxaban for stroke prevention.
The endocrinologist suspects post-RAI thyroiditis or ineffective RAI treatment. The patient is started on methimazole (10 mg bid), and his carvedilol is replaced with metoprolol (50 mg bid).
Two weeks postdischarge, the patient returns to the office. Although he says he’s doing better, he seems uneasy and agitated and has a pulse of 120 beats/min. His methimazole and metoprolol are increased (to 10 mg tid and 50 mg tid, respectively).
Another two weeks later, lab results still show elevated thyroid levels—now with increased enzyme levels on liver function testing. The patient reports worsening dizziness and shortness of breath. He is sent back to hospital and admitted for inpatient management, with urgent surgical consult for thyroidectomy. Total thyroidectomy is successfully performed, and the final pathology report shows a benign goiter.
DISCUSSION
Thyroid storm is an extreme form of thyrotoxicosis with an associated mortality rate of 8% to 25%.1 When thyroid hormone levels are elevated, adrenaline receptors are upregulated—but, while it is possible for persistent thyrotoxicosis to progress to thyroid storm on its own, a surge of adrenaline is usually needed. Most cases are triggered by acute stressors (ie, myocardial infarction, surgery, anesthesia, labor and delivery) in the context of underlying thyrotoxicosis.1
Diagnosis of thyroid storm is made clinically in patients who are thyrotoxic and present with systemic decompensation (ie, altered mental status, cardiovascular dysfunction, hyperpyrexia). Although no universally accepted criteria currently exist, the Burch-Wartofsky Point Scale (BWPS; see Table 2) can be used to assess disease severity and guide the extent of treatment and monitoring.2 However, this measure should not replace clinical judgment—the distinction between compensated thyrotoxicosis and decompensating thyrotoxicosis (thyroid storm) should be made by sound but prompt clinical assessment.
Once thyroid storm is suspected, aggressive treatment should be implemented to improve the systemic thyrotoxic state. Propylthiouracil (PTU) is preferred over methimazole, as it blocks T4 to T3 conversion in addition to blocking new hormone synthesis. Propranolol is the best choice of ß-blocker because it also blocks T4 to T3 conversion and controls cardiac rhythm.
Iodine can rapidly block new hormone synthesis and release; it is often used to reduce thyroid hormone levels prior to emergency thyroid surgery. However, it should be given at least one hour after a dose of PTU. Hydrocortisone is given prophylactically for relative adrenal insufficiency (due to rapid cortisol clearance during thyrotoxic state); it may block T4 to T3 conversion as well. Volume resuscitation, respiratory care, temperature control (eg, antipyretics, cooling blankets), and nutritional support should also be incorporated, ideally in the intensive care unit (ICU). During or after thyroid storm management, treatment of the precipitating event/illness and of hyperthyroidism should be initiated to prevent recurrence.1
The patient’s initial BWPS was 30 (gastrointestinal [GI] score 10 + central nervous system [CNS] score 10 + without precipitating factor 10), which put him in the “impending storm” category. At his second ED visit, his BWPS was 40 (cardiovascular score 10 + A-fib 10 + GI score 10 + CNS score 10 + precipitating factor [RAI ablation] score 0)—still in the “impending storm” category but certainly indicating a worsened state.
RAI for hyperthyroidism can transiently increase thyroid hormone levels due to inflammation of the gland. To prevent exacerbation of the thyrotoxic state, pretreatment with methimazole should be considered in patients with risk factors (eg, older age, cardiovascular complications, cerebrovascular disease, pulmonary disease, renal failure, infection, trauma, and poorly controlled diabetes). Patients should also be placed on ß-blockers prior to treatment, in anticipation of a transient rise in thyroid hormone levels.
Due to this patient’s age, severity of thyrotoxicosis, and multiple risk factors, strong consideration should have been given to pretreating him with antithyroid medication and a ß-blocker before definitive treatment was given. This would have potentially averted his subsequent hospital visits and urgent need for thyroidectomy.
CONCLUSION
Thyroid storm is an uncommon but serious medical condition with a high mortality rate. Prompt recognition and an aggressive multimodal treatment approach, ideally in the ICU, are paramount to stabilize patients and seek definitive treatment.
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
2. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993; 22(2):263-277.
A 73-year-old man is transported to the emergency department (ED) by ambulance for nausea, vomiting, diarrhea, and weakness of three days’ duration. Earlier today, he presented to his primary care provider with these symptoms and was found to be hypotensive; he was advised to go to the ED but instead went home against medical advice.
The patient’s medical history is significant for type 2 diabetes, stage 3b chronic kidney disease, dyslipidemia, hypertension, coronary artery disease, and benign prostatic hyperplasia. He has undergone stent placement and triple coronary artery bypass graft surgery. His medication list includes insulin glargine, glimepiride, liraglutide, atorvastatin, benazepril, carvedilol, amlodipine, clopidogrel, and tamsulosin.
Upon admission, the patient has a pulse of 98 beats/min; temperature, 98.2°F; respiratory rate, 18 breaths/min-1; and PO2, 98 mm Hg. An ECG, chest radiograph, and CT (without contrast) of the head, chest, and abdomen are all within normal limits. Lab evaluation is significant for severe thyrotoxicosis (see Table 1).
Endocrinology consult is requested. Further testing yields the following findings
- Thyroid-stimulating immunoglobulin: 309% (reference range, < 30%)
- Nuclear medicine thyroid scan with uptake: 6-hour uptake of 70.3% (10%-25%) and 24-hour uptake, 81.8% (15%-35%)
- Homogeneous radiotracer uptake within the thyroid gland: no evidence of hot or cold nodules
- Thyroid ultrasound: bilateral enlarged heterogeneous gland and multiple subcentimeter nodules (largest measuring 6 × 7 mm)
These results confirm a diagnosis of Graves’ disease. Treatment options, including antithyroid medications, radioactive iodine ablation (RAI), and surgery, are discussed. The patient is treated with RAI therapy (10 mCi) and discharged from the hospital.
Six days later, however, he returns to the ED with severe intermittent dizziness and lightheadedness of two hours’ duration, new-onset atrial fibrillation (A-fib), and mild shortness of breath. His vital signs include a pulse of 116 beats/min; temperature, 98.1°F; respiratory rate, 18 breaths/min-1, blood pressure, 154/88 mm Hg; and PO2, 100 mm Hg.
His lab values include
- TSH < 0.005 uIU/mL
- Free T4, 8.01 ng/dL
- Free T3, 3,701 pg/dL
- eGFR, 60 mL/min/1.73 m2
Cardiology consult is requested. A pacemaker is placed for bradycardia-tachycardia syndrome, and the patient is put on rivaroxaban for stroke prevention.
The endocrinologist suspects post-RAI thyroiditis or ineffective RAI treatment. The patient is started on methimazole (10 mg bid), and his carvedilol is replaced with metoprolol (50 mg bid).
Two weeks postdischarge, the patient returns to the office. Although he says he’s doing better, he seems uneasy and agitated and has a pulse of 120 beats/min. His methimazole and metoprolol are increased (to 10 mg tid and 50 mg tid, respectively).
Another two weeks later, lab results still show elevated thyroid levels—now with increased enzyme levels on liver function testing. The patient reports worsening dizziness and shortness of breath. He is sent back to hospital and admitted for inpatient management, with urgent surgical consult for thyroidectomy. Total thyroidectomy is successfully performed, and the final pathology report shows a benign goiter.
DISCUSSION
Thyroid storm is an extreme form of thyrotoxicosis with an associated mortality rate of 8% to 25%.1 When thyroid hormone levels are elevated, adrenaline receptors are upregulated—but, while it is possible for persistent thyrotoxicosis to progress to thyroid storm on its own, a surge of adrenaline is usually needed. Most cases are triggered by acute stressors (ie, myocardial infarction, surgery, anesthesia, labor and delivery) in the context of underlying thyrotoxicosis.1
Diagnosis of thyroid storm is made clinically in patients who are thyrotoxic and present with systemic decompensation (ie, altered mental status, cardiovascular dysfunction, hyperpyrexia). Although no universally accepted criteria currently exist, the Burch-Wartofsky Point Scale (BWPS; see Table 2) can be used to assess disease severity and guide the extent of treatment and monitoring.2 However, this measure should not replace clinical judgment—the distinction between compensated thyrotoxicosis and decompensating thyrotoxicosis (thyroid storm) should be made by sound but prompt clinical assessment.
Once thyroid storm is suspected, aggressive treatment should be implemented to improve the systemic thyrotoxic state. Propylthiouracil (PTU) is preferred over methimazole, as it blocks T4 to T3 conversion in addition to blocking new hormone synthesis. Propranolol is the best choice of ß-blocker because it also blocks T4 to T3 conversion and controls cardiac rhythm.
Iodine can rapidly block new hormone synthesis and release; it is often used to reduce thyroid hormone levels prior to emergency thyroid surgery. However, it should be given at least one hour after a dose of PTU. Hydrocortisone is given prophylactically for relative adrenal insufficiency (due to rapid cortisol clearance during thyrotoxic state); it may block T4 to T3 conversion as well. Volume resuscitation, respiratory care, temperature control (eg, antipyretics, cooling blankets), and nutritional support should also be incorporated, ideally in the intensive care unit (ICU). During or after thyroid storm management, treatment of the precipitating event/illness and of hyperthyroidism should be initiated to prevent recurrence.1
The patient’s initial BWPS was 30 (gastrointestinal [GI] score 10 + central nervous system [CNS] score 10 + without precipitating factor 10), which put him in the “impending storm” category. At his second ED visit, his BWPS was 40 (cardiovascular score 10 + A-fib 10 + GI score 10 + CNS score 10 + precipitating factor [RAI ablation] score 0)—still in the “impending storm” category but certainly indicating a worsened state.
RAI for hyperthyroidism can transiently increase thyroid hormone levels due to inflammation of the gland. To prevent exacerbation of the thyrotoxic state, pretreatment with methimazole should be considered in patients with risk factors (eg, older age, cardiovascular complications, cerebrovascular disease, pulmonary disease, renal failure, infection, trauma, and poorly controlled diabetes). Patients should also be placed on ß-blockers prior to treatment, in anticipation of a transient rise in thyroid hormone levels.
Due to this patient’s age, severity of thyrotoxicosis, and multiple risk factors, strong consideration should have been given to pretreating him with antithyroid medication and a ß-blocker before definitive treatment was given. This would have potentially averted his subsequent hospital visits and urgent need for thyroidectomy.
CONCLUSION
Thyroid storm is an uncommon but serious medical condition with a high mortality rate. Prompt recognition and an aggressive multimodal treatment approach, ideally in the ICU, are paramount to stabilize patients and seek definitive treatment.
A 73-year-old man is transported to the emergency department (ED) by ambulance for nausea, vomiting, diarrhea, and weakness of three days’ duration. Earlier today, he presented to his primary care provider with these symptoms and was found to be hypotensive; he was advised to go to the ED but instead went home against medical advice.
The patient’s medical history is significant for type 2 diabetes, stage 3b chronic kidney disease, dyslipidemia, hypertension, coronary artery disease, and benign prostatic hyperplasia. He has undergone stent placement and triple coronary artery bypass graft surgery. His medication list includes insulin glargine, glimepiride, liraglutide, atorvastatin, benazepril, carvedilol, amlodipine, clopidogrel, and tamsulosin.
Upon admission, the patient has a pulse of 98 beats/min; temperature, 98.2°F; respiratory rate, 18 breaths/min-1; and PO2, 98 mm Hg. An ECG, chest radiograph, and CT (without contrast) of the head, chest, and abdomen are all within normal limits. Lab evaluation is significant for severe thyrotoxicosis (see Table 1).
Endocrinology consult is requested. Further testing yields the following findings
- Thyroid-stimulating immunoglobulin: 309% (reference range, < 30%)
- Nuclear medicine thyroid scan with uptake: 6-hour uptake of 70.3% (10%-25%) and 24-hour uptake, 81.8% (15%-35%)
- Homogeneous radiotracer uptake within the thyroid gland: no evidence of hot or cold nodules
- Thyroid ultrasound: bilateral enlarged heterogeneous gland and multiple subcentimeter nodules (largest measuring 6 × 7 mm)
These results confirm a diagnosis of Graves’ disease. Treatment options, including antithyroid medications, radioactive iodine ablation (RAI), and surgery, are discussed. The patient is treated with RAI therapy (10 mCi) and discharged from the hospital.
Six days later, however, he returns to the ED with severe intermittent dizziness and lightheadedness of two hours’ duration, new-onset atrial fibrillation (A-fib), and mild shortness of breath. His vital signs include a pulse of 116 beats/min; temperature, 98.1°F; respiratory rate, 18 breaths/min-1, blood pressure, 154/88 mm Hg; and PO2, 100 mm Hg.
His lab values include
- TSH < 0.005 uIU/mL
- Free T4, 8.01 ng/dL
- Free T3, 3,701 pg/dL
- eGFR, 60 mL/min/1.73 m2
Cardiology consult is requested. A pacemaker is placed for bradycardia-tachycardia syndrome, and the patient is put on rivaroxaban for stroke prevention.
The endocrinologist suspects post-RAI thyroiditis or ineffective RAI treatment. The patient is started on methimazole (10 mg bid), and his carvedilol is replaced with metoprolol (50 mg bid).
Two weeks postdischarge, the patient returns to the office. Although he says he’s doing better, he seems uneasy and agitated and has a pulse of 120 beats/min. His methimazole and metoprolol are increased (to 10 mg tid and 50 mg tid, respectively).
Another two weeks later, lab results still show elevated thyroid levels—now with increased enzyme levels on liver function testing. The patient reports worsening dizziness and shortness of breath. He is sent back to hospital and admitted for inpatient management, with urgent surgical consult for thyroidectomy. Total thyroidectomy is successfully performed, and the final pathology report shows a benign goiter.
DISCUSSION
Thyroid storm is an extreme form of thyrotoxicosis with an associated mortality rate of 8% to 25%.1 When thyroid hormone levels are elevated, adrenaline receptors are upregulated—but, while it is possible for persistent thyrotoxicosis to progress to thyroid storm on its own, a surge of adrenaline is usually needed. Most cases are triggered by acute stressors (ie, myocardial infarction, surgery, anesthesia, labor and delivery) in the context of underlying thyrotoxicosis.1
Diagnosis of thyroid storm is made clinically in patients who are thyrotoxic and present with systemic decompensation (ie, altered mental status, cardiovascular dysfunction, hyperpyrexia). Although no universally accepted criteria currently exist, the Burch-Wartofsky Point Scale (BWPS; see Table 2) can be used to assess disease severity and guide the extent of treatment and monitoring.2 However, this measure should not replace clinical judgment—the distinction between compensated thyrotoxicosis and decompensating thyrotoxicosis (thyroid storm) should be made by sound but prompt clinical assessment.
Once thyroid storm is suspected, aggressive treatment should be implemented to improve the systemic thyrotoxic state. Propylthiouracil (PTU) is preferred over methimazole, as it blocks T4 to T3 conversion in addition to blocking new hormone synthesis. Propranolol is the best choice of ß-blocker because it also blocks T4 to T3 conversion and controls cardiac rhythm.
Iodine can rapidly block new hormone synthesis and release; it is often used to reduce thyroid hormone levels prior to emergency thyroid surgery. However, it should be given at least one hour after a dose of PTU. Hydrocortisone is given prophylactically for relative adrenal insufficiency (due to rapid cortisol clearance during thyrotoxic state); it may block T4 to T3 conversion as well. Volume resuscitation, respiratory care, temperature control (eg, antipyretics, cooling blankets), and nutritional support should also be incorporated, ideally in the intensive care unit (ICU). During or after thyroid storm management, treatment of the precipitating event/illness and of hyperthyroidism should be initiated to prevent recurrence.1
The patient’s initial BWPS was 30 (gastrointestinal [GI] score 10 + central nervous system [CNS] score 10 + without precipitating factor 10), which put him in the “impending storm” category. At his second ED visit, his BWPS was 40 (cardiovascular score 10 + A-fib 10 + GI score 10 + CNS score 10 + precipitating factor [RAI ablation] score 0)—still in the “impending storm” category but certainly indicating a worsened state.
RAI for hyperthyroidism can transiently increase thyroid hormone levels due to inflammation of the gland. To prevent exacerbation of the thyrotoxic state, pretreatment with methimazole should be considered in patients with risk factors (eg, older age, cardiovascular complications, cerebrovascular disease, pulmonary disease, renal failure, infection, trauma, and poorly controlled diabetes). Patients should also be placed on ß-blockers prior to treatment, in anticipation of a transient rise in thyroid hormone levels.
Due to this patient’s age, severity of thyrotoxicosis, and multiple risk factors, strong consideration should have been given to pretreating him with antithyroid medication and a ß-blocker before definitive treatment was given. This would have potentially averted his subsequent hospital visits and urgent need for thyroidectomy.
CONCLUSION
Thyroid storm is an uncommon but serious medical condition with a high mortality rate. Prompt recognition and an aggressive multimodal treatment approach, ideally in the ICU, are paramount to stabilize patients and seek definitive treatment.
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
2. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993; 22(2):263-277.
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
2. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993; 22(2):263-277.