User login
Monoclonal Antibodies in MS
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
Sexual Dysfunction in MS
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
MS: Partnering With Patients to Improve Health
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
Cognition and MS
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Identifying and Managing MS Relapse
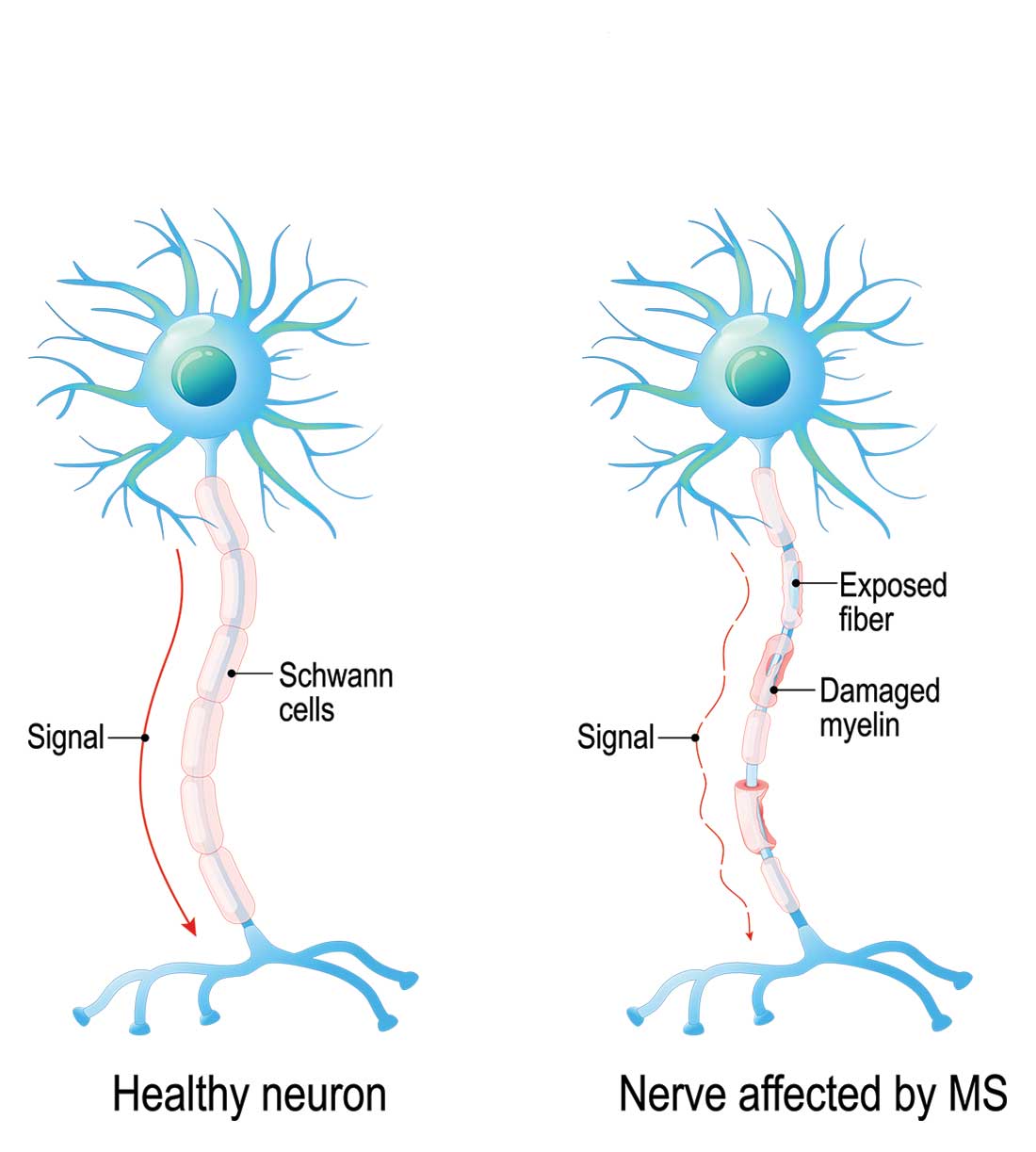
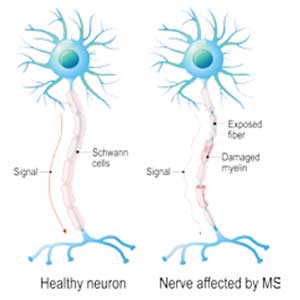
Multiple sclerosis (MS) is a chronic, autoimmune-mediated disorder of the central nervous system that affects more than 40,000 people in the United States. About 85% of cases are categorized as relapsing remitting multiple sclerosis (RRMS), based on the clinical and radiographic pattern of focal demyelination in different regions of the brain and spinal cord over time. Though not fully understood, the pathophysiology of RRMS involves axonal degeneration and inflammatory demyelination; the latter is considered a relapse in patients with an established MS diagnosis.
OVERVIEW
MS relapse can have a significant impact on patients’ short- and long-term function, quality of life, and finances. Relapse may be identified via
- New neurologic symptoms reported by the patient
- New neurologic findings on physical examination
- New radiographic findings on contrast-enhanced MRI of the central nervous system, or
- Abnormal results of cerebrospinal fluid analysis.
Patient-reported symptoms and abnormal signs identified on physical exam should correspond with the area of the central nervous system affected. In some cases, patients may have radiographic evidence of relapse without symptoms or signs.
It is essential for health care providers to identify relapse, as it is an important marker of disease activity that may warrant treatment—particularly if symptoms are impacting function or if there is optic neuritis. MS relapse is also an indicator of suboptimal response to disease-modifying therapies.
Treatment of relapse is one component of RRMS management, which also includes symptom management and use of disease-modifying therapy to reduce risk for disease activity and decline in function.
DIAGNOSING RELAPSE
Because risk for MS relapse cannot be predicted, both patients and providers need to have a high index of suspicion in the setting of new neurologic symptoms or decline in function. Relapse should be considered when these symptoms last longer than 24 hours in the absence of fever or infection. The clinical features of relapse should have corresponding radiographic evidence of active demyelination on contrast-enhanced MRI.
A pseudo-relapse is characterized by new or worsening neurologic symptoms lasting longer than 24 hours with concurrent fever, infection, or other metabolic derangement. Pseudo-relapse does not show radiographic evidence of active demyelination on contrast-enhanced MRI.
Continue to: Aggravation of longstanding neurologic symptoms...
Aggravation of longstanding neurologic symptoms is not considered a relapse, as no new radiographic evidence of disease progression will be seen on MRI. Factors that may contribute to aggravation of established symptoms include an increase in core body temperature, sleep deprivation, and psychosocial stress.
When a patient with suspected or diagnosed RRMS presents with new neurologic symptoms of more than 24 hours’ duration, the first step is to conduct a physical exam to assess for objective evidence of neurologic deficits and signs of infection, including fever. The provider should also order select laboratory testing—including a complete blood cell count and urinalysis with culture—to exclude infection. In certain cases, contrast-enhanced MRI of the brain and/or spine may be ordered; however, this may delay treatment initiation if the study cannot be promptly scheduled.
Evaluation and management should involve communication, if not face-to-face consultation, with the patient’s neurology provider who is responsible for MS management.
IMMEDIATE MANAGEMENT
Acute relapses are managed with anti-inflammatory agents. For some patients, treatment may provide symptomatic relief, shorten the recovery phase, and improve motor function. Long-term benefits have not been demonstrated, except in patients with optic neuritis.
Firstline therapy for MS relapse is high-dose corticosteroids, which can be administered at home, at an ambulatory infusion center, or (in some cases) in a hospital setting. The preferred regimen is methylprednisolone (1 g IV for 3-5 d), with or without prednisone taper. Another option is dexamethasone (80 mg bid for 3-5 d), with or without prednisone taper.1-3
Continue to: Common adverse effects of corticosteroids include...
Common adverse effects of corticosteroids include headache, emotional lability, insomnia, glucose intolerance, hypertension, dyspepsia, and exacerbation of psychiatric conditions; drug interactions should also be considered. Patients with diabetes may need to be admitted to the hospital for glycemic monitoring and control.
High-dose corticosteroids are associated with a rare, non–dose-dependent risk for aseptic femoral necrosis. For patients who are refractory to or not candidates for corticosteroids, adrenocorticotropic hormone (ACTH) gel (80 U/d IM or subQ daily for 10 d) is an option. This medication may be better tolerated, although it is much more expensive than corticosteroids. Plasmapheresis and IV immunoglobulin are also options for patients with refractory symptoms or contraindications to recommended therapies.1-3
ONGOING MANAGEMENT
Once a treatment plan is initiated, providers should carefully follow the patient’s response in terms of adverse effects, symptom improvement, and functional recovery. Those with refractory symptoms may need additional doses of the initial therapy or an alternative therapy.
The relapse recovery period may last several months and be complete or incomplete, so providers may also need to manage neurologic symptoms and functional deficits (with pharmacologic and/or nonpharmacologic options). Patients who have had a relapse should also meet with their neurology provider to discuss their disease-modifying therapy plan, since relapse indicates a suboptimal response to current therapy.
1. Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):17.
2. Frohman TC, O’Donoghue DL, Northrop D, eds. Multiple Sclerosis for the Physician Assistant. National Multiple Sclerosis Society. 2011.
3. Giesser B, ed. Primer on Multiple Sclerosis . 2nd ed. Oxford, UK: Oxford University Press ; 2015.
Multiple sclerosis (MS) is a chronic, autoimmune-mediated disorder of the central nervous system that affects more than 40,000 people in the United States. About 85% of cases are categorized as relapsing remitting multiple sclerosis (RRMS), based on the clinical and radiographic pattern of focal demyelination in different regions of the brain and spinal cord over time. Though not fully understood, the pathophysiology of RRMS involves axonal degeneration and inflammatory demyelination; the latter is considered a relapse in patients with an established MS diagnosis.
OVERVIEW
MS relapse can have a significant impact on patients’ short- and long-term function, quality of life, and finances. Relapse may be identified via
- New neurologic symptoms reported by the patient
- New neurologic findings on physical examination
- New radiographic findings on contrast-enhanced MRI of the central nervous system, or
- Abnormal results of cerebrospinal fluid analysis.
Patient-reported symptoms and abnormal signs identified on physical exam should correspond with the area of the central nervous system affected. In some cases, patients may have radiographic evidence of relapse without symptoms or signs.
It is essential for health care providers to identify relapse, as it is an important marker of disease activity that may warrant treatment—particularly if symptoms are impacting function or if there is optic neuritis. MS relapse is also an indicator of suboptimal response to disease-modifying therapies.
Treatment of relapse is one component of RRMS management, which also includes symptom management and use of disease-modifying therapy to reduce risk for disease activity and decline in function.
DIAGNOSING RELAPSE
Because risk for MS relapse cannot be predicted, both patients and providers need to have a high index of suspicion in the setting of new neurologic symptoms or decline in function. Relapse should be considered when these symptoms last longer than 24 hours in the absence of fever or infection. The clinical features of relapse should have corresponding radiographic evidence of active demyelination on contrast-enhanced MRI.
A pseudo-relapse is characterized by new or worsening neurologic symptoms lasting longer than 24 hours with concurrent fever, infection, or other metabolic derangement. Pseudo-relapse does not show radiographic evidence of active demyelination on contrast-enhanced MRI.
Continue to: Aggravation of longstanding neurologic symptoms...
Aggravation of longstanding neurologic symptoms is not considered a relapse, as no new radiographic evidence of disease progression will be seen on MRI. Factors that may contribute to aggravation of established symptoms include an increase in core body temperature, sleep deprivation, and psychosocial stress.
When a patient with suspected or diagnosed RRMS presents with new neurologic symptoms of more than 24 hours’ duration, the first step is to conduct a physical exam to assess for objective evidence of neurologic deficits and signs of infection, including fever. The provider should also order select laboratory testing—including a complete blood cell count and urinalysis with culture—to exclude infection. In certain cases, contrast-enhanced MRI of the brain and/or spine may be ordered; however, this may delay treatment initiation if the study cannot be promptly scheduled.
Evaluation and management should involve communication, if not face-to-face consultation, with the patient’s neurology provider who is responsible for MS management.
IMMEDIATE MANAGEMENT
Acute relapses are managed with anti-inflammatory agents. For some patients, treatment may provide symptomatic relief, shorten the recovery phase, and improve motor function. Long-term benefits have not been demonstrated, except in patients with optic neuritis.
Firstline therapy for MS relapse is high-dose corticosteroids, which can be administered at home, at an ambulatory infusion center, or (in some cases) in a hospital setting. The preferred regimen is methylprednisolone (1 g IV for 3-5 d), with or without prednisone taper. Another option is dexamethasone (80 mg bid for 3-5 d), with or without prednisone taper.1-3
Continue to: Common adverse effects of corticosteroids include...
Common adverse effects of corticosteroids include headache, emotional lability, insomnia, glucose intolerance, hypertension, dyspepsia, and exacerbation of psychiatric conditions; drug interactions should also be considered. Patients with diabetes may need to be admitted to the hospital for glycemic monitoring and control.
High-dose corticosteroids are associated with a rare, non–dose-dependent risk for aseptic femoral necrosis. For patients who are refractory to or not candidates for corticosteroids, adrenocorticotropic hormone (ACTH) gel (80 U/d IM or subQ daily for 10 d) is an option. This medication may be better tolerated, although it is much more expensive than corticosteroids. Plasmapheresis and IV immunoglobulin are also options for patients with refractory symptoms or contraindications to recommended therapies.1-3
ONGOING MANAGEMENT
Once a treatment plan is initiated, providers should carefully follow the patient’s response in terms of adverse effects, symptom improvement, and functional recovery. Those with refractory symptoms may need additional doses of the initial therapy or an alternative therapy.
The relapse recovery period may last several months and be complete or incomplete, so providers may also need to manage neurologic symptoms and functional deficits (with pharmacologic and/or nonpharmacologic options). Patients who have had a relapse should also meet with their neurology provider to discuss their disease-modifying therapy plan, since relapse indicates a suboptimal response to current therapy.
Multiple sclerosis (MS) is a chronic, autoimmune-mediated disorder of the central nervous system that affects more than 40,000 people in the United States. About 85% of cases are categorized as relapsing remitting multiple sclerosis (RRMS), based on the clinical and radiographic pattern of focal demyelination in different regions of the brain and spinal cord over time. Though not fully understood, the pathophysiology of RRMS involves axonal degeneration and inflammatory demyelination; the latter is considered a relapse in patients with an established MS diagnosis.
OVERVIEW
MS relapse can have a significant impact on patients’ short- and long-term function, quality of life, and finances. Relapse may be identified via
- New neurologic symptoms reported by the patient
- New neurologic findings on physical examination
- New radiographic findings on contrast-enhanced MRI of the central nervous system, or
- Abnormal results of cerebrospinal fluid analysis.
Patient-reported symptoms and abnormal signs identified on physical exam should correspond with the area of the central nervous system affected. In some cases, patients may have radiographic evidence of relapse without symptoms or signs.
It is essential for health care providers to identify relapse, as it is an important marker of disease activity that may warrant treatment—particularly if symptoms are impacting function or if there is optic neuritis. MS relapse is also an indicator of suboptimal response to disease-modifying therapies.
Treatment of relapse is one component of RRMS management, which also includes symptom management and use of disease-modifying therapy to reduce risk for disease activity and decline in function.
DIAGNOSING RELAPSE
Because risk for MS relapse cannot be predicted, both patients and providers need to have a high index of suspicion in the setting of new neurologic symptoms or decline in function. Relapse should be considered when these symptoms last longer than 24 hours in the absence of fever or infection. The clinical features of relapse should have corresponding radiographic evidence of active demyelination on contrast-enhanced MRI.
A pseudo-relapse is characterized by new or worsening neurologic symptoms lasting longer than 24 hours with concurrent fever, infection, or other metabolic derangement. Pseudo-relapse does not show radiographic evidence of active demyelination on contrast-enhanced MRI.
Continue to: Aggravation of longstanding neurologic symptoms...
Aggravation of longstanding neurologic symptoms is not considered a relapse, as no new radiographic evidence of disease progression will be seen on MRI. Factors that may contribute to aggravation of established symptoms include an increase in core body temperature, sleep deprivation, and psychosocial stress.
When a patient with suspected or diagnosed RRMS presents with new neurologic symptoms of more than 24 hours’ duration, the first step is to conduct a physical exam to assess for objective evidence of neurologic deficits and signs of infection, including fever. The provider should also order select laboratory testing—including a complete blood cell count and urinalysis with culture—to exclude infection. In certain cases, contrast-enhanced MRI of the brain and/or spine may be ordered; however, this may delay treatment initiation if the study cannot be promptly scheduled.
Evaluation and management should involve communication, if not face-to-face consultation, with the patient’s neurology provider who is responsible for MS management.
IMMEDIATE MANAGEMENT
Acute relapses are managed with anti-inflammatory agents. For some patients, treatment may provide symptomatic relief, shorten the recovery phase, and improve motor function. Long-term benefits have not been demonstrated, except in patients with optic neuritis.
Firstline therapy for MS relapse is high-dose corticosteroids, which can be administered at home, at an ambulatory infusion center, or (in some cases) in a hospital setting. The preferred regimen is methylprednisolone (1 g IV for 3-5 d), with or without prednisone taper. Another option is dexamethasone (80 mg bid for 3-5 d), with or without prednisone taper.1-3
Continue to: Common adverse effects of corticosteroids include...
Common adverse effects of corticosteroids include headache, emotional lability, insomnia, glucose intolerance, hypertension, dyspepsia, and exacerbation of psychiatric conditions; drug interactions should also be considered. Patients with diabetes may need to be admitted to the hospital for glycemic monitoring and control.
High-dose corticosteroids are associated with a rare, non–dose-dependent risk for aseptic femoral necrosis. For patients who are refractory to or not candidates for corticosteroids, adrenocorticotropic hormone (ACTH) gel (80 U/d IM or subQ daily for 10 d) is an option. This medication may be better tolerated, although it is much more expensive than corticosteroids. Plasmapheresis and IV immunoglobulin are also options for patients with refractory symptoms or contraindications to recommended therapies.1-3
ONGOING MANAGEMENT
Once a treatment plan is initiated, providers should carefully follow the patient’s response in terms of adverse effects, symptom improvement, and functional recovery. Those with refractory symptoms may need additional doses of the initial therapy or an alternative therapy.
The relapse recovery period may last several months and be complete or incomplete, so providers may also need to manage neurologic symptoms and functional deficits (with pharmacologic and/or nonpharmacologic options). Patients who have had a relapse should also meet with their neurology provider to discuss their disease-modifying therapy plan, since relapse indicates a suboptimal response to current therapy.
1. Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):17.
2. Frohman TC, O’Donoghue DL, Northrop D, eds. Multiple Sclerosis for the Physician Assistant. National Multiple Sclerosis Society. 2011.
3. Giesser B, ed. Primer on Multiple Sclerosis . 2nd ed. Oxford, UK: Oxford University Press ; 2015.
1. Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):17.
2. Frohman TC, O’Donoghue DL, Northrop D, eds. Multiple Sclerosis for the Physician Assistant. National Multiple Sclerosis Society. 2011.
3. Giesser B, ed. Primer on Multiple Sclerosis . 2nd ed. Oxford, UK: Oxford University Press ; 2015.
Neuropathic Pain in MS
Q) How do I assess for and treat neuropathic pain in MS?
In multiple sclerosis (MS), pain is a common symptom; patients may experience varying forms during their disease course. One type is neuropathic pain, which is initiated or caused by a demyelinating lesion in the central nervous system.1 It may occur spontaneously or be evoked, and it can be intermittent or steady. Given the nature of the disease course in MS, it is important to complete a pain assessment at each visit.
A patient experiencing neuropathic pain is likely to report abnormal sensations or hypersensitivity in the affected area. It is often combined with or adjacent to areas of sensory deficit.1 This includes altered sensations such as pins and needles, numbness, crawling, or burning. The most common MS-related neuropathic pain conditions are ongoing dysaesthetic extremity pain and paroxysmal pain, such as trigeminal neuralgia and Lhermitte phenomenon.1-3
Assessment. When assessing the history of neuropathic pain, it is beneficial to remember that abnormal sensory findings should be neuroanatomically aligned with a lesion site. The mnemonic OPQRST is a helpful reminder to ask about
Onset
Provoking/palliating factors
Quality of the sensation
If it radiates
Severity of the pain (using a scale of 0-10 can be helpful)
Time when the pain occurs.
These probing questions will aid diagnosis and uncover clues on areas to pay special attention to during the examination. For example, when a patient reports numbness of both feet, the clinician might suspect a lesion in the spinal cord and then can try to determine the level during the sensory exam.
Screening tools that capture the patient experience, such as the modified version of the Brief Pain Inventory (BPI), can assist in diagnosis as well as measure the impact of treatment.4
A physical assessment for neuropathic pain includes a full neurologic evaluation of motor, sensory, and autonomic systems to identify all signs of neurologic dysfunction. Attention should be paid to the possible types of negative sensory symptoms (eg, sensory loss) and positive findings (eg, paresthesia). When completing the sensory exam, the clinician can gauge pain by using a sharp object such as a toothpick. Tactile sense can be assessed with a piece of cotton, and temperature can be tested with warm and cold objects. A tuning fork can identify vibration sense. Body sensory maps, on which the clinician draws the sensory disturbance on schematic charts, can provide valuable information.
Diagnostic tests, such as MRI, can also assist in confirming the lesion of the somatosensory nervous system that explains the pain.
Continue to: Treatment
Treatment. Many patients who experience neuropathic pain require a multidisciplinary approach.5 Support from colleagues in rehabilitation can help the patient identify alternative approaches to functioning that avoid triggering or exacerbating the pain. Equipment can also maximize independence and improve quality of life. For example, a soft neck collar is often used to prevent the forward movement that triggers pain in Lhermitte phenomenon.6
When prescribing pain medication, it is important to understand that neuropathic pain is inadequately relieved or not relieved at all with conventional analgesics, such as NSAIDs, or opioid analgesics (eg, morphine).2,3
Dysesthesias are most frequently treated with medications that are categorized as antiseizure, such as gabapentin and pregabalin. Carbamazepam and phenytoin are used as secondline therapy. Sometimes, anti-anxiety medication (eg, duloxetine hydrochloride and clonazepam or tricyclic antidepressants, including amitriptyline or nortriptyline) can be helpful.7 When treating paroxysmal symptoms such as trigeminal neuralgia, antiseizure medications can be effective. Carbamazepine is often the firstline of treatment. As a secondline, oxcarbazepine, lamotrigine, and/or baclofen may be used. In some cases, a referral to neurosurgery for a procedure to reduce pressure on the trigeminal nerve is required.5,8
It is also important to treat any additional symptoms that the pain may be causing, such as depression or social isolation. Referral for counseling as well as integrative health and wellness services can support the patient through a difficult time.5 —RS
Rachael Stacom, MS, ANP-BC, MSCN
Independence Care System, New York, NY
1. Zagon IS, Mclaughlin PJ. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane, Australia: Codon Publications. 2017.
2. O’Connor AB, Schwid SR, Hermann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
3. Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. 2011;12(15):2355-2368.
4. Osborne TL, Raichle KA, Jensen MP, et al. The reliability and validity of pain interference measures in persons with multiple sclerosis. J Pain Symptom Manage. 2006;32(3):217-229.
5. Sullivan AB, Scheman J, Lopresti A, Prayor-Patterson H. Interdisciplinary treatment of patients with multiple sclerosis and chronic pain. Int J MS Care. 2012;14(4):216-220.
6. MS Australia. Pain and multiple sclerosis (MS). www.msaustralia.org.au/publications/pain-and-multiple-sclerosis-ms. Accessed May 15, 2018.
7. Maloni H; National Multiple Sclerosis Society. Clinical bulletin: pain in multiple sclerosis. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Clinical_Bulletin_Pain-in-MS.pdf. Accessed May 15, 2018.
8. Multiple Sclerosis Association of America (MSAA). (H. Maloni, Ed.) The Motivator Winter/Spring. Retrieved from https://mymsaa.org/publications/motivator/winter-spring13/cover-story/pain. Accessed May 15, 2018.
Q) How do I assess for and treat neuropathic pain in MS?
In multiple sclerosis (MS), pain is a common symptom; patients may experience varying forms during their disease course. One type is neuropathic pain, which is initiated or caused by a demyelinating lesion in the central nervous system.1 It may occur spontaneously or be evoked, and it can be intermittent or steady. Given the nature of the disease course in MS, it is important to complete a pain assessment at each visit.
A patient experiencing neuropathic pain is likely to report abnormal sensations or hypersensitivity in the affected area. It is often combined with or adjacent to areas of sensory deficit.1 This includes altered sensations such as pins and needles, numbness, crawling, or burning. The most common MS-related neuropathic pain conditions are ongoing dysaesthetic extremity pain and paroxysmal pain, such as trigeminal neuralgia and Lhermitte phenomenon.1-3
Assessment. When assessing the history of neuropathic pain, it is beneficial to remember that abnormal sensory findings should be neuroanatomically aligned with a lesion site. The mnemonic OPQRST is a helpful reminder to ask about
Onset
Provoking/palliating factors
Quality of the sensation
If it radiates
Severity of the pain (using a scale of 0-10 can be helpful)
Time when the pain occurs.
These probing questions will aid diagnosis and uncover clues on areas to pay special attention to during the examination. For example, when a patient reports numbness of both feet, the clinician might suspect a lesion in the spinal cord and then can try to determine the level during the sensory exam.
Screening tools that capture the patient experience, such as the modified version of the Brief Pain Inventory (BPI), can assist in diagnosis as well as measure the impact of treatment.4
A physical assessment for neuropathic pain includes a full neurologic evaluation of motor, sensory, and autonomic systems to identify all signs of neurologic dysfunction. Attention should be paid to the possible types of negative sensory symptoms (eg, sensory loss) and positive findings (eg, paresthesia). When completing the sensory exam, the clinician can gauge pain by using a sharp object such as a toothpick. Tactile sense can be assessed with a piece of cotton, and temperature can be tested with warm and cold objects. A tuning fork can identify vibration sense. Body sensory maps, on which the clinician draws the sensory disturbance on schematic charts, can provide valuable information.
Diagnostic tests, such as MRI, can also assist in confirming the lesion of the somatosensory nervous system that explains the pain.
Continue to: Treatment
Treatment. Many patients who experience neuropathic pain require a multidisciplinary approach.5 Support from colleagues in rehabilitation can help the patient identify alternative approaches to functioning that avoid triggering or exacerbating the pain. Equipment can also maximize independence and improve quality of life. For example, a soft neck collar is often used to prevent the forward movement that triggers pain in Lhermitte phenomenon.6
When prescribing pain medication, it is important to understand that neuropathic pain is inadequately relieved or not relieved at all with conventional analgesics, such as NSAIDs, or opioid analgesics (eg, morphine).2,3
Dysesthesias are most frequently treated with medications that are categorized as antiseizure, such as gabapentin and pregabalin. Carbamazepam and phenytoin are used as secondline therapy. Sometimes, anti-anxiety medication (eg, duloxetine hydrochloride and clonazepam or tricyclic antidepressants, including amitriptyline or nortriptyline) can be helpful.7 When treating paroxysmal symptoms such as trigeminal neuralgia, antiseizure medications can be effective. Carbamazepine is often the firstline of treatment. As a secondline, oxcarbazepine, lamotrigine, and/or baclofen may be used. In some cases, a referral to neurosurgery for a procedure to reduce pressure on the trigeminal nerve is required.5,8
It is also important to treat any additional symptoms that the pain may be causing, such as depression or social isolation. Referral for counseling as well as integrative health and wellness services can support the patient through a difficult time.5 —RS
Rachael Stacom, MS, ANP-BC, MSCN
Independence Care System, New York, NY
Q) How do I assess for and treat neuropathic pain in MS?
In multiple sclerosis (MS), pain is a common symptom; patients may experience varying forms during their disease course. One type is neuropathic pain, which is initiated or caused by a demyelinating lesion in the central nervous system.1 It may occur spontaneously or be evoked, and it can be intermittent or steady. Given the nature of the disease course in MS, it is important to complete a pain assessment at each visit.
A patient experiencing neuropathic pain is likely to report abnormal sensations or hypersensitivity in the affected area. It is often combined with or adjacent to areas of sensory deficit.1 This includes altered sensations such as pins and needles, numbness, crawling, or burning. The most common MS-related neuropathic pain conditions are ongoing dysaesthetic extremity pain and paroxysmal pain, such as trigeminal neuralgia and Lhermitte phenomenon.1-3
Assessment. When assessing the history of neuropathic pain, it is beneficial to remember that abnormal sensory findings should be neuroanatomically aligned with a lesion site. The mnemonic OPQRST is a helpful reminder to ask about
Onset
Provoking/palliating factors
Quality of the sensation
If it radiates
Severity of the pain (using a scale of 0-10 can be helpful)
Time when the pain occurs.
These probing questions will aid diagnosis and uncover clues on areas to pay special attention to during the examination. For example, when a patient reports numbness of both feet, the clinician might suspect a lesion in the spinal cord and then can try to determine the level during the sensory exam.
Screening tools that capture the patient experience, such as the modified version of the Brief Pain Inventory (BPI), can assist in diagnosis as well as measure the impact of treatment.4
A physical assessment for neuropathic pain includes a full neurologic evaluation of motor, sensory, and autonomic systems to identify all signs of neurologic dysfunction. Attention should be paid to the possible types of negative sensory symptoms (eg, sensory loss) and positive findings (eg, paresthesia). When completing the sensory exam, the clinician can gauge pain by using a sharp object such as a toothpick. Tactile sense can be assessed with a piece of cotton, and temperature can be tested with warm and cold objects. A tuning fork can identify vibration sense. Body sensory maps, on which the clinician draws the sensory disturbance on schematic charts, can provide valuable information.
Diagnostic tests, such as MRI, can also assist in confirming the lesion of the somatosensory nervous system that explains the pain.
Continue to: Treatment
Treatment. Many patients who experience neuropathic pain require a multidisciplinary approach.5 Support from colleagues in rehabilitation can help the patient identify alternative approaches to functioning that avoid triggering or exacerbating the pain. Equipment can also maximize independence and improve quality of life. For example, a soft neck collar is often used to prevent the forward movement that triggers pain in Lhermitte phenomenon.6
When prescribing pain medication, it is important to understand that neuropathic pain is inadequately relieved or not relieved at all with conventional analgesics, such as NSAIDs, or opioid analgesics (eg, morphine).2,3
Dysesthesias are most frequently treated with medications that are categorized as antiseizure, such as gabapentin and pregabalin. Carbamazepam and phenytoin are used as secondline therapy. Sometimes, anti-anxiety medication (eg, duloxetine hydrochloride and clonazepam or tricyclic antidepressants, including amitriptyline or nortriptyline) can be helpful.7 When treating paroxysmal symptoms such as trigeminal neuralgia, antiseizure medications can be effective. Carbamazepine is often the firstline of treatment. As a secondline, oxcarbazepine, lamotrigine, and/or baclofen may be used. In some cases, a referral to neurosurgery for a procedure to reduce pressure on the trigeminal nerve is required.5,8
It is also important to treat any additional symptoms that the pain may be causing, such as depression or social isolation. Referral for counseling as well as integrative health and wellness services can support the patient through a difficult time.5 —RS
Rachael Stacom, MS, ANP-BC, MSCN
Independence Care System, New York, NY
1. Zagon IS, Mclaughlin PJ. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane, Australia: Codon Publications. 2017.
2. O’Connor AB, Schwid SR, Hermann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
3. Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. 2011;12(15):2355-2368.
4. Osborne TL, Raichle KA, Jensen MP, et al. The reliability and validity of pain interference measures in persons with multiple sclerosis. J Pain Symptom Manage. 2006;32(3):217-229.
5. Sullivan AB, Scheman J, Lopresti A, Prayor-Patterson H. Interdisciplinary treatment of patients with multiple sclerosis and chronic pain. Int J MS Care. 2012;14(4):216-220.
6. MS Australia. Pain and multiple sclerosis (MS). www.msaustralia.org.au/publications/pain-and-multiple-sclerosis-ms. Accessed May 15, 2018.
7. Maloni H; National Multiple Sclerosis Society. Clinical bulletin: pain in multiple sclerosis. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Clinical_Bulletin_Pain-in-MS.pdf. Accessed May 15, 2018.
8. Multiple Sclerosis Association of America (MSAA). (H. Maloni, Ed.) The Motivator Winter/Spring. Retrieved from https://mymsaa.org/publications/motivator/winter-spring13/cover-story/pain. Accessed May 15, 2018.
1. Zagon IS, Mclaughlin PJ. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane, Australia: Codon Publications. 2017.
2. O’Connor AB, Schwid SR, Hermann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
3. Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. 2011;12(15):2355-2368.
4. Osborne TL, Raichle KA, Jensen MP, et al. The reliability and validity of pain interference measures in persons with multiple sclerosis. J Pain Symptom Manage. 2006;32(3):217-229.
5. Sullivan AB, Scheman J, Lopresti A, Prayor-Patterson H. Interdisciplinary treatment of patients with multiple sclerosis and chronic pain. Int J MS Care. 2012;14(4):216-220.
6. MS Australia. Pain and multiple sclerosis (MS). www.msaustralia.org.au/publications/pain-and-multiple-sclerosis-ms. Accessed May 15, 2018.
7. Maloni H; National Multiple Sclerosis Society. Clinical bulletin: pain in multiple sclerosis. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Clinical_Bulletin_Pain-in-MS.pdf. Accessed May 15, 2018.
8. Multiple Sclerosis Association of America (MSAA). (H. Maloni, Ed.) The Motivator Winter/Spring. Retrieved from https://mymsaa.org/publications/motivator/winter-spring13/cover-story/pain. Accessed May 15, 2018.
The ACA and Multiple Sclerosis
Q) How has the Affordable Care Act affected people living with multiple sclerosis—an Americans with Disabilities Act recognized disease?
The Affordable Care Act (ACA) has been a source of controversy since it became law in 2010. Perhaps some of the tension surrounding it stems from misunderstanding; however, it is clear that individual experiences and/or perceptions flavor the ongoing debate. Rather than perpetuate the contention, we’d simply like to outline some of the ways in which patients with multiple sclerosis (MS) have benefited from the ACA—and what we must do to ensure continued quality and affordability of care in the event of changes to the law.
Living with MS in the United States is costly. According to the National Multiple Sclerosis Society, average annual costs—both direct and indirect (ie, lost wages)—are about $69,000. Health care costs account for more than half of this total (about $39,000). Total costs for all people in the US living with MS are estimated at $28 billion per year.1
In 2016, according to the US Census Bureau, almost 13% of Americans lived below the federal poverty level, and 6% of Americans reported “deep poverty”—defined as household income below 50% of the poverty threshold for that year.2 It has been reported that while at least 90% of people living with MS are insured, 70% are struggling to pay for health care. In fact, 30% put off seeking care because of costs; one consequence is delay in filling prescriptions.3
The burden of expense for our MS patients is considerable. Here’s how the ACA has impacted our patients by attempting to minimize the devastating cost.
Guaranteed Health Insurance Coverage for Pre-existing Conditions. When the ACA became law in March 2010, there were three main goals: making affordable health insurance available to more people, expanding the Medicaid program to cover all adults with income below 138% of the federal poverty level, and supporting innovative medical care delivery methods to lower the cost of health care.4
Following the ACA’s full implementation in 2014, private health insurance companies were prevented from refusing coverage to those with pre-existing conditions, such as MS. This was a game changer, since patients, regardless of their MS diagnosis, were now guaranteed individual insurance. Furthermore, they could not be charged increased premiums based on their prior medical history.5
Preventive Services Covered Without Cost-sharing. Under the ACA, health plans generally must provide preventive services, such as those rated A or B by the US Preventive Services Task Force. This includes routine immunizations for both adults and children, which represents a cost savings to patients living with MS. Another advantage is that women, including those living with MS, have access to sexually transmitted infection screenings, breastfeeding support and supplies, domestic violence screening, and contraceptives.6
Improved Coverage Through Medicare. The ACA mandated improvement in coverage with Medicare Part D benefits. In addition to the preventive care benefits noted above, which apply to Medicare recipients as well, the ACA reduced federal payments to Medicare Advantage plans over time and provided bonus payments to plans with high quality ratings.7
Further changes in Medicare spending included the creation of a 15-person, by-appointment board (known as the Independent Payment Advisory Board) tasked with identifying ways to “modify benefits, eligibility, premiums, or taxes,” which will hopefully continue to optimize the cost of care for patients living with MS and utilizing Medicare.7
Cost Savings With Medicaid Expansion. Medicaid expansion was enacted to keep patients with a costly illness, such as MS, from financial destitution because of their condition. As of January 2018, 32 states and the District of Columbia have seen expansion of their programs.8 In those states, people with a household income below 138% of the poverty level (less than $27,000 for a family of three) can now qualify for Medicaid. States that have not expanded coverage include Idaho, Wyoming, Utah, South Dakota, Nebraska, Kansas, Oklahoma, Texas, Missouri, Wisconsin, Tennessee, Mississippi, Alabama, Georgia, Virginia, North Carolina, South Carolina, and Florida.8 The expansion of Medicaid helps MS patients by shrinking the ever-present gap that still prevents some from qualifying for the additional financial assistance they need due to their chronic illness.
One thing we have learned is that MS patients may not realize they have access to some of these services—particularly preventive care—or they may hesitate to obtain services due to a lack of clarity on whether they are covered. Health care providers can remind patients that they may qualify for “unrealized services,” which could provide value and optimize general preventive care. MS patients with Medicare and Medicaid, for example, may not know that they have access to colorectal cancer screenings via a waived deductible.6
Since last year, there has been vigorous discussion about repealing, replacing, or otherwise amending the ACA. While a political discussion is beyond the bounds of this column, we do need to be aware of how changes to the ACA would affect patients with MS.
Optimizing wellness and prevention and providing access to care to patients with a costly disease, such as MS, is important. In addition to ensuring ongoing access to affordable services, we need to do more to improve mental health access and reduce the cost of needed medications. We also need to close the insurance gap in all 50 states. Continued dialogue will be necessary to help government leaders understand the cost impact of MS (and other diseases), in order to keep our country moving in a positive direction that optimizes wellness and health care reform. —ALD
Amy L. Dix, MPAS, PA-C, MSCS
Department of Neurology at Kansas City Multiple Sclerosis Center in Overland Park, Kansas
1. National Multiple Sclerosis Society. Health Policy Fact Sheet #2: Financial burdens for people with MS, their families, and society. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Health-Policy-Fact-Sheet-2-Costs.pdf. Accessed February 8, 2018.
2. Center for Poverty Research, University of California—Davis. What is the current poverty rate in the United States? https://poverty.ucdavis.edu/faq/what-current-poverty-rate-united-states. Accessed February 8, 2018.
3. Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. 2007;13(4):534-546.
4. Centers for Medicare & Medicaid Services. Affordable Care Act (ACA). HealthCare.gov. www.healthcare.gov/glossary/affordable-care-act. Accessed February 8, 2018.
5. US Department of Health and Human Services. About the ACA: pre-existing conditions. www.hhs.gov/healthcare/about-the-aca/pre-existing-conditions/index.html. Accessed February 8, 2018.
6. Tolbert J. The coverage provisions in the Affordable Care Act: an update. Kaiser Family Foundation. www.kff.org/report-section/the-coverage-provisions-in-the-affordable-care-act-an-update-health-insurance-market-reforms. Accessed February 8, 2018.
7. Kaiser Family Foundation. Focus on health reform: summary of key changes to Medicare in 2010 health reform law. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7948-02.pdf. Accessed February 8, 2018.
8. Families USA. A 50-state look at Medicaid expansion. http://familiesusa.org/product/50-state-look-medicaid-expansion. Accessed February 8, 2018.
Q) How has the Affordable Care Act affected people living with multiple sclerosis—an Americans with Disabilities Act recognized disease?
The Affordable Care Act (ACA) has been a source of controversy since it became law in 2010. Perhaps some of the tension surrounding it stems from misunderstanding; however, it is clear that individual experiences and/or perceptions flavor the ongoing debate. Rather than perpetuate the contention, we’d simply like to outline some of the ways in which patients with multiple sclerosis (MS) have benefited from the ACA—and what we must do to ensure continued quality and affordability of care in the event of changes to the law.
Living with MS in the United States is costly. According to the National Multiple Sclerosis Society, average annual costs—both direct and indirect (ie, lost wages)—are about $69,000. Health care costs account for more than half of this total (about $39,000). Total costs for all people in the US living with MS are estimated at $28 billion per year.1
In 2016, according to the US Census Bureau, almost 13% of Americans lived below the federal poverty level, and 6% of Americans reported “deep poverty”—defined as household income below 50% of the poverty threshold for that year.2 It has been reported that while at least 90% of people living with MS are insured, 70% are struggling to pay for health care. In fact, 30% put off seeking care because of costs; one consequence is delay in filling prescriptions.3
The burden of expense for our MS patients is considerable. Here’s how the ACA has impacted our patients by attempting to minimize the devastating cost.
Guaranteed Health Insurance Coverage for Pre-existing Conditions. When the ACA became law in March 2010, there were three main goals: making affordable health insurance available to more people, expanding the Medicaid program to cover all adults with income below 138% of the federal poverty level, and supporting innovative medical care delivery methods to lower the cost of health care.4
Following the ACA’s full implementation in 2014, private health insurance companies were prevented from refusing coverage to those with pre-existing conditions, such as MS. This was a game changer, since patients, regardless of their MS diagnosis, were now guaranteed individual insurance. Furthermore, they could not be charged increased premiums based on their prior medical history.5
Preventive Services Covered Without Cost-sharing. Under the ACA, health plans generally must provide preventive services, such as those rated A or B by the US Preventive Services Task Force. This includes routine immunizations for both adults and children, which represents a cost savings to patients living with MS. Another advantage is that women, including those living with MS, have access to sexually transmitted infection screenings, breastfeeding support and supplies, domestic violence screening, and contraceptives.6
Improved Coverage Through Medicare. The ACA mandated improvement in coverage with Medicare Part D benefits. In addition to the preventive care benefits noted above, which apply to Medicare recipients as well, the ACA reduced federal payments to Medicare Advantage plans over time and provided bonus payments to plans with high quality ratings.7
Further changes in Medicare spending included the creation of a 15-person, by-appointment board (known as the Independent Payment Advisory Board) tasked with identifying ways to “modify benefits, eligibility, premiums, or taxes,” which will hopefully continue to optimize the cost of care for patients living with MS and utilizing Medicare.7
Cost Savings With Medicaid Expansion. Medicaid expansion was enacted to keep patients with a costly illness, such as MS, from financial destitution because of their condition. As of January 2018, 32 states and the District of Columbia have seen expansion of their programs.8 In those states, people with a household income below 138% of the poverty level (less than $27,000 for a family of three) can now qualify for Medicaid. States that have not expanded coverage include Idaho, Wyoming, Utah, South Dakota, Nebraska, Kansas, Oklahoma, Texas, Missouri, Wisconsin, Tennessee, Mississippi, Alabama, Georgia, Virginia, North Carolina, South Carolina, and Florida.8 The expansion of Medicaid helps MS patients by shrinking the ever-present gap that still prevents some from qualifying for the additional financial assistance they need due to their chronic illness.
One thing we have learned is that MS patients may not realize they have access to some of these services—particularly preventive care—or they may hesitate to obtain services due to a lack of clarity on whether they are covered. Health care providers can remind patients that they may qualify for “unrealized services,” which could provide value and optimize general preventive care. MS patients with Medicare and Medicaid, for example, may not know that they have access to colorectal cancer screenings via a waived deductible.6
Since last year, there has been vigorous discussion about repealing, replacing, or otherwise amending the ACA. While a political discussion is beyond the bounds of this column, we do need to be aware of how changes to the ACA would affect patients with MS.
Optimizing wellness and prevention and providing access to care to patients with a costly disease, such as MS, is important. In addition to ensuring ongoing access to affordable services, we need to do more to improve mental health access and reduce the cost of needed medications. We also need to close the insurance gap in all 50 states. Continued dialogue will be necessary to help government leaders understand the cost impact of MS (and other diseases), in order to keep our country moving in a positive direction that optimizes wellness and health care reform. —ALD
Amy L. Dix, MPAS, PA-C, MSCS
Department of Neurology at Kansas City Multiple Sclerosis Center in Overland Park, Kansas
Q) How has the Affordable Care Act affected people living with multiple sclerosis—an Americans with Disabilities Act recognized disease?
The Affordable Care Act (ACA) has been a source of controversy since it became law in 2010. Perhaps some of the tension surrounding it stems from misunderstanding; however, it is clear that individual experiences and/or perceptions flavor the ongoing debate. Rather than perpetuate the contention, we’d simply like to outline some of the ways in which patients with multiple sclerosis (MS) have benefited from the ACA—and what we must do to ensure continued quality and affordability of care in the event of changes to the law.
Living with MS in the United States is costly. According to the National Multiple Sclerosis Society, average annual costs—both direct and indirect (ie, lost wages)—are about $69,000. Health care costs account for more than half of this total (about $39,000). Total costs for all people in the US living with MS are estimated at $28 billion per year.1
In 2016, according to the US Census Bureau, almost 13% of Americans lived below the federal poverty level, and 6% of Americans reported “deep poverty”—defined as household income below 50% of the poverty threshold for that year.2 It has been reported that while at least 90% of people living with MS are insured, 70% are struggling to pay for health care. In fact, 30% put off seeking care because of costs; one consequence is delay in filling prescriptions.3
The burden of expense for our MS patients is considerable. Here’s how the ACA has impacted our patients by attempting to minimize the devastating cost.
Guaranteed Health Insurance Coverage for Pre-existing Conditions. When the ACA became law in March 2010, there were three main goals: making affordable health insurance available to more people, expanding the Medicaid program to cover all adults with income below 138% of the federal poverty level, and supporting innovative medical care delivery methods to lower the cost of health care.4
Following the ACA’s full implementation in 2014, private health insurance companies were prevented from refusing coverage to those with pre-existing conditions, such as MS. This was a game changer, since patients, regardless of their MS diagnosis, were now guaranteed individual insurance. Furthermore, they could not be charged increased premiums based on their prior medical history.5
Preventive Services Covered Without Cost-sharing. Under the ACA, health plans generally must provide preventive services, such as those rated A or B by the US Preventive Services Task Force. This includes routine immunizations for both adults and children, which represents a cost savings to patients living with MS. Another advantage is that women, including those living with MS, have access to sexually transmitted infection screenings, breastfeeding support and supplies, domestic violence screening, and contraceptives.6
Improved Coverage Through Medicare. The ACA mandated improvement in coverage with Medicare Part D benefits. In addition to the preventive care benefits noted above, which apply to Medicare recipients as well, the ACA reduced federal payments to Medicare Advantage plans over time and provided bonus payments to plans with high quality ratings.7
Further changes in Medicare spending included the creation of a 15-person, by-appointment board (known as the Independent Payment Advisory Board) tasked with identifying ways to “modify benefits, eligibility, premiums, or taxes,” which will hopefully continue to optimize the cost of care for patients living with MS and utilizing Medicare.7
Cost Savings With Medicaid Expansion. Medicaid expansion was enacted to keep patients with a costly illness, such as MS, from financial destitution because of their condition. As of January 2018, 32 states and the District of Columbia have seen expansion of their programs.8 In those states, people with a household income below 138% of the poverty level (less than $27,000 for a family of three) can now qualify for Medicaid. States that have not expanded coverage include Idaho, Wyoming, Utah, South Dakota, Nebraska, Kansas, Oklahoma, Texas, Missouri, Wisconsin, Tennessee, Mississippi, Alabama, Georgia, Virginia, North Carolina, South Carolina, and Florida.8 The expansion of Medicaid helps MS patients by shrinking the ever-present gap that still prevents some from qualifying for the additional financial assistance they need due to their chronic illness.
One thing we have learned is that MS patients may not realize they have access to some of these services—particularly preventive care—or they may hesitate to obtain services due to a lack of clarity on whether they are covered. Health care providers can remind patients that they may qualify for “unrealized services,” which could provide value and optimize general preventive care. MS patients with Medicare and Medicaid, for example, may not know that they have access to colorectal cancer screenings via a waived deductible.6
Since last year, there has been vigorous discussion about repealing, replacing, or otherwise amending the ACA. While a political discussion is beyond the bounds of this column, we do need to be aware of how changes to the ACA would affect patients with MS.
Optimizing wellness and prevention and providing access to care to patients with a costly disease, such as MS, is important. In addition to ensuring ongoing access to affordable services, we need to do more to improve mental health access and reduce the cost of needed medications. We also need to close the insurance gap in all 50 states. Continued dialogue will be necessary to help government leaders understand the cost impact of MS (and other diseases), in order to keep our country moving in a positive direction that optimizes wellness and health care reform. —ALD
Amy L. Dix, MPAS, PA-C, MSCS
Department of Neurology at Kansas City Multiple Sclerosis Center in Overland Park, Kansas
1. National Multiple Sclerosis Society. Health Policy Fact Sheet #2: Financial burdens for people with MS, their families, and society. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Health-Policy-Fact-Sheet-2-Costs.pdf. Accessed February 8, 2018.
2. Center for Poverty Research, University of California—Davis. What is the current poverty rate in the United States? https://poverty.ucdavis.edu/faq/what-current-poverty-rate-united-states. Accessed February 8, 2018.
3. Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. 2007;13(4):534-546.
4. Centers for Medicare & Medicaid Services. Affordable Care Act (ACA). HealthCare.gov. www.healthcare.gov/glossary/affordable-care-act. Accessed February 8, 2018.
5. US Department of Health and Human Services. About the ACA: pre-existing conditions. www.hhs.gov/healthcare/about-the-aca/pre-existing-conditions/index.html. Accessed February 8, 2018.
6. Tolbert J. The coverage provisions in the Affordable Care Act: an update. Kaiser Family Foundation. www.kff.org/report-section/the-coverage-provisions-in-the-affordable-care-act-an-update-health-insurance-market-reforms. Accessed February 8, 2018.
7. Kaiser Family Foundation. Focus on health reform: summary of key changes to Medicare in 2010 health reform law. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7948-02.pdf. Accessed February 8, 2018.
8. Families USA. A 50-state look at Medicaid expansion. http://familiesusa.org/product/50-state-look-medicaid-expansion. Accessed February 8, 2018.
1. National Multiple Sclerosis Society. Health Policy Fact Sheet #2: Financial burdens for people with MS, their families, and society. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Health-Policy-Fact-Sheet-2-Costs.pdf. Accessed February 8, 2018.
2. Center for Poverty Research, University of California—Davis. What is the current poverty rate in the United States? https://poverty.ucdavis.edu/faq/what-current-poverty-rate-united-states. Accessed February 8, 2018.
3. Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. 2007;13(4):534-546.
4. Centers for Medicare & Medicaid Services. Affordable Care Act (ACA). HealthCare.gov. www.healthcare.gov/glossary/affordable-care-act. Accessed February 8, 2018.
5. US Department of Health and Human Services. About the ACA: pre-existing conditions. www.hhs.gov/healthcare/about-the-aca/pre-existing-conditions/index.html. Accessed February 8, 2018.
6. Tolbert J. The coverage provisions in the Affordable Care Act: an update. Kaiser Family Foundation. www.kff.org/report-section/the-coverage-provisions-in-the-affordable-care-act-an-update-health-insurance-market-reforms. Accessed February 8, 2018.
7. Kaiser Family Foundation. Focus on health reform: summary of key changes to Medicare in 2010 health reform law. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7948-02.pdf. Accessed February 8, 2018.
8. Families USA. A 50-state look at Medicaid expansion. http://familiesusa.org/product/50-state-look-medicaid-expansion. Accessed February 8, 2018.
Monitoring for Infection in MS Patients
Q) How do you monitor for infection in patients with multiple sclerosis who take disease-modifying therapies?
The answer to this question is “it depends”—on several factors, including current and previous use of disease-modifying therapies (DMTs), concomitant medications, comorbidities, vaccination history, presence of John Cunningham virus (JCV) antibodies (in the case of natalizumab use), and prior or current use of immunosuppressive therapies.
There are many FDA-approved DMTs for multiple sclerosis (MS). Each has a different rate of infection occurring in clinical trials and varying requirements and/or recommendations for safety monitoring. The package inserts for each DMT offer some guidance for clinicians.
Injectable therapies. For two interferon therapies—interferon ß-1b SC and interferon ß-1a—it is recommended to order a complete blood count (CBC), blood chemistry, and liver function tests (LFTs) at baseline, then again at one, three, and six months, and then at clinician discretion thereafter.1,2 For peginterferon ß-1a, ordering a CBC, basic chemistry, and LFTs, at the clinician’s discretion, is advised.3 The package insert for interferon ß-1a IM does not offer specific recommendations for routine safety monitoring.4
The package insert for glatiramer acetate offers no recommendations for routine safety monitoring.5
In patients for whom two or more DMTs have failed to work, the monoclonal antibody daclizumab may be indicated. Compared to placebo and active comparator, this drug was associated with a higher risk for infection in clinical trials. The most commonly observed types were upper respiratory, urinary tract, and viral infections. There are no recommendations for CBC monitoring with daclizumab, but monthly LFTs are required due to increased risk for hepatic injury.6
Oral DMTs. Patients taking fingolimod, teriflunomide, and dimethyl fumarate have increased risk for infection; as a result, there are more safety monitoring recommendations for these medications.7-9
Prior to starting therapy with fingolimod, baseline CBC, blood chemistries, and varicella antibody testing should be done. During therapy, routine CBC testing and LFTs are advised at the clinician’s discretion or if the patient exhibits signs and symptoms of infection (see Table). In clinical trials, fingolimod use was interrupted if the lymphocyte count was sustained at < 200. In rare cases, progressive multifocal leukoencephalopathy (PML) has occurred—so the patient’s age, JCV antibody status, prior use of immunosuppressant therapy, and length of fingolimod treatment should be taken into consideration.7
Patients starting teriflunomide should have baseline LFTs and CBC and tuberculosis (TB) testing (either skin or serum), with subsequent monthly LFTs for the first six months on treatment. Some patients may experience neutropenia, thrombocytopenia, and lymphopenia. As a result, patients may have an increased risk for infection. Safety monitoring is at the clinician’s discretion.8
For patients initiating dimethyl fumarate, a baseline CBC is recommended, to be repeated every six to 12 months thereafter, and/or as clinically indicated. Since lymphopenia may occur, consider interruption of dimethyl fumarate in patients with lymphocyte counts < 0.5 persisting for more than six months. Rare cases of PML have also occurred; at the first suggestive sign or symptom, dimethyl fumarate should be withheld and appropriate diagnostic testing should be completed.9
Infusion therapies. There are four infusion therapies available for MS treatment. Mitoxantrone, though not commonly used, is still available for relapsing and secondary progressive forms of MS. Common infections seen in clinical trials include upper respiratory, urinary tract, and sinus infections. A CBC, including platelets, should be obtained prior to each course of mitoxantrone and again if signs and symptoms of infection develop.10
Natalizumab is an integrin receptor antagonist administered in monthly IV infusions. Patients receiving natalizumab may have increased risk for urinary tract infections, lower respiratory infections, gastroenteritis, vaginitis, and herpes infections. These risks should be monitored at the clinician’s discretion. There have been several cases of PML associated with natalizumab; risk factors include duration of therapy, prior use of immunosuppressants, and presence of JCV antibodies.11
Alemtuzumab is a CD52-directed monoclonal antibody indicated in patients with relapsing forms of MS who have had an inadequate response to at least two DMTs. In clinical trials, subjects had a higher risk for nasopharyngitis, urinary tract infections, upper respiratory infections, sinusitis, herpetic infections, influenza, and bronchitis. Due to the increased risk for infection and secondary autoimmunities, patients are required to have monthly CBC testing, LFTs, and urinalysis for up to 48 months after their last infusion.12
Lastly, ocrelizumab is a CD20-directed cytolytic antibody for the treatment of relapsing and progressive forms of MS. In clinical trials, there was a higher incidence of upper and lower respiratory infections, skin infections, and herpes-related infections. Prior to initiating ocrelizumab, hepatitis B virus screening should be completed. There are no specific recommendations for routine monitoring during therapy, although providers should monitor patients clinically for any signs and symptoms of infection.13
A word of caution: The common signs and symptoms of infection are listed in the Table. If these symptoms are present in your patient, consider ordering diagnostic testing to evaluate for infection.
Symptoms of PML include progressive unilateral weakness, clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. At the first sign or symptom suggestive of PML, the DMT should be discontinued and diagnostic testing performed.
Providers may contact the manufacturer directly for further guidance on DMT surveillance and treatment protocols. —CK
Christen Kutz, PhD, PA-C
Colorado Springs Neurological Associates
1. Betaseron (interferon [b]-1b) [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 1993.
2. Rebif (interferon [b]-1a) [package insert]. Rockland, MA: EMD Serono; revised 2015.
3. Plegridy (peginterferon [b]-1a) [package insert]. Cambridge, MA: Biogen Idec; 2013.
4. Avonex (interferon [b] -1a) [package insert]. Cambridge, MA: Biogen Inc.; 1996.
5. Copaxone (glatiramer acetate) [package insert]. Overland Park, KS: Teva Neuroscience; revised 2016.
6. Zinbryta (daclizumab) [package insert]. Cambridge, MA: Biogen Idec; 2016.
7. Gilenya (fingolimod) [package insert]. Hanover, NJ: Novartis; revised 2016.
8. Aubagio (teriflunomide) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2016.
9. Tecfidera (dimethyl fumarate) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
10. Novantrone (mitoxantrone) [package insert]. Rockland, MA: EMD Serono; 2008.
11. Tysabri (natalizumab) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
12. Lemtrada (alemtuzumab) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2017.
13. Ocrevus (ocrelizumab) [package insert]. San Francisco, CA: Genentech; 2017.
Q) How do you monitor for infection in patients with multiple sclerosis who take disease-modifying therapies?
The answer to this question is “it depends”—on several factors, including current and previous use of disease-modifying therapies (DMTs), concomitant medications, comorbidities, vaccination history, presence of John Cunningham virus (JCV) antibodies (in the case of natalizumab use), and prior or current use of immunosuppressive therapies.
There are many FDA-approved DMTs for multiple sclerosis (MS). Each has a different rate of infection occurring in clinical trials and varying requirements and/or recommendations for safety monitoring. The package inserts for each DMT offer some guidance for clinicians.
Injectable therapies. For two interferon therapies—interferon ß-1b SC and interferon ß-1a—it is recommended to order a complete blood count (CBC), blood chemistry, and liver function tests (LFTs) at baseline, then again at one, three, and six months, and then at clinician discretion thereafter.1,2 For peginterferon ß-1a, ordering a CBC, basic chemistry, and LFTs, at the clinician’s discretion, is advised.3 The package insert for interferon ß-1a IM does not offer specific recommendations for routine safety monitoring.4
The package insert for glatiramer acetate offers no recommendations for routine safety monitoring.5
In patients for whom two or more DMTs have failed to work, the monoclonal antibody daclizumab may be indicated. Compared to placebo and active comparator, this drug was associated with a higher risk for infection in clinical trials. The most commonly observed types were upper respiratory, urinary tract, and viral infections. There are no recommendations for CBC monitoring with daclizumab, but monthly LFTs are required due to increased risk for hepatic injury.6
Oral DMTs. Patients taking fingolimod, teriflunomide, and dimethyl fumarate have increased risk for infection; as a result, there are more safety monitoring recommendations for these medications.7-9
Prior to starting therapy with fingolimod, baseline CBC, blood chemistries, and varicella antibody testing should be done. During therapy, routine CBC testing and LFTs are advised at the clinician’s discretion or if the patient exhibits signs and symptoms of infection (see Table). In clinical trials, fingolimod use was interrupted if the lymphocyte count was sustained at < 200. In rare cases, progressive multifocal leukoencephalopathy (PML) has occurred—so the patient’s age, JCV antibody status, prior use of immunosuppressant therapy, and length of fingolimod treatment should be taken into consideration.7
Patients starting teriflunomide should have baseline LFTs and CBC and tuberculosis (TB) testing (either skin or serum), with subsequent monthly LFTs for the first six months on treatment. Some patients may experience neutropenia, thrombocytopenia, and lymphopenia. As a result, patients may have an increased risk for infection. Safety monitoring is at the clinician’s discretion.8
For patients initiating dimethyl fumarate, a baseline CBC is recommended, to be repeated every six to 12 months thereafter, and/or as clinically indicated. Since lymphopenia may occur, consider interruption of dimethyl fumarate in patients with lymphocyte counts < 0.5 persisting for more than six months. Rare cases of PML have also occurred; at the first suggestive sign or symptom, dimethyl fumarate should be withheld and appropriate diagnostic testing should be completed.9
Infusion therapies. There are four infusion therapies available for MS treatment. Mitoxantrone, though not commonly used, is still available for relapsing and secondary progressive forms of MS. Common infections seen in clinical trials include upper respiratory, urinary tract, and sinus infections. A CBC, including platelets, should be obtained prior to each course of mitoxantrone and again if signs and symptoms of infection develop.10
Natalizumab is an integrin receptor antagonist administered in monthly IV infusions. Patients receiving natalizumab may have increased risk for urinary tract infections, lower respiratory infections, gastroenteritis, vaginitis, and herpes infections. These risks should be monitored at the clinician’s discretion. There have been several cases of PML associated with natalizumab; risk factors include duration of therapy, prior use of immunosuppressants, and presence of JCV antibodies.11
Alemtuzumab is a CD52-directed monoclonal antibody indicated in patients with relapsing forms of MS who have had an inadequate response to at least two DMTs. In clinical trials, subjects had a higher risk for nasopharyngitis, urinary tract infections, upper respiratory infections, sinusitis, herpetic infections, influenza, and bronchitis. Due to the increased risk for infection and secondary autoimmunities, patients are required to have monthly CBC testing, LFTs, and urinalysis for up to 48 months after their last infusion.12
Lastly, ocrelizumab is a CD20-directed cytolytic antibody for the treatment of relapsing and progressive forms of MS. In clinical trials, there was a higher incidence of upper and lower respiratory infections, skin infections, and herpes-related infections. Prior to initiating ocrelizumab, hepatitis B virus screening should be completed. There are no specific recommendations for routine monitoring during therapy, although providers should monitor patients clinically for any signs and symptoms of infection.13
A word of caution: The common signs and symptoms of infection are listed in the Table. If these symptoms are present in your patient, consider ordering diagnostic testing to evaluate for infection.
Symptoms of PML include progressive unilateral weakness, clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. At the first sign or symptom suggestive of PML, the DMT should be discontinued and diagnostic testing performed.
Providers may contact the manufacturer directly for further guidance on DMT surveillance and treatment protocols. —CK
Christen Kutz, PhD, PA-C
Colorado Springs Neurological Associates
Q) How do you monitor for infection in patients with multiple sclerosis who take disease-modifying therapies?
The answer to this question is “it depends”—on several factors, including current and previous use of disease-modifying therapies (DMTs), concomitant medications, comorbidities, vaccination history, presence of John Cunningham virus (JCV) antibodies (in the case of natalizumab use), and prior or current use of immunosuppressive therapies.
There are many FDA-approved DMTs for multiple sclerosis (MS). Each has a different rate of infection occurring in clinical trials and varying requirements and/or recommendations for safety monitoring. The package inserts for each DMT offer some guidance for clinicians.
Injectable therapies. For two interferon therapies—interferon ß-1b SC and interferon ß-1a—it is recommended to order a complete blood count (CBC), blood chemistry, and liver function tests (LFTs) at baseline, then again at one, three, and six months, and then at clinician discretion thereafter.1,2 For peginterferon ß-1a, ordering a CBC, basic chemistry, and LFTs, at the clinician’s discretion, is advised.3 The package insert for interferon ß-1a IM does not offer specific recommendations for routine safety monitoring.4
The package insert for glatiramer acetate offers no recommendations for routine safety monitoring.5
In patients for whom two or more DMTs have failed to work, the monoclonal antibody daclizumab may be indicated. Compared to placebo and active comparator, this drug was associated with a higher risk for infection in clinical trials. The most commonly observed types were upper respiratory, urinary tract, and viral infections. There are no recommendations for CBC monitoring with daclizumab, but monthly LFTs are required due to increased risk for hepatic injury.6
Oral DMTs. Patients taking fingolimod, teriflunomide, and dimethyl fumarate have increased risk for infection; as a result, there are more safety monitoring recommendations for these medications.7-9
Prior to starting therapy with fingolimod, baseline CBC, blood chemistries, and varicella antibody testing should be done. During therapy, routine CBC testing and LFTs are advised at the clinician’s discretion or if the patient exhibits signs and symptoms of infection (see Table). In clinical trials, fingolimod use was interrupted if the lymphocyte count was sustained at < 200. In rare cases, progressive multifocal leukoencephalopathy (PML) has occurred—so the patient’s age, JCV antibody status, prior use of immunosuppressant therapy, and length of fingolimod treatment should be taken into consideration.7
Patients starting teriflunomide should have baseline LFTs and CBC and tuberculosis (TB) testing (either skin or serum), with subsequent monthly LFTs for the first six months on treatment. Some patients may experience neutropenia, thrombocytopenia, and lymphopenia. As a result, patients may have an increased risk for infection. Safety monitoring is at the clinician’s discretion.8
For patients initiating dimethyl fumarate, a baseline CBC is recommended, to be repeated every six to 12 months thereafter, and/or as clinically indicated. Since lymphopenia may occur, consider interruption of dimethyl fumarate in patients with lymphocyte counts < 0.5 persisting for more than six months. Rare cases of PML have also occurred; at the first suggestive sign or symptom, dimethyl fumarate should be withheld and appropriate diagnostic testing should be completed.9
Infusion therapies. There are four infusion therapies available for MS treatment. Mitoxantrone, though not commonly used, is still available for relapsing and secondary progressive forms of MS. Common infections seen in clinical trials include upper respiratory, urinary tract, and sinus infections. A CBC, including platelets, should be obtained prior to each course of mitoxantrone and again if signs and symptoms of infection develop.10
Natalizumab is an integrin receptor antagonist administered in monthly IV infusions. Patients receiving natalizumab may have increased risk for urinary tract infections, lower respiratory infections, gastroenteritis, vaginitis, and herpes infections. These risks should be monitored at the clinician’s discretion. There have been several cases of PML associated with natalizumab; risk factors include duration of therapy, prior use of immunosuppressants, and presence of JCV antibodies.11
Alemtuzumab is a CD52-directed monoclonal antibody indicated in patients with relapsing forms of MS who have had an inadequate response to at least two DMTs. In clinical trials, subjects had a higher risk for nasopharyngitis, urinary tract infections, upper respiratory infections, sinusitis, herpetic infections, influenza, and bronchitis. Due to the increased risk for infection and secondary autoimmunities, patients are required to have monthly CBC testing, LFTs, and urinalysis for up to 48 months after their last infusion.12
Lastly, ocrelizumab is a CD20-directed cytolytic antibody for the treatment of relapsing and progressive forms of MS. In clinical trials, there was a higher incidence of upper and lower respiratory infections, skin infections, and herpes-related infections. Prior to initiating ocrelizumab, hepatitis B virus screening should be completed. There are no specific recommendations for routine monitoring during therapy, although providers should monitor patients clinically for any signs and symptoms of infection.13
A word of caution: The common signs and symptoms of infection are listed in the Table. If these symptoms are present in your patient, consider ordering diagnostic testing to evaluate for infection.
Symptoms of PML include progressive unilateral weakness, clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. At the first sign or symptom suggestive of PML, the DMT should be discontinued and diagnostic testing performed.
Providers may contact the manufacturer directly for further guidance on DMT surveillance and treatment protocols. —CK
Christen Kutz, PhD, PA-C
Colorado Springs Neurological Associates
1. Betaseron (interferon [b]-1b) [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 1993.
2. Rebif (interferon [b]-1a) [package insert]. Rockland, MA: EMD Serono; revised 2015.
3. Plegridy (peginterferon [b]-1a) [package insert]. Cambridge, MA: Biogen Idec; 2013.
4. Avonex (interferon [b] -1a) [package insert]. Cambridge, MA: Biogen Inc.; 1996.
5. Copaxone (glatiramer acetate) [package insert]. Overland Park, KS: Teva Neuroscience; revised 2016.
6. Zinbryta (daclizumab) [package insert]. Cambridge, MA: Biogen Idec; 2016.
7. Gilenya (fingolimod) [package insert]. Hanover, NJ: Novartis; revised 2016.
8. Aubagio (teriflunomide) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2016.
9. Tecfidera (dimethyl fumarate) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
10. Novantrone (mitoxantrone) [package insert]. Rockland, MA: EMD Serono; 2008.
11. Tysabri (natalizumab) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
12. Lemtrada (alemtuzumab) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2017.
13. Ocrevus (ocrelizumab) [package insert]. San Francisco, CA: Genentech; 2017.
1. Betaseron (interferon [b]-1b) [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 1993.
2. Rebif (interferon [b]-1a) [package insert]. Rockland, MA: EMD Serono; revised 2015.
3. Plegridy (peginterferon [b]-1a) [package insert]. Cambridge, MA: Biogen Idec; 2013.
4. Avonex (interferon [b] -1a) [package insert]. Cambridge, MA: Biogen Inc.; 1996.
5. Copaxone (glatiramer acetate) [package insert]. Overland Park, KS: Teva Neuroscience; revised 2016.
6. Zinbryta (daclizumab) [package insert]. Cambridge, MA: Biogen Idec; 2016.
7. Gilenya (fingolimod) [package insert]. Hanover, NJ: Novartis; revised 2016.
8. Aubagio (teriflunomide) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2016.
9. Tecfidera (dimethyl fumarate) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
10. Novantrone (mitoxantrone) [package insert]. Rockland, MA: EMD Serono; 2008.
11. Tysabri (natalizumab) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
12. Lemtrada (alemtuzumab) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2017.
13. Ocrevus (ocrelizumab) [package insert]. San Francisco, CA: Genentech; 2017.
Diagnosing & Treating Neuromyelitis Optica Spectrum Disorder
Q) How do you know if a neurologic symptom is due to a relapse of neuromyelitis optica spectrum disorder? And how should a confirmed relapse be treated?
Neuromyelitis optica spectrum disorder (NMOSD) is a severe, relapsing autoimmune disease of the central nervous system (CNS) that targets the optic nerves and spinal cord, leading to blindness and paralysis.1,2 Whereas multiple sclerosis (MS) is characterized by demyelination, NMOSD is associated with astrocytic damage and tissue necrosis.3 Because longitudinally extensive inflammatory lesions are typical with NMOSD, permanent CNS damage is common with each relapse.4
Health care providers first need to determine whether a patient with NMOSD who presents with new or worsening symptoms is having a relapse. A relapse is caused by a breach of the blood-brain barrier by the peripheral immune system, which leads to inflammation and damage to the CNS.5 This causes neurologic symptoms that depend on the anatomic location. Once damage has occurred, symptoms may result either from a new relapse in the same location as a previous inflammatory event or from a pseudorelapse.6
Pseudorelapses are triggered by a systemic metabolic imbalance; they exacerbate symptoms from previous CNS damage. Differentiating between a true relapse and a pseudorelapse can be a diagnostic challenge for even the most seasoned of health care providers. Kessler et al retrospectively examined which clinical factors can distinguish relapses from pseudorelapses.6 Their findings suggest that while clinical examination alone may be effective in events involving vision loss, MRI may be necessary when signs and symptoms are attributable to a spinal cord lesion.
In fact, they found that the degree of clinical worsening in patients with spinal cord symptoms caused by a pseudorelapse was similar to that of a true relapse. The most common causes of pseudorelapse included infection, dysautonomia, metabolic abnormalities, and changes to medication regimens. Interestingly, the presence of infection did not rule out a relapse, as patients experiencing relapses were equally likely as those with pseudorelapse to have a urinary tract infection. The authors concluded, based on their data, that an MRI is warranted to verify a relapse in patients who experience worsening of symptoms localized to the spinal cord but is not necessary to rule out a pseudorelapse of optic neu
In contrast to MS, a progressive phase is not believed to be associated with NMOSD.7 Instead, accrual of disability occurs with each relapse. The majority of patients with NMOSD do not return to baseline following an untreated relapse, making it especially important that patients receive adequate acute treatment to mitigate the damage.8
Currently, there are no medications approved by the FDA for the acute or preventive treatment of NMOSD. However, off-label use of immunotherapies, including rituximab, mycophenolate mofetil, azathioprine, prednisone, methotrexate, tocilizumab, and mitoxantrone, have been studied for relapse prevention.2 In addition, there are three ongoing phase III trials investigating eculizumab (C5 complement inhibitor), inebilizumab (CD19 monoclonal antibody), and SA237 (IL6R blocker); results from these studies could potentially widen the landscape of immunotherapy use in NMOSD.2
Less investigation into appropriate acute treatment of new relapses has been conducted, however, leaving clinicians and patients uncertain about how to manage a new inflammatory event. Traditionally, firstline treatment for acute NMOSD relapses has been the same as for MS relapses—high-dose methylprednisolone. However, due to the severity of NMOSD relapses and the relative lack of response to steroids alone, methylprednisolone is commonly followed by plasma exchange (PLEX).2
Most data to guide clinical decision-making suggest that patients with NMOSD relapses recover better when PLEX is added to steroid treatment. Abboud et al found that 65% of patients who received both PLEX and methylprednisolone recovered to their prerelapse baseline, compared to 35% of those who received methylprednisolone alone.9 These findings were supported by a larger retrospective investigation by Kleiter et al, which found improved recovery with treatment escalation in their cohort.8 These data support the recommendation to use PLEX as an adjunct therapy in acute relapses—particularly in relapses with severe presentations.
Because diagnosis and treatment of relapses involve many factors, ranging from accrual of disability, long-term immunotherapy decisions, and medical costs, diligence in provider decision-making is essential when caring for patients with NMOSD. -MAM
Maureen A. Mealy, BSN, MSCN
Neuromyelitis Optica Research Program Manager, Senior Research Nurse of the Transverse Myelitis & Multiple Sclerosis Centers, PhD candidate at Johns Hopkins School of Nursing in Baltimore
1. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107-1114.
2. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
3. Popescu BF, Lucchinetti CF. Immunopathology: autoimmune glial diseases and differentiation from multiple sclerosis. Handb Clin Neurol. 2016;133:95-106.
4. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14.
5. Orman G, Wang KY, Pekcevik Y, et al. Enhancing brain lesions during acute optic neuritis and/or longitudinally extensive transverse myelitis may portend a higher relapse rate in neuromyelitis optica spectrum disorders. Am J Neuroradiol. 2017;38(5):949-953.
6. Kessler RA, Mealy MA, Levy M. Early indicators of relapses vs pseudorelapses in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e269.
7. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603-605.
8. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216.
9. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185-192.
Q) How do you know if a neurologic symptom is due to a relapse of neuromyelitis optica spectrum disorder? And how should a confirmed relapse be treated?
Neuromyelitis optica spectrum disorder (NMOSD) is a severe, relapsing autoimmune disease of the central nervous system (CNS) that targets the optic nerves and spinal cord, leading to blindness and paralysis.1,2 Whereas multiple sclerosis (MS) is characterized by demyelination, NMOSD is associated with astrocytic damage and tissue necrosis.3 Because longitudinally extensive inflammatory lesions are typical with NMOSD, permanent CNS damage is common with each relapse.4
Health care providers first need to determine whether a patient with NMOSD who presents with new or worsening symptoms is having a relapse. A relapse is caused by a breach of the blood-brain barrier by the peripheral immune system, which leads to inflammation and damage to the CNS.5 This causes neurologic symptoms that depend on the anatomic location. Once damage has occurred, symptoms may result either from a new relapse in the same location as a previous inflammatory event or from a pseudorelapse.6
Pseudorelapses are triggered by a systemic metabolic imbalance; they exacerbate symptoms from previous CNS damage. Differentiating between a true relapse and a pseudorelapse can be a diagnostic challenge for even the most seasoned of health care providers. Kessler et al retrospectively examined which clinical factors can distinguish relapses from pseudorelapses.6 Their findings suggest that while clinical examination alone may be effective in events involving vision loss, MRI may be necessary when signs and symptoms are attributable to a spinal cord lesion.
In fact, they found that the degree of clinical worsening in patients with spinal cord symptoms caused by a pseudorelapse was similar to that of a true relapse. The most common causes of pseudorelapse included infection, dysautonomia, metabolic abnormalities, and changes to medication regimens. Interestingly, the presence of infection did not rule out a relapse, as patients experiencing relapses were equally likely as those with pseudorelapse to have a urinary tract infection. The authors concluded, based on their data, that an MRI is warranted to verify a relapse in patients who experience worsening of symptoms localized to the spinal cord but is not necessary to rule out a pseudorelapse of optic neu
In contrast to MS, a progressive phase is not believed to be associated with NMOSD.7 Instead, accrual of disability occurs with each relapse. The majority of patients with NMOSD do not return to baseline following an untreated relapse, making it especially important that patients receive adequate acute treatment to mitigate the damage.8
Currently, there are no medications approved by the FDA for the acute or preventive treatment of NMOSD. However, off-label use of immunotherapies, including rituximab, mycophenolate mofetil, azathioprine, prednisone, methotrexate, tocilizumab, and mitoxantrone, have been studied for relapse prevention.2 In addition, there are three ongoing phase III trials investigating eculizumab (C5 complement inhibitor), inebilizumab (CD19 monoclonal antibody), and SA237 (IL6R blocker); results from these studies could potentially widen the landscape of immunotherapy use in NMOSD.2
Less investigation into appropriate acute treatment of new relapses has been conducted, however, leaving clinicians and patients uncertain about how to manage a new inflammatory event. Traditionally, firstline treatment for acute NMOSD relapses has been the same as for MS relapses—high-dose methylprednisolone. However, due to the severity of NMOSD relapses and the relative lack of response to steroids alone, methylprednisolone is commonly followed by plasma exchange (PLEX).2
Most data to guide clinical decision-making suggest that patients with NMOSD relapses recover better when PLEX is added to steroid treatment. Abboud et al found that 65% of patients who received both PLEX and methylprednisolone recovered to their prerelapse baseline, compared to 35% of those who received methylprednisolone alone.9 These findings were supported by a larger retrospective investigation by Kleiter et al, which found improved recovery with treatment escalation in their cohort.8 These data support the recommendation to use PLEX as an adjunct therapy in acute relapses—particularly in relapses with severe presentations.
Because diagnosis and treatment of relapses involve many factors, ranging from accrual of disability, long-term immunotherapy decisions, and medical costs, diligence in provider decision-making is essential when caring for patients with NMOSD. -MAM
Maureen A. Mealy, BSN, MSCN
Neuromyelitis Optica Research Program Manager, Senior Research Nurse of the Transverse Myelitis & Multiple Sclerosis Centers, PhD candidate at Johns Hopkins School of Nursing in Baltimore
Q) How do you know if a neurologic symptom is due to a relapse of neuromyelitis optica spectrum disorder? And how should a confirmed relapse be treated?
Neuromyelitis optica spectrum disorder (NMOSD) is a severe, relapsing autoimmune disease of the central nervous system (CNS) that targets the optic nerves and spinal cord, leading to blindness and paralysis.1,2 Whereas multiple sclerosis (MS) is characterized by demyelination, NMOSD is associated with astrocytic damage and tissue necrosis.3 Because longitudinally extensive inflammatory lesions are typical with NMOSD, permanent CNS damage is common with each relapse.4
Health care providers first need to determine whether a patient with NMOSD who presents with new or worsening symptoms is having a relapse. A relapse is caused by a breach of the blood-brain barrier by the peripheral immune system, which leads to inflammation and damage to the CNS.5 This causes neurologic symptoms that depend on the anatomic location. Once damage has occurred, symptoms may result either from a new relapse in the same location as a previous inflammatory event or from a pseudorelapse.6
Pseudorelapses are triggered by a systemic metabolic imbalance; they exacerbate symptoms from previous CNS damage. Differentiating between a true relapse and a pseudorelapse can be a diagnostic challenge for even the most seasoned of health care providers. Kessler et al retrospectively examined which clinical factors can distinguish relapses from pseudorelapses.6 Their findings suggest that while clinical examination alone may be effective in events involving vision loss, MRI may be necessary when signs and symptoms are attributable to a spinal cord lesion.
In fact, they found that the degree of clinical worsening in patients with spinal cord symptoms caused by a pseudorelapse was similar to that of a true relapse. The most common causes of pseudorelapse included infection, dysautonomia, metabolic abnormalities, and changes to medication regimens. Interestingly, the presence of infection did not rule out a relapse, as patients experiencing relapses were equally likely as those with pseudorelapse to have a urinary tract infection. The authors concluded, based on their data, that an MRI is warranted to verify a relapse in patients who experience worsening of symptoms localized to the spinal cord but is not necessary to rule out a pseudorelapse of optic neu
In contrast to MS, a progressive phase is not believed to be associated with NMOSD.7 Instead, accrual of disability occurs with each relapse. The majority of patients with NMOSD do not return to baseline following an untreated relapse, making it especially important that patients receive adequate acute treatment to mitigate the damage.8
Currently, there are no medications approved by the FDA for the acute or preventive treatment of NMOSD. However, off-label use of immunotherapies, including rituximab, mycophenolate mofetil, azathioprine, prednisone, methotrexate, tocilizumab, and mitoxantrone, have been studied for relapse prevention.2 In addition, there are three ongoing phase III trials investigating eculizumab (C5 complement inhibitor), inebilizumab (CD19 monoclonal antibody), and SA237 (IL6R blocker); results from these studies could potentially widen the landscape of immunotherapy use in NMOSD.2
Less investigation into appropriate acute treatment of new relapses has been conducted, however, leaving clinicians and patients uncertain about how to manage a new inflammatory event. Traditionally, firstline treatment for acute NMOSD relapses has been the same as for MS relapses—high-dose methylprednisolone. However, due to the severity of NMOSD relapses and the relative lack of response to steroids alone, methylprednisolone is commonly followed by plasma exchange (PLEX).2
Most data to guide clinical decision-making suggest that patients with NMOSD relapses recover better when PLEX is added to steroid treatment. Abboud et al found that 65% of patients who received both PLEX and methylprednisolone recovered to their prerelapse baseline, compared to 35% of those who received methylprednisolone alone.9 These findings were supported by a larger retrospective investigation by Kleiter et al, which found improved recovery with treatment escalation in their cohort.8 These data support the recommendation to use PLEX as an adjunct therapy in acute relapses—particularly in relapses with severe presentations.
Because diagnosis and treatment of relapses involve many factors, ranging from accrual of disability, long-term immunotherapy decisions, and medical costs, diligence in provider decision-making is essential when caring for patients with NMOSD. -MAM
Maureen A. Mealy, BSN, MSCN
Neuromyelitis Optica Research Program Manager, Senior Research Nurse of the Transverse Myelitis & Multiple Sclerosis Centers, PhD candidate at Johns Hopkins School of Nursing in Baltimore
1. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107-1114.
2. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
3. Popescu BF, Lucchinetti CF. Immunopathology: autoimmune glial diseases and differentiation from multiple sclerosis. Handb Clin Neurol. 2016;133:95-106.
4. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14.
5. Orman G, Wang KY, Pekcevik Y, et al. Enhancing brain lesions during acute optic neuritis and/or longitudinally extensive transverse myelitis may portend a higher relapse rate in neuromyelitis optica spectrum disorders. Am J Neuroradiol. 2017;38(5):949-953.
6. Kessler RA, Mealy MA, Levy M. Early indicators of relapses vs pseudorelapses in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e269.
7. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603-605.
8. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216.
9. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185-192.
1. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107-1114.
2. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
3. Popescu BF, Lucchinetti CF. Immunopathology: autoimmune glial diseases and differentiation from multiple sclerosis. Handb Clin Neurol. 2016;133:95-106.
4. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14.
5. Orman G, Wang KY, Pekcevik Y, et al. Enhancing brain lesions during acute optic neuritis and/or longitudinally extensive transverse myelitis may portend a higher relapse rate in neuromyelitis optica spectrum disorders. Am J Neuroradiol. 2017;38(5):949-953.
6. Kessler RA, Mealy MA, Levy M. Early indicators of relapses vs pseudorelapses in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e269.
7. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603-605.
8. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216.
9. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185-192.
Bladder Complications in MS
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.