User login
Identifying and Managing MS Relapse
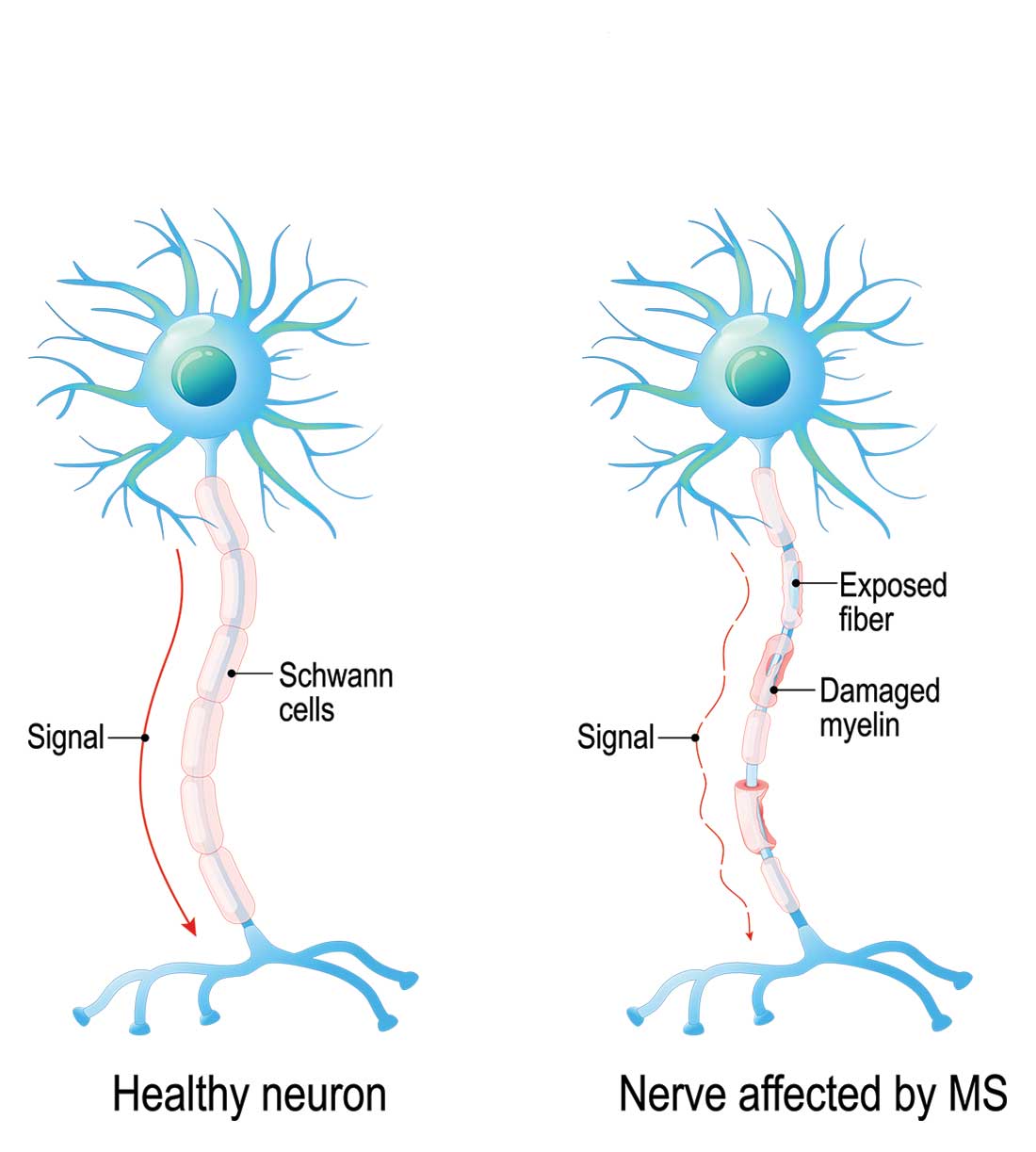
Multiple sclerosis (MS) is a chronic, autoimmune-mediated disorder of the central nervous system that affects more than 40,000 people in the United States. About 85% of cases are categorized as relapsing remitting multiple sclerosis (RRMS), based on the clinical and radiographic pattern of focal demyelination in different regions of the brain and spinal cord over time. Though not fully understood, the pathophysiology of RRMS involves axonal degeneration and inflammatory demyelination; the latter is considered a relapse in patients with an established MS diagnosis.
OVERVIEW
MS relapse can have a significant impact on patients’ short- and long-term function, quality of life, and finances. Relapse may be identified via
- New neurologic symptoms reported by the patient
- New neurologic findings on physical examination
- New radiographic findings on contrast-enhanced MRI of the central nervous system, or
- Abnormal results of cerebrospinal fluid analysis.
Patient-reported symptoms and abnormal signs identified on physical exam should correspond with the area of the central nervous system affected. In some cases, patients may have radiographic evidence of relapse without symptoms or signs.
It is essential for health care providers to identify relapse, as it is an important marker of disease activity that may warrant treatment—particularly if symptoms are impacting function or if there is optic neuritis. MS relapse is also an indicator of suboptimal response to disease-modifying therapies.
Treatment of relapse is one component of RRMS management, which also includes symptom management and use of disease-modifying therapy to reduce risk for disease activity and decline in function.
DIAGNOSING RELAPSE
Because risk for MS relapse cannot be predicted, both patients and providers need to have a high index of suspicion in the setting of new neurologic symptoms or decline in function. Relapse should be considered when these symptoms last longer than 24 hours in the absence of fever or infection. The clinical features of relapse should have corresponding radiographic evidence of active demyelination on contrast-enhanced MRI.
A pseudo-relapse is characterized by new or worsening neurologic symptoms lasting longer than 24 hours with concurrent fever, infection, or other metabolic derangement. Pseudo-relapse does not show radiographic evidence of active demyelination on contrast-enhanced MRI.
Continue to: Aggravation of longstanding neurologic symptoms...
Aggravation of longstanding neurologic symptoms is not considered a relapse, as no new radiographic evidence of disease progression will be seen on MRI. Factors that may contribute to aggravation of established symptoms include an increase in core body temperature, sleep deprivation, and psychosocial stress.
When a patient with suspected or diagnosed RRMS presents with new neurologic symptoms of more than 24 hours’ duration, the first step is to conduct a physical exam to assess for objective evidence of neurologic deficits and signs of infection, including fever. The provider should also order select laboratory testing—including a complete blood cell count and urinalysis with culture—to exclude infection. In certain cases, contrast-enhanced MRI of the brain and/or spine may be ordered; however, this may delay treatment initiation if the study cannot be promptly scheduled.
Evaluation and management should involve communication, if not face-to-face consultation, with the patient’s neurology provider who is responsible for MS management.
IMMEDIATE MANAGEMENT
Acute relapses are managed with anti-inflammatory agents. For some patients, treatment may provide symptomatic relief, shorten the recovery phase, and improve motor function. Long-term benefits have not been demonstrated, except in patients with optic neuritis.
Firstline therapy for MS relapse is high-dose corticosteroids, which can be administered at home, at an ambulatory infusion center, or (in some cases) in a hospital setting. The preferred regimen is methylprednisolone (1 g IV for 3-5 d), with or without prednisone taper. Another option is dexamethasone (80 mg bid for 3-5 d), with or without prednisone taper.1-3
Continue to: Common adverse effects of corticosteroids include...
Common adverse effects of corticosteroids include headache, emotional lability, insomnia, glucose intolerance, hypertension, dyspepsia, and exacerbation of psychiatric conditions; drug interactions should also be considered. Patients with diabetes may need to be admitted to the hospital for glycemic monitoring and control.
High-dose corticosteroids are associated with a rare, non–dose-dependent risk for aseptic femoral necrosis. For patients who are refractory to or not candidates for corticosteroids, adrenocorticotropic hormone (ACTH) gel (80 U/d IM or subQ daily for 10 d) is an option. This medication may be better tolerated, although it is much more expensive than corticosteroids. Plasmapheresis and IV immunoglobulin are also options for patients with refractory symptoms or contraindications to recommended therapies.1-3
ONGOING MANAGEMENT
Once a treatment plan is initiated, providers should carefully follow the patient’s response in terms of adverse effects, symptom improvement, and functional recovery. Those with refractory symptoms may need additional doses of the initial therapy or an alternative therapy.
The relapse recovery period may last several months and be complete or incomplete, so providers may also need to manage neurologic symptoms and functional deficits (with pharmacologic and/or nonpharmacologic options). Patients who have had a relapse should also meet with their neurology provider to discuss their disease-modifying therapy plan, since relapse indicates a suboptimal response to current therapy.
1. Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):17.
2. Frohman TC, O’Donoghue DL, Northrop D, eds. Multiple Sclerosis for the Physician Assistant. National Multiple Sclerosis Society. 2011.
3. Giesser B, ed. Primer on Multiple Sclerosis . 2nd ed. Oxford, UK: Oxford University Press ; 2015.
Multiple sclerosis (MS) is a chronic, autoimmune-mediated disorder of the central nervous system that affects more than 40,000 people in the United States. About 85% of cases are categorized as relapsing remitting multiple sclerosis (RRMS), based on the clinical and radiographic pattern of focal demyelination in different regions of the brain and spinal cord over time. Though not fully understood, the pathophysiology of RRMS involves axonal degeneration and inflammatory demyelination; the latter is considered a relapse in patients with an established MS diagnosis.
OVERVIEW
MS relapse can have a significant impact on patients’ short- and long-term function, quality of life, and finances. Relapse may be identified via
- New neurologic symptoms reported by the patient
- New neurologic findings on physical examination
- New radiographic findings on contrast-enhanced MRI of the central nervous system, or
- Abnormal results of cerebrospinal fluid analysis.
Patient-reported symptoms and abnormal signs identified on physical exam should correspond with the area of the central nervous system affected. In some cases, patients may have radiographic evidence of relapse without symptoms or signs.
It is essential for health care providers to identify relapse, as it is an important marker of disease activity that may warrant treatment—particularly if symptoms are impacting function or if there is optic neuritis. MS relapse is also an indicator of suboptimal response to disease-modifying therapies.
Treatment of relapse is one component of RRMS management, which also includes symptom management and use of disease-modifying therapy to reduce risk for disease activity and decline in function.
DIAGNOSING RELAPSE
Because risk for MS relapse cannot be predicted, both patients and providers need to have a high index of suspicion in the setting of new neurologic symptoms or decline in function. Relapse should be considered when these symptoms last longer than 24 hours in the absence of fever or infection. The clinical features of relapse should have corresponding radiographic evidence of active demyelination on contrast-enhanced MRI.
A pseudo-relapse is characterized by new or worsening neurologic symptoms lasting longer than 24 hours with concurrent fever, infection, or other metabolic derangement. Pseudo-relapse does not show radiographic evidence of active demyelination on contrast-enhanced MRI.
Continue to: Aggravation of longstanding neurologic symptoms...
Aggravation of longstanding neurologic symptoms is not considered a relapse, as no new radiographic evidence of disease progression will be seen on MRI. Factors that may contribute to aggravation of established symptoms include an increase in core body temperature, sleep deprivation, and psychosocial stress.
When a patient with suspected or diagnosed RRMS presents with new neurologic symptoms of more than 24 hours’ duration, the first step is to conduct a physical exam to assess for objective evidence of neurologic deficits and signs of infection, including fever. The provider should also order select laboratory testing—including a complete blood cell count and urinalysis with culture—to exclude infection. In certain cases, contrast-enhanced MRI of the brain and/or spine may be ordered; however, this may delay treatment initiation if the study cannot be promptly scheduled.
Evaluation and management should involve communication, if not face-to-face consultation, with the patient’s neurology provider who is responsible for MS management.
IMMEDIATE MANAGEMENT
Acute relapses are managed with anti-inflammatory agents. For some patients, treatment may provide symptomatic relief, shorten the recovery phase, and improve motor function. Long-term benefits have not been demonstrated, except in patients with optic neuritis.
Firstline therapy for MS relapse is high-dose corticosteroids, which can be administered at home, at an ambulatory infusion center, or (in some cases) in a hospital setting. The preferred regimen is methylprednisolone (1 g IV for 3-5 d), with or without prednisone taper. Another option is dexamethasone (80 mg bid for 3-5 d), with or without prednisone taper.1-3
Continue to: Common adverse effects of corticosteroids include...
Common adverse effects of corticosteroids include headache, emotional lability, insomnia, glucose intolerance, hypertension, dyspepsia, and exacerbation of psychiatric conditions; drug interactions should also be considered. Patients with diabetes may need to be admitted to the hospital for glycemic monitoring and control.
High-dose corticosteroids are associated with a rare, non–dose-dependent risk for aseptic femoral necrosis. For patients who are refractory to or not candidates for corticosteroids, adrenocorticotropic hormone (ACTH) gel (80 U/d IM or subQ daily for 10 d) is an option. This medication may be better tolerated, although it is much more expensive than corticosteroids. Plasmapheresis and IV immunoglobulin are also options for patients with refractory symptoms or contraindications to recommended therapies.1-3
ONGOING MANAGEMENT
Once a treatment plan is initiated, providers should carefully follow the patient’s response in terms of adverse effects, symptom improvement, and functional recovery. Those with refractory symptoms may need additional doses of the initial therapy or an alternative therapy.
The relapse recovery period may last several months and be complete or incomplete, so providers may also need to manage neurologic symptoms and functional deficits (with pharmacologic and/or nonpharmacologic options). Patients who have had a relapse should also meet with their neurology provider to discuss their disease-modifying therapy plan, since relapse indicates a suboptimal response to current therapy.
Multiple sclerosis (MS) is a chronic, autoimmune-mediated disorder of the central nervous system that affects more than 40,000 people in the United States. About 85% of cases are categorized as relapsing remitting multiple sclerosis (RRMS), based on the clinical and radiographic pattern of focal demyelination in different regions of the brain and spinal cord over time. Though not fully understood, the pathophysiology of RRMS involves axonal degeneration and inflammatory demyelination; the latter is considered a relapse in patients with an established MS diagnosis.
OVERVIEW
MS relapse can have a significant impact on patients’ short- and long-term function, quality of life, and finances. Relapse may be identified via
- New neurologic symptoms reported by the patient
- New neurologic findings on physical examination
- New radiographic findings on contrast-enhanced MRI of the central nervous system, or
- Abnormal results of cerebrospinal fluid analysis.
Patient-reported symptoms and abnormal signs identified on physical exam should correspond with the area of the central nervous system affected. In some cases, patients may have radiographic evidence of relapse without symptoms or signs.
It is essential for health care providers to identify relapse, as it is an important marker of disease activity that may warrant treatment—particularly if symptoms are impacting function or if there is optic neuritis. MS relapse is also an indicator of suboptimal response to disease-modifying therapies.
Treatment of relapse is one component of RRMS management, which also includes symptom management and use of disease-modifying therapy to reduce risk for disease activity and decline in function.
DIAGNOSING RELAPSE
Because risk for MS relapse cannot be predicted, both patients and providers need to have a high index of suspicion in the setting of new neurologic symptoms or decline in function. Relapse should be considered when these symptoms last longer than 24 hours in the absence of fever or infection. The clinical features of relapse should have corresponding radiographic evidence of active demyelination on contrast-enhanced MRI.
A pseudo-relapse is characterized by new or worsening neurologic symptoms lasting longer than 24 hours with concurrent fever, infection, or other metabolic derangement. Pseudo-relapse does not show radiographic evidence of active demyelination on contrast-enhanced MRI.
Continue to: Aggravation of longstanding neurologic symptoms...
Aggravation of longstanding neurologic symptoms is not considered a relapse, as no new radiographic evidence of disease progression will be seen on MRI. Factors that may contribute to aggravation of established symptoms include an increase in core body temperature, sleep deprivation, and psychosocial stress.
When a patient with suspected or diagnosed RRMS presents with new neurologic symptoms of more than 24 hours’ duration, the first step is to conduct a physical exam to assess for objective evidence of neurologic deficits and signs of infection, including fever. The provider should also order select laboratory testing—including a complete blood cell count and urinalysis with culture—to exclude infection. In certain cases, contrast-enhanced MRI of the brain and/or spine may be ordered; however, this may delay treatment initiation if the study cannot be promptly scheduled.
Evaluation and management should involve communication, if not face-to-face consultation, with the patient’s neurology provider who is responsible for MS management.
IMMEDIATE MANAGEMENT
Acute relapses are managed with anti-inflammatory agents. For some patients, treatment may provide symptomatic relief, shorten the recovery phase, and improve motor function. Long-term benefits have not been demonstrated, except in patients with optic neuritis.
Firstline therapy for MS relapse is high-dose corticosteroids, which can be administered at home, at an ambulatory infusion center, or (in some cases) in a hospital setting. The preferred regimen is methylprednisolone (1 g IV for 3-5 d), with or without prednisone taper. Another option is dexamethasone (80 mg bid for 3-5 d), with or without prednisone taper.1-3
Continue to: Common adverse effects of corticosteroids include...
Common adverse effects of corticosteroids include headache, emotional lability, insomnia, glucose intolerance, hypertension, dyspepsia, and exacerbation of psychiatric conditions; drug interactions should also be considered. Patients with diabetes may need to be admitted to the hospital for glycemic monitoring and control.
High-dose corticosteroids are associated with a rare, non–dose-dependent risk for aseptic femoral necrosis. For patients who are refractory to or not candidates for corticosteroids, adrenocorticotropic hormone (ACTH) gel (80 U/d IM or subQ daily for 10 d) is an option. This medication may be better tolerated, although it is much more expensive than corticosteroids. Plasmapheresis and IV immunoglobulin are also options for patients with refractory symptoms or contraindications to recommended therapies.1-3
ONGOING MANAGEMENT
Once a treatment plan is initiated, providers should carefully follow the patient’s response in terms of adverse effects, symptom improvement, and functional recovery. Those with refractory symptoms may need additional doses of the initial therapy or an alternative therapy.
The relapse recovery period may last several months and be complete or incomplete, so providers may also need to manage neurologic symptoms and functional deficits (with pharmacologic and/or nonpharmacologic options). Patients who have had a relapse should also meet with their neurology provider to discuss their disease-modifying therapy plan, since relapse indicates a suboptimal response to current therapy.
1. Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):17.
2. Frohman TC, O’Donoghue DL, Northrop D, eds. Multiple Sclerosis for the Physician Assistant. National Multiple Sclerosis Society. 2011.
3. Giesser B, ed. Primer on Multiple Sclerosis . 2nd ed. Oxford, UK: Oxford University Press ; 2015.
1. Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):17.
2. Frohman TC, O’Donoghue DL, Northrop D, eds. Multiple Sclerosis for the Physician Assistant. National Multiple Sclerosis Society. 2011.
3. Giesser B, ed. Primer on Multiple Sclerosis . 2nd ed. Oxford, UK: Oxford University Press ; 2015.
Hypertension and Diabetes: Addressing Common Comorbidities
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in patients with diabetes.1 ASCVD is defined by the American College of Cardiology and the American Heart Association (ACC/AHA) as acute coronary syndrome, myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin.2 Risk factors for ASCVD include hypertension, dyslipidemia, smoking, family history of premature coronary disease, chronic kidney disease, and albuminuria.3
Hypertension, a modifiable risk factor, is prevalent in patients with diabetes. Multiple studies have shown that antihypertensive therapy in these patients reduces ASCVD events; therefore, blood pressure control is necessary.1,3 The American Diabetes Association’s (ADA) 2018 Standards of Medical Care in Diabetes offers guidance on the assessment and treatment of hypertension in patients with diabetes—including the organization’s position statement on hypertensive treatment with comorbid diabetes.1,3 These guidelines are relevant and useful to both primary care and specialty providers who manage these complex patients.
Screening and Diagnosis
Every clinical care visit for patients with diabetes should include a blood pressure measurement. (Evaluation for orthostatic hypotension should also be performed at the initial visit, to help guide future treatment.1) For accuracy, blood pressure should be assessed
- By a trained individual using the appropriate size cuff
- In both arms on the initial visit
- With the patient seated, with feet on the floor and arm at heart level
- After five minutes of rest
- With two to three readings taken one to two minutes apart and results averaged.1
If blood pressure is found to be elevated and the patient has no known history of hypertension, the elevated blood pressure should be reassessed on another visit within one month to confirm the diagnosis.1 Patients should also monitor blood pressure at home to distinguish between white coat and masked hypertension.1 Home blood pressures should be measured with arm cuffs that are the appropriate size. The bladder of the cuff should encircle 80% of the arm, should not cover clothing, and should be placed on the upper arm at the midpoint of the sternum.1
The ACC/AHA’s 2017 guidelines define stage 1 hypertension as 130-139/80-89 mm Hg and stage 2 hypertension as ≥ 140/90 mm Hg.4 The ADA defines hypertension as a sustained blood pressure ≥ 140/90 mm Hg, noting that the definition is “based on unambiguous data that levels above this threshold are strongly associated with ASCVD, death, disability, and microvascular complications.”1
BLOOD PRESSURE TARGETS
Evidence has shown that treatment of blood pressure to a goal of ≤ 140/90 mm Hg reduces cardiovascular events as well as microvascular complications.1 For patients with diabetes, the ADA recommends treatment to a systolic blood pressure goal of < 140 mm Hg and a diastolic blood pressure goal of < 90 mm Hg, while the ACC/AHA guidelines recommend a goal of < 130/80 mm Hg.1,4
The ADA does note that lower blood pressure targets (eg, < 130/80 mm Hg) can be appropriate for individuals at high risk for cardiovascular disease if no treatment burdens (eg, adverse effects, costs) are imposed.1 This is important, since patients with diabetes often have multiple risk factors for ASCVD and will be considered high risk. Studies suggest lower blood pressure targets may decrease the risk for stroke and albuminuria but offer little to no effect on other ASCVD events, occurrence of heart failure, or other conditions associated with diabetes (eg, peripheral neuropathy).1
Continue to: LIFESTYLE MANAGEMENT
LIFESTYLE MANAGEMENT
Patients with diabetes and elevated blood pressure (> 120/80 mm Hg, per the 2017 ACC/AHA guidelines) are at high risk for hypertension and its complications.1,4 Lifestyle management—which includes weight loss, a healthy diet, increase in physical activity, and moderation in alcohol intake—is an important component of preventing or delaying a hypertension diagnosis.1,4
Both the ADA and the ACC/AHA recommend that patients with diabetes follow the Dietary Approaches to Stop Hypertension (DASH) diet.1,4 Guidelines include restricting sodium intake to < 2,300 mg/d, consuming 8-10 servings/d of fruits and vegetables and 2-3 servings/d of low-fat dairy products, limiting alcohol consumption to two servings/d for men and one serving/d for women, and increasing physical activity to include at least 30-45 min/d of aerobic exercise.1,4
PHARMACOLOGIC TREATMENT
Initial treatment for patients with hypertension and diabetes depends on the severity of the hypertension and should include drug classes that have demonstrated success in reducing ASCVD events: ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, and dihydropyridine calcium channel blockers. The ADA offers additional guidance:
Blood pressure ≥ 140/90 mm Hg should be treated with lifestyle modifications and simultaneous initiation of a single drug, with timely titration of pharmacologic therapy to achieve blood pressure goals.
Continue to: Blood pressure ≥ 160/100 mm Hg
Blood pressure ≥ 160/100 mm Hg should be treated with lifestyle therapy and prompt initiation and timely titration of two drugs or a single-pill combination of drugs.
Multidrug therapy is generally required to achieve blood pressure targets—but ACE inhibitors and ARBs should not be used in combination due to the increased risk for adverse effects.
Firstline therapy is an ACE inhibitor or an ARB, at the maximum tolerated dose, in patients with diabetes and a urine albumin-to-creatinine ratio ≥ 30 mg/g.
Monitoring of estimated glomerular filtration rate and serum potassium levels is needed in patients treated with an ACE inhibitor, ARB, or diuretic.1
RESISTANT HYPERTENSION
Patients with diabetes who have a blood pressure ≥ 140/90 mm Hg despite treatment that includes lifestyle management, two antihypertensives, and a diuretic, or who achieve blood pressure control with four or more medications, are considered to have resistant hypertension.1,5 Factors such as pseudoresistance (lack of medication adherence or poor measurement technique), masked hypertension, and white coat hypertension should be ruled out in making the diagnosis of resistant hypertension. Once these have been excluded, patients should be referred for a workup of their resistant hypertension to evaluate causes of secondary hypertension. These can include endocrine issues, renal arterial disease, edema in advanced kidney disease, hormones, and drugs such as NSAIDs and decongestants.1
Continue to: PATIENT-CENTERED CARE
PATIENT-CENTERED CARE
When evaluating and treating a patient with diabetes, it is important to consider
- What is the patient’s overall risk for atherosclerotic cardiovascular disease?
- Does he/she have an increased risk for stroke? If so, lower blood pressure targets may be appropriate.
- Is more than one antihypertensive agent (ACE inhibitor, ARB, or diuretic) being used? If so, close monitoring of estimated glomerular filtration rate and potassium (as well as other indications of adverse effects) is important.
The treatment regimen should be a shared decision-making process between the clinician and patient and should be individualized to each patient and his/her existing comorbidities.
CONCLUSION
Clinical trials and meta-analyses support target blood pressure management to < 140/90 mm Hg in most adults with diabetes, while lower targets (< 130/80 mm Hg) may be beneficial for patients with diabetes and a high risk for cardiovascular disease.1,5 Lifestyle management should be initiated and continued in patients with a blood pressure > 120/80 mm Hg and in those diagnosed with hypertension.1 Medications that reduce cardiovascular events should be used in management, with ACE inhibitors or ARBs being firstline treatment in patients with albuminuria.1
For more information on hypertensive treatment in special populations (eg, pregnant women and older adults), see the ADA’s full position statement.1
1. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014; 129(25 suppl 2):S1-S45.
3. American Diabetes Association. Position Statement 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324.
5. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916.
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in patients with diabetes.1 ASCVD is defined by the American College of Cardiology and the American Heart Association (ACC/AHA) as acute coronary syndrome, myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin.2 Risk factors for ASCVD include hypertension, dyslipidemia, smoking, family history of premature coronary disease, chronic kidney disease, and albuminuria.3
Hypertension, a modifiable risk factor, is prevalent in patients with diabetes. Multiple studies have shown that antihypertensive therapy in these patients reduces ASCVD events; therefore, blood pressure control is necessary.1,3 The American Diabetes Association’s (ADA) 2018 Standards of Medical Care in Diabetes offers guidance on the assessment and treatment of hypertension in patients with diabetes—including the organization’s position statement on hypertensive treatment with comorbid diabetes.1,3 These guidelines are relevant and useful to both primary care and specialty providers who manage these complex patients.
Screening and Diagnosis
Every clinical care visit for patients with diabetes should include a blood pressure measurement. (Evaluation for orthostatic hypotension should also be performed at the initial visit, to help guide future treatment.1) For accuracy, blood pressure should be assessed
- By a trained individual using the appropriate size cuff
- In both arms on the initial visit
- With the patient seated, with feet on the floor and arm at heart level
- After five minutes of rest
- With two to three readings taken one to two minutes apart and results averaged.1
If blood pressure is found to be elevated and the patient has no known history of hypertension, the elevated blood pressure should be reassessed on another visit within one month to confirm the diagnosis.1 Patients should also monitor blood pressure at home to distinguish between white coat and masked hypertension.1 Home blood pressures should be measured with arm cuffs that are the appropriate size. The bladder of the cuff should encircle 80% of the arm, should not cover clothing, and should be placed on the upper arm at the midpoint of the sternum.1
The ACC/AHA’s 2017 guidelines define stage 1 hypertension as 130-139/80-89 mm Hg and stage 2 hypertension as ≥ 140/90 mm Hg.4 The ADA defines hypertension as a sustained blood pressure ≥ 140/90 mm Hg, noting that the definition is “based on unambiguous data that levels above this threshold are strongly associated with ASCVD, death, disability, and microvascular complications.”1
BLOOD PRESSURE TARGETS
Evidence has shown that treatment of blood pressure to a goal of ≤ 140/90 mm Hg reduces cardiovascular events as well as microvascular complications.1 For patients with diabetes, the ADA recommends treatment to a systolic blood pressure goal of < 140 mm Hg and a diastolic blood pressure goal of < 90 mm Hg, while the ACC/AHA guidelines recommend a goal of < 130/80 mm Hg.1,4
The ADA does note that lower blood pressure targets (eg, < 130/80 mm Hg) can be appropriate for individuals at high risk for cardiovascular disease if no treatment burdens (eg, adverse effects, costs) are imposed.1 This is important, since patients with diabetes often have multiple risk factors for ASCVD and will be considered high risk. Studies suggest lower blood pressure targets may decrease the risk for stroke and albuminuria but offer little to no effect on other ASCVD events, occurrence of heart failure, or other conditions associated with diabetes (eg, peripheral neuropathy).1
Continue to: LIFESTYLE MANAGEMENT
LIFESTYLE MANAGEMENT
Patients with diabetes and elevated blood pressure (> 120/80 mm Hg, per the 2017 ACC/AHA guidelines) are at high risk for hypertension and its complications.1,4 Lifestyle management—which includes weight loss, a healthy diet, increase in physical activity, and moderation in alcohol intake—is an important component of preventing or delaying a hypertension diagnosis.1,4
Both the ADA and the ACC/AHA recommend that patients with diabetes follow the Dietary Approaches to Stop Hypertension (DASH) diet.1,4 Guidelines include restricting sodium intake to < 2,300 mg/d, consuming 8-10 servings/d of fruits and vegetables and 2-3 servings/d of low-fat dairy products, limiting alcohol consumption to two servings/d for men and one serving/d for women, and increasing physical activity to include at least 30-45 min/d of aerobic exercise.1,4
PHARMACOLOGIC TREATMENT
Initial treatment for patients with hypertension and diabetes depends on the severity of the hypertension and should include drug classes that have demonstrated success in reducing ASCVD events: ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, and dihydropyridine calcium channel blockers. The ADA offers additional guidance:
Blood pressure ≥ 140/90 mm Hg should be treated with lifestyle modifications and simultaneous initiation of a single drug, with timely titration of pharmacologic therapy to achieve blood pressure goals.
Continue to: Blood pressure ≥ 160/100 mm Hg
Blood pressure ≥ 160/100 mm Hg should be treated with lifestyle therapy and prompt initiation and timely titration of two drugs or a single-pill combination of drugs.
Multidrug therapy is generally required to achieve blood pressure targets—but ACE inhibitors and ARBs should not be used in combination due to the increased risk for adverse effects.
Firstline therapy is an ACE inhibitor or an ARB, at the maximum tolerated dose, in patients with diabetes and a urine albumin-to-creatinine ratio ≥ 30 mg/g.
Monitoring of estimated glomerular filtration rate and serum potassium levels is needed in patients treated with an ACE inhibitor, ARB, or diuretic.1
RESISTANT HYPERTENSION
Patients with diabetes who have a blood pressure ≥ 140/90 mm Hg despite treatment that includes lifestyle management, two antihypertensives, and a diuretic, or who achieve blood pressure control with four or more medications, are considered to have resistant hypertension.1,5 Factors such as pseudoresistance (lack of medication adherence or poor measurement technique), masked hypertension, and white coat hypertension should be ruled out in making the diagnosis of resistant hypertension. Once these have been excluded, patients should be referred for a workup of their resistant hypertension to evaluate causes of secondary hypertension. These can include endocrine issues, renal arterial disease, edema in advanced kidney disease, hormones, and drugs such as NSAIDs and decongestants.1
Continue to: PATIENT-CENTERED CARE
PATIENT-CENTERED CARE
When evaluating and treating a patient with diabetes, it is important to consider
- What is the patient’s overall risk for atherosclerotic cardiovascular disease?
- Does he/she have an increased risk for stroke? If so, lower blood pressure targets may be appropriate.
- Is more than one antihypertensive agent (ACE inhibitor, ARB, or diuretic) being used? If so, close monitoring of estimated glomerular filtration rate and potassium (as well as other indications of adverse effects) is important.
The treatment regimen should be a shared decision-making process between the clinician and patient and should be individualized to each patient and his/her existing comorbidities.
CONCLUSION
Clinical trials and meta-analyses support target blood pressure management to < 140/90 mm Hg in most adults with diabetes, while lower targets (< 130/80 mm Hg) may be beneficial for patients with diabetes and a high risk for cardiovascular disease.1,5 Lifestyle management should be initiated and continued in patients with a blood pressure > 120/80 mm Hg and in those diagnosed with hypertension.1 Medications that reduce cardiovascular events should be used in management, with ACE inhibitors or ARBs being firstline treatment in patients with albuminuria.1
For more information on hypertensive treatment in special populations (eg, pregnant women and older adults), see the ADA’s full position statement.1
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in patients with diabetes.1 ASCVD is defined by the American College of Cardiology and the American Heart Association (ACC/AHA) as acute coronary syndrome, myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin.2 Risk factors for ASCVD include hypertension, dyslipidemia, smoking, family history of premature coronary disease, chronic kidney disease, and albuminuria.3
Hypertension, a modifiable risk factor, is prevalent in patients with diabetes. Multiple studies have shown that antihypertensive therapy in these patients reduces ASCVD events; therefore, blood pressure control is necessary.1,3 The American Diabetes Association’s (ADA) 2018 Standards of Medical Care in Diabetes offers guidance on the assessment and treatment of hypertension in patients with diabetes—including the organization’s position statement on hypertensive treatment with comorbid diabetes.1,3 These guidelines are relevant and useful to both primary care and specialty providers who manage these complex patients.
Screening and Diagnosis
Every clinical care visit for patients with diabetes should include a blood pressure measurement. (Evaluation for orthostatic hypotension should also be performed at the initial visit, to help guide future treatment.1) For accuracy, blood pressure should be assessed
- By a trained individual using the appropriate size cuff
- In both arms on the initial visit
- With the patient seated, with feet on the floor and arm at heart level
- After five minutes of rest
- With two to three readings taken one to two minutes apart and results averaged.1
If blood pressure is found to be elevated and the patient has no known history of hypertension, the elevated blood pressure should be reassessed on another visit within one month to confirm the diagnosis.1 Patients should also monitor blood pressure at home to distinguish between white coat and masked hypertension.1 Home blood pressures should be measured with arm cuffs that are the appropriate size. The bladder of the cuff should encircle 80% of the arm, should not cover clothing, and should be placed on the upper arm at the midpoint of the sternum.1
The ACC/AHA’s 2017 guidelines define stage 1 hypertension as 130-139/80-89 mm Hg and stage 2 hypertension as ≥ 140/90 mm Hg.4 The ADA defines hypertension as a sustained blood pressure ≥ 140/90 mm Hg, noting that the definition is “based on unambiguous data that levels above this threshold are strongly associated with ASCVD, death, disability, and microvascular complications.”1
BLOOD PRESSURE TARGETS
Evidence has shown that treatment of blood pressure to a goal of ≤ 140/90 mm Hg reduces cardiovascular events as well as microvascular complications.1 For patients with diabetes, the ADA recommends treatment to a systolic blood pressure goal of < 140 mm Hg and a diastolic blood pressure goal of < 90 mm Hg, while the ACC/AHA guidelines recommend a goal of < 130/80 mm Hg.1,4
The ADA does note that lower blood pressure targets (eg, < 130/80 mm Hg) can be appropriate for individuals at high risk for cardiovascular disease if no treatment burdens (eg, adverse effects, costs) are imposed.1 This is important, since patients with diabetes often have multiple risk factors for ASCVD and will be considered high risk. Studies suggest lower blood pressure targets may decrease the risk for stroke and albuminuria but offer little to no effect on other ASCVD events, occurrence of heart failure, or other conditions associated with diabetes (eg, peripheral neuropathy).1
Continue to: LIFESTYLE MANAGEMENT
LIFESTYLE MANAGEMENT
Patients with diabetes and elevated blood pressure (> 120/80 mm Hg, per the 2017 ACC/AHA guidelines) are at high risk for hypertension and its complications.1,4 Lifestyle management—which includes weight loss, a healthy diet, increase in physical activity, and moderation in alcohol intake—is an important component of preventing or delaying a hypertension diagnosis.1,4
Both the ADA and the ACC/AHA recommend that patients with diabetes follow the Dietary Approaches to Stop Hypertension (DASH) diet.1,4 Guidelines include restricting sodium intake to < 2,300 mg/d, consuming 8-10 servings/d of fruits and vegetables and 2-3 servings/d of low-fat dairy products, limiting alcohol consumption to two servings/d for men and one serving/d for women, and increasing physical activity to include at least 30-45 min/d of aerobic exercise.1,4
PHARMACOLOGIC TREATMENT
Initial treatment for patients with hypertension and diabetes depends on the severity of the hypertension and should include drug classes that have demonstrated success in reducing ASCVD events: ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, and dihydropyridine calcium channel blockers. The ADA offers additional guidance:
Blood pressure ≥ 140/90 mm Hg should be treated with lifestyle modifications and simultaneous initiation of a single drug, with timely titration of pharmacologic therapy to achieve blood pressure goals.
Continue to: Blood pressure ≥ 160/100 mm Hg
Blood pressure ≥ 160/100 mm Hg should be treated with lifestyle therapy and prompt initiation and timely titration of two drugs or a single-pill combination of drugs.
Multidrug therapy is generally required to achieve blood pressure targets—but ACE inhibitors and ARBs should not be used in combination due to the increased risk for adverse effects.
Firstline therapy is an ACE inhibitor or an ARB, at the maximum tolerated dose, in patients with diabetes and a urine albumin-to-creatinine ratio ≥ 30 mg/g.
Monitoring of estimated glomerular filtration rate and serum potassium levels is needed in patients treated with an ACE inhibitor, ARB, or diuretic.1
RESISTANT HYPERTENSION
Patients with diabetes who have a blood pressure ≥ 140/90 mm Hg despite treatment that includes lifestyle management, two antihypertensives, and a diuretic, or who achieve blood pressure control with four or more medications, are considered to have resistant hypertension.1,5 Factors such as pseudoresistance (lack of medication adherence or poor measurement technique), masked hypertension, and white coat hypertension should be ruled out in making the diagnosis of resistant hypertension. Once these have been excluded, patients should be referred for a workup of their resistant hypertension to evaluate causes of secondary hypertension. These can include endocrine issues, renal arterial disease, edema in advanced kidney disease, hormones, and drugs such as NSAIDs and decongestants.1
Continue to: PATIENT-CENTERED CARE
PATIENT-CENTERED CARE
When evaluating and treating a patient with diabetes, it is important to consider
- What is the patient’s overall risk for atherosclerotic cardiovascular disease?
- Does he/she have an increased risk for stroke? If so, lower blood pressure targets may be appropriate.
- Is more than one antihypertensive agent (ACE inhibitor, ARB, or diuretic) being used? If so, close monitoring of estimated glomerular filtration rate and potassium (as well as other indications of adverse effects) is important.
The treatment regimen should be a shared decision-making process between the clinician and patient and should be individualized to each patient and his/her existing comorbidities.
CONCLUSION
Clinical trials and meta-analyses support target blood pressure management to < 140/90 mm Hg in most adults with diabetes, while lower targets (< 130/80 mm Hg) may be beneficial for patients with diabetes and a high risk for cardiovascular disease.1,5 Lifestyle management should be initiated and continued in patients with a blood pressure > 120/80 mm Hg and in those diagnosed with hypertension.1 Medications that reduce cardiovascular events should be used in management, with ACE inhibitors or ARBs being firstline treatment in patients with albuminuria.1
For more information on hypertensive treatment in special populations (eg, pregnant women and older adults), see the ADA’s full position statement.1
1. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014; 129(25 suppl 2):S1-S45.
3. American Diabetes Association. Position Statement 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324.
5. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916.
1. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014; 129(25 suppl 2):S1-S45.
3. American Diabetes Association. Position Statement 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324.
5. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916.
How the IHS Reduced Kidney Disease in the Highest-risk Population
Alaska is a vast state—larger than Texas, Montana, and California combined. It is also home to the highest percentage of American Indian (AI) and Alaska Native (AN) persons in the United States. These two populations—collectively referred to as Native Americans—have been served by the Indian Health Services (IHS) since it was established through the Snyder Act of 1921, in response to the dismal health conditions of the indigenous tribes in this country.1 Across the US (not only in Alaska), the IHS has partnered with AI/AN peoples to decrease health disparities in a culturally acceptable manner that honors and protects their traditions and values.
The IHS—which in 2016 comprised 2,500 nurses, 750 physicians, 700 pharmacists, 200 PAs and NPs, and 280 dentists, as well as nutritionists, diabetes educators, administrators, and other professionals—has made huge advances in decreasing health disparities in their populations. Among them: decreased rates of tuberculosis and of maternal and infant deaths.
However, life expectancy among Native Americans remains four years shorter than that of the rest of the US population. This disparity can be traced to three recalcitrant factors: unintentional injuries, liver disease, and diabetes.
The IHS practitioners decided to tackle diabetes with a multipronged approach. And what they achieved is astonishing.
WHAT THEY DID
Worldwide, diabetes is the most common cause of kidney failure; identifying patients with diabetes and early-stage chronic kidney disease allows for aggressive treatment that can slow progression to kidney failure and dialysis.
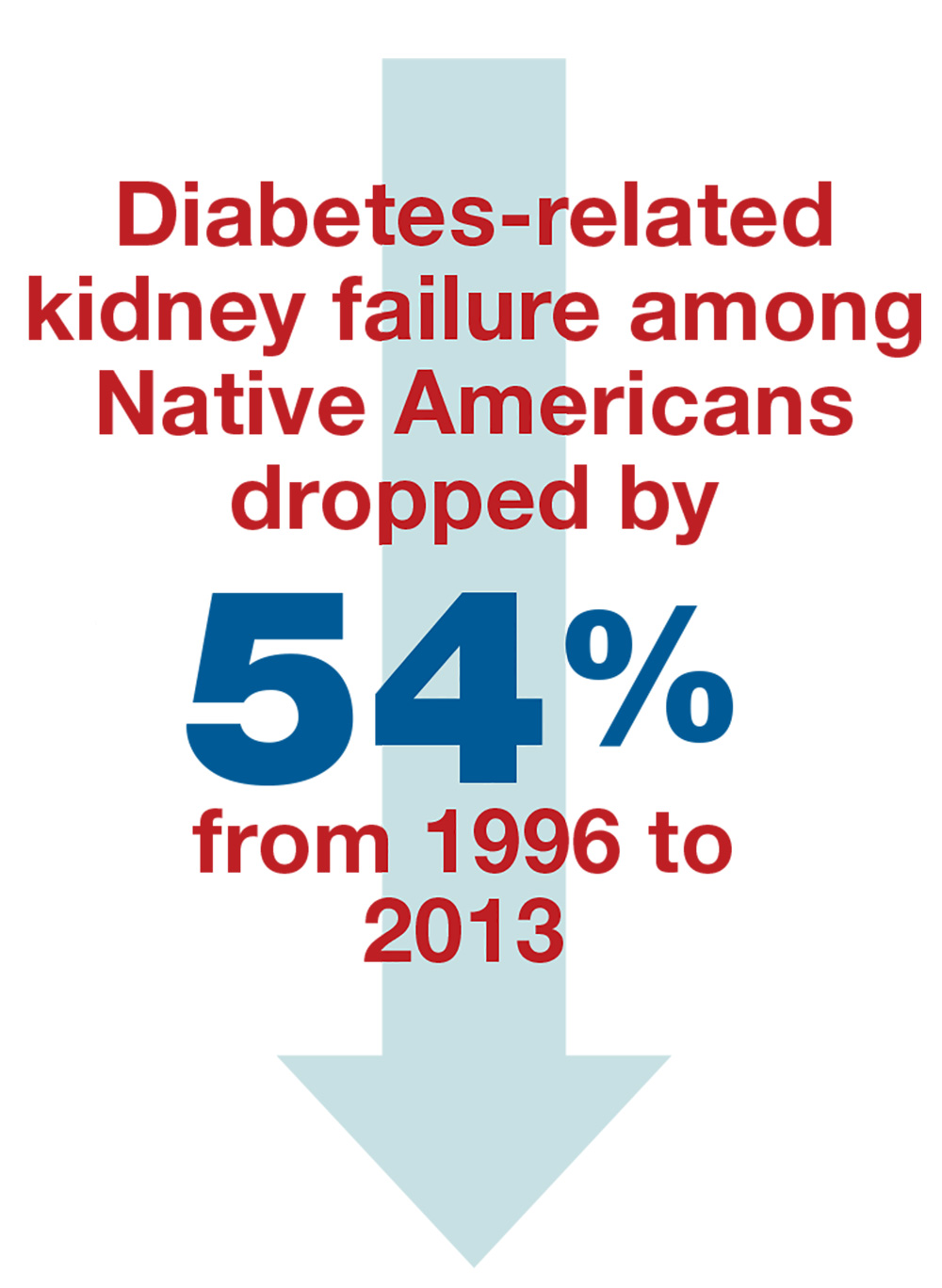
The IHS providers knew when they decided to tackle the problem of diabetes in the AI/AN population that the incidence was 16%—and the rate of diabetes leading to kidney failure in this population was the highest for any ethnic group in the US.2,3 And yet …
From 1996 to 2013, the rate of diabetes-related kidney failure among Native Americans dropped by 54%.3 Yes—the group of patients with the highest percentage of diabetes diagnoses has had the greatest improvement in prevention of kidney failure.4
Continue to: Some of the clinical achievements that contributed to...
Some of the clinical achievements that contributed to this significant change include
- Increased use of ACE inhibitors or angiotensin receptor blockers (ARBs) (from 42% to 74% over a five-year period)
- Reduced average blood pressure among hypertensive patients (to 133/76 mm Hg)
- Improved blood glucose control (by 10%)
- Increased testing for kidney disease among older patients (50% higher than the rest of the Medicare diabetes population).3
HOW THEY DID IT
This is not rocket science. The IHS staff integrated both population- and team-based approaches to achieve a more impressive decrease than ever could have been expected. In retrospect, perhaps this success should not come as such a surprise—many religious beliefs held by Native Americans focus around society, communal harmony, kinship, and cooperation.
The population health approach focused on promoting the wellness of the entire community and connecting people to local resources, including healthy food, transportation, housing, and mental health care. In the team approach, IHS medical experts implemented strategies to improve patient education, community outreach, care coordination, health outcome tracking, and access to a wide variety of health care providers.3,5
In a place like Alaska—where the northernmost city, Barrow, is more than 700 miles (two hours by plane) from Anchorage, and the southeastern Annette Island is more than 1,000 miles (six hours by plane) from the capital—this can be an especially challenging prospect. To reduce travel burden for rural patients, the IHS sponsors a diabetes team that travels from village to village. Nephrology services are not included in these field visits, however, so the kidney team relies heavily on telehealth. This requires extensive clinic staff coordination, as well as equipment and knowledgeable information systems support teams.
Other challenges require educational and logistical solutions. As noted, the use of ACE inhibitors and ARBs increased through the IHS’s efforts—and contributed to the delayed progression of diabetic kidney disease—but those additional prescriptions necessitate patient education. Understanding of these medications can be limited; many rural patients trust that when the bottle is empty, their practitioner has treated and cured their disease—mistakenly believing that no refills are needed. And even when the need to continue the prescription is understood, rural clinics may have difficulty tracking appointments and prescriptions written by providers at specialty clinics in Anchorage, making ongoing refills an issue.
Continue to: The necessary dietary changes can also be...
The necessary dietary changes can also be difficult for AI/AN populations. For example, in rural Alaska, tap water may not be safe to drink, and soda costs less than bottled water. Fresh produce is expensive and has often begun to spoil by the time it reaches local stores. The Native villagers often prefer their usual diets of gathered berries, fish, and red meat from subsistence hunting, making implementation of dietary changes difficult.
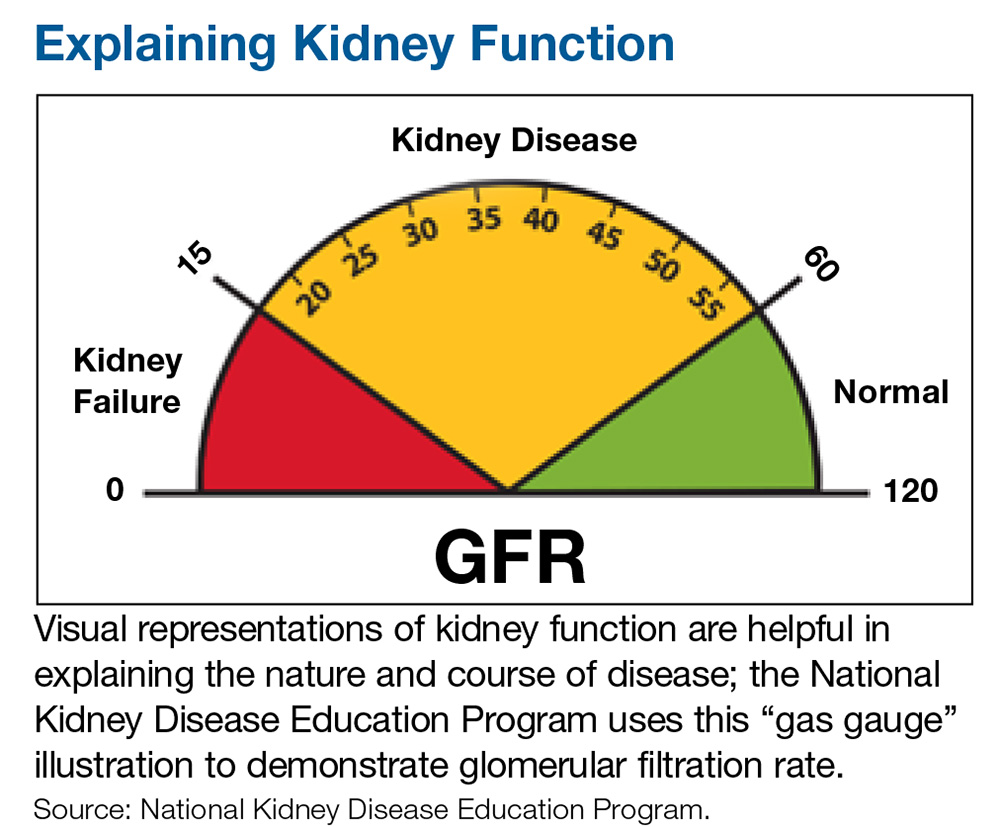
However, as the success of the IHS initiative shows, challenges can be met and overcome by practitioners who see a need, formulate a solution individualized to the circumstance, and think outside the box. One of the keys is developing a trusting relationship with patients. Another is to recognize informational needs and utilize available resources to educate patients. For example, visual representations of kidney function tend to be helpful in explaining the nature and course of disease; the National Kidney Disease Education Program uses an illustration similar to a gas gauge to demonstrate glomerular filtration rate (which would otherwise seem abstract and hard to understand for some patients; see below).6 When you understand your patient population and their needs, it makes addressing the challenging aspects of health care and prevention easier.
CONCLUSION
The results that the IHS achieved should serve as an example for all Americans with diabetes and their health care providers. We must be open to delivery of care via different approaches and practitioners in order to successfully help patients of different backgrounds and circumstances. This is the individualization of care that we hear so much about.
In 2016, the costs of caring for the kidney failure population were greater than the entire budget of the NIH. By aggressively identifying and treating patients at risk for kidney failure, we can slow disease progression—saving society money, but more importantly allowing our patients many more years of life free from the constraints of dialysis. —MET, RB
Mandy E. Thompson, PA-C
Kidney Center of Denver Health
Robin Bassett, DNP
Nephrology and Hypertension Associates, Anchorage
Adjunct Professor, NP program, University of Alaska, Anchorage
1. Indian Health Service. Legislation. www.ihs.gov/aboutihs/legislation. Accessed June 13, 2018.
2. National Health Interview Survey and Indian Health Service, 2010-2012.
3. CDC. Native Americans with diabetes. www.cdc.gov/vitalsigns/aian-diabetes/. Accessed June 13, 2018.
4. United States Renal Data System. Figure 1.5: Trends in adjusted* ESRD incidence rate (per million/year), by race, in the U.S. population, 1996-2014. In: 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
5. Indian Health Service. Special diabetes program for Indians. www.ihs.gov/newsroom/factsheets/diabetes. Accessed June 13, 2018.
6. National Kidney Disease Education Program. How well are your kidneys working? Explaining your kidney test results. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-education-outreach/explain-kidney-test-results. Accessed June 13, 2018.
Alaska is a vast state—larger than Texas, Montana, and California combined. It is also home to the highest percentage of American Indian (AI) and Alaska Native (AN) persons in the United States. These two populations—collectively referred to as Native Americans—have been served by the Indian Health Services (IHS) since it was established through the Snyder Act of 1921, in response to the dismal health conditions of the indigenous tribes in this country.1 Across the US (not only in Alaska), the IHS has partnered with AI/AN peoples to decrease health disparities in a culturally acceptable manner that honors and protects their traditions and values.
The IHS—which in 2016 comprised 2,500 nurses, 750 physicians, 700 pharmacists, 200 PAs and NPs, and 280 dentists, as well as nutritionists, diabetes educators, administrators, and other professionals—has made huge advances in decreasing health disparities in their populations. Among them: decreased rates of tuberculosis and of maternal and infant deaths.
However, life expectancy among Native Americans remains four years shorter than that of the rest of the US population. This disparity can be traced to three recalcitrant factors: unintentional injuries, liver disease, and diabetes.
The IHS practitioners decided to tackle diabetes with a multipronged approach. And what they achieved is astonishing.
WHAT THEY DID
Worldwide, diabetes is the most common cause of kidney failure; identifying patients with diabetes and early-stage chronic kidney disease allows for aggressive treatment that can slow progression to kidney failure and dialysis.
The IHS providers knew when they decided to tackle the problem of diabetes in the AI/AN population that the incidence was 16%—and the rate of diabetes leading to kidney failure in this population was the highest for any ethnic group in the US.2,3 And yet …
From 1996 to 2013, the rate of diabetes-related kidney failure among Native Americans dropped by 54%.3 Yes—the group of patients with the highest percentage of diabetes diagnoses has had the greatest improvement in prevention of kidney failure.4
Continue to: Some of the clinical achievements that contributed to...
Some of the clinical achievements that contributed to this significant change include
- Increased use of ACE inhibitors or angiotensin receptor blockers (ARBs) (from 42% to 74% over a five-year period)
- Reduced average blood pressure among hypertensive patients (to 133/76 mm Hg)
- Improved blood glucose control (by 10%)
- Increased testing for kidney disease among older patients (50% higher than the rest of the Medicare diabetes population).3
HOW THEY DID IT
This is not rocket science. The IHS staff integrated both population- and team-based approaches to achieve a more impressive decrease than ever could have been expected. In retrospect, perhaps this success should not come as such a surprise—many religious beliefs held by Native Americans focus around society, communal harmony, kinship, and cooperation.
The population health approach focused on promoting the wellness of the entire community and connecting people to local resources, including healthy food, transportation, housing, and mental health care. In the team approach, IHS medical experts implemented strategies to improve patient education, community outreach, care coordination, health outcome tracking, and access to a wide variety of health care providers.3,5
In a place like Alaska—where the northernmost city, Barrow, is more than 700 miles (two hours by plane) from Anchorage, and the southeastern Annette Island is more than 1,000 miles (six hours by plane) from the capital—this can be an especially challenging prospect. To reduce travel burden for rural patients, the IHS sponsors a diabetes team that travels from village to village. Nephrology services are not included in these field visits, however, so the kidney team relies heavily on telehealth. This requires extensive clinic staff coordination, as well as equipment and knowledgeable information systems support teams.
Other challenges require educational and logistical solutions. As noted, the use of ACE inhibitors and ARBs increased through the IHS’s efforts—and contributed to the delayed progression of diabetic kidney disease—but those additional prescriptions necessitate patient education. Understanding of these medications can be limited; many rural patients trust that when the bottle is empty, their practitioner has treated and cured their disease—mistakenly believing that no refills are needed. And even when the need to continue the prescription is understood, rural clinics may have difficulty tracking appointments and prescriptions written by providers at specialty clinics in Anchorage, making ongoing refills an issue.
Continue to: The necessary dietary changes can also be...
The necessary dietary changes can also be difficult for AI/AN populations. For example, in rural Alaska, tap water may not be safe to drink, and soda costs less than bottled water. Fresh produce is expensive and has often begun to spoil by the time it reaches local stores. The Native villagers often prefer their usual diets of gathered berries, fish, and red meat from subsistence hunting, making implementation of dietary changes difficult.
However, as the success of the IHS initiative shows, challenges can be met and overcome by practitioners who see a need, formulate a solution individualized to the circumstance, and think outside the box. One of the keys is developing a trusting relationship with patients. Another is to recognize informational needs and utilize available resources to educate patients. For example, visual representations of kidney function tend to be helpful in explaining the nature and course of disease; the National Kidney Disease Education Program uses an illustration similar to a gas gauge to demonstrate glomerular filtration rate (which would otherwise seem abstract and hard to understand for some patients; see below).6 When you understand your patient population and their needs, it makes addressing the challenging aspects of health care and prevention easier.
CONCLUSION
The results that the IHS achieved should serve as an example for all Americans with diabetes and their health care providers. We must be open to delivery of care via different approaches and practitioners in order to successfully help patients of different backgrounds and circumstances. This is the individualization of care that we hear so much about.
In 2016, the costs of caring for the kidney failure population were greater than the entire budget of the NIH. By aggressively identifying and treating patients at risk for kidney failure, we can slow disease progression—saving society money, but more importantly allowing our patients many more years of life free from the constraints of dialysis. —MET, RB
Mandy E. Thompson, PA-C
Kidney Center of Denver Health
Robin Bassett, DNP
Nephrology and Hypertension Associates, Anchorage
Adjunct Professor, NP program, University of Alaska, Anchorage
Alaska is a vast state—larger than Texas, Montana, and California combined. It is also home to the highest percentage of American Indian (AI) and Alaska Native (AN) persons in the United States. These two populations—collectively referred to as Native Americans—have been served by the Indian Health Services (IHS) since it was established through the Snyder Act of 1921, in response to the dismal health conditions of the indigenous tribes in this country.1 Across the US (not only in Alaska), the IHS has partnered with AI/AN peoples to decrease health disparities in a culturally acceptable manner that honors and protects their traditions and values.
The IHS—which in 2016 comprised 2,500 nurses, 750 physicians, 700 pharmacists, 200 PAs and NPs, and 280 dentists, as well as nutritionists, diabetes educators, administrators, and other professionals—has made huge advances in decreasing health disparities in their populations. Among them: decreased rates of tuberculosis and of maternal and infant deaths.
However, life expectancy among Native Americans remains four years shorter than that of the rest of the US population. This disparity can be traced to three recalcitrant factors: unintentional injuries, liver disease, and diabetes.
The IHS practitioners decided to tackle diabetes with a multipronged approach. And what they achieved is astonishing.
WHAT THEY DID
Worldwide, diabetes is the most common cause of kidney failure; identifying patients with diabetes and early-stage chronic kidney disease allows for aggressive treatment that can slow progression to kidney failure and dialysis.
The IHS providers knew when they decided to tackle the problem of diabetes in the AI/AN population that the incidence was 16%—and the rate of diabetes leading to kidney failure in this population was the highest for any ethnic group in the US.2,3 And yet …
From 1996 to 2013, the rate of diabetes-related kidney failure among Native Americans dropped by 54%.3 Yes—the group of patients with the highest percentage of diabetes diagnoses has had the greatest improvement in prevention of kidney failure.4
Continue to: Some of the clinical achievements that contributed to...
Some of the clinical achievements that contributed to this significant change include
- Increased use of ACE inhibitors or angiotensin receptor blockers (ARBs) (from 42% to 74% over a five-year period)
- Reduced average blood pressure among hypertensive patients (to 133/76 mm Hg)
- Improved blood glucose control (by 10%)
- Increased testing for kidney disease among older patients (50% higher than the rest of the Medicare diabetes population).3
HOW THEY DID IT
This is not rocket science. The IHS staff integrated both population- and team-based approaches to achieve a more impressive decrease than ever could have been expected. In retrospect, perhaps this success should not come as such a surprise—many religious beliefs held by Native Americans focus around society, communal harmony, kinship, and cooperation.
The population health approach focused on promoting the wellness of the entire community and connecting people to local resources, including healthy food, transportation, housing, and mental health care. In the team approach, IHS medical experts implemented strategies to improve patient education, community outreach, care coordination, health outcome tracking, and access to a wide variety of health care providers.3,5
In a place like Alaska—where the northernmost city, Barrow, is more than 700 miles (two hours by plane) from Anchorage, and the southeastern Annette Island is more than 1,000 miles (six hours by plane) from the capital—this can be an especially challenging prospect. To reduce travel burden for rural patients, the IHS sponsors a diabetes team that travels from village to village. Nephrology services are not included in these field visits, however, so the kidney team relies heavily on telehealth. This requires extensive clinic staff coordination, as well as equipment and knowledgeable information systems support teams.
Other challenges require educational and logistical solutions. As noted, the use of ACE inhibitors and ARBs increased through the IHS’s efforts—and contributed to the delayed progression of diabetic kidney disease—but those additional prescriptions necessitate patient education. Understanding of these medications can be limited; many rural patients trust that when the bottle is empty, their practitioner has treated and cured their disease—mistakenly believing that no refills are needed. And even when the need to continue the prescription is understood, rural clinics may have difficulty tracking appointments and prescriptions written by providers at specialty clinics in Anchorage, making ongoing refills an issue.
Continue to: The necessary dietary changes can also be...
The necessary dietary changes can also be difficult for AI/AN populations. For example, in rural Alaska, tap water may not be safe to drink, and soda costs less than bottled water. Fresh produce is expensive and has often begun to spoil by the time it reaches local stores. The Native villagers often prefer their usual diets of gathered berries, fish, and red meat from subsistence hunting, making implementation of dietary changes difficult.
However, as the success of the IHS initiative shows, challenges can be met and overcome by practitioners who see a need, formulate a solution individualized to the circumstance, and think outside the box. One of the keys is developing a trusting relationship with patients. Another is to recognize informational needs and utilize available resources to educate patients. For example, visual representations of kidney function tend to be helpful in explaining the nature and course of disease; the National Kidney Disease Education Program uses an illustration similar to a gas gauge to demonstrate glomerular filtration rate (which would otherwise seem abstract and hard to understand for some patients; see below).6 When you understand your patient population and their needs, it makes addressing the challenging aspects of health care and prevention easier.
CONCLUSION
The results that the IHS achieved should serve as an example for all Americans with diabetes and their health care providers. We must be open to delivery of care via different approaches and practitioners in order to successfully help patients of different backgrounds and circumstances. This is the individualization of care that we hear so much about.
In 2016, the costs of caring for the kidney failure population were greater than the entire budget of the NIH. By aggressively identifying and treating patients at risk for kidney failure, we can slow disease progression—saving society money, but more importantly allowing our patients many more years of life free from the constraints of dialysis. —MET, RB
Mandy E. Thompson, PA-C
Kidney Center of Denver Health
Robin Bassett, DNP
Nephrology and Hypertension Associates, Anchorage
Adjunct Professor, NP program, University of Alaska, Anchorage
1. Indian Health Service. Legislation. www.ihs.gov/aboutihs/legislation. Accessed June 13, 2018.
2. National Health Interview Survey and Indian Health Service, 2010-2012.
3. CDC. Native Americans with diabetes. www.cdc.gov/vitalsigns/aian-diabetes/. Accessed June 13, 2018.
4. United States Renal Data System. Figure 1.5: Trends in adjusted* ESRD incidence rate (per million/year), by race, in the U.S. population, 1996-2014. In: 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
5. Indian Health Service. Special diabetes program for Indians. www.ihs.gov/newsroom/factsheets/diabetes. Accessed June 13, 2018.
6. National Kidney Disease Education Program. How well are your kidneys working? Explaining your kidney test results. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-education-outreach/explain-kidney-test-results. Accessed June 13, 2018.
1. Indian Health Service. Legislation. www.ihs.gov/aboutihs/legislation. Accessed June 13, 2018.
2. National Health Interview Survey and Indian Health Service, 2010-2012.
3. CDC. Native Americans with diabetes. www.cdc.gov/vitalsigns/aian-diabetes/. Accessed June 13, 2018.
4. United States Renal Data System. Figure 1.5: Trends in adjusted* ESRD incidence rate (per million/year), by race, in the U.S. population, 1996-2014. In: 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
5. Indian Health Service. Special diabetes program for Indians. www.ihs.gov/newsroom/factsheets/diabetes. Accessed June 13, 2018.
6. National Kidney Disease Education Program. How well are your kidneys working? Explaining your kidney test results. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-education-outreach/explain-kidney-test-results. Accessed June 13, 2018.
Neuropathic Pain in MS
Q) How do I assess for and treat neuropathic pain in MS?
In multiple sclerosis (MS), pain is a common symptom; patients may experience varying forms during their disease course. One type is neuropathic pain, which is initiated or caused by a demyelinating lesion in the central nervous system.1 It may occur spontaneously or be evoked, and it can be intermittent or steady. Given the nature of the disease course in MS, it is important to complete a pain assessment at each visit.
A patient experiencing neuropathic pain is likely to report abnormal sensations or hypersensitivity in the affected area. It is often combined with or adjacent to areas of sensory deficit.1 This includes altered sensations such as pins and needles, numbness, crawling, or burning. The most common MS-related neuropathic pain conditions are ongoing dysaesthetic extremity pain and paroxysmal pain, such as trigeminal neuralgia and Lhermitte phenomenon.1-3
Assessment. When assessing the history of neuropathic pain, it is beneficial to remember that abnormal sensory findings should be neuroanatomically aligned with a lesion site. The mnemonic OPQRST is a helpful reminder to ask about
Onset
Provoking/palliating factors
Quality of the sensation
If it radiates
Severity of the pain (using a scale of 0-10 can be helpful)
Time when the pain occurs.
These probing questions will aid diagnosis and uncover clues on areas to pay special attention to during the examination. For example, when a patient reports numbness of both feet, the clinician might suspect a lesion in the spinal cord and then can try to determine the level during the sensory exam.
Screening tools that capture the patient experience, such as the modified version of the Brief Pain Inventory (BPI), can assist in diagnosis as well as measure the impact of treatment.4
A physical assessment for neuropathic pain includes a full neurologic evaluation of motor, sensory, and autonomic systems to identify all signs of neurologic dysfunction. Attention should be paid to the possible types of negative sensory symptoms (eg, sensory loss) and positive findings (eg, paresthesia). When completing the sensory exam, the clinician can gauge pain by using a sharp object such as a toothpick. Tactile sense can be assessed with a piece of cotton, and temperature can be tested with warm and cold objects. A tuning fork can identify vibration sense. Body sensory maps, on which the clinician draws the sensory disturbance on schematic charts, can provide valuable information.
Diagnostic tests, such as MRI, can also assist in confirming the lesion of the somatosensory nervous system that explains the pain.
Continue to: Treatment
Treatment. Many patients who experience neuropathic pain require a multidisciplinary approach.5 Support from colleagues in rehabilitation can help the patient identify alternative approaches to functioning that avoid triggering or exacerbating the pain. Equipment can also maximize independence and improve quality of life. For example, a soft neck collar is often used to prevent the forward movement that triggers pain in Lhermitte phenomenon.6
When prescribing pain medication, it is important to understand that neuropathic pain is inadequately relieved or not relieved at all with conventional analgesics, such as NSAIDs, or opioid analgesics (eg, morphine).2,3
Dysesthesias are most frequently treated with medications that are categorized as antiseizure, such as gabapentin and pregabalin. Carbamazepam and phenytoin are used as secondline therapy. Sometimes, anti-anxiety medication (eg, duloxetine hydrochloride and clonazepam or tricyclic antidepressants, including amitriptyline or nortriptyline) can be helpful.7 When treating paroxysmal symptoms such as trigeminal neuralgia, antiseizure medications can be effective. Carbamazepine is often the firstline of treatment. As a secondline, oxcarbazepine, lamotrigine, and/or baclofen may be used. In some cases, a referral to neurosurgery for a procedure to reduce pressure on the trigeminal nerve is required.5,8
It is also important to treat any additional symptoms that the pain may be causing, such as depression or social isolation. Referral for counseling as well as integrative health and wellness services can support the patient through a difficult time.5 —RS
Rachael Stacom, MS, ANP-BC, MSCN
Independence Care System, New York, NY
1. Zagon IS, Mclaughlin PJ. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane, Australia: Codon Publications. 2017.
2. O’Connor AB, Schwid SR, Hermann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
3. Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. 2011;12(15):2355-2368.
4. Osborne TL, Raichle KA, Jensen MP, et al. The reliability and validity of pain interference measures in persons with multiple sclerosis. J Pain Symptom Manage. 2006;32(3):217-229.
5. Sullivan AB, Scheman J, Lopresti A, Prayor-Patterson H. Interdisciplinary treatment of patients with multiple sclerosis and chronic pain. Int J MS Care. 2012;14(4):216-220.
6. MS Australia. Pain and multiple sclerosis (MS). www.msaustralia.org.au/publications/pain-and-multiple-sclerosis-ms. Accessed May 15, 2018.
7. Maloni H; National Multiple Sclerosis Society. Clinical bulletin: pain in multiple sclerosis. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Clinical_Bulletin_Pain-in-MS.pdf. Accessed May 15, 2018.
8. Multiple Sclerosis Association of America (MSAA). (H. Maloni, Ed.) The Motivator Winter/Spring. Retrieved from https://mymsaa.org/publications/motivator/winter-spring13/cover-story/pain. Accessed May 15, 2018.
Q) How do I assess for and treat neuropathic pain in MS?
In multiple sclerosis (MS), pain is a common symptom; patients may experience varying forms during their disease course. One type is neuropathic pain, which is initiated or caused by a demyelinating lesion in the central nervous system.1 It may occur spontaneously or be evoked, and it can be intermittent or steady. Given the nature of the disease course in MS, it is important to complete a pain assessment at each visit.
A patient experiencing neuropathic pain is likely to report abnormal sensations or hypersensitivity in the affected area. It is often combined with or adjacent to areas of sensory deficit.1 This includes altered sensations such as pins and needles, numbness, crawling, or burning. The most common MS-related neuropathic pain conditions are ongoing dysaesthetic extremity pain and paroxysmal pain, such as trigeminal neuralgia and Lhermitte phenomenon.1-3
Assessment. When assessing the history of neuropathic pain, it is beneficial to remember that abnormal sensory findings should be neuroanatomically aligned with a lesion site. The mnemonic OPQRST is a helpful reminder to ask about
Onset
Provoking/palliating factors
Quality of the sensation
If it radiates
Severity of the pain (using a scale of 0-10 can be helpful)
Time when the pain occurs.
These probing questions will aid diagnosis and uncover clues on areas to pay special attention to during the examination. For example, when a patient reports numbness of both feet, the clinician might suspect a lesion in the spinal cord and then can try to determine the level during the sensory exam.
Screening tools that capture the patient experience, such as the modified version of the Brief Pain Inventory (BPI), can assist in diagnosis as well as measure the impact of treatment.4
A physical assessment for neuropathic pain includes a full neurologic evaluation of motor, sensory, and autonomic systems to identify all signs of neurologic dysfunction. Attention should be paid to the possible types of negative sensory symptoms (eg, sensory loss) and positive findings (eg, paresthesia). When completing the sensory exam, the clinician can gauge pain by using a sharp object such as a toothpick. Tactile sense can be assessed with a piece of cotton, and temperature can be tested with warm and cold objects. A tuning fork can identify vibration sense. Body sensory maps, on which the clinician draws the sensory disturbance on schematic charts, can provide valuable information.
Diagnostic tests, such as MRI, can also assist in confirming the lesion of the somatosensory nervous system that explains the pain.
Continue to: Treatment
Treatment. Many patients who experience neuropathic pain require a multidisciplinary approach.5 Support from colleagues in rehabilitation can help the patient identify alternative approaches to functioning that avoid triggering or exacerbating the pain. Equipment can also maximize independence and improve quality of life. For example, a soft neck collar is often used to prevent the forward movement that triggers pain in Lhermitte phenomenon.6
When prescribing pain medication, it is important to understand that neuropathic pain is inadequately relieved or not relieved at all with conventional analgesics, such as NSAIDs, or opioid analgesics (eg, morphine).2,3
Dysesthesias are most frequently treated with medications that are categorized as antiseizure, such as gabapentin and pregabalin. Carbamazepam and phenytoin are used as secondline therapy. Sometimes, anti-anxiety medication (eg, duloxetine hydrochloride and clonazepam or tricyclic antidepressants, including amitriptyline or nortriptyline) can be helpful.7 When treating paroxysmal symptoms such as trigeminal neuralgia, antiseizure medications can be effective. Carbamazepine is often the firstline of treatment. As a secondline, oxcarbazepine, lamotrigine, and/or baclofen may be used. In some cases, a referral to neurosurgery for a procedure to reduce pressure on the trigeminal nerve is required.5,8
It is also important to treat any additional symptoms that the pain may be causing, such as depression or social isolation. Referral for counseling as well as integrative health and wellness services can support the patient through a difficult time.5 —RS
Rachael Stacom, MS, ANP-BC, MSCN
Independence Care System, New York, NY
Q) How do I assess for and treat neuropathic pain in MS?
In multiple sclerosis (MS), pain is a common symptom; patients may experience varying forms during their disease course. One type is neuropathic pain, which is initiated or caused by a demyelinating lesion in the central nervous system.1 It may occur spontaneously or be evoked, and it can be intermittent or steady. Given the nature of the disease course in MS, it is important to complete a pain assessment at each visit.
A patient experiencing neuropathic pain is likely to report abnormal sensations or hypersensitivity in the affected area. It is often combined with or adjacent to areas of sensory deficit.1 This includes altered sensations such as pins and needles, numbness, crawling, or burning. The most common MS-related neuropathic pain conditions are ongoing dysaesthetic extremity pain and paroxysmal pain, such as trigeminal neuralgia and Lhermitte phenomenon.1-3
Assessment. When assessing the history of neuropathic pain, it is beneficial to remember that abnormal sensory findings should be neuroanatomically aligned with a lesion site. The mnemonic OPQRST is a helpful reminder to ask about
Onset
Provoking/palliating factors
Quality of the sensation
If it radiates
Severity of the pain (using a scale of 0-10 can be helpful)
Time when the pain occurs.
These probing questions will aid diagnosis and uncover clues on areas to pay special attention to during the examination. For example, when a patient reports numbness of both feet, the clinician might suspect a lesion in the spinal cord and then can try to determine the level during the sensory exam.
Screening tools that capture the patient experience, such as the modified version of the Brief Pain Inventory (BPI), can assist in diagnosis as well as measure the impact of treatment.4
A physical assessment for neuropathic pain includes a full neurologic evaluation of motor, sensory, and autonomic systems to identify all signs of neurologic dysfunction. Attention should be paid to the possible types of negative sensory symptoms (eg, sensory loss) and positive findings (eg, paresthesia). When completing the sensory exam, the clinician can gauge pain by using a sharp object such as a toothpick. Tactile sense can be assessed with a piece of cotton, and temperature can be tested with warm and cold objects. A tuning fork can identify vibration sense. Body sensory maps, on which the clinician draws the sensory disturbance on schematic charts, can provide valuable information.
Diagnostic tests, such as MRI, can also assist in confirming the lesion of the somatosensory nervous system that explains the pain.
Continue to: Treatment
Treatment. Many patients who experience neuropathic pain require a multidisciplinary approach.5 Support from colleagues in rehabilitation can help the patient identify alternative approaches to functioning that avoid triggering or exacerbating the pain. Equipment can also maximize independence and improve quality of life. For example, a soft neck collar is often used to prevent the forward movement that triggers pain in Lhermitte phenomenon.6
When prescribing pain medication, it is important to understand that neuropathic pain is inadequately relieved or not relieved at all with conventional analgesics, such as NSAIDs, or opioid analgesics (eg, morphine).2,3
Dysesthesias are most frequently treated with medications that are categorized as antiseizure, such as gabapentin and pregabalin. Carbamazepam and phenytoin are used as secondline therapy. Sometimes, anti-anxiety medication (eg, duloxetine hydrochloride and clonazepam or tricyclic antidepressants, including amitriptyline or nortriptyline) can be helpful.7 When treating paroxysmal symptoms such as trigeminal neuralgia, antiseizure medications can be effective. Carbamazepine is often the firstline of treatment. As a secondline, oxcarbazepine, lamotrigine, and/or baclofen may be used. In some cases, a referral to neurosurgery for a procedure to reduce pressure on the trigeminal nerve is required.5,8
It is also important to treat any additional symptoms that the pain may be causing, such as depression or social isolation. Referral for counseling as well as integrative health and wellness services can support the patient through a difficult time.5 —RS
Rachael Stacom, MS, ANP-BC, MSCN
Independence Care System, New York, NY
1. Zagon IS, Mclaughlin PJ. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane, Australia: Codon Publications. 2017.
2. O’Connor AB, Schwid SR, Hermann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
3. Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. 2011;12(15):2355-2368.
4. Osborne TL, Raichle KA, Jensen MP, et al. The reliability and validity of pain interference measures in persons with multiple sclerosis. J Pain Symptom Manage. 2006;32(3):217-229.
5. Sullivan AB, Scheman J, Lopresti A, Prayor-Patterson H. Interdisciplinary treatment of patients with multiple sclerosis and chronic pain. Int J MS Care. 2012;14(4):216-220.
6. MS Australia. Pain and multiple sclerosis (MS). www.msaustralia.org.au/publications/pain-and-multiple-sclerosis-ms. Accessed May 15, 2018.
7. Maloni H; National Multiple Sclerosis Society. Clinical bulletin: pain in multiple sclerosis. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Clinical_Bulletin_Pain-in-MS.pdf. Accessed May 15, 2018.
8. Multiple Sclerosis Association of America (MSAA). (H. Maloni, Ed.) The Motivator Winter/Spring. Retrieved from https://mymsaa.org/publications/motivator/winter-spring13/cover-story/pain. Accessed May 15, 2018.
1. Zagon IS, Mclaughlin PJ. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane, Australia: Codon Publications. 2017.
2. O’Connor AB, Schwid SR, Hermann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
3. Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. 2011;12(15):2355-2368.
4. Osborne TL, Raichle KA, Jensen MP, et al. The reliability and validity of pain interference measures in persons with multiple sclerosis. J Pain Symptom Manage. 2006;32(3):217-229.
5. Sullivan AB, Scheman J, Lopresti A, Prayor-Patterson H. Interdisciplinary treatment of patients with multiple sclerosis and chronic pain. Int J MS Care. 2012;14(4):216-220.
6. MS Australia. Pain and multiple sclerosis (MS). www.msaustralia.org.au/publications/pain-and-multiple-sclerosis-ms. Accessed May 15, 2018.
7. Maloni H; National Multiple Sclerosis Society. Clinical bulletin: pain in multiple sclerosis. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Clinical_Bulletin_Pain-in-MS.pdf. Accessed May 15, 2018.
8. Multiple Sclerosis Association of America (MSAA). (H. Maloni, Ed.) The Motivator Winter/Spring. Retrieved from https://mymsaa.org/publications/motivator/winter-spring13/cover-story/pain. Accessed May 15, 2018.
What’s New in Diabetes Management: Psychosocial Care
The wide array of comorbidities and treatment variables can make diabetes a difficult disease to manage—and to live with. Providers must be equipped to address the complexities and complications that affect patients with diabetes. In December 2016, the American Diabetes Association published a position statement recognizing the psychosocial factors (environmental, social, behavioral, and emotional) that affect medical outcomes and psychological well-being in persons with diabetes. These include self-management, diabetes distress, psychological comorbidities, and life-course considerations.1
SELF-MANAGEMENT
A patient’s perception of his or her ability to self-manage diabetes is an important psychosocial factor in treatment and management outcomes. Training patients with diabetes in self-care skills and the use of technologies—at the time of diagnosis, annually, and/or when complications or transitions in care occur—can empower patients to assume an active role in their daily management. These interventions can be tailored to address specific, individualized problems that contribute to suboptimal glycemic outcomes, such as issues in numeracy or coping, food insecurity, or lack of support. Employing a nonjudgmental approach that normalizes periodic lapses in self-management may help encourage patients and minimize their resistance to self-management.
DIABETES DISTRESS
The frustration, worry, anger, guilt, and burnout imposed by diabetes and its management (via glucose monitoring, medication dosing, and insulin titration) is known as diabetes distress. With a reported prevalence of 18% to 45%, this disease burden is quite common.2 Because high levels of diabetes distress are associated with low self-efficacy, poor glycemic outcomes, and suboptimal exercise/dietary habits, referral for counseling should be considered if a patient expresses feelings of distress.
Use of validated screening tools, such as Problem Areas in Diabetes (PAID)3,4 or the Diabetes Distress Scale (DDS)5, can aid in routine monitoring for diabetes distress. (See the Table for more information.) If distress is identified in specific self-care areas, further patient education on self-management is appropriate.
PSYCHOLOGICAL COMORBIDITIES
Depression, anxiety, disordered eating, and serious mental illness (eg, schizophrenia) are known psychological comorbidities of diabetes. Screening for symptoms using patient-appropriate, standardized/validated tools should occur at initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance.
Depression
Patients with diabetes should be screened for depression when medical status worsens or when complications occur; it is recommended to include caregivers and family members in this assessment. Patients who screen positive for depression should be referred to mental health providers who have experience with cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches, and who can provide collaborative care alongside the diabetes treatment team. Once diagnosed with depression, patients should be screened annually.
Anxiety
Expression of fear, dread, or irrational thoughts, avoidant and/or repetitious behaviors, and social withdrawal are signs of anxiety that should prompt screening. Consider screening for anxiety in patients who express worry about diabetes complications, insulin injections or infusion, taking medications, and/or hypoglycemia that interferes with self-management behaviors.
Continue to: Patients with hypoglycemia unawareness...
Patients with hypoglycemia unawareness, which can co-occur with fear of hypoglycemia, can increase self-monitoring of glucose with a glucometer or continuous glucose monitor. Blood Glucose Awareness Training (or other similar evidence-based intervention) can be used to help reestablish awareness of hypoglycemia and reduce fear of hypoglycemia.6-8 Providers can deliver hypoglycemia awareness education in the clinic.
Disordered Eating
When hyperglycemia and weight loss are unexplained by self-reported medication dosing, diet, and exercise, consider screening for disordered or disrupted eating (see Table for screening tools). In addition, reviewing the medical regimen is recommended to identify potential treatment-related effects on hunger/caloric intake.
Cognitive Impairment
Since research has shown significantly increased rates of diabetes among persons with serious mental illness (eg, schizophrenia), annual screening for prediabetes and diabetes is recommended for those taking atypical antipsychotic medications. Furthermore, some of the effects of serious mental illness—such as disordered thinking and impaired judgment—make it difficult for a patient to engage in risk-reducing behaviors or (if diagnosed) to manage diabetes. Therefore, monitoring of diabetes self-care activities should be incorporated into treatment goals for persons with these comorbid conditions.
Continue to: CONCLUSION
CONCLUSION
As providers, we should be familiar with the evidence-based, validated tools available to identify the psychosocial comorbidities of diabetes. Screening and assessing patients for psychosocial/behavioral challenges should be performed at an initial visit, at periodic intervals, and whenever there is a change in disease, treatment, or life circumstances.
Health care alliances with behavioral/mental health providers who are knowledgeable about diabetes treatment and the psychosocial aspects of diabetes are key. Patient-centered care is essential to promote optimal medical outcomes and psychological well-being. As members of the health care team, we must be respectful and responsive to patient preferences, needs, and values; clinical decisions should be guided by patient values. If A1C is not at goal despite maximized medication therapy and lifestyle modification, consider identifying and addressing any psychosocial factors that may be involved.
1. Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140.
2. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472-2478.
3. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760.
4. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69-72.
5. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631.
6. Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood Glucose Awareness Training (BGAT-2). Diabetes Care. 2001;24(4):637-642.
7. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264.
8. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801-806.
The wide array of comorbidities and treatment variables can make diabetes a difficult disease to manage—and to live with. Providers must be equipped to address the complexities and complications that affect patients with diabetes. In December 2016, the American Diabetes Association published a position statement recognizing the psychosocial factors (environmental, social, behavioral, and emotional) that affect medical outcomes and psychological well-being in persons with diabetes. These include self-management, diabetes distress, psychological comorbidities, and life-course considerations.1
SELF-MANAGEMENT
A patient’s perception of his or her ability to self-manage diabetes is an important psychosocial factor in treatment and management outcomes. Training patients with diabetes in self-care skills and the use of technologies—at the time of diagnosis, annually, and/or when complications or transitions in care occur—can empower patients to assume an active role in their daily management. These interventions can be tailored to address specific, individualized problems that contribute to suboptimal glycemic outcomes, such as issues in numeracy or coping, food insecurity, or lack of support. Employing a nonjudgmental approach that normalizes periodic lapses in self-management may help encourage patients and minimize their resistance to self-management.
DIABETES DISTRESS
The frustration, worry, anger, guilt, and burnout imposed by diabetes and its management (via glucose monitoring, medication dosing, and insulin titration) is known as diabetes distress. With a reported prevalence of 18% to 45%, this disease burden is quite common.2 Because high levels of diabetes distress are associated with low self-efficacy, poor glycemic outcomes, and suboptimal exercise/dietary habits, referral for counseling should be considered if a patient expresses feelings of distress.
Use of validated screening tools, such as Problem Areas in Diabetes (PAID)3,4 or the Diabetes Distress Scale (DDS)5, can aid in routine monitoring for diabetes distress. (See the Table for more information.) If distress is identified in specific self-care areas, further patient education on self-management is appropriate.
PSYCHOLOGICAL COMORBIDITIES
Depression, anxiety, disordered eating, and serious mental illness (eg, schizophrenia) are known psychological comorbidities of diabetes. Screening for symptoms using patient-appropriate, standardized/validated tools should occur at initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance.
Depression
Patients with diabetes should be screened for depression when medical status worsens or when complications occur; it is recommended to include caregivers and family members in this assessment. Patients who screen positive for depression should be referred to mental health providers who have experience with cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches, and who can provide collaborative care alongside the diabetes treatment team. Once diagnosed with depression, patients should be screened annually.
Anxiety
Expression of fear, dread, or irrational thoughts, avoidant and/or repetitious behaviors, and social withdrawal are signs of anxiety that should prompt screening. Consider screening for anxiety in patients who express worry about diabetes complications, insulin injections or infusion, taking medications, and/or hypoglycemia that interferes with self-management behaviors.
Continue to: Patients with hypoglycemia unawareness...
Patients with hypoglycemia unawareness, which can co-occur with fear of hypoglycemia, can increase self-monitoring of glucose with a glucometer or continuous glucose monitor. Blood Glucose Awareness Training (or other similar evidence-based intervention) can be used to help reestablish awareness of hypoglycemia and reduce fear of hypoglycemia.6-8 Providers can deliver hypoglycemia awareness education in the clinic.
Disordered Eating
When hyperglycemia and weight loss are unexplained by self-reported medication dosing, diet, and exercise, consider screening for disordered or disrupted eating (see Table for screening tools). In addition, reviewing the medical regimen is recommended to identify potential treatment-related effects on hunger/caloric intake.
Cognitive Impairment
Since research has shown significantly increased rates of diabetes among persons with serious mental illness (eg, schizophrenia), annual screening for prediabetes and diabetes is recommended for those taking atypical antipsychotic medications. Furthermore, some of the effects of serious mental illness—such as disordered thinking and impaired judgment—make it difficult for a patient to engage in risk-reducing behaviors or (if diagnosed) to manage diabetes. Therefore, monitoring of diabetes self-care activities should be incorporated into treatment goals for persons with these comorbid conditions.
Continue to: CONCLUSION
CONCLUSION
As providers, we should be familiar with the evidence-based, validated tools available to identify the psychosocial comorbidities of diabetes. Screening and assessing patients for psychosocial/behavioral challenges should be performed at an initial visit, at periodic intervals, and whenever there is a change in disease, treatment, or life circumstances.
Health care alliances with behavioral/mental health providers who are knowledgeable about diabetes treatment and the psychosocial aspects of diabetes are key. Patient-centered care is essential to promote optimal medical outcomes and psychological well-being. As members of the health care team, we must be respectful and responsive to patient preferences, needs, and values; clinical decisions should be guided by patient values. If A1C is not at goal despite maximized medication therapy and lifestyle modification, consider identifying and addressing any psychosocial factors that may be involved.
The wide array of comorbidities and treatment variables can make diabetes a difficult disease to manage—and to live with. Providers must be equipped to address the complexities and complications that affect patients with diabetes. In December 2016, the American Diabetes Association published a position statement recognizing the psychosocial factors (environmental, social, behavioral, and emotional) that affect medical outcomes and psychological well-being in persons with diabetes. These include self-management, diabetes distress, psychological comorbidities, and life-course considerations.1
SELF-MANAGEMENT
A patient’s perception of his or her ability to self-manage diabetes is an important psychosocial factor in treatment and management outcomes. Training patients with diabetes in self-care skills and the use of technologies—at the time of diagnosis, annually, and/or when complications or transitions in care occur—can empower patients to assume an active role in their daily management. These interventions can be tailored to address specific, individualized problems that contribute to suboptimal glycemic outcomes, such as issues in numeracy or coping, food insecurity, or lack of support. Employing a nonjudgmental approach that normalizes periodic lapses in self-management may help encourage patients and minimize their resistance to self-management.
DIABETES DISTRESS
The frustration, worry, anger, guilt, and burnout imposed by diabetes and its management (via glucose monitoring, medication dosing, and insulin titration) is known as diabetes distress. With a reported prevalence of 18% to 45%, this disease burden is quite common.2 Because high levels of diabetes distress are associated with low self-efficacy, poor glycemic outcomes, and suboptimal exercise/dietary habits, referral for counseling should be considered if a patient expresses feelings of distress.
Use of validated screening tools, such as Problem Areas in Diabetes (PAID)3,4 or the Diabetes Distress Scale (DDS)5, can aid in routine monitoring for diabetes distress. (See the Table for more information.) If distress is identified in specific self-care areas, further patient education on self-management is appropriate.
PSYCHOLOGICAL COMORBIDITIES
Depression, anxiety, disordered eating, and serious mental illness (eg, schizophrenia) are known psychological comorbidities of diabetes. Screening for symptoms using patient-appropriate, standardized/validated tools should occur at initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance.
Depression
Patients with diabetes should be screened for depression when medical status worsens or when complications occur; it is recommended to include caregivers and family members in this assessment. Patients who screen positive for depression should be referred to mental health providers who have experience with cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches, and who can provide collaborative care alongside the diabetes treatment team. Once diagnosed with depression, patients should be screened annually.
Anxiety
Expression of fear, dread, or irrational thoughts, avoidant and/or repetitious behaviors, and social withdrawal are signs of anxiety that should prompt screening. Consider screening for anxiety in patients who express worry about diabetes complications, insulin injections or infusion, taking medications, and/or hypoglycemia that interferes with self-management behaviors.
Continue to: Patients with hypoglycemia unawareness...
Patients with hypoglycemia unawareness, which can co-occur with fear of hypoglycemia, can increase self-monitoring of glucose with a glucometer or continuous glucose monitor. Blood Glucose Awareness Training (or other similar evidence-based intervention) can be used to help reestablish awareness of hypoglycemia and reduce fear of hypoglycemia.6-8 Providers can deliver hypoglycemia awareness education in the clinic.
Disordered Eating
When hyperglycemia and weight loss are unexplained by self-reported medication dosing, diet, and exercise, consider screening for disordered or disrupted eating (see Table for screening tools). In addition, reviewing the medical regimen is recommended to identify potential treatment-related effects on hunger/caloric intake.
Cognitive Impairment
Since research has shown significantly increased rates of diabetes among persons with serious mental illness (eg, schizophrenia), annual screening for prediabetes and diabetes is recommended for those taking atypical antipsychotic medications. Furthermore, some of the effects of serious mental illness—such as disordered thinking and impaired judgment—make it difficult for a patient to engage in risk-reducing behaviors or (if diagnosed) to manage diabetes. Therefore, monitoring of diabetes self-care activities should be incorporated into treatment goals for persons with these comorbid conditions.
Continue to: CONCLUSION
CONCLUSION
As providers, we should be familiar with the evidence-based, validated tools available to identify the psychosocial comorbidities of diabetes. Screening and assessing patients for psychosocial/behavioral challenges should be performed at an initial visit, at periodic intervals, and whenever there is a change in disease, treatment, or life circumstances.
Health care alliances with behavioral/mental health providers who are knowledgeable about diabetes treatment and the psychosocial aspects of diabetes are key. Patient-centered care is essential to promote optimal medical outcomes and psychological well-being. As members of the health care team, we must be respectful and responsive to patient preferences, needs, and values; clinical decisions should be guided by patient values. If A1C is not at goal despite maximized medication therapy and lifestyle modification, consider identifying and addressing any psychosocial factors that may be involved.
1. Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140.
2. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472-2478.
3. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760.
4. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69-72.
5. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631.
6. Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood Glucose Awareness Training (BGAT-2). Diabetes Care. 2001;24(4):637-642.
7. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264.
8. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801-806.
1. Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140.
2. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472-2478.
3. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760.
4. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69-72.
5. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631.
6. Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood Glucose Awareness Training (BGAT-2). Diabetes Care. 2001;24(4):637-642.
7. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264.
8. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801-806.
Can African-American Patients Take Metoprolol?
Q) One of the physicians in my practice won't use metoprolol in African-American patients. He says it causes kidney disease. Is it right, or is this an old wives' tale?
There are multiple concerns with the use of metoprolol specifically—this does not apply to all ß-blockers—in the African-American population. The main concerns are
- Lack of effective blood pressure control, compared to angiotensin-converting enzyme (ACE) inhibitors and calcium channel blockers (CCBs)
- No observable reduction in proteinuria
- The possibility of a significant increase in uric acid.
Most of the evidence-based guidelines for care of hypertensive nephrosclerosis in the African-American population were derived from the African-American Study of Kidney Disease and Hypertension (AASK) trial. This large-scale, multicenter, randomized, double-blinded study from the National Institute of Health had multiple arms to compare an ACE inhibitor (ramipril) to a CCB (amlodipine) or a ß-blocker (metoprolol) in the nondiabetic African-American population.1
In a subgroup analysis, more than 1,000 subjects with hypertensive nephrosclerosis were followed for four years, with serial glomerular filtration rate (GFR) measurements taken. Treatment with ACE inhibitors was shown to be superior to CCB and ß-blockers for hypertension and proteinuria control.1
One important take-away from the AASK trial has been that strict blood pressure control is not enough to improve kidney outcomes. Proteinuria (albuminuria) must also be controlled.1
Continue to: In a subsequent secondary analysis
In a subsequent secondary analysis of data from the AASK study, Juraschek et al showed that metoprolol significantly increased serum uric acid in African-American adults.2 It is known that hyperuricemia (> 6 mg/dL) can cause a decline in kidney function.3
Furthermore, uric acid may be a strong prognostic factor for chronic kidney disease (CKD) progression. (This association, however, remains controversial. One recent study showed that, while hyperuricemia is associated with higher risk for kidney failure, the relationship was not parallel in CKD stage 3 or 4 [GFR ≤ 60 mL/min]).4 In fact, taking uric acid–lowering medications did not slow progression of kidney disease.
In other words, your colleague seems to believe that since A (metoprolol) leads to B (hyperuricemia) and B (hyperuricemia) leads to C (kidney disease), then A leads to C. While the theory is undoubtedly logical, we have no proof that metoprolol causes increased kidney disease in African-American patients.
What we do know, thanks to AASK, is that an African-American patient with kidney disease should be treated with a diuretic and/or an ACE inhibitor as initial therapy. Furthermore, we have a blood pressure goal: < 130/80 mm Hg. And we know that CCBs are most effective for African-American patients who do not have kidney disease.5—BWM
Barbara Weis Malone, DNP, FNP-C, FNKF
Assistant Professor
Adult/Gerontology NP Program, College of Nursing
Nurse Practitioner
School of Medicine, University of Colorado Anschutz Medical Campus
1. Toto RD. Lessons from the African-American Study of Kidney Disease and Hypertension: an update. Curr Hypertens Rep. 2006;8(5):409-412.
2. Juraschek SP, Appel LJ, Miller ER III. Metoprolol increases uric acid and risk of gout in African Americans with chronic kidney disease attributed to hypertension. Am J Hypertens. 2017; 30(9):871-875.
3. Tsai C-W, Lin S-Y, Kuo C-C, Huang C-C. Serum uric acid and progression of kidney disease: a longitudinal analysis and mini-review. PLoS One. 2017;12(1):e0170393.
4. Rincon-Choles H, Jolly SE, Arrigain S, et al. Impact of uric acid levels on kidney disease progression. Am J Nephrol. 2017;46(4):315-322.
5. Armstrong C. JNC8 guidelines for the management of hypertension in adults. Am Fam Physician. 2014;90(7):503-504.
Q) One of the physicians in my practice won't use metoprolol in African-American patients. He says it causes kidney disease. Is it right, or is this an old wives' tale?
There are multiple concerns with the use of metoprolol specifically—this does not apply to all ß-blockers—in the African-American population. The main concerns are
- Lack of effective blood pressure control, compared to angiotensin-converting enzyme (ACE) inhibitors and calcium channel blockers (CCBs)
- No observable reduction in proteinuria
- The possibility of a significant increase in uric acid.
Most of the evidence-based guidelines for care of hypertensive nephrosclerosis in the African-American population were derived from the African-American Study of Kidney Disease and Hypertension (AASK) trial. This large-scale, multicenter, randomized, double-blinded study from the National Institute of Health had multiple arms to compare an ACE inhibitor (ramipril) to a CCB (amlodipine) or a ß-blocker (metoprolol) in the nondiabetic African-American population.1
In a subgroup analysis, more than 1,000 subjects with hypertensive nephrosclerosis were followed for four years, with serial glomerular filtration rate (GFR) measurements taken. Treatment with ACE inhibitors was shown to be superior to CCB and ß-blockers for hypertension and proteinuria control.1
One important take-away from the AASK trial has been that strict blood pressure control is not enough to improve kidney outcomes. Proteinuria (albuminuria) must also be controlled.1
Continue to: In a subsequent secondary analysis
In a subsequent secondary analysis of data from the AASK study, Juraschek et al showed that metoprolol significantly increased serum uric acid in African-American adults.2 It is known that hyperuricemia (> 6 mg/dL) can cause a decline in kidney function.3
Furthermore, uric acid may be a strong prognostic factor for chronic kidney disease (CKD) progression. (This association, however, remains controversial. One recent study showed that, while hyperuricemia is associated with higher risk for kidney failure, the relationship was not parallel in CKD stage 3 or 4 [GFR ≤ 60 mL/min]).4 In fact, taking uric acid–lowering medications did not slow progression of kidney disease.
In other words, your colleague seems to believe that since A (metoprolol) leads to B (hyperuricemia) and B (hyperuricemia) leads to C (kidney disease), then A leads to C. While the theory is undoubtedly logical, we have no proof that metoprolol causes increased kidney disease in African-American patients.
What we do know, thanks to AASK, is that an African-American patient with kidney disease should be treated with a diuretic and/or an ACE inhibitor as initial therapy. Furthermore, we have a blood pressure goal: < 130/80 mm Hg. And we know that CCBs are most effective for African-American patients who do not have kidney disease.5—BWM
Barbara Weis Malone, DNP, FNP-C, FNKF
Assistant Professor
Adult/Gerontology NP Program, College of Nursing
Nurse Practitioner
School of Medicine, University of Colorado Anschutz Medical Campus
Q) One of the physicians in my practice won't use metoprolol in African-American patients. He says it causes kidney disease. Is it right, or is this an old wives' tale?
There are multiple concerns with the use of metoprolol specifically—this does not apply to all ß-blockers—in the African-American population. The main concerns are
- Lack of effective blood pressure control, compared to angiotensin-converting enzyme (ACE) inhibitors and calcium channel blockers (CCBs)
- No observable reduction in proteinuria
- The possibility of a significant increase in uric acid.
Most of the evidence-based guidelines for care of hypertensive nephrosclerosis in the African-American population were derived from the African-American Study of Kidney Disease and Hypertension (AASK) trial. This large-scale, multicenter, randomized, double-blinded study from the National Institute of Health had multiple arms to compare an ACE inhibitor (ramipril) to a CCB (amlodipine) or a ß-blocker (metoprolol) in the nondiabetic African-American population.1
In a subgroup analysis, more than 1,000 subjects with hypertensive nephrosclerosis were followed for four years, with serial glomerular filtration rate (GFR) measurements taken. Treatment with ACE inhibitors was shown to be superior to CCB and ß-blockers for hypertension and proteinuria control.1
One important take-away from the AASK trial has been that strict blood pressure control is not enough to improve kidney outcomes. Proteinuria (albuminuria) must also be controlled.1
Continue to: In a subsequent secondary analysis
In a subsequent secondary analysis of data from the AASK study, Juraschek et al showed that metoprolol significantly increased serum uric acid in African-American adults.2 It is known that hyperuricemia (> 6 mg/dL) can cause a decline in kidney function.3
Furthermore, uric acid may be a strong prognostic factor for chronic kidney disease (CKD) progression. (This association, however, remains controversial. One recent study showed that, while hyperuricemia is associated with higher risk for kidney failure, the relationship was not parallel in CKD stage 3 or 4 [GFR ≤ 60 mL/min]).4 In fact, taking uric acid–lowering medications did not slow progression of kidney disease.
In other words, your colleague seems to believe that since A (metoprolol) leads to B (hyperuricemia) and B (hyperuricemia) leads to C (kidney disease), then A leads to C. While the theory is undoubtedly logical, we have no proof that metoprolol causes increased kidney disease in African-American patients.
What we do know, thanks to AASK, is that an African-American patient with kidney disease should be treated with a diuretic and/or an ACE inhibitor as initial therapy. Furthermore, we have a blood pressure goal: < 130/80 mm Hg. And we know that CCBs are most effective for African-American patients who do not have kidney disease.5—BWM
Barbara Weis Malone, DNP, FNP-C, FNKF
Assistant Professor
Adult/Gerontology NP Program, College of Nursing
Nurse Practitioner
School of Medicine, University of Colorado Anschutz Medical Campus
1. Toto RD. Lessons from the African-American Study of Kidney Disease and Hypertension: an update. Curr Hypertens Rep. 2006;8(5):409-412.
2. Juraschek SP, Appel LJ, Miller ER III. Metoprolol increases uric acid and risk of gout in African Americans with chronic kidney disease attributed to hypertension. Am J Hypertens. 2017; 30(9):871-875.
3. Tsai C-W, Lin S-Y, Kuo C-C, Huang C-C. Serum uric acid and progression of kidney disease: a longitudinal analysis and mini-review. PLoS One. 2017;12(1):e0170393.
4. Rincon-Choles H, Jolly SE, Arrigain S, et al. Impact of uric acid levels on kidney disease progression. Am J Nephrol. 2017;46(4):315-322.
5. Armstrong C. JNC8 guidelines for the management of hypertension in adults. Am Fam Physician. 2014;90(7):503-504.
1. Toto RD. Lessons from the African-American Study of Kidney Disease and Hypertension: an update. Curr Hypertens Rep. 2006;8(5):409-412.
2. Juraschek SP, Appel LJ, Miller ER III. Metoprolol increases uric acid and risk of gout in African Americans with chronic kidney disease attributed to hypertension. Am J Hypertens. 2017; 30(9):871-875.
3. Tsai C-W, Lin S-Y, Kuo C-C, Huang C-C. Serum uric acid and progression of kidney disease: a longitudinal analysis and mini-review. PLoS One. 2017;12(1):e0170393.
4. Rincon-Choles H, Jolly SE, Arrigain S, et al. Impact of uric acid levels on kidney disease progression. Am J Nephrol. 2017;46(4):315-322.
5. Armstrong C. JNC8 guidelines for the management of hypertension in adults. Am Fam Physician. 2014;90(7):503-504.
Is “Runner’s Kidney” a Thing?
Q) Many of my patients are athletes. I recall reading something about kidney disease in marathon runners. Am I remembering correctly?
Although data on acute kidney injury (AKI) in marathon runners are limited, two recent studies have added to our knowledge. In 2017, Mansour et al studied 22 marathon runners, collecting urine and blood samples 24 hours before, immediately after, and 24 hours after a race. The results showed that in 82% of the subjects, serum creatinine increased to a level correlated with stage 1 or 2 AKI (as defined by the Acute Kidney Injury Network criteria).1
Based on urine microscopy results, as well as serum creatinine and novel biomarker levels, the researchers concluded that the runners’ AKI was caused by acute tubular injury—likely induced by ischemia. However, the subjects did not show any evidence of chronic kidney disease (CKD), despite years of running and intensive training. One theory: Habitual running might condition the kidneys to transient ischemic conditions—in other words, they build tolerance to repetitive injury over time.1
Continue to: The other recent study
The other recent study examined use of NSAIDs by ultramarathon
In summary: While marathon runners are prone to AKI, the injury seems to be transient and does not progress to CKD. Furthermore, use of NSAIDs during endurance running may contribute to AKI development, so patients should be advised to use caution with these analgesics. Finally, remind your endurance runners to stay hydrated, since it may help to limit kidney damage. As for the casual runner? The impact on the kidney remains unclear and needs further investigation. —DSW
Danielle S. Wentworth, MSN, FNP-BC
Division of Nephrology, University of Viriginia Health System, Charlottesville
1. Mansour SG, Verma G, Pata RW, et al. Kidney injury and repair biomarkers in marathon runners. Am J Kidney Dis. 2017;70(2):252-261.
2. Lipman GS, Shea K, Christensen M, et al. Ibuprofen versus placebo effect on acute kidney injury in ultramarathons: a randomised controlled trial. Emerg Med J. 2017;34(10):637-642.
Q) Many of my patients are athletes. I recall reading something about kidney disease in marathon runners. Am I remembering correctly?
Although data on acute kidney injury (AKI) in marathon runners are limited, two recent studies have added to our knowledge. In 2017, Mansour et al studied 22 marathon runners, collecting urine and blood samples 24 hours before, immediately after, and 24 hours after a race. The results showed that in 82% of the subjects, serum creatinine increased to a level correlated with stage 1 or 2 AKI (as defined by the Acute Kidney Injury Network criteria).1
Based on urine microscopy results, as well as serum creatinine and novel biomarker levels, the researchers concluded that the runners’ AKI was caused by acute tubular injury—likely induced by ischemia. However, the subjects did not show any evidence of chronic kidney disease (CKD), despite years of running and intensive training. One theory: Habitual running might condition the kidneys to transient ischemic conditions—in other words, they build tolerance to repetitive injury over time.1
Continue to: The other recent study
The other recent study examined use of NSAIDs by ultramarathon
In summary: While marathon runners are prone to AKI, the injury seems to be transient and does not progress to CKD. Furthermore, use of NSAIDs during endurance running may contribute to AKI development, so patients should be advised to use caution with these analgesics. Finally, remind your endurance runners to stay hydrated, since it may help to limit kidney damage. As for the casual runner? The impact on the kidney remains unclear and needs further investigation. —DSW
Danielle S. Wentworth, MSN, FNP-BC
Division of Nephrology, University of Viriginia Health System, Charlottesville
Q) Many of my patients are athletes. I recall reading something about kidney disease in marathon runners. Am I remembering correctly?
Although data on acute kidney injury (AKI) in marathon runners are limited, two recent studies have added to our knowledge. In 2017, Mansour et al studied 22 marathon runners, collecting urine and blood samples 24 hours before, immediately after, and 24 hours after a race. The results showed that in 82% of the subjects, serum creatinine increased to a level correlated with stage 1 or 2 AKI (as defined by the Acute Kidney Injury Network criteria).1
Based on urine microscopy results, as well as serum creatinine and novel biomarker levels, the researchers concluded that the runners’ AKI was caused by acute tubular injury—likely induced by ischemia. However, the subjects did not show any evidence of chronic kidney disease (CKD), despite years of running and intensive training. One theory: Habitual running might condition the kidneys to transient ischemic conditions—in other words, they build tolerance to repetitive injury over time.1
Continue to: The other recent study
The other recent study examined use of NSAIDs by ultramarathon
In summary: While marathon runners are prone to AKI, the injury seems to be transient and does not progress to CKD. Furthermore, use of NSAIDs during endurance running may contribute to AKI development, so patients should be advised to use caution with these analgesics. Finally, remind your endurance runners to stay hydrated, since it may help to limit kidney damage. As for the casual runner? The impact on the kidney remains unclear and needs further investigation. —DSW
Danielle S. Wentworth, MSN, FNP-BC
Division of Nephrology, University of Viriginia Health System, Charlottesville
1. Mansour SG, Verma G, Pata RW, et al. Kidney injury and repair biomarkers in marathon runners. Am J Kidney Dis. 2017;70(2):252-261.
2. Lipman GS, Shea K, Christensen M, et al. Ibuprofen versus placebo effect on acute kidney injury in ultramarathons: a randomised controlled trial. Emerg Med J. 2017;34(10):637-642.
1. Mansour SG, Verma G, Pata RW, et al. Kidney injury and repair biomarkers in marathon runners. Am J Kidney Dis. 2017;70(2):252-261.
2. Lipman GS, Shea K, Christensen M, et al. Ibuprofen versus placebo effect on acute kidney injury in ultramarathons: a randomised controlled trial. Emerg Med J. 2017;34(10):637-642.
The ACA and Multiple Sclerosis
Q) How has the Affordable Care Act affected people living with multiple sclerosis—an Americans with Disabilities Act recognized disease?
The Affordable Care Act (ACA) has been a source of controversy since it became law in 2010. Perhaps some of the tension surrounding it stems from misunderstanding; however, it is clear that individual experiences and/or perceptions flavor the ongoing debate. Rather than perpetuate the contention, we’d simply like to outline some of the ways in which patients with multiple sclerosis (MS) have benefited from the ACA—and what we must do to ensure continued quality and affordability of care in the event of changes to the law.
Living with MS in the United States is costly. According to the National Multiple Sclerosis Society, average annual costs—both direct and indirect (ie, lost wages)—are about $69,000. Health care costs account for more than half of this total (about $39,000). Total costs for all people in the US living with MS are estimated at $28 billion per year.1
In 2016, according to the US Census Bureau, almost 13% of Americans lived below the federal poverty level, and 6% of Americans reported “deep poverty”—defined as household income below 50% of the poverty threshold for that year.2 It has been reported that while at least 90% of people living with MS are insured, 70% are struggling to pay for health care. In fact, 30% put off seeking care because of costs; one consequence is delay in filling prescriptions.3
The burden of expense for our MS patients is considerable. Here’s how the ACA has impacted our patients by attempting to minimize the devastating cost.
Guaranteed Health Insurance Coverage for Pre-existing Conditions. When the ACA became law in March 2010, there were three main goals: making affordable health insurance available to more people, expanding the Medicaid program to cover all adults with income below 138% of the federal poverty level, and supporting innovative medical care delivery methods to lower the cost of health care.4
Following the ACA’s full implementation in 2014, private health insurance companies were prevented from refusing coverage to those with pre-existing conditions, such as MS. This was a game changer, since patients, regardless of their MS diagnosis, were now guaranteed individual insurance. Furthermore, they could not be charged increased premiums based on their prior medical history.5
Preventive Services Covered Without Cost-sharing. Under the ACA, health plans generally must provide preventive services, such as those rated A or B by the US Preventive Services Task Force. This includes routine immunizations for both adults and children, which represents a cost savings to patients living with MS. Another advantage is that women, including those living with MS, have access to sexually transmitted infection screenings, breastfeeding support and supplies, domestic violence screening, and contraceptives.6
Improved Coverage Through Medicare. The ACA mandated improvement in coverage with Medicare Part D benefits. In addition to the preventive care benefits noted above, which apply to Medicare recipients as well, the ACA reduced federal payments to Medicare Advantage plans over time and provided bonus payments to plans with high quality ratings.7
Further changes in Medicare spending included the creation of a 15-person, by-appointment board (known as the Independent Payment Advisory Board) tasked with identifying ways to “modify benefits, eligibility, premiums, or taxes,” which will hopefully continue to optimize the cost of care for patients living with MS and utilizing Medicare.7
Cost Savings With Medicaid Expansion. Medicaid expansion was enacted to keep patients with a costly illness, such as MS, from financial destitution because of their condition. As of January 2018, 32 states and the District of Columbia have seen expansion of their programs.8 In those states, people with a household income below 138% of the poverty level (less than $27,000 for a family of three) can now qualify for Medicaid. States that have not expanded coverage include Idaho, Wyoming, Utah, South Dakota, Nebraska, Kansas, Oklahoma, Texas, Missouri, Wisconsin, Tennessee, Mississippi, Alabama, Georgia, Virginia, North Carolina, South Carolina, and Florida.8 The expansion of Medicaid helps MS patients by shrinking the ever-present gap that still prevents some from qualifying for the additional financial assistance they need due to their chronic illness.
One thing we have learned is that MS patients may not realize they have access to some of these services—particularly preventive care—or they may hesitate to obtain services due to a lack of clarity on whether they are covered. Health care providers can remind patients that they may qualify for “unrealized services,” which could provide value and optimize general preventive care. MS patients with Medicare and Medicaid, for example, may not know that they have access to colorectal cancer screenings via a waived deductible.6
Since last year, there has been vigorous discussion about repealing, replacing, or otherwise amending the ACA. While a political discussion is beyond the bounds of this column, we do need to be aware of how changes to the ACA would affect patients with MS.
Optimizing wellness and prevention and providing access to care to patients with a costly disease, such as MS, is important. In addition to ensuring ongoing access to affordable services, we need to do more to improve mental health access and reduce the cost of needed medications. We also need to close the insurance gap in all 50 states. Continued dialogue will be necessary to help government leaders understand the cost impact of MS (and other diseases), in order to keep our country moving in a positive direction that optimizes wellness and health care reform. —ALD
Amy L. Dix, MPAS, PA-C, MSCS
Department of Neurology at Kansas City Multiple Sclerosis Center in Overland Park, Kansas
1. National Multiple Sclerosis Society. Health Policy Fact Sheet #2: Financial burdens for people with MS, their families, and society. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Health-Policy-Fact-Sheet-2-Costs.pdf. Accessed February 8, 2018.
2. Center for Poverty Research, University of California—Davis. What is the current poverty rate in the United States? https://poverty.ucdavis.edu/faq/what-current-poverty-rate-united-states. Accessed February 8, 2018.
3. Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. 2007;13(4):534-546.
4. Centers for Medicare & Medicaid Services. Affordable Care Act (ACA). HealthCare.gov. www.healthcare.gov/glossary/affordable-care-act. Accessed February 8, 2018.
5. US Department of Health and Human Services. About the ACA: pre-existing conditions. www.hhs.gov/healthcare/about-the-aca/pre-existing-conditions/index.html. Accessed February 8, 2018.
6. Tolbert J. The coverage provisions in the Affordable Care Act: an update. Kaiser Family Foundation. www.kff.org/report-section/the-coverage-provisions-in-the-affordable-care-act-an-update-health-insurance-market-reforms. Accessed February 8, 2018.
7. Kaiser Family Foundation. Focus on health reform: summary of key changes to Medicare in 2010 health reform law. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7948-02.pdf. Accessed February 8, 2018.
8. Families USA. A 50-state look at Medicaid expansion. http://familiesusa.org/product/50-state-look-medicaid-expansion. Accessed February 8, 2018.
Q) How has the Affordable Care Act affected people living with multiple sclerosis—an Americans with Disabilities Act recognized disease?
The Affordable Care Act (ACA) has been a source of controversy since it became law in 2010. Perhaps some of the tension surrounding it stems from misunderstanding; however, it is clear that individual experiences and/or perceptions flavor the ongoing debate. Rather than perpetuate the contention, we’d simply like to outline some of the ways in which patients with multiple sclerosis (MS) have benefited from the ACA—and what we must do to ensure continued quality and affordability of care in the event of changes to the law.
Living with MS in the United States is costly. According to the National Multiple Sclerosis Society, average annual costs—both direct and indirect (ie, lost wages)—are about $69,000. Health care costs account for more than half of this total (about $39,000). Total costs for all people in the US living with MS are estimated at $28 billion per year.1
In 2016, according to the US Census Bureau, almost 13% of Americans lived below the federal poverty level, and 6% of Americans reported “deep poverty”—defined as household income below 50% of the poverty threshold for that year.2 It has been reported that while at least 90% of people living with MS are insured, 70% are struggling to pay for health care. In fact, 30% put off seeking care because of costs; one consequence is delay in filling prescriptions.3
The burden of expense for our MS patients is considerable. Here’s how the ACA has impacted our patients by attempting to minimize the devastating cost.
Guaranteed Health Insurance Coverage for Pre-existing Conditions. When the ACA became law in March 2010, there were three main goals: making affordable health insurance available to more people, expanding the Medicaid program to cover all adults with income below 138% of the federal poverty level, and supporting innovative medical care delivery methods to lower the cost of health care.4
Following the ACA’s full implementation in 2014, private health insurance companies were prevented from refusing coverage to those with pre-existing conditions, such as MS. This was a game changer, since patients, regardless of their MS diagnosis, were now guaranteed individual insurance. Furthermore, they could not be charged increased premiums based on their prior medical history.5
Preventive Services Covered Without Cost-sharing. Under the ACA, health plans generally must provide preventive services, such as those rated A or B by the US Preventive Services Task Force. This includes routine immunizations for both adults and children, which represents a cost savings to patients living with MS. Another advantage is that women, including those living with MS, have access to sexually transmitted infection screenings, breastfeeding support and supplies, domestic violence screening, and contraceptives.6
Improved Coverage Through Medicare. The ACA mandated improvement in coverage with Medicare Part D benefits. In addition to the preventive care benefits noted above, which apply to Medicare recipients as well, the ACA reduced federal payments to Medicare Advantage plans over time and provided bonus payments to plans with high quality ratings.7
Further changes in Medicare spending included the creation of a 15-person, by-appointment board (known as the Independent Payment Advisory Board) tasked with identifying ways to “modify benefits, eligibility, premiums, or taxes,” which will hopefully continue to optimize the cost of care for patients living with MS and utilizing Medicare.7
Cost Savings With Medicaid Expansion. Medicaid expansion was enacted to keep patients with a costly illness, such as MS, from financial destitution because of their condition. As of January 2018, 32 states and the District of Columbia have seen expansion of their programs.8 In those states, people with a household income below 138% of the poverty level (less than $27,000 for a family of three) can now qualify for Medicaid. States that have not expanded coverage include Idaho, Wyoming, Utah, South Dakota, Nebraska, Kansas, Oklahoma, Texas, Missouri, Wisconsin, Tennessee, Mississippi, Alabama, Georgia, Virginia, North Carolina, South Carolina, and Florida.8 The expansion of Medicaid helps MS patients by shrinking the ever-present gap that still prevents some from qualifying for the additional financial assistance they need due to their chronic illness.
One thing we have learned is that MS patients may not realize they have access to some of these services—particularly preventive care—or they may hesitate to obtain services due to a lack of clarity on whether they are covered. Health care providers can remind patients that they may qualify for “unrealized services,” which could provide value and optimize general preventive care. MS patients with Medicare and Medicaid, for example, may not know that they have access to colorectal cancer screenings via a waived deductible.6
Since last year, there has been vigorous discussion about repealing, replacing, or otherwise amending the ACA. While a political discussion is beyond the bounds of this column, we do need to be aware of how changes to the ACA would affect patients with MS.
Optimizing wellness and prevention and providing access to care to patients with a costly disease, such as MS, is important. In addition to ensuring ongoing access to affordable services, we need to do more to improve mental health access and reduce the cost of needed medications. We also need to close the insurance gap in all 50 states. Continued dialogue will be necessary to help government leaders understand the cost impact of MS (and other diseases), in order to keep our country moving in a positive direction that optimizes wellness and health care reform. —ALD
Amy L. Dix, MPAS, PA-C, MSCS
Department of Neurology at Kansas City Multiple Sclerosis Center in Overland Park, Kansas
Q) How has the Affordable Care Act affected people living with multiple sclerosis—an Americans with Disabilities Act recognized disease?
The Affordable Care Act (ACA) has been a source of controversy since it became law in 2010. Perhaps some of the tension surrounding it stems from misunderstanding; however, it is clear that individual experiences and/or perceptions flavor the ongoing debate. Rather than perpetuate the contention, we’d simply like to outline some of the ways in which patients with multiple sclerosis (MS) have benefited from the ACA—and what we must do to ensure continued quality and affordability of care in the event of changes to the law.
Living with MS in the United States is costly. According to the National Multiple Sclerosis Society, average annual costs—both direct and indirect (ie, lost wages)—are about $69,000. Health care costs account for more than half of this total (about $39,000). Total costs for all people in the US living with MS are estimated at $28 billion per year.1
In 2016, according to the US Census Bureau, almost 13% of Americans lived below the federal poverty level, and 6% of Americans reported “deep poverty”—defined as household income below 50% of the poverty threshold for that year.2 It has been reported that while at least 90% of people living with MS are insured, 70% are struggling to pay for health care. In fact, 30% put off seeking care because of costs; one consequence is delay in filling prescriptions.3
The burden of expense for our MS patients is considerable. Here’s how the ACA has impacted our patients by attempting to minimize the devastating cost.
Guaranteed Health Insurance Coverage for Pre-existing Conditions. When the ACA became law in March 2010, there were three main goals: making affordable health insurance available to more people, expanding the Medicaid program to cover all adults with income below 138% of the federal poverty level, and supporting innovative medical care delivery methods to lower the cost of health care.4
Following the ACA’s full implementation in 2014, private health insurance companies were prevented from refusing coverage to those with pre-existing conditions, such as MS. This was a game changer, since patients, regardless of their MS diagnosis, were now guaranteed individual insurance. Furthermore, they could not be charged increased premiums based on their prior medical history.5
Preventive Services Covered Without Cost-sharing. Under the ACA, health plans generally must provide preventive services, such as those rated A or B by the US Preventive Services Task Force. This includes routine immunizations for both adults and children, which represents a cost savings to patients living with MS. Another advantage is that women, including those living with MS, have access to sexually transmitted infection screenings, breastfeeding support and supplies, domestic violence screening, and contraceptives.6
Improved Coverage Through Medicare. The ACA mandated improvement in coverage with Medicare Part D benefits. In addition to the preventive care benefits noted above, which apply to Medicare recipients as well, the ACA reduced federal payments to Medicare Advantage plans over time and provided bonus payments to plans with high quality ratings.7
Further changes in Medicare spending included the creation of a 15-person, by-appointment board (known as the Independent Payment Advisory Board) tasked with identifying ways to “modify benefits, eligibility, premiums, or taxes,” which will hopefully continue to optimize the cost of care for patients living with MS and utilizing Medicare.7
Cost Savings With Medicaid Expansion. Medicaid expansion was enacted to keep patients with a costly illness, such as MS, from financial destitution because of their condition. As of January 2018, 32 states and the District of Columbia have seen expansion of their programs.8 In those states, people with a household income below 138% of the poverty level (less than $27,000 for a family of three) can now qualify for Medicaid. States that have not expanded coverage include Idaho, Wyoming, Utah, South Dakota, Nebraska, Kansas, Oklahoma, Texas, Missouri, Wisconsin, Tennessee, Mississippi, Alabama, Georgia, Virginia, North Carolina, South Carolina, and Florida.8 The expansion of Medicaid helps MS patients by shrinking the ever-present gap that still prevents some from qualifying for the additional financial assistance they need due to their chronic illness.
One thing we have learned is that MS patients may not realize they have access to some of these services—particularly preventive care—or they may hesitate to obtain services due to a lack of clarity on whether they are covered. Health care providers can remind patients that they may qualify for “unrealized services,” which could provide value and optimize general preventive care. MS patients with Medicare and Medicaid, for example, may not know that they have access to colorectal cancer screenings via a waived deductible.6
Since last year, there has been vigorous discussion about repealing, replacing, or otherwise amending the ACA. While a political discussion is beyond the bounds of this column, we do need to be aware of how changes to the ACA would affect patients with MS.
Optimizing wellness and prevention and providing access to care to patients with a costly disease, such as MS, is important. In addition to ensuring ongoing access to affordable services, we need to do more to improve mental health access and reduce the cost of needed medications. We also need to close the insurance gap in all 50 states. Continued dialogue will be necessary to help government leaders understand the cost impact of MS (and other diseases), in order to keep our country moving in a positive direction that optimizes wellness and health care reform. —ALD
Amy L. Dix, MPAS, PA-C, MSCS
Department of Neurology at Kansas City Multiple Sclerosis Center in Overland Park, Kansas
1. National Multiple Sclerosis Society. Health Policy Fact Sheet #2: Financial burdens for people with MS, their families, and society. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Health-Policy-Fact-Sheet-2-Costs.pdf. Accessed February 8, 2018.
2. Center for Poverty Research, University of California—Davis. What is the current poverty rate in the United States? https://poverty.ucdavis.edu/faq/what-current-poverty-rate-united-states. Accessed February 8, 2018.
3. Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. 2007;13(4):534-546.
4. Centers for Medicare & Medicaid Services. Affordable Care Act (ACA). HealthCare.gov. www.healthcare.gov/glossary/affordable-care-act. Accessed February 8, 2018.
5. US Department of Health and Human Services. About the ACA: pre-existing conditions. www.hhs.gov/healthcare/about-the-aca/pre-existing-conditions/index.html. Accessed February 8, 2018.
6. Tolbert J. The coverage provisions in the Affordable Care Act: an update. Kaiser Family Foundation. www.kff.org/report-section/the-coverage-provisions-in-the-affordable-care-act-an-update-health-insurance-market-reforms. Accessed February 8, 2018.
7. Kaiser Family Foundation. Focus on health reform: summary of key changes to Medicare in 2010 health reform law. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7948-02.pdf. Accessed February 8, 2018.
8. Families USA. A 50-state look at Medicaid expansion. http://familiesusa.org/product/50-state-look-medicaid-expansion. Accessed February 8, 2018.
1. National Multiple Sclerosis Society. Health Policy Fact Sheet #2: Financial burdens for people with MS, their families, and society. www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Health-Policy-Fact-Sheet-2-Costs.pdf. Accessed February 8, 2018.
2. Center for Poverty Research, University of California—Davis. What is the current poverty rate in the United States? https://poverty.ucdavis.edu/faq/what-current-poverty-rate-united-states. Accessed February 8, 2018.
3. Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. 2007;13(4):534-546.
4. Centers for Medicare & Medicaid Services. Affordable Care Act (ACA). HealthCare.gov. www.healthcare.gov/glossary/affordable-care-act. Accessed February 8, 2018.
5. US Department of Health and Human Services. About the ACA: pre-existing conditions. www.hhs.gov/healthcare/about-the-aca/pre-existing-conditions/index.html. Accessed February 8, 2018.
6. Tolbert J. The coverage provisions in the Affordable Care Act: an update. Kaiser Family Foundation. www.kff.org/report-section/the-coverage-provisions-in-the-affordable-care-act-an-update-health-insurance-market-reforms. Accessed February 8, 2018.
7. Kaiser Family Foundation. Focus on health reform: summary of key changes to Medicare in 2010 health reform law. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7948-02.pdf. Accessed February 8, 2018.
8. Families USA. A 50-state look at Medicaid expansion. http://familiesusa.org/product/50-state-look-medicaid-expansion. Accessed February 8, 2018.
Monitoring for Infection in MS Patients
Q) How do you monitor for infection in patients with multiple sclerosis who take disease-modifying therapies?
The answer to this question is “it depends”—on several factors, including current and previous use of disease-modifying therapies (DMTs), concomitant medications, comorbidities, vaccination history, presence of John Cunningham virus (JCV) antibodies (in the case of natalizumab use), and prior or current use of immunosuppressive therapies.
There are many FDA-approved DMTs for multiple sclerosis (MS). Each has a different rate of infection occurring in clinical trials and varying requirements and/or recommendations for safety monitoring. The package inserts for each DMT offer some guidance for clinicians.
Injectable therapies. For two interferon therapies—interferon ß-1b SC and interferon ß-1a—it is recommended to order a complete blood count (CBC), blood chemistry, and liver function tests (LFTs) at baseline, then again at one, three, and six months, and then at clinician discretion thereafter.1,2 For peginterferon ß-1a, ordering a CBC, basic chemistry, and LFTs, at the clinician’s discretion, is advised.3 The package insert for interferon ß-1a IM does not offer specific recommendations for routine safety monitoring.4
The package insert for glatiramer acetate offers no recommendations for routine safety monitoring.5
In patients for whom two or more DMTs have failed to work, the monoclonal antibody daclizumab may be indicated. Compared to placebo and active comparator, this drug was associated with a higher risk for infection in clinical trials. The most commonly observed types were upper respiratory, urinary tract, and viral infections. There are no recommendations for CBC monitoring with daclizumab, but monthly LFTs are required due to increased risk for hepatic injury.6
Oral DMTs. Patients taking fingolimod, teriflunomide, and dimethyl fumarate have increased risk for infection; as a result, there are more safety monitoring recommendations for these medications.7-9
Prior to starting therapy with fingolimod, baseline CBC, blood chemistries, and varicella antibody testing should be done. During therapy, routine CBC testing and LFTs are advised at the clinician’s discretion or if the patient exhibits signs and symptoms of infection (see Table). In clinical trials, fingolimod use was interrupted if the lymphocyte count was sustained at < 200. In rare cases, progressive multifocal leukoencephalopathy (PML) has occurred—so the patient’s age, JCV antibody status, prior use of immunosuppressant therapy, and length of fingolimod treatment should be taken into consideration.7
Patients starting teriflunomide should have baseline LFTs and CBC and tuberculosis (TB) testing (either skin or serum), with subsequent monthly LFTs for the first six months on treatment. Some patients may experience neutropenia, thrombocytopenia, and lymphopenia. As a result, patients may have an increased risk for infection. Safety monitoring is at the clinician’s discretion.8
For patients initiating dimethyl fumarate, a baseline CBC is recommended, to be repeated every six to 12 months thereafter, and/or as clinically indicated. Since lymphopenia may occur, consider interruption of dimethyl fumarate in patients with lymphocyte counts < 0.5 persisting for more than six months. Rare cases of PML have also occurred; at the first suggestive sign or symptom, dimethyl fumarate should be withheld and appropriate diagnostic testing should be completed.9
Infusion therapies. There are four infusion therapies available for MS treatment. Mitoxantrone, though not commonly used, is still available for relapsing and secondary progressive forms of MS. Common infections seen in clinical trials include upper respiratory, urinary tract, and sinus infections. A CBC, including platelets, should be obtained prior to each course of mitoxantrone and again if signs and symptoms of infection develop.10
Natalizumab is an integrin receptor antagonist administered in monthly IV infusions. Patients receiving natalizumab may have increased risk for urinary tract infections, lower respiratory infections, gastroenteritis, vaginitis, and herpes infections. These risks should be monitored at the clinician’s discretion. There have been several cases of PML associated with natalizumab; risk factors include duration of therapy, prior use of immunosuppressants, and presence of JCV antibodies.11
Alemtuzumab is a CD52-directed monoclonal antibody indicated in patients with relapsing forms of MS who have had an inadequate response to at least two DMTs. In clinical trials, subjects had a higher risk for nasopharyngitis, urinary tract infections, upper respiratory infections, sinusitis, herpetic infections, influenza, and bronchitis. Due to the increased risk for infection and secondary autoimmunities, patients are required to have monthly CBC testing, LFTs, and urinalysis for up to 48 months after their last infusion.12
Lastly, ocrelizumab is a CD20-directed cytolytic antibody for the treatment of relapsing and progressive forms of MS. In clinical trials, there was a higher incidence of upper and lower respiratory infections, skin infections, and herpes-related infections. Prior to initiating ocrelizumab, hepatitis B virus screening should be completed. There are no specific recommendations for routine monitoring during therapy, although providers should monitor patients clinically for any signs and symptoms of infection.13
A word of caution: The common signs and symptoms of infection are listed in the Table. If these symptoms are present in your patient, consider ordering diagnostic testing to evaluate for infection.
Symptoms of PML include progressive unilateral weakness, clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. At the first sign or symptom suggestive of PML, the DMT should be discontinued and diagnostic testing performed.
Providers may contact the manufacturer directly for further guidance on DMT surveillance and treatment protocols. —CK
Christen Kutz, PhD, PA-C
Colorado Springs Neurological Associates
1. Betaseron (interferon [b]-1b) [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 1993.
2. Rebif (interferon [b]-1a) [package insert]. Rockland, MA: EMD Serono; revised 2015.
3. Plegridy (peginterferon [b]-1a) [package insert]. Cambridge, MA: Biogen Idec; 2013.
4. Avonex (interferon [b] -1a) [package insert]. Cambridge, MA: Biogen Inc.; 1996.
5. Copaxone (glatiramer acetate) [package insert]. Overland Park, KS: Teva Neuroscience; revised 2016.
6. Zinbryta (daclizumab) [package insert]. Cambridge, MA: Biogen Idec; 2016.
7. Gilenya (fingolimod) [package insert]. Hanover, NJ: Novartis; revised 2016.
8. Aubagio (teriflunomide) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2016.
9. Tecfidera (dimethyl fumarate) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
10. Novantrone (mitoxantrone) [package insert]. Rockland, MA: EMD Serono; 2008.
11. Tysabri (natalizumab) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
12. Lemtrada (alemtuzumab) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2017.
13. Ocrevus (ocrelizumab) [package insert]. San Francisco, CA: Genentech; 2017.
Q) How do you monitor for infection in patients with multiple sclerosis who take disease-modifying therapies?
The answer to this question is “it depends”—on several factors, including current and previous use of disease-modifying therapies (DMTs), concomitant medications, comorbidities, vaccination history, presence of John Cunningham virus (JCV) antibodies (in the case of natalizumab use), and prior or current use of immunosuppressive therapies.
There are many FDA-approved DMTs for multiple sclerosis (MS). Each has a different rate of infection occurring in clinical trials and varying requirements and/or recommendations for safety monitoring. The package inserts for each DMT offer some guidance for clinicians.
Injectable therapies. For two interferon therapies—interferon ß-1b SC and interferon ß-1a—it is recommended to order a complete blood count (CBC), blood chemistry, and liver function tests (LFTs) at baseline, then again at one, three, and six months, and then at clinician discretion thereafter.1,2 For peginterferon ß-1a, ordering a CBC, basic chemistry, and LFTs, at the clinician’s discretion, is advised.3 The package insert for interferon ß-1a IM does not offer specific recommendations for routine safety monitoring.4
The package insert for glatiramer acetate offers no recommendations for routine safety monitoring.5
In patients for whom two or more DMTs have failed to work, the monoclonal antibody daclizumab may be indicated. Compared to placebo and active comparator, this drug was associated with a higher risk for infection in clinical trials. The most commonly observed types were upper respiratory, urinary tract, and viral infections. There are no recommendations for CBC monitoring with daclizumab, but monthly LFTs are required due to increased risk for hepatic injury.6
Oral DMTs. Patients taking fingolimod, teriflunomide, and dimethyl fumarate have increased risk for infection; as a result, there are more safety monitoring recommendations for these medications.7-9
Prior to starting therapy with fingolimod, baseline CBC, blood chemistries, and varicella antibody testing should be done. During therapy, routine CBC testing and LFTs are advised at the clinician’s discretion or if the patient exhibits signs and symptoms of infection (see Table). In clinical trials, fingolimod use was interrupted if the lymphocyte count was sustained at < 200. In rare cases, progressive multifocal leukoencephalopathy (PML) has occurred—so the patient’s age, JCV antibody status, prior use of immunosuppressant therapy, and length of fingolimod treatment should be taken into consideration.7
Patients starting teriflunomide should have baseline LFTs and CBC and tuberculosis (TB) testing (either skin or serum), with subsequent monthly LFTs for the first six months on treatment. Some patients may experience neutropenia, thrombocytopenia, and lymphopenia. As a result, patients may have an increased risk for infection. Safety monitoring is at the clinician’s discretion.8
For patients initiating dimethyl fumarate, a baseline CBC is recommended, to be repeated every six to 12 months thereafter, and/or as clinically indicated. Since lymphopenia may occur, consider interruption of dimethyl fumarate in patients with lymphocyte counts < 0.5 persisting for more than six months. Rare cases of PML have also occurred; at the first suggestive sign or symptom, dimethyl fumarate should be withheld and appropriate diagnostic testing should be completed.9
Infusion therapies. There are four infusion therapies available for MS treatment. Mitoxantrone, though not commonly used, is still available for relapsing and secondary progressive forms of MS. Common infections seen in clinical trials include upper respiratory, urinary tract, and sinus infections. A CBC, including platelets, should be obtained prior to each course of mitoxantrone and again if signs and symptoms of infection develop.10
Natalizumab is an integrin receptor antagonist administered in monthly IV infusions. Patients receiving natalizumab may have increased risk for urinary tract infections, lower respiratory infections, gastroenteritis, vaginitis, and herpes infections. These risks should be monitored at the clinician’s discretion. There have been several cases of PML associated with natalizumab; risk factors include duration of therapy, prior use of immunosuppressants, and presence of JCV antibodies.11
Alemtuzumab is a CD52-directed monoclonal antibody indicated in patients with relapsing forms of MS who have had an inadequate response to at least two DMTs. In clinical trials, subjects had a higher risk for nasopharyngitis, urinary tract infections, upper respiratory infections, sinusitis, herpetic infections, influenza, and bronchitis. Due to the increased risk for infection and secondary autoimmunities, patients are required to have monthly CBC testing, LFTs, and urinalysis for up to 48 months after their last infusion.12
Lastly, ocrelizumab is a CD20-directed cytolytic antibody for the treatment of relapsing and progressive forms of MS. In clinical trials, there was a higher incidence of upper and lower respiratory infections, skin infections, and herpes-related infections. Prior to initiating ocrelizumab, hepatitis B virus screening should be completed. There are no specific recommendations for routine monitoring during therapy, although providers should monitor patients clinically for any signs and symptoms of infection.13
A word of caution: The common signs and symptoms of infection are listed in the Table. If these symptoms are present in your patient, consider ordering diagnostic testing to evaluate for infection.
Symptoms of PML include progressive unilateral weakness, clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. At the first sign or symptom suggestive of PML, the DMT should be discontinued and diagnostic testing performed.
Providers may contact the manufacturer directly for further guidance on DMT surveillance and treatment protocols. —CK
Christen Kutz, PhD, PA-C
Colorado Springs Neurological Associates
Q) How do you monitor for infection in patients with multiple sclerosis who take disease-modifying therapies?
The answer to this question is “it depends”—on several factors, including current and previous use of disease-modifying therapies (DMTs), concomitant medications, comorbidities, vaccination history, presence of John Cunningham virus (JCV) antibodies (in the case of natalizumab use), and prior or current use of immunosuppressive therapies.
There are many FDA-approved DMTs for multiple sclerosis (MS). Each has a different rate of infection occurring in clinical trials and varying requirements and/or recommendations for safety monitoring. The package inserts for each DMT offer some guidance for clinicians.
Injectable therapies. For two interferon therapies—interferon ß-1b SC and interferon ß-1a—it is recommended to order a complete blood count (CBC), blood chemistry, and liver function tests (LFTs) at baseline, then again at one, three, and six months, and then at clinician discretion thereafter.1,2 For peginterferon ß-1a, ordering a CBC, basic chemistry, and LFTs, at the clinician’s discretion, is advised.3 The package insert for interferon ß-1a IM does not offer specific recommendations for routine safety monitoring.4
The package insert for glatiramer acetate offers no recommendations for routine safety monitoring.5
In patients for whom two or more DMTs have failed to work, the monoclonal antibody daclizumab may be indicated. Compared to placebo and active comparator, this drug was associated with a higher risk for infection in clinical trials. The most commonly observed types were upper respiratory, urinary tract, and viral infections. There are no recommendations for CBC monitoring with daclizumab, but monthly LFTs are required due to increased risk for hepatic injury.6
Oral DMTs. Patients taking fingolimod, teriflunomide, and dimethyl fumarate have increased risk for infection; as a result, there are more safety monitoring recommendations for these medications.7-9
Prior to starting therapy with fingolimod, baseline CBC, blood chemistries, and varicella antibody testing should be done. During therapy, routine CBC testing and LFTs are advised at the clinician’s discretion or if the patient exhibits signs and symptoms of infection (see Table). In clinical trials, fingolimod use was interrupted if the lymphocyte count was sustained at < 200. In rare cases, progressive multifocal leukoencephalopathy (PML) has occurred—so the patient’s age, JCV antibody status, prior use of immunosuppressant therapy, and length of fingolimod treatment should be taken into consideration.7
Patients starting teriflunomide should have baseline LFTs and CBC and tuberculosis (TB) testing (either skin or serum), with subsequent monthly LFTs for the first six months on treatment. Some patients may experience neutropenia, thrombocytopenia, and lymphopenia. As a result, patients may have an increased risk for infection. Safety monitoring is at the clinician’s discretion.8
For patients initiating dimethyl fumarate, a baseline CBC is recommended, to be repeated every six to 12 months thereafter, and/or as clinically indicated. Since lymphopenia may occur, consider interruption of dimethyl fumarate in patients with lymphocyte counts < 0.5 persisting for more than six months. Rare cases of PML have also occurred; at the first suggestive sign or symptom, dimethyl fumarate should be withheld and appropriate diagnostic testing should be completed.9
Infusion therapies. There are four infusion therapies available for MS treatment. Mitoxantrone, though not commonly used, is still available for relapsing and secondary progressive forms of MS. Common infections seen in clinical trials include upper respiratory, urinary tract, and sinus infections. A CBC, including platelets, should be obtained prior to each course of mitoxantrone and again if signs and symptoms of infection develop.10
Natalizumab is an integrin receptor antagonist administered in monthly IV infusions. Patients receiving natalizumab may have increased risk for urinary tract infections, lower respiratory infections, gastroenteritis, vaginitis, and herpes infections. These risks should be monitored at the clinician’s discretion. There have been several cases of PML associated with natalizumab; risk factors include duration of therapy, prior use of immunosuppressants, and presence of JCV antibodies.11
Alemtuzumab is a CD52-directed monoclonal antibody indicated in patients with relapsing forms of MS who have had an inadequate response to at least two DMTs. In clinical trials, subjects had a higher risk for nasopharyngitis, urinary tract infections, upper respiratory infections, sinusitis, herpetic infections, influenza, and bronchitis. Due to the increased risk for infection and secondary autoimmunities, patients are required to have monthly CBC testing, LFTs, and urinalysis for up to 48 months after their last infusion.12
Lastly, ocrelizumab is a CD20-directed cytolytic antibody for the treatment of relapsing and progressive forms of MS. In clinical trials, there was a higher incidence of upper and lower respiratory infections, skin infections, and herpes-related infections. Prior to initiating ocrelizumab, hepatitis B virus screening should be completed. There are no specific recommendations for routine monitoring during therapy, although providers should monitor patients clinically for any signs and symptoms of infection.13
A word of caution: The common signs and symptoms of infection are listed in the Table. If these symptoms are present in your patient, consider ordering diagnostic testing to evaluate for infection.
Symptoms of PML include progressive unilateral weakness, clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. At the first sign or symptom suggestive of PML, the DMT should be discontinued and diagnostic testing performed.
Providers may contact the manufacturer directly for further guidance on DMT surveillance and treatment protocols. —CK
Christen Kutz, PhD, PA-C
Colorado Springs Neurological Associates
1. Betaseron (interferon [b]-1b) [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 1993.
2. Rebif (interferon [b]-1a) [package insert]. Rockland, MA: EMD Serono; revised 2015.
3. Plegridy (peginterferon [b]-1a) [package insert]. Cambridge, MA: Biogen Idec; 2013.
4. Avonex (interferon [b] -1a) [package insert]. Cambridge, MA: Biogen Inc.; 1996.
5. Copaxone (glatiramer acetate) [package insert]. Overland Park, KS: Teva Neuroscience; revised 2016.
6. Zinbryta (daclizumab) [package insert]. Cambridge, MA: Biogen Idec; 2016.
7. Gilenya (fingolimod) [package insert]. Hanover, NJ: Novartis; revised 2016.
8. Aubagio (teriflunomide) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2016.
9. Tecfidera (dimethyl fumarate) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
10. Novantrone (mitoxantrone) [package insert]. Rockland, MA: EMD Serono; 2008.
11. Tysabri (natalizumab) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
12. Lemtrada (alemtuzumab) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2017.
13. Ocrevus (ocrelizumab) [package insert]. San Francisco, CA: Genentech; 2017.
1. Betaseron (interferon [b]-1b) [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 1993.
2. Rebif (interferon [b]-1a) [package insert]. Rockland, MA: EMD Serono; revised 2015.
3. Plegridy (peginterferon [b]-1a) [package insert]. Cambridge, MA: Biogen Idec; 2013.
4. Avonex (interferon [b] -1a) [package insert]. Cambridge, MA: Biogen Inc.; 1996.
5. Copaxone (glatiramer acetate) [package insert]. Overland Park, KS: Teva Neuroscience; revised 2016.
6. Zinbryta (daclizumab) [package insert]. Cambridge, MA: Biogen Idec; 2016.
7. Gilenya (fingolimod) [package insert]. Hanover, NJ: Novartis; revised 2016.
8. Aubagio (teriflunomide) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2016.
9. Tecfidera (dimethyl fumarate) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
10. Novantrone (mitoxantrone) [package insert]. Rockland, MA: EMD Serono; 2008.
11. Tysabri (natalizumab) [package insert]. Cambridge, MA: Biogen Idec; revised 2017.
12. Lemtrada (alemtuzumab) [package insert]. Cambridge, MA: Genzyme Corporation; revised 2017.
13. Ocrevus (ocrelizumab) [package insert]. San Francisco, CA: Genentech; 2017.
The Gut Microbiome in Type 2 Diabetes
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.