User login
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
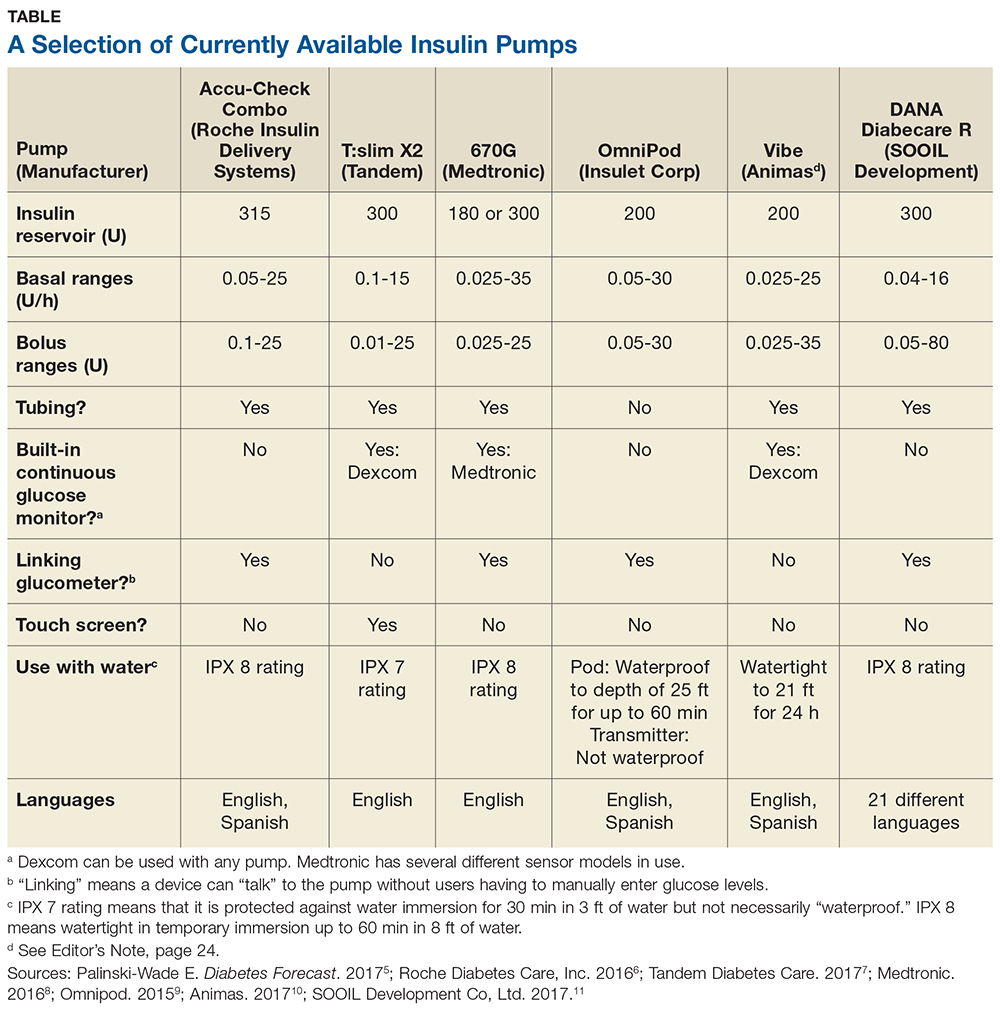
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .