User login
The Benefits of Exercise for Patients With Multiple Sclerosis
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
Does Diet Matter in Multiple Sclerosis?
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
To Vaccinate, or Not, in Patients With MS
Q) Are vaccines safe for patients with multiple sclerosis?
Vaccines are an important component of general disease prevention and are especially useful for patients with chronic illnesses, such as MS, who may be at elevated risk due to disability or medications that alter the immune system. Currently, there are many disease-modifying therapies that attempt to reduce relapses and impact the immune system, MRI activity, and disability. But is it safe for patients with MS to receive vaccines, given the multitude of studies suggesting that infections may increase relapse rate?
In 2002, the American Academy of Neurology published a summary of evidence and recommendations to provide guidance for practitioners.1 The data showed an increased risk for MS relapse during the weeks following infection.2,3 Therefore, preventing infections is beneficial for patients with MS. An analysis of studies in patients with MS who were vaccinated with inactivated vaccines (influenza, hepatitis B, tetanus) found sufficient evidence to support this practice. Studies of patients with MS who were given attenuated vaccines did not find enough evidence to support or reject these vaccines, except in the case of varicella. A study with sufficient follow-up concluded that varicella vaccination was safe for patients with MS who were not immunosuppressed. As a result of this effort, the MS Council for Clinical Practice Guidelines recommends that patients and health care providers follow the CDC’s indications for immunizations (www.cdc.gov/vaccines/schedules/hcp/adult.html).1
On the other hand, administration of the live-virus yellow fever vaccine in patients with clinically relapsing MS was correlated with an increased risk for disease progression in one study.4 The researchers followed disease progression, measured by relapses and MRI activity, in patients taking glatiramer acetate and interferon ß. Relapse rates reached 8.57 within three months after vaccination, compared to a rate of 0.67 the year prior to vaccine administration. Additionally, significant changes were seen on MRI; new or enlarging T2-weighted lesions and gadolinium-enhancing lesions were observed at three months, compared to 12 months prior and nine months after.4 Therefore, the researchers concluded that patients with MS traveling to endemic yellow fever areas should be cautioned regarding the risk for disease progression with vaccination, versus the risk for exposure to yellow fever.
Over the past decade, as newer therapies with different mechanisms of action have become available, concern has risen that patients may not respond to immunizations or may have a higher risk for infection after vaccination. For that reason, several studies have evaluated the ability of patients with MS to mount a normal antibody and cellular immune response after vaccine administration. In 2016, a study by Lin et al determined that patients who received daclizumab were able to mount a normal response after influenza vaccination.5
By contrast, Kappos et al, in a 2015 study, found that patients receiving fingolimod had lower response rates to influenza and tetanus booster vaccines than patients who took a placebo.6 Similarly, in a 2014 study, Olberg et al examined patients receiving interferon ß, glatiramer acetate, natalizumab, and mitoxantrone after receiving influenza and H1N1 vaccinations. The researchers found that those treated with any therapy other than interferon ß had a reduced rate of response and should therefore be considered for vaccine response analysis.7 Bar-Or et al also published data on response rates of patients treated with teriflunomide (7 mg or 14 mg) or interferon ß; rates were reduced with 14-mg teriflunomide compared to the other treatments—but most patients exhibited seroprotection regardless.8 Studying vaccine efficacy in 2013, McCarthy et al evaluated serum antibodies against common viruses before and after treatment with alemtuzumab and found that antibodies remained detectable six months post-alemtuzumab.9
In summary, most specialists agree that vaccines are helpful for patients with MS. However, due to the varied response rates among disease-modifying therapies and the correlation between infection and increased relapse rates, special care should be taken when treating this population. Generally, inactivated vaccines are safe, but seroprotection should be established to determine if a booster is necessary. Attenuated vaccines are generally safe for patients who are not immunosuppressed and can reduce the risk for infection if given prior to immunosuppression. After immunosuppression, attenuated vaccines should not be given until immune recovery has been established. —PP
Patricia Pagnotta, ARNP, MSN, CNRN, MSCN
Neurology Associates, PA
MS Center of Greater Orlando
1. Rutschmann OT, McCrory DC, Matchar DB. Immunization and MS: a summary of published evidence and recommendations. Neurology. 2002;59(12):1837-1843.
2. Anderson O, Lygner PE, Bergstrom T, et al. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. 1993;240(7):417-422.
3. Panitch HS, Bever CT, Katz E, Johnson KP. Upper respiratory tract infections trigger attacks of multiple sclerosis in patients treated with interferon. J Neuroimmunol. 1991; 36:125.
4. Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol. 2011;68(10):1267-1271.
5. Lin YC, Winokur P, Blake A, et al. Patients with MS under daclizumab therapy mount normal immune responses to influenza vaccine. Neurol Neuroimmunol Neuroinflamm. 2016;3(1):1-10.
6. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872-879.
7. Olberg HK, Cox RJ, Nostbakken JK, et al. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult Scler. 2014;20(8):1074-1080.
8. Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81(6):552-558.
9. McCarthy CL, Tuohy O, Compston DA, et al. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology. 2013;81(10):872-876.
Q) Are vaccines safe for patients with multiple sclerosis?
Vaccines are an important component of general disease prevention and are especially useful for patients with chronic illnesses, such as MS, who may be at elevated risk due to disability or medications that alter the immune system. Currently, there are many disease-modifying therapies that attempt to reduce relapses and impact the immune system, MRI activity, and disability. But is it safe for patients with MS to receive vaccines, given the multitude of studies suggesting that infections may increase relapse rate?
In 2002, the American Academy of Neurology published a summary of evidence and recommendations to provide guidance for practitioners.1 The data showed an increased risk for MS relapse during the weeks following infection.2,3 Therefore, preventing infections is beneficial for patients with MS. An analysis of studies in patients with MS who were vaccinated with inactivated vaccines (influenza, hepatitis B, tetanus) found sufficient evidence to support this practice. Studies of patients with MS who were given attenuated vaccines did not find enough evidence to support or reject these vaccines, except in the case of varicella. A study with sufficient follow-up concluded that varicella vaccination was safe for patients with MS who were not immunosuppressed. As a result of this effort, the MS Council for Clinical Practice Guidelines recommends that patients and health care providers follow the CDC’s indications for immunizations (www.cdc.gov/vaccines/schedules/hcp/adult.html).1
On the other hand, administration of the live-virus yellow fever vaccine in patients with clinically relapsing MS was correlated with an increased risk for disease progression in one study.4 The researchers followed disease progression, measured by relapses and MRI activity, in patients taking glatiramer acetate and interferon ß. Relapse rates reached 8.57 within three months after vaccination, compared to a rate of 0.67 the year prior to vaccine administration. Additionally, significant changes were seen on MRI; new or enlarging T2-weighted lesions and gadolinium-enhancing lesions were observed at three months, compared to 12 months prior and nine months after.4 Therefore, the researchers concluded that patients with MS traveling to endemic yellow fever areas should be cautioned regarding the risk for disease progression with vaccination, versus the risk for exposure to yellow fever.
Over the past decade, as newer therapies with different mechanisms of action have become available, concern has risen that patients may not respond to immunizations or may have a higher risk for infection after vaccination. For that reason, several studies have evaluated the ability of patients with MS to mount a normal antibody and cellular immune response after vaccine administration. In 2016, a study by Lin et al determined that patients who received daclizumab were able to mount a normal response after influenza vaccination.5
By contrast, Kappos et al, in a 2015 study, found that patients receiving fingolimod had lower response rates to influenza and tetanus booster vaccines than patients who took a placebo.6 Similarly, in a 2014 study, Olberg et al examined patients receiving interferon ß, glatiramer acetate, natalizumab, and mitoxantrone after receiving influenza and H1N1 vaccinations. The researchers found that those treated with any therapy other than interferon ß had a reduced rate of response and should therefore be considered for vaccine response analysis.7 Bar-Or et al also published data on response rates of patients treated with teriflunomide (7 mg or 14 mg) or interferon ß; rates were reduced with 14-mg teriflunomide compared to the other treatments—but most patients exhibited seroprotection regardless.8 Studying vaccine efficacy in 2013, McCarthy et al evaluated serum antibodies against common viruses before and after treatment with alemtuzumab and found that antibodies remained detectable six months post-alemtuzumab.9
In summary, most specialists agree that vaccines are helpful for patients with MS. However, due to the varied response rates among disease-modifying therapies and the correlation between infection and increased relapse rates, special care should be taken when treating this population. Generally, inactivated vaccines are safe, but seroprotection should be established to determine if a booster is necessary. Attenuated vaccines are generally safe for patients who are not immunosuppressed and can reduce the risk for infection if given prior to immunosuppression. After immunosuppression, attenuated vaccines should not be given until immune recovery has been established. —PP
Patricia Pagnotta, ARNP, MSN, CNRN, MSCN
Neurology Associates, PA
MS Center of Greater Orlando
Q) Are vaccines safe for patients with multiple sclerosis?
Vaccines are an important component of general disease prevention and are especially useful for patients with chronic illnesses, such as MS, who may be at elevated risk due to disability or medications that alter the immune system. Currently, there are many disease-modifying therapies that attempt to reduce relapses and impact the immune system, MRI activity, and disability. But is it safe for patients with MS to receive vaccines, given the multitude of studies suggesting that infections may increase relapse rate?
In 2002, the American Academy of Neurology published a summary of evidence and recommendations to provide guidance for practitioners.1 The data showed an increased risk for MS relapse during the weeks following infection.2,3 Therefore, preventing infections is beneficial for patients with MS. An analysis of studies in patients with MS who were vaccinated with inactivated vaccines (influenza, hepatitis B, tetanus) found sufficient evidence to support this practice. Studies of patients with MS who were given attenuated vaccines did not find enough evidence to support or reject these vaccines, except in the case of varicella. A study with sufficient follow-up concluded that varicella vaccination was safe for patients with MS who were not immunosuppressed. As a result of this effort, the MS Council for Clinical Practice Guidelines recommends that patients and health care providers follow the CDC’s indications for immunizations (www.cdc.gov/vaccines/schedules/hcp/adult.html).1
On the other hand, administration of the live-virus yellow fever vaccine in patients with clinically relapsing MS was correlated with an increased risk for disease progression in one study.4 The researchers followed disease progression, measured by relapses and MRI activity, in patients taking glatiramer acetate and interferon ß. Relapse rates reached 8.57 within three months after vaccination, compared to a rate of 0.67 the year prior to vaccine administration. Additionally, significant changes were seen on MRI; new or enlarging T2-weighted lesions and gadolinium-enhancing lesions were observed at three months, compared to 12 months prior and nine months after.4 Therefore, the researchers concluded that patients with MS traveling to endemic yellow fever areas should be cautioned regarding the risk for disease progression with vaccination, versus the risk for exposure to yellow fever.
Over the past decade, as newer therapies with different mechanisms of action have become available, concern has risen that patients may not respond to immunizations or may have a higher risk for infection after vaccination. For that reason, several studies have evaluated the ability of patients with MS to mount a normal antibody and cellular immune response after vaccine administration. In 2016, a study by Lin et al determined that patients who received daclizumab were able to mount a normal response after influenza vaccination.5
By contrast, Kappos et al, in a 2015 study, found that patients receiving fingolimod had lower response rates to influenza and tetanus booster vaccines than patients who took a placebo.6 Similarly, in a 2014 study, Olberg et al examined patients receiving interferon ß, glatiramer acetate, natalizumab, and mitoxantrone after receiving influenza and H1N1 vaccinations. The researchers found that those treated with any therapy other than interferon ß had a reduced rate of response and should therefore be considered for vaccine response analysis.7 Bar-Or et al also published data on response rates of patients treated with teriflunomide (7 mg or 14 mg) or interferon ß; rates were reduced with 14-mg teriflunomide compared to the other treatments—but most patients exhibited seroprotection regardless.8 Studying vaccine efficacy in 2013, McCarthy et al evaluated serum antibodies against common viruses before and after treatment with alemtuzumab and found that antibodies remained detectable six months post-alemtuzumab.9
In summary, most specialists agree that vaccines are helpful for patients with MS. However, due to the varied response rates among disease-modifying therapies and the correlation between infection and increased relapse rates, special care should be taken when treating this population. Generally, inactivated vaccines are safe, but seroprotection should be established to determine if a booster is necessary. Attenuated vaccines are generally safe for patients who are not immunosuppressed and can reduce the risk for infection if given prior to immunosuppression. After immunosuppression, attenuated vaccines should not be given until immune recovery has been established. —PP
Patricia Pagnotta, ARNP, MSN, CNRN, MSCN
Neurology Associates, PA
MS Center of Greater Orlando
1. Rutschmann OT, McCrory DC, Matchar DB. Immunization and MS: a summary of published evidence and recommendations. Neurology. 2002;59(12):1837-1843.
2. Anderson O, Lygner PE, Bergstrom T, et al. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. 1993;240(7):417-422.
3. Panitch HS, Bever CT, Katz E, Johnson KP. Upper respiratory tract infections trigger attacks of multiple sclerosis in patients treated with interferon. J Neuroimmunol. 1991; 36:125.
4. Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol. 2011;68(10):1267-1271.
5. Lin YC, Winokur P, Blake A, et al. Patients with MS under daclizumab therapy mount normal immune responses to influenza vaccine. Neurol Neuroimmunol Neuroinflamm. 2016;3(1):1-10.
6. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872-879.
7. Olberg HK, Cox RJ, Nostbakken JK, et al. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult Scler. 2014;20(8):1074-1080.
8. Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81(6):552-558.
9. McCarthy CL, Tuohy O, Compston DA, et al. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology. 2013;81(10):872-876.
1. Rutschmann OT, McCrory DC, Matchar DB. Immunization and MS: a summary of published evidence and recommendations. Neurology. 2002;59(12):1837-1843.
2. Anderson O, Lygner PE, Bergstrom T, et al. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. 1993;240(7):417-422.
3. Panitch HS, Bever CT, Katz E, Johnson KP. Upper respiratory tract infections trigger attacks of multiple sclerosis in patients treated with interferon. J Neuroimmunol. 1991; 36:125.
4. Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol. 2011;68(10):1267-1271.
5. Lin YC, Winokur P, Blake A, et al. Patients with MS under daclizumab therapy mount normal immune responses to influenza vaccine. Neurol Neuroimmunol Neuroinflamm. 2016;3(1):1-10.
6. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872-879.
7. Olberg HK, Cox RJ, Nostbakken JK, et al. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult Scler. 2014;20(8):1074-1080.
8. Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81(6):552-558.
9. McCarthy CL, Tuohy O, Compston DA, et al. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology. 2013;81(10):872-876.
Fighting Fatigue in MS
Q) Why do my patients with multiple sclerosis experience so much fatigue, and what can I do to help them?
Fatigue is an extremely common symptom of multiple sclerosis (MS) and one of the most disabling complications of the disease.1 More than 75% of patients with MS experience fatigue, which can worsen motor function, sleep quality, mood, and overall quality of life.1,2 Fatigue can also adversely affect employment; among patients with MS who reduce their work hours from full- to part-time, 90% do so because of fatigue.3
The
Patients with MS may have primary or secondary causes of fatigue. Primary fatigue is believed to result from the disease itself. Although it is not well understood, one hypothesis suggests that it is caused by an immune-related process involving inflammation and immune-mediated neurodegeneration.7 Another theory relates it to impaired nerve conduction.8
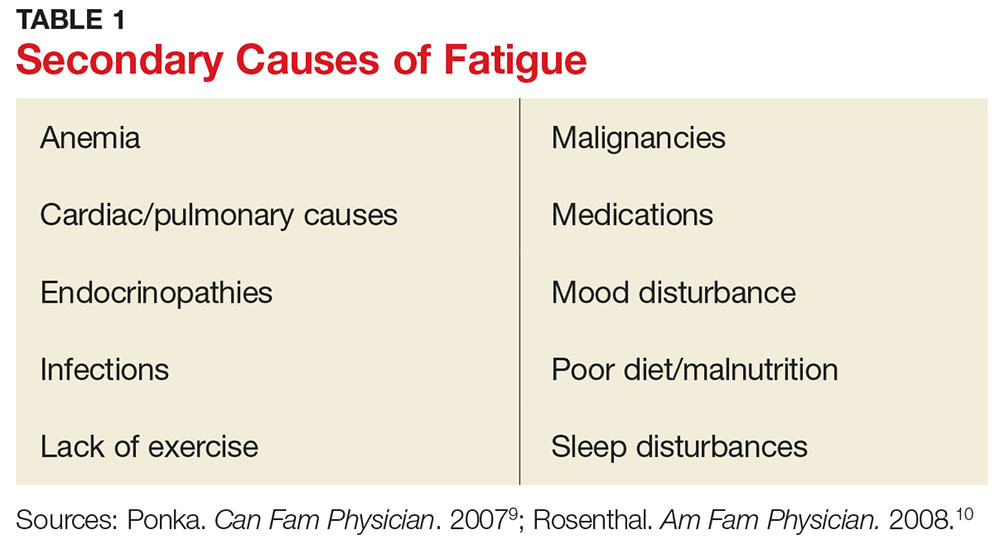
Secondary fatigue is unrelated to MS itself, and it is often treatable. Common causes include anemia, infection, or insomnia (see Table 1).9,10 These possibilities should be considered and ruled out in all patients with MS who complain of fatigue. A comprehensive history, exam, and evaluation performed by the clinician may help identify alternative reasons for fatigue.
Once any secondary causes have been addressed, primary fatigue should be evaluated and managed. One method for assessing the severity of fatigue and its impact on functional disability is to discuss it with the patient. The Fatigue Severity Scale can also be used as a measure; this self-assessment is quick, easy, and can be downloaded for free at www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf.11Identifying potential triggers of fatigue can help clinicians develop appropriate interventions. Heat intolerance is common and can precipitate or contribute to fatigue; cooling equipment can be a helpful solution (see Figure). Urinary tract infections frequently cause fatigue and can exacerbate many symptoms of MS. Bladder dysfunction and subsequent nocturnal wakening may contribute to the problem. Psychological stress is another common trigger; managing it can reduce fatigue.1,12 Screening for depression in patients with MS who complain of fatigue is imperative; if diagnosed, it must be addressed as the first line of treatment.1
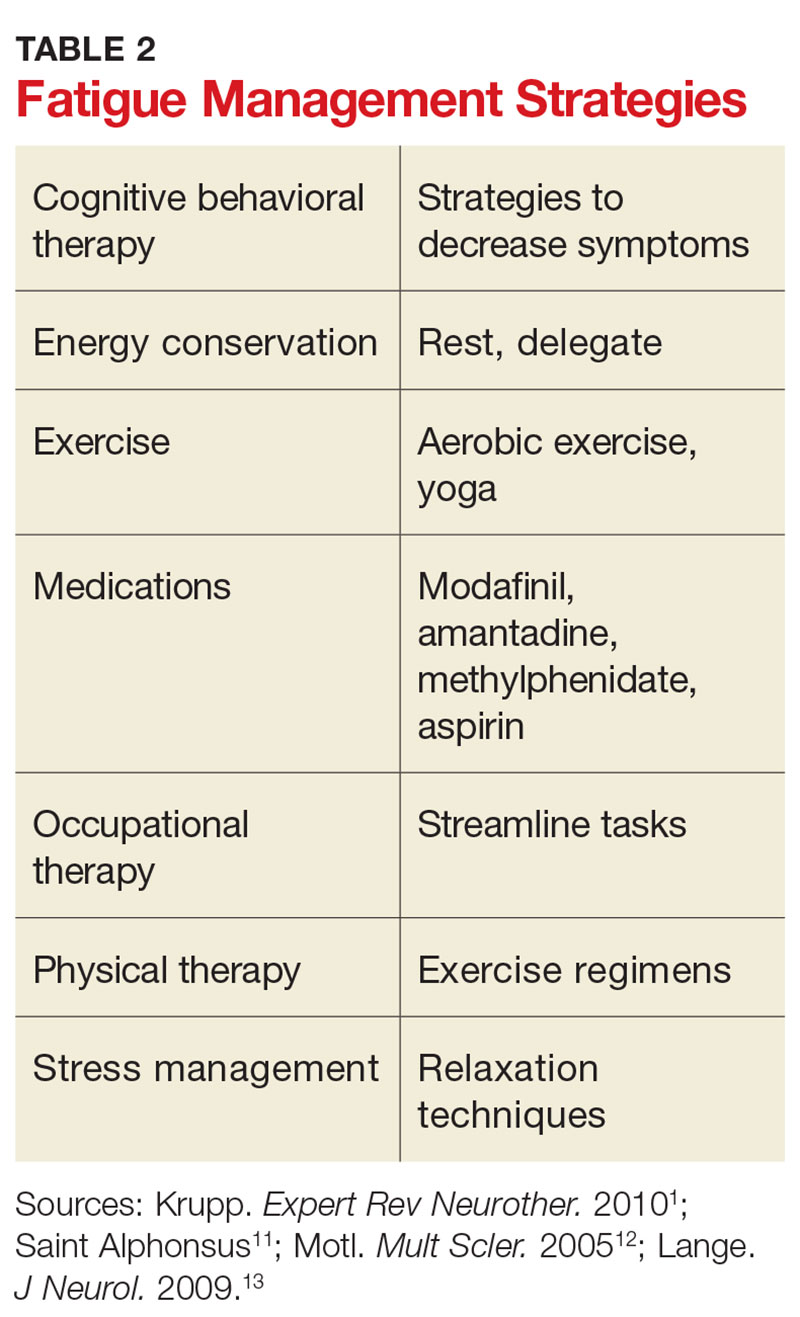
Other clinician-initiated intervention strategies include exercise, therapy, and medication. Modafinil is frequently prescribed for MS fatigue; small trials have demonstrated dramatic improvements with its use.13 Interestingly, aspirin has been shown to reduce fatigue in randomized controlled trials.14 This may be due to its indirect effects on neuroendocrine and autonomic responses, both of which are involved in the perception of fatigue.14 Additional interventions are listed in Table 2. As always, before prescribing any new medication, ensure that it is appropriate and that the patient’s other medical providers agree to the plan.
Counsel patients by emphasizing the importance of good sleep hygiene, a healthy diet, and avoidance of unhealthy habits. Taking an interdisciplinary approach can help patients with MS receive the best possible health care. While you may not be treating your patient’s disease, you will be managing much of his or her health care; treating the underlying causes of fatigue can significantly improve quality of life. —SA
Stephanie Agrella, MSN, RN, APRN, ANP-BC, MSCN
Director of Clinical Services, Multiple Scerlosis Clinic of Central Texas, Round Rock
1. Krupp B, Serafin D, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10(9):1437-1447.
2. Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367-368.
3. Dennett SL, Castelli-Haley J, Oleen-Burkey MK. The impact of multiple sclerosis on patient employment: a review of the medical literature. J Health Productivity. 2007;2(2):12-18.
4. Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
5. Kalb R. Multiple Sclerosis: The Questions You Have—The Answers You Need. New York, NY: Demos; 2012.
6. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435-437.
7. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15(3):210-220.
8. Davis S, Wilson T, White A, Frohman E. Thermoregulation in multiple sclerosis. J Appl Physiol. 2016;109(5):1531-1537.
9. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: fatigue. Can Fam Physician. 2007;53(5):892.
10. Rosenthal TC, Majeroni BA, Pretorius R, Malik K. Fatigue: an overview. Am Fam Physician. 2008;78(10):1173-1179.
11. Saint Alphonsus. Fatigue severity scale. www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf. Accessed May 16, 2017.
12. Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459-463.
13. Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009; 256(4):645-650.
14. Wingerchuk DM, Benarroch EE, O’Brien PC, et al. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005;64(7):1267-1269.
Q) Why do my patients with multiple sclerosis experience so much fatigue, and what can I do to help them?
Fatigue is an extremely common symptom of multiple sclerosis (MS) and one of the most disabling complications of the disease.1 More than 75% of patients with MS experience fatigue, which can worsen motor function, sleep quality, mood, and overall quality of life.1,2 Fatigue can also adversely affect employment; among patients with MS who reduce their work hours from full- to part-time, 90% do so because of fatigue.3
The
Patients with MS may have primary or secondary causes of fatigue. Primary fatigue is believed to result from the disease itself. Although it is not well understood, one hypothesis suggests that it is caused by an immune-related process involving inflammation and immune-mediated neurodegeneration.7 Another theory relates it to impaired nerve conduction.8
Secondary fatigue is unrelated to MS itself, and it is often treatable. Common causes include anemia, infection, or insomnia (see Table 1).9,10 These possibilities should be considered and ruled out in all patients with MS who complain of fatigue. A comprehensive history, exam, and evaluation performed by the clinician may help identify alternative reasons for fatigue.
Once any secondary causes have been addressed, primary fatigue should be evaluated and managed. One method for assessing the severity of fatigue and its impact on functional disability is to discuss it with the patient. The Fatigue Severity Scale can also be used as a measure; this self-assessment is quick, easy, and can be downloaded for free at www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf.11Identifying potential triggers of fatigue can help clinicians develop appropriate interventions. Heat intolerance is common and can precipitate or contribute to fatigue; cooling equipment can be a helpful solution (see Figure). Urinary tract infections frequently cause fatigue and can exacerbate many symptoms of MS. Bladder dysfunction and subsequent nocturnal wakening may contribute to the problem. Psychological stress is another common trigger; managing it can reduce fatigue.1,12 Screening for depression in patients with MS who complain of fatigue is imperative; if diagnosed, it must be addressed as the first line of treatment.1
Other clinician-initiated intervention strategies include exercise, therapy, and medication. Modafinil is frequently prescribed for MS fatigue; small trials have demonstrated dramatic improvements with its use.13 Interestingly, aspirin has been shown to reduce fatigue in randomized controlled trials.14 This may be due to its indirect effects on neuroendocrine and autonomic responses, both of which are involved in the perception of fatigue.14 Additional interventions are listed in Table 2. As always, before prescribing any new medication, ensure that it is appropriate and that the patient’s other medical providers agree to the plan.
Counsel patients by emphasizing the importance of good sleep hygiene, a healthy diet, and avoidance of unhealthy habits. Taking an interdisciplinary approach can help patients with MS receive the best possible health care. While you may not be treating your patient’s disease, you will be managing much of his or her health care; treating the underlying causes of fatigue can significantly improve quality of life. —SA
Stephanie Agrella, MSN, RN, APRN, ANP-BC, MSCN
Director of Clinical Services, Multiple Scerlosis Clinic of Central Texas, Round Rock
Q) Why do my patients with multiple sclerosis experience so much fatigue, and what can I do to help them?
Fatigue is an extremely common symptom of multiple sclerosis (MS) and one of the most disabling complications of the disease.1 More than 75% of patients with MS experience fatigue, which can worsen motor function, sleep quality, mood, and overall quality of life.1,2 Fatigue can also adversely affect employment; among patients with MS who reduce their work hours from full- to part-time, 90% do so because of fatigue.3
The
Patients with MS may have primary or secondary causes of fatigue. Primary fatigue is believed to result from the disease itself. Although it is not well understood, one hypothesis suggests that it is caused by an immune-related process involving inflammation and immune-mediated neurodegeneration.7 Another theory relates it to impaired nerve conduction.8
Secondary fatigue is unrelated to MS itself, and it is often treatable. Common causes include anemia, infection, or insomnia (see Table 1).9,10 These possibilities should be considered and ruled out in all patients with MS who complain of fatigue. A comprehensive history, exam, and evaluation performed by the clinician may help identify alternative reasons for fatigue.
Once any secondary causes have been addressed, primary fatigue should be evaluated and managed. One method for assessing the severity of fatigue and its impact on functional disability is to discuss it with the patient. The Fatigue Severity Scale can also be used as a measure; this self-assessment is quick, easy, and can be downloaded for free at www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf.11Identifying potential triggers of fatigue can help clinicians develop appropriate interventions. Heat intolerance is common and can precipitate or contribute to fatigue; cooling equipment can be a helpful solution (see Figure). Urinary tract infections frequently cause fatigue and can exacerbate many symptoms of MS. Bladder dysfunction and subsequent nocturnal wakening may contribute to the problem. Psychological stress is another common trigger; managing it can reduce fatigue.1,12 Screening for depression in patients with MS who complain of fatigue is imperative; if diagnosed, it must be addressed as the first line of treatment.1
Other clinician-initiated intervention strategies include exercise, therapy, and medication. Modafinil is frequently prescribed for MS fatigue; small trials have demonstrated dramatic improvements with its use.13 Interestingly, aspirin has been shown to reduce fatigue in randomized controlled trials.14 This may be due to its indirect effects on neuroendocrine and autonomic responses, both of which are involved in the perception of fatigue.14 Additional interventions are listed in Table 2. As always, before prescribing any new medication, ensure that it is appropriate and that the patient’s other medical providers agree to the plan.
Counsel patients by emphasizing the importance of good sleep hygiene, a healthy diet, and avoidance of unhealthy habits. Taking an interdisciplinary approach can help patients with MS receive the best possible health care. While you may not be treating your patient’s disease, you will be managing much of his or her health care; treating the underlying causes of fatigue can significantly improve quality of life. —SA
Stephanie Agrella, MSN, RN, APRN, ANP-BC, MSCN
Director of Clinical Services, Multiple Scerlosis Clinic of Central Texas, Round Rock
1. Krupp B, Serafin D, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10(9):1437-1447.
2. Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367-368.
3. Dennett SL, Castelli-Haley J, Oleen-Burkey MK. The impact of multiple sclerosis on patient employment: a review of the medical literature. J Health Productivity. 2007;2(2):12-18.
4. Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
5. Kalb R. Multiple Sclerosis: The Questions You Have—The Answers You Need. New York, NY: Demos; 2012.
6. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435-437.
7. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15(3):210-220.
8. Davis S, Wilson T, White A, Frohman E. Thermoregulation in multiple sclerosis. J Appl Physiol. 2016;109(5):1531-1537.
9. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: fatigue. Can Fam Physician. 2007;53(5):892.
10. Rosenthal TC, Majeroni BA, Pretorius R, Malik K. Fatigue: an overview. Am Fam Physician. 2008;78(10):1173-1179.
11. Saint Alphonsus. Fatigue severity scale. www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf. Accessed May 16, 2017.
12. Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459-463.
13. Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009; 256(4):645-650.
14. Wingerchuk DM, Benarroch EE, O’Brien PC, et al. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005;64(7):1267-1269.
1. Krupp B, Serafin D, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10(9):1437-1447.
2. Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367-368.
3. Dennett SL, Castelli-Haley J, Oleen-Burkey MK. The impact of multiple sclerosis on patient employment: a review of the medical literature. J Health Productivity. 2007;2(2):12-18.
4. Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
5. Kalb R. Multiple Sclerosis: The Questions You Have—The Answers You Need. New York, NY: Demos; 2012.
6. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435-437.
7. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15(3):210-220.
8. Davis S, Wilson T, White A, Frohman E. Thermoregulation in multiple sclerosis. J Appl Physiol. 2016;109(5):1531-1537.
9. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: fatigue. Can Fam Physician. 2007;53(5):892.
10. Rosenthal TC, Majeroni BA, Pretorius R, Malik K. Fatigue: an overview. Am Fam Physician. 2008;78(10):1173-1179.
11. Saint Alphonsus. Fatigue severity scale. www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf. Accessed May 16, 2017.
12. Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459-463.
13. Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009; 256(4):645-650.
14. Wingerchuk DM, Benarroch EE, O’Brien PC, et al. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005;64(7):1267-1269.
Is Vitamin D Beneficial for MS Patients?
Q) What is the role of vitamin D in multiple sclerosis? Is it beneficial?
The exact etiology and pathophysiology of multiple sclerosis (MS) is still not fully understood. Research strongly suggests that there are two major causative factors: one genetic, and the other, environmental. From an environmental standpoint, multiple studies have shown that living farther from the equator, not being exposed to sunlight, and having a low vitamin D level are all correlated with increased risk for MS and MS relapse.1
Our bodies need sunlight to successfully synthesize vitamin D in the skin. Research has found that individuals with lightly pigmented skin are five times more efficient at synthesizing vitamin D in the presence of sunlight than those with darker skin.2 However, the ability to absorb sunlight is also correlated with the earth’s latitude; worse absorption occurs in areas beyond the 40th parallel (in either hemisphere), where UVB levels are too low to synthesize vitamin D four to six months out of the year.2
When exposed to UVB rays, our bodies start to synthesize vitamin D; it undergoes a transformation in the liver and then the kidneys and ultimately becomes the hormonally active form of vitamin D, 1,25-dihydroxyvitamin D3 (calcitriol).2 Calcitriol is recognized by multiple tissues throughout the body that contain vitamin D receptors. Specifically, in the central nervous system, receptors are located on microglia, activated monocytes, and B and T lymphocytes.1 In MS, myelin (the coating around the nerves) is destroyed by an immune-mediated inflammatory process involving the microglia and B and T lymphocytes. Vitamin D quiets down this inflammation, thereby reducing disability accumulation and relapse risk and resulting in fewer changes on MRI.
Vitamin D is also believed to shift the immune response to an anti-inflammatory state by focusing the response on the cytotoxic T cells often found in MS lesions, which attack neurons and oligodendrocytes.2 This theory was tested by Munger and colleagues, who used a pooled cohort of 187,000 women from the Nurses’ Health Study and Nurses’ Health Study II to assess vitamin D intake and risk for MS. Compared to women with lower vitamin D intake, those who took 700 IU/d had a 41% lower incidence of MS. Women who took ≥ 400 IU/d had a 33% lower risk for MS, compared to nonusers.3 In another evaluation of 7 million US military personnel, individuals with a serum vitamin D level of 40 ng/mL were 62% less likely to develop MS.4
In light of the anti-inflammatory effects of vitamin D and its purported reduction of MS risk, it is possible that patients with MS should begin vitamin D supplementation early to obtain maximum anti-inflammatory effects. While an optimal vitamin D goal has not been established in the literature, some studies suggest 30 to 55 ng/mL as a target range for serum vitamin D level.1
While vitamin D has been found to be well-tolerated, patients should be cautioned that very high doses can cause fatigue, abdominal cramps, nausea, vomiting, kidney damage, hypertension, hypercalcemia, and oth
Lisa Marie Fox, MSPAS, PA-C
Division of Multiple Sclerosis, Department of Neurology, Johns Hopkins Hosptial, Baltimore
1. Waubant E, Mowry E, Bowling A. The role of vitamin D in multiple sclerosis pathology and treatment: answers and opportunities. Int J MS Care. 2015;17(2):1-24.
2. Pierrot-Deseilligny C. Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol. 2009;256(9):1468-1478.3. Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60-65.
4. Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006; 296(23):2832-2838.
5. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
Q) What is the role of vitamin D in multiple sclerosis? Is it beneficial?
The exact etiology and pathophysiology of multiple sclerosis (MS) is still not fully understood. Research strongly suggests that there are two major causative factors: one genetic, and the other, environmental. From an environmental standpoint, multiple studies have shown that living farther from the equator, not being exposed to sunlight, and having a low vitamin D level are all correlated with increased risk for MS and MS relapse.1
Our bodies need sunlight to successfully synthesize vitamin D in the skin. Research has found that individuals with lightly pigmented skin are five times more efficient at synthesizing vitamin D in the presence of sunlight than those with darker skin.2 However, the ability to absorb sunlight is also correlated with the earth’s latitude; worse absorption occurs in areas beyond the 40th parallel (in either hemisphere), where UVB levels are too low to synthesize vitamin D four to six months out of the year.2
When exposed to UVB rays, our bodies start to synthesize vitamin D; it undergoes a transformation in the liver and then the kidneys and ultimately becomes the hormonally active form of vitamin D, 1,25-dihydroxyvitamin D3 (calcitriol).2 Calcitriol is recognized by multiple tissues throughout the body that contain vitamin D receptors. Specifically, in the central nervous system, receptors are located on microglia, activated monocytes, and B and T lymphocytes.1 In MS, myelin (the coating around the nerves) is destroyed by an immune-mediated inflammatory process involving the microglia and B and T lymphocytes. Vitamin D quiets down this inflammation, thereby reducing disability accumulation and relapse risk and resulting in fewer changes on MRI.
Vitamin D is also believed to shift the immune response to an anti-inflammatory state by focusing the response on the cytotoxic T cells often found in MS lesions, which attack neurons and oligodendrocytes.2 This theory was tested by Munger and colleagues, who used a pooled cohort of 187,000 women from the Nurses’ Health Study and Nurses’ Health Study II to assess vitamin D intake and risk for MS. Compared to women with lower vitamin D intake, those who took 700 IU/d had a 41% lower incidence of MS. Women who took ≥ 400 IU/d had a 33% lower risk for MS, compared to nonusers.3 In another evaluation of 7 million US military personnel, individuals with a serum vitamin D level of 40 ng/mL were 62% less likely to develop MS.4
In light of the anti-inflammatory effects of vitamin D and its purported reduction of MS risk, it is possible that patients with MS should begin vitamin D supplementation early to obtain maximum anti-inflammatory effects. While an optimal vitamin D goal has not been established in the literature, some studies suggest 30 to 55 ng/mL as a target range for serum vitamin D level.1
While vitamin D has been found to be well-tolerated, patients should be cautioned that very high doses can cause fatigue, abdominal cramps, nausea, vomiting, kidney damage, hypertension, hypercalcemia, and oth
Lisa Marie Fox, MSPAS, PA-C
Division of Multiple Sclerosis, Department of Neurology, Johns Hopkins Hosptial, Baltimore
Q) What is the role of vitamin D in multiple sclerosis? Is it beneficial?
The exact etiology and pathophysiology of multiple sclerosis (MS) is still not fully understood. Research strongly suggests that there are two major causative factors: one genetic, and the other, environmental. From an environmental standpoint, multiple studies have shown that living farther from the equator, not being exposed to sunlight, and having a low vitamin D level are all correlated with increased risk for MS and MS relapse.1
Our bodies need sunlight to successfully synthesize vitamin D in the skin. Research has found that individuals with lightly pigmented skin are five times more efficient at synthesizing vitamin D in the presence of sunlight than those with darker skin.2 However, the ability to absorb sunlight is also correlated with the earth’s latitude; worse absorption occurs in areas beyond the 40th parallel (in either hemisphere), where UVB levels are too low to synthesize vitamin D four to six months out of the year.2
When exposed to UVB rays, our bodies start to synthesize vitamin D; it undergoes a transformation in the liver and then the kidneys and ultimately becomes the hormonally active form of vitamin D, 1,25-dihydroxyvitamin D3 (calcitriol).2 Calcitriol is recognized by multiple tissues throughout the body that contain vitamin D receptors. Specifically, in the central nervous system, receptors are located on microglia, activated monocytes, and B and T lymphocytes.1 In MS, myelin (the coating around the nerves) is destroyed by an immune-mediated inflammatory process involving the microglia and B and T lymphocytes. Vitamin D quiets down this inflammation, thereby reducing disability accumulation and relapse risk and resulting in fewer changes on MRI.
Vitamin D is also believed to shift the immune response to an anti-inflammatory state by focusing the response on the cytotoxic T cells often found in MS lesions, which attack neurons and oligodendrocytes.2 This theory was tested by Munger and colleagues, who used a pooled cohort of 187,000 women from the Nurses’ Health Study and Nurses’ Health Study II to assess vitamin D intake and risk for MS. Compared to women with lower vitamin D intake, those who took 700 IU/d had a 41% lower incidence of MS. Women who took ≥ 400 IU/d had a 33% lower risk for MS, compared to nonusers.3 In another evaluation of 7 million US military personnel, individuals with a serum vitamin D level of 40 ng/mL were 62% less likely to develop MS.4
In light of the anti-inflammatory effects of vitamin D and its purported reduction of MS risk, it is possible that patients with MS should begin vitamin D supplementation early to obtain maximum anti-inflammatory effects. While an optimal vitamin D goal has not been established in the literature, some studies suggest 30 to 55 ng/mL as a target range for serum vitamin D level.1
While vitamin D has been found to be well-tolerated, patients should be cautioned that very high doses can cause fatigue, abdominal cramps, nausea, vomiting, kidney damage, hypertension, hypercalcemia, and oth
Lisa Marie Fox, MSPAS, PA-C
Division of Multiple Sclerosis, Department of Neurology, Johns Hopkins Hosptial, Baltimore
1. Waubant E, Mowry E, Bowling A. The role of vitamin D in multiple sclerosis pathology and treatment: answers and opportunities. Int J MS Care. 2015;17(2):1-24.
2. Pierrot-Deseilligny C. Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol. 2009;256(9):1468-1478.3. Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60-65.
4. Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006; 296(23):2832-2838.
5. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
1. Waubant E, Mowry E, Bowling A. The role of vitamin D in multiple sclerosis pathology and treatment: answers and opportunities. Int J MS Care. 2015;17(2):1-24.
2. Pierrot-Deseilligny C. Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol. 2009;256(9):1468-1478.3. Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60-65.
4. Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006; 296(23):2832-2838.
5. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
Cigarette Smoking: Modifiable Risk Factor for MS
Q) What impact does cigarette smoking have on multiple sclerosis?
The development and disease course of multiple sclerosis (MS) are influenced by a variety of factors. Some—such as genetics, environmental exposure to viruses, and place of residence at an early age—cannot be modified. There are, however, other factors that can be modified, one of which is cigarette smoking.
Between 15% and 17% of patients with MS smoke cigarettes, a rate comparable to that of the general United States population; among US veterans with MS, prevalence can reach as high as 28.5%.1-4 Factors that correlate with smoking in patients with MS include younger age, lower economic/educational background, being single, and lack of available or affordable cessation strategies.1,2,4
Studies have found that in addition to contributing to the development of diseases (eg, cardiovascular and pulmonary) and certain cancers, smoking cigarettes may put individuals at higher risk for MS.5-7 Data also show that increased duration of smoking and/or increased quantity of cigarettes smoked may exacerbate this risk.5 While the mechanisms are not well understood, there appears to be a higher prevalence of MS in male smokers and in current smokers (compared with those who have already quit).6 Other studies have also suggested an increased risk with passive exposure to cigarette smoke.6,7
Current cigarette smoking accelerates the conversion from a relapsing to a progressive form of MS.8-11 One study demonstrated that after the diagnosis of MS had been made, continued smoking increased the rate of acceleration to a progressive form by 5% per year.9 Current smokers also had a higher disability rate attributable to their MS, but smoking cessation may improve disability outcomes.9-11
Current smokers, with or without other risk factors, therefore have incentive to quit smoking to reduce risk for MS. Patients with MS who smoke should be counseled on the increased risk associated with the combination of passive smoke exposure and other genetic and environmental factors, which may increase risk for MS in first-degree family members.3
While there is no one-size-fits-all strategy for smoking cessation in patients with MS, traditional behavioral and/or medication therapies should be offered. Some factors involved in cigarette smoking are variable and difficult to address, in addition to the physical dependence on nicotine. Many patients will require interventions to address related poor health behaviors (eg, lack of exercise) and comorbid factors (eg, depression).5,7 —BW
Bryan Walker, MHS, PA-C
Department of Neurology, Division of MS and Neuroimmunology, Duke University Medical Center, Durham, North Carolina
1. Friend KB, Mernoff ST, Block P, Reeve G. Smoking rates and smoking cessation among individuals with multiple sclerosis. Disabil Rehabil. 2006;28(18):1135-1141.
2. Marrie R, Horwitz R, Cutter G, et al. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1): 105-113.
3. CDC. Current cigarette smoking among adults in the United States. www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking. Accessed January 13, 2017.
4. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
5. Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2016; 36(2):103-114.
6. Zhang P, Wang R, Li Z, et al. The risk of smoking on multiple sclerosis: a meta-analysis based on 20,626 cases from case-control and cohort studies. PeerJ. 2016;4:e1797.
7. Hedström AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler. 2016; 22(8):1021-1026.
8. Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
9. Goldman MD, Stüve O. Smoking beyond multiple sclerosis diagnosis: a risk factor still worth modifying. JAMA Neurol. 2015;72(10):1105-1106.
10. Ramanujam R, Hedström A, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
11. Ben-Zacharia A. The effect of modifiable risk factors on multiple sclerosis progression. Neurology. 2016;86(16):Supplement P1.387.
Q) What impact does cigarette smoking have on multiple sclerosis?
The development and disease course of multiple sclerosis (MS) are influenced by a variety of factors. Some—such as genetics, environmental exposure to viruses, and place of residence at an early age—cannot be modified. There are, however, other factors that can be modified, one of which is cigarette smoking.
Between 15% and 17% of patients with MS smoke cigarettes, a rate comparable to that of the general United States population; among US veterans with MS, prevalence can reach as high as 28.5%.1-4 Factors that correlate with smoking in patients with MS include younger age, lower economic/educational background, being single, and lack of available or affordable cessation strategies.1,2,4
Studies have found that in addition to contributing to the development of diseases (eg, cardiovascular and pulmonary) and certain cancers, smoking cigarettes may put individuals at higher risk for MS.5-7 Data also show that increased duration of smoking and/or increased quantity of cigarettes smoked may exacerbate this risk.5 While the mechanisms are not well understood, there appears to be a higher prevalence of MS in male smokers and in current smokers (compared with those who have already quit).6 Other studies have also suggested an increased risk with passive exposure to cigarette smoke.6,7
Current cigarette smoking accelerates the conversion from a relapsing to a progressive form of MS.8-11 One study demonstrated that after the diagnosis of MS had been made, continued smoking increased the rate of acceleration to a progressive form by 5% per year.9 Current smokers also had a higher disability rate attributable to their MS, but smoking cessation may improve disability outcomes.9-11
Current smokers, with or without other risk factors, therefore have incentive to quit smoking to reduce risk for MS. Patients with MS who smoke should be counseled on the increased risk associated with the combination of passive smoke exposure and other genetic and environmental factors, which may increase risk for MS in first-degree family members.3
While there is no one-size-fits-all strategy for smoking cessation in patients with MS, traditional behavioral and/or medication therapies should be offered. Some factors involved in cigarette smoking are variable and difficult to address, in addition to the physical dependence on nicotine. Many patients will require interventions to address related poor health behaviors (eg, lack of exercise) and comorbid factors (eg, depression).5,7 —BW
Bryan Walker, MHS, PA-C
Department of Neurology, Division of MS and Neuroimmunology, Duke University Medical Center, Durham, North Carolina
Q) What impact does cigarette smoking have on multiple sclerosis?
The development and disease course of multiple sclerosis (MS) are influenced by a variety of factors. Some—such as genetics, environmental exposure to viruses, and place of residence at an early age—cannot be modified. There are, however, other factors that can be modified, one of which is cigarette smoking.
Between 15% and 17% of patients with MS smoke cigarettes, a rate comparable to that of the general United States population; among US veterans with MS, prevalence can reach as high as 28.5%.1-4 Factors that correlate with smoking in patients with MS include younger age, lower economic/educational background, being single, and lack of available or affordable cessation strategies.1,2,4
Studies have found that in addition to contributing to the development of diseases (eg, cardiovascular and pulmonary) and certain cancers, smoking cigarettes may put individuals at higher risk for MS.5-7 Data also show that increased duration of smoking and/or increased quantity of cigarettes smoked may exacerbate this risk.5 While the mechanisms are not well understood, there appears to be a higher prevalence of MS in male smokers and in current smokers (compared with those who have already quit).6 Other studies have also suggested an increased risk with passive exposure to cigarette smoke.6,7
Current cigarette smoking accelerates the conversion from a relapsing to a progressive form of MS.8-11 One study demonstrated that after the diagnosis of MS had been made, continued smoking increased the rate of acceleration to a progressive form by 5% per year.9 Current smokers also had a higher disability rate attributable to their MS, but smoking cessation may improve disability outcomes.9-11
Current smokers, with or without other risk factors, therefore have incentive to quit smoking to reduce risk for MS. Patients with MS who smoke should be counseled on the increased risk associated with the combination of passive smoke exposure and other genetic and environmental factors, which may increase risk for MS in first-degree family members.3
While there is no one-size-fits-all strategy for smoking cessation in patients with MS, traditional behavioral and/or medication therapies should be offered. Some factors involved in cigarette smoking are variable and difficult to address, in addition to the physical dependence on nicotine. Many patients will require interventions to address related poor health behaviors (eg, lack of exercise) and comorbid factors (eg, depression).5,7 —BW
Bryan Walker, MHS, PA-C
Department of Neurology, Division of MS and Neuroimmunology, Duke University Medical Center, Durham, North Carolina
1. Friend KB, Mernoff ST, Block P, Reeve G. Smoking rates and smoking cessation among individuals with multiple sclerosis. Disabil Rehabil. 2006;28(18):1135-1141.
2. Marrie R, Horwitz R, Cutter G, et al. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1): 105-113.
3. CDC. Current cigarette smoking among adults in the United States. www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking. Accessed January 13, 2017.
4. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
5. Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2016; 36(2):103-114.
6. Zhang P, Wang R, Li Z, et al. The risk of smoking on multiple sclerosis: a meta-analysis based on 20,626 cases from case-control and cohort studies. PeerJ. 2016;4:e1797.
7. Hedström AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler. 2016; 22(8):1021-1026.
8. Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
9. Goldman MD, Stüve O. Smoking beyond multiple sclerosis diagnosis: a risk factor still worth modifying. JAMA Neurol. 2015;72(10):1105-1106.
10. Ramanujam R, Hedström A, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
11. Ben-Zacharia A. The effect of modifiable risk factors on multiple sclerosis progression. Neurology. 2016;86(16):Supplement P1.387.
1. Friend KB, Mernoff ST, Block P, Reeve G. Smoking rates and smoking cessation among individuals with multiple sclerosis. Disabil Rehabil. 2006;28(18):1135-1141.
2. Marrie R, Horwitz R, Cutter G, et al. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1): 105-113.
3. CDC. Current cigarette smoking among adults in the United States. www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking. Accessed January 13, 2017.
4. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
5. Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2016; 36(2):103-114.
6. Zhang P, Wang R, Li Z, et al. The risk of smoking on multiple sclerosis: a meta-analysis based on 20,626 cases from case-control and cohort studies. PeerJ. 2016;4:e1797.
7. Hedström AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler. 2016; 22(8):1021-1026.
8. Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
9. Goldman MD, Stüve O. Smoking beyond multiple sclerosis diagnosis: a risk factor still worth modifying. JAMA Neurol. 2015;72(10):1105-1106.
10. Ramanujam R, Hedström A, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
11. Ben-Zacharia A. The effect of modifiable risk factors on multiple sclerosis progression. Neurology. 2016;86(16):Supplement P1.387.
MS & Pregnancy: What's Safe?
Q)What are the considerations and recommendations for pregnancy and breastfeeding in women with multiple sclerosis? When should women discontinue their disease-modifying therapies?
Multiple sclerosis (MS) is an inflammatory, demyelinating, degenerative disease. Three times more common in women than men, it may affect pregnancy planning and childbearing experiences.6 Evidence demonstrates a reduction in annualized relapse rate during pregnancy and the postpartum period with exclusive breastfeeding. Therefore, pregnancy and exclusive breastfeeding provide a favorable immunomodulatory effect in women with MS, which combats the increased relapse risk associated with the postpartum period.7,8
Reproductive education—including conception, pregnancy, and breastfeeding—is critical for patients with MS and their partners during a woman’s childbearing years. However, women with MS do not require special considerations during pregnancy unless they have remarkable disability. As soon as these women and their partners decide upon pregnancy, a plan should be established that includes a discussion about potential risks to the fetus due to drug exposure, as well as risks to the mother. The goal should be to minimize risk for disease activity and optimize the health of the baby.
Use of DMTs during conception, pregnancy, and breastfeeding. All disease-modifying therapies (DMTs) are usually discontinued during pregnancy and breastfeeding. Common practice among MS experts is to discontinue DMTs prior to conception, with a few exceptions. There is no consensus about timing of discontinuation and washout period for each DMT. The decision is based on the half-life of each DMT, the opinions of the woman and her partner, and risk tolerance. Note: For the purposes of this discussion, the old pregnancy category designations are used, since they are familiar to clinicians. New guidelines took effect in June 2015; Table 3 outlines the change in format.
Injectables. Glatiramer acetate (GA) is the safest drug in relation to pregnancy and breastfeeding (category B). There is no evidence of congenital malformation or spontaneous abortion. The common recommendation is to discontinue the drug one to two months before conception, although some clinicians allow continuation of the injections throughout pregnancy and into breastfeeding.
Interferon-ßs are category C and therefore pose minimal risk for the fetus. The washout period before conception is two to three months, varying among clinicians. Although there is no evidence of spontaneous abortion or birth defects in humans, animal data show increased risk for abortion.9
Both GA and interferon-ßs are large molecules; there is a very minimal chance that the medication will transmit to the baby via breast milk. Thus, both DMTs are considered safe during lactation.7
Oral MS medications. The three approved MS oral medications are fingolimod, dimethyl fumarate (DMF), and teriflunomide. Fingolimod and DMF are both category C. Women on DMF must discontinue use of the medication one month prior to conception due to its short half-life. There are no reports of birth defects or spontaneous abortion in women taking DMF. Fingolimod needs to be discontinued two months prior to conception. Animal data show evidence for teratogenicity and embryolethality at lower doses of fingolimod than those used in humans.7
Teriflunomide is category X, posing high risk for the health of the fetus. It stays in the blood for approximately eight months after discontinuation of use. Animal data show teratogenicity and embryotoxicity; therefore, teriflunomide is contraindicated in pregnancy. Women on teriflunomide who plan to become pregnant need to undergo an elimination procedure with cholestyramine or charcoal.
Infusions and injections (monoclonal antibodies). The approved monoclonal antibodies include natalizumab, alemtuzumab, and daclizumab. (Currently, use of rituximab in MS is off label, and the FDA is reviewing the efficacy and safety data for ocrelizumab.) The monoclonal antibodies are category C. The recommendation is to discontinue natalizumab one to two months prior to conception and discontinue alemtuzumab four months preconception.10 There is no evidence of spontaneous abortion or birth defects in women on alemtuzumab, but there is potentially increased risk for spontaneous abortion in those on natalizumab.
The spontaneous abortion rate in daclizumab-exposed women is consistent with early pregnancy loss in the general population (12% to 26%). Data on a small number of pregnancies exposed to daclizumab did not suggest an increased risk for adverse fetal or maternal outcomes.11 However, the recommendation is to discontinue daclizumab four months prior to conception. Rituximab should be discontinued 12 months prior to conception, based on the manufacturer recommendation, although it is potentially safe to conceive when the B cell counts return to normal.
Chemotherapy agents. Mitoxantrone is the only FDA-approved chemotherapeutic drug used in MS. However, a few chemotherapy drugs—among them, azathioprine, methotrexate, and cyclophosphamide—are used off label. Chemotherapeutic agents are category D, except methotrexate (category X). Women on category D medications must use a method of birth control for three months after stopping the DMT. Often, clinicians will recommend women initiate GA or interferon during this period, in the hope of minimizing disease activity. Women on mitoxantrone and methotrexate need to use birth control for six months after stopping these immunosuppressive medications, before conceiving.12 These women likely need to switch to a safer pregnancy DMT during the long washout period.
Pregnancy and breastfeeding among women with MS require planning and decision making. The recommendations differ among clinicians and MS experts since there is no definitive evidence about the risks of the DMTs on the mother and/or the fetus. Clinicians should discuss the potential risks with women based on their knowledge and experience, and the data available based on animal research and the pregnancy registries. —ABB-Z
Aliza Bitton Ben-Zacharia, DNP, ANP
President-Elect of IOMSN
Neurology Faculty, Icahn School of Medicine, Mount Sinai Hospital System, New York, New York
6. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2):S15-S20.
7. Fabian M. Pregnancy in the setting of multiple sclerosis. Continuum (Minneap Minn). 2016; 22(3):837-850.
8. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(6):1353-1360.
9. Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011; 17(4):423-430.
10. Coyle PK. Multiple sclerosis in pregnancy. Continuum (Minneap Minn). 2014;20(1):42-59.
11. Gold SM, Voskuhl RR. Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol. 2016;38:709.
12. Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the ‘treatment era’. Nat Rev Neurol. 2015;11(5):280-289.
13. FDA. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Accessed November 2, 2016.
14. Whyte J. FDA implements new labeling for medications used during pregnancy and lactation. Am Fam Physician. 2016;94(1):12-13.
Q)What are the considerations and recommendations for pregnancy and breastfeeding in women with multiple sclerosis? When should women discontinue their disease-modifying therapies?
Multiple sclerosis (MS) is an inflammatory, demyelinating, degenerative disease. Three times more common in women than men, it may affect pregnancy planning and childbearing experiences.6 Evidence demonstrates a reduction in annualized relapse rate during pregnancy and the postpartum period with exclusive breastfeeding. Therefore, pregnancy and exclusive breastfeeding provide a favorable immunomodulatory effect in women with MS, which combats the increased relapse risk associated with the postpartum period.7,8
Reproductive education—including conception, pregnancy, and breastfeeding—is critical for patients with MS and their partners during a woman’s childbearing years. However, women with MS do not require special considerations during pregnancy unless they have remarkable disability. As soon as these women and their partners decide upon pregnancy, a plan should be established that includes a discussion about potential risks to the fetus due to drug exposure, as well as risks to the mother. The goal should be to minimize risk for disease activity and optimize the health of the baby.
Use of DMTs during conception, pregnancy, and breastfeeding. All disease-modifying therapies (DMTs) are usually discontinued during pregnancy and breastfeeding. Common practice among MS experts is to discontinue DMTs prior to conception, with a few exceptions. There is no consensus about timing of discontinuation and washout period for each DMT. The decision is based on the half-life of each DMT, the opinions of the woman and her partner, and risk tolerance. Note: For the purposes of this discussion, the old pregnancy category designations are used, since they are familiar to clinicians. New guidelines took effect in June 2015; Table 3 outlines the change in format.
Injectables. Glatiramer acetate (GA) is the safest drug in relation to pregnancy and breastfeeding (category B). There is no evidence of congenital malformation or spontaneous abortion. The common recommendation is to discontinue the drug one to two months before conception, although some clinicians allow continuation of the injections throughout pregnancy and into breastfeeding.
Interferon-ßs are category C and therefore pose minimal risk for the fetus. The washout period before conception is two to three months, varying among clinicians. Although there is no evidence of spontaneous abortion or birth defects in humans, animal data show increased risk for abortion.9
Both GA and interferon-ßs are large molecules; there is a very minimal chance that the medication will transmit to the baby via breast milk. Thus, both DMTs are considered safe during lactation.7
Oral MS medications. The three approved MS oral medications are fingolimod, dimethyl fumarate (DMF), and teriflunomide. Fingolimod and DMF are both category C. Women on DMF must discontinue use of the medication one month prior to conception due to its short half-life. There are no reports of birth defects or spontaneous abortion in women taking DMF. Fingolimod needs to be discontinued two months prior to conception. Animal data show evidence for teratogenicity and embryolethality at lower doses of fingolimod than those used in humans.7
Teriflunomide is category X, posing high risk for the health of the fetus. It stays in the blood for approximately eight months after discontinuation of use. Animal data show teratogenicity and embryotoxicity; therefore, teriflunomide is contraindicated in pregnancy. Women on teriflunomide who plan to become pregnant need to undergo an elimination procedure with cholestyramine or charcoal.
Infusions and injections (monoclonal antibodies). The approved monoclonal antibodies include natalizumab, alemtuzumab, and daclizumab. (Currently, use of rituximab in MS is off label, and the FDA is reviewing the efficacy and safety data for ocrelizumab.) The monoclonal antibodies are category C. The recommendation is to discontinue natalizumab one to two months prior to conception and discontinue alemtuzumab four months preconception.10 There is no evidence of spontaneous abortion or birth defects in women on alemtuzumab, but there is potentially increased risk for spontaneous abortion in those on natalizumab.
The spontaneous abortion rate in daclizumab-exposed women is consistent with early pregnancy loss in the general population (12% to 26%). Data on a small number of pregnancies exposed to daclizumab did not suggest an increased risk for adverse fetal or maternal outcomes.11 However, the recommendation is to discontinue daclizumab four months prior to conception. Rituximab should be discontinued 12 months prior to conception, based on the manufacturer recommendation, although it is potentially safe to conceive when the B cell counts return to normal.
Chemotherapy agents. Mitoxantrone is the only FDA-approved chemotherapeutic drug used in MS. However, a few chemotherapy drugs—among them, azathioprine, methotrexate, and cyclophosphamide—are used off label. Chemotherapeutic agents are category D, except methotrexate (category X). Women on category D medications must use a method of birth control for three months after stopping the DMT. Often, clinicians will recommend women initiate GA or interferon during this period, in the hope of minimizing disease activity. Women on mitoxantrone and methotrexate need to use birth control for six months after stopping these immunosuppressive medications, before conceiving.12 These women likely need to switch to a safer pregnancy DMT during the long washout period.
Pregnancy and breastfeeding among women with MS require planning and decision making. The recommendations differ among clinicians and MS experts since there is no definitive evidence about the risks of the DMTs on the mother and/or the fetus. Clinicians should discuss the potential risks with women based on their knowledge and experience, and the data available based on animal research and the pregnancy registries. —ABB-Z
Aliza Bitton Ben-Zacharia, DNP, ANP
President-Elect of IOMSN
Neurology Faculty, Icahn School of Medicine, Mount Sinai Hospital System, New York, New York
Q)What are the considerations and recommendations for pregnancy and breastfeeding in women with multiple sclerosis? When should women discontinue their disease-modifying therapies?
Multiple sclerosis (MS) is an inflammatory, demyelinating, degenerative disease. Three times more common in women than men, it may affect pregnancy planning and childbearing experiences.6 Evidence demonstrates a reduction in annualized relapse rate during pregnancy and the postpartum period with exclusive breastfeeding. Therefore, pregnancy and exclusive breastfeeding provide a favorable immunomodulatory effect in women with MS, which combats the increased relapse risk associated with the postpartum period.7,8
Reproductive education—including conception, pregnancy, and breastfeeding—is critical for patients with MS and their partners during a woman’s childbearing years. However, women with MS do not require special considerations during pregnancy unless they have remarkable disability. As soon as these women and their partners decide upon pregnancy, a plan should be established that includes a discussion about potential risks to the fetus due to drug exposure, as well as risks to the mother. The goal should be to minimize risk for disease activity and optimize the health of the baby.
Use of DMTs during conception, pregnancy, and breastfeeding. All disease-modifying therapies (DMTs) are usually discontinued during pregnancy and breastfeeding. Common practice among MS experts is to discontinue DMTs prior to conception, with a few exceptions. There is no consensus about timing of discontinuation and washout period for each DMT. The decision is based on the half-life of each DMT, the opinions of the woman and her partner, and risk tolerance. Note: For the purposes of this discussion, the old pregnancy category designations are used, since they are familiar to clinicians. New guidelines took effect in June 2015; Table 3 outlines the change in format.
Injectables. Glatiramer acetate (GA) is the safest drug in relation to pregnancy and breastfeeding (category B). There is no evidence of congenital malformation or spontaneous abortion. The common recommendation is to discontinue the drug one to two months before conception, although some clinicians allow continuation of the injections throughout pregnancy and into breastfeeding.
Interferon-ßs are category C and therefore pose minimal risk for the fetus. The washout period before conception is two to three months, varying among clinicians. Although there is no evidence of spontaneous abortion or birth defects in humans, animal data show increased risk for abortion.9
Both GA and interferon-ßs are large molecules; there is a very minimal chance that the medication will transmit to the baby via breast milk. Thus, both DMTs are considered safe during lactation.7
Oral MS medications. The three approved MS oral medications are fingolimod, dimethyl fumarate (DMF), and teriflunomide. Fingolimod and DMF are both category C. Women on DMF must discontinue use of the medication one month prior to conception due to its short half-life. There are no reports of birth defects or spontaneous abortion in women taking DMF. Fingolimod needs to be discontinued two months prior to conception. Animal data show evidence for teratogenicity and embryolethality at lower doses of fingolimod than those used in humans.7
Teriflunomide is category X, posing high risk for the health of the fetus. It stays in the blood for approximately eight months after discontinuation of use. Animal data show teratogenicity and embryotoxicity; therefore, teriflunomide is contraindicated in pregnancy. Women on teriflunomide who plan to become pregnant need to undergo an elimination procedure with cholestyramine or charcoal.
Infusions and injections (monoclonal antibodies). The approved monoclonal antibodies include natalizumab, alemtuzumab, and daclizumab. (Currently, use of rituximab in MS is off label, and the FDA is reviewing the efficacy and safety data for ocrelizumab.) The monoclonal antibodies are category C. The recommendation is to discontinue natalizumab one to two months prior to conception and discontinue alemtuzumab four months preconception.10 There is no evidence of spontaneous abortion or birth defects in women on alemtuzumab, but there is potentially increased risk for spontaneous abortion in those on natalizumab.
The spontaneous abortion rate in daclizumab-exposed women is consistent with early pregnancy loss in the general population (12% to 26%). Data on a small number of pregnancies exposed to daclizumab did not suggest an increased risk for adverse fetal or maternal outcomes.11 However, the recommendation is to discontinue daclizumab four months prior to conception. Rituximab should be discontinued 12 months prior to conception, based on the manufacturer recommendation, although it is potentially safe to conceive when the B cell counts return to normal.
Chemotherapy agents. Mitoxantrone is the only FDA-approved chemotherapeutic drug used in MS. However, a few chemotherapy drugs—among them, azathioprine, methotrexate, and cyclophosphamide—are used off label. Chemotherapeutic agents are category D, except methotrexate (category X). Women on category D medications must use a method of birth control for three months after stopping the DMT. Often, clinicians will recommend women initiate GA or interferon during this period, in the hope of minimizing disease activity. Women on mitoxantrone and methotrexate need to use birth control for six months after stopping these immunosuppressive medications, before conceiving.12 These women likely need to switch to a safer pregnancy DMT during the long washout period.
Pregnancy and breastfeeding among women with MS require planning and decision making. The recommendations differ among clinicians and MS experts since there is no definitive evidence about the risks of the DMTs on the mother and/or the fetus. Clinicians should discuss the potential risks with women based on their knowledge and experience, and the data available based on animal research and the pregnancy registries. —ABB-Z
Aliza Bitton Ben-Zacharia, DNP, ANP
President-Elect of IOMSN
Neurology Faculty, Icahn School of Medicine, Mount Sinai Hospital System, New York, New York
6. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2):S15-S20.
7. Fabian M. Pregnancy in the setting of multiple sclerosis. Continuum (Minneap Minn). 2016; 22(3):837-850.
8. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(6):1353-1360.
9. Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011; 17(4):423-430.
10. Coyle PK. Multiple sclerosis in pregnancy. Continuum (Minneap Minn). 2014;20(1):42-59.
11. Gold SM, Voskuhl RR. Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol. 2016;38:709.
12. Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the ‘treatment era’. Nat Rev Neurol. 2015;11(5):280-289.
13. FDA. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Accessed November 2, 2016.
14. Whyte J. FDA implements new labeling for medications used during pregnancy and lactation. Am Fam Physician. 2016;94(1):12-13.
6. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2):S15-S20.
7. Fabian M. Pregnancy in the setting of multiple sclerosis. Continuum (Minneap Minn). 2016; 22(3):837-850.
8. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(6):1353-1360.
9. Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011; 17(4):423-430.
10. Coyle PK. Multiple sclerosis in pregnancy. Continuum (Minneap Minn). 2014;20(1):42-59.
11. Gold SM, Voskuhl RR. Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol. 2016;38:709.
12. Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the ‘treatment era’. Nat Rev Neurol. 2015;11(5):280-289.
13. FDA. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Accessed November 2, 2016.
14. Whyte J. FDA implements new labeling for medications used during pregnancy and lactation. Am Fam Physician. 2016;94(1):12-13.
CAM in MS: What Works?
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.
Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.
Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.
Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.
Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.
Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.
Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.