User login
Brazilian Peppertree: Watch Out for This Lesser-Known Relative of Poison Ivy
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
Practice Points
- The Anacardiaceae family contains several plants, including Brazilian peppertree and poison ivy, that have the potential to cause allergic contact dermatitis (ACD).
- Hot spots for Brazilian peppertree include Florida and California, though it also has been reported in Texas, Hawaii, Georgia, Alabama, Arkansas, Nevada, and Arizona.
- Alkenyl phenols (eg, cardol, cardanol) are the key sensitizers found in Brazilian peppertree.
- Treatment consists of supportive care and either topical, oral, or intramuscular steroids depending on the extent and severity of the ACD.
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
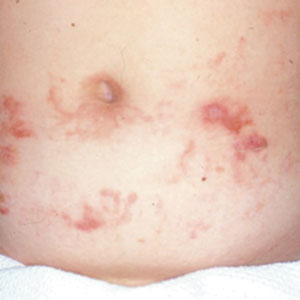
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10
magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).
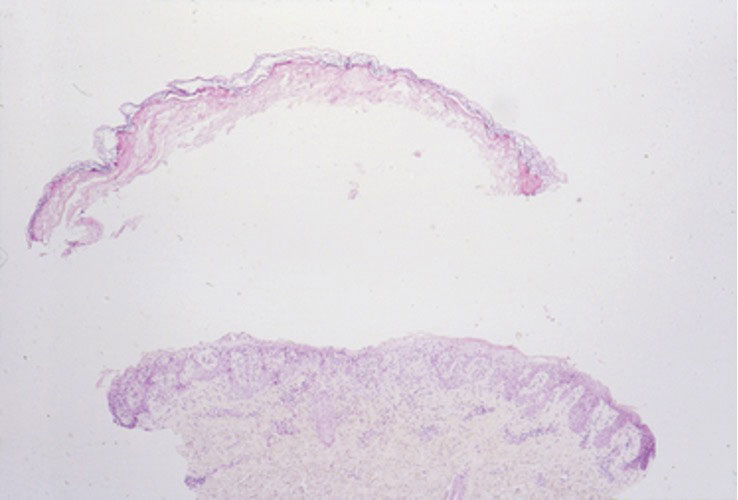
Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
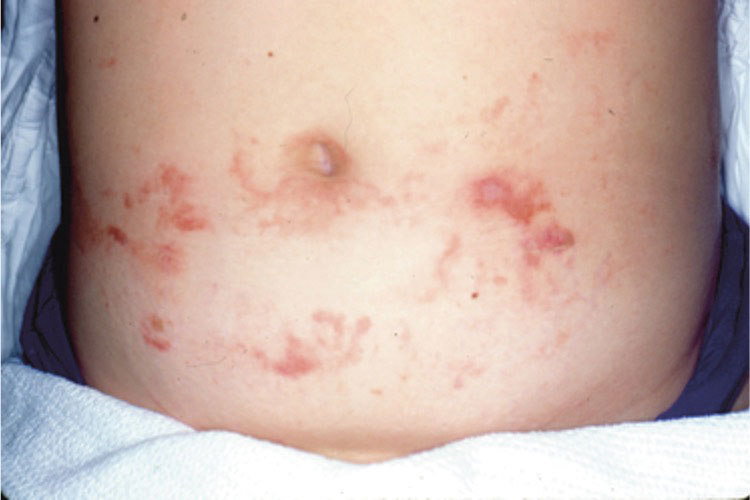
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
PRACTICE POINTS
- Lyngbya majuscula causes seaweed dermatitis in swimmers and can be prevented by avoiding rough turbid waters in areas known to have L majuscula blooms.
- Seaweed dermatitis should be included in the differential diagnosis for erythematous papulovesicular rashes manifesting in patients who recently have spent time in the ocean.
Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Practice Points
- Exposure to the components of the common fig tree (Ficus carica) can induce phytophotodermatitis.
- Notable postinflammatory hyperpigmentation typically occurs in the healing stage of fig phytophotodermatitis.
Botanical Briefs: Contact Dermatitis Induced by Western Poison Ivy (Toxicodendron rydbergii)
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
PRACTICE POINTS
- Western poison ivy (Toxicodendron rydbergii) accounts for many of the cases of Toxicodendron contact dermatitis (TCD) in the western and northern United States. Individuals in these regions should be educated on how to identify T rydbergii to avoid TCD.
- Dermatologists should include TCD in the differential diagnosis when a patient presents with an erythematous pruritic rash in a linear pattern with sharp borders.
- Most patients who experience intense itching and pain from TCD benefit greatly from prompt treatment with an oral or intramuscular corticosteroid. Topical steroids rarely provide relief; oral antihistamines provide no benefit.
Botanical Briefs: Australian Stinging Tree (Dendrocnide moroides)
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6
The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Practice Points
- Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.
- Clinical observations after contact reveal immediate piloerection and local swelling, which may disappear after 1 hour or last as long as 24 hours, but subjective pain, pruritus, and burning can persist for months.
- The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.