User login
23rd World Congress of Dermatology
WCD: Touch Avoidance Is a New Dimension of Psoriatic Impairment
VANCOUVER – Touch impairment is an important but largely unappreciated aspect of quality-of-life impairment in psoriasis, Dr. April Armstrong said at the World Congress of Dermatology.
“Touch avoidance is a new concept in dermatology, and I think it’s great because it really brings home a humanistic aspect to research,” observed Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
“Touch is a fundamental human gesture that conveys understanding, connection, validation, as well as being cared for or caring for someone,” she added.
She presented an online study of 1,109 psoriasis patients, 70% of whom were female. When asked whether within the past 2 weeks they had avoided hugging, shaking hands, or other touching of others, or of being touched, because of how their skin looks or feels, 48% responded yes. On a 0-10 scale, 27% of the overall patient population rated themselves as having touch avoidance at the level of 4-10, corresponding to moderate to severe.
In a multivariate analysis, the prevalence or severity of touch avoidance didn’t vary by gender. However, touch avoidance was reported most often by patients under age 25 years. And touch avoidance was strongly associated with disease severity: Those with mild psoriasis as defined by body surface area of involvement were only about one-third as likely to report touch avoidance, compared with those with severe disease. Also, psoriatic itching was an important correlate of touch avoidance: Those with itch were roughly fivefold more likely to report recent touch avoidance.
In addition, patients with psoriasis on exposed areas such as the face and neck or in sensitive areas such as the genitalia were more likely to report touch avoidance.
Touch avoidance had a strong negative impact on quality of life. Scores on the Dermatology Life Quality Index averaged nearly threefold higher – indicating worse quality of life – in participants who reported touch avoidance. They were at similarly increased risk for mild or greater depressive symptoms based upon their scores on the Quick Inventory of Depressive Symptoms.
Thus, touch avoidance is an important new patient-reported outcome, and these study results open up a new area of psoriasis research, according to Dr. Armstrong.
“Future research may reveal that therapies offering clearance of psoriasis, particularly in sensitive areas, could lead to large improvements in the initiation and acceptance of interpersonal touch by patients with psoriasis,” she noted.
This study was supported by the National Psoriasis Foundation. Dr. Armstrong reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies as well as the National Institutes of Health.
VANCOUVER – Touch impairment is an important but largely unappreciated aspect of quality-of-life impairment in psoriasis, Dr. April Armstrong said at the World Congress of Dermatology.
“Touch avoidance is a new concept in dermatology, and I think it’s great because it really brings home a humanistic aspect to research,” observed Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
“Touch is a fundamental human gesture that conveys understanding, connection, validation, as well as being cared for or caring for someone,” she added.
She presented an online study of 1,109 psoriasis patients, 70% of whom were female. When asked whether within the past 2 weeks they had avoided hugging, shaking hands, or other touching of others, or of being touched, because of how their skin looks or feels, 48% responded yes. On a 0-10 scale, 27% of the overall patient population rated themselves as having touch avoidance at the level of 4-10, corresponding to moderate to severe.
In a multivariate analysis, the prevalence or severity of touch avoidance didn’t vary by gender. However, touch avoidance was reported most often by patients under age 25 years. And touch avoidance was strongly associated with disease severity: Those with mild psoriasis as defined by body surface area of involvement were only about one-third as likely to report touch avoidance, compared with those with severe disease. Also, psoriatic itching was an important correlate of touch avoidance: Those with itch were roughly fivefold more likely to report recent touch avoidance.
In addition, patients with psoriasis on exposed areas such as the face and neck or in sensitive areas such as the genitalia were more likely to report touch avoidance.
Touch avoidance had a strong negative impact on quality of life. Scores on the Dermatology Life Quality Index averaged nearly threefold higher – indicating worse quality of life – in participants who reported touch avoidance. They were at similarly increased risk for mild or greater depressive symptoms based upon their scores on the Quick Inventory of Depressive Symptoms.
Thus, touch avoidance is an important new patient-reported outcome, and these study results open up a new area of psoriasis research, according to Dr. Armstrong.
“Future research may reveal that therapies offering clearance of psoriasis, particularly in sensitive areas, could lead to large improvements in the initiation and acceptance of interpersonal touch by patients with psoriasis,” she noted.
This study was supported by the National Psoriasis Foundation. Dr. Armstrong reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies as well as the National Institutes of Health.
VANCOUVER – Touch impairment is an important but largely unappreciated aspect of quality-of-life impairment in psoriasis, Dr. April Armstrong said at the World Congress of Dermatology.
“Touch avoidance is a new concept in dermatology, and I think it’s great because it really brings home a humanistic aspect to research,” observed Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
“Touch is a fundamental human gesture that conveys understanding, connection, validation, as well as being cared for or caring for someone,” she added.
She presented an online study of 1,109 psoriasis patients, 70% of whom were female. When asked whether within the past 2 weeks they had avoided hugging, shaking hands, or other touching of others, or of being touched, because of how their skin looks or feels, 48% responded yes. On a 0-10 scale, 27% of the overall patient population rated themselves as having touch avoidance at the level of 4-10, corresponding to moderate to severe.
In a multivariate analysis, the prevalence or severity of touch avoidance didn’t vary by gender. However, touch avoidance was reported most often by patients under age 25 years. And touch avoidance was strongly associated with disease severity: Those with mild psoriasis as defined by body surface area of involvement were only about one-third as likely to report touch avoidance, compared with those with severe disease. Also, psoriatic itching was an important correlate of touch avoidance: Those with itch were roughly fivefold more likely to report recent touch avoidance.
In addition, patients with psoriasis on exposed areas such as the face and neck or in sensitive areas such as the genitalia were more likely to report touch avoidance.
Touch avoidance had a strong negative impact on quality of life. Scores on the Dermatology Life Quality Index averaged nearly threefold higher – indicating worse quality of life – in participants who reported touch avoidance. They were at similarly increased risk for mild or greater depressive symptoms based upon their scores on the Quick Inventory of Depressive Symptoms.
Thus, touch avoidance is an important new patient-reported outcome, and these study results open up a new area of psoriasis research, according to Dr. Armstrong.
“Future research may reveal that therapies offering clearance of psoriasis, particularly in sensitive areas, could lead to large improvements in the initiation and acceptance of interpersonal touch by patients with psoriasis,” she noted.
This study was supported by the National Psoriasis Foundation. Dr. Armstrong reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies as well as the National Institutes of Health.
AT WCD 2015
WCD: Touch avoidance is a new dimension of psoriatic impairment
VANCOUVER – Touch impairment is an important but largely unappreciated aspect of quality-of-life impairment in psoriasis, Dr. April Armstrong said at the World Congress of Dermatology.
“Touch avoidance is a new concept in dermatology, and I think it’s great because it really brings home a humanistic aspect to research,” observed Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
“Touch is a fundamental human gesture that conveys understanding, connection, validation, as well as being cared for or caring for someone,” she added.
She presented an online study of 1,109 psoriasis patients, 70% of whom were female. When asked whether within the past 2 weeks they had avoided hugging, shaking hands, or other touching of others, or of being touched, because of how their skin looks or feels, 48% responded yes. On a 0-10 scale, 27% of the overall patient population rated themselves as having touch avoidance at the level of 4-10, corresponding to moderate to severe.
In a multivariate analysis, the prevalence or severity of touch avoidance didn’t vary by gender. However, touch avoidance was reported most often by patients under age 25 years. And touch avoidance was strongly associated with disease severity: Those with mild psoriasis as defined by body surface area of involvement were only about one-third as likely to report touch avoidance, compared with those with severe disease. Also, psoriatic itching was an important correlate of touch avoidance: Those with itch were roughly fivefold more likely to report recent touch avoidance.
In addition, patients with psoriasis on exposed areas such as the face and neck or in sensitive areas such as the genitalia were more likely to report touch avoidance.
Touch avoidance had a strong negative impact on quality of life. Scores on the Dermatology Life Quality Index averaged nearly threefold higher – indicating worse quality of life – in participants who reported touch avoidance. They were at similarly increased risk for mild or greater depressive symptoms based upon their scores on the Quick Inventory of Depressive Symptoms.
Thus, touch avoidance is an important new patient-reported outcome, and these study results open up a new area of psoriasis research, according to Dr. Armstrong.
“Future research may reveal that therapies offering clearance of psoriasis, particularly in sensitive areas, could lead to large improvements in the initiation and acceptance of interpersonal touch by patients with psoriasis,” she noted.
This study was supported by the National Psoriasis Foundation. Dr. Armstrong reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies as well as the National Institutes of Health.
VANCOUVER – Touch impairment is an important but largely unappreciated aspect of quality-of-life impairment in psoriasis, Dr. April Armstrong said at the World Congress of Dermatology.
“Touch avoidance is a new concept in dermatology, and I think it’s great because it really brings home a humanistic aspect to research,” observed Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
“Touch is a fundamental human gesture that conveys understanding, connection, validation, as well as being cared for or caring for someone,” she added.
She presented an online study of 1,109 psoriasis patients, 70% of whom were female. When asked whether within the past 2 weeks they had avoided hugging, shaking hands, or other touching of others, or of being touched, because of how their skin looks or feels, 48% responded yes. On a 0-10 scale, 27% of the overall patient population rated themselves as having touch avoidance at the level of 4-10, corresponding to moderate to severe.
In a multivariate analysis, the prevalence or severity of touch avoidance didn’t vary by gender. However, touch avoidance was reported most often by patients under age 25 years. And touch avoidance was strongly associated with disease severity: Those with mild psoriasis as defined by body surface area of involvement were only about one-third as likely to report touch avoidance, compared with those with severe disease. Also, psoriatic itching was an important correlate of touch avoidance: Those with itch were roughly fivefold more likely to report recent touch avoidance.
In addition, patients with psoriasis on exposed areas such as the face and neck or in sensitive areas such as the genitalia were more likely to report touch avoidance.
Touch avoidance had a strong negative impact on quality of life. Scores on the Dermatology Life Quality Index averaged nearly threefold higher – indicating worse quality of life – in participants who reported touch avoidance. They were at similarly increased risk for mild or greater depressive symptoms based upon their scores on the Quick Inventory of Depressive Symptoms.
Thus, touch avoidance is an important new patient-reported outcome, and these study results open up a new area of psoriasis research, according to Dr. Armstrong.
“Future research may reveal that therapies offering clearance of psoriasis, particularly in sensitive areas, could lead to large improvements in the initiation and acceptance of interpersonal touch by patients with psoriasis,” she noted.
This study was supported by the National Psoriasis Foundation. Dr. Armstrong reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies as well as the National Institutes of Health.
VANCOUVER – Touch impairment is an important but largely unappreciated aspect of quality-of-life impairment in psoriasis, Dr. April Armstrong said at the World Congress of Dermatology.
“Touch avoidance is a new concept in dermatology, and I think it’s great because it really brings home a humanistic aspect to research,” observed Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
“Touch is a fundamental human gesture that conveys understanding, connection, validation, as well as being cared for or caring for someone,” she added.
She presented an online study of 1,109 psoriasis patients, 70% of whom were female. When asked whether within the past 2 weeks they had avoided hugging, shaking hands, or other touching of others, or of being touched, because of how their skin looks or feels, 48% responded yes. On a 0-10 scale, 27% of the overall patient population rated themselves as having touch avoidance at the level of 4-10, corresponding to moderate to severe.
In a multivariate analysis, the prevalence or severity of touch avoidance didn’t vary by gender. However, touch avoidance was reported most often by patients under age 25 years. And touch avoidance was strongly associated with disease severity: Those with mild psoriasis as defined by body surface area of involvement were only about one-third as likely to report touch avoidance, compared with those with severe disease. Also, psoriatic itching was an important correlate of touch avoidance: Those with itch were roughly fivefold more likely to report recent touch avoidance.
In addition, patients with psoriasis on exposed areas such as the face and neck or in sensitive areas such as the genitalia were more likely to report touch avoidance.
Touch avoidance had a strong negative impact on quality of life. Scores on the Dermatology Life Quality Index averaged nearly threefold higher – indicating worse quality of life – in participants who reported touch avoidance. They were at similarly increased risk for mild or greater depressive symptoms based upon their scores on the Quick Inventory of Depressive Symptoms.
Thus, touch avoidance is an important new patient-reported outcome, and these study results open up a new area of psoriasis research, according to Dr. Armstrong.
“Future research may reveal that therapies offering clearance of psoriasis, particularly in sensitive areas, could lead to large improvements in the initiation and acceptance of interpersonal touch by patients with psoriasis,” she noted.
This study was supported by the National Psoriasis Foundation. Dr. Armstrong reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies as well as the National Institutes of Health.
AT WCD 2015
Key clinical point: Touch avoidance among psoriasis patients is an important new patient-reported outcome having a major negative quality-of-life impact.
Major finding: A total of 48 of a large group of psoriasis patients reported having avoided touching others, or having others touching them, within the prior 2 weeks because of how their skin looks or feels.
Data source: This was an online survey of 1,109 psoriasis patients.
Disclosures: The study was supported by the National Psoriasis Foundation. Dr. April Armstrong reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies as well as the National Institutes of Health.
Double chin therapy scores high in patient satisfaction
VANCOUVER – Patients who underwent injections of ATX-101 (Kybella) for treatment of an unsightly double chin reported a high degree of satisfaction with the results, according to a pooled analysis of four large, double-blind, placebo-controlled, phase III trials.
Two of the trials were conducted in North America, two were conducted in Europe. The patient-reported outcomes were consistently favorable across this wide geographic and cultural range, Dr. Nowell Solish reported at the World Congress of Dermatology.
ATX-101 is a proprietary formulation of nonhuman, nonanimal deoxycholic acid. Injected directly into the excess submental fat also known as a double chin, it causes lysis of adipocytes. Earlier this year the product became the first Food and Drug Administration–approved nonsurgical treatment for double chins.
Although physician-scored ratings of improvement played a key role in obtaining regulatory approval, Dr. Solish, a dermatologist at the University of Toronto, focused on the patient-reported outcomes, which are particularly germane given that this is an aesthetic treatment.
The pooled analysis was restricted to 315 placebo-treated controls and 413 participants who received the FDA-approved dosing regimen, in which a single treatment consists of up to 50 injections of 0.2 mL each, placed 1 cm apart, which translates to 2 mg/cm2. The pooled analysis included patients who had up to four treatment sessions placed at least 1 month apart. All subjects had to have moderate to severe excess submental fat and dissatisfaction with the appearance of their face and chin.
A variety of outcome measures collectively painted a picture of improved patient satisfaction with face and chin appearance, significant psychological improvement, and satisfaction with the therapy itself.
For example, on the Subject Self-Rating Scale, which assesses satisfaction with face and chin appearance, 70% of North American and 72% of European ATX-101–treated patients were classified as satisfied as defined by a score of 4 or more on the 0-6 scale 12 weeks after the final treatment. This was the case for only 27% of North American and 32% of European placebo-treated controls.
On the 0-10 Patient-Response Submental Fat Impact Scale, which assesses the psychological impact of the visual appearance of the chin as well as emotional self-perception, average scores in the active treatment arms improved by 3-4 points from a baseline of 7, compared with 1- to 2-point improvements in the control groups.
Roughly 80% of patients who received the chin-fat buster in the double-blind studies declared themselves to be “a little,” “moderately,” or “extremely” satisfied with the posttreatment definition between their chin and neck as well as with the treatment itself, as did 25%-45% of controls.
Pain, burning, swelling, and/or numbness occurred in 60%-84% of ATX-101–treated patients. These adverse events were mostly mild in intensity and short lived.
The pooled analysis was sponsored by Kythera Biopharmaceuticals, which markets ATX-101 as Kybella. Dr. Solish reported serving as a consultant to Allergan, which in June announced a definitive agreement to acquire Kythera.
VANCOUVER – Patients who underwent injections of ATX-101 (Kybella) for treatment of an unsightly double chin reported a high degree of satisfaction with the results, according to a pooled analysis of four large, double-blind, placebo-controlled, phase III trials.
Two of the trials were conducted in North America, two were conducted in Europe. The patient-reported outcomes were consistently favorable across this wide geographic and cultural range, Dr. Nowell Solish reported at the World Congress of Dermatology.
ATX-101 is a proprietary formulation of nonhuman, nonanimal deoxycholic acid. Injected directly into the excess submental fat also known as a double chin, it causes lysis of adipocytes. Earlier this year the product became the first Food and Drug Administration–approved nonsurgical treatment for double chins.
Although physician-scored ratings of improvement played a key role in obtaining regulatory approval, Dr. Solish, a dermatologist at the University of Toronto, focused on the patient-reported outcomes, which are particularly germane given that this is an aesthetic treatment.
The pooled analysis was restricted to 315 placebo-treated controls and 413 participants who received the FDA-approved dosing regimen, in which a single treatment consists of up to 50 injections of 0.2 mL each, placed 1 cm apart, which translates to 2 mg/cm2. The pooled analysis included patients who had up to four treatment sessions placed at least 1 month apart. All subjects had to have moderate to severe excess submental fat and dissatisfaction with the appearance of their face and chin.
A variety of outcome measures collectively painted a picture of improved patient satisfaction with face and chin appearance, significant psychological improvement, and satisfaction with the therapy itself.
For example, on the Subject Self-Rating Scale, which assesses satisfaction with face and chin appearance, 70% of North American and 72% of European ATX-101–treated patients were classified as satisfied as defined by a score of 4 or more on the 0-6 scale 12 weeks after the final treatment. This was the case for only 27% of North American and 32% of European placebo-treated controls.
On the 0-10 Patient-Response Submental Fat Impact Scale, which assesses the psychological impact of the visual appearance of the chin as well as emotional self-perception, average scores in the active treatment arms improved by 3-4 points from a baseline of 7, compared with 1- to 2-point improvements in the control groups.
Roughly 80% of patients who received the chin-fat buster in the double-blind studies declared themselves to be “a little,” “moderately,” or “extremely” satisfied with the posttreatment definition between their chin and neck as well as with the treatment itself, as did 25%-45% of controls.
Pain, burning, swelling, and/or numbness occurred in 60%-84% of ATX-101–treated patients. These adverse events were mostly mild in intensity and short lived.
The pooled analysis was sponsored by Kythera Biopharmaceuticals, which markets ATX-101 as Kybella. Dr. Solish reported serving as a consultant to Allergan, which in June announced a definitive agreement to acquire Kythera.
VANCOUVER – Patients who underwent injections of ATX-101 (Kybella) for treatment of an unsightly double chin reported a high degree of satisfaction with the results, according to a pooled analysis of four large, double-blind, placebo-controlled, phase III trials.
Two of the trials were conducted in North America, two were conducted in Europe. The patient-reported outcomes were consistently favorable across this wide geographic and cultural range, Dr. Nowell Solish reported at the World Congress of Dermatology.
ATX-101 is a proprietary formulation of nonhuman, nonanimal deoxycholic acid. Injected directly into the excess submental fat also known as a double chin, it causes lysis of adipocytes. Earlier this year the product became the first Food and Drug Administration–approved nonsurgical treatment for double chins.
Although physician-scored ratings of improvement played a key role in obtaining regulatory approval, Dr. Solish, a dermatologist at the University of Toronto, focused on the patient-reported outcomes, which are particularly germane given that this is an aesthetic treatment.
The pooled analysis was restricted to 315 placebo-treated controls and 413 participants who received the FDA-approved dosing regimen, in which a single treatment consists of up to 50 injections of 0.2 mL each, placed 1 cm apart, which translates to 2 mg/cm2. The pooled analysis included patients who had up to four treatment sessions placed at least 1 month apart. All subjects had to have moderate to severe excess submental fat and dissatisfaction with the appearance of their face and chin.
A variety of outcome measures collectively painted a picture of improved patient satisfaction with face and chin appearance, significant psychological improvement, and satisfaction with the therapy itself.
For example, on the Subject Self-Rating Scale, which assesses satisfaction with face and chin appearance, 70% of North American and 72% of European ATX-101–treated patients were classified as satisfied as defined by a score of 4 or more on the 0-6 scale 12 weeks after the final treatment. This was the case for only 27% of North American and 32% of European placebo-treated controls.
On the 0-10 Patient-Response Submental Fat Impact Scale, which assesses the psychological impact of the visual appearance of the chin as well as emotional self-perception, average scores in the active treatment arms improved by 3-4 points from a baseline of 7, compared with 1- to 2-point improvements in the control groups.
Roughly 80% of patients who received the chin-fat buster in the double-blind studies declared themselves to be “a little,” “moderately,” or “extremely” satisfied with the posttreatment definition between their chin and neck as well as with the treatment itself, as did 25%-45% of controls.
Pain, burning, swelling, and/or numbness occurred in 60%-84% of ATX-101–treated patients. These adverse events were mostly mild in intensity and short lived.
The pooled analysis was sponsored by Kythera Biopharmaceuticals, which markets ATX-101 as Kybella. Dr. Solish reported serving as a consultant to Allergan, which in June announced a definitive agreement to acquire Kythera.
AT WCD 2015
Key clinical point: ATX-101, the first FDA-approved nonsurgical treatment for double chins, is a hit with patients who’ve undergone the injections in double-blind trials.
Major finding: A total of 70% of North American and 72% of European patients declared satisfaction with their face and chin appearance following a course of injections of the fat-busting agent ATX-101, compared with 30% of placebo-treated controls.
Data source: This was a pooled analysis of patient-reported outcomes in four large, double-blind, placebo-controlled randomized phase III trials conducted in North America and Europe.
Disclosures: The pooled analysis was funded by Kythera Biopharmaceuticals. Dr. Nowell Solish reported serving as a consultant to Allergan, which in June announced a definitive agreement to acquire Kythera.
WCD: How to submit a proper nail specimen
VANCOUVER – Dr. Curtis T. Thompson is on a mission: to improve the often-shoddy quality of nail biopsy specimens submitted to pathologists.
No standardized protocols for nail specimens exist. The quality of pathologic diagnosis often suffers as a result, Dr. Thompson said at the World Congress of Dermatology.
“What often happens is the nail specimens get put into a bottle of formaldehyde, they float around and get torn up, and then when they come to the lab, we have no idea what’s proximal and dorsal. This is an issue. We’re all used to just putting a nail specimen in a bottle and sending it away, so all the grossing happens in the laboratory. What I submit to you is you need to be more involved in the grossing side so the specimen can be properly processed,” said Dr. Thompson, a dermatopathologist in group practice in Tigard, Ore.
He added that clear and concise guidelines for standardized specimen submission are needed, and he offered several specific suggestions regarding the orientation of the tissue and securing it for transport.
“Careful submission of tissue specimens is of great importance and allows for better diagnostics,” Dr. Thompson stressed. “There’s really nothing more terrifying than to be told you’re being sent a pigmented lesion and then not being able to find anything at all in the specimen. You really worry that it’s ended up in the trash can through leveling. This is why dermatopathologists don’t want to read nail biopsies very much.”
When a nail specimen is submitted properly, such mix-ups become “almost impossible,” according to the dermatopathologist.
Dr. Thompson borrowed one of his key ideas on efficient handling of nail specimens from opthalmologic pathology. Ophthalmologists routinely send delicate tissue segments and margins from the operating room, and they do so with consistent success because they place the segments on a cartoon of the eye so the pathologist can see exactly where the tissue was located on the patient.
Dermatologists and surgeons can do the same after printing out a sheaf of nail diagrams gratis at the Website for Dr. Thompson’s dermatopathology practice.
The rest of the necessary equipment is similarly simple and readily obtainable from any pathology laboratory, which routinely purchases small plastic cassettes by the tens of thousands for handling of tissue specimens.
“You don’t need to go out and buy them; just ask the lab you work with to send over 10 or so,” Dr. Thompson advised.
The cassette comes with a small fitted sponge to be placed over the tissue to keep it securely in place on the nail diagram rather than floating off. Ink one end of the specimen using the wooden end of a cotton-tip applicator so the lab knows which end is proximal and which is distal. The wooden tip provides more precise inking than the cotton-tip end. Then place the closed cassette in a larger bottle of formaldehyde for shipping.
One more thing: Separate the nail plate from the nail bed or matrix whenever possible, and place them in separate cassettes. Lab technicians typically devote a lot of attention to trying to get the nail plate to stick to a slide, but the diagnostic material is usually present in the nail bed or matrix, and keeping those soft tissues separate makes it less likely they’ll get lost in the shuffle.
“I recommend putting the nail plate cassette and the lesional tissue in the same bottle because then you don’t have two specimens with double the charge for the patient,” Dr. Thompson said.
He reported having no relevant financial conflicts.
VANCOUVER – Dr. Curtis T. Thompson is on a mission: to improve the often-shoddy quality of nail biopsy specimens submitted to pathologists.
No standardized protocols for nail specimens exist. The quality of pathologic diagnosis often suffers as a result, Dr. Thompson said at the World Congress of Dermatology.
“What often happens is the nail specimens get put into a bottle of formaldehyde, they float around and get torn up, and then when they come to the lab, we have no idea what’s proximal and dorsal. This is an issue. We’re all used to just putting a nail specimen in a bottle and sending it away, so all the grossing happens in the laboratory. What I submit to you is you need to be more involved in the grossing side so the specimen can be properly processed,” said Dr. Thompson, a dermatopathologist in group practice in Tigard, Ore.
He added that clear and concise guidelines for standardized specimen submission are needed, and he offered several specific suggestions regarding the orientation of the tissue and securing it for transport.
“Careful submission of tissue specimens is of great importance and allows for better diagnostics,” Dr. Thompson stressed. “There’s really nothing more terrifying than to be told you’re being sent a pigmented lesion and then not being able to find anything at all in the specimen. You really worry that it’s ended up in the trash can through leveling. This is why dermatopathologists don’t want to read nail biopsies very much.”
When a nail specimen is submitted properly, such mix-ups become “almost impossible,” according to the dermatopathologist.
Dr. Thompson borrowed one of his key ideas on efficient handling of nail specimens from opthalmologic pathology. Ophthalmologists routinely send delicate tissue segments and margins from the operating room, and they do so with consistent success because they place the segments on a cartoon of the eye so the pathologist can see exactly where the tissue was located on the patient.
Dermatologists and surgeons can do the same after printing out a sheaf of nail diagrams gratis at the Website for Dr. Thompson’s dermatopathology practice.
The rest of the necessary equipment is similarly simple and readily obtainable from any pathology laboratory, which routinely purchases small plastic cassettes by the tens of thousands for handling of tissue specimens.
“You don’t need to go out and buy them; just ask the lab you work with to send over 10 or so,” Dr. Thompson advised.
The cassette comes with a small fitted sponge to be placed over the tissue to keep it securely in place on the nail diagram rather than floating off. Ink one end of the specimen using the wooden end of a cotton-tip applicator so the lab knows which end is proximal and which is distal. The wooden tip provides more precise inking than the cotton-tip end. Then place the closed cassette in a larger bottle of formaldehyde for shipping.
One more thing: Separate the nail plate from the nail bed or matrix whenever possible, and place them in separate cassettes. Lab technicians typically devote a lot of attention to trying to get the nail plate to stick to a slide, but the diagnostic material is usually present in the nail bed or matrix, and keeping those soft tissues separate makes it less likely they’ll get lost in the shuffle.
“I recommend putting the nail plate cassette and the lesional tissue in the same bottle because then you don’t have two specimens with double the charge for the patient,” Dr. Thompson said.
He reported having no relevant financial conflicts.
VANCOUVER – Dr. Curtis T. Thompson is on a mission: to improve the often-shoddy quality of nail biopsy specimens submitted to pathologists.
No standardized protocols for nail specimens exist. The quality of pathologic diagnosis often suffers as a result, Dr. Thompson said at the World Congress of Dermatology.
“What often happens is the nail specimens get put into a bottle of formaldehyde, they float around and get torn up, and then when they come to the lab, we have no idea what’s proximal and dorsal. This is an issue. We’re all used to just putting a nail specimen in a bottle and sending it away, so all the grossing happens in the laboratory. What I submit to you is you need to be more involved in the grossing side so the specimen can be properly processed,” said Dr. Thompson, a dermatopathologist in group practice in Tigard, Ore.
He added that clear and concise guidelines for standardized specimen submission are needed, and he offered several specific suggestions regarding the orientation of the tissue and securing it for transport.
“Careful submission of tissue specimens is of great importance and allows for better diagnostics,” Dr. Thompson stressed. “There’s really nothing more terrifying than to be told you’re being sent a pigmented lesion and then not being able to find anything at all in the specimen. You really worry that it’s ended up in the trash can through leveling. This is why dermatopathologists don’t want to read nail biopsies very much.”
When a nail specimen is submitted properly, such mix-ups become “almost impossible,” according to the dermatopathologist.
Dr. Thompson borrowed one of his key ideas on efficient handling of nail specimens from opthalmologic pathology. Ophthalmologists routinely send delicate tissue segments and margins from the operating room, and they do so with consistent success because they place the segments on a cartoon of the eye so the pathologist can see exactly where the tissue was located on the patient.
Dermatologists and surgeons can do the same after printing out a sheaf of nail diagrams gratis at the Website for Dr. Thompson’s dermatopathology practice.
The rest of the necessary equipment is similarly simple and readily obtainable from any pathology laboratory, which routinely purchases small plastic cassettes by the tens of thousands for handling of tissue specimens.
“You don’t need to go out and buy them; just ask the lab you work with to send over 10 or so,” Dr. Thompson advised.
The cassette comes with a small fitted sponge to be placed over the tissue to keep it securely in place on the nail diagram rather than floating off. Ink one end of the specimen using the wooden end of a cotton-tip applicator so the lab knows which end is proximal and which is distal. The wooden tip provides more precise inking than the cotton-tip end. Then place the closed cassette in a larger bottle of formaldehyde for shipping.
One more thing: Separate the nail plate from the nail bed or matrix whenever possible, and place them in separate cassettes. Lab technicians typically devote a lot of attention to trying to get the nail plate to stick to a slide, but the diagnostic material is usually present in the nail bed or matrix, and keeping those soft tissues separate makes it less likely they’ll get lost in the shuffle.
“I recommend putting the nail plate cassette and the lesional tissue in the same bottle because then you don’t have two specimens with double the charge for the patient,” Dr. Thompson said.
He reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM WCD 2015
WCD: Dapsone Gel Effective for Acne in Women of Color
VANCOUVER – Dapsone gel 5% proved effective and well tolerated for facial acne in women with skin of color in a multicenter pilot study.
The study was conducted because even though dapsone gel 5% (Aczone) is approved for the treatment of acne on the strength of two pivotal randomized, double-blind clinical trials totaling more than 3,000 patients, scant data exist on the topical agent’s performance in women with skin of color, Dr. Andrew F. Alexis explained at the World Congress of Dermatology.
He presented an open-label, seven-center, 12-week, single-arm study involving 68 women of color – three-quarters of whom were black – who treated their facial acne with dapsone gel 5% twice daily as monotherapy.
Participants averaged a mean baseline score of 2.6 on the 0-4 Global Acne Assessment Score (GAAS), with a mean total of 50 inflammatory and noninflammatory acne lesions on the face.
The primary endpoint was change in GAAS at 12 weeks, although patients also were formally assessed at 2 and 6 weeks. The average reduction in GAAS was 8.8% at 2 weeks, 20% at 6 weeks, and 39% at 12 weeks. At week 12, 43% of the women were categorized as responders, meaning they had a GAAS of 0 (meaning no acne lesions) or 1 (indicating mild disease), reported Dr. Alexis of Mt. Sinai Hospital in New York.
Total lesion counts dropped steadily throughout the 12-week trial: by 16% from baseline to week 2, 30% at week 6, and 52% at week 12. Inflammatory lesions responded best, with a 65% reduction in number at week 12.
Patient-reported outcomes on the validated, 17-item Acne Symptom and Impact Scale were favorable: Reductions of roughly 50% were documented over 12 weeks on the scale’s two domains, acne signs and quality of life impact.
No clinically meaningful treatment-related adverse events were reported in the study, although a handful of women reported trace levels of redness, burning, dryness, and/or oiliness.
Acne is more common among African American than white women. In a large epidemiologic study of adolescent and adult women, the prevalence of acne vulgaris was 37% in African Americans, compared with 24% in whites (J. Eur. Acad. Dermatol. Venereol. 2011;25:1054-60).
Dr. Alexis’ study was sponsored by Allergan. He reported serving as a consultant to and receiving research grants from the company.
VANCOUVER – Dapsone gel 5% proved effective and well tolerated for facial acne in women with skin of color in a multicenter pilot study.
The study was conducted because even though dapsone gel 5% (Aczone) is approved for the treatment of acne on the strength of two pivotal randomized, double-blind clinical trials totaling more than 3,000 patients, scant data exist on the topical agent’s performance in women with skin of color, Dr. Andrew F. Alexis explained at the World Congress of Dermatology.
He presented an open-label, seven-center, 12-week, single-arm study involving 68 women of color – three-quarters of whom were black – who treated their facial acne with dapsone gel 5% twice daily as monotherapy.
Participants averaged a mean baseline score of 2.6 on the 0-4 Global Acne Assessment Score (GAAS), with a mean total of 50 inflammatory and noninflammatory acne lesions on the face.
The primary endpoint was change in GAAS at 12 weeks, although patients also were formally assessed at 2 and 6 weeks. The average reduction in GAAS was 8.8% at 2 weeks, 20% at 6 weeks, and 39% at 12 weeks. At week 12, 43% of the women were categorized as responders, meaning they had a GAAS of 0 (meaning no acne lesions) or 1 (indicating mild disease), reported Dr. Alexis of Mt. Sinai Hospital in New York.
Total lesion counts dropped steadily throughout the 12-week trial: by 16% from baseline to week 2, 30% at week 6, and 52% at week 12. Inflammatory lesions responded best, with a 65% reduction in number at week 12.
Patient-reported outcomes on the validated, 17-item Acne Symptom and Impact Scale were favorable: Reductions of roughly 50% were documented over 12 weeks on the scale’s two domains, acne signs and quality of life impact.
No clinically meaningful treatment-related adverse events were reported in the study, although a handful of women reported trace levels of redness, burning, dryness, and/or oiliness.
Acne is more common among African American than white women. In a large epidemiologic study of adolescent and adult women, the prevalence of acne vulgaris was 37% in African Americans, compared with 24% in whites (J. Eur. Acad. Dermatol. Venereol. 2011;25:1054-60).
Dr. Alexis’ study was sponsored by Allergan. He reported serving as a consultant to and receiving research grants from the company.
VANCOUVER – Dapsone gel 5% proved effective and well tolerated for facial acne in women with skin of color in a multicenter pilot study.
The study was conducted because even though dapsone gel 5% (Aczone) is approved for the treatment of acne on the strength of two pivotal randomized, double-blind clinical trials totaling more than 3,000 patients, scant data exist on the topical agent’s performance in women with skin of color, Dr. Andrew F. Alexis explained at the World Congress of Dermatology.
He presented an open-label, seven-center, 12-week, single-arm study involving 68 women of color – three-quarters of whom were black – who treated their facial acne with dapsone gel 5% twice daily as monotherapy.
Participants averaged a mean baseline score of 2.6 on the 0-4 Global Acne Assessment Score (GAAS), with a mean total of 50 inflammatory and noninflammatory acne lesions on the face.
The primary endpoint was change in GAAS at 12 weeks, although patients also were formally assessed at 2 and 6 weeks. The average reduction in GAAS was 8.8% at 2 weeks, 20% at 6 weeks, and 39% at 12 weeks. At week 12, 43% of the women were categorized as responders, meaning they had a GAAS of 0 (meaning no acne lesions) or 1 (indicating mild disease), reported Dr. Alexis of Mt. Sinai Hospital in New York.
Total lesion counts dropped steadily throughout the 12-week trial: by 16% from baseline to week 2, 30% at week 6, and 52% at week 12. Inflammatory lesions responded best, with a 65% reduction in number at week 12.
Patient-reported outcomes on the validated, 17-item Acne Symptom and Impact Scale were favorable: Reductions of roughly 50% were documented over 12 weeks on the scale’s two domains, acne signs and quality of life impact.
No clinically meaningful treatment-related adverse events were reported in the study, although a handful of women reported trace levels of redness, burning, dryness, and/or oiliness.
Acne is more common among African American than white women. In a large epidemiologic study of adolescent and adult women, the prevalence of acne vulgaris was 37% in African Americans, compared with 24% in whites (J. Eur. Acad. Dermatol. Venereol. 2011;25:1054-60).
Dr. Alexis’ study was sponsored by Allergan. He reported serving as a consultant to and receiving research grants from the company.
AT WCD 2015
WCD: Dapsone gel effective for acne in women of color
VANCOUVER – Dapsone gel 5% proved effective and well tolerated for facial acne in women with skin of color in a multicenter pilot study.
The study was conducted because even though dapsone gel 5% (Aczone) is approved for the treatment of acne on the strength of two pivotal randomized, double-blind clinical trials totaling more than 3,000 patients, scant data exist on the topical agent’s performance in women with skin of color, Dr. Andrew F. Alexis explained at the World Congress of Dermatology.
He presented an open-label, seven-center, 12-week, single-arm study involving 68 women of color – three-quarters of whom were black – who treated their facial acne with dapsone gel 5% twice daily as monotherapy.
Participants averaged a mean baseline score of 2.6 on the 0-4 Global Acne Assessment Score (GAAS), with a mean total of 50 inflammatory and noninflammatory acne lesions on the face.
The primary endpoint was change in GAAS at 12 weeks, although patients also were formally assessed at 2 and 6 weeks. The average reduction in GAAS was 8.8% at 2 weeks, 20% at 6 weeks, and 39% at 12 weeks. At week 12, 43% of the women were categorized as responders, meaning they had a GAAS of 0 (meaning no acne lesions) or 1 (indicating mild disease), reported Dr. Alexis of Mt. Sinai Hospital in New York.
Total lesion counts dropped steadily throughout the 12-week trial: by 16% from baseline to week 2, 30% at week 6, and 52% at week 12. Inflammatory lesions responded best, with a 65% reduction in number at week 12.
Patient-reported outcomes on the validated, 17-item Acne Symptom and Impact Scale were favorable: Reductions of roughly 50% were documented over 12 weeks on the scale’s two domains, acne signs and quality of life impact.
No clinically meaningful treatment-related adverse events were reported in the study, although a handful of women reported trace levels of redness, burning, dryness, and/or oiliness.
Acne is more common among African American than white women. In a large epidemiologic study of adolescent and adult women, the prevalence of acne vulgaris was 37% in African Americans, compared with 24% in whites (J. Eur. Acad. Dermatol. Venereol. 2011;25:1054-60).
Dr. Alexis’ study was sponsored by Allergan. He reported serving as a consultant to and receiving research grants from the company.
VANCOUVER – Dapsone gel 5% proved effective and well tolerated for facial acne in women with skin of color in a multicenter pilot study.
The study was conducted because even though dapsone gel 5% (Aczone) is approved for the treatment of acne on the strength of two pivotal randomized, double-blind clinical trials totaling more than 3,000 patients, scant data exist on the topical agent’s performance in women with skin of color, Dr. Andrew F. Alexis explained at the World Congress of Dermatology.
He presented an open-label, seven-center, 12-week, single-arm study involving 68 women of color – three-quarters of whom were black – who treated their facial acne with dapsone gel 5% twice daily as monotherapy.
Participants averaged a mean baseline score of 2.6 on the 0-4 Global Acne Assessment Score (GAAS), with a mean total of 50 inflammatory and noninflammatory acne lesions on the face.
The primary endpoint was change in GAAS at 12 weeks, although patients also were formally assessed at 2 and 6 weeks. The average reduction in GAAS was 8.8% at 2 weeks, 20% at 6 weeks, and 39% at 12 weeks. At week 12, 43% of the women were categorized as responders, meaning they had a GAAS of 0 (meaning no acne lesions) or 1 (indicating mild disease), reported Dr. Alexis of Mt. Sinai Hospital in New York.
Total lesion counts dropped steadily throughout the 12-week trial: by 16% from baseline to week 2, 30% at week 6, and 52% at week 12. Inflammatory lesions responded best, with a 65% reduction in number at week 12.
Patient-reported outcomes on the validated, 17-item Acne Symptom and Impact Scale were favorable: Reductions of roughly 50% were documented over 12 weeks on the scale’s two domains, acne signs and quality of life impact.
No clinically meaningful treatment-related adverse events were reported in the study, although a handful of women reported trace levels of redness, burning, dryness, and/or oiliness.
Acne is more common among African American than white women. In a large epidemiologic study of adolescent and adult women, the prevalence of acne vulgaris was 37% in African Americans, compared with 24% in whites (J. Eur. Acad. Dermatol. Venereol. 2011;25:1054-60).
Dr. Alexis’ study was sponsored by Allergan. He reported serving as a consultant to and receiving research grants from the company.
VANCOUVER – Dapsone gel 5% proved effective and well tolerated for facial acne in women with skin of color in a multicenter pilot study.
The study was conducted because even though dapsone gel 5% (Aczone) is approved for the treatment of acne on the strength of two pivotal randomized, double-blind clinical trials totaling more than 3,000 patients, scant data exist on the topical agent’s performance in women with skin of color, Dr. Andrew F. Alexis explained at the World Congress of Dermatology.
He presented an open-label, seven-center, 12-week, single-arm study involving 68 women of color – three-quarters of whom were black – who treated their facial acne with dapsone gel 5% twice daily as monotherapy.
Participants averaged a mean baseline score of 2.6 on the 0-4 Global Acne Assessment Score (GAAS), with a mean total of 50 inflammatory and noninflammatory acne lesions on the face.
The primary endpoint was change in GAAS at 12 weeks, although patients also were formally assessed at 2 and 6 weeks. The average reduction in GAAS was 8.8% at 2 weeks, 20% at 6 weeks, and 39% at 12 weeks. At week 12, 43% of the women were categorized as responders, meaning they had a GAAS of 0 (meaning no acne lesions) or 1 (indicating mild disease), reported Dr. Alexis of Mt. Sinai Hospital in New York.
Total lesion counts dropped steadily throughout the 12-week trial: by 16% from baseline to week 2, 30% at week 6, and 52% at week 12. Inflammatory lesions responded best, with a 65% reduction in number at week 12.
Patient-reported outcomes on the validated, 17-item Acne Symptom and Impact Scale were favorable: Reductions of roughly 50% were documented over 12 weeks on the scale’s two domains, acne signs and quality of life impact.
No clinically meaningful treatment-related adverse events were reported in the study, although a handful of women reported trace levels of redness, burning, dryness, and/or oiliness.
Acne is more common among African American than white women. In a large epidemiologic study of adolescent and adult women, the prevalence of acne vulgaris was 37% in African Americans, compared with 24% in whites (J. Eur. Acad. Dermatol. Venereol. 2011;25:1054-60).
Dr. Alexis’ study was sponsored by Allergan. He reported serving as a consultant to and receiving research grants from the company.
AT WCD 2015
Key clinical point: Dapsone gel 5% is effective and well tolerated for treatment of facial acne in women with skin of color.
Major finding: Women of color experienced a mean 39% reduction in Global Acne Assessment Scores after 12 weeks of self-treatment of facial acne using dapsone gel 5% twice daily as monotherapy.
Data source: This was a 68-patient, open-label, seven-site, single-arm, 12-week study.
Disclosures: The study was sponsored by Allergan. Dr. Andrew F. Alexis reported serving as a consultant to and receiving research grants from the company.
WCD: Topical Tofacitinib Hits Marks in Atopic Dermatitis
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
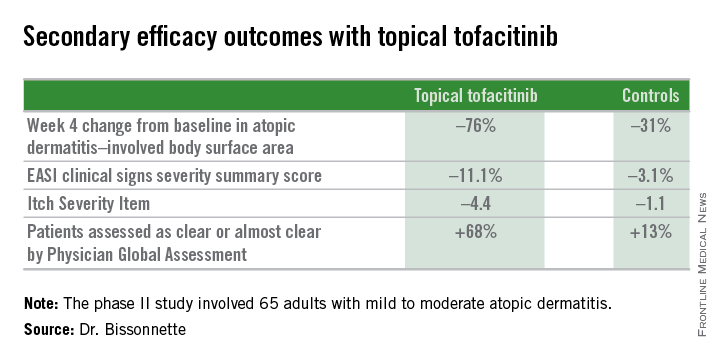
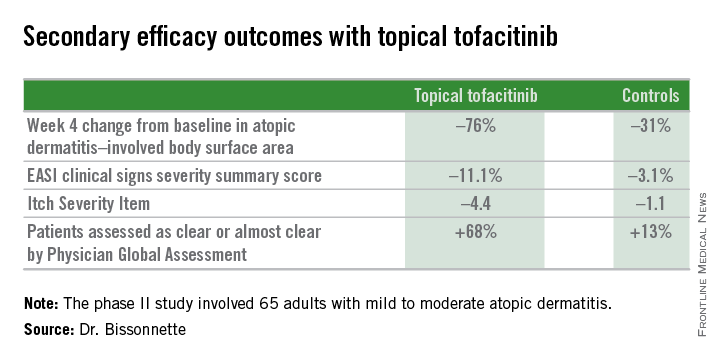
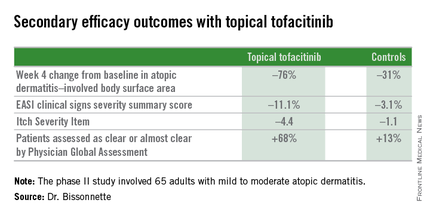
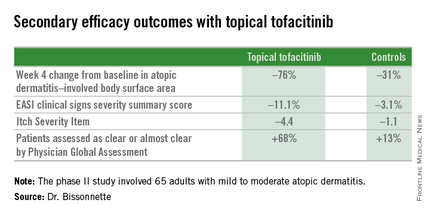
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

AT WCD 2015
WCD: Topical tofacitinib hits marks in atopic dermatitis
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

AT WCD 2015
Key clinical point: Topical tofacitinib, a Janus kinase inhibitor, may provide a novel safe and effective therapy for atopic dermatitis.
Major finding: After 4 weeks of topical tofacitinib, atopic dermatitis patients experienced a mean 82% reduction in their Eczema Area and Severity Index total score, compared with a 30% decrease in vehicle-treated controls.
Data source: A five-center, randomized, double-blind, prospective, vehicle-controlled phase II study involving 65 adults with atopic dermatitis.
Disclosures: Dr. Robert Bissonnette disclosed ties with Pfizer – the study sponsor – and more than a dozen other pharmaceutical companies.
Elderly-onset atopic dermatitis is on the rise
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
EXPERT ANALYSIS FROM WCD 2015
WCD: As MRSA Situation Worsens, Don’t Overlook Strep
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
EXPERT ANALYSIS FROM WCD 2015