User login
FIT unfit for inpatient, emergency settings
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Black patients less likely to receive H. pylori eradication testing
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
FROM ACG 2020
Cirrhosis, Child-Pugh score predict ERCP complications
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
FROM ACG 2020
Antibiotics fail to improve colon ischemia outcomes
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
FROM ACG 2020
Probiotic blend may help patients with GI symptoms
A novel five-strain probiotic blend could provide relief for patients with functional GI disorders, a new study shows.
The combination “improved patient’s functional GI symptoms and displayed a favorable safety profile,” said lead study investigator Lucinda A. Harris, MD, MS, from the Mayo Clinic School of Medicine in Scottsdale, Ariz.
“Results of this study are promising, and additional studies would support the novel probiotic blend’s efficacy, safety, and durability of effect,” said Dr. Harris during her presentation at the virtual American College of Gastroenterology 2020 Annual Scientific Meeting.
Treatment with probiotics, such as Bifidobacterium lactis strains Bl-04, Bi-07, and HN019 and Lactobacillus strains L. acidophilus NCFM and L. paracasei Lpc-37 – administered alone or in multistrain blends – has been shown to improve diarrhea, abdominal pain, bloating, and constipation symptoms in patients with GI disturbances, she reported.
“Multiple pathophysiologic processes may cause functional GI symptoms, including altered gut microbiota,” she said. “The administration of probiotics can impact intestinal microbial balance, thereby contributing to improvement in functional GI symptoms.”
In their study, Dr. Harris and her colleagues evaluated the safety and efficacy of a five-strain probiotic blend – composed of Bl-04, Bi-07, HN019, NCFM, and Lpc-37 – in people with functional GI disturbances.
In the open-label, multicenter study, all 188 adult participants (mean age, 44.1 years; 72.3% female) demonstrated symptoms of functional GI disturbances. Each received an oral capsule of the probiotic blend once daily for 30 days.
Patients were assessed at multiple time points: screening (days –15 to –1), baseline (day 1), day 14, day 30, and a follow-up visit (day 42). The study’s primary efficacy endpoint was patient-reported improvement in overall GI well-being at day 30. Secondary outcomes included changes in GI symptoms, assessed with the 11-point GI Health Symptom Questionnaire. The incidence of treatment-emergent adverse events was assessed during all patient visits.
By day 30, 85.1% of patients had achieved the primary endpoint and indicated a positive response when asked about their overall GI well-being. All of the improvements reported at day 30 were generally observed at day 14 as well.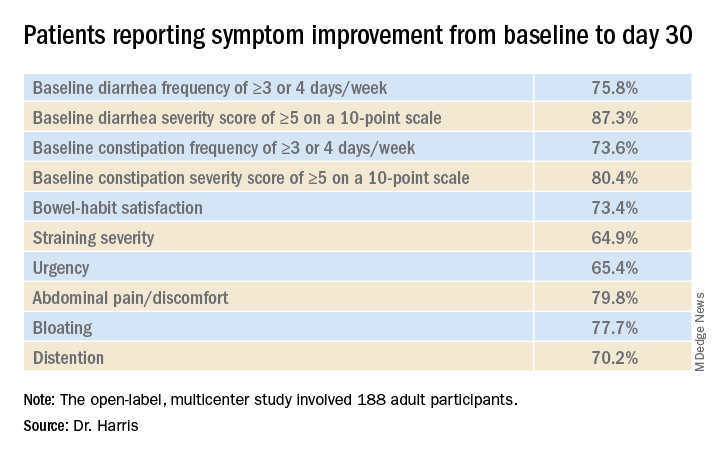
“In addition, we observed a mean decrease in I-FABP [intestinal fatty-acid binding protein] of 32.7% in patients with the highest quartile of baseline I-FABP levels,” Dr. Harris reported.
With respect to tolerability, adverse events were reported by 18.6% of participants and treatment-related adverse events were reported by 8.0%.
“Overall, 35 patients experienced a treatment-emergent adverse event,” she said. “Six patients experienced flatulence and five patients had a cough.” There were no deaths, no serious treatment-emergent adverse events, and no drug-related discontinuations
Placebo effect?
“We know that the biome has a role in modulating a number of physiologic processes, so looking at biomic influence for functional disease makes sense,” said David A. Johnson, MD, from the Eastern Virginia Medical School in Norfolk, who was not involved in the study.
However, one of the limitations of this study is the potential for a marked placebo effect, he said in an interview. “When you do an open-label trial in functional diseases, there’s a high placebo rate response. This effect is less pronounced in longer trials, but shorter trials like this one definitely carry the risk of increased placebo responses.”
“Although promising, a randomized control trial evaluating the microbiome as a response to the treatment intervention would be extremely helpful in defining the true role of effect,” he added.
This study was funded by Bausch Health Americas, Inc. Harris reports financial relationships with Allergan, Ironwood, and Takeda. Johnson has disclosed no relevant financial relationships; he writes the Johnson on Gastroenterology blog on Medscape.
A version of this article originally appeared on Medscape.com.
A novel five-strain probiotic blend could provide relief for patients with functional GI disorders, a new study shows.
The combination “improved patient’s functional GI symptoms and displayed a favorable safety profile,” said lead study investigator Lucinda A. Harris, MD, MS, from the Mayo Clinic School of Medicine in Scottsdale, Ariz.
“Results of this study are promising, and additional studies would support the novel probiotic blend’s efficacy, safety, and durability of effect,” said Dr. Harris during her presentation at the virtual American College of Gastroenterology 2020 Annual Scientific Meeting.
Treatment with probiotics, such as Bifidobacterium lactis strains Bl-04, Bi-07, and HN019 and Lactobacillus strains L. acidophilus NCFM and L. paracasei Lpc-37 – administered alone or in multistrain blends – has been shown to improve diarrhea, abdominal pain, bloating, and constipation symptoms in patients with GI disturbances, she reported.
“Multiple pathophysiologic processes may cause functional GI symptoms, including altered gut microbiota,” she said. “The administration of probiotics can impact intestinal microbial balance, thereby contributing to improvement in functional GI symptoms.”
In their study, Dr. Harris and her colleagues evaluated the safety and efficacy of a five-strain probiotic blend – composed of Bl-04, Bi-07, HN019, NCFM, and Lpc-37 – in people with functional GI disturbances.
In the open-label, multicenter study, all 188 adult participants (mean age, 44.1 years; 72.3% female) demonstrated symptoms of functional GI disturbances. Each received an oral capsule of the probiotic blend once daily for 30 days.
Patients were assessed at multiple time points: screening (days –15 to –1), baseline (day 1), day 14, day 30, and a follow-up visit (day 42). The study’s primary efficacy endpoint was patient-reported improvement in overall GI well-being at day 30. Secondary outcomes included changes in GI symptoms, assessed with the 11-point GI Health Symptom Questionnaire. The incidence of treatment-emergent adverse events was assessed during all patient visits.
By day 30, 85.1% of patients had achieved the primary endpoint and indicated a positive response when asked about their overall GI well-being. All of the improvements reported at day 30 were generally observed at day 14 as well.
“In addition, we observed a mean decrease in I-FABP [intestinal fatty-acid binding protein] of 32.7% in patients with the highest quartile of baseline I-FABP levels,” Dr. Harris reported.
With respect to tolerability, adverse events were reported by 18.6% of participants and treatment-related adverse events were reported by 8.0%.
“Overall, 35 patients experienced a treatment-emergent adverse event,” she said. “Six patients experienced flatulence and five patients had a cough.” There were no deaths, no serious treatment-emergent adverse events, and no drug-related discontinuations
Placebo effect?
“We know that the biome has a role in modulating a number of physiologic processes, so looking at biomic influence for functional disease makes sense,” said David A. Johnson, MD, from the Eastern Virginia Medical School in Norfolk, who was not involved in the study.
However, one of the limitations of this study is the potential for a marked placebo effect, he said in an interview. “When you do an open-label trial in functional diseases, there’s a high placebo rate response. This effect is less pronounced in longer trials, but shorter trials like this one definitely carry the risk of increased placebo responses.”
“Although promising, a randomized control trial evaluating the microbiome as a response to the treatment intervention would be extremely helpful in defining the true role of effect,” he added.
This study was funded by Bausch Health Americas, Inc. Harris reports financial relationships with Allergan, Ironwood, and Takeda. Johnson has disclosed no relevant financial relationships; he writes the Johnson on Gastroenterology blog on Medscape.
A version of this article originally appeared on Medscape.com.
A novel five-strain probiotic blend could provide relief for patients with functional GI disorders, a new study shows.
The combination “improved patient’s functional GI symptoms and displayed a favorable safety profile,” said lead study investigator Lucinda A. Harris, MD, MS, from the Mayo Clinic School of Medicine in Scottsdale, Ariz.
“Results of this study are promising, and additional studies would support the novel probiotic blend’s efficacy, safety, and durability of effect,” said Dr. Harris during her presentation at the virtual American College of Gastroenterology 2020 Annual Scientific Meeting.
Treatment with probiotics, such as Bifidobacterium lactis strains Bl-04, Bi-07, and HN019 and Lactobacillus strains L. acidophilus NCFM and L. paracasei Lpc-37 – administered alone or in multistrain blends – has been shown to improve diarrhea, abdominal pain, bloating, and constipation symptoms in patients with GI disturbances, she reported.
“Multiple pathophysiologic processes may cause functional GI symptoms, including altered gut microbiota,” she said. “The administration of probiotics can impact intestinal microbial balance, thereby contributing to improvement in functional GI symptoms.”
In their study, Dr. Harris and her colleagues evaluated the safety and efficacy of a five-strain probiotic blend – composed of Bl-04, Bi-07, HN019, NCFM, and Lpc-37 – in people with functional GI disturbances.
In the open-label, multicenter study, all 188 adult participants (mean age, 44.1 years; 72.3% female) demonstrated symptoms of functional GI disturbances. Each received an oral capsule of the probiotic blend once daily for 30 days.
Patients were assessed at multiple time points: screening (days –15 to –1), baseline (day 1), day 14, day 30, and a follow-up visit (day 42). The study’s primary efficacy endpoint was patient-reported improvement in overall GI well-being at day 30. Secondary outcomes included changes in GI symptoms, assessed with the 11-point GI Health Symptom Questionnaire. The incidence of treatment-emergent adverse events was assessed during all patient visits.
By day 30, 85.1% of patients had achieved the primary endpoint and indicated a positive response when asked about their overall GI well-being. All of the improvements reported at day 30 were generally observed at day 14 as well.
“In addition, we observed a mean decrease in I-FABP [intestinal fatty-acid binding protein] of 32.7% in patients with the highest quartile of baseline I-FABP levels,” Dr. Harris reported.
With respect to tolerability, adverse events were reported by 18.6% of participants and treatment-related adverse events were reported by 8.0%.
“Overall, 35 patients experienced a treatment-emergent adverse event,” she said. “Six patients experienced flatulence and five patients had a cough.” There were no deaths, no serious treatment-emergent adverse events, and no drug-related discontinuations
Placebo effect?
“We know that the biome has a role in modulating a number of physiologic processes, so looking at biomic influence for functional disease makes sense,” said David A. Johnson, MD, from the Eastern Virginia Medical School in Norfolk, who was not involved in the study.
However, one of the limitations of this study is the potential for a marked placebo effect, he said in an interview. “When you do an open-label trial in functional diseases, there’s a high placebo rate response. This effect is less pronounced in longer trials, but shorter trials like this one definitely carry the risk of increased placebo responses.”
“Although promising, a randomized control trial evaluating the microbiome as a response to the treatment intervention would be extremely helpful in defining the true role of effect,” he added.
This study was funded by Bausch Health Americas, Inc. Harris reports financial relationships with Allergan, Ironwood, and Takeda. Johnson has disclosed no relevant financial relationships; he writes the Johnson on Gastroenterology blog on Medscape.
A version of this article originally appeared on Medscape.com.
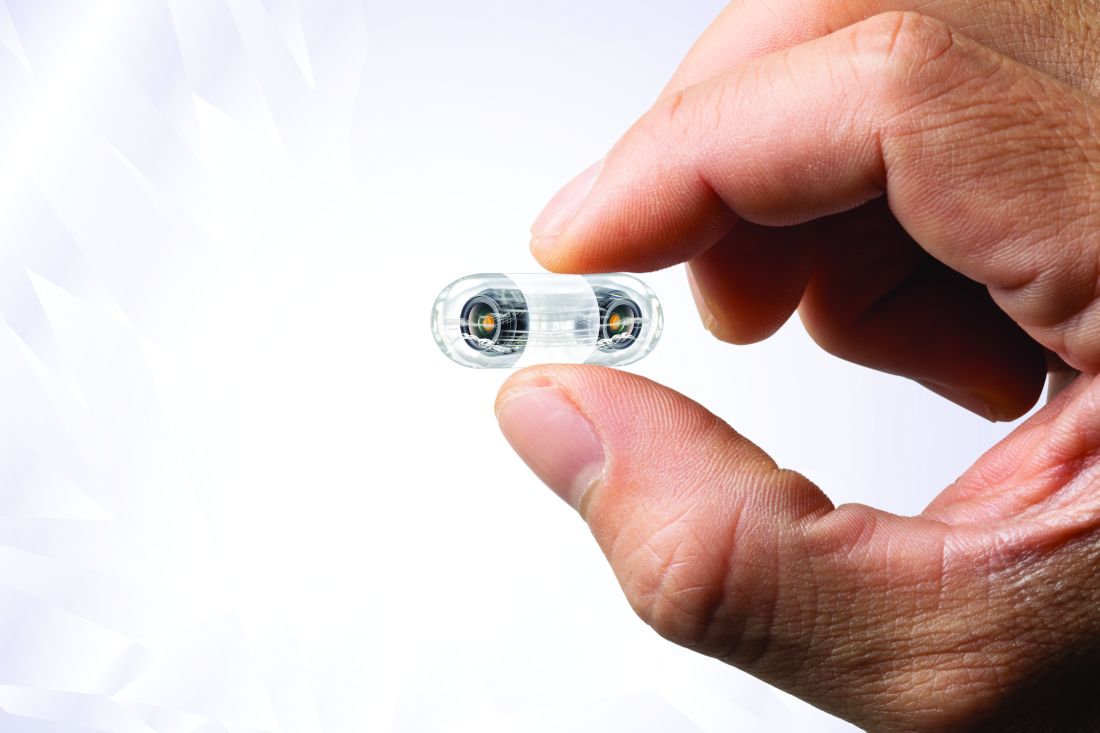
Video capsule endoscopy shows superiority, may reduce coronavirus exposure
Video capsule endoscopy (VCE) offers an alternative triage tool for acute GI bleeding that may reduce personnel exposure to SARS-CoV-2, based on a cohort study with historical controls.
VCE should be considered even when rapid coronavirus testing is available, as active bleeding is more likely to be detected when evaluated sooner, potentially sparing patients from invasive procedures, reported lead author Shahrad Hakimian, MD, of the University of Massachusetts Medical Center, Worchester, and colleagues.
“Endoscopists and staff are at high risk of exposure to coronavirus through aerosols, as well as unintended, unrecognized splashes that are well known to occur frequently during routine endoscopy,” Dr. Hakimian said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
Although pretesting and delaying procedures as needed may mitigate risks of viral exposure, “many urgent procedures, such as endoscopic evaluation of gastrointestinal bleeding, can’t really wait,” Dr. Hakimian said.
Current guidelines recommend early upper endoscopy and/or colonoscopy for evaluation of GI bleeding, but Dr. Hakimian noted that two out of three initial tests are nondiagnostic, so multiple procedures are often needed to find an answer.
In 2018, a randomized, controlled trial coauthored by Dr. Hakimian’s colleagues demonstrated how VCE may be a better approach, as it more frequently detected active bleeding than standard of care (adjusted hazard ratio, 2.77; 95% confidence interval, 1.36-5.64).
The present study built on these findings in the context of the COVID-19 pandemic.
Dr. Hakimian and colleagues analyzed data from 50 consecutive, hemodynamically stable patients with severe anemia or suspected GI bleeding who underwent VCE as a first-line diagnostic modality from mid-March to mid-May 2020 (COVID arm). These patients were compared with 57 consecutive patients who were evaluated for acute GI bleeding or severe anemia with standard of care prior to the COVID-19 pandemic (pre-COVID arm).
Characteristics of the two cohorts were generally similar, although the COVID arm included a slightly older population, with a median age of 68 years, compared with a median age of 61.8 years for the pre-COVID arm (P = .03). Among presenting symptoms, hematochezia was less common in the COVID group (4% vs. 18%; P = .03). Comorbidities were not significantly different between cohorts.
Per the study design, 100% of patients in the COVID arm underwent VCE as their first diagnostic modality. In the pre-COVID arm, 82% of patients first underwent upper endoscopy, followed by colonoscopy (12%) and VCE (5%).
The main outcome, bleeding localization, did not differ between groups, whether this was confined to the first test, or in terms of final localization. But VCE was significantly better at detecting active bleeding or stigmata of bleeding, at a rate of 66%, compared with 28% in the pre-COVID group (P < .001). Patients in the COVID arm were also significantly less likely to need any invasive procedures (44% vs. 96%; P < .001).
No intergroup differences were observed in rates of blood transfusion, in-hospital or GI-bleed mortality, rebleeding, or readmission for bleeding.
“VCE appears to be a safe alternative to traditional diagnostic evaluation of GI bleeding in the era of COVID,” Dr. Hakimian concluded, noting that “the VCE-first strategy reduces the risk of staff exposure to endoscopic aerosols, conserves personal protective equipment, and reduces staff utilization.”
According to Neil Sengupta, MD, of the University of Chicago, “a VCE-first strategy in GI bleeding may be a useful triage tool in the COVID-19 era to determine which patients truly benefit from invasive endoscopy,” although he also noted that “further data are needed to determine the efficacy and safety of this approach.”
Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., had a similar opinion.
“VCE appears to be a reasonable alternative in this patient group, at least as a first step,” Dr. Hookey said. “However, whether it truly makes a difference in the decision making process would have to be assessed prospectively via a randomized controlled trial or a decision analysis done in real time at various steps of the patient’s care path.”
Erik A. Holzwanger, MD, a gastroenterology fellow at Tufts Medical Center in Boston, suggested that these findings may “serve as a foundation” for similar studies, “as it appears COVID-19 will be an ongoing obstacle in endoscopy for the foreseeable future.”
“It would be interesting to have further discussion of timing of VCE, any COVID-19 transmission to staff during the VCE placement, and discussion of what constituted proceeding with endoscopic intervention [high-risk lesion, active bleeding] in both groups,” he added.
David Cave, MD, PhD, coauthor of the present study and the 2015 ACG clinical guideline for small bowel bleeding, said that VCE is gaining ground as the diagnostic of choice for GI bleeding, and patients prefer it, since it does not require anesthesia.
“This abstract is another clear pointer to the way in which, we should in the future, investigate gastrointestinal bleeding, both acute and chronic,” Dr. Cave said. “We are at an inflection point of transition to a new technology.”
Dr. Cave disclosed relationships with Medtronic and Olympus. The other investigators and interviewees reported no conflicts of interest.
Video capsule endoscopy (VCE) offers an alternative triage tool for acute GI bleeding that may reduce personnel exposure to SARS-CoV-2, based on a cohort study with historical controls.
VCE should be considered even when rapid coronavirus testing is available, as active bleeding is more likely to be detected when evaluated sooner, potentially sparing patients from invasive procedures, reported lead author Shahrad Hakimian, MD, of the University of Massachusetts Medical Center, Worchester, and colleagues.
“Endoscopists and staff are at high risk of exposure to coronavirus through aerosols, as well as unintended, unrecognized splashes that are well known to occur frequently during routine endoscopy,” Dr. Hakimian said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
Although pretesting and delaying procedures as needed may mitigate risks of viral exposure, “many urgent procedures, such as endoscopic evaluation of gastrointestinal bleeding, can’t really wait,” Dr. Hakimian said.
Current guidelines recommend early upper endoscopy and/or colonoscopy for evaluation of GI bleeding, but Dr. Hakimian noted that two out of three initial tests are nondiagnostic, so multiple procedures are often needed to find an answer.
In 2018, a randomized, controlled trial coauthored by Dr. Hakimian’s colleagues demonstrated how VCE may be a better approach, as it more frequently detected active bleeding than standard of care (adjusted hazard ratio, 2.77; 95% confidence interval, 1.36-5.64).
The present study built on these findings in the context of the COVID-19 pandemic.
Dr. Hakimian and colleagues analyzed data from 50 consecutive, hemodynamically stable patients with severe anemia or suspected GI bleeding who underwent VCE as a first-line diagnostic modality from mid-March to mid-May 2020 (COVID arm). These patients were compared with 57 consecutive patients who were evaluated for acute GI bleeding or severe anemia with standard of care prior to the COVID-19 pandemic (pre-COVID arm).
Characteristics of the two cohorts were generally similar, although the COVID arm included a slightly older population, with a median age of 68 years, compared with a median age of 61.8 years for the pre-COVID arm (P = .03). Among presenting symptoms, hematochezia was less common in the COVID group (4% vs. 18%; P = .03). Comorbidities were not significantly different between cohorts.
Per the study design, 100% of patients in the COVID arm underwent VCE as their first diagnostic modality. In the pre-COVID arm, 82% of patients first underwent upper endoscopy, followed by colonoscopy (12%) and VCE (5%).
The main outcome, bleeding localization, did not differ between groups, whether this was confined to the first test, or in terms of final localization. But VCE was significantly better at detecting active bleeding or stigmata of bleeding, at a rate of 66%, compared with 28% in the pre-COVID group (P < .001). Patients in the COVID arm were also significantly less likely to need any invasive procedures (44% vs. 96%; P < .001).
No intergroup differences were observed in rates of blood transfusion, in-hospital or GI-bleed mortality, rebleeding, or readmission for bleeding.
“VCE appears to be a safe alternative to traditional diagnostic evaluation of GI bleeding in the era of COVID,” Dr. Hakimian concluded, noting that “the VCE-first strategy reduces the risk of staff exposure to endoscopic aerosols, conserves personal protective equipment, and reduces staff utilization.”
According to Neil Sengupta, MD, of the University of Chicago, “a VCE-first strategy in GI bleeding may be a useful triage tool in the COVID-19 era to determine which patients truly benefit from invasive endoscopy,” although he also noted that “further data are needed to determine the efficacy and safety of this approach.”
Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., had a similar opinion.
“VCE appears to be a reasonable alternative in this patient group, at least as a first step,” Dr. Hookey said. “However, whether it truly makes a difference in the decision making process would have to be assessed prospectively via a randomized controlled trial or a decision analysis done in real time at various steps of the patient’s care path.”
Erik A. Holzwanger, MD, a gastroenterology fellow at Tufts Medical Center in Boston, suggested that these findings may “serve as a foundation” for similar studies, “as it appears COVID-19 will be an ongoing obstacle in endoscopy for the foreseeable future.”
“It would be interesting to have further discussion of timing of VCE, any COVID-19 transmission to staff during the VCE placement, and discussion of what constituted proceeding with endoscopic intervention [high-risk lesion, active bleeding] in both groups,” he added.
David Cave, MD, PhD, coauthor of the present study and the 2015 ACG clinical guideline for small bowel bleeding, said that VCE is gaining ground as the diagnostic of choice for GI bleeding, and patients prefer it, since it does not require anesthesia.
“This abstract is another clear pointer to the way in which, we should in the future, investigate gastrointestinal bleeding, both acute and chronic,” Dr. Cave said. “We are at an inflection point of transition to a new technology.”
Dr. Cave disclosed relationships with Medtronic and Olympus. The other investigators and interviewees reported no conflicts of interest.
Video capsule endoscopy (VCE) offers an alternative triage tool for acute GI bleeding that may reduce personnel exposure to SARS-CoV-2, based on a cohort study with historical controls.
VCE should be considered even when rapid coronavirus testing is available, as active bleeding is more likely to be detected when evaluated sooner, potentially sparing patients from invasive procedures, reported lead author Shahrad Hakimian, MD, of the University of Massachusetts Medical Center, Worchester, and colleagues.
“Endoscopists and staff are at high risk of exposure to coronavirus through aerosols, as well as unintended, unrecognized splashes that are well known to occur frequently during routine endoscopy,” Dr. Hakimian said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
Although pretesting and delaying procedures as needed may mitigate risks of viral exposure, “many urgent procedures, such as endoscopic evaluation of gastrointestinal bleeding, can’t really wait,” Dr. Hakimian said.
Current guidelines recommend early upper endoscopy and/or colonoscopy for evaluation of GI bleeding, but Dr. Hakimian noted that two out of three initial tests are nondiagnostic, so multiple procedures are often needed to find an answer.
In 2018, a randomized, controlled trial coauthored by Dr. Hakimian’s colleagues demonstrated how VCE may be a better approach, as it more frequently detected active bleeding than standard of care (adjusted hazard ratio, 2.77; 95% confidence interval, 1.36-5.64).
The present study built on these findings in the context of the COVID-19 pandemic.
Dr. Hakimian and colleagues analyzed data from 50 consecutive, hemodynamically stable patients with severe anemia or suspected GI bleeding who underwent VCE as a first-line diagnostic modality from mid-March to mid-May 2020 (COVID arm). These patients were compared with 57 consecutive patients who were evaluated for acute GI bleeding or severe anemia with standard of care prior to the COVID-19 pandemic (pre-COVID arm).
Characteristics of the two cohorts were generally similar, although the COVID arm included a slightly older population, with a median age of 68 years, compared with a median age of 61.8 years for the pre-COVID arm (P = .03). Among presenting symptoms, hematochezia was less common in the COVID group (4% vs. 18%; P = .03). Comorbidities were not significantly different between cohorts.
Per the study design, 100% of patients in the COVID arm underwent VCE as their first diagnostic modality. In the pre-COVID arm, 82% of patients first underwent upper endoscopy, followed by colonoscopy (12%) and VCE (5%).
The main outcome, bleeding localization, did not differ between groups, whether this was confined to the first test, or in terms of final localization. But VCE was significantly better at detecting active bleeding or stigmata of bleeding, at a rate of 66%, compared with 28% in the pre-COVID group (P < .001). Patients in the COVID arm were also significantly less likely to need any invasive procedures (44% vs. 96%; P < .001).
No intergroup differences were observed in rates of blood transfusion, in-hospital or GI-bleed mortality, rebleeding, or readmission for bleeding.
“VCE appears to be a safe alternative to traditional diagnostic evaluation of GI bleeding in the era of COVID,” Dr. Hakimian concluded, noting that “the VCE-first strategy reduces the risk of staff exposure to endoscopic aerosols, conserves personal protective equipment, and reduces staff utilization.”
According to Neil Sengupta, MD, of the University of Chicago, “a VCE-first strategy in GI bleeding may be a useful triage tool in the COVID-19 era to determine which patients truly benefit from invasive endoscopy,” although he also noted that “further data are needed to determine the efficacy and safety of this approach.”
Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., had a similar opinion.
“VCE appears to be a reasonable alternative in this patient group, at least as a first step,” Dr. Hookey said. “However, whether it truly makes a difference in the decision making process would have to be assessed prospectively via a randomized controlled trial or a decision analysis done in real time at various steps of the patient’s care path.”
Erik A. Holzwanger, MD, a gastroenterology fellow at Tufts Medical Center in Boston, suggested that these findings may “serve as a foundation” for similar studies, “as it appears COVID-19 will be an ongoing obstacle in endoscopy for the foreseeable future.”
“It would be interesting to have further discussion of timing of VCE, any COVID-19 transmission to staff during the VCE placement, and discussion of what constituted proceeding with endoscopic intervention [high-risk lesion, active bleeding] in both groups,” he added.
David Cave, MD, PhD, coauthor of the present study and the 2015 ACG clinical guideline for small bowel bleeding, said that VCE is gaining ground as the diagnostic of choice for GI bleeding, and patients prefer it, since it does not require anesthesia.
“This abstract is another clear pointer to the way in which, we should in the future, investigate gastrointestinal bleeding, both acute and chronic,” Dr. Cave said. “We are at an inflection point of transition to a new technology.”
Dr. Cave disclosed relationships with Medtronic and Olympus. The other investigators and interviewees reported no conflicts of interest.
FROM ACG 2020
Statins may lower risk of colorectal cancer
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
Statin use may significantly lower the risk of colorectal cancer (CRC) in patients with or without inflammatory bowel disease (IBD), based on a meta-analysis and systematic review.
In more than 15,000 patients with IBD, statin use was associated with a 60% reduced risk of CRC, reported lead author Kevin N. Singh, MD, of NYU Langone Medical Center in New York, and colleagues.
“Statin use has been linked with a risk reduction for cancers including hepatocellular carcinoma, breast, gastric, pancreatic, and biliary tract cancers, but data supporting the use of statins for chemoprevention against CRC is conflicting,” Dr. Singh said during a virtual presentation at the annual meeting of the American College of Gastroenterology.
He noted a 2014 meta-analysis by Lytras and colleagues that reported a 9% CRC risk reduction in statin users who did not have IBD. In patients with IBD, data are scarce, according to Dr. Singh.
To further explore the relationship between statin use and CRC in patients without IBD, the investigators analyzed data from 52 studies, including 8 randomized clinical trials, 17 cohort studies, and 27 case-control studies. Of the 11,459,306 patients involved, approximately 2 million used statins and roughly 9 million did not.
To evaluate the same relationship in patients with IBD, the investigators conducted a separate meta-analysis involving 15,342 patients from 5 observational studies, 1 of which was an unpublished abstract. In the 4 published studies, 1,161 patients used statins while 12,145 did not.
In the non-IBD population, statin use was associated with a 20% reduced risk of CRC (pooled odds ratio, 0.80; 95% confidence interval, 0.73-0.88; P less than .001). In patients with IBD, statin use was associated with a 60% CRC risk reduction (pooled OR, 0.40; 95% CI, 0.19-0.86, P = .019).
Dr. Singh noted “significant heterogeneity” in both analyses (I2 greater than 75), most prominently in the IBD populations, which he ascribed to “differences in demographic features, ethnic groups, and risk factors for CRC.”
While publication bias was absent from the non-IBD analysis, it was detected in the IBD portion of the study. Dr. Singh said that selection bias may also have been present in the IBD analysis, due to exclusive use of observational studies.
“Prospective trials are needed to confirm the risk reduction of CRC in the IBD population, including whether the effects of statins differ between ulcerative colitis and Crohn’s disease patients,” Dr. Singh said.
Additional analyses are underway, he added, including one that will account for the potentially confounding effect of aspirin use.
According to David E. Kaplan, MD, of the University of Pennsylvania, Philadelphia, “The finding that statins are associated with reduced CRC in IBD provides additional support for the clinical importance of the antineoplastic effects of statins. This effect has been strongly observed in liver cancer, and is pending prospective validation.”
Dr. Kaplan also offered some mechanistic insight into why statins have an anticancer effect, pointing to “the centrality of cholesterol biosynthesis for development and/or progression of malignancy.”
The investigators and Dr. Kaplan reported no relevant conflicts of interest.
FROM ACG 2020
Tofacitinib retreatment effective for ulcerative colitis
Retreatment with tofacitinib after a period of treatment interruption was well tolerated and effective in patients with ulcerative colitis who had shown a previous response to tofacitinib induction, according to an analysis of data from the OCTAVE extension trial.
“Clinical response was recaptured in most patients by month 2, and about half of patients by month 36, irrespective of prior anti–[tumor necrosis factor] status,” said lead researcher Edward V. Loftus Jr, MD, from the Mayo Medical School, Rochester, Minn.
A temporary suspension of treatment with the oral, small-molecule Janus kinase (JAK) inhibitor might be necessary for a number of reasons, such as if a patient has to undergo surgery, experiences adverse events, or becomes pregnant.
For their study, Dr. Loftus and colleagues set out to assess the safety and efficacy of retreatment after a period of interruption.
“The population we’re interested in are patients who received tofacitinib during induction and placebo during maintenance” in the original OCTAVE trials, said Dr. Loftus. “They then either completed the trial or flared and rolled over to the open-label extension.”
The researchers looked at the 100 patients who had achieved a clinical response after 8 weeks of treatment with tofacitinib 10 mg twice-daily in the OCTAVE Induction 1 and OCTAVE Induction 2 trials and then received placebo in the OCTAVE Sustain trial and experienced treatment failure between week 8 and week 52. These patients went on to receive tofacitinib 10 mg twice daily as part of the ongoing, open-label, long-term extension OCTAVE Open trial.
Treatment failure was defined as an increase of at least 3 points from the baseline total Mayo score achieved in OCTAVE Sustain, plus an increase of at least 1 point in rectal bleeding and endoscopic subscores and an absolute endoscopic subscore of at least 2 points after at least 8 weeks of treatment. Efficacy was evaluated for up to 36 months in the open-label extension; adverse events were assessed throughout the study period.
The median time to treatment failure was 135 days, Dr. Loftus reported during his award-winning presentation at the virtual annual meeting of the American College of Gastroenterology.
On last observation carried forward (LOCF) analysis, or observed data, 85.2% of the patients had recaptured clinical response by month 2. That rate fell to 74.3% when the analysis was modified for nonresponder imputation (NRI).
“The truth lies somewhere in between,” Dr. Loftus said.
Of interest, a clinical response to tofacitinib retreatment at month 2 was achieved by 92.5% (observed data) and 80.4% (NRI-LOCF) of patients who experienced treatment failure after tumor necrosis factor inhibitor therapy.
“Many patients were able to regain response with tofacitinib and then maintain that over time,” said Dr. Loftus.
Study supports retreatment, which is good news for patients
Incidence rates of adverse events were comparable in the retreatment population and in the overall extension cohort. “There are no signals jumping out, saying that safety events were higher or more frequent in this retreatment population, which is reassuring,” Dr. Loftus added.
Findings such as these are to be expected given the mechanism of action and pharmacologic features of tofacitinib, said Gionata Fiorino, MD, from Humanitas University in Milan, who was not involved in the study.
“I think this is important for patients who need to stop therapy for several reasons – pregnancy, adverse events that do not require permanent withdrawal of the drug, or surgical interventions – and experience a flare after drug withdrawal,” he said in an interview.
“There are several other therapeutic options for these patients, but I have experienced many patients who do not respond to other mechanisms of action apart from JAK [inhibitors],” he added. “And, in the case of a patient who has stopped the drug after having achieved remission, this study clearly supports retreatment, which is good news, especially for patients.”
This study was funded by Pfizer. Dr. Loftus reported financial relationships with AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Exact Sciences, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda, and UCB. Dr. Fiorino reports financial relationships with MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung, Amgen, Roche, and Ferring.
A version of this article originally appeared on Medscape.com.
Retreatment with tofacitinib after a period of treatment interruption was well tolerated and effective in patients with ulcerative colitis who had shown a previous response to tofacitinib induction, according to an analysis of data from the OCTAVE extension trial.
“Clinical response was recaptured in most patients by month 2, and about half of patients by month 36, irrespective of prior anti–[tumor necrosis factor] status,” said lead researcher Edward V. Loftus Jr, MD, from the Mayo Medical School, Rochester, Minn.
A temporary suspension of treatment with the oral, small-molecule Janus kinase (JAK) inhibitor might be necessary for a number of reasons, such as if a patient has to undergo surgery, experiences adverse events, or becomes pregnant.
For their study, Dr. Loftus and colleagues set out to assess the safety and efficacy of retreatment after a period of interruption.
“The population we’re interested in are patients who received tofacitinib during induction and placebo during maintenance” in the original OCTAVE trials, said Dr. Loftus. “They then either completed the trial or flared and rolled over to the open-label extension.”
The researchers looked at the 100 patients who had achieved a clinical response after 8 weeks of treatment with tofacitinib 10 mg twice-daily in the OCTAVE Induction 1 and OCTAVE Induction 2 trials and then received placebo in the OCTAVE Sustain trial and experienced treatment failure between week 8 and week 52. These patients went on to receive tofacitinib 10 mg twice daily as part of the ongoing, open-label, long-term extension OCTAVE Open trial.
Treatment failure was defined as an increase of at least 3 points from the baseline total Mayo score achieved in OCTAVE Sustain, plus an increase of at least 1 point in rectal bleeding and endoscopic subscores and an absolute endoscopic subscore of at least 2 points after at least 8 weeks of treatment. Efficacy was evaluated for up to 36 months in the open-label extension; adverse events were assessed throughout the study period.
The median time to treatment failure was 135 days, Dr. Loftus reported during his award-winning presentation at the virtual annual meeting of the American College of Gastroenterology.
On last observation carried forward (LOCF) analysis, or observed data, 85.2% of the patients had recaptured clinical response by month 2. That rate fell to 74.3% when the analysis was modified for nonresponder imputation (NRI).
“The truth lies somewhere in between,” Dr. Loftus said.
Of interest, a clinical response to tofacitinib retreatment at month 2 was achieved by 92.5% (observed data) and 80.4% (NRI-LOCF) of patients who experienced treatment failure after tumor necrosis factor inhibitor therapy.
“Many patients were able to regain response with tofacitinib and then maintain that over time,” said Dr. Loftus.
Study supports retreatment, which is good news for patients
Incidence rates of adverse events were comparable in the retreatment population and in the overall extension cohort. “There are no signals jumping out, saying that safety events were higher or more frequent in this retreatment population, which is reassuring,” Dr. Loftus added.
Findings such as these are to be expected given the mechanism of action and pharmacologic features of tofacitinib, said Gionata Fiorino, MD, from Humanitas University in Milan, who was not involved in the study.
“I think this is important for patients who need to stop therapy for several reasons – pregnancy, adverse events that do not require permanent withdrawal of the drug, or surgical interventions – and experience a flare after drug withdrawal,” he said in an interview.
“There are several other therapeutic options for these patients, but I have experienced many patients who do not respond to other mechanisms of action apart from JAK [inhibitors],” he added. “And, in the case of a patient who has stopped the drug after having achieved remission, this study clearly supports retreatment, which is good news, especially for patients.”
This study was funded by Pfizer. Dr. Loftus reported financial relationships with AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Exact Sciences, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda, and UCB. Dr. Fiorino reports financial relationships with MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung, Amgen, Roche, and Ferring.
A version of this article originally appeared on Medscape.com.
Retreatment with tofacitinib after a period of treatment interruption was well tolerated and effective in patients with ulcerative colitis who had shown a previous response to tofacitinib induction, according to an analysis of data from the OCTAVE extension trial.
“Clinical response was recaptured in most patients by month 2, and about half of patients by month 36, irrespective of prior anti–[tumor necrosis factor] status,” said lead researcher Edward V. Loftus Jr, MD, from the Mayo Medical School, Rochester, Minn.
A temporary suspension of treatment with the oral, small-molecule Janus kinase (JAK) inhibitor might be necessary for a number of reasons, such as if a patient has to undergo surgery, experiences adverse events, or becomes pregnant.
For their study, Dr. Loftus and colleagues set out to assess the safety and efficacy of retreatment after a period of interruption.
“The population we’re interested in are patients who received tofacitinib during induction and placebo during maintenance” in the original OCTAVE trials, said Dr. Loftus. “They then either completed the trial or flared and rolled over to the open-label extension.”
The researchers looked at the 100 patients who had achieved a clinical response after 8 weeks of treatment with tofacitinib 10 mg twice-daily in the OCTAVE Induction 1 and OCTAVE Induction 2 trials and then received placebo in the OCTAVE Sustain trial and experienced treatment failure between week 8 and week 52. These patients went on to receive tofacitinib 10 mg twice daily as part of the ongoing, open-label, long-term extension OCTAVE Open trial.
Treatment failure was defined as an increase of at least 3 points from the baseline total Mayo score achieved in OCTAVE Sustain, plus an increase of at least 1 point in rectal bleeding and endoscopic subscores and an absolute endoscopic subscore of at least 2 points after at least 8 weeks of treatment. Efficacy was evaluated for up to 36 months in the open-label extension; adverse events were assessed throughout the study period.
The median time to treatment failure was 135 days, Dr. Loftus reported during his award-winning presentation at the virtual annual meeting of the American College of Gastroenterology.
On last observation carried forward (LOCF) analysis, or observed data, 85.2% of the patients had recaptured clinical response by month 2. That rate fell to 74.3% when the analysis was modified for nonresponder imputation (NRI).
“The truth lies somewhere in between,” Dr. Loftus said.
Of interest, a clinical response to tofacitinib retreatment at month 2 was achieved by 92.5% (observed data) and 80.4% (NRI-LOCF) of patients who experienced treatment failure after tumor necrosis factor inhibitor therapy.
“Many patients were able to regain response with tofacitinib and then maintain that over time,” said Dr. Loftus.
Study supports retreatment, which is good news for patients
Incidence rates of adverse events were comparable in the retreatment population and in the overall extension cohort. “There are no signals jumping out, saying that safety events were higher or more frequent in this retreatment population, which is reassuring,” Dr. Loftus added.
Findings such as these are to be expected given the mechanism of action and pharmacologic features of tofacitinib, said Gionata Fiorino, MD, from Humanitas University in Milan, who was not involved in the study.
“I think this is important for patients who need to stop therapy for several reasons – pregnancy, adverse events that do not require permanent withdrawal of the drug, or surgical interventions – and experience a flare after drug withdrawal,” he said in an interview.
“There are several other therapeutic options for these patients, but I have experienced many patients who do not respond to other mechanisms of action apart from JAK [inhibitors],” he added. “And, in the case of a patient who has stopped the drug after having achieved remission, this study clearly supports retreatment, which is good news, especially for patients.”
This study was funded by Pfizer. Dr. Loftus reported financial relationships with AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Exact Sciences, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda, and UCB. Dr. Fiorino reports financial relationships with MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung, Amgen, Roche, and Ferring.
A version of this article originally appeared on Medscape.com.
Cannabis may improve liver function in patients with obesity
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
FROM ACG 2020