User login
Infectious Diseases Society of America (IDSA)/ Society for Healthcare Epidemiology of America (SHEA)/ HIV Medicine Association (HIVMA)/ Pediatric Infectious Diseases Society (PIDS): IDWeek 2014
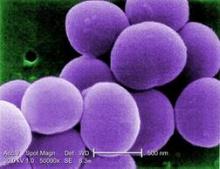
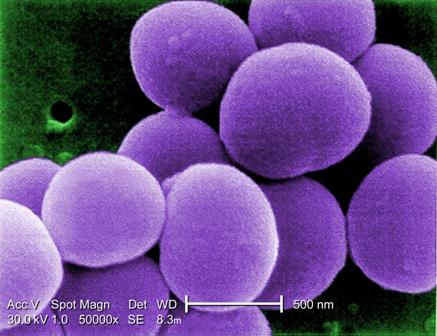
Bundled intervention tackles S. aureus SSIs
PHILADELPHIA – A bundled intervention including Staphylococcus aureus screening, decolonization, and targeted perioperative prophylaxis significantly decreased the rate of complex S. aureus surgical site infections in a multicenter quasi-experimental effectiveness study of patients undergoing cardiac operations or total joint arthroplasty.
The pooled rate of complex S. aureus surgical site infections (SSIs) decreased from 0.36% following 28,218 procedures performed during the preintervention period to 0.20% after 14,316 procedures performed during the intervention period (rate ratio, 0.58), Dr. Loreen A. Herwaldt of the University of Iowa, Iowa City, reported at an annual scientific meeting on infectious diseases.
Further, the number of months with no complex S. aureus SSIs increased from 2 of 39 months (5.1%) to 8 of 22 months (36.4%) Dr. Herwaldt said, noting that the median rate and range of complex SSIs became zero by intervention month 4.
The decrease in SSIs was greatest for joint arthroplasties, she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Subgroup analyses also demonstrated significantly lower rates of complex SSIs for scheduled vs. nonscheduled or emergent operations (rate ratio, 0.55), fully adherent vs. partially or nonadherent operations (rate ratio, 0.26), and for operations in which the surgeon (in accordance with hospital participation) implemented at least some bundle elements vs. no bundle elements (rate ratio, 0.54), she said, explaining that surgeons could opt out of the study even if a hospital was participating.
The rate of complex SSIs caused by any pathogen also was reduced (rate ratio, 0.67).
“We were very pleased to note that gram negative SSIs did not increase. The rate ratio was 0.86, and the confidence interval did cross 1 and the P value was 0.67,” she said.
The study, known as STOP SSI, was conducted at 20 Hospital Corporation of America (HCA) hospitals in nine states from March 1, 2009, to March 31, 2014. Patients who tested positive for methicillin-resistant or methicillin-susceptible S. aureus on a preoperative nares screen within 30 days before surgery were asked to apply mupirocin intranasally twice daily for 5 days and to bathe with chlorhexidine gluconate once daily for 5 days prior to their operation, including on the night before and the morning of surgery. Those who tested negative for MRSA and MSSA bathed with chlorhexidine gluconate only on the night before surgery and the morning of surgery.
Those with MRSA were treated with vancomycin and cefazolin perioperatively, and those without MRSA received only cefazolin.
If the patient’s status was unknown at the time of the operation, the goal was to have the patient bathe in chlorhexidine and to give as many intranasal doses of mupirocin as possible before surgery. The patient was treated perioperatively with vancomycin and cefazolin, and if it was later determined that the patient was positive for MRSA, the mupirocin was continued after surgery until the patient had been treated for 5 days.
After a 3-month phase-in period, 48% of the hospitals were fully compliant with this protocol, and 20% were partially compliant.
The use of a bundled intervention similar to the one used in this study was shown in a recent meta-analysis (BMJ 2013;346:f2743) to be likely to reduce the rate of S. aureus SSIs, but the approach had not been studied in a multicenter trial, Dr. Herwaldt said.
“Implementation of this SSI bundle was associated with significantly lower rates of complex S. aureus SSIs in the total cohort and in the hip and knee arthroplasty group. It was not associated with an increase in gram-negative SSIs, and thus we feel that if people actually did implement this bundle, it could substantially reduce patient morbidity and the cost of care,” she concluded, noting that the effect was seen only with implementation of the full bundle.
The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.
PHILADELPHIA – A bundled intervention including Staphylococcus aureus screening, decolonization, and targeted perioperative prophylaxis significantly decreased the rate of complex S. aureus surgical site infections in a multicenter quasi-experimental effectiveness study of patients undergoing cardiac operations or total joint arthroplasty.
The pooled rate of complex S. aureus surgical site infections (SSIs) decreased from 0.36% following 28,218 procedures performed during the preintervention period to 0.20% after 14,316 procedures performed during the intervention period (rate ratio, 0.58), Dr. Loreen A. Herwaldt of the University of Iowa, Iowa City, reported at an annual scientific meeting on infectious diseases.
Further, the number of months with no complex S. aureus SSIs increased from 2 of 39 months (5.1%) to 8 of 22 months (36.4%) Dr. Herwaldt said, noting that the median rate and range of complex SSIs became zero by intervention month 4.
The decrease in SSIs was greatest for joint arthroplasties, she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Subgroup analyses also demonstrated significantly lower rates of complex SSIs for scheduled vs. nonscheduled or emergent operations (rate ratio, 0.55), fully adherent vs. partially or nonadherent operations (rate ratio, 0.26), and for operations in which the surgeon (in accordance with hospital participation) implemented at least some bundle elements vs. no bundle elements (rate ratio, 0.54), she said, explaining that surgeons could opt out of the study even if a hospital was participating.
The rate of complex SSIs caused by any pathogen also was reduced (rate ratio, 0.67).
“We were very pleased to note that gram negative SSIs did not increase. The rate ratio was 0.86, and the confidence interval did cross 1 and the P value was 0.67,” she said.
The study, known as STOP SSI, was conducted at 20 Hospital Corporation of America (HCA) hospitals in nine states from March 1, 2009, to March 31, 2014. Patients who tested positive for methicillin-resistant or methicillin-susceptible S. aureus on a preoperative nares screen within 30 days before surgery were asked to apply mupirocin intranasally twice daily for 5 days and to bathe with chlorhexidine gluconate once daily for 5 days prior to their operation, including on the night before and the morning of surgery. Those who tested negative for MRSA and MSSA bathed with chlorhexidine gluconate only on the night before surgery and the morning of surgery.
Those with MRSA were treated with vancomycin and cefazolin perioperatively, and those without MRSA received only cefazolin.
If the patient’s status was unknown at the time of the operation, the goal was to have the patient bathe in chlorhexidine and to give as many intranasal doses of mupirocin as possible before surgery. The patient was treated perioperatively with vancomycin and cefazolin, and if it was later determined that the patient was positive for MRSA, the mupirocin was continued after surgery until the patient had been treated for 5 days.
After a 3-month phase-in period, 48% of the hospitals were fully compliant with this protocol, and 20% were partially compliant.
The use of a bundled intervention similar to the one used in this study was shown in a recent meta-analysis (BMJ 2013;346:f2743) to be likely to reduce the rate of S. aureus SSIs, but the approach had not been studied in a multicenter trial, Dr. Herwaldt said.
“Implementation of this SSI bundle was associated with significantly lower rates of complex S. aureus SSIs in the total cohort and in the hip and knee arthroplasty group. It was not associated with an increase in gram-negative SSIs, and thus we feel that if people actually did implement this bundle, it could substantially reduce patient morbidity and the cost of care,” she concluded, noting that the effect was seen only with implementation of the full bundle.
The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.
PHILADELPHIA – A bundled intervention including Staphylococcus aureus screening, decolonization, and targeted perioperative prophylaxis significantly decreased the rate of complex S. aureus surgical site infections in a multicenter quasi-experimental effectiveness study of patients undergoing cardiac operations or total joint arthroplasty.
The pooled rate of complex S. aureus surgical site infections (SSIs) decreased from 0.36% following 28,218 procedures performed during the preintervention period to 0.20% after 14,316 procedures performed during the intervention period (rate ratio, 0.58), Dr. Loreen A. Herwaldt of the University of Iowa, Iowa City, reported at an annual scientific meeting on infectious diseases.
Further, the number of months with no complex S. aureus SSIs increased from 2 of 39 months (5.1%) to 8 of 22 months (36.4%) Dr. Herwaldt said, noting that the median rate and range of complex SSIs became zero by intervention month 4.
The decrease in SSIs was greatest for joint arthroplasties, she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Subgroup analyses also demonstrated significantly lower rates of complex SSIs for scheduled vs. nonscheduled or emergent operations (rate ratio, 0.55), fully adherent vs. partially or nonadherent operations (rate ratio, 0.26), and for operations in which the surgeon (in accordance with hospital participation) implemented at least some bundle elements vs. no bundle elements (rate ratio, 0.54), she said, explaining that surgeons could opt out of the study even if a hospital was participating.
The rate of complex SSIs caused by any pathogen also was reduced (rate ratio, 0.67).
“We were very pleased to note that gram negative SSIs did not increase. The rate ratio was 0.86, and the confidence interval did cross 1 and the P value was 0.67,” she said.
The study, known as STOP SSI, was conducted at 20 Hospital Corporation of America (HCA) hospitals in nine states from March 1, 2009, to March 31, 2014. Patients who tested positive for methicillin-resistant or methicillin-susceptible S. aureus on a preoperative nares screen within 30 days before surgery were asked to apply mupirocin intranasally twice daily for 5 days and to bathe with chlorhexidine gluconate once daily for 5 days prior to their operation, including on the night before and the morning of surgery. Those who tested negative for MRSA and MSSA bathed with chlorhexidine gluconate only on the night before surgery and the morning of surgery.
Those with MRSA were treated with vancomycin and cefazolin perioperatively, and those without MRSA received only cefazolin.
If the patient’s status was unknown at the time of the operation, the goal was to have the patient bathe in chlorhexidine and to give as many intranasal doses of mupirocin as possible before surgery. The patient was treated perioperatively with vancomycin and cefazolin, and if it was later determined that the patient was positive for MRSA, the mupirocin was continued after surgery until the patient had been treated for 5 days.
After a 3-month phase-in period, 48% of the hospitals were fully compliant with this protocol, and 20% were partially compliant.
The use of a bundled intervention similar to the one used in this study was shown in a recent meta-analysis (BMJ 2013;346:f2743) to be likely to reduce the rate of S. aureus SSIs, but the approach had not been studied in a multicenter trial, Dr. Herwaldt said.
“Implementation of this SSI bundle was associated with significantly lower rates of complex S. aureus SSIs in the total cohort and in the hip and knee arthroplasty group. It was not associated with an increase in gram-negative SSIs, and thus we feel that if people actually did implement this bundle, it could substantially reduce patient morbidity and the cost of care,” she concluded, noting that the effect was seen only with implementation of the full bundle.
The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.
AT IDWEEK 2014
Key clinical point: Implementing a bundled intervention reduced S. aureus SSIs and could reduce patient morbidity and costs.
Major finding: The pooled S. aureus SSI rate decreased from 0.36% to 0.20% (rate ratio, 0.58).
Data source: A multicenter quasi-experimental effectiveness study of 42,534 procedures.
Disclosures: The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.
Nursing home-onset C. difficile infections cut wide swath
PHILADELPHIA – Clostridium difficile infections among nursing home residents resulted in some 31,000 hospitalizations and 9,000 deaths nationally in 2012, population-based surveillance data suggest.
“Nursing home-onset CDI is associated with substantial morbidity and mortality,” Dr. Fernanda Lessa reported at an annual scientific meeting on infectious diseases.
Nursing home residents are at higher risk of C. difficile infections (CDI) because of advanced age, frequent healthcare utilization, extended lengths of stay, and exposure to antimicrobials. The national burden of CDI in this setting, however, is not well characterized, said Dr. Lessa of the Centers for Disease Control and Prevention, Atlanta.
The investigators, lead by the CDC’s Dr. Jennifer C. Hunter analyzed surveillance data from 10 states in the Emerging Infections Program (EIP), representing a population of 11.4 million Americans and encompassing 382 nursing homes in 2012. A regression model was used to calculate incidence controlling for identified predictors of high nursing home-onset CDI incidence that vary by region. Sampling weights were used to estimate the national burden of infections and numbers of hospitalizations, recurrences, and deaths.
Nursing home-onset CDI cases were defined as a C. difficile-positive stool sample by toxin or molecular assay during 2012 in a surveillance area resident at least one year of age without a positive test in the prior 8 weeks and a positive stool sample collected in a nursing home resident or within 3 days after hospital admission from a nursing home.
A total of 16,565 CDI cases were identified, of which 3,513 (21%) were nursing home-onset CDI, she said.
A full medical record review of 272 random nursing home-onset CDI cases revealed that most patients received antibiotics in the 12 weeks before testing positive (77%) and had onset of the disease within a month after hospital discharge (76%), Dr. Lessa observed.
Approximately 13% required hospitalization within 7 days of testing positive, 2% required ICU admission, 20% had recurrent disease within 2-8 weeks, and 8% died within 30 days of a positive specimen.
After adjusting for age and diagnostic testing methods, an estimated 115,811 cases of nursing home-onset CDI occurred in 2012, with the highest rate among those aged 65-84 years (58,857 cases; rate 157.97/100,000 population).
Of these, an estimated 31,644 patients required hospitalization, 21,103 experienced recurrence, and 9,053 died within 30 days, Dr. Lessa reported.
“Strategies to reduce unnecessary antibiotic use in both acute and long-term care settings may lead to decreases in CDI onset in nursing homes in the U.S.,” she suggested.
During a discussion of the results, it was noted that the data are very consistent with multiple other studies showing that CDI in long-term care facilities is occurring within 30 days of transfer to the hospital, raising the question of whether the major problem isn’t antibiotic stewardship in the hospital setting.
Dr. Lessa pointed out that 30% of cases were not hospitalized in the 12 weeks prior to CDI onset, but went on to say, “I think we should focus on both [settings], but I completely agree that antimicrobial stewardship in the acute care setting will likely have a major impact in reducing nursing home C. difficile rates.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Clostridium difficile infections among nursing home residents resulted in some 31,000 hospitalizations and 9,000 deaths nationally in 2012, population-based surveillance data suggest.
“Nursing home-onset CDI is associated with substantial morbidity and mortality,” Dr. Fernanda Lessa reported at an annual scientific meeting on infectious diseases.
Nursing home residents are at higher risk of C. difficile infections (CDI) because of advanced age, frequent healthcare utilization, extended lengths of stay, and exposure to antimicrobials. The national burden of CDI in this setting, however, is not well characterized, said Dr. Lessa of the Centers for Disease Control and Prevention, Atlanta.
The investigators, lead by the CDC’s Dr. Jennifer C. Hunter analyzed surveillance data from 10 states in the Emerging Infections Program (EIP), representing a population of 11.4 million Americans and encompassing 382 nursing homes in 2012. A regression model was used to calculate incidence controlling for identified predictors of high nursing home-onset CDI incidence that vary by region. Sampling weights were used to estimate the national burden of infections and numbers of hospitalizations, recurrences, and deaths.
Nursing home-onset CDI cases were defined as a C. difficile-positive stool sample by toxin or molecular assay during 2012 in a surveillance area resident at least one year of age without a positive test in the prior 8 weeks and a positive stool sample collected in a nursing home resident or within 3 days after hospital admission from a nursing home.
A total of 16,565 CDI cases were identified, of which 3,513 (21%) were nursing home-onset CDI, she said.
A full medical record review of 272 random nursing home-onset CDI cases revealed that most patients received antibiotics in the 12 weeks before testing positive (77%) and had onset of the disease within a month after hospital discharge (76%), Dr. Lessa observed.
Approximately 13% required hospitalization within 7 days of testing positive, 2% required ICU admission, 20% had recurrent disease within 2-8 weeks, and 8% died within 30 days of a positive specimen.
After adjusting for age and diagnostic testing methods, an estimated 115,811 cases of nursing home-onset CDI occurred in 2012, with the highest rate among those aged 65-84 years (58,857 cases; rate 157.97/100,000 population).
Of these, an estimated 31,644 patients required hospitalization, 21,103 experienced recurrence, and 9,053 died within 30 days, Dr. Lessa reported.
“Strategies to reduce unnecessary antibiotic use in both acute and long-term care settings may lead to decreases in CDI onset in nursing homes in the U.S.,” she suggested.
During a discussion of the results, it was noted that the data are very consistent with multiple other studies showing that CDI in long-term care facilities is occurring within 30 days of transfer to the hospital, raising the question of whether the major problem isn’t antibiotic stewardship in the hospital setting.
Dr. Lessa pointed out that 30% of cases were not hospitalized in the 12 weeks prior to CDI onset, but went on to say, “I think we should focus on both [settings], but I completely agree that antimicrobial stewardship in the acute care setting will likely have a major impact in reducing nursing home C. difficile rates.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Clostridium difficile infections among nursing home residents resulted in some 31,000 hospitalizations and 9,000 deaths nationally in 2012, population-based surveillance data suggest.
“Nursing home-onset CDI is associated with substantial morbidity and mortality,” Dr. Fernanda Lessa reported at an annual scientific meeting on infectious diseases.
Nursing home residents are at higher risk of C. difficile infections (CDI) because of advanced age, frequent healthcare utilization, extended lengths of stay, and exposure to antimicrobials. The national burden of CDI in this setting, however, is not well characterized, said Dr. Lessa of the Centers for Disease Control and Prevention, Atlanta.
The investigators, lead by the CDC’s Dr. Jennifer C. Hunter analyzed surveillance data from 10 states in the Emerging Infections Program (EIP), representing a population of 11.4 million Americans and encompassing 382 nursing homes in 2012. A regression model was used to calculate incidence controlling for identified predictors of high nursing home-onset CDI incidence that vary by region. Sampling weights were used to estimate the national burden of infections and numbers of hospitalizations, recurrences, and deaths.
Nursing home-onset CDI cases were defined as a C. difficile-positive stool sample by toxin or molecular assay during 2012 in a surveillance area resident at least one year of age without a positive test in the prior 8 weeks and a positive stool sample collected in a nursing home resident or within 3 days after hospital admission from a nursing home.
A total of 16,565 CDI cases were identified, of which 3,513 (21%) were nursing home-onset CDI, she said.
A full medical record review of 272 random nursing home-onset CDI cases revealed that most patients received antibiotics in the 12 weeks before testing positive (77%) and had onset of the disease within a month after hospital discharge (76%), Dr. Lessa observed.
Approximately 13% required hospitalization within 7 days of testing positive, 2% required ICU admission, 20% had recurrent disease within 2-8 weeks, and 8% died within 30 days of a positive specimen.
After adjusting for age and diagnostic testing methods, an estimated 115,811 cases of nursing home-onset CDI occurred in 2012, with the highest rate among those aged 65-84 years (58,857 cases; rate 157.97/100,000 population).
Of these, an estimated 31,644 patients required hospitalization, 21,103 experienced recurrence, and 9,053 died within 30 days, Dr. Lessa reported.
“Strategies to reduce unnecessary antibiotic use in both acute and long-term care settings may lead to decreases in CDI onset in nursing homes in the U.S.,” she suggested.
During a discussion of the results, it was noted that the data are very consistent with multiple other studies showing that CDI in long-term care facilities is occurring within 30 days of transfer to the hospital, raising the question of whether the major problem isn’t antibiotic stewardship in the hospital setting.
Dr. Lessa pointed out that 30% of cases were not hospitalized in the 12 weeks prior to CDI onset, but went on to say, “I think we should focus on both [settings], but I completely agree that antimicrobial stewardship in the acute care setting will likely have a major impact in reducing nursing home C. difficile rates.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
AT ID WEEK 2014
Key clinical point: Nursing home-onset C. difficile infections are associated with significant morbidity and mortality.
Major finding: An estimated 115,811 cases of nursing home-onset C. difficile occurred in 2012 nationally.
Data source: Population-based surveillance data from 10 states and 382 nursing homes.
Disclosures: Dr. Lessa reported having no financial disclosures.
Reduced sedation during ventilation lowered ventilator-associated events
PHILADELPHIA – A nurse- and respiratory therapist–led opt-out protocol for coordinated daily spontaneous awakening trials and spontaneous breathing trials was associated with significant reductions in hospital length of stay and ventilator-associated events in a multicenter quality improvement collaborative nested within a prospective study of ventilator-associated events.
The protocol led to significant increases – after adjustment for age, sex, Sequential Organ Failure Assessment score, reason for intubation, comorbidity score, and unit ID – in spontaneous awakening trials (SATs), spontaneous breathing trials (SBTs), and in the percentage of SBTs performed without sedation among 3,425 episodes and 22,991days of mechanical ventilation in the collaborative units, Dr. Deverick Anderson of Duke University Medical Center, Durham, N.C., reported at an annual scientific meeting on infectious diseases.
The SAT performance rate increased from 30% to 70% during the course of the study, and the SBT performance rate also increased, though more modestly, from about 55% to nearly 70%. The performance rate of SBTs performed with sedatives off – an intervention that improves the ability to be extubated – increased from nearly 55% to more than 95%.
The mean duration of mechanical ventilation decreased by 2.4 days, mean ICU stay decreased by 3 days, and mean hospital length of stay decreased by 6.3 days, Dr. Anderson said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Further, ventilator-associated conditions and infection-related ventilator-associated complications significantly decreased (odds ratio, 0.63 and 0.35, respectively). However, there was no decrease in possible or probable pneumonia (OR, 0.51).
Self-extubations increased (OR, 2.1, but there was no change in reintubations within 24 hours (OR, 0.96), Dr. Anderson said
“When we put all of this together, we were able to show a decrease in our rates of VAEs [ventilator-associated events] per 100 episodes. Over the course of the entire study, we calculated a 37% decrease in the risk of VAEs,” Dr. Anderson said.
However, the number of VAEs per 1,000 days didn’t change, because both the denominator and the numerator changed with the intervention. This finding raises questions about determining the right denominator to use. Based on the findings, it appears that ventilator episodes, rather than ventilator days, might be the best denominators, he said.
The study was conducted at 12 adult intensive care units at seven hospitals participating in the Centers for Disease Control and Prevention’s Prevention Epicenters Wake Up and Breathe Collaborative between November 2011 and May 2013. The collaborative was designed to prevent VAEs by decreasing patients’ sedative and ventilator exposures.
The collaborative was developed after early 2013 when the CDC replaced its ventilator-associated pneumonia (VAP) definitions with VAE definitions, expanding surveillance to VAEs in an effort to improve the objectivity of the definitions, to improve the ease of performing surveillance, and to try to improve the ability to make interhospital comparisons, Dr. Anderson explained, adding that VAEs include VAP, but also include pulmonary edema, atelectasis, and acute respiratory distress syndrome.
Thus, interventions aimed simply at reducing VAP may not change the rate of VAEs, he said.
Patients with VAEs stay on ventilators longer, stay in the ICU longer, are exposed to more antibiotic, and have two- to threefold increased rates of mortality, compared with those on ventilators but without VAEs, but little is known about preventing VAEs.
A larger study suggested that about a third of cases might be preventable, but no intervention has been tested and found to have an effect on the rate of VAEs. The Wake Up and Breathe Collaborative was tasked with answering the question of whether VAEs are preventable, and the investigators thought the best opportunity for prevention was to decrease the amount of sedation that ventilated patients received, Dr. Anderson said.
“More specifically – to decrease sedation through daily SATs and SBTs,” he added.
The opt-out protocol called for SATs and SBTs in all ventilated patients unless they met specific safety criteria or a physician wrote a specific opt-out order.
Though limited by the quasi-experimental open label study design, the findings are consistent with those from prior studies of such protocols.
“We felt that our multicenter prospective collaborative study was a success. … putting it all together, we conclude that VAEs are preventable when we improve compliance with evidence-based practice for our ventilated patients,” he said.Dr. Anderson reported receiving royalties from UpToDate and receiving research support from the CDC and the National Institutes of Health/National Institute of Allergy and Infectious Diseases.
 |
| Dr. Vera DePalo |
Dr. Vera DePalo, FCCP, comments: The results of this collaborative underscore an important point in patient-focused care, namely that participation of the patient in his or her own care is often able to accelerate a patient's recovery.
It seems that with a protocol for coordinated daily spontaneous awakening trials, patients were more likely to be able to have success with a spontaneous breathing trial. A more awake state enables the patient to have a stronger cough, do a better job of clearing secretions, and take deeper breaths.
In this study, these interventions resulted in a reduction in mechanical ventilator days, a reduction in ICU days, and a decrease in mean hospital length of stay. The partnership between patient, physician and care team has enhanced the care delivery and improved health in many chronic conditions. With the current focus on population health, engaging patients in improving their health will be a win for all. As care providers, we should continue to look for every opportunity to engage our patients to participate actively in their health care.
Dr. DePalo is CMO, Chief of Medicine, for Signature Healthcare Brockton Hospital in Brockton, MA.
 |
| Dr. Vera DePalo |
Dr. Vera DePalo, FCCP, comments: The results of this collaborative underscore an important point in patient-focused care, namely that participation of the patient in his or her own care is often able to accelerate a patient's recovery.
It seems that with a protocol for coordinated daily spontaneous awakening trials, patients were more likely to be able to have success with a spontaneous breathing trial. A more awake state enables the patient to have a stronger cough, do a better job of clearing secretions, and take deeper breaths.
In this study, these interventions resulted in a reduction in mechanical ventilator days, a reduction in ICU days, and a decrease in mean hospital length of stay. The partnership between patient, physician and care team has enhanced the care delivery and improved health in many chronic conditions. With the current focus on population health, engaging patients in improving their health will be a win for all. As care providers, we should continue to look for every opportunity to engage our patients to participate actively in their health care.
Dr. DePalo is CMO, Chief of Medicine, for Signature Healthcare Brockton Hospital in Brockton, MA.
 |
| Dr. Vera DePalo |
Dr. Vera DePalo, FCCP, comments: The results of this collaborative underscore an important point in patient-focused care, namely that participation of the patient in his or her own care is often able to accelerate a patient's recovery.
It seems that with a protocol for coordinated daily spontaneous awakening trials, patients were more likely to be able to have success with a spontaneous breathing trial. A more awake state enables the patient to have a stronger cough, do a better job of clearing secretions, and take deeper breaths.
In this study, these interventions resulted in a reduction in mechanical ventilator days, a reduction in ICU days, and a decrease in mean hospital length of stay. The partnership between patient, physician and care team has enhanced the care delivery and improved health in many chronic conditions. With the current focus on population health, engaging patients in improving their health will be a win for all. As care providers, we should continue to look for every opportunity to engage our patients to participate actively in their health care.
Dr. DePalo is CMO, Chief of Medicine, for Signature Healthcare Brockton Hospital in Brockton, MA.
PHILADELPHIA – A nurse- and respiratory therapist–led opt-out protocol for coordinated daily spontaneous awakening trials and spontaneous breathing trials was associated with significant reductions in hospital length of stay and ventilator-associated events in a multicenter quality improvement collaborative nested within a prospective study of ventilator-associated events.
The protocol led to significant increases – after adjustment for age, sex, Sequential Organ Failure Assessment score, reason for intubation, comorbidity score, and unit ID – in spontaneous awakening trials (SATs), spontaneous breathing trials (SBTs), and in the percentage of SBTs performed without sedation among 3,425 episodes and 22,991days of mechanical ventilation in the collaborative units, Dr. Deverick Anderson of Duke University Medical Center, Durham, N.C., reported at an annual scientific meeting on infectious diseases.
The SAT performance rate increased from 30% to 70% during the course of the study, and the SBT performance rate also increased, though more modestly, from about 55% to nearly 70%. The performance rate of SBTs performed with sedatives off – an intervention that improves the ability to be extubated – increased from nearly 55% to more than 95%.
The mean duration of mechanical ventilation decreased by 2.4 days, mean ICU stay decreased by 3 days, and mean hospital length of stay decreased by 6.3 days, Dr. Anderson said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Further, ventilator-associated conditions and infection-related ventilator-associated complications significantly decreased (odds ratio, 0.63 and 0.35, respectively). However, there was no decrease in possible or probable pneumonia (OR, 0.51).
Self-extubations increased (OR, 2.1, but there was no change in reintubations within 24 hours (OR, 0.96), Dr. Anderson said
“When we put all of this together, we were able to show a decrease in our rates of VAEs [ventilator-associated events] per 100 episodes. Over the course of the entire study, we calculated a 37% decrease in the risk of VAEs,” Dr. Anderson said.
However, the number of VAEs per 1,000 days didn’t change, because both the denominator and the numerator changed with the intervention. This finding raises questions about determining the right denominator to use. Based on the findings, it appears that ventilator episodes, rather than ventilator days, might be the best denominators, he said.
The study was conducted at 12 adult intensive care units at seven hospitals participating in the Centers for Disease Control and Prevention’s Prevention Epicenters Wake Up and Breathe Collaborative between November 2011 and May 2013. The collaborative was designed to prevent VAEs by decreasing patients’ sedative and ventilator exposures.
The collaborative was developed after early 2013 when the CDC replaced its ventilator-associated pneumonia (VAP) definitions with VAE definitions, expanding surveillance to VAEs in an effort to improve the objectivity of the definitions, to improve the ease of performing surveillance, and to try to improve the ability to make interhospital comparisons, Dr. Anderson explained, adding that VAEs include VAP, but also include pulmonary edema, atelectasis, and acute respiratory distress syndrome.
Thus, interventions aimed simply at reducing VAP may not change the rate of VAEs, he said.
Patients with VAEs stay on ventilators longer, stay in the ICU longer, are exposed to more antibiotic, and have two- to threefold increased rates of mortality, compared with those on ventilators but without VAEs, but little is known about preventing VAEs.
A larger study suggested that about a third of cases might be preventable, but no intervention has been tested and found to have an effect on the rate of VAEs. The Wake Up and Breathe Collaborative was tasked with answering the question of whether VAEs are preventable, and the investigators thought the best opportunity for prevention was to decrease the amount of sedation that ventilated patients received, Dr. Anderson said.
“More specifically – to decrease sedation through daily SATs and SBTs,” he added.
The opt-out protocol called for SATs and SBTs in all ventilated patients unless they met specific safety criteria or a physician wrote a specific opt-out order.
Though limited by the quasi-experimental open label study design, the findings are consistent with those from prior studies of such protocols.
“We felt that our multicenter prospective collaborative study was a success. … putting it all together, we conclude that VAEs are preventable when we improve compliance with evidence-based practice for our ventilated patients,” he said.Dr. Anderson reported receiving royalties from UpToDate and receiving research support from the CDC and the National Institutes of Health/National Institute of Allergy and Infectious Diseases.
PHILADELPHIA – A nurse- and respiratory therapist–led opt-out protocol for coordinated daily spontaneous awakening trials and spontaneous breathing trials was associated with significant reductions in hospital length of stay and ventilator-associated events in a multicenter quality improvement collaborative nested within a prospective study of ventilator-associated events.
The protocol led to significant increases – after adjustment for age, sex, Sequential Organ Failure Assessment score, reason for intubation, comorbidity score, and unit ID – in spontaneous awakening trials (SATs), spontaneous breathing trials (SBTs), and in the percentage of SBTs performed without sedation among 3,425 episodes and 22,991days of mechanical ventilation in the collaborative units, Dr. Deverick Anderson of Duke University Medical Center, Durham, N.C., reported at an annual scientific meeting on infectious diseases.
The SAT performance rate increased from 30% to 70% during the course of the study, and the SBT performance rate also increased, though more modestly, from about 55% to nearly 70%. The performance rate of SBTs performed with sedatives off – an intervention that improves the ability to be extubated – increased from nearly 55% to more than 95%.
The mean duration of mechanical ventilation decreased by 2.4 days, mean ICU stay decreased by 3 days, and mean hospital length of stay decreased by 6.3 days, Dr. Anderson said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Further, ventilator-associated conditions and infection-related ventilator-associated complications significantly decreased (odds ratio, 0.63 and 0.35, respectively). However, there was no decrease in possible or probable pneumonia (OR, 0.51).
Self-extubations increased (OR, 2.1, but there was no change in reintubations within 24 hours (OR, 0.96), Dr. Anderson said
“When we put all of this together, we were able to show a decrease in our rates of VAEs [ventilator-associated events] per 100 episodes. Over the course of the entire study, we calculated a 37% decrease in the risk of VAEs,” Dr. Anderson said.
However, the number of VAEs per 1,000 days didn’t change, because both the denominator and the numerator changed with the intervention. This finding raises questions about determining the right denominator to use. Based on the findings, it appears that ventilator episodes, rather than ventilator days, might be the best denominators, he said.
The study was conducted at 12 adult intensive care units at seven hospitals participating in the Centers for Disease Control and Prevention’s Prevention Epicenters Wake Up and Breathe Collaborative between November 2011 and May 2013. The collaborative was designed to prevent VAEs by decreasing patients’ sedative and ventilator exposures.
The collaborative was developed after early 2013 when the CDC replaced its ventilator-associated pneumonia (VAP) definitions with VAE definitions, expanding surveillance to VAEs in an effort to improve the objectivity of the definitions, to improve the ease of performing surveillance, and to try to improve the ability to make interhospital comparisons, Dr. Anderson explained, adding that VAEs include VAP, but also include pulmonary edema, atelectasis, and acute respiratory distress syndrome.
Thus, interventions aimed simply at reducing VAP may not change the rate of VAEs, he said.
Patients with VAEs stay on ventilators longer, stay in the ICU longer, are exposed to more antibiotic, and have two- to threefold increased rates of mortality, compared with those on ventilators but without VAEs, but little is known about preventing VAEs.
A larger study suggested that about a third of cases might be preventable, but no intervention has been tested and found to have an effect on the rate of VAEs. The Wake Up and Breathe Collaborative was tasked with answering the question of whether VAEs are preventable, and the investigators thought the best opportunity for prevention was to decrease the amount of sedation that ventilated patients received, Dr. Anderson said.
“More specifically – to decrease sedation through daily SATs and SBTs,” he added.
The opt-out protocol called for SATs and SBTs in all ventilated patients unless they met specific safety criteria or a physician wrote a specific opt-out order.
Though limited by the quasi-experimental open label study design, the findings are consistent with those from prior studies of such protocols.
“We felt that our multicenter prospective collaborative study was a success. … putting it all together, we conclude that VAEs are preventable when we improve compliance with evidence-based practice for our ventilated patients,” he said.Dr. Anderson reported receiving royalties from UpToDate and receiving research support from the CDC and the National Institutes of Health/National Institute of Allergy and Infectious Diseases.
Key clinical point: Reducing ventilated patients’ sedation time through a nurse- and respiratory therapist–led opt-out protocol reduces the risk of ventilator-associated events.
Major finding: Protocol implementation was associated with a 37% decrease in VAE risk.
Data source: A multicenter quasi-experimental open-label study of 3,425 mechanical ventilation episodes.
Disclosures: Dr. Anderson reported receiving royalties from UpToDate, Online, and receiving research support from the CDC and the NIH/NIAID.
Brincidofovir promising for adenovirus infection in early data
PHILADELPHIA – Brincidofovir, an orally available lipid conjugate of cidofovir, appears promising for the treatment of adenovirus infection in children and young adults, according to findings from the open-label pilot portion of a phase III study.
Of 26 patients from birth through age 29 years who were enrolled in the study as of July 15, 13 discontinued treatment prematurely, 4 completed treatment, and 9 continued with treatment. Plasma viral load decreased over time in most of the 13 patients who either completed or remained on treatment, Dr. Jo-Anne Young reported at an annual scientific meeting on infectious diseases.
After a median treatment duration of 54 days, clearance of adenovirus from respiratory secretions, urine, and stool among those patients in whom these values were measured at baseline was 42%, 33%, and 27%, respectively. Mortality was 46% among all 26 patients and was 38% among patients with disseminated disease. In a prior expanded-access trial of the drug, mortality was 51% in treated patients with disseminated disease, and in a historical cohort from a 2003 study of patients with disseminated disease, mortality at 1 year was 82%.
Of note, one patient in the current study experienced an increase in viral load; that patient had received prior treatment with brincidofovir, and was found to have a T87I mutation with known resistance to cidofovir and brincidofovir. Six other patients with prior cidofovir exposure reached undetectable levels of adenovirus, and one had a greater than 2-log decline, so the effect of prior exposure on treatment response remains uncertain, Dr. Young of the University of Minnesota Medical Center, Minneapolis, said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Another trend that was noted in the current study was a possible increase in viral load prior to a decline in viral load. In one case this occurred in association with treatment interruption due to moderate hyperbilirubinemia, but the viral load declined again once treatment was restarted, and was undetectable by the 8th week of treatment.
Most of the patients in the study to date (80%) were children, 65% were males, 65% were white, and 65% had disseminated infection with two or more body systems affected. Most of those were high-risk patients, who had received hematopoietic stem cell transplants from unrelated donors, Dr. Young noted.
Half of the patients had a high viral copy load in their blood, and many had multiple viruses detected. For example, 27% had BK virus in their urine, 19% had cytomegalovirus detected in their plasma, and 8% had Epstein-Barr virus detected in their plasma .
Treatment was given as a twice-weekly 100-mg dose in adults weighing at least 50 kg, and as a twice-weekly, 2-mg/kg dose in children under age 12 or weighing less than 50 kg. Treatment duration was 12 weeks, and patients were followed for an additional 24 weeks.
Of the 13 patients who discontinued treatment, 4 died, 3 discontinued because of an adverse event (including 2 cases of diarrhea considered treatment related and 1 case of liver function testing abnormalities deemed unrelated to treatment), 2 discontinued on physician advice, 2 started other therapy, 1 experienced progression of transplant qualifying disease, and 1 withdrew consent for study participation. Among those who continued, median treatment duration was 54 days at the time the data were presented.
“Adenovirus is an infection with a high unmet medical treatment need,” Dr. Young said, noting that it is associated with a wide spectrum of disease, ranging from asymptomatic viremia to disseminated disease, particularly among hematopoietic stem cell transplant recipients.
The reported incidence varies from 5% to 50%, and mortality ranges from 26% for untreated symptomatic infection to 80% for disseminated disease.
Risk factors include pediatric age, high-risk types of allogeneic transplants, and the presence of acute graft vs. host disease in any transplant recipient.
Current treatment strategies involve supportive care with a reduction in immune suppression and/or initiation of direct antiviral treatment – typically intravenous cidofovir, which is associated with a risk of significant renal injury, Dr. Young said.
Brincidofovir, however, allows for oral dosing and high intracellular uptake, delivering high intracellular levels of the active antiviral, she noted, adding that there is no evidence of nephrotoxicity or hematologic toxicity associated with brincidofovir.
Antiadenovirus activity was confirmed in a previous study of the drug, and in the expanded-access study, treatment was associated with improved survival vs. historical data.
Up to 100 patients will be enrolled in the current pilot portion of the study, and the findings will be used to guide the second half of the phase III study, she said, noting that as of September, 48 subjects from 17 centers had been enrolled.
“Brincidofovir demonstrated potent virologic activity in patients with adenovirus disease. Subjects treated with brincidofovir appeared to have improved survival vs. historical controls, and there were no new safety signals identified. The data from the pilot portion of this study clearly support progression to the design of the next half of this phase III study for brincidofovir among adenovirus-infected patients,” she concluded.
Dr. Young reported being a clinical investigator and/or receiving research support from Chimerix, GlaxoSmithKline, Merck, and ViroPharma.
PHILADELPHIA – Brincidofovir, an orally available lipid conjugate of cidofovir, appears promising for the treatment of adenovirus infection in children and young adults, according to findings from the open-label pilot portion of a phase III study.
Of 26 patients from birth through age 29 years who were enrolled in the study as of July 15, 13 discontinued treatment prematurely, 4 completed treatment, and 9 continued with treatment. Plasma viral load decreased over time in most of the 13 patients who either completed or remained on treatment, Dr. Jo-Anne Young reported at an annual scientific meeting on infectious diseases.
After a median treatment duration of 54 days, clearance of adenovirus from respiratory secretions, urine, and stool among those patients in whom these values were measured at baseline was 42%, 33%, and 27%, respectively. Mortality was 46% among all 26 patients and was 38% among patients with disseminated disease. In a prior expanded-access trial of the drug, mortality was 51% in treated patients with disseminated disease, and in a historical cohort from a 2003 study of patients with disseminated disease, mortality at 1 year was 82%.
Of note, one patient in the current study experienced an increase in viral load; that patient had received prior treatment with brincidofovir, and was found to have a T87I mutation with known resistance to cidofovir and brincidofovir. Six other patients with prior cidofovir exposure reached undetectable levels of adenovirus, and one had a greater than 2-log decline, so the effect of prior exposure on treatment response remains uncertain, Dr. Young of the University of Minnesota Medical Center, Minneapolis, said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Another trend that was noted in the current study was a possible increase in viral load prior to a decline in viral load. In one case this occurred in association with treatment interruption due to moderate hyperbilirubinemia, but the viral load declined again once treatment was restarted, and was undetectable by the 8th week of treatment.
Most of the patients in the study to date (80%) were children, 65% were males, 65% were white, and 65% had disseminated infection with two or more body systems affected. Most of those were high-risk patients, who had received hematopoietic stem cell transplants from unrelated donors, Dr. Young noted.
Half of the patients had a high viral copy load in their blood, and many had multiple viruses detected. For example, 27% had BK virus in their urine, 19% had cytomegalovirus detected in their plasma, and 8% had Epstein-Barr virus detected in their plasma .
Treatment was given as a twice-weekly 100-mg dose in adults weighing at least 50 kg, and as a twice-weekly, 2-mg/kg dose in children under age 12 or weighing less than 50 kg. Treatment duration was 12 weeks, and patients were followed for an additional 24 weeks.
Of the 13 patients who discontinued treatment, 4 died, 3 discontinued because of an adverse event (including 2 cases of diarrhea considered treatment related and 1 case of liver function testing abnormalities deemed unrelated to treatment), 2 discontinued on physician advice, 2 started other therapy, 1 experienced progression of transplant qualifying disease, and 1 withdrew consent for study participation. Among those who continued, median treatment duration was 54 days at the time the data were presented.
“Adenovirus is an infection with a high unmet medical treatment need,” Dr. Young said, noting that it is associated with a wide spectrum of disease, ranging from asymptomatic viremia to disseminated disease, particularly among hematopoietic stem cell transplant recipients.
The reported incidence varies from 5% to 50%, and mortality ranges from 26% for untreated symptomatic infection to 80% for disseminated disease.
Risk factors include pediatric age, high-risk types of allogeneic transplants, and the presence of acute graft vs. host disease in any transplant recipient.
Current treatment strategies involve supportive care with a reduction in immune suppression and/or initiation of direct antiviral treatment – typically intravenous cidofovir, which is associated with a risk of significant renal injury, Dr. Young said.
Brincidofovir, however, allows for oral dosing and high intracellular uptake, delivering high intracellular levels of the active antiviral, she noted, adding that there is no evidence of nephrotoxicity or hematologic toxicity associated with brincidofovir.
Antiadenovirus activity was confirmed in a previous study of the drug, and in the expanded-access study, treatment was associated with improved survival vs. historical data.
Up to 100 patients will be enrolled in the current pilot portion of the study, and the findings will be used to guide the second half of the phase III study, she said, noting that as of September, 48 subjects from 17 centers had been enrolled.
“Brincidofovir demonstrated potent virologic activity in patients with adenovirus disease. Subjects treated with brincidofovir appeared to have improved survival vs. historical controls, and there were no new safety signals identified. The data from the pilot portion of this study clearly support progression to the design of the next half of this phase III study for brincidofovir among adenovirus-infected patients,” she concluded.
Dr. Young reported being a clinical investigator and/or receiving research support from Chimerix, GlaxoSmithKline, Merck, and ViroPharma.
PHILADELPHIA – Brincidofovir, an orally available lipid conjugate of cidofovir, appears promising for the treatment of adenovirus infection in children and young adults, according to findings from the open-label pilot portion of a phase III study.
Of 26 patients from birth through age 29 years who were enrolled in the study as of July 15, 13 discontinued treatment prematurely, 4 completed treatment, and 9 continued with treatment. Plasma viral load decreased over time in most of the 13 patients who either completed or remained on treatment, Dr. Jo-Anne Young reported at an annual scientific meeting on infectious diseases.
After a median treatment duration of 54 days, clearance of adenovirus from respiratory secretions, urine, and stool among those patients in whom these values were measured at baseline was 42%, 33%, and 27%, respectively. Mortality was 46% among all 26 patients and was 38% among patients with disseminated disease. In a prior expanded-access trial of the drug, mortality was 51% in treated patients with disseminated disease, and in a historical cohort from a 2003 study of patients with disseminated disease, mortality at 1 year was 82%.
Of note, one patient in the current study experienced an increase in viral load; that patient had received prior treatment with brincidofovir, and was found to have a T87I mutation with known resistance to cidofovir and brincidofovir. Six other patients with prior cidofovir exposure reached undetectable levels of adenovirus, and one had a greater than 2-log decline, so the effect of prior exposure on treatment response remains uncertain, Dr. Young of the University of Minnesota Medical Center, Minneapolis, said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Another trend that was noted in the current study was a possible increase in viral load prior to a decline in viral load. In one case this occurred in association with treatment interruption due to moderate hyperbilirubinemia, but the viral load declined again once treatment was restarted, and was undetectable by the 8th week of treatment.
Most of the patients in the study to date (80%) were children, 65% were males, 65% were white, and 65% had disseminated infection with two or more body systems affected. Most of those were high-risk patients, who had received hematopoietic stem cell transplants from unrelated donors, Dr. Young noted.
Half of the patients had a high viral copy load in their blood, and many had multiple viruses detected. For example, 27% had BK virus in their urine, 19% had cytomegalovirus detected in their plasma, and 8% had Epstein-Barr virus detected in their plasma .
Treatment was given as a twice-weekly 100-mg dose in adults weighing at least 50 kg, and as a twice-weekly, 2-mg/kg dose in children under age 12 or weighing less than 50 kg. Treatment duration was 12 weeks, and patients were followed for an additional 24 weeks.
Of the 13 patients who discontinued treatment, 4 died, 3 discontinued because of an adverse event (including 2 cases of diarrhea considered treatment related and 1 case of liver function testing abnormalities deemed unrelated to treatment), 2 discontinued on physician advice, 2 started other therapy, 1 experienced progression of transplant qualifying disease, and 1 withdrew consent for study participation. Among those who continued, median treatment duration was 54 days at the time the data were presented.
“Adenovirus is an infection with a high unmet medical treatment need,” Dr. Young said, noting that it is associated with a wide spectrum of disease, ranging from asymptomatic viremia to disseminated disease, particularly among hematopoietic stem cell transplant recipients.
The reported incidence varies from 5% to 50%, and mortality ranges from 26% for untreated symptomatic infection to 80% for disseminated disease.
Risk factors include pediatric age, high-risk types of allogeneic transplants, and the presence of acute graft vs. host disease in any transplant recipient.
Current treatment strategies involve supportive care with a reduction in immune suppression and/or initiation of direct antiviral treatment – typically intravenous cidofovir, which is associated with a risk of significant renal injury, Dr. Young said.
Brincidofovir, however, allows for oral dosing and high intracellular uptake, delivering high intracellular levels of the active antiviral, she noted, adding that there is no evidence of nephrotoxicity or hematologic toxicity associated with brincidofovir.
Antiadenovirus activity was confirmed in a previous study of the drug, and in the expanded-access study, treatment was associated with improved survival vs. historical data.
Up to 100 patients will be enrolled in the current pilot portion of the study, and the findings will be used to guide the second half of the phase III study, she said, noting that as of September, 48 subjects from 17 centers had been enrolled.
“Brincidofovir demonstrated potent virologic activity in patients with adenovirus disease. Subjects treated with brincidofovir appeared to have improved survival vs. historical controls, and there were no new safety signals identified. The data from the pilot portion of this study clearly support progression to the design of the next half of this phase III study for brincidofovir among adenovirus-infected patients,” she concluded.
Dr. Young reported being a clinical investigator and/or receiving research support from Chimerix, GlaxoSmithKline, Merck, and ViroPharma.
Key clinical point: Brincidofovir looks promising as a treatment for adenovirus infection.
Major finding: A majority of patients experience clearance of adenovirus from plasma, and 42%, 33%, and 27% of those with virus detected in respiratory secretions, urine, and stool, respectively, had clearance.
Data source: 26 patients in an open-label pilot portion of a phase III study.
Disclosures: Dr. Young reported being a clinical investigator and/or receiving research support from Chimerix, GlaxoSmithKline, Merck, and ViroPharma.
Pentavalent Meningococcal Vaccine Makes Headway
PHILADELPHIA – An investigational meningococcal vaccine that covers the five predominant serogroups, including serogroup B, showed early promise in a phase II study in adolescents and young adults.
“As a pediatrician giving shots all day long, giving kids the quadrivalent vaccine and a monovalent serogroup B vaccine at two separate time points can be a real challenge. This combination vaccine gets around that problem by combining all five serogroups,” Dr. Stanley Block said in an interview at an annual scientific meeting on infectious diseases.
Two quadrivalent polysaccharide-protein conjugate meningococcal vaccines against serogroups A, C, W, and Y are available including Menveo from study sponsor, Novartis. It uses the mutated diphtheria toxin, CRM₁₉₇, as a carrier protein (MenACWY-CRM), and is licensed in the United States for persons aged 2 months to 55 years.
Novartis’ serogroup B vaccine, Bexsero (4CMenB), is licensed for use in Europe, Canada, and Australia, but was rejected in the United Kingdom and is being evaluated under fast track designation in the United States. It consists of three recombinant membrane-bound proteins and one outer membrane vesicle (OMV) protein from a New Zealand serogroup B strain, explained Dr. Block, who is a pediatrician in private practice and also works with Kentucky Pediatric and Adult Research in Bardstown, Ky.
The phase II, investigator-blinded study randomly assigned 480 participants, aged 10-25 years, to experimental MenABCWY plus full dose OMV, experimental MenABCWY plus one-quarter dose OMV, MenACWY-CRM plus a placebo vaccination, or 4CMenB. The vaccines were given as a 2-dose series at 0 and 2 months. The per-protocol immunogenicity analysis included 343 participants.
As expected, the percentage of participants with seroresponses against serogroups A, C, W, and Y was notably higher after two doses of combination MenABCWY with full OMV or quarter OMV than after a single dose of the licensed MenACWY-CRM vaccine, Dr. Block reported. Seroresponses were: 90% and 92% vs. 73% for A, 95% and 93% vs. 63% for C, 80% and 84% vs. 65% for W, and 92% and 90% vs. 75% for Y, respectively, likely demonstrating no MenB component interference.
Dr. Block noted that a single dose of MenACWY-CRM vaccine is currently the standard dosage given to all 11- and 12-year-olds, although studies from the Centers for Disease Control and Prevention suggest that immune protection from a single dose may wane after several years.
Two doses of 4CMenB in the study induced immune responses against serogroups A (90%), C (57%), and W (86%), but not against serogroup Y (18%). The 4CMenB vaccine may contribute immune protection against other serotypes because, unlike the polysaccharides in the ACWY conjugate vaccines, the 4CMenB proteins are not unique to a single serogroup, the authors noted in the poster.
Both MenABCWY formulations stimulated robust immune responses to four distinct serogroup B test strains, but they were less than those elicited by the licensed 4CMenB vaccine. This pattern was true regardless of whether response was measured as percentage of subjects with a human serum bactericidal activity titer ≥ 5 or by geometric mean titers.
No overall difference was seen between the MenABCWY formulations in terms of reactogenicity or safety profile, Dr. Block said. Ten serious adverse events were reported by nine subjects, but none were thought to be related to vaccination. The OMV-containing MenABCWY groups, however, had higher frequencies of local reactions, myalgia, and arthralgia.
A phase III trial is being planned, but it is unclear which MenABCWY formulation will be selected for testing, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
PHILADELPHIA – An investigational meningococcal vaccine that covers the five predominant serogroups, including serogroup B, showed early promise in a phase II study in adolescents and young adults.
“As a pediatrician giving shots all day long, giving kids the quadrivalent vaccine and a monovalent serogroup B vaccine at two separate time points can be a real challenge. This combination vaccine gets around that problem by combining all five serogroups,” Dr. Stanley Block said in an interview at an annual scientific meeting on infectious diseases.
Two quadrivalent polysaccharide-protein conjugate meningococcal vaccines against serogroups A, C, W, and Y are available including Menveo from study sponsor, Novartis. It uses the mutated diphtheria toxin, CRM₁₉₇, as a carrier protein (MenACWY-CRM), and is licensed in the United States for persons aged 2 months to 55 years.
Novartis’ serogroup B vaccine, Bexsero (4CMenB), is licensed for use in Europe, Canada, and Australia, but was rejected in the United Kingdom and is being evaluated under fast track designation in the United States. It consists of three recombinant membrane-bound proteins and one outer membrane vesicle (OMV) protein from a New Zealand serogroup B strain, explained Dr. Block, who is a pediatrician in private practice and also works with Kentucky Pediatric and Adult Research in Bardstown, Ky.
The phase II, investigator-blinded study randomly assigned 480 participants, aged 10-25 years, to experimental MenABCWY plus full dose OMV, experimental MenABCWY plus one-quarter dose OMV, MenACWY-CRM plus a placebo vaccination, or 4CMenB. The vaccines were given as a 2-dose series at 0 and 2 months. The per-protocol immunogenicity analysis included 343 participants.
As expected, the percentage of participants with seroresponses against serogroups A, C, W, and Y was notably higher after two doses of combination MenABCWY with full OMV or quarter OMV than after a single dose of the licensed MenACWY-CRM vaccine, Dr. Block reported. Seroresponses were: 90% and 92% vs. 73% for A, 95% and 93% vs. 63% for C, 80% and 84% vs. 65% for W, and 92% and 90% vs. 75% for Y, respectively, likely demonstrating no MenB component interference.
Dr. Block noted that a single dose of MenACWY-CRM vaccine is currently the standard dosage given to all 11- and 12-year-olds, although studies from the Centers for Disease Control and Prevention suggest that immune protection from a single dose may wane after several years.
Two doses of 4CMenB in the study induced immune responses against serogroups A (90%), C (57%), and W (86%), but not against serogroup Y (18%). The 4CMenB vaccine may contribute immune protection against other serotypes because, unlike the polysaccharides in the ACWY conjugate vaccines, the 4CMenB proteins are not unique to a single serogroup, the authors noted in the poster.
Both MenABCWY formulations stimulated robust immune responses to four distinct serogroup B test strains, but they were less than those elicited by the licensed 4CMenB vaccine. This pattern was true regardless of whether response was measured as percentage of subjects with a human serum bactericidal activity titer ≥ 5 or by geometric mean titers.
No overall difference was seen between the MenABCWY formulations in terms of reactogenicity or safety profile, Dr. Block said. Ten serious adverse events were reported by nine subjects, but none were thought to be related to vaccination. The OMV-containing MenABCWY groups, however, had higher frequencies of local reactions, myalgia, and arthralgia.
A phase III trial is being planned, but it is unclear which MenABCWY formulation will be selected for testing, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
PHILADELPHIA – An investigational meningococcal vaccine that covers the five predominant serogroups, including serogroup B, showed early promise in a phase II study in adolescents and young adults.
“As a pediatrician giving shots all day long, giving kids the quadrivalent vaccine and a monovalent serogroup B vaccine at two separate time points can be a real challenge. This combination vaccine gets around that problem by combining all five serogroups,” Dr. Stanley Block said in an interview at an annual scientific meeting on infectious diseases.
Two quadrivalent polysaccharide-protein conjugate meningococcal vaccines against serogroups A, C, W, and Y are available including Menveo from study sponsor, Novartis. It uses the mutated diphtheria toxin, CRM₁₉₇, as a carrier protein (MenACWY-CRM), and is licensed in the United States for persons aged 2 months to 55 years.
Novartis’ serogroup B vaccine, Bexsero (4CMenB), is licensed for use in Europe, Canada, and Australia, but was rejected in the United Kingdom and is being evaluated under fast track designation in the United States. It consists of three recombinant membrane-bound proteins and one outer membrane vesicle (OMV) protein from a New Zealand serogroup B strain, explained Dr. Block, who is a pediatrician in private practice and also works with Kentucky Pediatric and Adult Research in Bardstown, Ky.
The phase II, investigator-blinded study randomly assigned 480 participants, aged 10-25 years, to experimental MenABCWY plus full dose OMV, experimental MenABCWY plus one-quarter dose OMV, MenACWY-CRM plus a placebo vaccination, or 4CMenB. The vaccines were given as a 2-dose series at 0 and 2 months. The per-protocol immunogenicity analysis included 343 participants.
As expected, the percentage of participants with seroresponses against serogroups A, C, W, and Y was notably higher after two doses of combination MenABCWY with full OMV or quarter OMV than after a single dose of the licensed MenACWY-CRM vaccine, Dr. Block reported. Seroresponses were: 90% and 92% vs. 73% for A, 95% and 93% vs. 63% for C, 80% and 84% vs. 65% for W, and 92% and 90% vs. 75% for Y, respectively, likely demonstrating no MenB component interference.
Dr. Block noted that a single dose of MenACWY-CRM vaccine is currently the standard dosage given to all 11- and 12-year-olds, although studies from the Centers for Disease Control and Prevention suggest that immune protection from a single dose may wane after several years.
Two doses of 4CMenB in the study induced immune responses against serogroups A (90%), C (57%), and W (86%), but not against serogroup Y (18%). The 4CMenB vaccine may contribute immune protection against other serotypes because, unlike the polysaccharides in the ACWY conjugate vaccines, the 4CMenB proteins are not unique to a single serogroup, the authors noted in the poster.
Both MenABCWY formulations stimulated robust immune responses to four distinct serogroup B test strains, but they were less than those elicited by the licensed 4CMenB vaccine. This pattern was true regardless of whether response was measured as percentage of subjects with a human serum bactericidal activity titer ≥ 5 or by geometric mean titers.
No overall difference was seen between the MenABCWY formulations in terms of reactogenicity or safety profile, Dr. Block said. Ten serious adverse events were reported by nine subjects, but none were thought to be related to vaccination. The OMV-containing MenABCWY groups, however, had higher frequencies of local reactions, myalgia, and arthralgia.
A phase III trial is being planned, but it is unclear which MenABCWY formulation will be selected for testing, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
AT IDWEEK 2014
Pentavalent meningococcal vaccine makes headway
PHILADELPHIA – An investigational meningococcal vaccine that covers the five predominant serogroups, including serogroup B, showed early promise in a phase II study in adolescents and young adults.
“As a pediatrician giving shots all day long, giving kids the quadrivalent vaccine and a monovalent serogroup B vaccine at two separate time points can be a real challenge. This combination vaccine gets around that problem by combining all five serogroups,” Dr. Stanley Block said in an interview at an annual scientific meeting on infectious diseases.
Two quadrivalent polysaccharide-protein conjugate meningococcal vaccines against serogroups A, C, W, and Y are available including Menveo from study sponsor, Novartis. It uses the mutated diphtheria toxin, CRM₁₉₇, as a carrier protein (MenACWY-CRM), and is licensed in the United States for persons aged 2 months to 55 years.
Novartis’ serogroup B vaccine, Bexsero (4CMenB), is licensed for use in Europe, Canada, and Australia, but was rejected in the United Kingdom and is being evaluated under fast track designation in the United States. It consists of three recombinant membrane-bound proteins and one outer membrane vesicle (OMV) protein from a New Zealand serogroup B strain, explained Dr. Block, who is a pediatrician in private practice and also works with Kentucky Pediatric and Adult Research in Bardstown, Ky.
The phase II, investigator-blinded study randomly assigned 480 participants, aged 10-25 years, to experimental MenABCWY plus full dose OMV, experimental MenABCWY plus one-quarter dose OMV, MenACWY-CRM plus a placebo vaccination, or 4CMenB. The vaccines were given as a 2-dose series at 0 and 2 months. The per-protocol immunogenicity analysis included 343 participants.
As expected, the percentage of participants with seroresponses against serogroups A, C, W, and Y was notably higher after two doses of combination MenABCWY with full OMV or quarter OMV than after a single dose of the licensed MenACWY-CRM vaccine, Dr. Block reported. Seroresponses were: 90% and 92% vs. 73% for A, 95% and 93% vs. 63% for C, 80% and 84% vs. 65% for W, and 92% and 90% vs. 75% for Y, respectively, likely demonstrating no MenB component interference.
Dr. Block noted that a single dose of MenACWY-CRM vaccine is currently the standard dosage given to all 11- and 12-year-olds, although studies from the Centers for Disease Control and Prevention suggest that immune protection from a single dose may wane after several years.
Two doses of 4CMenB in the study induced immune responses against serogroups A (90%), C (57%), and W (86%), but not against serogroup Y (18%). The 4CMenB vaccine may contribute immune protection against other serotypes because, unlike the polysaccharides in the ACWY conjugate vaccines, the 4CMenB proteins are not unique to a single serogroup, the authors noted in the poster.
Both MenABCWY formulations stimulated robust immune responses to four distinct serogroup B test strains, but they were less than those elicited by the licensed 4CMenB vaccine. This pattern was true regardless of whether response was measured as percentage of subjects with a human serum bactericidal activity titer ≥ 5 or by geometric mean titers.
No overall difference was seen between the MenABCWY formulations in terms of reactogenicity or safety profile, Dr. Block said. Ten serious adverse events were reported by nine subjects, but none were thought to be related to vaccination. The OMV-containing MenABCWY groups, however, had higher frequencies of local reactions, myalgia, and arthralgia.
A phase III trial is being planned, but it is unclear which MenABCWY formulation will be selected for testing, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
PHILADELPHIA – An investigational meningococcal vaccine that covers the five predominant serogroups, including serogroup B, showed early promise in a phase II study in adolescents and young adults.
“As a pediatrician giving shots all day long, giving kids the quadrivalent vaccine and a monovalent serogroup B vaccine at two separate time points can be a real challenge. This combination vaccine gets around that problem by combining all five serogroups,” Dr. Stanley Block said in an interview at an annual scientific meeting on infectious diseases.
Two quadrivalent polysaccharide-protein conjugate meningococcal vaccines against serogroups A, C, W, and Y are available including Menveo from study sponsor, Novartis. It uses the mutated diphtheria toxin, CRM₁₉₇, as a carrier protein (MenACWY-CRM), and is licensed in the United States for persons aged 2 months to 55 years.
Novartis’ serogroup B vaccine, Bexsero (4CMenB), is licensed for use in Europe, Canada, and Australia, but was rejected in the United Kingdom and is being evaluated under fast track designation in the United States. It consists of three recombinant membrane-bound proteins and one outer membrane vesicle (OMV) protein from a New Zealand serogroup B strain, explained Dr. Block, who is a pediatrician in private practice and also works with Kentucky Pediatric and Adult Research in Bardstown, Ky.
The phase II, investigator-blinded study randomly assigned 480 participants, aged 10-25 years, to experimental MenABCWY plus full dose OMV, experimental MenABCWY plus one-quarter dose OMV, MenACWY-CRM plus a placebo vaccination, or 4CMenB. The vaccines were given as a 2-dose series at 0 and 2 months. The per-protocol immunogenicity analysis included 343 participants.
As expected, the percentage of participants with seroresponses against serogroups A, C, W, and Y was notably higher after two doses of combination MenABCWY with full OMV or quarter OMV than after a single dose of the licensed MenACWY-CRM vaccine, Dr. Block reported. Seroresponses were: 90% and 92% vs. 73% for A, 95% and 93% vs. 63% for C, 80% and 84% vs. 65% for W, and 92% and 90% vs. 75% for Y, respectively, likely demonstrating no MenB component interference.
Dr. Block noted that a single dose of MenACWY-CRM vaccine is currently the standard dosage given to all 11- and 12-year-olds, although studies from the Centers for Disease Control and Prevention suggest that immune protection from a single dose may wane after several years.
Two doses of 4CMenB in the study induced immune responses against serogroups A (90%), C (57%), and W (86%), but not against serogroup Y (18%). The 4CMenB vaccine may contribute immune protection against other serotypes because, unlike the polysaccharides in the ACWY conjugate vaccines, the 4CMenB proteins are not unique to a single serogroup, the authors noted in the poster.
Both MenABCWY formulations stimulated robust immune responses to four distinct serogroup B test strains, but they were less than those elicited by the licensed 4CMenB vaccine. This pattern was true regardless of whether response was measured as percentage of subjects with a human serum bactericidal activity titer ≥ 5 or by geometric mean titers.
No overall difference was seen between the MenABCWY formulations in terms of reactogenicity or safety profile, Dr. Block said. Ten serious adverse events were reported by nine subjects, but none were thought to be related to vaccination. The OMV-containing MenABCWY groups, however, had higher frequencies of local reactions, myalgia, and arthralgia.
A phase III trial is being planned, but it is unclear which MenABCWY formulation will be selected for testing, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
PHILADELPHIA – An investigational meningococcal vaccine that covers the five predominant serogroups, including serogroup B, showed early promise in a phase II study in adolescents and young adults.
“As a pediatrician giving shots all day long, giving kids the quadrivalent vaccine and a monovalent serogroup B vaccine at two separate time points can be a real challenge. This combination vaccine gets around that problem by combining all five serogroups,” Dr. Stanley Block said in an interview at an annual scientific meeting on infectious diseases.
Two quadrivalent polysaccharide-protein conjugate meningococcal vaccines against serogroups A, C, W, and Y are available including Menveo from study sponsor, Novartis. It uses the mutated diphtheria toxin, CRM₁₉₇, as a carrier protein (MenACWY-CRM), and is licensed in the United States for persons aged 2 months to 55 years.
Novartis’ serogroup B vaccine, Bexsero (4CMenB), is licensed for use in Europe, Canada, and Australia, but was rejected in the United Kingdom and is being evaluated under fast track designation in the United States. It consists of three recombinant membrane-bound proteins and one outer membrane vesicle (OMV) protein from a New Zealand serogroup B strain, explained Dr. Block, who is a pediatrician in private practice and also works with Kentucky Pediatric and Adult Research in Bardstown, Ky.
The phase II, investigator-blinded study randomly assigned 480 participants, aged 10-25 years, to experimental MenABCWY plus full dose OMV, experimental MenABCWY plus one-quarter dose OMV, MenACWY-CRM plus a placebo vaccination, or 4CMenB. The vaccines were given as a 2-dose series at 0 and 2 months. The per-protocol immunogenicity analysis included 343 participants.
As expected, the percentage of participants with seroresponses against serogroups A, C, W, and Y was notably higher after two doses of combination MenABCWY with full OMV or quarter OMV than after a single dose of the licensed MenACWY-CRM vaccine, Dr. Block reported. Seroresponses were: 90% and 92% vs. 73% for A, 95% and 93% vs. 63% for C, 80% and 84% vs. 65% for W, and 92% and 90% vs. 75% for Y, respectively, likely demonstrating no MenB component interference.
Dr. Block noted that a single dose of MenACWY-CRM vaccine is currently the standard dosage given to all 11- and 12-year-olds, although studies from the Centers for Disease Control and Prevention suggest that immune protection from a single dose may wane after several years.
Two doses of 4CMenB in the study induced immune responses against serogroups A (90%), C (57%), and W (86%), but not against serogroup Y (18%). The 4CMenB vaccine may contribute immune protection against other serotypes because, unlike the polysaccharides in the ACWY conjugate vaccines, the 4CMenB proteins are not unique to a single serogroup, the authors noted in the poster.
Both MenABCWY formulations stimulated robust immune responses to four distinct serogroup B test strains, but they were less than those elicited by the licensed 4CMenB vaccine. This pattern was true regardless of whether response was measured as percentage of subjects with a human serum bactericidal activity titer ≥ 5 or by geometric mean titers.
No overall difference was seen between the MenABCWY formulations in terms of reactogenicity or safety profile, Dr. Block said. Ten serious adverse events were reported by nine subjects, but none were thought to be related to vaccination. The OMV-containing MenABCWY groups, however, had higher frequencies of local reactions, myalgia, and arthralgia.
A phase III trial is being planned, but it is unclear which MenABCWY formulation will be selected for testing, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
AT IDWEEK 2014
Key clinical point: An experimental meningococcal vaccine that targets all five serogroups A, C, W, Y, and B could reduce the need for two separate vaccines.
Major finding: The percentage of participants with seroresponses against serogroups A, C, W, and Y was higher after two doses of experimental MenABCWY than after a single dose of the licensed MenACWY-CRM vaccine.
Data source: Phase II study in 480 healthy 10- to 25-year-olds.
Disclosures: Novartis Vaccines sponsored the study. Dr. Block reported serving as an investigator for Novartis. Several coauthors are Novartis employees.
Novel nucleoside analog rapidly reduces RSV viral load
PHILADELPHIA – ALS-008176, an oral prodrug of the novel cytidine nucleoside analog, rapidly reduced respiratory syncitial virus load and clinical disease severity in healthy adult volunteers infected with a clinical strain of the virus as part of a double-blind, placebo-controlled phase IIa study.
Of 62 adults aged 18-45 years with preexisting RSV-A specific antibody titers who were inoculated with RSV-A Memphis 37b virus and randomized to receive placebo or one of three ALS-008176 dosing regimens, 35 met the criteria for infection. The viral load area under the curve (AUC) was significantly and rapidly reduced in all volunteers who received ALS-008176, compared with those who received placebo; the reduction in AUC, compared with placebo, ranged from 73% to 88% for the treatment groups at day 12 , Dr. John DeVincenzo of the University of Tennessee Health Science Center, Memphis, reported at an annual scientific meeting on infectious diseases.
Further, the AUC for symptom scores and mucous quantity as measured by weight were also reduced for all treatment groups, compared with placebo, and no subjects exhibited viral load rebound at days 16 or 28 after dosing, Dr. DeVincenzo said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The study subjects, who had RSV-A specific antibody titers in the lowest 25th percentile of the population, were inoculated at baseline and monitored for RSV infection in nasal wash every 12 hours. At either 12 hours after infection was detected or 6 days following inoculation – whichever came first – they were randomized to receive either placebo or ALS-008176 given as a loading dose of 750 mg followed by 500-mg maintenance doses, a loading dose of 750 mg followed by 150-mg maintenance doses, or 375 mg every 12 hours for 5 days. The treatment and placebo groups were well balanced with respect to age, body mass index, sex, and viral load. Assessment of viral load, RSV symptom scores, and mucous weight were evaluated multiple times daily for 12 days, then again on days 16 and 28.
A decrease in viral load in the treatment groups occurred as early as day 0.5 after the initial dose, whereas the viral load in the placebo group increased to more than 4 logs, peaking at day 3.5 and then dropping. The most dramatic decrease in viral load was seen in the highest-dose group and was better in both groups that received a loading dose than in the treatment group that did not.
Viral load in the treatment groups became undetectable very quickly, Dr. DeVincenzo said, noting that levels remained undetectable at days 16 and 28, indicating there was no rebound after dosing was stopped.
No viral resistance mutations were noted.
Treatment with ALS-008176, which is a potent and selective inhibitor of RSV RNA-dependent RNA polymerase that is active against multiple A and B strains of RSV, was well tolerated; no clinically significant laboratory abnormalities occurred, and adverse events all were mild or moderate and were generally balanced between the placebo and treatment groups. None of the volunteers discontinued the study because of adverse events, Dr. DeVincenzo said.
The findings are encouraging given the lack of an effective treatment or vaccine for RSV, which is an important cause of morbidity and mortality worldwide – particularly in infants.
“Further studies of ALS-008176 in natural infections are warranted,” he said, noting that such studies are currently ongoing, including one in otherwise healthy infants hospitalized with RSV.
Dr. DeVincenzo is a consultant for, and/or has received research support from, Abbvie, Alios Biopharma, Alnylam, Ark Pharma, Astra Zeneca, Biota, Crucell, the Genomics Institute of the Novartis Research Foundation, Gilead Sciences, Janssen, MedImmune, Retroscreen Virology, Teva, and Novartis.
PHILADELPHIA – ALS-008176, an oral prodrug of the novel cytidine nucleoside analog, rapidly reduced respiratory syncitial virus load and clinical disease severity in healthy adult volunteers infected with a clinical strain of the virus as part of a double-blind, placebo-controlled phase IIa study.
Of 62 adults aged 18-45 years with preexisting RSV-A specific antibody titers who were inoculated with RSV-A Memphis 37b virus and randomized to receive placebo or one of three ALS-008176 dosing regimens, 35 met the criteria for infection. The viral load area under the curve (AUC) was significantly and rapidly reduced in all volunteers who received ALS-008176, compared with those who received placebo; the reduction in AUC, compared with placebo, ranged from 73% to 88% for the treatment groups at day 12 , Dr. John DeVincenzo of the University of Tennessee Health Science Center, Memphis, reported at an annual scientific meeting on infectious diseases.
Further, the AUC for symptom scores and mucous quantity as measured by weight were also reduced for all treatment groups, compared with placebo, and no subjects exhibited viral load rebound at days 16 or 28 after dosing, Dr. DeVincenzo said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The study subjects, who had RSV-A specific antibody titers in the lowest 25th percentile of the population, were inoculated at baseline and monitored for RSV infection in nasal wash every 12 hours. At either 12 hours after infection was detected or 6 days following inoculation – whichever came first – they were randomized to receive either placebo or ALS-008176 given as a loading dose of 750 mg followed by 500-mg maintenance doses, a loading dose of 750 mg followed by 150-mg maintenance doses, or 375 mg every 12 hours for 5 days. The treatment and placebo groups were well balanced with respect to age, body mass index, sex, and viral load. Assessment of viral load, RSV symptom scores, and mucous weight were evaluated multiple times daily for 12 days, then again on days 16 and 28.
A decrease in viral load in the treatment groups occurred as early as day 0.5 after the initial dose, whereas the viral load in the placebo group increased to more than 4 logs, peaking at day 3.5 and then dropping. The most dramatic decrease in viral load was seen in the highest-dose group and was better in both groups that received a loading dose than in the treatment group that did not.
Viral load in the treatment groups became undetectable very quickly, Dr. DeVincenzo said, noting that levels remained undetectable at days 16 and 28, indicating there was no rebound after dosing was stopped.
No viral resistance mutations were noted.
Treatment with ALS-008176, which is a potent and selective inhibitor of RSV RNA-dependent RNA polymerase that is active against multiple A and B strains of RSV, was well tolerated; no clinically significant laboratory abnormalities occurred, and adverse events all were mild or moderate and were generally balanced between the placebo and treatment groups. None of the volunteers discontinued the study because of adverse events, Dr. DeVincenzo said.
The findings are encouraging given the lack of an effective treatment or vaccine for RSV, which is an important cause of morbidity and mortality worldwide – particularly in infants.
“Further studies of ALS-008176 in natural infections are warranted,” he said, noting that such studies are currently ongoing, including one in otherwise healthy infants hospitalized with RSV.
Dr. DeVincenzo is a consultant for, and/or has received research support from, Abbvie, Alios Biopharma, Alnylam, Ark Pharma, Astra Zeneca, Biota, Crucell, the Genomics Institute of the Novartis Research Foundation, Gilead Sciences, Janssen, MedImmune, Retroscreen Virology, Teva, and Novartis.
PHILADELPHIA – ALS-008176, an oral prodrug of the novel cytidine nucleoside analog, rapidly reduced respiratory syncitial virus load and clinical disease severity in healthy adult volunteers infected with a clinical strain of the virus as part of a double-blind, placebo-controlled phase IIa study.
Of 62 adults aged 18-45 years with preexisting RSV-A specific antibody titers who were inoculated with RSV-A Memphis 37b virus and randomized to receive placebo or one of three ALS-008176 dosing regimens, 35 met the criteria for infection. The viral load area under the curve (AUC) was significantly and rapidly reduced in all volunteers who received ALS-008176, compared with those who received placebo; the reduction in AUC, compared with placebo, ranged from 73% to 88% for the treatment groups at day 12 , Dr. John DeVincenzo of the University of Tennessee Health Science Center, Memphis, reported at an annual scientific meeting on infectious diseases.
Further, the AUC for symptom scores and mucous quantity as measured by weight were also reduced for all treatment groups, compared with placebo, and no subjects exhibited viral load rebound at days 16 or 28 after dosing, Dr. DeVincenzo said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The study subjects, who had RSV-A specific antibody titers in the lowest 25th percentile of the population, were inoculated at baseline and monitored for RSV infection in nasal wash every 12 hours. At either 12 hours after infection was detected or 6 days following inoculation – whichever came first – they were randomized to receive either placebo or ALS-008176 given as a loading dose of 750 mg followed by 500-mg maintenance doses, a loading dose of 750 mg followed by 150-mg maintenance doses, or 375 mg every 12 hours for 5 days. The treatment and placebo groups were well balanced with respect to age, body mass index, sex, and viral load. Assessment of viral load, RSV symptom scores, and mucous weight were evaluated multiple times daily for 12 days, then again on days 16 and 28.
A decrease in viral load in the treatment groups occurred as early as day 0.5 after the initial dose, whereas the viral load in the placebo group increased to more than 4 logs, peaking at day 3.5 and then dropping. The most dramatic decrease in viral load was seen in the highest-dose group and was better in both groups that received a loading dose than in the treatment group that did not.
Viral load in the treatment groups became undetectable very quickly, Dr. DeVincenzo said, noting that levels remained undetectable at days 16 and 28, indicating there was no rebound after dosing was stopped.
No viral resistance mutations were noted.
Treatment with ALS-008176, which is a potent and selective inhibitor of RSV RNA-dependent RNA polymerase that is active against multiple A and B strains of RSV, was well tolerated; no clinically significant laboratory abnormalities occurred, and adverse events all were mild or moderate and were generally balanced between the placebo and treatment groups. None of the volunteers discontinued the study because of adverse events, Dr. DeVincenzo said.
The findings are encouraging given the lack of an effective treatment or vaccine for RSV, which is an important cause of morbidity and mortality worldwide – particularly in infants.
“Further studies of ALS-008176 in natural infections are warranted,” he said, noting that such studies are currently ongoing, including one in otherwise healthy infants hospitalized with RSV.
Dr. DeVincenzo is a consultant for, and/or has received research support from, Abbvie, Alios Biopharma, Alnylam, Ark Pharma, Astra Zeneca, Biota, Crucell, the Genomics Institute of the Novartis Research Foundation, Gilead Sciences, Janssen, MedImmune, Retroscreen Virology, Teva, and Novartis.
Key clinical point: ALS-008176 shows promise for the treatment of RSV
Major finding: The viral load AUC was significantly and rapidly reduced by 73%-88% at day 12, compared with placebo, in those who received ALS-008176.
Data source: A double-blind, placebo-controlled phase IIa study of 62 adults.
Disclosures: Dr. DeVincenzo is a consultant for, and/or has received research support from, Abbvie, Alios Biopharma, Alnylam, Ark Pharma, Astra Zeneca, Biota, Crucell, the Genomics Institute of the Novartis Research Foundation, Gilead Sciences, Janssen, MedImmune, Retroscreen Virology, Teva, and Novartis.
Obesity, Diabetes Spur on Group A Strep Infections
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
AT ID WEEK 2014
Obesity, diabetes spur on group A strep infections
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
AT ID WEEK 2014
Key clinical point: Patients with diabetes and obesity are at increased risk for invasive group A Streptococcus infection.
Major finding: The relative risk of invasive group A Streptococcus (iGAS) infection was 15.6 in diabetics with grade 3 morbid obesity vs. normal-weight diabetics.
Data source: Active surveillance data from 2,927 cases of iGAS.
Disclosures: Dr. Langley reported having no financial disclosures. The CDC funds the ABC surveillance sites.
Vaccine slashes odds of flu hospitalizations for older adults
PHILADELPHIA – Receiving a flu shot cut the odds of influenza hospitalizations by 56% among older adults during the 2010-2011 flu season, surveillance data show.
Vaccine effectiveness was consistent across all age groups, even among those aged 75 years or older.
“Continued emphasis on the importance of influenza vaccination among older adults is crucial,” Dr. Fiona Havers said at an annual scientific meeting on infectious diseases.
She reported on 364 adults, aged 50 years and older, who received a flu shot at least 14 days prior to hospital admission for laboratory-confirmed influenza during the 2010-2011 season. Cases were admitted to one of 11 sites participating in the Emerging Infections Program, now FluSurv-Net, and were matched by age and county to 773 controls.
Cases were significantly more likely than were controls to be of nonwhite race (31% vs. 15%), to be Hispanic (7% vs. 2%), to have an annual income less than $35,000 (52% vs. 33%), to have high school or lower-level education (44% vs. 27%), and to have at least two chronic health conditions (72% vs. 36%) (P values < .01 for all).
Estimates of influenza vaccine effectiveness in preventing hospitalization was 33% for all ages, 33% for ages 50-64 years, 45% for ages 65-74 years, and 21% for those 75 years and older, reported Dr. Havers of the influenza division of the Centers for Disease Control and Prevention in Atlanta.
After adjustment for age, gender, race/ethnicity, income, education, recent hospitalization, functional status, and chronic medical conditions, vaccine effectiveness estimates were 56%, 64%, 61%, and 57%, respectively, (P values < .05 for all).
The investigators also looked at influenza subtype and found that after adjustment, vaccination was associated with a significant reduction in the risk of hospitalizations for influenza A H3N2 (51%) and influenza B (95%), but not influenza A H1N1 (46%), likely because of the small number of H1N1 cases, said Dr. Havers, who urged older adults to seek care if they develop symptoms, even if they were vaccinated.
“We know that antiviral treatment is recommended for all older adults with suspected influenza, as they are at high risk for influenza complications, and antiviral drugs work best if given promptly after symptoms develop,” she said in an interview. ID Week comprises the combined annual meetings of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Receiving a flu shot cut the odds of influenza hospitalizations by 56% among older adults during the 2010-2011 flu season, surveillance data show.
Vaccine effectiveness was consistent across all age groups, even among those aged 75 years or older.
“Continued emphasis on the importance of influenza vaccination among older adults is crucial,” Dr. Fiona Havers said at an annual scientific meeting on infectious diseases.
She reported on 364 adults, aged 50 years and older, who received a flu shot at least 14 days prior to hospital admission for laboratory-confirmed influenza during the 2010-2011 season. Cases were admitted to one of 11 sites participating in the Emerging Infections Program, now FluSurv-Net, and were matched by age and county to 773 controls.
Cases were significantly more likely than were controls to be of nonwhite race (31% vs. 15%), to be Hispanic (7% vs. 2%), to have an annual income less than $35,000 (52% vs. 33%), to have high school or lower-level education (44% vs. 27%), and to have at least two chronic health conditions (72% vs. 36%) (P values < .01 for all).
Estimates of influenza vaccine effectiveness in preventing hospitalization was 33% for all ages, 33% for ages 50-64 years, 45% for ages 65-74 years, and 21% for those 75 years and older, reported Dr. Havers of the influenza division of the Centers for Disease Control and Prevention in Atlanta.
After adjustment for age, gender, race/ethnicity, income, education, recent hospitalization, functional status, and chronic medical conditions, vaccine effectiveness estimates were 56%, 64%, 61%, and 57%, respectively, (P values < .05 for all).
The investigators also looked at influenza subtype and found that after adjustment, vaccination was associated with a significant reduction in the risk of hospitalizations for influenza A H3N2 (51%) and influenza B (95%), but not influenza A H1N1 (46%), likely because of the small number of H1N1 cases, said Dr. Havers, who urged older adults to seek care if they develop symptoms, even if they were vaccinated.
“We know that antiviral treatment is recommended for all older adults with suspected influenza, as they are at high risk for influenza complications, and antiviral drugs work best if given promptly after symptoms develop,” she said in an interview. ID Week comprises the combined annual meetings of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Receiving a flu shot cut the odds of influenza hospitalizations by 56% among older adults during the 2010-2011 flu season, surveillance data show.
Vaccine effectiveness was consistent across all age groups, even among those aged 75 years or older.
“Continued emphasis on the importance of influenza vaccination among older adults is crucial,” Dr. Fiona Havers said at an annual scientific meeting on infectious diseases.
She reported on 364 adults, aged 50 years and older, who received a flu shot at least 14 days prior to hospital admission for laboratory-confirmed influenza during the 2010-2011 season. Cases were admitted to one of 11 sites participating in the Emerging Infections Program, now FluSurv-Net, and were matched by age and county to 773 controls.
Cases were significantly more likely than were controls to be of nonwhite race (31% vs. 15%), to be Hispanic (7% vs. 2%), to have an annual income less than $35,000 (52% vs. 33%), to have high school or lower-level education (44% vs. 27%), and to have at least two chronic health conditions (72% vs. 36%) (P values < .01 for all).
Estimates of influenza vaccine effectiveness in preventing hospitalization was 33% for all ages, 33% for ages 50-64 years, 45% for ages 65-74 years, and 21% for those 75 years and older, reported Dr. Havers of the influenza division of the Centers for Disease Control and Prevention in Atlanta.
After adjustment for age, gender, race/ethnicity, income, education, recent hospitalization, functional status, and chronic medical conditions, vaccine effectiveness estimates were 56%, 64%, 61%, and 57%, respectively, (P values < .05 for all).
The investigators also looked at influenza subtype and found that after adjustment, vaccination was associated with a significant reduction in the risk of hospitalizations for influenza A H3N2 (51%) and influenza B (95%), but not influenza A H1N1 (46%), likely because of the small number of H1N1 cases, said Dr. Havers, who urged older adults to seek care if they develop symptoms, even if they were vaccinated.
“We know that antiviral treatment is recommended for all older adults with suspected influenza, as they are at high risk for influenza complications, and antiviral drugs work best if given promptly after symptoms develop,” she said in an interview. ID Week comprises the combined annual meetings of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
AT ID WEEK 2014
Key clinical point: Influenza vaccination significantly reduced the risk of influenza hospitalization in older adults, regardless of age.
Major finding: Adjusted vaccine effectiveness was 56% for all ages, 64% for ages 50-64 years, 61% for ages 65-74 years, and 57% for ≥ 75 years.
Data source: Case-control study of 1,141 adults vaccinated for influenza during the 2010-2011 season.
Disclosures: Dr. Havers declared no financial conflicts.