User login
Interscience Conference on Antimicrobial Agents & Chemotherapy (ICAAC)/ International Congress of Chemotherapy (ICC)
Long-term ceftaroline use associated with neutropenia
SAN DIEGO – The long-term use of ceftaroline is associated with neutropenia, results from a single-center retrospective study showed.
A fifth-generation cephalosporin antibiotic with activity against methicillin-resistant Staphylococcus aureus, ceftaroline is approved for the treatment of community-acquired pneumonia and skin and skin structure infections. It’s also an option for treating orthopedic and endovascular infections when primary therapy fails or is contraindicated, according to one of the study authors, Dr. Hollis R. O’Neal Jr. “There are small case reports of associations between ceftaroline use and neutropenia, and we were noticing that many patients in our clinical practice were developing neutropenia,” Dr. O’Neal, a pulmonologist at Louisiana State University Health Baton Rouge, said in an interview at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In an effort to determine the incidence, severity, and outcome of neutropenia in patients receiving long-term ceftaroline therapy, Dr. O’Neal and his associates retrospectively evaluated 38 patients who received at least 7 days of ceftaroline initiated at LSU Health Baton Rouge between June 2012 and December 2014. They defined clinically significant neutropenia as having an absolute neutrophil count (ANC) below 2500 cells/mm3 and gathered pertinent data from medical records including comorbidities, chronic medications, and diagnoses.
The median age of the 38 patients was 47 years and their median body mass index was 28 kg/m2. Of the 38 patients, 10 (26%) developed neutropenia. “That was surprising to me,” Dr. O’Neal said. “I thought we would find three or four cases.” Compared with those who were nonneutropenic, those who developed neutropenia tended to be younger (a median of 44 years vs. 51 years), have a lower body mass index (a median of 25 kg/m2 vs. 32 kg/m2), and be more likely to have a longer duration of ceftaroline therapy (a median of 36 days vs. 26 days).
Of the 10 neutropenic patients, 7 had an ANC below 1,500 cells/mm3, and 4 had an ANC below 500 cells/mm3. The median time to first neutropenic day was day 21, with a median nadir of 1,156 cells/mm3. One hospitalization occurred that was believed to be due to neutropenia, but all 30 patients were alive at least 30 days after completing ceftaroline therapy.
“Once the ANC falls below 2,500 cells/mm3, the risk of developing true neutropenia is high,” Dr. O’Neal said. “So what we’re doing is monitoring ANC levels weekly. When they fall below 2,500 we monitor [ANC levels] twice weekly. When they reach 1,500 we stop the drug. So it’s really changed how we monitor the drug’s use.”
The study’s lead author is Dr. Katherine W. Lavie, an infectious diseases fellow at LSU Health Baton Rouge. The researchers reported having no financial disclosures.
SAN DIEGO – The long-term use of ceftaroline is associated with neutropenia, results from a single-center retrospective study showed.
A fifth-generation cephalosporin antibiotic with activity against methicillin-resistant Staphylococcus aureus, ceftaroline is approved for the treatment of community-acquired pneumonia and skin and skin structure infections. It’s also an option for treating orthopedic and endovascular infections when primary therapy fails or is contraindicated, according to one of the study authors, Dr. Hollis R. O’Neal Jr. “There are small case reports of associations between ceftaroline use and neutropenia, and we were noticing that many patients in our clinical practice were developing neutropenia,” Dr. O’Neal, a pulmonologist at Louisiana State University Health Baton Rouge, said in an interview at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In an effort to determine the incidence, severity, and outcome of neutropenia in patients receiving long-term ceftaroline therapy, Dr. O’Neal and his associates retrospectively evaluated 38 patients who received at least 7 days of ceftaroline initiated at LSU Health Baton Rouge between June 2012 and December 2014. They defined clinically significant neutropenia as having an absolute neutrophil count (ANC) below 2500 cells/mm3 and gathered pertinent data from medical records including comorbidities, chronic medications, and diagnoses.
The median age of the 38 patients was 47 years and their median body mass index was 28 kg/m2. Of the 38 patients, 10 (26%) developed neutropenia. “That was surprising to me,” Dr. O’Neal said. “I thought we would find three or four cases.” Compared with those who were nonneutropenic, those who developed neutropenia tended to be younger (a median of 44 years vs. 51 years), have a lower body mass index (a median of 25 kg/m2 vs. 32 kg/m2), and be more likely to have a longer duration of ceftaroline therapy (a median of 36 days vs. 26 days).
Of the 10 neutropenic patients, 7 had an ANC below 1,500 cells/mm3, and 4 had an ANC below 500 cells/mm3. The median time to first neutropenic day was day 21, with a median nadir of 1,156 cells/mm3. One hospitalization occurred that was believed to be due to neutropenia, but all 30 patients were alive at least 30 days after completing ceftaroline therapy.
“Once the ANC falls below 2,500 cells/mm3, the risk of developing true neutropenia is high,” Dr. O’Neal said. “So what we’re doing is monitoring ANC levels weekly. When they fall below 2,500 we monitor [ANC levels] twice weekly. When they reach 1,500 we stop the drug. So it’s really changed how we monitor the drug’s use.”
The study’s lead author is Dr. Katherine W. Lavie, an infectious diseases fellow at LSU Health Baton Rouge. The researchers reported having no financial disclosures.
SAN DIEGO – The long-term use of ceftaroline is associated with neutropenia, results from a single-center retrospective study showed.
A fifth-generation cephalosporin antibiotic with activity against methicillin-resistant Staphylococcus aureus, ceftaroline is approved for the treatment of community-acquired pneumonia and skin and skin structure infections. It’s also an option for treating orthopedic and endovascular infections when primary therapy fails or is contraindicated, according to one of the study authors, Dr. Hollis R. O’Neal Jr. “There are small case reports of associations between ceftaroline use and neutropenia, and we were noticing that many patients in our clinical practice were developing neutropenia,” Dr. O’Neal, a pulmonologist at Louisiana State University Health Baton Rouge, said in an interview at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In an effort to determine the incidence, severity, and outcome of neutropenia in patients receiving long-term ceftaroline therapy, Dr. O’Neal and his associates retrospectively evaluated 38 patients who received at least 7 days of ceftaroline initiated at LSU Health Baton Rouge between June 2012 and December 2014. They defined clinically significant neutropenia as having an absolute neutrophil count (ANC) below 2500 cells/mm3 and gathered pertinent data from medical records including comorbidities, chronic medications, and diagnoses.
The median age of the 38 patients was 47 years and their median body mass index was 28 kg/m2. Of the 38 patients, 10 (26%) developed neutropenia. “That was surprising to me,” Dr. O’Neal said. “I thought we would find three or four cases.” Compared with those who were nonneutropenic, those who developed neutropenia tended to be younger (a median of 44 years vs. 51 years), have a lower body mass index (a median of 25 kg/m2 vs. 32 kg/m2), and be more likely to have a longer duration of ceftaroline therapy (a median of 36 days vs. 26 days).
Of the 10 neutropenic patients, 7 had an ANC below 1,500 cells/mm3, and 4 had an ANC below 500 cells/mm3. The median time to first neutropenic day was day 21, with a median nadir of 1,156 cells/mm3. One hospitalization occurred that was believed to be due to neutropenia, but all 30 patients were alive at least 30 days after completing ceftaroline therapy.
“Once the ANC falls below 2,500 cells/mm3, the risk of developing true neutropenia is high,” Dr. O’Neal said. “So what we’re doing is monitoring ANC levels weekly. When they fall below 2,500 we monitor [ANC levels] twice weekly. When they reach 1,500 we stop the drug. So it’s really changed how we monitor the drug’s use.”
The study’s lead author is Dr. Katherine W. Lavie, an infectious diseases fellow at LSU Health Baton Rouge. The researchers reported having no financial disclosures.
AT ICAAC 2015
Key clinical point: Long-term ceftaroline use is associated with neutropenia.
Major finding: Of 38 patients who received at least 7 days of ceftaroline, 10 (26%) developed neutropenia.
Data source: A retrospective cohort study of 38 patients who received long-term ceftaroline therapy between June 2012 and December 2014.
Disclosures: The researchers reported having no financial disclosures.
Reported pertussis underestimates true incidence by up to 93-fold
SAN DIEGO – The true incidence of pertussis in recent years in Americans less than 50 years old is estimated to be 58- to 93-fold greater than the laboratory-confirmed reported case count, Philip O. Buck, Ph.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
It’s widely accepted that national surveillance systems vastly underestimate the incidence of pertussis because most cases don’t get reported. In order to obtain a more complete picture of the situation, Dr. Buck utilized a regression equation to estimate the proportion of cough illnesses attributable to laboratory-confirmed pertussis.
A closely similar regression model has previously been utilized by other investigators in published studies that provided estimates of the true burdens of influenza (Epidemiol Infect. 2002 Aug;129:99-106) and respiratory syncytial virus (Eur J Pediatr. 2010 Aug;169:997-1008), noted Dr. Buck, director of U.S. Health Outcomes at GlaxoSmithKline in Philadelphia.
He applied the regression model to medical claims for ICD-9-CM–diagnosed pertussis in individuals under age 50 in the IMS PharmMetric Plus claims database for the years 2008-2013. The database includes more than 150 million enrollees. The average reported incidence of pertussis in individuals less than 50 years old during the study years was 9 cases per 100,000 per year; however, the average regression-estimated incidence was 649 per 100,000, a 72-fold greater figure.
During 2011-2013, the 3 most recent years covered by the study, the regression-estimated incidence of pertussis was 93-fold, 62-fold, and 87-fold greater than the reported rates.
Dr. Buck is employed by GlaxoSmithKline, which funded the study and markets pertussis vaccine.
SAN DIEGO – The true incidence of pertussis in recent years in Americans less than 50 years old is estimated to be 58- to 93-fold greater than the laboratory-confirmed reported case count, Philip O. Buck, Ph.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
It’s widely accepted that national surveillance systems vastly underestimate the incidence of pertussis because most cases don’t get reported. In order to obtain a more complete picture of the situation, Dr. Buck utilized a regression equation to estimate the proportion of cough illnesses attributable to laboratory-confirmed pertussis.
A closely similar regression model has previously been utilized by other investigators in published studies that provided estimates of the true burdens of influenza (Epidemiol Infect. 2002 Aug;129:99-106) and respiratory syncytial virus (Eur J Pediatr. 2010 Aug;169:997-1008), noted Dr. Buck, director of U.S. Health Outcomes at GlaxoSmithKline in Philadelphia.
He applied the regression model to medical claims for ICD-9-CM–diagnosed pertussis in individuals under age 50 in the IMS PharmMetric Plus claims database for the years 2008-2013. The database includes more than 150 million enrollees. The average reported incidence of pertussis in individuals less than 50 years old during the study years was 9 cases per 100,000 per year; however, the average regression-estimated incidence was 649 per 100,000, a 72-fold greater figure.
During 2011-2013, the 3 most recent years covered by the study, the regression-estimated incidence of pertussis was 93-fold, 62-fold, and 87-fold greater than the reported rates.
Dr. Buck is employed by GlaxoSmithKline, which funded the study and markets pertussis vaccine.
SAN DIEGO – The true incidence of pertussis in recent years in Americans less than 50 years old is estimated to be 58- to 93-fold greater than the laboratory-confirmed reported case count, Philip O. Buck, Ph.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
It’s widely accepted that national surveillance systems vastly underestimate the incidence of pertussis because most cases don’t get reported. In order to obtain a more complete picture of the situation, Dr. Buck utilized a regression equation to estimate the proportion of cough illnesses attributable to laboratory-confirmed pertussis.
A closely similar regression model has previously been utilized by other investigators in published studies that provided estimates of the true burdens of influenza (Epidemiol Infect. 2002 Aug;129:99-106) and respiratory syncytial virus (Eur J Pediatr. 2010 Aug;169:997-1008), noted Dr. Buck, director of U.S. Health Outcomes at GlaxoSmithKline in Philadelphia.
He applied the regression model to medical claims for ICD-9-CM–diagnosed pertussis in individuals under age 50 in the IMS PharmMetric Plus claims database for the years 2008-2013. The database includes more than 150 million enrollees. The average reported incidence of pertussis in individuals less than 50 years old during the study years was 9 cases per 100,000 per year; however, the average regression-estimated incidence was 649 per 100,000, a 72-fold greater figure.
During 2011-2013, the 3 most recent years covered by the study, the regression-estimated incidence of pertussis was 93-fold, 62-fold, and 87-fold greater than the reported rates.
Dr. Buck is employed by GlaxoSmithKline, which funded the study and markets pertussis vaccine.
AT ICAAC 2015
Key clinical point: The annual rate of pertussis in Americans less than 50 years old is up to 93-fold greater than the reported incidence.
Major finding: The true incidence of pertussis in Americans less than 50 years old is estimated to be 58-93 times greater per year than the reported annual rates during recent years.
Data source: This was a retrospective cohort study which utilized a regression model to estimate the true fraction of cough illness attributable to laboratory-confirmed pertussis through analysis of 6 years worth of medical claims data from a large national database.
Disclosures: The study was funded by GlaxoSmithKline. The presenter is a company employee.
Monitoring microbiome may help reduce infection in AML

Photo by Rhoda Baer
SAN DIEGO—Monitoring the microbiome during chemotherapy might help reduce infections in leukemia patients, according to research presented at ICAAC/ICC 2015.
The researchers studied buccal and fecal samples from patients with acute myeloid leukemia (AML) who were undergoing induction chemotherapy.
This revealed that decreased microbial diversity was associated with an increased risk of infection.
Jessica Galloway-Peña, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and her colleagues described this work in a poster presentation at the meeting (poster B-993).
The team analyzed samples from 34 AML patients. All of the patients received prophylactic antimicrobials, and 91% received systemic antibiotics. The patients received an average of 5.4 different antibiotics for an average duration of 6.5 days.
The researchers collected buccal and fecal specimens from the patients every 96 hours over the course of induction chemotherapy. This yielded 276 buccal and 202 fecal samples—an average of 8 oral and 6 stool samples per patient.
The team used 16S rRNA V4 region sequencing to assign bacterial taxa and calculate α- and β-diversities. They had a total of 16,082,550 high-quality reads.
Analyzing these data, the researchers found that decreased microbial diversity, both at baseline and throughout induction, was associated with an increased risk of infection.
“We found the baseline microbial diversities from stool samples were significantly lower in patients that developed infections during chemotherapy compared to those that did not [P=0.006],” Dr Galloway-Peña said.
She and her colleagues also found that, overall, there was a significant decrease in oral (P=0.006) and intestinal (P<0.001) microbial diversity over the course of chemotherapy, although not all patients experienced decreases. There was a linear correlation between oral and stool microbiome changes (P=0.004).
In addition, over the course of induction, there was a significant increase (P=0.02) in the rates of bacterial domination (>30% of the microbiome dominated by 1 organism) by common causes of bacteremia, such as Streptococcus, Bacteriodes, Rothia, and Staphylococcus.
However, if patients were able to maintain a healthy microbiome overall or if they experienced an increase in microbial diversity over the induction course, they remained infection-free in the 90 days after induction.
Dr Galloway-Peña and her colleagues also assessed the role common antibiotics play in microbial diversity. And they found that carbapenems significantly decreased diversity.
There was a significant difference in oral and stool diversity when patients received carbapenems for at least 72 hours and when they did not (P=0.03). But there was no significant difference for piperacillin-tazobactam (P=1.0) or cefepime (P=0.48).
“This study shows that, in the future, doctors could use microbiome sampling in order to predict the chance of infectious complications during chemotherapy and that monitoring of a patient’s microbiome during induction chemotherapy could also predict their risk for microbial-related illness during subsequent treatments,” Dr Galloway-Peña said.
In addition, monitoring the microbiome could potentially mitigate the overuse of antimicrobials by allowing physicians to stratify patients according to their risk of developing an infection. ![]()

Photo by Rhoda Baer
SAN DIEGO—Monitoring the microbiome during chemotherapy might help reduce infections in leukemia patients, according to research presented at ICAAC/ICC 2015.
The researchers studied buccal and fecal samples from patients with acute myeloid leukemia (AML) who were undergoing induction chemotherapy.
This revealed that decreased microbial diversity was associated with an increased risk of infection.
Jessica Galloway-Peña, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and her colleagues described this work in a poster presentation at the meeting (poster B-993).
The team analyzed samples from 34 AML patients. All of the patients received prophylactic antimicrobials, and 91% received systemic antibiotics. The patients received an average of 5.4 different antibiotics for an average duration of 6.5 days.
The researchers collected buccal and fecal specimens from the patients every 96 hours over the course of induction chemotherapy. This yielded 276 buccal and 202 fecal samples—an average of 8 oral and 6 stool samples per patient.
The team used 16S rRNA V4 region sequencing to assign bacterial taxa and calculate α- and β-diversities. They had a total of 16,082,550 high-quality reads.
Analyzing these data, the researchers found that decreased microbial diversity, both at baseline and throughout induction, was associated with an increased risk of infection.
“We found the baseline microbial diversities from stool samples were significantly lower in patients that developed infections during chemotherapy compared to those that did not [P=0.006],” Dr Galloway-Peña said.
She and her colleagues also found that, overall, there was a significant decrease in oral (P=0.006) and intestinal (P<0.001) microbial diversity over the course of chemotherapy, although not all patients experienced decreases. There was a linear correlation between oral and stool microbiome changes (P=0.004).
In addition, over the course of induction, there was a significant increase (P=0.02) in the rates of bacterial domination (>30% of the microbiome dominated by 1 organism) by common causes of bacteremia, such as Streptococcus, Bacteriodes, Rothia, and Staphylococcus.
However, if patients were able to maintain a healthy microbiome overall or if they experienced an increase in microbial diversity over the induction course, they remained infection-free in the 90 days after induction.
Dr Galloway-Peña and her colleagues also assessed the role common antibiotics play in microbial diversity. And they found that carbapenems significantly decreased diversity.
There was a significant difference in oral and stool diversity when patients received carbapenems for at least 72 hours and when they did not (P=0.03). But there was no significant difference for piperacillin-tazobactam (P=1.0) or cefepime (P=0.48).
“This study shows that, in the future, doctors could use microbiome sampling in order to predict the chance of infectious complications during chemotherapy and that monitoring of a patient’s microbiome during induction chemotherapy could also predict their risk for microbial-related illness during subsequent treatments,” Dr Galloway-Peña said.
In addition, monitoring the microbiome could potentially mitigate the overuse of antimicrobials by allowing physicians to stratify patients according to their risk of developing an infection. ![]()

Photo by Rhoda Baer
SAN DIEGO—Monitoring the microbiome during chemotherapy might help reduce infections in leukemia patients, according to research presented at ICAAC/ICC 2015.
The researchers studied buccal and fecal samples from patients with acute myeloid leukemia (AML) who were undergoing induction chemotherapy.
This revealed that decreased microbial diversity was associated with an increased risk of infection.
Jessica Galloway-Peña, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and her colleagues described this work in a poster presentation at the meeting (poster B-993).
The team analyzed samples from 34 AML patients. All of the patients received prophylactic antimicrobials, and 91% received systemic antibiotics. The patients received an average of 5.4 different antibiotics for an average duration of 6.5 days.
The researchers collected buccal and fecal specimens from the patients every 96 hours over the course of induction chemotherapy. This yielded 276 buccal and 202 fecal samples—an average of 8 oral and 6 stool samples per patient.
The team used 16S rRNA V4 region sequencing to assign bacterial taxa and calculate α- and β-diversities. They had a total of 16,082,550 high-quality reads.
Analyzing these data, the researchers found that decreased microbial diversity, both at baseline and throughout induction, was associated with an increased risk of infection.
“We found the baseline microbial diversities from stool samples were significantly lower in patients that developed infections during chemotherapy compared to those that did not [P=0.006],” Dr Galloway-Peña said.
She and her colleagues also found that, overall, there was a significant decrease in oral (P=0.006) and intestinal (P<0.001) microbial diversity over the course of chemotherapy, although not all patients experienced decreases. There was a linear correlation between oral and stool microbiome changes (P=0.004).
In addition, over the course of induction, there was a significant increase (P=0.02) in the rates of bacterial domination (>30% of the microbiome dominated by 1 organism) by common causes of bacteremia, such as Streptococcus, Bacteriodes, Rothia, and Staphylococcus.
However, if patients were able to maintain a healthy microbiome overall or if they experienced an increase in microbial diversity over the induction course, they remained infection-free in the 90 days after induction.
Dr Galloway-Peña and her colleagues also assessed the role common antibiotics play in microbial diversity. And they found that carbapenems significantly decreased diversity.
There was a significant difference in oral and stool diversity when patients received carbapenems for at least 72 hours and when they did not (P=0.03). But there was no significant difference for piperacillin-tazobactam (P=1.0) or cefepime (P=0.48).
“This study shows that, in the future, doctors could use microbiome sampling in order to predict the chance of infectious complications during chemotherapy and that monitoring of a patient’s microbiome during induction chemotherapy could also predict their risk for microbial-related illness during subsequent treatments,” Dr Galloway-Peña said.
In addition, monitoring the microbiome could potentially mitigate the overuse of antimicrobials by allowing physicians to stratify patients according to their risk of developing an infection. ![]()
New assay may be a game changer in invasive candidiasis
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
EXPERT ANALYSIS FROM ICAAC 2015
ICAAC: Prescribing guide successful in a children’s hospital
SAN DIEGO – The introduction and dissemination of an antibiotic prescribing guide in a children’s hospital resulted in a statistically significant increase in appropriate prescribing, a prospective study showed.
“Antimicrobial drugs are one of the largest groups of medications prescribed to hospitalized children worldwide,” Dr. Jacqueline Wong wrote in an abstract presented at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “Inappropriate antibiotic use is a major factor driving increasing bacterial antibiotic resistance. There are few published data on the effectiveness of stewardship strategies in a pediatric setting.”
In a prospective observational study conducted during Dr. Wong’s pediatrics residency at British Columbia Children’s Hospital, Vancouver, she and her associates set out to compare the proportion of patients who received appropriate antimicrobials before and after the introduction of a new empiric antibiotic guide that was gradually rolled out at the hospital beginning in April of 2012 via lectures, paper copies, and pocket-sized and electronic versions. The time period studied was January 2012 to June 2013. All children admitted and treated with antibiotics were included in the analysis. Exclusion criteria included patients who were admitted to the neonatal unit or hematology/oncology ward, patients with cystic fibrosis, patients who were immunocompromised, and those receiving antibiotics solely for prophylaxis.
The researchers obtained prescribing information from hospital pharmacy data and electronic medical records, and two members of the study team used the updated empiric antibiotic guidelines to determine if the empiric prescribing was appropriate or not.
A total of 1,815 admissions were initially studied. Of these, 63% of the patients were younger than 3 months old, 16% were 4-6 months old, and 21% were 7-11 months old. These percentages did not vary significantly during the study time frame (P greater than .05). The five most common clinical syndromes were septicemia/meningitis (n = 556), respiratory tract infections (n = 532), intra-abdominal infections (n = 195), skin and soft tissue infections (n = 193), and urinary tract infections (n = 184).
Next, the researchers reviewed 752 admissions: 430 prior to introduction of the guidelines and 322 afterward. When they combined the five most common clinical syndromes, they observed a statistically significant increase in appropriate empiric antibiotic therapy between the preintervention period and postintervention period, from 65% to 74%, respectively (P = .035).
“What was interesting to learn from this study was the distribution of the various syndromes and distribution of the age groups of the children that were admitted,” Dr. Wong, now an infectious diseases fellow at The Hospital for Sick Children, Toronto, said in an interview. “The vast majority were 1 year and under.”
While the findings represent success in terms of patient care and cost savings, “the overall limited impact of such a passive antimicrobial stewardship program intervention suggests additional active strategies may be required to further enhance implementation, such as prospective audit with intervention, and feedback may be required to further enhance implementation of antimicrobial guidelines,” the researchers wrote in their abstract. For example, in response to the study findings, British Columbia Children’s Hospital implemented a formal multidisciplinary antimicrobial stewardship program including daily audit and feedback of all admitted patients on antimicrobials within 24 hours. “Data from this program are currently awaiting analysis,” they wrote.
The researchers reported having no financial disclosures.
SAN DIEGO – The introduction and dissemination of an antibiotic prescribing guide in a children’s hospital resulted in a statistically significant increase in appropriate prescribing, a prospective study showed.
“Antimicrobial drugs are one of the largest groups of medications prescribed to hospitalized children worldwide,” Dr. Jacqueline Wong wrote in an abstract presented at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “Inappropriate antibiotic use is a major factor driving increasing bacterial antibiotic resistance. There are few published data on the effectiveness of stewardship strategies in a pediatric setting.”
In a prospective observational study conducted during Dr. Wong’s pediatrics residency at British Columbia Children’s Hospital, Vancouver, she and her associates set out to compare the proportion of patients who received appropriate antimicrobials before and after the introduction of a new empiric antibiotic guide that was gradually rolled out at the hospital beginning in April of 2012 via lectures, paper copies, and pocket-sized and electronic versions. The time period studied was January 2012 to June 2013. All children admitted and treated with antibiotics were included in the analysis. Exclusion criteria included patients who were admitted to the neonatal unit or hematology/oncology ward, patients with cystic fibrosis, patients who were immunocompromised, and those receiving antibiotics solely for prophylaxis.
The researchers obtained prescribing information from hospital pharmacy data and electronic medical records, and two members of the study team used the updated empiric antibiotic guidelines to determine if the empiric prescribing was appropriate or not.
A total of 1,815 admissions were initially studied. Of these, 63% of the patients were younger than 3 months old, 16% were 4-6 months old, and 21% were 7-11 months old. These percentages did not vary significantly during the study time frame (P greater than .05). The five most common clinical syndromes were septicemia/meningitis (n = 556), respiratory tract infections (n = 532), intra-abdominal infections (n = 195), skin and soft tissue infections (n = 193), and urinary tract infections (n = 184).
Next, the researchers reviewed 752 admissions: 430 prior to introduction of the guidelines and 322 afterward. When they combined the five most common clinical syndromes, they observed a statistically significant increase in appropriate empiric antibiotic therapy between the preintervention period and postintervention period, from 65% to 74%, respectively (P = .035).
“What was interesting to learn from this study was the distribution of the various syndromes and distribution of the age groups of the children that were admitted,” Dr. Wong, now an infectious diseases fellow at The Hospital for Sick Children, Toronto, said in an interview. “The vast majority were 1 year and under.”
While the findings represent success in terms of patient care and cost savings, “the overall limited impact of such a passive antimicrobial stewardship program intervention suggests additional active strategies may be required to further enhance implementation, such as prospective audit with intervention, and feedback may be required to further enhance implementation of antimicrobial guidelines,” the researchers wrote in their abstract. For example, in response to the study findings, British Columbia Children’s Hospital implemented a formal multidisciplinary antimicrobial stewardship program including daily audit and feedback of all admitted patients on antimicrobials within 24 hours. “Data from this program are currently awaiting analysis,” they wrote.
The researchers reported having no financial disclosures.
SAN DIEGO – The introduction and dissemination of an antibiotic prescribing guide in a children’s hospital resulted in a statistically significant increase in appropriate prescribing, a prospective study showed.
“Antimicrobial drugs are one of the largest groups of medications prescribed to hospitalized children worldwide,” Dr. Jacqueline Wong wrote in an abstract presented at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “Inappropriate antibiotic use is a major factor driving increasing bacterial antibiotic resistance. There are few published data on the effectiveness of stewardship strategies in a pediatric setting.”
In a prospective observational study conducted during Dr. Wong’s pediatrics residency at British Columbia Children’s Hospital, Vancouver, she and her associates set out to compare the proportion of patients who received appropriate antimicrobials before and after the introduction of a new empiric antibiotic guide that was gradually rolled out at the hospital beginning in April of 2012 via lectures, paper copies, and pocket-sized and electronic versions. The time period studied was January 2012 to June 2013. All children admitted and treated with antibiotics were included in the analysis. Exclusion criteria included patients who were admitted to the neonatal unit or hematology/oncology ward, patients with cystic fibrosis, patients who were immunocompromised, and those receiving antibiotics solely for prophylaxis.
The researchers obtained prescribing information from hospital pharmacy data and electronic medical records, and two members of the study team used the updated empiric antibiotic guidelines to determine if the empiric prescribing was appropriate or not.
A total of 1,815 admissions were initially studied. Of these, 63% of the patients were younger than 3 months old, 16% were 4-6 months old, and 21% were 7-11 months old. These percentages did not vary significantly during the study time frame (P greater than .05). The five most common clinical syndromes were septicemia/meningitis (n = 556), respiratory tract infections (n = 532), intra-abdominal infections (n = 195), skin and soft tissue infections (n = 193), and urinary tract infections (n = 184).
Next, the researchers reviewed 752 admissions: 430 prior to introduction of the guidelines and 322 afterward. When they combined the five most common clinical syndromes, they observed a statistically significant increase in appropriate empiric antibiotic therapy between the preintervention period and postintervention period, from 65% to 74%, respectively (P = .035).
“What was interesting to learn from this study was the distribution of the various syndromes and distribution of the age groups of the children that were admitted,” Dr. Wong, now an infectious diseases fellow at The Hospital for Sick Children, Toronto, said in an interview. “The vast majority were 1 year and under.”
While the findings represent success in terms of patient care and cost savings, “the overall limited impact of such a passive antimicrobial stewardship program intervention suggests additional active strategies may be required to further enhance implementation, such as prospective audit with intervention, and feedback may be required to further enhance implementation of antimicrobial guidelines,” the researchers wrote in their abstract. For example, in response to the study findings, British Columbia Children’s Hospital implemented a formal multidisciplinary antimicrobial stewardship program including daily audit and feedback of all admitted patients on antimicrobials within 24 hours. “Data from this program are currently awaiting analysis,” they wrote.
The researchers reported having no financial disclosures.
AT ICAAC 2015
Key clinical point: A new antibiotic prescribing guide introduced at a children’s hospital resulted in a statistically significant increase in appropriate prescribing.
Major finding: Following introduction of a new antibiotic prescribing guide, the rate of appropriate empiric antibiotic therapy between rose significantly from 65% to 74% (P = .035).
Data source: A prospective observational study of 752 pediatric hospital admissions.
Disclosures: The researchers reported having no financial disclosures.
Readmissions due to infection after HSCT

Photo courtesy of the CDC
SAN DIEGO—A retrospective study has provided insight into hospital readmissions related to opportunistic infection following hematopoietic stem cell transplant (HSCT).
Of the roughly 4200 HSCT recipients studied, 26% were readmitted to the hospital due to opportunistic infection.
About 1 in 3 infection-related readmissions were due to double-stranded DNA (dsDNA) viral infections, and cytomegalovirus (CMV) infections were the most common.
Nearly half of the dsDNA viral infections occurred within the first month of HSCT discharge.
These findings were presented at ICAAC/ICC 2015 (poster T-1360). The study was sponsored by Chimerix, Inc.
Investigators searched the Premier hospital database for patients who underwent HSCT between January 2009 and September 2013. The team identified 4393 patients with a mean age of 50.4 years. Most were adults (91.2%), most were male (57.9%), and most received an autologous HSCT (63.2%).
About 42% (n=1841) of patients had a diagnostic code for opportunistic infection in their HSCT discharge records. Overall, 7.3% (n=319) of patients had dsDNA virus infections, including 13.4% (n=216) of patients who received an allogeneic HSCT.
One hundred and fifty-seven patients died during HSCT hospitalization, leaving 4236 patients evaluable for readmission analysis.
In all, 37.7% (n=1595) of the surviving patients were readmitted to the hospital for any reason during the 12 months after HSCT discharge. And 65.6% of the readmissions occurred within the first 3 months of HSCT discharge.
Readmissions were most frequently related to opportunistic infections (25.8%, n=1091), followed by graft-versus-host disease (13.7%, n=579), renal impairment (11.1%, n=470), and neutropenia (10.0%, n=422).
The investigators noted that patients may have had multiple readmissions or readmission with multiple diagnoses.
Of the hospital readmissions related to opportunistic infections, 32.0% (n=349) were related to dsDNA virus infections. This included CMV (65.9%, n=230), BK virus (13.8%, n=48), adenovirus (5.2%, n=18), and other dsDNA virus infections (32.7%, n=114).
Patients may have experienced more than one viral infection, so the number of hospital readmissions related to each dsDNA virus was not mutually exclusive.
Readmission within the first month of HSCT discharge occurred in 41.8% of patients with any dsDNA virus infection, 49.6% with CMV infection, and 56.3% with BK virus infection. More than half (55.6%) of readmissions related to adenovirus infection occurred within the first 3 months of HSCT discharge.
Taking these results together, the investigators concluded that hospital readmissions related to opportunistic infections were relatively common among HSCT recipients. So strategies that minimize the risks of these infections might have significant clinical and economic advantages. ![]()

Photo courtesy of the CDC
SAN DIEGO—A retrospective study has provided insight into hospital readmissions related to opportunistic infection following hematopoietic stem cell transplant (HSCT).
Of the roughly 4200 HSCT recipients studied, 26% were readmitted to the hospital due to opportunistic infection.
About 1 in 3 infection-related readmissions were due to double-stranded DNA (dsDNA) viral infections, and cytomegalovirus (CMV) infections were the most common.
Nearly half of the dsDNA viral infections occurred within the first month of HSCT discharge.
These findings were presented at ICAAC/ICC 2015 (poster T-1360). The study was sponsored by Chimerix, Inc.
Investigators searched the Premier hospital database for patients who underwent HSCT between January 2009 and September 2013. The team identified 4393 patients with a mean age of 50.4 years. Most were adults (91.2%), most were male (57.9%), and most received an autologous HSCT (63.2%).
About 42% (n=1841) of patients had a diagnostic code for opportunistic infection in their HSCT discharge records. Overall, 7.3% (n=319) of patients had dsDNA virus infections, including 13.4% (n=216) of patients who received an allogeneic HSCT.
One hundred and fifty-seven patients died during HSCT hospitalization, leaving 4236 patients evaluable for readmission analysis.
In all, 37.7% (n=1595) of the surviving patients were readmitted to the hospital for any reason during the 12 months after HSCT discharge. And 65.6% of the readmissions occurred within the first 3 months of HSCT discharge.
Readmissions were most frequently related to opportunistic infections (25.8%, n=1091), followed by graft-versus-host disease (13.7%, n=579), renal impairment (11.1%, n=470), and neutropenia (10.0%, n=422).
The investigators noted that patients may have had multiple readmissions or readmission with multiple diagnoses.
Of the hospital readmissions related to opportunistic infections, 32.0% (n=349) were related to dsDNA virus infections. This included CMV (65.9%, n=230), BK virus (13.8%, n=48), adenovirus (5.2%, n=18), and other dsDNA virus infections (32.7%, n=114).
Patients may have experienced more than one viral infection, so the number of hospital readmissions related to each dsDNA virus was not mutually exclusive.
Readmission within the first month of HSCT discharge occurred in 41.8% of patients with any dsDNA virus infection, 49.6% with CMV infection, and 56.3% with BK virus infection. More than half (55.6%) of readmissions related to adenovirus infection occurred within the first 3 months of HSCT discharge.
Taking these results together, the investigators concluded that hospital readmissions related to opportunistic infections were relatively common among HSCT recipients. So strategies that minimize the risks of these infections might have significant clinical and economic advantages. ![]()

Photo courtesy of the CDC
SAN DIEGO—A retrospective study has provided insight into hospital readmissions related to opportunistic infection following hematopoietic stem cell transplant (HSCT).
Of the roughly 4200 HSCT recipients studied, 26% were readmitted to the hospital due to opportunistic infection.
About 1 in 3 infection-related readmissions were due to double-stranded DNA (dsDNA) viral infections, and cytomegalovirus (CMV) infections were the most common.
Nearly half of the dsDNA viral infections occurred within the first month of HSCT discharge.
These findings were presented at ICAAC/ICC 2015 (poster T-1360). The study was sponsored by Chimerix, Inc.
Investigators searched the Premier hospital database for patients who underwent HSCT between January 2009 and September 2013. The team identified 4393 patients with a mean age of 50.4 years. Most were adults (91.2%), most were male (57.9%), and most received an autologous HSCT (63.2%).
About 42% (n=1841) of patients had a diagnostic code for opportunistic infection in their HSCT discharge records. Overall, 7.3% (n=319) of patients had dsDNA virus infections, including 13.4% (n=216) of patients who received an allogeneic HSCT.
One hundred and fifty-seven patients died during HSCT hospitalization, leaving 4236 patients evaluable for readmission analysis.
In all, 37.7% (n=1595) of the surviving patients were readmitted to the hospital for any reason during the 12 months after HSCT discharge. And 65.6% of the readmissions occurred within the first 3 months of HSCT discharge.
Readmissions were most frequently related to opportunistic infections (25.8%, n=1091), followed by graft-versus-host disease (13.7%, n=579), renal impairment (11.1%, n=470), and neutropenia (10.0%, n=422).
The investigators noted that patients may have had multiple readmissions or readmission with multiple diagnoses.
Of the hospital readmissions related to opportunistic infections, 32.0% (n=349) were related to dsDNA virus infections. This included CMV (65.9%, n=230), BK virus (13.8%, n=48), adenovirus (5.2%, n=18), and other dsDNA virus infections (32.7%, n=114).
Patients may have experienced more than one viral infection, so the number of hospital readmissions related to each dsDNA virus was not mutually exclusive.
Readmission within the first month of HSCT discharge occurred in 41.8% of patients with any dsDNA virus infection, 49.6% with CMV infection, and 56.3% with BK virus infection. More than half (55.6%) of readmissions related to adenovirus infection occurred within the first 3 months of HSCT discharge.
Taking these results together, the investigators concluded that hospital readmissions related to opportunistic infections were relatively common among HSCT recipients. So strategies that minimize the risks of these infections might have significant clinical and economic advantages. ![]()
Diabetic foot ulcer: Early closure post debridement best
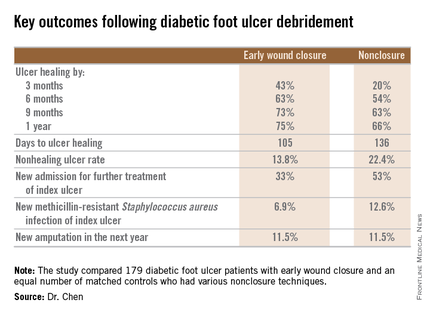
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
AT ICAAC 2015
Key clinical point: Diabetic foot ulcers are more likely to heal with early wound closure following surgical debridement than with nonclosure techniques.
Major finding: Healing of diabetic foot ulcers after surgical debridement took an average of 105 days in patients who underwent early wound closure prior to hospital discharge, compared with 136 days in those whose wounds were managed with nonclosure techniques.
Data source: A retrospective, nonrandomized study featuring two propensity score–matched groups, with 179 patients in each, who were followed for 1 year post discharge for surgical debridement of a diabetic foot ulcer.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
Septicemia due to ‘ESKAPE’ pathogens rose between 2008 and 2012
SAN DIEGO – The incidence of septicemia due to antibiotic-resistant bacteria (referred to as the ESKAPE pathogens) increased in United States hospitals between 2008 and 2012, yet length of stay and in-hospital mortality both decreased over the same time period.
“We don’t know what’s going on here,” lead study author Dana R. Bowers, Pharm.D., said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “No new drugs [for septicemia] came out during that time frame. Maybe we’re getting better at identifying these patients and treating them earlier; maybe that’s impacting mortality.”
According to the Infectious Diseases Society of America, ESKAPE pathogens include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. “Septicemia caused by these pathogens is particularly concerning because of their propensity for antimicrobial resistance, limited treatment options, and elevated risk of death,” the researchers wrote in their abstract. “However, the burden of ESKAPE septicemia in the United States is relatively unknown.”
In an effort to provide national estimates for ESKAPE septicemia incidence over 5 years and to identify trends related to in-hospital mortality and hospital length of stay, Dr. Bowers, a clinical specialist in infectious diseases at Kingman (Ariz.) Regional Medical Center and her associates retrospectively evaluated the Nationwide Inpatient Sample between 2008 and 2012.
They used clinical classification software to identify patients with septicemia who were at least 18 years of age, and followed the International Classification of Diseases, Ninth Revision, Clinical Modification to identify codes for E. faecium [EF], Staphylococcus aureus [MRSA], Klebsiella pneumonia [KP], Acinetobacter baumannii [AB], Pseudomonas aeruginosa [PA] and Enterobacter spp. [EB].
In all, 7,668,636 patients acquired septicemia during the study period. Of these, 951,677 acquired septicemia due to ESKAPE pathogens. Patients in this cohort had a median age of 67 years, 47% were women, and 66% were white.
The researchers observed that the incidence for septicemia caused by ESKAPE pathogens increased over the time period, especially for MRSA (from 3.8 per 10,000 hospital discharges in 2008 to 14.6 per 10,000 discharges in 2012) and EF (from 6.2 per 10,000 discharges in 2008 to 10.4 per 10,000 discharges in 2012). They also observed a slight decline in median LOS for patients with MRSA (from 11 days in 2008 to 10 days in 2012), PA (from 10 days in 2008 to 9 days in 2012), as well as among those with KP/AB/EB (from 9 days in 2008 to 7 days in 2012).
In-hospital mortality declined over the study period among all pathogen groups and was greatest for those with KP/AB/EB septicemia (from 16.3% in 2008 to 12.1% in 2012).
The researchers concluded that additional research is needed to assess the clinical impact of these findings at the population level. They reported having no financial disclosures.
SAN DIEGO – The incidence of septicemia due to antibiotic-resistant bacteria (referred to as the ESKAPE pathogens) increased in United States hospitals between 2008 and 2012, yet length of stay and in-hospital mortality both decreased over the same time period.
“We don’t know what’s going on here,” lead study author Dana R. Bowers, Pharm.D., said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “No new drugs [for septicemia] came out during that time frame. Maybe we’re getting better at identifying these patients and treating them earlier; maybe that’s impacting mortality.”
According to the Infectious Diseases Society of America, ESKAPE pathogens include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. “Septicemia caused by these pathogens is particularly concerning because of their propensity for antimicrobial resistance, limited treatment options, and elevated risk of death,” the researchers wrote in their abstract. “However, the burden of ESKAPE septicemia in the United States is relatively unknown.”
In an effort to provide national estimates for ESKAPE septicemia incidence over 5 years and to identify trends related to in-hospital mortality and hospital length of stay, Dr. Bowers, a clinical specialist in infectious diseases at Kingman (Ariz.) Regional Medical Center and her associates retrospectively evaluated the Nationwide Inpatient Sample between 2008 and 2012.
They used clinical classification software to identify patients with septicemia who were at least 18 years of age, and followed the International Classification of Diseases, Ninth Revision, Clinical Modification to identify codes for E. faecium [EF], Staphylococcus aureus [MRSA], Klebsiella pneumonia [KP], Acinetobacter baumannii [AB], Pseudomonas aeruginosa [PA] and Enterobacter spp. [EB].
In all, 7,668,636 patients acquired septicemia during the study period. Of these, 951,677 acquired septicemia due to ESKAPE pathogens. Patients in this cohort had a median age of 67 years, 47% were women, and 66% were white.
The researchers observed that the incidence for septicemia caused by ESKAPE pathogens increased over the time period, especially for MRSA (from 3.8 per 10,000 hospital discharges in 2008 to 14.6 per 10,000 discharges in 2012) and EF (from 6.2 per 10,000 discharges in 2008 to 10.4 per 10,000 discharges in 2012). They also observed a slight decline in median LOS for patients with MRSA (from 11 days in 2008 to 10 days in 2012), PA (from 10 days in 2008 to 9 days in 2012), as well as among those with KP/AB/EB (from 9 days in 2008 to 7 days in 2012).
In-hospital mortality declined over the study period among all pathogen groups and was greatest for those with KP/AB/EB septicemia (from 16.3% in 2008 to 12.1% in 2012).
The researchers concluded that additional research is needed to assess the clinical impact of these findings at the population level. They reported having no financial disclosures.
SAN DIEGO – The incidence of septicemia due to antibiotic-resistant bacteria (referred to as the ESKAPE pathogens) increased in United States hospitals between 2008 and 2012, yet length of stay and in-hospital mortality both decreased over the same time period.
“We don’t know what’s going on here,” lead study author Dana R. Bowers, Pharm.D., said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “No new drugs [for septicemia] came out during that time frame. Maybe we’re getting better at identifying these patients and treating them earlier; maybe that’s impacting mortality.”
According to the Infectious Diseases Society of America, ESKAPE pathogens include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. “Septicemia caused by these pathogens is particularly concerning because of their propensity for antimicrobial resistance, limited treatment options, and elevated risk of death,” the researchers wrote in their abstract. “However, the burden of ESKAPE septicemia in the United States is relatively unknown.”
In an effort to provide national estimates for ESKAPE septicemia incidence over 5 years and to identify trends related to in-hospital mortality and hospital length of stay, Dr. Bowers, a clinical specialist in infectious diseases at Kingman (Ariz.) Regional Medical Center and her associates retrospectively evaluated the Nationwide Inpatient Sample between 2008 and 2012.
They used clinical classification software to identify patients with septicemia who were at least 18 years of age, and followed the International Classification of Diseases, Ninth Revision, Clinical Modification to identify codes for E. faecium [EF], Staphylococcus aureus [MRSA], Klebsiella pneumonia [KP], Acinetobacter baumannii [AB], Pseudomonas aeruginosa [PA] and Enterobacter spp. [EB].
In all, 7,668,636 patients acquired septicemia during the study period. Of these, 951,677 acquired septicemia due to ESKAPE pathogens. Patients in this cohort had a median age of 67 years, 47% were women, and 66% were white.
The researchers observed that the incidence for septicemia caused by ESKAPE pathogens increased over the time period, especially for MRSA (from 3.8 per 10,000 hospital discharges in 2008 to 14.6 per 10,000 discharges in 2012) and EF (from 6.2 per 10,000 discharges in 2008 to 10.4 per 10,000 discharges in 2012). They also observed a slight decline in median LOS for patients with MRSA (from 11 days in 2008 to 10 days in 2012), PA (from 10 days in 2008 to 9 days in 2012), as well as among those with KP/AB/EB (from 9 days in 2008 to 7 days in 2012).
In-hospital mortality declined over the study period among all pathogen groups and was greatest for those with KP/AB/EB septicemia (from 16.3% in 2008 to 12.1% in 2012).
The researchers concluded that additional research is needed to assess the clinical impact of these findings at the population level. They reported having no financial disclosures.
AT ICAAC 2015
Key clinical point: The incidence of ESKAPE septicemia in U.S. hospitals increased between 2008 and 2012.
Major finding: The incidence of septicemia caused by ESKAPE pathogens increased between 2008 and 2012, especially for Methicillin-resistant Staphylococcus aureus (from 3.8 per 10,000 hospital discharges in 2008 to 14.6 per 10,000 discharges in 2012) and Enterococcus faecium (from 6.2 per 10,000 discharges in 2008 to 10.4 per 10,000 discharges in 2012).
Data source: A retrospective study of 951,677 patients who acquired septicemia due to ESKAPE pathogens.
Disclosures: The researchers reported having no financial disclosures.
Community hospitals harness rapid testing to slash bacteremia mortality
SAN DIEGO – Adoption of rapid diagnostic testing in conjunction with an antimicrobial stewardship program reduced time to initiation of optimal antibiotic therapy and markedly improved clinical outcomes in patients admitted with Gram-negative bacteremia at two Houston-area community hospitals.
Indeed, in-hospital mortality in patients with Gram-negative bacteremia plummeted from 26% immediately prior to acquisition of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectrometry, when lab testing of bloodstream organisms was done the traditional way, to just 2% post intervention, Ashley M. Lockwood, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Moreover, average overall hospital costs dropped from $18,644 to $15,234, for a savings of $3,410 per patient attributable to MALDI-TOF plus the pharmacist-led antimicrobial stewardship program, added Dr. Lockwood, who performed the before-and-after comparison study while at Houston Methodist Hospital System and is now the infectious disease pharmacist at Bayfront Health in St. Petersburg, Fla.
Prior studies have shown big benefits for MALDI-TOF and other rapid diagnostic tests in combination with antimicrobial stewardship, but all the research was done at tertiary medical centers. It was unclear whether the results were generalizable to the thousands of community hospitals across the nation. The new study removes any doubts on that score, Dr. Lockwood said in an interview.
“The use of MALDI-TOF coupled with near real-time antimicrobial stewardship should be incorporated in the community setting to improve time to therapeutic optimization in patients with Gram-negative bacteremia,” she declared.
The study compared outcomes in 149 patients with Gram-negative bacteremia admitted to the two community hospitals prior to acquisition of a shared MALDI-TOF system and 241 admitted post acquisition. Prior to MALDI-TOF, when the traditional culture and susceptibility results came back, the information would simply be entered into the electronic medical record. Post-MALDI-TOF, when the lab results get entered into the EMR, a third-party surveillance program known as Vigilanz Real-Time Surveillance notifies a clinical pharmacist, who then goes into the EMR to make sure the patient is on appropriate antibiotic therapy. If not, a call is made to the attending physician, who can either accept or reject the pharmacist’s recommended medication change.
Both pre- and post-MALDI-TOF it took about 20 hours from the time of the blood draw to a positive culture. But the time from that point to identification of the infecting pathogen dropped from 32 hours using the traditional method to 6.5 hours with MALDI-TOF. Time to results of susceptibility testing decreased from 48 to 22 hours. Time to antibiotic optimization for patients who weren’t already on effective therapy dropped from an average of 71 hours to 30 hours – and that’s what accounts for the dramatic reduction in mortality, according to Dr. Lockwood.
“With Gram-negative bacteremia, every hour counts. The mortality increases by 7.6% each hour, so I definitely think that reduction in mortality is real,” she said.
Although Dr. Lockwood and her coinvestigators expected to see a reduction in hospital length of stay post-MALDI-TOF, the average stay turned out to be 6.4 days in both groups. That’s probably because 6 days is already at the low end of the spectrum for patients admitted to a community hospital for bacteremia, she observed.
The initial investment for MALDI-TOF technology is roughly $250,000. That’s a drop in the bucket considering the reduced overall per-patient hospital cost and marked reduction in mortality, Dr. Lockwood observed.
She reported having no financial conflicts regarding her study, which was an unfunded research project.
SAN DIEGO – Adoption of rapid diagnostic testing in conjunction with an antimicrobial stewardship program reduced time to initiation of optimal antibiotic therapy and markedly improved clinical outcomes in patients admitted with Gram-negative bacteremia at two Houston-area community hospitals.
Indeed, in-hospital mortality in patients with Gram-negative bacteremia plummeted from 26% immediately prior to acquisition of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectrometry, when lab testing of bloodstream organisms was done the traditional way, to just 2% post intervention, Ashley M. Lockwood, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Moreover, average overall hospital costs dropped from $18,644 to $15,234, for a savings of $3,410 per patient attributable to MALDI-TOF plus the pharmacist-led antimicrobial stewardship program, added Dr. Lockwood, who performed the before-and-after comparison study while at Houston Methodist Hospital System and is now the infectious disease pharmacist at Bayfront Health in St. Petersburg, Fla.
Prior studies have shown big benefits for MALDI-TOF and other rapid diagnostic tests in combination with antimicrobial stewardship, but all the research was done at tertiary medical centers. It was unclear whether the results were generalizable to the thousands of community hospitals across the nation. The new study removes any doubts on that score, Dr. Lockwood said in an interview.
“The use of MALDI-TOF coupled with near real-time antimicrobial stewardship should be incorporated in the community setting to improve time to therapeutic optimization in patients with Gram-negative bacteremia,” she declared.
The study compared outcomes in 149 patients with Gram-negative bacteremia admitted to the two community hospitals prior to acquisition of a shared MALDI-TOF system and 241 admitted post acquisition. Prior to MALDI-TOF, when the traditional culture and susceptibility results came back, the information would simply be entered into the electronic medical record. Post-MALDI-TOF, when the lab results get entered into the EMR, a third-party surveillance program known as Vigilanz Real-Time Surveillance notifies a clinical pharmacist, who then goes into the EMR to make sure the patient is on appropriate antibiotic therapy. If not, a call is made to the attending physician, who can either accept or reject the pharmacist’s recommended medication change.
Both pre- and post-MALDI-TOF it took about 20 hours from the time of the blood draw to a positive culture. But the time from that point to identification of the infecting pathogen dropped from 32 hours using the traditional method to 6.5 hours with MALDI-TOF. Time to results of susceptibility testing decreased from 48 to 22 hours. Time to antibiotic optimization for patients who weren’t already on effective therapy dropped from an average of 71 hours to 30 hours – and that’s what accounts for the dramatic reduction in mortality, according to Dr. Lockwood.
“With Gram-negative bacteremia, every hour counts. The mortality increases by 7.6% each hour, so I definitely think that reduction in mortality is real,” she said.
Although Dr. Lockwood and her coinvestigators expected to see a reduction in hospital length of stay post-MALDI-TOF, the average stay turned out to be 6.4 days in both groups. That’s probably because 6 days is already at the low end of the spectrum for patients admitted to a community hospital for bacteremia, she observed.
The initial investment for MALDI-TOF technology is roughly $250,000. That’s a drop in the bucket considering the reduced overall per-patient hospital cost and marked reduction in mortality, Dr. Lockwood observed.
She reported having no financial conflicts regarding her study, which was an unfunded research project.
SAN DIEGO – Adoption of rapid diagnostic testing in conjunction with an antimicrobial stewardship program reduced time to initiation of optimal antibiotic therapy and markedly improved clinical outcomes in patients admitted with Gram-negative bacteremia at two Houston-area community hospitals.
Indeed, in-hospital mortality in patients with Gram-negative bacteremia plummeted from 26% immediately prior to acquisition of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectrometry, when lab testing of bloodstream organisms was done the traditional way, to just 2% post intervention, Ashley M. Lockwood, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Moreover, average overall hospital costs dropped from $18,644 to $15,234, for a savings of $3,410 per patient attributable to MALDI-TOF plus the pharmacist-led antimicrobial stewardship program, added Dr. Lockwood, who performed the before-and-after comparison study while at Houston Methodist Hospital System and is now the infectious disease pharmacist at Bayfront Health in St. Petersburg, Fla.
Prior studies have shown big benefits for MALDI-TOF and other rapid diagnostic tests in combination with antimicrobial stewardship, but all the research was done at tertiary medical centers. It was unclear whether the results were generalizable to the thousands of community hospitals across the nation. The new study removes any doubts on that score, Dr. Lockwood said in an interview.
“The use of MALDI-TOF coupled with near real-time antimicrobial stewardship should be incorporated in the community setting to improve time to therapeutic optimization in patients with Gram-negative bacteremia,” she declared.
The study compared outcomes in 149 patients with Gram-negative bacteremia admitted to the two community hospitals prior to acquisition of a shared MALDI-TOF system and 241 admitted post acquisition. Prior to MALDI-TOF, when the traditional culture and susceptibility results came back, the information would simply be entered into the electronic medical record. Post-MALDI-TOF, when the lab results get entered into the EMR, a third-party surveillance program known as Vigilanz Real-Time Surveillance notifies a clinical pharmacist, who then goes into the EMR to make sure the patient is on appropriate antibiotic therapy. If not, a call is made to the attending physician, who can either accept or reject the pharmacist’s recommended medication change.
Both pre- and post-MALDI-TOF it took about 20 hours from the time of the blood draw to a positive culture. But the time from that point to identification of the infecting pathogen dropped from 32 hours using the traditional method to 6.5 hours with MALDI-TOF. Time to results of susceptibility testing decreased from 48 to 22 hours. Time to antibiotic optimization for patients who weren’t already on effective therapy dropped from an average of 71 hours to 30 hours – and that’s what accounts for the dramatic reduction in mortality, according to Dr. Lockwood.
“With Gram-negative bacteremia, every hour counts. The mortality increases by 7.6% each hour, so I definitely think that reduction in mortality is real,” she said.
Although Dr. Lockwood and her coinvestigators expected to see a reduction in hospital length of stay post-MALDI-TOF, the average stay turned out to be 6.4 days in both groups. That’s probably because 6 days is already at the low end of the spectrum for patients admitted to a community hospital for bacteremia, she observed.
The initial investment for MALDI-TOF technology is roughly $250,000. That’s a drop in the bucket considering the reduced overall per-patient hospital cost and marked reduction in mortality, Dr. Lockwood observed.
She reported having no financial conflicts regarding her study, which was an unfunded research project.
AT ICAAC 2015
Key clinical point: The use of rapid diagnostic testing in community hospitals sharply cuts time to antibiotic optimization and saves lives in patients with Gram-negative bacteremia.
Major finding: Hospital mortality in patients with Gram-negative bacteremia not on active empiric therapy prior to the results of susceptibility testing dropped from 26% to 2.1% following adoption of rapid diagnostic testing plus an antimicrobial stewardship program at two community hospitals.
Data source: This case-control study included 149 patients admitted with Gram-negative bacteremia prior to the intervention and 241 others admitted afterwards.
Disclosures: The presenter reported having no financial conflicts regarding this unfunded study.
Delayed treatment associated with higher mortality from VRE bloodstream infections
SAN DIEGO – Among patients with vancomycin-resistant Enterococcus bloodstream infections, delay of treatment with linezolid or daptomycin for three days or more increased the morality risk by 39%, a nationwide retrospective cohort study demonstrated.
In addition, propensity score matching revealed that treatment with linezolid was associated with an increased mortality, compared with daptomycin.
“Based on these findings, we believe daptomycin should be the treatment of choice for patients with vancomycin-resistant Enterococcus (VRE) bacteremia and that early initiation is associated with improved survival,” lead study author Nicholas S. Britt, Pharm.D., said in an interview in advance of the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Prior to the current study, linezolid and daptomycin were considered equivalent in terms of clinical outcomes for vancomycin-resistant Enterococcal (VRE) bacteremia, said Dr. Britt, of Barnes-Jewish Hospital/Washington University Medical Center, St. Louis, Mo. “However, the studies that had compared these agents were small and may not have had enough patients enrolled to find an outcomes difference between the two agents if one existed.”
In an effort to evaluate the effect of time to treatment with linezolid or daptomycin on 30-day mortality and to compare clinical outcomes between linezolid and daptomycin while accounting for time to treatment and potential treatment selection bias, the researchers conducted a nationwide retrospective cohort study of hospitalized Veterans Affairs patients treated with linezolid or daptomycin for VRE BSI between 2004 and 2014. They included all adult patients with at least one blood culture positive for VRE who were treated with at least one dose of linezolid or daptomycin. Classification and regression tree (CART) analysis was performed to identify the most significant time to treatment breakpoint for the primary outcome, which was 30-day mortality. To account for treatment selection bias, propensity scores were derived and 1:1 matching was performed.
A total of 851 patients were evaluated: 426 in the linezolid group and 425 in the daptomycin group. CART analysis demonstrated a significant time to treatment breakpoint for 30-day mortality of 80 hours or more (RR 1.39; P less than .001). After propensity score matching, treatment with linezolid was significantly associated with 30-day mortality (RR 1.16; P =.018). This association persisted in propensity score adjusted binomial regression (adjusted RR, 1.25; P =.014) and Cox regression (hazard ratio 1.73; P less than .001). There was no difference between the two agents in terms of adverse effects.
“In this evaluation, we found that every hour delay in initiation of anti-VRE therapy was associated with an increased risk of death,” Dr. Britt said. “Delays in therapy of greater than 3 days were associated with the greatest risk of mortality, but overall, the sooner anti-VRE therapy was initiated, the better the patients did.” He went on to note that some debate exists among clinicians “as far as the virulence of VRE and the true impact of VRE bacteremia on patient outcomes. We were surprised that the time to treatment effect was so pronounced and that this effect persisted independent of other patient factors.”
Dr. Britt acknowledged certain limitations of the study, including the potential for bias due to its retrospective design. “However, attempts at clinical trials comparing these two agents have failed in the past due to poor enrollment,” he said. “Other potential limitations are with regard to generalizability to non-VA patient populations. This study featured a relatively low number of stem cell and solid organ transplant recipients and further research in these populations are warranted.”
The study was funded in part by the National Institutes of Health and the Department of Veterans Affairs. The researchers reported having no financial disclosures.
SAN DIEGO – Among patients with vancomycin-resistant Enterococcus bloodstream infections, delay of treatment with linezolid or daptomycin for three days or more increased the morality risk by 39%, a nationwide retrospective cohort study demonstrated.
In addition, propensity score matching revealed that treatment with linezolid was associated with an increased mortality, compared with daptomycin.
“Based on these findings, we believe daptomycin should be the treatment of choice for patients with vancomycin-resistant Enterococcus (VRE) bacteremia and that early initiation is associated with improved survival,” lead study author Nicholas S. Britt, Pharm.D., said in an interview in advance of the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Prior to the current study, linezolid and daptomycin were considered equivalent in terms of clinical outcomes for vancomycin-resistant Enterococcal (VRE) bacteremia, said Dr. Britt, of Barnes-Jewish Hospital/Washington University Medical Center, St. Louis, Mo. “However, the studies that had compared these agents were small and may not have had enough patients enrolled to find an outcomes difference between the two agents if one existed.”
In an effort to evaluate the effect of time to treatment with linezolid or daptomycin on 30-day mortality and to compare clinical outcomes between linezolid and daptomycin while accounting for time to treatment and potential treatment selection bias, the researchers conducted a nationwide retrospective cohort study of hospitalized Veterans Affairs patients treated with linezolid or daptomycin for VRE BSI between 2004 and 2014. They included all adult patients with at least one blood culture positive for VRE who were treated with at least one dose of linezolid or daptomycin. Classification and regression tree (CART) analysis was performed to identify the most significant time to treatment breakpoint for the primary outcome, which was 30-day mortality. To account for treatment selection bias, propensity scores were derived and 1:1 matching was performed.
A total of 851 patients were evaluated: 426 in the linezolid group and 425 in the daptomycin group. CART analysis demonstrated a significant time to treatment breakpoint for 30-day mortality of 80 hours or more (RR 1.39; P less than .001). After propensity score matching, treatment with linezolid was significantly associated with 30-day mortality (RR 1.16; P =.018). This association persisted in propensity score adjusted binomial regression (adjusted RR, 1.25; P =.014) and Cox regression (hazard ratio 1.73; P less than .001). There was no difference between the two agents in terms of adverse effects.
“In this evaluation, we found that every hour delay in initiation of anti-VRE therapy was associated with an increased risk of death,” Dr. Britt said. “Delays in therapy of greater than 3 days were associated with the greatest risk of mortality, but overall, the sooner anti-VRE therapy was initiated, the better the patients did.” He went on to note that some debate exists among clinicians “as far as the virulence of VRE and the true impact of VRE bacteremia on patient outcomes. We were surprised that the time to treatment effect was so pronounced and that this effect persisted independent of other patient factors.”
Dr. Britt acknowledged certain limitations of the study, including the potential for bias due to its retrospective design. “However, attempts at clinical trials comparing these two agents have failed in the past due to poor enrollment,” he said. “Other potential limitations are with regard to generalizability to non-VA patient populations. This study featured a relatively low number of stem cell and solid organ transplant recipients and further research in these populations are warranted.”
The study was funded in part by the National Institutes of Health and the Department of Veterans Affairs. The researchers reported having no financial disclosures.
SAN DIEGO – Among patients with vancomycin-resistant Enterococcus bloodstream infections, delay of treatment with linezolid or daptomycin for three days or more increased the morality risk by 39%, a nationwide retrospective cohort study demonstrated.
In addition, propensity score matching revealed that treatment with linezolid was associated with an increased mortality, compared with daptomycin.
“Based on these findings, we believe daptomycin should be the treatment of choice for patients with vancomycin-resistant Enterococcus (VRE) bacteremia and that early initiation is associated with improved survival,” lead study author Nicholas S. Britt, Pharm.D., said in an interview in advance of the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Prior to the current study, linezolid and daptomycin were considered equivalent in terms of clinical outcomes for vancomycin-resistant Enterococcal (VRE) bacteremia, said Dr. Britt, of Barnes-Jewish Hospital/Washington University Medical Center, St. Louis, Mo. “However, the studies that had compared these agents were small and may not have had enough patients enrolled to find an outcomes difference between the two agents if one existed.”
In an effort to evaluate the effect of time to treatment with linezolid or daptomycin on 30-day mortality and to compare clinical outcomes between linezolid and daptomycin while accounting for time to treatment and potential treatment selection bias, the researchers conducted a nationwide retrospective cohort study of hospitalized Veterans Affairs patients treated with linezolid or daptomycin for VRE BSI between 2004 and 2014. They included all adult patients with at least one blood culture positive for VRE who were treated with at least one dose of linezolid or daptomycin. Classification and regression tree (CART) analysis was performed to identify the most significant time to treatment breakpoint for the primary outcome, which was 30-day mortality. To account for treatment selection bias, propensity scores were derived and 1:1 matching was performed.
A total of 851 patients were evaluated: 426 in the linezolid group and 425 in the daptomycin group. CART analysis demonstrated a significant time to treatment breakpoint for 30-day mortality of 80 hours or more (RR 1.39; P less than .001). After propensity score matching, treatment with linezolid was significantly associated with 30-day mortality (RR 1.16; P =.018). This association persisted in propensity score adjusted binomial regression (adjusted RR, 1.25; P =.014) and Cox regression (hazard ratio 1.73; P less than .001). There was no difference between the two agents in terms of adverse effects.
“In this evaluation, we found that every hour delay in initiation of anti-VRE therapy was associated with an increased risk of death,” Dr. Britt said. “Delays in therapy of greater than 3 days were associated with the greatest risk of mortality, but overall, the sooner anti-VRE therapy was initiated, the better the patients did.” He went on to note that some debate exists among clinicians “as far as the virulence of VRE and the true impact of VRE bacteremia on patient outcomes. We were surprised that the time to treatment effect was so pronounced and that this effect persisted independent of other patient factors.”
Dr. Britt acknowledged certain limitations of the study, including the potential for bias due to its retrospective design. “However, attempts at clinical trials comparing these two agents have failed in the past due to poor enrollment,” he said. “Other potential limitations are with regard to generalizability to non-VA patient populations. This study featured a relatively low number of stem cell and solid organ transplant recipients and further research in these populations are warranted.”
The study was funded in part by the National Institutes of Health and the Department of Veterans Affairs. The researchers reported having no financial disclosures.
AT ICAAC 2015
Key clinical point: Among patients with vancomycin-resistant Enterococcus bloodstream infections, early initiation of treatment with linezolid or daptomycin is associated with improved survival, especially with daptomycin.
Major finding: The study demonstrated a significant time to treatment breakpoint for 30-day mortality of 80 hours or more.
Data source: A retrospective study of 851 hospitalized Veterans Affairs patients treated with linezolid or daptomycin for Vancomycin-resistant Enterococcus bloodstream infections between 2004 and 2014.
Disclosures: The study was funded in part by the National Institutes of Health and the Department of Veterans Affairs. The researchers reported having no financial disclosures.