User login
Endocrine Society: Annual Meeting (ENDO 2014)
Testosterone replacement enhances aerobic capacity in mobility-limited men
CHICAGO – Testosterone replacement therapy in mobility-impaired, sedentary older men with low testosterone levels improved two different measures of aerobic capacity in a placebo-controlled, randomized 6-month clinical trial.
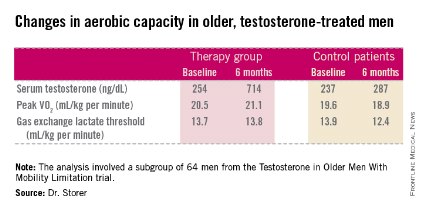
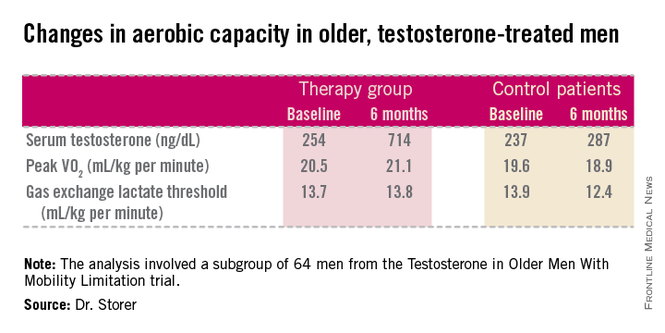
The age-related decline in peak oxygen uptake during exercise, or peak VO2, was 3.4-fold less in the testosterone-treated men than would be expected based on published population norms. Moreover, the rate of decline in peak VO2 in placebo-treated controls was nearly twice the expected rate for the age-matched general population; this accelerated decline was probably due to their limited mobility and low testosterone levels, Thomas W. Storer, Ph.D., said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"This study is the first to show enhanced endurance performance as a result of testosterone therapy in men who have difficulty performing some physical tasks but are otherwise healthy. This is something we think is going to be clinically meaningful," said Dr. Storer, director of the exercise physiology and physical performance laboratory at Brigham and Women’s Hospital, Boston.
He presented an analysis of a subset of participants in the prospective, randomized Testosterone in Older Men With Mobility Limitation (TOM) trial, in which subjects with low total or free testosterone levels were placed on 10 mg/day of testosterone gel or placebo gel for 6 months. The subgroup consisted of 64 men, mean age 73, who underwent formal testing of aerobic capacity via measurement of changes over time in peak VO2 and gas exchange lactate threshold during symptom-limited exercise cycling.
The gas exchange lactate threshold is a good functional measure of the ability to do work over a prolonged period. The rate remained steady during the 6-month study in the testosterone-treated men but declined significantly – and to a greater-than-expected extent based on normative values – in the placebo-treated controls (see chart).
"We think the mechanisms involved in this benefit are many," Dr. Storer said in an interview. Among them are testosterone’s demonstrated ability to increase muscle mass and thereby generate more force during exercise; increased RBC formation; stimulation of tissue capillarity in order to allow more blood flow to the exercising muscle; and stimulation of mitochondrial biogenesis, which increases oxygen uptake by muscle tissue.
However, he added that although these results are quite promising, he doesn’t think this work is ready for prime time application in daily clinical practice. He plans to further evaluate the safety of this treatment and the durability of the effects in a study with larger patient numbers and longer treatment.
The study was funded by the National Institute on Aging, the Claude D. Pepper Older Americans Independence Center, and Boston University. Dr. Storer reported having no relevant financial conflicts.
CHICAGO – Testosterone replacement therapy in mobility-impaired, sedentary older men with low testosterone levels improved two different measures of aerobic capacity in a placebo-controlled, randomized 6-month clinical trial.
The age-related decline in peak oxygen uptake during exercise, or peak VO2, was 3.4-fold less in the testosterone-treated men than would be expected based on published population norms. Moreover, the rate of decline in peak VO2 in placebo-treated controls was nearly twice the expected rate for the age-matched general population; this accelerated decline was probably due to their limited mobility and low testosterone levels, Thomas W. Storer, Ph.D., said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"This study is the first to show enhanced endurance performance as a result of testosterone therapy in men who have difficulty performing some physical tasks but are otherwise healthy. This is something we think is going to be clinically meaningful," said Dr. Storer, director of the exercise physiology and physical performance laboratory at Brigham and Women’s Hospital, Boston.
He presented an analysis of a subset of participants in the prospective, randomized Testosterone in Older Men With Mobility Limitation (TOM) trial, in which subjects with low total or free testosterone levels were placed on 10 mg/day of testosterone gel or placebo gel for 6 months. The subgroup consisted of 64 men, mean age 73, who underwent formal testing of aerobic capacity via measurement of changes over time in peak VO2 and gas exchange lactate threshold during symptom-limited exercise cycling.
The gas exchange lactate threshold is a good functional measure of the ability to do work over a prolonged period. The rate remained steady during the 6-month study in the testosterone-treated men but declined significantly – and to a greater-than-expected extent based on normative values – in the placebo-treated controls (see chart).
"We think the mechanisms involved in this benefit are many," Dr. Storer said in an interview. Among them are testosterone’s demonstrated ability to increase muscle mass and thereby generate more force during exercise; increased RBC formation; stimulation of tissue capillarity in order to allow more blood flow to the exercising muscle; and stimulation of mitochondrial biogenesis, which increases oxygen uptake by muscle tissue.
However, he added that although these results are quite promising, he doesn’t think this work is ready for prime time application in daily clinical practice. He plans to further evaluate the safety of this treatment and the durability of the effects in a study with larger patient numbers and longer treatment.
The study was funded by the National Institute on Aging, the Claude D. Pepper Older Americans Independence Center, and Boston University. Dr. Storer reported having no relevant financial conflicts.
CHICAGO – Testosterone replacement therapy in mobility-impaired, sedentary older men with low testosterone levels improved two different measures of aerobic capacity in a placebo-controlled, randomized 6-month clinical trial.
The age-related decline in peak oxygen uptake during exercise, or peak VO2, was 3.4-fold less in the testosterone-treated men than would be expected based on published population norms. Moreover, the rate of decline in peak VO2 in placebo-treated controls was nearly twice the expected rate for the age-matched general population; this accelerated decline was probably due to their limited mobility and low testosterone levels, Thomas W. Storer, Ph.D., said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"This study is the first to show enhanced endurance performance as a result of testosterone therapy in men who have difficulty performing some physical tasks but are otherwise healthy. This is something we think is going to be clinically meaningful," said Dr. Storer, director of the exercise physiology and physical performance laboratory at Brigham and Women’s Hospital, Boston.
He presented an analysis of a subset of participants in the prospective, randomized Testosterone in Older Men With Mobility Limitation (TOM) trial, in which subjects with low total or free testosterone levels were placed on 10 mg/day of testosterone gel or placebo gel for 6 months. The subgroup consisted of 64 men, mean age 73, who underwent formal testing of aerobic capacity via measurement of changes over time in peak VO2 and gas exchange lactate threshold during symptom-limited exercise cycling.
The gas exchange lactate threshold is a good functional measure of the ability to do work over a prolonged period. The rate remained steady during the 6-month study in the testosterone-treated men but declined significantly – and to a greater-than-expected extent based on normative values – in the placebo-treated controls (see chart).
"We think the mechanisms involved in this benefit are many," Dr. Storer said in an interview. Among them are testosterone’s demonstrated ability to increase muscle mass and thereby generate more force during exercise; increased RBC formation; stimulation of tissue capillarity in order to allow more blood flow to the exercising muscle; and stimulation of mitochondrial biogenesis, which increases oxygen uptake by muscle tissue.
However, he added that although these results are quite promising, he doesn’t think this work is ready for prime time application in daily clinical practice. He plans to further evaluate the safety of this treatment and the durability of the effects in a study with larger patient numbers and longer treatment.
The study was funded by the National Institute on Aging, the Claude D. Pepper Older Americans Independence Center, and Boston University. Dr. Storer reported having no relevant financial conflicts.
AT ICE/ENDO 2014
Key clinical point: Testosterone replacement therapy neutralizes the age-related decline in aerobic capacity in older men with low testosterone and mobility limitation.
Major finding: The age-related decline in peak VO2 in testosterone takers was 3.4-fold less than expected based on age-related norms.
Data source: A subanalysis of data from the randomized, prospective TOM trial, in which participants with low testosterone were randomized to 10 mg/day of testosterone gel or placebo for 6 months.
Disclosures: TOM was funded by the National Institute on Aging, the Claude D. Pepper Older Americans Independence Center, and Boston University.
Mifepristone for Cushing’s brings sustained weight loss
CHICAGO – Mifepristone therapy for Cushing’s syndrome provides an important side benefit: clinically meaningful weight loss that persists over time.
That’s a key finding from the long-term extension phase of the SEISMIC study, a 24-week, multicenter study that led to Food and Drug Administration approval of mifepristone (Korlym) in 2012 as the first and only medication indicated for the treatment of endogenous Cushing’s syndrome.
In the long-term extension phase of the SEISMIC study, mifepristone therapy for up to 3.5 years was associated with a mean 9.3% weight loss from baseline, Dr. Henry G. Fein reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Of patients who lost at least 5% of their body weight during the initial 24-week treatment period in the SEISMIC study, 83% maintained that amount of weight loss through the long-term extension phase. A sustained weight loss of that scope is likely to translate into a reduced risk of cardiovascular disease and metabolic dysfunction, said Dr. Fein of Johns Hopkins University, Baltimore.
Endogenous Cushing’s syndrome is a rare disease, and the phase III SEISMIC study (J. Clin. Endocrinol. Metab. 2012;97:2039-49), while pivotal, was small, involving 50 patients treated with the glucocorticoid receptor antagonist at 300-1,200 mg once daily for 24 weeks. Afterward, 29 patients underwent a 6-week period off drug for safety assessment and then went back on mifepristone for a median of 29.2 months and a maximum of 3.5 years in the extension study.
At baseline, the mean body weight in these 29 patients was 105.4 kg. It dropped to 97.2 kg by week 24. Six weeks later, when patients went back on mifepristone, their mean weight was 98.6 kg. At last follow-up – with patients still on the drug – their mean weight was 95.1 kg, a 9.3% decrease from baseline.
Eighteen of 29 patients achieved a 5% or better weight loss by week 24; at the most recent follow-up, 15 of the 18 (83%) maintained or improved upon that degree of weight loss. Moreover, of the 10 patients who lost at least 10% of their body weight during the first 24 weeks of treatment, 8 maintained that amount of weight loss by study’s end.
Surgery is accepted as the treatment of choice for most patients with Cushing’s syndrome. However, various studies have shown that 10%-45% of surgically treated patients have persistent or recurrent hypercortisolism postoperatively, with accompanying weight gain. That’s where mifepristone plays a key role. In addition, the drug is valuable in patients who aren’t surgical candidates, the endocrinologist noted.
Mifepristone was formerly known as RU-486, "the abortion pill."
The SEISMIC study and its long-term extension were funded by Corcept Therapeutics. Dr. Fein is on the company’s speakers bureau.
CHICAGO – Mifepristone therapy for Cushing’s syndrome provides an important side benefit: clinically meaningful weight loss that persists over time.
That’s a key finding from the long-term extension phase of the SEISMIC study, a 24-week, multicenter study that led to Food and Drug Administration approval of mifepristone (Korlym) in 2012 as the first and only medication indicated for the treatment of endogenous Cushing’s syndrome.
In the long-term extension phase of the SEISMIC study, mifepristone therapy for up to 3.5 years was associated with a mean 9.3% weight loss from baseline, Dr. Henry G. Fein reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Of patients who lost at least 5% of their body weight during the initial 24-week treatment period in the SEISMIC study, 83% maintained that amount of weight loss through the long-term extension phase. A sustained weight loss of that scope is likely to translate into a reduced risk of cardiovascular disease and metabolic dysfunction, said Dr. Fein of Johns Hopkins University, Baltimore.
Endogenous Cushing’s syndrome is a rare disease, and the phase III SEISMIC study (J. Clin. Endocrinol. Metab. 2012;97:2039-49), while pivotal, was small, involving 50 patients treated with the glucocorticoid receptor antagonist at 300-1,200 mg once daily for 24 weeks. Afterward, 29 patients underwent a 6-week period off drug for safety assessment and then went back on mifepristone for a median of 29.2 months and a maximum of 3.5 years in the extension study.
At baseline, the mean body weight in these 29 patients was 105.4 kg. It dropped to 97.2 kg by week 24. Six weeks later, when patients went back on mifepristone, their mean weight was 98.6 kg. At last follow-up – with patients still on the drug – their mean weight was 95.1 kg, a 9.3% decrease from baseline.
Eighteen of 29 patients achieved a 5% or better weight loss by week 24; at the most recent follow-up, 15 of the 18 (83%) maintained or improved upon that degree of weight loss. Moreover, of the 10 patients who lost at least 10% of their body weight during the first 24 weeks of treatment, 8 maintained that amount of weight loss by study’s end.
Surgery is accepted as the treatment of choice for most patients with Cushing’s syndrome. However, various studies have shown that 10%-45% of surgically treated patients have persistent or recurrent hypercortisolism postoperatively, with accompanying weight gain. That’s where mifepristone plays a key role. In addition, the drug is valuable in patients who aren’t surgical candidates, the endocrinologist noted.
Mifepristone was formerly known as RU-486, "the abortion pill."
The SEISMIC study and its long-term extension were funded by Corcept Therapeutics. Dr. Fein is on the company’s speakers bureau.
CHICAGO – Mifepristone therapy for Cushing’s syndrome provides an important side benefit: clinically meaningful weight loss that persists over time.
That’s a key finding from the long-term extension phase of the SEISMIC study, a 24-week, multicenter study that led to Food and Drug Administration approval of mifepristone (Korlym) in 2012 as the first and only medication indicated for the treatment of endogenous Cushing’s syndrome.
In the long-term extension phase of the SEISMIC study, mifepristone therapy for up to 3.5 years was associated with a mean 9.3% weight loss from baseline, Dr. Henry G. Fein reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Of patients who lost at least 5% of their body weight during the initial 24-week treatment period in the SEISMIC study, 83% maintained that amount of weight loss through the long-term extension phase. A sustained weight loss of that scope is likely to translate into a reduced risk of cardiovascular disease and metabolic dysfunction, said Dr. Fein of Johns Hopkins University, Baltimore.
Endogenous Cushing’s syndrome is a rare disease, and the phase III SEISMIC study (J. Clin. Endocrinol. Metab. 2012;97:2039-49), while pivotal, was small, involving 50 patients treated with the glucocorticoid receptor antagonist at 300-1,200 mg once daily for 24 weeks. Afterward, 29 patients underwent a 6-week period off drug for safety assessment and then went back on mifepristone for a median of 29.2 months and a maximum of 3.5 years in the extension study.
At baseline, the mean body weight in these 29 patients was 105.4 kg. It dropped to 97.2 kg by week 24. Six weeks later, when patients went back on mifepristone, their mean weight was 98.6 kg. At last follow-up – with patients still on the drug – their mean weight was 95.1 kg, a 9.3% decrease from baseline.
Eighteen of 29 patients achieved a 5% or better weight loss by week 24; at the most recent follow-up, 15 of the 18 (83%) maintained or improved upon that degree of weight loss. Moreover, of the 10 patients who lost at least 10% of their body weight during the first 24 weeks of treatment, 8 maintained that amount of weight loss by study’s end.
Surgery is accepted as the treatment of choice for most patients with Cushing’s syndrome. However, various studies have shown that 10%-45% of surgically treated patients have persistent or recurrent hypercortisolism postoperatively, with accompanying weight gain. That’s where mifepristone plays a key role. In addition, the drug is valuable in patients who aren’t surgical candidates, the endocrinologist noted.
Mifepristone was formerly known as RU-486, "the abortion pill."
The SEISMIC study and its long-term extension were funded by Corcept Therapeutics. Dr. Fein is on the company’s speakers bureau.
AT ICE/ENDO 2014
Key clinical point: Treatment of Cushing’s syndrome with the glucocorticoid receptor antagonist mifepristone results in sustained significant weight loss, with its attendant cardiometabolic benefits.
Major finding: More than 80% of patients with Cushing’s syndrome who lost at least 5% of their initial body weight during 24 weeks of mifepristone therapy maintained that degree of weight loss for up to 3.5 years.
Data source: The long-term extension study of SEISMIC, an open-label, multicenter study involving 50 mifepristone-treated patients with Cushing’s syndrome.
Disclosures: The study was funded by Corcept Therapeutics, and its presenter is a member of the company’s speakers bureau.
New Clinical Practice Guidelines on Pheochromocytomas
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
AT ICE/ENDO 2014
A third of follicular thyroid lesions of undetermined significance were malignant
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
AT ICE/ENDO 2014
Key clinical point: National estimates of FLUS malignancy may not apply to your institution.
Major finding: Among 80 patients diagnosed with FLUS who opted for surgery, the lesion turned out to be differentiated thyroid cancer in 27 (34%).
Data source: Retrospective study of outcomes for 134 FLUS nodules.
Disclosures: The investigators had no disclosures and had no outside funding for their study.
Aspirin’s benefits may be blunted in black women
CHICAGO – Postmenopausal African American women with subclinical atherosclerosis appear to be more resistant to the anti-inflammatory effects of daily aspirin than their white counterparts.
In a 6-month, double-blind, placebo-controlled pilot study, daily aspirin at 325 mg showed essentially no impact on high-sensitivity C-reactive protein (hsCRP ) levels in the African American women. Moreover, their levels of interleukin-6 (IL-6) shot up while on aspirin. In contrast, levels of both proinflammatory markers declined markedly with aspirin therapy in the white women, Dr. Nora Alghothani reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"Given apparent ethnic differences in response to aspirin-mediated anti-inflammatory benefits, perhaps a higher dose of aspirin may be required in African American women already at higher risk of inflammatory disease processes in order to reduce cardiovascular disease outcomes and lessen disparities," concluded Dr. Alghothani, of the department of endocrinology at the Ohio State University in Columbus.
This remark lit a four-alarm fire among audience members. They were quick to emphasize that aspirin at doses greater than 325 mg/day is associated with a sharply increased risk of bleeding and should thus not be considered as part of an individualized cardioprevention strategy for African American women unless and until there is solid evidence that the benefits outweigh the risks.
Dr. Alghothani concurred that a large-scale dose-response study is needed. In the meantime, though, the take home message of her pilot study is that physicians should not necessarily expect the same robust cardiovascular benefits with daily aspirin in their postmenopausal African American patients as in other populations, she added.
The pilot study included 42 postmenopausal, nondiabetic women with evidence of subclinical atherosclerosis based upon carotid intimal medial thickness measurements. Half were African American; half were white. Participants in each group were randomized in double-blind fashion to 6 months of aspirin at 325 mg/day or placebo, with fasting blood samples and anthropomorphic measurements obtained at baseline and 6 months. Consistent with findings from much larger studies, the African American women were heavier, with a mean body mass index of 32.8 kg/m2, compared with 27.8 kg/m2 for the white women. The African Americans also had significantly lower triglycerides and higher apolopoprotein A-I levels; however, the two groups didn’t differ in terms of fasting insulin or glucose, high-density lipoprotein, low-density lipoprotein, or blood pressure.
In the aspirin-treated African American women, levels of hsCRP remained static over time, going from a mean of 4.53 mg/L at baseline to 4.62 mg/L at 6 months. In placebo-treated African American women, however, hsCRP jumped from 3.34 mg/L at baseline to 8.36 mg/L at follow-up.
The mean hsCRP in white women on aspirin dropped from 2.13 to 1.6 mg/L over the course of 6 months, while with placebo it went from 2.19 to 2.69 mg/L.
Levels of IL-6 in aspirin-treated African American women climbed from 0.93 pg/mL at baseline to 2.56 pg/mL at 6 months. In contrast, mean IL-6 levels in white women on daily aspirin fell from 2.69 to 1.39 pg/mL. White women on placebo experienced a rise in IL-6 from 0.58 to 2.97 pg/mL.
Most of these differences didn’t achieve statistical significance because of the small sample size, but the consistent trends suggest an overall blunted response to the anti-inflammatory effects of aspirin among African Americans, according to Dr. Alghothani. She added that these findings might help explain the well-documented ethnic disparities in cardiovascular outcomes, whereby African American women have a significantly higher cardiovascular mortality rate than white women despite on average having higher HDL and lower triglycerides.
Her study was funded by the university’s Center for Women’s Health. She reported no financial conflicts.
Dr. Jennifer Cox, FCCP, comments: Although most results were not statistically significant, Dr. Alghothani presents an interesting pilot study that revealed the blunted response in anti-inflammatory markers to aspirin between African American women and white women. More studies are needed before changing prescribing practices, but it is clear that genetics, ethnicity, and drug metabolism should be on the forefront of drug research for more that just antineoplastic therapies.
Dr. Jennifer Cox, FCCP, comments: Although most results were not statistically significant, Dr. Alghothani presents an interesting pilot study that revealed the blunted response in anti-inflammatory markers to aspirin between African American women and white women. More studies are needed before changing prescribing practices, but it is clear that genetics, ethnicity, and drug metabolism should be on the forefront of drug research for more that just antineoplastic therapies.
Dr. Jennifer Cox, FCCP, comments: Although most results were not statistically significant, Dr. Alghothani presents an interesting pilot study that revealed the blunted response in anti-inflammatory markers to aspirin between African American women and white women. More studies are needed before changing prescribing practices, but it is clear that genetics, ethnicity, and drug metabolism should be on the forefront of drug research for more that just antineoplastic therapies.
CHICAGO – Postmenopausal African American women with subclinical atherosclerosis appear to be more resistant to the anti-inflammatory effects of daily aspirin than their white counterparts.
In a 6-month, double-blind, placebo-controlled pilot study, daily aspirin at 325 mg showed essentially no impact on high-sensitivity C-reactive protein (hsCRP ) levels in the African American women. Moreover, their levels of interleukin-6 (IL-6) shot up while on aspirin. In contrast, levels of both proinflammatory markers declined markedly with aspirin therapy in the white women, Dr. Nora Alghothani reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"Given apparent ethnic differences in response to aspirin-mediated anti-inflammatory benefits, perhaps a higher dose of aspirin may be required in African American women already at higher risk of inflammatory disease processes in order to reduce cardiovascular disease outcomes and lessen disparities," concluded Dr. Alghothani, of the department of endocrinology at the Ohio State University in Columbus.
This remark lit a four-alarm fire among audience members. They were quick to emphasize that aspirin at doses greater than 325 mg/day is associated with a sharply increased risk of bleeding and should thus not be considered as part of an individualized cardioprevention strategy for African American women unless and until there is solid evidence that the benefits outweigh the risks.
Dr. Alghothani concurred that a large-scale dose-response study is needed. In the meantime, though, the take home message of her pilot study is that physicians should not necessarily expect the same robust cardiovascular benefits with daily aspirin in their postmenopausal African American patients as in other populations, she added.
The pilot study included 42 postmenopausal, nondiabetic women with evidence of subclinical atherosclerosis based upon carotid intimal medial thickness measurements. Half were African American; half were white. Participants in each group were randomized in double-blind fashion to 6 months of aspirin at 325 mg/day or placebo, with fasting blood samples and anthropomorphic measurements obtained at baseline and 6 months. Consistent with findings from much larger studies, the African American women were heavier, with a mean body mass index of 32.8 kg/m2, compared with 27.8 kg/m2 for the white women. The African Americans also had significantly lower triglycerides and higher apolopoprotein A-I levels; however, the two groups didn’t differ in terms of fasting insulin or glucose, high-density lipoprotein, low-density lipoprotein, or blood pressure.
In the aspirin-treated African American women, levels of hsCRP remained static over time, going from a mean of 4.53 mg/L at baseline to 4.62 mg/L at 6 months. In placebo-treated African American women, however, hsCRP jumped from 3.34 mg/L at baseline to 8.36 mg/L at follow-up.
The mean hsCRP in white women on aspirin dropped from 2.13 to 1.6 mg/L over the course of 6 months, while with placebo it went from 2.19 to 2.69 mg/L.
Levels of IL-6 in aspirin-treated African American women climbed from 0.93 pg/mL at baseline to 2.56 pg/mL at 6 months. In contrast, mean IL-6 levels in white women on daily aspirin fell from 2.69 to 1.39 pg/mL. White women on placebo experienced a rise in IL-6 from 0.58 to 2.97 pg/mL.
Most of these differences didn’t achieve statistical significance because of the small sample size, but the consistent trends suggest an overall blunted response to the anti-inflammatory effects of aspirin among African Americans, according to Dr. Alghothani. She added that these findings might help explain the well-documented ethnic disparities in cardiovascular outcomes, whereby African American women have a significantly higher cardiovascular mortality rate than white women despite on average having higher HDL and lower triglycerides.
Her study was funded by the university’s Center for Women’s Health. She reported no financial conflicts.
CHICAGO – Postmenopausal African American women with subclinical atherosclerosis appear to be more resistant to the anti-inflammatory effects of daily aspirin than their white counterparts.
In a 6-month, double-blind, placebo-controlled pilot study, daily aspirin at 325 mg showed essentially no impact on high-sensitivity C-reactive protein (hsCRP ) levels in the African American women. Moreover, their levels of interleukin-6 (IL-6) shot up while on aspirin. In contrast, levels of both proinflammatory markers declined markedly with aspirin therapy in the white women, Dr. Nora Alghothani reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"Given apparent ethnic differences in response to aspirin-mediated anti-inflammatory benefits, perhaps a higher dose of aspirin may be required in African American women already at higher risk of inflammatory disease processes in order to reduce cardiovascular disease outcomes and lessen disparities," concluded Dr. Alghothani, of the department of endocrinology at the Ohio State University in Columbus.
This remark lit a four-alarm fire among audience members. They were quick to emphasize that aspirin at doses greater than 325 mg/day is associated with a sharply increased risk of bleeding and should thus not be considered as part of an individualized cardioprevention strategy for African American women unless and until there is solid evidence that the benefits outweigh the risks.
Dr. Alghothani concurred that a large-scale dose-response study is needed. In the meantime, though, the take home message of her pilot study is that physicians should not necessarily expect the same robust cardiovascular benefits with daily aspirin in their postmenopausal African American patients as in other populations, she added.
The pilot study included 42 postmenopausal, nondiabetic women with evidence of subclinical atherosclerosis based upon carotid intimal medial thickness measurements. Half were African American; half were white. Participants in each group were randomized in double-blind fashion to 6 months of aspirin at 325 mg/day or placebo, with fasting blood samples and anthropomorphic measurements obtained at baseline and 6 months. Consistent with findings from much larger studies, the African American women were heavier, with a mean body mass index of 32.8 kg/m2, compared with 27.8 kg/m2 for the white women. The African Americans also had significantly lower triglycerides and higher apolopoprotein A-I levels; however, the two groups didn’t differ in terms of fasting insulin or glucose, high-density lipoprotein, low-density lipoprotein, or blood pressure.
In the aspirin-treated African American women, levels of hsCRP remained static over time, going from a mean of 4.53 mg/L at baseline to 4.62 mg/L at 6 months. In placebo-treated African American women, however, hsCRP jumped from 3.34 mg/L at baseline to 8.36 mg/L at follow-up.
The mean hsCRP in white women on aspirin dropped from 2.13 to 1.6 mg/L over the course of 6 months, while with placebo it went from 2.19 to 2.69 mg/L.
Levels of IL-6 in aspirin-treated African American women climbed from 0.93 pg/mL at baseline to 2.56 pg/mL at 6 months. In contrast, mean IL-6 levels in white women on daily aspirin fell from 2.69 to 1.39 pg/mL. White women on placebo experienced a rise in IL-6 from 0.58 to 2.97 pg/mL.
Most of these differences didn’t achieve statistical significance because of the small sample size, but the consistent trends suggest an overall blunted response to the anti-inflammatory effects of aspirin among African Americans, according to Dr. Alghothani. She added that these findings might help explain the well-documented ethnic disparities in cardiovascular outcomes, whereby African American women have a significantly higher cardiovascular mortality rate than white women despite on average having higher HDL and lower triglycerides.
Her study was funded by the university’s Center for Women’s Health. She reported no financial conflicts.
Key clinical point: Postmenopausal African American women may have a blunted response to the anti-inflammatory effects of aspirin at 325 mg/day.
Major finding: Mean levels of hsCRP dropped by 25% and IL-6 decreased by 48% in white women over the course of 6 months of aspirin at 325 mg/day. In contrast, CRP remained essentially unchanged despite daily aspirin in African American women, and their IL-6 levels rose.
Data source: This randomized, double-blind, placebo-controlled pilot study included 42 postmenopausal, nondiabetic women; half were African American, half were white. All had documented subclinical atherosclerosis. Subjects received aspirin at 325 mg/day and were studied over the course of 6 months.
Disclosures: The study was supported by the Ohio State University Center for Women’s Health. The presenter reported having no financial conflicts.
Reassuring results on cardiovascular safety of DPP-4 inhibitors
CHICAGO – Treatment with a dipeptidyl peptidase-4 inhibitor in real-world community practice doesn’t increase the risk of cardiovascular events in type 2 diabetic patients; in fact, the opposite was true in a very large population-based cohort study.
The study, hailed by audience members as "a tour de force" and "a really excellent study," involved 39,769 type 2 diabetes patients who initiated treatment with a dipeptidyl peptidase-4 inhibitor (DPP-4i), either as monotherapy or, far more commonly, on top of metformin, and another 39,769 patients with type 2 diabetes on metformin who initiated combination therapy with a non–DPP-4i hypoglycemic agent. The two groups were closely matched using propensity scores that incorporated 50 variables, including comorbid conditions, medications, hemoglobin A1c, and health care utilization.
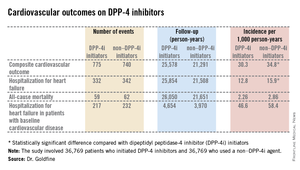
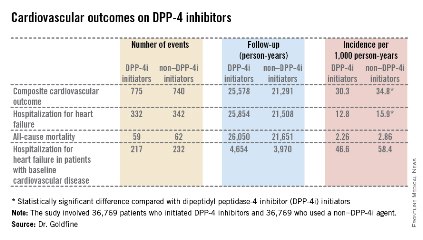
The primary endpoint was a composite cardiovascular outcome comprising acute MI, stroke, coronary revascularization, and hospitalization for heart failure. The rate was 30.3 per 1,000 person-years in patients after initiating DPP-4i therapy and 34.76 per 1,000 in controls who initiated treatment with a non–DPP-4i, for a statistically significant 13% relative risk reduction, Dr. Allison B. Goldfine reported at a joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Also noteworthy was the finding that patients who initiated treatment with a DPP-4i had a 19% lower subsequent risk of hospitalization for heart failure than did those who initiated therapy with another glucose-lowering agent (see chart). This statistically significant reduction in heart failure hospitalization was particularly reassuring. Earlier, the roughly 16,500-subject, randomized, SAVOR-TIMI 53 trial showed a significant 27% increased risk of hospitalization for heart failure in patients assigned to saxagliptin, compared with placebo-treated controls (N. Engl. J. Med. 2013;369:1317-26).
Moreover, the EXAMINE study, another randomized trial, albeit smaller, reported a nonsignificant 19% increased relative risk of hospitalization for heart failure, compared with placebo (N. Engl. J. Med. 2013;369:1327-35). These findings raised a red flag at the Food and Drug Administration, which sought additional information.
Both SAVOR-TIMI 53 and EXAMINE involved type 2 diabetes patients with known cardiovascular disease at enrollment. In the new population-based cohort study, 7,293 of the 39,769 patient pairs had baseline cardiovascular disease. Reassuringly, those with baseline cardiovascular disease had a 12% relative risk reduction for the composite cardiovascular endpoint and a 16% lower risk of hospitalization for heart failure after going on a DPP-4i, compared with controls who initiated treatment with a non–DPP-4i. Both risk reductions barely missed achieving statistical significance because of insufficient patient numbers, said Dr. Goldfine, head of the section of clinical, behavioral, and outcomes research at the Joslin Diabetes Center, Boston, and an endocrinologist at Harvard University.
To investigate the additional possibility that going on DPP-4i therapy might somehow predispose to mild heart failure not severe enough to result in hospitalization, she and her coinvestigators looked at new use of loop diuretics among matched patient pairs not on that medication class at baseline. The risk of getting a new prescription for a loop diuretic during follow-up turned out to be lower by a statistically significant 26% in DPP-4i users, compared with nonusers.
The study utilized the UnitedHealthcare insurance claims database for 2005-2012. From an initial population of 4.35 million patients with type 2 diabetes, Dr. Goldfine and her coworkers utilized propensity scores to whittle down to a study population of just less than 40,000 very closely matched patient pairs.
The great majority of DPP-4i users in the study were on sitagliptin (Januvia), the first-in-class agent to reach the market.
The study was funded by the National Institutes of Health. Dr. Goldfine reported serving as an investigator on studies funded by Daiichi Sankyo, NovoNordisk, and other companies.
CHICAGO – Treatment with a dipeptidyl peptidase-4 inhibitor in real-world community practice doesn’t increase the risk of cardiovascular events in type 2 diabetic patients; in fact, the opposite was true in a very large population-based cohort study.
The study, hailed by audience members as "a tour de force" and "a really excellent study," involved 39,769 type 2 diabetes patients who initiated treatment with a dipeptidyl peptidase-4 inhibitor (DPP-4i), either as monotherapy or, far more commonly, on top of metformin, and another 39,769 patients with type 2 diabetes on metformin who initiated combination therapy with a non–DPP-4i hypoglycemic agent. The two groups were closely matched using propensity scores that incorporated 50 variables, including comorbid conditions, medications, hemoglobin A1c, and health care utilization.

The primary endpoint was a composite cardiovascular outcome comprising acute MI, stroke, coronary revascularization, and hospitalization for heart failure. The rate was 30.3 per 1,000 person-years in patients after initiating DPP-4i therapy and 34.76 per 1,000 in controls who initiated treatment with a non–DPP-4i, for a statistically significant 13% relative risk reduction, Dr. Allison B. Goldfine reported at a joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Also noteworthy was the finding that patients who initiated treatment with a DPP-4i had a 19% lower subsequent risk of hospitalization for heart failure than did those who initiated therapy with another glucose-lowering agent (see chart). This statistically significant reduction in heart failure hospitalization was particularly reassuring. Earlier, the roughly 16,500-subject, randomized, SAVOR-TIMI 53 trial showed a significant 27% increased risk of hospitalization for heart failure in patients assigned to saxagliptin, compared with placebo-treated controls (N. Engl. J. Med. 2013;369:1317-26).
Moreover, the EXAMINE study, another randomized trial, albeit smaller, reported a nonsignificant 19% increased relative risk of hospitalization for heart failure, compared with placebo (N. Engl. J. Med. 2013;369:1327-35). These findings raised a red flag at the Food and Drug Administration, which sought additional information.
Both SAVOR-TIMI 53 and EXAMINE involved type 2 diabetes patients with known cardiovascular disease at enrollment. In the new population-based cohort study, 7,293 of the 39,769 patient pairs had baseline cardiovascular disease. Reassuringly, those with baseline cardiovascular disease had a 12% relative risk reduction for the composite cardiovascular endpoint and a 16% lower risk of hospitalization for heart failure after going on a DPP-4i, compared with controls who initiated treatment with a non–DPP-4i. Both risk reductions barely missed achieving statistical significance because of insufficient patient numbers, said Dr. Goldfine, head of the section of clinical, behavioral, and outcomes research at the Joslin Diabetes Center, Boston, and an endocrinologist at Harvard University.
To investigate the additional possibility that going on DPP-4i therapy might somehow predispose to mild heart failure not severe enough to result in hospitalization, she and her coinvestigators looked at new use of loop diuretics among matched patient pairs not on that medication class at baseline. The risk of getting a new prescription for a loop diuretic during follow-up turned out to be lower by a statistically significant 26% in DPP-4i users, compared with nonusers.
The study utilized the UnitedHealthcare insurance claims database for 2005-2012. From an initial population of 4.35 million patients with type 2 diabetes, Dr. Goldfine and her coworkers utilized propensity scores to whittle down to a study population of just less than 40,000 very closely matched patient pairs.
The great majority of DPP-4i users in the study were on sitagliptin (Januvia), the first-in-class agent to reach the market.
The study was funded by the National Institutes of Health. Dr. Goldfine reported serving as an investigator on studies funded by Daiichi Sankyo, NovoNordisk, and other companies.
CHICAGO – Treatment with a dipeptidyl peptidase-4 inhibitor in real-world community practice doesn’t increase the risk of cardiovascular events in type 2 diabetic patients; in fact, the opposite was true in a very large population-based cohort study.
The study, hailed by audience members as "a tour de force" and "a really excellent study," involved 39,769 type 2 diabetes patients who initiated treatment with a dipeptidyl peptidase-4 inhibitor (DPP-4i), either as monotherapy or, far more commonly, on top of metformin, and another 39,769 patients with type 2 diabetes on metformin who initiated combination therapy with a non–DPP-4i hypoglycemic agent. The two groups were closely matched using propensity scores that incorporated 50 variables, including comorbid conditions, medications, hemoglobin A1c, and health care utilization.

The primary endpoint was a composite cardiovascular outcome comprising acute MI, stroke, coronary revascularization, and hospitalization for heart failure. The rate was 30.3 per 1,000 person-years in patients after initiating DPP-4i therapy and 34.76 per 1,000 in controls who initiated treatment with a non–DPP-4i, for a statistically significant 13% relative risk reduction, Dr. Allison B. Goldfine reported at a joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Also noteworthy was the finding that patients who initiated treatment with a DPP-4i had a 19% lower subsequent risk of hospitalization for heart failure than did those who initiated therapy with another glucose-lowering agent (see chart). This statistically significant reduction in heart failure hospitalization was particularly reassuring. Earlier, the roughly 16,500-subject, randomized, SAVOR-TIMI 53 trial showed a significant 27% increased risk of hospitalization for heart failure in patients assigned to saxagliptin, compared with placebo-treated controls (N. Engl. J. Med. 2013;369:1317-26).
Moreover, the EXAMINE study, another randomized trial, albeit smaller, reported a nonsignificant 19% increased relative risk of hospitalization for heart failure, compared with placebo (N. Engl. J. Med. 2013;369:1327-35). These findings raised a red flag at the Food and Drug Administration, which sought additional information.
Both SAVOR-TIMI 53 and EXAMINE involved type 2 diabetes patients with known cardiovascular disease at enrollment. In the new population-based cohort study, 7,293 of the 39,769 patient pairs had baseline cardiovascular disease. Reassuringly, those with baseline cardiovascular disease had a 12% relative risk reduction for the composite cardiovascular endpoint and a 16% lower risk of hospitalization for heart failure after going on a DPP-4i, compared with controls who initiated treatment with a non–DPP-4i. Both risk reductions barely missed achieving statistical significance because of insufficient patient numbers, said Dr. Goldfine, head of the section of clinical, behavioral, and outcomes research at the Joslin Diabetes Center, Boston, and an endocrinologist at Harvard University.
To investigate the additional possibility that going on DPP-4i therapy might somehow predispose to mild heart failure not severe enough to result in hospitalization, she and her coinvestigators looked at new use of loop diuretics among matched patient pairs not on that medication class at baseline. The risk of getting a new prescription for a loop diuretic during follow-up turned out to be lower by a statistically significant 26% in DPP-4i users, compared with nonusers.
The study utilized the UnitedHealthcare insurance claims database for 2005-2012. From an initial population of 4.35 million patients with type 2 diabetes, Dr. Goldfine and her coworkers utilized propensity scores to whittle down to a study population of just less than 40,000 very closely matched patient pairs.
The great majority of DPP-4i users in the study were on sitagliptin (Januvia), the first-in-class agent to reach the market.
The study was funded by the National Institutes of Health. Dr. Goldfine reported serving as an investigator on studies funded by Daiichi Sankyo, NovoNordisk, and other companies.
AT ICE/ENDO 2014
Key clinical point: Prescribing a DPP-4 inhibitor in type 2 diabetes appears safe from a cardiovascular risk standpoint in community practice, contrary to earlier concerns.
Major finding: Patients with type 2 diabetes who initiated therapy with a DPP-4 inhibitor had a 13% reduction in the subsequent risk of a composite cardiovascular endpoint and a 19% reduction in hospitalization for heart failure, compared with closely-matched patients who initiated treatment with hypoglycemic agents from other classes.
Data source: A population-based cohort study of 39,769 pairs of adults with type 2 diabetes. Half initiated treatment with a dipeptidyl peptidase-4 inhibitor, the other half with a drug from a different class of antihyperglycemic agents.
Disclosures: The study was sponsored by the National Institutes of Health. Dr. Goldfine reported serving as an investigator on studies funded by Daiichi Sankyo, NovoNordisk, and other companies.
Etomidate drip quickly curbs severe hypercortisolism
CHICAGO – Continuous intravenous infusion of etomidate safely and swiftly gains control of severe hypercortisolism in patients with adrenocorticotropic hormone–dependent Cushing’s syndrome when conventional presurgical oral treatment is problematic.
"From our cumulative experience, we have now developed a standardized titrated etomidate infusion protocol, which should provide clinicians with a simple, safe, and effective means to lower serum cortisol in patients with severe clinical, metabolic, and neuropsychiatric consequences of prodigious hypercortisolism as a bridge to definitive medical or surgical therapy," explained Dr. Katarzyna G. Zarnecki at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Etomidate (Amidate) is a sedative hypnotic agent with an excellent cardiovascular safety profile. It is widely used in emergency settings, such as reduction of dislocated joints and cardioversion. It suppresses adrenal steroidogenesis by potently inhibiting 11-beta hydroxylase. Fortunately for endocrinologic purposes, etomidate suppresses cortisol synthesis even at subhypnotic doses. In using it off label for management of severe hypercortisolism, it’s essential to keep the drug at subhypnotic doses, meaning not more than 0.3 mg/kg per hour, emphasized Dr. Zarnecki of the University of Wisconsin, Milwaukee.
Dr. Zarnecki and her coworkers utilize as their standard etomidate infusion protocol an initial 5-mg bolus followed by an infusion at 0.02 mg/kg per hour, with dose titration in increments of 0.01-0.02 mg/kg per hour every 4-6 hours based on changes in serum cortisol level. The goal is to bring the cortisol down to a target range of 10-20 mcg/dL.
She presented an illustrative six-patient series in which she and her colleagues turned to continuous infusion of etomidate because conventional oral therapy would have taken too long to rein in the severe hypercortisolism or because medication side effects were intolerable.
Mean baseline pretreatment serum cortisol was 138 mcg/dL, with an adrenocorticotropic hormone level of 419 pg/mL. Five of the six patients reached the goal of 10-20 mcg/dL in an average time of 64 hours. The mean rate of serum cortisol reduction was 1.93 mcg/dL per hour. The average etomidate infusion rate at the time the target level was reached was 0.07 mg/kg per hour, with a maximum rate of 0.1 mg/kg per hour. Monitoring via the Richmond Agitation Sedation Scale confirmed that none of the patients experienced sedative effects.
In the sole patient who didn’t reach goal, etomidate therapy was suspended because the patient entered palliative care because of extensive tumor progression.
Dr. Zarnecki reported having no financial conflicts of interest.
CHICAGO – Continuous intravenous infusion of etomidate safely and swiftly gains control of severe hypercortisolism in patients with adrenocorticotropic hormone–dependent Cushing’s syndrome when conventional presurgical oral treatment is problematic.
"From our cumulative experience, we have now developed a standardized titrated etomidate infusion protocol, which should provide clinicians with a simple, safe, and effective means to lower serum cortisol in patients with severe clinical, metabolic, and neuropsychiatric consequences of prodigious hypercortisolism as a bridge to definitive medical or surgical therapy," explained Dr. Katarzyna G. Zarnecki at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Etomidate (Amidate) is a sedative hypnotic agent with an excellent cardiovascular safety profile. It is widely used in emergency settings, such as reduction of dislocated joints and cardioversion. It suppresses adrenal steroidogenesis by potently inhibiting 11-beta hydroxylase. Fortunately for endocrinologic purposes, etomidate suppresses cortisol synthesis even at subhypnotic doses. In using it off label for management of severe hypercortisolism, it’s essential to keep the drug at subhypnotic doses, meaning not more than 0.3 mg/kg per hour, emphasized Dr. Zarnecki of the University of Wisconsin, Milwaukee.
Dr. Zarnecki and her coworkers utilize as their standard etomidate infusion protocol an initial 5-mg bolus followed by an infusion at 0.02 mg/kg per hour, with dose titration in increments of 0.01-0.02 mg/kg per hour every 4-6 hours based on changes in serum cortisol level. The goal is to bring the cortisol down to a target range of 10-20 mcg/dL.
She presented an illustrative six-patient series in which she and her colleagues turned to continuous infusion of etomidate because conventional oral therapy would have taken too long to rein in the severe hypercortisolism or because medication side effects were intolerable.
Mean baseline pretreatment serum cortisol was 138 mcg/dL, with an adrenocorticotropic hormone level of 419 pg/mL. Five of the six patients reached the goal of 10-20 mcg/dL in an average time of 64 hours. The mean rate of serum cortisol reduction was 1.93 mcg/dL per hour. The average etomidate infusion rate at the time the target level was reached was 0.07 mg/kg per hour, with a maximum rate of 0.1 mg/kg per hour. Monitoring via the Richmond Agitation Sedation Scale confirmed that none of the patients experienced sedative effects.
In the sole patient who didn’t reach goal, etomidate therapy was suspended because the patient entered palliative care because of extensive tumor progression.
Dr. Zarnecki reported having no financial conflicts of interest.
CHICAGO – Continuous intravenous infusion of etomidate safely and swiftly gains control of severe hypercortisolism in patients with adrenocorticotropic hormone–dependent Cushing’s syndrome when conventional presurgical oral treatment is problematic.
"From our cumulative experience, we have now developed a standardized titrated etomidate infusion protocol, which should provide clinicians with a simple, safe, and effective means to lower serum cortisol in patients with severe clinical, metabolic, and neuropsychiatric consequences of prodigious hypercortisolism as a bridge to definitive medical or surgical therapy," explained Dr. Katarzyna G. Zarnecki at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Etomidate (Amidate) is a sedative hypnotic agent with an excellent cardiovascular safety profile. It is widely used in emergency settings, such as reduction of dislocated joints and cardioversion. It suppresses adrenal steroidogenesis by potently inhibiting 11-beta hydroxylase. Fortunately for endocrinologic purposes, etomidate suppresses cortisol synthesis even at subhypnotic doses. In using it off label for management of severe hypercortisolism, it’s essential to keep the drug at subhypnotic doses, meaning not more than 0.3 mg/kg per hour, emphasized Dr. Zarnecki of the University of Wisconsin, Milwaukee.
Dr. Zarnecki and her coworkers utilize as their standard etomidate infusion protocol an initial 5-mg bolus followed by an infusion at 0.02 mg/kg per hour, with dose titration in increments of 0.01-0.02 mg/kg per hour every 4-6 hours based on changes in serum cortisol level. The goal is to bring the cortisol down to a target range of 10-20 mcg/dL.
She presented an illustrative six-patient series in which she and her colleagues turned to continuous infusion of etomidate because conventional oral therapy would have taken too long to rein in the severe hypercortisolism or because medication side effects were intolerable.
Mean baseline pretreatment serum cortisol was 138 mcg/dL, with an adrenocorticotropic hormone level of 419 pg/mL. Five of the six patients reached the goal of 10-20 mcg/dL in an average time of 64 hours. The mean rate of serum cortisol reduction was 1.93 mcg/dL per hour. The average etomidate infusion rate at the time the target level was reached was 0.07 mg/kg per hour, with a maximum rate of 0.1 mg/kg per hour. Monitoring via the Richmond Agitation Sedation Scale confirmed that none of the patients experienced sedative effects.
In the sole patient who didn’t reach goal, etomidate therapy was suspended because the patient entered palliative care because of extensive tumor progression.
Dr. Zarnecki reported having no financial conflicts of interest.
AT ICE/ENDO 2014
Key clinical point: The anesthetic induction agent etomidate is a potent suppressor of cortisol synthesis in the adrenal cortex at subhypnotic doses, making it a safe and effective agent for management of severe hypercortisolism in Cushing’s syndrome.
Major finding: Continuous infusion of etomidate using a standardized protocol resulted in a reduction in serum cortisol from a mean of 138 mcg/dL to a goal range of 10-20 mcg/dL in an average of 64 hours.
Data source: This was a retrospective case series involving six patients with severe hypercortisolism caused by adrenocorticotropic hormone–dependent Cushing’s syndrome.
Disclosures: The study was carried out with institutional funds. The presenter reported having no financial conflicts.
Novel oral testosterone undecanoate under FDA review
CHICAGO – A unique investigational oral testosterone replacement product for hypogonadal men showed efficacy and safety comparable to a commercially available testosterone gel in two phase III clinical trials, which drew considerable interest when presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
This formulation of oral testosterone undecanoate, known as Rextoro, uses proprietary self-emulsifying technology to provide more consistent absorption and a longer half-life than oral testosterone undecanoate products long available outside the United States.
The new product fulfills an unmet need for an effective and convenient treatment for male hypogonadism that avoids the risk of unintended testosterone transference to women and children inherent in currently marketed topical testosterone products, said lead investigator Dr. Ronald S. Swerdloff, professor of medicine at the University of California, Los Angeles, and chief of the division of endocrinology at Harbor-UCLA Medical Center.
"An oral testosterone replacement therapy would be the preferred choice for many hypogonal men," he said.
Clarus Therapeutics filed for marketing approval for Rextoro with the Food and Drug Administration earlier this year on the strength of the two phase III studies. In addition, the company is sponsoring a supplemental phase IV, open-label, 12-month safety study to be reported later this summer.
The phase IV study is being conducted to resolve safety questions raised in one of the two phase III studies presented at ICE/ENDO 2014. In this 325-patient, open-label, 12-month multicenter trial in which patients were randomized to the investigational oral testosterone or to 1% AndroGel, the oral therapy group experienced a 22% reduction from baseline in HDL, compared with a 12% decrease with the testosterone gel. In addition, two patients in the oral testosterone group had an acute MI; none did in the topical therapy group. Hematocrit levels rose by 3% in oral testosterone–treated patients, although mean values remained within the normal range.
LDL and total cholesterol, triglyceride, C-reactive protein, lipoprotein-associated phospholipase A2, and prostate-specific antigen levels did not differ between the two treatment groups.
Moreover, in the second and pivotal phase III trial, which utilized a revised Rextoro dose-titration algorithm, the reduction in HDL over the course of 114 days of treatment was a more modest 9%. This second trial was a single-arm study involving 144 Rextoro-treated patients.
The revised dose-adjustment algorithm involved starting treatment at 200 mg of oral Rextoro twice daily, then measuring serum testosterone 3-5 hours after the morning dose on days 30 and 72. A value outside the range of 200-750 ng/dL triggered a 50-mg increase or decrease. When this algorithm was used, 75% of treated patients were maintained within the target eugonadal range, compared with 84% of the oral testosterone group and 79% of the testosterone gel–treated patients in the first phase III trial. Under the revised algorithm, only 3% of patients experienced a transient serum peak testosterone excursion greater than 2,500 ng/dL, Dr. Swedloff reported.
The phase III trials were funded by Clarus. Dr. Swerdloff is a consultant to Clarus and Endo Pharmaceuticals.
CHICAGO – A unique investigational oral testosterone replacement product for hypogonadal men showed efficacy and safety comparable to a commercially available testosterone gel in two phase III clinical trials, which drew considerable interest when presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
This formulation of oral testosterone undecanoate, known as Rextoro, uses proprietary self-emulsifying technology to provide more consistent absorption and a longer half-life than oral testosterone undecanoate products long available outside the United States.
The new product fulfills an unmet need for an effective and convenient treatment for male hypogonadism that avoids the risk of unintended testosterone transference to women and children inherent in currently marketed topical testosterone products, said lead investigator Dr. Ronald S. Swerdloff, professor of medicine at the University of California, Los Angeles, and chief of the division of endocrinology at Harbor-UCLA Medical Center.
"An oral testosterone replacement therapy would be the preferred choice for many hypogonal men," he said.
Clarus Therapeutics filed for marketing approval for Rextoro with the Food and Drug Administration earlier this year on the strength of the two phase III studies. In addition, the company is sponsoring a supplemental phase IV, open-label, 12-month safety study to be reported later this summer.
The phase IV study is being conducted to resolve safety questions raised in one of the two phase III studies presented at ICE/ENDO 2014. In this 325-patient, open-label, 12-month multicenter trial in which patients were randomized to the investigational oral testosterone or to 1% AndroGel, the oral therapy group experienced a 22% reduction from baseline in HDL, compared with a 12% decrease with the testosterone gel. In addition, two patients in the oral testosterone group had an acute MI; none did in the topical therapy group. Hematocrit levels rose by 3% in oral testosterone–treated patients, although mean values remained within the normal range.
LDL and total cholesterol, triglyceride, C-reactive protein, lipoprotein-associated phospholipase A2, and prostate-specific antigen levels did not differ between the two treatment groups.
Moreover, in the second and pivotal phase III trial, which utilized a revised Rextoro dose-titration algorithm, the reduction in HDL over the course of 114 days of treatment was a more modest 9%. This second trial was a single-arm study involving 144 Rextoro-treated patients.
The revised dose-adjustment algorithm involved starting treatment at 200 mg of oral Rextoro twice daily, then measuring serum testosterone 3-5 hours after the morning dose on days 30 and 72. A value outside the range of 200-750 ng/dL triggered a 50-mg increase or decrease. When this algorithm was used, 75% of treated patients were maintained within the target eugonadal range, compared with 84% of the oral testosterone group and 79% of the testosterone gel–treated patients in the first phase III trial. Under the revised algorithm, only 3% of patients experienced a transient serum peak testosterone excursion greater than 2,500 ng/dL, Dr. Swedloff reported.
The phase III trials were funded by Clarus. Dr. Swerdloff is a consultant to Clarus and Endo Pharmaceuticals.
CHICAGO – A unique investigational oral testosterone replacement product for hypogonadal men showed efficacy and safety comparable to a commercially available testosterone gel in two phase III clinical trials, which drew considerable interest when presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
This formulation of oral testosterone undecanoate, known as Rextoro, uses proprietary self-emulsifying technology to provide more consistent absorption and a longer half-life than oral testosterone undecanoate products long available outside the United States.
The new product fulfills an unmet need for an effective and convenient treatment for male hypogonadism that avoids the risk of unintended testosterone transference to women and children inherent in currently marketed topical testosterone products, said lead investigator Dr. Ronald S. Swerdloff, professor of medicine at the University of California, Los Angeles, and chief of the division of endocrinology at Harbor-UCLA Medical Center.
"An oral testosterone replacement therapy would be the preferred choice for many hypogonal men," he said.
Clarus Therapeutics filed for marketing approval for Rextoro with the Food and Drug Administration earlier this year on the strength of the two phase III studies. In addition, the company is sponsoring a supplemental phase IV, open-label, 12-month safety study to be reported later this summer.
The phase IV study is being conducted to resolve safety questions raised in one of the two phase III studies presented at ICE/ENDO 2014. In this 325-patient, open-label, 12-month multicenter trial in which patients were randomized to the investigational oral testosterone or to 1% AndroGel, the oral therapy group experienced a 22% reduction from baseline in HDL, compared with a 12% decrease with the testosterone gel. In addition, two patients in the oral testosterone group had an acute MI; none did in the topical therapy group. Hematocrit levels rose by 3% in oral testosterone–treated patients, although mean values remained within the normal range.
LDL and total cholesterol, triglyceride, C-reactive protein, lipoprotein-associated phospholipase A2, and prostate-specific antigen levels did not differ between the two treatment groups.
Moreover, in the second and pivotal phase III trial, which utilized a revised Rextoro dose-titration algorithm, the reduction in HDL over the course of 114 days of treatment was a more modest 9%. This second trial was a single-arm study involving 144 Rextoro-treated patients.
The revised dose-adjustment algorithm involved starting treatment at 200 mg of oral Rextoro twice daily, then measuring serum testosterone 3-5 hours after the morning dose on days 30 and 72. A value outside the range of 200-750 ng/dL triggered a 50-mg increase or decrease. When this algorithm was used, 75% of treated patients were maintained within the target eugonadal range, compared with 84% of the oral testosterone group and 79% of the testosterone gel–treated patients in the first phase III trial. Under the revised algorithm, only 3% of patients experienced a transient serum peak testosterone excursion greater than 2,500 ng/dL, Dr. Swedloff reported.
The phase III trials were funded by Clarus. Dr. Swerdloff is a consultant to Clarus and Endo Pharmaceuticals.
AT ICE/ENDO 2014
Key clinical point: Hypogonadal men may gain access to a convenient oral form of testosterone replacement therapy that avoids the risk of medication transference to women and children posed by current topical testosterone products.
Major finding: An investigational oral testosterone undecanoate showed safety and efficacy comparable to a commercially available testosterone gel in two phase III clinical trials.
Data source: One open-label, multicenter phase III study in 325 hypogonadal men randomized to 12 months of daily investigational oral testosterone replacement or commercially available 1% testosterone gel, and a second phase III single-arm trial in which 144 subjects with male hypogonadism were treated with the oral agent for 114 days using a revised dose-adjustment scheme.
Disclosures: The studies were sponsored by Clarus Therapeutics. The presenter is a consultant to the company.
New agent builds bone bigger, faster
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
AT ICE/ENDO 2014
Key clinical point: A novel anabolic agent being developed for the treatment of postmenopausal osteoporosis increased BMD faster and to a greater extent than did teriparatide.
Major finding: Total hip bone mineral density increased by 2.6% over baseline after 24 weeks of abaloparatide at 80 mcg daily, compared with 0.45% with teriparatide at 20 mcg daily and 0.65% with placebo.
Data source: This phase II randomized trial included 222 postmenopausal women with osteoporosis.
Disclosures: The study was sponsored by Radius Health. The presenter is the company’s chief medical officer.
Aspirin’s benefits may be blunted in African American women
CHICAGO – Postmenopausal African American women with subclinical atherosclerosis appear to be more resistant to the anti-inflammatory effects of daily aspirin than their white counterparts.
In a 6-month, double-blind, placebo-controlled pilot study, daily aspirin at 325 mg showed essentially no impact on high-sensitivity C-reactive protein (hsCRP ) levels in the African American women. Moreover, their levels of interleukin-6 (IL-6) actually shot up while on aspirin. In contrast, levels of both proinflammatory markers declined markedly with aspirin therapy in the white women, Dr. Nora Alghothani reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"Given apparent ethnic differences in response to aspirin-mediated anti-inflammatory benefits, perhaps a higher dose of aspirin may be required in African American women already at higher risk of inflammatory disease processes in order to reduce cardiovascular disease outcomes and lessen disparities," concluded Dr. Alghothani, of the department of endocrinology at the Ohio State University in Columbus.
This remark lit a four-alarm fire among audience members. They were quick to emphasize that aspirin at doses greater than 325 mg/day is associated with a sharply increased risk of bleeding and should thus not be considered as part of an individualized cardioprevention strategy for African American women unless and until there is solid evidence that the benefits outweigh the risks.
Dr. Alghothani concurred that a large-scale dose-response study is needed. In the meantime, though, the take home message of her pilot study is that physicians should not necessarily expect the same robust cardiovascular benefits with daily aspirin in their postmenopausal African American patients as in other populations, she added.
The pilot study included 42 postmenopausal, nondiabetic women with evidence of subclinical atherosclerosis based upon carotid intimal medial thickness measurements. Half were African American; half were white. Participants in each group were randomized in double-blind fashion to 6 months of aspirin at 325 mg/day or placebo, with fasting blood samples and anthropomorphic measurements obtained at baseline and 6 months. Consistent with findings from much larger studies, the African American women were heavier, with a mean body mass index of 32.8 kg/m2, compared with 27.8 kg/m2 for the white women. The African Americans also had significantly lower triglycerides and higher apolopoprotein A-I levels; however, the two groups didn’t differ in terms of fasting insulin or glucose, high-density lipoprotein, low-density lipoprotein, or blood pressure.
In the aspirin-treated African American women, levels of hsCRP remained static over time, going from a mean of 4.53 mg/L at baseline to 4.62 mg/L at 6 months. In placebo-treated African American women, however, hsCRP jumped from 3.34 mg/L at baseline to 8.36 mg/L at follow-up.
The mean hsCRP in white women on aspirin dropped from 2.13 to 1.6 mg/L over the course of 6 months, while with placebo it went from 2.19 to 2.69 mg/L.
Levels of IL-6 in aspirin-treated African American women climbed from 0.93 pg/mL at baseline to 2.56 pg/mL at 6 months. In contrast, mean IL-6 levels in white women on daily aspirin fell from 2.69 to 1.39 pg/mL. White women on placebo experienced a rise in IL-6 from 0.58 to 2.97 pg/mL.
Most of these differences didn’t achieve statistical significance because of the small sample size, but the consistent trends suggest an overall blunted response to the anti-inflammatory effects of aspirin among African Americans, according to Dr. Alghothani. She added that these findings might help explain the well-documented ethnic disparities in cardiovascular outcomes, whereby African American women have a significantly higher cardiovascular mortality rate than white women despite on average having higher HDL and lower triglycerides.
Her study was funded by the Ohio State University Center for Women’s Health. She reported having no financial conflicts.
 |
|
There has been a significant amount of research focused on understanding the relationship between inflammation and cardiovascular disease, but how this relationship is impacted by gender and race has been less studied. Although the sample size here was small, the key point is that more work is needed in the critical area of ethnic differences. Also, the correlation between anti-inflammatory levels and outcomes in this study needs more clarity, and although the results are intriguing, we should not change current practice until more research is undertaken in a larger population and linked to clear cardiovascular outcomes. Finally, there may be additional confounders such as body weight or other modifiable cardiovascular risk factors that may have led to the corresponding differences in these markers.
In summary, this study does hint at the notion that when designing interventions to reduce cardiovascular risks for elderly women, clearly "one size may not fit all."
Dr. Hiren Shah is with Northwestern University, Chicago, and medical director of the Medicine and Cardiac Telemetry Hospitalist Unit at Northwestern Memorial Hospital.
 |
|
There has been a significant amount of research focused on understanding the relationship between inflammation and cardiovascular disease, but how this relationship is impacted by gender and race has been less studied. Although the sample size here was small, the key point is that more work is needed in the critical area of ethnic differences. Also, the correlation between anti-inflammatory levels and outcomes in this study needs more clarity, and although the results are intriguing, we should not change current practice until more research is undertaken in a larger population and linked to clear cardiovascular outcomes. Finally, there may be additional confounders such as body weight or other modifiable cardiovascular risk factors that may have led to the corresponding differences in these markers.
In summary, this study does hint at the notion that when designing interventions to reduce cardiovascular risks for elderly women, clearly "one size may not fit all."
Dr. Hiren Shah is with Northwestern University, Chicago, and medical director of the Medicine and Cardiac Telemetry Hospitalist Unit at Northwestern Memorial Hospital.
 |
|
There has been a significant amount of research focused on understanding the relationship between inflammation and cardiovascular disease, but how this relationship is impacted by gender and race has been less studied. Although the sample size here was small, the key point is that more work is needed in the critical area of ethnic differences. Also, the correlation between anti-inflammatory levels and outcomes in this study needs more clarity, and although the results are intriguing, we should not change current practice until more research is undertaken in a larger population and linked to clear cardiovascular outcomes. Finally, there may be additional confounders such as body weight or other modifiable cardiovascular risk factors that may have led to the corresponding differences in these markers.
In summary, this study does hint at the notion that when designing interventions to reduce cardiovascular risks for elderly women, clearly "one size may not fit all."
Dr. Hiren Shah is with Northwestern University, Chicago, and medical director of the Medicine and Cardiac Telemetry Hospitalist Unit at Northwestern Memorial Hospital.
CHICAGO – Postmenopausal African American women with subclinical atherosclerosis appear to be more resistant to the anti-inflammatory effects of daily aspirin than their white counterparts.
In a 6-month, double-blind, placebo-controlled pilot study, daily aspirin at 325 mg showed essentially no impact on high-sensitivity C-reactive protein (hsCRP ) levels in the African American women. Moreover, their levels of interleukin-6 (IL-6) actually shot up while on aspirin. In contrast, levels of both proinflammatory markers declined markedly with aspirin therapy in the white women, Dr. Nora Alghothani reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"Given apparent ethnic differences in response to aspirin-mediated anti-inflammatory benefits, perhaps a higher dose of aspirin may be required in African American women already at higher risk of inflammatory disease processes in order to reduce cardiovascular disease outcomes and lessen disparities," concluded Dr. Alghothani, of the department of endocrinology at the Ohio State University in Columbus.
This remark lit a four-alarm fire among audience members. They were quick to emphasize that aspirin at doses greater than 325 mg/day is associated with a sharply increased risk of bleeding and should thus not be considered as part of an individualized cardioprevention strategy for African American women unless and until there is solid evidence that the benefits outweigh the risks.
Dr. Alghothani concurred that a large-scale dose-response study is needed. In the meantime, though, the take home message of her pilot study is that physicians should not necessarily expect the same robust cardiovascular benefits with daily aspirin in their postmenopausal African American patients as in other populations, she added.
The pilot study included 42 postmenopausal, nondiabetic women with evidence of subclinical atherosclerosis based upon carotid intimal medial thickness measurements. Half were African American; half were white. Participants in each group were randomized in double-blind fashion to 6 months of aspirin at 325 mg/day or placebo, with fasting blood samples and anthropomorphic measurements obtained at baseline and 6 months. Consistent with findings from much larger studies, the African American women were heavier, with a mean body mass index of 32.8 kg/m2, compared with 27.8 kg/m2 for the white women. The African Americans also had significantly lower triglycerides and higher apolopoprotein A-I levels; however, the two groups didn’t differ in terms of fasting insulin or glucose, high-density lipoprotein, low-density lipoprotein, or blood pressure.
In the aspirin-treated African American women, levels of hsCRP remained static over time, going from a mean of 4.53 mg/L at baseline to 4.62 mg/L at 6 months. In placebo-treated African American women, however, hsCRP jumped from 3.34 mg/L at baseline to 8.36 mg/L at follow-up.
The mean hsCRP in white women on aspirin dropped from 2.13 to 1.6 mg/L over the course of 6 months, while with placebo it went from 2.19 to 2.69 mg/L.
Levels of IL-6 in aspirin-treated African American women climbed from 0.93 pg/mL at baseline to 2.56 pg/mL at 6 months. In contrast, mean IL-6 levels in white women on daily aspirin fell from 2.69 to 1.39 pg/mL. White women on placebo experienced a rise in IL-6 from 0.58 to 2.97 pg/mL.
Most of these differences didn’t achieve statistical significance because of the small sample size, but the consistent trends suggest an overall blunted response to the anti-inflammatory effects of aspirin among African Americans, according to Dr. Alghothani. She added that these findings might help explain the well-documented ethnic disparities in cardiovascular outcomes, whereby African American women have a significantly higher cardiovascular mortality rate than white women despite on average having higher HDL and lower triglycerides.
Her study was funded by the Ohio State University Center for Women’s Health. She reported having no financial conflicts.
CHICAGO – Postmenopausal African American women with subclinical atherosclerosis appear to be more resistant to the anti-inflammatory effects of daily aspirin than their white counterparts.
In a 6-month, double-blind, placebo-controlled pilot study, daily aspirin at 325 mg showed essentially no impact on high-sensitivity C-reactive protein (hsCRP ) levels in the African American women. Moreover, their levels of interleukin-6 (IL-6) actually shot up while on aspirin. In contrast, levels of both proinflammatory markers declined markedly with aspirin therapy in the white women, Dr. Nora Alghothani reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"Given apparent ethnic differences in response to aspirin-mediated anti-inflammatory benefits, perhaps a higher dose of aspirin may be required in African American women already at higher risk of inflammatory disease processes in order to reduce cardiovascular disease outcomes and lessen disparities," concluded Dr. Alghothani, of the department of endocrinology at the Ohio State University in Columbus.
This remark lit a four-alarm fire among audience members. They were quick to emphasize that aspirin at doses greater than 325 mg/day is associated with a sharply increased risk of bleeding and should thus not be considered as part of an individualized cardioprevention strategy for African American women unless and until there is solid evidence that the benefits outweigh the risks.
Dr. Alghothani concurred that a large-scale dose-response study is needed. In the meantime, though, the take home message of her pilot study is that physicians should not necessarily expect the same robust cardiovascular benefits with daily aspirin in their postmenopausal African American patients as in other populations, she added.
The pilot study included 42 postmenopausal, nondiabetic women with evidence of subclinical atherosclerosis based upon carotid intimal medial thickness measurements. Half were African American; half were white. Participants in each group were randomized in double-blind fashion to 6 months of aspirin at 325 mg/day or placebo, with fasting blood samples and anthropomorphic measurements obtained at baseline and 6 months. Consistent with findings from much larger studies, the African American women were heavier, with a mean body mass index of 32.8 kg/m2, compared with 27.8 kg/m2 for the white women. The African Americans also had significantly lower triglycerides and higher apolopoprotein A-I levels; however, the two groups didn’t differ in terms of fasting insulin or glucose, high-density lipoprotein, low-density lipoprotein, or blood pressure.
In the aspirin-treated African American women, levels of hsCRP remained static over time, going from a mean of 4.53 mg/L at baseline to 4.62 mg/L at 6 months. In placebo-treated African American women, however, hsCRP jumped from 3.34 mg/L at baseline to 8.36 mg/L at follow-up.
The mean hsCRP in white women on aspirin dropped from 2.13 to 1.6 mg/L over the course of 6 months, while with placebo it went from 2.19 to 2.69 mg/L.
Levels of IL-6 in aspirin-treated African American women climbed from 0.93 pg/mL at baseline to 2.56 pg/mL at 6 months. In contrast, mean IL-6 levels in white women on daily aspirin fell from 2.69 to 1.39 pg/mL. White women on placebo experienced a rise in IL-6 from 0.58 to 2.97 pg/mL.
Most of these differences didn’t achieve statistical significance because of the small sample size, but the consistent trends suggest an overall blunted response to the anti-inflammatory effects of aspirin among African Americans, according to Dr. Alghothani. She added that these findings might help explain the well-documented ethnic disparities in cardiovascular outcomes, whereby African American women have a significantly higher cardiovascular mortality rate than white women despite on average having higher HDL and lower triglycerides.
Her study was funded by the Ohio State University Center for Women’s Health. She reported having no financial conflicts.
AT ICE/ENDO 2014
Key clinical point: Postmenopausal African American women may have a blunted response to the anti-inflammatory effects of aspirin at 325 mg/day.
Major finding: Mean levels of hsCRP dropped by 25% and IL-6 decreased by 48% in white women over the course of 6 months of aspirin at 325 mg/day. In contrast, CRP remained essentially unchanged despite daily aspirin in African American women, and their IL-6 levels rose.
Data source: This randomized, double-blind, placebo-controlled pilot study included 42 postmenopausal, nondiabetic women; half were African American, half were white. All had documented subclinical atherosclerosis. Subjects received aspirin at 325 mg/day and were studied over the course of 6 months.
Disclosures: The study was supported by the Ohio State University Center for Women’s Health. The presenter reported having no financial conflicts.