User login
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
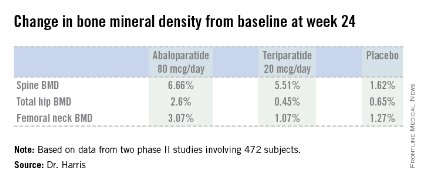
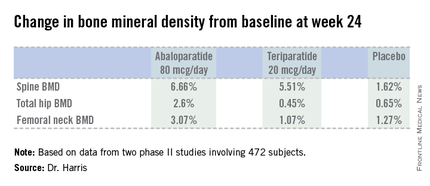
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
AT ICE/ENDO 2014
Key clinical point: A novel anabolic agent being developed for the treatment of postmenopausal osteoporosis increased BMD faster and to a greater extent than did teriparatide.
Major finding: Total hip bone mineral density increased by 2.6% over baseline after 24 weeks of abaloparatide at 80 mcg daily, compared with 0.45% with teriparatide at 20 mcg daily and 0.65% with placebo.
Data source: This phase II randomized trial included 222 postmenopausal women with osteoporosis.
Disclosures: The study was sponsored by Radius Health. The presenter is the company’s chief medical officer.