User login
High-Intensity Focused Ultrasound Slims Waists
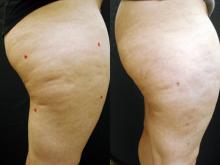
GRAPEVINE, TEX. – High-intensity focused ultrasound body contouring was both objectively and subjectively superior to sham treatment for reducing waist circumference in a randomized controlled trial of 180 adults who sought removal of excess abdominal fat.
"High-intensity focused ultrasound is a noninvasive, effective, and well-tolerated option for body contouring," said Dr. Jeremy B. Green of Skin Care Physicians, Chestnut Hill, Mass.
The patients were enrolled at nine clinical sites. They were aged 18-65 years, with subcutaneous abdominal adipose tissue depth of 2.5 cm or greater but with body mass indexes no greater than 30 kg/m2.
The majority (80%) were white women, with an average weight of 70 kg and BMI of approximately 25 kg/m2. They were randomized to one of three groups: a "low-energy" HIFU dose of 141 J/cm2 (three passes of 47 J/cm2), a "high-energy" dose of 177 J/cm2 (three passes of 59 J/cm2), or a sham control using three passes with 0 J/cm2.
The primary outcome was mean change from baseline waist circumference at 12 weeks post treatment.
In the per protocol population of 168 patients, there were significantly greater least squares mean reductions of 2.5 cm with the high-energy treatment and 2.1 cm with the low-energy treatment, compared with a drop of 1.2 cm in the sham group. (Subjects had agreed not to change their diet or exercise.)
There were also significantly greater waist circumference reductions for the high-energy HIFU at 4 weeks (1.8 cm vs. 0.2 cm with sham) and for both energy levels at week 8 (2.6 cm with high-energy and 2.6 cm with low-energy vs. 0.9 cm with sham).
Significance was achieved for the primary outcome with the high-energy treatment and some of the secondary outcomes in the intent-to-treat population of all 180 enrolled patients, he noted.
Subjective aesthetic assessments by both investigators and patients were consistent with the primary outcome measure. Investigator Global Aesthetic Improvement Scale (GAIS) ratings were significantly improved compared with sham treatments for both energy levels at weeks 4-8.
At 8 weeks, investigators deemed two-thirds of the patients as "much improved" or "improved" with both high- and low-energy HIFU, compared with just 19% for sham treatment, Dr. Green reported.
On patient satisfaction questionnaires, 58% treated with the low-energy and 67% with high-energy HIFU reported being "satisfied" or "very satisfied" at week 12, compared with 48% of those who received sham treatment. The difference was statistically significant for the high-energy HIFU.
Adverse events included pain during and after the procedure, ecchymosis, and edema. Six of the 180 enrolled patients did not complete the treatment because of pain. However, more than two-thirds of each group reported just mild or no pain, and all resolved by 16 days.
Half of the active treatment patients had mild bruising, all of which resolved in 10-14 days. There were no unanticipated adverse events. Clinical laboratory tests did not reveal any abnormalities or fluctuations with regard to lipid profiles, markers of inflammation, coagulation, hepatic or renal function, hematologic assessments, or blood chemistry, he reported.
Patients were compliant in maintaining their pretreatment diet and exercise habits, and there were minimal weight fluctuations during the study.
GRAPEVINE, TEX. – High-intensity focused ultrasound body contouring was both objectively and subjectively superior to sham treatment for reducing waist circumference in a randomized controlled trial of 180 adults who sought removal of excess abdominal fat.
"High-intensity focused ultrasound is a noninvasive, effective, and well-tolerated option for body contouring," said Dr. Jeremy B. Green of Skin Care Physicians, Chestnut Hill, Mass.
The patients were enrolled at nine clinical sites. They were aged 18-65 years, with subcutaneous abdominal adipose tissue depth of 2.5 cm or greater but with body mass indexes no greater than 30 kg/m2.
The majority (80%) were white women, with an average weight of 70 kg and BMI of approximately 25 kg/m2. They were randomized to one of three groups: a "low-energy" HIFU dose of 141 J/cm2 (three passes of 47 J/cm2), a "high-energy" dose of 177 J/cm2 (three passes of 59 J/cm2), or a sham control using three passes with 0 J/cm2.
The primary outcome was mean change from baseline waist circumference at 12 weeks post treatment.
In the per protocol population of 168 patients, there were significantly greater least squares mean reductions of 2.5 cm with the high-energy treatment and 2.1 cm with the low-energy treatment, compared with a drop of 1.2 cm in the sham group. (Subjects had agreed not to change their diet or exercise.)
There were also significantly greater waist circumference reductions for the high-energy HIFU at 4 weeks (1.8 cm vs. 0.2 cm with sham) and for both energy levels at week 8 (2.6 cm with high-energy and 2.6 cm with low-energy vs. 0.9 cm with sham).
Significance was achieved for the primary outcome with the high-energy treatment and some of the secondary outcomes in the intent-to-treat population of all 180 enrolled patients, he noted.
Subjective aesthetic assessments by both investigators and patients were consistent with the primary outcome measure. Investigator Global Aesthetic Improvement Scale (GAIS) ratings were significantly improved compared with sham treatments for both energy levels at weeks 4-8.
At 8 weeks, investigators deemed two-thirds of the patients as "much improved" or "improved" with both high- and low-energy HIFU, compared with just 19% for sham treatment, Dr. Green reported.
On patient satisfaction questionnaires, 58% treated with the low-energy and 67% with high-energy HIFU reported being "satisfied" or "very satisfied" at week 12, compared with 48% of those who received sham treatment. The difference was statistically significant for the high-energy HIFU.
Adverse events included pain during and after the procedure, ecchymosis, and edema. Six of the 180 enrolled patients did not complete the treatment because of pain. However, more than two-thirds of each group reported just mild or no pain, and all resolved by 16 days.
Half of the active treatment patients had mild bruising, all of which resolved in 10-14 days. There were no unanticipated adverse events. Clinical laboratory tests did not reveal any abnormalities or fluctuations with regard to lipid profiles, markers of inflammation, coagulation, hepatic or renal function, hematologic assessments, or blood chemistry, he reported.
Patients were compliant in maintaining their pretreatment diet and exercise habits, and there were minimal weight fluctuations during the study.
GRAPEVINE, TEX. – High-intensity focused ultrasound body contouring was both objectively and subjectively superior to sham treatment for reducing waist circumference in a randomized controlled trial of 180 adults who sought removal of excess abdominal fat.
"High-intensity focused ultrasound is a noninvasive, effective, and well-tolerated option for body contouring," said Dr. Jeremy B. Green of Skin Care Physicians, Chestnut Hill, Mass.
The patients were enrolled at nine clinical sites. They were aged 18-65 years, with subcutaneous abdominal adipose tissue depth of 2.5 cm or greater but with body mass indexes no greater than 30 kg/m2.
The majority (80%) were white women, with an average weight of 70 kg and BMI of approximately 25 kg/m2. They were randomized to one of three groups: a "low-energy" HIFU dose of 141 J/cm2 (three passes of 47 J/cm2), a "high-energy" dose of 177 J/cm2 (three passes of 59 J/cm2), or a sham control using three passes with 0 J/cm2.
The primary outcome was mean change from baseline waist circumference at 12 weeks post treatment.
In the per protocol population of 168 patients, there were significantly greater least squares mean reductions of 2.5 cm with the high-energy treatment and 2.1 cm with the low-energy treatment, compared with a drop of 1.2 cm in the sham group. (Subjects had agreed not to change their diet or exercise.)
There were also significantly greater waist circumference reductions for the high-energy HIFU at 4 weeks (1.8 cm vs. 0.2 cm with sham) and for both energy levels at week 8 (2.6 cm with high-energy and 2.6 cm with low-energy vs. 0.9 cm with sham).
Significance was achieved for the primary outcome with the high-energy treatment and some of the secondary outcomes in the intent-to-treat population of all 180 enrolled patients, he noted.
Subjective aesthetic assessments by both investigators and patients were consistent with the primary outcome measure. Investigator Global Aesthetic Improvement Scale (GAIS) ratings were significantly improved compared with sham treatments for both energy levels at weeks 4-8.
At 8 weeks, investigators deemed two-thirds of the patients as "much improved" or "improved" with both high- and low-energy HIFU, compared with just 19% for sham treatment, Dr. Green reported.
On patient satisfaction questionnaires, 58% treated with the low-energy and 67% with high-energy HIFU reported being "satisfied" or "very satisfied" at week 12, compared with 48% of those who received sham treatment. The difference was statistically significant for the high-energy HIFU.
Adverse events included pain during and after the procedure, ecchymosis, and edema. Six of the 180 enrolled patients did not complete the treatment because of pain. However, more than two-thirds of each group reported just mild or no pain, and all resolved by 16 days.
Half of the active treatment patients had mild bruising, all of which resolved in 10-14 days. There were no unanticipated adverse events. Clinical laboratory tests did not reveal any abnormalities or fluctuations with regard to lipid profiles, markers of inflammation, coagulation, hepatic or renal function, hematologic assessments, or blood chemistry, he reported.
Patients were compliant in maintaining their pretreatment diet and exercise habits, and there were minimal weight fluctuations during the study.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: In the per protocol population of 168 patients, there was a significantly greater least squares mean drop of 2.5 cm with the high-energy treatment, compared with a drop of just 1.2 cm in the sham group.
Data Source: A randomized controlled trial of 180 adults with excess abdominal fat.
Disclosures: The study was funded by Medicis Technologies, which manufactures the HIFU device called Liposonix.
Microdermabrasion Plus Nd:YAG Laser Improves Melasma
GRAPEVINE, TEX. – The combination of microdermabrasion and low-fluence Q-switched neodymium: YAG laser treatment in conjunction with pigment-reducing skin care produced consistent improvement in two or three treatments for 27 female patients with refractory facial melasma.
Previous attempts to treat melasma using various types of lasers have been associated with significant downtime, punctate hypopigmentation, melasma recurrence, and rebound hyperpigmentation.
Fractional lasers require four to six treatments and are associated with treatment pain, several days of recovery, and a high risk of rebound melasma. Higher-fluence Q-switched Nd:YAG laser therapy, performed with multiple laser passes during weekly treatments, is associated with pain, hair whitening, urticaria, punctuate hypopigmentation, and rebound melasma, said Dr. Arielle N.B. Kauvar at the annual meeting of the American Society for Laser Medicine and Surgery.
In this observational study, the 27 women had phototypes II-V with refractory mixed-type or dermal melasma. Their skin was first cleansed and then treated with diamond-chip microdermabrasion. Immediately after the microdermabrasion, 17 of the women received treatment with the Candela TriVantage (wavelength 1064 nm, nominal pulse width 50 ns, spot size 5 mm, fluence 1.6 J/cm2), while the other 10 women were treated with the Palomar Q-YAG (1064 nm, 5-7 ns, 6 mm, 1.8-2.0 J/cm2).
Patients began using a broad-spectrum sunscreen SPF 40 or higher immediately after treatment with the microdermabrasion and laser. For 2 days after each laser treatment, 4% hydroquinone twice daily plus 0.05% tretinoin at bedtime was applied until the day of the next microdermabrasion/laser treatment.
For patients with sensitive skin, 15% L-ascorbic acid was substituted for the tretinoin and used in the morning. Patients were maintained on skin care long term, said Dr. Kauvar, who is director of New York Laser & Skin Care.
The 27 women had a mean age of 37 years (range, 26-54 years). Four had skin type II, 11 type III, 7 type IV, and 5 type V. The mean number of treatments was 2.6. Mean clearance scores were 3.3 at 3 months, 3.2 at 6 months (25 patients), and 3.3 at 12 months (9); a score of 0 indicates less than 25% clearance, 1 = 25%-50% clearance, 2 = 51%-75%, 3 = 75%-95%, and 4 = greater than 95%.
Of the 27 patients, 22 had greater than 27% clearance of their melasma, while 11 had more than 95% clearance of pigmented patches. Most showed greater than 50% clearance at 1 month after the first treatment session, and only one patient had less than 25% clearance after one treatment, said Dr. Kauvar, who is in the department of dermatology at New York University.
The procedure was not associated with pain. All of the patients experienced very faint erythema that developed after the microdermabrasion, which lasted 30-60 minutes. Seven of the 27 had significant irritation from the skin care regimen, which resolved when the retinoid was discontinued. Another four had mild irritation from the skin care, which was successfully managed with reduction of the hydroquinone and retinoid applications. There was no incidence of hyperpigmentation or hypopigmentation.
Microdermabrasion decreases the scattering of laser light and increases epidermal cell turnover, while the low-fluence Q-switched YAG laser directly damages the melanocytes and melanosomes. The skin care regimen suppresses melanin production and protects against ultraviolet exposure, Dr. Kauvar explained.
"The combination of microdermabrasion and low-fluence Q-switched YAG laser treatment in conjunction with pigment-reducing skin care is a safe and effective treatment for melasma with minimal risks. This treatment offers substantial benefits over more invasive, higher-risk, costly procedures such as nonablative or ablative fractional laser treatment," she said.
Dr. Kauver stated that she has no disclosures.
GRAPEVINE, TEX. – The combination of microdermabrasion and low-fluence Q-switched neodymium: YAG laser treatment in conjunction with pigment-reducing skin care produced consistent improvement in two or three treatments for 27 female patients with refractory facial melasma.
Previous attempts to treat melasma using various types of lasers have been associated with significant downtime, punctate hypopigmentation, melasma recurrence, and rebound hyperpigmentation.
Fractional lasers require four to six treatments and are associated with treatment pain, several days of recovery, and a high risk of rebound melasma. Higher-fluence Q-switched Nd:YAG laser therapy, performed with multiple laser passes during weekly treatments, is associated with pain, hair whitening, urticaria, punctuate hypopigmentation, and rebound melasma, said Dr. Arielle N.B. Kauvar at the annual meeting of the American Society for Laser Medicine and Surgery.
In this observational study, the 27 women had phototypes II-V with refractory mixed-type or dermal melasma. Their skin was first cleansed and then treated with diamond-chip microdermabrasion. Immediately after the microdermabrasion, 17 of the women received treatment with the Candela TriVantage (wavelength 1064 nm, nominal pulse width 50 ns, spot size 5 mm, fluence 1.6 J/cm2), while the other 10 women were treated with the Palomar Q-YAG (1064 nm, 5-7 ns, 6 mm, 1.8-2.0 J/cm2).
Patients began using a broad-spectrum sunscreen SPF 40 or higher immediately after treatment with the microdermabrasion and laser. For 2 days after each laser treatment, 4% hydroquinone twice daily plus 0.05% tretinoin at bedtime was applied until the day of the next microdermabrasion/laser treatment.
For patients with sensitive skin, 15% L-ascorbic acid was substituted for the tretinoin and used in the morning. Patients were maintained on skin care long term, said Dr. Kauvar, who is director of New York Laser & Skin Care.
The 27 women had a mean age of 37 years (range, 26-54 years). Four had skin type II, 11 type III, 7 type IV, and 5 type V. The mean number of treatments was 2.6. Mean clearance scores were 3.3 at 3 months, 3.2 at 6 months (25 patients), and 3.3 at 12 months (9); a score of 0 indicates less than 25% clearance, 1 = 25%-50% clearance, 2 = 51%-75%, 3 = 75%-95%, and 4 = greater than 95%.
Of the 27 patients, 22 had greater than 27% clearance of their melasma, while 11 had more than 95% clearance of pigmented patches. Most showed greater than 50% clearance at 1 month after the first treatment session, and only one patient had less than 25% clearance after one treatment, said Dr. Kauvar, who is in the department of dermatology at New York University.
The procedure was not associated with pain. All of the patients experienced very faint erythema that developed after the microdermabrasion, which lasted 30-60 minutes. Seven of the 27 had significant irritation from the skin care regimen, which resolved when the retinoid was discontinued. Another four had mild irritation from the skin care, which was successfully managed with reduction of the hydroquinone and retinoid applications. There was no incidence of hyperpigmentation or hypopigmentation.
Microdermabrasion decreases the scattering of laser light and increases epidermal cell turnover, while the low-fluence Q-switched YAG laser directly damages the melanocytes and melanosomes. The skin care regimen suppresses melanin production and protects against ultraviolet exposure, Dr. Kauvar explained.
"The combination of microdermabrasion and low-fluence Q-switched YAG laser treatment in conjunction with pigment-reducing skin care is a safe and effective treatment for melasma with minimal risks. This treatment offers substantial benefits over more invasive, higher-risk, costly procedures such as nonablative or ablative fractional laser treatment," she said.
Dr. Kauver stated that she has no disclosures.
GRAPEVINE, TEX. – The combination of microdermabrasion and low-fluence Q-switched neodymium: YAG laser treatment in conjunction with pigment-reducing skin care produced consistent improvement in two or three treatments for 27 female patients with refractory facial melasma.
Previous attempts to treat melasma using various types of lasers have been associated with significant downtime, punctate hypopigmentation, melasma recurrence, and rebound hyperpigmentation.
Fractional lasers require four to six treatments and are associated with treatment pain, several days of recovery, and a high risk of rebound melasma. Higher-fluence Q-switched Nd:YAG laser therapy, performed with multiple laser passes during weekly treatments, is associated with pain, hair whitening, urticaria, punctuate hypopigmentation, and rebound melasma, said Dr. Arielle N.B. Kauvar at the annual meeting of the American Society for Laser Medicine and Surgery.
In this observational study, the 27 women had phototypes II-V with refractory mixed-type or dermal melasma. Their skin was first cleansed and then treated with diamond-chip microdermabrasion. Immediately after the microdermabrasion, 17 of the women received treatment with the Candela TriVantage (wavelength 1064 nm, nominal pulse width 50 ns, spot size 5 mm, fluence 1.6 J/cm2), while the other 10 women were treated with the Palomar Q-YAG (1064 nm, 5-7 ns, 6 mm, 1.8-2.0 J/cm2).
Patients began using a broad-spectrum sunscreen SPF 40 or higher immediately after treatment with the microdermabrasion and laser. For 2 days after each laser treatment, 4% hydroquinone twice daily plus 0.05% tretinoin at bedtime was applied until the day of the next microdermabrasion/laser treatment.
For patients with sensitive skin, 15% L-ascorbic acid was substituted for the tretinoin and used in the morning. Patients were maintained on skin care long term, said Dr. Kauvar, who is director of New York Laser & Skin Care.
The 27 women had a mean age of 37 years (range, 26-54 years). Four had skin type II, 11 type III, 7 type IV, and 5 type V. The mean number of treatments was 2.6. Mean clearance scores were 3.3 at 3 months, 3.2 at 6 months (25 patients), and 3.3 at 12 months (9); a score of 0 indicates less than 25% clearance, 1 = 25%-50% clearance, 2 = 51%-75%, 3 = 75%-95%, and 4 = greater than 95%.
Of the 27 patients, 22 had greater than 27% clearance of their melasma, while 11 had more than 95% clearance of pigmented patches. Most showed greater than 50% clearance at 1 month after the first treatment session, and only one patient had less than 25% clearance after one treatment, said Dr. Kauvar, who is in the department of dermatology at New York University.
The procedure was not associated with pain. All of the patients experienced very faint erythema that developed after the microdermabrasion, which lasted 30-60 minutes. Seven of the 27 had significant irritation from the skin care regimen, which resolved when the retinoid was discontinued. Another four had mild irritation from the skin care, which was successfully managed with reduction of the hydroquinone and retinoid applications. There was no incidence of hyperpigmentation or hypopigmentation.
Microdermabrasion decreases the scattering of laser light and increases epidermal cell turnover, while the low-fluence Q-switched YAG laser directly damages the melanocytes and melanosomes. The skin care regimen suppresses melanin production and protects against ultraviolet exposure, Dr. Kauvar explained.
"The combination of microdermabrasion and low-fluence Q-switched YAG laser treatment in conjunction with pigment-reducing skin care is a safe and effective treatment for melasma with minimal risks. This treatment offers substantial benefits over more invasive, higher-risk, costly procedures such as nonablative or ablative fractional laser treatment," she said.
Dr. Kauver stated that she has no disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: Of a total of 27 patients, 22 had greater than 27% clearance of their melasma while 11 had more than 95% clearance of pigmented patches.
Data Source: Observational study of 27 women with refractory mixed-type or dermal melasma.
Disclosures: Dr. Kauver stated that she had no disclosures.
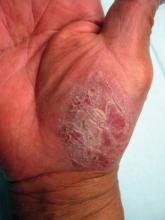
308-nm Excimer Laser Effective for Treating Palmoplantar Psoriasis
GRAPEVINE, TEX. – The 308-nm excimer laser significantly reduced palmoplantar psoriasis severity in a study of 30 patients.
Multiple studies have demonstrated the efficacy of the excimer laser in the treatment of plaque psoriasis, but few have investigated its use for palmoplantar psoriasis. "Palmoplantar psoriasis is difficult to treat and often recalcitrant to traditional therapies such as corticosteroids. Since the excimer laser can selectively treat psoriatic plaque with higher fluences than is tolerated with traditional phototherapy, it could be a therapeutic modality for the thicker skin on the palms and soles," said Dr. David Goldberg, director of dermatologic laser research at Mount Sinai School of Medicine, New York.
The 30 patients were aged 18-75 years, with mild to severe psoriasis on their hands and/or feet. All discontinued other treatments 4 weeks prior to starting the study. By study design, patients could receive up to 16 biweekly laser treatments over the course of 3 months with an excimer laser (PHAROS EX-308, RA Medical Systems). Fluences ranged from 400-600 mJ/cm2 depending on disease severity. Short pulses were delivered with a flexible handpiece, with a maximal output of 15 mJ/pulse (J. Cosmet. Laser Ther. 2011;13:47-9).
Each treatment was tailored to the individual patient’s response from the previous session. If there was no response or minimal erythema, the dose was increased by 30%. If there was moderate erythema, the dose was increased by 20%, and if significant erythema, the dose was increased by 10% until the patient could not tolerate further increases. For severe reactions or blistering, the dose was decreased by 20%. The number of sessions ranged from 7 to 14, with a mean of 11.
All of the subjects had some improvement by week 5, as measured by the Psoriasis Area and Severity Index (PASI). At the end of the treatments, all showed 50%-100% reductions in scaling, erythema, and flattened plaques. No patient had a relapse detected at the 3-month follow-up, but two-thirds of the patients had relapses by 6 months. There was no evidence of persistent pigmentary changes or scarring. Periodic retreatments will be required, Dr. Goldberg said.
"Although no treatment can be expected to ‘cure’ palmoplantar psoriasis, our data do support the use of the excimer laser to treat patients with hand and foot psoriasis. The excimer laser should be strongly considered for patients with palmoplantar psoriasis unresponsive to other treatments," he concluded.
The excimer laser study was provided on loan by the manufacturer during the course of the study. Dr. Goldberg stated that he had no other disclosures.
GRAPEVINE, TEX. – The 308-nm excimer laser significantly reduced palmoplantar psoriasis severity in a study of 30 patients.
Multiple studies have demonstrated the efficacy of the excimer laser in the treatment of plaque psoriasis, but few have investigated its use for palmoplantar psoriasis. "Palmoplantar psoriasis is difficult to treat and often recalcitrant to traditional therapies such as corticosteroids. Since the excimer laser can selectively treat psoriatic plaque with higher fluences than is tolerated with traditional phototherapy, it could be a therapeutic modality for the thicker skin on the palms and soles," said Dr. David Goldberg, director of dermatologic laser research at Mount Sinai School of Medicine, New York.
The 30 patients were aged 18-75 years, with mild to severe psoriasis on their hands and/or feet. All discontinued other treatments 4 weeks prior to starting the study. By study design, patients could receive up to 16 biweekly laser treatments over the course of 3 months with an excimer laser (PHAROS EX-308, RA Medical Systems). Fluences ranged from 400-600 mJ/cm2 depending on disease severity. Short pulses were delivered with a flexible handpiece, with a maximal output of 15 mJ/pulse (J. Cosmet. Laser Ther. 2011;13:47-9).
Each treatment was tailored to the individual patient’s response from the previous session. If there was no response or minimal erythema, the dose was increased by 30%. If there was moderate erythema, the dose was increased by 20%, and if significant erythema, the dose was increased by 10% until the patient could not tolerate further increases. For severe reactions or blistering, the dose was decreased by 20%. The number of sessions ranged from 7 to 14, with a mean of 11.
All of the subjects had some improvement by week 5, as measured by the Psoriasis Area and Severity Index (PASI). At the end of the treatments, all showed 50%-100% reductions in scaling, erythema, and flattened plaques. No patient had a relapse detected at the 3-month follow-up, but two-thirds of the patients had relapses by 6 months. There was no evidence of persistent pigmentary changes or scarring. Periodic retreatments will be required, Dr. Goldberg said.
"Although no treatment can be expected to ‘cure’ palmoplantar psoriasis, our data do support the use of the excimer laser to treat patients with hand and foot psoriasis. The excimer laser should be strongly considered for patients with palmoplantar psoriasis unresponsive to other treatments," he concluded.
The excimer laser study was provided on loan by the manufacturer during the course of the study. Dr. Goldberg stated that he had no other disclosures.
GRAPEVINE, TEX. – The 308-nm excimer laser significantly reduced palmoplantar psoriasis severity in a study of 30 patients.
Multiple studies have demonstrated the efficacy of the excimer laser in the treatment of plaque psoriasis, but few have investigated its use for palmoplantar psoriasis. "Palmoplantar psoriasis is difficult to treat and often recalcitrant to traditional therapies such as corticosteroids. Since the excimer laser can selectively treat psoriatic plaque with higher fluences than is tolerated with traditional phototherapy, it could be a therapeutic modality for the thicker skin on the palms and soles," said Dr. David Goldberg, director of dermatologic laser research at Mount Sinai School of Medicine, New York.
The 30 patients were aged 18-75 years, with mild to severe psoriasis on their hands and/or feet. All discontinued other treatments 4 weeks prior to starting the study. By study design, patients could receive up to 16 biweekly laser treatments over the course of 3 months with an excimer laser (PHAROS EX-308, RA Medical Systems). Fluences ranged from 400-600 mJ/cm2 depending on disease severity. Short pulses were delivered with a flexible handpiece, with a maximal output of 15 mJ/pulse (J. Cosmet. Laser Ther. 2011;13:47-9).
Each treatment was tailored to the individual patient’s response from the previous session. If there was no response or minimal erythema, the dose was increased by 30%. If there was moderate erythema, the dose was increased by 20%, and if significant erythema, the dose was increased by 10% until the patient could not tolerate further increases. For severe reactions or blistering, the dose was decreased by 20%. The number of sessions ranged from 7 to 14, with a mean of 11.
All of the subjects had some improvement by week 5, as measured by the Psoriasis Area and Severity Index (PASI). At the end of the treatments, all showed 50%-100% reductions in scaling, erythema, and flattened plaques. No patient had a relapse detected at the 3-month follow-up, but two-thirds of the patients had relapses by 6 months. There was no evidence of persistent pigmentary changes or scarring. Periodic retreatments will be required, Dr. Goldberg said.
"Although no treatment can be expected to ‘cure’ palmoplantar psoriasis, our data do support the use of the excimer laser to treat patients with hand and foot psoriasis. The excimer laser should be strongly considered for patients with palmoplantar psoriasis unresponsive to other treatments," he concluded.
The excimer laser study was provided on loan by the manufacturer during the course of the study. Dr. Goldberg stated that he had no other disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: At the end of the treatments, all patients showed 50%-100% reductions in scaling, erythema, and flattened plaques
Data Source: The 30 patients were aged 18-75 years, with mild to severe psoriasis on their hands and/or feet.
Disclosures: The excimer laser study was provided on-loan by the manufacturer during the course of the study. Dr. Goldberg stated that he had no other disclosures.
Nonablative Fractional Laser Proves Effective for Actinic Cheilitis
GRAPEVINE, TEX. – The nonablative fractional thulium 1927-nm laser effectively treated actinic cheilitis in 15 patients, without subsequent downtime or significant side effects, Dr. Robert Anolik reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Current treatments for actinic cheilitis – including surgery, carbon dioxide/erbium laser ablation, electrodesiccation, and 5-fluorouracil – typically involve significant pain, edema, and other adverse effects, including permanent scarring. The 1927-nm thulium laser, which is effective and well tolerated for superficial resurfacing, has been approved by the Food and Drug Administration for treating actinic keratoses, said Dr. Anolik of the Laser and Skin Surgery Center of New York.
Charts were reviewed for the 15 patients with actinic cheilitis who had been treated with the nonablative fractional 1,927-nm laser at two private laser and skin surgery centers. All were pretreated with topical anesthetic creams and given oral antiviral prophylaxis. Treatment parameters were 10-20 mJ per MTZ, 65%-70% coverage density, and total delivered energy of 0.08-0.1 kJ.
In blinded assessments of before and after photographs using a quartile improvement scale, all 15 patients had improvements of either 76%-100% (9 patients) or 51%-75% (6 patients) after 1-2 treatments. No adverse events occurred, and the only side effects were transient erythema for 1-4 days and edema for 1-3 days. "This is in stark contrast to the wounding, pain, and downtime expected with the other common treatment strategies," Dr. Anolik commented.
Planned next steps include increasing the patient pool, trial treatment of patients with a range of actinic cheilitis severity, assessment of before/after or left/right specimens for molecular features of actinic cheilitis such as p53, and an evaluation of long term benefit, he noted.
Dr. Anolik stated that he has no relevant relationships with industry.
GRAPEVINE, TEX. – The nonablative fractional thulium 1927-nm laser effectively treated actinic cheilitis in 15 patients, without subsequent downtime or significant side effects, Dr. Robert Anolik reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Current treatments for actinic cheilitis – including surgery, carbon dioxide/erbium laser ablation, electrodesiccation, and 5-fluorouracil – typically involve significant pain, edema, and other adverse effects, including permanent scarring. The 1927-nm thulium laser, which is effective and well tolerated for superficial resurfacing, has been approved by the Food and Drug Administration for treating actinic keratoses, said Dr. Anolik of the Laser and Skin Surgery Center of New York.
Charts were reviewed for the 15 patients with actinic cheilitis who had been treated with the nonablative fractional 1,927-nm laser at two private laser and skin surgery centers. All were pretreated with topical anesthetic creams and given oral antiviral prophylaxis. Treatment parameters were 10-20 mJ per MTZ, 65%-70% coverage density, and total delivered energy of 0.08-0.1 kJ.
In blinded assessments of before and after photographs using a quartile improvement scale, all 15 patients had improvements of either 76%-100% (9 patients) or 51%-75% (6 patients) after 1-2 treatments. No adverse events occurred, and the only side effects were transient erythema for 1-4 days and edema for 1-3 days. "This is in stark contrast to the wounding, pain, and downtime expected with the other common treatment strategies," Dr. Anolik commented.
Planned next steps include increasing the patient pool, trial treatment of patients with a range of actinic cheilitis severity, assessment of before/after or left/right specimens for molecular features of actinic cheilitis such as p53, and an evaluation of long term benefit, he noted.
Dr. Anolik stated that he has no relevant relationships with industry.
GRAPEVINE, TEX. – The nonablative fractional thulium 1927-nm laser effectively treated actinic cheilitis in 15 patients, without subsequent downtime or significant side effects, Dr. Robert Anolik reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Current treatments for actinic cheilitis – including surgery, carbon dioxide/erbium laser ablation, electrodesiccation, and 5-fluorouracil – typically involve significant pain, edema, and other adverse effects, including permanent scarring. The 1927-nm thulium laser, which is effective and well tolerated for superficial resurfacing, has been approved by the Food and Drug Administration for treating actinic keratoses, said Dr. Anolik of the Laser and Skin Surgery Center of New York.
Charts were reviewed for the 15 patients with actinic cheilitis who had been treated with the nonablative fractional 1,927-nm laser at two private laser and skin surgery centers. All were pretreated with topical anesthetic creams and given oral antiviral prophylaxis. Treatment parameters were 10-20 mJ per MTZ, 65%-70% coverage density, and total delivered energy of 0.08-0.1 kJ.
In blinded assessments of before and after photographs using a quartile improvement scale, all 15 patients had improvements of either 76%-100% (9 patients) or 51%-75% (6 patients) after 1-2 treatments. No adverse events occurred, and the only side effects were transient erythema for 1-4 days and edema for 1-3 days. "This is in stark contrast to the wounding, pain, and downtime expected with the other common treatment strategies," Dr. Anolik commented.
Planned next steps include increasing the patient pool, trial treatment of patients with a range of actinic cheilitis severity, assessment of before/after or left/right specimens for molecular features of actinic cheilitis such as p53, and an evaluation of long term benefit, he noted.
Dr. Anolik stated that he has no relevant relationships with industry.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: All 15 patients had improvements of either 76%-100% (9) or 51%-75% (6) after one or two treatments with a nonablative fractional thulium 1927-nm laser.
Data Source: Chart review of 15 patients with actinic cheilitis.
Disclosures: Dr. Anolik stated that he has no relevant relationships with industry.
Cryolipolysis Side Effects Mostly Mild and Transient, "Severe Pain" Rare
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system. The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq’s advisory board and have received educational and research support and honoraria from the company.
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system. The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq’s advisory board and have received educational and research support and honoraria from the company.
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system. The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq’s advisory board and have received educational and research support and honoraria from the company.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Cryolipolysis Side Effects Mostly Mild, "Severe Pain" Rare
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system.
The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq's advisory board and have received educational and research support and honoraria from the company.
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system.
The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq's advisory board and have received educational and research support and honoraria from the company.
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system.
The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq's advisory board and have received educational and research support and honoraria from the company.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Microwave Device Offers Long-Term Treatment for Hyperhidrosis
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: First study: Efficacy of treatment in 81 patients as determined by HDSS scores was 89% at 30 days and 67% at 6 months; efficacy of sham treatment in 39 patients was 54% and 44%, respectively. Second study: Of 28 patients seen at 30 days after treatment, all but one had HDSS scores of 1 or 2 (96% efficacy). Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments at 3 months showed that 94% had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Data Source: Two studies of 120 and 31 patients, respectively, with axillary hyperhidrosis
Disclosures: The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
Microwave Device Offers Long-Term Treatment for Hyperhidrosis
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: First study: Efficacy of treatment in 81 patients as determined by HDSS scores was 89% at 30 days and 67% at 6 months; efficacy of sham treatment in 39 patients was 54% and 44%, respectively. Second study: Of 28 patients seen at 30 days after treatment, all but one had HDSS scores of 1 or 2 (96% efficacy). Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments at 3 months showed that 94% had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Data Source: Two studies of 120 and 31 patients, respectively, with axillary hyperhidrosis
Disclosures: The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
Microwave Device Offers Long-Term Treatment for Hyperhidrosis
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs' miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs' miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs' miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: First study: Efficacy of treatment in 81 patients as determined by HDSS scores was 89% at 30 days and 67% at 6 months. Second study: Of 25 patients seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy).
Data Source: Two studies of 120 and 31 patients, respectively, with axillary hyperhidrosis
Disclosures: The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
Investigational Nd:YAG Laser Plus 3-D Optical Fiber Targets Cellulite
GRAPEVINE, TEX. – An investigational sidelight 3-D optical fiber and 1440-nm Nd:YAG laser produced significant improvement of cellulite with one treatment at 6 months in a study of 15 healthy women.
Cynosure Inc.'s Cellulaze Cellulite Laser Workstation's 1440 wavelength is well absorbed by adipose tissue and water. The side-firing SideLight 3-D optical fiber thermally subcises subcutaneous septa, deplanes fat cells, and heats dermal tissue to promote skin thickening and tightening, said Dr. Bruce E. Katz of the department of dermatology at Mount Sinai School of Medicine, New York.
The 15 women (aged 20-55 years) all had cellulite on their lateral or posterior thighs or buttocks, body mass indexes less than 30 kg/m2, and skin types I-V. Following local anesthesia, two 1.5-mm incisions were made, and the probe was inserted. Subcutaneous temperature was kept at lower than 47° C and surface temperature at lower than 40° C. The Nd:YAG laser delivered 1,000 J per 5- x 5-cm square.
Digital photographic evaluation by two independent observers at 6 months found that 68% of the women had excellent improvement in cellulite; Vectra 3-D imaging demonstrated significant improvement in 65% of them. By physician evaluation, 76% had good or excellent results (69% by patient evaluation). Vectra analysis of skin contour demonstrated an average 47% reduction in depth and 32% reduction in height of skin bulges, Dr. Katz reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Histologically, there was an increase in coarser collagen and elastic fibers in the dermis, noted Dr. Katz, who is also director of the cosmetic surgery and laser clinic at Mount Sinai Medical Center.
Three patients experienced mild ecchymoses, and four had edema lasting less than a week. There were no other adverse events.
"This may be a game changer for the treatment of cellulite," Dr. Katz said.
Cynosure received CE marking certification for Cellulaze in the European Union in February. The U.S. Food and Drug Administration approved an Investigational Device Exemption (IDE) for the device, and a clinical IDE study is currently underway. Regulatory action on a 510(k) submission, filed in late 2010, is currently expected in the first half of 2011, according to a company statement.
Dr. Katz disclosed that he is a Cynosure stockholder.
GRAPEVINE, TEX. – An investigational sidelight 3-D optical fiber and 1440-nm Nd:YAG laser produced significant improvement of cellulite with one treatment at 6 months in a study of 15 healthy women.
Cynosure Inc.'s Cellulaze Cellulite Laser Workstation's 1440 wavelength is well absorbed by adipose tissue and water. The side-firing SideLight 3-D optical fiber thermally subcises subcutaneous septa, deplanes fat cells, and heats dermal tissue to promote skin thickening and tightening, said Dr. Bruce E. Katz of the department of dermatology at Mount Sinai School of Medicine, New York.
The 15 women (aged 20-55 years) all had cellulite on their lateral or posterior thighs or buttocks, body mass indexes less than 30 kg/m2, and skin types I-V. Following local anesthesia, two 1.5-mm incisions were made, and the probe was inserted. Subcutaneous temperature was kept at lower than 47° C and surface temperature at lower than 40° C. The Nd:YAG laser delivered 1,000 J per 5- x 5-cm square.
Digital photographic evaluation by two independent observers at 6 months found that 68% of the women had excellent improvement in cellulite; Vectra 3-D imaging demonstrated significant improvement in 65% of them. By physician evaluation, 76% had good or excellent results (69% by patient evaluation). Vectra analysis of skin contour demonstrated an average 47% reduction in depth and 32% reduction in height of skin bulges, Dr. Katz reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Histologically, there was an increase in coarser collagen and elastic fibers in the dermis, noted Dr. Katz, who is also director of the cosmetic surgery and laser clinic at Mount Sinai Medical Center.
Three patients experienced mild ecchymoses, and four had edema lasting less than a week. There were no other adverse events.
"This may be a game changer for the treatment of cellulite," Dr. Katz said.
Cynosure received CE marking certification for Cellulaze in the European Union in February. The U.S. Food and Drug Administration approved an Investigational Device Exemption (IDE) for the device, and a clinical IDE study is currently underway. Regulatory action on a 510(k) submission, filed in late 2010, is currently expected in the first half of 2011, according to a company statement.
Dr. Katz disclosed that he is a Cynosure stockholder.
GRAPEVINE, TEX. – An investigational sidelight 3-D optical fiber and 1440-nm Nd:YAG laser produced significant improvement of cellulite with one treatment at 6 months in a study of 15 healthy women.
Cynosure Inc.'s Cellulaze Cellulite Laser Workstation's 1440 wavelength is well absorbed by adipose tissue and water. The side-firing SideLight 3-D optical fiber thermally subcises subcutaneous septa, deplanes fat cells, and heats dermal tissue to promote skin thickening and tightening, said Dr. Bruce E. Katz of the department of dermatology at Mount Sinai School of Medicine, New York.
The 15 women (aged 20-55 years) all had cellulite on their lateral or posterior thighs or buttocks, body mass indexes less than 30 kg/m2, and skin types I-V. Following local anesthesia, two 1.5-mm incisions were made, and the probe was inserted. Subcutaneous temperature was kept at lower than 47° C and surface temperature at lower than 40° C. The Nd:YAG laser delivered 1,000 J per 5- x 5-cm square.
Digital photographic evaluation by two independent observers at 6 months found that 68% of the women had excellent improvement in cellulite; Vectra 3-D imaging demonstrated significant improvement in 65% of them. By physician evaluation, 76% had good or excellent results (69% by patient evaluation). Vectra analysis of skin contour demonstrated an average 47% reduction in depth and 32% reduction in height of skin bulges, Dr. Katz reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Histologically, there was an increase in coarser collagen and elastic fibers in the dermis, noted Dr. Katz, who is also director of the cosmetic surgery and laser clinic at Mount Sinai Medical Center.
Three patients experienced mild ecchymoses, and four had edema lasting less than a week. There were no other adverse events.
"This may be a game changer for the treatment of cellulite," Dr. Katz said.
Cynosure received CE marking certification for Cellulaze in the European Union in February. The U.S. Food and Drug Administration approved an Investigational Device Exemption (IDE) for the device, and a clinical IDE study is currently underway. Regulatory action on a 510(k) submission, filed in late 2010, is currently expected in the first half of 2011, according to a company statement.
Dr. Katz disclosed that he is a Cynosure stockholder.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: Digital photographic evaluation by two independent observers at 6 months found that 68% had excellent improvement in cellulite, and Vectra 3-D imaging demonstrated significant improvement in 65%. By physician evaluation, 76% had good or excellent results (69% by patient evaluation).
Data Source: A 6-month study of 15 women aged 20-55 years with cellulite on their lateral or posterior thighs or buttocks.
Disclosures: The study was funded by Cynosure, of which Dr. Katz is a stockholder.