User login
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
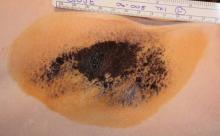
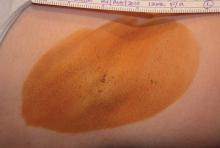
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs’ miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.
"The study is continuing out to 12 months, and at 6 months the preliminary data so far are showing sustained positive improvements in all measures of quality of life, reduction of sweat, and impact on daily living," Dr. Lupin said in an interview. Many patients also reported reduction of axillary hair, and a few patients noted improvement in odor, he said.
Overall, patient satisfaction, as measured by the Dermatology Life Quality Index (DLQI), was 96% at 3 months.
When Dr. Lupin was asked whether miraDry had any disadvantages compared with botulinum toxin type A, he said that there are several short-term advantages to botulinum toxin type A: It is a quicker and easier procedure (about 5-10 minutes) with sweat reduction occurring in just a few days, versus 1 hour for the miraDry procedure, which requires anesthesia and can take a week or longer to produce results. Moreover, botulinum toxin type A is a single treatment and its benefit lasts about 6-8 months, whereas miraDry takes 1-3 sessions for a benefit of at least a year and possibly longer.
Indeed, Dr. Kilmer said in an interview, duration of benefit is an advantage of miraDry over just about every current hyperhidrosis treatment other than sympathectomy, a procedure that is rarely done. So far, the longest miraDry has been studied is 12 months, so "we can’t say for sure, but it didn’t drop off much during that time, so we expect it will last much longer," she said.
Dr. Mathew Avram, director of the Massachusetts General Hospital Dermatology Laser and Cosmetic Center, Boston, said, "This is innovative and interesting technology to address an issue that is very problematic for patients. We need to learn a little more about the duration of these benefits and what if any side effects may be created with repeated treatments over time."
The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: First study: Efficacy of treatment in 81 patients as determined by HDSS scores was 89% at 30 days and 67% at 6 months; efficacy of sham treatment in 39 patients was 54% and 44%, respectively. Second study: Of 28 patients seen at 30 days after treatment, all but one had HDSS scores of 1 or 2 (96% efficacy). Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments at 3 months showed that 94% had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Data Source: Two studies of 120 and 31 patients, respectively, with axillary hyperhidrosis
Disclosures: The studies were sponsored by Miramar Labs. Dr. Lupin disclosed that he received a research grant for the study and travel expenses from Miramar, and also received honoraria and travel expenses from Allergan. Dr. Kilmer disclosed that she received research support from Miramar. Dr. Avram is a stockholder in Zeltiq.