User login
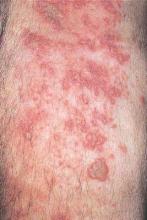
Risk for IBD Doubled in Hidradenitis Suppurativa
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
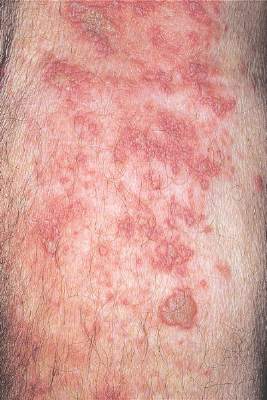
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
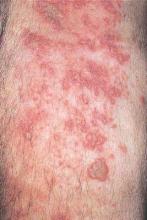
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
AT AAD 16
Risk of IBD doubled in hidradenitis suppurativa
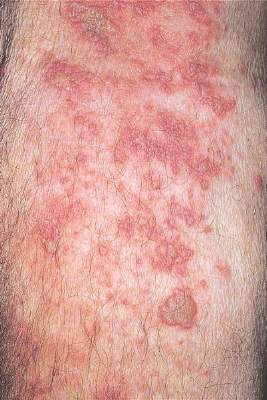
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
AT AAD 16
Key clinical point: Hidradenitis suppurativa may be considered a risk factor for inflammatory bowel disease.
Major finding: Patients with hidradenitis suppurativa were twice as likely as controls to have a concurrent or subsequent diagnosis of inflammatory bowel disease.
Data source: A case-control study of 1,332 patients and about 2,600 age-matched controls.
Disclosures: Ms. Cices had no relevant financial disclosures.
Topical allantoin cream speeds wound healing in epidermolysis bullosa
WASHINGTON – A 6% allantoin cream has shown good results in healing wounds caused by epidermolysis bullosa (EB), with 82% of patients getting a complete closure by 2 months, in a phase IIb study.
The results of the 3 month study were good enough to propel that dose into both an open label and a phase III study, Dr. Amy Paller said at the annual meeting of the American Academy of Dermatology.
SD-101 (Zorblisa) may not be a “game-changer” in terms of disease modification, but it’s an enormous step forward in symptomatic treatment, Dr. Paller said in an interview. The active ingredient, allantoin – used for years at low concentrations in cosmetics and emollients – imparts virtually no adverse effects, and may, at this higher dosage, actually be working on a cellular level, she added.
Although its mechanism has not been fully elucidated, allantoin seems to help reduce inflammation, loosen protein bonds, and promote collagen formation. It also has some bactericidal effects, she noted.
“We see that healing is improved and inflammation is decreased. Many patients also report less itching and pain, which can speed dressing changes, and that is a huge quality of life issue for families. Although these results are still early stage, and in a small number of patients, they were enough for us to move forward,” said Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University, Chicago.
At the meeting, she discussed the phase IIb study, which compared allantoin 6%, allantoin 3%, and a vehicle cream, in 48 patients aged 6 months to 44 years old. Most had the recessive dystrophic form of EB, which is associated with skin and mucosal blistering; orofacial and esophageal narrowing; anemia; and growth restriction. Older patients have a high risk of aggressive skin cancers.
The less severe EB simplex form was present in 11 patients; 8 had the most severe junctional form. The median size of the target lesion was about 8 cm2; the largest was 39 cm2. The baseline body surface area of lesional skin was 19%, with the largest area encompassing 48%. The target wounds were a median of 182 days in age, although this varied widely, from 21 to 1,600 days.
The cream was applied to the target lesion once a day. The study’s primary endpoint was target wound healing at 1 month; secondary endpoints included time to wound closure and the change in total body surface area of lesional skin. Assessments were conducted at baseline and at 14, 30, 60, and 90 days.
In the evaluable population of 45 patients, the 6% cream was most successful in effecting complete target wound closure at 1 month: 67%, compared with 38% for allantoin 3% and 41% for the vehicle (P = .165 for allantoin 6% vs. placebo).
And at 2 months, complete closure of the target wound occurred in 82% of the high dose group, compared with 44% of the low dose group and 41% of the vehicle group (P = .04 for allantoin 6% vs. placebo).
Results were similar at 3 months (82% for the high dose group, vs. 56% and 53% for the 3% and vehicle groups, respectively); this difference was not statistically significant (P = .124 for allantoin 6% vs. placebo).
These results equated to a significantly faster time to total wound closure in the allantoin 6% group (a median of 30 days vs. 86 days in the low dose group and 91 days for placebo).
Treatment emergent adverse events were low and were similar between the groups. Itching occurred in 13% of those in each active group and in 6% of those in the placebo group. Fever was most common in the high dose group (33% vs. 19% of the low dose group, and 12% of the placebo group.) However, those in the placebo group experienced more rash, erythematous rash, and oropharyngeal pain.
There were no deaths or severe adverse events in any group, according to Dr. Paller.
The study has evolved into an open label extension study that includes 42 patients in the original cohort. To date, 28 patients have completed 12 months of treatment. The time line shows a steady decrease in the body surface area of lesional skin, with a mean 3.4% decline from baseline to 1 year, she noted. Patient baselines were reset at 0, so these improvements were measured on top of any that may have occurred during the IIb study, she pointed out.
Scioderm has started a phase III double-blind, randomized placebo-controlled trial evaluating the 6% concentration, which is being conducted in the United States and Europe and is still recruiting participants. Endpoints are similar to the phase IIb study.
Although not a disease modifying agent, allantoin 6% cream is the biggest treatment advance so far for these patients, Dr. Paller commented. “There is lots of research going on,” including stem cell therapy leading to fibroblast differentiation, grafting cultured keratinocytes onto skin, and intravenous collagen, she said.
But these approaches are in the early stages of research, she added, “and so far we either don’t know their effect or [if] they offer minimal assistance. The concept of using something that has virtually no risk, even if it simply helps make someone more comfortable with less itching and pain, or which starts to enable some healing, is really important to a child with one of these devastating disorders.”
Dr. Paller is also excited about the possibility that starting the treatment earlier, or continuing for a longer time, could even have a better result. While she cautioned that this is a “very small study of patients with mixed EB types,” she said, “still, it’s the best thing we have ever seen with anything topically.”
Dr. Paller is a consultant for Scioderm, which is developing Zorblisa and sponsoring the studies. Northwestern was one of the study sites for the phase IIb study.
WASHINGTON – A 6% allantoin cream has shown good results in healing wounds caused by epidermolysis bullosa (EB), with 82% of patients getting a complete closure by 2 months, in a phase IIb study.
The results of the 3 month study were good enough to propel that dose into both an open label and a phase III study, Dr. Amy Paller said at the annual meeting of the American Academy of Dermatology.
SD-101 (Zorblisa) may not be a “game-changer” in terms of disease modification, but it’s an enormous step forward in symptomatic treatment, Dr. Paller said in an interview. The active ingredient, allantoin – used for years at low concentrations in cosmetics and emollients – imparts virtually no adverse effects, and may, at this higher dosage, actually be working on a cellular level, she added.
Although its mechanism has not been fully elucidated, allantoin seems to help reduce inflammation, loosen protein bonds, and promote collagen formation. It also has some bactericidal effects, she noted.
“We see that healing is improved and inflammation is decreased. Many patients also report less itching and pain, which can speed dressing changes, and that is a huge quality of life issue for families. Although these results are still early stage, and in a small number of patients, they were enough for us to move forward,” said Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University, Chicago.
At the meeting, she discussed the phase IIb study, which compared allantoin 6%, allantoin 3%, and a vehicle cream, in 48 patients aged 6 months to 44 years old. Most had the recessive dystrophic form of EB, which is associated with skin and mucosal blistering; orofacial and esophageal narrowing; anemia; and growth restriction. Older patients have a high risk of aggressive skin cancers.
The less severe EB simplex form was present in 11 patients; 8 had the most severe junctional form. The median size of the target lesion was about 8 cm2; the largest was 39 cm2. The baseline body surface area of lesional skin was 19%, with the largest area encompassing 48%. The target wounds were a median of 182 days in age, although this varied widely, from 21 to 1,600 days.
The cream was applied to the target lesion once a day. The study’s primary endpoint was target wound healing at 1 month; secondary endpoints included time to wound closure and the change in total body surface area of lesional skin. Assessments were conducted at baseline and at 14, 30, 60, and 90 days.
In the evaluable population of 45 patients, the 6% cream was most successful in effecting complete target wound closure at 1 month: 67%, compared with 38% for allantoin 3% and 41% for the vehicle (P = .165 for allantoin 6% vs. placebo).
And at 2 months, complete closure of the target wound occurred in 82% of the high dose group, compared with 44% of the low dose group and 41% of the vehicle group (P = .04 for allantoin 6% vs. placebo).
Results were similar at 3 months (82% for the high dose group, vs. 56% and 53% for the 3% and vehicle groups, respectively); this difference was not statistically significant (P = .124 for allantoin 6% vs. placebo).
These results equated to a significantly faster time to total wound closure in the allantoin 6% group (a median of 30 days vs. 86 days in the low dose group and 91 days for placebo).
Treatment emergent adverse events were low and were similar between the groups. Itching occurred in 13% of those in each active group and in 6% of those in the placebo group. Fever was most common in the high dose group (33% vs. 19% of the low dose group, and 12% of the placebo group.) However, those in the placebo group experienced more rash, erythematous rash, and oropharyngeal pain.
There were no deaths or severe adverse events in any group, according to Dr. Paller.
The study has evolved into an open label extension study that includes 42 patients in the original cohort. To date, 28 patients have completed 12 months of treatment. The time line shows a steady decrease in the body surface area of lesional skin, with a mean 3.4% decline from baseline to 1 year, she noted. Patient baselines were reset at 0, so these improvements were measured on top of any that may have occurred during the IIb study, she pointed out.
Scioderm has started a phase III double-blind, randomized placebo-controlled trial evaluating the 6% concentration, which is being conducted in the United States and Europe and is still recruiting participants. Endpoints are similar to the phase IIb study.
Although not a disease modifying agent, allantoin 6% cream is the biggest treatment advance so far for these patients, Dr. Paller commented. “There is lots of research going on,” including stem cell therapy leading to fibroblast differentiation, grafting cultured keratinocytes onto skin, and intravenous collagen, she said.
But these approaches are in the early stages of research, she added, “and so far we either don’t know their effect or [if] they offer minimal assistance. The concept of using something that has virtually no risk, even if it simply helps make someone more comfortable with less itching and pain, or which starts to enable some healing, is really important to a child with one of these devastating disorders.”
Dr. Paller is also excited about the possibility that starting the treatment earlier, or continuing for a longer time, could even have a better result. While she cautioned that this is a “very small study of patients with mixed EB types,” she said, “still, it’s the best thing we have ever seen with anything topically.”
Dr. Paller is a consultant for Scioderm, which is developing Zorblisa and sponsoring the studies. Northwestern was one of the study sites for the phase IIb study.
WASHINGTON – A 6% allantoin cream has shown good results in healing wounds caused by epidermolysis bullosa (EB), with 82% of patients getting a complete closure by 2 months, in a phase IIb study.
The results of the 3 month study were good enough to propel that dose into both an open label and a phase III study, Dr. Amy Paller said at the annual meeting of the American Academy of Dermatology.
SD-101 (Zorblisa) may not be a “game-changer” in terms of disease modification, but it’s an enormous step forward in symptomatic treatment, Dr. Paller said in an interview. The active ingredient, allantoin – used for years at low concentrations in cosmetics and emollients – imparts virtually no adverse effects, and may, at this higher dosage, actually be working on a cellular level, she added.
Although its mechanism has not been fully elucidated, allantoin seems to help reduce inflammation, loosen protein bonds, and promote collagen formation. It also has some bactericidal effects, she noted.
“We see that healing is improved and inflammation is decreased. Many patients also report less itching and pain, which can speed dressing changes, and that is a huge quality of life issue for families. Although these results are still early stage, and in a small number of patients, they were enough for us to move forward,” said Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University, Chicago.
At the meeting, she discussed the phase IIb study, which compared allantoin 6%, allantoin 3%, and a vehicle cream, in 48 patients aged 6 months to 44 years old. Most had the recessive dystrophic form of EB, which is associated with skin and mucosal blistering; orofacial and esophageal narrowing; anemia; and growth restriction. Older patients have a high risk of aggressive skin cancers.
The less severe EB simplex form was present in 11 patients; 8 had the most severe junctional form. The median size of the target lesion was about 8 cm2; the largest was 39 cm2. The baseline body surface area of lesional skin was 19%, with the largest area encompassing 48%. The target wounds were a median of 182 days in age, although this varied widely, from 21 to 1,600 days.
The cream was applied to the target lesion once a day. The study’s primary endpoint was target wound healing at 1 month; secondary endpoints included time to wound closure and the change in total body surface area of lesional skin. Assessments were conducted at baseline and at 14, 30, 60, and 90 days.
In the evaluable population of 45 patients, the 6% cream was most successful in effecting complete target wound closure at 1 month: 67%, compared with 38% for allantoin 3% and 41% for the vehicle (P = .165 for allantoin 6% vs. placebo).
And at 2 months, complete closure of the target wound occurred in 82% of the high dose group, compared with 44% of the low dose group and 41% of the vehicle group (P = .04 for allantoin 6% vs. placebo).
Results were similar at 3 months (82% for the high dose group, vs. 56% and 53% for the 3% and vehicle groups, respectively); this difference was not statistically significant (P = .124 for allantoin 6% vs. placebo).
These results equated to a significantly faster time to total wound closure in the allantoin 6% group (a median of 30 days vs. 86 days in the low dose group and 91 days for placebo).
Treatment emergent adverse events were low and were similar between the groups. Itching occurred in 13% of those in each active group and in 6% of those in the placebo group. Fever was most common in the high dose group (33% vs. 19% of the low dose group, and 12% of the placebo group.) However, those in the placebo group experienced more rash, erythematous rash, and oropharyngeal pain.
There were no deaths or severe adverse events in any group, according to Dr. Paller.
The study has evolved into an open label extension study that includes 42 patients in the original cohort. To date, 28 patients have completed 12 months of treatment. The time line shows a steady decrease in the body surface area of lesional skin, with a mean 3.4% decline from baseline to 1 year, she noted. Patient baselines were reset at 0, so these improvements were measured on top of any that may have occurred during the IIb study, she pointed out.
Scioderm has started a phase III double-blind, randomized placebo-controlled trial evaluating the 6% concentration, which is being conducted in the United States and Europe and is still recruiting participants. Endpoints are similar to the phase IIb study.
Although not a disease modifying agent, allantoin 6% cream is the biggest treatment advance so far for these patients, Dr. Paller commented. “There is lots of research going on,” including stem cell therapy leading to fibroblast differentiation, grafting cultured keratinocytes onto skin, and intravenous collagen, she said.
But these approaches are in the early stages of research, she added, “and so far we either don’t know their effect or [if] they offer minimal assistance. The concept of using something that has virtually no risk, even if it simply helps make someone more comfortable with less itching and pain, or which starts to enable some healing, is really important to a child with one of these devastating disorders.”
Dr. Paller is also excited about the possibility that starting the treatment earlier, or continuing for a longer time, could even have a better result. While she cautioned that this is a “very small study of patients with mixed EB types,” she said, “still, it’s the best thing we have ever seen with anything topically.”
Dr. Paller is a consultant for Scioderm, which is developing Zorblisa and sponsoring the studies. Northwestern was one of the study sites for the phase IIb study.
AT AAD 16
Key clinical point: A 6% allantoin cream shows promise at healing wounds in patients with epidermolysis bullosa.
Major finding: By 2 months, 82% of patients treated with allantoin 6% achieved complete closure of the target lesion, vs. 41% of the vehicle group (P = .04).
Data source: The phase IIb dose-ranging study compared healing rates with two concentrations of allantoin cream and a vehicle cream in patients with different EB types.
Disclosures: The manufacturer, Scioderm, sponsored the study; Dr. Amy Paller is a company consultant.
Severe Psoriasis, Kidney Disease Linked
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
AT AAD 16
Severe psoriasis, kidney disease linked
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
AT AAD 16
Key clinical point: Severe psoriasis appears to increase the risk of both immunoglobulin A glomerulonephritis and glomerular disease.
Major finding: The risk of glomerulonephritis was five-fold higher and the risk of glomerular disease doubled in those with severe psoriasis.
Data source: A population based cohort study comprised about 1.2 million subjects.
Disclosures: Ms. Sungat Grewal had no financial disclosures.
New topical anticholinergic beats axillary sweat
WASHINGTON – Sofpironium bromide, a newly developed “soft” anticholinergic gel, improved clinical outcomes and quality of life in patients with severe hyperhidrosis.
The drug is designed to work as an effective anticholinergic, but is metabolized and excreted much more quickly. This limits the possibility of troublesome systemic anticholinergic side effects, Dr. David M. Pariser said at the annual meeting of the American Academy of Dermatology.
“The main one we saw was vision issues, which occurred in about 8% of the patients,” who participated in a phase IIb randomized study, said Dr. Pariser, a dermatologist in Norfolk, Va. “We also saw urinary hesitancy, dry eyes, and dry lips. But these were nothing unexpected, and didn’t occur at an unexpected rate,” he added.
Sofpironium bromide (BBI-4000; BrickellBiotech) is a new molecular entity designed to bind to the M3 AC (acetylcholine) receptor on sweat glands – inhibiting sweat production – but to incompletely bind to systemic AC receptors. Thus, it exerts most of its effects locally, is rapidly metabolized, and exhibits very low systemic absorption, according to the company website.
The phase IIb study randomized 189 subjects to BBI-4000 in 5%, 10%, and 15% concentrations, or a placebo gel. All of the subjects had axillary hyperhidrosis scores of 3 or 4 on the Hyperhidrosis Disease Severity Scale (HDSS) – a 1-4 scale – and/or sweat at least 50 mg per 5 minutes per axilla.
Subjects applied the gel to underarms once daily for 28 days. Key endpoints were significant changes on the HDSS scale and the Hyperhidrosis Disease Severity Measure Axillary (HDSM-Ax), an axillary-specific scale developed by the company.
Positive results occurred in a dose-dependent outcome, with the 15% gel being the most effective. At that concentration, 38% of subjects achieved at least a 2 point improvement on the HDSS scale vs. 12% of those using the placebo (P less than .01).
On the HDSM-Ax, almost 45% of the high dose group and 19.5% of the placebo group achieved at least a 2 point improvement (P = .01). Those in the high dose group also produced significantly less sweat by day 29 than those in the placebo group.
All of these results were considered clinically significant, Dr. Pariser said.
About 10% of subjects in both groups experienced an application site reaction. Another 10% experienced a systemic anticholinergic adverse event.
Based on the positive results, BrickellBiotech intends to evaluate the 15% gel in a phase III study, Dr. Pariser said.
He is a consultant for the company. BrickellBiotech sponsored the study.
WASHINGTON – Sofpironium bromide, a newly developed “soft” anticholinergic gel, improved clinical outcomes and quality of life in patients with severe hyperhidrosis.
The drug is designed to work as an effective anticholinergic, but is metabolized and excreted much more quickly. This limits the possibility of troublesome systemic anticholinergic side effects, Dr. David M. Pariser said at the annual meeting of the American Academy of Dermatology.
“The main one we saw was vision issues, which occurred in about 8% of the patients,” who participated in a phase IIb randomized study, said Dr. Pariser, a dermatologist in Norfolk, Va. “We also saw urinary hesitancy, dry eyes, and dry lips. But these were nothing unexpected, and didn’t occur at an unexpected rate,” he added.
Sofpironium bromide (BBI-4000; BrickellBiotech) is a new molecular entity designed to bind to the M3 AC (acetylcholine) receptor on sweat glands – inhibiting sweat production – but to incompletely bind to systemic AC receptors. Thus, it exerts most of its effects locally, is rapidly metabolized, and exhibits very low systemic absorption, according to the company website.
The phase IIb study randomized 189 subjects to BBI-4000 in 5%, 10%, and 15% concentrations, or a placebo gel. All of the subjects had axillary hyperhidrosis scores of 3 or 4 on the Hyperhidrosis Disease Severity Scale (HDSS) – a 1-4 scale – and/or sweat at least 50 mg per 5 minutes per axilla.
Subjects applied the gel to underarms once daily for 28 days. Key endpoints were significant changes on the HDSS scale and the Hyperhidrosis Disease Severity Measure Axillary (HDSM-Ax), an axillary-specific scale developed by the company.
Positive results occurred in a dose-dependent outcome, with the 15% gel being the most effective. At that concentration, 38% of subjects achieved at least a 2 point improvement on the HDSS scale vs. 12% of those using the placebo (P less than .01).
On the HDSM-Ax, almost 45% of the high dose group and 19.5% of the placebo group achieved at least a 2 point improvement (P = .01). Those in the high dose group also produced significantly less sweat by day 29 than those in the placebo group.
All of these results were considered clinically significant, Dr. Pariser said.
About 10% of subjects in both groups experienced an application site reaction. Another 10% experienced a systemic anticholinergic adverse event.
Based on the positive results, BrickellBiotech intends to evaluate the 15% gel in a phase III study, Dr. Pariser said.
He is a consultant for the company. BrickellBiotech sponsored the study.
WASHINGTON – Sofpironium bromide, a newly developed “soft” anticholinergic gel, improved clinical outcomes and quality of life in patients with severe hyperhidrosis.
The drug is designed to work as an effective anticholinergic, but is metabolized and excreted much more quickly. This limits the possibility of troublesome systemic anticholinergic side effects, Dr. David M. Pariser said at the annual meeting of the American Academy of Dermatology.
“The main one we saw was vision issues, which occurred in about 8% of the patients,” who participated in a phase IIb randomized study, said Dr. Pariser, a dermatologist in Norfolk, Va. “We also saw urinary hesitancy, dry eyes, and dry lips. But these were nothing unexpected, and didn’t occur at an unexpected rate,” he added.
Sofpironium bromide (BBI-4000; BrickellBiotech) is a new molecular entity designed to bind to the M3 AC (acetylcholine) receptor on sweat glands – inhibiting sweat production – but to incompletely bind to systemic AC receptors. Thus, it exerts most of its effects locally, is rapidly metabolized, and exhibits very low systemic absorption, according to the company website.
The phase IIb study randomized 189 subjects to BBI-4000 in 5%, 10%, and 15% concentrations, or a placebo gel. All of the subjects had axillary hyperhidrosis scores of 3 or 4 on the Hyperhidrosis Disease Severity Scale (HDSS) – a 1-4 scale – and/or sweat at least 50 mg per 5 minutes per axilla.
Subjects applied the gel to underarms once daily for 28 days. Key endpoints were significant changes on the HDSS scale and the Hyperhidrosis Disease Severity Measure Axillary (HDSM-Ax), an axillary-specific scale developed by the company.
Positive results occurred in a dose-dependent outcome, with the 15% gel being the most effective. At that concentration, 38% of subjects achieved at least a 2 point improvement on the HDSS scale vs. 12% of those using the placebo (P less than .01).
On the HDSM-Ax, almost 45% of the high dose group and 19.5% of the placebo group achieved at least a 2 point improvement (P = .01). Those in the high dose group also produced significantly less sweat by day 29 than those in the placebo group.
All of these results were considered clinically significant, Dr. Pariser said.
About 10% of subjects in both groups experienced an application site reaction. Another 10% experienced a systemic anticholinergic adverse event.
Based on the positive results, BrickellBiotech intends to evaluate the 15% gel in a phase III study, Dr. Pariser said.
He is a consultant for the company. BrickellBiotech sponsored the study.
AT AAD 16
Key clinical point: An investigational anticholinergic gel had a significant impact on the severity of hyperhidrosis in individuals with severe axillary hyperhidrosis.
Major finding: Almost 40% of patients treated with the 15% concentration of sofpironium bromide gel experienced an improvement of at least 2 points on a scale measuring hyperhidrosis severity, vs. 12% of those using the placebo (P less than .01).
Data source: The 28-day study evaluated the impact of treatment in 189 subjects with severe hyperhidrosis.
Disclosures: BrickellBiotech sponsored the study. Dr. Pariser is a consultant for the company.
Topical Steroid Alleviates Tretinoin Irritation in Small Study
WASHINGTON – Adding a topical steroid to tretinoin may be useful in reducing some of the retinoid-induced dermal irritation commonly seen with this treatment in patients with acne.
The reduction in dryness and peeling could, potentially, increase compliance with tretinoin treatment, especially in the first few weeks when retinoid reactions are most likely to occur, Dr. Garrett Coman said at the annual meeting of the American Academy of Dermatology.
“Patients definitely preferred adding the topical steroid to tretinoin-alone treatment,” said Dr. Coman, a clinical research fellow in the department of dermatology at the Virginia Tech Carilion School of Medicine and Research Institute, Roanoke. “At week 2, 64% already exhibited a strong preference, and that had increased to 86% by week 4.”
He and his colleagues conducted the 8-week, randomized, split-face trial of 20 patients who were treated with tretinoin for acne. In addition to 0.05% tretinoin, they applied 0.025% triamcinolone to one side of the face and a vehicle emollient to the other side.
Patients were a typical acne treatment population, with a mean age of 18 years and most were female. They had already tried a number of acne treatments, including benzoyl peroxide, topical and oral antibiotics, and tretinoin.
They were assessed at baseline and at weeks 1, 2, 4, and 8. The outcome measures were acne severity; dryness, peeling, and erythema; and overall irritation.
Patients said that irritation increased significantly on the tretinoin-only (control) side by the end of week 1, while dryness did not change on the steroid combination side. By week 4 those scores were converging; by week 8 they were similar.
Physicians scored three categories of irritation: dryness, peeling, and erythema. They felt that dryness was significantly worse on the control side, peaked at week 2, and then slowly decreased, while dryness on the steroid side slowly increased and the scores converged by week 8. Peeling followed a similar pattern. Erythema was not significantly different at any time point.
Overall, acne severity decreased more quickly and dramatically on the steroid side, according to physicians. This finding, combined with the early decreases in symptoms of irritation, might improve compliance. “We have all noticed that the early irritation can be a significant inhibitor of compliance. Our results suggest that topical corticosteroids reduce peeling and dryness early in treatment. We think this could increase compliance and boost outcomes in patients starting topical retinoids,” Dr. Coman commented.
He had no disclosures. The Carilion Clinic funded the study.
WASHINGTON – Adding a topical steroid to tretinoin may be useful in reducing some of the retinoid-induced dermal irritation commonly seen with this treatment in patients with acne.
The reduction in dryness and peeling could, potentially, increase compliance with tretinoin treatment, especially in the first few weeks when retinoid reactions are most likely to occur, Dr. Garrett Coman said at the annual meeting of the American Academy of Dermatology.
“Patients definitely preferred adding the topical steroid to tretinoin-alone treatment,” said Dr. Coman, a clinical research fellow in the department of dermatology at the Virginia Tech Carilion School of Medicine and Research Institute, Roanoke. “At week 2, 64% already exhibited a strong preference, and that had increased to 86% by week 4.”
He and his colleagues conducted the 8-week, randomized, split-face trial of 20 patients who were treated with tretinoin for acne. In addition to 0.05% tretinoin, they applied 0.025% triamcinolone to one side of the face and a vehicle emollient to the other side.
Patients were a typical acne treatment population, with a mean age of 18 years and most were female. They had already tried a number of acne treatments, including benzoyl peroxide, topical and oral antibiotics, and tretinoin.
They were assessed at baseline and at weeks 1, 2, 4, and 8. The outcome measures were acne severity; dryness, peeling, and erythema; and overall irritation.
Patients said that irritation increased significantly on the tretinoin-only (control) side by the end of week 1, while dryness did not change on the steroid combination side. By week 4 those scores were converging; by week 8 they were similar.
Physicians scored three categories of irritation: dryness, peeling, and erythema. They felt that dryness was significantly worse on the control side, peaked at week 2, and then slowly decreased, while dryness on the steroid side slowly increased and the scores converged by week 8. Peeling followed a similar pattern. Erythema was not significantly different at any time point.
Overall, acne severity decreased more quickly and dramatically on the steroid side, according to physicians. This finding, combined with the early decreases in symptoms of irritation, might improve compliance. “We have all noticed that the early irritation can be a significant inhibitor of compliance. Our results suggest that topical corticosteroids reduce peeling and dryness early in treatment. We think this could increase compliance and boost outcomes in patients starting topical retinoids,” Dr. Coman commented.
He had no disclosures. The Carilion Clinic funded the study.
WASHINGTON – Adding a topical steroid to tretinoin may be useful in reducing some of the retinoid-induced dermal irritation commonly seen with this treatment in patients with acne.
The reduction in dryness and peeling could, potentially, increase compliance with tretinoin treatment, especially in the first few weeks when retinoid reactions are most likely to occur, Dr. Garrett Coman said at the annual meeting of the American Academy of Dermatology.
“Patients definitely preferred adding the topical steroid to tretinoin-alone treatment,” said Dr. Coman, a clinical research fellow in the department of dermatology at the Virginia Tech Carilion School of Medicine and Research Institute, Roanoke. “At week 2, 64% already exhibited a strong preference, and that had increased to 86% by week 4.”
He and his colleagues conducted the 8-week, randomized, split-face trial of 20 patients who were treated with tretinoin for acne. In addition to 0.05% tretinoin, they applied 0.025% triamcinolone to one side of the face and a vehicle emollient to the other side.
Patients were a typical acne treatment population, with a mean age of 18 years and most were female. They had already tried a number of acne treatments, including benzoyl peroxide, topical and oral antibiotics, and tretinoin.
They were assessed at baseline and at weeks 1, 2, 4, and 8. The outcome measures were acne severity; dryness, peeling, and erythema; and overall irritation.
Patients said that irritation increased significantly on the tretinoin-only (control) side by the end of week 1, while dryness did not change on the steroid combination side. By week 4 those scores were converging; by week 8 they were similar.
Physicians scored three categories of irritation: dryness, peeling, and erythema. They felt that dryness was significantly worse on the control side, peaked at week 2, and then slowly decreased, while dryness on the steroid side slowly increased and the scores converged by week 8. Peeling followed a similar pattern. Erythema was not significantly different at any time point.
Overall, acne severity decreased more quickly and dramatically on the steroid side, according to physicians. This finding, combined with the early decreases in symptoms of irritation, might improve compliance. “We have all noticed that the early irritation can be a significant inhibitor of compliance. Our results suggest that topical corticosteroids reduce peeling and dryness early in treatment. We think this could increase compliance and boost outcomes in patients starting topical retinoids,” Dr. Coman commented.
He had no disclosures. The Carilion Clinic funded the study.
AT AAD 16
Topical steroid alleviates tretinoin irritation in small study
WASHINGTON – Adding a topical steroid to tretinoin may be useful in reducing some of the retinoid-induced dermal irritation commonly seen with this treatment in patients with acne.
The reduction in dryness and peeling could, potentially, increase compliance with tretinoin treatment, especially in the first few weeks when retinoid reactions are most likely to occur, Dr. Garrett Coman said at the annual meeting of the American Academy of Dermatology.
“Patients definitely preferred adding the topical steroid to tretinoin-alone treatment,” said Dr. Coman, a clinical research fellow in the department of dermatology at the Virginia Tech Carilion School of Medicine and Research Institute, Roanoke. “At week 2, 64% already exhibited a strong preference, and that had increased to 86% by week 4.”
He and his colleagues conducted the 8-week, randomized, split-face trial of 20 patients who were treated with tretinoin for acne. In addition to 0.05% tretinoin, they applied 0.025% triamcinolone to one side of the face and a vehicle emollient to the other side.
Patients were a typical acne treatment population, with a mean age of 18 years and most were female. They had already tried a number of acne treatments, including benzoyl peroxide, topical and oral antibiotics, and tretinoin.
They were assessed at baseline and at weeks 1, 2, 4, and 8. The outcome measures were acne severity; dryness, peeling, and erythema; and overall irritation.
Patients said that irritation increased significantly on the tretinoin-only (control) side by the end of week 1, while dryness did not change on the steroid combination side. By week 4 those scores were converging; by week 8 they were similar.
Physicians scored three categories of irritation: dryness, peeling, and erythema. They felt that dryness was significantly worse on the control side, peaked at week 2, and then slowly decreased, while dryness on the steroid side slowly increased and the scores converged by week 8. Peeling followed a similar pattern. Erythema was not significantly different at any time point.
Overall, acne severity decreased more quickly and dramatically on the steroid side, according to physicians. This finding, combined with the early decreases in symptoms of irritation, might improve compliance. “We have all noticed that the early irritation can be a significant inhibitor of compliance. Our results suggest that topical corticosteroids reduce peeling and dryness early in treatment. We think this could increase compliance and boost outcomes in patients starting topical retinoids,” Dr. Coman commented.
He had no disclosures. The Carilion Clinic funded the study.
On Twitter @Alz_Gal
WASHINGTON – Adding a topical steroid to tretinoin may be useful in reducing some of the retinoid-induced dermal irritation commonly seen with this treatment in patients with acne.
The reduction in dryness and peeling could, potentially, increase compliance with tretinoin treatment, especially in the first few weeks when retinoid reactions are most likely to occur, Dr. Garrett Coman said at the annual meeting of the American Academy of Dermatology.
“Patients definitely preferred adding the topical steroid to tretinoin-alone treatment,” said Dr. Coman, a clinical research fellow in the department of dermatology at the Virginia Tech Carilion School of Medicine and Research Institute, Roanoke. “At week 2, 64% already exhibited a strong preference, and that had increased to 86% by week 4.”
He and his colleagues conducted the 8-week, randomized, split-face trial of 20 patients who were treated with tretinoin for acne. In addition to 0.05% tretinoin, they applied 0.025% triamcinolone to one side of the face and a vehicle emollient to the other side.
Patients were a typical acne treatment population, with a mean age of 18 years and most were female. They had already tried a number of acne treatments, including benzoyl peroxide, topical and oral antibiotics, and tretinoin.
They were assessed at baseline and at weeks 1, 2, 4, and 8. The outcome measures were acne severity; dryness, peeling, and erythema; and overall irritation.
Patients said that irritation increased significantly on the tretinoin-only (control) side by the end of week 1, while dryness did not change on the steroid combination side. By week 4 those scores were converging; by week 8 they were similar.
Physicians scored three categories of irritation: dryness, peeling, and erythema. They felt that dryness was significantly worse on the control side, peaked at week 2, and then slowly decreased, while dryness on the steroid side slowly increased and the scores converged by week 8. Peeling followed a similar pattern. Erythema was not significantly different at any time point.
Overall, acne severity decreased more quickly and dramatically on the steroid side, according to physicians. This finding, combined with the early decreases in symptoms of irritation, might improve compliance. “We have all noticed that the early irritation can be a significant inhibitor of compliance. Our results suggest that topical corticosteroids reduce peeling and dryness early in treatment. We think this could increase compliance and boost outcomes in patients starting topical retinoids,” Dr. Coman commented.
He had no disclosures. The Carilion Clinic funded the study.
On Twitter @Alz_Gal
WASHINGTON – Adding a topical steroid to tretinoin may be useful in reducing some of the retinoid-induced dermal irritation commonly seen with this treatment in patients with acne.
The reduction in dryness and peeling could, potentially, increase compliance with tretinoin treatment, especially in the first few weeks when retinoid reactions are most likely to occur, Dr. Garrett Coman said at the annual meeting of the American Academy of Dermatology.
“Patients definitely preferred adding the topical steroid to tretinoin-alone treatment,” said Dr. Coman, a clinical research fellow in the department of dermatology at the Virginia Tech Carilion School of Medicine and Research Institute, Roanoke. “At week 2, 64% already exhibited a strong preference, and that had increased to 86% by week 4.”
He and his colleagues conducted the 8-week, randomized, split-face trial of 20 patients who were treated with tretinoin for acne. In addition to 0.05% tretinoin, they applied 0.025% triamcinolone to one side of the face and a vehicle emollient to the other side.
Patients were a typical acne treatment population, with a mean age of 18 years and most were female. They had already tried a number of acne treatments, including benzoyl peroxide, topical and oral antibiotics, and tretinoin.
They were assessed at baseline and at weeks 1, 2, 4, and 8. The outcome measures were acne severity; dryness, peeling, and erythema; and overall irritation.
Patients said that irritation increased significantly on the tretinoin-only (control) side by the end of week 1, while dryness did not change on the steroid combination side. By week 4 those scores were converging; by week 8 they were similar.
Physicians scored three categories of irritation: dryness, peeling, and erythema. They felt that dryness was significantly worse on the control side, peaked at week 2, and then slowly decreased, while dryness on the steroid side slowly increased and the scores converged by week 8. Peeling followed a similar pattern. Erythema was not significantly different at any time point.
Overall, acne severity decreased more quickly and dramatically on the steroid side, according to physicians. This finding, combined with the early decreases in symptoms of irritation, might improve compliance. “We have all noticed that the early irritation can be a significant inhibitor of compliance. Our results suggest that topical corticosteroids reduce peeling and dryness early in treatment. We think this could increase compliance and boost outcomes in patients starting topical retinoids,” Dr. Coman commented.
He had no disclosures. The Carilion Clinic funded the study.
On Twitter @Alz_Gal
AT AAD 16
Key clinical point: Dryness and peeling were less pronounced when triamcinolone was applied with tretinoin in patients treated for acne.
Major finding: By week 2, physicians scored both dryness and peeling as significantly worse on the side of the face treated with tretinoin alone.
Data source: The 8-week, randomized, split-face trial evaluated 20 patients.
Disclosures: Dr. Coman had no disclosures. The Carilion Clinic funded the study.
As Varicella Recedes, Zoster Rises: The Question Is "Why?"
WASHINGTON – As the number of children vaccinated against varicella has risen, the number of chicken pox cases has proportionally declined.
No news there.
But, as the number of kids getting chickenpox gets smaller, the number of adults getting shingles is growing – and no one really knows why, Dr. Vikash Oza said at the annual meeting of the American Academy of Dermatology.
“Varicella vaccination has had a dramatic impact on the incidence of chickenpox,” said Dr. Oza, a pediatric dermatologist at New York University Langone Medical Center. “In fact, as a resident I saw not one case of chickenpox. That’s pretty astounding considering how common it used to be.”
Introduced in 1995, the vaccine now covers about 90% of children in this country – an achievement almost exactly mirrored by the decrease in disease incidence. In fact, according to a 2013 study, the bang for the vaccine buck may be even bigger, achieving 98% decreases in two regions examined (Pediatrics. 2013 Nov;132[5]:e1134-40).
That study also found that vaccinated children who did get chickenpox had much milder infections that resolved more quickly.
Again, that’s good news, but not particularly surprising, said Dr. Oza. The more intriguing change is the steady increase in herpes zoster among older adults occurring in tandem with the decreased incidence of chickenpox. Cases were up a total of 39% from 1992 to 2010 among adults 65 and older, according to the Centers for Disease Control and Prevention (Ann Intern Med. 2013;159[11]:739-45).
“This thing that’s happening in adults is quite interesting,” Dr. Oza said. “Although zoster in children is uncommon, we do know that children are much less likely to develop it if they’ve been vaccinated against varicella. What we don’t really know is what this means for adults. One of the effects of the vaccine, of course, is less circulating varicella zoster virus in our communities. It’s possible that a benefit of having some circulating VZV gives adults an immune boost that keeps zoster in check, and without that, developing shingles is more likely.”
Several recent studies have documented the association. A 2005 study found that during 1998-2003, the incidence of chickenpox in Massachusetts went down by 79%, while the incidence of shingles increased by 90% (BMC Public Health 2005;5:68. doi: 10.1186/1471-2458-5-68). But a shifting age-related immune response is only one possible explanation, the authors wrote. “There are several possible explanations, which may be operating in combination. Other possible explanations include increases in the proportion of people with immunosuppressive conditions and therapies, in the duration of those conditions and treatments, and/or in the prevalence of other triggering factors.”
The shifting incidences of chickenpox and shingles, however, were predicted years before they occurred. A mathematical model from 1992 predicted a long-term elevation of up to 20% in the shingles rate, relative to pre-vaccination incidence (Epidemiol Infect. 1992 Jun;108[3]:513–28). A 2001 paper predicted that shingles would increase for 30-50 years after mass varicella vaccination, rising to a maximum of about 50% above pre-vaccination rates, before falling below baseline levels (Epidemiol Infect. 2001 Oct;127[2]:305–14).
The CDC refutes any causative link between varicella vaccinations and shingles. “This proposed explanation seems unlikely based on two CDC studies which found that shingles rates started increasing before chickenpox vaccine was introduced in the United States, and after the routine chickenpox vaccination program started,” the agency’s surveillance pagenotes. “Other countries without routine chickenpox vaccination programs, have observed similar increases in shingles rates.”
Regardless of the “why” behind the association, Dr. Oza expects it to level out as vaccinated children come of shingles age. “As the Millenials take over, with their heightened immunity to varicella virus, we will eventually get to the point where zoster has less of an impact on clinical practice and the healthcare industry.”
He had no financial disclosures.
WASHINGTON – As the number of children vaccinated against varicella has risen, the number of chicken pox cases has proportionally declined.
No news there.
But, as the number of kids getting chickenpox gets smaller, the number of adults getting shingles is growing – and no one really knows why, Dr. Vikash Oza said at the annual meeting of the American Academy of Dermatology.
“Varicella vaccination has had a dramatic impact on the incidence of chickenpox,” said Dr. Oza, a pediatric dermatologist at New York University Langone Medical Center. “In fact, as a resident I saw not one case of chickenpox. That’s pretty astounding considering how common it used to be.”
Introduced in 1995, the vaccine now covers about 90% of children in this country – an achievement almost exactly mirrored by the decrease in disease incidence. In fact, according to a 2013 study, the bang for the vaccine buck may be even bigger, achieving 98% decreases in two regions examined (Pediatrics. 2013 Nov;132[5]:e1134-40).
That study also found that vaccinated children who did get chickenpox had much milder infections that resolved more quickly.
Again, that’s good news, but not particularly surprising, said Dr. Oza. The more intriguing change is the steady increase in herpes zoster among older adults occurring in tandem with the decreased incidence of chickenpox. Cases were up a total of 39% from 1992 to 2010 among adults 65 and older, according to the Centers for Disease Control and Prevention (Ann Intern Med. 2013;159[11]:739-45).
“This thing that’s happening in adults is quite interesting,” Dr. Oza said. “Although zoster in children is uncommon, we do know that children are much less likely to develop it if they’ve been vaccinated against varicella. What we don’t really know is what this means for adults. One of the effects of the vaccine, of course, is less circulating varicella zoster virus in our communities. It’s possible that a benefit of having some circulating VZV gives adults an immune boost that keeps zoster in check, and without that, developing shingles is more likely.”
Several recent studies have documented the association. A 2005 study found that during 1998-2003, the incidence of chickenpox in Massachusetts went down by 79%, while the incidence of shingles increased by 90% (BMC Public Health 2005;5:68. doi: 10.1186/1471-2458-5-68). But a shifting age-related immune response is only one possible explanation, the authors wrote. “There are several possible explanations, which may be operating in combination. Other possible explanations include increases in the proportion of people with immunosuppressive conditions and therapies, in the duration of those conditions and treatments, and/or in the prevalence of other triggering factors.”
The shifting incidences of chickenpox and shingles, however, were predicted years before they occurred. A mathematical model from 1992 predicted a long-term elevation of up to 20% in the shingles rate, relative to pre-vaccination incidence (Epidemiol Infect. 1992 Jun;108[3]:513–28). A 2001 paper predicted that shingles would increase for 30-50 years after mass varicella vaccination, rising to a maximum of about 50% above pre-vaccination rates, before falling below baseline levels (Epidemiol Infect. 2001 Oct;127[2]:305–14).
The CDC refutes any causative link between varicella vaccinations and shingles. “This proposed explanation seems unlikely based on two CDC studies which found that shingles rates started increasing before chickenpox vaccine was introduced in the United States, and after the routine chickenpox vaccination program started,” the agency’s surveillance pagenotes. “Other countries without routine chickenpox vaccination programs, have observed similar increases in shingles rates.”
Regardless of the “why” behind the association, Dr. Oza expects it to level out as vaccinated children come of shingles age. “As the Millenials take over, with their heightened immunity to varicella virus, we will eventually get to the point where zoster has less of an impact on clinical practice and the healthcare industry.”
He had no financial disclosures.
WASHINGTON – As the number of children vaccinated against varicella has risen, the number of chicken pox cases has proportionally declined.
No news there.
But, as the number of kids getting chickenpox gets smaller, the number of adults getting shingles is growing – and no one really knows why, Dr. Vikash Oza said at the annual meeting of the American Academy of Dermatology.
“Varicella vaccination has had a dramatic impact on the incidence of chickenpox,” said Dr. Oza, a pediatric dermatologist at New York University Langone Medical Center. “In fact, as a resident I saw not one case of chickenpox. That’s pretty astounding considering how common it used to be.”
Introduced in 1995, the vaccine now covers about 90% of children in this country – an achievement almost exactly mirrored by the decrease in disease incidence. In fact, according to a 2013 study, the bang for the vaccine buck may be even bigger, achieving 98% decreases in two regions examined (Pediatrics. 2013 Nov;132[5]:e1134-40).
That study also found that vaccinated children who did get chickenpox had much milder infections that resolved more quickly.
Again, that’s good news, but not particularly surprising, said Dr. Oza. The more intriguing change is the steady increase in herpes zoster among older adults occurring in tandem with the decreased incidence of chickenpox. Cases were up a total of 39% from 1992 to 2010 among adults 65 and older, according to the Centers for Disease Control and Prevention (Ann Intern Med. 2013;159[11]:739-45).
“This thing that’s happening in adults is quite interesting,” Dr. Oza said. “Although zoster in children is uncommon, we do know that children are much less likely to develop it if they’ve been vaccinated against varicella. What we don’t really know is what this means for adults. One of the effects of the vaccine, of course, is less circulating varicella zoster virus in our communities. It’s possible that a benefit of having some circulating VZV gives adults an immune boost that keeps zoster in check, and without that, developing shingles is more likely.”
Several recent studies have documented the association. A 2005 study found that during 1998-2003, the incidence of chickenpox in Massachusetts went down by 79%, while the incidence of shingles increased by 90% (BMC Public Health 2005;5:68. doi: 10.1186/1471-2458-5-68). But a shifting age-related immune response is only one possible explanation, the authors wrote. “There are several possible explanations, which may be operating in combination. Other possible explanations include increases in the proportion of people with immunosuppressive conditions and therapies, in the duration of those conditions and treatments, and/or in the prevalence of other triggering factors.”
The shifting incidences of chickenpox and shingles, however, were predicted years before they occurred. A mathematical model from 1992 predicted a long-term elevation of up to 20% in the shingles rate, relative to pre-vaccination incidence (Epidemiol Infect. 1992 Jun;108[3]:513–28). A 2001 paper predicted that shingles would increase for 30-50 years after mass varicella vaccination, rising to a maximum of about 50% above pre-vaccination rates, before falling below baseline levels (Epidemiol Infect. 2001 Oct;127[2]:305–14).
The CDC refutes any causative link between varicella vaccinations and shingles. “This proposed explanation seems unlikely based on two CDC studies which found that shingles rates started increasing before chickenpox vaccine was introduced in the United States, and after the routine chickenpox vaccination program started,” the agency’s surveillance pagenotes. “Other countries without routine chickenpox vaccination programs, have observed similar increases in shingles rates.”
Regardless of the “why” behind the association, Dr. Oza expects it to level out as vaccinated children come of shingles age. “As the Millenials take over, with their heightened immunity to varicella virus, we will eventually get to the point where zoster has less of an impact on clinical practice and the healthcare industry.”
He had no financial disclosures.
EXPERT ANALYSIS FROM AAD 2016
As varicella recedes, zoster rises: The question is ‘why?’
WASHINGTON – As the number of children vaccinated against varicella has risen, the number of chicken pox cases has proportionally declined.
No news there.
But, as the number of kids getting chickenpox gets smaller, the number of adults getting shingles is growing – and no one really knows why, Dr. Vikash Oza said at the annual meeting of the American Academy of Dermatology.
“Varicella vaccination has had a dramatic impact on the incidence of chickenpox,” said Dr. Oza, a pediatric dermatologist at New York University Langone Medical Center. “In fact, as a resident I saw not one case of chickenpox. That’s pretty astounding considering how common it used to be.”
Introduced in 1995, the vaccine now covers about 90% of children in this country – an achievement almost exactly mirrored by the decrease in disease incidence. In fact, according to a 2013 study, the bang for the vaccine buck may be even bigger, achieving 98% decreases in two regions examined (Pediatrics. 2013 Nov;132[5]:e1134-40).
That study also found that vaccinated children who did get chickenpox had much milder infections that resolved more quickly.
Again, that’s good news, but not particularly surprising, said Dr. Oza. The more intriguing change is the steady increase in herpes zoster among older adults occurring in tandem with the decreased incidence of chickenpox. Cases were up a total of 39% from 1992 to 2010 among adults 65 and older, according to the Centers for Disease Control and Prevention (Ann Intern Med. 2013;159[11]:739-45).
“This thing that’s happening in adults is quite interesting,” Dr. Oza said. “Although zoster in children is uncommon, we do know that children are much less likely to develop it if they’ve been vaccinated against varicella. What we don’t really know is what this means for adults. One of the effects of the vaccine, of course, is less circulating varicella zoster virus in our communities. It’s possible that a benefit of having some circulating VZV gives adults an immune boost that keeps zoster in check, and without that, developing shingles is more likely.”
Several recent studies have documented the association. A 2005 study found that during 1998-2003, the incidence of chickenpox in Massachusetts went down by 79%, while the incidence of shingles increased by 90% (BMC Public Health 2005;5:68. doi: 10.1186/1471-2458-5-68). But a shifting age-related immune response is only one possible explanation, the authors wrote. “There are several possible explanations, which may be operating in combination. Other possible explanations include increases in the proportion of people with immunosuppressive conditions and therapies, in the duration of those conditions and treatments, and/or in the prevalence of other triggering factors.”
The shifting incidences of chickenpox and shingles, however, were predicted years before they occurred. A mathematical model from 1992 predicted a long-term elevation of up to 20% in the shingles rate, relative to pre-vaccination incidence (Epidemiol Infect. 1992 Jun;108[3]:513–28). A 2001 paper predicted that shingles would increase for 30-50 years after mass varicella vaccination, rising to a maximum of about 50% above pre-vaccination rates, before falling below baseline levels (Epidemiol Infect. 2001 Oct;127[2]:305–14).
The CDC refutes any causative link between varicella vaccinations and shingles. “This proposed explanation seems unlikely based on two CDC studies which found that shingles rates started increasing before chickenpox vaccine was introduced in the United States, and after the routine chickenpox vaccination program started,” the agency’s surveillance pagenotes. “Other countries without routine chickenpox vaccination programs, have observed similar increases in shingles rates.”
Regardless of the “why” behind the association, Dr. Oza expects it to level out as vaccinated children come of shingles age. “As the Millenials take over, with their heightened immunity to varicella virus, we will eventually get to the point where zoster has less of an impact on clinical practice and the healthcare industry.”
He had no financial disclosures.
On Twitter @Alz_Gal
WASHINGTON – As the number of children vaccinated against varicella has risen, the number of chicken pox cases has proportionally declined.
No news there.
But, as the number of kids getting chickenpox gets smaller, the number of adults getting shingles is growing – and no one really knows why, Dr. Vikash Oza said at the annual meeting of the American Academy of Dermatology.
“Varicella vaccination has had a dramatic impact on the incidence of chickenpox,” said Dr. Oza, a pediatric dermatologist at New York University Langone Medical Center. “In fact, as a resident I saw not one case of chickenpox. That’s pretty astounding considering how common it used to be.”
Introduced in 1995, the vaccine now covers about 90% of children in this country – an achievement almost exactly mirrored by the decrease in disease incidence. In fact, according to a 2013 study, the bang for the vaccine buck may be even bigger, achieving 98% decreases in two regions examined (Pediatrics. 2013 Nov;132[5]:e1134-40).
That study also found that vaccinated children who did get chickenpox had much milder infections that resolved more quickly.
Again, that’s good news, but not particularly surprising, said Dr. Oza. The more intriguing change is the steady increase in herpes zoster among older adults occurring in tandem with the decreased incidence of chickenpox. Cases were up a total of 39% from 1992 to 2010 among adults 65 and older, according to the Centers for Disease Control and Prevention (Ann Intern Med. 2013;159[11]:739-45).
“This thing that’s happening in adults is quite interesting,” Dr. Oza said. “Although zoster in children is uncommon, we do know that children are much less likely to develop it if they’ve been vaccinated against varicella. What we don’t really know is what this means for adults. One of the effects of the vaccine, of course, is less circulating varicella zoster virus in our communities. It’s possible that a benefit of having some circulating VZV gives adults an immune boost that keeps zoster in check, and without that, developing shingles is more likely.”
Several recent studies have documented the association. A 2005 study found that during 1998-2003, the incidence of chickenpox in Massachusetts went down by 79%, while the incidence of shingles increased by 90% (BMC Public Health 2005;5:68. doi: 10.1186/1471-2458-5-68). But a shifting age-related immune response is only one possible explanation, the authors wrote. “There are several possible explanations, which may be operating in combination. Other possible explanations include increases in the proportion of people with immunosuppressive conditions and therapies, in the duration of those conditions and treatments, and/or in the prevalence of other triggering factors.”
The shifting incidences of chickenpox and shingles, however, were predicted years before they occurred. A mathematical model from 1992 predicted a long-term elevation of up to 20% in the shingles rate, relative to pre-vaccination incidence (Epidemiol Infect. 1992 Jun;108[3]:513–28). A 2001 paper predicted that shingles would increase for 30-50 years after mass varicella vaccination, rising to a maximum of about 50% above pre-vaccination rates, before falling below baseline levels (Epidemiol Infect. 2001 Oct;127[2]:305–14).
The CDC refutes any causative link between varicella vaccinations and shingles. “This proposed explanation seems unlikely based on two CDC studies which found that shingles rates started increasing before chickenpox vaccine was introduced in the United States, and after the routine chickenpox vaccination program started,” the agency’s surveillance pagenotes. “Other countries without routine chickenpox vaccination programs, have observed similar increases in shingles rates.”
Regardless of the “why” behind the association, Dr. Oza expects it to level out as vaccinated children come of shingles age. “As the Millenials take over, with their heightened immunity to varicella virus, we will eventually get to the point where zoster has less of an impact on clinical practice and the healthcare industry.”
He had no financial disclosures.
On Twitter @Alz_Gal
WASHINGTON – As the number of children vaccinated against varicella has risen, the number of chicken pox cases has proportionally declined.
No news there.
But, as the number of kids getting chickenpox gets smaller, the number of adults getting shingles is growing – and no one really knows why, Dr. Vikash Oza said at the annual meeting of the American Academy of Dermatology.
“Varicella vaccination has had a dramatic impact on the incidence of chickenpox,” said Dr. Oza, a pediatric dermatologist at New York University Langone Medical Center. “In fact, as a resident I saw not one case of chickenpox. That’s pretty astounding considering how common it used to be.”
Introduced in 1995, the vaccine now covers about 90% of children in this country – an achievement almost exactly mirrored by the decrease in disease incidence. In fact, according to a 2013 study, the bang for the vaccine buck may be even bigger, achieving 98% decreases in two regions examined (Pediatrics. 2013 Nov;132[5]:e1134-40).
That study also found that vaccinated children who did get chickenpox had much milder infections that resolved more quickly.
Again, that’s good news, but not particularly surprising, said Dr. Oza. The more intriguing change is the steady increase in herpes zoster among older adults occurring in tandem with the decreased incidence of chickenpox. Cases were up a total of 39% from 1992 to 2010 among adults 65 and older, according to the Centers for Disease Control and Prevention (Ann Intern Med. 2013;159[11]:739-45).
“This thing that’s happening in adults is quite interesting,” Dr. Oza said. “Although zoster in children is uncommon, we do know that children are much less likely to develop it if they’ve been vaccinated against varicella. What we don’t really know is what this means for adults. One of the effects of the vaccine, of course, is less circulating varicella zoster virus in our communities. It’s possible that a benefit of having some circulating VZV gives adults an immune boost that keeps zoster in check, and without that, developing shingles is more likely.”
Several recent studies have documented the association. A 2005 study found that during 1998-2003, the incidence of chickenpox in Massachusetts went down by 79%, while the incidence of shingles increased by 90% (BMC Public Health 2005;5:68. doi: 10.1186/1471-2458-5-68). But a shifting age-related immune response is only one possible explanation, the authors wrote. “There are several possible explanations, which may be operating in combination. Other possible explanations include increases in the proportion of people with immunosuppressive conditions and therapies, in the duration of those conditions and treatments, and/or in the prevalence of other triggering factors.”
The shifting incidences of chickenpox and shingles, however, were predicted years before they occurred. A mathematical model from 1992 predicted a long-term elevation of up to 20% in the shingles rate, relative to pre-vaccination incidence (Epidemiol Infect. 1992 Jun;108[3]:513–28). A 2001 paper predicted that shingles would increase for 30-50 years after mass varicella vaccination, rising to a maximum of about 50% above pre-vaccination rates, before falling below baseline levels (Epidemiol Infect. 2001 Oct;127[2]:305–14).
The CDC refutes any causative link between varicella vaccinations and shingles. “This proposed explanation seems unlikely based on two CDC studies which found that shingles rates started increasing before chickenpox vaccine was introduced in the United States, and after the routine chickenpox vaccination program started,” the agency’s surveillance pagenotes. “Other countries without routine chickenpox vaccination programs, have observed similar increases in shingles rates.”
Regardless of the “why” behind the association, Dr. Oza expects it to level out as vaccinated children come of shingles age. “As the Millenials take over, with their heightened immunity to varicella virus, we will eventually get to the point where zoster has less of an impact on clinical practice and the healthcare industry.”
He had no financial disclosures.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM AAD 2016