User login
American Academy of Dermatology (AAD): Annual Meeting
Check sweat glands, hair follicles in mycosis fungoides
SAN FRANCISCO – Check for syringotropism and folliculotropism in biopsies when managing mycosis fungoides, based on data from an ongoing observational, prospective study at Thomas Jefferson University in Philadelphia.
The presence of syringotropism and folliculotropism indicates the need for more aggressive treatment, according to lead investigator Dr. Joya Sahu, of the department of dermatology at the university.
Mycosis fungoides – the most common form of cutaneous T-cell lymphoma – is usually thought to favor the epidermis, but investigators at Thomas Jefferson University have found that it often works its tentacles deeper into the skin to attack hair follicles (folliculotropism) or eccrine glands (syringotropism), Dr. Sahu said at the annual meeting of the American Academy of Dermatology.
The researchers checked biopsy samples to see how common those variants were in 34 new patients with mycosis fungoides (most with stage 1 disease). Overall, 18 (52.9%) had folliculotropism, 22 (64.7%) had syringotropism, and 15 (44.1%) had both.
Not surprisingly, deeper penetration indicated worse disease, Dr. Sahu said. On the modified Severity Weighted Assessment tool (mSWAT) – a measure of surface area involvement and lesion severity – the mean scores were 57.51 in patients with folliculotropism, 59.4 in patients with syringotropism, and 66.4 in patients with both. The higher mSWAT scores also correlated with more severe pruritus and the likelihood that the patient had tried four or more treatments. By contrast, the nine patients without folliculotropism or syringotropism, who had a mean mSWAT score of 16.85, had tried only one or two treatments.
Almost all of the cases presented classically; two had head and neck lesions or other signs of folliculotropic disease, and both of these patients had folliculotropism and syringotropism on biopsy. None of the patients had a syringotropic presentation.
“The majority of patients studied exhibited either folliculotropism or syringotropism, implying greater prevalence,” Dr. Sahu said. “These presentations also have findings indicative of more severe disease. We propose that histopathology reports on patients with suspected [mycosis fungoides] should document the presence of folliculotropism and syringotropism as they may aid in diagnosis and in predicting severity and progression risk,” she noted.
She cautioned, however, that her clinic is a tertiary referral center, and as such might see patients with more severe disease, compared with other clinics.
The patients were otherwise typical of the mycosis fungoides population, she said. About two-thirds were men, and the average age was 63 years.
Dr. Sahu said she had no relevant financial conflicts of interest.
SAN FRANCISCO – Check for syringotropism and folliculotropism in biopsies when managing mycosis fungoides, based on data from an ongoing observational, prospective study at Thomas Jefferson University in Philadelphia.
The presence of syringotropism and folliculotropism indicates the need for more aggressive treatment, according to lead investigator Dr. Joya Sahu, of the department of dermatology at the university.
Mycosis fungoides – the most common form of cutaneous T-cell lymphoma – is usually thought to favor the epidermis, but investigators at Thomas Jefferson University have found that it often works its tentacles deeper into the skin to attack hair follicles (folliculotropism) or eccrine glands (syringotropism), Dr. Sahu said at the annual meeting of the American Academy of Dermatology.
The researchers checked biopsy samples to see how common those variants were in 34 new patients with mycosis fungoides (most with stage 1 disease). Overall, 18 (52.9%) had folliculotropism, 22 (64.7%) had syringotropism, and 15 (44.1%) had both.
Not surprisingly, deeper penetration indicated worse disease, Dr. Sahu said. On the modified Severity Weighted Assessment tool (mSWAT) – a measure of surface area involvement and lesion severity – the mean scores were 57.51 in patients with folliculotropism, 59.4 in patients with syringotropism, and 66.4 in patients with both. The higher mSWAT scores also correlated with more severe pruritus and the likelihood that the patient had tried four or more treatments. By contrast, the nine patients without folliculotropism or syringotropism, who had a mean mSWAT score of 16.85, had tried only one or two treatments.
Almost all of the cases presented classically; two had head and neck lesions or other signs of folliculotropic disease, and both of these patients had folliculotropism and syringotropism on biopsy. None of the patients had a syringotropic presentation.
“The majority of patients studied exhibited either folliculotropism or syringotropism, implying greater prevalence,” Dr. Sahu said. “These presentations also have findings indicative of more severe disease. We propose that histopathology reports on patients with suspected [mycosis fungoides] should document the presence of folliculotropism and syringotropism as they may aid in diagnosis and in predicting severity and progression risk,” she noted.
She cautioned, however, that her clinic is a tertiary referral center, and as such might see patients with more severe disease, compared with other clinics.
The patients were otherwise typical of the mycosis fungoides population, she said. About two-thirds were men, and the average age was 63 years.
Dr. Sahu said she had no relevant financial conflicts of interest.
SAN FRANCISCO – Check for syringotropism and folliculotropism in biopsies when managing mycosis fungoides, based on data from an ongoing observational, prospective study at Thomas Jefferson University in Philadelphia.
The presence of syringotropism and folliculotropism indicates the need for more aggressive treatment, according to lead investigator Dr. Joya Sahu, of the department of dermatology at the university.
Mycosis fungoides – the most common form of cutaneous T-cell lymphoma – is usually thought to favor the epidermis, but investigators at Thomas Jefferson University have found that it often works its tentacles deeper into the skin to attack hair follicles (folliculotropism) or eccrine glands (syringotropism), Dr. Sahu said at the annual meeting of the American Academy of Dermatology.
The researchers checked biopsy samples to see how common those variants were in 34 new patients with mycosis fungoides (most with stage 1 disease). Overall, 18 (52.9%) had folliculotropism, 22 (64.7%) had syringotropism, and 15 (44.1%) had both.
Not surprisingly, deeper penetration indicated worse disease, Dr. Sahu said. On the modified Severity Weighted Assessment tool (mSWAT) – a measure of surface area involvement and lesion severity – the mean scores were 57.51 in patients with folliculotropism, 59.4 in patients with syringotropism, and 66.4 in patients with both. The higher mSWAT scores also correlated with more severe pruritus and the likelihood that the patient had tried four or more treatments. By contrast, the nine patients without folliculotropism or syringotropism, who had a mean mSWAT score of 16.85, had tried only one or two treatments.
Almost all of the cases presented classically; two had head and neck lesions or other signs of folliculotropic disease, and both of these patients had folliculotropism and syringotropism on biopsy. None of the patients had a syringotropic presentation.
“The majority of patients studied exhibited either folliculotropism or syringotropism, implying greater prevalence,” Dr. Sahu said. “These presentations also have findings indicative of more severe disease. We propose that histopathology reports on patients with suspected [mycosis fungoides] should document the presence of folliculotropism and syringotropism as they may aid in diagnosis and in predicting severity and progression risk,” she noted.
She cautioned, however, that her clinic is a tertiary referral center, and as such might see patients with more severe disease, compared with other clinics.
The patients were otherwise typical of the mycosis fungoides population, she said. About two-thirds were men, and the average age was 63 years.
Dr. Sahu said she had no relevant financial conflicts of interest.
AT THE AAD ANNUAL MEETING
Key clinical point: Routinely check mycosis fungoides patients for sweat gland and hair follicle involvement.
Major finding: Among 34mycosis fungoides patients with mostly stage 1 disease, 18 (52.9%) had folliculotropism, 22 (64.7%) had syringotropism, and 15 (44.1%) had both on biopsy.
Data source: An observational, prospective study of 34 adults with mycosis fungoides.
Disclosures: The lead investigator declared no relevant financial conflicts.
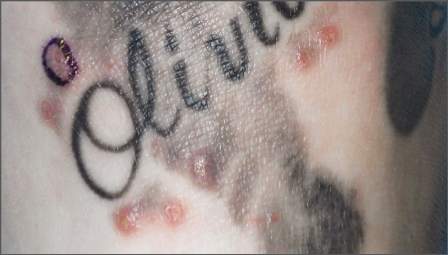
Teenage tattoos are most often regretted
SAN FRANCISCO – The younger people are when they get a tattoo, the more likely they are to regret it later, according to a survey of 501 people in the French Quarter of New Orleans.
All the participants were aged 18 years or older and had at least one tattoo. Overall, 16.2% said they regretted at least one tattoo, but that number rose to more than a third (35%) among the 77 people who got their first tattoo when they were 17 years old or younger. Among the 257 people who waited until they were 18-20 years old, 13.6% regretted one or more of their tattoos.
The rest got their first tattoo when they were at least 21 years old; 11.4% had regrets.
About one in five Americans have a tattoo, with the number increasing to about a third or more in people 18-30 years old, according to Walter Liszewski, a medical student at Tulane University in New Orleans.
Previous studies have found a regret rate of 16%-44%. The association with age suggests that it might be wise, when possible, to counsel teenagers to hold off for a while, Mr. Liszewski said. There’s a chance they will listen to that message, too, if it comes from a dermatologist. In the survey, 93% of people thought that tattoo artists were the best source of information on tattoo complications and how to treat them, but dermatologists weren’t far behind, with about 80% of respondents saying that dermatologists also were a trusted source for reliable information about tattoos. “So don’t hesitate to engage with patients about tattoos, complications, and other questions they might have,” Mr. Liszewski said at the annual meeting of the American Academy of Dermatology.
About 70% of the survey participants said they felt comfortable discussing such issues with primary care providers, and 40% said they felt comfortable discussing such issues with pharmacists.
Age requirements for tattoos vary widely from state to state, but some do allow individuals under 18 years of age to get a tattoo, even without parent permission.
In the first few days of 2015, the investigators asked passersby in the French Quarter’s Jackson Square if they had at least one tattoo, then offered the 18-question survey. They ran the project between 11 a.m. and 3 p.m., when people were less likely to be tipsy, Mr. Liszewski said. Respondents came from 38 states, plus the District of Columbia and Puerto Rico; most were from the Southeast, including 39% from Louisiana. Just over half were women, and the majority were white.
“We were also concerned that 21.2% received a tattoo while intoxicated, and 17.6% had at least one tattoo” done at someplace other than a tattoo parlor, he said. That means that if “patients have a lot of tattoos and you are trying to make small talk, feel free to ask them where they’re getting their tattoos done,” he added.
If tattoos were received at a party, or in prison or someplace else, “you may want to consider counseling them on HIV and hepatitis C testing,” Mr. Liszewski noted.
Just over 3% of the participants reported having had an infected tattoo, and while it’s normal for tattoos to be itchy and a little bit painful for the first week or two, 22.6% of the respondents said they had a pruritic tattoo, and 3.8% said they’d had a painful one a month or more after it was done.
“Tattoo complications are not uncommon,” Mr. Liszewski said. Given how much tattooed people seem to trust dermatologists, “there is an opportunity for dermatologists to manage these complications,” he added.
Mr. Liszewski said he had no disclosures.
SAN FRANCISCO – The younger people are when they get a tattoo, the more likely they are to regret it later, according to a survey of 501 people in the French Quarter of New Orleans.
All the participants were aged 18 years or older and had at least one tattoo. Overall, 16.2% said they regretted at least one tattoo, but that number rose to more than a third (35%) among the 77 people who got their first tattoo when they were 17 years old or younger. Among the 257 people who waited until they were 18-20 years old, 13.6% regretted one or more of their tattoos.
The rest got their first tattoo when they were at least 21 years old; 11.4% had regrets.
About one in five Americans have a tattoo, with the number increasing to about a third or more in people 18-30 years old, according to Walter Liszewski, a medical student at Tulane University in New Orleans.
Previous studies have found a regret rate of 16%-44%. The association with age suggests that it might be wise, when possible, to counsel teenagers to hold off for a while, Mr. Liszewski said. There’s a chance they will listen to that message, too, if it comes from a dermatologist. In the survey, 93% of people thought that tattoo artists were the best source of information on tattoo complications and how to treat them, but dermatologists weren’t far behind, with about 80% of respondents saying that dermatologists also were a trusted source for reliable information about tattoos. “So don’t hesitate to engage with patients about tattoos, complications, and other questions they might have,” Mr. Liszewski said at the annual meeting of the American Academy of Dermatology.
About 70% of the survey participants said they felt comfortable discussing such issues with primary care providers, and 40% said they felt comfortable discussing such issues with pharmacists.
Age requirements for tattoos vary widely from state to state, but some do allow individuals under 18 years of age to get a tattoo, even without parent permission.
In the first few days of 2015, the investigators asked passersby in the French Quarter’s Jackson Square if they had at least one tattoo, then offered the 18-question survey. They ran the project between 11 a.m. and 3 p.m., when people were less likely to be tipsy, Mr. Liszewski said. Respondents came from 38 states, plus the District of Columbia and Puerto Rico; most were from the Southeast, including 39% from Louisiana. Just over half were women, and the majority were white.
“We were also concerned that 21.2% received a tattoo while intoxicated, and 17.6% had at least one tattoo” done at someplace other than a tattoo parlor, he said. That means that if “patients have a lot of tattoos and you are trying to make small talk, feel free to ask them where they’re getting their tattoos done,” he added.
If tattoos were received at a party, or in prison or someplace else, “you may want to consider counseling them on HIV and hepatitis C testing,” Mr. Liszewski noted.
Just over 3% of the participants reported having had an infected tattoo, and while it’s normal for tattoos to be itchy and a little bit painful for the first week or two, 22.6% of the respondents said they had a pruritic tattoo, and 3.8% said they’d had a painful one a month or more after it was done.
“Tattoo complications are not uncommon,” Mr. Liszewski said. Given how much tattooed people seem to trust dermatologists, “there is an opportunity for dermatologists to manage these complications,” he added.
Mr. Liszewski said he had no disclosures.
SAN FRANCISCO – The younger people are when they get a tattoo, the more likely they are to regret it later, according to a survey of 501 people in the French Quarter of New Orleans.
All the participants were aged 18 years or older and had at least one tattoo. Overall, 16.2% said they regretted at least one tattoo, but that number rose to more than a third (35%) among the 77 people who got their first tattoo when they were 17 years old or younger. Among the 257 people who waited until they were 18-20 years old, 13.6% regretted one or more of their tattoos.
The rest got their first tattoo when they were at least 21 years old; 11.4% had regrets.
About one in five Americans have a tattoo, with the number increasing to about a third or more in people 18-30 years old, according to Walter Liszewski, a medical student at Tulane University in New Orleans.
Previous studies have found a regret rate of 16%-44%. The association with age suggests that it might be wise, when possible, to counsel teenagers to hold off for a while, Mr. Liszewski said. There’s a chance they will listen to that message, too, if it comes from a dermatologist. In the survey, 93% of people thought that tattoo artists were the best source of information on tattoo complications and how to treat them, but dermatologists weren’t far behind, with about 80% of respondents saying that dermatologists also were a trusted source for reliable information about tattoos. “So don’t hesitate to engage with patients about tattoos, complications, and other questions they might have,” Mr. Liszewski said at the annual meeting of the American Academy of Dermatology.
About 70% of the survey participants said they felt comfortable discussing such issues with primary care providers, and 40% said they felt comfortable discussing such issues with pharmacists.
Age requirements for tattoos vary widely from state to state, but some do allow individuals under 18 years of age to get a tattoo, even without parent permission.
In the first few days of 2015, the investigators asked passersby in the French Quarter’s Jackson Square if they had at least one tattoo, then offered the 18-question survey. They ran the project between 11 a.m. and 3 p.m., when people were less likely to be tipsy, Mr. Liszewski said. Respondents came from 38 states, plus the District of Columbia and Puerto Rico; most were from the Southeast, including 39% from Louisiana. Just over half were women, and the majority were white.
“We were also concerned that 21.2% received a tattoo while intoxicated, and 17.6% had at least one tattoo” done at someplace other than a tattoo parlor, he said. That means that if “patients have a lot of tattoos and you are trying to make small talk, feel free to ask them where they’re getting their tattoos done,” he added.
If tattoos were received at a party, or in prison or someplace else, “you may want to consider counseling them on HIV and hepatitis C testing,” Mr. Liszewski noted.
Just over 3% of the participants reported having had an infected tattoo, and while it’s normal for tattoos to be itchy and a little bit painful for the first week or two, 22.6% of the respondents said they had a pruritic tattoo, and 3.8% said they’d had a painful one a month or more after it was done.
“Tattoo complications are not uncommon,” Mr. Liszewski said. Given how much tattooed people seem to trust dermatologists, “there is an opportunity for dermatologists to manage these complications,” he added.
Mr. Liszewski said he had no disclosures.
AT THE AAD ANNUAL MEETING
Key clinical point: It might be wise, when possible, to counsel teenagers to postpone tattoos.
Major finding: Overall, 16.2% of tattooed adults said they regretted at least one, but that number rose to 35% among people who got their first tattoo when they were 17 years old or younger.
Data source: An in-person survey of 501 people with at least one tattoo.
Disclosures: The lead investigator had no disclosures.
Apremilast meets psoriasis endpoints at week 32
SAN FRANCISCO – Apremilast met its primary endpoint at week 32 with no new or serious safety signals among patients with moderate to severe plaque psoriasis, according to phase III data from the ongoing LIBERATE study.
At week 16, 40% of patients who received oral apremilast achieved PASI-75, compared with 12% of the placebo group, Dr. Kristian Reich reported at the annual meeting of the American Academy of Dermatology.
By week 32, PASI-75 response for apremilast had further risen to 53%, he added. “This drug may not be a quick fix, but the longer you give it, the higher the response,” said Dr. Reich of SCIderm Research Institute and Dermatologikum Hamburg in Germany.
The randomized, double-blind LIBERATE study compared the safety and efficacy of apremilast (30 mg twice daily) and injectable etanercept (50 mg weekly) with placebo among 250 patients with plaque psoriasis who had not previously received biologic therapy. Patients received one of the three treatments through week 16, and then all were switched to or continued apremilast.
At week 16, PASI-75 response rates were 40% for apremilast, 48% for etanercept, and 12% for placebo (P < .0001 for apremilast versus placebo), reported Dr. Reich. The high rate of response to placebo might have occurred because patients in the study were treatment naive, he said. At week 32, PASI-75 response rates were 53% for patients who received apremilast from baseline, and 61% for patients who switched from etanercept to apremilast at week 16.
Based on the results, “I would probably use apremilast more in the moderate [psoriasis] space than in the severe space, but its efficacy does not correlate with baseline disease severity, as far as I know,” said Dr. Reich. Apremilast also beat placebo in analyses of secondary endpoints, including static physician global assessment of clear or almost clear and the Dermatology Quality of Life Index, he added.
Safety analyses showed that the drug was generally well tolerated. Fewer than 4% of patients discontinued treatment because of adverse events, the most common of which were diarrhea, nausea, vomiting, and headache (including tension headache). No new side effects emerged after patients switched from etanercept to apremilast at week 16, he said.
A post hoc analysis found that apremilast was noninferior to etanercept (P > .05) in terms of PASI-75, although the study was not powered to directly compare the two biologics, Dr. Reich noted.
Celgene Corporation makes apremilast and sponsored the study. Dr. Reich reported serving as a speaker for and receiving honoraria from Celgene.
SAN FRANCISCO – Apremilast met its primary endpoint at week 32 with no new or serious safety signals among patients with moderate to severe plaque psoriasis, according to phase III data from the ongoing LIBERATE study.
At week 16, 40% of patients who received oral apremilast achieved PASI-75, compared with 12% of the placebo group, Dr. Kristian Reich reported at the annual meeting of the American Academy of Dermatology.
By week 32, PASI-75 response for apremilast had further risen to 53%, he added. “This drug may not be a quick fix, but the longer you give it, the higher the response,” said Dr. Reich of SCIderm Research Institute and Dermatologikum Hamburg in Germany.
The randomized, double-blind LIBERATE study compared the safety and efficacy of apremilast (30 mg twice daily) and injectable etanercept (50 mg weekly) with placebo among 250 patients with plaque psoriasis who had not previously received biologic therapy. Patients received one of the three treatments through week 16, and then all were switched to or continued apremilast.
At week 16, PASI-75 response rates were 40% for apremilast, 48% for etanercept, and 12% for placebo (P < .0001 for apremilast versus placebo), reported Dr. Reich. The high rate of response to placebo might have occurred because patients in the study were treatment naive, he said. At week 32, PASI-75 response rates were 53% for patients who received apremilast from baseline, and 61% for patients who switched from etanercept to apremilast at week 16.
Based on the results, “I would probably use apremilast more in the moderate [psoriasis] space than in the severe space, but its efficacy does not correlate with baseline disease severity, as far as I know,” said Dr. Reich. Apremilast also beat placebo in analyses of secondary endpoints, including static physician global assessment of clear or almost clear and the Dermatology Quality of Life Index, he added.
Safety analyses showed that the drug was generally well tolerated. Fewer than 4% of patients discontinued treatment because of adverse events, the most common of which were diarrhea, nausea, vomiting, and headache (including tension headache). No new side effects emerged after patients switched from etanercept to apremilast at week 16, he said.
A post hoc analysis found that apremilast was noninferior to etanercept (P > .05) in terms of PASI-75, although the study was not powered to directly compare the two biologics, Dr. Reich noted.
Celgene Corporation makes apremilast and sponsored the study. Dr. Reich reported serving as a speaker for and receiving honoraria from Celgene.
SAN FRANCISCO – Apremilast met its primary endpoint at week 32 with no new or serious safety signals among patients with moderate to severe plaque psoriasis, according to phase III data from the ongoing LIBERATE study.
At week 16, 40% of patients who received oral apremilast achieved PASI-75, compared with 12% of the placebo group, Dr. Kristian Reich reported at the annual meeting of the American Academy of Dermatology.
By week 32, PASI-75 response for apremilast had further risen to 53%, he added. “This drug may not be a quick fix, but the longer you give it, the higher the response,” said Dr. Reich of SCIderm Research Institute and Dermatologikum Hamburg in Germany.
The randomized, double-blind LIBERATE study compared the safety and efficacy of apremilast (30 mg twice daily) and injectable etanercept (50 mg weekly) with placebo among 250 patients with plaque psoriasis who had not previously received biologic therapy. Patients received one of the three treatments through week 16, and then all were switched to or continued apremilast.
At week 16, PASI-75 response rates were 40% for apremilast, 48% for etanercept, and 12% for placebo (P < .0001 for apremilast versus placebo), reported Dr. Reich. The high rate of response to placebo might have occurred because patients in the study were treatment naive, he said. At week 32, PASI-75 response rates were 53% for patients who received apremilast from baseline, and 61% for patients who switched from etanercept to apremilast at week 16.
Based on the results, “I would probably use apremilast more in the moderate [psoriasis] space than in the severe space, but its efficacy does not correlate with baseline disease severity, as far as I know,” said Dr. Reich. Apremilast also beat placebo in analyses of secondary endpoints, including static physician global assessment of clear or almost clear and the Dermatology Quality of Life Index, he added.
Safety analyses showed that the drug was generally well tolerated. Fewer than 4% of patients discontinued treatment because of adverse events, the most common of which were diarrhea, nausea, vomiting, and headache (including tension headache). No new side effects emerged after patients switched from etanercept to apremilast at week 16, he said.
A post hoc analysis found that apremilast was noninferior to etanercept (P > .05) in terms of PASI-75, although the study was not powered to directly compare the two biologics, Dr. Reich noted.
Celgene Corporation makes apremilast and sponsored the study. Dr. Reich reported serving as a speaker for and receiving honoraria from Celgene.
AT THE AAD ANNUAL MEETING
Key clinical point: Oral apremilast beat placebo and showed durable efficacy in patients with moderate to severe plaque psoriasis.
Major finding: PASI-75 response rates were 40% at week 16 (compared with 12% for placebo) and 53% at week 32.
Data source: Ongoing phase IIIb study of apremilast, etanercept, and placebo among 250 patients with moderate to severe plaque psoriasis.
Disclosures: Celgene Corporation makes apremilast and sponsored the study. Dr. Reich reported serving as a speaker for and receiving honoraria from Celgene.
Ixekizumab met psoriasis endpoints by week 12, with durable response at 60 weeks
SAN FRANCISCO – More than 80% of psoriasis patients who received ixekizumab achieved a 75% reduction in the Psoriasis Area and Severity Index score and static Physician Global Assessment scores of clear or almost clear skin at 12 weeks, based on the results of a phase III trial.
In addition, 35% of patients who received 80 mg ixekizumab twice monthly for 12 weeks achieved complete resolution of their psoriasis plaques (PASI 100), reported Dr. Kenneth Gordon of Northwestern University in Chicago. “The overall safety profile for ixekizumab was acceptable during both the induction and maintenance phases,” Dr. Gordon said at the annual meeting of the American Academy of Dermatology.
Ixekizumab is a monoclonal antibody that targets interleukin (IL)-17A, a major cytokine in the pathogenesis of psoriasis. Dr. Gordon and his associates conducted a randomized induction and withdrawal trial that compared twice-monthly or monthly treatment of the biologic with placebo among 1,296 patients. During the 12-week induction phase, 431 patients received placebo, 433 received 80 mg ixekizumab every 2 weeks, and 432 received 80 mg ixekizumab every 4 weeks, said Dr. Gordon. He and his associates then rerandomized the responders (based on PASI 75 and static Patient Global Assessment [sPGA] scores) to one of the three protocols, and followed these patients until week 60.
About 80% of patients who received ixekizumab every 2 or 4 weeks achieved sPGA scores of 0 or 1 (clear or almost clear) at 12 weeks, compared with 3% of the placebo group, said Dr. Gordon. Rates of PASI 75 response at 12 weeks were 89% for patients treated twice monthly, 83% for patients treated monthly, and 4% for the placebo group. Rates of PASI 100 were 35%, 33%, and 0%, respectively.
Among initial responders who were rerandomized to monthly ixekizumab, 73% maintained sPGA scores of clear or almost clear at week 60, while 78% maintained or achieved PASI 75, and 52% maintained or achieved PASI 100, said Dr. Gordon. The investigators also found a significant positive linear correlation between PASI response and scores on the Dermatology Quality of Life Index (DLQI), with P values ranging from less than .001 to less than .002, Dr. Gordon reported. “We are seeing that clearance is very important in quality of life,” he added. “More patients reported no itching or other negative impact on quality of life with higher levels of response.”
Serious adverse events at week 12 affected 1.4% of the twice-monthly ixekizumab group, 2.8% of the monthly ixekizumab group, and 1.2% of the placebo group, Dr. Gordon reported. Rates of candidiasis were similar among all three arms. Between weeks 12 and 60, the monthly treatment group had three major adverse cardiac events, but exposure-adjusted rates of adverse events for this group were similar to those during the induction period, he added.
Eli Lilly sponsored the trial. Dr. Gordon reported receiving research funding from Eli Lilly and several other pharmaceutical companies.
SAN FRANCISCO – More than 80% of psoriasis patients who received ixekizumab achieved a 75% reduction in the Psoriasis Area and Severity Index score and static Physician Global Assessment scores of clear or almost clear skin at 12 weeks, based on the results of a phase III trial.
In addition, 35% of patients who received 80 mg ixekizumab twice monthly for 12 weeks achieved complete resolution of their psoriasis plaques (PASI 100), reported Dr. Kenneth Gordon of Northwestern University in Chicago. “The overall safety profile for ixekizumab was acceptable during both the induction and maintenance phases,” Dr. Gordon said at the annual meeting of the American Academy of Dermatology.
Ixekizumab is a monoclonal antibody that targets interleukin (IL)-17A, a major cytokine in the pathogenesis of psoriasis. Dr. Gordon and his associates conducted a randomized induction and withdrawal trial that compared twice-monthly or monthly treatment of the biologic with placebo among 1,296 patients. During the 12-week induction phase, 431 patients received placebo, 433 received 80 mg ixekizumab every 2 weeks, and 432 received 80 mg ixekizumab every 4 weeks, said Dr. Gordon. He and his associates then rerandomized the responders (based on PASI 75 and static Patient Global Assessment [sPGA] scores) to one of the three protocols, and followed these patients until week 60.
About 80% of patients who received ixekizumab every 2 or 4 weeks achieved sPGA scores of 0 or 1 (clear or almost clear) at 12 weeks, compared with 3% of the placebo group, said Dr. Gordon. Rates of PASI 75 response at 12 weeks were 89% for patients treated twice monthly, 83% for patients treated monthly, and 4% for the placebo group. Rates of PASI 100 were 35%, 33%, and 0%, respectively.
Among initial responders who were rerandomized to monthly ixekizumab, 73% maintained sPGA scores of clear or almost clear at week 60, while 78% maintained or achieved PASI 75, and 52% maintained or achieved PASI 100, said Dr. Gordon. The investigators also found a significant positive linear correlation between PASI response and scores on the Dermatology Quality of Life Index (DLQI), with P values ranging from less than .001 to less than .002, Dr. Gordon reported. “We are seeing that clearance is very important in quality of life,” he added. “More patients reported no itching or other negative impact on quality of life with higher levels of response.”
Serious adverse events at week 12 affected 1.4% of the twice-monthly ixekizumab group, 2.8% of the monthly ixekizumab group, and 1.2% of the placebo group, Dr. Gordon reported. Rates of candidiasis were similar among all three arms. Between weeks 12 and 60, the monthly treatment group had three major adverse cardiac events, but exposure-adjusted rates of adverse events for this group were similar to those during the induction period, he added.
Eli Lilly sponsored the trial. Dr. Gordon reported receiving research funding from Eli Lilly and several other pharmaceutical companies.
SAN FRANCISCO – More than 80% of psoriasis patients who received ixekizumab achieved a 75% reduction in the Psoriasis Area and Severity Index score and static Physician Global Assessment scores of clear or almost clear skin at 12 weeks, based on the results of a phase III trial.
In addition, 35% of patients who received 80 mg ixekizumab twice monthly for 12 weeks achieved complete resolution of their psoriasis plaques (PASI 100), reported Dr. Kenneth Gordon of Northwestern University in Chicago. “The overall safety profile for ixekizumab was acceptable during both the induction and maintenance phases,” Dr. Gordon said at the annual meeting of the American Academy of Dermatology.
Ixekizumab is a monoclonal antibody that targets interleukin (IL)-17A, a major cytokine in the pathogenesis of psoriasis. Dr. Gordon and his associates conducted a randomized induction and withdrawal trial that compared twice-monthly or monthly treatment of the biologic with placebo among 1,296 patients. During the 12-week induction phase, 431 patients received placebo, 433 received 80 mg ixekizumab every 2 weeks, and 432 received 80 mg ixekizumab every 4 weeks, said Dr. Gordon. He and his associates then rerandomized the responders (based on PASI 75 and static Patient Global Assessment [sPGA] scores) to one of the three protocols, and followed these patients until week 60.
About 80% of patients who received ixekizumab every 2 or 4 weeks achieved sPGA scores of 0 or 1 (clear or almost clear) at 12 weeks, compared with 3% of the placebo group, said Dr. Gordon. Rates of PASI 75 response at 12 weeks were 89% for patients treated twice monthly, 83% for patients treated monthly, and 4% for the placebo group. Rates of PASI 100 were 35%, 33%, and 0%, respectively.
Among initial responders who were rerandomized to monthly ixekizumab, 73% maintained sPGA scores of clear or almost clear at week 60, while 78% maintained or achieved PASI 75, and 52% maintained or achieved PASI 100, said Dr. Gordon. The investigators also found a significant positive linear correlation between PASI response and scores on the Dermatology Quality of Life Index (DLQI), with P values ranging from less than .001 to less than .002, Dr. Gordon reported. “We are seeing that clearance is very important in quality of life,” he added. “More patients reported no itching or other negative impact on quality of life with higher levels of response.”
Serious adverse events at week 12 affected 1.4% of the twice-monthly ixekizumab group, 2.8% of the monthly ixekizumab group, and 1.2% of the placebo group, Dr. Gordon reported. Rates of candidiasis were similar among all three arms. Between weeks 12 and 60, the monthly treatment group had three major adverse cardiac events, but exposure-adjusted rates of adverse events for this group were similar to those during the induction period, he added.
Eli Lilly sponsored the trial. Dr. Gordon reported receiving research funding from Eli Lilly and several other pharmaceutical companies.
AT THE AAD ANNUAL MEETING
Key clinical point: The investigational anti-IL-17A antibody ixekizumab met its endpoints at week 12 in patients with plaque psoriasis, and showed durability of response at 60 weeks.
Major finding: More than 80% of treated patients achieved PASI 75 and static Physician Global Assessment scores of clear or almost clear skin at 12 weeks.
Data source: A phase III trial of 1,296 patients with plaque psoriasis.
Disclosures: Eli Lilly sponsored the trial. Dr. Gordon reported receiving research funding from Eli Lilly and several other pharmaceutical companies.
Worse melanoma outcomes found in pregnant women
SAN FRANCISCO – Pregnancy increases the risk of poor outcomes in melanoma, according to a review of melanoma cases at the Cleveland Clinic.
The effect of pregnancy on melanoma has been debated for more than a decade. Some studies have found evidence of worse outcomes, but others have not. That prompted Dr. Natasha Mesinkovska, a dermatologist at the clinic, and her colleagues to review their own melanoma outcomes over the past 20 years. They compared 49 women who were pregnant or within a year of pregnancy at diagnosis, with 418 women of childbearing age who were not pregnant. All the patients had at least 2 years follow-up.
Mortality (20% vs. 10.3%; P = .06); recurrence (12.5% vs. 1.4%; P < .001); metastasis (25% vs. 12.7%; P = .03); and the use of radiation and chemotherapy were all more common in the pregnancy group. On logistic regression, women who were pregnant or recently pregnant at the time of melanoma diagnosis were 5.1 times more likely to die of the disease than those who weren’t (P = .03), Dr. Mesinkovska reported at the annual meeting of the American Academy of Dermatology.
The findings prompted the investigators to compare histologic specimens from 17 pregnant and 14 nonpregnant women to find an explanation.
Pregnancy melanomas had reduced PD-1 [programmed cell death] expression (18.3 vs. 45 cells per high-power field [hpf]); decreased CD-3 [cluster of differentiation 3] (191.7 vs. 265.7 cells/hpf); and increased CD-3/PD-1 ratios (57.4 vs. 8.3).
The findings were statistically significant and may have treatment implications for the use in recently pregnant women of antibodies against PD-1 cell-surface receptors, a class of biologics that include nivolumab and pembrolizumab, both approved in 2014 for advanced melanoma. With reduced expression of PD-1, tumors arising around pregnancy might not be as sensitive to such agents.
Labeling for both biologics, however, note the risk of fetal harm, and advise the use of effective contraception while on the agents and for several months after their discontinuation. Both agents should also be discontinued during breastfeeding.
“Immune ignorance may predominate in melanoma specimens in pregnancy. Most novel melanoma therapies target immune modulation. There’s a need in some pregnant women for neoadjuvant treatment prior to immunotherapy to convert them to an inflamed phenotype,” Dr. Mesinkovska said.
Dr. Mesinkovska said she has no relevant financial disclosures.
SAN FRANCISCO – Pregnancy increases the risk of poor outcomes in melanoma, according to a review of melanoma cases at the Cleveland Clinic.
The effect of pregnancy on melanoma has been debated for more than a decade. Some studies have found evidence of worse outcomes, but others have not. That prompted Dr. Natasha Mesinkovska, a dermatologist at the clinic, and her colleagues to review their own melanoma outcomes over the past 20 years. They compared 49 women who were pregnant or within a year of pregnancy at diagnosis, with 418 women of childbearing age who were not pregnant. All the patients had at least 2 years follow-up.
Mortality (20% vs. 10.3%; P = .06); recurrence (12.5% vs. 1.4%; P < .001); metastasis (25% vs. 12.7%; P = .03); and the use of radiation and chemotherapy were all more common in the pregnancy group. On logistic regression, women who were pregnant or recently pregnant at the time of melanoma diagnosis were 5.1 times more likely to die of the disease than those who weren’t (P = .03), Dr. Mesinkovska reported at the annual meeting of the American Academy of Dermatology.
The findings prompted the investigators to compare histologic specimens from 17 pregnant and 14 nonpregnant women to find an explanation.
Pregnancy melanomas had reduced PD-1 [programmed cell death] expression (18.3 vs. 45 cells per high-power field [hpf]); decreased CD-3 [cluster of differentiation 3] (191.7 vs. 265.7 cells/hpf); and increased CD-3/PD-1 ratios (57.4 vs. 8.3).
The findings were statistically significant and may have treatment implications for the use in recently pregnant women of antibodies against PD-1 cell-surface receptors, a class of biologics that include nivolumab and pembrolizumab, both approved in 2014 for advanced melanoma. With reduced expression of PD-1, tumors arising around pregnancy might not be as sensitive to such agents.
Labeling for both biologics, however, note the risk of fetal harm, and advise the use of effective contraception while on the agents and for several months after their discontinuation. Both agents should also be discontinued during breastfeeding.
“Immune ignorance may predominate in melanoma specimens in pregnancy. Most novel melanoma therapies target immune modulation. There’s a need in some pregnant women for neoadjuvant treatment prior to immunotherapy to convert them to an inflamed phenotype,” Dr. Mesinkovska said.
Dr. Mesinkovska said she has no relevant financial disclosures.
SAN FRANCISCO – Pregnancy increases the risk of poor outcomes in melanoma, according to a review of melanoma cases at the Cleveland Clinic.
The effect of pregnancy on melanoma has been debated for more than a decade. Some studies have found evidence of worse outcomes, but others have not. That prompted Dr. Natasha Mesinkovska, a dermatologist at the clinic, and her colleagues to review their own melanoma outcomes over the past 20 years. They compared 49 women who were pregnant or within a year of pregnancy at diagnosis, with 418 women of childbearing age who were not pregnant. All the patients had at least 2 years follow-up.
Mortality (20% vs. 10.3%; P = .06); recurrence (12.5% vs. 1.4%; P < .001); metastasis (25% vs. 12.7%; P = .03); and the use of radiation and chemotherapy were all more common in the pregnancy group. On logistic regression, women who were pregnant or recently pregnant at the time of melanoma diagnosis were 5.1 times more likely to die of the disease than those who weren’t (P = .03), Dr. Mesinkovska reported at the annual meeting of the American Academy of Dermatology.
The findings prompted the investigators to compare histologic specimens from 17 pregnant and 14 nonpregnant women to find an explanation.
Pregnancy melanomas had reduced PD-1 [programmed cell death] expression (18.3 vs. 45 cells per high-power field [hpf]); decreased CD-3 [cluster of differentiation 3] (191.7 vs. 265.7 cells/hpf); and increased CD-3/PD-1 ratios (57.4 vs. 8.3).
The findings were statistically significant and may have treatment implications for the use in recently pregnant women of antibodies against PD-1 cell-surface receptors, a class of biologics that include nivolumab and pembrolizumab, both approved in 2014 for advanced melanoma. With reduced expression of PD-1, tumors arising around pregnancy might not be as sensitive to such agents.
Labeling for both biologics, however, note the risk of fetal harm, and advise the use of effective contraception while on the agents and for several months after their discontinuation. Both agents should also be discontinued during breastfeeding.
“Immune ignorance may predominate in melanoma specimens in pregnancy. Most novel melanoma therapies target immune modulation. There’s a need in some pregnant women for neoadjuvant treatment prior to immunotherapy to convert them to an inflamed phenotype,” Dr. Mesinkovska said.
Dr. Mesinkovska said she has no relevant financial disclosures.
AT AAD 2015
Key clinical point: Heighten melanoma surveillance in and around pregnancy, and treat lesions aggressively.
Major finding: Melanoma mortality (20% vs. 10.3%; P = .06); recurrence (12.5% vs. 1.4%; P < .001); and metastasis (25% vs. 12.7%; P = .03) were higher in 49 women diagnosed with melanoma while pregnant or within a year of pregnancy, compared with 418 women of childbearing age who were not pregnant.
Data source: Review of 467 melanoma cases at the Cleveland Clinic.
Disclosures: The lead investigator has no relevant financial disclosures.
Avoid voriconazole in transplant patients at risk for skin cancer
SAN FRANCISCO – Voriconazole increased the risk of squamous cell carcinoma by 73% in a review of 455 lung transplant patients at the University of California, San Francisco.
The increase was for any exposure to the drug after transplant (adjusted hazard ratio, 1.73; P = .03). The investigators also found that each additional 30-day exposure at 200 mg of voriconazole twice daily increased the risk of squamous cell carcinoma (SCC) by 3.0% (HR, 1.03; P < .001). The results were adjusted for age at transplant, sex, and race. Overall, SCC risk was highest among white men aged 50 years or older at the time of transplant.
Although voriconazole did protect against posttransplant Aspergillus colonization (aHR, 0.50; P < .001), it did not reduce the risk of invasive aspergillosis. The drug reduced all-cause mortality only among colonized subjects (aHR, 0.34; P = .03), and offered no mortality benefit among those who were not colonized.
There was no difference in all-cause mortality between patients who had any exposure to voriconazole and those who did not, “but we actually found a 2% increased risk of death for each 1 month on the medication. Patients who weren’t colonized were the ones contributing to this increased risk of death,” said lead investigator Matthew Mansh, now a medical student at Stanford (Calif.) University.
There was no increased risk of SCC with alternative antifungals, including inhaled amphotericin and posaconazole. These alternatives should be considered instead of voriconazole in people at higher risk for skin cancer after lung transplants, according to the study, Mr. Mansh noted.
Voriconazole, which is widely used for antifungal prophylaxis after solid organ transplants, has been linked to skin cancer. The reason for the carcinogenic effect is not known; researchers are working to unravel the molecular mechanisms.
“Physicians should be cautious when using voriconazole in the care of transplant recipients. If you see a patient who is developing phototoxicity” with voriconazole, “and if they don’t have evidence of Aspergillus colonization, you may want to limit exposure to high doses of this drug or suggest an alternative,” Mr. Mansh said.
“We have now demonstrated that the alternatives “don’t carry this increased risk of cutaneous SCC,” Mr. Mansh said at the American Academy of Dermatology annual meeting.
The mean age of the study patients at transplant was 52 years, and the majority of patients were white; slightly more than half were men. Most had bilateral lung transplants, with pulmonary fibrosis at the leading indication.
Voriconazole was used in 85% of the patients for an average of 10 months. A quarter of voriconazole patients developed SCC within 5 years of transplant, and 43% within 10 years. Among patients who did not receive the drug, 15% developed SCC within 5 years of transplant, and 28% developed SCC within 10 years of transplant.
“The benefit of voriconazole in terms of death was limited to patients with evidence of Aspergillus colonization, and it wasn’t dose dependent. Patients who had a higher cumulative exposure did not get more benefit,” Mr. Mansh said.
Mr. Mansh had no relevant disclosures.
 |
Dr. Paul T. Nghiem |
This is a carefully done study with a practical message: voriconazole patients are at a prolonged increased risk for squamous cell carcinoma. If patients develop phototoxicity or are fair-skinned, have sun damage, a history of squamous cell carcinoma or other risk factors, I think it’s highly appropriate to suggest an alternative. The alternatives are not at all associated with phototoxicity or squamous cell carcinoma.
Dr. Paul T. Nghiem moderated the late-breaker presentation in which the study was presented and is a professor of dermatology at the University of Washington, Seattle. Dr. Nghiem had no disclosures related to the study.
 |
Dr. Paul T. Nghiem |
This is a carefully done study with a practical message: voriconazole patients are at a prolonged increased risk for squamous cell carcinoma. If patients develop phototoxicity or are fair-skinned, have sun damage, a history of squamous cell carcinoma or other risk factors, I think it’s highly appropriate to suggest an alternative. The alternatives are not at all associated with phototoxicity or squamous cell carcinoma.
Dr. Paul T. Nghiem moderated the late-breaker presentation in which the study was presented and is a professor of dermatology at the University of Washington, Seattle. Dr. Nghiem had no disclosures related to the study.
 |
Dr. Paul T. Nghiem |
This is a carefully done study with a practical message: voriconazole patients are at a prolonged increased risk for squamous cell carcinoma. If patients develop phototoxicity or are fair-skinned, have sun damage, a history of squamous cell carcinoma or other risk factors, I think it’s highly appropriate to suggest an alternative. The alternatives are not at all associated with phototoxicity or squamous cell carcinoma.
Dr. Paul T. Nghiem moderated the late-breaker presentation in which the study was presented and is a professor of dermatology at the University of Washington, Seattle. Dr. Nghiem had no disclosures related to the study.
SAN FRANCISCO – Voriconazole increased the risk of squamous cell carcinoma by 73% in a review of 455 lung transplant patients at the University of California, San Francisco.
The increase was for any exposure to the drug after transplant (adjusted hazard ratio, 1.73; P = .03). The investigators also found that each additional 30-day exposure at 200 mg of voriconazole twice daily increased the risk of squamous cell carcinoma (SCC) by 3.0% (HR, 1.03; P < .001). The results were adjusted for age at transplant, sex, and race. Overall, SCC risk was highest among white men aged 50 years or older at the time of transplant.
Although voriconazole did protect against posttransplant Aspergillus colonization (aHR, 0.50; P < .001), it did not reduce the risk of invasive aspergillosis. The drug reduced all-cause mortality only among colonized subjects (aHR, 0.34; P = .03), and offered no mortality benefit among those who were not colonized.
There was no difference in all-cause mortality between patients who had any exposure to voriconazole and those who did not, “but we actually found a 2% increased risk of death for each 1 month on the medication. Patients who weren’t colonized were the ones contributing to this increased risk of death,” said lead investigator Matthew Mansh, now a medical student at Stanford (Calif.) University.
There was no increased risk of SCC with alternative antifungals, including inhaled amphotericin and posaconazole. These alternatives should be considered instead of voriconazole in people at higher risk for skin cancer after lung transplants, according to the study, Mr. Mansh noted.
Voriconazole, which is widely used for antifungal prophylaxis after solid organ transplants, has been linked to skin cancer. The reason for the carcinogenic effect is not known; researchers are working to unravel the molecular mechanisms.
“Physicians should be cautious when using voriconazole in the care of transplant recipients. If you see a patient who is developing phototoxicity” with voriconazole, “and if they don’t have evidence of Aspergillus colonization, you may want to limit exposure to high doses of this drug or suggest an alternative,” Mr. Mansh said.
“We have now demonstrated that the alternatives “don’t carry this increased risk of cutaneous SCC,” Mr. Mansh said at the American Academy of Dermatology annual meeting.
The mean age of the study patients at transplant was 52 years, and the majority of patients were white; slightly more than half were men. Most had bilateral lung transplants, with pulmonary fibrosis at the leading indication.
Voriconazole was used in 85% of the patients for an average of 10 months. A quarter of voriconazole patients developed SCC within 5 years of transplant, and 43% within 10 years. Among patients who did not receive the drug, 15% developed SCC within 5 years of transplant, and 28% developed SCC within 10 years of transplant.
“The benefit of voriconazole in terms of death was limited to patients with evidence of Aspergillus colonization, and it wasn’t dose dependent. Patients who had a higher cumulative exposure did not get more benefit,” Mr. Mansh said.
Mr. Mansh had no relevant disclosures.
SAN FRANCISCO – Voriconazole increased the risk of squamous cell carcinoma by 73% in a review of 455 lung transplant patients at the University of California, San Francisco.
The increase was for any exposure to the drug after transplant (adjusted hazard ratio, 1.73; P = .03). The investigators also found that each additional 30-day exposure at 200 mg of voriconazole twice daily increased the risk of squamous cell carcinoma (SCC) by 3.0% (HR, 1.03; P < .001). The results were adjusted for age at transplant, sex, and race. Overall, SCC risk was highest among white men aged 50 years or older at the time of transplant.
Although voriconazole did protect against posttransplant Aspergillus colonization (aHR, 0.50; P < .001), it did not reduce the risk of invasive aspergillosis. The drug reduced all-cause mortality only among colonized subjects (aHR, 0.34; P = .03), and offered no mortality benefit among those who were not colonized.
There was no difference in all-cause mortality between patients who had any exposure to voriconazole and those who did not, “but we actually found a 2% increased risk of death for each 1 month on the medication. Patients who weren’t colonized were the ones contributing to this increased risk of death,” said lead investigator Matthew Mansh, now a medical student at Stanford (Calif.) University.
There was no increased risk of SCC with alternative antifungals, including inhaled amphotericin and posaconazole. These alternatives should be considered instead of voriconazole in people at higher risk for skin cancer after lung transplants, according to the study, Mr. Mansh noted.
Voriconazole, which is widely used for antifungal prophylaxis after solid organ transplants, has been linked to skin cancer. The reason for the carcinogenic effect is not known; researchers are working to unravel the molecular mechanisms.
“Physicians should be cautious when using voriconazole in the care of transplant recipients. If you see a patient who is developing phototoxicity” with voriconazole, “and if they don’t have evidence of Aspergillus colonization, you may want to limit exposure to high doses of this drug or suggest an alternative,” Mr. Mansh said.
“We have now demonstrated that the alternatives “don’t carry this increased risk of cutaneous SCC,” Mr. Mansh said at the American Academy of Dermatology annual meeting.
The mean age of the study patients at transplant was 52 years, and the majority of patients were white; slightly more than half were men. Most had bilateral lung transplants, with pulmonary fibrosis at the leading indication.
Voriconazole was used in 85% of the patients for an average of 10 months. A quarter of voriconazole patients developed SCC within 5 years of transplant, and 43% within 10 years. Among patients who did not receive the drug, 15% developed SCC within 5 years of transplant, and 28% developed SCC within 10 years of transplant.
“The benefit of voriconazole in terms of death was limited to patients with evidence of Aspergillus colonization, and it wasn’t dose dependent. Patients who had a higher cumulative exposure did not get more benefit,” Mr. Mansh said.
Mr. Mansh had no relevant disclosures.
AT AAD 2015
Key clinical point: Use an alternative antifungal after lung transplant in white men aged 50 years and older.
Major finding: Exposure to voriconazole increased the risk of squamous cell carcinoma by 73% after lung transplant (aHR, 1.73; P = .03); each additional 30‐day exposure at 200 mg twice daily increased the risk by 3.0% (HR 1.03; P < .001).
Data source: Retrospective cohort study of 455 lung transplant patients
Disclosures: The lead investigator had no relevant disclosures.
Investigational topical sebum inhibitor reduced acne lesions
SAN FRANCISCO – An investigational topical sebum inhibitor met its primary endpoints in a phase IIa trial, significantly reducing inflammatory and noninflammatory lesions in patients with facial acne vulgaris, researchers reported at the annual meeting of the American Academy of Dermatology.
“This study demonstrated that DRM01, belonging to a novel class of topical acne therapeutic agents, is well tolerated and shows evidence of efficacy for treatment of facial acne vulgaris,” said Dr. Robert Bissonnette of Innovaderm Research. Patients had no serious adverse events related to treatment, although the topical product caused erythema at the application site, he said.
The DRM01 agent inhibits sebum production by competing with acetyl coenzyme A carboxylase, which facilitates the first and rate-limiting step in fatty acid synthesis. “It has shown pronounced impact on triacylglycerols, which are considered the most important component of sebum in acne vulgaris,” said Dr. Bissonnette.
The researchers randomized 108 patients with moderate to severe facial acne to 12 weeks of twice-daily treatment with either 7.5% DRM01 or the vehicle gel. On average, patients had 29 inflammatory lesions and 40 noninflammatory lesions at baseline, and approximately two-thirds were women.
At week 12, the DRM01 group had an average 63.9% reduction in inflammatory lesions, compared with 45.9% for the vehicle control group (P < .001), Dr. Bissonnette said. The DRM01 group also had a 48.1% reduction in non-inflammatory lesions, compared with 28.8% for the control group (P =.003). Furthermore, 24.5% of patients who received DRM01 improved by at least two grades on the Investigator’s Global Assessment score, compared with only 7.3% of the control group (P = .007).
Most adverse events were mild or moderate and affected similar proportions of patients in each group, said Dr. Bissonnette. More than 5% of patients in both groups experienced application site dryness, burning, and stinging, but only patients in the treatment group reported application site erythema, he said. Nasopharyngitis affected 24.5% of patients treated with DRM01 and 12.7% of those treated with the vehicle, but was considered unrelated to treatment.
Researchers do not know how or whether DRM01 reduces inflammation, Dr. Bissonnette said in response to a question from an audience member. The study also was not able to show that DRM01 significantly reduced sebum production compared with the gel vehicle. “It could be a data quality problem, as the sites had not all been fully trained in doing those measurements,” he said.
Dermira manufactures DRM01 and sponsored the study. Dr. Bissonnette reported having served as a researcher for Dermira.
SAN FRANCISCO – An investigational topical sebum inhibitor met its primary endpoints in a phase IIa trial, significantly reducing inflammatory and noninflammatory lesions in patients with facial acne vulgaris, researchers reported at the annual meeting of the American Academy of Dermatology.
“This study demonstrated that DRM01, belonging to a novel class of topical acne therapeutic agents, is well tolerated and shows evidence of efficacy for treatment of facial acne vulgaris,” said Dr. Robert Bissonnette of Innovaderm Research. Patients had no serious adverse events related to treatment, although the topical product caused erythema at the application site, he said.
The DRM01 agent inhibits sebum production by competing with acetyl coenzyme A carboxylase, which facilitates the first and rate-limiting step in fatty acid synthesis. “It has shown pronounced impact on triacylglycerols, which are considered the most important component of sebum in acne vulgaris,” said Dr. Bissonnette.
The researchers randomized 108 patients with moderate to severe facial acne to 12 weeks of twice-daily treatment with either 7.5% DRM01 or the vehicle gel. On average, patients had 29 inflammatory lesions and 40 noninflammatory lesions at baseline, and approximately two-thirds were women.
At week 12, the DRM01 group had an average 63.9% reduction in inflammatory lesions, compared with 45.9% for the vehicle control group (P < .001), Dr. Bissonnette said. The DRM01 group also had a 48.1% reduction in non-inflammatory lesions, compared with 28.8% for the control group (P =.003). Furthermore, 24.5% of patients who received DRM01 improved by at least two grades on the Investigator’s Global Assessment score, compared with only 7.3% of the control group (P = .007).
Most adverse events were mild or moderate and affected similar proportions of patients in each group, said Dr. Bissonnette. More than 5% of patients in both groups experienced application site dryness, burning, and stinging, but only patients in the treatment group reported application site erythema, he said. Nasopharyngitis affected 24.5% of patients treated with DRM01 and 12.7% of those treated with the vehicle, but was considered unrelated to treatment.
Researchers do not know how or whether DRM01 reduces inflammation, Dr. Bissonnette said in response to a question from an audience member. The study also was not able to show that DRM01 significantly reduced sebum production compared with the gel vehicle. “It could be a data quality problem, as the sites had not all been fully trained in doing those measurements,” he said.
Dermira manufactures DRM01 and sponsored the study. Dr. Bissonnette reported having served as a researcher for Dermira.
SAN FRANCISCO – An investigational topical sebum inhibitor met its primary endpoints in a phase IIa trial, significantly reducing inflammatory and noninflammatory lesions in patients with facial acne vulgaris, researchers reported at the annual meeting of the American Academy of Dermatology.
“This study demonstrated that DRM01, belonging to a novel class of topical acne therapeutic agents, is well tolerated and shows evidence of efficacy for treatment of facial acne vulgaris,” said Dr. Robert Bissonnette of Innovaderm Research. Patients had no serious adverse events related to treatment, although the topical product caused erythema at the application site, he said.
The DRM01 agent inhibits sebum production by competing with acetyl coenzyme A carboxylase, which facilitates the first and rate-limiting step in fatty acid synthesis. “It has shown pronounced impact on triacylglycerols, which are considered the most important component of sebum in acne vulgaris,” said Dr. Bissonnette.
The researchers randomized 108 patients with moderate to severe facial acne to 12 weeks of twice-daily treatment with either 7.5% DRM01 or the vehicle gel. On average, patients had 29 inflammatory lesions and 40 noninflammatory lesions at baseline, and approximately two-thirds were women.
At week 12, the DRM01 group had an average 63.9% reduction in inflammatory lesions, compared with 45.9% for the vehicle control group (P < .001), Dr. Bissonnette said. The DRM01 group also had a 48.1% reduction in non-inflammatory lesions, compared with 28.8% for the control group (P =.003). Furthermore, 24.5% of patients who received DRM01 improved by at least two grades on the Investigator’s Global Assessment score, compared with only 7.3% of the control group (P = .007).
Most adverse events were mild or moderate and affected similar proportions of patients in each group, said Dr. Bissonnette. More than 5% of patients in both groups experienced application site dryness, burning, and stinging, but only patients in the treatment group reported application site erythema, he said. Nasopharyngitis affected 24.5% of patients treated with DRM01 and 12.7% of those treated with the vehicle, but was considered unrelated to treatment.
Researchers do not know how or whether DRM01 reduces inflammation, Dr. Bissonnette said in response to a question from an audience member. The study also was not able to show that DRM01 significantly reduced sebum production compared with the gel vehicle. “It could be a data quality problem, as the sites had not all been fully trained in doing those measurements,” he said.
Dermira manufactures DRM01 and sponsored the study. Dr. Bissonnette reported having served as a researcher for Dermira.
AT THE AAD ANNUAL MEETING
Key clinical point: The investigational agent DRM01 showed promise in treating moderate to severe facial acne vulgaris.
Major Finding: The DRM01 group had a 63.9% average reduction in inflammatory facial lesions, compared with 45.9% for the control group (P < .001).
Data source: Phase IIa trial of 108 patients with moderate to severe facial acne vulgaris.
Disclosures: Dermira makes DRM01 and sponsored the study. Dr. Bissonnette reported having served as a researcher for Dermira.
IL-23 inhibitor topped ustekinumab against psoriasis
SAN FRANCISCO – An investigational interleukin-23 inhibitor for moderate to severe plaque psoriasis achieved almost twice the PASI 90 response rate of ustekinumab and generated no major safety signals, based on data from randomized trial of 166 patients.
At 12 weeks, 77% of patients treated with 90- or 180-mg doses of BI 655066 were clear or almost clear, compared with 40% of patients treated with weight-based ustekinumab (P < .0001), Dr. Kim A. Papp reported at the annual meeting of the American Academy of Dermatology.
Response to the 180-mg dose of BI 655066 was both early and durable, he noted. “At 4 weeks, we were starting to see these patients do very well. At week 12, almost 80% of patients who received the 180-mg dose achieved PASI 90, and that response was maintained through 24 weeks,” he said. In contrast, the 90-mg dose of BI 65506 was associated with a decline in PASI 90 response after week 16, added Dr. Papp of Probity Medical Research in Waterloo, Ontario.
Past studies have shown that IL-23 plays a key role in psoriasis by mediating the Th17 cell pathway. The BI 655066 agent is a monoclonal antibody that selectively targets IL23p19, while ustekinumab (STELARA) is an IL12/23 antagonist approved for treatment of moderate or severe plaque psoriasis or active psoriatic arthritis. To compare the two biologics, Dr. Papp and his associates randomized 166 patients with moderate or severe plaque psoriasis to subcutaneous treatment with BI 655066 (18 mg at baseline, 90 mg at baseline and week 4, or 180 mg at baseline and week 4) or weight-based ustekinumab (45 or 90 mg at baseline and week 4.) The patient cohorts were demographically similar, had comparable histories of exposure to anti–tumor necrosis factor agents, and their average baseline PASI scores were about 19 to 20, said Dr. Papp.
In all, 90% of patients treated with 90- or 180-mg doses of BI 655066 had achieved static Physicians Global Assessment scores of clear or almost clear at week 12, compared with 67.5% of patients who received ustekinumab, Dr. Papp reported. Furthermore, 46% of BI 655066 patients achieved PASI 100 (complete clearing) at week 12, compared with 17.5% of ustekinumab patients. At week 20, PASI 100 response rates were 62% for the 180-mg group, 53% for the 90-mg group, and 30% for the ustekinumab group, he said.
Rates of adverse events were low and similar for the treatment groups, said Dr. Papp. The most common adverse events were nasopharyngitis and headache, although some patients reported arthralgia and myalgia. The investigators identified no severe or serious treatment-related side effects, nor any sign that adverse effects were more common at higher treatment doses, Dr. Papp said.
Researchers are continuing to assess the safety and efficacy of BI 655066 in an open-label extension trial of patients with plaque psoriasis. That study is using week 48 PASI 90 response as its primary endpoint and will track adverse events for 24 months.
Boehringer Ingelheim funded the study. Dr. Papp disclosed reported relevant relationships with Boehringer Ingelheim and with several other pharmaceutical companies.
SAN FRANCISCO – An investigational interleukin-23 inhibitor for moderate to severe plaque psoriasis achieved almost twice the PASI 90 response rate of ustekinumab and generated no major safety signals, based on data from randomized trial of 166 patients.
At 12 weeks, 77% of patients treated with 90- or 180-mg doses of BI 655066 were clear or almost clear, compared with 40% of patients treated with weight-based ustekinumab (P < .0001), Dr. Kim A. Papp reported at the annual meeting of the American Academy of Dermatology.
Response to the 180-mg dose of BI 655066 was both early and durable, he noted. “At 4 weeks, we were starting to see these patients do very well. At week 12, almost 80% of patients who received the 180-mg dose achieved PASI 90, and that response was maintained through 24 weeks,” he said. In contrast, the 90-mg dose of BI 65506 was associated with a decline in PASI 90 response after week 16, added Dr. Papp of Probity Medical Research in Waterloo, Ontario.
Past studies have shown that IL-23 plays a key role in psoriasis by mediating the Th17 cell pathway. The BI 655066 agent is a monoclonal antibody that selectively targets IL23p19, while ustekinumab (STELARA) is an IL12/23 antagonist approved for treatment of moderate or severe plaque psoriasis or active psoriatic arthritis. To compare the two biologics, Dr. Papp and his associates randomized 166 patients with moderate or severe plaque psoriasis to subcutaneous treatment with BI 655066 (18 mg at baseline, 90 mg at baseline and week 4, or 180 mg at baseline and week 4) or weight-based ustekinumab (45 or 90 mg at baseline and week 4.) The patient cohorts were demographically similar, had comparable histories of exposure to anti–tumor necrosis factor agents, and their average baseline PASI scores were about 19 to 20, said Dr. Papp.
In all, 90% of patients treated with 90- or 180-mg doses of BI 655066 had achieved static Physicians Global Assessment scores of clear or almost clear at week 12, compared with 67.5% of patients who received ustekinumab, Dr. Papp reported. Furthermore, 46% of BI 655066 patients achieved PASI 100 (complete clearing) at week 12, compared with 17.5% of ustekinumab patients. At week 20, PASI 100 response rates were 62% for the 180-mg group, 53% for the 90-mg group, and 30% for the ustekinumab group, he said.
Rates of adverse events were low and similar for the treatment groups, said Dr. Papp. The most common adverse events were nasopharyngitis and headache, although some patients reported arthralgia and myalgia. The investigators identified no severe or serious treatment-related side effects, nor any sign that adverse effects were more common at higher treatment doses, Dr. Papp said.
Researchers are continuing to assess the safety and efficacy of BI 655066 in an open-label extension trial of patients with plaque psoriasis. That study is using week 48 PASI 90 response as its primary endpoint and will track adverse events for 24 months.
Boehringer Ingelheim funded the study. Dr. Papp disclosed reported relevant relationships with Boehringer Ingelheim and with several other pharmaceutical companies.
SAN FRANCISCO – An investigational interleukin-23 inhibitor for moderate to severe plaque psoriasis achieved almost twice the PASI 90 response rate of ustekinumab and generated no major safety signals, based on data from randomized trial of 166 patients.
At 12 weeks, 77% of patients treated with 90- or 180-mg doses of BI 655066 were clear or almost clear, compared with 40% of patients treated with weight-based ustekinumab (P < .0001), Dr. Kim A. Papp reported at the annual meeting of the American Academy of Dermatology.
Response to the 180-mg dose of BI 655066 was both early and durable, he noted. “At 4 weeks, we were starting to see these patients do very well. At week 12, almost 80% of patients who received the 180-mg dose achieved PASI 90, and that response was maintained through 24 weeks,” he said. In contrast, the 90-mg dose of BI 65506 was associated with a decline in PASI 90 response after week 16, added Dr. Papp of Probity Medical Research in Waterloo, Ontario.
Past studies have shown that IL-23 plays a key role in psoriasis by mediating the Th17 cell pathway. The BI 655066 agent is a monoclonal antibody that selectively targets IL23p19, while ustekinumab (STELARA) is an IL12/23 antagonist approved for treatment of moderate or severe plaque psoriasis or active psoriatic arthritis. To compare the two biologics, Dr. Papp and his associates randomized 166 patients with moderate or severe plaque psoriasis to subcutaneous treatment with BI 655066 (18 mg at baseline, 90 mg at baseline and week 4, or 180 mg at baseline and week 4) or weight-based ustekinumab (45 or 90 mg at baseline and week 4.) The patient cohorts were demographically similar, had comparable histories of exposure to anti–tumor necrosis factor agents, and their average baseline PASI scores were about 19 to 20, said Dr. Papp.
In all, 90% of patients treated with 90- or 180-mg doses of BI 655066 had achieved static Physicians Global Assessment scores of clear or almost clear at week 12, compared with 67.5% of patients who received ustekinumab, Dr. Papp reported. Furthermore, 46% of BI 655066 patients achieved PASI 100 (complete clearing) at week 12, compared with 17.5% of ustekinumab patients. At week 20, PASI 100 response rates were 62% for the 180-mg group, 53% for the 90-mg group, and 30% for the ustekinumab group, he said.
Rates of adverse events were low and similar for the treatment groups, said Dr. Papp. The most common adverse events were nasopharyngitis and headache, although some patients reported arthralgia and myalgia. The investigators identified no severe or serious treatment-related side effects, nor any sign that adverse effects were more common at higher treatment doses, Dr. Papp said.
Researchers are continuing to assess the safety and efficacy of BI 655066 in an open-label extension trial of patients with plaque psoriasis. That study is using week 48 PASI 90 response as its primary endpoint and will track adverse events for 24 months.
Boehringer Ingelheim funded the study. Dr. Papp disclosed reported relevant relationships with Boehringer Ingelheim and with several other pharmaceutical companies.
AT THE AAD ANNUAL MEETING
Key clinical point: The interleukin-23 inhibitor BI 655066 outperformed ustekinumab and generated no concerning safety signals in a small phase II trial.
Major finding: 77.1% of BI 655066 patients achieved PASI 90 at week 12, compared with 40% of ustekinumab patients, and response was maintained at 24 weeks.
Data source: Randomized trial of 166 patients with moderate to severe plaque psoriasis.
Disclosures: Boehringer Ingelheim funded the study. Dr. Papp disclosed reported relevant relationships with Boehringer Ingelheim and several other pharmaceutical companies.
VIDEO: Nanodermatology Society roundtable tackles tough questions on photoprotection
SAN FRANCISCO – What role does nanotechnology play in photoprotection? How is the current regulatory climate helping and hurting innovation and product development? What are the real and perceived safety issues? These and other questions were addressed in a roundtable discussion hosted by the Nanodermatology Society at the annual meeting of the American Academy of Dermatology. Participants included dermatology practitioners and researchers, along with representatives of industry and the media.
“We have good data on a lot of the filters that are out there,” emphasized Dr. Adnan Nasir, president of the Nanodermatology Society and moderator of the roundtable.
Given the epidemic of skin cancer in the United States, “I hope that we can target our message as a public health message” and increase the confidence of physicians in discussing photoprotection and the confidence of patients in their doctors’ opinions on this important topic, Dr. Nasir said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – What role does nanotechnology play in photoprotection? How is the current regulatory climate helping and hurting innovation and product development? What are the real and perceived safety issues? These and other questions were addressed in a roundtable discussion hosted by the Nanodermatology Society at the annual meeting of the American Academy of Dermatology. Participants included dermatology practitioners and researchers, along with representatives of industry and the media.
“We have good data on a lot of the filters that are out there,” emphasized Dr. Adnan Nasir, president of the Nanodermatology Society and moderator of the roundtable.
Given the epidemic of skin cancer in the United States, “I hope that we can target our message as a public health message” and increase the confidence of physicians in discussing photoprotection and the confidence of patients in their doctors’ opinions on this important topic, Dr. Nasir said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – What role does nanotechnology play in photoprotection? How is the current regulatory climate helping and hurting innovation and product development? What are the real and perceived safety issues? These and other questions were addressed in a roundtable discussion hosted by the Nanodermatology Society at the annual meeting of the American Academy of Dermatology. Participants included dermatology practitioners and researchers, along with representatives of industry and the media.
“We have good data on a lot of the filters that are out there,” emphasized Dr. Adnan Nasir, president of the Nanodermatology Society and moderator of the roundtable.
Given the epidemic of skin cancer in the United States, “I hope that we can target our message as a public health message” and increase the confidence of physicians in discussing photoprotection and the confidence of patients in their doctors’ opinions on this important topic, Dr. Nasir said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AAD ANNUAL MEETING
Weekly adalimumab at 40 mg works best for hidradenitis suppurativa
SAN FRANCISCO – Weekly treatment with 40 mg of adalimumab yielded the strongest clinical responses in adults with moderate to severe hidradenitis suppurativa with a safety profile similar to less intensive doses, according to data from two phase III trials.
Overall, 43% of adalimumab-treated patients achieved the predefined clinical response at 36 weeks, compared with 28% of patients who first received adalimumab and switched to placebo, Dr. Alexa Kimball said at the annual meeting of the American Academy of Dermatology. The 40-mg weekly dose also beat placebo in demographic subgroup analyses, said Dr. Kimball, professor of dermatology at Harvard Medical School in Boston. “Greater responses rates with this dose, combined with no difference in safety across the three treatment arms, suggests that weekly dosing was the optimal medium-term dosing strategy in hidradenitis suppurativa,” she added.
Hidradenitis suppurativa (also called acne inversa) is a chronic follicular occlusive skin disease that affects about 1%-4% of adults, particularly women, smokers, and obese or overweight patients. The disease has very few treatment options. Patients can develop recurrent abscesses, draining sinuses, and scarring, all of which negatively affect quality of life.
Adalimumab is an anti–tumor necrosis factor–alpha agent that previously bested placebo for hidradenitis suppurativa in phase II trials. Dr. Kimball and associates analyzed data on 633 patients with moderate to severe disease from two phase III, placebo-controlled trials (PIONEER I and II). For the first 12 weeks, patients were randomized to placebo or to 40 mg of adalimumab weekly. At week 12, patients were rerandomized to 40 mg adalimumab weekly, 40 mg of adalimumab every other week, or to placebo. The researchers defined treatment response as at least a 50% drop in abscesses and inflammatory nodules, with no numerical increase in individual numbers of abscesses or draining fistulas.
At week 12, 50.6% of patients who had received 40 mg of adalimumab weekly had achieved treatment response, compared with 26.8% of the placebo group (P < .001), with no new or unexpected adverse events, said Dr. Kimball. The weekly 40-mg dose also beat placebo in subgroups stratified by smoking status, sex, disease duration, disease severity, and age, she said. Adalimumab outperformed placebo for patients of black race or who had a body mass index (BMI) of at least 40 kg/m2, although the differences did not reach statistical significance. Treated patients also had 23% fewer flares (defined as a 25% increase in lesion count; P = .001), and 18.9 average days of flare, compared with 32 days for the placebo group (P = .001), Dr. Kimball reported.
Past studies have linked adalimumab to serious systemic infections, demyelinating disorders, and lymphoma and other malignancies. No patients in the PIONEER trials developed these adverse events except for nonmelanoma skin cancer, said Dr. Kimball. In all, 2% of patients in each arm developed adverse events that led them to stop treatment, she added. Rates of infection were similar among the treatment groups. Although one patient (1%) on 40 mg weekly for 36 weeks developed pneumonia, none of the patients in the other arms developed serious infections.
Abbvie is the manufacturer of adalimumab and sponsored the trials. Dr. Kimball reported receiving consulting honoraria and research grants from Abbvie and several other pharmaceutical companies.
SAN FRANCISCO – Weekly treatment with 40 mg of adalimumab yielded the strongest clinical responses in adults with moderate to severe hidradenitis suppurativa with a safety profile similar to less intensive doses, according to data from two phase III trials.
Overall, 43% of adalimumab-treated patients achieved the predefined clinical response at 36 weeks, compared with 28% of patients who first received adalimumab and switched to placebo, Dr. Alexa Kimball said at the annual meeting of the American Academy of Dermatology. The 40-mg weekly dose also beat placebo in demographic subgroup analyses, said Dr. Kimball, professor of dermatology at Harvard Medical School in Boston. “Greater responses rates with this dose, combined with no difference in safety across the three treatment arms, suggests that weekly dosing was the optimal medium-term dosing strategy in hidradenitis suppurativa,” she added.
Hidradenitis suppurativa (also called acne inversa) is a chronic follicular occlusive skin disease that affects about 1%-4% of adults, particularly women, smokers, and obese or overweight patients. The disease has very few treatment options. Patients can develop recurrent abscesses, draining sinuses, and scarring, all of which negatively affect quality of life.
Adalimumab is an anti–tumor necrosis factor–alpha agent that previously bested placebo for hidradenitis suppurativa in phase II trials. Dr. Kimball and associates analyzed data on 633 patients with moderate to severe disease from two phase III, placebo-controlled trials (PIONEER I and II). For the first 12 weeks, patients were randomized to placebo or to 40 mg of adalimumab weekly. At week 12, patients were rerandomized to 40 mg adalimumab weekly, 40 mg of adalimumab every other week, or to placebo. The researchers defined treatment response as at least a 50% drop in abscesses and inflammatory nodules, with no numerical increase in individual numbers of abscesses or draining fistulas.
At week 12, 50.6% of patients who had received 40 mg of adalimumab weekly had achieved treatment response, compared with 26.8% of the placebo group (P < .001), with no new or unexpected adverse events, said Dr. Kimball. The weekly 40-mg dose also beat placebo in subgroups stratified by smoking status, sex, disease duration, disease severity, and age, she said. Adalimumab outperformed placebo for patients of black race or who had a body mass index (BMI) of at least 40 kg/m2, although the differences did not reach statistical significance. Treated patients also had 23% fewer flares (defined as a 25% increase in lesion count; P = .001), and 18.9 average days of flare, compared with 32 days for the placebo group (P = .001), Dr. Kimball reported.
Past studies have linked adalimumab to serious systemic infections, demyelinating disorders, and lymphoma and other malignancies. No patients in the PIONEER trials developed these adverse events except for nonmelanoma skin cancer, said Dr. Kimball. In all, 2% of patients in each arm developed adverse events that led them to stop treatment, she added. Rates of infection were similar among the treatment groups. Although one patient (1%) on 40 mg weekly for 36 weeks developed pneumonia, none of the patients in the other arms developed serious infections.
Abbvie is the manufacturer of adalimumab and sponsored the trials. Dr. Kimball reported receiving consulting honoraria and research grants from Abbvie and several other pharmaceutical companies.
SAN FRANCISCO – Weekly treatment with 40 mg of adalimumab yielded the strongest clinical responses in adults with moderate to severe hidradenitis suppurativa with a safety profile similar to less intensive doses, according to data from two phase III trials.
Overall, 43% of adalimumab-treated patients achieved the predefined clinical response at 36 weeks, compared with 28% of patients who first received adalimumab and switched to placebo, Dr. Alexa Kimball said at the annual meeting of the American Academy of Dermatology. The 40-mg weekly dose also beat placebo in demographic subgroup analyses, said Dr. Kimball, professor of dermatology at Harvard Medical School in Boston. “Greater responses rates with this dose, combined with no difference in safety across the three treatment arms, suggests that weekly dosing was the optimal medium-term dosing strategy in hidradenitis suppurativa,” she added.
Hidradenitis suppurativa (also called acne inversa) is a chronic follicular occlusive skin disease that affects about 1%-4% of adults, particularly women, smokers, and obese or overweight patients. The disease has very few treatment options. Patients can develop recurrent abscesses, draining sinuses, and scarring, all of which negatively affect quality of life.
Adalimumab is an anti–tumor necrosis factor–alpha agent that previously bested placebo for hidradenitis suppurativa in phase II trials. Dr. Kimball and associates analyzed data on 633 patients with moderate to severe disease from two phase III, placebo-controlled trials (PIONEER I and II). For the first 12 weeks, patients were randomized to placebo or to 40 mg of adalimumab weekly. At week 12, patients were rerandomized to 40 mg adalimumab weekly, 40 mg of adalimumab every other week, or to placebo. The researchers defined treatment response as at least a 50% drop in abscesses and inflammatory nodules, with no numerical increase in individual numbers of abscesses or draining fistulas.
At week 12, 50.6% of patients who had received 40 mg of adalimumab weekly had achieved treatment response, compared with 26.8% of the placebo group (P < .001), with no new or unexpected adverse events, said Dr. Kimball. The weekly 40-mg dose also beat placebo in subgroups stratified by smoking status, sex, disease duration, disease severity, and age, she said. Adalimumab outperformed placebo for patients of black race or who had a body mass index (BMI) of at least 40 kg/m2, although the differences did not reach statistical significance. Treated patients also had 23% fewer flares (defined as a 25% increase in lesion count; P = .001), and 18.9 average days of flare, compared with 32 days for the placebo group (P = .001), Dr. Kimball reported.
Past studies have linked adalimumab to serious systemic infections, demyelinating disorders, and lymphoma and other malignancies. No patients in the PIONEER trials developed these adverse events except for nonmelanoma skin cancer, said Dr. Kimball. In all, 2% of patients in each arm developed adverse events that led them to stop treatment, she added. Rates of infection were similar among the treatment groups. Although one patient (1%) on 40 mg weekly for 36 weeks developed pneumonia, none of the patients in the other arms developed serious infections.
Abbvie is the manufacturer of adalimumab and sponsored the trials. Dr. Kimball reported receiving consulting honoraria and research grants from Abbvie and several other pharmaceutical companies.
AT THE AAD ANNUAL MEETING
Key clinical point: Weekly treatment with 40 mg adalimumab was the best medium-term dose for hidradenitis suppurativa.
Major finding: 43.4% of patients who received 40 mg adalimumab weekly for 36 weeks achieved the predefined clinical response, compared with 28% of patients who received adalimumab for 12 weeks but then switched to placebo.
Data source: Integrated analysis of data from two phase III, randomized, placebo-controlled trials of 633 patients.
Disclosures: Abbvie is the marker of adalimumab and sponsored the trials. Dr. Kimball reported receiving consulting honoraria and research grants from Abbvie and several other pharmaceutical companies.