User login
Biologic agents have provided patients with moderate to severe psoriasis with treatment alternatives that have improved systemic safety profiles and disease control1; however, case reports of associated neurologic complications have been emerging. Tumor necrosis factor α (TNF-α) inhibitors have been associated with central and peripheral demyelinating disorders. Notably, efalizumab was withdrawn from the market for its association with fatal cases of progressive multifocal leukoencephalopathy (PML).2,3 It is imperative for dermatologists to be familiar with the clinical presentation, evaluation, and diagnostic criteria of neurologic complications of biologic agents used in the treatment of psoriasis.
Leukoencephalopathy
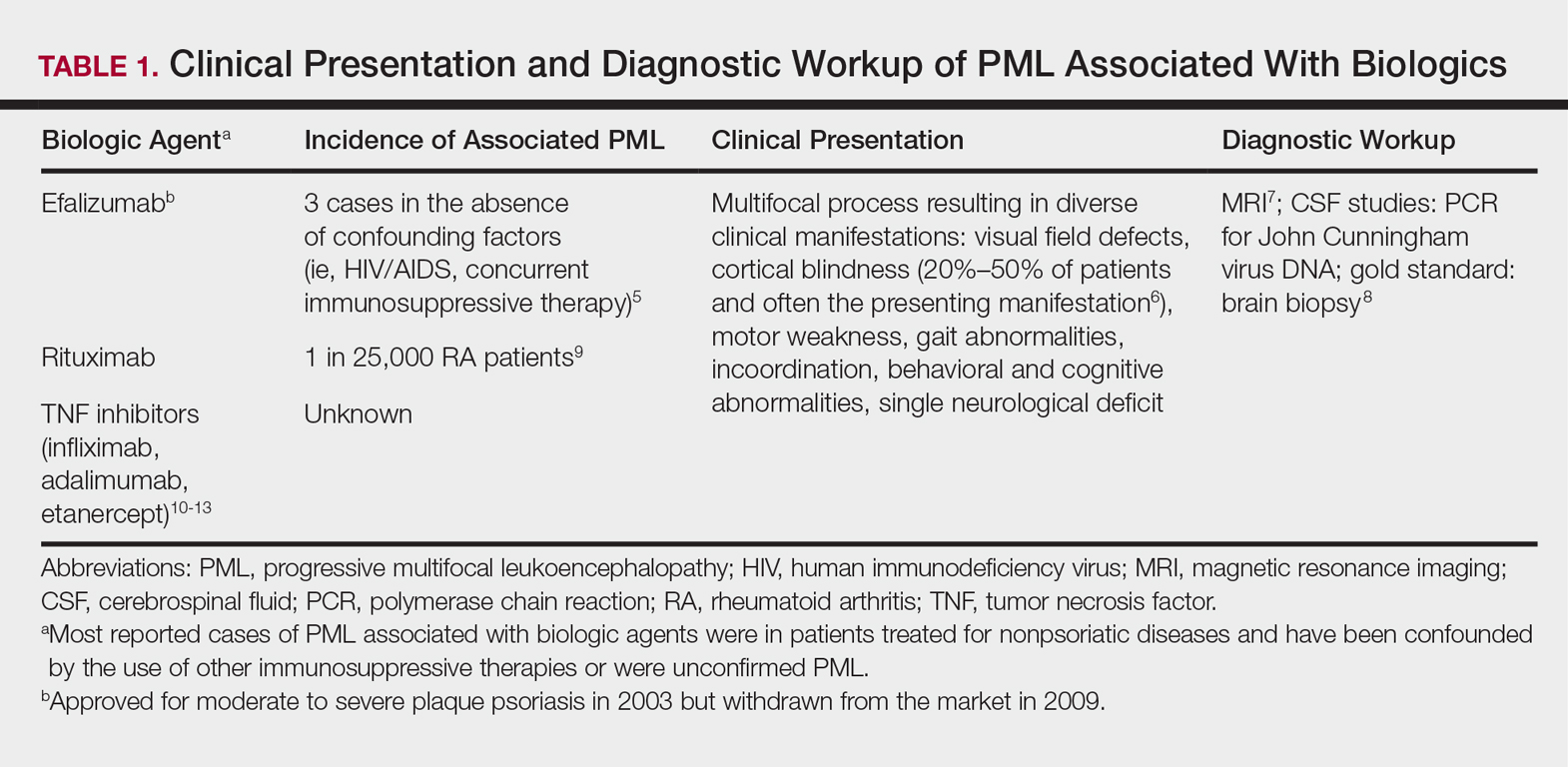
Progressive multifocal leukoencephalopathy is a fatal demyelinating neurodegenerative disease caused by reactivation of the ubiquitous John Cunningham virus. Primary asymptomatic infection is thought to occur during childhood, then the virus remains latent. Reactivation usually occurs during severe immunosuppression and is classically described in human immunodeficiency virus infection, lymphoproliferative disorders, and other forms of cancer.4 A summary of PML and its association with biologics is found in Table 1.5-13 Few case reports of TNF-α inhibitor–associated PML exist, mostly in the presence of confounding factors such as immunosuppression or underlying autoimmune disease.10-13 Presenting symptoms of PML often are subacute, rapidly progressive, and can be focal or multifocal and include motor, cognitive, and visual deficits. Of note, there are 2 reported cases of ustekinumab associated with reversible posterior leukoencephalopathy syndrome, which is a hypertensive encephalopathy characterized by headache, altered mental status, vision abnormalities, and seizures.14,15 Fortunately, this disease is reversible with blood pressure control and removal of the immunosuppressive agent.16
Demyelinating Disorders
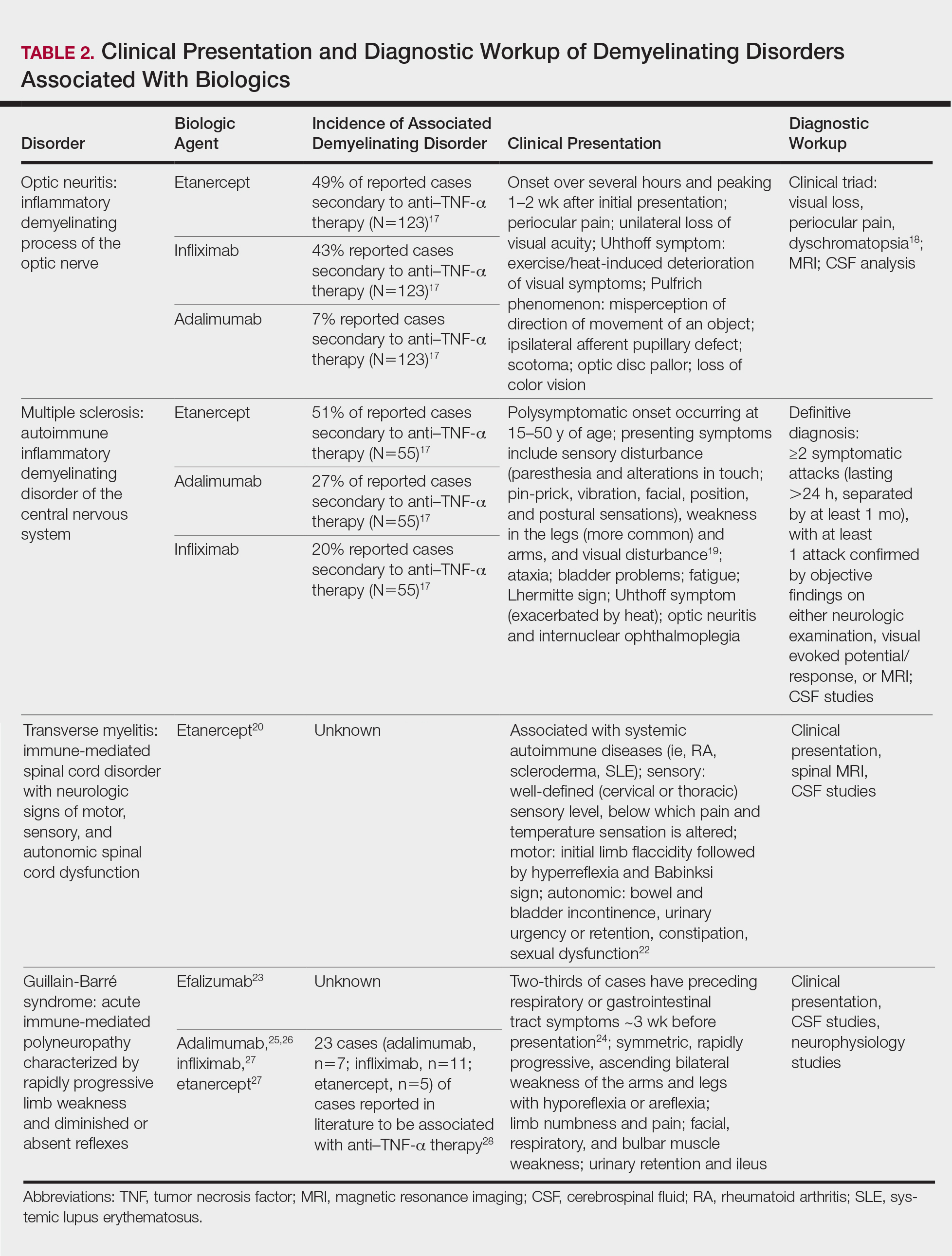
Clinical presentation of demyelinating events associated with biologic agents are varied but include optic neuritis, multiple sclerosis, transverse myelitis, and Guillain-Barré syndrome, among others.17-28 These demyelinating disorders with their salient features and associated biologics are summarized in Table 2.17-20,22-28 Patients on biologic agents, especially TNF-α inhibitors, with new-onset visual, motor, or sensory changes warrant closer inspection. Currently, there are no data on any neurologic side effects occurring with the new biologic secukinumab.29
Conclusion
Biologic agents are effective in treating moderate to severe plaque psoriasis, but awareness of associated neurological adverse effects, though rare, is important to consider. Physicians need to be able to counsel patients concerning these risks and promote informed decision-making prior to initiating biologics. Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–TNF-α therapy. Since the withdrawal of efalizumab, no new cases of PML have been reported in patients who were previously on a long-term course. Dermatologists should be vigilant in detecting signs of neurological complications so that an expedited evaluation and neurology referral may prevent progression of disease.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- FDA Statement on the Voluntary Withdrawal of Raptiva From the U.S. Market. US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrug-SafetyInformationforPatientsandProviders/ucm143347.htm. Published April 8, 2009. Accessed December 21, 2017.
- Kothary N, Diak IL, Brinker A, et al. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546-551.
- Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776-1780.
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937-942.
- Sudhakar P, Bachman DM, Mark AS, et al. Progressive multifocal leukoencephalopathy: recent advances and a neuro-ophthalmological review. J Neuroophthalmol. 2015;35:296-305.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430-1438.
- Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253-260.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156-1164.
- Babi MA, Pendlebury W, Braff S, et al. JC virus PCR detection is not infallible: a fulminant case of progressive multifocal leukoencephalopathy with false-negative cerebrospinal fluid studies despite progressive clinical course and radiological findings [published online March 12, 2015]. Case Rep Neurol Med. 2015;2015:643216.
- Ray M, Curtis JR, Baddley JW. A case report of progressive multifocal leucoencephalopathy (PML) associated with adalimumab. Ann Rheum Dis. 2014;73:1429-1430.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191-3195.
- Graff-Radford J, Robinson MT, Warsame RM, et al. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist. 2012;18:85-87.
- Dickson L, Menter A. Reversible posterior leukoencephalopathy syndrome (RPLS) in a psoriasis patient treated with ustekinumab. J Drugs Dermatol. 2017;16:177-179.
- Gratton D, Szapary P, Goyal K, et al. Reversible posterior leukoencephalopathy syndrome in a patient treated with ustekinumab: case report and review of the literature. Arch Dermatol. 2011;147:1197-1202.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- Ramos-Casals M, Roberto-Perez A, Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188-193.
- Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72.
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6:1-73.
- Caracseghi F, Izquierdo-Blasco J, Sanchez-Montanez A, et al. Etanercept-induced myelopathy in a pediatric case of blau syndrome [published online January 15, 2012]. Case Rep Rheumatol. 2011;2011:134106.
- Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45:55-57.
- Sellner J, Lüthi N, Schüpbach WM, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2009;47:312-317.
- Turatti M, Tamburin S, Idone D, et al. Guillain-Barré syndrome after short-course efalizumab treatment. J Neurol. 2010;257:1404-1405.
- Koga M, Yuki N, Hirata K. Antecedent symptoms in Guillain-Barré syndrome: an important indicator for clinical and serological subgroups. Acta Neurol Scand. 2001;103:278-287.
- Cesarini M, Angelucci E, Foglietta T, et al. Guillain-Barré syndrome after treatment with human anti-tumor necrosis factor alpha (adalimumab) in a Crohn’s disease patient: case report and literature review [published online July 28, 2011]. J Crohns Colitis. 2011;5:619-622.
- Soto-Cabrera E, Hernández-Martínez A, Yañez H, et al. Guillain-Barré syndrome. Its association with alpha tumor necrosis factor [in Spanish]. Rev Med Inst Mex Seguro Soc. 2012;50:565-567.
- Shin IS, Baer AN, Kwon HJ, et al. Guillain-Barré and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429-1434.
- Alvarez-Lario B, Prieto-Tejedo R, Colazo-Burlato M, et al. Severe Guillain-Barré syndrome in a patient receiving anti-TNF therapy. consequence or coincidence. a case-based review. Clin Rheumatol. 2013;32:1407-1412.
- Garnock-Jones KP. Secukinumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2015;16:323-330.
Biologic agents have provided patients with moderate to severe psoriasis with treatment alternatives that have improved systemic safety profiles and disease control1; however, case reports of associated neurologic complications have been emerging. Tumor necrosis factor α (TNF-α) inhibitors have been associated with central and peripheral demyelinating disorders. Notably, efalizumab was withdrawn from the market for its association with fatal cases of progressive multifocal leukoencephalopathy (PML).2,3 It is imperative for dermatologists to be familiar with the clinical presentation, evaluation, and diagnostic criteria of neurologic complications of biologic agents used in the treatment of psoriasis.
Leukoencephalopathy
Progressive multifocal leukoencephalopathy is a fatal demyelinating neurodegenerative disease caused by reactivation of the ubiquitous John Cunningham virus. Primary asymptomatic infection is thought to occur during childhood, then the virus remains latent. Reactivation usually occurs during severe immunosuppression and is classically described in human immunodeficiency virus infection, lymphoproliferative disorders, and other forms of cancer.4 A summary of PML and its association with biologics is found in Table 1.5-13 Few case reports of TNF-α inhibitor–associated PML exist, mostly in the presence of confounding factors such as immunosuppression or underlying autoimmune disease.10-13 Presenting symptoms of PML often are subacute, rapidly progressive, and can be focal or multifocal and include motor, cognitive, and visual deficits. Of note, there are 2 reported cases of ustekinumab associated with reversible posterior leukoencephalopathy syndrome, which is a hypertensive encephalopathy characterized by headache, altered mental status, vision abnormalities, and seizures.14,15 Fortunately, this disease is reversible with blood pressure control and removal of the immunosuppressive agent.16
Demyelinating Disorders
Clinical presentation of demyelinating events associated with biologic agents are varied but include optic neuritis, multiple sclerosis, transverse myelitis, and Guillain-Barré syndrome, among others.17-28 These demyelinating disorders with their salient features and associated biologics are summarized in Table 2.17-20,22-28 Patients on biologic agents, especially TNF-α inhibitors, with new-onset visual, motor, or sensory changes warrant closer inspection. Currently, there are no data on any neurologic side effects occurring with the new biologic secukinumab.29

Conclusion
Biologic agents are effective in treating moderate to severe plaque psoriasis, but awareness of associated neurological adverse effects, though rare, is important to consider. Physicians need to be able to counsel patients concerning these risks and promote informed decision-making prior to initiating biologics. Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–TNF-α therapy. Since the withdrawal of efalizumab, no new cases of PML have been reported in patients who were previously on a long-term course. Dermatologists should be vigilant in detecting signs of neurological complications so that an expedited evaluation and neurology referral may prevent progression of disease.
Biologic agents have provided patients with moderate to severe psoriasis with treatment alternatives that have improved systemic safety profiles and disease control1; however, case reports of associated neurologic complications have been emerging. Tumor necrosis factor α (TNF-α) inhibitors have been associated with central and peripheral demyelinating disorders. Notably, efalizumab was withdrawn from the market for its association with fatal cases of progressive multifocal leukoencephalopathy (PML).2,3 It is imperative for dermatologists to be familiar with the clinical presentation, evaluation, and diagnostic criteria of neurologic complications of biologic agents used in the treatment of psoriasis.
Leukoencephalopathy
Progressive multifocal leukoencephalopathy is a fatal demyelinating neurodegenerative disease caused by reactivation of the ubiquitous John Cunningham virus. Primary asymptomatic infection is thought to occur during childhood, then the virus remains latent. Reactivation usually occurs during severe immunosuppression and is classically described in human immunodeficiency virus infection, lymphoproliferative disorders, and other forms of cancer.4 A summary of PML and its association with biologics is found in Table 1.5-13 Few case reports of TNF-α inhibitor–associated PML exist, mostly in the presence of confounding factors such as immunosuppression or underlying autoimmune disease.10-13 Presenting symptoms of PML often are subacute, rapidly progressive, and can be focal or multifocal and include motor, cognitive, and visual deficits. Of note, there are 2 reported cases of ustekinumab associated with reversible posterior leukoencephalopathy syndrome, which is a hypertensive encephalopathy characterized by headache, altered mental status, vision abnormalities, and seizures.14,15 Fortunately, this disease is reversible with blood pressure control and removal of the immunosuppressive agent.16
Demyelinating Disorders
Clinical presentation of demyelinating events associated with biologic agents are varied but include optic neuritis, multiple sclerosis, transverse myelitis, and Guillain-Barré syndrome, among others.17-28 These demyelinating disorders with their salient features and associated biologics are summarized in Table 2.17-20,22-28 Patients on biologic agents, especially TNF-α inhibitors, with new-onset visual, motor, or sensory changes warrant closer inspection. Currently, there are no data on any neurologic side effects occurring with the new biologic secukinumab.29

Conclusion
Biologic agents are effective in treating moderate to severe plaque psoriasis, but awareness of associated neurological adverse effects, though rare, is important to consider. Physicians need to be able to counsel patients concerning these risks and promote informed decision-making prior to initiating biologics. Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–TNF-α therapy. Since the withdrawal of efalizumab, no new cases of PML have been reported in patients who were previously on a long-term course. Dermatologists should be vigilant in detecting signs of neurological complications so that an expedited evaluation and neurology referral may prevent progression of disease.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- FDA Statement on the Voluntary Withdrawal of Raptiva From the U.S. Market. US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrug-SafetyInformationforPatientsandProviders/ucm143347.htm. Published April 8, 2009. Accessed December 21, 2017.
- Kothary N, Diak IL, Brinker A, et al. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546-551.
- Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776-1780.
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937-942.
- Sudhakar P, Bachman DM, Mark AS, et al. Progressive multifocal leukoencephalopathy: recent advances and a neuro-ophthalmological review. J Neuroophthalmol. 2015;35:296-305.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430-1438.
- Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253-260.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156-1164.
- Babi MA, Pendlebury W, Braff S, et al. JC virus PCR detection is not infallible: a fulminant case of progressive multifocal leukoencephalopathy with false-negative cerebrospinal fluid studies despite progressive clinical course and radiological findings [published online March 12, 2015]. Case Rep Neurol Med. 2015;2015:643216.
- Ray M, Curtis JR, Baddley JW. A case report of progressive multifocal leucoencephalopathy (PML) associated with adalimumab. Ann Rheum Dis. 2014;73:1429-1430.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191-3195.
- Graff-Radford J, Robinson MT, Warsame RM, et al. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist. 2012;18:85-87.
- Dickson L, Menter A. Reversible posterior leukoencephalopathy syndrome (RPLS) in a psoriasis patient treated with ustekinumab. J Drugs Dermatol. 2017;16:177-179.
- Gratton D, Szapary P, Goyal K, et al. Reversible posterior leukoencephalopathy syndrome in a patient treated with ustekinumab: case report and review of the literature. Arch Dermatol. 2011;147:1197-1202.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- Ramos-Casals M, Roberto-Perez A, Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188-193.
- Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72.
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6:1-73.
- Caracseghi F, Izquierdo-Blasco J, Sanchez-Montanez A, et al. Etanercept-induced myelopathy in a pediatric case of blau syndrome [published online January 15, 2012]. Case Rep Rheumatol. 2011;2011:134106.
- Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45:55-57.
- Sellner J, Lüthi N, Schüpbach WM, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2009;47:312-317.
- Turatti M, Tamburin S, Idone D, et al. Guillain-Barré syndrome after short-course efalizumab treatment. J Neurol. 2010;257:1404-1405.
- Koga M, Yuki N, Hirata K. Antecedent symptoms in Guillain-Barré syndrome: an important indicator for clinical and serological subgroups. Acta Neurol Scand. 2001;103:278-287.
- Cesarini M, Angelucci E, Foglietta T, et al. Guillain-Barré syndrome after treatment with human anti-tumor necrosis factor alpha (adalimumab) in a Crohn’s disease patient: case report and literature review [published online July 28, 2011]. J Crohns Colitis. 2011;5:619-622.
- Soto-Cabrera E, Hernández-Martínez A, Yañez H, et al. Guillain-Barré syndrome. Its association with alpha tumor necrosis factor [in Spanish]. Rev Med Inst Mex Seguro Soc. 2012;50:565-567.
- Shin IS, Baer AN, Kwon HJ, et al. Guillain-Barré and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429-1434.
- Alvarez-Lario B, Prieto-Tejedo R, Colazo-Burlato M, et al. Severe Guillain-Barré syndrome in a patient receiving anti-TNF therapy. consequence or coincidence. a case-based review. Clin Rheumatol. 2013;32:1407-1412.
- Garnock-Jones KP. Secukinumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2015;16:323-330.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- FDA Statement on the Voluntary Withdrawal of Raptiva From the U.S. Market. US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrug-SafetyInformationforPatientsandProviders/ucm143347.htm. Published April 8, 2009. Accessed December 21, 2017.
- Kothary N, Diak IL, Brinker A, et al. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546-551.
- Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776-1780.
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937-942.
- Sudhakar P, Bachman DM, Mark AS, et al. Progressive multifocal leukoencephalopathy: recent advances and a neuro-ophthalmological review. J Neuroophthalmol. 2015;35:296-305.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430-1438.
- Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253-260.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156-1164.
- Babi MA, Pendlebury W, Braff S, et al. JC virus PCR detection is not infallible: a fulminant case of progressive multifocal leukoencephalopathy with false-negative cerebrospinal fluid studies despite progressive clinical course and radiological findings [published online March 12, 2015]. Case Rep Neurol Med. 2015;2015:643216.
- Ray M, Curtis JR, Baddley JW. A case report of progressive multifocal leucoencephalopathy (PML) associated with adalimumab. Ann Rheum Dis. 2014;73:1429-1430.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191-3195.
- Graff-Radford J, Robinson MT, Warsame RM, et al. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist. 2012;18:85-87.
- Dickson L, Menter A. Reversible posterior leukoencephalopathy syndrome (RPLS) in a psoriasis patient treated with ustekinumab. J Drugs Dermatol. 2017;16:177-179.
- Gratton D, Szapary P, Goyal K, et al. Reversible posterior leukoencephalopathy syndrome in a patient treated with ustekinumab: case report and review of the literature. Arch Dermatol. 2011;147:1197-1202.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- Ramos-Casals M, Roberto-Perez A, Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188-193.
- Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72.
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6:1-73.
- Caracseghi F, Izquierdo-Blasco J, Sanchez-Montanez A, et al. Etanercept-induced myelopathy in a pediatric case of blau syndrome [published online January 15, 2012]. Case Rep Rheumatol. 2011;2011:134106.
- Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45:55-57.
- Sellner J, Lüthi N, Schüpbach WM, et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord. 2009;47:312-317.
- Turatti M, Tamburin S, Idone D, et al. Guillain-Barré syndrome after short-course efalizumab treatment. J Neurol. 2010;257:1404-1405.
- Koga M, Yuki N, Hirata K. Antecedent symptoms in Guillain-Barré syndrome: an important indicator for clinical and serological subgroups. Acta Neurol Scand. 2001;103:278-287.
- Cesarini M, Angelucci E, Foglietta T, et al. Guillain-Barré syndrome after treatment with human anti-tumor necrosis factor alpha (adalimumab) in a Crohn’s disease patient: case report and literature review [published online July 28, 2011]. J Crohns Colitis. 2011;5:619-622.
- Soto-Cabrera E, Hernández-Martínez A, Yañez H, et al. Guillain-Barré syndrome. Its association with alpha tumor necrosis factor [in Spanish]. Rev Med Inst Mex Seguro Soc. 2012;50:565-567.
- Shin IS, Baer AN, Kwon HJ, et al. Guillain-Barré and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429-1434.
- Alvarez-Lario B, Prieto-Tejedo R, Colazo-Burlato M, et al. Severe Guillain-Barré syndrome in a patient receiving anti-TNF therapy. consequence or coincidence. a case-based review. Clin Rheumatol. 2013;32:1407-1412.
- Garnock-Jones KP. Secukinumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2015;16:323-330.
Practice Points
- Patients with a personal or strong family history of demyelinating disease should be considered for alternative treatment options before initiating anti–tumor necrosis factor (TNF) α therapy.
- Patients on biologic agents, especially TNF-α inhibitors, with subacute or rapidly progressive visual, motor, or sensory changes or a single neurologic deficit may warrant referral to neurology and/or neuroimaging.