User login
When and how to evaluate mildly elevated liver enzymes in apparently healthy patients
In seemingly healthy patients, abnormal liver enzyme levels challenge even the most experienced clinicians in deciding what further evaluation to pursue, if any. Automated laboratory testing has made serum liver enzyme levels very easy to obtain, leading to an increase in testing and also in the number of incidental abnormal findings. An estimated 1% to 9% of people who have no symptoms have high liver enzyme levels when screened with standard biochemistry panels.1,2 A US survey2 showed elevated alanine aminotransferase (ALT) in 8.9% of surveyed people from 1999 to 2002, an increase from previous reports.
An extensive evaluation can be costly, anxiety-provoking, and risky, especially if it leads to unnecessary invasive procedures such as liver biopsy or endoscopic retrograde cholangiopancreatography (ERCP). Not all people with a single, isolated, mildly elevated liver enzyme value have underlying liver disease, nor do they require an extensive evaluation. Factors to consider when deciding whether to evaluate include:
- The patient’s overall health, including chronic illness
- The duration and pattern of enzyme elevation
- Patient characteristics such as age, a personal or family history of liver, lung, or neurologic disease, risk factors for viral hepatitis, amount of alcohol consumption, use of prescribed or over-the-counter drugs or dietary supplements
- The costs and risks associated with additional evaluation.
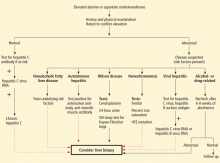
This article reviews the most likely causes of elevated aminotransferase, alkaline phosphatase, and gamma-glutamyl transferase (GGT) levels. It also provides an algorithm for evaluating mildly abnormal liver enzyme values in apparently healthy people. Patients with signs of hepatic decompensation need a more concise and urgent evaluation.
PATTERNS OF LIVER ENZYME ELEVATION
“Liver function test” is commonly used to describe liver enzyme measurements, but the term should be reserved for tests of the functional hepatic reserve—traditionally, the albumin level and the prothrombin time.3
On the other hand, elevated serum liver enzymes (aminotransferases, alkaline phosphatase, and GGT) can reflect abnormalities in liver cells or in the bile duct. For example, predominant elevation of aminotransferases typically indicates hepatocellular injury, whereas elevated alkaline phosphatase and GGT indicates cholestatic injury. Elevated alkaline phosphatase and aminotransferases can indicate a mixed pattern of injury.
High AST, ALT suggest liver cell damage
Both aspartate aminotransferase (AST) and ALT are normally present in serum at low levels, usually less than 30 to 40 U/L. Although the actual values may differ from laboratory to laboratory, normal serum levels are usually less than 40 U/L for AST and less than 50 U/L for ALT. On the other hand, some experts have suggested lowering the upper limit of normal because of the increasing rate of obesity and associated nonalcoholic fatty liver disease, which may not be detected using the traditional, higher normal values. Acceptance is growing for using ALT levels less than 40 U/L in men and less than 31 U/L in women, and AST levels less than 37 U/L in men and less than 31 U/L in women, as normal thresholds.
Although ALT is present in several organs and in muscle, the highest levels are in the liver, which makes this enzyme a more specific indicator of liver injury. Both AST and ALT are released into the blood in greater amounts when hepatocytes are damaged.
Alkaline phosphatase suggests cholestasis
Alkaline phosphatase comes mostly from the liver and bone. In general, normal serum alkaline phosphatase levels in adults range between 20 and 120 U/L. When bone disease is excluded, an elevation suggests biliary obstruction, injury to the bile duct epithelium, or cholestasis. Additionally, there are rare cases of benign familial elevation of serum alkaline phosphatase, mainly of intestinal origin.
GGT is not specific
GGT is present in hepatocytes and biliary epithelial cells. The normal range is 0 U/L to 50 U/L in men, and 0 U/L to 35 U/L in women. GGT elevation is the most sensitive marker of hepatobiliary disease. However, its routine clinical use is not recommended, as it cannot by itself indicate a specific cause of liver disease, although measuring the GGT level can help determine a hepatic origin for an isolated elevation of alkaline phosphatase.
RISK FACTORS GUIDE THE WORKUP OF ELEVATED ENZYMES
Chronic viral hepatitis
Prevalence. Hepatitis C virus infection affects an estimated 1.8% of the general population, but the rate is much higher in people with known risk factors (see below), and those with ALT levels greater than 40 U/L.
Hepatitis B virus infection is somewhat less common: between 0.2% and 0.9% of the general US population have positive results on tests for hepatitis B surface antigen. However, the prevalence of this antigen in the United States can be as high as 20% in patients who have emigrated from endemic areas of the world. The risk factors described below dramatically increase the prevalence of both viruses.
Risk factors. Risk factors include bloodproduct transfusions (especially before 1992), intravenous drug use, intranasal cocaine use, hemodialysis, organ transplantation, and birth in an endemic region. Although both viruses can be transmitted sexually, hepatitis B is more readily transmitted by this route than hepatitis C. Worldwide, transmission of hepatitis B virus usually occurs shortly after birth or at a young age.
Comments. Most patients with chronic viral hepatitis have no symptoms or only mild symptoms and minimally elevated ALT and AST levels, ie, two to five times higher than the upper limit of normal. Given the relatively high prevalence of hepatitis C, serologic testing for it should be done early in the evaluation of chronically elevated liver enzyme levels.4,5
Hereditary hemochromatosis
Prevalence. The prevalence of the major HFE-gene mutations that cause hereditary hemochromatosis is 0.25% to 0.5% in people of northern European descent. In northern Europe, about 1 person in 10 is heterozygous and 1 in 200 to 400 is homozygous for the mutated gene.
Risk factors. Northern European ancestry is the primary risk factor. In men, the onset of disease is usually in the third and fourth decades of life, while menses protects women until menopause. From 83% to 85% of people with clinically defined hemochromatosis are homozygous for the C282Y mutation in the HFE gene.
Comments. Hereditary hemochromatosis should be considered early in the evaluation of men of northern European descent. Patients usually have no symptoms until iron overload causes significant end-organ damage. Phlebotomy can be an effective treatment for this potentially fatal disease.6
Alcoholic liver disease
Risk factors. The degree of alcohol-related liver disease depends on a variety of factors, including the volume and duration of alcohol ingestion, the type of liver disease, genetics, and the coexistence of viral hepatitis and obesity.
Alcohol-related liver disease can range from simple fatty liver to alcoholic hepatitis with or without cirrhosis. Cirrhosis develops in only 20% to 30% of patients who consume a substantial amount of alcohol, defined as more than a decade of 60 g/day to 80 g/day of alcohol in men and as little as 20 g/day in women. (A standard drink, ie, a 12-ounce beer, a 5-ounce glass of wine, or 1.5 ounces of distilled spirits, contains 12 g of alcohol.) Factors that potentiate alcohol’s harmful effects include female sex, chronic viral hepatitis (especially hepatitis C), obesity, hereditary hemochromatosis, and use of drugs such as methotrexate (Trexall) and acetaminophen (Tylenol).
Comments. Although cirrhosis affects fewer than one-third of long-term heavy drinkers, early detection and treatment can potentially reduce morbidity and prevent early death. Alcoholic liver disease should be suspected in patients with elevated ALT and AST levels (if the AST level is two to three times higher than normal7) and with a history of excessive alcohol use.
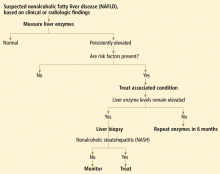
Nonalcoholic fatty liver disease
Prevalence. Nonalcoholic fatty liver disease is a spectrum that ranges from simple steatosis to nonalcoholic steatohepatitis to cirrhosis. Its prevalence in the general US population is about 25%, but is much higher in groups at risk, such as patients with type 2 diabetes (50% to 60%), and morbidly obese patients undergoing bariatric surgery (90% to 95%).
On the other hand, the prevalence of the potentially progressive form of nonalcoholic fatty liver disease, ie, nonalcoholic steatohepatitis, is estimated to be 3% to 5%. Nonalcoholic fatty liver disease is perhaps the most common cause of mildly elevated liver enzymes in the United States.
Comments. Nonalcoholic steatohepatitis and steatonecrosis describe a potentially progressive form of nonalcoholic fatty liver disease. Although these disorders are histologically indistinguishable from alcohol-induced liver disease, their mechanism is related to insulin resistance, abnormalities of lipid metabolism, increased hepatic lipid peroxidation, activated fibrocytes, and abnormal patterns of adipokine and cytokine production related to visceral obesity. Results from a few natural history studies suggest that simple steatosis has a benign course, whereas nonalcoholic steatohepatitis can progress to cirrhosis in 10% to 20% of patients.8
Treatment of diabetes, obesity, hypertension, and hyperlipidemia has potential benefit and should be undertaken regardless of liver test abnormalities in any patient with underlying nonalcoholic fatty liver disease.
Autoimmune hepatitis
Prevalence. The prevalence of autoimmune hepatitis varies, depending on geographic location and on the extent of viral hepatitis in the community. In Hong Kong, only 1% of all people with chronic hepatitis have autoimmune hepatitis. By contrast, in Germany and Austria, 34% and 62% of patients with chronic hepatitis may have autoimmune hepatitis.9 In North America, the prevalence of autoimmune hepatitis in patients with chronic liver disease is estimated to be 11% to 23%, and the incidence is about 0.68 per 100,000 individuals per year. In addition to underlying genetic differences, detection bias can explain the variability in prevalence rates.
Risk factors. Autoimmune hepatitis occurs predominantly in women and can be associated with other autoimmune disorders.
Comments. The diagnosis of autoimmune hepatitis is suggested by exclusion of viral causes of chronic hepatitis, by pathologic findings, and by the presence of autoimmune markers such as antinuclear antibody, smooth muscle antibody, and liver-kidney microsomal antibody. Hypergammaglobulinemia is present in most patients, and serum protein electrophoresis may be helpful as part of the initial evaluation of autoimmune hepatitis.
Liver biopsy is usually needed to confirm the diagnosis and to stage the extent of fibrosis. The International Autoimmune Hepatitis Group Scoring System is based on clinical, laboratory, and pathologic data and can be very helpful in establishing the diagnosis.
Treatment of autoimmune hepatitis with immunosuppression is effective. Most patients may need long-term maintenance treatment.
Wilson disease
Prevalence. The estimated prevalence of Wilson disease is 1 in 40,000 to 1 in 100,000. It has been reported in most populations worldwide.
Risk factors. Anyone under age 40 with abnormal liver enzyme levels (including mild elevations) should be evaluated for Wilson disease, even in the absence of neurologic or ocular findings. However, such routine screening is rarely helpful in patients over age 50. Genetic testing is of limited value because of the large number of potential mutations of the ATP7B gene, the gene responsible for Wilson disease. However, if a a person is known to have Wilson disease, genetic screening of family members is useful.
Comments. Effective therapy is available (ie, d-penicillamine, trientine, zinc). For Wilson disease, alpha-1-antitrypsin deficiency, and genetic hemochromatosis, establishing the diagnosis is not only important to the individual patient; it also may prompt the screening of asymptomatic members of the proband’s family.10–12
Alpha-1-antitrypsin deficiency
Prevalence. Alpha-1-antitrypsin deficiency is present in 1 of every 1,600 to 1,800 live births.11
Risk factors. Patients with emphysema or with a young sibling with liver failure should undergo an investigation for alpha-1-antitrypsin deficiency, consisting of a measurement of the alpha-1-antitrypsin level and an assessment for the PiZZ genotype (the most severe form, because homozygous for the abnormal Z allele).
Comments. Although alpha-1-antitrypsin deficiency is a common cause of liver disease in the very young, it is important to remember that a small number of these patients develop end-stage liver disease in adulthood. Liver transplantation is the only effective treatment for end-stage liver disease associated with alpha-1-antitrypsin deficiency.
Primary biliary cirrhosis
Prevalence. In one study of urban-dwelling women in northeast England, the prevalence of primary biliary cirrhosis was estimated at 0.10%.13
Risk factors. Like autoimmune hepatitis, primary biliary cirrhosis mainly affects women and can be associated with other autoimmune disorders.
Comments. A cholestatic pattern of injury is predominant in primary biliary cirrhosis. Treatment of primary biliary cirrhosis with the cytoprotective agent ursodeoxycholic acid improves liver enzyme levels, may lead to histologic improvement and increased survival, and may also delay the need for liver transplantation. 9,13
Drug- and toxin-related liver diseases
Nonsteroidal anti-inflammatory drugs and penicillin-derived antibiotics are the drugs that most commonly cause abnormal serum liver enzyme levels. The mechanisms of druginduced liver disease include induction of hepatic enzymes (antiepileptic drugs), allergic reactions, autoimmunity (nitrofurantoin [Furadantin, Macrobid]), idiosyncratic reactions, and veno-occlusive injury.14 Drugs that are potentially hepatotoxic are listed in Table 2 and are classified as causing hepatocellular damage, cholestatic damage, or steatosis.
MILD ENZYME ELEVATIONS AS INDICATORS OF SPECIFIC DISEASES
Mildly elevated liver enzymes are common and potentially important, yet very few welldesigned prospective studies have addressed the issue of what should be done once they are identified. Most current data are from small retrospective studies that lack accurate information on the important causes of liver diseases such as hepatitis C and nonalcoholic steatohepatitis.
Despite these shortcomings, the literature delineates the three patterns of mild liver enzyme elevations discussed earlier: hepatocellular injury pattern (elevated ALT or AST), cholestatic pattern (elevated alkaline phosphatase or GGT, or both), and mixed pattern (elevation of ALT, AST, and alkaline phosphatase). The following paragraphs focus on the causes of elevation of specific liver enzymes.
AMINOTRANSFERASE ELEVATION
Causes
Aminotransferases are commonly used markers of hepatocyte injury. AST is present in blood cells and many tissues, including liver, muscle, brain, pancreas, and lung. ALT is a cytosolic enzyme found primarily in hepatocytes, making it a more specific indicator of liver disease.
Acute viral hepatitis, toxins, and liver ischemia can markedly raise serum aminotransferase levels (often into the thousands of units per liter). On the other hand, these enzymes are only mildly elevated (< 300 U/L) in nonalcoholic steatohepatitis, chronic hepatitis, cholestatic liver conditions, drug-induced hepatotoxicity, and liver tumors.
Because AST is a mitochondrial enzyme and is affected by alcohol ingestion, an AST level more than twice that of the ALT suggests hepatic damage due to alcohol.
Of note, aminotransferase elevation can also be due to nonhepatic causes. For example, muscle necrosis can result in mild elevation of these enzymes, especially AST, and an elevated creatine kinase can help confirm that the source is muscle tissue.
Undiagnosed celiac disease has been associated with abnormal liver enzyme levels when all other causes have been ruled out, but the mechanism is not yet understood. In this situation, ALT and AST levels typically return to normal with a gluten-free diet.15
Studies of mild aminotransferase elevations
Only a few studies have documented the results of a thorough evaluation of patients with mildly elevated aminotransferase levels:
Hultcrantz et al1 performed a full evaluation, including liver biopsy, in 149 consecutive patients with chronic, asymptomatic, mild elevations of AST or ALT. Of these patients, 63% had “fatty liver,” 20% had “chronic hepatitis,” and 17% had miscellaneous diagnoses. Whether patients in the “chronic hepatitis” group had hepatitis C was not determined because serologic testing was not available at the time.
Friedman et al16 studied 100 healthy blood donors with elevated ALT levels and found that in 33% of patients the elevation occurred once, in 36% it was intermittent, and in 28% it was persistent. In this series, 45% of patients had no diagnosis, 22% were obese (presumed to have nonalcoholic steatohepatitis), 5% had alcoholic liver disease, 3% had “resolving hepatitis,” 1% had hemochromatosis, and 1% had “cytomegalovirus hepatitis.”16 Although the patients underwent a complete history, physical examination, and serologic testing, liver biopsy was not done to confirm the clinical diagnosis.
Hay et al17 described 47 patients with chronically elevated aminotransferases (three to eight times higher than normal levels) who underwent full evaluation and liver biopsy and who had no clinical symptoms of alcoholic, viral, or drug-induced liver disease. A diagnosis of steatohepatitis was given in 10 patients, another 34 were diagnosed with “chronic hepatitis,” and 3 had miscellaneous diagnoses. Of patients with chronic hepatitis, 16 had evidence of cirrhosis on biopsy, and 18 tested positive for at least one autoimmune marker (antinuclear antibody or smooth muscle antibody).
Daniel et al18 performed biopsy in 81 of 1,124 asymptomatic and symptomatic patients with chronically elevated aminotransferases in whom a cause was not identified via noninvasive studies. Liver biopsy showed that 67 (83%) of the 81 patients had steatosis or steatohepatitis, while 8 (10%) had normal histologic findings. Of note, 6 patients had underlying fibrosis or cirrhosis and some degree of fatty infiltration.
Together, these studies suggest that fatty liver, resulting either from alcohol use or from nonalcoholic fatty liver disease, is the major cause of mildly elevated aminotransferases. Two major drawbacks of the earlier studies include the lack of data on the hepatitis C serologic status of patients with the diagnosis of “chronic hepatitis” and the lack of a uniform approach to the pathologic diagnosis of nonalcoholic steatohepatitis. With serologic testing for hepatitis C virus infection now widely available, it is possible that a substantial portion of patients with “chronic hepatitis“ can further be classified as having chronic hepatitis C infection.
Workup of aminotransferase elevations
- Viral hepatitis (intravenous drug use, intranasal cocaine use, native of an endemic area of the world, unsafe sexual activity, blood product transfusions)
- Nonalcoholic fatty liver disease (components of the metabolic syndrome, including visceral obesity)
- Alcoholic liver disease (smaller amounts are needed to cause liver disease in women)
- Medication exposure (prescription, overthe-counter, and herbal medications)
- Genetic liver disorders (family history of liver disease)
- Possible coexisting disease (diabetes and obesity in nonalcoholic steatohepatitis, neurologic disorders in Wilson disease, emphysema in alpha-1-antitrypsin deficiency, thyroid disease in autoimmune hepatitis and primary biliary cirrhosis, and diabetes and impotence in genetic hemochromatosis).
Although the physical signs of chronic liver disease (eg, spider angiomata, palmar erythema, caput medusae, and gynecomastia) are nonspecific and are usually observed in advanced liver disease, some physical findings suggest potential causes (eg, Kayser-Fleischer rings on slit-lamp examination for Wilson disease, hypertrophy of the second and third metacarpophalangeal joints for hemochromatosis). Iron studies for middleaged men, autoimmune markers for women, and screening for Wilson disease in young patients are helpful when the clinical information points to one of these entities as a potential diagnosis.
If medication or alcohol is a suspected cause, aminotransferase levels should be repeated after 6 to 8 weeks of abstinence. If nonalcoholic steatohepatitis is suspected, testing should be repeated after treating the potential risk factor (eg, obesity, diabetes, hyperlipidemia), but the levels may remain elevated for a period of time. An imaging study (ultrasonography, computed tomography, or magnetic resonance imaging) may be helpful; eg, abdominal ultrasonography may show increased hepatic echogenicity, suggesting increased fatty infiltration, in addition to excluding most hepatic tumors.
If the history and physical do not suggest a specific condition, serologic testing for hepatitis C should be done. If negative, other testing can be helpful (iron studies in men, autoimmune markers in women, ceruloplasmin and slit-lamp examination in young patients). If the preliminary workup remains negative and the aminotransferase levels remain elevated for 6 months (ie, chronic elevation), liver biopsy may establish the diagnosis. Features in the biopsy specimens may further confirm the diagnosis: eg, globules positive on periodic acid-Schiff testing in alpha-1-antitrypsin deficiency; hepatic iron index for hemochromatosis; hepatic copper content for Wilson disease.
ALKALINE PHOSPHATASE ELEVATION
Causes
Alkaline phosphatase is active in many organs, mainly the liver and bones, but is also found in the small bowel, kidneys, and placenta. Diseases of the hepatobiliary system can cause moderate to marked elevations of alkaline phosphatase. Conditions with bone involvement, such as Paget disease of the bone, sarcoma, metastatic disease, hyperparathyroidism, and rickets, can raise alkaline phosphatase levels. Elevated GGT in conjunction with elevated alkaline phosphatase usually points to hepatobiliary injury. Clinically, isoenzyme fractionation of alkaline phosphatase may help distinguish the source of the elevation, but this is often not needed if the GGT is also elevated.
Hepatobiliary causes of alkaline phosphatase elevation can be divided into four categories:
- Chronic inflammation involving the bile ducts (eg, as in primary biliary cirrhosis and primary sclerosing cholangitis)
- Infiltrative process (eg, neoplasm, tuberculosis, sarcoidosis)
- Cholestatic disorders (eg, drug hepatotoxicity)
- Biliary obstruction (eg, in neoplasia or cholelithiasis).
Only a few studies have investigated the significance of a mild, isolated elevation of alkaline phosphatase. Lieberman and Phillips20 evaluated 87 patients and found that the abnormality resolved completely in less than 3 months in 28 patients, and resolved in 3 to 12 months in another 17 patients. Of the other 42 patients, 24 did not undergo further evaluation because they had significant coexisting disease. Of the remaining 18 patients, 5 had phenytoin-related hepatotoxicity, 3 had congestive heart failure, 3 had metabolic bone disease, 2 had hepatobiliary disease, and 1 had metastatic bone disease; in 4 patients, no explanation was determined. Follow-up was 1.5 to 3 years.
Workup of alkaline phosphatase elevation
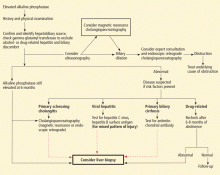
An isolated elevated alkaline phosphatase level should always be confirmed and a hepatic origin suspected if the GGT level is also elevated (Figure 2).
A history of recent drug exposure usually points to drug hepatotoxicity as the source of this abnormality. Similarly, other information from the history can point to a potential underlying pathologic process causing the rise in alkaline phosphatase. For example, a history of ulcerative colitis suggests primary sclerosing cholangitis, and a history of previous cancer or sarcoidosis suggests liver involvement.
As part of the initial evaluation, an imaging study (eg, ultrasonography) can exclude biliary obstruction or suggest an infiltrative process.
If a drug is the suspected cause, the alkaline phosphatase level should be repeated after the patient has abstained from the drug for 6 to 8 weeks. If the initial examination suggests a specific disease, disease-specific markers (eg, antimitochondrial antibody for primary biliary cirrhosis, or viral serology) can confirm the suspected diagnosis. If the disease-specific markers are negative and the alkaline phosphatase level does not return to normal, further studies should be considered, including liver biopsy and endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography. Given the invasive nature of biopsy and pancreatography, an expert consultation should be done before ordering these tests.
GGT ELEVATION
GGT is a membrane enzyme that is a marker of hepatobiliary disease. Elevations usually parallel the elevation of alkaline phosphatase, confirming a hepatic source for the latter. GGT is the most sensitive marker of biliary tract disease but is not very specific. Alcohol and drugs (eg, phenytoin, phenobarbital) induce GGT. In one study,21 GGT was elevated in 52% of patients without known liver disease. The GGT level can be used to monitor abstinence from alcohol in patients with alcoholic liver disease.21
Workup of GGT elevation
Because GGT lacks specificity as a marker and is highly inducible, an extensive evaluation of an isolated GGT elevation in an otherwise asymptomatic patient is not warranted.
WHEN TO CONSULT
Many of the evaluations discussed in this paper and elsewhere22–27 can be carried out by primary care providers following a systematic approach. Input from a gastroenterologist or hepatologist can be valuable if the initial workup fails to establish the diagnosis, as well as in assuring that the most effective therapy for a specific disease is initiated. Reassurance, patient education, and a systematic approach for evaluating these abnormalities can identify most treatable causes of liver disease in the most cost-effective and efficient manner.
- Hultcrantz R, Glaumann H, Lindberg G, Nilsson LH. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol 1986; 21:109–113.
- Ioanou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol 2006; 101:76–82.
- Flora KD, Keeffe EB. Evaluation of mildly abnormal liver tests in asymptomatic patients. J Insur Med 1990; 22:264–267.
- Alter HJ. To C or not to C: these are the questions. Blood 1995; 85:1681–1695.
- Everhart JE, editor. Digestive diseases in the United States: epidemiology and impact. Washington, DC: US Government Printing Office, 1994; NIH Publication No. 94–1447.
- Bacon BR, Tavill AS. Hemochromatosis and the iron overload syndromes. In:Zakim D, Boyer TD, editors. Hepatology. A Textbook of Liver Disease, 3rd ed. Philadelphia: WB Saunders, 1996:1439–1472.
- Lieber CS. Alcoholic liver disease. Curr Opin Gastroenterol 1994; 10:319–330.
- Seth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med 1997; 126:137–145.
- Czaja A. Autoimmune liver disease. In: Zakim D, Boyer TD, editors. Hepatolology: A Textbook of Liver Disease, 3rd ed. Philadelphia: WB Saunders, 1996:1259–1292.
- Bull PC, Cox DW. Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet 1994; 10:246–252.
- Perlmutter DH. Clinical manifestations of alpha-1-antitrypsin deficiency. Gastroenterol Clin North Am 1995; 24:27–43.
- Olivarez L, Caggana M, Pass KA, Ferguson P, Brewer GJ. Estimate of the frequency of Wilson’s disease in the US Caucasian population: a mutation analysis approach. Ann Hum Genet 2001; 65:459–463.
- Metcalf JV, Howel D, James OF, Bhopal R. Primary biliary cirrhosis: epidemiology helping the clinician. BMJ 1996; 312:1181–1182.
- Farrell GC. Liver disease caused by drugs, anesthetics, and toxins. In:Feldman M, Friedman LS, Sleisenger MH, editors. Gastrointestinal and Liver Disease, 7th ed. Philadelphia: WB Saunders, 2002;1403–1447.
- Abdo A, Meddings J, Swain M. Liver abnormalities in celiac disease. Clin Gastroenterol Hepatol 2004; 2:107–112.
- Friedman LS, Dienstag JL, Watkins E, et al. Evaluation of blood donors with elevated serum alanine aminotransferase. Ann Intern Med 1987; 107:137–144.
- Hay JE, Czaja AJ, Rakela J, Ludwig J. The nature of unexplained chronic aminotransferase elevations of a mild to moderate degree in asymptomatic patients. Hepatology 1989; 9:193–197.
- Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol 1999; 94:3010–3014.
- Van Ness MM, Diehl AM. Is liver biopsy useful in the evaluation of patients with chronically elevated liver enzymes? Ann Intern Med 1989; 111:473–478.
- Lieberman D, Phillips D. “Isolated” elevation of alkaline phosphatase: significance in hospitalized patients. J Clin Gastroenterol 1990; 12:415–419.
- Margarian GJ, Lucas LM, Kumar KL. Clinical significance in alcoholic patients of commonly encountered laboratory test results. West J Med 1992; 156:287–294.
- Fregia A, Jensen DM. Evaluation of abnromal liver tests. Compr Ther 1994; 20:50–54.
- Goddard CJ, Warens TW. Raised liver enzymes in asymptomatic patients: investigation and outcome. Dig Dis 1992; 10:218–226.
- Gitlin N. The differential of elevated liver enzymes. Contemporary Internal Medicine 1993:44–56.
- Keefe EB. Diagnostic approach to mild elevation of liver enzyme levels. Gastrointestinal Diseases Today 1994; 3:1–9.
- Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000; 342:1266–1271.
- American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology 2002; 123:1364–1366.
In seemingly healthy patients, abnormal liver enzyme levels challenge even the most experienced clinicians in deciding what further evaluation to pursue, if any. Automated laboratory testing has made serum liver enzyme levels very easy to obtain, leading to an increase in testing and also in the number of incidental abnormal findings. An estimated 1% to 9% of people who have no symptoms have high liver enzyme levels when screened with standard biochemistry panels.1,2 A US survey2 showed elevated alanine aminotransferase (ALT) in 8.9% of surveyed people from 1999 to 2002, an increase from previous reports.
An extensive evaluation can be costly, anxiety-provoking, and risky, especially if it leads to unnecessary invasive procedures such as liver biopsy or endoscopic retrograde cholangiopancreatography (ERCP). Not all people with a single, isolated, mildly elevated liver enzyme value have underlying liver disease, nor do they require an extensive evaluation. Factors to consider when deciding whether to evaluate include:
- The patient’s overall health, including chronic illness
- The duration and pattern of enzyme elevation
- Patient characteristics such as age, a personal or family history of liver, lung, or neurologic disease, risk factors for viral hepatitis, amount of alcohol consumption, use of prescribed or over-the-counter drugs or dietary supplements
- The costs and risks associated with additional evaluation.
This article reviews the most likely causes of elevated aminotransferase, alkaline phosphatase, and gamma-glutamyl transferase (GGT) levels. It also provides an algorithm for evaluating mildly abnormal liver enzyme values in apparently healthy people. Patients with signs of hepatic decompensation need a more concise and urgent evaluation.
PATTERNS OF LIVER ENZYME ELEVATION
“Liver function test” is commonly used to describe liver enzyme measurements, but the term should be reserved for tests of the functional hepatic reserve—traditionally, the albumin level and the prothrombin time.3
On the other hand, elevated serum liver enzymes (aminotransferases, alkaline phosphatase, and GGT) can reflect abnormalities in liver cells or in the bile duct. For example, predominant elevation of aminotransferases typically indicates hepatocellular injury, whereas elevated alkaline phosphatase and GGT indicates cholestatic injury. Elevated alkaline phosphatase and aminotransferases can indicate a mixed pattern of injury.
High AST, ALT suggest liver cell damage
Both aspartate aminotransferase (AST) and ALT are normally present in serum at low levels, usually less than 30 to 40 U/L. Although the actual values may differ from laboratory to laboratory, normal serum levels are usually less than 40 U/L for AST and less than 50 U/L for ALT. On the other hand, some experts have suggested lowering the upper limit of normal because of the increasing rate of obesity and associated nonalcoholic fatty liver disease, which may not be detected using the traditional, higher normal values. Acceptance is growing for using ALT levels less than 40 U/L in men and less than 31 U/L in women, and AST levels less than 37 U/L in men and less than 31 U/L in women, as normal thresholds.
Although ALT is present in several organs and in muscle, the highest levels are in the liver, which makes this enzyme a more specific indicator of liver injury. Both AST and ALT are released into the blood in greater amounts when hepatocytes are damaged.
Alkaline phosphatase suggests cholestasis
Alkaline phosphatase comes mostly from the liver and bone. In general, normal serum alkaline phosphatase levels in adults range between 20 and 120 U/L. When bone disease is excluded, an elevation suggests biliary obstruction, injury to the bile duct epithelium, or cholestasis. Additionally, there are rare cases of benign familial elevation of serum alkaline phosphatase, mainly of intestinal origin.
GGT is not specific
GGT is present in hepatocytes and biliary epithelial cells. The normal range is 0 U/L to 50 U/L in men, and 0 U/L to 35 U/L in women. GGT elevation is the most sensitive marker of hepatobiliary disease. However, its routine clinical use is not recommended, as it cannot by itself indicate a specific cause of liver disease, although measuring the GGT level can help determine a hepatic origin for an isolated elevation of alkaline phosphatase.
RISK FACTORS GUIDE THE WORKUP OF ELEVATED ENZYMES
Chronic viral hepatitis
Prevalence. Hepatitis C virus infection affects an estimated 1.8% of the general population, but the rate is much higher in people with known risk factors (see below), and those with ALT levels greater than 40 U/L.
Hepatitis B virus infection is somewhat less common: between 0.2% and 0.9% of the general US population have positive results on tests for hepatitis B surface antigen. However, the prevalence of this antigen in the United States can be as high as 20% in patients who have emigrated from endemic areas of the world. The risk factors described below dramatically increase the prevalence of both viruses.
Risk factors. Risk factors include bloodproduct transfusions (especially before 1992), intravenous drug use, intranasal cocaine use, hemodialysis, organ transplantation, and birth in an endemic region. Although both viruses can be transmitted sexually, hepatitis B is more readily transmitted by this route than hepatitis C. Worldwide, transmission of hepatitis B virus usually occurs shortly after birth or at a young age.
Comments. Most patients with chronic viral hepatitis have no symptoms or only mild symptoms and minimally elevated ALT and AST levels, ie, two to five times higher than the upper limit of normal. Given the relatively high prevalence of hepatitis C, serologic testing for it should be done early in the evaluation of chronically elevated liver enzyme levels.4,5
Hereditary hemochromatosis
Prevalence. The prevalence of the major HFE-gene mutations that cause hereditary hemochromatosis is 0.25% to 0.5% in people of northern European descent. In northern Europe, about 1 person in 10 is heterozygous and 1 in 200 to 400 is homozygous for the mutated gene.
Risk factors. Northern European ancestry is the primary risk factor. In men, the onset of disease is usually in the third and fourth decades of life, while menses protects women until menopause. From 83% to 85% of people with clinically defined hemochromatosis are homozygous for the C282Y mutation in the HFE gene.
Comments. Hereditary hemochromatosis should be considered early in the evaluation of men of northern European descent. Patients usually have no symptoms until iron overload causes significant end-organ damage. Phlebotomy can be an effective treatment for this potentially fatal disease.6
Alcoholic liver disease
Risk factors. The degree of alcohol-related liver disease depends on a variety of factors, including the volume and duration of alcohol ingestion, the type of liver disease, genetics, and the coexistence of viral hepatitis and obesity.
Alcohol-related liver disease can range from simple fatty liver to alcoholic hepatitis with or without cirrhosis. Cirrhosis develops in only 20% to 30% of patients who consume a substantial amount of alcohol, defined as more than a decade of 60 g/day to 80 g/day of alcohol in men and as little as 20 g/day in women. (A standard drink, ie, a 12-ounce beer, a 5-ounce glass of wine, or 1.5 ounces of distilled spirits, contains 12 g of alcohol.) Factors that potentiate alcohol’s harmful effects include female sex, chronic viral hepatitis (especially hepatitis C), obesity, hereditary hemochromatosis, and use of drugs such as methotrexate (Trexall) and acetaminophen (Tylenol).
Comments. Although cirrhosis affects fewer than one-third of long-term heavy drinkers, early detection and treatment can potentially reduce morbidity and prevent early death. Alcoholic liver disease should be suspected in patients with elevated ALT and AST levels (if the AST level is two to three times higher than normal7) and with a history of excessive alcohol use.
Nonalcoholic fatty liver disease
Prevalence. Nonalcoholic fatty liver disease is a spectrum that ranges from simple steatosis to nonalcoholic steatohepatitis to cirrhosis. Its prevalence in the general US population is about 25%, but is much higher in groups at risk, such as patients with type 2 diabetes (50% to 60%), and morbidly obese patients undergoing bariatric surgery (90% to 95%).
On the other hand, the prevalence of the potentially progressive form of nonalcoholic fatty liver disease, ie, nonalcoholic steatohepatitis, is estimated to be 3% to 5%. Nonalcoholic fatty liver disease is perhaps the most common cause of mildly elevated liver enzymes in the United States.
Comments. Nonalcoholic steatohepatitis and steatonecrosis describe a potentially progressive form of nonalcoholic fatty liver disease. Although these disorders are histologically indistinguishable from alcohol-induced liver disease, their mechanism is related to insulin resistance, abnormalities of lipid metabolism, increased hepatic lipid peroxidation, activated fibrocytes, and abnormal patterns of adipokine and cytokine production related to visceral obesity. Results from a few natural history studies suggest that simple steatosis has a benign course, whereas nonalcoholic steatohepatitis can progress to cirrhosis in 10% to 20% of patients.8
Treatment of diabetes, obesity, hypertension, and hyperlipidemia has potential benefit and should be undertaken regardless of liver test abnormalities in any patient with underlying nonalcoholic fatty liver disease.
Autoimmune hepatitis
Prevalence. The prevalence of autoimmune hepatitis varies, depending on geographic location and on the extent of viral hepatitis in the community. In Hong Kong, only 1% of all people with chronic hepatitis have autoimmune hepatitis. By contrast, in Germany and Austria, 34% and 62% of patients with chronic hepatitis may have autoimmune hepatitis.9 In North America, the prevalence of autoimmune hepatitis in patients with chronic liver disease is estimated to be 11% to 23%, and the incidence is about 0.68 per 100,000 individuals per year. In addition to underlying genetic differences, detection bias can explain the variability in prevalence rates.
Risk factors. Autoimmune hepatitis occurs predominantly in women and can be associated with other autoimmune disorders.
Comments. The diagnosis of autoimmune hepatitis is suggested by exclusion of viral causes of chronic hepatitis, by pathologic findings, and by the presence of autoimmune markers such as antinuclear antibody, smooth muscle antibody, and liver-kidney microsomal antibody. Hypergammaglobulinemia is present in most patients, and serum protein electrophoresis may be helpful as part of the initial evaluation of autoimmune hepatitis.
Liver biopsy is usually needed to confirm the diagnosis and to stage the extent of fibrosis. The International Autoimmune Hepatitis Group Scoring System is based on clinical, laboratory, and pathologic data and can be very helpful in establishing the diagnosis.
Treatment of autoimmune hepatitis with immunosuppression is effective. Most patients may need long-term maintenance treatment.
Wilson disease
Prevalence. The estimated prevalence of Wilson disease is 1 in 40,000 to 1 in 100,000. It has been reported in most populations worldwide.
Risk factors. Anyone under age 40 with abnormal liver enzyme levels (including mild elevations) should be evaluated for Wilson disease, even in the absence of neurologic or ocular findings. However, such routine screening is rarely helpful in patients over age 50. Genetic testing is of limited value because of the large number of potential mutations of the ATP7B gene, the gene responsible for Wilson disease. However, if a a person is known to have Wilson disease, genetic screening of family members is useful.
Comments. Effective therapy is available (ie, d-penicillamine, trientine, zinc). For Wilson disease, alpha-1-antitrypsin deficiency, and genetic hemochromatosis, establishing the diagnosis is not only important to the individual patient; it also may prompt the screening of asymptomatic members of the proband’s family.10–12
Alpha-1-antitrypsin deficiency
Prevalence. Alpha-1-antitrypsin deficiency is present in 1 of every 1,600 to 1,800 live births.11
Risk factors. Patients with emphysema or with a young sibling with liver failure should undergo an investigation for alpha-1-antitrypsin deficiency, consisting of a measurement of the alpha-1-antitrypsin level and an assessment for the PiZZ genotype (the most severe form, because homozygous for the abnormal Z allele).
Comments. Although alpha-1-antitrypsin deficiency is a common cause of liver disease in the very young, it is important to remember that a small number of these patients develop end-stage liver disease in adulthood. Liver transplantation is the only effective treatment for end-stage liver disease associated with alpha-1-antitrypsin deficiency.
Primary biliary cirrhosis
Prevalence. In one study of urban-dwelling women in northeast England, the prevalence of primary biliary cirrhosis was estimated at 0.10%.13
Risk factors. Like autoimmune hepatitis, primary biliary cirrhosis mainly affects women and can be associated with other autoimmune disorders.
Comments. A cholestatic pattern of injury is predominant in primary biliary cirrhosis. Treatment of primary biliary cirrhosis with the cytoprotective agent ursodeoxycholic acid improves liver enzyme levels, may lead to histologic improvement and increased survival, and may also delay the need for liver transplantation. 9,13
Drug- and toxin-related liver diseases
Nonsteroidal anti-inflammatory drugs and penicillin-derived antibiotics are the drugs that most commonly cause abnormal serum liver enzyme levels. The mechanisms of druginduced liver disease include induction of hepatic enzymes (antiepileptic drugs), allergic reactions, autoimmunity (nitrofurantoin [Furadantin, Macrobid]), idiosyncratic reactions, and veno-occlusive injury.14 Drugs that are potentially hepatotoxic are listed in Table 2 and are classified as causing hepatocellular damage, cholestatic damage, or steatosis.
MILD ENZYME ELEVATIONS AS INDICATORS OF SPECIFIC DISEASES
Mildly elevated liver enzymes are common and potentially important, yet very few welldesigned prospective studies have addressed the issue of what should be done once they are identified. Most current data are from small retrospective studies that lack accurate information on the important causes of liver diseases such as hepatitis C and nonalcoholic steatohepatitis.
Despite these shortcomings, the literature delineates the three patterns of mild liver enzyme elevations discussed earlier: hepatocellular injury pattern (elevated ALT or AST), cholestatic pattern (elevated alkaline phosphatase or GGT, or both), and mixed pattern (elevation of ALT, AST, and alkaline phosphatase). The following paragraphs focus on the causes of elevation of specific liver enzymes.
AMINOTRANSFERASE ELEVATION
Causes
Aminotransferases are commonly used markers of hepatocyte injury. AST is present in blood cells and many tissues, including liver, muscle, brain, pancreas, and lung. ALT is a cytosolic enzyme found primarily in hepatocytes, making it a more specific indicator of liver disease.
Acute viral hepatitis, toxins, and liver ischemia can markedly raise serum aminotransferase levels (often into the thousands of units per liter). On the other hand, these enzymes are only mildly elevated (< 300 U/L) in nonalcoholic steatohepatitis, chronic hepatitis, cholestatic liver conditions, drug-induced hepatotoxicity, and liver tumors.
Because AST is a mitochondrial enzyme and is affected by alcohol ingestion, an AST level more than twice that of the ALT suggests hepatic damage due to alcohol.
Of note, aminotransferase elevation can also be due to nonhepatic causes. For example, muscle necrosis can result in mild elevation of these enzymes, especially AST, and an elevated creatine kinase can help confirm that the source is muscle tissue.
Undiagnosed celiac disease has been associated with abnormal liver enzyme levels when all other causes have been ruled out, but the mechanism is not yet understood. In this situation, ALT and AST levels typically return to normal with a gluten-free diet.15
Studies of mild aminotransferase elevations
Only a few studies have documented the results of a thorough evaluation of patients with mildly elevated aminotransferase levels:
Hultcrantz et al1 performed a full evaluation, including liver biopsy, in 149 consecutive patients with chronic, asymptomatic, mild elevations of AST or ALT. Of these patients, 63% had “fatty liver,” 20% had “chronic hepatitis,” and 17% had miscellaneous diagnoses. Whether patients in the “chronic hepatitis” group had hepatitis C was not determined because serologic testing was not available at the time.
Friedman et al16 studied 100 healthy blood donors with elevated ALT levels and found that in 33% of patients the elevation occurred once, in 36% it was intermittent, and in 28% it was persistent. In this series, 45% of patients had no diagnosis, 22% were obese (presumed to have nonalcoholic steatohepatitis), 5% had alcoholic liver disease, 3% had “resolving hepatitis,” 1% had hemochromatosis, and 1% had “cytomegalovirus hepatitis.”16 Although the patients underwent a complete history, physical examination, and serologic testing, liver biopsy was not done to confirm the clinical diagnosis.
Hay et al17 described 47 patients with chronically elevated aminotransferases (three to eight times higher than normal levels) who underwent full evaluation and liver biopsy and who had no clinical symptoms of alcoholic, viral, or drug-induced liver disease. A diagnosis of steatohepatitis was given in 10 patients, another 34 were diagnosed with “chronic hepatitis,” and 3 had miscellaneous diagnoses. Of patients with chronic hepatitis, 16 had evidence of cirrhosis on biopsy, and 18 tested positive for at least one autoimmune marker (antinuclear antibody or smooth muscle antibody).
Daniel et al18 performed biopsy in 81 of 1,124 asymptomatic and symptomatic patients with chronically elevated aminotransferases in whom a cause was not identified via noninvasive studies. Liver biopsy showed that 67 (83%) of the 81 patients had steatosis or steatohepatitis, while 8 (10%) had normal histologic findings. Of note, 6 patients had underlying fibrosis or cirrhosis and some degree of fatty infiltration.
Together, these studies suggest that fatty liver, resulting either from alcohol use or from nonalcoholic fatty liver disease, is the major cause of mildly elevated aminotransferases. Two major drawbacks of the earlier studies include the lack of data on the hepatitis C serologic status of patients with the diagnosis of “chronic hepatitis” and the lack of a uniform approach to the pathologic diagnosis of nonalcoholic steatohepatitis. With serologic testing for hepatitis C virus infection now widely available, it is possible that a substantial portion of patients with “chronic hepatitis“ can further be classified as having chronic hepatitis C infection.
Workup of aminotransferase elevations
- Viral hepatitis (intravenous drug use, intranasal cocaine use, native of an endemic area of the world, unsafe sexual activity, blood product transfusions)
- Nonalcoholic fatty liver disease (components of the metabolic syndrome, including visceral obesity)
- Alcoholic liver disease (smaller amounts are needed to cause liver disease in women)
- Medication exposure (prescription, overthe-counter, and herbal medications)
- Genetic liver disorders (family history of liver disease)
- Possible coexisting disease (diabetes and obesity in nonalcoholic steatohepatitis, neurologic disorders in Wilson disease, emphysema in alpha-1-antitrypsin deficiency, thyroid disease in autoimmune hepatitis and primary biliary cirrhosis, and diabetes and impotence in genetic hemochromatosis).
Although the physical signs of chronic liver disease (eg, spider angiomata, palmar erythema, caput medusae, and gynecomastia) are nonspecific and are usually observed in advanced liver disease, some physical findings suggest potential causes (eg, Kayser-Fleischer rings on slit-lamp examination for Wilson disease, hypertrophy of the second and third metacarpophalangeal joints for hemochromatosis). Iron studies for middleaged men, autoimmune markers for women, and screening for Wilson disease in young patients are helpful when the clinical information points to one of these entities as a potential diagnosis.
If medication or alcohol is a suspected cause, aminotransferase levels should be repeated after 6 to 8 weeks of abstinence. If nonalcoholic steatohepatitis is suspected, testing should be repeated after treating the potential risk factor (eg, obesity, diabetes, hyperlipidemia), but the levels may remain elevated for a period of time. An imaging study (ultrasonography, computed tomography, or magnetic resonance imaging) may be helpful; eg, abdominal ultrasonography may show increased hepatic echogenicity, suggesting increased fatty infiltration, in addition to excluding most hepatic tumors.
If the history and physical do not suggest a specific condition, serologic testing for hepatitis C should be done. If negative, other testing can be helpful (iron studies in men, autoimmune markers in women, ceruloplasmin and slit-lamp examination in young patients). If the preliminary workup remains negative and the aminotransferase levels remain elevated for 6 months (ie, chronic elevation), liver biopsy may establish the diagnosis. Features in the biopsy specimens may further confirm the diagnosis: eg, globules positive on periodic acid-Schiff testing in alpha-1-antitrypsin deficiency; hepatic iron index for hemochromatosis; hepatic copper content for Wilson disease.
ALKALINE PHOSPHATASE ELEVATION
Causes
Alkaline phosphatase is active in many organs, mainly the liver and bones, but is also found in the small bowel, kidneys, and placenta. Diseases of the hepatobiliary system can cause moderate to marked elevations of alkaline phosphatase. Conditions with bone involvement, such as Paget disease of the bone, sarcoma, metastatic disease, hyperparathyroidism, and rickets, can raise alkaline phosphatase levels. Elevated GGT in conjunction with elevated alkaline phosphatase usually points to hepatobiliary injury. Clinically, isoenzyme fractionation of alkaline phosphatase may help distinguish the source of the elevation, but this is often not needed if the GGT is also elevated.
Hepatobiliary causes of alkaline phosphatase elevation can be divided into four categories:
- Chronic inflammation involving the bile ducts (eg, as in primary biliary cirrhosis and primary sclerosing cholangitis)
- Infiltrative process (eg, neoplasm, tuberculosis, sarcoidosis)
- Cholestatic disorders (eg, drug hepatotoxicity)
- Biliary obstruction (eg, in neoplasia or cholelithiasis).
Only a few studies have investigated the significance of a mild, isolated elevation of alkaline phosphatase. Lieberman and Phillips20 evaluated 87 patients and found that the abnormality resolved completely in less than 3 months in 28 patients, and resolved in 3 to 12 months in another 17 patients. Of the other 42 patients, 24 did not undergo further evaluation because they had significant coexisting disease. Of the remaining 18 patients, 5 had phenytoin-related hepatotoxicity, 3 had congestive heart failure, 3 had metabolic bone disease, 2 had hepatobiliary disease, and 1 had metastatic bone disease; in 4 patients, no explanation was determined. Follow-up was 1.5 to 3 years.
Workup of alkaline phosphatase elevation
An isolated elevated alkaline phosphatase level should always be confirmed and a hepatic origin suspected if the GGT level is also elevated (Figure 2).
A history of recent drug exposure usually points to drug hepatotoxicity as the source of this abnormality. Similarly, other information from the history can point to a potential underlying pathologic process causing the rise in alkaline phosphatase. For example, a history of ulcerative colitis suggests primary sclerosing cholangitis, and a history of previous cancer or sarcoidosis suggests liver involvement.
As part of the initial evaluation, an imaging study (eg, ultrasonography) can exclude biliary obstruction or suggest an infiltrative process.
If a drug is the suspected cause, the alkaline phosphatase level should be repeated after the patient has abstained from the drug for 6 to 8 weeks. If the initial examination suggests a specific disease, disease-specific markers (eg, antimitochondrial antibody for primary biliary cirrhosis, or viral serology) can confirm the suspected diagnosis. If the disease-specific markers are negative and the alkaline phosphatase level does not return to normal, further studies should be considered, including liver biopsy and endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography. Given the invasive nature of biopsy and pancreatography, an expert consultation should be done before ordering these tests.
GGT ELEVATION
GGT is a membrane enzyme that is a marker of hepatobiliary disease. Elevations usually parallel the elevation of alkaline phosphatase, confirming a hepatic source for the latter. GGT is the most sensitive marker of biliary tract disease but is not very specific. Alcohol and drugs (eg, phenytoin, phenobarbital) induce GGT. In one study,21 GGT was elevated in 52% of patients without known liver disease. The GGT level can be used to monitor abstinence from alcohol in patients with alcoholic liver disease.21
Workup of GGT elevation
Because GGT lacks specificity as a marker and is highly inducible, an extensive evaluation of an isolated GGT elevation in an otherwise asymptomatic patient is not warranted.
WHEN TO CONSULT
Many of the evaluations discussed in this paper and elsewhere22–27 can be carried out by primary care providers following a systematic approach. Input from a gastroenterologist or hepatologist can be valuable if the initial workup fails to establish the diagnosis, as well as in assuring that the most effective therapy for a specific disease is initiated. Reassurance, patient education, and a systematic approach for evaluating these abnormalities can identify most treatable causes of liver disease in the most cost-effective and efficient manner.
In seemingly healthy patients, abnormal liver enzyme levels challenge even the most experienced clinicians in deciding what further evaluation to pursue, if any. Automated laboratory testing has made serum liver enzyme levels very easy to obtain, leading to an increase in testing and also in the number of incidental abnormal findings. An estimated 1% to 9% of people who have no symptoms have high liver enzyme levels when screened with standard biochemistry panels.1,2 A US survey2 showed elevated alanine aminotransferase (ALT) in 8.9% of surveyed people from 1999 to 2002, an increase from previous reports.
An extensive evaluation can be costly, anxiety-provoking, and risky, especially if it leads to unnecessary invasive procedures such as liver biopsy or endoscopic retrograde cholangiopancreatography (ERCP). Not all people with a single, isolated, mildly elevated liver enzyme value have underlying liver disease, nor do they require an extensive evaluation. Factors to consider when deciding whether to evaluate include:
- The patient’s overall health, including chronic illness
- The duration and pattern of enzyme elevation
- Patient characteristics such as age, a personal or family history of liver, lung, or neurologic disease, risk factors for viral hepatitis, amount of alcohol consumption, use of prescribed or over-the-counter drugs or dietary supplements
- The costs and risks associated with additional evaluation.
This article reviews the most likely causes of elevated aminotransferase, alkaline phosphatase, and gamma-glutamyl transferase (GGT) levels. It also provides an algorithm for evaluating mildly abnormal liver enzyme values in apparently healthy people. Patients with signs of hepatic decompensation need a more concise and urgent evaluation.
PATTERNS OF LIVER ENZYME ELEVATION
“Liver function test” is commonly used to describe liver enzyme measurements, but the term should be reserved for tests of the functional hepatic reserve—traditionally, the albumin level and the prothrombin time.3
On the other hand, elevated serum liver enzymes (aminotransferases, alkaline phosphatase, and GGT) can reflect abnormalities in liver cells or in the bile duct. For example, predominant elevation of aminotransferases typically indicates hepatocellular injury, whereas elevated alkaline phosphatase and GGT indicates cholestatic injury. Elevated alkaline phosphatase and aminotransferases can indicate a mixed pattern of injury.
High AST, ALT suggest liver cell damage
Both aspartate aminotransferase (AST) and ALT are normally present in serum at low levels, usually less than 30 to 40 U/L. Although the actual values may differ from laboratory to laboratory, normal serum levels are usually less than 40 U/L for AST and less than 50 U/L for ALT. On the other hand, some experts have suggested lowering the upper limit of normal because of the increasing rate of obesity and associated nonalcoholic fatty liver disease, which may not be detected using the traditional, higher normal values. Acceptance is growing for using ALT levels less than 40 U/L in men and less than 31 U/L in women, and AST levels less than 37 U/L in men and less than 31 U/L in women, as normal thresholds.
Although ALT is present in several organs and in muscle, the highest levels are in the liver, which makes this enzyme a more specific indicator of liver injury. Both AST and ALT are released into the blood in greater amounts when hepatocytes are damaged.
Alkaline phosphatase suggests cholestasis
Alkaline phosphatase comes mostly from the liver and bone. In general, normal serum alkaline phosphatase levels in adults range between 20 and 120 U/L. When bone disease is excluded, an elevation suggests biliary obstruction, injury to the bile duct epithelium, or cholestasis. Additionally, there are rare cases of benign familial elevation of serum alkaline phosphatase, mainly of intestinal origin.
GGT is not specific
GGT is present in hepatocytes and biliary epithelial cells. The normal range is 0 U/L to 50 U/L in men, and 0 U/L to 35 U/L in women. GGT elevation is the most sensitive marker of hepatobiliary disease. However, its routine clinical use is not recommended, as it cannot by itself indicate a specific cause of liver disease, although measuring the GGT level can help determine a hepatic origin for an isolated elevation of alkaline phosphatase.
RISK FACTORS GUIDE THE WORKUP OF ELEVATED ENZYMES
Chronic viral hepatitis
Prevalence. Hepatitis C virus infection affects an estimated 1.8% of the general population, but the rate is much higher in people with known risk factors (see below), and those with ALT levels greater than 40 U/L.
Hepatitis B virus infection is somewhat less common: between 0.2% and 0.9% of the general US population have positive results on tests for hepatitis B surface antigen. However, the prevalence of this antigen in the United States can be as high as 20% in patients who have emigrated from endemic areas of the world. The risk factors described below dramatically increase the prevalence of both viruses.
Risk factors. Risk factors include bloodproduct transfusions (especially before 1992), intravenous drug use, intranasal cocaine use, hemodialysis, organ transplantation, and birth in an endemic region. Although both viruses can be transmitted sexually, hepatitis B is more readily transmitted by this route than hepatitis C. Worldwide, transmission of hepatitis B virus usually occurs shortly after birth or at a young age.
Comments. Most patients with chronic viral hepatitis have no symptoms or only mild symptoms and minimally elevated ALT and AST levels, ie, two to five times higher than the upper limit of normal. Given the relatively high prevalence of hepatitis C, serologic testing for it should be done early in the evaluation of chronically elevated liver enzyme levels.4,5
Hereditary hemochromatosis
Prevalence. The prevalence of the major HFE-gene mutations that cause hereditary hemochromatosis is 0.25% to 0.5% in people of northern European descent. In northern Europe, about 1 person in 10 is heterozygous and 1 in 200 to 400 is homozygous for the mutated gene.
Risk factors. Northern European ancestry is the primary risk factor. In men, the onset of disease is usually in the third and fourth decades of life, while menses protects women until menopause. From 83% to 85% of people with clinically defined hemochromatosis are homozygous for the C282Y mutation in the HFE gene.
Comments. Hereditary hemochromatosis should be considered early in the evaluation of men of northern European descent. Patients usually have no symptoms until iron overload causes significant end-organ damage. Phlebotomy can be an effective treatment for this potentially fatal disease.6
Alcoholic liver disease
Risk factors. The degree of alcohol-related liver disease depends on a variety of factors, including the volume and duration of alcohol ingestion, the type of liver disease, genetics, and the coexistence of viral hepatitis and obesity.
Alcohol-related liver disease can range from simple fatty liver to alcoholic hepatitis with or without cirrhosis. Cirrhosis develops in only 20% to 30% of patients who consume a substantial amount of alcohol, defined as more than a decade of 60 g/day to 80 g/day of alcohol in men and as little as 20 g/day in women. (A standard drink, ie, a 12-ounce beer, a 5-ounce glass of wine, or 1.5 ounces of distilled spirits, contains 12 g of alcohol.) Factors that potentiate alcohol’s harmful effects include female sex, chronic viral hepatitis (especially hepatitis C), obesity, hereditary hemochromatosis, and use of drugs such as methotrexate (Trexall) and acetaminophen (Tylenol).
Comments. Although cirrhosis affects fewer than one-third of long-term heavy drinkers, early detection and treatment can potentially reduce morbidity and prevent early death. Alcoholic liver disease should be suspected in patients with elevated ALT and AST levels (if the AST level is two to three times higher than normal7) and with a history of excessive alcohol use.
Nonalcoholic fatty liver disease
Prevalence. Nonalcoholic fatty liver disease is a spectrum that ranges from simple steatosis to nonalcoholic steatohepatitis to cirrhosis. Its prevalence in the general US population is about 25%, but is much higher in groups at risk, such as patients with type 2 diabetes (50% to 60%), and morbidly obese patients undergoing bariatric surgery (90% to 95%).
On the other hand, the prevalence of the potentially progressive form of nonalcoholic fatty liver disease, ie, nonalcoholic steatohepatitis, is estimated to be 3% to 5%. Nonalcoholic fatty liver disease is perhaps the most common cause of mildly elevated liver enzymes in the United States.
Comments. Nonalcoholic steatohepatitis and steatonecrosis describe a potentially progressive form of nonalcoholic fatty liver disease. Although these disorders are histologically indistinguishable from alcohol-induced liver disease, their mechanism is related to insulin resistance, abnormalities of lipid metabolism, increased hepatic lipid peroxidation, activated fibrocytes, and abnormal patterns of adipokine and cytokine production related to visceral obesity. Results from a few natural history studies suggest that simple steatosis has a benign course, whereas nonalcoholic steatohepatitis can progress to cirrhosis in 10% to 20% of patients.8
Treatment of diabetes, obesity, hypertension, and hyperlipidemia has potential benefit and should be undertaken regardless of liver test abnormalities in any patient with underlying nonalcoholic fatty liver disease.
Autoimmune hepatitis
Prevalence. The prevalence of autoimmune hepatitis varies, depending on geographic location and on the extent of viral hepatitis in the community. In Hong Kong, only 1% of all people with chronic hepatitis have autoimmune hepatitis. By contrast, in Germany and Austria, 34% and 62% of patients with chronic hepatitis may have autoimmune hepatitis.9 In North America, the prevalence of autoimmune hepatitis in patients with chronic liver disease is estimated to be 11% to 23%, and the incidence is about 0.68 per 100,000 individuals per year. In addition to underlying genetic differences, detection bias can explain the variability in prevalence rates.
Risk factors. Autoimmune hepatitis occurs predominantly in women and can be associated with other autoimmune disorders.
Comments. The diagnosis of autoimmune hepatitis is suggested by exclusion of viral causes of chronic hepatitis, by pathologic findings, and by the presence of autoimmune markers such as antinuclear antibody, smooth muscle antibody, and liver-kidney microsomal antibody. Hypergammaglobulinemia is present in most patients, and serum protein electrophoresis may be helpful as part of the initial evaluation of autoimmune hepatitis.
Liver biopsy is usually needed to confirm the diagnosis and to stage the extent of fibrosis. The International Autoimmune Hepatitis Group Scoring System is based on clinical, laboratory, and pathologic data and can be very helpful in establishing the diagnosis.
Treatment of autoimmune hepatitis with immunosuppression is effective. Most patients may need long-term maintenance treatment.
Wilson disease
Prevalence. The estimated prevalence of Wilson disease is 1 in 40,000 to 1 in 100,000. It has been reported in most populations worldwide.
Risk factors. Anyone under age 40 with abnormal liver enzyme levels (including mild elevations) should be evaluated for Wilson disease, even in the absence of neurologic or ocular findings. However, such routine screening is rarely helpful in patients over age 50. Genetic testing is of limited value because of the large number of potential mutations of the ATP7B gene, the gene responsible for Wilson disease. However, if a a person is known to have Wilson disease, genetic screening of family members is useful.
Comments. Effective therapy is available (ie, d-penicillamine, trientine, zinc). For Wilson disease, alpha-1-antitrypsin deficiency, and genetic hemochromatosis, establishing the diagnosis is not only important to the individual patient; it also may prompt the screening of asymptomatic members of the proband’s family.10–12
Alpha-1-antitrypsin deficiency
Prevalence. Alpha-1-antitrypsin deficiency is present in 1 of every 1,600 to 1,800 live births.11
Risk factors. Patients with emphysema or with a young sibling with liver failure should undergo an investigation for alpha-1-antitrypsin deficiency, consisting of a measurement of the alpha-1-antitrypsin level and an assessment for the PiZZ genotype (the most severe form, because homozygous for the abnormal Z allele).
Comments. Although alpha-1-antitrypsin deficiency is a common cause of liver disease in the very young, it is important to remember that a small number of these patients develop end-stage liver disease in adulthood. Liver transplantation is the only effective treatment for end-stage liver disease associated with alpha-1-antitrypsin deficiency.
Primary biliary cirrhosis
Prevalence. In one study of urban-dwelling women in northeast England, the prevalence of primary biliary cirrhosis was estimated at 0.10%.13
Risk factors. Like autoimmune hepatitis, primary biliary cirrhosis mainly affects women and can be associated with other autoimmune disorders.
Comments. A cholestatic pattern of injury is predominant in primary biliary cirrhosis. Treatment of primary biliary cirrhosis with the cytoprotective agent ursodeoxycholic acid improves liver enzyme levels, may lead to histologic improvement and increased survival, and may also delay the need for liver transplantation. 9,13
Drug- and toxin-related liver diseases
Nonsteroidal anti-inflammatory drugs and penicillin-derived antibiotics are the drugs that most commonly cause abnormal serum liver enzyme levels. The mechanisms of druginduced liver disease include induction of hepatic enzymes (antiepileptic drugs), allergic reactions, autoimmunity (nitrofurantoin [Furadantin, Macrobid]), idiosyncratic reactions, and veno-occlusive injury.14 Drugs that are potentially hepatotoxic are listed in Table 2 and are classified as causing hepatocellular damage, cholestatic damage, or steatosis.
MILD ENZYME ELEVATIONS AS INDICATORS OF SPECIFIC DISEASES
Mildly elevated liver enzymes are common and potentially important, yet very few welldesigned prospective studies have addressed the issue of what should be done once they are identified. Most current data are from small retrospective studies that lack accurate information on the important causes of liver diseases such as hepatitis C and nonalcoholic steatohepatitis.
Despite these shortcomings, the literature delineates the three patterns of mild liver enzyme elevations discussed earlier: hepatocellular injury pattern (elevated ALT or AST), cholestatic pattern (elevated alkaline phosphatase or GGT, or both), and mixed pattern (elevation of ALT, AST, and alkaline phosphatase). The following paragraphs focus on the causes of elevation of specific liver enzymes.
AMINOTRANSFERASE ELEVATION
Causes
Aminotransferases are commonly used markers of hepatocyte injury. AST is present in blood cells and many tissues, including liver, muscle, brain, pancreas, and lung. ALT is a cytosolic enzyme found primarily in hepatocytes, making it a more specific indicator of liver disease.
Acute viral hepatitis, toxins, and liver ischemia can markedly raise serum aminotransferase levels (often into the thousands of units per liter). On the other hand, these enzymes are only mildly elevated (< 300 U/L) in nonalcoholic steatohepatitis, chronic hepatitis, cholestatic liver conditions, drug-induced hepatotoxicity, and liver tumors.
Because AST is a mitochondrial enzyme and is affected by alcohol ingestion, an AST level more than twice that of the ALT suggests hepatic damage due to alcohol.
Of note, aminotransferase elevation can also be due to nonhepatic causes. For example, muscle necrosis can result in mild elevation of these enzymes, especially AST, and an elevated creatine kinase can help confirm that the source is muscle tissue.
Undiagnosed celiac disease has been associated with abnormal liver enzyme levels when all other causes have been ruled out, but the mechanism is not yet understood. In this situation, ALT and AST levels typically return to normal with a gluten-free diet.15
Studies of mild aminotransferase elevations
Only a few studies have documented the results of a thorough evaluation of patients with mildly elevated aminotransferase levels:
Hultcrantz et al1 performed a full evaluation, including liver biopsy, in 149 consecutive patients with chronic, asymptomatic, mild elevations of AST or ALT. Of these patients, 63% had “fatty liver,” 20% had “chronic hepatitis,” and 17% had miscellaneous diagnoses. Whether patients in the “chronic hepatitis” group had hepatitis C was not determined because serologic testing was not available at the time.
Friedman et al16 studied 100 healthy blood donors with elevated ALT levels and found that in 33% of patients the elevation occurred once, in 36% it was intermittent, and in 28% it was persistent. In this series, 45% of patients had no diagnosis, 22% were obese (presumed to have nonalcoholic steatohepatitis), 5% had alcoholic liver disease, 3% had “resolving hepatitis,” 1% had hemochromatosis, and 1% had “cytomegalovirus hepatitis.”16 Although the patients underwent a complete history, physical examination, and serologic testing, liver biopsy was not done to confirm the clinical diagnosis.
Hay et al17 described 47 patients with chronically elevated aminotransferases (three to eight times higher than normal levels) who underwent full evaluation and liver biopsy and who had no clinical symptoms of alcoholic, viral, or drug-induced liver disease. A diagnosis of steatohepatitis was given in 10 patients, another 34 were diagnosed with “chronic hepatitis,” and 3 had miscellaneous diagnoses. Of patients with chronic hepatitis, 16 had evidence of cirrhosis on biopsy, and 18 tested positive for at least one autoimmune marker (antinuclear antibody or smooth muscle antibody).
Daniel et al18 performed biopsy in 81 of 1,124 asymptomatic and symptomatic patients with chronically elevated aminotransferases in whom a cause was not identified via noninvasive studies. Liver biopsy showed that 67 (83%) of the 81 patients had steatosis or steatohepatitis, while 8 (10%) had normal histologic findings. Of note, 6 patients had underlying fibrosis or cirrhosis and some degree of fatty infiltration.
Together, these studies suggest that fatty liver, resulting either from alcohol use or from nonalcoholic fatty liver disease, is the major cause of mildly elevated aminotransferases. Two major drawbacks of the earlier studies include the lack of data on the hepatitis C serologic status of patients with the diagnosis of “chronic hepatitis” and the lack of a uniform approach to the pathologic diagnosis of nonalcoholic steatohepatitis. With serologic testing for hepatitis C virus infection now widely available, it is possible that a substantial portion of patients with “chronic hepatitis“ can further be classified as having chronic hepatitis C infection.
Workup of aminotransferase elevations
- Viral hepatitis (intravenous drug use, intranasal cocaine use, native of an endemic area of the world, unsafe sexual activity, blood product transfusions)
- Nonalcoholic fatty liver disease (components of the metabolic syndrome, including visceral obesity)
- Alcoholic liver disease (smaller amounts are needed to cause liver disease in women)
- Medication exposure (prescription, overthe-counter, and herbal medications)
- Genetic liver disorders (family history of liver disease)
- Possible coexisting disease (diabetes and obesity in nonalcoholic steatohepatitis, neurologic disorders in Wilson disease, emphysema in alpha-1-antitrypsin deficiency, thyroid disease in autoimmune hepatitis and primary biliary cirrhosis, and diabetes and impotence in genetic hemochromatosis).
Although the physical signs of chronic liver disease (eg, spider angiomata, palmar erythema, caput medusae, and gynecomastia) are nonspecific and are usually observed in advanced liver disease, some physical findings suggest potential causes (eg, Kayser-Fleischer rings on slit-lamp examination for Wilson disease, hypertrophy of the second and third metacarpophalangeal joints for hemochromatosis). Iron studies for middleaged men, autoimmune markers for women, and screening for Wilson disease in young patients are helpful when the clinical information points to one of these entities as a potential diagnosis.
If medication or alcohol is a suspected cause, aminotransferase levels should be repeated after 6 to 8 weeks of abstinence. If nonalcoholic steatohepatitis is suspected, testing should be repeated after treating the potential risk factor (eg, obesity, diabetes, hyperlipidemia), but the levels may remain elevated for a period of time. An imaging study (ultrasonography, computed tomography, or magnetic resonance imaging) may be helpful; eg, abdominal ultrasonography may show increased hepatic echogenicity, suggesting increased fatty infiltration, in addition to excluding most hepatic tumors.
If the history and physical do not suggest a specific condition, serologic testing for hepatitis C should be done. If negative, other testing can be helpful (iron studies in men, autoimmune markers in women, ceruloplasmin and slit-lamp examination in young patients). If the preliminary workup remains negative and the aminotransferase levels remain elevated for 6 months (ie, chronic elevation), liver biopsy may establish the diagnosis. Features in the biopsy specimens may further confirm the diagnosis: eg, globules positive on periodic acid-Schiff testing in alpha-1-antitrypsin deficiency; hepatic iron index for hemochromatosis; hepatic copper content for Wilson disease.
ALKALINE PHOSPHATASE ELEVATION
Causes
Alkaline phosphatase is active in many organs, mainly the liver and bones, but is also found in the small bowel, kidneys, and placenta. Diseases of the hepatobiliary system can cause moderate to marked elevations of alkaline phosphatase. Conditions with bone involvement, such as Paget disease of the bone, sarcoma, metastatic disease, hyperparathyroidism, and rickets, can raise alkaline phosphatase levels. Elevated GGT in conjunction with elevated alkaline phosphatase usually points to hepatobiliary injury. Clinically, isoenzyme fractionation of alkaline phosphatase may help distinguish the source of the elevation, but this is often not needed if the GGT is also elevated.
Hepatobiliary causes of alkaline phosphatase elevation can be divided into four categories:
- Chronic inflammation involving the bile ducts (eg, as in primary biliary cirrhosis and primary sclerosing cholangitis)
- Infiltrative process (eg, neoplasm, tuberculosis, sarcoidosis)
- Cholestatic disorders (eg, drug hepatotoxicity)
- Biliary obstruction (eg, in neoplasia or cholelithiasis).
Only a few studies have investigated the significance of a mild, isolated elevation of alkaline phosphatase. Lieberman and Phillips20 evaluated 87 patients and found that the abnormality resolved completely in less than 3 months in 28 patients, and resolved in 3 to 12 months in another 17 patients. Of the other 42 patients, 24 did not undergo further evaluation because they had significant coexisting disease. Of the remaining 18 patients, 5 had phenytoin-related hepatotoxicity, 3 had congestive heart failure, 3 had metabolic bone disease, 2 had hepatobiliary disease, and 1 had metastatic bone disease; in 4 patients, no explanation was determined. Follow-up was 1.5 to 3 years.
Workup of alkaline phosphatase elevation
An isolated elevated alkaline phosphatase level should always be confirmed and a hepatic origin suspected if the GGT level is also elevated (Figure 2).
A history of recent drug exposure usually points to drug hepatotoxicity as the source of this abnormality. Similarly, other information from the history can point to a potential underlying pathologic process causing the rise in alkaline phosphatase. For example, a history of ulcerative colitis suggests primary sclerosing cholangitis, and a history of previous cancer or sarcoidosis suggests liver involvement.
As part of the initial evaluation, an imaging study (eg, ultrasonography) can exclude biliary obstruction or suggest an infiltrative process.
If a drug is the suspected cause, the alkaline phosphatase level should be repeated after the patient has abstained from the drug for 6 to 8 weeks. If the initial examination suggests a specific disease, disease-specific markers (eg, antimitochondrial antibody for primary biliary cirrhosis, or viral serology) can confirm the suspected diagnosis. If the disease-specific markers are negative and the alkaline phosphatase level does not return to normal, further studies should be considered, including liver biopsy and endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography. Given the invasive nature of biopsy and pancreatography, an expert consultation should be done before ordering these tests.
GGT ELEVATION
GGT is a membrane enzyme that is a marker of hepatobiliary disease. Elevations usually parallel the elevation of alkaline phosphatase, confirming a hepatic source for the latter. GGT is the most sensitive marker of biliary tract disease but is not very specific. Alcohol and drugs (eg, phenytoin, phenobarbital) induce GGT. In one study,21 GGT was elevated in 52% of patients without known liver disease. The GGT level can be used to monitor abstinence from alcohol in patients with alcoholic liver disease.21
Workup of GGT elevation
Because GGT lacks specificity as a marker and is highly inducible, an extensive evaluation of an isolated GGT elevation in an otherwise asymptomatic patient is not warranted.
WHEN TO CONSULT
Many of the evaluations discussed in this paper and elsewhere22–27 can be carried out by primary care providers following a systematic approach. Input from a gastroenterologist or hepatologist can be valuable if the initial workup fails to establish the diagnosis, as well as in assuring that the most effective therapy for a specific disease is initiated. Reassurance, patient education, and a systematic approach for evaluating these abnormalities can identify most treatable causes of liver disease in the most cost-effective and efficient manner.
- Hultcrantz R, Glaumann H, Lindberg G, Nilsson LH. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol 1986; 21:109–113.
- Ioanou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol 2006; 101:76–82.
- Flora KD, Keeffe EB. Evaluation of mildly abnormal liver tests in asymptomatic patients. J Insur Med 1990; 22:264–267.
- Alter HJ. To C or not to C: these are the questions. Blood 1995; 85:1681–1695.
- Everhart JE, editor. Digestive diseases in the United States: epidemiology and impact. Washington, DC: US Government Printing Office, 1994; NIH Publication No. 94–1447.
- Bacon BR, Tavill AS. Hemochromatosis and the iron overload syndromes. In:Zakim D, Boyer TD, editors. Hepatology. A Textbook of Liver Disease, 3rd ed. Philadelphia: WB Saunders, 1996:1439–1472.
- Lieber CS. Alcoholic liver disease. Curr Opin Gastroenterol 1994; 10:319–330.
- Seth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med 1997; 126:137–145.
- Czaja A. Autoimmune liver disease. In: Zakim D, Boyer TD, editors. Hepatolology: A Textbook of Liver Disease, 3rd ed. Philadelphia: WB Saunders, 1996:1259–1292.
- Bull PC, Cox DW. Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet 1994; 10:246–252.
- Perlmutter DH. Clinical manifestations of alpha-1-antitrypsin deficiency. Gastroenterol Clin North Am 1995; 24:27–43.
- Olivarez L, Caggana M, Pass KA, Ferguson P, Brewer GJ. Estimate of the frequency of Wilson’s disease in the US Caucasian population: a mutation analysis approach. Ann Hum Genet 2001; 65:459–463.
- Metcalf JV, Howel D, James OF, Bhopal R. Primary biliary cirrhosis: epidemiology helping the clinician. BMJ 1996; 312:1181–1182.
- Farrell GC. Liver disease caused by drugs, anesthetics, and toxins. In:Feldman M, Friedman LS, Sleisenger MH, editors. Gastrointestinal and Liver Disease, 7th ed. Philadelphia: WB Saunders, 2002;1403–1447.
- Abdo A, Meddings J, Swain M. Liver abnormalities in celiac disease. Clin Gastroenterol Hepatol 2004; 2:107–112.
- Friedman LS, Dienstag JL, Watkins E, et al. Evaluation of blood donors with elevated serum alanine aminotransferase. Ann Intern Med 1987; 107:137–144.
- Hay JE, Czaja AJ, Rakela J, Ludwig J. The nature of unexplained chronic aminotransferase elevations of a mild to moderate degree in asymptomatic patients. Hepatology 1989; 9:193–197.
- Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol 1999; 94:3010–3014.
- Van Ness MM, Diehl AM. Is liver biopsy useful in the evaluation of patients with chronically elevated liver enzymes? Ann Intern Med 1989; 111:473–478.
- Lieberman D, Phillips D. “Isolated” elevation of alkaline phosphatase: significance in hospitalized patients. J Clin Gastroenterol 1990; 12:415–419.
- Margarian GJ, Lucas LM, Kumar KL. Clinical significance in alcoholic patients of commonly encountered laboratory test results. West J Med 1992; 156:287–294.
- Fregia A, Jensen DM. Evaluation of abnromal liver tests. Compr Ther 1994; 20:50–54.
- Goddard CJ, Warens TW. Raised liver enzymes in asymptomatic patients: investigation and outcome. Dig Dis 1992; 10:218–226.
- Gitlin N. The differential of elevated liver enzymes. Contemporary Internal Medicine 1993:44–56.
- Keefe EB. Diagnostic approach to mild elevation of liver enzyme levels. Gastrointestinal Diseases Today 1994; 3:1–9.
- Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000; 342:1266–1271.
- American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology 2002; 123:1364–1366.
- Hultcrantz R, Glaumann H, Lindberg G, Nilsson LH. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol 1986; 21:109–113.
- Ioanou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol 2006; 101:76–82.
- Flora KD, Keeffe EB. Evaluation of mildly abnormal liver tests in asymptomatic patients. J Insur Med 1990; 22:264–267.
- Alter HJ. To C or not to C: these are the questions. Blood 1995; 85:1681–1695.
- Everhart JE, editor. Digestive diseases in the United States: epidemiology and impact. Washington, DC: US Government Printing Office, 1994; NIH Publication No. 94–1447.
- Bacon BR, Tavill AS. Hemochromatosis and the iron overload syndromes. In:Zakim D, Boyer TD, editors. Hepatology. A Textbook of Liver Disease, 3rd ed. Philadelphia: WB Saunders, 1996:1439–1472.
- Lieber CS. Alcoholic liver disease. Curr Opin Gastroenterol 1994; 10:319–330.
- Seth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med 1997; 126:137–145.
- Czaja A. Autoimmune liver disease. In: Zakim D, Boyer TD, editors. Hepatolology: A Textbook of Liver Disease, 3rd ed. Philadelphia: WB Saunders, 1996:1259–1292.
- Bull PC, Cox DW. Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet 1994; 10:246–252.
- Perlmutter DH. Clinical manifestations of alpha-1-antitrypsin deficiency. Gastroenterol Clin North Am 1995; 24:27–43.
- Olivarez L, Caggana M, Pass KA, Ferguson P, Brewer GJ. Estimate of the frequency of Wilson’s disease in the US Caucasian population: a mutation analysis approach. Ann Hum Genet 2001; 65:459–463.
- Metcalf JV, Howel D, James OF, Bhopal R. Primary biliary cirrhosis: epidemiology helping the clinician. BMJ 1996; 312:1181–1182.
- Farrell GC. Liver disease caused by drugs, anesthetics, and toxins. In:Feldman M, Friedman LS, Sleisenger MH, editors. Gastrointestinal and Liver Disease, 7th ed. Philadelphia: WB Saunders, 2002;1403–1447.
- Abdo A, Meddings J, Swain M. Liver abnormalities in celiac disease. Clin Gastroenterol Hepatol 2004; 2:107–112.
- Friedman LS, Dienstag JL, Watkins E, et al. Evaluation of blood donors with elevated serum alanine aminotransferase. Ann Intern Med 1987; 107:137–144.
- Hay JE, Czaja AJ, Rakela J, Ludwig J. The nature of unexplained chronic aminotransferase elevations of a mild to moderate degree in asymptomatic patients. Hepatology 1989; 9:193–197.
- Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol 1999; 94:3010–3014.
- Van Ness MM, Diehl AM. Is liver biopsy useful in the evaluation of patients with chronically elevated liver enzymes? Ann Intern Med 1989; 111:473–478.
- Lieberman D, Phillips D. “Isolated” elevation of alkaline phosphatase: significance in hospitalized patients. J Clin Gastroenterol 1990; 12:415–419.
- Margarian GJ, Lucas LM, Kumar KL. Clinical significance in alcoholic patients of commonly encountered laboratory test results. West J Med 1992; 156:287–294.
- Fregia A, Jensen DM. Evaluation of abnromal liver tests. Compr Ther 1994; 20:50–54.
- Goddard CJ, Warens TW. Raised liver enzymes in asymptomatic patients: investigation and outcome. Dig Dis 1992; 10:218–226.
- Gitlin N. The differential of elevated liver enzymes. Contemporary Internal Medicine 1993:44–56.
- Keefe EB. Diagnostic approach to mild elevation of liver enzyme levels. Gastrointestinal Diseases Today 1994; 3:1–9.
- Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000; 342:1266–1271.
- American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology 2002; 123:1364–1366.
KEY POINTS
- Nonalcoholic fatty liver disease is the most common cause of asymptomatic elevated aminotransferase levels.
- Suspect alcoholic liver disease when the aminotransferases are elevated and the aspartate aminotransferase level is two to three times higher than the alanine aminotransferase level, especially when gamma-glutamyl transferase levels are elevated.
- If medications or alcohol is a suspected cause of elevated aminotransferase levels, remeasure the levels after 6 to 8 weeks of abstinence.
Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
KEY POINTS
- The clinical spectrum of NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma.
- NAFLD is closely associated with metabolic syndrome, insulin resistance, and obesity.
- Weight loss and treating components of the metabolic syndrome are central to the treatment of NAFLD. Insulin sensitizers such as biguanides and glitazones, antioxidants such as vitamin E, and lipid-lowering agents have shown promise in small clinical trials, but the evidence remains preliminary.