User login
Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
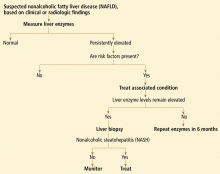
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
KEY POINTS
- The clinical spectrum of NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma.
- NAFLD is closely associated with metabolic syndrome, insulin resistance, and obesity.
- Weight loss and treating components of the metabolic syndrome are central to the treatment of NAFLD. Insulin sensitizers such as biguanides and glitazones, antioxidants such as vitamin E, and lipid-lowering agents have shown promise in small clinical trials, but the evidence remains preliminary.