User login
Laparoscopic myomectomy: Tips for patient selection and technique
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
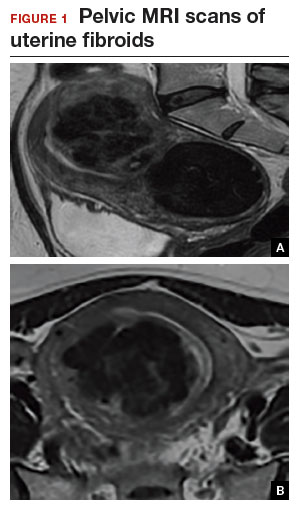
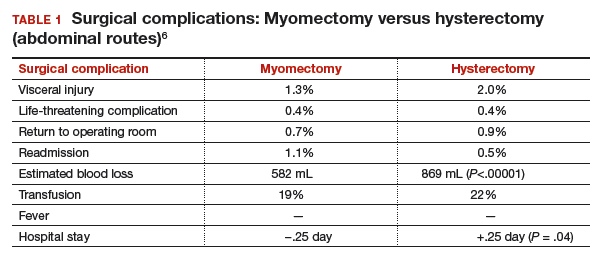
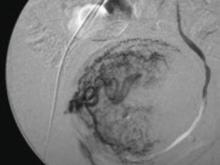
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.
Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.
Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
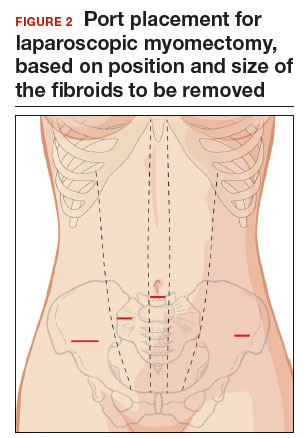
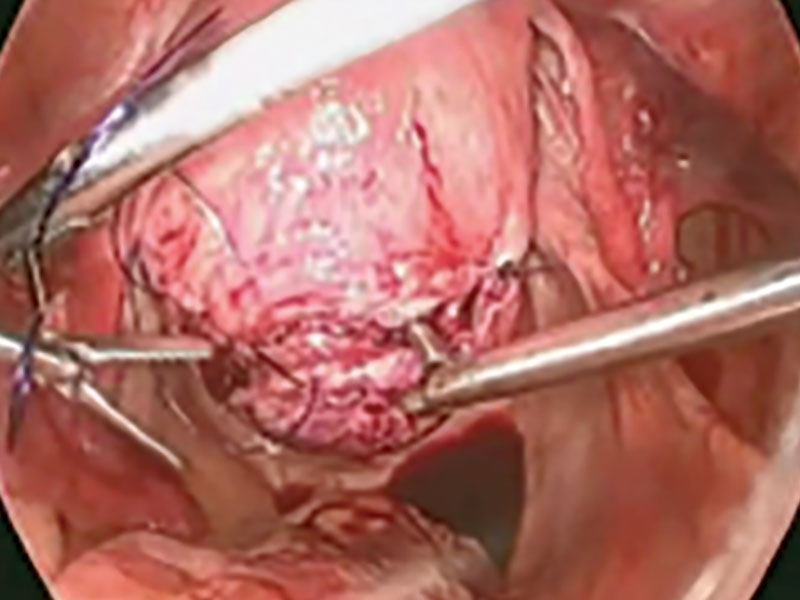
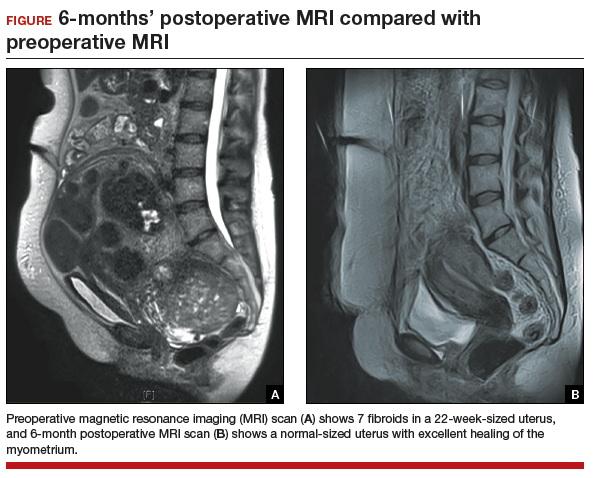
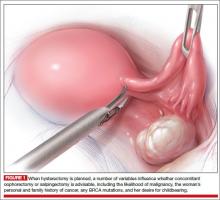
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
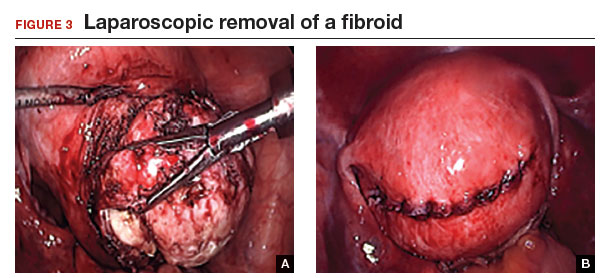
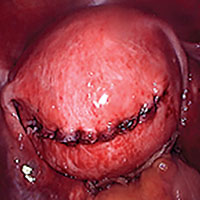
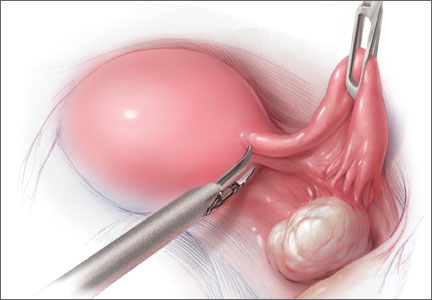
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.
The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.
Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.
Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.
The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.
Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.
Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.
The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
Laparoscopic myomectomy technique
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Abdominal myomectomy: Patient and surgical technique considerations
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
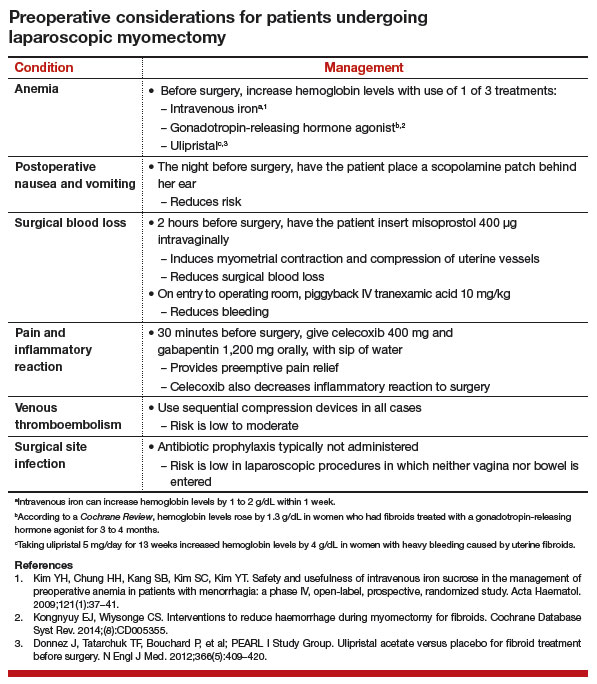
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
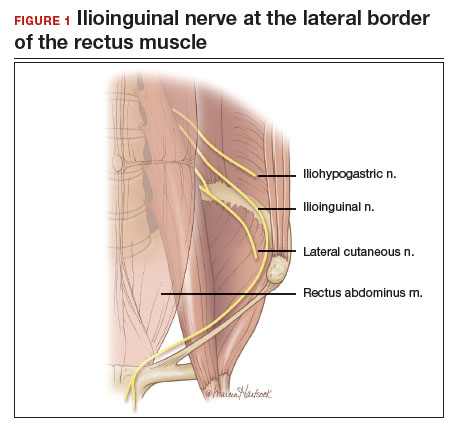
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
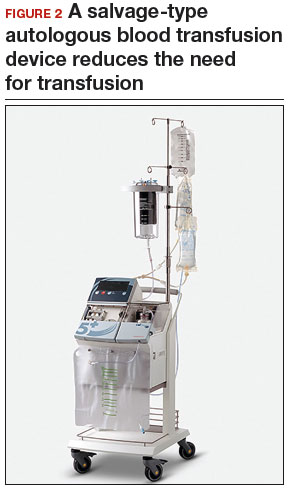
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
Additional surgical considerations
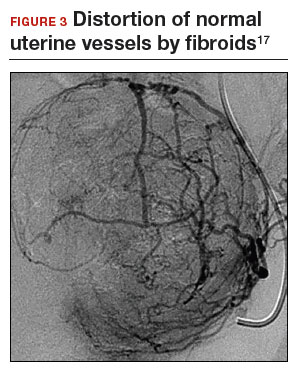
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
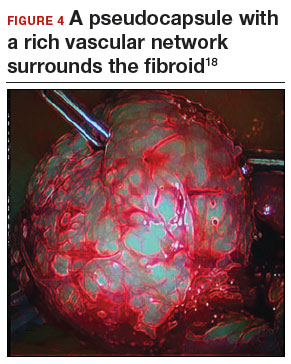
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
7 Myomectomy myths debunked
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15
Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15
Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15
Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Oophorectomy or salpingectomy—which makes more sense?
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
Can a novel risk-scoring system for ovarian cancer predict who is most likely to develop disease?
The risks and benefits of bilateral oophorectomy at the time of hysterectomy for benign disease are the subject of ongoing discussion. (See, for example, an earlier article on the subject, “Remove the ovaries at hysterectomy? Here’s the lowdown on risks and benefits,” in the February 2010 issue of OBG MANAGEMENT.) There is uniform agreement that women who are at high risk of ovarian and breast cancer because of a significant family history or known BRCA mutation should strongly consider bilateral oophorectomy after completing childbearing. For women at average risk of ovarian or breast cancer, individualization of elective oophorectomy is recommended—but how can you do this for the patient sitting in your office?
Vitonis and colleagues analyzed multiple risk factors associated with ovarian cancer and developed a scoring system to help provide guidance for average-risk women and their physicians who need to make this important decision. This is the kind of mental modeling clinicians do daily in an abstract way, but this scoring system helps frame the associated risks and gives a mathematical value to inform the decision.
Risk factors in the scoring system are:
- Jewish ethnicity
- less than 1 year of oral contraceptive use
- nulliparity
- no breastfeeding
- no tubal ligation
- painful periods or endometriosis
- polycystic ovary syndrome or obesity
- talc use.
Subjects who had none or one of these risk factors were calculated to have a 1.2% lifetime risk of ovarian cancer (98.8% will not get ovarian cancer); the risk was 6.6% with a score of 5 or higher (93.4% will not get ovarian cancer).
Risk equation wasn’t fully explored
Noted by the authors, but not studied here, is the other side of this equation: namely, a woman’s risk factors for medical conditions that might be exacerbated by oophorectomy—including bone fracture, neurologic conditions, and, most important, cardiovascular disease. These conditions appear to be more common after oophorectomy and are considerably more prevalent causes of morbidity and mortality among women than is ovarian cancer.
Case-control design is a weakness
Vitonis and colleagues chose exclusion criteria wisely, but the case-control design of the study is a weakness because of inherent recall and selection biases. The authors should be commended for stating calculated risks as absolute risk rather than relative risk, which is usually misunderstood by the media and patients alike.
As the authors point out, their prototype needs to be validated in other populations and data sets, but it begins to frame the decision regarding oophorectomy for women undergoing hysterectomy for benign disease. However, we won’t have the complete picture until the other side of the equation is similarly analyzed—and that side concerns an individual woman’s risks for cardiovascular disease, neurologic conditions, and bone fracture.
Women who have a significant family history of breast or ovarian cancer or a documented BRCA mutation should be offered salpingo-oophorectomy once they have completed childbearing. Women who have an average risk of ovarian cancer should be counseled about risks and benefits as they apply in their particular case. The study by Vitonis and colleagues may be helpful in this regard. The decision to preserve or remove the ovaries and fallopian tubes should be made according to these risk factors and individual preference.—William H. Parker, MD
We want to hear from you! Tell us what you think.
The risks and benefits of bilateral oophorectomy at the time of hysterectomy for benign disease are the subject of ongoing discussion. (See, for example, an earlier article on the subject, “Remove the ovaries at hysterectomy? Here’s the lowdown on risks and benefits,” in the February 2010 issue of OBG MANAGEMENT.) There is uniform agreement that women who are at high risk of ovarian and breast cancer because of a significant family history or known BRCA mutation should strongly consider bilateral oophorectomy after completing childbearing. For women at average risk of ovarian or breast cancer, individualization of elective oophorectomy is recommended—but how can you do this for the patient sitting in your office?
Vitonis and colleagues analyzed multiple risk factors associated with ovarian cancer and developed a scoring system to help provide guidance for average-risk women and their physicians who need to make this important decision. This is the kind of mental modeling clinicians do daily in an abstract way, but this scoring system helps frame the associated risks and gives a mathematical value to inform the decision.
Risk factors in the scoring system are:
- Jewish ethnicity
- less than 1 year of oral contraceptive use
- nulliparity
- no breastfeeding
- no tubal ligation
- painful periods or endometriosis
- polycystic ovary syndrome or obesity
- talc use.
Subjects who had none or one of these risk factors were calculated to have a 1.2% lifetime risk of ovarian cancer (98.8% will not get ovarian cancer); the risk was 6.6% with a score of 5 or higher (93.4% will not get ovarian cancer).
Risk equation wasn’t fully explored
Noted by the authors, but not studied here, is the other side of this equation: namely, a woman’s risk factors for medical conditions that might be exacerbated by oophorectomy—including bone fracture, neurologic conditions, and, most important, cardiovascular disease. These conditions appear to be more common after oophorectomy and are considerably more prevalent causes of morbidity and mortality among women than is ovarian cancer.
Case-control design is a weakness
Vitonis and colleagues chose exclusion criteria wisely, but the case-control design of the study is a weakness because of inherent recall and selection biases. The authors should be commended for stating calculated risks as absolute risk rather than relative risk, which is usually misunderstood by the media and patients alike.
As the authors point out, their prototype needs to be validated in other populations and data sets, but it begins to frame the decision regarding oophorectomy for women undergoing hysterectomy for benign disease. However, we won’t have the complete picture until the other side of the equation is similarly analyzed—and that side concerns an individual woman’s risks for cardiovascular disease, neurologic conditions, and bone fracture.
Women who have a significant family history of breast or ovarian cancer or a documented BRCA mutation should be offered salpingo-oophorectomy once they have completed childbearing. Women who have an average risk of ovarian cancer should be counseled about risks and benefits as they apply in their particular case. The study by Vitonis and colleagues may be helpful in this regard. The decision to preserve or remove the ovaries and fallopian tubes should be made according to these risk factors and individual preference.—William H. Parker, MD
We want to hear from you! Tell us what you think.
The risks and benefits of bilateral oophorectomy at the time of hysterectomy for benign disease are the subject of ongoing discussion. (See, for example, an earlier article on the subject, “Remove the ovaries at hysterectomy? Here’s the lowdown on risks and benefits,” in the February 2010 issue of OBG MANAGEMENT.) There is uniform agreement that women who are at high risk of ovarian and breast cancer because of a significant family history or known BRCA mutation should strongly consider bilateral oophorectomy after completing childbearing. For women at average risk of ovarian or breast cancer, individualization of elective oophorectomy is recommended—but how can you do this for the patient sitting in your office?
Vitonis and colleagues analyzed multiple risk factors associated with ovarian cancer and developed a scoring system to help provide guidance for average-risk women and their physicians who need to make this important decision. This is the kind of mental modeling clinicians do daily in an abstract way, but this scoring system helps frame the associated risks and gives a mathematical value to inform the decision.
Risk factors in the scoring system are:
- Jewish ethnicity
- less than 1 year of oral contraceptive use
- nulliparity
- no breastfeeding
- no tubal ligation
- painful periods or endometriosis
- polycystic ovary syndrome or obesity
- talc use.
Subjects who had none or one of these risk factors were calculated to have a 1.2% lifetime risk of ovarian cancer (98.8% will not get ovarian cancer); the risk was 6.6% with a score of 5 or higher (93.4% will not get ovarian cancer).
Risk equation wasn’t fully explored
Noted by the authors, but not studied here, is the other side of this equation: namely, a woman’s risk factors for medical conditions that might be exacerbated by oophorectomy—including bone fracture, neurologic conditions, and, most important, cardiovascular disease. These conditions appear to be more common after oophorectomy and are considerably more prevalent causes of morbidity and mortality among women than is ovarian cancer.
Case-control design is a weakness
Vitonis and colleagues chose exclusion criteria wisely, but the case-control design of the study is a weakness because of inherent recall and selection biases. The authors should be commended for stating calculated risks as absolute risk rather than relative risk, which is usually misunderstood by the media and patients alike.
As the authors point out, their prototype needs to be validated in other populations and data sets, but it begins to frame the decision regarding oophorectomy for women undergoing hysterectomy for benign disease. However, we won’t have the complete picture until the other side of the equation is similarly analyzed—and that side concerns an individual woman’s risks for cardiovascular disease, neurologic conditions, and bone fracture.
Women who have a significant family history of breast or ovarian cancer or a documented BRCA mutation should be offered salpingo-oophorectomy once they have completed childbearing. Women who have an average risk of ovarian cancer should be counseled about risks and benefits as they apply in their particular case. The study by Vitonis and colleagues may be helpful in this regard. The decision to preserve or remove the ovaries and fallopian tubes should be made according to these risk factors and individual preference.—William H. Parker, MD
We want to hear from you! Tell us what you think.
Remove the ovaries at hysterectomy? Here’s the lowdown on risks and benefits
CASE: Hysterectomy candidate asks about her ovaries
A 51-year-old premenopausal woman complains of severe menorrhagia that often causes her to miss work. Although she is taking an iron supplement, her hemoglobin level often drops below 10 g/dL. She has already been identified as having fibroids, with a uterine size of 14 weeks. You order ultra-sonography, which reveals an enlarged uterus with multiple fibroids and normal endometrial thickness, but no intracavitary lesions.
After you describe the treatment options, including uterine artery embolization, the patient requests a hysterectomy as a reasonably low-risk means of cure. During informed consent, she asks whether she should have her ovaries removed during the surgery. Further discussion reveals that her father died of a myocardial infarction when he was 64 years old, but there is no family or personal history of ovarian or breast cancer.
How do you advise this patient, based on her history and recent findings from medical research?
Many gynecologists have been trained to recommend bilateral oophorectomy for women older than 45 or 50 years who request a hysterectomy for benign disease. In these women, oophorectomy is recommended to prevent ovarian cancer and avert the potential for other ovarian pathology that might require later surgery.
In the United States, 78% of women 45 to 64 years old and 55% of women overall undergo bilateral oophorectomy at the time of hysterectomy.1 These percentages mean that almost 300,000 women undergo bilateral oophorectomy each year.1
Hysterectomy alone can sometimes lead to early ovarian failure, but this phenomenon is infrequent. A prospective study of premenopausal women found that, after 5 years of follow-up, 20% of women who underwent simple hysterectomy reached menopause, compared with 7% of matched women who did not undergo hysterectomy.2
In this article, I explore the risks and benefits associated with bilateral oophorectomy and present an algorithm to aid in deciding whether the patient should keep her ovaries—and when oophorectomy might be a better option ( FIGURE ).
Among the hazards associated with bilateral oophorectomy are:
- an increased risk of death from coronary artery disease (CAD), lung cancer, all cancers (except ovarian), and all causes3,4
- an increased risk of osteoporosis and hip fracture5
- when performed before the onset of menopause, an increased risk of parkinsonism, cognitive impairment, dementia, anxiety, and depression.6-8
Benefits include a reduced risk of ovarian cancer, particularly among women who have a BRCA gene mutation or strong family history of ovarian or breast cancer.
Although ovarian cancer causes 15,000 deaths each year in the United States, that figure pales when compared with heart disease, which accounts for 350,000 deaths. In addition, hip fracture may cause approximately 66,000 deaths each year, and dementia attributable to bilateral oophorectomy may affect 100,000 to 200,000 women.9 Reoperation for adnexal pathology or pain after hysterectomy is rare, occurring in only 2.8% of women. Therefore, the benefits of oophorectomy are often outweighed by the risks of CAD, hip fracture, and neurologic conditions.
The assumption that medical treatment will ameliorate the risks associated with oophorectomy is unrealistic. Estrogen may mitigate some risks, but many women avoid hormone therapy. This avoidance can be especially problematic in young women.
In the Nurses’ Health Study, a separate analysis focused on women who had never used postmenopausal hormone therapy.4 In this analysis, all women who underwent bilateral oophorectomy had a greater risk of stroke (HR, 1.85; 95% CI, 1.09, 3.16) and lung cancer (HR, 2.09; 95% CI, 1.01, 4.33) than did women who retained their ovaries. Among women who underwent oophorectomy before age 50 and who did not take estrogen, the risk of coronary artery disease (CAD) was higher (HR, 1.98; 95% CI, 1.18, 3.32), as was the risk of death from all causes (HR, 1.40; 95% CI, 1.01, 1.96), compared with women who retained their ovaries.
Despite estrogen’s proven benefit among oophorectomized women, usage rates continue to decline. In the 6 months after publication of Women’s Health Initiative findings on estrogen–progestin therapy, the continuation rate of estrogen therapy decreased from 12.6% to 9.1%, and new starts also declined significantly.47
Among women who had a diagnosis of osteoporosis and who began treatment with estrogen, estrogen plus progestin, a bisphosphonate, or raloxifene, medication continuation rates were less than 25% at 12 months.48 Moreover, only 18% of women started on a statin to reduce the risk of cardiovascular disease were still taking the drug after 1 year.49
FIGURE Conservation vs oophorectomy: A guide to decision-making
* Estrogen replacement is recommended for women younger than 45 years who opt for oophorectomy
Ovarian cancer is a real, but relatively low, risk
In 2008, an estimated 21,650 new cases of ovarian cancer were diagnosed (age at diagnosis: mean, 63 years), and 15,520 women died from the disease.10 Because we lack a reliable screening test to detect early-stage ovarian cancer in the general population, most women are given a diagnosis when disease is advanced and the 5-year survival rate is 15% to 25%.
There is agreement that women who are known to have a BRCA mutation, which increases the risk of ovarian and breast cancer, should strongly consider oophorectomy once childbearing is complete.11 In the general population, however, the outlook is different.
In the United States, the lifetime risk of ovarian cancer is 1.4% overall. Among white women who have had three or more term pregnancies and who have used an oral contraceptive for at least 4 years, the lifetime risk of ovarian cancer drops to 0.3%.12
Need for reoperation is very low
The percentage of women who require reoperation after ovarian conservation—2.8%—may surprise you.13 That figure is lower than once thought because many studies were performed before asymptomatic, benign ovarian cysts were determined to be a fairly common phenomenon in postmenopausal women (prevalence, 6.6%). These cysts do not undergo transformation to cancer and, therefore, do not need to be removed.14
In addition, studies indicate that only 0.1% to 0.75% of women who retain their ovaries at the time of hysterectomy develop ovarian cancer.15,16 Therefore, the rationale of performing oophorectomy to avoid future surgery appears to be unfounded.
CAD risk rises sharply after oophorectomy
A recent systematic review found mixed evidence concerning the risk of CAD following bilateral salpingo-oophorectomy.17 In observational studies, however, earlier age of surgical or natural menopause has been associated with a higher risk of cardiovascular mortality.18-20 Early reports from the Nurses’ Health Study found that the risk of myocardial infarction doubled among women who underwent oophorectomy and never used estrogen, compared with age-matched premenopausal women (relative risk [RR], 2.2; 95% confidence interval [CI], 1.2, 4.2).3 Even after age 50, the risk of a first myocardial infarction is increased among oophorectomized women, compared with women who retain their ovaries (RR, 1.4; 95% CI, 1.0–2.0).21
A study by researchers from the Mayo Clinic, who examined all causes listed on the death certificate, found a significant association between bilateral oophorectomy before the age of 45 years and cardiovascular mortality (hazard ratio [HR], 1.44; 95% CI, 1.01–2.05).22 This risk was significantly increased among women who were not treated with estrogen through at least age 45, compared with estrogen-treated women.
Oophorectomy may impair bone health
After menopause, ovaries continue to produce significant amounts of the androgens testosterone and androstenedione, which are converted to estrone peripherally by skin, muscle, and fat cells.23,24 The levels of these hormones remain consistent and have been documented to age 80.25
Both estrogens and androgens inhibit bone resorption, and androgens also stimulate bone formation.26 Low levels of androgens and estrogens are linked to lower bone density and a higher risk of hip and vertebral fracture in postmenopausal women.27-29
Postmenopausal women who have been oophorectomized may have an even greater risk of osteoporosis. Over 16 years of follow-up, 340 women who had undergone oophorectomy at a median age of 62 years had 54% more osteoporotic fractures than women who had intact ovaries.5 Two other studies found no association between oophorectomy and bone loss or fracture risk, however.30,31
Hip fracture is a well-documented cause of increased morbidity and mortality in older women. One study found that, before hip fracture, 28% of patients were housebound; 1 year after hip fracture, the percentage was 46%.32 Women older than 60 who underwent oophorectomy had a doubled risk of mortality after low-trauma hip fracture, compared with women who had intact ovaries (odds ratio [OR], 2.18; 95% CI, 2.03–2.32).5
Loss of ovaries may affect mental health and sexuality
In a premenopausal woman, oophorectomy causes a sudden loss of estrogen and often triggers hot flashes, mood changes, sleep disturbances, headaches, and a decline in feelings of well-being.33,34 Over time, vaginal dryness, painful intercourse, loss of libido, bladder dysfunction, and depression may occur.35,36
Evidence suggests that, in women, sexual desire, sexual sensation, and orgasmic response are influenced by androgens. After elective oophorectomy, declines in sexual desire have been reported.37-39
Mental health and sexuality may rebound over time, however. One study found less improvement in mental health measures and body image 6 months after hysterectomy among women who were oophorectomized, compared with those who retained their ovaries. After 2 years, improvement levels were similar between groups.40
Cognitive function may suffer
Analysis of data from the Mayo Clinic Cohort Study of Oophorectomy and Aging found that bilateral oophorectomy before the onset of menopause increased the risk of parkinsonism, cognitive impairment or dementia, and anxiety or depression, particularly if estrogen was not replaced.6-8 These risks increased with younger age at oophorectomy.
The Women’s Health Initiative found an increased risk of dementia or mild cognitive impairment in women who were treated by estrogen alone or estrogen plus progestin after age 65.41-44
These disparate conclusions suggest that estrogen may have a protective effect on the brain if it is given right after natural menopause or premenopausal oophorectomy, but deleterious effects if it is started years later.45
Other studies of endogenous estrogen and cognitive function are few and yield inconsistent results.
Ovarian conservation boosts long-term survival
When there is no family history of ovarian cancer, ovarian conservation appears to maximize survival among healthy women 40 to 65 years old who undergo hysterectomy for benign disease.46 Among healthy women hysterectomized before the age of 55, calculations suggest that 8.6% more would be alive at age 80 if their ovaries were conserved than if they were removed.46
A study from the Mayo Clinic found that all-cause mortality was significantly higher among women who underwent prophylactic bilateral oophorectomy before the age of 45 than it was among women in the control group (HR, 1.67; 95% CI, 1.16–2.40); it was particularly high in women who did not receive estrogen treatment before age 45 (HR, 1.93; 95% CI, 1.25–2.96).22
In a recent study, investigators used the Nurses’ Health Study database to explore the long-term health outcomes of 29,380 women who underwent hysterectomy.4 Of these women, 13,035 (44.4%) had their ovaries conserved, and 16,345 (55.6%) underwent bilateral oophorectomy. Follow-up was 24 years. Oophorectomy was associated with an increased risk of nonfatal CAD among all women (HR, 1.17; 95% CI, 1.02, 1.35), especially those who underwent the procedure before age 45 (HR, 1.26; 95% CI, 1.04, 1.54). Oophorectomy was associated with a markedly reduced risk of ovarian cancer but an increased risk of lung cancer (HR, 1.26; 95% CI, 1.02–1.56).
In regard to fatal events, oophorectomy increased the risk of death from all causes (HR, 1.12; 95% CI, 1.03, 1.21). Specifically, there was an increased risk of death from CAD (HR, 1.28; 95% CI, 1.00, 1.64), lung cancer (HR, 1.31; 95% CI, 1.02, 1.68), and all cancers (HR, 1.17; 95% CI, 1.04, 1.32). There was no overall difference in the risk of death from stroke, breast cancer, and colorectal cancer between women who underwent oophorectomy and those who retained their ovaries.
During the 24 years of follow-up, 37 women died from ovarian cancer, accounting for 1.2% of all deaths. At no age did oophorectomy show a survival benefit.
How this evidence should inform your practice
It is unfortunate that the entire body of evidence on the risks and benefits of bilateral salpingo-oophorectomy consists of observational studies, which have significant inherent limitations. Although the Nurses’ Health Study was the largest prospective study to examine the effect of oophorectomy on women’s health, and involved the longest follow-up, the study was observational, and oophorectomy and ovarian conservation were self-selected. Nevertheless, recent data suggest that a more detailed informed-consent process is warranted than the process in place. Informed consent should cover the risks and benefits of both oophorectomy and ovarian conservation.
Prophylactic oophorectomy is recommended only if a preponderance of the evidence establishes that it clearly benefits the patient. The studies described in this article suggest that bilateral oophorectomy does harm more often than it does good. Therefore, a cautious approach to oophorectomy at the time of hysterectomy is advised.
CASE RESOLVED
After you describe the risks and benefits of oophorectomy, and address the patient’s concerns about her family history of heart disease, she decides to keep her ovaries.
1. Healthcare Cost and Utilization Project (HCUP), 1988–2001: A Federal–State–Industry Partnership in Health Data. Rockville, Md: Agency for Healthcare Research and Quality; July 2003.
2. Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962.
3. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105-1110.
4. Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027-1037.
5. Melton LJ 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900-905.
6. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074.-
7. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200-209.
8. Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050-1059.
9. Bennett DA. Editorial comment on “Prevalence of dementia in the United States: the aging, demographics, and memory study” by Plassman et al. Neuroepidemiology. 2007;29:133-135.
10. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
11. Armstrong K, Schwartz J, Randall T, Rubin S, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
12. Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84:760-764.
13. Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68:159-164.
14. Bailey CL, Ueland FR, Land GL, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998;69:3-7.
15. Naylor AC. Hysterectomy—analysis of 2,901 personally performed procedures. S Afr Med J. 1984;65:242-245.
16. Christ JE, Lotze EC. The residual ovary syndrome. Obstet Gynecol. 1975;46:551-556.
17. Jacoby VL, Grady D, Sawaya GF. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol. 2009;200:140.e1-140.e9.
18. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714-718.
19. Løkkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226-233.
20. de Kleijn MJJ, van der Schouw YT, Verbeek ALM, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339.-
21. Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53:832-837.
22. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821-828.
23. Judd H, Judd G, Lucas W, Yen S. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020-1024.
24. Fogle R, Stanczyk F, Zhang X, Paulson R. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040-3043.
25. Meldrum D, Davidson B, Tataryn I, Judd H. Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol. 1981;57:624-628.
26. Raisz LG, Wilta B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
27. Johansson C, Mellstrom D, Milsom I. Reproductive factors as predictors of bone density and fractures in women at age 70. Maturitas. 1993;17:39-50.
28. Davidson BJ, Ross RK, Paganini-Hill A, et al. Total and free estrogens and androgens in postmenopausal women with hip fractures. J Clin Endocrinol Metab. 1982;54:115-120.
29. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733-738.
30. Kritz-Silverstein D, von Mühlen D, Barrett-Connor E. Hysterectomy and oophorectomy are unrelated to bone loss in older women. Maturitas. 2004;47:61-69.
31. Antoniucci DM, Sellmeyer DE, Cauley JA, et al. Postmenopausal bilateral oophorectomy is not associated with increased fracture risk in older women. J Bone Miner Res. 2005;20:741-747.
32. Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248-1250.
33. Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy—effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283-293.
34. Nieman L. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
35. Shifren J. Androgen deficiency in the oophorectomized woman. Fertil Steril. 2002;77 Suppl 4:s60-s62.
36. Sherwin B, Gelfan M. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1978;49:397-409.
37. Shifren JL, Avis NE. Surgical menopause: effects on psychological well-being and sexuality. Menopause. 2007;14:586.-
38. Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149-156.
39. Sherwin BB, Gelfan MM. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1978;49:397-409.
40. Teplin V, Vittinghoff E, Lin F, Learman L, Richter H, Kuppermann M. Oophorectomy in premenopausal women: health-related quality of life and sexual functioning. Obstet Gynecol. 2007;109:347-354.
41. Shumaker SA, Legault C, Rapp SR, et al. WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
42. Rapp SR, Espeland MA, Shumaker SA, et al. WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663-2672.
43. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959-2968.
44. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
45. Siegfried T. Neuroscience: it’s all in the timing. Nature. 2007;445:359-361.
46. Parker W, Broder M, Liu Z, Shoupe D, Farquhar C, Berek J. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
47. Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042-1050.
48. McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271-287.
49. Huser MA, Evans TS, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22:163-171.
CASE: Hysterectomy candidate asks about her ovaries
A 51-year-old premenopausal woman complains of severe menorrhagia that often causes her to miss work. Although she is taking an iron supplement, her hemoglobin level often drops below 10 g/dL. She has already been identified as having fibroids, with a uterine size of 14 weeks. You order ultra-sonography, which reveals an enlarged uterus with multiple fibroids and normal endometrial thickness, but no intracavitary lesions.
After you describe the treatment options, including uterine artery embolization, the patient requests a hysterectomy as a reasonably low-risk means of cure. During informed consent, she asks whether she should have her ovaries removed during the surgery. Further discussion reveals that her father died of a myocardial infarction when he was 64 years old, but there is no family or personal history of ovarian or breast cancer.
How do you advise this patient, based on her history and recent findings from medical research?
Many gynecologists have been trained to recommend bilateral oophorectomy for women older than 45 or 50 years who request a hysterectomy for benign disease. In these women, oophorectomy is recommended to prevent ovarian cancer and avert the potential for other ovarian pathology that might require later surgery.
In the United States, 78% of women 45 to 64 years old and 55% of women overall undergo bilateral oophorectomy at the time of hysterectomy.1 These percentages mean that almost 300,000 women undergo bilateral oophorectomy each year.1
Hysterectomy alone can sometimes lead to early ovarian failure, but this phenomenon is infrequent. A prospective study of premenopausal women found that, after 5 years of follow-up, 20% of women who underwent simple hysterectomy reached menopause, compared with 7% of matched women who did not undergo hysterectomy.2
In this article, I explore the risks and benefits associated with bilateral oophorectomy and present an algorithm to aid in deciding whether the patient should keep her ovaries—and when oophorectomy might be a better option ( FIGURE ).
Among the hazards associated with bilateral oophorectomy are:
- an increased risk of death from coronary artery disease (CAD), lung cancer, all cancers (except ovarian), and all causes3,4
- an increased risk of osteoporosis and hip fracture5
- when performed before the onset of menopause, an increased risk of parkinsonism, cognitive impairment, dementia, anxiety, and depression.6-8
Benefits include a reduced risk of ovarian cancer, particularly among women who have a BRCA gene mutation or strong family history of ovarian or breast cancer.
Although ovarian cancer causes 15,000 deaths each year in the United States, that figure pales when compared with heart disease, which accounts for 350,000 deaths. In addition, hip fracture may cause approximately 66,000 deaths each year, and dementia attributable to bilateral oophorectomy may affect 100,000 to 200,000 women.9 Reoperation for adnexal pathology or pain after hysterectomy is rare, occurring in only 2.8% of women. Therefore, the benefits of oophorectomy are often outweighed by the risks of CAD, hip fracture, and neurologic conditions.
The assumption that medical treatment will ameliorate the risks associated with oophorectomy is unrealistic. Estrogen may mitigate some risks, but many women avoid hormone therapy. This avoidance can be especially problematic in young women.
In the Nurses’ Health Study, a separate analysis focused on women who had never used postmenopausal hormone therapy.4 In this analysis, all women who underwent bilateral oophorectomy had a greater risk of stroke (HR, 1.85; 95% CI, 1.09, 3.16) and lung cancer (HR, 2.09; 95% CI, 1.01, 4.33) than did women who retained their ovaries. Among women who underwent oophorectomy before age 50 and who did not take estrogen, the risk of coronary artery disease (CAD) was higher (HR, 1.98; 95% CI, 1.18, 3.32), as was the risk of death from all causes (HR, 1.40; 95% CI, 1.01, 1.96), compared with women who retained their ovaries.
Despite estrogen’s proven benefit among oophorectomized women, usage rates continue to decline. In the 6 months after publication of Women’s Health Initiative findings on estrogen–progestin therapy, the continuation rate of estrogen therapy decreased from 12.6% to 9.1%, and new starts also declined significantly.47
Among women who had a diagnosis of osteoporosis and who began treatment with estrogen, estrogen plus progestin, a bisphosphonate, or raloxifene, medication continuation rates were less than 25% at 12 months.48 Moreover, only 18% of women started on a statin to reduce the risk of cardiovascular disease were still taking the drug after 1 year.49

FIGURE Conservation vs oophorectomy: A guide to decision-making
* Estrogen replacement is recommended for women younger than 45 years who opt for oophorectomy
Ovarian cancer is a real, but relatively low, risk
In 2008, an estimated 21,650 new cases of ovarian cancer were diagnosed (age at diagnosis: mean, 63 years), and 15,520 women died from the disease.10 Because we lack a reliable screening test to detect early-stage ovarian cancer in the general population, most women are given a diagnosis when disease is advanced and the 5-year survival rate is 15% to 25%.
There is agreement that women who are known to have a BRCA mutation, which increases the risk of ovarian and breast cancer, should strongly consider oophorectomy once childbearing is complete.11 In the general population, however, the outlook is different.
In the United States, the lifetime risk of ovarian cancer is 1.4% overall. Among white women who have had three or more term pregnancies and who have used an oral contraceptive for at least 4 years, the lifetime risk of ovarian cancer drops to 0.3%.12
Need for reoperation is very low
The percentage of women who require reoperation after ovarian conservation—2.8%—may surprise you.13 That figure is lower than once thought because many studies were performed before asymptomatic, benign ovarian cysts were determined to be a fairly common phenomenon in postmenopausal women (prevalence, 6.6%). These cysts do not undergo transformation to cancer and, therefore, do not need to be removed.14
In addition, studies indicate that only 0.1% to 0.75% of women who retain their ovaries at the time of hysterectomy develop ovarian cancer.15,16 Therefore, the rationale of performing oophorectomy to avoid future surgery appears to be unfounded.
CAD risk rises sharply after oophorectomy
A recent systematic review found mixed evidence concerning the risk of CAD following bilateral salpingo-oophorectomy.17 In observational studies, however, earlier age of surgical or natural menopause has been associated with a higher risk of cardiovascular mortality.18-20 Early reports from the Nurses’ Health Study found that the risk of myocardial infarction doubled among women who underwent oophorectomy and never used estrogen, compared with age-matched premenopausal women (relative risk [RR], 2.2; 95% confidence interval [CI], 1.2, 4.2).3 Even after age 50, the risk of a first myocardial infarction is increased among oophorectomized women, compared with women who retain their ovaries (RR, 1.4; 95% CI, 1.0–2.0).21
A study by researchers from the Mayo Clinic, who examined all causes listed on the death certificate, found a significant association between bilateral oophorectomy before the age of 45 years and cardiovascular mortality (hazard ratio [HR], 1.44; 95% CI, 1.01–2.05).22 This risk was significantly increased among women who were not treated with estrogen through at least age 45, compared with estrogen-treated women.
Oophorectomy may impair bone health
After menopause, ovaries continue to produce significant amounts of the androgens testosterone and androstenedione, which are converted to estrone peripherally by skin, muscle, and fat cells.23,24 The levels of these hormones remain consistent and have been documented to age 80.25
Both estrogens and androgens inhibit bone resorption, and androgens also stimulate bone formation.26 Low levels of androgens and estrogens are linked to lower bone density and a higher risk of hip and vertebral fracture in postmenopausal women.27-29
Postmenopausal women who have been oophorectomized may have an even greater risk of osteoporosis. Over 16 years of follow-up, 340 women who had undergone oophorectomy at a median age of 62 years had 54% more osteoporotic fractures than women who had intact ovaries.5 Two other studies found no association between oophorectomy and bone loss or fracture risk, however.30,31
Hip fracture is a well-documented cause of increased morbidity and mortality in older women. One study found that, before hip fracture, 28% of patients were housebound; 1 year after hip fracture, the percentage was 46%.32 Women older than 60 who underwent oophorectomy had a doubled risk of mortality after low-trauma hip fracture, compared with women who had intact ovaries (odds ratio [OR], 2.18; 95% CI, 2.03–2.32).5
Loss of ovaries may affect mental health and sexuality
In a premenopausal woman, oophorectomy causes a sudden loss of estrogen and often triggers hot flashes, mood changes, sleep disturbances, headaches, and a decline in feelings of well-being.33,34 Over time, vaginal dryness, painful intercourse, loss of libido, bladder dysfunction, and depression may occur.35,36
Evidence suggests that, in women, sexual desire, sexual sensation, and orgasmic response are influenced by androgens. After elective oophorectomy, declines in sexual desire have been reported.37-39
Mental health and sexuality may rebound over time, however. One study found less improvement in mental health measures and body image 6 months after hysterectomy among women who were oophorectomized, compared with those who retained their ovaries. After 2 years, improvement levels were similar between groups.40
Cognitive function may suffer
Analysis of data from the Mayo Clinic Cohort Study of Oophorectomy and Aging found that bilateral oophorectomy before the onset of menopause increased the risk of parkinsonism, cognitive impairment or dementia, and anxiety or depression, particularly if estrogen was not replaced.6-8 These risks increased with younger age at oophorectomy.
The Women’s Health Initiative found an increased risk of dementia or mild cognitive impairment in women who were treated by estrogen alone or estrogen plus progestin after age 65.41-44
These disparate conclusions suggest that estrogen may have a protective effect on the brain if it is given right after natural menopause or premenopausal oophorectomy, but deleterious effects if it is started years later.45
Other studies of endogenous estrogen and cognitive function are few and yield inconsistent results.
Ovarian conservation boosts long-term survival
When there is no family history of ovarian cancer, ovarian conservation appears to maximize survival among healthy women 40 to 65 years old who undergo hysterectomy for benign disease.46 Among healthy women hysterectomized before the age of 55, calculations suggest that 8.6% more would be alive at age 80 if their ovaries were conserved than if they were removed.46
A study from the Mayo Clinic found that all-cause mortality was significantly higher among women who underwent prophylactic bilateral oophorectomy before the age of 45 than it was among women in the control group (HR, 1.67; 95% CI, 1.16–2.40); it was particularly high in women who did not receive estrogen treatment before age 45 (HR, 1.93; 95% CI, 1.25–2.96).22
In a recent study, investigators used the Nurses’ Health Study database to explore the long-term health outcomes of 29,380 women who underwent hysterectomy.4 Of these women, 13,035 (44.4%) had their ovaries conserved, and 16,345 (55.6%) underwent bilateral oophorectomy. Follow-up was 24 years. Oophorectomy was associated with an increased risk of nonfatal CAD among all women (HR, 1.17; 95% CI, 1.02, 1.35), especially those who underwent the procedure before age 45 (HR, 1.26; 95% CI, 1.04, 1.54). Oophorectomy was associated with a markedly reduced risk of ovarian cancer but an increased risk of lung cancer (HR, 1.26; 95% CI, 1.02–1.56).
In regard to fatal events, oophorectomy increased the risk of death from all causes (HR, 1.12; 95% CI, 1.03, 1.21). Specifically, there was an increased risk of death from CAD (HR, 1.28; 95% CI, 1.00, 1.64), lung cancer (HR, 1.31; 95% CI, 1.02, 1.68), and all cancers (HR, 1.17; 95% CI, 1.04, 1.32). There was no overall difference in the risk of death from stroke, breast cancer, and colorectal cancer between women who underwent oophorectomy and those who retained their ovaries.
During the 24 years of follow-up, 37 women died from ovarian cancer, accounting for 1.2% of all deaths. At no age did oophorectomy show a survival benefit.
How this evidence should inform your practice
It is unfortunate that the entire body of evidence on the risks and benefits of bilateral salpingo-oophorectomy consists of observational studies, which have significant inherent limitations. Although the Nurses’ Health Study was the largest prospective study to examine the effect of oophorectomy on women’s health, and involved the longest follow-up, the study was observational, and oophorectomy and ovarian conservation were self-selected. Nevertheless, recent data suggest that a more detailed informed-consent process is warranted than the process in place. Informed consent should cover the risks and benefits of both oophorectomy and ovarian conservation.
Prophylactic oophorectomy is recommended only if a preponderance of the evidence establishes that it clearly benefits the patient. The studies described in this article suggest that bilateral oophorectomy does harm more often than it does good. Therefore, a cautious approach to oophorectomy at the time of hysterectomy is advised.
CASE RESOLVED
After you describe the risks and benefits of oophorectomy, and address the patient’s concerns about her family history of heart disease, she decides to keep her ovaries.
CASE: Hysterectomy candidate asks about her ovaries
A 51-year-old premenopausal woman complains of severe menorrhagia that often causes her to miss work. Although she is taking an iron supplement, her hemoglobin level often drops below 10 g/dL. She has already been identified as having fibroids, with a uterine size of 14 weeks. You order ultra-sonography, which reveals an enlarged uterus with multiple fibroids and normal endometrial thickness, but no intracavitary lesions.
After you describe the treatment options, including uterine artery embolization, the patient requests a hysterectomy as a reasonably low-risk means of cure. During informed consent, she asks whether she should have her ovaries removed during the surgery. Further discussion reveals that her father died of a myocardial infarction when he was 64 years old, but there is no family or personal history of ovarian or breast cancer.
How do you advise this patient, based on her history and recent findings from medical research?
Many gynecologists have been trained to recommend bilateral oophorectomy for women older than 45 or 50 years who request a hysterectomy for benign disease. In these women, oophorectomy is recommended to prevent ovarian cancer and avert the potential for other ovarian pathology that might require later surgery.
In the United States, 78% of women 45 to 64 years old and 55% of women overall undergo bilateral oophorectomy at the time of hysterectomy.1 These percentages mean that almost 300,000 women undergo bilateral oophorectomy each year.1
Hysterectomy alone can sometimes lead to early ovarian failure, but this phenomenon is infrequent. A prospective study of premenopausal women found that, after 5 years of follow-up, 20% of women who underwent simple hysterectomy reached menopause, compared with 7% of matched women who did not undergo hysterectomy.2
In this article, I explore the risks and benefits associated with bilateral oophorectomy and present an algorithm to aid in deciding whether the patient should keep her ovaries—and when oophorectomy might be a better option ( FIGURE ).
Among the hazards associated with bilateral oophorectomy are:
- an increased risk of death from coronary artery disease (CAD), lung cancer, all cancers (except ovarian), and all causes3,4
- an increased risk of osteoporosis and hip fracture5
- when performed before the onset of menopause, an increased risk of parkinsonism, cognitive impairment, dementia, anxiety, and depression.6-8
Benefits include a reduced risk of ovarian cancer, particularly among women who have a BRCA gene mutation or strong family history of ovarian or breast cancer.
Although ovarian cancer causes 15,000 deaths each year in the United States, that figure pales when compared with heart disease, which accounts for 350,000 deaths. In addition, hip fracture may cause approximately 66,000 deaths each year, and dementia attributable to bilateral oophorectomy may affect 100,000 to 200,000 women.9 Reoperation for adnexal pathology or pain after hysterectomy is rare, occurring in only 2.8% of women. Therefore, the benefits of oophorectomy are often outweighed by the risks of CAD, hip fracture, and neurologic conditions.
The assumption that medical treatment will ameliorate the risks associated with oophorectomy is unrealistic. Estrogen may mitigate some risks, but many women avoid hormone therapy. This avoidance can be especially problematic in young women.
In the Nurses’ Health Study, a separate analysis focused on women who had never used postmenopausal hormone therapy.4 In this analysis, all women who underwent bilateral oophorectomy had a greater risk of stroke (HR, 1.85; 95% CI, 1.09, 3.16) and lung cancer (HR, 2.09; 95% CI, 1.01, 4.33) than did women who retained their ovaries. Among women who underwent oophorectomy before age 50 and who did not take estrogen, the risk of coronary artery disease (CAD) was higher (HR, 1.98; 95% CI, 1.18, 3.32), as was the risk of death from all causes (HR, 1.40; 95% CI, 1.01, 1.96), compared with women who retained their ovaries.
Despite estrogen’s proven benefit among oophorectomized women, usage rates continue to decline. In the 6 months after publication of Women’s Health Initiative findings on estrogen–progestin therapy, the continuation rate of estrogen therapy decreased from 12.6% to 9.1%, and new starts also declined significantly.47
Among women who had a diagnosis of osteoporosis and who began treatment with estrogen, estrogen plus progestin, a bisphosphonate, or raloxifene, medication continuation rates were less than 25% at 12 months.48 Moreover, only 18% of women started on a statin to reduce the risk of cardiovascular disease were still taking the drug after 1 year.49

FIGURE Conservation vs oophorectomy: A guide to decision-making
* Estrogen replacement is recommended for women younger than 45 years who opt for oophorectomy
Ovarian cancer is a real, but relatively low, risk
In 2008, an estimated 21,650 new cases of ovarian cancer were diagnosed (age at diagnosis: mean, 63 years), and 15,520 women died from the disease.10 Because we lack a reliable screening test to detect early-stage ovarian cancer in the general population, most women are given a diagnosis when disease is advanced and the 5-year survival rate is 15% to 25%.
There is agreement that women who are known to have a BRCA mutation, which increases the risk of ovarian and breast cancer, should strongly consider oophorectomy once childbearing is complete.11 In the general population, however, the outlook is different.
In the United States, the lifetime risk of ovarian cancer is 1.4% overall. Among white women who have had three or more term pregnancies and who have used an oral contraceptive for at least 4 years, the lifetime risk of ovarian cancer drops to 0.3%.12
Need for reoperation is very low
The percentage of women who require reoperation after ovarian conservation—2.8%—may surprise you.13 That figure is lower than once thought because many studies were performed before asymptomatic, benign ovarian cysts were determined to be a fairly common phenomenon in postmenopausal women (prevalence, 6.6%). These cysts do not undergo transformation to cancer and, therefore, do not need to be removed.14
In addition, studies indicate that only 0.1% to 0.75% of women who retain their ovaries at the time of hysterectomy develop ovarian cancer.15,16 Therefore, the rationale of performing oophorectomy to avoid future surgery appears to be unfounded.
CAD risk rises sharply after oophorectomy
A recent systematic review found mixed evidence concerning the risk of CAD following bilateral salpingo-oophorectomy.17 In observational studies, however, earlier age of surgical or natural menopause has been associated with a higher risk of cardiovascular mortality.18-20 Early reports from the Nurses’ Health Study found that the risk of myocardial infarction doubled among women who underwent oophorectomy and never used estrogen, compared with age-matched premenopausal women (relative risk [RR], 2.2; 95% confidence interval [CI], 1.2, 4.2).3 Even after age 50, the risk of a first myocardial infarction is increased among oophorectomized women, compared with women who retain their ovaries (RR, 1.4; 95% CI, 1.0–2.0).21
A study by researchers from the Mayo Clinic, who examined all causes listed on the death certificate, found a significant association between bilateral oophorectomy before the age of 45 years and cardiovascular mortality (hazard ratio [HR], 1.44; 95% CI, 1.01–2.05).22 This risk was significantly increased among women who were not treated with estrogen through at least age 45, compared with estrogen-treated women.
Oophorectomy may impair bone health
After menopause, ovaries continue to produce significant amounts of the androgens testosterone and androstenedione, which are converted to estrone peripherally by skin, muscle, and fat cells.23,24 The levels of these hormones remain consistent and have been documented to age 80.25
Both estrogens and androgens inhibit bone resorption, and androgens also stimulate bone formation.26 Low levels of androgens and estrogens are linked to lower bone density and a higher risk of hip and vertebral fracture in postmenopausal women.27-29
Postmenopausal women who have been oophorectomized may have an even greater risk of osteoporosis. Over 16 years of follow-up, 340 women who had undergone oophorectomy at a median age of 62 years had 54% more osteoporotic fractures than women who had intact ovaries.5 Two other studies found no association between oophorectomy and bone loss or fracture risk, however.30,31
Hip fracture is a well-documented cause of increased morbidity and mortality in older women. One study found that, before hip fracture, 28% of patients were housebound; 1 year after hip fracture, the percentage was 46%.32 Women older than 60 who underwent oophorectomy had a doubled risk of mortality after low-trauma hip fracture, compared with women who had intact ovaries (odds ratio [OR], 2.18; 95% CI, 2.03–2.32).5
Loss of ovaries may affect mental health and sexuality
In a premenopausal woman, oophorectomy causes a sudden loss of estrogen and often triggers hot flashes, mood changes, sleep disturbances, headaches, and a decline in feelings of well-being.33,34 Over time, vaginal dryness, painful intercourse, loss of libido, bladder dysfunction, and depression may occur.35,36
Evidence suggests that, in women, sexual desire, sexual sensation, and orgasmic response are influenced by androgens. After elective oophorectomy, declines in sexual desire have been reported.37-39
Mental health and sexuality may rebound over time, however. One study found less improvement in mental health measures and body image 6 months after hysterectomy among women who were oophorectomized, compared with those who retained their ovaries. After 2 years, improvement levels were similar between groups.40
Cognitive function may suffer
Analysis of data from the Mayo Clinic Cohort Study of Oophorectomy and Aging found that bilateral oophorectomy before the onset of menopause increased the risk of parkinsonism, cognitive impairment or dementia, and anxiety or depression, particularly if estrogen was not replaced.6-8 These risks increased with younger age at oophorectomy.
The Women’s Health Initiative found an increased risk of dementia or mild cognitive impairment in women who were treated by estrogen alone or estrogen plus progestin after age 65.41-44
These disparate conclusions suggest that estrogen may have a protective effect on the brain if it is given right after natural menopause or premenopausal oophorectomy, but deleterious effects if it is started years later.45
Other studies of endogenous estrogen and cognitive function are few and yield inconsistent results.
Ovarian conservation boosts long-term survival
When there is no family history of ovarian cancer, ovarian conservation appears to maximize survival among healthy women 40 to 65 years old who undergo hysterectomy for benign disease.46 Among healthy women hysterectomized before the age of 55, calculations suggest that 8.6% more would be alive at age 80 if their ovaries were conserved than if they were removed.46
A study from the Mayo Clinic found that all-cause mortality was significantly higher among women who underwent prophylactic bilateral oophorectomy before the age of 45 than it was among women in the control group (HR, 1.67; 95% CI, 1.16–2.40); it was particularly high in women who did not receive estrogen treatment before age 45 (HR, 1.93; 95% CI, 1.25–2.96).22
In a recent study, investigators used the Nurses’ Health Study database to explore the long-term health outcomes of 29,380 women who underwent hysterectomy.4 Of these women, 13,035 (44.4%) had their ovaries conserved, and 16,345 (55.6%) underwent bilateral oophorectomy. Follow-up was 24 years. Oophorectomy was associated with an increased risk of nonfatal CAD among all women (HR, 1.17; 95% CI, 1.02, 1.35), especially those who underwent the procedure before age 45 (HR, 1.26; 95% CI, 1.04, 1.54). Oophorectomy was associated with a markedly reduced risk of ovarian cancer but an increased risk of lung cancer (HR, 1.26; 95% CI, 1.02–1.56).
In regard to fatal events, oophorectomy increased the risk of death from all causes (HR, 1.12; 95% CI, 1.03, 1.21). Specifically, there was an increased risk of death from CAD (HR, 1.28; 95% CI, 1.00, 1.64), lung cancer (HR, 1.31; 95% CI, 1.02, 1.68), and all cancers (HR, 1.17; 95% CI, 1.04, 1.32). There was no overall difference in the risk of death from stroke, breast cancer, and colorectal cancer between women who underwent oophorectomy and those who retained their ovaries.
During the 24 years of follow-up, 37 women died from ovarian cancer, accounting for 1.2% of all deaths. At no age did oophorectomy show a survival benefit.
How this evidence should inform your practice
It is unfortunate that the entire body of evidence on the risks and benefits of bilateral salpingo-oophorectomy consists of observational studies, which have significant inherent limitations. Although the Nurses’ Health Study was the largest prospective study to examine the effect of oophorectomy on women’s health, and involved the longest follow-up, the study was observational, and oophorectomy and ovarian conservation were self-selected. Nevertheless, recent data suggest that a more detailed informed-consent process is warranted than the process in place. Informed consent should cover the risks and benefits of both oophorectomy and ovarian conservation.
Prophylactic oophorectomy is recommended only if a preponderance of the evidence establishes that it clearly benefits the patient. The studies described in this article suggest that bilateral oophorectomy does harm more often than it does good. Therefore, a cautious approach to oophorectomy at the time of hysterectomy is advised.
CASE RESOLVED
After you describe the risks and benefits of oophorectomy, and address the patient’s concerns about her family history of heart disease, she decides to keep her ovaries.
1. Healthcare Cost and Utilization Project (HCUP), 1988–2001: A Federal–State–Industry Partnership in Health Data. Rockville, Md: Agency for Healthcare Research and Quality; July 2003.
2. Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962.
3. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105-1110.
4. Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027-1037.
5. Melton LJ 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900-905.
6. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074.-
7. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200-209.
8. Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050-1059.
9. Bennett DA. Editorial comment on “Prevalence of dementia in the United States: the aging, demographics, and memory study” by Plassman et al. Neuroepidemiology. 2007;29:133-135.
10. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
11. Armstrong K, Schwartz J, Randall T, Rubin S, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
12. Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84:760-764.
13. Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68:159-164.
14. Bailey CL, Ueland FR, Land GL, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998;69:3-7.
15. Naylor AC. Hysterectomy—analysis of 2,901 personally performed procedures. S Afr Med J. 1984;65:242-245.
16. Christ JE, Lotze EC. The residual ovary syndrome. Obstet Gynecol. 1975;46:551-556.
17. Jacoby VL, Grady D, Sawaya GF. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol. 2009;200:140.e1-140.e9.
18. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714-718.
19. Løkkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226-233.
20. de Kleijn MJJ, van der Schouw YT, Verbeek ALM, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339.-
21. Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53:832-837.
22. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821-828.
23. Judd H, Judd G, Lucas W, Yen S. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020-1024.
24. Fogle R, Stanczyk F, Zhang X, Paulson R. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040-3043.
25. Meldrum D, Davidson B, Tataryn I, Judd H. Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol. 1981;57:624-628.
26. Raisz LG, Wilta B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
27. Johansson C, Mellstrom D, Milsom I. Reproductive factors as predictors of bone density and fractures in women at age 70. Maturitas. 1993;17:39-50.
28. Davidson BJ, Ross RK, Paganini-Hill A, et al. Total and free estrogens and androgens in postmenopausal women with hip fractures. J Clin Endocrinol Metab. 1982;54:115-120.
29. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733-738.
30. Kritz-Silverstein D, von Mühlen D, Barrett-Connor E. Hysterectomy and oophorectomy are unrelated to bone loss in older women. Maturitas. 2004;47:61-69.
31. Antoniucci DM, Sellmeyer DE, Cauley JA, et al. Postmenopausal bilateral oophorectomy is not associated with increased fracture risk in older women. J Bone Miner Res. 2005;20:741-747.
32. Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248-1250.
33. Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy—effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283-293.
34. Nieman L. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
35. Shifren J. Androgen deficiency in the oophorectomized woman. Fertil Steril. 2002;77 Suppl 4:s60-s62.
36. Sherwin B, Gelfan M. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1978;49:397-409.
37. Shifren JL, Avis NE. Surgical menopause: effects on psychological well-being and sexuality. Menopause. 2007;14:586.-
38. Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149-156.
39. Sherwin BB, Gelfan MM. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1978;49:397-409.
40. Teplin V, Vittinghoff E, Lin F, Learman L, Richter H, Kuppermann M. Oophorectomy in premenopausal women: health-related quality of life and sexual functioning. Obstet Gynecol. 2007;109:347-354.
41. Shumaker SA, Legault C, Rapp SR, et al. WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
42. Rapp SR, Espeland MA, Shumaker SA, et al. WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663-2672.
43. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959-2968.
44. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
45. Siegfried T. Neuroscience: it’s all in the timing. Nature. 2007;445:359-361.
46. Parker W, Broder M, Liu Z, Shoupe D, Farquhar C, Berek J. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
47. Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042-1050.
48. McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271-287.
49. Huser MA, Evans TS, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22:163-171.
1. Healthcare Cost and Utilization Project (HCUP), 1988–2001: A Federal–State–Industry Partnership in Health Data. Rockville, Md: Agency for Healthcare Research and Quality; July 2003.
2. Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962.
3. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105-1110.
4. Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027-1037.
5. Melton LJ 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900-905.
6. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074.-
7. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200-209.
8. Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050-1059.
9. Bennett DA. Editorial comment on “Prevalence of dementia in the United States: the aging, demographics, and memory study” by Plassman et al. Neuroepidemiology. 2007;29:133-135.
10. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
11. Armstrong K, Schwartz J, Randall T, Rubin S, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
12. Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84:760-764.
13. Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68:159-164.
14. Bailey CL, Ueland FR, Land GL, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998;69:3-7.
15. Naylor AC. Hysterectomy—analysis of 2,901 personally performed procedures. S Afr Med J. 1984;65:242-245.
16. Christ JE, Lotze EC. The residual ovary syndrome. Obstet Gynecol. 1975;46:551-556.
17. Jacoby VL, Grady D, Sawaya GF. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol. 2009;200:140.e1-140.e9.
18. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714-718.
19. Løkkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226-233.
20. de Kleijn MJJ, van der Schouw YT, Verbeek ALM, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339.-
21. Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53:832-837.
22. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821-828.
23. Judd H, Judd G, Lucas W, Yen S. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020-1024.
24. Fogle R, Stanczyk F, Zhang X, Paulson R. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040-3043.
25. Meldrum D, Davidson B, Tataryn I, Judd H. Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol. 1981;57:624-628.
26. Raisz LG, Wilta B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
27. Johansson C, Mellstrom D, Milsom I. Reproductive factors as predictors of bone density and fractures in women at age 70. Maturitas. 1993;17:39-50.
28. Davidson BJ, Ross RK, Paganini-Hill A, et al. Total and free estrogens and androgens in postmenopausal women with hip fractures. J Clin Endocrinol Metab. 1982;54:115-120.
29. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733-738.
30. Kritz-Silverstein D, von Mühlen D, Barrett-Connor E. Hysterectomy and oophorectomy are unrelated to bone loss in older women. Maturitas. 2004;47:61-69.
31. Antoniucci DM, Sellmeyer DE, Cauley JA, et al. Postmenopausal bilateral oophorectomy is not associated with increased fracture risk in older women. J Bone Miner Res. 2005;20:741-747.
32. Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248-1250.
33. Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy—effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283-293.
34. Nieman L. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
35. Shifren J. Androgen deficiency in the oophorectomized woman. Fertil Steril. 2002;77 Suppl 4:s60-s62.
36. Sherwin B, Gelfan M. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1978;49:397-409.
37. Shifren JL, Avis NE. Surgical menopause: effects on psychological well-being and sexuality. Menopause. 2007;14:586.-
38. Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149-156.
39. Sherwin BB, Gelfan MM. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1978;49:397-409.
40. Teplin V, Vittinghoff E, Lin F, Learman L, Richter H, Kuppermann M. Oophorectomy in premenopausal women: health-related quality of life and sexual functioning. Obstet Gynecol. 2007;109:347-354.
41. Shumaker SA, Legault C, Rapp SR, et al. WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
42. Rapp SR, Espeland MA, Shumaker SA, et al. WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663-2672.
43. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959-2968.
44. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
45. Siegfried T. Neuroscience: it’s all in the timing. Nature. 2007;445:359-361.
46. Parker W, Broder M, Liu Z, Shoupe D, Farquhar C, Berek J. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
47. Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042-1050.
48. McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271-287.
49. Huser MA, Evans TS, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22:163-171.
When necessity calls for treating uterine fibroids
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
PART 1: Advising your patients Uterine fibroids: Childbearing, cancer, and hormone effects
The author reports no financial relationships relevant to this article.
CASE 1 Rapid growth=cancer?
Mrs. G., 47 years old, has had uterine fibroids the size of a 12-week pregnancy for about 6 years. At today’s examination, however, her uterus feels about the size of a 16-week pregnancy.
She is aware that her abdomen is bigger, and she complains of some abdominal pressure and urinary frequency. She reports no abnormal bleeding and no abdominal pain.
Mrs. G. is upset because another physician told her she might have cancer and needs a hysterectomy immediately. She tells you that she does not want a hysterectomy unless “it’s absolutely necessary.”
Ultrasonography at this visit reveals that two of the three fibroids noted on a previous sonogram have grown—one from 6 cm to 9 cm in diameter; the other from 5 cm to 8 cm.
What do you tell Mrs. G.?
Understanding myomas
Uterine fibroids, also called myomas, are benign, monoclonal tumors of the myometrium that contain collagen, fibronectin, and proteoglycan. The collagen fibrils are abnormally formed and in disarray; they look like the collagen found in keloids.1 Although the precise causes of fibroids are unknown, hormonal, genetic, and growth factors appear to be involved in their development and growth.2,3
About 40% of fibroids are chromosomally abnormal; the remaining 60% may have undetected mutations. More than 100 genes have been found to be up-regulated or down-regulated in fibroid cells. Many of these genes appear to regulate cell growth, differentiation, proliferation, and mitogenesis.
- A myoma is benign tumor of the myometrium
- In a premenopausal woman, rapid uterine growth almost never indicates the presence of uterine sarcoma
- In an older woman who experiences uterine growth, abdominal pain, and irregular vaginal bleeding, pelvic malignancy may be suspected; an increased level of LDH isoenzyme 3 with increased gadolinium uptake on MRI within 40 to 60 seconds suggests a diagnosis of leiomyosarcoma
- Most fibroids have no impact on fertility, but submucosal fibroids that distort the uterine cavity decrease fertility; removing them increases fertility
- Location, size, number, and extent of myoma penetration into the myometrium can be evaluated by pelvic MRI, with coronal, axial, and sagittal images without gadolinium contrast
- Given the risks associated with surgery and the lack of proof of efficacy, myomectomy to improve fertility should be undertaken with caution
- Most myomas do not grow during pregnancy. Unfavorable pregnancy outcomes are very rare in women with myomas.
- Oral contraceptives and postmenopausal hormone therapy almost never influence fibroid growth. Women with fibroids can usually use these therapies safely.
Differentiating benign myoma from uterine sarcoma
Myomas have chromosomal rearrangements similar to other benign lesions, whereas leiomyosarcomas are undifferentiated and have complex chromosomal rearrangements not seen in myomas.4 Genetic differences between myomas and leiomyosarcomas indicate they most likely have distinct origins, and that leiomyosarcomas do not result from malignant degeneration of myomas.2
In premenopausal women, rapid uterine growth almost never indicates uterine sarcoma: One study found only one sarcoma among 371 (0.26%) women operated on for rapid growth of a presumed myoma, and no sarcomas were found in the 198 women who had a 6-week-pregnancy-equivalent increase in uterine size over 1 year.5
Clinical indications. The clinical signs that would lead to suspicion of pelvic malignancy are:
- older age
- abdominal pain
- irregular vaginal bleeding.6
The average age of 2,098 women with uterine sarcoma reported in the SEER (Surveillance Epidemiology and End Results) cancer database from 1989 to 1999 was 63 years, whereas a review of the literature found a mean age of 36 years in women subjected to myomectomy who did not have sarcoma.3,7
Diagnostic tests. The distinction between benign myoma and leiomyosarcoma need not be based on clinical signs alone. Preoperative diagnosis of leiomyosarcoma may be possible, using laboratory values of total serum lactate dehydrogenase (LDH) and LDH isoenzyme 3 plus gadolinium-enhanced magnetic resonance imaging (MRI) scan (Gd-DTPA), with initial images taken 40 to 60 seconds after injection of gadolinium. A study of 87 women with fibroids, 10 women with leiomyosarcoma, and 130 women with degenerating fibroids reported 100% specificity, 100% positive predictive value, 100% negative predictive value, and 100% diagnostic accuracy for leiomyosarcoma with this combination diagnostic procedure.8
CASE 1 RESOLVED What advice for Mrs. G.?
You order a gadolinium-enhanced, dynamic MRI scan and have blood drawn for total LDH and LDH isoenzymes. Total LDH and isoenzyme 3 are normal. MRI shows no increased enhancement of the fibroids on images taken 40 to 60 seconds after injection of gadolinium. You advise the patient that there is no evidence of cancer (sarcoma) and no urgent need for hysterectomy. You also tell her that, because she is symptomatic, myomectomy is an option that will preserve her uterus.
CASE 2 Fibroids, and contemplating childbearing
Mrs. H., a 35-year-old nulligravida, comes to the office for her first visit with you. She has no complaints, but your pelvic examination reveals a 10 week-size enlarged uterus. A sonogram shows a 6-cm subserosal fibroid and a 3-cm intramural fibroid near the endometrial cavity (see FIGURE). This patient was recently married and wants to become pregnant within the coming year.
When you tell Mrs. H. that she has fibroids, she grows concerned. She asks: “Will this affect my fertility, or a pregnancy?” and “Do I need surgery?”
You explain that the larger, subserosal fibroid is not a concern. The smaller, intramural fibroid could, however, have an impact on fertility and pregnancy if it distorts the uterine cavity.
To find out if that is the case, you order a saline infusion sonogram, which demonstrates, clearly, a normal cavity without distortion by the fibroid.
What is your advice to this woman?
Submucous fibroids that distort the uterine cavity decrease fertility; removal increases fertility. Otherwise, neither intramural nor subserosal fibroids appear to affect the fertility rate; removal has not been shown to increase fertility. Meta-analysis of 11 studies found that submucous myomas that distort the uterine cavity appear to decrease the pregnancy rate by 70% (relative risk [RR], 0.32; confidence interval [CI], 0.13–0.70).9
Assessing the uterine cavity. Evaluating a woman with fibroids for fertility requires reliable assessment of the uterine cavity. Hysteroscopy and saline infusion sonography have been shown to be far superior to transvaginal sonography or hysterosalpingography for detecting submucosal fibroids.10
The best modality for determining the extent of submucosal penetration of a myoma into the myometrium is MRI. It is also an excellent modality for evaluating the size, position, and number of multiple fibroids.11 The drawback to using MRI? It is more costly than other modalities.
Note: Classification of submucosal fibroids is based on the fraction of the mass within the cavity:
- Class 0 myomas are entirely intracavitary
- Class I myomas have 50% or more of the fibroid within the cavity
- Class II myomas have less than 50% within the cavity.12
Resection and fertility. Submucous fibroids can often be removed hysteroscopically. Systematic review of the evidence found that resection-restored fertility is equal to that of infertile controls undergoing in vitro fertilization who do not have fibroids (RR, 1.72; CI, 1.13–2.58).9 In that review, the presence of neither intramural nor subserosal fibroids decreased fertility (intramural: RR, 0.94, and CI, 0.73–1.20; subserosal: RR, 1.1, and CI, 0.06–1.72). Furthermore, removal of intramural and subserosal myomas by abdominal or laparoscopic myomectomy did not improve fertility. An updated unpublished meta-analysis, including studies published after 2001, came to the same conclusion (Pritts E, personal communication, 2008).
CASE 2: Subserosal, intramural myomas cause concern
In a woman contemplating childbearing, does a subserosal (left) or intramural (right) myoma present a problem because of potential to distort the uterine cavity?
Fibroids and pregnancy
The incidence of sonographically detected fibroids during pregnancy is low.13 Among 12,600 women at a prenatal clinic, routine second-trimester sonography identified myomas in 183 (mean age, 33 years)—an incidence of 1.5%.
Pregnancy has a variable and unpredictable effect on myoma growth, likely dependent on individual differences in genetics, circulating growth factors, and myoma-localized receptors. Most myomas do not, however, grow during pregnancy. A prospective study of pregnant women who had a single myoma found that 69% had no increase in volume throughout their pregnancy. In women who were noted to have an increase in the volume of their myoma, the greatest growth occurred before 10 weeks’ gestation. No relationship was found between initial myoma volume and myoma growth during the gestational period. 14
Do myomas complicate pregnancy?
Very rarely. Two studies reported on outcomes in large populations of pregnant women who were examined with routine second-trimester ultrasonography, with follow-up and delivery at the same institution.
In one of those studies, 12,600 pregnant women were evaluated, and the outcome in 167 women who were given a diagnosis of myoma was compared with the outcome in women who did not have a myoma.15 Despite similar clinical management between the two groups, no significant differences were seen in regard to the incidence of:
- preterm delivery
- premature rupture of membranes
- fetal growth restriction
- placenta previa
- placental abruption
- postpartum hemorrhage
- retained placenta.
Only cesarean section was more common among women with fibroids (23% vs 12%).
The second study reviewed 15,104 pregnancies and compared 401 women found to have myomas and the remaining women who did not.16 Although the presence of myoma did not increase the risk of premature rupture of membranes, operative vaginal delivery, chorioamnionitis, or endomyometritis, there was some increased risk of pre-term delivery (19.2% vs 12.7%), placenta previa (3.5% vs 1.8%), and post-partum hemorrhage (8.3% vs 2.9%). Cesarean section was, again, more common (49.1% vs 21.4%).
Do myomas injure the fetus?
Fetal injury as a consequence of fibroids has been reported very infrequently. A review of the literature from 1980 to 2005 revealed only four cases—one each of:
- fetal head anomalies with fetal growth restriction
- postural deformity
- limb reduction
- fetal head deformation with torticollis.17-19
CASE 2 RESOLVED Should Mrs. H. have a myomectomy?
Probably not. Abdominal and laparoscopic myomectomy involve substantial operative and anesthetic risks, including infection, postoperative adhesions, a very small risk of uterine rupture during pregnancy, and increased likelihood of cesarean section. Costs are also substantial, involving not only the expense of surgery, but also patient discomfort and time for recovery. Therefore, until it is proved that intramural myomas decrease fertility and myomectomy increases fertility, surgery should be undertaken with caution. As far as the effects of myoma on pregnancy are concerned, no data are available by which to compare pregnancy outcomes following myomectomy with pregnancy outcomes in women whose myomas are untreated. Randomized studies are needed to clarify these important issues.
CASES 3 & 4 The effects of oral contraceptives and hormone replacement therapy
Mrs. J. is a 32-year-old G0P0 woman who has a 5-cm fundal myoma. She is sexually active and wants to use an oral contraceptive (OC). She has heard from friends, however, that taking an OC makes fibroids grow, and she asks for your advice.
The same day, you see Mrs. K., a 54-year-old, recently menopausal woman. She complains of severe hot flashes and night sweats that disturb her sleep. She has had asymptomatic uterine fibroids for about 10 years and, although she would like to take menopausal hormone therapy, she is worried that the medication will make the fibroids larger.
How do you advise these two women?
OCs. OCs do not appear to influence the growth of fibroids. One study found a slightly increased risk of fibroids, another study found no increased risk, and a third found a decreased risk.18,19 These studies are retrospective, however, and may be marked by selection bias.
Postmenopausal hormone replacement therapy. Postmenopausal hormone therapy does not ordinarily cause fibroid growth. After 3 years, only three of 34 (8%) post-menopausal women who had fibroids and were treated with 0.625 mg of conjugated equine estrogen (CEE) and 5 mg of medroxyprogesterone acetate (MPA) a day had any increase in the size of fibroids.20 If any increase in the size of the uterus is noted, it is likely related to progestins.
A study found that 23% of women taking oral estrogen plus 2.5 mg of MPA a day for 1 year had a slight increase in the size of fibroids, whereas 50% of women taking 5 mg of MPA had an increase in size (mean increase in diameter, 3.2 cm).21 Transdermal estrogen plus oral MPA was shown, after 1 year, to cause, on average, a 0.5-cm increase in the diameter of fibroids; oral estrogen and MPA caused no increase in size.22
CASES RESOLVED Rx: Reassurance
Advise Mrs. J. that taking an OC is unlikely to make her fibroids grow larger.
Mrs. K., who is older, can seek relief from postmenopausal symptoms by taking hormone therapy without fear of her fibroids being stimulated to grow.
1. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900-906.
2. Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037-1054.
3. Parker W. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725-736.
4. Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97-108.
5. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
6. Boutselis JG, Ullery JC. Sarcoma of the uterus. Obstet Gynecol. 1962;20:23-35.
7. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204-208.
8. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12:354-361.
9. Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483-491.
10. Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76:350-357.
11. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:388-403.
12. Cohen LS, Valle RF. Role of vaginal sonography and hysterosonography in the endoscopic treatment of uterine myomas. Fertil Steril. 2000;73:197-204.
13. Cooper NP, Okolo S. Fibroids in pregnancy—common but poorly understood. Obstet Gynecol Surv. 2005;60:132-138.
14. Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med. 1992;11:511-515.
15. Vergani P, Ghidini A, Strobelt N, et al. Do uterine leiomyomas influence pregnancy outcome? Am J Perinatol. 1994;11:356-358.
16. Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376-382.
17. Joo JG, Inovay J, Silhavy M, Papp Z. Successful enucleation of a necrotizing fibroid causing oligohydramnios and fetal postural deformity in the 25th week of gestation. A case report. J Reprod Med. 2001;46:923-925.
18. Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:359-362.
19. Ratner H. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:1027.-
20. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas. 2002;43:35-39.
21. Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2002;102:199-201.
22. Sener AB, Seçkin NC, Ozmen S, Gökmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354-357.
The author reports no financial relationships relevant to this article.
CASE 1 Rapid growth=cancer?
Mrs. G., 47 years old, has had uterine fibroids the size of a 12-week pregnancy for about 6 years. At today’s examination, however, her uterus feels about the size of a 16-week pregnancy.
She is aware that her abdomen is bigger, and she complains of some abdominal pressure and urinary frequency. She reports no abnormal bleeding and no abdominal pain.
Mrs. G. is upset because another physician told her she might have cancer and needs a hysterectomy immediately. She tells you that she does not want a hysterectomy unless “it’s absolutely necessary.”
Ultrasonography at this visit reveals that two of the three fibroids noted on a previous sonogram have grown—one from 6 cm to 9 cm in diameter; the other from 5 cm to 8 cm.
What do you tell Mrs. G.?
Understanding myomas
Uterine fibroids, also called myomas, are benign, monoclonal tumors of the myometrium that contain collagen, fibronectin, and proteoglycan. The collagen fibrils are abnormally formed and in disarray; they look like the collagen found in keloids.1 Although the precise causes of fibroids are unknown, hormonal, genetic, and growth factors appear to be involved in their development and growth.2,3
About 40% of fibroids are chromosomally abnormal; the remaining 60% may have undetected mutations. More than 100 genes have been found to be up-regulated or down-regulated in fibroid cells. Many of these genes appear to regulate cell growth, differentiation, proliferation, and mitogenesis.
- A myoma is benign tumor of the myometrium
- In a premenopausal woman, rapid uterine growth almost never indicates the presence of uterine sarcoma
- In an older woman who experiences uterine growth, abdominal pain, and irregular vaginal bleeding, pelvic malignancy may be suspected; an increased level of LDH isoenzyme 3 with increased gadolinium uptake on MRI within 40 to 60 seconds suggests a diagnosis of leiomyosarcoma
- Most fibroids have no impact on fertility, but submucosal fibroids that distort the uterine cavity decrease fertility; removing them increases fertility
- Location, size, number, and extent of myoma penetration into the myometrium can be evaluated by pelvic MRI, with coronal, axial, and sagittal images without gadolinium contrast
- Given the risks associated with surgery and the lack of proof of efficacy, myomectomy to improve fertility should be undertaken with caution
- Most myomas do not grow during pregnancy. Unfavorable pregnancy outcomes are very rare in women with myomas.
- Oral contraceptives and postmenopausal hormone therapy almost never influence fibroid growth. Women with fibroids can usually use these therapies safely.
Differentiating benign myoma from uterine sarcoma
Myomas have chromosomal rearrangements similar to other benign lesions, whereas leiomyosarcomas are undifferentiated and have complex chromosomal rearrangements not seen in myomas.4 Genetic differences between myomas and leiomyosarcomas indicate they most likely have distinct origins, and that leiomyosarcomas do not result from malignant degeneration of myomas.2
In premenopausal women, rapid uterine growth almost never indicates uterine sarcoma: One study found only one sarcoma among 371 (0.26%) women operated on for rapid growth of a presumed myoma, and no sarcomas were found in the 198 women who had a 6-week-pregnancy-equivalent increase in uterine size over 1 year.5
Clinical indications. The clinical signs that would lead to suspicion of pelvic malignancy are:
- older age
- abdominal pain
- irregular vaginal bleeding.6
The average age of 2,098 women with uterine sarcoma reported in the SEER (Surveillance Epidemiology and End Results) cancer database from 1989 to 1999 was 63 years, whereas a review of the literature found a mean age of 36 years in women subjected to myomectomy who did not have sarcoma.3,7
Diagnostic tests. The distinction between benign myoma and leiomyosarcoma need not be based on clinical signs alone. Preoperative diagnosis of leiomyosarcoma may be possible, using laboratory values of total serum lactate dehydrogenase (LDH) and LDH isoenzyme 3 plus gadolinium-enhanced magnetic resonance imaging (MRI) scan (Gd-DTPA), with initial images taken 40 to 60 seconds after injection of gadolinium. A study of 87 women with fibroids, 10 women with leiomyosarcoma, and 130 women with degenerating fibroids reported 100% specificity, 100% positive predictive value, 100% negative predictive value, and 100% diagnostic accuracy for leiomyosarcoma with this combination diagnostic procedure.8
CASE 1 RESOLVED What advice for Mrs. G.?
You order a gadolinium-enhanced, dynamic MRI scan and have blood drawn for total LDH and LDH isoenzymes. Total LDH and isoenzyme 3 are normal. MRI shows no increased enhancement of the fibroids on images taken 40 to 60 seconds after injection of gadolinium. You advise the patient that there is no evidence of cancer (sarcoma) and no urgent need for hysterectomy. You also tell her that, because she is symptomatic, myomectomy is an option that will preserve her uterus.
CASE 2 Fibroids, and contemplating childbearing
Mrs. H., a 35-year-old nulligravida, comes to the office for her first visit with you. She has no complaints, but your pelvic examination reveals a 10 week-size enlarged uterus. A sonogram shows a 6-cm subserosal fibroid and a 3-cm intramural fibroid near the endometrial cavity (see FIGURE). This patient was recently married and wants to become pregnant within the coming year.
When you tell Mrs. H. that she has fibroids, she grows concerned. She asks: “Will this affect my fertility, or a pregnancy?” and “Do I need surgery?”
You explain that the larger, subserosal fibroid is not a concern. The smaller, intramural fibroid could, however, have an impact on fertility and pregnancy if it distorts the uterine cavity.
To find out if that is the case, you order a saline infusion sonogram, which demonstrates, clearly, a normal cavity without distortion by the fibroid.
What is your advice to this woman?
Submucous fibroids that distort the uterine cavity decrease fertility; removal increases fertility. Otherwise, neither intramural nor subserosal fibroids appear to affect the fertility rate; removal has not been shown to increase fertility. Meta-analysis of 11 studies found that submucous myomas that distort the uterine cavity appear to decrease the pregnancy rate by 70% (relative risk [RR], 0.32; confidence interval [CI], 0.13–0.70).9
Assessing the uterine cavity. Evaluating a woman with fibroids for fertility requires reliable assessment of the uterine cavity. Hysteroscopy and saline infusion sonography have been shown to be far superior to transvaginal sonography or hysterosalpingography for detecting submucosal fibroids.10
The best modality for determining the extent of submucosal penetration of a myoma into the myometrium is MRI. It is also an excellent modality for evaluating the size, position, and number of multiple fibroids.11 The drawback to using MRI? It is more costly than other modalities.
Note: Classification of submucosal fibroids is based on the fraction of the mass within the cavity:
- Class 0 myomas are entirely intracavitary
- Class I myomas have 50% or more of the fibroid within the cavity
- Class II myomas have less than 50% within the cavity.12
Resection and fertility. Submucous fibroids can often be removed hysteroscopically. Systematic review of the evidence found that resection-restored fertility is equal to that of infertile controls undergoing in vitro fertilization who do not have fibroids (RR, 1.72; CI, 1.13–2.58).9 In that review, the presence of neither intramural nor subserosal fibroids decreased fertility (intramural: RR, 0.94, and CI, 0.73–1.20; subserosal: RR, 1.1, and CI, 0.06–1.72). Furthermore, removal of intramural and subserosal myomas by abdominal or laparoscopic myomectomy did not improve fertility. An updated unpublished meta-analysis, including studies published after 2001, came to the same conclusion (Pritts E, personal communication, 2008).
CASE 2: Subserosal, intramural myomas cause concern
In a woman contemplating childbearing, does a subserosal (left) or intramural (right) myoma present a problem because of potential to distort the uterine cavity?
Fibroids and pregnancy
The incidence of sonographically detected fibroids during pregnancy is low.13 Among 12,600 women at a prenatal clinic, routine second-trimester sonography identified myomas in 183 (mean age, 33 years)—an incidence of 1.5%.
Pregnancy has a variable and unpredictable effect on myoma growth, likely dependent on individual differences in genetics, circulating growth factors, and myoma-localized receptors. Most myomas do not, however, grow during pregnancy. A prospective study of pregnant women who had a single myoma found that 69% had no increase in volume throughout their pregnancy. In women who were noted to have an increase in the volume of their myoma, the greatest growth occurred before 10 weeks’ gestation. No relationship was found between initial myoma volume and myoma growth during the gestational period. 14
Do myomas complicate pregnancy?
Very rarely. Two studies reported on outcomes in large populations of pregnant women who were examined with routine second-trimester ultrasonography, with follow-up and delivery at the same institution.
In one of those studies, 12,600 pregnant women were evaluated, and the outcome in 167 women who were given a diagnosis of myoma was compared with the outcome in women who did not have a myoma.15 Despite similar clinical management between the two groups, no significant differences were seen in regard to the incidence of:
- preterm delivery
- premature rupture of membranes
- fetal growth restriction
- placenta previa
- placental abruption
- postpartum hemorrhage
- retained placenta.
Only cesarean section was more common among women with fibroids (23% vs 12%).
The second study reviewed 15,104 pregnancies and compared 401 women found to have myomas and the remaining women who did not.16 Although the presence of myoma did not increase the risk of premature rupture of membranes, operative vaginal delivery, chorioamnionitis, or endomyometritis, there was some increased risk of pre-term delivery (19.2% vs 12.7%), placenta previa (3.5% vs 1.8%), and post-partum hemorrhage (8.3% vs 2.9%). Cesarean section was, again, more common (49.1% vs 21.4%).
Do myomas injure the fetus?
Fetal injury as a consequence of fibroids has been reported very infrequently. A review of the literature from 1980 to 2005 revealed only four cases—one each of:
- fetal head anomalies with fetal growth restriction
- postural deformity
- limb reduction
- fetal head deformation with torticollis.17-19
CASE 2 RESOLVED Should Mrs. H. have a myomectomy?
Probably not. Abdominal and laparoscopic myomectomy involve substantial operative and anesthetic risks, including infection, postoperative adhesions, a very small risk of uterine rupture during pregnancy, and increased likelihood of cesarean section. Costs are also substantial, involving not only the expense of surgery, but also patient discomfort and time for recovery. Therefore, until it is proved that intramural myomas decrease fertility and myomectomy increases fertility, surgery should be undertaken with caution. As far as the effects of myoma on pregnancy are concerned, no data are available by which to compare pregnancy outcomes following myomectomy with pregnancy outcomes in women whose myomas are untreated. Randomized studies are needed to clarify these important issues.
CASES 3 & 4 The effects of oral contraceptives and hormone replacement therapy
Mrs. J. is a 32-year-old G0P0 woman who has a 5-cm fundal myoma. She is sexually active and wants to use an oral contraceptive (OC). She has heard from friends, however, that taking an OC makes fibroids grow, and she asks for your advice.
The same day, you see Mrs. K., a 54-year-old, recently menopausal woman. She complains of severe hot flashes and night sweats that disturb her sleep. She has had asymptomatic uterine fibroids for about 10 years and, although she would like to take menopausal hormone therapy, she is worried that the medication will make the fibroids larger.
How do you advise these two women?
OCs. OCs do not appear to influence the growth of fibroids. One study found a slightly increased risk of fibroids, another study found no increased risk, and a third found a decreased risk.18,19 These studies are retrospective, however, and may be marked by selection bias.
Postmenopausal hormone replacement therapy. Postmenopausal hormone therapy does not ordinarily cause fibroid growth. After 3 years, only three of 34 (8%) post-menopausal women who had fibroids and were treated with 0.625 mg of conjugated equine estrogen (CEE) and 5 mg of medroxyprogesterone acetate (MPA) a day had any increase in the size of fibroids.20 If any increase in the size of the uterus is noted, it is likely related to progestins.
A study found that 23% of women taking oral estrogen plus 2.5 mg of MPA a day for 1 year had a slight increase in the size of fibroids, whereas 50% of women taking 5 mg of MPA had an increase in size (mean increase in diameter, 3.2 cm).21 Transdermal estrogen plus oral MPA was shown, after 1 year, to cause, on average, a 0.5-cm increase in the diameter of fibroids; oral estrogen and MPA caused no increase in size.22
CASES RESOLVED Rx: Reassurance
Advise Mrs. J. that taking an OC is unlikely to make her fibroids grow larger.
Mrs. K., who is older, can seek relief from postmenopausal symptoms by taking hormone therapy without fear of her fibroids being stimulated to grow.
The author reports no financial relationships relevant to this article.
CASE 1 Rapid growth=cancer?
Mrs. G., 47 years old, has had uterine fibroids the size of a 12-week pregnancy for about 6 years. At today’s examination, however, her uterus feels about the size of a 16-week pregnancy.
She is aware that her abdomen is bigger, and she complains of some abdominal pressure and urinary frequency. She reports no abnormal bleeding and no abdominal pain.
Mrs. G. is upset because another physician told her she might have cancer and needs a hysterectomy immediately. She tells you that she does not want a hysterectomy unless “it’s absolutely necessary.”
Ultrasonography at this visit reveals that two of the three fibroids noted on a previous sonogram have grown—one from 6 cm to 9 cm in diameter; the other from 5 cm to 8 cm.
What do you tell Mrs. G.?
Understanding myomas
Uterine fibroids, also called myomas, are benign, monoclonal tumors of the myometrium that contain collagen, fibronectin, and proteoglycan. The collagen fibrils are abnormally formed and in disarray; they look like the collagen found in keloids.1 Although the precise causes of fibroids are unknown, hormonal, genetic, and growth factors appear to be involved in their development and growth.2,3
About 40% of fibroids are chromosomally abnormal; the remaining 60% may have undetected mutations. More than 100 genes have been found to be up-regulated or down-regulated in fibroid cells. Many of these genes appear to regulate cell growth, differentiation, proliferation, and mitogenesis.
- A myoma is benign tumor of the myometrium
- In a premenopausal woman, rapid uterine growth almost never indicates the presence of uterine sarcoma
- In an older woman who experiences uterine growth, abdominal pain, and irregular vaginal bleeding, pelvic malignancy may be suspected; an increased level of LDH isoenzyme 3 with increased gadolinium uptake on MRI within 40 to 60 seconds suggests a diagnosis of leiomyosarcoma
- Most fibroids have no impact on fertility, but submucosal fibroids that distort the uterine cavity decrease fertility; removing them increases fertility
- Location, size, number, and extent of myoma penetration into the myometrium can be evaluated by pelvic MRI, with coronal, axial, and sagittal images without gadolinium contrast
- Given the risks associated with surgery and the lack of proof of efficacy, myomectomy to improve fertility should be undertaken with caution
- Most myomas do not grow during pregnancy. Unfavorable pregnancy outcomes are very rare in women with myomas.
- Oral contraceptives and postmenopausal hormone therapy almost never influence fibroid growth. Women with fibroids can usually use these therapies safely.
Differentiating benign myoma from uterine sarcoma
Myomas have chromosomal rearrangements similar to other benign lesions, whereas leiomyosarcomas are undifferentiated and have complex chromosomal rearrangements not seen in myomas.4 Genetic differences between myomas and leiomyosarcomas indicate they most likely have distinct origins, and that leiomyosarcomas do not result from malignant degeneration of myomas.2
In premenopausal women, rapid uterine growth almost never indicates uterine sarcoma: One study found only one sarcoma among 371 (0.26%) women operated on for rapid growth of a presumed myoma, and no sarcomas were found in the 198 women who had a 6-week-pregnancy-equivalent increase in uterine size over 1 year.5
Clinical indications. The clinical signs that would lead to suspicion of pelvic malignancy are:
- older age
- abdominal pain
- irregular vaginal bleeding.6
The average age of 2,098 women with uterine sarcoma reported in the SEER (Surveillance Epidemiology and End Results) cancer database from 1989 to 1999 was 63 years, whereas a review of the literature found a mean age of 36 years in women subjected to myomectomy who did not have sarcoma.3,7
Diagnostic tests. The distinction between benign myoma and leiomyosarcoma need not be based on clinical signs alone. Preoperative diagnosis of leiomyosarcoma may be possible, using laboratory values of total serum lactate dehydrogenase (LDH) and LDH isoenzyme 3 plus gadolinium-enhanced magnetic resonance imaging (MRI) scan (Gd-DTPA), with initial images taken 40 to 60 seconds after injection of gadolinium. A study of 87 women with fibroids, 10 women with leiomyosarcoma, and 130 women with degenerating fibroids reported 100% specificity, 100% positive predictive value, 100% negative predictive value, and 100% diagnostic accuracy for leiomyosarcoma with this combination diagnostic procedure.8
CASE 1 RESOLVED What advice for Mrs. G.?
You order a gadolinium-enhanced, dynamic MRI scan and have blood drawn for total LDH and LDH isoenzymes. Total LDH and isoenzyme 3 are normal. MRI shows no increased enhancement of the fibroids on images taken 40 to 60 seconds after injection of gadolinium. You advise the patient that there is no evidence of cancer (sarcoma) and no urgent need for hysterectomy. You also tell her that, because she is symptomatic, myomectomy is an option that will preserve her uterus.
CASE 2 Fibroids, and contemplating childbearing
Mrs. H., a 35-year-old nulligravida, comes to the office for her first visit with you. She has no complaints, but your pelvic examination reveals a 10 week-size enlarged uterus. A sonogram shows a 6-cm subserosal fibroid and a 3-cm intramural fibroid near the endometrial cavity (see FIGURE). This patient was recently married and wants to become pregnant within the coming year.
When you tell Mrs. H. that she has fibroids, she grows concerned. She asks: “Will this affect my fertility, or a pregnancy?” and “Do I need surgery?”
You explain that the larger, subserosal fibroid is not a concern. The smaller, intramural fibroid could, however, have an impact on fertility and pregnancy if it distorts the uterine cavity.
To find out if that is the case, you order a saline infusion sonogram, which demonstrates, clearly, a normal cavity without distortion by the fibroid.
What is your advice to this woman?
Submucous fibroids that distort the uterine cavity decrease fertility; removal increases fertility. Otherwise, neither intramural nor subserosal fibroids appear to affect the fertility rate; removal has not been shown to increase fertility. Meta-analysis of 11 studies found that submucous myomas that distort the uterine cavity appear to decrease the pregnancy rate by 70% (relative risk [RR], 0.32; confidence interval [CI], 0.13–0.70).9
Assessing the uterine cavity. Evaluating a woman with fibroids for fertility requires reliable assessment of the uterine cavity. Hysteroscopy and saline infusion sonography have been shown to be far superior to transvaginal sonography or hysterosalpingography for detecting submucosal fibroids.10
The best modality for determining the extent of submucosal penetration of a myoma into the myometrium is MRI. It is also an excellent modality for evaluating the size, position, and number of multiple fibroids.11 The drawback to using MRI? It is more costly than other modalities.
Note: Classification of submucosal fibroids is based on the fraction of the mass within the cavity:
- Class 0 myomas are entirely intracavitary
- Class I myomas have 50% or more of the fibroid within the cavity
- Class II myomas have less than 50% within the cavity.12
Resection and fertility. Submucous fibroids can often be removed hysteroscopically. Systematic review of the evidence found that resection-restored fertility is equal to that of infertile controls undergoing in vitro fertilization who do not have fibroids (RR, 1.72; CI, 1.13–2.58).9 In that review, the presence of neither intramural nor subserosal fibroids decreased fertility (intramural: RR, 0.94, and CI, 0.73–1.20; subserosal: RR, 1.1, and CI, 0.06–1.72). Furthermore, removal of intramural and subserosal myomas by abdominal or laparoscopic myomectomy did not improve fertility. An updated unpublished meta-analysis, including studies published after 2001, came to the same conclusion (Pritts E, personal communication, 2008).
CASE 2: Subserosal, intramural myomas cause concern
In a woman contemplating childbearing, does a subserosal (left) or intramural (right) myoma present a problem because of potential to distort the uterine cavity?
Fibroids and pregnancy
The incidence of sonographically detected fibroids during pregnancy is low.13 Among 12,600 women at a prenatal clinic, routine second-trimester sonography identified myomas in 183 (mean age, 33 years)—an incidence of 1.5%.
Pregnancy has a variable and unpredictable effect on myoma growth, likely dependent on individual differences in genetics, circulating growth factors, and myoma-localized receptors. Most myomas do not, however, grow during pregnancy. A prospective study of pregnant women who had a single myoma found that 69% had no increase in volume throughout their pregnancy. In women who were noted to have an increase in the volume of their myoma, the greatest growth occurred before 10 weeks’ gestation. No relationship was found between initial myoma volume and myoma growth during the gestational period. 14
Do myomas complicate pregnancy?
Very rarely. Two studies reported on outcomes in large populations of pregnant women who were examined with routine second-trimester ultrasonography, with follow-up and delivery at the same institution.
In one of those studies, 12,600 pregnant women were evaluated, and the outcome in 167 women who were given a diagnosis of myoma was compared with the outcome in women who did not have a myoma.15 Despite similar clinical management between the two groups, no significant differences were seen in regard to the incidence of:
- preterm delivery
- premature rupture of membranes
- fetal growth restriction
- placenta previa
- placental abruption
- postpartum hemorrhage
- retained placenta.
Only cesarean section was more common among women with fibroids (23% vs 12%).
The second study reviewed 15,104 pregnancies and compared 401 women found to have myomas and the remaining women who did not.16 Although the presence of myoma did not increase the risk of premature rupture of membranes, operative vaginal delivery, chorioamnionitis, or endomyometritis, there was some increased risk of pre-term delivery (19.2% vs 12.7%), placenta previa (3.5% vs 1.8%), and post-partum hemorrhage (8.3% vs 2.9%). Cesarean section was, again, more common (49.1% vs 21.4%).
Do myomas injure the fetus?
Fetal injury as a consequence of fibroids has been reported very infrequently. A review of the literature from 1980 to 2005 revealed only four cases—one each of:
- fetal head anomalies with fetal growth restriction
- postural deformity
- limb reduction
- fetal head deformation with torticollis.17-19
CASE 2 RESOLVED Should Mrs. H. have a myomectomy?
Probably not. Abdominal and laparoscopic myomectomy involve substantial operative and anesthetic risks, including infection, postoperative adhesions, a very small risk of uterine rupture during pregnancy, and increased likelihood of cesarean section. Costs are also substantial, involving not only the expense of surgery, but also patient discomfort and time for recovery. Therefore, until it is proved that intramural myomas decrease fertility and myomectomy increases fertility, surgery should be undertaken with caution. As far as the effects of myoma on pregnancy are concerned, no data are available by which to compare pregnancy outcomes following myomectomy with pregnancy outcomes in women whose myomas are untreated. Randomized studies are needed to clarify these important issues.
CASES 3 & 4 The effects of oral contraceptives and hormone replacement therapy
Mrs. J. is a 32-year-old G0P0 woman who has a 5-cm fundal myoma. She is sexually active and wants to use an oral contraceptive (OC). She has heard from friends, however, that taking an OC makes fibroids grow, and she asks for your advice.
The same day, you see Mrs. K., a 54-year-old, recently menopausal woman. She complains of severe hot flashes and night sweats that disturb her sleep. She has had asymptomatic uterine fibroids for about 10 years and, although she would like to take menopausal hormone therapy, she is worried that the medication will make the fibroids larger.
How do you advise these two women?
OCs. OCs do not appear to influence the growth of fibroids. One study found a slightly increased risk of fibroids, another study found no increased risk, and a third found a decreased risk.18,19 These studies are retrospective, however, and may be marked by selection bias.
Postmenopausal hormone replacement therapy. Postmenopausal hormone therapy does not ordinarily cause fibroid growth. After 3 years, only three of 34 (8%) post-menopausal women who had fibroids and were treated with 0.625 mg of conjugated equine estrogen (CEE) and 5 mg of medroxyprogesterone acetate (MPA) a day had any increase in the size of fibroids.20 If any increase in the size of the uterus is noted, it is likely related to progestins.
A study found that 23% of women taking oral estrogen plus 2.5 mg of MPA a day for 1 year had a slight increase in the size of fibroids, whereas 50% of women taking 5 mg of MPA had an increase in size (mean increase in diameter, 3.2 cm).21 Transdermal estrogen plus oral MPA was shown, after 1 year, to cause, on average, a 0.5-cm increase in the diameter of fibroids; oral estrogen and MPA caused no increase in size.22
CASES RESOLVED Rx: Reassurance
Advise Mrs. J. that taking an OC is unlikely to make her fibroids grow larger.
Mrs. K., who is older, can seek relief from postmenopausal symptoms by taking hormone therapy without fear of her fibroids being stimulated to grow.
1. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900-906.
2. Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037-1054.
3. Parker W. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725-736.
4. Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97-108.
5. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
6. Boutselis JG, Ullery JC. Sarcoma of the uterus. Obstet Gynecol. 1962;20:23-35.
7. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204-208.
8. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12:354-361.
9. Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483-491.
10. Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76:350-357.
11. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:388-403.
12. Cohen LS, Valle RF. Role of vaginal sonography and hysterosonography in the endoscopic treatment of uterine myomas. Fertil Steril. 2000;73:197-204.
13. Cooper NP, Okolo S. Fibroids in pregnancy—common but poorly understood. Obstet Gynecol Surv. 2005;60:132-138.
14. Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med. 1992;11:511-515.
15. Vergani P, Ghidini A, Strobelt N, et al. Do uterine leiomyomas influence pregnancy outcome? Am J Perinatol. 1994;11:356-358.
16. Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376-382.
17. Joo JG, Inovay J, Silhavy M, Papp Z. Successful enucleation of a necrotizing fibroid causing oligohydramnios and fetal postural deformity in the 25th week of gestation. A case report. J Reprod Med. 2001;46:923-925.
18. Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:359-362.
19. Ratner H. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:1027.-
20. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas. 2002;43:35-39.
21. Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2002;102:199-201.
22. Sener AB, Seçkin NC, Ozmen S, Gökmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354-357.
1. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900-906.
2. Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037-1054.
3. Parker W. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725-736.
4. Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97-108.
5. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
6. Boutselis JG, Ullery JC. Sarcoma of the uterus. Obstet Gynecol. 1962;20:23-35.
7. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204-208.
8. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12:354-361.
9. Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483-491.
10. Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76:350-357.
11. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:388-403.
12. Cohen LS, Valle RF. Role of vaginal sonography and hysterosonography in the endoscopic treatment of uterine myomas. Fertil Steril. 2000;73:197-204.
13. Cooper NP, Okolo S. Fibroids in pregnancy—common but poorly understood. Obstet Gynecol Surv. 2005;60:132-138.
14. Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med. 1992;11:511-515.
15. Vergani P, Ghidini A, Strobelt N, et al. Do uterine leiomyomas influence pregnancy outcome? Am J Perinatol. 1994;11:356-358.
16. Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376-382.
17. Joo JG, Inovay J, Silhavy M, Papp Z. Successful enucleation of a necrotizing fibroid causing oligohydramnios and fetal postural deformity in the 25th week of gestation. A case report. J Reprod Med. 2001;46:923-925.
18. Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:359-362.
19. Ratner H. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293:1027.-
20. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas. 2002;43:35-39.
21. Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2002;102:199-201.
22. Sener AB, Seçkin NC, Ozmen S, Gökmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354-357.
IN THIS ARTICLE