User login
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
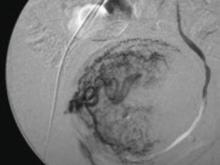
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
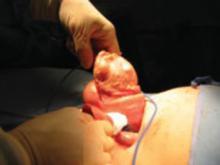
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.