User login
CASE: Hysterectomy candidate asks about her ovaries
A 51-year-old premenopausal woman complains of severe menorrhagia that often causes her to miss work. Although she is taking an iron supplement, her hemoglobin level often drops below 10 g/dL. She has already been identified as having fibroids, with a uterine size of 14 weeks. You order ultra-sonography, which reveals an enlarged uterus with multiple fibroids and normal endometrial thickness, but no intracavitary lesions.
After you describe the treatment options, including uterine artery embolization, the patient requests a hysterectomy as a reasonably low-risk means of cure. During informed consent, she asks whether she should have her ovaries removed during the surgery. Further discussion reveals that her father died of a myocardial infarction when he was 64 years old, but there is no family or personal history of ovarian or breast cancer.
How do you advise this patient, based on her history and recent findings from medical research?
Many gynecologists have been trained to recommend bilateral oophorectomy for women older than 45 or 50 years who request a hysterectomy for benign disease. In these women, oophorectomy is recommended to prevent ovarian cancer and avert the potential for other ovarian pathology that might require later surgery.
In the United States, 78% of women 45 to 64 years old and 55% of women overall undergo bilateral oophorectomy at the time of hysterectomy.1 These percentages mean that almost 300,000 women undergo bilateral oophorectomy each year.1
Hysterectomy alone can sometimes lead to early ovarian failure, but this phenomenon is infrequent. A prospective study of premenopausal women found that, after 5 years of follow-up, 20% of women who underwent simple hysterectomy reached menopause, compared with 7% of matched women who did not undergo hysterectomy.2
In this article, I explore the risks and benefits associated with bilateral oophorectomy and present an algorithm to aid in deciding whether the patient should keep her ovaries—and when oophorectomy might be a better option ( FIGURE ).
Among the hazards associated with bilateral oophorectomy are:
- an increased risk of death from coronary artery disease (CAD), lung cancer, all cancers (except ovarian), and all causes3,4
- an increased risk of osteoporosis and hip fracture5
- when performed before the onset of menopause, an increased risk of parkinsonism, cognitive impairment, dementia, anxiety, and depression.6-8
Benefits include a reduced risk of ovarian cancer, particularly among women who have a BRCA gene mutation or strong family history of ovarian or breast cancer.
Although ovarian cancer causes 15,000 deaths each year in the United States, that figure pales when compared with heart disease, which accounts for 350,000 deaths. In addition, hip fracture may cause approximately 66,000 deaths each year, and dementia attributable to bilateral oophorectomy may affect 100,000 to 200,000 women.9 Reoperation for adnexal pathology or pain after hysterectomy is rare, occurring in only 2.8% of women. Therefore, the benefits of oophorectomy are often outweighed by the risks of CAD, hip fracture, and neurologic conditions.
The assumption that medical treatment will ameliorate the risks associated with oophorectomy is unrealistic. Estrogen may mitigate some risks, but many women avoid hormone therapy. This avoidance can be especially problematic in young women.
In the Nurses’ Health Study, a separate analysis focused on women who had never used postmenopausal hormone therapy.4 In this analysis, all women who underwent bilateral oophorectomy had a greater risk of stroke (HR, 1.85; 95% CI, 1.09, 3.16) and lung cancer (HR, 2.09; 95% CI, 1.01, 4.33) than did women who retained their ovaries. Among women who underwent oophorectomy before age 50 and who did not take estrogen, the risk of coronary artery disease (CAD) was higher (HR, 1.98; 95% CI, 1.18, 3.32), as was the risk of death from all causes (HR, 1.40; 95% CI, 1.01, 1.96), compared with women who retained their ovaries.
Despite estrogen’s proven benefit among oophorectomized women, usage rates continue to decline. In the 6 months after publication of Women’s Health Initiative findings on estrogen–progestin therapy, the continuation rate of estrogen therapy decreased from 12.6% to 9.1%, and new starts also declined significantly.47
Among women who had a diagnosis of osteoporosis and who began treatment with estrogen, estrogen plus progestin, a bisphosphonate, or raloxifene, medication continuation rates were less than 25% at 12 months.48 Moreover, only 18% of women started on a statin to reduce the risk of cardiovascular disease were still taking the drug after 1 year.49
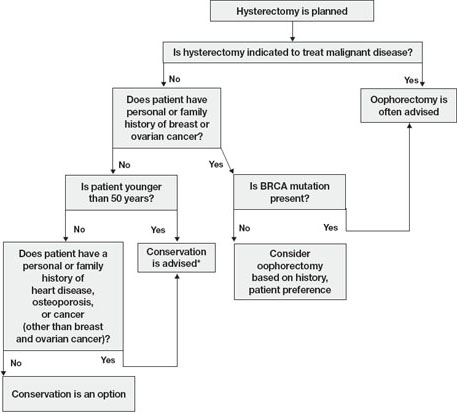
FIGURE Conservation vs oophorectomy: A guide to decision-making
* Estrogen replacement is recommended for women younger than 45 years who opt for oophorectomy
Ovarian cancer is a real, but relatively low, risk
In 2008, an estimated 21,650 new cases of ovarian cancer were diagnosed (age at diagnosis: mean, 63 years), and 15,520 women died from the disease.10 Because we lack a reliable screening test to detect early-stage ovarian cancer in the general population, most women are given a diagnosis when disease is advanced and the 5-year survival rate is 15% to 25%.
There is agreement that women who are known to have a BRCA mutation, which increases the risk of ovarian and breast cancer, should strongly consider oophorectomy once childbearing is complete.11 In the general population, however, the outlook is different.
In the United States, the lifetime risk of ovarian cancer is 1.4% overall. Among white women who have had three or more term pregnancies and who have used an oral contraceptive for at least 4 years, the lifetime risk of ovarian cancer drops to 0.3%.12
Need for reoperation is very low
The percentage of women who require reoperation after ovarian conservation—2.8%—may surprise you.13 That figure is lower than once thought because many studies were performed before asymptomatic, benign ovarian cysts were determined to be a fairly common phenomenon in postmenopausal women (prevalence, 6.6%). These cysts do not undergo transformation to cancer and, therefore, do not need to be removed.14
In addition, studies indicate that only 0.1% to 0.75% of women who retain their ovaries at the time of hysterectomy develop ovarian cancer.15,16 Therefore, the rationale of performing oophorectomy to avoid future surgery appears to be unfounded.
CAD risk rises sharply after oophorectomy
A recent systematic review found mixed evidence concerning the risk of CAD following bilateral salpingo-oophorectomy.17 In observational studies, however, earlier age of surgical or natural menopause has been associated with a higher risk of cardiovascular mortality.18-20 Early reports from the Nurses’ Health Study found that the risk of myocardial infarction doubled among women who underwent oophorectomy and never used estrogen, compared with age-matched premenopausal women (relative risk [RR], 2.2; 95% confidence interval [CI], 1.2, 4.2).3 Even after age 50, the risk of a first myocardial infarction is increased among oophorectomized women, compared with women who retain their ovaries (RR, 1.4; 95% CI, 1.0–2.0).21
A study by researchers from the Mayo Clinic, who examined all causes listed on the death certificate, found a significant association between bilateral oophorectomy before the age of 45 years and cardiovascular mortality (hazard ratio [HR], 1.44; 95% CI, 1.01–2.05).22 This risk was significantly increased among women who were not treated with estrogen through at least age 45, compared with estrogen-treated women.
Oophorectomy may impair bone health
After menopause, ovaries continue to produce significant amounts of the androgens testosterone and androstenedione, which are converted to estrone peripherally by skin, muscle, and fat cells.23,24 The levels of these hormones remain consistent and have been documented to age 80.25
Both estrogens and androgens inhibit bone resorption, and androgens also stimulate bone formation.26 Low levels of androgens and estrogens are linked to lower bone density and a higher risk of hip and vertebral fracture in postmenopausal women.27-29
Postmenopausal women who have been oophorectomized may have an even greater risk of osteoporosis. Over 16 years of follow-up, 340 women who had undergone oophorectomy at a median age of 62 years had 54% more osteoporotic fractures than women who had intact ovaries.5 Two other studies found no association between oophorectomy and bone loss or fracture risk, however.30,31
Hip fracture is a well-documented cause of increased morbidity and mortality in older women. One study found that, before hip fracture, 28% of patients were housebound; 1 year after hip fracture, the percentage was 46%.32 Women older than 60 who underwent oophorectomy had a doubled risk of mortality after low-trauma hip fracture, compared with women who had intact ovaries (odds ratio [OR], 2.18; 95% CI, 2.03–2.32).5
Loss of ovaries may affect mental health and sexuality
In a premenopausal woman, oophorectomy causes a sudden loss of estrogen and often triggers hot flashes, mood changes, sleep disturbances, headaches, and a decline in feelings of well-being.33,34 Over time, vaginal dryness, painful intercourse, loss of libido, bladder dysfunction, and depression may occur.35,36
Evidence suggests that, in women, sexual desire, sexual sensation, and orgasmic response are influenced by androgens. After elective oophorectomy, declines in sexual desire have been reported.37-39
Mental health and sexuality may rebound over time, however. One study found less improvement in mental health measures and body image 6 months after hysterectomy among women who were oophorectomized, compared with those who retained their ovaries. After 2 years, improvement levels were similar between groups.40
Cognitive function may suffer
Analysis of data from the Mayo Clinic Cohort Study of Oophorectomy and Aging found that bilateral oophorectomy before the onset of menopause increased the risk of parkinsonism, cognitive impairment or dementia, and anxiety or depression, particularly if estrogen was not replaced.6-8 These risks increased with younger age at oophorectomy.
The Women’s Health Initiative found an increased risk of dementia or mild cognitive impairment in women who were treated by estrogen alone or estrogen plus progestin after age 65.41-44
These disparate conclusions suggest that estrogen may have a protective effect on the brain if it is given right after natural menopause or premenopausal oophorectomy, but deleterious effects if it is started years later.45
Other studies of endogenous estrogen and cognitive function are few and yield inconsistent results.
Ovarian conservation boosts long-term survival
When there is no family history of ovarian cancer, ovarian conservation appears to maximize survival among healthy women 40 to 65 years old who undergo hysterectomy for benign disease.46 Among healthy women hysterectomized before the age of 55, calculations suggest that 8.6% more would be alive at age 80 if their ovaries were conserved than if they were removed.46
A study from the Mayo Clinic found that all-cause mortality was significantly higher among women who underwent prophylactic bilateral oophorectomy before the age of 45 than it was among women in the control group (HR, 1.67; 95% CI, 1.16–2.40); it was particularly high in women who did not receive estrogen treatment before age 45 (HR, 1.93; 95% CI, 1.25–2.96).22
In a recent study, investigators used the Nurses’ Health Study database to explore the long-term health outcomes of 29,380 women who underwent hysterectomy.4 Of these women, 13,035 (44.4%) had their ovaries conserved, and 16,345 (55.6%) underwent bilateral oophorectomy. Follow-up was 24 years. Oophorectomy was associated with an increased risk of nonfatal CAD among all women (HR, 1.17; 95% CI, 1.02, 1.35), especially those who underwent the procedure before age 45 (HR, 1.26; 95% CI, 1.04, 1.54). Oophorectomy was associated with a markedly reduced risk of ovarian cancer but an increased risk of lung cancer (HR, 1.26; 95% CI, 1.02–1.56).
In regard to fatal events, oophorectomy increased the risk of death from all causes (HR, 1.12; 95% CI, 1.03, 1.21). Specifically, there was an increased risk of death from CAD (HR, 1.28; 95% CI, 1.00, 1.64), lung cancer (HR, 1.31; 95% CI, 1.02, 1.68), and all cancers (HR, 1.17; 95% CI, 1.04, 1.32). There was no overall difference in the risk of death from stroke, breast cancer, and colorectal cancer between women who underwent oophorectomy and those who retained their ovaries.
During the 24 years of follow-up, 37 women died from ovarian cancer, accounting for 1.2% of all deaths. At no age did oophorectomy show a survival benefit.
How this evidence should inform your practice
It is unfortunate that the entire body of evidence on the risks and benefits of bilateral salpingo-oophorectomy consists of observational studies, which have significant inherent limitations. Although the Nurses’ Health Study was the largest prospective study to examine the effect of oophorectomy on women’s health, and involved the longest follow-up, the study was observational, and oophorectomy and ovarian conservation were self-selected. Nevertheless, recent data suggest that a more detailed informed-consent process is warranted than the process in place. Informed consent should cover the risks and benefits of both oophorectomy and ovarian conservation.
Prophylactic oophorectomy is recommended only if a preponderance of the evidence establishes that it clearly benefits the patient. The studies described in this article suggest that bilateral oophorectomy does harm more often than it does good. Therefore, a cautious approach to oophorectomy at the time of hysterectomy is advised.
CASE RESOLVED
After you describe the risks and benefits of oophorectomy, and address the patient’s concerns about her family history of heart disease, she decides to keep her ovaries.
1. Healthcare Cost and Utilization Project (HCUP), 1988–2001: A Federal–State–Industry Partnership in Health Data. Rockville, Md: Agency for Healthcare Research and Quality; July 2003.
2. Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962.
3. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105-1110.
4. Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027-1037.
5. Melton LJ 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900-905.
6. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074.-
7. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200-209.
8. Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050-1059.
9. Bennett DA. Editorial comment on “Prevalence of dementia in the United States: the aging, demographics, and memory study” by Plassman et al. Neuroepidemiology. 2007;29:133-135.
10. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
11. Armstrong K, Schwartz J, Randall T, Rubin S, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
12. Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84:760-764.
13. Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68:159-164.
14. Bailey CL, Ueland FR, Land GL, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998;69:3-7.
15. Naylor AC. Hysterectomy—analysis of 2,901 personally performed procedures. S Afr Med J. 1984;65:242-245.
16. Christ JE, Lotze EC. The residual ovary syndrome. Obstet Gynecol. 1975;46:551-556.
17. Jacoby VL, Grady D, Sawaya GF. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol. 2009;200:140.e1-140.e9.
18. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714-718.
19. Løkkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226-233.
20. de Kleijn MJJ, van der Schouw YT, Verbeek ALM, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339.-
21. Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53:832-837.
22. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821-828.
23. Judd H, Judd G, Lucas W, Yen S. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020-1024.
24. Fogle R, Stanczyk F, Zhang X, Paulson R. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040-3043.
25. Meldrum D, Davidson B, Tataryn I, Judd H. Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol. 1981;57:624-628.
26. Raisz LG, Wilta B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
27. Johansson C, Mellstrom D, Milsom I. Reproductive factors as predictors of bone density and fractures in women at age 70. Maturitas. 1993;17:39-50.
28. Davidson BJ, Ross RK, Paganini-Hill A, et al. Total and free estrogens and androgens in postmenopausal women with hip fractures. J Clin Endocrinol Metab. 1982;54:115-120.
29. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733-738.
30. Kritz-Silverstein D, von Mühlen D, Barrett-Connor E. Hysterectomy and oophorectomy are unrelated to bone loss in older women. Maturitas. 2004;47:61-69.
31. Antoniucci DM, Sellmeyer DE, Cauley JA, et al. Postmenopausal bilateral oophorectomy is not associated with increased fracture risk in older women. J Bone Miner Res. 2005;20:741-747.
32. Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248-1250.
33. Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy—effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283-293.
34. Nieman L. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
35. Shifren J. Androgen deficiency in the oophorectomized woman. Fertil Steril. 2002;77 Suppl 4:s60-s62.
36. Sherwin B, Gelfan M. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1978;49:397-409.
37. Shifren JL, Avis NE. Surgical menopause: effects on psychological well-being and sexuality. Menopause. 2007;14:586.-
38. Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149-156.
39. Sherwin BB, Gelfan MM. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1978;49:397-409.
40. Teplin V, Vittinghoff E, Lin F, Learman L, Richter H, Kuppermann M. Oophorectomy in premenopausal women: health-related quality of life and sexual functioning. Obstet Gynecol. 2007;109:347-354.
41. Shumaker SA, Legault C, Rapp SR, et al. WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
42. Rapp SR, Espeland MA, Shumaker SA, et al. WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663-2672.
43. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959-2968.
44. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
45. Siegfried T. Neuroscience: it’s all in the timing. Nature. 2007;445:359-361.
46. Parker W, Broder M, Liu Z, Shoupe D, Farquhar C, Berek J. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
47. Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042-1050.
48. McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271-287.
49. Huser MA, Evans TS, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22:163-171.
CASE: Hysterectomy candidate asks about her ovaries
A 51-year-old premenopausal woman complains of severe menorrhagia that often causes her to miss work. Although she is taking an iron supplement, her hemoglobin level often drops below 10 g/dL. She has already been identified as having fibroids, with a uterine size of 14 weeks. You order ultra-sonography, which reveals an enlarged uterus with multiple fibroids and normal endometrial thickness, but no intracavitary lesions.
After you describe the treatment options, including uterine artery embolization, the patient requests a hysterectomy as a reasonably low-risk means of cure. During informed consent, she asks whether she should have her ovaries removed during the surgery. Further discussion reveals that her father died of a myocardial infarction when he was 64 years old, but there is no family or personal history of ovarian or breast cancer.
How do you advise this patient, based on her history and recent findings from medical research?
Many gynecologists have been trained to recommend bilateral oophorectomy for women older than 45 or 50 years who request a hysterectomy for benign disease. In these women, oophorectomy is recommended to prevent ovarian cancer and avert the potential for other ovarian pathology that might require later surgery.
In the United States, 78% of women 45 to 64 years old and 55% of women overall undergo bilateral oophorectomy at the time of hysterectomy.1 These percentages mean that almost 300,000 women undergo bilateral oophorectomy each year.1
Hysterectomy alone can sometimes lead to early ovarian failure, but this phenomenon is infrequent. A prospective study of premenopausal women found that, after 5 years of follow-up, 20% of women who underwent simple hysterectomy reached menopause, compared with 7% of matched women who did not undergo hysterectomy.2
In this article, I explore the risks and benefits associated with bilateral oophorectomy and present an algorithm to aid in deciding whether the patient should keep her ovaries—and when oophorectomy might be a better option ( FIGURE ).
Among the hazards associated with bilateral oophorectomy are:
- an increased risk of death from coronary artery disease (CAD), lung cancer, all cancers (except ovarian), and all causes3,4
- an increased risk of osteoporosis and hip fracture5
- when performed before the onset of menopause, an increased risk of parkinsonism, cognitive impairment, dementia, anxiety, and depression.6-8
Benefits include a reduced risk of ovarian cancer, particularly among women who have a BRCA gene mutation or strong family history of ovarian or breast cancer.
Although ovarian cancer causes 15,000 deaths each year in the United States, that figure pales when compared with heart disease, which accounts for 350,000 deaths. In addition, hip fracture may cause approximately 66,000 deaths each year, and dementia attributable to bilateral oophorectomy may affect 100,000 to 200,000 women.9 Reoperation for adnexal pathology or pain after hysterectomy is rare, occurring in only 2.8% of women. Therefore, the benefits of oophorectomy are often outweighed by the risks of CAD, hip fracture, and neurologic conditions.
The assumption that medical treatment will ameliorate the risks associated with oophorectomy is unrealistic. Estrogen may mitigate some risks, but many women avoid hormone therapy. This avoidance can be especially problematic in young women.
In the Nurses’ Health Study, a separate analysis focused on women who had never used postmenopausal hormone therapy.4 In this analysis, all women who underwent bilateral oophorectomy had a greater risk of stroke (HR, 1.85; 95% CI, 1.09, 3.16) and lung cancer (HR, 2.09; 95% CI, 1.01, 4.33) than did women who retained their ovaries. Among women who underwent oophorectomy before age 50 and who did not take estrogen, the risk of coronary artery disease (CAD) was higher (HR, 1.98; 95% CI, 1.18, 3.32), as was the risk of death from all causes (HR, 1.40; 95% CI, 1.01, 1.96), compared with women who retained their ovaries.
Despite estrogen’s proven benefit among oophorectomized women, usage rates continue to decline. In the 6 months after publication of Women’s Health Initiative findings on estrogen–progestin therapy, the continuation rate of estrogen therapy decreased from 12.6% to 9.1%, and new starts also declined significantly.47
Among women who had a diagnosis of osteoporosis and who began treatment with estrogen, estrogen plus progestin, a bisphosphonate, or raloxifene, medication continuation rates were less than 25% at 12 months.48 Moreover, only 18% of women started on a statin to reduce the risk of cardiovascular disease were still taking the drug after 1 year.49

FIGURE Conservation vs oophorectomy: A guide to decision-making
* Estrogen replacement is recommended for women younger than 45 years who opt for oophorectomy
Ovarian cancer is a real, but relatively low, risk
In 2008, an estimated 21,650 new cases of ovarian cancer were diagnosed (age at diagnosis: mean, 63 years), and 15,520 women died from the disease.10 Because we lack a reliable screening test to detect early-stage ovarian cancer in the general population, most women are given a diagnosis when disease is advanced and the 5-year survival rate is 15% to 25%.
There is agreement that women who are known to have a BRCA mutation, which increases the risk of ovarian and breast cancer, should strongly consider oophorectomy once childbearing is complete.11 In the general population, however, the outlook is different.
In the United States, the lifetime risk of ovarian cancer is 1.4% overall. Among white women who have had three or more term pregnancies and who have used an oral contraceptive for at least 4 years, the lifetime risk of ovarian cancer drops to 0.3%.12
Need for reoperation is very low
The percentage of women who require reoperation after ovarian conservation—2.8%—may surprise you.13 That figure is lower than once thought because many studies were performed before asymptomatic, benign ovarian cysts were determined to be a fairly common phenomenon in postmenopausal women (prevalence, 6.6%). These cysts do not undergo transformation to cancer and, therefore, do not need to be removed.14
In addition, studies indicate that only 0.1% to 0.75% of women who retain their ovaries at the time of hysterectomy develop ovarian cancer.15,16 Therefore, the rationale of performing oophorectomy to avoid future surgery appears to be unfounded.
CAD risk rises sharply after oophorectomy
A recent systematic review found mixed evidence concerning the risk of CAD following bilateral salpingo-oophorectomy.17 In observational studies, however, earlier age of surgical or natural menopause has been associated with a higher risk of cardiovascular mortality.18-20 Early reports from the Nurses’ Health Study found that the risk of myocardial infarction doubled among women who underwent oophorectomy and never used estrogen, compared with age-matched premenopausal women (relative risk [RR], 2.2; 95% confidence interval [CI], 1.2, 4.2).3 Even after age 50, the risk of a first myocardial infarction is increased among oophorectomized women, compared with women who retain their ovaries (RR, 1.4; 95% CI, 1.0–2.0).21
A study by researchers from the Mayo Clinic, who examined all causes listed on the death certificate, found a significant association between bilateral oophorectomy before the age of 45 years and cardiovascular mortality (hazard ratio [HR], 1.44; 95% CI, 1.01–2.05).22 This risk was significantly increased among women who were not treated with estrogen through at least age 45, compared with estrogen-treated women.
Oophorectomy may impair bone health
After menopause, ovaries continue to produce significant amounts of the androgens testosterone and androstenedione, which are converted to estrone peripherally by skin, muscle, and fat cells.23,24 The levels of these hormones remain consistent and have been documented to age 80.25
Both estrogens and androgens inhibit bone resorption, and androgens also stimulate bone formation.26 Low levels of androgens and estrogens are linked to lower bone density and a higher risk of hip and vertebral fracture in postmenopausal women.27-29
Postmenopausal women who have been oophorectomized may have an even greater risk of osteoporosis. Over 16 years of follow-up, 340 women who had undergone oophorectomy at a median age of 62 years had 54% more osteoporotic fractures than women who had intact ovaries.5 Two other studies found no association between oophorectomy and bone loss or fracture risk, however.30,31
Hip fracture is a well-documented cause of increased morbidity and mortality in older women. One study found that, before hip fracture, 28% of patients were housebound; 1 year after hip fracture, the percentage was 46%.32 Women older than 60 who underwent oophorectomy had a doubled risk of mortality after low-trauma hip fracture, compared with women who had intact ovaries (odds ratio [OR], 2.18; 95% CI, 2.03–2.32).5
Loss of ovaries may affect mental health and sexuality
In a premenopausal woman, oophorectomy causes a sudden loss of estrogen and often triggers hot flashes, mood changes, sleep disturbances, headaches, and a decline in feelings of well-being.33,34 Over time, vaginal dryness, painful intercourse, loss of libido, bladder dysfunction, and depression may occur.35,36
Evidence suggests that, in women, sexual desire, sexual sensation, and orgasmic response are influenced by androgens. After elective oophorectomy, declines in sexual desire have been reported.37-39
Mental health and sexuality may rebound over time, however. One study found less improvement in mental health measures and body image 6 months after hysterectomy among women who were oophorectomized, compared with those who retained their ovaries. After 2 years, improvement levels were similar between groups.40
Cognitive function may suffer
Analysis of data from the Mayo Clinic Cohort Study of Oophorectomy and Aging found that bilateral oophorectomy before the onset of menopause increased the risk of parkinsonism, cognitive impairment or dementia, and anxiety or depression, particularly if estrogen was not replaced.6-8 These risks increased with younger age at oophorectomy.
The Women’s Health Initiative found an increased risk of dementia or mild cognitive impairment in women who were treated by estrogen alone or estrogen plus progestin after age 65.41-44
These disparate conclusions suggest that estrogen may have a protective effect on the brain if it is given right after natural menopause or premenopausal oophorectomy, but deleterious effects if it is started years later.45
Other studies of endogenous estrogen and cognitive function are few and yield inconsistent results.
Ovarian conservation boosts long-term survival
When there is no family history of ovarian cancer, ovarian conservation appears to maximize survival among healthy women 40 to 65 years old who undergo hysterectomy for benign disease.46 Among healthy women hysterectomized before the age of 55, calculations suggest that 8.6% more would be alive at age 80 if their ovaries were conserved than if they were removed.46
A study from the Mayo Clinic found that all-cause mortality was significantly higher among women who underwent prophylactic bilateral oophorectomy before the age of 45 than it was among women in the control group (HR, 1.67; 95% CI, 1.16–2.40); it was particularly high in women who did not receive estrogen treatment before age 45 (HR, 1.93; 95% CI, 1.25–2.96).22
In a recent study, investigators used the Nurses’ Health Study database to explore the long-term health outcomes of 29,380 women who underwent hysterectomy.4 Of these women, 13,035 (44.4%) had their ovaries conserved, and 16,345 (55.6%) underwent bilateral oophorectomy. Follow-up was 24 years. Oophorectomy was associated with an increased risk of nonfatal CAD among all women (HR, 1.17; 95% CI, 1.02, 1.35), especially those who underwent the procedure before age 45 (HR, 1.26; 95% CI, 1.04, 1.54). Oophorectomy was associated with a markedly reduced risk of ovarian cancer but an increased risk of lung cancer (HR, 1.26; 95% CI, 1.02–1.56).
In regard to fatal events, oophorectomy increased the risk of death from all causes (HR, 1.12; 95% CI, 1.03, 1.21). Specifically, there was an increased risk of death from CAD (HR, 1.28; 95% CI, 1.00, 1.64), lung cancer (HR, 1.31; 95% CI, 1.02, 1.68), and all cancers (HR, 1.17; 95% CI, 1.04, 1.32). There was no overall difference in the risk of death from stroke, breast cancer, and colorectal cancer between women who underwent oophorectomy and those who retained their ovaries.
During the 24 years of follow-up, 37 women died from ovarian cancer, accounting for 1.2% of all deaths. At no age did oophorectomy show a survival benefit.
How this evidence should inform your practice
It is unfortunate that the entire body of evidence on the risks and benefits of bilateral salpingo-oophorectomy consists of observational studies, which have significant inherent limitations. Although the Nurses’ Health Study was the largest prospective study to examine the effect of oophorectomy on women’s health, and involved the longest follow-up, the study was observational, and oophorectomy and ovarian conservation were self-selected. Nevertheless, recent data suggest that a more detailed informed-consent process is warranted than the process in place. Informed consent should cover the risks and benefits of both oophorectomy and ovarian conservation.
Prophylactic oophorectomy is recommended only if a preponderance of the evidence establishes that it clearly benefits the patient. The studies described in this article suggest that bilateral oophorectomy does harm more often than it does good. Therefore, a cautious approach to oophorectomy at the time of hysterectomy is advised.
CASE RESOLVED
After you describe the risks and benefits of oophorectomy, and address the patient’s concerns about her family history of heart disease, she decides to keep her ovaries.
CASE: Hysterectomy candidate asks about her ovaries
A 51-year-old premenopausal woman complains of severe menorrhagia that often causes her to miss work. Although she is taking an iron supplement, her hemoglobin level often drops below 10 g/dL. She has already been identified as having fibroids, with a uterine size of 14 weeks. You order ultra-sonography, which reveals an enlarged uterus with multiple fibroids and normal endometrial thickness, but no intracavitary lesions.
After you describe the treatment options, including uterine artery embolization, the patient requests a hysterectomy as a reasonably low-risk means of cure. During informed consent, she asks whether she should have her ovaries removed during the surgery. Further discussion reveals that her father died of a myocardial infarction when he was 64 years old, but there is no family or personal history of ovarian or breast cancer.
How do you advise this patient, based on her history and recent findings from medical research?
Many gynecologists have been trained to recommend bilateral oophorectomy for women older than 45 or 50 years who request a hysterectomy for benign disease. In these women, oophorectomy is recommended to prevent ovarian cancer and avert the potential for other ovarian pathology that might require later surgery.
In the United States, 78% of women 45 to 64 years old and 55% of women overall undergo bilateral oophorectomy at the time of hysterectomy.1 These percentages mean that almost 300,000 women undergo bilateral oophorectomy each year.1
Hysterectomy alone can sometimes lead to early ovarian failure, but this phenomenon is infrequent. A prospective study of premenopausal women found that, after 5 years of follow-up, 20% of women who underwent simple hysterectomy reached menopause, compared with 7% of matched women who did not undergo hysterectomy.2
In this article, I explore the risks and benefits associated with bilateral oophorectomy and present an algorithm to aid in deciding whether the patient should keep her ovaries—and when oophorectomy might be a better option ( FIGURE ).
Among the hazards associated with bilateral oophorectomy are:
- an increased risk of death from coronary artery disease (CAD), lung cancer, all cancers (except ovarian), and all causes3,4
- an increased risk of osteoporosis and hip fracture5
- when performed before the onset of menopause, an increased risk of parkinsonism, cognitive impairment, dementia, anxiety, and depression.6-8
Benefits include a reduced risk of ovarian cancer, particularly among women who have a BRCA gene mutation or strong family history of ovarian or breast cancer.
Although ovarian cancer causes 15,000 deaths each year in the United States, that figure pales when compared with heart disease, which accounts for 350,000 deaths. In addition, hip fracture may cause approximately 66,000 deaths each year, and dementia attributable to bilateral oophorectomy may affect 100,000 to 200,000 women.9 Reoperation for adnexal pathology or pain after hysterectomy is rare, occurring in only 2.8% of women. Therefore, the benefits of oophorectomy are often outweighed by the risks of CAD, hip fracture, and neurologic conditions.
The assumption that medical treatment will ameliorate the risks associated with oophorectomy is unrealistic. Estrogen may mitigate some risks, but many women avoid hormone therapy. This avoidance can be especially problematic in young women.
In the Nurses’ Health Study, a separate analysis focused on women who had never used postmenopausal hormone therapy.4 In this analysis, all women who underwent bilateral oophorectomy had a greater risk of stroke (HR, 1.85; 95% CI, 1.09, 3.16) and lung cancer (HR, 2.09; 95% CI, 1.01, 4.33) than did women who retained their ovaries. Among women who underwent oophorectomy before age 50 and who did not take estrogen, the risk of coronary artery disease (CAD) was higher (HR, 1.98; 95% CI, 1.18, 3.32), as was the risk of death from all causes (HR, 1.40; 95% CI, 1.01, 1.96), compared with women who retained their ovaries.
Despite estrogen’s proven benefit among oophorectomized women, usage rates continue to decline. In the 6 months after publication of Women’s Health Initiative findings on estrogen–progestin therapy, the continuation rate of estrogen therapy decreased from 12.6% to 9.1%, and new starts also declined significantly.47
Among women who had a diagnosis of osteoporosis and who began treatment with estrogen, estrogen plus progestin, a bisphosphonate, or raloxifene, medication continuation rates were less than 25% at 12 months.48 Moreover, only 18% of women started on a statin to reduce the risk of cardiovascular disease were still taking the drug after 1 year.49

FIGURE Conservation vs oophorectomy: A guide to decision-making
* Estrogen replacement is recommended for women younger than 45 years who opt for oophorectomy
Ovarian cancer is a real, but relatively low, risk
In 2008, an estimated 21,650 new cases of ovarian cancer were diagnosed (age at diagnosis: mean, 63 years), and 15,520 women died from the disease.10 Because we lack a reliable screening test to detect early-stage ovarian cancer in the general population, most women are given a diagnosis when disease is advanced and the 5-year survival rate is 15% to 25%.
There is agreement that women who are known to have a BRCA mutation, which increases the risk of ovarian and breast cancer, should strongly consider oophorectomy once childbearing is complete.11 In the general population, however, the outlook is different.
In the United States, the lifetime risk of ovarian cancer is 1.4% overall. Among white women who have had three or more term pregnancies and who have used an oral contraceptive for at least 4 years, the lifetime risk of ovarian cancer drops to 0.3%.12
Need for reoperation is very low
The percentage of women who require reoperation after ovarian conservation—2.8%—may surprise you.13 That figure is lower than once thought because many studies were performed before asymptomatic, benign ovarian cysts were determined to be a fairly common phenomenon in postmenopausal women (prevalence, 6.6%). These cysts do not undergo transformation to cancer and, therefore, do not need to be removed.14
In addition, studies indicate that only 0.1% to 0.75% of women who retain their ovaries at the time of hysterectomy develop ovarian cancer.15,16 Therefore, the rationale of performing oophorectomy to avoid future surgery appears to be unfounded.
CAD risk rises sharply after oophorectomy
A recent systematic review found mixed evidence concerning the risk of CAD following bilateral salpingo-oophorectomy.17 In observational studies, however, earlier age of surgical or natural menopause has been associated with a higher risk of cardiovascular mortality.18-20 Early reports from the Nurses’ Health Study found that the risk of myocardial infarction doubled among women who underwent oophorectomy and never used estrogen, compared with age-matched premenopausal women (relative risk [RR], 2.2; 95% confidence interval [CI], 1.2, 4.2).3 Even after age 50, the risk of a first myocardial infarction is increased among oophorectomized women, compared with women who retain their ovaries (RR, 1.4; 95% CI, 1.0–2.0).21
A study by researchers from the Mayo Clinic, who examined all causes listed on the death certificate, found a significant association between bilateral oophorectomy before the age of 45 years and cardiovascular mortality (hazard ratio [HR], 1.44; 95% CI, 1.01–2.05).22 This risk was significantly increased among women who were not treated with estrogen through at least age 45, compared with estrogen-treated women.
Oophorectomy may impair bone health
After menopause, ovaries continue to produce significant amounts of the androgens testosterone and androstenedione, which are converted to estrone peripherally by skin, muscle, and fat cells.23,24 The levels of these hormones remain consistent and have been documented to age 80.25
Both estrogens and androgens inhibit bone resorption, and androgens also stimulate bone formation.26 Low levels of androgens and estrogens are linked to lower bone density and a higher risk of hip and vertebral fracture in postmenopausal women.27-29
Postmenopausal women who have been oophorectomized may have an even greater risk of osteoporosis. Over 16 years of follow-up, 340 women who had undergone oophorectomy at a median age of 62 years had 54% more osteoporotic fractures than women who had intact ovaries.5 Two other studies found no association between oophorectomy and bone loss or fracture risk, however.30,31
Hip fracture is a well-documented cause of increased morbidity and mortality in older women. One study found that, before hip fracture, 28% of patients were housebound; 1 year after hip fracture, the percentage was 46%.32 Women older than 60 who underwent oophorectomy had a doubled risk of mortality after low-trauma hip fracture, compared with women who had intact ovaries (odds ratio [OR], 2.18; 95% CI, 2.03–2.32).5
Loss of ovaries may affect mental health and sexuality
In a premenopausal woman, oophorectomy causes a sudden loss of estrogen and often triggers hot flashes, mood changes, sleep disturbances, headaches, and a decline in feelings of well-being.33,34 Over time, vaginal dryness, painful intercourse, loss of libido, bladder dysfunction, and depression may occur.35,36
Evidence suggests that, in women, sexual desire, sexual sensation, and orgasmic response are influenced by androgens. After elective oophorectomy, declines in sexual desire have been reported.37-39
Mental health and sexuality may rebound over time, however. One study found less improvement in mental health measures and body image 6 months after hysterectomy among women who were oophorectomized, compared with those who retained their ovaries. After 2 years, improvement levels were similar between groups.40
Cognitive function may suffer
Analysis of data from the Mayo Clinic Cohort Study of Oophorectomy and Aging found that bilateral oophorectomy before the onset of menopause increased the risk of parkinsonism, cognitive impairment or dementia, and anxiety or depression, particularly if estrogen was not replaced.6-8 These risks increased with younger age at oophorectomy.
The Women’s Health Initiative found an increased risk of dementia or mild cognitive impairment in women who were treated by estrogen alone or estrogen plus progestin after age 65.41-44
These disparate conclusions suggest that estrogen may have a protective effect on the brain if it is given right after natural menopause or premenopausal oophorectomy, but deleterious effects if it is started years later.45
Other studies of endogenous estrogen and cognitive function are few and yield inconsistent results.
Ovarian conservation boosts long-term survival
When there is no family history of ovarian cancer, ovarian conservation appears to maximize survival among healthy women 40 to 65 years old who undergo hysterectomy for benign disease.46 Among healthy women hysterectomized before the age of 55, calculations suggest that 8.6% more would be alive at age 80 if their ovaries were conserved than if they were removed.46
A study from the Mayo Clinic found that all-cause mortality was significantly higher among women who underwent prophylactic bilateral oophorectomy before the age of 45 than it was among women in the control group (HR, 1.67; 95% CI, 1.16–2.40); it was particularly high in women who did not receive estrogen treatment before age 45 (HR, 1.93; 95% CI, 1.25–2.96).22
In a recent study, investigators used the Nurses’ Health Study database to explore the long-term health outcomes of 29,380 women who underwent hysterectomy.4 Of these women, 13,035 (44.4%) had their ovaries conserved, and 16,345 (55.6%) underwent bilateral oophorectomy. Follow-up was 24 years. Oophorectomy was associated with an increased risk of nonfatal CAD among all women (HR, 1.17; 95% CI, 1.02, 1.35), especially those who underwent the procedure before age 45 (HR, 1.26; 95% CI, 1.04, 1.54). Oophorectomy was associated with a markedly reduced risk of ovarian cancer but an increased risk of lung cancer (HR, 1.26; 95% CI, 1.02–1.56).
In regard to fatal events, oophorectomy increased the risk of death from all causes (HR, 1.12; 95% CI, 1.03, 1.21). Specifically, there was an increased risk of death from CAD (HR, 1.28; 95% CI, 1.00, 1.64), lung cancer (HR, 1.31; 95% CI, 1.02, 1.68), and all cancers (HR, 1.17; 95% CI, 1.04, 1.32). There was no overall difference in the risk of death from stroke, breast cancer, and colorectal cancer between women who underwent oophorectomy and those who retained their ovaries.
During the 24 years of follow-up, 37 women died from ovarian cancer, accounting for 1.2% of all deaths. At no age did oophorectomy show a survival benefit.
How this evidence should inform your practice
It is unfortunate that the entire body of evidence on the risks and benefits of bilateral salpingo-oophorectomy consists of observational studies, which have significant inherent limitations. Although the Nurses’ Health Study was the largest prospective study to examine the effect of oophorectomy on women’s health, and involved the longest follow-up, the study was observational, and oophorectomy and ovarian conservation were self-selected. Nevertheless, recent data suggest that a more detailed informed-consent process is warranted than the process in place. Informed consent should cover the risks and benefits of both oophorectomy and ovarian conservation.
Prophylactic oophorectomy is recommended only if a preponderance of the evidence establishes that it clearly benefits the patient. The studies described in this article suggest that bilateral oophorectomy does harm more often than it does good. Therefore, a cautious approach to oophorectomy at the time of hysterectomy is advised.
CASE RESOLVED
After you describe the risks and benefits of oophorectomy, and address the patient’s concerns about her family history of heart disease, she decides to keep her ovaries.
1. Healthcare Cost and Utilization Project (HCUP), 1988–2001: A Federal–State–Industry Partnership in Health Data. Rockville, Md: Agency for Healthcare Research and Quality; July 2003.
2. Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962.
3. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105-1110.
4. Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027-1037.
5. Melton LJ 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900-905.
6. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074.-
7. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200-209.
8. Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050-1059.
9. Bennett DA. Editorial comment on “Prevalence of dementia in the United States: the aging, demographics, and memory study” by Plassman et al. Neuroepidemiology. 2007;29:133-135.
10. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
11. Armstrong K, Schwartz J, Randall T, Rubin S, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
12. Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84:760-764.
13. Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68:159-164.
14. Bailey CL, Ueland FR, Land GL, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998;69:3-7.
15. Naylor AC. Hysterectomy—analysis of 2,901 personally performed procedures. S Afr Med J. 1984;65:242-245.
16. Christ JE, Lotze EC. The residual ovary syndrome. Obstet Gynecol. 1975;46:551-556.
17. Jacoby VL, Grady D, Sawaya GF. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol. 2009;200:140.e1-140.e9.
18. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714-718.
19. Løkkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226-233.
20. de Kleijn MJJ, van der Schouw YT, Verbeek ALM, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339.-
21. Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53:832-837.
22. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821-828.
23. Judd H, Judd G, Lucas W, Yen S. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020-1024.
24. Fogle R, Stanczyk F, Zhang X, Paulson R. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040-3043.
25. Meldrum D, Davidson B, Tataryn I, Judd H. Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol. 1981;57:624-628.
26. Raisz LG, Wilta B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
27. Johansson C, Mellstrom D, Milsom I. Reproductive factors as predictors of bone density and fractures in women at age 70. Maturitas. 1993;17:39-50.
28. Davidson BJ, Ross RK, Paganini-Hill A, et al. Total and free estrogens and androgens in postmenopausal women with hip fractures. J Clin Endocrinol Metab. 1982;54:115-120.
29. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733-738.
30. Kritz-Silverstein D, von Mühlen D, Barrett-Connor E. Hysterectomy and oophorectomy are unrelated to bone loss in older women. Maturitas. 2004;47:61-69.
31. Antoniucci DM, Sellmeyer DE, Cauley JA, et al. Postmenopausal bilateral oophorectomy is not associated with increased fracture risk in older women. J Bone Miner Res. 2005;20:741-747.
32. Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248-1250.
33. Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy—effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283-293.
34. Nieman L. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
35. Shifren J. Androgen deficiency in the oophorectomized woman. Fertil Steril. 2002;77 Suppl 4:s60-s62.
36. Sherwin B, Gelfan M. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1978;49:397-409.
37. Shifren JL, Avis NE. Surgical menopause: effects on psychological well-being and sexuality. Menopause. 2007;14:586.-
38. Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149-156.
39. Sherwin BB, Gelfan MM. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1978;49:397-409.
40. Teplin V, Vittinghoff E, Lin F, Learman L, Richter H, Kuppermann M. Oophorectomy in premenopausal women: health-related quality of life and sexual functioning. Obstet Gynecol. 2007;109:347-354.
41. Shumaker SA, Legault C, Rapp SR, et al. WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
42. Rapp SR, Espeland MA, Shumaker SA, et al. WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663-2672.
43. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959-2968.
44. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
45. Siegfried T. Neuroscience: it’s all in the timing. Nature. 2007;445:359-361.
46. Parker W, Broder M, Liu Z, Shoupe D, Farquhar C, Berek J. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
47. Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042-1050.
48. McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271-287.
49. Huser MA, Evans TS, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22:163-171.
1. Healthcare Cost and Utilization Project (HCUP), 1988–2001: A Federal–State–Industry Partnership in Health Data. Rockville, Md: Agency for Healthcare Research and Quality; July 2003.
2. Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962.
3. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105-1110.
4. Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027-1037.
5. Melton LJ 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900-905.
6. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074.-
7. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200-209.
8. Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050-1059.
9. Bennett DA. Editorial comment on “Prevalence of dementia in the United States: the aging, demographics, and memory study” by Plassman et al. Neuroepidemiology. 2007;29:133-135.
10. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
11. Armstrong K, Schwartz J, Randall T, Rubin S, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
12. Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84:760-764.
13. Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68:159-164.
14. Bailey CL, Ueland FR, Land GL, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998;69:3-7.
15. Naylor AC. Hysterectomy—analysis of 2,901 personally performed procedures. S Afr Med J. 1984;65:242-245.
16. Christ JE, Lotze EC. The residual ovary syndrome. Obstet Gynecol. 1975;46:551-556.
17. Jacoby VL, Grady D, Sawaya GF. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol. 2009;200:140.e1-140.e9.
18. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714-718.
19. Løkkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226-233.
20. de Kleijn MJJ, van der Schouw YT, Verbeek ALM, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339.-
21. Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53:832-837.
22. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821-828.
23. Judd H, Judd G, Lucas W, Yen S. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020-1024.
24. Fogle R, Stanczyk F, Zhang X, Paulson R. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040-3043.
25. Meldrum D, Davidson B, Tataryn I, Judd H. Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol. 1981;57:624-628.
26. Raisz LG, Wilta B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
27. Johansson C, Mellstrom D, Milsom I. Reproductive factors as predictors of bone density and fractures in women at age 70. Maturitas. 1993;17:39-50.
28. Davidson BJ, Ross RK, Paganini-Hill A, et al. Total and free estrogens and androgens in postmenopausal women with hip fractures. J Clin Endocrinol Metab. 1982;54:115-120.
29. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733-738.
30. Kritz-Silverstein D, von Mühlen D, Barrett-Connor E. Hysterectomy and oophorectomy are unrelated to bone loss in older women. Maturitas. 2004;47:61-69.
31. Antoniucci DM, Sellmeyer DE, Cauley JA, et al. Postmenopausal bilateral oophorectomy is not associated with increased fracture risk in older women. J Bone Miner Res. 2005;20:741-747.
32. Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248-1250.
33. Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy—effects on sexual life, psychological well-being and androgen status. J Psychosom Obstet Gynaecol. 1993;14:283-293.
34. Nieman L. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
35. Shifren J. Androgen deficiency in the oophorectomized woman. Fertil Steril. 2002;77 Suppl 4:s60-s62.
36. Sherwin B, Gelfan M. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1978;49:397-409.
37. Shifren JL, Avis NE. Surgical menopause: effects on psychological well-being and sexuality. Menopause. 2007;14:586.-
38. Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149-156.
39. Sherwin BB, Gelfan MM. The role of androgens in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1978;49:397-409.
40. Teplin V, Vittinghoff E, Lin F, Learman L, Richter H, Kuppermann M. Oophorectomy in premenopausal women: health-related quality of life and sexual functioning. Obstet Gynecol. 2007;109:347-354.
41. Shumaker SA, Legault C, Rapp SR, et al. WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
42. Rapp SR, Espeland MA, Shumaker SA, et al. WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663-2672.
43. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959-2968.
44. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
45. Siegfried T. Neuroscience: it’s all in the timing. Nature. 2007;445:359-361.
46. Parker W, Broder M, Liu Z, Shoupe D, Farquhar C, Berek J. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
47. Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042-1050.
48. McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271-287.
49. Huser MA, Evans TS, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22:163-171.