User login
EBER-Negative, Double-Hit High-Grade B-Cell Lymphoma Responding to Methotrexate Discontinuation
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
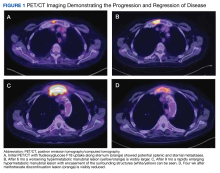
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
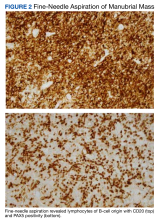
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
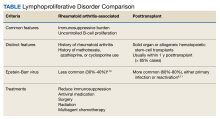
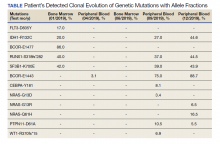
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
A Case Series of Rare Immune-Mediated Adverse Reactions at the New Mexico Veterans Affairs Medical Center
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
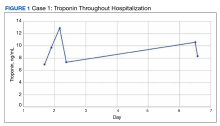
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
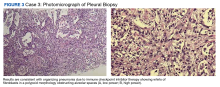
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
Sequential Targeted Treatment for a Geriatric Patient with Acute Myeloid Leukemia with Concurrent FLT3-TKD and IDH1 Mutations
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
Sequential Targeted Treatment of an Elderly Patient With Acute Myeloid Leukemia Harboring Concurrent FLT3-TKD and IDH1 Mutations: A Case Report
INTRODUCTION: With the increasing availability of novel targeted therapies and next-generation sequencing (NGS) hematology panels, the treatment paradigm for patients with acute myeloid leukemia (AML) has recently been altered. Specifically, patients who bear mutations within the FMS-like tyrosine kinase (FLT3) gene or the isocitrate dehydrogenase (IDH) 1 or IDH2 genes may now be candidates for targeted treatments either in the frontline or relapsed or refractory (R/R) settings. The sequential targeted approach to AML patients who harbor mutations within both FLT3 and IDH genes has yet to be elucidated.
CASE PRESENTATION: Herein, we report a case of an elderly patient with FLT3 and IDH1 mutations who underwent induction chemotherapy in combination with midostaurin, and subsequently, ivosidenib in the R/R setting. Clonal evaluation was demonstrated with repeated cytogenetic analysis and NGS of blood and bone marrow specimens. At diagnosis, the patient’s AML harbored several pathogenic gene variants, including FLT3 and IDH1 mutations. Following induction chemotherapy with midostaurin, the patient’s FLT3 mutation was no longer detected. Upon relapse, the FLT3 mutation was still undetectable, however the IDH1 mutation remained. Unfortunately, the patient’s AML did not respond to ivosidenib, and expansion of a leukemic clone with a BCOR mutation was observed.
CONCLUSION: This case conveys the use of multiple targeted therapies in a sequential fashion for an AML patient with frequent completion of NGS panels to monitor clonal evolution. Given that a considerable minority of patients harbor both FLT3 and IDH mutations, further investigations evaluating optimal sequencing or combinations of targeted therapies are required.
INTRODUCTION: With the increasing availability of novel targeted therapies and next-generation sequencing (NGS) hematology panels, the treatment paradigm for patients with acute myeloid leukemia (AML) has recently been altered. Specifically, patients who bear mutations within the FMS-like tyrosine kinase (FLT3) gene or the isocitrate dehydrogenase (IDH) 1 or IDH2 genes may now be candidates for targeted treatments either in the frontline or relapsed or refractory (R/R) settings. The sequential targeted approach to AML patients who harbor mutations within both FLT3 and IDH genes has yet to be elucidated.
CASE PRESENTATION: Herein, we report a case of an elderly patient with FLT3 and IDH1 mutations who underwent induction chemotherapy in combination with midostaurin, and subsequently, ivosidenib in the R/R setting. Clonal evaluation was demonstrated with repeated cytogenetic analysis and NGS of blood and bone marrow specimens. At diagnosis, the patient’s AML harbored several pathogenic gene variants, including FLT3 and IDH1 mutations. Following induction chemotherapy with midostaurin, the patient’s FLT3 mutation was no longer detected. Upon relapse, the FLT3 mutation was still undetectable, however the IDH1 mutation remained. Unfortunately, the patient’s AML did not respond to ivosidenib, and expansion of a leukemic clone with a BCOR mutation was observed.
CONCLUSION: This case conveys the use of multiple targeted therapies in a sequential fashion for an AML patient with frequent completion of NGS panels to monitor clonal evolution. Given that a considerable minority of patients harbor both FLT3 and IDH mutations, further investigations evaluating optimal sequencing or combinations of targeted therapies are required.
INTRODUCTION: With the increasing availability of novel targeted therapies and next-generation sequencing (NGS) hematology panels, the treatment paradigm for patients with acute myeloid leukemia (AML) has recently been altered. Specifically, patients who bear mutations within the FMS-like tyrosine kinase (FLT3) gene or the isocitrate dehydrogenase (IDH) 1 or IDH2 genes may now be candidates for targeted treatments either in the frontline or relapsed or refractory (R/R) settings. The sequential targeted approach to AML patients who harbor mutations within both FLT3 and IDH genes has yet to be elucidated.
CASE PRESENTATION: Herein, we report a case of an elderly patient with FLT3 and IDH1 mutations who underwent induction chemotherapy in combination with midostaurin, and subsequently, ivosidenib in the R/R setting. Clonal evaluation was demonstrated with repeated cytogenetic analysis and NGS of blood and bone marrow specimens. At diagnosis, the patient’s AML harbored several pathogenic gene variants, including FLT3 and IDH1 mutations. Following induction chemotherapy with midostaurin, the patient’s FLT3 mutation was no longer detected. Upon relapse, the FLT3 mutation was still undetectable, however the IDH1 mutation remained. Unfortunately, the patient’s AML did not respond to ivosidenib, and expansion of a leukemic clone with a BCOR mutation was observed.
CONCLUSION: This case conveys the use of multiple targeted therapies in a sequential fashion for an AML patient with frequent completion of NGS panels to monitor clonal evolution. Given that a considerable minority of patients harbor both FLT3 and IDH mutations, further investigations evaluating optimal sequencing or combinations of targeted therapies are required.
DNA Repair Gene Variants in Patients With Prostate Cancer Achieving Durable Clinical Benefit With PARP Inhibitors
BACKGROUND: PARP inhibitors (PARPi’s) were recently approved for the treatment of metastatic prostate cancer among patients harboring mutations in an array of genes responsible for DNA repair. We sought to identify whether a subset of these genes correlates with response to treatment more frequently than others. Consequently, an evaluation of the specific DNA repair genotypes associated with durable clinical benefit (DCB) using real-world patient data was undertaken.
METHODS: The U.S. Department of Veterans Affairs (VA) National Precision Oncology Program’s (NPOP) database and Corporate Data Warehouse (CDW) were reviewed to select patients who (1) carried a diagnosis of prostate cancer, (2) successfully underwent tumor DNA sequencing through NPOP, (3) were prescribed olaparib, rucaparib, nirapib, and/or talazaporib for their prostate cancer between July 2016 and February 2020, and (4) and achieved DCB, defined as no progression in prostate-specific antigen (PSA) for at least 6 months following PARPi initiation without concurrent systemic or non-systemic therapies other than androgen-deprivation. The DNA repair gene variants and orders placed for NPOP consultative support were reviewed.
RESULTS: Of the 44 prostate cancer patients treated with a PARPi, 6 (13.6%) had tumor DNA sequencing through NPOP and had achieved DCB. Five patients were treated with olaparib and 1 with rucaparib. The median PSA progression-free survival was 8.9 (interquartile range = 8.5 – 11.2) months among these selected patients. Regarding gene variants, 5 patients had 7 BRCA2 mutations, including 4 frameshift, 1 nonsense, 1 single nucleotide variant, and 1 splice site. One patient had frameshift and missense ATM mutations. Referrals to the NPOP consult service were ordered for 2 out of the 5 patients with BRCA2 mutations achieving DCB.
CONCLUSIONS: Within the VA’s NPOP, the presence of BRCA2 gene variants was the most common finding from tumor DNA sequencing among patients with prostate cancer achieving DCB with a PARPi. Further analysis of the genotypes of all patients treated with PARPi in NPOP to assess the differential impact of BRCA2 mutations is needed to confirm the clinical implication of this finding.
BACKGROUND: PARP inhibitors (PARPi’s) were recently approved for the treatment of metastatic prostate cancer among patients harboring mutations in an array of genes responsible for DNA repair. We sought to identify whether a subset of these genes correlates with response to treatment more frequently than others. Consequently, an evaluation of the specific DNA repair genotypes associated with durable clinical benefit (DCB) using real-world patient data was undertaken.
METHODS: The U.S. Department of Veterans Affairs (VA) National Precision Oncology Program’s (NPOP) database and Corporate Data Warehouse (CDW) were reviewed to select patients who (1) carried a diagnosis of prostate cancer, (2) successfully underwent tumor DNA sequencing through NPOP, (3) were prescribed olaparib, rucaparib, nirapib, and/or talazaporib for their prostate cancer between July 2016 and February 2020, and (4) and achieved DCB, defined as no progression in prostate-specific antigen (PSA) for at least 6 months following PARPi initiation without concurrent systemic or non-systemic therapies other than androgen-deprivation. The DNA repair gene variants and orders placed for NPOP consultative support were reviewed.
RESULTS: Of the 44 prostate cancer patients treated with a PARPi, 6 (13.6%) had tumor DNA sequencing through NPOP and had achieved DCB. Five patients were treated with olaparib and 1 with rucaparib. The median PSA progression-free survival was 8.9 (interquartile range = 8.5 – 11.2) months among these selected patients. Regarding gene variants, 5 patients had 7 BRCA2 mutations, including 4 frameshift, 1 nonsense, 1 single nucleotide variant, and 1 splice site. One patient had frameshift and missense ATM mutations. Referrals to the NPOP consult service were ordered for 2 out of the 5 patients with BRCA2 mutations achieving DCB.
CONCLUSIONS: Within the VA’s NPOP, the presence of BRCA2 gene variants was the most common finding from tumor DNA sequencing among patients with prostate cancer achieving DCB with a PARPi. Further analysis of the genotypes of all patients treated with PARPi in NPOP to assess the differential impact of BRCA2 mutations is needed to confirm the clinical implication of this finding.
BACKGROUND: PARP inhibitors (PARPi’s) were recently approved for the treatment of metastatic prostate cancer among patients harboring mutations in an array of genes responsible for DNA repair. We sought to identify whether a subset of these genes correlates with response to treatment more frequently than others. Consequently, an evaluation of the specific DNA repair genotypes associated with durable clinical benefit (DCB) using real-world patient data was undertaken.
METHODS: The U.S. Department of Veterans Affairs (VA) National Precision Oncology Program’s (NPOP) database and Corporate Data Warehouse (CDW) were reviewed to select patients who (1) carried a diagnosis of prostate cancer, (2) successfully underwent tumor DNA sequencing through NPOP, (3) were prescribed olaparib, rucaparib, nirapib, and/or talazaporib for their prostate cancer between July 2016 and February 2020, and (4) and achieved DCB, defined as no progression in prostate-specific antigen (PSA) for at least 6 months following PARPi initiation without concurrent systemic or non-systemic therapies other than androgen-deprivation. The DNA repair gene variants and orders placed for NPOP consultative support were reviewed.
RESULTS: Of the 44 prostate cancer patients treated with a PARPi, 6 (13.6%) had tumor DNA sequencing through NPOP and had achieved DCB. Five patients were treated with olaparib and 1 with rucaparib. The median PSA progression-free survival was 8.9 (interquartile range = 8.5 – 11.2) months among these selected patients. Regarding gene variants, 5 patients had 7 BRCA2 mutations, including 4 frameshift, 1 nonsense, 1 single nucleotide variant, and 1 splice site. One patient had frameshift and missense ATM mutations. Referrals to the NPOP consult service were ordered for 2 out of the 5 patients with BRCA2 mutations achieving DCB.
CONCLUSIONS: Within the VA’s NPOP, the presence of BRCA2 gene variants was the most common finding from tumor DNA sequencing among patients with prostate cancer achieving DCB with a PARPi. Further analysis of the genotypes of all patients treated with PARPi in NPOP to assess the differential impact of BRCA2 mutations is needed to confirm the clinical implication of this finding.
In reply: Serotonin syndrome
In Reply: The questions posed by Dr. Rose reflect critical issues primary care physicians encounter when prescribing medications for patients who are taking serotonergic agents. “Switching strategies” have been described for starting or discontinuing serotonergic antidepressants.1 Options range from conservative exchanges requiring 5 half-lives between discontinuation of 1 antidepressant and initiation of another vs a direct cross-taper exchange. Decisions regarding specific patients should take into account previous adverse effects from serotonergic medications and half-lives of discontinued antidepressants. To our knowledge, switching strategies have not been validated and are based on expert opinion. Scenarios are complicated further if patients have already been prescribed 2 or more antidepressants and 1 medication is exchanged or dose-adjusted while another is added. With this degree of complexity, we recommend referral to a psychiatrist.
Dr. Rose’s questions on prescribing nonpsychiatric serotonergic drugs concurrently with antidepressants broaches a topic with even less evidence. Some data exist about nonpsychiatric serotonergic drugs given in combination with triptans. Soldin et al2 reviewed the US Food and Drug Administration’s Adverse Event Reporting System and discovered 38 cases of serotonin syndrome in patients using triptans. Eleven of these patients were using triptans without concomitant antidepressants. Though definitive evidence is lacking for safe prescribing practice with triptans, the authors noted that most cases of triptan-induced serotonin toxicity occur within hours of triptan ingestion.2
The evidence on the risk of serotonin syndrome with other medications is limited to case reports. In regard to linezolid, a review suggested that when linezolid was administered to a patient on long-term citalopram, a prolonged serotonin syndrome was precipitated, which is not an issue with other antidepressants.3 The World Health Organization has issued warnings for serotonin toxicity with ondansetron and other 5-HT3 receptor antagonists based on case reports.4,5 No data are available for the appropriate prescribing of 5-HT3 antagonists with antidepressants. A review of cases suggests a link between fluconazole and severe serotonin toxicity in patients taking citalopram; however, no prescribing guidelines have been established for fluconazole either.6
Dr. Rose asks important clinical questions, but evidence-based answers are not available. We can only recommend that patients be advised to report symptoms immediately after starting any medication associated with serotonin syndrome. For patients on multiple antidepressants, psychiatric assistance is advised. An observational cohort study of patients using antidepressants while exposed to other suspect drugs may better delineate effects of several pharmaceuticals on the serotonergic axis. Only then may safe prescribing practices be validated with evidence.
- Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr 2016; 39:76–83.
- Soldin OP, Tonning JM; Obstetric-Fetal Pharmacology Research Unit Network. Serotonin syndrome associated with triptan monotherapy (letter). N Engl J Med 2008; 15:2185–2186.
- Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Grau S. Linezolid-associated serotonin syndrome: what we can learn from cases reported so far. J Antimicrob Chemother 2005; 56:1176–1178.
- World Health Organization. Ondansetron and serotonin syndrome. WHO Pharmaceuticals Newsletter 2012; 3:16–21.
- Rojas-Fernandez CH. Can 5-HT3 antagonists really contribute to serotonin toxicity? A call for clarity and pharmacological law and order. Drugs Real World Outcomes 2014; 1:3–5.
- Levin TT, Cortes-Ladino A, Weiss M, Palomba ML. Life-threatening serotonin toxicity due to a citalopram-fluconazole drug interaction: case reports and discussion. Gen Hosp Psychiatry 2008; 30:372–377.
In Reply: The questions posed by Dr. Rose reflect critical issues primary care physicians encounter when prescribing medications for patients who are taking serotonergic agents. “Switching strategies” have been described for starting or discontinuing serotonergic antidepressants.1 Options range from conservative exchanges requiring 5 half-lives between discontinuation of 1 antidepressant and initiation of another vs a direct cross-taper exchange. Decisions regarding specific patients should take into account previous adverse effects from serotonergic medications and half-lives of discontinued antidepressants. To our knowledge, switching strategies have not been validated and are based on expert opinion. Scenarios are complicated further if patients have already been prescribed 2 or more antidepressants and 1 medication is exchanged or dose-adjusted while another is added. With this degree of complexity, we recommend referral to a psychiatrist.
Dr. Rose’s questions on prescribing nonpsychiatric serotonergic drugs concurrently with antidepressants broaches a topic with even less evidence. Some data exist about nonpsychiatric serotonergic drugs given in combination with triptans. Soldin et al2 reviewed the US Food and Drug Administration’s Adverse Event Reporting System and discovered 38 cases of serotonin syndrome in patients using triptans. Eleven of these patients were using triptans without concomitant antidepressants. Though definitive evidence is lacking for safe prescribing practice with triptans, the authors noted that most cases of triptan-induced serotonin toxicity occur within hours of triptan ingestion.2
The evidence on the risk of serotonin syndrome with other medications is limited to case reports. In regard to linezolid, a review suggested that when linezolid was administered to a patient on long-term citalopram, a prolonged serotonin syndrome was precipitated, which is not an issue with other antidepressants.3 The World Health Organization has issued warnings for serotonin toxicity with ondansetron and other 5-HT3 receptor antagonists based on case reports.4,5 No data are available for the appropriate prescribing of 5-HT3 antagonists with antidepressants. A review of cases suggests a link between fluconazole and severe serotonin toxicity in patients taking citalopram; however, no prescribing guidelines have been established for fluconazole either.6
Dr. Rose asks important clinical questions, but evidence-based answers are not available. We can only recommend that patients be advised to report symptoms immediately after starting any medication associated with serotonin syndrome. For patients on multiple antidepressants, psychiatric assistance is advised. An observational cohort study of patients using antidepressants while exposed to other suspect drugs may better delineate effects of several pharmaceuticals on the serotonergic axis. Only then may safe prescribing practices be validated with evidence.
In Reply: The questions posed by Dr. Rose reflect critical issues primary care physicians encounter when prescribing medications for patients who are taking serotonergic agents. “Switching strategies” have been described for starting or discontinuing serotonergic antidepressants.1 Options range from conservative exchanges requiring 5 half-lives between discontinuation of 1 antidepressant and initiation of another vs a direct cross-taper exchange. Decisions regarding specific patients should take into account previous adverse effects from serotonergic medications and half-lives of discontinued antidepressants. To our knowledge, switching strategies have not been validated and are based on expert opinion. Scenarios are complicated further if patients have already been prescribed 2 or more antidepressants and 1 medication is exchanged or dose-adjusted while another is added. With this degree of complexity, we recommend referral to a psychiatrist.
Dr. Rose’s questions on prescribing nonpsychiatric serotonergic drugs concurrently with antidepressants broaches a topic with even less evidence. Some data exist about nonpsychiatric serotonergic drugs given in combination with triptans. Soldin et al2 reviewed the US Food and Drug Administration’s Adverse Event Reporting System and discovered 38 cases of serotonin syndrome in patients using triptans. Eleven of these patients were using triptans without concomitant antidepressants. Though definitive evidence is lacking for safe prescribing practice with triptans, the authors noted that most cases of triptan-induced serotonin toxicity occur within hours of triptan ingestion.2
The evidence on the risk of serotonin syndrome with other medications is limited to case reports. In regard to linezolid, a review suggested that when linezolid was administered to a patient on long-term citalopram, a prolonged serotonin syndrome was precipitated, which is not an issue with other antidepressants.3 The World Health Organization has issued warnings for serotonin toxicity with ondansetron and other 5-HT3 receptor antagonists based on case reports.4,5 No data are available for the appropriate prescribing of 5-HT3 antagonists with antidepressants. A review of cases suggests a link between fluconazole and severe serotonin toxicity in patients taking citalopram; however, no prescribing guidelines have been established for fluconazole either.6
Dr. Rose asks important clinical questions, but evidence-based answers are not available. We can only recommend that patients be advised to report symptoms immediately after starting any medication associated with serotonin syndrome. For patients on multiple antidepressants, psychiatric assistance is advised. An observational cohort study of patients using antidepressants while exposed to other suspect drugs may better delineate effects of several pharmaceuticals on the serotonergic axis. Only then may safe prescribing practices be validated with evidence.
- Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr 2016; 39:76–83.
- Soldin OP, Tonning JM; Obstetric-Fetal Pharmacology Research Unit Network. Serotonin syndrome associated with triptan monotherapy (letter). N Engl J Med 2008; 15:2185–2186.
- Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Grau S. Linezolid-associated serotonin syndrome: what we can learn from cases reported so far. J Antimicrob Chemother 2005; 56:1176–1178.
- World Health Organization. Ondansetron and serotonin syndrome. WHO Pharmaceuticals Newsletter 2012; 3:16–21.
- Rojas-Fernandez CH. Can 5-HT3 antagonists really contribute to serotonin toxicity? A call for clarity and pharmacological law and order. Drugs Real World Outcomes 2014; 1:3–5.
- Levin TT, Cortes-Ladino A, Weiss M, Palomba ML. Life-threatening serotonin toxicity due to a citalopram-fluconazole drug interaction: case reports and discussion. Gen Hosp Psychiatry 2008; 30:372–377.
- Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr 2016; 39:76–83.
- Soldin OP, Tonning JM; Obstetric-Fetal Pharmacology Research Unit Network. Serotonin syndrome associated with triptan monotherapy (letter). N Engl J Med 2008; 15:2185–2186.
- Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Grau S. Linezolid-associated serotonin syndrome: what we can learn from cases reported so far. J Antimicrob Chemother 2005; 56:1176–1178.
- World Health Organization. Ondansetron and serotonin syndrome. WHO Pharmaceuticals Newsletter 2012; 3:16–21.
- Rojas-Fernandez CH. Can 5-HT3 antagonists really contribute to serotonin toxicity? A call for clarity and pharmacological law and order. Drugs Real World Outcomes 2014; 1:3–5.
- Levin TT, Cortes-Ladino A, Weiss M, Palomba ML. Life-threatening serotonin toxicity due to a citalopram-fluconazole drug interaction: case reports and discussion. Gen Hosp Psychiatry 2008; 30:372–377.
Serotonin syndrome: Preventing, recognizing, and treating it
With a substantial increase in antidepressant use in the United States over the last 2 decades, serotonin syndrome has become an increasingly common and significant clinical concern. In 1999, 6.5% of adults age 18 and older were taking antidepressants; by 2010, the percentage had increased to 10.4%.1 Though the true incidence of serotonin syndrome is difficult to determine, the number of ingestions of selective serotonin reuptake inhibitors (SSRIs) associated with moderate to major effects reported to US poison control centers increased from 7,349 in 20022 to 8,585 in 2005.3
Though the clinical manifestations are often mild to moderate, patients with serotonin syndrome can deteriorate rapidly and require intensive care. Unlike neuroleptic malignant syndrome, serotonin syndrome should not be considered an extremely rare idiosyncratic reaction to medication, but rather a progression of serotonergic toxicity based on increasing concentration levels that can occur in any patient regardless of age.4
Because it has a nonspecific prodrome and protean manifestations, serotonin syndrome can easily be overlooked, misdiagnosed, or exacerbated if not carefully assessed. Diagnosis requires a low threshold for suspicion and a meticulous history and physical examination. In the syndrome’s mildest stage, symptoms are often misattributed to other causes, and in its most severe form, it can easily be mistaken for neuroleptic malignant syndrome.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome classically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms are a result of increased serotonin levels affecting the central and peripheral nervous systems. Serotonin affects a family of receptors that has seven members, of which 5-HT1A and 5-HT2A are most often responsible for serotonin syndrome.5
Conditions that can alter the regulation of serotonin include therapeutic doses, drug interactions, intentional or unintentional overdoses, and overlapping transitions between medications. As a result, drugs that have been associated with serotonin syndrome can be classified into the following five categories as shown below and in Table 1:
Drugs that decrease serotonin breakdown include monoamine oxidase inhibitors (MAOIs), linezolid,6 methylene blue, procarbazine, and Syrian rue.
Drugs that decrease serotonin reuptake include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, opioids (meperidine, buprenorphine, tramadol, tapentadol, dextromethorphan), antiepileptics (carbamazepine, valproate), and antiemetics (ondansetron, granisetron, metoclopramide), and the herbal preparation St. John’s wort.
Drugs that increase serotonin precursors or agonists include tryptophan, lithium, fentanyl, and lysergic acid diethylamide (LSD).
Drugs that increase serotonin release include fenfluramine, amphetamines, and methylenedioxymethamphetamine (ecstasy).
Drugs that prevent breakdown of the agents listed above are CYP2D6 and CYP3A4 inhibitors, eg, erythromycin,7 ciprofloxacin, fluconazole, ritonavir, and grapefruit juice.
However, the only drugs that have been reliably confirmed to precipitate serotonin syndrome are MAOIs, SSRIs, SNRIs, and serotonin releasers. Other listed drug interactions are based on case reports and have not been thoroughly evaluated.6–9
Currently, SSRIs are the most commonly prescribed antidepressant medications and, consequently, they are the ones most often implicated in serotonergic toxicity.1,10 An estimated 15% of SSRI overdoses lead to mild or moderate serotonin toxicity.11 Serotonergic agents used in conjunction can increase the risk for severe serotonin syndrome; an SSRI and an MAOI in combination poses the greatest risk.5
Ultimately, the incidence of serotonin syndrome is difficult to assess, but it is believed to be underreported because it is easy to misdiagnose and mild symptoms may be dismissed.
WHO IS AT RISK OF SEROTONIN SYNDROME?
Long-term antidepressant use has disproportionately increased in middle-aged and older adults and non-Hispanic whites.1,12,13 Intuitively, as the risk for depression increases dramatically in patients with chronic medical conditions, serotonin syndrome should be more prevalent among the elderly. In addition, patients with multiple comorbidities take more medications, increasing the risk of polypharmacy and adverse drug reactions.14
Although the epidemiology of serotonin syndrome has yet to be extensively studied, the combination of age and comorbidities may increase the risk for this condition.
HOW DOES IT PRESENT?
Serotonin syndrome characteristically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. However, these symptoms may not occur simultaneously: autonomic dysfunction is present in 40% of patients, neuromuscular excitation in 50%, and altered mental status in 40%.15 The symptoms can range from mild to life-threatening (Table 2).16
Autonomic dysfunction. Diaphoresis is present in 48.8% of cases, tachycardia in 44%, nausea and vomiting in 26.8%, and mydriasis in 19.5%. Other signs are hyperactive bowel sounds, diarrhea, and flushing.16
Neuromuscular excitation. Myoclonus is present in 48.8%, hyperreflexia in 41%, hyperthermia in 26.8%, and hypertonicity and rigidity in 19.5%. Other signs are spontaneous or inducible clonus, ocular clonus (continuous rhythmic oscillations of gaze), and tremor.
Altered mental status. Confusion is present in 41.2% and agitation in 36.5%. Other signs are anxiety, lethargy, and coma.
Symptoms of serotonin toxicity arise within an hour of a precipitating event (eg, ingestion) in approximately 28% of patients, and within 6 hours in 61%.16 Highly diagnostic features include hyperreflexia and induced or spontaneous clonus that are generally more pronounced in the lower limbs.11 Clonus can be elicited with ankle dorsiflexion.
In mild toxicity, patients may present with tremor or twitching and anxiety, as well as with hyperreflexia, tachycardia, diaphoresis, and mydriasis. Further investigation may uncover a recently initiated antidepressant or a cold-and-cough medication that contains dextromethorphan.15,17
In moderate toxicity, patients present in significant distress, with agitation and restlessness. Features may include hyperreflexia and clonus of the lower extremities, opsoclonus, hyperactive bowel sounds, diarrhea, nausea, vomiting, tachycardia, hypertension, diaphoresis, mydriasis, and hyperthermia (< 40°C, 104°F). The patient’s history may reveal use of ecstasy or combined treatment with serotonin-potentiating agents such as an antidepressant with a proserotonergic opioid, antiepileptic, or CYP2D6 or CYP3A4 inhibitor.15
Severe serotonin toxicity is a life-threatening condition that can lead to multiorgan failure within hours. It can be characterized by muscle rigidity, which can cause the body temperature to elevate rapidly to over 40°C. This hypertonicity can mask the classic and diagnostic signs of hyperreflexia and clonus. Patients may have unstable and dynamic vital signs with confusion or delirium and can experience tonic-clonic seizures.
If the muscle rigidity and resulting hyperthermia are not managed properly, patients can develop cellular damage and enzyme dysfunction leading to rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress syndrome, and disseminated intravascular coagulation.16,18
Serotonin crisis is usually caused by the co-ingestion of multiple serotonergic agents, such as an antidepressant with an aforementioned opioid and antiemetic19; combining an SSRI and an MAOI poses the greatest risk. Alternatively, patients may have recently switched antidepressants without observing a safe washout period, leading to an overlap of serotonin levels.16
HOW DO WE DIAGNOSE SEROTONIN SYNDROME?
Serotonin syndrome is a clinical diagnosis and therefore requires a thorough review of medications and physical examination. Serum serotonin levels are an unreliable indicator of toxicity and do not correlate well with the clinical presentation.16
(based on information in reference 9).
Currently, there are two clinical tools for diagnosing serotonin syndrome: the Hunter serotonin toxicity criteria (Figure 1) and the Sternbach criteria.
The Hunter criteria are based more heavily on physical findings. The patient must have taken a serotonergic agent and have one of the following:
- Spontaneous clonus
- Inducible clonus plus agitation or diaphoresis
- Ocular clonus plus agitation or diaphoresis
- Inducible clonus or ocular clonus, plus hypertonia and hyperthermia
- Tremor plus hyperreflexia.
The Sternbach criteria. The patient must be using a serotonergic agent, must have no other causes of symptoms, must not have recently used a neuroleptic agent, and must have three of the following:
- Mental status changes
- Agitation
- Hyperreflexia
- Myoclonus
- Diaphoresis
- Shivering
- Tremor
- Diarrhea
- Incoordination
- Fever
The Hunter criteria are recommended and are more specific (97% vs 96%) and more sensitive (84% vs 75%) than the Sternbach criteria when compared with the gold standard of diagnosis by a clinical toxicologist.1 The Hunter criteria are also less likely to yield false-positive results.11
Differential diagnosis
The differential diagnosis for serotonin syndrome includes neuroleptic malignant syndrome, anticholinergic poisoning (Table 3), metastatic carcinoma, central nervous system infection, gastroenteritis, and sepsis.
Neuroleptic malignant syndrome, the disorder most often misdiagnosed as serotonin syndrome, is an idiosyncratic reaction to a dopamine antagonist (eg, haloperidol, fluphenazine) that develops over days to weeks.20 In 70% of patients, agitated delirium with confusion appears first, followed by lead pipe rigidity and cogwheel tremor, then hyperthermia with body temperature greater than 40°C, and finally, profuse diaphoresis, tachycardia, hypertension, and tachypnea.21
Key elements that distinguish neuroleptic malignant syndrome are the timeline of the clinical course, bradyreflexia, and the absence of clonus. Prodromal symptoms of nausea, vomiting, and diarrhea are also rare in neuroleptic malignant syndrome. Neuroleptic malignant syndrome typically requires an average of 9 days to resolve.
Anticholinergic poisoning usually develops within 1 to 2 hours of oral ingestion. Symptoms include flushing, anhidrosis, anhidrotic hyperthermia, mydriasis, urinary retention, decreased bowel sounds, agitated delirium, and visual hallucinations. In contrast to serotonin syndrome, reflexes and muscle tone are normal with anticholinergic poisoning.
HOW CAN WE TREAT SEROTONIN SYNDROME?
The two mainstays of serotonin syndrome management are to discontinue the serotonergic agent and to give supportive care. Most patients improve within 24 hours of stopping the precipitating drug and starting therapy.16
For mild serotonin syndrome, treatment involves discontinuing the offending agent and supportive therapy with intravenous fluids, correction of vital signs, and symptomatic treatment with a benzodiazepine. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For moderate serotonin syndrome, treatment also involves stopping the serotonergic agent and giving supportive care. Symptomatic treatment with a benzodiazepine and nonserotonergic antiemetics is recommended, and standard cooling measures should be implemented for hyperthermia. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For severe serotonin toxicity, treatment should focus on management of airway, breathing, and circulation—ie, the “ABCs.” The two primary life-threatening concerns are hyperthermia (temperature > 40°C or 104°F) and rigidity, which can lead to hypoventilation.1,22 Controlling hyperthermia and rigidity can prevent other grave complications. Patients with severe serotonin toxicity should be sedated, paralyzed, and intubated.21 This will reverse ventilatory hypertonia and allow for mechanical ventilation. Paralysis will also prevent the exacerbation of hyperthermia, which is caused by muscle rigidity. Antipyretics have no role in the treatment of serotonin syndrome since the hyperthermia is not caused by a change in the hypothalamic temperature set point.21 Standard cooling measures should be used to manage hyperthermia.
Serotonin antagonists
Serotonin antagonists have had some success in case reports, but further studies are needed to confirm this.4,23,24
Cyproheptadine is a potent 5-HT2A antagonist; patients usually respond within 1 to 2 hours of administration. Signs and symptoms have resolved completely within times ranging from 20 minutes to 48 hours, depending on the severity of toxicity.
The recommended initial dose of cyproheptadine is 12 mg, followed by 2 mg every 2 hours if symptoms continue.16 Maintenance dosing with 8 mg every 6 hours should be prescribed once stabilization is achieved. The total daily dose for adults should not exceed 0.5 mg/kg/day. Cyproheptadine is available only in oral form but can be crushed and administered via a nasogastric tube.21
Chlorpromazine is a 5-HT1A and 5-HT2A antagonist and can be given intramuscularly. Despite case reports citing its effectiveness, the risk of hypotension, dystonic reactions, and neuroleptic malignant syndrome may make it a less desirable option.4,25
Cyproheptadine, chlorpromazine, and other serotonin receptor antagonists require further investigation beyond individual case reports to determine their effectiveness and reliability in treating serotonin syndrome.
Other agents
Benzodiazepines are considered a mainstay for symptomatic relief because of their anxiolytic and muscle relaxant effects.26 However, animal studies showed that treatment with benzodiazepines attenuated hyperthermia but had no effect on time to recovery or outcome.27
Neuromuscular blocking agents. The suggested neuromuscular blocking agent for severe toxicity is a nondepolarizing agent such as vecuronium. Succinylcholine should be avoided, as it can exacerbate rhabdomyolysis and hyperkalemia.21
Dantrolene has also been suggested for its muscle-relaxing effects and use in malignant hyperthermia. However, this treatment has not been successful in isolated case reports and has been ineffective in animal models.4,28
Physical restraints are ill-advised, since isometric muscle contractions can exacerbate hyperthermia and lactic acidosis in agitated patients.21 If physical restraints are necessary to deliver medications, they should be removed as soon as possible.
HOW CAN WE PREVENT SEROTONIN SYNDROME?
Prevention of serotonin syndrome begins with improving education and awareness in patients and healthcare providers. Patients should be primarily concerned with taking their medications carefully as prescribed and recognizing early signs and symptoms of serotonin toxicity.
As use of antidepressants among an aging population continues to increase, and as physicians in multiple disciplines prescribe them for evolving indications (eg, duloxetine to treat osteoarthritis, diabetic neuropathy, fibromyalgia, and chemotherapy-induced peripheral neuropathy), healthcare providers need to be prepared to see more cases of serotonin syndrome and its deleterious effects.29–31 Physicians should be vigilant in minimizing unnecessary use of serotonergic agents and reviewing drug regimens regularly to limit polypharmacy.
Electronic ordering systems should be designed to detect and alert the prescriber to possible interactions that can potentiate serotonin syndrome, and to not place the order until the prescriber overrides the alert. Combinations of SSRIs and MAOIs have the highest risk for inducing severe serotonin syndrome and should always be avoided.
If a patient is transitioning between serotonergic agents, physicians should observe a safe washout period to prevent overlap.16,32 Washout periods may differ among medications depending on their half-lives. For example, sertraline has a washout period of 2 weeks, while fluoxetine requires a washout period of 5 to 6 weeks.33 Consulting a pharmacist may be helpful when considering half-lives and washout periods.
We believe that educating both patients and physicians regarding prevention will help minimize the risk for serotonergic syndrome and will improve efficiency in assessment and management should toxicity develop.
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry 2014; 75:169–177.
- Watson WA, Litovitz TL, Rodgers GC Jr, et al. 2002 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2003;21:353–421.
- Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exopsure database. Clin Toxicol (Phila) 2006; 44:803–932.
- Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999; 13:100–109.
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28:205–214.
- Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 2013; 47:388–397.
- Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999; 19:894–896.
- Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache 2010; 50:264–272.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust 2007; 187:361–365.
- Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014; 52:1032–1283.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–642.
- Karkare SU, Bhattacharjee S, Kamble P, Aparasu R. Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. Am J Geriatr Pharmacother 2011; 9:109–119.
- Weissman J, Meyers BS, Ghosh S, Bruce ML. Demographic, clinical, and functional factors associated with antidepressant use in the home healthcare elderly. Am J Geriatr Psychiatry 2011; 19:1042–1045.
- Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Co-morbidity and potential treatment conflicts in elderly heart failure patients: a retrospective, cross-sectional study of administrative claims data. Drugs Aging 2011; 28:575–581.
- Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Ann Clin Psychiatry 2012; 24:310–318.
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2000; 79:201–209.
- Prakash S, Patel V, Kakked S, Patel I, Yadav R. Mild serotonin syndrome: a report of 12 cases. Ann Indian Acad Neurol 2015; 18:226–230.
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung 2014; 43:117–119.
- Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care 2014; 21:108–113.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
- Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994; 182:168–173.
- Isbister GK, Hackett LP, Dawson AH, Whyte IM, Smith AJ. Moclobemide poisoning: toxicokinetics and occurrence of serotonin toxicity. Br J Clin Pharmacol 2003; 56:441–450.
- Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16:615–619.
- Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med 1994; 331:1021–1022.
- Gillman PK. Successful treatment of serotonin syndrome with chlorpromazine. Med J Aust 1996; 165:345–346.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ 2014; 348:g1626.
- Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43:155–164.
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001; 890:23–31.
- Micca JL, Ruff D, Ahl J, Wohlreich MM. Safety and efficacy of duloxetine treatment in older and younger patients with osteoarthritis knee pain: a post hoc, subgroup analysis of two randomized, placebo-controlled trials. BMC Musculoskelet Disord 2013; 14:137.
- Smith EM, Pang H, Cirrincione C, et al; Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309:1359–1367.
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 2014; 1:CD007115.
- Caughey GE, Roughead EE, Shakib S, McDermott RA, Vitry AI, Gilbert AL. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010; 39:488–494.
- Gury C, Cousin F. Pharmacokinetics of SSRI antidepressants: half-life and clinical applicability. Encephale 1999; 25:470–476. French.
With a substantial increase in antidepressant use in the United States over the last 2 decades, serotonin syndrome has become an increasingly common and significant clinical concern. In 1999, 6.5% of adults age 18 and older were taking antidepressants; by 2010, the percentage had increased to 10.4%.1 Though the true incidence of serotonin syndrome is difficult to determine, the number of ingestions of selective serotonin reuptake inhibitors (SSRIs) associated with moderate to major effects reported to US poison control centers increased from 7,349 in 20022 to 8,585 in 2005.3
Though the clinical manifestations are often mild to moderate, patients with serotonin syndrome can deteriorate rapidly and require intensive care. Unlike neuroleptic malignant syndrome, serotonin syndrome should not be considered an extremely rare idiosyncratic reaction to medication, but rather a progression of serotonergic toxicity based on increasing concentration levels that can occur in any patient regardless of age.4
Because it has a nonspecific prodrome and protean manifestations, serotonin syndrome can easily be overlooked, misdiagnosed, or exacerbated if not carefully assessed. Diagnosis requires a low threshold for suspicion and a meticulous history and physical examination. In the syndrome’s mildest stage, symptoms are often misattributed to other causes, and in its most severe form, it can easily be mistaken for neuroleptic malignant syndrome.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome classically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms are a result of increased serotonin levels affecting the central and peripheral nervous systems. Serotonin affects a family of receptors that has seven members, of which 5-HT1A and 5-HT2A are most often responsible for serotonin syndrome.5
Conditions that can alter the regulation of serotonin include therapeutic doses, drug interactions, intentional or unintentional overdoses, and overlapping transitions between medications. As a result, drugs that have been associated with serotonin syndrome can be classified into the following five categories as shown below and in Table 1:
Drugs that decrease serotonin breakdown include monoamine oxidase inhibitors (MAOIs), linezolid,6 methylene blue, procarbazine, and Syrian rue.
Drugs that decrease serotonin reuptake include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, opioids (meperidine, buprenorphine, tramadol, tapentadol, dextromethorphan), antiepileptics (carbamazepine, valproate), and antiemetics (ondansetron, granisetron, metoclopramide), and the herbal preparation St. John’s wort.
Drugs that increase serotonin precursors or agonists include tryptophan, lithium, fentanyl, and lysergic acid diethylamide (LSD).
Drugs that increase serotonin release include fenfluramine, amphetamines, and methylenedioxymethamphetamine (ecstasy).
Drugs that prevent breakdown of the agents listed above are CYP2D6 and CYP3A4 inhibitors, eg, erythromycin,7 ciprofloxacin, fluconazole, ritonavir, and grapefruit juice.
However, the only drugs that have been reliably confirmed to precipitate serotonin syndrome are MAOIs, SSRIs, SNRIs, and serotonin releasers. Other listed drug interactions are based on case reports and have not been thoroughly evaluated.6–9
Currently, SSRIs are the most commonly prescribed antidepressant medications and, consequently, they are the ones most often implicated in serotonergic toxicity.1,10 An estimated 15% of SSRI overdoses lead to mild or moderate serotonin toxicity.11 Serotonergic agents used in conjunction can increase the risk for severe serotonin syndrome; an SSRI and an MAOI in combination poses the greatest risk.5
Ultimately, the incidence of serotonin syndrome is difficult to assess, but it is believed to be underreported because it is easy to misdiagnose and mild symptoms may be dismissed.
WHO IS AT RISK OF SEROTONIN SYNDROME?
Long-term antidepressant use has disproportionately increased in middle-aged and older adults and non-Hispanic whites.1,12,13 Intuitively, as the risk for depression increases dramatically in patients with chronic medical conditions, serotonin syndrome should be more prevalent among the elderly. In addition, patients with multiple comorbidities take more medications, increasing the risk of polypharmacy and adverse drug reactions.14
Although the epidemiology of serotonin syndrome has yet to be extensively studied, the combination of age and comorbidities may increase the risk for this condition.
HOW DOES IT PRESENT?
Serotonin syndrome characteristically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. However, these symptoms may not occur simultaneously: autonomic dysfunction is present in 40% of patients, neuromuscular excitation in 50%, and altered mental status in 40%.15 The symptoms can range from mild to life-threatening (Table 2).16
Autonomic dysfunction. Diaphoresis is present in 48.8% of cases, tachycardia in 44%, nausea and vomiting in 26.8%, and mydriasis in 19.5%. Other signs are hyperactive bowel sounds, diarrhea, and flushing.16
Neuromuscular excitation. Myoclonus is present in 48.8%, hyperreflexia in 41%, hyperthermia in 26.8%, and hypertonicity and rigidity in 19.5%. Other signs are spontaneous or inducible clonus, ocular clonus (continuous rhythmic oscillations of gaze), and tremor.
Altered mental status. Confusion is present in 41.2% and agitation in 36.5%. Other signs are anxiety, lethargy, and coma.
Symptoms of serotonin toxicity arise within an hour of a precipitating event (eg, ingestion) in approximately 28% of patients, and within 6 hours in 61%.16 Highly diagnostic features include hyperreflexia and induced or spontaneous clonus that are generally more pronounced in the lower limbs.11 Clonus can be elicited with ankle dorsiflexion.
In mild toxicity, patients may present with tremor or twitching and anxiety, as well as with hyperreflexia, tachycardia, diaphoresis, and mydriasis. Further investigation may uncover a recently initiated antidepressant or a cold-and-cough medication that contains dextromethorphan.15,17
In moderate toxicity, patients present in significant distress, with agitation and restlessness. Features may include hyperreflexia and clonus of the lower extremities, opsoclonus, hyperactive bowel sounds, diarrhea, nausea, vomiting, tachycardia, hypertension, diaphoresis, mydriasis, and hyperthermia (< 40°C, 104°F). The patient’s history may reveal use of ecstasy or combined treatment with serotonin-potentiating agents such as an antidepressant with a proserotonergic opioid, antiepileptic, or CYP2D6 or CYP3A4 inhibitor.15
Severe serotonin toxicity is a life-threatening condition that can lead to multiorgan failure within hours. It can be characterized by muscle rigidity, which can cause the body temperature to elevate rapidly to over 40°C. This hypertonicity can mask the classic and diagnostic signs of hyperreflexia and clonus. Patients may have unstable and dynamic vital signs with confusion or delirium and can experience tonic-clonic seizures.
If the muscle rigidity and resulting hyperthermia are not managed properly, patients can develop cellular damage and enzyme dysfunction leading to rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress syndrome, and disseminated intravascular coagulation.16,18
Serotonin crisis is usually caused by the co-ingestion of multiple serotonergic agents, such as an antidepressant with an aforementioned opioid and antiemetic19; combining an SSRI and an MAOI poses the greatest risk. Alternatively, patients may have recently switched antidepressants without observing a safe washout period, leading to an overlap of serotonin levels.16
HOW DO WE DIAGNOSE SEROTONIN SYNDROME?
Serotonin syndrome is a clinical diagnosis and therefore requires a thorough review of medications and physical examination. Serum serotonin levels are an unreliable indicator of toxicity and do not correlate well with the clinical presentation.16
(based on information in reference 9).
Currently, there are two clinical tools for diagnosing serotonin syndrome: the Hunter serotonin toxicity criteria (Figure 1) and the Sternbach criteria.
The Hunter criteria are based more heavily on physical findings. The patient must have taken a serotonergic agent and have one of the following:
- Spontaneous clonus
- Inducible clonus plus agitation or diaphoresis
- Ocular clonus plus agitation or diaphoresis
- Inducible clonus or ocular clonus, plus hypertonia and hyperthermia
- Tremor plus hyperreflexia.
The Sternbach criteria. The patient must be using a serotonergic agent, must have no other causes of symptoms, must not have recently used a neuroleptic agent, and must have three of the following:
- Mental status changes
- Agitation
- Hyperreflexia
- Myoclonus
- Diaphoresis
- Shivering
- Tremor
- Diarrhea
- Incoordination
- Fever
The Hunter criteria are recommended and are more specific (97% vs 96%) and more sensitive (84% vs 75%) than the Sternbach criteria when compared with the gold standard of diagnosis by a clinical toxicologist.1 The Hunter criteria are also less likely to yield false-positive results.11
Differential diagnosis
The differential diagnosis for serotonin syndrome includes neuroleptic malignant syndrome, anticholinergic poisoning (Table 3), metastatic carcinoma, central nervous system infection, gastroenteritis, and sepsis.
Neuroleptic malignant syndrome, the disorder most often misdiagnosed as serotonin syndrome, is an idiosyncratic reaction to a dopamine antagonist (eg, haloperidol, fluphenazine) that develops over days to weeks.20 In 70% of patients, agitated delirium with confusion appears first, followed by lead pipe rigidity and cogwheel tremor, then hyperthermia with body temperature greater than 40°C, and finally, profuse diaphoresis, tachycardia, hypertension, and tachypnea.21
Key elements that distinguish neuroleptic malignant syndrome are the timeline of the clinical course, bradyreflexia, and the absence of clonus. Prodromal symptoms of nausea, vomiting, and diarrhea are also rare in neuroleptic malignant syndrome. Neuroleptic malignant syndrome typically requires an average of 9 days to resolve.
Anticholinergic poisoning usually develops within 1 to 2 hours of oral ingestion. Symptoms include flushing, anhidrosis, anhidrotic hyperthermia, mydriasis, urinary retention, decreased bowel sounds, agitated delirium, and visual hallucinations. In contrast to serotonin syndrome, reflexes and muscle tone are normal with anticholinergic poisoning.
HOW CAN WE TREAT SEROTONIN SYNDROME?
The two mainstays of serotonin syndrome management are to discontinue the serotonergic agent and to give supportive care. Most patients improve within 24 hours of stopping the precipitating drug and starting therapy.16
For mild serotonin syndrome, treatment involves discontinuing the offending agent and supportive therapy with intravenous fluids, correction of vital signs, and symptomatic treatment with a benzodiazepine. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For moderate serotonin syndrome, treatment also involves stopping the serotonergic agent and giving supportive care. Symptomatic treatment with a benzodiazepine and nonserotonergic antiemetics is recommended, and standard cooling measures should be implemented for hyperthermia. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For severe serotonin toxicity, treatment should focus on management of airway, breathing, and circulation—ie, the “ABCs.” The two primary life-threatening concerns are hyperthermia (temperature > 40°C or 104°F) and rigidity, which can lead to hypoventilation.1,22 Controlling hyperthermia and rigidity can prevent other grave complications. Patients with severe serotonin toxicity should be sedated, paralyzed, and intubated.21 This will reverse ventilatory hypertonia and allow for mechanical ventilation. Paralysis will also prevent the exacerbation of hyperthermia, which is caused by muscle rigidity. Antipyretics have no role in the treatment of serotonin syndrome since the hyperthermia is not caused by a change in the hypothalamic temperature set point.21 Standard cooling measures should be used to manage hyperthermia.
Serotonin antagonists
Serotonin antagonists have had some success in case reports, but further studies are needed to confirm this.4,23,24
Cyproheptadine is a potent 5-HT2A antagonist; patients usually respond within 1 to 2 hours of administration. Signs and symptoms have resolved completely within times ranging from 20 minutes to 48 hours, depending on the severity of toxicity.
The recommended initial dose of cyproheptadine is 12 mg, followed by 2 mg every 2 hours if symptoms continue.16 Maintenance dosing with 8 mg every 6 hours should be prescribed once stabilization is achieved. The total daily dose for adults should not exceed 0.5 mg/kg/day. Cyproheptadine is available only in oral form but can be crushed and administered via a nasogastric tube.21
Chlorpromazine is a 5-HT1A and 5-HT2A antagonist and can be given intramuscularly. Despite case reports citing its effectiveness, the risk of hypotension, dystonic reactions, and neuroleptic malignant syndrome may make it a less desirable option.4,25
Cyproheptadine, chlorpromazine, and other serotonin receptor antagonists require further investigation beyond individual case reports to determine their effectiveness and reliability in treating serotonin syndrome.
Other agents
Benzodiazepines are considered a mainstay for symptomatic relief because of their anxiolytic and muscle relaxant effects.26 However, animal studies showed that treatment with benzodiazepines attenuated hyperthermia but had no effect on time to recovery or outcome.27
Neuromuscular blocking agents. The suggested neuromuscular blocking agent for severe toxicity is a nondepolarizing agent such as vecuronium. Succinylcholine should be avoided, as it can exacerbate rhabdomyolysis and hyperkalemia.21
Dantrolene has also been suggested for its muscle-relaxing effects and use in malignant hyperthermia. However, this treatment has not been successful in isolated case reports and has been ineffective in animal models.4,28
Physical restraints are ill-advised, since isometric muscle contractions can exacerbate hyperthermia and lactic acidosis in agitated patients.21 If physical restraints are necessary to deliver medications, they should be removed as soon as possible.
HOW CAN WE PREVENT SEROTONIN SYNDROME?
Prevention of serotonin syndrome begins with improving education and awareness in patients and healthcare providers. Patients should be primarily concerned with taking their medications carefully as prescribed and recognizing early signs and symptoms of serotonin toxicity.
As use of antidepressants among an aging population continues to increase, and as physicians in multiple disciplines prescribe them for evolving indications (eg, duloxetine to treat osteoarthritis, diabetic neuropathy, fibromyalgia, and chemotherapy-induced peripheral neuropathy), healthcare providers need to be prepared to see more cases of serotonin syndrome and its deleterious effects.29–31 Physicians should be vigilant in minimizing unnecessary use of serotonergic agents and reviewing drug regimens regularly to limit polypharmacy.
Electronic ordering systems should be designed to detect and alert the prescriber to possible interactions that can potentiate serotonin syndrome, and to not place the order until the prescriber overrides the alert. Combinations of SSRIs and MAOIs have the highest risk for inducing severe serotonin syndrome and should always be avoided.
If a patient is transitioning between serotonergic agents, physicians should observe a safe washout period to prevent overlap.16,32 Washout periods may differ among medications depending on their half-lives. For example, sertraline has a washout period of 2 weeks, while fluoxetine requires a washout period of 5 to 6 weeks.33 Consulting a pharmacist may be helpful when considering half-lives and washout periods.
We believe that educating both patients and physicians regarding prevention will help minimize the risk for serotonergic syndrome and will improve efficiency in assessment and management should toxicity develop.
With a substantial increase in antidepressant use in the United States over the last 2 decades, serotonin syndrome has become an increasingly common and significant clinical concern. In 1999, 6.5% of adults age 18 and older were taking antidepressants; by 2010, the percentage had increased to 10.4%.1 Though the true incidence of serotonin syndrome is difficult to determine, the number of ingestions of selective serotonin reuptake inhibitors (SSRIs) associated with moderate to major effects reported to US poison control centers increased from 7,349 in 20022 to 8,585 in 2005.3
Though the clinical manifestations are often mild to moderate, patients with serotonin syndrome can deteriorate rapidly and require intensive care. Unlike neuroleptic malignant syndrome, serotonin syndrome should not be considered an extremely rare idiosyncratic reaction to medication, but rather a progression of serotonergic toxicity based on increasing concentration levels that can occur in any patient regardless of age.4
Because it has a nonspecific prodrome and protean manifestations, serotonin syndrome can easily be overlooked, misdiagnosed, or exacerbated if not carefully assessed. Diagnosis requires a low threshold for suspicion and a meticulous history and physical examination. In the syndrome’s mildest stage, symptoms are often misattributed to other causes, and in its most severe form, it can easily be mistaken for neuroleptic malignant syndrome.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome classically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms are a result of increased serotonin levels affecting the central and peripheral nervous systems. Serotonin affects a family of receptors that has seven members, of which 5-HT1A and 5-HT2A are most often responsible for serotonin syndrome.5
Conditions that can alter the regulation of serotonin include therapeutic doses, drug interactions, intentional or unintentional overdoses, and overlapping transitions between medications. As a result, drugs that have been associated with serotonin syndrome can be classified into the following five categories as shown below and in Table 1:
Drugs that decrease serotonin breakdown include monoamine oxidase inhibitors (MAOIs), linezolid,6 methylene blue, procarbazine, and Syrian rue.
Drugs that decrease serotonin reuptake include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, opioids (meperidine, buprenorphine, tramadol, tapentadol, dextromethorphan), antiepileptics (carbamazepine, valproate), and antiemetics (ondansetron, granisetron, metoclopramide), and the herbal preparation St. John’s wort.
Drugs that increase serotonin precursors or agonists include tryptophan, lithium, fentanyl, and lysergic acid diethylamide (LSD).
Drugs that increase serotonin release include fenfluramine, amphetamines, and methylenedioxymethamphetamine (ecstasy).
Drugs that prevent breakdown of the agents listed above are CYP2D6 and CYP3A4 inhibitors, eg, erythromycin,7 ciprofloxacin, fluconazole, ritonavir, and grapefruit juice.
However, the only drugs that have been reliably confirmed to precipitate serotonin syndrome are MAOIs, SSRIs, SNRIs, and serotonin releasers. Other listed drug interactions are based on case reports and have not been thoroughly evaluated.6–9
Currently, SSRIs are the most commonly prescribed antidepressant medications and, consequently, they are the ones most often implicated in serotonergic toxicity.1,10 An estimated 15% of SSRI overdoses lead to mild or moderate serotonin toxicity.11 Serotonergic agents used in conjunction can increase the risk for severe serotonin syndrome; an SSRI and an MAOI in combination poses the greatest risk.5
Ultimately, the incidence of serotonin syndrome is difficult to assess, but it is believed to be underreported because it is easy to misdiagnose and mild symptoms may be dismissed.
WHO IS AT RISK OF SEROTONIN SYNDROME?
Long-term antidepressant use has disproportionately increased in middle-aged and older adults and non-Hispanic whites.1,12,13 Intuitively, as the risk for depression increases dramatically in patients with chronic medical conditions, serotonin syndrome should be more prevalent among the elderly. In addition, patients with multiple comorbidities take more medications, increasing the risk of polypharmacy and adverse drug reactions.14
Although the epidemiology of serotonin syndrome has yet to be extensively studied, the combination of age and comorbidities may increase the risk for this condition.
HOW DOES IT PRESENT?
Serotonin syndrome characteristically presents as the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. However, these symptoms may not occur simultaneously: autonomic dysfunction is present in 40% of patients, neuromuscular excitation in 50%, and altered mental status in 40%.15 The symptoms can range from mild to life-threatening (Table 2).16
Autonomic dysfunction. Diaphoresis is present in 48.8% of cases, tachycardia in 44%, nausea and vomiting in 26.8%, and mydriasis in 19.5%. Other signs are hyperactive bowel sounds, diarrhea, and flushing.16
Neuromuscular excitation. Myoclonus is present in 48.8%, hyperreflexia in 41%, hyperthermia in 26.8%, and hypertonicity and rigidity in 19.5%. Other signs are spontaneous or inducible clonus, ocular clonus (continuous rhythmic oscillations of gaze), and tremor.
Altered mental status. Confusion is present in 41.2% and agitation in 36.5%. Other signs are anxiety, lethargy, and coma.
Symptoms of serotonin toxicity arise within an hour of a precipitating event (eg, ingestion) in approximately 28% of patients, and within 6 hours in 61%.16 Highly diagnostic features include hyperreflexia and induced or spontaneous clonus that are generally more pronounced in the lower limbs.11 Clonus can be elicited with ankle dorsiflexion.
In mild toxicity, patients may present with tremor or twitching and anxiety, as well as with hyperreflexia, tachycardia, diaphoresis, and mydriasis. Further investigation may uncover a recently initiated antidepressant or a cold-and-cough medication that contains dextromethorphan.15,17
In moderate toxicity, patients present in significant distress, with agitation and restlessness. Features may include hyperreflexia and clonus of the lower extremities, opsoclonus, hyperactive bowel sounds, diarrhea, nausea, vomiting, tachycardia, hypertension, diaphoresis, mydriasis, and hyperthermia (< 40°C, 104°F). The patient’s history may reveal use of ecstasy or combined treatment with serotonin-potentiating agents such as an antidepressant with a proserotonergic opioid, antiepileptic, or CYP2D6 or CYP3A4 inhibitor.15
Severe serotonin toxicity is a life-threatening condition that can lead to multiorgan failure within hours. It can be characterized by muscle rigidity, which can cause the body temperature to elevate rapidly to over 40°C. This hypertonicity can mask the classic and diagnostic signs of hyperreflexia and clonus. Patients may have unstable and dynamic vital signs with confusion or delirium and can experience tonic-clonic seizures.
If the muscle rigidity and resulting hyperthermia are not managed properly, patients can develop cellular damage and enzyme dysfunction leading to rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress syndrome, and disseminated intravascular coagulation.16,18
Serotonin crisis is usually caused by the co-ingestion of multiple serotonergic agents, such as an antidepressant with an aforementioned opioid and antiemetic19; combining an SSRI and an MAOI poses the greatest risk. Alternatively, patients may have recently switched antidepressants without observing a safe washout period, leading to an overlap of serotonin levels.16
HOW DO WE DIAGNOSE SEROTONIN SYNDROME?
Serotonin syndrome is a clinical diagnosis and therefore requires a thorough review of medications and physical examination. Serum serotonin levels are an unreliable indicator of toxicity and do not correlate well with the clinical presentation.16
(based on information in reference 9).
Currently, there are two clinical tools for diagnosing serotonin syndrome: the Hunter serotonin toxicity criteria (Figure 1) and the Sternbach criteria.
The Hunter criteria are based more heavily on physical findings. The patient must have taken a serotonergic agent and have one of the following:
- Spontaneous clonus
- Inducible clonus plus agitation or diaphoresis
- Ocular clonus plus agitation or diaphoresis
- Inducible clonus or ocular clonus, plus hypertonia and hyperthermia
- Tremor plus hyperreflexia.
The Sternbach criteria. The patient must be using a serotonergic agent, must have no other causes of symptoms, must not have recently used a neuroleptic agent, and must have three of the following:
- Mental status changes
- Agitation
- Hyperreflexia
- Myoclonus
- Diaphoresis
- Shivering
- Tremor
- Diarrhea
- Incoordination
- Fever
The Hunter criteria are recommended and are more specific (97% vs 96%) and more sensitive (84% vs 75%) than the Sternbach criteria when compared with the gold standard of diagnosis by a clinical toxicologist.1 The Hunter criteria are also less likely to yield false-positive results.11
Differential diagnosis
The differential diagnosis for serotonin syndrome includes neuroleptic malignant syndrome, anticholinergic poisoning (Table 3), metastatic carcinoma, central nervous system infection, gastroenteritis, and sepsis.
Neuroleptic malignant syndrome, the disorder most often misdiagnosed as serotonin syndrome, is an idiosyncratic reaction to a dopamine antagonist (eg, haloperidol, fluphenazine) that develops over days to weeks.20 In 70% of patients, agitated delirium with confusion appears first, followed by lead pipe rigidity and cogwheel tremor, then hyperthermia with body temperature greater than 40°C, and finally, profuse diaphoresis, tachycardia, hypertension, and tachypnea.21
Key elements that distinguish neuroleptic malignant syndrome are the timeline of the clinical course, bradyreflexia, and the absence of clonus. Prodromal symptoms of nausea, vomiting, and diarrhea are also rare in neuroleptic malignant syndrome. Neuroleptic malignant syndrome typically requires an average of 9 days to resolve.
Anticholinergic poisoning usually develops within 1 to 2 hours of oral ingestion. Symptoms include flushing, anhidrosis, anhidrotic hyperthermia, mydriasis, urinary retention, decreased bowel sounds, agitated delirium, and visual hallucinations. In contrast to serotonin syndrome, reflexes and muscle tone are normal with anticholinergic poisoning.
HOW CAN WE TREAT SEROTONIN SYNDROME?
The two mainstays of serotonin syndrome management are to discontinue the serotonergic agent and to give supportive care. Most patients improve within 24 hours of stopping the precipitating drug and starting therapy.16
For mild serotonin syndrome, treatment involves discontinuing the offending agent and supportive therapy with intravenous fluids, correction of vital signs, and symptomatic treatment with a benzodiazepine. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For moderate serotonin syndrome, treatment also involves stopping the serotonergic agent and giving supportive care. Symptomatic treatment with a benzodiazepine and nonserotonergic antiemetics is recommended, and standard cooling measures should be implemented for hyperthermia. Patients should be admitted and observed for 12 to 24 hours to prevent exacerbation.
For severe serotonin toxicity, treatment should focus on management of airway, breathing, and circulation—ie, the “ABCs.” The two primary life-threatening concerns are hyperthermia (temperature > 40°C or 104°F) and rigidity, which can lead to hypoventilation.1,22 Controlling hyperthermia and rigidity can prevent other grave complications. Patients with severe serotonin toxicity should be sedated, paralyzed, and intubated.21 This will reverse ventilatory hypertonia and allow for mechanical ventilation. Paralysis will also prevent the exacerbation of hyperthermia, which is caused by muscle rigidity. Antipyretics have no role in the treatment of serotonin syndrome since the hyperthermia is not caused by a change in the hypothalamic temperature set point.21 Standard cooling measures should be used to manage hyperthermia.
Serotonin antagonists
Serotonin antagonists have had some success in case reports, but further studies are needed to confirm this.4,23,24
Cyproheptadine is a potent 5-HT2A antagonist; patients usually respond within 1 to 2 hours of administration. Signs and symptoms have resolved completely within times ranging from 20 minutes to 48 hours, depending on the severity of toxicity.
The recommended initial dose of cyproheptadine is 12 mg, followed by 2 mg every 2 hours if symptoms continue.16 Maintenance dosing with 8 mg every 6 hours should be prescribed once stabilization is achieved. The total daily dose for adults should not exceed 0.5 mg/kg/day. Cyproheptadine is available only in oral form but can be crushed and administered via a nasogastric tube.21
Chlorpromazine is a 5-HT1A and 5-HT2A antagonist and can be given intramuscularly. Despite case reports citing its effectiveness, the risk of hypotension, dystonic reactions, and neuroleptic malignant syndrome may make it a less desirable option.4,25
Cyproheptadine, chlorpromazine, and other serotonin receptor antagonists require further investigation beyond individual case reports to determine their effectiveness and reliability in treating serotonin syndrome.
Other agents
Benzodiazepines are considered a mainstay for symptomatic relief because of their anxiolytic and muscle relaxant effects.26 However, animal studies showed that treatment with benzodiazepines attenuated hyperthermia but had no effect on time to recovery or outcome.27
Neuromuscular blocking agents. The suggested neuromuscular blocking agent for severe toxicity is a nondepolarizing agent such as vecuronium. Succinylcholine should be avoided, as it can exacerbate rhabdomyolysis and hyperkalemia.21
Dantrolene has also been suggested for its muscle-relaxing effects and use in malignant hyperthermia. However, this treatment has not been successful in isolated case reports and has been ineffective in animal models.4,28
Physical restraints are ill-advised, since isometric muscle contractions can exacerbate hyperthermia and lactic acidosis in agitated patients.21 If physical restraints are necessary to deliver medications, they should be removed as soon as possible.
HOW CAN WE PREVENT SEROTONIN SYNDROME?
Prevention of serotonin syndrome begins with improving education and awareness in patients and healthcare providers. Patients should be primarily concerned with taking their medications carefully as prescribed and recognizing early signs and symptoms of serotonin toxicity.
As use of antidepressants among an aging population continues to increase, and as physicians in multiple disciplines prescribe them for evolving indications (eg, duloxetine to treat osteoarthritis, diabetic neuropathy, fibromyalgia, and chemotherapy-induced peripheral neuropathy), healthcare providers need to be prepared to see more cases of serotonin syndrome and its deleterious effects.29–31 Physicians should be vigilant in minimizing unnecessary use of serotonergic agents and reviewing drug regimens regularly to limit polypharmacy.
Electronic ordering systems should be designed to detect and alert the prescriber to possible interactions that can potentiate serotonin syndrome, and to not place the order until the prescriber overrides the alert. Combinations of SSRIs and MAOIs have the highest risk for inducing severe serotonin syndrome and should always be avoided.
If a patient is transitioning between serotonergic agents, physicians should observe a safe washout period to prevent overlap.16,32 Washout periods may differ among medications depending on their half-lives. For example, sertraline has a washout period of 2 weeks, while fluoxetine requires a washout period of 5 to 6 weeks.33 Consulting a pharmacist may be helpful when considering half-lives and washout periods.
We believe that educating both patients and physicians regarding prevention will help minimize the risk for serotonergic syndrome and will improve efficiency in assessment and management should toxicity develop.
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry 2014; 75:169–177.
- Watson WA, Litovitz TL, Rodgers GC Jr, et al. 2002 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2003;21:353–421.
- Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exopsure database. Clin Toxicol (Phila) 2006; 44:803–932.
- Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999; 13:100–109.
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28:205–214.
- Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 2013; 47:388–397.
- Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999; 19:894–896.
- Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache 2010; 50:264–272.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust 2007; 187:361–365.
- Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014; 52:1032–1283.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–642.
- Karkare SU, Bhattacharjee S, Kamble P, Aparasu R. Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. Am J Geriatr Pharmacother 2011; 9:109–119.
- Weissman J, Meyers BS, Ghosh S, Bruce ML. Demographic, clinical, and functional factors associated with antidepressant use in the home healthcare elderly. Am J Geriatr Psychiatry 2011; 19:1042–1045.
- Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Co-morbidity and potential treatment conflicts in elderly heart failure patients: a retrospective, cross-sectional study of administrative claims data. Drugs Aging 2011; 28:575–581.
- Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Ann Clin Psychiatry 2012; 24:310–318.
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2000; 79:201–209.
- Prakash S, Patel V, Kakked S, Patel I, Yadav R. Mild serotonin syndrome: a report of 12 cases. Ann Indian Acad Neurol 2015; 18:226–230.
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung 2014; 43:117–119.
- Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care 2014; 21:108–113.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
- Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994; 182:168–173.
- Isbister GK, Hackett LP, Dawson AH, Whyte IM, Smith AJ. Moclobemide poisoning: toxicokinetics and occurrence of serotonin toxicity. Br J Clin Pharmacol 2003; 56:441–450.
- Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16:615–619.
- Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med 1994; 331:1021–1022.
- Gillman PK. Successful treatment of serotonin syndrome with chlorpromazine. Med J Aust 1996; 165:345–346.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ 2014; 348:g1626.
- Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43:155–164.
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001; 890:23–31.
- Micca JL, Ruff D, Ahl J, Wohlreich MM. Safety and efficacy of duloxetine treatment in older and younger patients with osteoarthritis knee pain: a post hoc, subgroup analysis of two randomized, placebo-controlled trials. BMC Musculoskelet Disord 2013; 14:137.
- Smith EM, Pang H, Cirrincione C, et al; Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309:1359–1367.
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 2014; 1:CD007115.
- Caughey GE, Roughead EE, Shakib S, McDermott RA, Vitry AI, Gilbert AL. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010; 39:488–494.
- Gury C, Cousin F. Pharmacokinetics of SSRI antidepressants: half-life and clinical applicability. Encephale 1999; 25:470–476. French.
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry 2014; 75:169–177.
- Watson WA, Litovitz TL, Rodgers GC Jr, et al. 2002 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2003;21:353–421.
- Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exopsure database. Clin Toxicol (Phila) 2006; 44:803–932.
- Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999; 13:100–109.
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28:205–214.
- Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 2013; 47:388–397.
- Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999; 19:894–896.
- Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache 2010; 50:264–272.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust 2007; 187:361–365.
- Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014; 52:1032–1283.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–642.
- Karkare SU, Bhattacharjee S, Kamble P, Aparasu R. Prevalence and predictors of antidepressant prescribing in nursing home residents in the United States. Am J Geriatr Pharmacother 2011; 9:109–119.
- Weissman J, Meyers BS, Ghosh S, Bruce ML. Demographic, clinical, and functional factors associated with antidepressant use in the home healthcare elderly. Am J Geriatr Psychiatry 2011; 19:1042–1045.
- Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Co-morbidity and potential treatment conflicts in elderly heart failure patients: a retrospective, cross-sectional study of administrative claims data. Drugs Aging 2011; 28:575–581.
- Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Ann Clin Psychiatry 2012; 24:310–318.
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2000; 79:201–209.
- Prakash S, Patel V, Kakked S, Patel I, Yadav R. Mild serotonin syndrome: a report of 12 cases. Ann Indian Acad Neurol 2015; 18:226–230.
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung 2014; 43:117–119.
- Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care 2014; 21:108–113.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
- Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994; 182:168–173.
- Isbister GK, Hackett LP, Dawson AH, Whyte IM, Smith AJ. Moclobemide poisoning: toxicokinetics and occurrence of serotonin toxicity. Br J Clin Pharmacol 2003; 56:441–450.
- Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16:615–619.
- Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med 1994; 331:1021–1022.
- Gillman PK. Successful treatment of serotonin syndrome with chlorpromazine. Med J Aust 1996; 165:345–346.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ 2014; 348:g1626.
- Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003; 43:155–164.
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001; 890:23–31.
- Micca JL, Ruff D, Ahl J, Wohlreich MM. Safety and efficacy of duloxetine treatment in older and younger patients with osteoarthritis knee pain: a post hoc, subgroup analysis of two randomized, placebo-controlled trials. BMC Musculoskelet Disord 2013; 14:137.
- Smith EM, Pang H, Cirrincione C, et al; Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309:1359–1367.
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 2014; 1:CD007115.
- Caughey GE, Roughead EE, Shakib S, McDermott RA, Vitry AI, Gilbert AL. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010; 39:488–494.
- Gury C, Cousin F. Pharmacokinetics of SSRI antidepressants: half-life and clinical applicability. Encephale 1999; 25:470–476. French.
KEY POINTS
- Serotonin syndrome is caused by elevated serotonin levels in the central and peripheral nervous systems.
- The classic presentation is the triad of autonomic dysfunction, neuromuscular excitation, and altered mental status. These symptoms vary based on the severity of serotonergic toxicity and often do not present concomitantly.
- Early recognition is critical to ensure appropriate resuscitative measures and to limit further use of drugs that can exacerbate symptoms.