User login
VTE Diagnosis and Treatment
Despite the availability of effective thromboprophylaxis, the prevalence of venous thromboembolism (VTE) is increasing in the hospital setting. In 2008, the Fifth Annual Health Grades Patient Safety in American Hospitals Study reported on key patient safety incidents among nearly 41 million hospitalizations in the Medicare population between 2004 and 2006. Although many areas showed improvementincluding reduced rates of hospital‐related infections, postoperative bleeding, transfusion reactions, and other injuriesthe number of cases of postoperative VTE increased by 11% during this period.1
Even with optimal thromboprophylaxis, VTE will develop in some at‐risk patients. Early diagnosis and treatment of VTE is critical to reduce morbidity and mortality, but no single tool can definitively confirm its presence. Consequently, the detection of deep vein thrombosis (DVT) and pulmonary embolism (PE) requires a stepwise diagnostic strategy that combines clinical, biochemical, and imaging modalities.
In addition to outlining diagnostic strategies for DVT and PE, this article summarizes VTE treatment guidelines from various organizations and societies and discusses long‐term management strategies to prevent recurrent VTE and other complications.
Diagnosis of DVT
The clinical symptoms and signs of DVT are nonspecific and include unilateral calf, leg, or thigh swelling and pain. Despite the limited sensitivity and specificity of individual signs and symptoms of DVT, the combination of these variables can be useful in assessing the probability of VTE. Patients can be risk stratified according to the likelihood of DVT, as determined by implicit clinical judgment or by a validated prediction rule.2
Assessment of Clinical Probability
The Wells prediction rule is used in assessing the probability of DVT.3 It incorporates signs, symptoms, and risk factors of DVT to calculate a clinical probability rating. Specifically, 1 point is assigned to each of the following factors, if present:3
-
Active cancer (treatment ongoing, within 6 months, or palliative)
-
Calf swelling >3 cm asymptomatic side (measured 10 cm below tibial tuberosity)
-
Collateral superficial veins (nonvaricose)
-
Entire leg swelling
-
Localized tenderness along the distribution of the deep venous system
-
Paralysis, paresis, or recent plaster immobilization of the lower extremities
-
Pitting edema confined to the symptomatic leg
-
Recently bedridden more than 3 days or major surgery within 4 weeks
In addition, 2 points are subtracted if an alternative diagnosis is as likely as or more likely than DVT. In patients with symptoms in both legs, the more symptomatic leg is used.
Patients with low (score <1), moderate (score 1‐2), and high (score 3) pretest probability of DVT have been shown to have DVT prevalence rates of 3%, 17%, and 75%, respectively.3
D‐Dimer Testing
D‐dimer testing measures the small protein fragments remaining in the blood after a cross‐linked fibrin clot is degraded by fibrinolysis. A low clinical probability assessment combined with a negative result in a highly sensitive, enzyme‐linked immunosorbent assay (ELISA)‐based D‐dimer test can safely exclude DVT, with a negative predictive value of 99.1% (95% confidence interval [CI]; 96.7‐99.9).4
Due to its poor specificity, D‐dimer testing has limited utility in unselected inpatients, especially older patients and those who have undergone prolonged hospitalization.5 However, it is reasonable to obtain a highly sensitive, ELISA‐based D‐dimer test in carefully selected inpatients with a low pretest probability of DVT.5, 6 In such patients, a negative result indicates that DVT is highly unlikely, while a positive result indicates a need for further testing. D‐dimer testing is likely not helpful in moderate‐risk or high‐risk patients.
Diagnostic Imaging
For patients with a moderate to high pretest probability of DVT, ultrasound is recommended.6 Compression ultrasonography (CUS) is currently the preferred imaging tool in patients with suspected DVT because it is noninvasive, can be repeated serially, and offers high sensitivity (+90%) and high specificity (95%) for detecting proximal vein thrombosis.7, 8 If the clinical suspicion of DVT persists after an initial negative CUS study, imaging can be repeated after 3 to 7 days to detect the propagation of any thrombosis to the proximal veins. Limitations of CUS include poor visualization of deep iliac and pelvic veins and poor sensitivity in isolated or nonocclusive calf vein thrombi.2
Contrast venography was considered the gold standard for the detection of DVT of the lower extremity, but this modality is invasive, painful, and offers poor visualization of the deep femoral vein and the internal iliac vein. In addition, contrast venography is associated with an increased risk of new thrombosis, renal failure, and hypersensitivity reaction to contrast media. Consequently, contrast venography is currently used in symptomatic patients only when noninvasive testing is inconclusive or unavailable.2 Other second‐line diagnostic tools include computed tomography venography (CTV) and magnetic resonance venography (MRV).9
Diagnostic Strategy
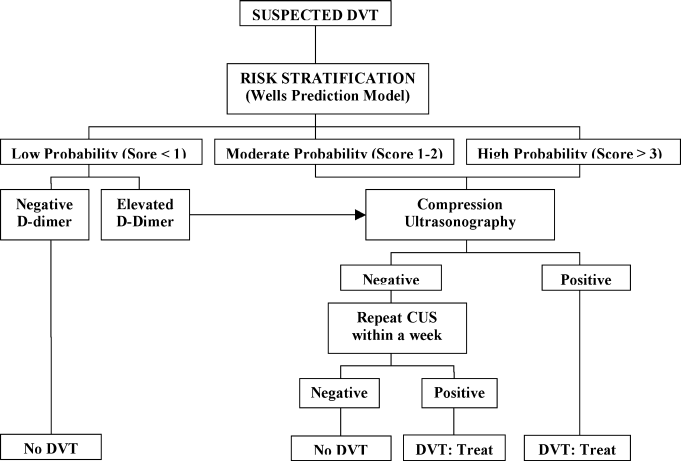
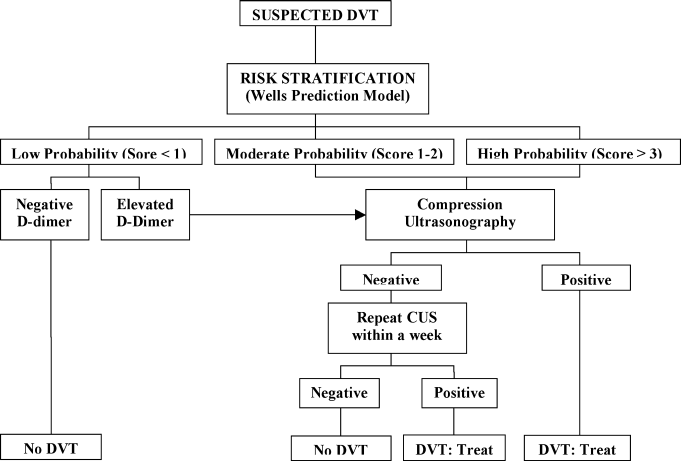
A diagnostic algorithm for DVT is presented in Figure 1. First, a validated clinical prediction scale such as the Wells prediction rule should be used to estimate the pretest probability of DVT, and the result of the clinical assessment should influence the choice and interpretation of subsequent testing.
Abbreviations: CUS, compression ultrasonography; DVT, deep vein thrombosis (DVT).
Diagnosis of PE
Clinical symptoms and signs such as dyspnea, chest pain, tachycardia, tachypnea, and syncope raise the suspicion of PE. Individual signs and symptoms, however, cannot confirm or exclude acute PE, as they are neither sensitive nor specific.10 Furthermore, although the likelihood of PE increases with the number of predisposing risk factors, approximately 30% of PE cases are unprovoked or idiopathic, meaning that they occur in the absence of predisposing factors. Diagnosis, therefore, depends on an integrated strategy involving similar tools as those used in diagnosing DVT.
Assessing Clinical Probability
Wells et al.11 also developed a clinical prediction rule for the risk stratification of patients with suspected PE. In this model, 7 specified variables are assigned different scores: clinical signs and symptoms of DVT (3.0); lack of a likely alternative diagnosis (3.0); heart rate greater than 100 beats per minute (1.5); immobilization for more than 3 days or surgery in the previous 4 weeks (1.5); previous DVT/PE (1.5); hemoptysis (1.0); and malignancy (1.0). Although the Wells prediction rule initially categorized 3 levels of probability for PE (low, moderate, or high), a revised model uses a simplified, dichotomized approach to determine whether PE is likely (Wells score >4) or unlikely (4 Wells score).11 An independent, prospective observational study found that the Wells prediction model reliably risk‐stratified pretest probability in patients with suspected PE.12
For patients who are stratified into the low‐risk category, the pulmonary embolism rule‐out criteria (PERC) rule may be helpful in reducing unnecessary diagnostic testing for PE.13 The PERC rule consists of 8 variables designed to offer a pretest probability of PE of less than 1.8%, a probability at which further testing is unnecessary. If the clinical gestalt is that PE is unlikely and all of the following variables are present, further testing can be safely discontinued: (1) pulse <100; (2) age <50; (3) oxygen saturation (SaO2) >94%; (4) no unilateral leg swelling; (5) no hemoptysis; (6) no recent trauma or surgery; (7) no prior DVT or PE; and (8) no hormone use.13 In a large, multicenter study, these criteria combined with a gestalt interpretation of low risk were shown to select a subgroup of patients with a very low probability of PE (<2%).14
D‐Dimer Testing
Evidence suggests that the combination of a low clinical probability assessment and a normal result in a highly sensitive, ELISA‐based D‐dimer test can safely exclude PE in hospitalized patients.15 Due to the large number of comorbidities among hospitalized patients, however, this combination occurs in only approximately 10% of inpatients.15 D‐dimer levels may be elevated in patients with a variety of nonthrombotic conditions, and it is therefore most useful in the diagnosis of otherwise healthy patients who have symptoms of PE. D‐dimer testing is not appropriate in moderate‐risk or high‐risk patients.
Diagnostic Imaging
Computed tomography (CT) is a leading imaging modality for the exclusion or confirmation of PE, as well as for the detection of alternative diagnoses. The diagnostic algorithms endorsed by the European Society of Cardiology (ESC) rely on both single‐detector and multidetector CT. However, multidetector CT scanners are now preferred because, in contrast to single‐detector CT, they can detect pulmonary emboli in smaller pulmonary arteries.10 Because single‐detector CT has a limited sensitivity of approximately 70%, it must be used in conjunction with lower limb venous CUS.16 In contrast, multidetector CT angiography has high sensitivity (83%) and specificity (96%) for the detection of PE and does not require the additional use of lower limb venous CUS.16, 17
Diagnostic Strategy
The Christopher Study demonstrated the utility of a diagnostic algorithm that incorporates a dichotomized decision rule, D‐dimer testing, and CT. In this approach, PE is excluded in patients with an unlikely clinical probability score (Wells score 4) and a normal D‐dimer test result. In all other patients, CT is the sole imaging method used to make management decisions.18 However, in patients with massive pulmonary embolism, if CT angiography is not immediately available, selective pulmonary angiography has been performed to identify and localize the emboli before aggressive therapy is instituted (Figure 2).19 If the patient is critically ill (hypotensive, severely hypoxemic), empiric treatment is appropriate while diagnostic strategy is being formulated.
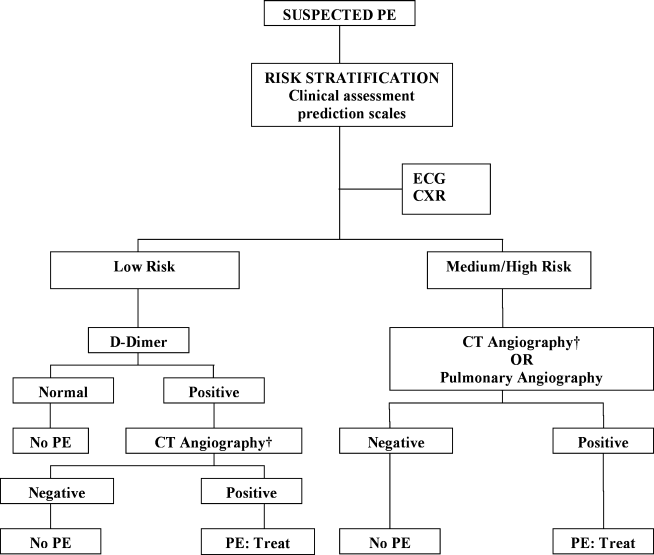
Abbreviations: CT, computed tomography; CXR, plain chest X‐ray; ECG, electrocardiogram; PE, pulmonary embolism. †CT angiography using multidetector instruments.
Treatment Options for VTE
For patients with VTE, the American College of Chest Physicians (ACCP) guidelines recommend initial treatment with low‐molecular‐weight heparin (LMWH), intravenous unfractionated heparin (UFH), or adjusted‐dose subcutaneous UFH, followed by at least 3 months of oral anticoagulation therapy.20
When VTE is diagnosed, anticoagulation should be initiated immediately unless contraindications are present. In addition, patients without contraindications to anticoagulation should receive treatment before diagnostic testing if such testing is delayed or if the clinical suspicion of VTE is high.20
Anticoagulant Treatment
For decades, parenteral administration of UFH for 5 to 7 days followed by long‐term warfarin therapy has been the conventional treatment of patients with VTE. Although UFH can be administered subcutaneously or by intravenous (IV) infusion, continuous IV infusion has been preferred because of superior dosing precision. The anticoagulation effect of intravenous UFH must be monitored to ensure a therapeutic activated partial thromboplastin time (aPTT). Consequently, the use of intravenous UFH requires frequent aPTT assessment and dose adjustment.20
Given their ease of use and improved pharmacokinetic and pharmacodynamic profiles, LMWHs have replaced UFH for the treatment of VTE in many institutions. Fondaparinux is also a safe and effective alternative to both intravenous UFH and LMWH in the treatment of VTE.20 It has a longer half‐life (15‐20 hours) than LMWH, permitting a once‐daily administration, and in patients with submassive PE, its efficacy and safety are comparable to UFH.21 Platelet count monitoring is not necessary with fondaparinux because it is given at weight‐adjusted doses, and only 1 case of heparin‐induced thrombocytopenia (HIT) has been reported.22 It is, however, contraindicated in renal failure with a creatinine clearance of <30 mL/minute.10
Warfarin is very effective in the long‐term management of VTE and should be started concurrently with rapid‐acting injectable anticoagulation therapy. Warfarin requires overlap with injectable anticoagulants for a minimum of 5 days until a therapeutic international normalized ratio (INR) has been achieved.20
Other Treatments
Most patients with VTE can be treated effectively with only anticoagulation therapy. However, in cases of massive PE (with or without systemic arterial hypotension and usually with significant hypoxemia not generally responsive to supplemental oxygen), removal of the occluding thrombus by thrombolytic agents, special clot‐removing catheters, or surgical procedures may be necessary to prevent or ameliorate shock and subsequent death.23 In other cases, such as when anticoagulants are ineffective or contraindicated, an inferior vena cava (IVC) filter may be an appropriate option for VTE treatment. Importantly, guidelines do not recommend filters in patients who can tolerate anticoagulation.
Permanent and retrievable IVC filters are effective at preventing PE and are generally associated with a low complication rate.24 However, nonfatal complications are relatively common with permanent IVC filters. One early complication is insertion‐site thrombosis, which occurs in about 10% of patients. Subsequent complications are more frequent and include recurrent DVT and post‐thrombotic syndrome (PTS), which occur in approximately 20% and 40% of patients, respectively. At 5 and 9 years, about 22% and 33% of the filters are occluded, regardless of the use and duration of anticoagulation.2527 To minimize these complications, retrievable filters have been increasingly used, but most filters are not retrieved and are subject to the same complications as permanent IVC filters.28
Catheter‐directed thrombolysis, with or without IVC filter placement, is safe and effective in treating acute DVT.29 Additional measures, such as the use of graduated compression stockings, can reduce the risk of developing PTS.20
Guideline Recommendations
Guidelines from the ACCP, the American College of Physicians (ACP), and the American Academy of Family Physicians (AAFP) address the treatment of VTE in a broad spectrum of patients. Additional guidelines provide recommendations for specific presentations or patient groups. For example, the ESC guidelines address the treatment of acute PE, and several groupsthe American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the French Working Group (FWG)have published guidelines for the treatment of VTE in patients with cancer. The following sections summarize the most important recommendations from several of these organizations and societies.
ACCP Guidelines
The ACCP guideline recommendations are assigned grades of 1 or 2, denoting a stronger or weaker recommendation, as well as a grade of A, B, or C, indicating high‐quality evidence, moderate‐quality evidence, and low‐quality evidence, respectively. Physicians must supplement the guideline recommendations with informed clinical judgment to ensure proper use of treatment in at‐risk hospitalized patients.20
The 2008 ACCP guidelines suggest several options for the initial treatment of VTE, which are listed, along with acceptable dosing regimens, in Table 1.20, 30, 31 Fixed‐dose, unmonitored, subcutaneous UFH and fondaparinux are new Grade 1A additions to the 2008 update. In general, LMWH is preferred over intravenous UFH, except in patients with severe renal failure.20
| Initial Anticoagulation Therapy | Grade | Acceptable Treatment Regimen* |
|---|---|---|
| ||
| SC LMWH | 1A | Enoxaparin: 1 mg/kg every 12 hours or 1.5 mg/kg once daily; Dalteparin: 200 IU/kg once daily (can be administered out of hospital) |
| Intravenous UFH | 1A | Get baseline aPTT, PT, and platelet count; if no abnormalities, proceed with a weight‐based heparin infusion protocol such as: |
| Bolus of 80 U/kg, followed by an infusion of 18 U/kg per hour (treatment duration 72 days); check aPTT every 4‐6 hours and adjust according to the normogram; monitor platelet count every 3‐4 days for HIT | ||
| Monitored SC UFH; fixed‐dose, unmonitored, SC UFH | 1A; 1A | Initial dose of 333 U/kg, followed by a fixed dose of 250 U/kg every 12 hours (can be administered out of hospital) |
| SC fondaparinux | 1A | 5 mg (body weight <50 kg), 7.5 mg (body weight 50‐100 kg), or 10 mg (body weight >100 kg) once daily (treatment duration 72 days) |
| IVC filter if anticoagulation contraindicated | 1C | |
Warfarin should also be initiated on the same day as UFH or LMWH and adjusted to a target INR of 2.5 (range, 2.0‐3.0). Treatment with UFH or LMWH should be continued concomitantly for a minimum of 5 days and should not be discontinued until the INR has been over 2.0 for 24 hours. The ACCP guidelines also recommend systematic follow‐up of oral anticoagulation therapy.
ACP/AAFP Guidelines
In 2007, the ACP and the AAFP collaborated to develop joint guidelines for the management of VTE.32 Several of their key recommendations are the following:32
-
LMWH, rather than UFH, should be used whenever possible for the initial inpatient treatment of DVT
-
Either UFH or LMWH is appropriate for the initial treatment of PE
-
Anticoagulation should be continued for 3 to 6 months for VTE secondary to transient risk factors, and for more than 12 months for recurrent VTE
-
LMWH is safe and effective for the long‐term treatment of VTE in selected patients (and may be preferable for patients with cancer)
ESC Guidelines for the Treatment of PE
According to the 2008 ESC guidelines, anticoagulation with UFH, LMWH, or fondaparinux should be initiated immediately in patients with confirmed PE, as well as in those with a high or intermediate clinical probability of PE while the diagnostic workup is ongoing. Subcutaneous LMWH or fondaparinux is preferable to intravenous UFH for initial treatment in most patients. UFH, however, should be used in patients with a high risk of bleeding due to its capacity for reversal and short half‐life, as well as in those with severe renal dysfunction.10
According to the ESC, patients with high‐risk PE (presenting with cardiogenic shock or persistent arterial hypotension) should receive thrombolytic therapy as first‐line therapy. Hemodynamic and respiratory support is also necessary for these patients.10 Routine thrombolysis is not recommended in patients with non‐high‐risk PE, but it may be considered in select patients with intermediate‐risk PE (characterized by severe right ventricular dysfunction on echocardiography and/or myocardial injury), depending on the patient's risk of bleeding. Thrombolytic therapy should not be used in patients with low‐risk PE (presenting without shock, hypotension, right ventricular dysfunction, or myocardial injury).10
Like the ESC guidelines, the ACCP guidelines recommend against the use of thrombolytic therapy for the majority of patients with PE (Grade 1B), but they do recommend its use in patients with evidence of hemodynamic compromise and no major contraindications owing to bleeding risk (Grade 1B) and in certain other high‐risk patients (Grade 2B).20
The ESC states that pulmonary embolectomy has recently become a reasonable option for patients with massive, high‐risk PE and an absolute contraindication to thrombolysis, or in whom thrombolysis has failed, when appropriate expertise is available. In the past, it was performed as a last resort in patients with massive PE who were in shock and conferred a high risk of mortality (+50%). Recently, however, the procedure has been revived and performed immediately in patients with confirmed massive PE (with severe right ventricular dysfunction but before shock), with mortality rates of less than 10%.19 It should be noted, however, that the ACCP guidelines consider embolectomy a Grade 2C recommendation.20 Alternatively, catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in these patients.10
NCCN Guidelines: Oncology Patients
The NCCN has provided treatment algorithms for the management of DVT and PE in patients with cancer, which are available online at
FWG Guidelines: Oncology Patients
At the 2008 ASH annual meeting, the FWG presented updated guidelines for the treatment of VTE in cancer patients.34 The FWG guidelines contain the following key recommendations:
-
The treatment of VTE should be based on LMWH at curative doses for at least 3 months
-
During the initial treatment (up to 10 days), any approved drug (including LMWH, UFH, and fondaparinux) may be used
-
Beyond the first 10 days, VTE treatment should be based on LMWH at curative doses for at least 3 months and optimally 6 months, as validated with the following drugs and dosage regimens:
-
Dalteparin 200 IU/kg once daily for 1 month, then 150 IU/kg once daily
-
Enoxaparin 150 IU/kg (1.5 mg/kg) once daily
-
Tinzaparin 175 IU/kg once daily
-
Special treatment considerations include the following:
-
In severe renal impairment, UFH should be used and rapidly followed by a vitamin K agonist (VKA) for at least 3 months
-
In severe PE (representing hemodynamic failure), the indications and recommended uses of thrombolytic drugs in noncancer patients apply
-
In patients with an absolute contraindication to anticoagulation or VTE recurrence despite optimal anticoagulation, vena cava filters should be considered
-
In patients with intracranial malignancies, VTE treatment is the same as in cancer patients with nonintracranial tumors
The treatment of central venous catheter thrombosis requires the long‐term use of LMWH according to the FWG guidelines. In patients with severe renal failure, UFH with early VKA must be used as an alternative treatment. Regardless of the therapy used, treatment should be continued as long as the catheter is maintained.34
Long‐Term Management of VTE
The high rate of recurrent VTE after a first episode of DVT or PEapproximately 8% within 90 daysunderscores the importance of maintaining effective prophylaxis postdischarge.35 Inadequate prophylaxis following discharge from the hospital can have severe consequences. In a recent study of 10,744 patients who were discharged from the hospital following hip or knee replacement surgery, fewer than 1 in 5 received postdischarge thromboprophylaxis. The 3‐month risk of mortality was significantly lower among those who received thromboprophylaxis at discharge (adjusted hazard ratio, 0.34; 95% CI, 0.20‐0.57).36
Detailed patient education at the time of discharge may be one of the most effective ways to prevent or minimize the burden of long‐term complications such as PTS or recurrent VTE. Accordingly, proper discharge planning and postdischarge support, including an appropriate anticoagulant, are critical steps toward reducing mortality, morbidity, and healthcare costs.
PTS
As many as 50% of patients with VTE will develop PTS, a serious but preventable complication that leads to pain, swelling, and skin changes in the affected limb. Female gender, older age, higher body mass index (BMI), and DVT of the common femoral or iliac vein (vs. distal DVT) are associated with an increased risk of PTS.37 To prevent PTS in a patient who has had a symptomatic proximal DVT, current guidelines recommend the use of graduated elastic compression stockings with an ankle pressure of 30 to 40 mm Hg, if feasible. Compression therapy should start as soon as possible after the initiation of anticoagulation therapy and be encouraged for a minimum of 2 years.20
Recurrent VTE
After discontinuing anticoagulation, the risk of recurrent VTE increases steadily over time. In a recent long‐term study of patients with acute proximal DVT or PE, the risk of recurrent VTE was 11% after 1 year, 20% after 3 years, 30% after 5 years, and 40% after 10 years. In this study, risk factors for recurrent VTE included unprovoked initial VTE, thrombophilia, increasing age, and a shorter duration of anticoagulation (6 months or less).38 Another study identified residual venous thrombosis as an important risk factor for recurrent VTE.39 In addition, 1 meta‐analysis found that men had a 50% higher risk of recurrent VTE than women.40 Recurrent DVT events are associated with a 21% greater cost than the initial event, suggesting that recurrent VTE is a preventable drain on healthcare resources.41
Secondary Prevention
The risk of recurrent VTE is determined by the effectiveness of treatment for the acute episode of VTE and by the patient's intrinsic risk of thromboembolism. The ACCP recommends different durations of warfarin or LMWH anticoagulant therapy according to these features (Table 2).20 Attaching a high value to prevention of recurrent VTE and a lower value to the burden of long‐term anticoagulant treatment, the ACCP recommends long‐term treatment for patients with a first unprovoked proximal DVT, no risk factors for bleeding, and the ability to monitor the anticoagulant effectively (Grade 1A).20
| Clinical Features | Duration | Grade |
|---|---|---|
| ||
| First episode and transient risk factors | 3 months | 1A |
| Unprovoked episode | 3 months | 1A |
| Unprovoked proximal DVT with low bleed risk | Long‐term | 1A |
| Cancer | 3 to 6 months with LMWH; then with a VKA or a LMWH indefinitely or until cancer is resolved | 1A; 1C |
| Second unprovoked episode | Indefinite | 2A |
Transition to Outpatient Therapy
The use of outpatient LMWH has changed the course of long‐term anticoagulation therapy and is listed as the preferred option for anticoagulation in the ACCP guidelines.20 With the availability of subcutaneous LMWHs, patients with acute VTE no longer have to be hospitalized for the initiation of oral therapy. In addition, patients undergoing invasive procedures that require temporary discontinuation of warfarin can opt for bridge therapy with LMWH.42
Conclusions
The diagnosis of VTE is challenging and depends on the integration of clinical, biochemical, and imaging modalities. In the absence of contraindications, treatment should be initiated immediately after a diagnosis of VTE is confirmed. Anticoagulant therapy alone is sufficient for most patients, but some patients may require thrombolytics or other strategies. Various societies and organizations have issued recommendations regarding the optimal use of these therapies in specific patient populations. Following these recommendations carefully may reduce the risk of complications in patients with VTE.
- Health Grades, Inc. The Fifth Annual Health Grades Patient Safety in American Hospitals Study. Available at: http://www.healthgrades.com/media/dms/pdf/PatientSafetyInAmericanHospitalsStudy2008.pdf. Accessed August2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest2008;133(6 suppl):381S–453S.
- , , , et al.Value of assessment of pretest probability of deep‐vein thrombosis in clinical management.Lancet.1997;350(9094):1795–1798.
- , , , et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- , , , et al.Limitations of D‐dimer testing in unselected inpatients with suspected venous thromboembolism.Am J Med.2003;114(4):276–282.
- , , , et al.Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians.Ann Fam Med.2007;5(1):57–62.
- , , .The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism.Ann Intern Med.1998;129:1044–1049.
- , .Ultrasonography of leg veins in patients suspected of having pulmonary embolism.Ann Intern Med.1998;128:243–245.
- , .Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism.Circulation.2004;109(12 suppl 1):I15–I21.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology.Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer.Thromb Haemost.2000;83(3):416–420.
- , , , et al.Prospective validation of Wells criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44(5):503–510.
- , , , et al.Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism.J Thromb Haemost.2004;2(8):1247–1255.
- , , , et al.Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria.J Thromb Haemost.2008;6(5):772–780.
- , , , et al.A simple diagnostic strategy in hospitalized patients with clinically suspected pulmonary embolism.J Intern Med.2006;260(5):459–466.
- , .Is computed tomographic venography of lower limbs useful in suspected pulmonary embolism?Rev Med Suisse.2008;4(143):354,356–359.
- , , , et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354(22):2317–2327.
- , , , et al.Christopher Study Investigators.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D‐dimer testing, and computed tomography.JAMA.2006;295(2):172–179.
- , , , .Acute pulmonary embolectomy: a contemporary approach.Circulation.2002;105:1416–1419.
- , , , et al.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 suppl):454S–545S.
- , , , et al.Subcutaneous fondaparinux versus unfractionated heparin in the initial treatment of pulmonary embolism.N Engl J Med.2003;349:1695–1702.
- .Heparin‐induced thrombocytopenia associated with fondaparinux.N Engl J Med.2007;356:2653–2655.
- , , , .Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- , , , et al.Ins and outs of inferior vena cava filters in patients with venous thromboembolism: the experience at Monash Medical Centre and review of the published reports.Intern Med J.2008;38(1):38–43.
- PREPIC Study Group.Eight‐year follow‐up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study.Circulation.2005;112:416–422.
- , , , .Inferior vena cava filters: key considerations.Am J Med Sci.2005;330:82–87.
- , , , .Percutaneous inferior vena cava filters: follow‐up of 7 designs in 320 patients.Radiology.1993;188:851–856.
- , , , et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24.
- , , , , , .Long‐term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement.J Vasc Surg.2007;45(5):992–997.
- , , , et al.Comparison of fixed‐dose weight‐adjusted unfractionated heparin and low‐molecular‐weight heparin for acute treatment of venous thromboembolism.JAMA.2006;296(8):935–942.
- Arixtra prescribing information. Last updated October 2008. Research Triangle Park, NC: GlaxoSmithKline. Available at: http://us.gsk.com/products/assets/us_arixtra.pdf. Accessed August2009.
- , , , et al.American College of Physicians;American Academy of Family Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Ann Intern Med.2007;146(3):204–210.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice Guidelines in Oncology. V.1.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2009.
- , , , et al. Guidelines for the treatment of venous thromboembolism in cancer patients: report from the French Working Group. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1284.
- , , , et al.Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study.Arch Intern Med.2000;160(6):761–768.
- , , , et al.Postdischarge thromboprophylaxis and mortality risk after hip‐or knee‐replacement surgery.CMAJ.2008;178(12):1545–1554.
- , , , et al.Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis.Ann Intern Med.2008;149(10):698–707.
- , , , et al.The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients.Haematologica.2007;92(2):199–205.
- .Risk factors of recurrent venous thromboembolism: the role of residual vein thrombosis.Pathophysiol Haemost Thromb.2003/2004;33(5‐6):351–353.
- , , , et al.Effect of patient's sex on risk of recurrent venous thromboembolism: a meta‐analysis.Lancet.2006;368:371–378.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
Despite the availability of effective thromboprophylaxis, the prevalence of venous thromboembolism (VTE) is increasing in the hospital setting. In 2008, the Fifth Annual Health Grades Patient Safety in American Hospitals Study reported on key patient safety incidents among nearly 41 million hospitalizations in the Medicare population between 2004 and 2006. Although many areas showed improvementincluding reduced rates of hospital‐related infections, postoperative bleeding, transfusion reactions, and other injuriesthe number of cases of postoperative VTE increased by 11% during this period.1
Even with optimal thromboprophylaxis, VTE will develop in some at‐risk patients. Early diagnosis and treatment of VTE is critical to reduce morbidity and mortality, but no single tool can definitively confirm its presence. Consequently, the detection of deep vein thrombosis (DVT) and pulmonary embolism (PE) requires a stepwise diagnostic strategy that combines clinical, biochemical, and imaging modalities.
In addition to outlining diagnostic strategies for DVT and PE, this article summarizes VTE treatment guidelines from various organizations and societies and discusses long‐term management strategies to prevent recurrent VTE and other complications.
Diagnosis of DVT
The clinical symptoms and signs of DVT are nonspecific and include unilateral calf, leg, or thigh swelling and pain. Despite the limited sensitivity and specificity of individual signs and symptoms of DVT, the combination of these variables can be useful in assessing the probability of VTE. Patients can be risk stratified according to the likelihood of DVT, as determined by implicit clinical judgment or by a validated prediction rule.2
Assessment of Clinical Probability
The Wells prediction rule is used in assessing the probability of DVT.3 It incorporates signs, symptoms, and risk factors of DVT to calculate a clinical probability rating. Specifically, 1 point is assigned to each of the following factors, if present:3
-
Active cancer (treatment ongoing, within 6 months, or palliative)
-
Calf swelling >3 cm asymptomatic side (measured 10 cm below tibial tuberosity)
-
Collateral superficial veins (nonvaricose)
-
Entire leg swelling
-
Localized tenderness along the distribution of the deep venous system
-
Paralysis, paresis, or recent plaster immobilization of the lower extremities
-
Pitting edema confined to the symptomatic leg
-
Recently bedridden more than 3 days or major surgery within 4 weeks
In addition, 2 points are subtracted if an alternative diagnosis is as likely as or more likely than DVT. In patients with symptoms in both legs, the more symptomatic leg is used.
Patients with low (score <1), moderate (score 1‐2), and high (score 3) pretest probability of DVT have been shown to have DVT prevalence rates of 3%, 17%, and 75%, respectively.3
D‐Dimer Testing
D‐dimer testing measures the small protein fragments remaining in the blood after a cross‐linked fibrin clot is degraded by fibrinolysis. A low clinical probability assessment combined with a negative result in a highly sensitive, enzyme‐linked immunosorbent assay (ELISA)‐based D‐dimer test can safely exclude DVT, with a negative predictive value of 99.1% (95% confidence interval [CI]; 96.7‐99.9).4
Due to its poor specificity, D‐dimer testing has limited utility in unselected inpatients, especially older patients and those who have undergone prolonged hospitalization.5 However, it is reasonable to obtain a highly sensitive, ELISA‐based D‐dimer test in carefully selected inpatients with a low pretest probability of DVT.5, 6 In such patients, a negative result indicates that DVT is highly unlikely, while a positive result indicates a need for further testing. D‐dimer testing is likely not helpful in moderate‐risk or high‐risk patients.
Diagnostic Imaging
For patients with a moderate to high pretest probability of DVT, ultrasound is recommended.6 Compression ultrasonography (CUS) is currently the preferred imaging tool in patients with suspected DVT because it is noninvasive, can be repeated serially, and offers high sensitivity (+90%) and high specificity (95%) for detecting proximal vein thrombosis.7, 8 If the clinical suspicion of DVT persists after an initial negative CUS study, imaging can be repeated after 3 to 7 days to detect the propagation of any thrombosis to the proximal veins. Limitations of CUS include poor visualization of deep iliac and pelvic veins and poor sensitivity in isolated or nonocclusive calf vein thrombi.2
Contrast venography was considered the gold standard for the detection of DVT of the lower extremity, but this modality is invasive, painful, and offers poor visualization of the deep femoral vein and the internal iliac vein. In addition, contrast venography is associated with an increased risk of new thrombosis, renal failure, and hypersensitivity reaction to contrast media. Consequently, contrast venography is currently used in symptomatic patients only when noninvasive testing is inconclusive or unavailable.2 Other second‐line diagnostic tools include computed tomography venography (CTV) and magnetic resonance venography (MRV).9
Diagnostic Strategy
A diagnostic algorithm for DVT is presented in Figure 1. First, a validated clinical prediction scale such as the Wells prediction rule should be used to estimate the pretest probability of DVT, and the result of the clinical assessment should influence the choice and interpretation of subsequent testing.

Abbreviations: CUS, compression ultrasonography; DVT, deep vein thrombosis (DVT).
Diagnosis of PE
Clinical symptoms and signs such as dyspnea, chest pain, tachycardia, tachypnea, and syncope raise the suspicion of PE. Individual signs and symptoms, however, cannot confirm or exclude acute PE, as they are neither sensitive nor specific.10 Furthermore, although the likelihood of PE increases with the number of predisposing risk factors, approximately 30% of PE cases are unprovoked or idiopathic, meaning that they occur in the absence of predisposing factors. Diagnosis, therefore, depends on an integrated strategy involving similar tools as those used in diagnosing DVT.
Assessing Clinical Probability
Wells et al.11 also developed a clinical prediction rule for the risk stratification of patients with suspected PE. In this model, 7 specified variables are assigned different scores: clinical signs and symptoms of DVT (3.0); lack of a likely alternative diagnosis (3.0); heart rate greater than 100 beats per minute (1.5); immobilization for more than 3 days or surgery in the previous 4 weeks (1.5); previous DVT/PE (1.5); hemoptysis (1.0); and malignancy (1.0). Although the Wells prediction rule initially categorized 3 levels of probability for PE (low, moderate, or high), a revised model uses a simplified, dichotomized approach to determine whether PE is likely (Wells score >4) or unlikely (4 Wells score).11 An independent, prospective observational study found that the Wells prediction model reliably risk‐stratified pretest probability in patients with suspected PE.12
For patients who are stratified into the low‐risk category, the pulmonary embolism rule‐out criteria (PERC) rule may be helpful in reducing unnecessary diagnostic testing for PE.13 The PERC rule consists of 8 variables designed to offer a pretest probability of PE of less than 1.8%, a probability at which further testing is unnecessary. If the clinical gestalt is that PE is unlikely and all of the following variables are present, further testing can be safely discontinued: (1) pulse <100; (2) age <50; (3) oxygen saturation (SaO2) >94%; (4) no unilateral leg swelling; (5) no hemoptysis; (6) no recent trauma or surgery; (7) no prior DVT or PE; and (8) no hormone use.13 In a large, multicenter study, these criteria combined with a gestalt interpretation of low risk were shown to select a subgroup of patients with a very low probability of PE (<2%).14
D‐Dimer Testing
Evidence suggests that the combination of a low clinical probability assessment and a normal result in a highly sensitive, ELISA‐based D‐dimer test can safely exclude PE in hospitalized patients.15 Due to the large number of comorbidities among hospitalized patients, however, this combination occurs in only approximately 10% of inpatients.15 D‐dimer levels may be elevated in patients with a variety of nonthrombotic conditions, and it is therefore most useful in the diagnosis of otherwise healthy patients who have symptoms of PE. D‐dimer testing is not appropriate in moderate‐risk or high‐risk patients.
Diagnostic Imaging
Computed tomography (CT) is a leading imaging modality for the exclusion or confirmation of PE, as well as for the detection of alternative diagnoses. The diagnostic algorithms endorsed by the European Society of Cardiology (ESC) rely on both single‐detector and multidetector CT. However, multidetector CT scanners are now preferred because, in contrast to single‐detector CT, they can detect pulmonary emboli in smaller pulmonary arteries.10 Because single‐detector CT has a limited sensitivity of approximately 70%, it must be used in conjunction with lower limb venous CUS.16 In contrast, multidetector CT angiography has high sensitivity (83%) and specificity (96%) for the detection of PE and does not require the additional use of lower limb venous CUS.16, 17
Diagnostic Strategy
The Christopher Study demonstrated the utility of a diagnostic algorithm that incorporates a dichotomized decision rule, D‐dimer testing, and CT. In this approach, PE is excluded in patients with an unlikely clinical probability score (Wells score 4) and a normal D‐dimer test result. In all other patients, CT is the sole imaging method used to make management decisions.18 However, in patients with massive pulmonary embolism, if CT angiography is not immediately available, selective pulmonary angiography has been performed to identify and localize the emboli before aggressive therapy is instituted (Figure 2).19 If the patient is critically ill (hypotensive, severely hypoxemic), empiric treatment is appropriate while diagnostic strategy is being formulated.

Abbreviations: CT, computed tomography; CXR, plain chest X‐ray; ECG, electrocardiogram; PE, pulmonary embolism. †CT angiography using multidetector instruments.
Treatment Options for VTE
For patients with VTE, the American College of Chest Physicians (ACCP) guidelines recommend initial treatment with low‐molecular‐weight heparin (LMWH), intravenous unfractionated heparin (UFH), or adjusted‐dose subcutaneous UFH, followed by at least 3 months of oral anticoagulation therapy.20
When VTE is diagnosed, anticoagulation should be initiated immediately unless contraindications are present. In addition, patients without contraindications to anticoagulation should receive treatment before diagnostic testing if such testing is delayed or if the clinical suspicion of VTE is high.20
Anticoagulant Treatment
For decades, parenteral administration of UFH for 5 to 7 days followed by long‐term warfarin therapy has been the conventional treatment of patients with VTE. Although UFH can be administered subcutaneously or by intravenous (IV) infusion, continuous IV infusion has been preferred because of superior dosing precision. The anticoagulation effect of intravenous UFH must be monitored to ensure a therapeutic activated partial thromboplastin time (aPTT). Consequently, the use of intravenous UFH requires frequent aPTT assessment and dose adjustment.20
Given their ease of use and improved pharmacokinetic and pharmacodynamic profiles, LMWHs have replaced UFH for the treatment of VTE in many institutions. Fondaparinux is also a safe and effective alternative to both intravenous UFH and LMWH in the treatment of VTE.20 It has a longer half‐life (15‐20 hours) than LMWH, permitting a once‐daily administration, and in patients with submassive PE, its efficacy and safety are comparable to UFH.21 Platelet count monitoring is not necessary with fondaparinux because it is given at weight‐adjusted doses, and only 1 case of heparin‐induced thrombocytopenia (HIT) has been reported.22 It is, however, contraindicated in renal failure with a creatinine clearance of <30 mL/minute.10
Warfarin is very effective in the long‐term management of VTE and should be started concurrently with rapid‐acting injectable anticoagulation therapy. Warfarin requires overlap with injectable anticoagulants for a minimum of 5 days until a therapeutic international normalized ratio (INR) has been achieved.20
Other Treatments
Most patients with VTE can be treated effectively with only anticoagulation therapy. However, in cases of massive PE (with or without systemic arterial hypotension and usually with significant hypoxemia not generally responsive to supplemental oxygen), removal of the occluding thrombus by thrombolytic agents, special clot‐removing catheters, or surgical procedures may be necessary to prevent or ameliorate shock and subsequent death.23 In other cases, such as when anticoagulants are ineffective or contraindicated, an inferior vena cava (IVC) filter may be an appropriate option for VTE treatment. Importantly, guidelines do not recommend filters in patients who can tolerate anticoagulation.
Permanent and retrievable IVC filters are effective at preventing PE and are generally associated with a low complication rate.24 However, nonfatal complications are relatively common with permanent IVC filters. One early complication is insertion‐site thrombosis, which occurs in about 10% of patients. Subsequent complications are more frequent and include recurrent DVT and post‐thrombotic syndrome (PTS), which occur in approximately 20% and 40% of patients, respectively. At 5 and 9 years, about 22% and 33% of the filters are occluded, regardless of the use and duration of anticoagulation.2527 To minimize these complications, retrievable filters have been increasingly used, but most filters are not retrieved and are subject to the same complications as permanent IVC filters.28
Catheter‐directed thrombolysis, with or without IVC filter placement, is safe and effective in treating acute DVT.29 Additional measures, such as the use of graduated compression stockings, can reduce the risk of developing PTS.20
Guideline Recommendations
Guidelines from the ACCP, the American College of Physicians (ACP), and the American Academy of Family Physicians (AAFP) address the treatment of VTE in a broad spectrum of patients. Additional guidelines provide recommendations for specific presentations or patient groups. For example, the ESC guidelines address the treatment of acute PE, and several groupsthe American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the French Working Group (FWG)have published guidelines for the treatment of VTE in patients with cancer. The following sections summarize the most important recommendations from several of these organizations and societies.
ACCP Guidelines
The ACCP guideline recommendations are assigned grades of 1 or 2, denoting a stronger or weaker recommendation, as well as a grade of A, B, or C, indicating high‐quality evidence, moderate‐quality evidence, and low‐quality evidence, respectively. Physicians must supplement the guideline recommendations with informed clinical judgment to ensure proper use of treatment in at‐risk hospitalized patients.20
The 2008 ACCP guidelines suggest several options for the initial treatment of VTE, which are listed, along with acceptable dosing regimens, in Table 1.20, 30, 31 Fixed‐dose, unmonitored, subcutaneous UFH and fondaparinux are new Grade 1A additions to the 2008 update. In general, LMWH is preferred over intravenous UFH, except in patients with severe renal failure.20
| Initial Anticoagulation Therapy | Grade | Acceptable Treatment Regimen* |
|---|---|---|
| ||
| SC LMWH | 1A | Enoxaparin: 1 mg/kg every 12 hours or 1.5 mg/kg once daily; Dalteparin: 200 IU/kg once daily (can be administered out of hospital) |
| Intravenous UFH | 1A | Get baseline aPTT, PT, and platelet count; if no abnormalities, proceed with a weight‐based heparin infusion protocol such as: |
| Bolus of 80 U/kg, followed by an infusion of 18 U/kg per hour (treatment duration 72 days); check aPTT every 4‐6 hours and adjust according to the normogram; monitor platelet count every 3‐4 days for HIT | ||
| Monitored SC UFH; fixed‐dose, unmonitored, SC UFH | 1A; 1A | Initial dose of 333 U/kg, followed by a fixed dose of 250 U/kg every 12 hours (can be administered out of hospital) |
| SC fondaparinux | 1A | 5 mg (body weight <50 kg), 7.5 mg (body weight 50‐100 kg), or 10 mg (body weight >100 kg) once daily (treatment duration 72 days) |
| IVC filter if anticoagulation contraindicated | 1C | |
Warfarin should also be initiated on the same day as UFH or LMWH and adjusted to a target INR of 2.5 (range, 2.0‐3.0). Treatment with UFH or LMWH should be continued concomitantly for a minimum of 5 days and should not be discontinued until the INR has been over 2.0 for 24 hours. The ACCP guidelines also recommend systematic follow‐up of oral anticoagulation therapy.
ACP/AAFP Guidelines
In 2007, the ACP and the AAFP collaborated to develop joint guidelines for the management of VTE.32 Several of their key recommendations are the following:32
-
LMWH, rather than UFH, should be used whenever possible for the initial inpatient treatment of DVT
-
Either UFH or LMWH is appropriate for the initial treatment of PE
-
Anticoagulation should be continued for 3 to 6 months for VTE secondary to transient risk factors, and for more than 12 months for recurrent VTE
-
LMWH is safe and effective for the long‐term treatment of VTE in selected patients (and may be preferable for patients with cancer)
ESC Guidelines for the Treatment of PE
According to the 2008 ESC guidelines, anticoagulation with UFH, LMWH, or fondaparinux should be initiated immediately in patients with confirmed PE, as well as in those with a high or intermediate clinical probability of PE while the diagnostic workup is ongoing. Subcutaneous LMWH or fondaparinux is preferable to intravenous UFH for initial treatment in most patients. UFH, however, should be used in patients with a high risk of bleeding due to its capacity for reversal and short half‐life, as well as in those with severe renal dysfunction.10
According to the ESC, patients with high‐risk PE (presenting with cardiogenic shock or persistent arterial hypotension) should receive thrombolytic therapy as first‐line therapy. Hemodynamic and respiratory support is also necessary for these patients.10 Routine thrombolysis is not recommended in patients with non‐high‐risk PE, but it may be considered in select patients with intermediate‐risk PE (characterized by severe right ventricular dysfunction on echocardiography and/or myocardial injury), depending on the patient's risk of bleeding. Thrombolytic therapy should not be used in patients with low‐risk PE (presenting without shock, hypotension, right ventricular dysfunction, or myocardial injury).10
Like the ESC guidelines, the ACCP guidelines recommend against the use of thrombolytic therapy for the majority of patients with PE (Grade 1B), but they do recommend its use in patients with evidence of hemodynamic compromise and no major contraindications owing to bleeding risk (Grade 1B) and in certain other high‐risk patients (Grade 2B).20
The ESC states that pulmonary embolectomy has recently become a reasonable option for patients with massive, high‐risk PE and an absolute contraindication to thrombolysis, or in whom thrombolysis has failed, when appropriate expertise is available. In the past, it was performed as a last resort in patients with massive PE who were in shock and conferred a high risk of mortality (+50%). Recently, however, the procedure has been revived and performed immediately in patients with confirmed massive PE (with severe right ventricular dysfunction but before shock), with mortality rates of less than 10%.19 It should be noted, however, that the ACCP guidelines consider embolectomy a Grade 2C recommendation.20 Alternatively, catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in these patients.10
NCCN Guidelines: Oncology Patients
The NCCN has provided treatment algorithms for the management of DVT and PE in patients with cancer, which are available online at
FWG Guidelines: Oncology Patients
At the 2008 ASH annual meeting, the FWG presented updated guidelines for the treatment of VTE in cancer patients.34 The FWG guidelines contain the following key recommendations:
-
The treatment of VTE should be based on LMWH at curative doses for at least 3 months
-
During the initial treatment (up to 10 days), any approved drug (including LMWH, UFH, and fondaparinux) may be used
-
Beyond the first 10 days, VTE treatment should be based on LMWH at curative doses for at least 3 months and optimally 6 months, as validated with the following drugs and dosage regimens:
-
Dalteparin 200 IU/kg once daily for 1 month, then 150 IU/kg once daily
-
Enoxaparin 150 IU/kg (1.5 mg/kg) once daily
-
Tinzaparin 175 IU/kg once daily
-
Special treatment considerations include the following:
-
In severe renal impairment, UFH should be used and rapidly followed by a vitamin K agonist (VKA) for at least 3 months
-
In severe PE (representing hemodynamic failure), the indications and recommended uses of thrombolytic drugs in noncancer patients apply
-
In patients with an absolute contraindication to anticoagulation or VTE recurrence despite optimal anticoagulation, vena cava filters should be considered
-
In patients with intracranial malignancies, VTE treatment is the same as in cancer patients with nonintracranial tumors
The treatment of central venous catheter thrombosis requires the long‐term use of LMWH according to the FWG guidelines. In patients with severe renal failure, UFH with early VKA must be used as an alternative treatment. Regardless of the therapy used, treatment should be continued as long as the catheter is maintained.34
Long‐Term Management of VTE
The high rate of recurrent VTE after a first episode of DVT or PEapproximately 8% within 90 daysunderscores the importance of maintaining effective prophylaxis postdischarge.35 Inadequate prophylaxis following discharge from the hospital can have severe consequences. In a recent study of 10,744 patients who were discharged from the hospital following hip or knee replacement surgery, fewer than 1 in 5 received postdischarge thromboprophylaxis. The 3‐month risk of mortality was significantly lower among those who received thromboprophylaxis at discharge (adjusted hazard ratio, 0.34; 95% CI, 0.20‐0.57).36
Detailed patient education at the time of discharge may be one of the most effective ways to prevent or minimize the burden of long‐term complications such as PTS or recurrent VTE. Accordingly, proper discharge planning and postdischarge support, including an appropriate anticoagulant, are critical steps toward reducing mortality, morbidity, and healthcare costs.
PTS
As many as 50% of patients with VTE will develop PTS, a serious but preventable complication that leads to pain, swelling, and skin changes in the affected limb. Female gender, older age, higher body mass index (BMI), and DVT of the common femoral or iliac vein (vs. distal DVT) are associated with an increased risk of PTS.37 To prevent PTS in a patient who has had a symptomatic proximal DVT, current guidelines recommend the use of graduated elastic compression stockings with an ankle pressure of 30 to 40 mm Hg, if feasible. Compression therapy should start as soon as possible after the initiation of anticoagulation therapy and be encouraged for a minimum of 2 years.20
Recurrent VTE
After discontinuing anticoagulation, the risk of recurrent VTE increases steadily over time. In a recent long‐term study of patients with acute proximal DVT or PE, the risk of recurrent VTE was 11% after 1 year, 20% after 3 years, 30% after 5 years, and 40% after 10 years. In this study, risk factors for recurrent VTE included unprovoked initial VTE, thrombophilia, increasing age, and a shorter duration of anticoagulation (6 months or less).38 Another study identified residual venous thrombosis as an important risk factor for recurrent VTE.39 In addition, 1 meta‐analysis found that men had a 50% higher risk of recurrent VTE than women.40 Recurrent DVT events are associated with a 21% greater cost than the initial event, suggesting that recurrent VTE is a preventable drain on healthcare resources.41
Secondary Prevention
The risk of recurrent VTE is determined by the effectiveness of treatment for the acute episode of VTE and by the patient's intrinsic risk of thromboembolism. The ACCP recommends different durations of warfarin or LMWH anticoagulant therapy according to these features (Table 2).20 Attaching a high value to prevention of recurrent VTE and a lower value to the burden of long‐term anticoagulant treatment, the ACCP recommends long‐term treatment for patients with a first unprovoked proximal DVT, no risk factors for bleeding, and the ability to monitor the anticoagulant effectively (Grade 1A).20
| Clinical Features | Duration | Grade |
|---|---|---|
| ||
| First episode and transient risk factors | 3 months | 1A |
| Unprovoked episode | 3 months | 1A |
| Unprovoked proximal DVT with low bleed risk | Long‐term | 1A |
| Cancer | 3 to 6 months with LMWH; then with a VKA or a LMWH indefinitely or until cancer is resolved | 1A; 1C |
| Second unprovoked episode | Indefinite | 2A |
Transition to Outpatient Therapy
The use of outpatient LMWH has changed the course of long‐term anticoagulation therapy and is listed as the preferred option for anticoagulation in the ACCP guidelines.20 With the availability of subcutaneous LMWHs, patients with acute VTE no longer have to be hospitalized for the initiation of oral therapy. In addition, patients undergoing invasive procedures that require temporary discontinuation of warfarin can opt for bridge therapy with LMWH.42
Conclusions
The diagnosis of VTE is challenging and depends on the integration of clinical, biochemical, and imaging modalities. In the absence of contraindications, treatment should be initiated immediately after a diagnosis of VTE is confirmed. Anticoagulant therapy alone is sufficient for most patients, but some patients may require thrombolytics or other strategies. Various societies and organizations have issued recommendations regarding the optimal use of these therapies in specific patient populations. Following these recommendations carefully may reduce the risk of complications in patients with VTE.
Despite the availability of effective thromboprophylaxis, the prevalence of venous thromboembolism (VTE) is increasing in the hospital setting. In 2008, the Fifth Annual Health Grades Patient Safety in American Hospitals Study reported on key patient safety incidents among nearly 41 million hospitalizations in the Medicare population between 2004 and 2006. Although many areas showed improvementincluding reduced rates of hospital‐related infections, postoperative bleeding, transfusion reactions, and other injuriesthe number of cases of postoperative VTE increased by 11% during this period.1
Even with optimal thromboprophylaxis, VTE will develop in some at‐risk patients. Early diagnosis and treatment of VTE is critical to reduce morbidity and mortality, but no single tool can definitively confirm its presence. Consequently, the detection of deep vein thrombosis (DVT) and pulmonary embolism (PE) requires a stepwise diagnostic strategy that combines clinical, biochemical, and imaging modalities.
In addition to outlining diagnostic strategies for DVT and PE, this article summarizes VTE treatment guidelines from various organizations and societies and discusses long‐term management strategies to prevent recurrent VTE and other complications.
Diagnosis of DVT
The clinical symptoms and signs of DVT are nonspecific and include unilateral calf, leg, or thigh swelling and pain. Despite the limited sensitivity and specificity of individual signs and symptoms of DVT, the combination of these variables can be useful in assessing the probability of VTE. Patients can be risk stratified according to the likelihood of DVT, as determined by implicit clinical judgment or by a validated prediction rule.2
Assessment of Clinical Probability
The Wells prediction rule is used in assessing the probability of DVT.3 It incorporates signs, symptoms, and risk factors of DVT to calculate a clinical probability rating. Specifically, 1 point is assigned to each of the following factors, if present:3
-
Active cancer (treatment ongoing, within 6 months, or palliative)
-
Calf swelling >3 cm asymptomatic side (measured 10 cm below tibial tuberosity)
-
Collateral superficial veins (nonvaricose)
-
Entire leg swelling
-
Localized tenderness along the distribution of the deep venous system
-
Paralysis, paresis, or recent plaster immobilization of the lower extremities
-
Pitting edema confined to the symptomatic leg
-
Recently bedridden more than 3 days or major surgery within 4 weeks
In addition, 2 points are subtracted if an alternative diagnosis is as likely as or more likely than DVT. In patients with symptoms in both legs, the more symptomatic leg is used.
Patients with low (score <1), moderate (score 1‐2), and high (score 3) pretest probability of DVT have been shown to have DVT prevalence rates of 3%, 17%, and 75%, respectively.3
D‐Dimer Testing
D‐dimer testing measures the small protein fragments remaining in the blood after a cross‐linked fibrin clot is degraded by fibrinolysis. A low clinical probability assessment combined with a negative result in a highly sensitive, enzyme‐linked immunosorbent assay (ELISA)‐based D‐dimer test can safely exclude DVT, with a negative predictive value of 99.1% (95% confidence interval [CI]; 96.7‐99.9).4
Due to its poor specificity, D‐dimer testing has limited utility in unselected inpatients, especially older patients and those who have undergone prolonged hospitalization.5 However, it is reasonable to obtain a highly sensitive, ELISA‐based D‐dimer test in carefully selected inpatients with a low pretest probability of DVT.5, 6 In such patients, a negative result indicates that DVT is highly unlikely, while a positive result indicates a need for further testing. D‐dimer testing is likely not helpful in moderate‐risk or high‐risk patients.
Diagnostic Imaging
For patients with a moderate to high pretest probability of DVT, ultrasound is recommended.6 Compression ultrasonography (CUS) is currently the preferred imaging tool in patients with suspected DVT because it is noninvasive, can be repeated serially, and offers high sensitivity (+90%) and high specificity (95%) for detecting proximal vein thrombosis.7, 8 If the clinical suspicion of DVT persists after an initial negative CUS study, imaging can be repeated after 3 to 7 days to detect the propagation of any thrombosis to the proximal veins. Limitations of CUS include poor visualization of deep iliac and pelvic veins and poor sensitivity in isolated or nonocclusive calf vein thrombi.2
Contrast venography was considered the gold standard for the detection of DVT of the lower extremity, but this modality is invasive, painful, and offers poor visualization of the deep femoral vein and the internal iliac vein. In addition, contrast venography is associated with an increased risk of new thrombosis, renal failure, and hypersensitivity reaction to contrast media. Consequently, contrast venography is currently used in symptomatic patients only when noninvasive testing is inconclusive or unavailable.2 Other second‐line diagnostic tools include computed tomography venography (CTV) and magnetic resonance venography (MRV).9
Diagnostic Strategy
A diagnostic algorithm for DVT is presented in Figure 1. First, a validated clinical prediction scale such as the Wells prediction rule should be used to estimate the pretest probability of DVT, and the result of the clinical assessment should influence the choice and interpretation of subsequent testing.

Abbreviations: CUS, compression ultrasonography; DVT, deep vein thrombosis (DVT).
Diagnosis of PE
Clinical symptoms and signs such as dyspnea, chest pain, tachycardia, tachypnea, and syncope raise the suspicion of PE. Individual signs and symptoms, however, cannot confirm or exclude acute PE, as they are neither sensitive nor specific.10 Furthermore, although the likelihood of PE increases with the number of predisposing risk factors, approximately 30% of PE cases are unprovoked or idiopathic, meaning that they occur in the absence of predisposing factors. Diagnosis, therefore, depends on an integrated strategy involving similar tools as those used in diagnosing DVT.
Assessing Clinical Probability
Wells et al.11 also developed a clinical prediction rule for the risk stratification of patients with suspected PE. In this model, 7 specified variables are assigned different scores: clinical signs and symptoms of DVT (3.0); lack of a likely alternative diagnosis (3.0); heart rate greater than 100 beats per minute (1.5); immobilization for more than 3 days or surgery in the previous 4 weeks (1.5); previous DVT/PE (1.5); hemoptysis (1.0); and malignancy (1.0). Although the Wells prediction rule initially categorized 3 levels of probability for PE (low, moderate, or high), a revised model uses a simplified, dichotomized approach to determine whether PE is likely (Wells score >4) or unlikely (4 Wells score).11 An independent, prospective observational study found that the Wells prediction model reliably risk‐stratified pretest probability in patients with suspected PE.12
For patients who are stratified into the low‐risk category, the pulmonary embolism rule‐out criteria (PERC) rule may be helpful in reducing unnecessary diagnostic testing for PE.13 The PERC rule consists of 8 variables designed to offer a pretest probability of PE of less than 1.8%, a probability at which further testing is unnecessary. If the clinical gestalt is that PE is unlikely and all of the following variables are present, further testing can be safely discontinued: (1) pulse <100; (2) age <50; (3) oxygen saturation (SaO2) >94%; (4) no unilateral leg swelling; (5) no hemoptysis; (6) no recent trauma or surgery; (7) no prior DVT or PE; and (8) no hormone use.13 In a large, multicenter study, these criteria combined with a gestalt interpretation of low risk were shown to select a subgroup of patients with a very low probability of PE (<2%).14
D‐Dimer Testing
Evidence suggests that the combination of a low clinical probability assessment and a normal result in a highly sensitive, ELISA‐based D‐dimer test can safely exclude PE in hospitalized patients.15 Due to the large number of comorbidities among hospitalized patients, however, this combination occurs in only approximately 10% of inpatients.15 D‐dimer levels may be elevated in patients with a variety of nonthrombotic conditions, and it is therefore most useful in the diagnosis of otherwise healthy patients who have symptoms of PE. D‐dimer testing is not appropriate in moderate‐risk or high‐risk patients.
Diagnostic Imaging
Computed tomography (CT) is a leading imaging modality for the exclusion or confirmation of PE, as well as for the detection of alternative diagnoses. The diagnostic algorithms endorsed by the European Society of Cardiology (ESC) rely on both single‐detector and multidetector CT. However, multidetector CT scanners are now preferred because, in contrast to single‐detector CT, they can detect pulmonary emboli in smaller pulmonary arteries.10 Because single‐detector CT has a limited sensitivity of approximately 70%, it must be used in conjunction with lower limb venous CUS.16 In contrast, multidetector CT angiography has high sensitivity (83%) and specificity (96%) for the detection of PE and does not require the additional use of lower limb venous CUS.16, 17
Diagnostic Strategy
The Christopher Study demonstrated the utility of a diagnostic algorithm that incorporates a dichotomized decision rule, D‐dimer testing, and CT. In this approach, PE is excluded in patients with an unlikely clinical probability score (Wells score 4) and a normal D‐dimer test result. In all other patients, CT is the sole imaging method used to make management decisions.18 However, in patients with massive pulmonary embolism, if CT angiography is not immediately available, selective pulmonary angiography has been performed to identify and localize the emboli before aggressive therapy is instituted (Figure 2).19 If the patient is critically ill (hypotensive, severely hypoxemic), empiric treatment is appropriate while diagnostic strategy is being formulated.

Abbreviations: CT, computed tomography; CXR, plain chest X‐ray; ECG, electrocardiogram; PE, pulmonary embolism. †CT angiography using multidetector instruments.
Treatment Options for VTE
For patients with VTE, the American College of Chest Physicians (ACCP) guidelines recommend initial treatment with low‐molecular‐weight heparin (LMWH), intravenous unfractionated heparin (UFH), or adjusted‐dose subcutaneous UFH, followed by at least 3 months of oral anticoagulation therapy.20
When VTE is diagnosed, anticoagulation should be initiated immediately unless contraindications are present. In addition, patients without contraindications to anticoagulation should receive treatment before diagnostic testing if such testing is delayed or if the clinical suspicion of VTE is high.20
Anticoagulant Treatment
For decades, parenteral administration of UFH for 5 to 7 days followed by long‐term warfarin therapy has been the conventional treatment of patients with VTE. Although UFH can be administered subcutaneously or by intravenous (IV) infusion, continuous IV infusion has been preferred because of superior dosing precision. The anticoagulation effect of intravenous UFH must be monitored to ensure a therapeutic activated partial thromboplastin time (aPTT). Consequently, the use of intravenous UFH requires frequent aPTT assessment and dose adjustment.20
Given their ease of use and improved pharmacokinetic and pharmacodynamic profiles, LMWHs have replaced UFH for the treatment of VTE in many institutions. Fondaparinux is also a safe and effective alternative to both intravenous UFH and LMWH in the treatment of VTE.20 It has a longer half‐life (15‐20 hours) than LMWH, permitting a once‐daily administration, and in patients with submassive PE, its efficacy and safety are comparable to UFH.21 Platelet count monitoring is not necessary with fondaparinux because it is given at weight‐adjusted doses, and only 1 case of heparin‐induced thrombocytopenia (HIT) has been reported.22 It is, however, contraindicated in renal failure with a creatinine clearance of <30 mL/minute.10
Warfarin is very effective in the long‐term management of VTE and should be started concurrently with rapid‐acting injectable anticoagulation therapy. Warfarin requires overlap with injectable anticoagulants for a minimum of 5 days until a therapeutic international normalized ratio (INR) has been achieved.20
Other Treatments
Most patients with VTE can be treated effectively with only anticoagulation therapy. However, in cases of massive PE (with or without systemic arterial hypotension and usually with significant hypoxemia not generally responsive to supplemental oxygen), removal of the occluding thrombus by thrombolytic agents, special clot‐removing catheters, or surgical procedures may be necessary to prevent or ameliorate shock and subsequent death.23 In other cases, such as when anticoagulants are ineffective or contraindicated, an inferior vena cava (IVC) filter may be an appropriate option for VTE treatment. Importantly, guidelines do not recommend filters in patients who can tolerate anticoagulation.
Permanent and retrievable IVC filters are effective at preventing PE and are generally associated with a low complication rate.24 However, nonfatal complications are relatively common with permanent IVC filters. One early complication is insertion‐site thrombosis, which occurs in about 10% of patients. Subsequent complications are more frequent and include recurrent DVT and post‐thrombotic syndrome (PTS), which occur in approximately 20% and 40% of patients, respectively. At 5 and 9 years, about 22% and 33% of the filters are occluded, regardless of the use and duration of anticoagulation.2527 To minimize these complications, retrievable filters have been increasingly used, but most filters are not retrieved and are subject to the same complications as permanent IVC filters.28
Catheter‐directed thrombolysis, with or without IVC filter placement, is safe and effective in treating acute DVT.29 Additional measures, such as the use of graduated compression stockings, can reduce the risk of developing PTS.20
Guideline Recommendations
Guidelines from the ACCP, the American College of Physicians (ACP), and the American Academy of Family Physicians (AAFP) address the treatment of VTE in a broad spectrum of patients. Additional guidelines provide recommendations for specific presentations or patient groups. For example, the ESC guidelines address the treatment of acute PE, and several groupsthe American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the French Working Group (FWG)have published guidelines for the treatment of VTE in patients with cancer. The following sections summarize the most important recommendations from several of these organizations and societies.
ACCP Guidelines
The ACCP guideline recommendations are assigned grades of 1 or 2, denoting a stronger or weaker recommendation, as well as a grade of A, B, or C, indicating high‐quality evidence, moderate‐quality evidence, and low‐quality evidence, respectively. Physicians must supplement the guideline recommendations with informed clinical judgment to ensure proper use of treatment in at‐risk hospitalized patients.20
The 2008 ACCP guidelines suggest several options for the initial treatment of VTE, which are listed, along with acceptable dosing regimens, in Table 1.20, 30, 31 Fixed‐dose, unmonitored, subcutaneous UFH and fondaparinux are new Grade 1A additions to the 2008 update. In general, LMWH is preferred over intravenous UFH, except in patients with severe renal failure.20
| Initial Anticoagulation Therapy | Grade | Acceptable Treatment Regimen* |
|---|---|---|
| ||
| SC LMWH | 1A | Enoxaparin: 1 mg/kg every 12 hours or 1.5 mg/kg once daily; Dalteparin: 200 IU/kg once daily (can be administered out of hospital) |
| Intravenous UFH | 1A | Get baseline aPTT, PT, and platelet count; if no abnormalities, proceed with a weight‐based heparin infusion protocol such as: |
| Bolus of 80 U/kg, followed by an infusion of 18 U/kg per hour (treatment duration 72 days); check aPTT every 4‐6 hours and adjust according to the normogram; monitor platelet count every 3‐4 days for HIT | ||
| Monitored SC UFH; fixed‐dose, unmonitored, SC UFH | 1A; 1A | Initial dose of 333 U/kg, followed by a fixed dose of 250 U/kg every 12 hours (can be administered out of hospital) |
| SC fondaparinux | 1A | 5 mg (body weight <50 kg), 7.5 mg (body weight 50‐100 kg), or 10 mg (body weight >100 kg) once daily (treatment duration 72 days) |
| IVC filter if anticoagulation contraindicated | 1C | |
Warfarin should also be initiated on the same day as UFH or LMWH and adjusted to a target INR of 2.5 (range, 2.0‐3.0). Treatment with UFH or LMWH should be continued concomitantly for a minimum of 5 days and should not be discontinued until the INR has been over 2.0 for 24 hours. The ACCP guidelines also recommend systematic follow‐up of oral anticoagulation therapy.
ACP/AAFP Guidelines
In 2007, the ACP and the AAFP collaborated to develop joint guidelines for the management of VTE.32 Several of their key recommendations are the following:32
-
LMWH, rather than UFH, should be used whenever possible for the initial inpatient treatment of DVT
-
Either UFH or LMWH is appropriate for the initial treatment of PE
-
Anticoagulation should be continued for 3 to 6 months for VTE secondary to transient risk factors, and for more than 12 months for recurrent VTE
-
LMWH is safe and effective for the long‐term treatment of VTE in selected patients (and may be preferable for patients with cancer)
ESC Guidelines for the Treatment of PE
According to the 2008 ESC guidelines, anticoagulation with UFH, LMWH, or fondaparinux should be initiated immediately in patients with confirmed PE, as well as in those with a high or intermediate clinical probability of PE while the diagnostic workup is ongoing. Subcutaneous LMWH or fondaparinux is preferable to intravenous UFH for initial treatment in most patients. UFH, however, should be used in patients with a high risk of bleeding due to its capacity for reversal and short half‐life, as well as in those with severe renal dysfunction.10
According to the ESC, patients with high‐risk PE (presenting with cardiogenic shock or persistent arterial hypotension) should receive thrombolytic therapy as first‐line therapy. Hemodynamic and respiratory support is also necessary for these patients.10 Routine thrombolysis is not recommended in patients with non‐high‐risk PE, but it may be considered in select patients with intermediate‐risk PE (characterized by severe right ventricular dysfunction on echocardiography and/or myocardial injury), depending on the patient's risk of bleeding. Thrombolytic therapy should not be used in patients with low‐risk PE (presenting without shock, hypotension, right ventricular dysfunction, or myocardial injury).10
Like the ESC guidelines, the ACCP guidelines recommend against the use of thrombolytic therapy for the majority of patients with PE (Grade 1B), but they do recommend its use in patients with evidence of hemodynamic compromise and no major contraindications owing to bleeding risk (Grade 1B) and in certain other high‐risk patients (Grade 2B).20
The ESC states that pulmonary embolectomy has recently become a reasonable option for patients with massive, high‐risk PE and an absolute contraindication to thrombolysis, or in whom thrombolysis has failed, when appropriate expertise is available. In the past, it was performed as a last resort in patients with massive PE who were in shock and conferred a high risk of mortality (+50%). Recently, however, the procedure has been revived and performed immediately in patients with confirmed massive PE (with severe right ventricular dysfunction but before shock), with mortality rates of less than 10%.19 It should be noted, however, that the ACCP guidelines consider embolectomy a Grade 2C recommendation.20 Alternatively, catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in these patients.10
NCCN Guidelines: Oncology Patients
The NCCN has provided treatment algorithms for the management of DVT and PE in patients with cancer, which are available online at
FWG Guidelines: Oncology Patients
At the 2008 ASH annual meeting, the FWG presented updated guidelines for the treatment of VTE in cancer patients.34 The FWG guidelines contain the following key recommendations:
-
The treatment of VTE should be based on LMWH at curative doses for at least 3 months
-
During the initial treatment (up to 10 days), any approved drug (including LMWH, UFH, and fondaparinux) may be used
-
Beyond the first 10 days, VTE treatment should be based on LMWH at curative doses for at least 3 months and optimally 6 months, as validated with the following drugs and dosage regimens:
-
Dalteparin 200 IU/kg once daily for 1 month, then 150 IU/kg once daily
-
Enoxaparin 150 IU/kg (1.5 mg/kg) once daily
-
Tinzaparin 175 IU/kg once daily
-
Special treatment considerations include the following:
-
In severe renal impairment, UFH should be used and rapidly followed by a vitamin K agonist (VKA) for at least 3 months
-
In severe PE (representing hemodynamic failure), the indications and recommended uses of thrombolytic drugs in noncancer patients apply
-
In patients with an absolute contraindication to anticoagulation or VTE recurrence despite optimal anticoagulation, vena cava filters should be considered
-
In patients with intracranial malignancies, VTE treatment is the same as in cancer patients with nonintracranial tumors
The treatment of central venous catheter thrombosis requires the long‐term use of LMWH according to the FWG guidelines. In patients with severe renal failure, UFH with early VKA must be used as an alternative treatment. Regardless of the therapy used, treatment should be continued as long as the catheter is maintained.34
Long‐Term Management of VTE
The high rate of recurrent VTE after a first episode of DVT or PEapproximately 8% within 90 daysunderscores the importance of maintaining effective prophylaxis postdischarge.35 Inadequate prophylaxis following discharge from the hospital can have severe consequences. In a recent study of 10,744 patients who were discharged from the hospital following hip or knee replacement surgery, fewer than 1 in 5 received postdischarge thromboprophylaxis. The 3‐month risk of mortality was significantly lower among those who received thromboprophylaxis at discharge (adjusted hazard ratio, 0.34; 95% CI, 0.20‐0.57).36
Detailed patient education at the time of discharge may be one of the most effective ways to prevent or minimize the burden of long‐term complications such as PTS or recurrent VTE. Accordingly, proper discharge planning and postdischarge support, including an appropriate anticoagulant, are critical steps toward reducing mortality, morbidity, and healthcare costs.
PTS
As many as 50% of patients with VTE will develop PTS, a serious but preventable complication that leads to pain, swelling, and skin changes in the affected limb. Female gender, older age, higher body mass index (BMI), and DVT of the common femoral or iliac vein (vs. distal DVT) are associated with an increased risk of PTS.37 To prevent PTS in a patient who has had a symptomatic proximal DVT, current guidelines recommend the use of graduated elastic compression stockings with an ankle pressure of 30 to 40 mm Hg, if feasible. Compression therapy should start as soon as possible after the initiation of anticoagulation therapy and be encouraged for a minimum of 2 years.20
Recurrent VTE
After discontinuing anticoagulation, the risk of recurrent VTE increases steadily over time. In a recent long‐term study of patients with acute proximal DVT or PE, the risk of recurrent VTE was 11% after 1 year, 20% after 3 years, 30% after 5 years, and 40% after 10 years. In this study, risk factors for recurrent VTE included unprovoked initial VTE, thrombophilia, increasing age, and a shorter duration of anticoagulation (6 months or less).38 Another study identified residual venous thrombosis as an important risk factor for recurrent VTE.39 In addition, 1 meta‐analysis found that men had a 50% higher risk of recurrent VTE than women.40 Recurrent DVT events are associated with a 21% greater cost than the initial event, suggesting that recurrent VTE is a preventable drain on healthcare resources.41
Secondary Prevention
The risk of recurrent VTE is determined by the effectiveness of treatment for the acute episode of VTE and by the patient's intrinsic risk of thromboembolism. The ACCP recommends different durations of warfarin or LMWH anticoagulant therapy according to these features (Table 2).20 Attaching a high value to prevention of recurrent VTE and a lower value to the burden of long‐term anticoagulant treatment, the ACCP recommends long‐term treatment for patients with a first unprovoked proximal DVT, no risk factors for bleeding, and the ability to monitor the anticoagulant effectively (Grade 1A).20
| Clinical Features | Duration | Grade |
|---|---|---|
| ||
| First episode and transient risk factors | 3 months | 1A |
| Unprovoked episode | 3 months | 1A |
| Unprovoked proximal DVT with low bleed risk | Long‐term | 1A |
| Cancer | 3 to 6 months with LMWH; then with a VKA or a LMWH indefinitely or until cancer is resolved | 1A; 1C |
| Second unprovoked episode | Indefinite | 2A |
Transition to Outpatient Therapy
The use of outpatient LMWH has changed the course of long‐term anticoagulation therapy and is listed as the preferred option for anticoagulation in the ACCP guidelines.20 With the availability of subcutaneous LMWHs, patients with acute VTE no longer have to be hospitalized for the initiation of oral therapy. In addition, patients undergoing invasive procedures that require temporary discontinuation of warfarin can opt for bridge therapy with LMWH.42
Conclusions
The diagnosis of VTE is challenging and depends on the integration of clinical, biochemical, and imaging modalities. In the absence of contraindications, treatment should be initiated immediately after a diagnosis of VTE is confirmed. Anticoagulant therapy alone is sufficient for most patients, but some patients may require thrombolytics or other strategies. Various societies and organizations have issued recommendations regarding the optimal use of these therapies in specific patient populations. Following these recommendations carefully may reduce the risk of complications in patients with VTE.
- Health Grades, Inc. The Fifth Annual Health Grades Patient Safety in American Hospitals Study. Available at: http://www.healthgrades.com/media/dms/pdf/PatientSafetyInAmericanHospitalsStudy2008.pdf. Accessed August2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest2008;133(6 suppl):381S–453S.
- , , , et al.Value of assessment of pretest probability of deep‐vein thrombosis in clinical management.Lancet.1997;350(9094):1795–1798.
- , , , et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- , , , et al.Limitations of D‐dimer testing in unselected inpatients with suspected venous thromboembolism.Am J Med.2003;114(4):276–282.
- , , , et al.Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians.Ann Fam Med.2007;5(1):57–62.
- , , .The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism.Ann Intern Med.1998;129:1044–1049.
- , .Ultrasonography of leg veins in patients suspected of having pulmonary embolism.Ann Intern Med.1998;128:243–245.
- , .Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism.Circulation.2004;109(12 suppl 1):I15–I21.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology.Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer.Thromb Haemost.2000;83(3):416–420.
- , , , et al.Prospective validation of Wells criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44(5):503–510.
- , , , et al.Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism.J Thromb Haemost.2004;2(8):1247–1255.
- , , , et al.Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria.J Thromb Haemost.2008;6(5):772–780.
- , , , et al.A simple diagnostic strategy in hospitalized patients with clinically suspected pulmonary embolism.J Intern Med.2006;260(5):459–466.
- , .Is computed tomographic venography of lower limbs useful in suspected pulmonary embolism?Rev Med Suisse.2008;4(143):354,356–359.
- , , , et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354(22):2317–2327.
- , , , et al.Christopher Study Investigators.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D‐dimer testing, and computed tomography.JAMA.2006;295(2):172–179.
- , , , .Acute pulmonary embolectomy: a contemporary approach.Circulation.2002;105:1416–1419.
- , , , et al.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 suppl):454S–545S.
- , , , et al.Subcutaneous fondaparinux versus unfractionated heparin in the initial treatment of pulmonary embolism.N Engl J Med.2003;349:1695–1702.
- .Heparin‐induced thrombocytopenia associated with fondaparinux.N Engl J Med.2007;356:2653–2655.
- , , , .Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- , , , et al.Ins and outs of inferior vena cava filters in patients with venous thromboembolism: the experience at Monash Medical Centre and review of the published reports.Intern Med J.2008;38(1):38–43.
- PREPIC Study Group.Eight‐year follow‐up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study.Circulation.2005;112:416–422.
- , , , .Inferior vena cava filters: key considerations.Am J Med Sci.2005;330:82–87.
- , , , .Percutaneous inferior vena cava filters: follow‐up of 7 designs in 320 patients.Radiology.1993;188:851–856.
- , , , et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24.
- , , , , , .Long‐term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement.J Vasc Surg.2007;45(5):992–997.
- , , , et al.Comparison of fixed‐dose weight‐adjusted unfractionated heparin and low‐molecular‐weight heparin for acute treatment of venous thromboembolism.JAMA.2006;296(8):935–942.
- Arixtra prescribing information. Last updated October 2008. Research Triangle Park, NC: GlaxoSmithKline. Available at: http://us.gsk.com/products/assets/us_arixtra.pdf. Accessed August2009.
- , , , et al.American College of Physicians;American Academy of Family Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Ann Intern Med.2007;146(3):204–210.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice Guidelines in Oncology. V.1.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2009.
- , , , et al. Guidelines for the treatment of venous thromboembolism in cancer patients: report from the French Working Group. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1284.
- , , , et al.Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study.Arch Intern Med.2000;160(6):761–768.
- , , , et al.Postdischarge thromboprophylaxis and mortality risk after hip‐or knee‐replacement surgery.CMAJ.2008;178(12):1545–1554.
- , , , et al.Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis.Ann Intern Med.2008;149(10):698–707.
- , , , et al.The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients.Haematologica.2007;92(2):199–205.
- .Risk factors of recurrent venous thromboembolism: the role of residual vein thrombosis.Pathophysiol Haemost Thromb.2003/2004;33(5‐6):351–353.
- , , , et al.Effect of patient's sex on risk of recurrent venous thromboembolism: a meta‐analysis.Lancet.2006;368:371–378.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
- Health Grades, Inc. The Fifth Annual Health Grades Patient Safety in American Hospitals Study. Available at: http://www.healthgrades.com/media/dms/pdf/PatientSafetyInAmericanHospitalsStudy2008.pdf. Accessed August2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest2008;133(6 suppl):381S–453S.
- , , , et al.Value of assessment of pretest probability of deep‐vein thrombosis in clinical management.Lancet.1997;350(9094):1795–1798.
- , , , et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- , , , et al.Limitations of D‐dimer testing in unselected inpatients with suspected venous thromboembolism.Am J Med.2003;114(4):276–282.
- , , , et al.Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians.Ann Fam Med.2007;5(1):57–62.
- , , .The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism.Ann Intern Med.1998;129:1044–1049.
- , .Ultrasonography of leg veins in patients suspected of having pulmonary embolism.Ann Intern Med.1998;128:243–245.
- , .Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism.Circulation.2004;109(12 suppl 1):I15–I21.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology.Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer.Thromb Haemost.2000;83(3):416–420.
- , , , et al.Prospective validation of Wells criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44(5):503–510.
- , , , et al.Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism.J Thromb Haemost.2004;2(8):1247–1255.
- , , , et al.Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria.J Thromb Haemost.2008;6(5):772–780.
- , , , et al.A simple diagnostic strategy in hospitalized patients with clinically suspected pulmonary embolism.J Intern Med.2006;260(5):459–466.
- , .Is computed tomographic venography of lower limbs useful in suspected pulmonary embolism?Rev Med Suisse.2008;4(143):354,356–359.
- , , , et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354(22):2317–2327.
- , , , et al.Christopher Study Investigators.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D‐dimer testing, and computed tomography.JAMA.2006;295(2):172–179.
- , , , .Acute pulmonary embolectomy: a contemporary approach.Circulation.2002;105:1416–1419.
- , , , et al.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 suppl):454S–545S.
- , , , et al.Subcutaneous fondaparinux versus unfractionated heparin in the initial treatment of pulmonary embolism.N Engl J Med.2003;349:1695–1702.
- .Heparin‐induced thrombocytopenia associated with fondaparinux.N Engl J Med.2007;356:2653–2655.
- , , , .Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- , , , et al.Ins and outs of inferior vena cava filters in patients with venous thromboembolism: the experience at Monash Medical Centre and review of the published reports.Intern Med J.2008;38(1):38–43.
- PREPIC Study Group.Eight‐year follow‐up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study.Circulation.2005;112:416–422.
- , , , .Inferior vena cava filters: key considerations.Am J Med Sci.2005;330:82–87.
- , , , .Percutaneous inferior vena cava filters: follow‐up of 7 designs in 320 patients.Radiology.1993;188:851–856.
- , , , et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24.
- , , , , , .Long‐term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement.J Vasc Surg.2007;45(5):992–997.
- , , , et al.Comparison of fixed‐dose weight‐adjusted unfractionated heparin and low‐molecular‐weight heparin for acute treatment of venous thromboembolism.JAMA.2006;296(8):935–942.
- Arixtra prescribing information. Last updated October 2008. Research Triangle Park, NC: GlaxoSmithKline. Available at: http://us.gsk.com/products/assets/us_arixtra.pdf. Accessed August2009.
- , , , et al.American College of Physicians;American Academy of Family Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Ann Intern Med.2007;146(3):204–210.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice Guidelines in Oncology. V.1.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2009.
- , , , et al. Guidelines for the treatment of venous thromboembolism in cancer patients: report from the French Working Group. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1284.
- , , , et al.Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study.Arch Intern Med.2000;160(6):761–768.
- , , , et al.Postdischarge thromboprophylaxis and mortality risk after hip‐or knee‐replacement surgery.CMAJ.2008;178(12):1545–1554.
- , , , et al.Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis.Ann Intern Med.2008;149(10):698–707.
- , , , et al.The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients.Haematologica.2007;92(2):199–205.
- .Risk factors of recurrent venous thromboembolism: the role of residual vein thrombosis.Pathophysiol Haemost Thromb.2003/2004;33(5‐6):351–353.
- , , , et al.Effect of patient's sex on risk of recurrent venous thromboembolism: a meta‐analysis.Lancet.2006;368:371–378.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
VTE Performance Measures and Strategies
Despite the availability of evidence‐based guidelines for the prevention of thromboembolic morbidity and mortality, venous thromboembolism (VTE) remains a pervasive threat to public health. Prophylaxis is underused for a variety of reasons, which were summarized in the first article of this Supplement. Overcoming these barriers and reducing the incidence of VTE has become a major priority for public health policy.
The Office of the Surgeon General released a report in September 2008 that reflects this sense of urgency and national focus by calling for a coordinated, multifaceted plan to reduce the incidence of VTE in the United States.1 The Surgeon General report is one of the latest in a string of national initiatives designed to improve outcomes in patients at risk of VTE. In the past several years, public and private agencies have launched a range of programs aimed at improving deficiencies in the awareness, prevention, and treatment of VTE in hospitalized patients (these are summarized in Table 1). New performance measures and improvement initiatives may reduce the discrepancies between recommendations and practice, ultimately improving patient outcomes. These measures may possibly become benchmarks for pay‐for‐performance initiatives or future hospital accreditation.
| Measure/Initiative | Description |
|---|---|
| |
| National Quality Forum/The Joint Commission (NQF/TJC) | Public reporting of hospital performance in 6 performance measures; will apply to all medical and surgical patients |
| Surgical Care Improvement Project (SCIP) | Two performance measures enacted with reimbursement implications; 2 outcomes measures |
| American Medical Association Physician Consortium for Performance Improvement (PCPI) | Medical societies collaborating to identify gaps in care and develop performance measures; 1 measure has been endorsed |
| Leapfrog Hospital Quality and Safety Survey | Web database allowing consumers to compare performance among participating hospitals; includes 2 NQF safe practices |
| TJC National Patient Safety Goals (NPSG) | Goals for solving patient safety problems; compliance required for Joint Commission accreditation, with online reporting of results (Quality Check website) |
| North American Thrombosis Forum (NATF) | Nonprofit organization addressing unmet needs related to VTE and other thrombotic disorders |
| American Venous Forum National Venous Screening Program | National VTE awareness campaign; promotes compliance with protocols |
Herein, we review a variety of VTE performance measures, including those from the National Quality Forum (NQF), The Joint Commission (TJC), and the Surgical Care Improvement Project (SCIP). To illustrate how performance measures may be applied in the hospital setting to improve patient care, performance improvement programs that have proven effective in select hospitals across the United States are described.
Performance Measures and Initiatives
National Quality Forum Performance Measures
The NQF and TJC (formerly known as the Joint Commission on Accreditation of Healthcare Organizations) have already enacted performance measures for pneumonia, heart failure, acute myocardial infarction (MI), and other conditions. Since 2005, the NQF and TJC have been collaborating to develop national consensus performance measures for the prevention and care of VTE. The VTE performance measures will apply to all medical and surgical patients and include process measures in the areas of prevention and treatment, as well as outcome measures. After pilot‐testing a range of measures for 3 years, TJC recommended 7 candidate measures in November 2007. In May 2008, the NQF endorsed 6 of these, embracing all TJC recommendations except one relating to the use and documentation of vena cava filter quality improvement (Table 2).2
|
| Risk assessment and prophylaxis |
| 1. Documentation of VTE risk/prophylaxis within 24 hours of hospital admission or surgery end‐time |
| 2. Documentation of VTE risk/prophylaxis within 24 hours after ICU admission, transfer to ICU, or surgery end‐time |
| Treatment |
| 3. Patients with VTE with overlap of parenteral and warfarin anticoagulation therapy for at least 5 days with an INR 2 before discontinuation of parenteral therapy; for > 5 days with an INR < 2 and discharged on overlap therapy; or discharged in < 5 days on overlap therapy |
| 4. Patients with VTE receiving UFH with dosages/platelet count monitoring by protocol or nomogram |
| 5. Patients with VTE or their caregivers are given written discharge instructions or other educational material addressing all of the following: follow‐up monitoring, compliance issues, dietary restrictions, and potential for adverse drug reactions and interactions |
| Outcomes |
| 6. Incidence of potentially preventable hospital‐acquired VTE measured by patients who received no VTE prophylaxis before VTE diagnosis |
The next step is for the NQF to develop a specification manual that defines which patients should be given prophylaxis using International Classification of Diseases, 9th edition (ICD‐9) codes and identifies which interventions are appropriate for each patient population. Current clinical guidelines provide important guidance for appropriate inclusion and exclusion criteria for medical and surgical prophylaxis, as well as evidence‐based recommendations for the treatment of VTE.3, 4
SCIP
The SCIP has a stated goal of reducing surgical complications by 25% by 2010.5 To accomplish this, the SCIP is targeting improvement in 4 areas: surgical‐site infection, cardiac events, postoperative pneumonia, and VTE prophylaxis. The SCIP performance measures for VTE prophylaxis in surgical patients are as follows:
-
Recommended VTE prophylaxis ordered during admission; and
-
Appropriate VTE prophylaxis received within 24 hours prior to surgical incision time to 24 hours after surgery end time.
After the success seen by a core group of hospitals who volunteered to participate, all Medicare‐accredited hospitals were required to submit SCIP data beginning with discharges in the first quarter of 2007 to obtain full reimbursement from the Centers for Medicare and Medicaid Services (CMS). Institutions can gauge whether they are in compliance with the SCIP VTE measures by answering a series of yes or no questions about whether prophylaxis has been ordered and received for specific patient groups and procedures. In a recent study, almost one‐half of all surgical patients at risk of VTE did not receive recommended and timely prophylaxis as specified by the SCIP performance measures.6
In addition to the 2 enacted SCIP performance measures for VTE prophylaxis, 2 outcome measures are under development. These measures address the rate at which intraoperative or postoperative pulmonary embolism (PE; SCIP VTE‐3) and deep vein thrombosis (DVT; SCIP VTE‐4) are diagnosed during the index hospitalization and within 30 days after surgery. If implemented, these measures will capture the efficacy of thromboprophylaxis.5
Other VTE Performance Initiatives
Several professional and consumer organizations are developing standards and compiling performance data for public reporting and other purposes:
-
The American Medical Association Physician Consortium for Performance Improvement (PCPI) comprises more than 100 national medical specialty and state medical societies working to identify gaps in care that can be addressed with evidence‐based medicine and formal performance measures. The PCPI has endorsed a measure requiring low‐molecular‐weight heparin (LMWH), low‐dose unfractionated heparin (UFH), adjusted‐dose warfarin, fondaparinux, or mechanical prophylaxis to be given within 24 hours prior to incision time or within 24 hours after surgery end‐time for adults undergoing a procedure for which prophylaxis is indicated.7
-
The Leapfrog Hospital Quality and Safety Survey hosts a searchable web‐based database that consumers can use to compare performance among participating hospitals in specific geographic regions. The Leapfrog survey includes NQF safe practices #28 (reduce occurrence of VTE) and #29 (ensure long‐term anticoagulation is effective and safe).8
-
TJC National Patient Safety Goals (NPSG) target specific improvements in patient safety by providing healthcare organizations with solutions to prevalent patient safety problems. Compliance is necessary for Joint Commission accreditation, and results are reported on the Quality Check website. NPSG Goal 3 is focused on improving the safety of medications, and Goal 3E specifically addresses patient harm associated with the use of anticoagulation therapy. The 2008 NPSG goals must be implemented by January 2009.2
-
The North American Thrombosis Forum (NATF), a nonprofit organization, was recently organized to address unmet needs in North America related to VTE and other thrombotic disorders. It is designed to complement existing organizations dealing with thrombosis‐related issues, with 5 major focus areas: basic translational research; clinical research; prevention and education; public policy; and advocacy. Each month, its website (
http://www.natfonline.org ) features several scientific papers dealing with venous and arterial thrombosis‐related issues. -
The American Venous Forum National Venous Screening Program is a national campaign designed to increase VTE awareness and promote the importance of compliance with prophylaxis protocols.9
As different organizations work to develop performance measures for VTE, conflicting standards have emerged. Although this remains a major challenge, the NQF is attempting to develop voluntary consensus standards that will harmonize VTE performance measures across all sites of care, including the acute medical, surgical, and oncology settings. Major clinical guidelines from the American College of Physicians (ACP), American College of Chest Physicians (ACCP), the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), the European Society of Cardiology (ESC), and other organizations provide data to support standardized, evidence‐based measures for VTE.
Implications of Performance Data
Hospital‐Level Performance Reporting
Performance results may affect an institution's ability to contract best rates with payors, obtain full reimbursement for services, and be eligible for bonus payments. For example, pay‐for‐reporting legislation from CMS provides targeted financial incentives to improve the rates at which hospitals report data on quality measures. The current legislation stipulates that hospitals must submit performance data, including data on compliance with the 2 SCIP‐VTE measures, or lose 2% of their annual CMS payment update. For a 500‐bed hospital with 80% occupancy and 50% CMS patients, failure to report data on SCIP‐VTE measures would result in an estimated annual loss of $2.6 million.10
In 2007, the first year of the CMS pay‐for‐reporting program, 93% of hospitals met the reporting goals. As penalties for nonreporting increase, an even higher compliance rate may be expected. CMS is proposing a new system that would withhold 5% of the base operating diagnosis‐related group payment from a hospital's budget; hospitals would be required to earn this back through reporting and meeting specific performance goals. Using a phase‐in system, CMS would reimburse 2.5% in the first year for pay‐for‐reporting and 2.5% for pay‐for‐performance. Ultimately, the full 5% bonus would be based on performance results.11
Performance ratings play a central role in hospital accreditation, which is critical for negotiating terms for tiered contracting arrangements with private insurers. In addition, hospital performance rankings are becoming more publicly accessible. TJC reports hospital performance in meeting the SCIP measures on its website (
Physician‐Level Performance Reporting
In new quality assessment programs, physicians will also be rewarded or penalized according to their individual performance. The CMS Physician Quality Reporting Initiative (PQRI) is a claims‐based, voluntary, pay‐for‐reporting initiative targeted to Medicare providers. The PQRI program currently pays physicians 2% of total charges for covered services in exchange for voluntary reporting, and it is moving toward results‐based reimbursement.12 The 2009 PQRI Measures List describes 186 quality measures, including 2 related to VTE:13
-
Quality Measure 23: Percentage of patients aged 18 years and older undergoing procedures for which VTE prophylaxis is indicated in all patients, who had an order for LMWH, low‐dose UFH, adjusted‐dose warfarin, fondaparinux, or mechanical prophylaxis to be given within 24 hours prior to incision time or within 24 hours after surgery end‐time; and
-
Quality Measure 31: Percentage of patients aged 18 years and older with a diagnosis of ischemic stroke or intracranial hemorrhage who received DVT prophylaxis by end of hospital day 2.
In the PQRI, physicians report quality measures on process and patient outcomes to CMS using G‐codes or current procedural terminology (CPT)‐II codes. Approximately one‐half of the 100,000 providers who submitted quality codes during the first PQRI reporting period (July 1 to December 31, 2007) qualified for the incentive payment, totaling $36 million.12
More stringent pay‐for‐performance initiatives that hold physicians personally accountable for performance results are being developed in the private sector. For example, the Consumer‐Purchaser Disclosure Project (CPDP) is a consumer‐advocacy group that aims to improve healthcare and lower costs by holding healthcare providers publicly accountable for their quality of treatment. The CPDP has partnered with the National Committee for Quality Assurance to develop guidelines for reporting NQF performance measures.14
VTE as a Nonreimbursable Never Event
In a program that began with hospital discharges on October 1, 2008, hospitals will not receive CMS payment for 12 selected conditions that were not present on admission and were caused by medical error. These hospital‐acquired conditions (HAC), commonly known as never events, include pressure ulcers, catheter‐associated urinary tract infections, postoperative infections, and other complications. Beginning in fiscal year 2009, CMS has added hospital‐acquired VTE following hip or knee replacement surgery as a nonreimbursable never event.15 While CMS acknowledges that prophylaxis will not prevent every occurrence of DVT/PE, they feel it is a reasonably preventable HAC.15 Similar policies are expanding to state and private payor programs that require neither the patient nor the payor to reimburse the hospital for care related to reasonably preventable complications.
Improving Performance and Patient Outcomes
Despite the growing volume of evidence supporting the use of thromboprophylaxis, its use remains inadequate. The consequences are clear: between 2004 and 2006, the number of cases of postoperative VTE increased by 11%.1 This lack of progress may be due to clinicians' lack of awareness of evidence‐based interventions and to hospitals' lack of protocols for the provision of high‐quality preventive treatment.1 Successful strategies for improving thromboprophylaxis and other VTE performance measures are urgently needed. Over the past several years, researchers have been evaluating the utility of different strategies for improving guideline compliance, such as computer‐aided decision‐making and auditing and feedback programs.
Several initiatives seem to have been successful. In one review, Tooher et al.16 found that computerized reminders are, in general, one of the most effective strategies for improving prescribing practice. Paper‐based systems are easier to ignore without a challenge, while electronic systems may force users to acknowledge alerts. Stand‐alone protocols and reminder systems at the point of care can improve prophylaxis rates by about 50%, and decision‐support systems that integrate orders for prophylaxis can increase rates by up to 85%. Importantly, education‐only programs have not been sufficiently effective.16
Regardless of the strategy chosen, Tooher et al identified.16 several general features that, when included as part of the initiative, increase the likelihood of program success:
-
A process for demonstrating the importance and relevance of VTE prophylaxis in the local clinical setting (eg, presenting findings of a local audit of current practice to clinical staff);
-
A process for improving clinician knowledge about VTE risk assessment and prophylaxis practice, such as through a continuing education program;
-
A method of reminding clinicians to assess patients for VTE risk, accompanied by aids to assist in the documentation of patient risk;
-
A process for assisting clinicians in prescribing the appropriate prophylaxis; and
-
A method for assessing the effectiveness of any changes and for refining local policy to further improve practice, such as through clinical audit and feedback.
Table 3 lists several resources and tools that may be useful when designing and implementing strategies to improve performance and quality of care for hospitalized patients at risk of VTE.
| Resource | Description |
|---|---|
| |
| Society of Hospital Medicine, VTE Resource Room* | A website with educational resources, prophylaxis and treatment algorithms, and sample VTE protocols for various patient populations |
| American Society of Clinical Oncology; VTE Prophylaxis Orders and Flow Sheet | A sheet to consult and fill out when prescribing pharmacologic VTE prophylaxis for cancer patients; includes justifications for use, contraindications, anticoagulant options and doses, and other important details |
| American College of Chest Physicians | A source of guidelines, clinical research, education, and other resources for building an evidence‐based VTE protocol |
| National Comprehensive Cancer Network, Clinical Practice GuidelinesVTE | A concise source of algorithms for VTE prophylaxis, diagnosis, and treatment in cancer patients; also includes tables detailing recommended prevention/treatment regimens and warnings/contraindications |
Case Studies in Performance Improvement
Several institutions have reported success stories and shared details of their quality improvement initiatives. Whether paper‐based, electronic, physician‐targeted, or pharmacist‐led, these programs were designed to meet the unique needs of each institution and can serve as models for other hospitals wishing to implement similar programs to improve VTE prophylaxis rates and patient outcomes.
Brigham and Women's Hospital, Boston, MA
In 2005, Kucher et al.17 published a landmark report illustrating the benefits of an electronic alert system in increasing thromboprophylaxis and reducing VTE rates among hospitalized patients. The randomized trial identified high‐risk patients who were not receiving prophylaxis and assigned them to the intervention group, in which the treating physician was alerted to the VTE risk (n = 1255), or to the control group, in which no alert was made (n = 1251). Compared with patients in the control arm, those in the intervention arm were more than twice as likely to receive mechanical or pharmacologic prophylaxis (14.5% vs. 33.5%) and 41% less likely to develop VTE within 90 days (P < 0.001).17
In 2008, this system was evaluated in a new cohort study to determine the ongoing effectiveness of electronic alerts in a real hospital setting.18 The following steps were taken:
-
Alerts were dispatched for all high‐risk cases; and
-
The responsible physician for each high‐risk patient not receiving prophylaxis was issued a single alert detailing the patient's risk and encouraging the use of thromboprophylaxis
During the study period, the use of prophylaxis increased by 50% (P < 0.001). Still, nearly two‐thirds of physicians ignored the electronic alerts.18 Thus, while computer alert systems are helpful, other strategies must be employed to further improve prophylaxis rates in high‐risk medical patients.18
Roswell Park Cancer Institute, Buffalo, NY
Roswell Park Cancer Institute (RPCI), a Comprehensive Cancer Center with 24,000 active patients, initiated an institute‐wide quality improvement initiative in 2006 to improve the rates of VTE prophylaxis for all adult inpatients.19 This initiative included efforts to improve compliance with NCCN guidelines on all medical services and follow guidelines in accordance with NCCN, surgical best practices, and published standards on all surgical services. To accomplish this objective, RPCI:
-
Implemented mandatory, computerized physician order entry forms;
-
Promoted VTE awareness via staff education, field in‐services, and seminars; and
-
Tracked compliance with manual audits of patient charts every 3 months.
When the initiative began in the fourth quarter of 2006, the rate of NCCN‐recommended VTE prophylaxis was 61% with the medical services and 86% with the surgical services. As of the second quarter of 2008, guideline compliance had increased to 90% and 100% with the medical and surgical services, respectively. Accompanying this increase in compliance was a corresponding decrease in the incidence of VTE, from 0.39% in the fourth quarter of 2006 to 0.08% in the second quarter of 2008 (P < 0.0001). The most pronounced reductions in VTE incidence were observed within the medical services and among outpatients.19
Hartford Hospital, Hartford, CT
Hartford Hospital is an 819‐bed acute‐care community hospital with 300 designated medical beds. In an effort to improve thromboprophylaxis rates among medical patients, the pharmacy, medicine, and information technology departments collaborated to develop an alert within the computerized prescriber‐order‐entry system that reminded clinicians to assess patients for VTE risk factors and the need for prophylaxis.20 When a patient met predefined criteria for VTE risk, the message was displayed until either mechanical or pharmacologic VTE prophylaxis was an active order on the patient's treatment profile (Figure 1). The program was implemented in conjunction with an extensive educational program targeting hospital staff, pharmacists, physicians, nurse practitioners, physician assistants, and nurses.20
Compliance with institutional prophylaxis guidelines increased from 49% to 93% following implementation (P < 0.001). Interestingly, the initiative at Hartford Hospital was able to increase the use of mechanical prophylaxis among patients with a contradiction to pharmacologic therapy from 25% prior to the program to 100% after its implementation (P < 0.001).20
Saint Elizabeth's Hospital, Collinsville, IL
In 2008, Bauer et al.21 reported the benefits of a pharmacist‐led program for VTE prevention in Saint Elizabeth's Hospital, a 278‐bed hospital with more than 13,000 admissions per year. As part of the initiative, hospital pharmacists:
-
Received daily reports of all new admissions cross‐referenced with an accounting of patients currently prescribed UFH or LMWH;
-
Assessed the remaining patients at risk of VTE; and
-
Placed recommendations in patient charts in the form of a bold sticker alerting the physician to the patient's risk factors, their overall risk of VTE, and treatment recommendations (Figure 2).

The program ran 7 days per week, involved 1 pharmacist per day, and required an average of 4 hours per day. Patients in the maternity, nursery, pediatric, and psychiatric units were excluded from the program.
The program led to a significant increase in the use of VTE prophylaxis and a significant reduction in the rate of DVT (P < 0.002).21 These findings suggest that innovative programs tailored to the needs of individual institutions can dramatically increase thromboprophylaxis rates and decrease the incidence of VTE in at‐risk hospitalized patients.
Conclusions
VTE is a serious disease that leads to excess morbidity and mortality among hospitalized patients. The impact of hospital reporting on reimbursement and patient outcomes necessitates the adoption of strategies and protocols proven to enhance the management of VTE and improve patient outcomes. Several successful VTE initiatives have been described in the literature and can serve as models for institutions wishing to develop policies and procedures for preventing VTE. In addition, a number of online resources exist that can aid in the development of VTE protocols.
- U.S. Department of Health and Human Services. The surgeon general's call to action to prevent deep vein thrombosis and pulmonary embolism. Available at: http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call‐to‐action‐on‐dvt‐2008.pdf. Accessed June2009.
- The Joint Commission. National consensus standards for prevention and care of venous thromboembolism (VTE). Last updated April 2009. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition).Chest2008;133(6 suppl):381S–453S.
- , , , , , ;American College of Chest Physicians.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(6 suppl):454S–545S.
- The Joint Commission. Surgical Care Improvement Project Core Measure Set. Updated November 2008. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/SCIP+Core+Measure+Set.htm. Accessed June2009.
- , , , et al.Are surgical patients at risk of venous thromboembolism current meeting the surgical care improvement performance target goal for appropriate and timely prophylaxis?Chest.2008;134:s46003.
- American Medical Association. Perioperative Care: Physician Performance Measurement Set. October 2006. Available at http://www.ama‐assn.org/ama1/pub/upload/mm/370/perioperativews1206.pdf. Accessed June2009.
- Leapfrog Group. The Leapfrog Group Hospital Quality and Safety Survey: What's New in the 2009 Survey (Version 5.1). Available at: https://leapfrog.medstat.com/pdf/final.pdf. Accessed June2009.
- , , , et al.Increasing awareness about venous disease: The American Venous Forum expands the National Venous Screening Program.J Vasc Surg.2008;48(2):394–399.
- Federal Register. Medicare Program; Proposed Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2007 Rates. Available at: http://edocket.access.gpo.gov/2006/pdf/06‐3629.pdf. Accessed December 10,2008.
- Department of Health and Human Services, Centers for Medicare 241(3):397–415.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- , , , et al.Electronic alerts for hospitalized high‐VTE risk patients not receiving prophylaxis: a cohort study.J Thromb Thrombolysis.2008;25(2):146–150.
- , , , et al. Improving compliance with guidelines for venous thromboembolism (VTE) prophylaxis significantly reduces VTE events. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1288.
- .Development and implementation of a program to assess medical patients' need for venous thromboembolism prophylaxis.Am J Health Syst Pharm.2008;65(18):1755–1760.
- , , .Pharmacist‐led program to improve venous thromboembolism prophylaxis in a community hospital.Am J Health Syst Pharm.2008;65(17):1643–1647.
Despite the availability of evidence‐based guidelines for the prevention of thromboembolic morbidity and mortality, venous thromboembolism (VTE) remains a pervasive threat to public health. Prophylaxis is underused for a variety of reasons, which were summarized in the first article of this Supplement. Overcoming these barriers and reducing the incidence of VTE has become a major priority for public health policy.
The Office of the Surgeon General released a report in September 2008 that reflects this sense of urgency and national focus by calling for a coordinated, multifaceted plan to reduce the incidence of VTE in the United States.1 The Surgeon General report is one of the latest in a string of national initiatives designed to improve outcomes in patients at risk of VTE. In the past several years, public and private agencies have launched a range of programs aimed at improving deficiencies in the awareness, prevention, and treatment of VTE in hospitalized patients (these are summarized in Table 1). New performance measures and improvement initiatives may reduce the discrepancies between recommendations and practice, ultimately improving patient outcomes. These measures may possibly become benchmarks for pay‐for‐performance initiatives or future hospital accreditation.
| Measure/Initiative | Description |
|---|---|
| |
| National Quality Forum/The Joint Commission (NQF/TJC) | Public reporting of hospital performance in 6 performance measures; will apply to all medical and surgical patients |
| Surgical Care Improvement Project (SCIP) | Two performance measures enacted with reimbursement implications; 2 outcomes measures |
| American Medical Association Physician Consortium for Performance Improvement (PCPI) | Medical societies collaborating to identify gaps in care and develop performance measures; 1 measure has been endorsed |
| Leapfrog Hospital Quality and Safety Survey | Web database allowing consumers to compare performance among participating hospitals; includes 2 NQF safe practices |
| TJC National Patient Safety Goals (NPSG) | Goals for solving patient safety problems; compliance required for Joint Commission accreditation, with online reporting of results (Quality Check website) |
| North American Thrombosis Forum (NATF) | Nonprofit organization addressing unmet needs related to VTE and other thrombotic disorders |
| American Venous Forum National Venous Screening Program | National VTE awareness campaign; promotes compliance with protocols |
Herein, we review a variety of VTE performance measures, including those from the National Quality Forum (NQF), The Joint Commission (TJC), and the Surgical Care Improvement Project (SCIP). To illustrate how performance measures may be applied in the hospital setting to improve patient care, performance improvement programs that have proven effective in select hospitals across the United States are described.
Performance Measures and Initiatives
National Quality Forum Performance Measures
The NQF and TJC (formerly known as the Joint Commission on Accreditation of Healthcare Organizations) have already enacted performance measures for pneumonia, heart failure, acute myocardial infarction (MI), and other conditions. Since 2005, the NQF and TJC have been collaborating to develop national consensus performance measures for the prevention and care of VTE. The VTE performance measures will apply to all medical and surgical patients and include process measures in the areas of prevention and treatment, as well as outcome measures. After pilot‐testing a range of measures for 3 years, TJC recommended 7 candidate measures in November 2007. In May 2008, the NQF endorsed 6 of these, embracing all TJC recommendations except one relating to the use and documentation of vena cava filter quality improvement (Table 2).2
|
| Risk assessment and prophylaxis |
| 1. Documentation of VTE risk/prophylaxis within 24 hours of hospital admission or surgery end‐time |
| 2. Documentation of VTE risk/prophylaxis within 24 hours after ICU admission, transfer to ICU, or surgery end‐time |
| Treatment |
| 3. Patients with VTE with overlap of parenteral and warfarin anticoagulation therapy for at least 5 days with an INR 2 before discontinuation of parenteral therapy; for > 5 days with an INR < 2 and discharged on overlap therapy; or discharged in < 5 days on overlap therapy |
| 4. Patients with VTE receiving UFH with dosages/platelet count monitoring by protocol or nomogram |
| 5. Patients with VTE or their caregivers are given written discharge instructions or other educational material addressing all of the following: follow‐up monitoring, compliance issues, dietary restrictions, and potential for adverse drug reactions and interactions |
| Outcomes |
| 6. Incidence of potentially preventable hospital‐acquired VTE measured by patients who received no VTE prophylaxis before VTE diagnosis |
The next step is for the NQF to develop a specification manual that defines which patients should be given prophylaxis using International Classification of Diseases, 9th edition (ICD‐9) codes and identifies which interventions are appropriate for each patient population. Current clinical guidelines provide important guidance for appropriate inclusion and exclusion criteria for medical and surgical prophylaxis, as well as evidence‐based recommendations for the treatment of VTE.3, 4
SCIP
The SCIP has a stated goal of reducing surgical complications by 25% by 2010.5 To accomplish this, the SCIP is targeting improvement in 4 areas: surgical‐site infection, cardiac events, postoperative pneumonia, and VTE prophylaxis. The SCIP performance measures for VTE prophylaxis in surgical patients are as follows:
-
Recommended VTE prophylaxis ordered during admission; and
-
Appropriate VTE prophylaxis received within 24 hours prior to surgical incision time to 24 hours after surgery end time.
After the success seen by a core group of hospitals who volunteered to participate, all Medicare‐accredited hospitals were required to submit SCIP data beginning with discharges in the first quarter of 2007 to obtain full reimbursement from the Centers for Medicare and Medicaid Services (CMS). Institutions can gauge whether they are in compliance with the SCIP VTE measures by answering a series of yes or no questions about whether prophylaxis has been ordered and received for specific patient groups and procedures. In a recent study, almost one‐half of all surgical patients at risk of VTE did not receive recommended and timely prophylaxis as specified by the SCIP performance measures.6
In addition to the 2 enacted SCIP performance measures for VTE prophylaxis, 2 outcome measures are under development. These measures address the rate at which intraoperative or postoperative pulmonary embolism (PE; SCIP VTE‐3) and deep vein thrombosis (DVT; SCIP VTE‐4) are diagnosed during the index hospitalization and within 30 days after surgery. If implemented, these measures will capture the efficacy of thromboprophylaxis.5
Other VTE Performance Initiatives
Several professional and consumer organizations are developing standards and compiling performance data for public reporting and other purposes:
-
The American Medical Association Physician Consortium for Performance Improvement (PCPI) comprises more than 100 national medical specialty and state medical societies working to identify gaps in care that can be addressed with evidence‐based medicine and formal performance measures. The PCPI has endorsed a measure requiring low‐molecular‐weight heparin (LMWH), low‐dose unfractionated heparin (UFH), adjusted‐dose warfarin, fondaparinux, or mechanical prophylaxis to be given within 24 hours prior to incision time or within 24 hours after surgery end‐time for adults undergoing a procedure for which prophylaxis is indicated.7
-
The Leapfrog Hospital Quality and Safety Survey hosts a searchable web‐based database that consumers can use to compare performance among participating hospitals in specific geographic regions. The Leapfrog survey includes NQF safe practices #28 (reduce occurrence of VTE) and #29 (ensure long‐term anticoagulation is effective and safe).8
-
TJC National Patient Safety Goals (NPSG) target specific improvements in patient safety by providing healthcare organizations with solutions to prevalent patient safety problems. Compliance is necessary for Joint Commission accreditation, and results are reported on the Quality Check website. NPSG Goal 3 is focused on improving the safety of medications, and Goal 3E specifically addresses patient harm associated with the use of anticoagulation therapy. The 2008 NPSG goals must be implemented by January 2009.2
-
The North American Thrombosis Forum (NATF), a nonprofit organization, was recently organized to address unmet needs in North America related to VTE and other thrombotic disorders. It is designed to complement existing organizations dealing with thrombosis‐related issues, with 5 major focus areas: basic translational research; clinical research; prevention and education; public policy; and advocacy. Each month, its website (
http://www.natfonline.org ) features several scientific papers dealing with venous and arterial thrombosis‐related issues. -
The American Venous Forum National Venous Screening Program is a national campaign designed to increase VTE awareness and promote the importance of compliance with prophylaxis protocols.9
As different organizations work to develop performance measures for VTE, conflicting standards have emerged. Although this remains a major challenge, the NQF is attempting to develop voluntary consensus standards that will harmonize VTE performance measures across all sites of care, including the acute medical, surgical, and oncology settings. Major clinical guidelines from the American College of Physicians (ACP), American College of Chest Physicians (ACCP), the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), the European Society of Cardiology (ESC), and other organizations provide data to support standardized, evidence‐based measures for VTE.
Implications of Performance Data
Hospital‐Level Performance Reporting
Performance results may affect an institution's ability to contract best rates with payors, obtain full reimbursement for services, and be eligible for bonus payments. For example, pay‐for‐reporting legislation from CMS provides targeted financial incentives to improve the rates at which hospitals report data on quality measures. The current legislation stipulates that hospitals must submit performance data, including data on compliance with the 2 SCIP‐VTE measures, or lose 2% of their annual CMS payment update. For a 500‐bed hospital with 80% occupancy and 50% CMS patients, failure to report data on SCIP‐VTE measures would result in an estimated annual loss of $2.6 million.10
In 2007, the first year of the CMS pay‐for‐reporting program, 93% of hospitals met the reporting goals. As penalties for nonreporting increase, an even higher compliance rate may be expected. CMS is proposing a new system that would withhold 5% of the base operating diagnosis‐related group payment from a hospital's budget; hospitals would be required to earn this back through reporting and meeting specific performance goals. Using a phase‐in system, CMS would reimburse 2.5% in the first year for pay‐for‐reporting and 2.5% for pay‐for‐performance. Ultimately, the full 5% bonus would be based on performance results.11
Performance ratings play a central role in hospital accreditation, which is critical for negotiating terms for tiered contracting arrangements with private insurers. In addition, hospital performance rankings are becoming more publicly accessible. TJC reports hospital performance in meeting the SCIP measures on its website (
Physician‐Level Performance Reporting
In new quality assessment programs, physicians will also be rewarded or penalized according to their individual performance. The CMS Physician Quality Reporting Initiative (PQRI) is a claims‐based, voluntary, pay‐for‐reporting initiative targeted to Medicare providers. The PQRI program currently pays physicians 2% of total charges for covered services in exchange for voluntary reporting, and it is moving toward results‐based reimbursement.12 The 2009 PQRI Measures List describes 186 quality measures, including 2 related to VTE:13
-
Quality Measure 23: Percentage of patients aged 18 years and older undergoing procedures for which VTE prophylaxis is indicated in all patients, who had an order for LMWH, low‐dose UFH, adjusted‐dose warfarin, fondaparinux, or mechanical prophylaxis to be given within 24 hours prior to incision time or within 24 hours after surgery end‐time; and
-
Quality Measure 31: Percentage of patients aged 18 years and older with a diagnosis of ischemic stroke or intracranial hemorrhage who received DVT prophylaxis by end of hospital day 2.
In the PQRI, physicians report quality measures on process and patient outcomes to CMS using G‐codes or current procedural terminology (CPT)‐II codes. Approximately one‐half of the 100,000 providers who submitted quality codes during the first PQRI reporting period (July 1 to December 31, 2007) qualified for the incentive payment, totaling $36 million.12
More stringent pay‐for‐performance initiatives that hold physicians personally accountable for performance results are being developed in the private sector. For example, the Consumer‐Purchaser Disclosure Project (CPDP) is a consumer‐advocacy group that aims to improve healthcare and lower costs by holding healthcare providers publicly accountable for their quality of treatment. The CPDP has partnered with the National Committee for Quality Assurance to develop guidelines for reporting NQF performance measures.14
VTE as a Nonreimbursable Never Event
In a program that began with hospital discharges on October 1, 2008, hospitals will not receive CMS payment for 12 selected conditions that were not present on admission and were caused by medical error. These hospital‐acquired conditions (HAC), commonly known as never events, include pressure ulcers, catheter‐associated urinary tract infections, postoperative infections, and other complications. Beginning in fiscal year 2009, CMS has added hospital‐acquired VTE following hip or knee replacement surgery as a nonreimbursable never event.15 While CMS acknowledges that prophylaxis will not prevent every occurrence of DVT/PE, they feel it is a reasonably preventable HAC.15 Similar policies are expanding to state and private payor programs that require neither the patient nor the payor to reimburse the hospital for care related to reasonably preventable complications.
Improving Performance and Patient Outcomes
Despite the growing volume of evidence supporting the use of thromboprophylaxis, its use remains inadequate. The consequences are clear: between 2004 and 2006, the number of cases of postoperative VTE increased by 11%.1 This lack of progress may be due to clinicians' lack of awareness of evidence‐based interventions and to hospitals' lack of protocols for the provision of high‐quality preventive treatment.1 Successful strategies for improving thromboprophylaxis and other VTE performance measures are urgently needed. Over the past several years, researchers have been evaluating the utility of different strategies for improving guideline compliance, such as computer‐aided decision‐making and auditing and feedback programs.
Several initiatives seem to have been successful. In one review, Tooher et al.16 found that computerized reminders are, in general, one of the most effective strategies for improving prescribing practice. Paper‐based systems are easier to ignore without a challenge, while electronic systems may force users to acknowledge alerts. Stand‐alone protocols and reminder systems at the point of care can improve prophylaxis rates by about 50%, and decision‐support systems that integrate orders for prophylaxis can increase rates by up to 85%. Importantly, education‐only programs have not been sufficiently effective.16
Regardless of the strategy chosen, Tooher et al identified.16 several general features that, when included as part of the initiative, increase the likelihood of program success:
-
A process for demonstrating the importance and relevance of VTE prophylaxis in the local clinical setting (eg, presenting findings of a local audit of current practice to clinical staff);
-
A process for improving clinician knowledge about VTE risk assessment and prophylaxis practice, such as through a continuing education program;
-
A method of reminding clinicians to assess patients for VTE risk, accompanied by aids to assist in the documentation of patient risk;
-
A process for assisting clinicians in prescribing the appropriate prophylaxis; and
-
A method for assessing the effectiveness of any changes and for refining local policy to further improve practice, such as through clinical audit and feedback.
Table 3 lists several resources and tools that may be useful when designing and implementing strategies to improve performance and quality of care for hospitalized patients at risk of VTE.
| Resource | Description |
|---|---|
| |
| Society of Hospital Medicine, VTE Resource Room* | A website with educational resources, prophylaxis and treatment algorithms, and sample VTE protocols for various patient populations |
| American Society of Clinical Oncology; VTE Prophylaxis Orders and Flow Sheet | A sheet to consult and fill out when prescribing pharmacologic VTE prophylaxis for cancer patients; includes justifications for use, contraindications, anticoagulant options and doses, and other important details |
| American College of Chest Physicians | A source of guidelines, clinical research, education, and other resources for building an evidence‐based VTE protocol |
| National Comprehensive Cancer Network, Clinical Practice GuidelinesVTE | A concise source of algorithms for VTE prophylaxis, diagnosis, and treatment in cancer patients; also includes tables detailing recommended prevention/treatment regimens and warnings/contraindications |
Case Studies in Performance Improvement
Several institutions have reported success stories and shared details of their quality improvement initiatives. Whether paper‐based, electronic, physician‐targeted, or pharmacist‐led, these programs were designed to meet the unique needs of each institution and can serve as models for other hospitals wishing to implement similar programs to improve VTE prophylaxis rates and patient outcomes.
Brigham and Women's Hospital, Boston, MA
In 2005, Kucher et al.17 published a landmark report illustrating the benefits of an electronic alert system in increasing thromboprophylaxis and reducing VTE rates among hospitalized patients. The randomized trial identified high‐risk patients who were not receiving prophylaxis and assigned them to the intervention group, in which the treating physician was alerted to the VTE risk (n = 1255), or to the control group, in which no alert was made (n = 1251). Compared with patients in the control arm, those in the intervention arm were more than twice as likely to receive mechanical or pharmacologic prophylaxis (14.5% vs. 33.5%) and 41% less likely to develop VTE within 90 days (P < 0.001).17
In 2008, this system was evaluated in a new cohort study to determine the ongoing effectiveness of electronic alerts in a real hospital setting.18 The following steps were taken:
-
Alerts were dispatched for all high‐risk cases; and
-
The responsible physician for each high‐risk patient not receiving prophylaxis was issued a single alert detailing the patient's risk and encouraging the use of thromboprophylaxis
During the study period, the use of prophylaxis increased by 50% (P < 0.001). Still, nearly two‐thirds of physicians ignored the electronic alerts.18 Thus, while computer alert systems are helpful, other strategies must be employed to further improve prophylaxis rates in high‐risk medical patients.18
Roswell Park Cancer Institute, Buffalo, NY
Roswell Park Cancer Institute (RPCI), a Comprehensive Cancer Center with 24,000 active patients, initiated an institute‐wide quality improvement initiative in 2006 to improve the rates of VTE prophylaxis for all adult inpatients.19 This initiative included efforts to improve compliance with NCCN guidelines on all medical services and follow guidelines in accordance with NCCN, surgical best practices, and published standards on all surgical services. To accomplish this objective, RPCI:
-
Implemented mandatory, computerized physician order entry forms;
-
Promoted VTE awareness via staff education, field in‐services, and seminars; and
-
Tracked compliance with manual audits of patient charts every 3 months.
When the initiative began in the fourth quarter of 2006, the rate of NCCN‐recommended VTE prophylaxis was 61% with the medical services and 86% with the surgical services. As of the second quarter of 2008, guideline compliance had increased to 90% and 100% with the medical and surgical services, respectively. Accompanying this increase in compliance was a corresponding decrease in the incidence of VTE, from 0.39% in the fourth quarter of 2006 to 0.08% in the second quarter of 2008 (P < 0.0001). The most pronounced reductions in VTE incidence were observed within the medical services and among outpatients.19
Hartford Hospital, Hartford, CT
Hartford Hospital is an 819‐bed acute‐care community hospital with 300 designated medical beds. In an effort to improve thromboprophylaxis rates among medical patients, the pharmacy, medicine, and information technology departments collaborated to develop an alert within the computerized prescriber‐order‐entry system that reminded clinicians to assess patients for VTE risk factors and the need for prophylaxis.20 When a patient met predefined criteria for VTE risk, the message was displayed until either mechanical or pharmacologic VTE prophylaxis was an active order on the patient's treatment profile (Figure 1). The program was implemented in conjunction with an extensive educational program targeting hospital staff, pharmacists, physicians, nurse practitioners, physician assistants, and nurses.20

Compliance with institutional prophylaxis guidelines increased from 49% to 93% following implementation (P < 0.001). Interestingly, the initiative at Hartford Hospital was able to increase the use of mechanical prophylaxis among patients with a contradiction to pharmacologic therapy from 25% prior to the program to 100% after its implementation (P < 0.001).20
Saint Elizabeth's Hospital, Collinsville, IL
In 2008, Bauer et al.21 reported the benefits of a pharmacist‐led program for VTE prevention in Saint Elizabeth's Hospital, a 278‐bed hospital with more than 13,000 admissions per year. As part of the initiative, hospital pharmacists:
-
Received daily reports of all new admissions cross‐referenced with an accounting of patients currently prescribed UFH or LMWH;
-
Assessed the remaining patients at risk of VTE; and
-
Placed recommendations in patient charts in the form of a bold sticker alerting the physician to the patient's risk factors, their overall risk of VTE, and treatment recommendations (Figure 2).

The program ran 7 days per week, involved 1 pharmacist per day, and required an average of 4 hours per day. Patients in the maternity, nursery, pediatric, and psychiatric units were excluded from the program.
The program led to a significant increase in the use of VTE prophylaxis and a significant reduction in the rate of DVT (P < 0.002).21 These findings suggest that innovative programs tailored to the needs of individual institutions can dramatically increase thromboprophylaxis rates and decrease the incidence of VTE in at‐risk hospitalized patients.
Conclusions
VTE is a serious disease that leads to excess morbidity and mortality among hospitalized patients. The impact of hospital reporting on reimbursement and patient outcomes necessitates the adoption of strategies and protocols proven to enhance the management of VTE and improve patient outcomes. Several successful VTE initiatives have been described in the literature and can serve as models for institutions wishing to develop policies and procedures for preventing VTE. In addition, a number of online resources exist that can aid in the development of VTE protocols.
Despite the availability of evidence‐based guidelines for the prevention of thromboembolic morbidity and mortality, venous thromboembolism (VTE) remains a pervasive threat to public health. Prophylaxis is underused for a variety of reasons, which were summarized in the first article of this Supplement. Overcoming these barriers and reducing the incidence of VTE has become a major priority for public health policy.
The Office of the Surgeon General released a report in September 2008 that reflects this sense of urgency and national focus by calling for a coordinated, multifaceted plan to reduce the incidence of VTE in the United States.1 The Surgeon General report is one of the latest in a string of national initiatives designed to improve outcomes in patients at risk of VTE. In the past several years, public and private agencies have launched a range of programs aimed at improving deficiencies in the awareness, prevention, and treatment of VTE in hospitalized patients (these are summarized in Table 1). New performance measures and improvement initiatives may reduce the discrepancies between recommendations and practice, ultimately improving patient outcomes. These measures may possibly become benchmarks for pay‐for‐performance initiatives or future hospital accreditation.
| Measure/Initiative | Description |
|---|---|
| |
| National Quality Forum/The Joint Commission (NQF/TJC) | Public reporting of hospital performance in 6 performance measures; will apply to all medical and surgical patients |
| Surgical Care Improvement Project (SCIP) | Two performance measures enacted with reimbursement implications; 2 outcomes measures |
| American Medical Association Physician Consortium for Performance Improvement (PCPI) | Medical societies collaborating to identify gaps in care and develop performance measures; 1 measure has been endorsed |
| Leapfrog Hospital Quality and Safety Survey | Web database allowing consumers to compare performance among participating hospitals; includes 2 NQF safe practices |
| TJC National Patient Safety Goals (NPSG) | Goals for solving patient safety problems; compliance required for Joint Commission accreditation, with online reporting of results (Quality Check website) |
| North American Thrombosis Forum (NATF) | Nonprofit organization addressing unmet needs related to VTE and other thrombotic disorders |
| American Venous Forum National Venous Screening Program | National VTE awareness campaign; promotes compliance with protocols |
Herein, we review a variety of VTE performance measures, including those from the National Quality Forum (NQF), The Joint Commission (TJC), and the Surgical Care Improvement Project (SCIP). To illustrate how performance measures may be applied in the hospital setting to improve patient care, performance improvement programs that have proven effective in select hospitals across the United States are described.
Performance Measures and Initiatives
National Quality Forum Performance Measures
The NQF and TJC (formerly known as the Joint Commission on Accreditation of Healthcare Organizations) have already enacted performance measures for pneumonia, heart failure, acute myocardial infarction (MI), and other conditions. Since 2005, the NQF and TJC have been collaborating to develop national consensus performance measures for the prevention and care of VTE. The VTE performance measures will apply to all medical and surgical patients and include process measures in the areas of prevention and treatment, as well as outcome measures. After pilot‐testing a range of measures for 3 years, TJC recommended 7 candidate measures in November 2007. In May 2008, the NQF endorsed 6 of these, embracing all TJC recommendations except one relating to the use and documentation of vena cava filter quality improvement (Table 2).2
|
| Risk assessment and prophylaxis |
| 1. Documentation of VTE risk/prophylaxis within 24 hours of hospital admission or surgery end‐time |
| 2. Documentation of VTE risk/prophylaxis within 24 hours after ICU admission, transfer to ICU, or surgery end‐time |
| Treatment |
| 3. Patients with VTE with overlap of parenteral and warfarin anticoagulation therapy for at least 5 days with an INR 2 before discontinuation of parenteral therapy; for > 5 days with an INR < 2 and discharged on overlap therapy; or discharged in < 5 days on overlap therapy |
| 4. Patients with VTE receiving UFH with dosages/platelet count monitoring by protocol or nomogram |
| 5. Patients with VTE or their caregivers are given written discharge instructions or other educational material addressing all of the following: follow‐up monitoring, compliance issues, dietary restrictions, and potential for adverse drug reactions and interactions |
| Outcomes |
| 6. Incidence of potentially preventable hospital‐acquired VTE measured by patients who received no VTE prophylaxis before VTE diagnosis |
The next step is for the NQF to develop a specification manual that defines which patients should be given prophylaxis using International Classification of Diseases, 9th edition (ICD‐9) codes and identifies which interventions are appropriate for each patient population. Current clinical guidelines provide important guidance for appropriate inclusion and exclusion criteria for medical and surgical prophylaxis, as well as evidence‐based recommendations for the treatment of VTE.3, 4
SCIP
The SCIP has a stated goal of reducing surgical complications by 25% by 2010.5 To accomplish this, the SCIP is targeting improvement in 4 areas: surgical‐site infection, cardiac events, postoperative pneumonia, and VTE prophylaxis. The SCIP performance measures for VTE prophylaxis in surgical patients are as follows:
-
Recommended VTE prophylaxis ordered during admission; and
-
Appropriate VTE prophylaxis received within 24 hours prior to surgical incision time to 24 hours after surgery end time.
After the success seen by a core group of hospitals who volunteered to participate, all Medicare‐accredited hospitals were required to submit SCIP data beginning with discharges in the first quarter of 2007 to obtain full reimbursement from the Centers for Medicare and Medicaid Services (CMS). Institutions can gauge whether they are in compliance with the SCIP VTE measures by answering a series of yes or no questions about whether prophylaxis has been ordered and received for specific patient groups and procedures. In a recent study, almost one‐half of all surgical patients at risk of VTE did not receive recommended and timely prophylaxis as specified by the SCIP performance measures.6
In addition to the 2 enacted SCIP performance measures for VTE prophylaxis, 2 outcome measures are under development. These measures address the rate at which intraoperative or postoperative pulmonary embolism (PE; SCIP VTE‐3) and deep vein thrombosis (DVT; SCIP VTE‐4) are diagnosed during the index hospitalization and within 30 days after surgery. If implemented, these measures will capture the efficacy of thromboprophylaxis.5
Other VTE Performance Initiatives
Several professional and consumer organizations are developing standards and compiling performance data for public reporting and other purposes:
-
The American Medical Association Physician Consortium for Performance Improvement (PCPI) comprises more than 100 national medical specialty and state medical societies working to identify gaps in care that can be addressed with evidence‐based medicine and formal performance measures. The PCPI has endorsed a measure requiring low‐molecular‐weight heparin (LMWH), low‐dose unfractionated heparin (UFH), adjusted‐dose warfarin, fondaparinux, or mechanical prophylaxis to be given within 24 hours prior to incision time or within 24 hours after surgery end‐time for adults undergoing a procedure for which prophylaxis is indicated.7
-
The Leapfrog Hospital Quality and Safety Survey hosts a searchable web‐based database that consumers can use to compare performance among participating hospitals in specific geographic regions. The Leapfrog survey includes NQF safe practices #28 (reduce occurrence of VTE) and #29 (ensure long‐term anticoagulation is effective and safe).8
-
TJC National Patient Safety Goals (NPSG) target specific improvements in patient safety by providing healthcare organizations with solutions to prevalent patient safety problems. Compliance is necessary for Joint Commission accreditation, and results are reported on the Quality Check website. NPSG Goal 3 is focused on improving the safety of medications, and Goal 3E specifically addresses patient harm associated with the use of anticoagulation therapy. The 2008 NPSG goals must be implemented by January 2009.2
-
The North American Thrombosis Forum (NATF), a nonprofit organization, was recently organized to address unmet needs in North America related to VTE and other thrombotic disorders. It is designed to complement existing organizations dealing with thrombosis‐related issues, with 5 major focus areas: basic translational research; clinical research; prevention and education; public policy; and advocacy. Each month, its website (
http://www.natfonline.org ) features several scientific papers dealing with venous and arterial thrombosis‐related issues. -
The American Venous Forum National Venous Screening Program is a national campaign designed to increase VTE awareness and promote the importance of compliance with prophylaxis protocols.9
As different organizations work to develop performance measures for VTE, conflicting standards have emerged. Although this remains a major challenge, the NQF is attempting to develop voluntary consensus standards that will harmonize VTE performance measures across all sites of care, including the acute medical, surgical, and oncology settings. Major clinical guidelines from the American College of Physicians (ACP), American College of Chest Physicians (ACCP), the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), the European Society of Cardiology (ESC), and other organizations provide data to support standardized, evidence‐based measures for VTE.
Implications of Performance Data
Hospital‐Level Performance Reporting
Performance results may affect an institution's ability to contract best rates with payors, obtain full reimbursement for services, and be eligible for bonus payments. For example, pay‐for‐reporting legislation from CMS provides targeted financial incentives to improve the rates at which hospitals report data on quality measures. The current legislation stipulates that hospitals must submit performance data, including data on compliance with the 2 SCIP‐VTE measures, or lose 2% of their annual CMS payment update. For a 500‐bed hospital with 80% occupancy and 50% CMS patients, failure to report data on SCIP‐VTE measures would result in an estimated annual loss of $2.6 million.10
In 2007, the first year of the CMS pay‐for‐reporting program, 93% of hospitals met the reporting goals. As penalties for nonreporting increase, an even higher compliance rate may be expected. CMS is proposing a new system that would withhold 5% of the base operating diagnosis‐related group payment from a hospital's budget; hospitals would be required to earn this back through reporting and meeting specific performance goals. Using a phase‐in system, CMS would reimburse 2.5% in the first year for pay‐for‐reporting and 2.5% for pay‐for‐performance. Ultimately, the full 5% bonus would be based on performance results.11
Performance ratings play a central role in hospital accreditation, which is critical for negotiating terms for tiered contracting arrangements with private insurers. In addition, hospital performance rankings are becoming more publicly accessible. TJC reports hospital performance in meeting the SCIP measures on its website (
Physician‐Level Performance Reporting
In new quality assessment programs, physicians will also be rewarded or penalized according to their individual performance. The CMS Physician Quality Reporting Initiative (PQRI) is a claims‐based, voluntary, pay‐for‐reporting initiative targeted to Medicare providers. The PQRI program currently pays physicians 2% of total charges for covered services in exchange for voluntary reporting, and it is moving toward results‐based reimbursement.12 The 2009 PQRI Measures List describes 186 quality measures, including 2 related to VTE:13
-
Quality Measure 23: Percentage of patients aged 18 years and older undergoing procedures for which VTE prophylaxis is indicated in all patients, who had an order for LMWH, low‐dose UFH, adjusted‐dose warfarin, fondaparinux, or mechanical prophylaxis to be given within 24 hours prior to incision time or within 24 hours after surgery end‐time; and
-
Quality Measure 31: Percentage of patients aged 18 years and older with a diagnosis of ischemic stroke or intracranial hemorrhage who received DVT prophylaxis by end of hospital day 2.
In the PQRI, physicians report quality measures on process and patient outcomes to CMS using G‐codes or current procedural terminology (CPT)‐II codes. Approximately one‐half of the 100,000 providers who submitted quality codes during the first PQRI reporting period (July 1 to December 31, 2007) qualified for the incentive payment, totaling $36 million.12
More stringent pay‐for‐performance initiatives that hold physicians personally accountable for performance results are being developed in the private sector. For example, the Consumer‐Purchaser Disclosure Project (CPDP) is a consumer‐advocacy group that aims to improve healthcare and lower costs by holding healthcare providers publicly accountable for their quality of treatment. The CPDP has partnered with the National Committee for Quality Assurance to develop guidelines for reporting NQF performance measures.14
VTE as a Nonreimbursable Never Event
In a program that began with hospital discharges on October 1, 2008, hospitals will not receive CMS payment for 12 selected conditions that were not present on admission and were caused by medical error. These hospital‐acquired conditions (HAC), commonly known as never events, include pressure ulcers, catheter‐associated urinary tract infections, postoperative infections, and other complications. Beginning in fiscal year 2009, CMS has added hospital‐acquired VTE following hip or knee replacement surgery as a nonreimbursable never event.15 While CMS acknowledges that prophylaxis will not prevent every occurrence of DVT/PE, they feel it is a reasonably preventable HAC.15 Similar policies are expanding to state and private payor programs that require neither the patient nor the payor to reimburse the hospital for care related to reasonably preventable complications.
Improving Performance and Patient Outcomes
Despite the growing volume of evidence supporting the use of thromboprophylaxis, its use remains inadequate. The consequences are clear: between 2004 and 2006, the number of cases of postoperative VTE increased by 11%.1 This lack of progress may be due to clinicians' lack of awareness of evidence‐based interventions and to hospitals' lack of protocols for the provision of high‐quality preventive treatment.1 Successful strategies for improving thromboprophylaxis and other VTE performance measures are urgently needed. Over the past several years, researchers have been evaluating the utility of different strategies for improving guideline compliance, such as computer‐aided decision‐making and auditing and feedback programs.
Several initiatives seem to have been successful. In one review, Tooher et al.16 found that computerized reminders are, in general, one of the most effective strategies for improving prescribing practice. Paper‐based systems are easier to ignore without a challenge, while electronic systems may force users to acknowledge alerts. Stand‐alone protocols and reminder systems at the point of care can improve prophylaxis rates by about 50%, and decision‐support systems that integrate orders for prophylaxis can increase rates by up to 85%. Importantly, education‐only programs have not been sufficiently effective.16
Regardless of the strategy chosen, Tooher et al identified.16 several general features that, when included as part of the initiative, increase the likelihood of program success:
-
A process for demonstrating the importance and relevance of VTE prophylaxis in the local clinical setting (eg, presenting findings of a local audit of current practice to clinical staff);
-
A process for improving clinician knowledge about VTE risk assessment and prophylaxis practice, such as through a continuing education program;
-
A method of reminding clinicians to assess patients for VTE risk, accompanied by aids to assist in the documentation of patient risk;
-
A process for assisting clinicians in prescribing the appropriate prophylaxis; and
-
A method for assessing the effectiveness of any changes and for refining local policy to further improve practice, such as through clinical audit and feedback.
Table 3 lists several resources and tools that may be useful when designing and implementing strategies to improve performance and quality of care for hospitalized patients at risk of VTE.
| Resource | Description |
|---|---|
| |
| Society of Hospital Medicine, VTE Resource Room* | A website with educational resources, prophylaxis and treatment algorithms, and sample VTE protocols for various patient populations |
| American Society of Clinical Oncology; VTE Prophylaxis Orders and Flow Sheet | A sheet to consult and fill out when prescribing pharmacologic VTE prophylaxis for cancer patients; includes justifications for use, contraindications, anticoagulant options and doses, and other important details |
| American College of Chest Physicians | A source of guidelines, clinical research, education, and other resources for building an evidence‐based VTE protocol |
| National Comprehensive Cancer Network, Clinical Practice GuidelinesVTE | A concise source of algorithms for VTE prophylaxis, diagnosis, and treatment in cancer patients; also includes tables detailing recommended prevention/treatment regimens and warnings/contraindications |
Case Studies in Performance Improvement
Several institutions have reported success stories and shared details of their quality improvement initiatives. Whether paper‐based, electronic, physician‐targeted, or pharmacist‐led, these programs were designed to meet the unique needs of each institution and can serve as models for other hospitals wishing to implement similar programs to improve VTE prophylaxis rates and patient outcomes.
Brigham and Women's Hospital, Boston, MA
In 2005, Kucher et al.17 published a landmark report illustrating the benefits of an electronic alert system in increasing thromboprophylaxis and reducing VTE rates among hospitalized patients. The randomized trial identified high‐risk patients who were not receiving prophylaxis and assigned them to the intervention group, in which the treating physician was alerted to the VTE risk (n = 1255), or to the control group, in which no alert was made (n = 1251). Compared with patients in the control arm, those in the intervention arm were more than twice as likely to receive mechanical or pharmacologic prophylaxis (14.5% vs. 33.5%) and 41% less likely to develop VTE within 90 days (P < 0.001).17
In 2008, this system was evaluated in a new cohort study to determine the ongoing effectiveness of electronic alerts in a real hospital setting.18 The following steps were taken:
-
Alerts were dispatched for all high‐risk cases; and
-
The responsible physician for each high‐risk patient not receiving prophylaxis was issued a single alert detailing the patient's risk and encouraging the use of thromboprophylaxis
During the study period, the use of prophylaxis increased by 50% (P < 0.001). Still, nearly two‐thirds of physicians ignored the electronic alerts.18 Thus, while computer alert systems are helpful, other strategies must be employed to further improve prophylaxis rates in high‐risk medical patients.18
Roswell Park Cancer Institute, Buffalo, NY
Roswell Park Cancer Institute (RPCI), a Comprehensive Cancer Center with 24,000 active patients, initiated an institute‐wide quality improvement initiative in 2006 to improve the rates of VTE prophylaxis for all adult inpatients.19 This initiative included efforts to improve compliance with NCCN guidelines on all medical services and follow guidelines in accordance with NCCN, surgical best practices, and published standards on all surgical services. To accomplish this objective, RPCI:
-
Implemented mandatory, computerized physician order entry forms;
-
Promoted VTE awareness via staff education, field in‐services, and seminars; and
-
Tracked compliance with manual audits of patient charts every 3 months.
When the initiative began in the fourth quarter of 2006, the rate of NCCN‐recommended VTE prophylaxis was 61% with the medical services and 86% with the surgical services. As of the second quarter of 2008, guideline compliance had increased to 90% and 100% with the medical and surgical services, respectively. Accompanying this increase in compliance was a corresponding decrease in the incidence of VTE, from 0.39% in the fourth quarter of 2006 to 0.08% in the second quarter of 2008 (P < 0.0001). The most pronounced reductions in VTE incidence were observed within the medical services and among outpatients.19
Hartford Hospital, Hartford, CT
Hartford Hospital is an 819‐bed acute‐care community hospital with 300 designated medical beds. In an effort to improve thromboprophylaxis rates among medical patients, the pharmacy, medicine, and information technology departments collaborated to develop an alert within the computerized prescriber‐order‐entry system that reminded clinicians to assess patients for VTE risk factors and the need for prophylaxis.20 When a patient met predefined criteria for VTE risk, the message was displayed until either mechanical or pharmacologic VTE prophylaxis was an active order on the patient's treatment profile (Figure 1). The program was implemented in conjunction with an extensive educational program targeting hospital staff, pharmacists, physicians, nurse practitioners, physician assistants, and nurses.20

Compliance with institutional prophylaxis guidelines increased from 49% to 93% following implementation (P < 0.001). Interestingly, the initiative at Hartford Hospital was able to increase the use of mechanical prophylaxis among patients with a contradiction to pharmacologic therapy from 25% prior to the program to 100% after its implementation (P < 0.001).20
Saint Elizabeth's Hospital, Collinsville, IL
In 2008, Bauer et al.21 reported the benefits of a pharmacist‐led program for VTE prevention in Saint Elizabeth's Hospital, a 278‐bed hospital with more than 13,000 admissions per year. As part of the initiative, hospital pharmacists:
-
Received daily reports of all new admissions cross‐referenced with an accounting of patients currently prescribed UFH or LMWH;
-
Assessed the remaining patients at risk of VTE; and
-
Placed recommendations in patient charts in the form of a bold sticker alerting the physician to the patient's risk factors, their overall risk of VTE, and treatment recommendations (Figure 2).

The program ran 7 days per week, involved 1 pharmacist per day, and required an average of 4 hours per day. Patients in the maternity, nursery, pediatric, and psychiatric units were excluded from the program.
The program led to a significant increase in the use of VTE prophylaxis and a significant reduction in the rate of DVT (P < 0.002).21 These findings suggest that innovative programs tailored to the needs of individual institutions can dramatically increase thromboprophylaxis rates and decrease the incidence of VTE in at‐risk hospitalized patients.
Conclusions
VTE is a serious disease that leads to excess morbidity and mortality among hospitalized patients. The impact of hospital reporting on reimbursement and patient outcomes necessitates the adoption of strategies and protocols proven to enhance the management of VTE and improve patient outcomes. Several successful VTE initiatives have been described in the literature and can serve as models for institutions wishing to develop policies and procedures for preventing VTE. In addition, a number of online resources exist that can aid in the development of VTE protocols.
- U.S. Department of Health and Human Services. The surgeon general's call to action to prevent deep vein thrombosis and pulmonary embolism. Available at: http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call‐to‐action‐on‐dvt‐2008.pdf. Accessed June2009.
- The Joint Commission. National consensus standards for prevention and care of venous thromboembolism (VTE). Last updated April 2009. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition).Chest2008;133(6 suppl):381S–453S.
- , , , , , ;American College of Chest Physicians.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(6 suppl):454S–545S.
- The Joint Commission. Surgical Care Improvement Project Core Measure Set. Updated November 2008. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/SCIP+Core+Measure+Set.htm. Accessed June2009.
- , , , et al.Are surgical patients at risk of venous thromboembolism current meeting the surgical care improvement performance target goal for appropriate and timely prophylaxis?Chest.2008;134:s46003.
- American Medical Association. Perioperative Care: Physician Performance Measurement Set. October 2006. Available at http://www.ama‐assn.org/ama1/pub/upload/mm/370/perioperativews1206.pdf. Accessed June2009.
- Leapfrog Group. The Leapfrog Group Hospital Quality and Safety Survey: What's New in the 2009 Survey (Version 5.1). Available at: https://leapfrog.medstat.com/pdf/final.pdf. Accessed June2009.
- , , , et al.Increasing awareness about venous disease: The American Venous Forum expands the National Venous Screening Program.J Vasc Surg.2008;48(2):394–399.
- Federal Register. Medicare Program; Proposed Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2007 Rates. Available at: http://edocket.access.gpo.gov/2006/pdf/06‐3629.pdf. Accessed December 10,2008.
- Department of Health and Human Services, Centers for Medicare 241(3):397–415.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- , , , et al.Electronic alerts for hospitalized high‐VTE risk patients not receiving prophylaxis: a cohort study.J Thromb Thrombolysis.2008;25(2):146–150.
- , , , et al. Improving compliance with guidelines for venous thromboembolism (VTE) prophylaxis significantly reduces VTE events. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1288.
- .Development and implementation of a program to assess medical patients' need for venous thromboembolism prophylaxis.Am J Health Syst Pharm.2008;65(18):1755–1760.
- , , .Pharmacist‐led program to improve venous thromboembolism prophylaxis in a community hospital.Am J Health Syst Pharm.2008;65(17):1643–1647.
- U.S. Department of Health and Human Services. The surgeon general's call to action to prevent deep vein thrombosis and pulmonary embolism. Available at: http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call‐to‐action‐on‐dvt‐2008.pdf. Accessed June2009.
- The Joint Commission. National consensus standards for prevention and care of venous thromboembolism (VTE). Last updated April 2009. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition).Chest2008;133(6 suppl):381S–453S.
- , , , , , ;American College of Chest Physicians.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(6 suppl):454S–545S.
- The Joint Commission. Surgical Care Improvement Project Core Measure Set. Updated November 2008. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/SCIP+Core+Measure+Set.htm. Accessed June2009.
- , , , et al.Are surgical patients at risk of venous thromboembolism current meeting the surgical care improvement performance target goal for appropriate and timely prophylaxis?Chest.2008;134:s46003.
- American Medical Association. Perioperative Care: Physician Performance Measurement Set. October 2006. Available at http://www.ama‐assn.org/ama1/pub/upload/mm/370/perioperativews1206.pdf. Accessed June2009.
- Leapfrog Group. The Leapfrog Group Hospital Quality and Safety Survey: What's New in the 2009 Survey (Version 5.1). Available at: https://leapfrog.medstat.com/pdf/final.pdf. Accessed June2009.
- , , , et al.Increasing awareness about venous disease: The American Venous Forum expands the National Venous Screening Program.J Vasc Surg.2008;48(2):394–399.
- Federal Register. Medicare Program; Proposed Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2007 Rates. Available at: http://edocket.access.gpo.gov/2006/pdf/06‐3629.pdf. Accessed December 10,2008.
- Department of Health and Human Services, Centers for Medicare 241(3):397–415.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- , , , et al.Electronic alerts for hospitalized high‐VTE risk patients not receiving prophylaxis: a cohort study.J Thromb Thrombolysis.2008;25(2):146–150.
- , , , et al. Improving compliance with guidelines for venous thromboembolism (VTE) prophylaxis significantly reduces VTE events. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1288.
- .Development and implementation of a program to assess medical patients' need for venous thromboembolism prophylaxis.Am J Health Syst Pharm.2008;65(18):1755–1760.
- , , .Pharmacist‐led program to improve venous thromboembolism prophylaxis in a community hospital.Am J Health Syst Pharm.2008;65(17):1643–1647.
VTE Risk Factors and Barriers
Venous thromboembolism (VTE) is a common and potentially devastating complication of medical illness and surgical intervention. Among patients discharged from acute‐care hospitals in 2003, more than 12 million (31%) had a moderate or high risk of VTE during hospitalization, including 11% at risk due to surgical procedures and 20% at risk due to medical illnesses.1 The incidence of VTEwhich can present as deep vein thrombosis (DVT) or pulmonary embolism (PE)is rising in hospitalized patients.2 Despite the availability of effective prophylaxis, VTE is the third most common cause of hospital‐related death and the most common preventable cause of hospital mortality.3
The clinical impact of VTE is significant. While total incidence, prevalence, and mortality rates of VTE are elusive, the annual incidence of DVT is thought to be as high as 2 million.4 The most serious complication of DVT is acute PE, which occurs in approximately 600,000 patients per year, one‐third of whom die.5 DVT may also be complicated by recurrent episodes of VTE and postthrombotic sequelae such as chronic venous stasis, venous ulceration, debilitating pain, and intractable edema.6 One prospective cohort study found that 30% of patients who had experienced a first episode of DVT developed recurrent VTE within 8 years, and the incidence of postthrombotic syndrome (PTS) was 23% after just 2 years.6
The economic burden of VTE is substantial, due both to the initial event and to the high rate of hospital readmission. The estimated average cost of DVT management (including initial acute care and 6 months of follow‐up care) is $10,072 per patient, and the corresponding cost for PE is $14,649.7 Hospital readmission occurs in 5% to 14% of patients, more than one‐half of whom are readmitted within 90 days.8 For patients with recurrent DVT or PE, the mean total hospitalization costs of readmission are $11,862 and $14,722, respectively.8
The clinical and economic burden of VTE can be significantly mitigated by the use of effective prophylaxis. Because VTE is difficult to diagnose antemortem, it is easier and safer to prevent with appropriate prophylaxis than to diagnose after it has occurred. Unfortunately, VTE prophylaxis is markedly underused, particularly among high‐risk, hospitalized medical patients who would most benefit from it.9
This article summarizes specific risk factors for VTE and provides guidance in identifying patients who may require thromboprophylaxis. Barriers to optimal VTE prophylaxis in the hospital setting will also be explored.
Methodology
For this article and the ones that follow, relevant literature was identified through a Medline search (January 1980 to December 2008) using the following search terms: venous thromboembolism, pulmonary embolism, deep vein thrombosis, epidemiology, risk factors, prophylaxis, mechanical prophylaxis, diagnosis, treatment, anticoagulants, monitoring, secondary prevention, guideline, adherence, treatment protocol, performance measure, and quality improvement. The bibliographies of all key texts were searched for additional relevant articles. The websites of the American College of Chest Physicians (ACCP), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and Society of Hospital Medicine (SHM) were also searched for annual meeting abstracts, position statements, and other key publications.
Evidence‐based clinical guidelines were identified through a search of the National Guideline Clearinghouse (
Pathogenesis of VTE
Venous thrombosis occurs as a result of at least 1 of 3 underlying factors: alterations in blood flow, vascular endothelial injury, and alterations in the constitution of the blood.10 Each potential underlying factor encompasses a wide range of risk factors and clinical scenarios. Alterations in venous blood flow can include several situations, including venous stasis, venous hypertension, and valvular incompetence. Endothelial injury can arise from shear stress, direct trauma, infection, hypertension, or other sources of endothelial damage. Hypercoagulability from alterations in the constitution of the blood may be due to antithrombin deficiency, cancer, surgery, pregnancy, or other risk factors. The presence of any of these factors indicates an elevated risk of VTE, and the presence of multiple factors further increases risk.10
Risk Factors for VTE
VTE can occur in a wide variety of clinical circumstances. Recognized risk factors for VTE include hospitalization for an acute medical illness, cardiovascular disease, pulmonary disease, major surgery, multiple trauma, obesity, and increasing age.10 Additional factors that place patients at increased risk of VTE (independent of age) include a history of prior VTE, known hypercoagulable states, active cancer, and acute infection.11 Hospital‐acquired risk factors such as immobility, acute illness, or medical interventions may lead to the development of VTE in these patients. Severity of illness must be factored into the risk assessment, and all patients need to be assessed for VTE risk at the time of hospital admission and daily thereafter if pharmacologic therapy is not initiated.
In a review of 1231 consecutive patients treated for acute DVT and/or PE, 96.3% had at least 1 risk factor for VTE, and more than one‐third (39%) had 3 or more risk factors (Table 1).10 The incidence of VTE in hospitalized patients is directly related to the number of risk factors present:10
-
1 risk factor: 11%
-
2 risk factors: 24%
-
3 risk factors: 36%
-
4 risk factors: 50%
-
5 risk factors: > 90%
| Risk Factor | Patients (%) |
|---|---|
| |
| Age 40 years | 88.5 |
| Obesity | 37.8 |
| History of VTE | 26.0 |
| Cancer | 22.3 |
| Bed rest 5 days | 12.0 |
| Major surgery | 11.2 |
| Congestive heart failure | 8.2 |
| Varicose veins | 5.8 |
| Fracture (hip or leg) | 3.7 |
| Estrogen treatment | 2.0 |
| Stroke | 1.8 |
| Multiple trauma | 1.1 |
| Childbirth | 1.1 |
| Myocardial infarction | 0.7 |
Current or Recent Prior Hospitalization
The risk of VTE is elevated among hospitalized patients. The prevalence of DVT varies across hospital specialties, reaching up to 80% in major trauma, spinal cord injury, and critical care.3 The Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study evaluated the prevalence of VTE risk factors in the acute hospital care setting.12 Among the 68,183 patients enrolled, more than one‐half (52%) were judged to be at risk of VTE. In a case‐control study examining 625 patients with a first lifetime VTE, confinement to a hospital (among other risk factors) was found to be an independent and important predictor of VTE (odds ratio [OR], 8.0; 95% confidence interval [CI], 4.514.2) (Figure 1).13 Recent hospitalization is also an important risk factor for VTE, and patients who are readmitted to the hospital should be considered moderate or high risk.13
Age
Patient age must be considered when assessing VTE risk. VTE is predominantly a disease of older age, and age older than 75 years is an important risk factor for the condition.11 In general, patients older than 40 years have a significantly increased risk compared with younger patients, and the risk approximately doubles with each additional decade.10 Given the aging population, the prevalence of VTE and its complications are expected to increase.
Women of childbearing age experience VTE more frequently than men of the same age, due to pregnancy and exposure to contraceptive therapy.14 This risk, however, is modest compared with the risk among older patients. After age 45 years, the incidence of VTE increases markedly for both sexes, becoming more prominent in men.14 Compared with women, men also have an increased risk of recurrent VTE.15
Despite the effect of age on VTE risk, the risk among patients younger than 40 years may be underestimated because this subgroup has not been extensively studied. For reasons that are not well‐understood, the risk of VTE associated with heart failure is higher in patients younger than 40 years, and the relative risk of PE in patients with chronic obstructive pulmonary disease (COPD) is also higher in younger patients.16, 17
Cancer and Its Treatment
Cancer patients, on average, have twice the risk of VTE compared with noncancer patients.18 This risk, however, varies considerably by cancer type. According to an assessment of nearly 41 million hospitalized patients in the National Hospital Discharge Survey (NHDS), the relative risk of VTE varied from 1.02 in patients with bladder cancer to 4.34 in patients with cancer of the pancreas.18
VTE is one of the most common complications of cancer and cancer therapy, and it is the second leading cause of death among hospitalized cancer patients.19 Molecular mechanisms underlying thromboembolic events in cancer patients include tumor cell procoagulants, inflammatory cell cytokines, mediators of platelet adhesion, and tumor‐related stasis and endothelial damage.20 The clinical implications of these processes are severe. Cancer exacerbates the natural course of VTE, increasing the risk of recurrent VTE and major bleeding, and VTE worsens the prognosis of cancer, increasing the risk of death among cancer patients.
Various cancer therapiesincluding surgery, chemotherapy, hematopoietic stem cell transplantation, and even growth factor supportalso increase the risk of VTE, in part because extrinsic factors such as surgery or chemotherapy can intensify the hypercoagulable process.18, 2123 In the NHDS, cancer patients undergoing surgery had at least twice the risk of postoperative DVT and more than 3 times the risk of PE compared with noncancer patients undergoing similar procedures.18
Cancer is an independent predictor of thromboprophylaxis failure following surgery. The @RISTOS Project found that VTE was the most common cause of death among 2373 patients undergoing general, urologic, or gynecologic surgery for cancer.24 A multivariate analysis identified 5 independent risk factors for VTE after cancer surgery:
-
Previous VTE (OR, 5.98; 95% CI, 2.1316.80)
-
Anesthesia 2 hours (OR, 4.50; 95% CI, 1.0619.04)
-
Bed rest 4 days (OR, 4.37; 95% CI, 2.457.78)
-
Age 60 years (OR, 2.63; 95% CI, 1.215.71)
-
Advanced‐stage cancer (OR, 2.68; 95% CI, 1.375.24)
Cardiovascular Disease
The risk of VTE is pronounced among patients with cardiovascular disease. After stroke and coronary disease, VTE is the third most common cardiovascular disorder, and PE causes more deaths each year than myocardial infarction (MI).25 Several cardiovascular diseases, including hypertension, stroke, acute MI, and heart failure, are independently associated with VTE.10, 2627 Related disorders, including diabetes and the metabolic syndrome, also increase the risk of VTE.26, 28
Congestive heart failure (CHF) is a risk factor for VTE, and the severity of illness increases risk. In the DVT‐Free Prospective Registry, 13% of patients with ultrasound‐confirmed DVT had CHF.29 In a subgroup analysis of patients of the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, the incidence of VTE exceeded 20% in patients with New York Heart Association (NYHA) class IV heart failure, compared with 12% in patients with NYHA class III heart failure.30 Another study found that VTE risk increases as left ventricular ejection fraction (LVEF) decreases, with an LVEF of less than 20% associated with a VTE OR of 38.3 (95% CI, 9.6152.5).31
Infectious Disease
Acute infection may increase the relative risk of VTE by as much as 50% and is associated with VTE event rates of up to 26%.11 Acute infections may be associated with acute inflammation, adverse effects on cardiac or pulmonary function, and prolonged immobilization.30, 32, 33 Human immunodeficiency virus (HIV) patients may also have an increased risk of VTE due to a circulating lupus anticoagulant and/or the presence of acute infection.34
Obesity
The 2008 ACCP guideline update recognizes obesity, for the first time, as a risk factor for VTE.3 Obesity was 1 of the 5 most frequent comorbidities found in patients with DVT in the DVT‐Free Prospective Registry.29 It increases the risk of both incident and recurrent VTE, with every 1‐point increase in body mass index (BMI) increasing the risk of recurrent VTE by 4.4% (95% CI, 1.37.6%; P < 0.001).35
Pregnancy and Puerperium
Pregnancy, particularly the postpartum period, is associated with an increased risk of VTE in women, even though the absolute risk is small.36 Still, PE is one of the leading causes of maternal death following childbirth.10 Smoking, prior VTE, and inherited thrombophilias all increase the risk of VTE in pregnant women.10 The risk begins to rise in the first trimester, and when prophylaxis is needed, it should be started early in gestation.37
Pulmonary Disease
COPD is another risk factor for the development of VTE. COPD patients who develop VTE tend to be older, hospitalized in the intensive care unit (ICU), and on mechanical ventilation.38 In the DVT‐Free Prospective Registry, 12% of patients with ultrasound‐confirmed DVT had COPD.29
Trauma and Surgery
Injury to the body tissue, via trauma or surgery, stimulates the body's clotting mechanism and increases the risk of thromboembolic complications. During the perioperative period, the circulatory system must balance a variety of assaults: an immune response to surgical stress, prolonged immobilization during surgery and recovery, vasodilation associated with general or regional anesthesia, and hypercoagulability due to venous stasis and vascular injury.39 Renal transplant recipients have an increased risk of VTE due to a chronic hypercoagulable state.40 In surgery patients, perioperative complications such as dehydration and acute infection increase the risk of VTE beyond the risk associated with the surgical procedure itself.10
VTE risk is increased approximately 13‐fold by recent major trauma or lower‐extremity injury.13 In the absence of prophylaxis, the overall risk of VTE among patients undergoing major surgery is increased nearly 22‐fold.13 After controlling for the type of surgery, additional independent risk factors for VTE within 3 months of major surgery include:41, 42
-
Obesity
-
Central venous catheter placement
-
Malignancy
-
Smoking
-
Heart failure
-
Previous DVT
-
Prolonged immobility
-
Infection
Many surgical and medical inpatients share common risk factors, and without prophylaxis, the incidence of hospital‐acquired DVT ranges from 10% to 40% for both groups.3
Inherited or Acquired Risk Factors
VTE is a multifactorial disease, and recent evidence indicates that some heritable traits may be potent risk factors for VTE.43 Approximately 35% of patients with DVT will have at least 1 of 5 traits related to an inherited blood clotting disorder:43
-
Deficiencies in the anticoagulation factors protein C, protein S, or antithrombin, or
-
Mutations in the factor V and prothrombin genes, resulting in Factor V Leiden and prothrombin G20210A, respectively.
Certain inherited traits and genetic polymorphisms increase the risk of VTE by interacting with clinical risk factors such as contraceptive use, pregnancy, surgery, trauma, and cancer. One recent study found that oral estrogen therapy among women with the CYP3A5*1 allele was associated with a particularly high risk of VTE.44 Although widespread screening for inherited risk factors is not currently practical, future tools may incorporate genetic polymorphisms to more precisely identify patients who would benefit from aggressive prophylaxis.
Lifestyle Factors
Lifestyle factors have a significant effect on VTE risk. Smoking increases the risk of VTE by 20% to 30%, and a sedentary lifestyle also increases the risk of VTE.26, 45 In fact, women who exercise regularly and consume alcohol in moderation have one‐half the risk of VTE as women who have a sedentary lifestyle and drink little or no alcohol.42 For both men and women, a diet high in fruits, vegetables, and fish is associated with a lower lifetime risk of VTE.46
Medications
Medications may also increase the risk of VTE. In cancer patients with anemia, for example, the use of erythropoiesis‐stimulating agents such as recombinant erythropoietin and darbepoetin was recently shown to increase the risk of VTE by 57% (95% CI, 3187%) and increase mortality risk by 10% (95% CI, 120%).23 In addition, combination hormone replacement therapy in women is associated with a higher risk of VTE compared with estrogen monotherapy, and transdermal contraceptive systems more than double the risk of VTE compared with oral contraceptives (95% CI, 1.33.8).47, 48 Recent studies have also reported an increased risk of VTE with some psychiatric drugs, including amitriptyline, clozapine, olanzapine, and risperidone.4952
Thromboprophylaxis in the Hospital Setting
Despite the prevalence of risk factors and compelling evidence regarding the value of prophylaxis, VTE prophylaxis is suboptimal in hospitalized medical and surgical patients. In a study of 123,304 hospitalized patients who were determined to be at risk of VTE, only 13.3% received prophylaxis in accordance with ACCP guidelines.53 Compliance ranged from a high of 52.4% among patients undergoing orthopedic surgery to a low of 2.8% among patients undergoing neurosurgery.53 Results from several other large trials echo these findings (Table 2).12, 5456
| Trial | Patient Type | Total Patients | Patients at Risk of VTE (Based on ACCP Criteria) (%) | At‐Risk Patients Receiving Recommended Prophylaxis | |
|---|---|---|---|---|---|
| Medical Patients (%) | Surgical Patients (%) | ||||
| |||||
| IMPROVE | Medical patients | 15,156 | 52 | 61 | n/a |
| ENDORSE | Medical and surgical patients | 68,183 | 51.8 | 39.5 | 58.5 |
| ADHERE | Hospitalized heart failure patients | 155,073 | 46 | 30.6 | n/a |
| Amin et al.56 (2008) | Medical and surgical patients | 258,556 | 26.4 | 9.8 | 17.9 |
Reasons for Inadequate Prophylaxis
Researchers have identified a range of barriers to adequate VTE prophylaxis (Table 3).57 Some of these barriers are outlined below.
|
| Variability in clinician knowledge of risk assessment and appropriate prophylaxis |
| Lack of agreement with, and inconsistency between, guidelines in certain patient populations |
| Perceived lack of need |
| Concerns about adverse effects |
| Lack of hospital support systems and policies |
| Lack of established responsibilities for prophylaxis |
Underestimation of Risk of Clotting
VTE is often clinically silent, leading some physicians to mistakenly believe that it is rare.58 In hospitalized surgical patients, for example, the incidence of thromboembolic complications during a short postoperative stay may be low. Given that many cases of symptomatic VTE occur after hospital discharge, hospitalists and surgeons may be unaware of the true incidence of DVT.59
Overestimation of the Risk of Bleeding
Physicians may also overestimate the risk of possible side effects of prophylaxis, such as major bleeding or heparin‐induced thrombocytopenia (HIT).58 Fear of excess bleeding has been cited by physicians as a leading reason for their decision to withhold thromboprophylaxis from at‐risk hospitalized patients.60 Physicians are particularly fearful of complications among elderly patients, who are less likely to receive adequate prophylaxis than younger patients with a similar risk of VTE.61 When bleeding does occur, it rarely results in death. On the other hand, PE may account for as many as 10% of hospital deaths.9
Guideline Confusion and Complexity
Discrepancies between guidelines published by different medical societies contribute to confusion in choosing a management approach. The American Academy of Orthopedic Surgeons (AAOS), for example, describes aspirin alone as a reasonable choice for VTE prophylaxis in some patients, but the ACCP guidelines advise against the use of aspirin monotherapy.58 The cumbersome nature of multiple risk‐assessment and treatment algorithms can also be problematic.61 Furthermore, certain patient subgroups, such as those with cirrhosis, severe renal failure, and epidural catheters, have been excluded from randomized controlled trials, and the management of such patients is not straightforward.
Absence of Institutional Protocols and Information Technology Support
The lack of institution‐level guidance and support can have a detrimental effect on patient care. In a 2007 survey of 127 community hospitals, the prevalence of institutional protocols related to VTE was low: only 60% had protocols to encourage prophylaxis in at‐risk patients, 54% had guidelines to assist in appropriate drug selection, and 43% had guidelines for the dosing of prophylaxis regimens.62 A lack of systems for data collection and audit has also been identified as a barrier to the implementation of prophylaxis guidelines.57 Thus, hospitals need to adopt protocols such as:3
-
Written, institution‐wide thromboprophylaxis policies
-
Preprinted order forms and computer decision‐support systems
-
Policies specifying responsibilities for assessing VTE risk and prescribing prophylaxis
Conclusions
VTE is the most common preventable cause of hospital death, and prophylaxis is underused in hospitalized patients. Although VTE risk factors are numerous and complex, deciding whether to use prophylaxis need not be complicated. In general, elderly patients, medically‐ill patients, and patients undergoing surgery will benefit from prophylaxis, as well as those who are hospitalized for more than 1 night. Hospitalized patients with at least 1 risk factor should be considered for pharmacologic prophylaxis. In general, the risk of hospital‐acquired VTE greatly exceeds the risk of bleeding with prophylactic doses of anticoagulation. A patient's risk of VTE may change, and regular assessment of this risk should be mandated if pharmacologic therapy is not initiated at the time of admission.
Numerous barriers to the optimal use of VTE prophylaxis exist, and hospitals must implement systems changes and multidisciplinary approaches to overcome these barriers. The fourth article in this supplement provides detailed strategies for meeting VTE performance measures and overcoming barriers to the optimal use of prophylaxis.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82(9):777–782.
- , , .Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients.Am J Cardiol.2005;95(12):1525–1526.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th ed.Chest.2008;133(6 suppl):381S–453S.
- , .Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association.Circulation.1996;93(12)2212–2245.
- , , , et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- , , , et al.The long‐term clinical course of acute deep venous thrombosis.Ann Intern Med.1996;125(1):1–7.
- , .Direct medical cost of managing deep vein thrombosis according to the occurrence of complications.Pharmacoeconomics.2002;20(9):603–615.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , .Risk factors for venous thromboembolism.Circulation.2003;107(23 suppl 1):I9–I16.
- , , , et al.Risk factors for VTE in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- , , , et al.;ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , et al.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160(6):809–815.
- , .Range of published estimates of venous thromboembolism incidence in young women.Contraception.2007;75(5):328–336.
- , , , , , .The risk of recurrent venous thromboembolism in men and women.N Engl J Med.2004;350(25):2558–2563.
- , , , et al.Risk of venous thromboembolism in patients hospitalized with heart failure.Am J Cardiol.2006;98(6):793–795.
- , , , et al.Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease.J Cardiovasc Med.2007;8(4):253–257.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.Causes of death in cancer patients.J Med.1975;6(1):61–64.
- , .Tissue factor and angiogenesis in cancer.Curr Opin Hematol.2002;9(5):401–406.
- , , , et al.Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel‐containing first‐line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group.J Clin Oncol.2008;26(16):2683–2689.
- , , , , , .The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention.Blood.2008;112(3):504–510.
- , , , et al.Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia.JAMA.2008;299(8):914–924.
- , , , et al.A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project.Ann Surg.2006;243(1):89–95.
- .Pulmonary embolism in thrombolysis: a clarion call for international collaboration.J Am Coll Cardiol.1992;19:246–247.
- , , , et al.Cardiovascular risk factors and venous thromboembolism: a meta‐analysis.Circulation.2008;117(1):93–102.
- , , , , , .Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study.J Thromb Haemost.2008;6(11):1851–1857.
- , , , et al.Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome—the Tromsø study.J Thromb Haemost.2009;7(5):739–745.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , , .Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case‐control study.J Clin Epidemiol.2001;54(8):810–816.
- , , , et al;ARTEMIS Investigators.Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- , , , et al.HIV infection is a risk factor for venous thromboembolism.AIDS Patient Care STDS.2002;16(5):205–209.
- , , , et al.Overweight, obesity, and the risk of recurrent venous thromboembolism.Arch Intern Med.2008;168(15):1678–1683.
- .Venous thromboembolism in women.Vasc Med.2008;13(3):255–266.
- , , .Thrombosis during pregnancy and the postpartum period.Am J Obstet Gynecol.2005;193(1):216–219.
- , , , et al.Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination.J Thromb Thrombolysis.2008;26(1):35–40.
- , , .Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability?J Trauma.2003;54(2):224–231.
- , .Acquired hypercoagulable state in renal transplant recipients.Thromb Haemost.2004;91(4):646–654.
- , , , .Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion.Thromb Haemost.2007;98(6):1220–1225.
- , , , , , .A 10‐year analysis of venous thromboembolism on the surgical service: the effect of practice guidelines for prophylaxis.Surgery.2008;144(1):3–11.
- , , , et al;EDITH Collaborative Study Group.Family history as a risk factor for venous thromboembolism.Thromb Res.2008;122(5):624–629.
- , , , et al;Estrogen and Thromboembolism Risk (ESTHER) Study Group.Synergism between oral estrogen therapy and cytochrome P450 3A5*1 allele on the risk of venous thromboembolism among postmenopausal women.J Clin Endocrinol Metab.2008;93(8):3082–3087.
- , , .The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study.Br J Haematol.2009;144(2):234–240.
- , , , , .Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology.Circulation.2007;115(2):188–195.
- , , .Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta‐analysis.Eur Heart J.2008;29(16):2031–2041.
- , , , .Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users.Obstet Gynecol.2007;109(2 Pt 1):339–346.
- , , , , , .Pulmonary thromboembolism associated with olanzapine and risperidone.J Emerg Med.2008;35(2):159–161.
- , , , .Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions.Drug Saf.2008;31(8):685–694.
- .Evaluating the association between clozapine and venous thromboembolism.Am J Health Syst Pharm.2008;65(19):1825–1829.
- , .Antidepressant drug use and risk of venous thromboembolism.Pharmacotherapy.2008;28(2):144–150.
- , , , .Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64(1):69–76.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients.Chest.2007;132:936–945.
- , , , et al.Adhere Scientific Advisory Committee and Investigators. Venous thromboembolism prophylaxis in hospitalized heart failure patients.J Card Fail.2008;14(2):127–132.
- , , , et al. Are hospitals following guidelines for VTE Prevention? The Venous Thromboembolism Study to Assess the Rate of Thromboprophylaxis. Presented at the 50th Annual Meeting of the American College of Hematology, San Francisco, CA, December 6–9, 2008. Abstract 1286.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- .A need for a simplified approach to venous thromboembolism prophylaxis in acute medical inpatients.Int J Clin Pract.2007;61(2):336–340.
- .Barriers to the optimal use of anticoagulants after orthopaedic surgery.Arch Orthop Trauma Surg.2008 (Published online Oct, 8.). [http://dx.doi.org/DOI 10.1007/s00402‐008‐0765‐9]
- , , , , .Venous thrombosis in cancer patients: Insights from the FRONTLINE survey.Oncologist.2003;8:381–388.
- , , , et al.Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross‐sectional study in Brazil.J Thromb Haemost.2006;4(6):1266–1270.
- , , , , .Survey of hospitals for guidelines, policies, and protocols for anticoagulants.Am J Health Syst Pharm.2007;64(11):1203–1208.
Venous thromboembolism (VTE) is a common and potentially devastating complication of medical illness and surgical intervention. Among patients discharged from acute‐care hospitals in 2003, more than 12 million (31%) had a moderate or high risk of VTE during hospitalization, including 11% at risk due to surgical procedures and 20% at risk due to medical illnesses.1 The incidence of VTEwhich can present as deep vein thrombosis (DVT) or pulmonary embolism (PE)is rising in hospitalized patients.2 Despite the availability of effective prophylaxis, VTE is the third most common cause of hospital‐related death and the most common preventable cause of hospital mortality.3
The clinical impact of VTE is significant. While total incidence, prevalence, and mortality rates of VTE are elusive, the annual incidence of DVT is thought to be as high as 2 million.4 The most serious complication of DVT is acute PE, which occurs in approximately 600,000 patients per year, one‐third of whom die.5 DVT may also be complicated by recurrent episodes of VTE and postthrombotic sequelae such as chronic venous stasis, venous ulceration, debilitating pain, and intractable edema.6 One prospective cohort study found that 30% of patients who had experienced a first episode of DVT developed recurrent VTE within 8 years, and the incidence of postthrombotic syndrome (PTS) was 23% after just 2 years.6
The economic burden of VTE is substantial, due both to the initial event and to the high rate of hospital readmission. The estimated average cost of DVT management (including initial acute care and 6 months of follow‐up care) is $10,072 per patient, and the corresponding cost for PE is $14,649.7 Hospital readmission occurs in 5% to 14% of patients, more than one‐half of whom are readmitted within 90 days.8 For patients with recurrent DVT or PE, the mean total hospitalization costs of readmission are $11,862 and $14,722, respectively.8
The clinical and economic burden of VTE can be significantly mitigated by the use of effective prophylaxis. Because VTE is difficult to diagnose antemortem, it is easier and safer to prevent with appropriate prophylaxis than to diagnose after it has occurred. Unfortunately, VTE prophylaxis is markedly underused, particularly among high‐risk, hospitalized medical patients who would most benefit from it.9
This article summarizes specific risk factors for VTE and provides guidance in identifying patients who may require thromboprophylaxis. Barriers to optimal VTE prophylaxis in the hospital setting will also be explored.
Methodology
For this article and the ones that follow, relevant literature was identified through a Medline search (January 1980 to December 2008) using the following search terms: venous thromboembolism, pulmonary embolism, deep vein thrombosis, epidemiology, risk factors, prophylaxis, mechanical prophylaxis, diagnosis, treatment, anticoagulants, monitoring, secondary prevention, guideline, adherence, treatment protocol, performance measure, and quality improvement. The bibliographies of all key texts were searched for additional relevant articles. The websites of the American College of Chest Physicians (ACCP), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and Society of Hospital Medicine (SHM) were also searched for annual meeting abstracts, position statements, and other key publications.
Evidence‐based clinical guidelines were identified through a search of the National Guideline Clearinghouse (
Pathogenesis of VTE
Venous thrombosis occurs as a result of at least 1 of 3 underlying factors: alterations in blood flow, vascular endothelial injury, and alterations in the constitution of the blood.10 Each potential underlying factor encompasses a wide range of risk factors and clinical scenarios. Alterations in venous blood flow can include several situations, including venous stasis, venous hypertension, and valvular incompetence. Endothelial injury can arise from shear stress, direct trauma, infection, hypertension, or other sources of endothelial damage. Hypercoagulability from alterations in the constitution of the blood may be due to antithrombin deficiency, cancer, surgery, pregnancy, or other risk factors. The presence of any of these factors indicates an elevated risk of VTE, and the presence of multiple factors further increases risk.10
Risk Factors for VTE
VTE can occur in a wide variety of clinical circumstances. Recognized risk factors for VTE include hospitalization for an acute medical illness, cardiovascular disease, pulmonary disease, major surgery, multiple trauma, obesity, and increasing age.10 Additional factors that place patients at increased risk of VTE (independent of age) include a history of prior VTE, known hypercoagulable states, active cancer, and acute infection.11 Hospital‐acquired risk factors such as immobility, acute illness, or medical interventions may lead to the development of VTE in these patients. Severity of illness must be factored into the risk assessment, and all patients need to be assessed for VTE risk at the time of hospital admission and daily thereafter if pharmacologic therapy is not initiated.
In a review of 1231 consecutive patients treated for acute DVT and/or PE, 96.3% had at least 1 risk factor for VTE, and more than one‐third (39%) had 3 or more risk factors (Table 1).10 The incidence of VTE in hospitalized patients is directly related to the number of risk factors present:10
-
1 risk factor: 11%
-
2 risk factors: 24%
-
3 risk factors: 36%
-
4 risk factors: 50%
-
5 risk factors: > 90%
| Risk Factor | Patients (%) |
|---|---|
| |
| Age 40 years | 88.5 |
| Obesity | 37.8 |
| History of VTE | 26.0 |
| Cancer | 22.3 |
| Bed rest 5 days | 12.0 |
| Major surgery | 11.2 |
| Congestive heart failure | 8.2 |
| Varicose veins | 5.8 |
| Fracture (hip or leg) | 3.7 |
| Estrogen treatment | 2.0 |
| Stroke | 1.8 |
| Multiple trauma | 1.1 |
| Childbirth | 1.1 |
| Myocardial infarction | 0.7 |
Current or Recent Prior Hospitalization
The risk of VTE is elevated among hospitalized patients. The prevalence of DVT varies across hospital specialties, reaching up to 80% in major trauma, spinal cord injury, and critical care.3 The Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study evaluated the prevalence of VTE risk factors in the acute hospital care setting.12 Among the 68,183 patients enrolled, more than one‐half (52%) were judged to be at risk of VTE. In a case‐control study examining 625 patients with a first lifetime VTE, confinement to a hospital (among other risk factors) was found to be an independent and important predictor of VTE (odds ratio [OR], 8.0; 95% confidence interval [CI], 4.514.2) (Figure 1).13 Recent hospitalization is also an important risk factor for VTE, and patients who are readmitted to the hospital should be considered moderate or high risk.13

Age
Patient age must be considered when assessing VTE risk. VTE is predominantly a disease of older age, and age older than 75 years is an important risk factor for the condition.11 In general, patients older than 40 years have a significantly increased risk compared with younger patients, and the risk approximately doubles with each additional decade.10 Given the aging population, the prevalence of VTE and its complications are expected to increase.
Women of childbearing age experience VTE more frequently than men of the same age, due to pregnancy and exposure to contraceptive therapy.14 This risk, however, is modest compared with the risk among older patients. After age 45 years, the incidence of VTE increases markedly for both sexes, becoming more prominent in men.14 Compared with women, men also have an increased risk of recurrent VTE.15
Despite the effect of age on VTE risk, the risk among patients younger than 40 years may be underestimated because this subgroup has not been extensively studied. For reasons that are not well‐understood, the risk of VTE associated with heart failure is higher in patients younger than 40 years, and the relative risk of PE in patients with chronic obstructive pulmonary disease (COPD) is also higher in younger patients.16, 17
Cancer and Its Treatment
Cancer patients, on average, have twice the risk of VTE compared with noncancer patients.18 This risk, however, varies considerably by cancer type. According to an assessment of nearly 41 million hospitalized patients in the National Hospital Discharge Survey (NHDS), the relative risk of VTE varied from 1.02 in patients with bladder cancer to 4.34 in patients with cancer of the pancreas.18
VTE is one of the most common complications of cancer and cancer therapy, and it is the second leading cause of death among hospitalized cancer patients.19 Molecular mechanisms underlying thromboembolic events in cancer patients include tumor cell procoagulants, inflammatory cell cytokines, mediators of platelet adhesion, and tumor‐related stasis and endothelial damage.20 The clinical implications of these processes are severe. Cancer exacerbates the natural course of VTE, increasing the risk of recurrent VTE and major bleeding, and VTE worsens the prognosis of cancer, increasing the risk of death among cancer patients.
Various cancer therapiesincluding surgery, chemotherapy, hematopoietic stem cell transplantation, and even growth factor supportalso increase the risk of VTE, in part because extrinsic factors such as surgery or chemotherapy can intensify the hypercoagulable process.18, 2123 In the NHDS, cancer patients undergoing surgery had at least twice the risk of postoperative DVT and more than 3 times the risk of PE compared with noncancer patients undergoing similar procedures.18
Cancer is an independent predictor of thromboprophylaxis failure following surgery. The @RISTOS Project found that VTE was the most common cause of death among 2373 patients undergoing general, urologic, or gynecologic surgery for cancer.24 A multivariate analysis identified 5 independent risk factors for VTE after cancer surgery:
-
Previous VTE (OR, 5.98; 95% CI, 2.1316.80)
-
Anesthesia 2 hours (OR, 4.50; 95% CI, 1.0619.04)
-
Bed rest 4 days (OR, 4.37; 95% CI, 2.457.78)
-
Age 60 years (OR, 2.63; 95% CI, 1.215.71)
-
Advanced‐stage cancer (OR, 2.68; 95% CI, 1.375.24)
Cardiovascular Disease
The risk of VTE is pronounced among patients with cardiovascular disease. After stroke and coronary disease, VTE is the third most common cardiovascular disorder, and PE causes more deaths each year than myocardial infarction (MI).25 Several cardiovascular diseases, including hypertension, stroke, acute MI, and heart failure, are independently associated with VTE.10, 2627 Related disorders, including diabetes and the metabolic syndrome, also increase the risk of VTE.26, 28
Congestive heart failure (CHF) is a risk factor for VTE, and the severity of illness increases risk. In the DVT‐Free Prospective Registry, 13% of patients with ultrasound‐confirmed DVT had CHF.29 In a subgroup analysis of patients of the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, the incidence of VTE exceeded 20% in patients with New York Heart Association (NYHA) class IV heart failure, compared with 12% in patients with NYHA class III heart failure.30 Another study found that VTE risk increases as left ventricular ejection fraction (LVEF) decreases, with an LVEF of less than 20% associated with a VTE OR of 38.3 (95% CI, 9.6152.5).31
Infectious Disease
Acute infection may increase the relative risk of VTE by as much as 50% and is associated with VTE event rates of up to 26%.11 Acute infections may be associated with acute inflammation, adverse effects on cardiac or pulmonary function, and prolonged immobilization.30, 32, 33 Human immunodeficiency virus (HIV) patients may also have an increased risk of VTE due to a circulating lupus anticoagulant and/or the presence of acute infection.34
Obesity
The 2008 ACCP guideline update recognizes obesity, for the first time, as a risk factor for VTE.3 Obesity was 1 of the 5 most frequent comorbidities found in patients with DVT in the DVT‐Free Prospective Registry.29 It increases the risk of both incident and recurrent VTE, with every 1‐point increase in body mass index (BMI) increasing the risk of recurrent VTE by 4.4% (95% CI, 1.37.6%; P < 0.001).35
Pregnancy and Puerperium
Pregnancy, particularly the postpartum period, is associated with an increased risk of VTE in women, even though the absolute risk is small.36 Still, PE is one of the leading causes of maternal death following childbirth.10 Smoking, prior VTE, and inherited thrombophilias all increase the risk of VTE in pregnant women.10 The risk begins to rise in the first trimester, and when prophylaxis is needed, it should be started early in gestation.37
Pulmonary Disease
COPD is another risk factor for the development of VTE. COPD patients who develop VTE tend to be older, hospitalized in the intensive care unit (ICU), and on mechanical ventilation.38 In the DVT‐Free Prospective Registry, 12% of patients with ultrasound‐confirmed DVT had COPD.29
Trauma and Surgery
Injury to the body tissue, via trauma or surgery, stimulates the body's clotting mechanism and increases the risk of thromboembolic complications. During the perioperative period, the circulatory system must balance a variety of assaults: an immune response to surgical stress, prolonged immobilization during surgery and recovery, vasodilation associated with general or regional anesthesia, and hypercoagulability due to venous stasis and vascular injury.39 Renal transplant recipients have an increased risk of VTE due to a chronic hypercoagulable state.40 In surgery patients, perioperative complications such as dehydration and acute infection increase the risk of VTE beyond the risk associated with the surgical procedure itself.10
VTE risk is increased approximately 13‐fold by recent major trauma or lower‐extremity injury.13 In the absence of prophylaxis, the overall risk of VTE among patients undergoing major surgery is increased nearly 22‐fold.13 After controlling for the type of surgery, additional independent risk factors for VTE within 3 months of major surgery include:41, 42
-
Obesity
-
Central venous catheter placement
-
Malignancy
-
Smoking
-
Heart failure
-
Previous DVT
-
Prolonged immobility
-
Infection
Many surgical and medical inpatients share common risk factors, and without prophylaxis, the incidence of hospital‐acquired DVT ranges from 10% to 40% for both groups.3
Inherited or Acquired Risk Factors
VTE is a multifactorial disease, and recent evidence indicates that some heritable traits may be potent risk factors for VTE.43 Approximately 35% of patients with DVT will have at least 1 of 5 traits related to an inherited blood clotting disorder:43
-
Deficiencies in the anticoagulation factors protein C, protein S, or antithrombin, or
-
Mutations in the factor V and prothrombin genes, resulting in Factor V Leiden and prothrombin G20210A, respectively.
Certain inherited traits and genetic polymorphisms increase the risk of VTE by interacting with clinical risk factors such as contraceptive use, pregnancy, surgery, trauma, and cancer. One recent study found that oral estrogen therapy among women with the CYP3A5*1 allele was associated with a particularly high risk of VTE.44 Although widespread screening for inherited risk factors is not currently practical, future tools may incorporate genetic polymorphisms to more precisely identify patients who would benefit from aggressive prophylaxis.
Lifestyle Factors
Lifestyle factors have a significant effect on VTE risk. Smoking increases the risk of VTE by 20% to 30%, and a sedentary lifestyle also increases the risk of VTE.26, 45 In fact, women who exercise regularly and consume alcohol in moderation have one‐half the risk of VTE as women who have a sedentary lifestyle and drink little or no alcohol.42 For both men and women, a diet high in fruits, vegetables, and fish is associated with a lower lifetime risk of VTE.46
Medications
Medications may also increase the risk of VTE. In cancer patients with anemia, for example, the use of erythropoiesis‐stimulating agents such as recombinant erythropoietin and darbepoetin was recently shown to increase the risk of VTE by 57% (95% CI, 3187%) and increase mortality risk by 10% (95% CI, 120%).23 In addition, combination hormone replacement therapy in women is associated with a higher risk of VTE compared with estrogen monotherapy, and transdermal contraceptive systems more than double the risk of VTE compared with oral contraceptives (95% CI, 1.33.8).47, 48 Recent studies have also reported an increased risk of VTE with some psychiatric drugs, including amitriptyline, clozapine, olanzapine, and risperidone.4952
Thromboprophylaxis in the Hospital Setting
Despite the prevalence of risk factors and compelling evidence regarding the value of prophylaxis, VTE prophylaxis is suboptimal in hospitalized medical and surgical patients. In a study of 123,304 hospitalized patients who were determined to be at risk of VTE, only 13.3% received prophylaxis in accordance with ACCP guidelines.53 Compliance ranged from a high of 52.4% among patients undergoing orthopedic surgery to a low of 2.8% among patients undergoing neurosurgery.53 Results from several other large trials echo these findings (Table 2).12, 5456
| Trial | Patient Type | Total Patients | Patients at Risk of VTE (Based on ACCP Criteria) (%) | At‐Risk Patients Receiving Recommended Prophylaxis | |
|---|---|---|---|---|---|
| Medical Patients (%) | Surgical Patients (%) | ||||
| |||||
| IMPROVE | Medical patients | 15,156 | 52 | 61 | n/a |
| ENDORSE | Medical and surgical patients | 68,183 | 51.8 | 39.5 | 58.5 |
| ADHERE | Hospitalized heart failure patients | 155,073 | 46 | 30.6 | n/a |
| Amin et al.56 (2008) | Medical and surgical patients | 258,556 | 26.4 | 9.8 | 17.9 |
Reasons for Inadequate Prophylaxis
Researchers have identified a range of barriers to adequate VTE prophylaxis (Table 3).57 Some of these barriers are outlined below.
|
| Variability in clinician knowledge of risk assessment and appropriate prophylaxis |
| Lack of agreement with, and inconsistency between, guidelines in certain patient populations |
| Perceived lack of need |
| Concerns about adverse effects |
| Lack of hospital support systems and policies |
| Lack of established responsibilities for prophylaxis |
Underestimation of Risk of Clotting
VTE is often clinically silent, leading some physicians to mistakenly believe that it is rare.58 In hospitalized surgical patients, for example, the incidence of thromboembolic complications during a short postoperative stay may be low. Given that many cases of symptomatic VTE occur after hospital discharge, hospitalists and surgeons may be unaware of the true incidence of DVT.59
Overestimation of the Risk of Bleeding
Physicians may also overestimate the risk of possible side effects of prophylaxis, such as major bleeding or heparin‐induced thrombocytopenia (HIT).58 Fear of excess bleeding has been cited by physicians as a leading reason for their decision to withhold thromboprophylaxis from at‐risk hospitalized patients.60 Physicians are particularly fearful of complications among elderly patients, who are less likely to receive adequate prophylaxis than younger patients with a similar risk of VTE.61 When bleeding does occur, it rarely results in death. On the other hand, PE may account for as many as 10% of hospital deaths.9
Guideline Confusion and Complexity
Discrepancies between guidelines published by different medical societies contribute to confusion in choosing a management approach. The American Academy of Orthopedic Surgeons (AAOS), for example, describes aspirin alone as a reasonable choice for VTE prophylaxis in some patients, but the ACCP guidelines advise against the use of aspirin monotherapy.58 The cumbersome nature of multiple risk‐assessment and treatment algorithms can also be problematic.61 Furthermore, certain patient subgroups, such as those with cirrhosis, severe renal failure, and epidural catheters, have been excluded from randomized controlled trials, and the management of such patients is not straightforward.
Absence of Institutional Protocols and Information Technology Support
The lack of institution‐level guidance and support can have a detrimental effect on patient care. In a 2007 survey of 127 community hospitals, the prevalence of institutional protocols related to VTE was low: only 60% had protocols to encourage prophylaxis in at‐risk patients, 54% had guidelines to assist in appropriate drug selection, and 43% had guidelines for the dosing of prophylaxis regimens.62 A lack of systems for data collection and audit has also been identified as a barrier to the implementation of prophylaxis guidelines.57 Thus, hospitals need to adopt protocols such as:3
-
Written, institution‐wide thromboprophylaxis policies
-
Preprinted order forms and computer decision‐support systems
-
Policies specifying responsibilities for assessing VTE risk and prescribing prophylaxis
Conclusions
VTE is the most common preventable cause of hospital death, and prophylaxis is underused in hospitalized patients. Although VTE risk factors are numerous and complex, deciding whether to use prophylaxis need not be complicated. In general, elderly patients, medically‐ill patients, and patients undergoing surgery will benefit from prophylaxis, as well as those who are hospitalized for more than 1 night. Hospitalized patients with at least 1 risk factor should be considered for pharmacologic prophylaxis. In general, the risk of hospital‐acquired VTE greatly exceeds the risk of bleeding with prophylactic doses of anticoagulation. A patient's risk of VTE may change, and regular assessment of this risk should be mandated if pharmacologic therapy is not initiated at the time of admission.
Numerous barriers to the optimal use of VTE prophylaxis exist, and hospitals must implement systems changes and multidisciplinary approaches to overcome these barriers. The fourth article in this supplement provides detailed strategies for meeting VTE performance measures and overcoming barriers to the optimal use of prophylaxis.
Venous thromboembolism (VTE) is a common and potentially devastating complication of medical illness and surgical intervention. Among patients discharged from acute‐care hospitals in 2003, more than 12 million (31%) had a moderate or high risk of VTE during hospitalization, including 11% at risk due to surgical procedures and 20% at risk due to medical illnesses.1 The incidence of VTEwhich can present as deep vein thrombosis (DVT) or pulmonary embolism (PE)is rising in hospitalized patients.2 Despite the availability of effective prophylaxis, VTE is the third most common cause of hospital‐related death and the most common preventable cause of hospital mortality.3
The clinical impact of VTE is significant. While total incidence, prevalence, and mortality rates of VTE are elusive, the annual incidence of DVT is thought to be as high as 2 million.4 The most serious complication of DVT is acute PE, which occurs in approximately 600,000 patients per year, one‐third of whom die.5 DVT may also be complicated by recurrent episodes of VTE and postthrombotic sequelae such as chronic venous stasis, venous ulceration, debilitating pain, and intractable edema.6 One prospective cohort study found that 30% of patients who had experienced a first episode of DVT developed recurrent VTE within 8 years, and the incidence of postthrombotic syndrome (PTS) was 23% after just 2 years.6
The economic burden of VTE is substantial, due both to the initial event and to the high rate of hospital readmission. The estimated average cost of DVT management (including initial acute care and 6 months of follow‐up care) is $10,072 per patient, and the corresponding cost for PE is $14,649.7 Hospital readmission occurs in 5% to 14% of patients, more than one‐half of whom are readmitted within 90 days.8 For patients with recurrent DVT or PE, the mean total hospitalization costs of readmission are $11,862 and $14,722, respectively.8
The clinical and economic burden of VTE can be significantly mitigated by the use of effective prophylaxis. Because VTE is difficult to diagnose antemortem, it is easier and safer to prevent with appropriate prophylaxis than to diagnose after it has occurred. Unfortunately, VTE prophylaxis is markedly underused, particularly among high‐risk, hospitalized medical patients who would most benefit from it.9
This article summarizes specific risk factors for VTE and provides guidance in identifying patients who may require thromboprophylaxis. Barriers to optimal VTE prophylaxis in the hospital setting will also be explored.
Methodology
For this article and the ones that follow, relevant literature was identified through a Medline search (January 1980 to December 2008) using the following search terms: venous thromboembolism, pulmonary embolism, deep vein thrombosis, epidemiology, risk factors, prophylaxis, mechanical prophylaxis, diagnosis, treatment, anticoagulants, monitoring, secondary prevention, guideline, adherence, treatment protocol, performance measure, and quality improvement. The bibliographies of all key texts were searched for additional relevant articles. The websites of the American College of Chest Physicians (ACCP), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and Society of Hospital Medicine (SHM) were also searched for annual meeting abstracts, position statements, and other key publications.
Evidence‐based clinical guidelines were identified through a search of the National Guideline Clearinghouse (
Pathogenesis of VTE
Venous thrombosis occurs as a result of at least 1 of 3 underlying factors: alterations in blood flow, vascular endothelial injury, and alterations in the constitution of the blood.10 Each potential underlying factor encompasses a wide range of risk factors and clinical scenarios. Alterations in venous blood flow can include several situations, including venous stasis, venous hypertension, and valvular incompetence. Endothelial injury can arise from shear stress, direct trauma, infection, hypertension, or other sources of endothelial damage. Hypercoagulability from alterations in the constitution of the blood may be due to antithrombin deficiency, cancer, surgery, pregnancy, or other risk factors. The presence of any of these factors indicates an elevated risk of VTE, and the presence of multiple factors further increases risk.10
Risk Factors for VTE
VTE can occur in a wide variety of clinical circumstances. Recognized risk factors for VTE include hospitalization for an acute medical illness, cardiovascular disease, pulmonary disease, major surgery, multiple trauma, obesity, and increasing age.10 Additional factors that place patients at increased risk of VTE (independent of age) include a history of prior VTE, known hypercoagulable states, active cancer, and acute infection.11 Hospital‐acquired risk factors such as immobility, acute illness, or medical interventions may lead to the development of VTE in these patients. Severity of illness must be factored into the risk assessment, and all patients need to be assessed for VTE risk at the time of hospital admission and daily thereafter if pharmacologic therapy is not initiated.
In a review of 1231 consecutive patients treated for acute DVT and/or PE, 96.3% had at least 1 risk factor for VTE, and more than one‐third (39%) had 3 or more risk factors (Table 1).10 The incidence of VTE in hospitalized patients is directly related to the number of risk factors present:10
-
1 risk factor: 11%
-
2 risk factors: 24%
-
3 risk factors: 36%
-
4 risk factors: 50%
-
5 risk factors: > 90%
| Risk Factor | Patients (%) |
|---|---|
| |
| Age 40 years | 88.5 |
| Obesity | 37.8 |
| History of VTE | 26.0 |
| Cancer | 22.3 |
| Bed rest 5 days | 12.0 |
| Major surgery | 11.2 |
| Congestive heart failure | 8.2 |
| Varicose veins | 5.8 |
| Fracture (hip or leg) | 3.7 |
| Estrogen treatment | 2.0 |
| Stroke | 1.8 |
| Multiple trauma | 1.1 |
| Childbirth | 1.1 |
| Myocardial infarction | 0.7 |
Current or Recent Prior Hospitalization
The risk of VTE is elevated among hospitalized patients. The prevalence of DVT varies across hospital specialties, reaching up to 80% in major trauma, spinal cord injury, and critical care.3 The Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study evaluated the prevalence of VTE risk factors in the acute hospital care setting.12 Among the 68,183 patients enrolled, more than one‐half (52%) were judged to be at risk of VTE. In a case‐control study examining 625 patients with a first lifetime VTE, confinement to a hospital (among other risk factors) was found to be an independent and important predictor of VTE (odds ratio [OR], 8.0; 95% confidence interval [CI], 4.514.2) (Figure 1).13 Recent hospitalization is also an important risk factor for VTE, and patients who are readmitted to the hospital should be considered moderate or high risk.13

Age
Patient age must be considered when assessing VTE risk. VTE is predominantly a disease of older age, and age older than 75 years is an important risk factor for the condition.11 In general, patients older than 40 years have a significantly increased risk compared with younger patients, and the risk approximately doubles with each additional decade.10 Given the aging population, the prevalence of VTE and its complications are expected to increase.
Women of childbearing age experience VTE more frequently than men of the same age, due to pregnancy and exposure to contraceptive therapy.14 This risk, however, is modest compared with the risk among older patients. After age 45 years, the incidence of VTE increases markedly for both sexes, becoming more prominent in men.14 Compared with women, men also have an increased risk of recurrent VTE.15
Despite the effect of age on VTE risk, the risk among patients younger than 40 years may be underestimated because this subgroup has not been extensively studied. For reasons that are not well‐understood, the risk of VTE associated with heart failure is higher in patients younger than 40 years, and the relative risk of PE in patients with chronic obstructive pulmonary disease (COPD) is also higher in younger patients.16, 17
Cancer and Its Treatment
Cancer patients, on average, have twice the risk of VTE compared with noncancer patients.18 This risk, however, varies considerably by cancer type. According to an assessment of nearly 41 million hospitalized patients in the National Hospital Discharge Survey (NHDS), the relative risk of VTE varied from 1.02 in patients with bladder cancer to 4.34 in patients with cancer of the pancreas.18
VTE is one of the most common complications of cancer and cancer therapy, and it is the second leading cause of death among hospitalized cancer patients.19 Molecular mechanisms underlying thromboembolic events in cancer patients include tumor cell procoagulants, inflammatory cell cytokines, mediators of platelet adhesion, and tumor‐related stasis and endothelial damage.20 The clinical implications of these processes are severe. Cancer exacerbates the natural course of VTE, increasing the risk of recurrent VTE and major bleeding, and VTE worsens the prognosis of cancer, increasing the risk of death among cancer patients.
Various cancer therapiesincluding surgery, chemotherapy, hematopoietic stem cell transplantation, and even growth factor supportalso increase the risk of VTE, in part because extrinsic factors such as surgery or chemotherapy can intensify the hypercoagulable process.18, 2123 In the NHDS, cancer patients undergoing surgery had at least twice the risk of postoperative DVT and more than 3 times the risk of PE compared with noncancer patients undergoing similar procedures.18
Cancer is an independent predictor of thromboprophylaxis failure following surgery. The @RISTOS Project found that VTE was the most common cause of death among 2373 patients undergoing general, urologic, or gynecologic surgery for cancer.24 A multivariate analysis identified 5 independent risk factors for VTE after cancer surgery:
-
Previous VTE (OR, 5.98; 95% CI, 2.1316.80)
-
Anesthesia 2 hours (OR, 4.50; 95% CI, 1.0619.04)
-
Bed rest 4 days (OR, 4.37; 95% CI, 2.457.78)
-
Age 60 years (OR, 2.63; 95% CI, 1.215.71)
-
Advanced‐stage cancer (OR, 2.68; 95% CI, 1.375.24)
Cardiovascular Disease
The risk of VTE is pronounced among patients with cardiovascular disease. After stroke and coronary disease, VTE is the third most common cardiovascular disorder, and PE causes more deaths each year than myocardial infarction (MI).25 Several cardiovascular diseases, including hypertension, stroke, acute MI, and heart failure, are independently associated with VTE.10, 2627 Related disorders, including diabetes and the metabolic syndrome, also increase the risk of VTE.26, 28
Congestive heart failure (CHF) is a risk factor for VTE, and the severity of illness increases risk. In the DVT‐Free Prospective Registry, 13% of patients with ultrasound‐confirmed DVT had CHF.29 In a subgroup analysis of patients of the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, the incidence of VTE exceeded 20% in patients with New York Heart Association (NYHA) class IV heart failure, compared with 12% in patients with NYHA class III heart failure.30 Another study found that VTE risk increases as left ventricular ejection fraction (LVEF) decreases, with an LVEF of less than 20% associated with a VTE OR of 38.3 (95% CI, 9.6152.5).31
Infectious Disease
Acute infection may increase the relative risk of VTE by as much as 50% and is associated with VTE event rates of up to 26%.11 Acute infections may be associated with acute inflammation, adverse effects on cardiac or pulmonary function, and prolonged immobilization.30, 32, 33 Human immunodeficiency virus (HIV) patients may also have an increased risk of VTE due to a circulating lupus anticoagulant and/or the presence of acute infection.34
Obesity
The 2008 ACCP guideline update recognizes obesity, for the first time, as a risk factor for VTE.3 Obesity was 1 of the 5 most frequent comorbidities found in patients with DVT in the DVT‐Free Prospective Registry.29 It increases the risk of both incident and recurrent VTE, with every 1‐point increase in body mass index (BMI) increasing the risk of recurrent VTE by 4.4% (95% CI, 1.37.6%; P < 0.001).35
Pregnancy and Puerperium
Pregnancy, particularly the postpartum period, is associated with an increased risk of VTE in women, even though the absolute risk is small.36 Still, PE is one of the leading causes of maternal death following childbirth.10 Smoking, prior VTE, and inherited thrombophilias all increase the risk of VTE in pregnant women.10 The risk begins to rise in the first trimester, and when prophylaxis is needed, it should be started early in gestation.37
Pulmonary Disease
COPD is another risk factor for the development of VTE. COPD patients who develop VTE tend to be older, hospitalized in the intensive care unit (ICU), and on mechanical ventilation.38 In the DVT‐Free Prospective Registry, 12% of patients with ultrasound‐confirmed DVT had COPD.29
Trauma and Surgery
Injury to the body tissue, via trauma or surgery, stimulates the body's clotting mechanism and increases the risk of thromboembolic complications. During the perioperative period, the circulatory system must balance a variety of assaults: an immune response to surgical stress, prolonged immobilization during surgery and recovery, vasodilation associated with general or regional anesthesia, and hypercoagulability due to venous stasis and vascular injury.39 Renal transplant recipients have an increased risk of VTE due to a chronic hypercoagulable state.40 In surgery patients, perioperative complications such as dehydration and acute infection increase the risk of VTE beyond the risk associated with the surgical procedure itself.10
VTE risk is increased approximately 13‐fold by recent major trauma or lower‐extremity injury.13 In the absence of prophylaxis, the overall risk of VTE among patients undergoing major surgery is increased nearly 22‐fold.13 After controlling for the type of surgery, additional independent risk factors for VTE within 3 months of major surgery include:41, 42
-
Obesity
-
Central venous catheter placement
-
Malignancy
-
Smoking
-
Heart failure
-
Previous DVT
-
Prolonged immobility
-
Infection
Many surgical and medical inpatients share common risk factors, and without prophylaxis, the incidence of hospital‐acquired DVT ranges from 10% to 40% for both groups.3
Inherited or Acquired Risk Factors
VTE is a multifactorial disease, and recent evidence indicates that some heritable traits may be potent risk factors for VTE.43 Approximately 35% of patients with DVT will have at least 1 of 5 traits related to an inherited blood clotting disorder:43
-
Deficiencies in the anticoagulation factors protein C, protein S, or antithrombin, or
-
Mutations in the factor V and prothrombin genes, resulting in Factor V Leiden and prothrombin G20210A, respectively.
Certain inherited traits and genetic polymorphisms increase the risk of VTE by interacting with clinical risk factors such as contraceptive use, pregnancy, surgery, trauma, and cancer. One recent study found that oral estrogen therapy among women with the CYP3A5*1 allele was associated with a particularly high risk of VTE.44 Although widespread screening for inherited risk factors is not currently practical, future tools may incorporate genetic polymorphisms to more precisely identify patients who would benefit from aggressive prophylaxis.
Lifestyle Factors
Lifestyle factors have a significant effect on VTE risk. Smoking increases the risk of VTE by 20% to 30%, and a sedentary lifestyle also increases the risk of VTE.26, 45 In fact, women who exercise regularly and consume alcohol in moderation have one‐half the risk of VTE as women who have a sedentary lifestyle and drink little or no alcohol.42 For both men and women, a diet high in fruits, vegetables, and fish is associated with a lower lifetime risk of VTE.46
Medications
Medications may also increase the risk of VTE. In cancer patients with anemia, for example, the use of erythropoiesis‐stimulating agents such as recombinant erythropoietin and darbepoetin was recently shown to increase the risk of VTE by 57% (95% CI, 3187%) and increase mortality risk by 10% (95% CI, 120%).23 In addition, combination hormone replacement therapy in women is associated with a higher risk of VTE compared with estrogen monotherapy, and transdermal contraceptive systems more than double the risk of VTE compared with oral contraceptives (95% CI, 1.33.8).47, 48 Recent studies have also reported an increased risk of VTE with some psychiatric drugs, including amitriptyline, clozapine, olanzapine, and risperidone.4952
Thromboprophylaxis in the Hospital Setting
Despite the prevalence of risk factors and compelling evidence regarding the value of prophylaxis, VTE prophylaxis is suboptimal in hospitalized medical and surgical patients. In a study of 123,304 hospitalized patients who were determined to be at risk of VTE, only 13.3% received prophylaxis in accordance with ACCP guidelines.53 Compliance ranged from a high of 52.4% among patients undergoing orthopedic surgery to a low of 2.8% among patients undergoing neurosurgery.53 Results from several other large trials echo these findings (Table 2).12, 5456
| Trial | Patient Type | Total Patients | Patients at Risk of VTE (Based on ACCP Criteria) (%) | At‐Risk Patients Receiving Recommended Prophylaxis | |
|---|---|---|---|---|---|
| Medical Patients (%) | Surgical Patients (%) | ||||
| |||||
| IMPROVE | Medical patients | 15,156 | 52 | 61 | n/a |
| ENDORSE | Medical and surgical patients | 68,183 | 51.8 | 39.5 | 58.5 |
| ADHERE | Hospitalized heart failure patients | 155,073 | 46 | 30.6 | n/a |
| Amin et al.56 (2008) | Medical and surgical patients | 258,556 | 26.4 | 9.8 | 17.9 |
Reasons for Inadequate Prophylaxis
Researchers have identified a range of barriers to adequate VTE prophylaxis (Table 3).57 Some of these barriers are outlined below.
|
| Variability in clinician knowledge of risk assessment and appropriate prophylaxis |
| Lack of agreement with, and inconsistency between, guidelines in certain patient populations |
| Perceived lack of need |
| Concerns about adverse effects |
| Lack of hospital support systems and policies |
| Lack of established responsibilities for prophylaxis |
Underestimation of Risk of Clotting
VTE is often clinically silent, leading some physicians to mistakenly believe that it is rare.58 In hospitalized surgical patients, for example, the incidence of thromboembolic complications during a short postoperative stay may be low. Given that many cases of symptomatic VTE occur after hospital discharge, hospitalists and surgeons may be unaware of the true incidence of DVT.59
Overestimation of the Risk of Bleeding
Physicians may also overestimate the risk of possible side effects of prophylaxis, such as major bleeding or heparin‐induced thrombocytopenia (HIT).58 Fear of excess bleeding has been cited by physicians as a leading reason for their decision to withhold thromboprophylaxis from at‐risk hospitalized patients.60 Physicians are particularly fearful of complications among elderly patients, who are less likely to receive adequate prophylaxis than younger patients with a similar risk of VTE.61 When bleeding does occur, it rarely results in death. On the other hand, PE may account for as many as 10% of hospital deaths.9
Guideline Confusion and Complexity
Discrepancies between guidelines published by different medical societies contribute to confusion in choosing a management approach. The American Academy of Orthopedic Surgeons (AAOS), for example, describes aspirin alone as a reasonable choice for VTE prophylaxis in some patients, but the ACCP guidelines advise against the use of aspirin monotherapy.58 The cumbersome nature of multiple risk‐assessment and treatment algorithms can also be problematic.61 Furthermore, certain patient subgroups, such as those with cirrhosis, severe renal failure, and epidural catheters, have been excluded from randomized controlled trials, and the management of such patients is not straightforward.
Absence of Institutional Protocols and Information Technology Support
The lack of institution‐level guidance and support can have a detrimental effect on patient care. In a 2007 survey of 127 community hospitals, the prevalence of institutional protocols related to VTE was low: only 60% had protocols to encourage prophylaxis in at‐risk patients, 54% had guidelines to assist in appropriate drug selection, and 43% had guidelines for the dosing of prophylaxis regimens.62 A lack of systems for data collection and audit has also been identified as a barrier to the implementation of prophylaxis guidelines.57 Thus, hospitals need to adopt protocols such as:3
-
Written, institution‐wide thromboprophylaxis policies
-
Preprinted order forms and computer decision‐support systems
-
Policies specifying responsibilities for assessing VTE risk and prescribing prophylaxis
Conclusions
VTE is the most common preventable cause of hospital death, and prophylaxis is underused in hospitalized patients. Although VTE risk factors are numerous and complex, deciding whether to use prophylaxis need not be complicated. In general, elderly patients, medically‐ill patients, and patients undergoing surgery will benefit from prophylaxis, as well as those who are hospitalized for more than 1 night. Hospitalized patients with at least 1 risk factor should be considered for pharmacologic prophylaxis. In general, the risk of hospital‐acquired VTE greatly exceeds the risk of bleeding with prophylactic doses of anticoagulation. A patient's risk of VTE may change, and regular assessment of this risk should be mandated if pharmacologic therapy is not initiated at the time of admission.
Numerous barriers to the optimal use of VTE prophylaxis exist, and hospitals must implement systems changes and multidisciplinary approaches to overcome these barriers. The fourth article in this supplement provides detailed strategies for meeting VTE performance measures and overcoming barriers to the optimal use of prophylaxis.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82(9):777–782.
- , , .Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients.Am J Cardiol.2005;95(12):1525–1526.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th ed.Chest.2008;133(6 suppl):381S–453S.
- , .Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association.Circulation.1996;93(12)2212–2245.
- , , , et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- , , , et al.The long‐term clinical course of acute deep venous thrombosis.Ann Intern Med.1996;125(1):1–7.
- , .Direct medical cost of managing deep vein thrombosis according to the occurrence of complications.Pharmacoeconomics.2002;20(9):603–615.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , .Risk factors for venous thromboembolism.Circulation.2003;107(23 suppl 1):I9–I16.
- , , , et al.Risk factors for VTE in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- , , , et al.;ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , et al.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160(6):809–815.
- , .Range of published estimates of venous thromboembolism incidence in young women.Contraception.2007;75(5):328–336.
- , , , , , .The risk of recurrent venous thromboembolism in men and women.N Engl J Med.2004;350(25):2558–2563.
- , , , et al.Risk of venous thromboembolism in patients hospitalized with heart failure.Am J Cardiol.2006;98(6):793–795.
- , , , et al.Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease.J Cardiovasc Med.2007;8(4):253–257.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.Causes of death in cancer patients.J Med.1975;6(1):61–64.
- , .Tissue factor and angiogenesis in cancer.Curr Opin Hematol.2002;9(5):401–406.
- , , , et al.Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel‐containing first‐line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group.J Clin Oncol.2008;26(16):2683–2689.
- , , , , , .The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention.Blood.2008;112(3):504–510.
- , , , et al.Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia.JAMA.2008;299(8):914–924.
- , , , et al.A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project.Ann Surg.2006;243(1):89–95.
- .Pulmonary embolism in thrombolysis: a clarion call for international collaboration.J Am Coll Cardiol.1992;19:246–247.
- , , , et al.Cardiovascular risk factors and venous thromboembolism: a meta‐analysis.Circulation.2008;117(1):93–102.
- , , , , , .Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study.J Thromb Haemost.2008;6(11):1851–1857.
- , , , et al.Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome—the Tromsø study.J Thromb Haemost.2009;7(5):739–745.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , , .Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case‐control study.J Clin Epidemiol.2001;54(8):810–816.
- , , , et al;ARTEMIS Investigators.Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- , , , et al.HIV infection is a risk factor for venous thromboembolism.AIDS Patient Care STDS.2002;16(5):205–209.
- , , , et al.Overweight, obesity, and the risk of recurrent venous thromboembolism.Arch Intern Med.2008;168(15):1678–1683.
- .Venous thromboembolism in women.Vasc Med.2008;13(3):255–266.
- , , .Thrombosis during pregnancy and the postpartum period.Am J Obstet Gynecol.2005;193(1):216–219.
- , , , et al.Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination.J Thromb Thrombolysis.2008;26(1):35–40.
- , , .Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability?J Trauma.2003;54(2):224–231.
- , .Acquired hypercoagulable state in renal transplant recipients.Thromb Haemost.2004;91(4):646–654.
- , , , .Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion.Thromb Haemost.2007;98(6):1220–1225.
- , , , , , .A 10‐year analysis of venous thromboembolism on the surgical service: the effect of practice guidelines for prophylaxis.Surgery.2008;144(1):3–11.
- , , , et al;EDITH Collaborative Study Group.Family history as a risk factor for venous thromboembolism.Thromb Res.2008;122(5):624–629.
- , , , et al;Estrogen and Thromboembolism Risk (ESTHER) Study Group.Synergism between oral estrogen therapy and cytochrome P450 3A5*1 allele on the risk of venous thromboembolism among postmenopausal women.J Clin Endocrinol Metab.2008;93(8):3082–3087.
- , , .The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study.Br J Haematol.2009;144(2):234–240.
- , , , , .Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology.Circulation.2007;115(2):188–195.
- , , .Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta‐analysis.Eur Heart J.2008;29(16):2031–2041.
- , , , .Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users.Obstet Gynecol.2007;109(2 Pt 1):339–346.
- , , , , , .Pulmonary thromboembolism associated with olanzapine and risperidone.J Emerg Med.2008;35(2):159–161.
- , , , .Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions.Drug Saf.2008;31(8):685–694.
- .Evaluating the association between clozapine and venous thromboembolism.Am J Health Syst Pharm.2008;65(19):1825–1829.
- , .Antidepressant drug use and risk of venous thromboembolism.Pharmacotherapy.2008;28(2):144–150.
- , , , .Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64(1):69–76.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients.Chest.2007;132:936–945.
- , , , et al.Adhere Scientific Advisory Committee and Investigators. Venous thromboembolism prophylaxis in hospitalized heart failure patients.J Card Fail.2008;14(2):127–132.
- , , , et al. Are hospitals following guidelines for VTE Prevention? The Venous Thromboembolism Study to Assess the Rate of Thromboprophylaxis. Presented at the 50th Annual Meeting of the American College of Hematology, San Francisco, CA, December 6–9, 2008. Abstract 1286.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- .A need for a simplified approach to venous thromboembolism prophylaxis in acute medical inpatients.Int J Clin Pract.2007;61(2):336–340.
- .Barriers to the optimal use of anticoagulants after orthopaedic surgery.Arch Orthop Trauma Surg.2008 (Published online Oct, 8.). [http://dx.doi.org/DOI 10.1007/s00402‐008‐0765‐9]
- , , , , .Venous thrombosis in cancer patients: Insights from the FRONTLINE survey.Oncologist.2003;8:381–388.
- , , , et al.Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross‐sectional study in Brazil.J Thromb Haemost.2006;4(6):1266–1270.
- , , , , .Survey of hospitals for guidelines, policies, and protocols for anticoagulants.Am J Health Syst Pharm.2007;64(11):1203–1208.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82(9):777–782.
- , , .Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients.Am J Cardiol.2005;95(12):1525–1526.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th ed.Chest.2008;133(6 suppl):381S–453S.
- , .Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association.Circulation.1996;93(12)2212–2245.
- , , , et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- , , , et al.The long‐term clinical course of acute deep venous thrombosis.Ann Intern Med.1996;125(1):1–7.
- , .Direct medical cost of managing deep vein thrombosis according to the occurrence of complications.Pharmacoeconomics.2002;20(9):603–615.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , .Risk factors for venous thromboembolism.Circulation.2003;107(23 suppl 1):I9–I16.
- , , , et al.Risk factors for VTE in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- , , , et al.;ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , et al.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160(6):809–815.
- , .Range of published estimates of venous thromboembolism incidence in young women.Contraception.2007;75(5):328–336.
- , , , , , .The risk of recurrent venous thromboembolism in men and women.N Engl J Med.2004;350(25):2558–2563.
- , , , et al.Risk of venous thromboembolism in patients hospitalized with heart failure.Am J Cardiol.2006;98(6):793–795.
- , , , et al.Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease.J Cardiovasc Med.2007;8(4):253–257.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.Causes of death in cancer patients.J Med.1975;6(1):61–64.
- , .Tissue factor and angiogenesis in cancer.Curr Opin Hematol.2002;9(5):401–406.
- , , , et al.Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel‐containing first‐line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group.J Clin Oncol.2008;26(16):2683–2689.
- , , , , , .The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention.Blood.2008;112(3):504–510.
- , , , et al.Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia.JAMA.2008;299(8):914–924.
- , , , et al.A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project.Ann Surg.2006;243(1):89–95.
- .Pulmonary embolism in thrombolysis: a clarion call for international collaboration.J Am Coll Cardiol.1992;19:246–247.
- , , , et al.Cardiovascular risk factors and venous thromboembolism: a meta‐analysis.Circulation.2008;117(1):93–102.
- , , , , , .Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study.J Thromb Haemost.2008;6(11):1851–1857.
- , , , et al.Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome—the Tromsø study.J Thromb Haemost.2009;7(5):739–745.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , , .Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case‐control study.J Clin Epidemiol.2001;54(8):810–816.
- , , , et al;ARTEMIS Investigators.Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- , , , et al.HIV infection is a risk factor for venous thromboembolism.AIDS Patient Care STDS.2002;16(5):205–209.
- , , , et al.Overweight, obesity, and the risk of recurrent venous thromboembolism.Arch Intern Med.2008;168(15):1678–1683.
- .Venous thromboembolism in women.Vasc Med.2008;13(3):255–266.
- , , .Thrombosis during pregnancy and the postpartum period.Am J Obstet Gynecol.2005;193(1):216–219.
- , , , et al.Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination.J Thromb Thrombolysis.2008;26(1):35–40.
- , , .Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability?J Trauma.2003;54(2):224–231.
- , .Acquired hypercoagulable state in renal transplant recipients.Thromb Haemost.2004;91(4):646–654.
- , , , .Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion.Thromb Haemost.2007;98(6):1220–1225.
- , , , , , .A 10‐year analysis of venous thromboembolism on the surgical service: the effect of practice guidelines for prophylaxis.Surgery.2008;144(1):3–11.
- , , , et al;EDITH Collaborative Study Group.Family history as a risk factor for venous thromboembolism.Thromb Res.2008;122(5):624–629.
- , , , et al;Estrogen and Thromboembolism Risk (ESTHER) Study Group.Synergism between oral estrogen therapy and cytochrome P450 3A5*1 allele on the risk of venous thromboembolism among postmenopausal women.J Clin Endocrinol Metab.2008;93(8):3082–3087.
- , , .The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study.Br J Haematol.2009;144(2):234–240.
- , , , , .Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology.Circulation.2007;115(2):188–195.
- , , .Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta‐analysis.Eur Heart J.2008;29(16):2031–2041.
- , , , .Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users.Obstet Gynecol.2007;109(2 Pt 1):339–346.
- , , , , , .Pulmonary thromboembolism associated with olanzapine and risperidone.J Emerg Med.2008;35(2):159–161.
- , , , .Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions.Drug Saf.2008;31(8):685–694.
- .Evaluating the association between clozapine and venous thromboembolism.Am J Health Syst Pharm.2008;65(19):1825–1829.
- , .Antidepressant drug use and risk of venous thromboembolism.Pharmacotherapy.2008;28(2):144–150.
- , , , .Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64(1):69–76.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients.Chest.2007;132:936–945.
- , , , et al.Adhere Scientific Advisory Committee and Investigators. Venous thromboembolism prophylaxis in hospitalized heart failure patients.J Card Fail.2008;14(2):127–132.
- , , , et al. Are hospitals following guidelines for VTE Prevention? The Venous Thromboembolism Study to Assess the Rate of Thromboprophylaxis. Presented at the 50th Annual Meeting of the American College of Hematology, San Francisco, CA, December 6–9, 2008. Abstract 1286.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- .A need for a simplified approach to venous thromboembolism prophylaxis in acute medical inpatients.Int J Clin Pract.2007;61(2):336–340.
- .Barriers to the optimal use of anticoagulants after orthopaedic surgery.Arch Orthop Trauma Surg.2008 (Published online Oct, 8.). [http://dx.doi.org/DOI 10.1007/s00402‐008‐0765‐9]
- , , , , .Venous thrombosis in cancer patients: Insights from the FRONTLINE survey.Oncologist.2003;8:381–388.
- , , , et al.Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross‐sectional study in Brazil.J Thromb Haemost.2006;4(6):1266–1270.
- , , , , .Survey of hospitals for guidelines, policies, and protocols for anticoagulants.Am J Health Syst Pharm.2007;64(11):1203–1208.
Guidelines‐Based Thromboprophylaxis
Thromboprophylaxis with anticoagulants has proven benefits in hospitalized patients. Despite this, venous thromboembolism (VTE) prophylaxis is underused and VTE remains the leading cause of preventable hospital mortality.1 Medical patients have a particularly high risk; those who develop a deep vein thrombosis (DVT) are significantly less likely to have received prophylaxis prior to the diagnosis of DVT than nonmedical patients. Even within the high‐risk setting of the intensive care unit (ICU), medical patients receive thromboprophylaxis only two‐thirds as often as nonmedical patients.2
In this article we summarize the evidence concerning the various prophylaxis options, including current guideline recommendations for VTE prevention in medical and surgical patients. We also discuss strategies for thromboprophylaxis in special populations and potential complications of prophylaxis.
Efficacy of Prophylaxis in Medical Patients
Several meta‐analyses have demonstrated the marked benefits of anticoagulant prophylaxis in medical patients. Dentali et al3 conducted a meta‐analysis of 9 randomized controlled trials enrolling a total of 19,958 at‐risk hospitalized medical patients. The selected trials compared standard anticoagulant regimens with no treatment and only included studies with objectively documented and independently adjudicated outcomes. Compared with patients receiving placebo, those receiving thromboprophylaxis had significant reductions in any PE by 57% (95% CI, 0.26‐0.71; absolute risk reduction, 0.29%) and fatal pulmonary embolism (PE) by 62% (95% CI, 0.21‐0.69; absolute risk reduction, 0.25%), with a nonsignificant reduction in symptomatic DVT (relative risk [RR], 0.47; 95% CI, 0.22‐1.00) and a nonsignificant increase in major bleeding (RR, 1.32; 95% CI, 0.73‐2.37). The researchers concluded that anticoagulant prophylaxis is effective in preventing symptomatic VTE in medical patients, though the optimal duration of therapy is not yet defined.3
Another meta‐analysis focusing on subclinical DVT in acutely ill medical patients examined the therapeutic effects of various prophylaxis regimens. Overall, anticoagulant prophylaxis reduced the risk of any asymptomatic DVT (assessed by venogram or ultrasound) by 49% (95% CI, 0.39‐0.67) and asymptomatic proximal DVT by 55% (95% CI, 0.31‐0.65) compared with placebo (absolute risk reduction, 2.6% and 1.8%, respectively). Although prophylaxis was associated with a 0.5% absolute risk increase in major bleeding, the authors concluded that the benefits of prophylaxis outweighed the risks of bleeding.4
Anticoagulant Agents in the Prevention of VTE
Currently available anticoagulants for the prevention of VTE include unfractionated heparin (UFH), low‐molecular‐weight heparins (LMWHs), fondaparinux, and warfarin. These agents interrupt thrombus formation, either indirectly (through interaction with antithrombin) or directly (by inhibiting the action of thrombin). Each class of therapy has advantages and limitations. Table 1 lists common anticoagulant options for VTE prophylaxis, along with dosing information and other important information.510
| Prophylactic Dose | Warnings/Contraindications/Adverse Reactions | |
|---|---|---|
| ||
| Warfarin | 5 to 10 mg daily initially;* adjust dose based on INR; therapeutic INR goal: 2.5 (2‐3) | Warning: bleeding risk; requires frequent monitoring; contraindicated in patients for whom hazard of hemorrhage outweighs potential benefit (eg, in pregnant women) |
| UFH | 5000 IU every 8‐12 hours subcutaneously | Contraindicated in the presence of active bleeding, uncontrolled hypertension, or severe thrombocytopenia; monitor platelet count every 4‐7 days for HIT |
| Dalteparin | 5000 IU daily subcutaneously | Warning: spinal/epidural hematoma; monitor for signs and symptoms of neurological impairment. LMWHs should be used with caution in renal impairment; anti‐Xa monitoring and dose adjustments may be required. Follow prescribing information for dose adjustments and body weight‐based dosing. Most common adverse reactions: bleeding, anemia, thrombocytopenia, elevation of serum aminotransferase, diarrhea, and nausea |
| Enoxaparin | 40 mg daily subcutaneously; reduce to 30 mg daily in renal impairment | |
| Tinzaparin | 3500 IU daily subcutaneously | |
| Fondaparinux | 2.5 mg daily subcutaneously | Warning: spinal/epidural hematoma; monitor for signs and symptoms of neurological impairment; contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/minute) and in patients < 50 kg |
UFH
UFH, which is typically administered by subcutaneous injection, has the longest history as an anticoagulant in the prevention and treatment of VTE. It is an attractive option in patients with severe renal failure or those who may require a procedure in the near future. Although UFH is partially cleared by the kidney, its short half‐life can be perceived as a safety advantage in patients with severe renal impairment and an increased risk of bleeding. For most other patients, UFH holds several disadvantages compared with newer therapies, including the need for injections to be administered 3 times a day to be optimally effective, its effect on platelets, and its association with heparin‐induced thrombocytopenia (HIT).1 Given the costs of administration and potential complications, it is not less expensive than LMWHs, and it appears to be less cost‐effective.11
LMWHs
LMWHs have a higher bioavailability and longer half‐life than UFH, which translates to reliable anticoagulation levels when given subcutaneously on a weight‐based dosing schedule. Unlike UFH, LMWHs do not require laboratory tests to monitor the intensity of anticoagulation, except in special circumstances.1 The LMWHs dalteparin, enoxaparin, and tinzaparin are widely used for the prevention and treatment of VTE in the United States.
Two landmark clinical trials demonstrated the efficacy of appropriate thromboprophylaxis with LMWHs in reducing the burden of VTE in acutely ill, hospitalized medical patients. The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial and Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT) demonstrated the benefits of enoxaparin and dalteparin, respectively, in reducing the risk of VTE. As shown in Table 2, thromboprophylaxis with these agents was associated with a 45% to 63% relative reduction in the risk of VTE compared with placebo.12, 13
| Trial | Number of Patients | Agent (vs. placebo) | Detection of VTE | Relative Risk Reduction | Number Needed to Treat |
|---|---|---|---|---|---|
| |||||
| MEDENOX | 866 | Enoxaparin 20 mg or 40 mg SC daily for 6‐14 days | Distal and proximal venographic DVT or documented PE | 63% (with 40 mg; 97.6 CI, 0.22‐0.63; P <0.001) | 10 |
| PREVENT | 3706 | Dalteparin 5000 IU SC daily for up to 14 days | CUS DVT, symptomatic VTE, and fatal PE | 45% (95% CI, 0.38‐0.80; P = 0.0015) | 45 |
| ARTEMIS | 849 | Fondaparinux 2.5 mg SC daily for 6‐14 days | Distal and proximal venographic DVT, symptomatic VTE, and fatal PE | 47% (95% CI, 0.077‐0.693) | 20 |
Pentasaccharides
Fondaparinux is a synthetic factor Xa antagonist that shares many features of LMWHs, including a high bioavailability and long half‐life. Fondaparinux does not require monitoring, but it is contraindicated in patients with renal failure (CrCl < 30 mL/minute) and in patients weighing less than 50 kg.1 Although PF4 antibodies have been associated with fondaparinux administration, this drug has not, to date, been associated with HIT.14 The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS) trial demonstrated the advantage of fondaparinux over placebo in reducing the risk of VTE (Table 2).15 The American College of Chest Physicians (ACCP) guidelines state that fondaparinux appears to be as effective and safe as LMWH.1
Vitamin K Antagonists
Vitamin K antagonists (VKAs) such as warfarin inhibit the production of prothrombin, clotting factors VII, IX, and X, and the anticoagulants protein C and protein S. Warfarin is challenging to manage because of its narrow therapeutic window, its tendency to exhibit considerable variability in dose‐response, the time required to reach target international normalized ratio (INR), its potential for interaction with diet and concomitant medications, and its need for ongoing monitoring.5 Warfarin should usually be initiated within the same 24 hours as parenteral anticoagulation, with a goal of achieving INR results between 2.0 and 3.0. An initial dose of 5 to 10 mg for the first 1 or 2 days is appropriate for most patients, and subsequent dosing should be based on INR response.5 Warfarin prophylaxis is primarily used in patients in the US undergoing orthopedic surgery, including total hip replacement and hip and knee arthroplasty.1
Future Anticoagulants
New oral agents have the potential to improve the management of patients who have a moderate to high risk of thromboembolic disease.
Rivaroxaban
This oral factor Xa inhibitor is showing promise in patients undergoing major orthopedic surgery. A prespecified pooled analysis was performed on data from the four Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism (RECORD) trials to evaluate the effect of rivaroxaban on the composite of symptomatic VTE (DVT or PE) and death, and bleeding. In the analysis, patients undergoing hip or knee arthroplasty had a VTE rate of 0.8% with rivaroxaban vs. 1.6% with enoxaparin, the current gold standard for surgical prophylaxis (P < 0.001). Bleeding rates were not significantly different between treatment arms (P = 0.376).16
Apixaban
This oral, direct, reversible factor Xa inhibitor is under evaluation for the prevention and treatment of VTE. In the Apixaban Prophylaxis in Patients Undergoing Total Knee Replacement Surgery (APROPOS) study of patients undergoing knee replacement, apixaban had a lower composite rate of DVT, PE, and all‐cause mortality when compared with enoxaparin or warfarin.17 In the ADVANCE‐1 study of patients undergoing knee surgery, however, apixaban failed to meet criteria for noninferiority when compared with enoxaparin.18 Apixaban is now being evaluated for VTE prophylaxis in acutely ill medical patients.
Dabigatran
This oral direct thrombin inhibitor reversibly binds to free and fibrin‐bound thrombin. In the RE‐NOVATE trial, dabigatran was noninferior to enoxaparin in reducing the events of DVT, PE, and all‐cause mortality following total hip replacement surgery.19 In a Phase II dose‐ranging trial in patients with atrial fibrillation (Prevention of Embolic and Thrombotic Events in Patients with Persistent [AFPETRO]), dabigatran with or without aspirin was as effective as warfarin in reducing embolic events.20 In the RE‐MODEL study, dabigatran was as effective as enoxaparin in preventing VTE and all‐cause mortality following knee replacement surgery, but failed to show equivalence to a higher dose of enoxaparin in the RE‐MOBILIZE trial.21, 22 It should be noted that in the RE‐MODEL study, enoxaparin was not administered at the dosage recommended by the U.S. Food and Drug Administration (FDA) for knee replacement surgery.
Mechanical Prophylaxis
Mechanical methods of thromboprophylaxis include graduated compression stockings (GCS), intermittent pneumatic compression (IPC) devices, and the venous foot pump (VFP). Mechanical approaches to thromboprophylaxis should be used primarily in patients who have a high risk of bleeding or as an adjunct to pharmacotherapeutic prophylaxis.1 The ACCP guidelines summarize the advantages and limitations of mechanical prophylaxis in patients at risk of developing VTE (Table 3).1
| Advantages | Limitations |
|---|---|
| |
| Does not increase the risk of bleeding | Not as intensively studied as pharmacologic thromboprophylaxis (fewer studies and smaller) |
| Can be used in patients who have a high risk of bleeding | No established standards for size, pressure, or physiologic features |
| Efficacy has been demonstrated in a number of patient groups | Many specific mechanical devices have never been assessed in any clinical trial |
| May enhance the effectiveness of anticoagulant thromboprophylaxis | Almost all mechanical thromboprophylaxis trials were unblinded and therefore have a potential for bias |
| May reduce leg swelling | Are less effective in high‐risk groups than anticoagulant thromboprophylaxis |
| Greater effect in reducing calf DVT than proximal DVT | |
| Effect on PE and death unknown | |
| May reduce or delay the use of more effective anticoagulant thromboprophylaxis | |
| Compliance by patients and staff is often poor | |
| Trials may overestimate the protection compared with routine use | |
| Cost associated with purchase, storage, dispensing, and cleaning of the devices, as well as ensuring optimal compliance | |
When properly fitted, GCS increase venous blood return through external pressure, thereby reducing venous stasis. IPC devices or sequential compression devices are usually applied over compression stockings. In addition to improving venous blood flow, these devices stimulate endogenous fibrinolysis. Compliance is often a problem in medical patients, who may not use the devices properly. Furthermore, for patients with severe vascular insufficiency (ankle brachial index <0.05), IPC may worsen vascular insufficiency and digital gangrene.
Inferior vena cava (IVC) filters are barrier devices that may benefit patients with major bleeding risk in the acute VTE setting by preventing PE. These devices, however, do not prevent DVT and may promote further venous stasis and clotting below the device. Importantly, patients with HIT should not have IVC filters placed due to a very high thrombogenic state that could lead to limb ischemia or cerulea phlegmasia dolens.23
Thromboprophylaxis in Medical Patients
Duration
Although major trials support the use of short‐term prophylaxistypically 6 to 14 daysin‐hospital for acutely ill medical patients, the optimal duration of thromboprophylaxis in these patients is unclear.24 The Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial is the first randomized trial to evaluate the potential benefits of extended prophylaxis in acutely ill medical patients. In this study, 5101 hospitalized patients with varying levels of reduced mobility due to cancer, ischemic stroke, heart failure, respiratory failure, infection, and other acute medical conditions received open‐label enoxaparin 40 mg daily for a mean duration of 10 days. Patients were then randomly assigned to additional therapy with enoxaparin or placebo for a mean duration of 28 additional days.25 Preliminary findings from this trial suggest that high‐risk medical patients can benefit from extended thromboprophylaxis following hospital discharge, with significantly reduced VTE events (RR reduction, 44%; P = 0.0011). The benefits of thromboprophylaxis were apparent during the extended treatment period and persistent through 90 days.26
Guideline Recommendations
Incorrect use of thromboprophylaxis does not stem from a lack of evidence‐based recommendations. Within the past year, the ACCP, the American Society of Clinical Oncology (ASCO), and the National Comprehensive Cancer Network (NCCN) have published updated guidelines for thromboprophylaxis in hospitalized patients at risk of VTE.1, 27, 28 The 2008 ACCP guidelines include more than 700 recommendations for VTE risk assessment and management, to be implemented by a variety of physicians, including pulmonologists, cardiologists, cardiothoracic surgeons, and critical care medicine specialists.1
The ACCP guidelines organize prophylaxis recommendations on the basis of patient risk (Table 4).1 Risk assessment remains relatively subjective, however, and validated risk assessment models are not yet widely available. The prudent approach is to consider thromboprophylaxis for all hospitalized medically ill patients who do not have a specific contraindication.
| Levels of Risk | Approximate DVT Risk Without Thromboprophylaxis (%) | Suggested Thromboprophylaxis Options |
|---|---|---|
| ||
| Low risk | ||
| Minor surgery in mobile patients | < 10 | No specific thromboprophylaxis; early and aggressive ambulation |
| Medical patients who are fully mobile | ||
| Moderate risk | ||
| Most general, open gynecologic, or urologic surgery patients | 10‐40 | LMWH (at recommended doses), low‐dose UFH bid or tid, fondaparinux |
| Medical patients, bed rest or sick | ||
| Moderate VTE risk plus high bleeding risk | Mechanical thromboprophylaxis | |
| High risk | ||
| Hip or knee arthroplasty, HFS | 40‐80 | LMWH (at recommended doses), fondaparinux, oral vitamin K antagonist (INR 2‐3) |
| Major trauma, SCI | ||
| High VTE risk plus high bleeding risk | Mechanical thromboprophylaxis | |
Key evidence‐based recommendations regarding thromboprophylaxis for hospitalized, acutely ill patients include the following:1
-
Every hospital should develop a formal strategy to addresses VTE prophylaxis;
-
Aspirin alone is not recommended to prevent VTE for any patient group;
-
Mechanical methods of thromboprophylaxis should be used primarily for patients who have a high bleeding risk or possibly as an adjunct to anticoagulant thromboprophylaxis;
-
Thromboprophylaxis with LMWH, UFH, or fondaparinux is recommended for patients admitted to hospital with an acute medical illness (Note: fondaparinux is recommended, but not FDA‐approved, for this indication in the United States);
-
On admission to the ICU, all patients should be assessed for risk of VTE, and most should receive thromboprophylaxis;
-
All major trauma and all spinal‐cord injury patients should receive thromboprophylaxis.
Thromboprophylaxis in Surgical Patients
For hospitalized surgical patients, the ACCP guidelines indicate the importance of the type of surgery (eg, gynecologic, urologic, or neurologic) in determining the appropriate prophylaxis strategy. In general, routine thromboprophylaxis is recommended for patients undergoing major general, gynecologic, or orthopedic surgery, as well as bariatric and coronary artery bypass surgery.1 Some specific recommendations regarding thromboprophylaxis for surgical patients include the following:
-
Major general surgery: LMWH, low‐dose UFH, or fondaparinux;
-
Major gynecologic surgery and major open urologic procedures: LMWH, low‐dose UFH, fondaparinux, and/or a mechanical device;
-
Elective hip or knee arthroplasty: Anticoagulant therapy (LMWH, fondaparinux, or a VKA);
-
Hip‐fracture surgery: Fondaparinux, LMWH, a VKA, or low‐dose UFH;
-
Patients undergoing hip or knee arthroplasty or hip‐fracture surgery should receive thromboprophylaxis for a minimum of 10 days; for hip arthroplasty and hip‐fracture surgery, thromboprophylaxis should continue for more than 10 days and up to 35 days.
Although the ACCP guidelines recommend against aspirin monotherapy for any patient group, the American Academy of Orthopaedic Surgeons (AAOS) guidelines state that aspirin alone is an effective option in preventing VTE in standard‐risk patients who are undergoing hip or knee replacement surgery.29 However, evidence for aspirin monotherapy is currently limited.1
The 2008 ACCP guidelines include a new chapter on the perioperative management of patients receiving long‐term antithrombotic treatment who must undergo surgery or other invasive procedures. To minimize surgical bleeding, the ACCP recommends the temporary discontinuation of antithrombotic treatment immediately before and during surgery for most patients. Discontinuing antithrombotics can increase the risk of a thromboembolic event, but this risk must be weighed against the risk of bleeding.1 The guidelines also offer specific recommendations for the use of perioperative bridging therapy in patients receiving VKAs based on the risk of VTE and whether the patient has a mechanical heart valve or atrial fibrillation. Guidelines recommend discontinuing bridging anticoagulation 24 hours prior to surgery if therapeutic subcutaneous LMWH is the agent used and approximately 4 hours prior to surgery if intravenous UFH is the agent used.
Thromboprophylaxis in Special Populations
Care must be taken when using thromboprophylaxis in certain high‐risk populations. The following section provides recommendations regarding prophylaxis in the presence of cancer, pregnancy, renal insufficiency, and epidural anesthesia.
Cancer Patients
The ASCO and NCCN guidelines endorse the use of VTE prophylaxis with anticoagulants in all hospitalized patients with active cancer or suspicion of cancer in the absence of contraindications.27, 28 The ACCP guidelines restrict this recommendation to hospitalized cancer patients who are bedridden.1 Thromboprophylaxis should continue at least through the duration of the hospital stay. Acceptable subcutaneous regimens include fondaparinux, dalteparin, or enoxaparin at the doses presented in Table 1; if UFH is chosen, the dose should be 5000 units every 8 hours.
Cancer patients who are scheduled to undergo major surgery require a different prophylaxis strategy. Even with prophylaxis, cancer patients have a 2‐fold higher risk of postoperative VTE compared with noncancer patients and more than a 3‐fold higher risk of fatal PE.30 To manage this risk, the ASCO, NCCN, and ACCP guidelines recommend extended prophylaxis in patients undergoing major cancer surgery.1, 27, 28 Specific recommendations include the following:
-
All patients undergoing major surgical intervention for malignant disease should be considered for VTE prophylaxis with anticoagulants, with or without mechanical prophylaxis;
-
Thromboprophylaxis should be initiated prior to the start of surgery or as early as possible following surgery;
-
Mechanical interventions may supplement pharmacologic prophylaxis, especially in patients who have the highest risk;
-
Prophylaxis with a LMWH should be initiated 12 to 24 hours after the surgical procedure;
-
Continue prophylaxis at least 7 to 10 days postoperatively;
-
Consider prolonged prophylaxis (ie, up to 4 weeks) with a LMWH for high‐risk patients (eg, patients undergoing major abdominal or pelvic surgery, those with residual malignant disease after surgery, obese patients, and patients with a history of VTE).
Routine prophylaxis with anticoagulants is not recommended for most outpatients, except for those with high‐risk factors (eg, thrombogenic chemotherapy or a central venous catheter). The strategy of restricting thromboprophylaxis to cancer outpatients with specific indications, however, may miss an opportunity to reduce VTE in this vulnerable patient population. In the PROTECHT study, 1166 ambulatory cancer patients were randomly assigned to placebo or the LMWH nadroparin for the duration of their chemotherapy. Treatment with nadroparin reduced the rate of clinical thrombosis by 47.2% compared with placebo (3.9% vs. 2.1%; P = 0.033). The risk reduction was consistent across all measured events, including DVT, PE, stroke, and visceral venous thrombosis.31
Pregnancy
Prophylaxis should be considered in pregnant women with known risk factors for VTE such as prior VTE, thrombophilia, and a history of prolonged immobility. In addition, women with a moderate to high risk of VTE associated with a cesarean section should be considered for postpartum thromboprophylaxis. For example, 1 of the following regimens may be appropriate for high‐risk women following a cesarean section:32
-
UFH 5000 units subcutaneously every 12 hours until fully mobile;
-
LMWH subcutaneously once daily for 5 days (such as enoxaparin 20 mg daily).
For pregnant women already receiving anticoagulant prophylaxis (eg, for hypercoagulable state, structural heart disease, or prior DVT/PE), ACCP guidelines recommend discontinuing VKAs before 6 weeks of fetal gestation to minimize the risk of birth defects and miscarriage. In general, a LMWH should be substituted for VKAs as soon as pregnancy is confirmed or prior to conception in preparation for pregnancy, as VKAs cross the placental barrier, but LMWH and UFH do not.1, 33
Renal Insufficiency
The ACCP guidelines recommend that renal function be considered when making decisions about the use and/or dose of LMWHs and fondaparinux. Because these agents are eliminated primarily via renal clearance, changes in renal function can reduce drug clearance, prolong the half‐life, and increase plasma concentrations. Consequently, the risk of treatment‐related bleeding complications is elevated in patients with renal impairment.1 Depending on the circumstances, one of the following options should be considered1:
-
Avoid using an anticoagulant that bioaccumulates in the presence of renal impairment;
-
Use a lower dose of the agent;
-
Monitor the drug level or its anticoagulant effect.
In severe renal impairment (creatinine clearance < 30 mL/minute):710
-
The prophylactic dose of enoxaparin should be adjusted to 30 mg subcutaneously once daily; no specific dosing adjustments have been recommended for dalteparin or tinzaparin;
-
Fondaparinux is contraindicated.
Epidural Anesthesia
Neuraxial blockade has several advantages over systemic opioids, but the risk of spinal or epidural hematoma may be increased with the concomitant use of antithrombotic drugs. Therefore, these agents must be used cautiously in patients with neuraxial blockade.1 Guidelines from the American Society of Regional Anesthesia and Pain Medicine (ASRA) contain the following recommendations:34
-
Subcutaneous UFH: No contraindication, consider delaying heparin until after block if technical difficulty is anticipated;
-
LMWH: Since twice daily dosing may be associated with an increased risk of spinal hematoma, delay initiation of LMWH until at least 24 hours after surgery, regardless of anesthetic technique; for single daily dosing, administer the first dose of LMWH 6 to 8 hours postoperatively and second dose no sooner than 24 hours after the first dose;
-
Warfarin: Document normal INR after discontinuation (prior to neuraxial technique); remove catheter when INR 1.5 (initiation of therapy).
Complications of Thromboprophylaxis
Before initiating thromboprophylaxis, it is important to evaluate the risk of bleeding, and patients should be assessed for contraindications that could increase that risk. HIT should also be considered.
Bleeding Risk
The ACCP and ASCO guidelines emphasize the importance of weighing the potential benefits of thromboprophylaxis against the potential risks of bleeding in individual patients. According to the ACCP, the overall risk of bleeding with intravenous UFH in patients with VTE is less than 3%, and thromboprophylaxis has not been shown to increase the risk of bleeding compared with placebo in major clinical trials.13, 15, 35 However, bleeding risk may increase in older patients and with higher doses of heparin. Warfarin therapy can be monitored with an INR to reduce the risk of bleeding during thromboprophylaxis.1
Anticoagulation therapy may be contraindicated in patients with certain factors and conditions that increase the risk of bleeding. These include:
-
Clinically significant active or chronic bleeding;
-
Recent central nervous system or spinal surgery with increased risk of bleeding;
-
Thrombocytopenia (excluding HIT) or severe platelet dysfunction;
-
Abnormalities associated with clotting factors.
The NCCN provides specific contraindications to anticoagulation therapy for the prevention and treatment of VTE in cancer patients.28 These include:
-
Recent central nervous system bleed; intracranial, or spinal lesions at high risk of bleeding;
-
Active major bleeding (> 2 units transfused in 24 hours);
-
Chronic, clinically significant measurable bleeding for more than 48 hours;
-
Thrombocytopenia (platelets < 50,000/L);
-
Severe platelet dysfunction;
-
Recent major operation with high risk of bleeding;
-
Underlying coagulopathy (eg, clotting factor abnormalities or elevated prothrombin time or activated partial thromboplastin time [aPTT]);
-
Spinal anesthesia or lumbar puncture;
-
High risk of falls.
HIT
HIT is a serious complication that can occur as a result of exposure to heparin. It is an immune response that causes platelet activation and platelet aggregation, among other effects, and is capable of leading to severe thrombosis, amputation, or death.36 The incidence of HIT varies with subpopulations of patients and more commonly develops in patients receiving heparin in therapeutic doses. Early diagnosis (through an interpretation of clinical and laboratory information) is important to improve clinical outcomes, but difficult to achieve.36 The ACCP guidelines note that enzyme‐linked immunosorbent assay (ELISA)‐based tests for HIT are often falsely positive after surgery. As an alternative, serotonin‐release tests are more specific, although they are not as widely available.1
Substantial clinical evidence suggests that LMWH poses less of a risk of HIT than UFH. Martel et al,37 for example, conducted a meta‐analysis of 15 randomized and nonrandomized controlled trials (a total of 7287 patients) that included studies that compared prophylactic doses of UFH and LMWH and assessed postoperative or medical inpatients who received prophylaxis. The analysis revealed that the risk of HIT was 2.6% following UFH use compared with 0.2% following LMWH use.37 Despite the inclusion of UFH in the ASCO guidelines, ASCO acknowledges that a lower risk of HIT is one of the potential advantages of LMWH over UFH in cancer surgery prophylaxis.27 In addition, the recommendation to transition to outpatient therapy as soon as possible is an indirect way of stating a preference for LMWH. For cancer patients with established VTE, the recommendation is more direct: LMWH is clearly preferred over UFH for both initial and continuing antithrombotic therapy.27
Conclusions
Thromboprophylaxis should be considered in all hospitalized patients who have a risk of VTE. Anticoagulants are the mainstays of prophylaxis, and recent clinical trials have clearly demonstrated the efficacy of LMWHs and fondaparinux in preventing VTE. Each class of anticoagulant carries a number of side effects and contraindications, and frequent patient evaluation and monitoring may be required. This is especially true in those with renal impairment, for whom UFH may be a logical choice. A number of organizations have released guidelines for VTE prophylaxis that provide specific recommendations regarding thromboprophylaxis in special patient populations and scenarios.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest.2008;133(6 suppl):381S–453S.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , , , , .Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- , , , , .Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta‐analysis.J Thromb Haemost.2008;6:405–414.
- , , , et al.Pharmacology and Management of the Vitamin K Antagonists. American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. 8th Edition.Chest.2008;133:160S–198S.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
- Fragmin prescribing information.Last updated April 2007.Woodcliff Lake, NJ:Eisai, Inc.;2007.
- Lovenox prescribing information.Last updated 2008.Bridgewater, NJ:sanofi‐aventis U.S.;2008.
- Innohep prescribing information.Last updated July 2000.Wilmington, DE:DuPont Pharma;2000.
- Arixtra prescribing information.Last updated October 2008.Research Triangle Park, NC:GlaxoSmithKline;2008.
- , , , et al.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10(9):632–642.
- , , , et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341(11):793–800.
- , , , et al.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- , , , et al.A pooled analysis of four pivotal studies of rivaroxaban for the prevention of venous thromboembolism after orthopaedic surgery: effect on symptomatic venous thromboembolism, death, and bleeding.Blood.2008;112:19–20.
- , , , , , .The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement.J Thromb Haemost.2007;5:2368–2375.
- Bristol‐Myers Squibb and Pfizer. Bristol‐Myers Squibb and Pfizer provide update on apixaban clinical development program [Press release]. August 26, 2008. Available at: http://www.pfizer.com. Accessed August2009.
- , , , et al.Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double blind, non‐inferiority trial.Lancet.2007;370:949–956.
- , , , et al.Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with non‐valvular atrial fibrillation (PETRO Study).Am J Cardiol.2007;100:1419–1426.
- , , , et al.Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE‐MODEL randomized trial.J Thromb Haemost.2007;5:2178–2185.
- , , , et al.Dabigatran etexilate versus enoxaparin in preventing venous thromboembolism following total knee arthroplasty.J Thromb Haemost.2007;5(suppl 2):Abstract O‐W‐051.
- , , , .Treatment and prevention of heparin‐induced thrombocytopenia: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th edition.Chest.2008;133:340–380.
- .Extended duration of thromboprophylaxis in acutely ill medical patients: optimizing therapy?J Thromb Haemost.2007;5:5–11.
- , , , et al.Extended‐duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study.J Thromb Thrombolysis.2006;22:31–38.
- , , , et al.Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. 2007 Congress of the International Society on Thrombosis and Hemostasis; July 7–13,2007; Geneva, Switzerland. Abstract O‐S‐001.
- , , , et al.American Society of Clinical Oncology Guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer.J Clin Oncol.2007;25:5490–5505.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice guidelines in oncology. V.1. 2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2006.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty. May 18, 2007. Available at http://www.aaos.org/research/guidelines/PE_guideline.pdf. Accessed August2009.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.A randomized double‐blind placebo‐controlled study on nadroparin for prophylaxis of thromboembolic events in cancer patients receiving chemotherapy: The PROTECHT Study.Blood.2008;112(11):Abstract 6.
- , , , et al.Prevention and treatment of venous thromboembolism (VTE) in obstetrics.J SOGC.2000;22(9):736–749.
- , , , et al.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Am Fam Med.2007;5:74–80.
- , , , et al.Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation).Reg Anesth Pain Med.2003;28(3):172–197.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , .The laboratory diagnosis and clinical management of patients with HIT: an update.Semin Thromb Hemost.2008;34(1):86–93.
- , , .Risk for heparin‐induced thrombocytopenia with unfractionated and low‐molecular‐weight heparin thromboprophylaxis: a meta‐analysis.Blood.2005;106(8):2710–2715.
Thromboprophylaxis with anticoagulants has proven benefits in hospitalized patients. Despite this, venous thromboembolism (VTE) prophylaxis is underused and VTE remains the leading cause of preventable hospital mortality.1 Medical patients have a particularly high risk; those who develop a deep vein thrombosis (DVT) are significantly less likely to have received prophylaxis prior to the diagnosis of DVT than nonmedical patients. Even within the high‐risk setting of the intensive care unit (ICU), medical patients receive thromboprophylaxis only two‐thirds as often as nonmedical patients.2
In this article we summarize the evidence concerning the various prophylaxis options, including current guideline recommendations for VTE prevention in medical and surgical patients. We also discuss strategies for thromboprophylaxis in special populations and potential complications of prophylaxis.
Efficacy of Prophylaxis in Medical Patients
Several meta‐analyses have demonstrated the marked benefits of anticoagulant prophylaxis in medical patients. Dentali et al3 conducted a meta‐analysis of 9 randomized controlled trials enrolling a total of 19,958 at‐risk hospitalized medical patients. The selected trials compared standard anticoagulant regimens with no treatment and only included studies with objectively documented and independently adjudicated outcomes. Compared with patients receiving placebo, those receiving thromboprophylaxis had significant reductions in any PE by 57% (95% CI, 0.26‐0.71; absolute risk reduction, 0.29%) and fatal pulmonary embolism (PE) by 62% (95% CI, 0.21‐0.69; absolute risk reduction, 0.25%), with a nonsignificant reduction in symptomatic DVT (relative risk [RR], 0.47; 95% CI, 0.22‐1.00) and a nonsignificant increase in major bleeding (RR, 1.32; 95% CI, 0.73‐2.37). The researchers concluded that anticoagulant prophylaxis is effective in preventing symptomatic VTE in medical patients, though the optimal duration of therapy is not yet defined.3
Another meta‐analysis focusing on subclinical DVT in acutely ill medical patients examined the therapeutic effects of various prophylaxis regimens. Overall, anticoagulant prophylaxis reduced the risk of any asymptomatic DVT (assessed by venogram or ultrasound) by 49% (95% CI, 0.39‐0.67) and asymptomatic proximal DVT by 55% (95% CI, 0.31‐0.65) compared with placebo (absolute risk reduction, 2.6% and 1.8%, respectively). Although prophylaxis was associated with a 0.5% absolute risk increase in major bleeding, the authors concluded that the benefits of prophylaxis outweighed the risks of bleeding.4
Anticoagulant Agents in the Prevention of VTE
Currently available anticoagulants for the prevention of VTE include unfractionated heparin (UFH), low‐molecular‐weight heparins (LMWHs), fondaparinux, and warfarin. These agents interrupt thrombus formation, either indirectly (through interaction with antithrombin) or directly (by inhibiting the action of thrombin). Each class of therapy has advantages and limitations. Table 1 lists common anticoagulant options for VTE prophylaxis, along with dosing information and other important information.510
| Prophylactic Dose | Warnings/Contraindications/Adverse Reactions | |
|---|---|---|
| ||
| Warfarin | 5 to 10 mg daily initially;* adjust dose based on INR; therapeutic INR goal: 2.5 (2‐3) | Warning: bleeding risk; requires frequent monitoring; contraindicated in patients for whom hazard of hemorrhage outweighs potential benefit (eg, in pregnant women) |
| UFH | 5000 IU every 8‐12 hours subcutaneously | Contraindicated in the presence of active bleeding, uncontrolled hypertension, or severe thrombocytopenia; monitor platelet count every 4‐7 days for HIT |
| Dalteparin | 5000 IU daily subcutaneously | Warning: spinal/epidural hematoma; monitor for signs and symptoms of neurological impairment. LMWHs should be used with caution in renal impairment; anti‐Xa monitoring and dose adjustments may be required. Follow prescribing information for dose adjustments and body weight‐based dosing. Most common adverse reactions: bleeding, anemia, thrombocytopenia, elevation of serum aminotransferase, diarrhea, and nausea |
| Enoxaparin | 40 mg daily subcutaneously; reduce to 30 mg daily in renal impairment | |
| Tinzaparin | 3500 IU daily subcutaneously | |
| Fondaparinux | 2.5 mg daily subcutaneously | Warning: spinal/epidural hematoma; monitor for signs and symptoms of neurological impairment; contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/minute) and in patients < 50 kg |
UFH
UFH, which is typically administered by subcutaneous injection, has the longest history as an anticoagulant in the prevention and treatment of VTE. It is an attractive option in patients with severe renal failure or those who may require a procedure in the near future. Although UFH is partially cleared by the kidney, its short half‐life can be perceived as a safety advantage in patients with severe renal impairment and an increased risk of bleeding. For most other patients, UFH holds several disadvantages compared with newer therapies, including the need for injections to be administered 3 times a day to be optimally effective, its effect on platelets, and its association with heparin‐induced thrombocytopenia (HIT).1 Given the costs of administration and potential complications, it is not less expensive than LMWHs, and it appears to be less cost‐effective.11
LMWHs
LMWHs have a higher bioavailability and longer half‐life than UFH, which translates to reliable anticoagulation levels when given subcutaneously on a weight‐based dosing schedule. Unlike UFH, LMWHs do not require laboratory tests to monitor the intensity of anticoagulation, except in special circumstances.1 The LMWHs dalteparin, enoxaparin, and tinzaparin are widely used for the prevention and treatment of VTE in the United States.
Two landmark clinical trials demonstrated the efficacy of appropriate thromboprophylaxis with LMWHs in reducing the burden of VTE in acutely ill, hospitalized medical patients. The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial and Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT) demonstrated the benefits of enoxaparin and dalteparin, respectively, in reducing the risk of VTE. As shown in Table 2, thromboprophylaxis with these agents was associated with a 45% to 63% relative reduction in the risk of VTE compared with placebo.12, 13
| Trial | Number of Patients | Agent (vs. placebo) | Detection of VTE | Relative Risk Reduction | Number Needed to Treat |
|---|---|---|---|---|---|
| |||||
| MEDENOX | 866 | Enoxaparin 20 mg or 40 mg SC daily for 6‐14 days | Distal and proximal venographic DVT or documented PE | 63% (with 40 mg; 97.6 CI, 0.22‐0.63; P <0.001) | 10 |
| PREVENT | 3706 | Dalteparin 5000 IU SC daily for up to 14 days | CUS DVT, symptomatic VTE, and fatal PE | 45% (95% CI, 0.38‐0.80; P = 0.0015) | 45 |
| ARTEMIS | 849 | Fondaparinux 2.5 mg SC daily for 6‐14 days | Distal and proximal venographic DVT, symptomatic VTE, and fatal PE | 47% (95% CI, 0.077‐0.693) | 20 |
Pentasaccharides
Fondaparinux is a synthetic factor Xa antagonist that shares many features of LMWHs, including a high bioavailability and long half‐life. Fondaparinux does not require monitoring, but it is contraindicated in patients with renal failure (CrCl < 30 mL/minute) and in patients weighing less than 50 kg.1 Although PF4 antibodies have been associated with fondaparinux administration, this drug has not, to date, been associated with HIT.14 The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS) trial demonstrated the advantage of fondaparinux over placebo in reducing the risk of VTE (Table 2).15 The American College of Chest Physicians (ACCP) guidelines state that fondaparinux appears to be as effective and safe as LMWH.1
Vitamin K Antagonists
Vitamin K antagonists (VKAs) such as warfarin inhibit the production of prothrombin, clotting factors VII, IX, and X, and the anticoagulants protein C and protein S. Warfarin is challenging to manage because of its narrow therapeutic window, its tendency to exhibit considerable variability in dose‐response, the time required to reach target international normalized ratio (INR), its potential for interaction with diet and concomitant medications, and its need for ongoing monitoring.5 Warfarin should usually be initiated within the same 24 hours as parenteral anticoagulation, with a goal of achieving INR results between 2.0 and 3.0. An initial dose of 5 to 10 mg for the first 1 or 2 days is appropriate for most patients, and subsequent dosing should be based on INR response.5 Warfarin prophylaxis is primarily used in patients in the US undergoing orthopedic surgery, including total hip replacement and hip and knee arthroplasty.1
Future Anticoagulants
New oral agents have the potential to improve the management of patients who have a moderate to high risk of thromboembolic disease.
Rivaroxaban
This oral factor Xa inhibitor is showing promise in patients undergoing major orthopedic surgery. A prespecified pooled analysis was performed on data from the four Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism (RECORD) trials to evaluate the effect of rivaroxaban on the composite of symptomatic VTE (DVT or PE) and death, and bleeding. In the analysis, patients undergoing hip or knee arthroplasty had a VTE rate of 0.8% with rivaroxaban vs. 1.6% with enoxaparin, the current gold standard for surgical prophylaxis (P < 0.001). Bleeding rates were not significantly different between treatment arms (P = 0.376).16
Apixaban
This oral, direct, reversible factor Xa inhibitor is under evaluation for the prevention and treatment of VTE. In the Apixaban Prophylaxis in Patients Undergoing Total Knee Replacement Surgery (APROPOS) study of patients undergoing knee replacement, apixaban had a lower composite rate of DVT, PE, and all‐cause mortality when compared with enoxaparin or warfarin.17 In the ADVANCE‐1 study of patients undergoing knee surgery, however, apixaban failed to meet criteria for noninferiority when compared with enoxaparin.18 Apixaban is now being evaluated for VTE prophylaxis in acutely ill medical patients.
Dabigatran
This oral direct thrombin inhibitor reversibly binds to free and fibrin‐bound thrombin. In the RE‐NOVATE trial, dabigatran was noninferior to enoxaparin in reducing the events of DVT, PE, and all‐cause mortality following total hip replacement surgery.19 In a Phase II dose‐ranging trial in patients with atrial fibrillation (Prevention of Embolic and Thrombotic Events in Patients with Persistent [AFPETRO]), dabigatran with or without aspirin was as effective as warfarin in reducing embolic events.20 In the RE‐MODEL study, dabigatran was as effective as enoxaparin in preventing VTE and all‐cause mortality following knee replacement surgery, but failed to show equivalence to a higher dose of enoxaparin in the RE‐MOBILIZE trial.21, 22 It should be noted that in the RE‐MODEL study, enoxaparin was not administered at the dosage recommended by the U.S. Food and Drug Administration (FDA) for knee replacement surgery.
Mechanical Prophylaxis
Mechanical methods of thromboprophylaxis include graduated compression stockings (GCS), intermittent pneumatic compression (IPC) devices, and the venous foot pump (VFP). Mechanical approaches to thromboprophylaxis should be used primarily in patients who have a high risk of bleeding or as an adjunct to pharmacotherapeutic prophylaxis.1 The ACCP guidelines summarize the advantages and limitations of mechanical prophylaxis in patients at risk of developing VTE (Table 3).1
| Advantages | Limitations |
|---|---|
| |
| Does not increase the risk of bleeding | Not as intensively studied as pharmacologic thromboprophylaxis (fewer studies and smaller) |
| Can be used in patients who have a high risk of bleeding | No established standards for size, pressure, or physiologic features |
| Efficacy has been demonstrated in a number of patient groups | Many specific mechanical devices have never been assessed in any clinical trial |
| May enhance the effectiveness of anticoagulant thromboprophylaxis | Almost all mechanical thromboprophylaxis trials were unblinded and therefore have a potential for bias |
| May reduce leg swelling | Are less effective in high‐risk groups than anticoagulant thromboprophylaxis |
| Greater effect in reducing calf DVT than proximal DVT | |
| Effect on PE and death unknown | |
| May reduce or delay the use of more effective anticoagulant thromboprophylaxis | |
| Compliance by patients and staff is often poor | |
| Trials may overestimate the protection compared with routine use | |
| Cost associated with purchase, storage, dispensing, and cleaning of the devices, as well as ensuring optimal compliance | |
When properly fitted, GCS increase venous blood return through external pressure, thereby reducing venous stasis. IPC devices or sequential compression devices are usually applied over compression stockings. In addition to improving venous blood flow, these devices stimulate endogenous fibrinolysis. Compliance is often a problem in medical patients, who may not use the devices properly. Furthermore, for patients with severe vascular insufficiency (ankle brachial index <0.05), IPC may worsen vascular insufficiency and digital gangrene.
Inferior vena cava (IVC) filters are barrier devices that may benefit patients with major bleeding risk in the acute VTE setting by preventing PE. These devices, however, do not prevent DVT and may promote further venous stasis and clotting below the device. Importantly, patients with HIT should not have IVC filters placed due to a very high thrombogenic state that could lead to limb ischemia or cerulea phlegmasia dolens.23
Thromboprophylaxis in Medical Patients
Duration
Although major trials support the use of short‐term prophylaxistypically 6 to 14 daysin‐hospital for acutely ill medical patients, the optimal duration of thromboprophylaxis in these patients is unclear.24 The Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial is the first randomized trial to evaluate the potential benefits of extended prophylaxis in acutely ill medical patients. In this study, 5101 hospitalized patients with varying levels of reduced mobility due to cancer, ischemic stroke, heart failure, respiratory failure, infection, and other acute medical conditions received open‐label enoxaparin 40 mg daily for a mean duration of 10 days. Patients were then randomly assigned to additional therapy with enoxaparin or placebo for a mean duration of 28 additional days.25 Preliminary findings from this trial suggest that high‐risk medical patients can benefit from extended thromboprophylaxis following hospital discharge, with significantly reduced VTE events (RR reduction, 44%; P = 0.0011). The benefits of thromboprophylaxis were apparent during the extended treatment period and persistent through 90 days.26
Guideline Recommendations
Incorrect use of thromboprophylaxis does not stem from a lack of evidence‐based recommendations. Within the past year, the ACCP, the American Society of Clinical Oncology (ASCO), and the National Comprehensive Cancer Network (NCCN) have published updated guidelines for thromboprophylaxis in hospitalized patients at risk of VTE.1, 27, 28 The 2008 ACCP guidelines include more than 700 recommendations for VTE risk assessment and management, to be implemented by a variety of physicians, including pulmonologists, cardiologists, cardiothoracic surgeons, and critical care medicine specialists.1
The ACCP guidelines organize prophylaxis recommendations on the basis of patient risk (Table 4).1 Risk assessment remains relatively subjective, however, and validated risk assessment models are not yet widely available. The prudent approach is to consider thromboprophylaxis for all hospitalized medically ill patients who do not have a specific contraindication.
| Levels of Risk | Approximate DVT Risk Without Thromboprophylaxis (%) | Suggested Thromboprophylaxis Options |
|---|---|---|
| ||
| Low risk | ||
| Minor surgery in mobile patients | < 10 | No specific thromboprophylaxis; early and aggressive ambulation |
| Medical patients who are fully mobile | ||
| Moderate risk | ||
| Most general, open gynecologic, or urologic surgery patients | 10‐40 | LMWH (at recommended doses), low‐dose UFH bid or tid, fondaparinux |
| Medical patients, bed rest or sick | ||
| Moderate VTE risk plus high bleeding risk | Mechanical thromboprophylaxis | |
| High risk | ||
| Hip or knee arthroplasty, HFS | 40‐80 | LMWH (at recommended doses), fondaparinux, oral vitamin K antagonist (INR 2‐3) |
| Major trauma, SCI | ||
| High VTE risk plus high bleeding risk | Mechanical thromboprophylaxis | |
Key evidence‐based recommendations regarding thromboprophylaxis for hospitalized, acutely ill patients include the following:1
-
Every hospital should develop a formal strategy to addresses VTE prophylaxis;
-
Aspirin alone is not recommended to prevent VTE for any patient group;
-
Mechanical methods of thromboprophylaxis should be used primarily for patients who have a high bleeding risk or possibly as an adjunct to anticoagulant thromboprophylaxis;
-
Thromboprophylaxis with LMWH, UFH, or fondaparinux is recommended for patients admitted to hospital with an acute medical illness (Note: fondaparinux is recommended, but not FDA‐approved, for this indication in the United States);
-
On admission to the ICU, all patients should be assessed for risk of VTE, and most should receive thromboprophylaxis;
-
All major trauma and all spinal‐cord injury patients should receive thromboprophylaxis.
Thromboprophylaxis in Surgical Patients
For hospitalized surgical patients, the ACCP guidelines indicate the importance of the type of surgery (eg, gynecologic, urologic, or neurologic) in determining the appropriate prophylaxis strategy. In general, routine thromboprophylaxis is recommended for patients undergoing major general, gynecologic, or orthopedic surgery, as well as bariatric and coronary artery bypass surgery.1 Some specific recommendations regarding thromboprophylaxis for surgical patients include the following:
-
Major general surgery: LMWH, low‐dose UFH, or fondaparinux;
-
Major gynecologic surgery and major open urologic procedures: LMWH, low‐dose UFH, fondaparinux, and/or a mechanical device;
-
Elective hip or knee arthroplasty: Anticoagulant therapy (LMWH, fondaparinux, or a VKA);
-
Hip‐fracture surgery: Fondaparinux, LMWH, a VKA, or low‐dose UFH;
-
Patients undergoing hip or knee arthroplasty or hip‐fracture surgery should receive thromboprophylaxis for a minimum of 10 days; for hip arthroplasty and hip‐fracture surgery, thromboprophylaxis should continue for more than 10 days and up to 35 days.
Although the ACCP guidelines recommend against aspirin monotherapy for any patient group, the American Academy of Orthopaedic Surgeons (AAOS) guidelines state that aspirin alone is an effective option in preventing VTE in standard‐risk patients who are undergoing hip or knee replacement surgery.29 However, evidence for aspirin monotherapy is currently limited.1
The 2008 ACCP guidelines include a new chapter on the perioperative management of patients receiving long‐term antithrombotic treatment who must undergo surgery or other invasive procedures. To minimize surgical bleeding, the ACCP recommends the temporary discontinuation of antithrombotic treatment immediately before and during surgery for most patients. Discontinuing antithrombotics can increase the risk of a thromboembolic event, but this risk must be weighed against the risk of bleeding.1 The guidelines also offer specific recommendations for the use of perioperative bridging therapy in patients receiving VKAs based on the risk of VTE and whether the patient has a mechanical heart valve or atrial fibrillation. Guidelines recommend discontinuing bridging anticoagulation 24 hours prior to surgery if therapeutic subcutaneous LMWH is the agent used and approximately 4 hours prior to surgery if intravenous UFH is the agent used.
Thromboprophylaxis in Special Populations
Care must be taken when using thromboprophylaxis in certain high‐risk populations. The following section provides recommendations regarding prophylaxis in the presence of cancer, pregnancy, renal insufficiency, and epidural anesthesia.
Cancer Patients
The ASCO and NCCN guidelines endorse the use of VTE prophylaxis with anticoagulants in all hospitalized patients with active cancer or suspicion of cancer in the absence of contraindications.27, 28 The ACCP guidelines restrict this recommendation to hospitalized cancer patients who are bedridden.1 Thromboprophylaxis should continue at least through the duration of the hospital stay. Acceptable subcutaneous regimens include fondaparinux, dalteparin, or enoxaparin at the doses presented in Table 1; if UFH is chosen, the dose should be 5000 units every 8 hours.
Cancer patients who are scheduled to undergo major surgery require a different prophylaxis strategy. Even with prophylaxis, cancer patients have a 2‐fold higher risk of postoperative VTE compared with noncancer patients and more than a 3‐fold higher risk of fatal PE.30 To manage this risk, the ASCO, NCCN, and ACCP guidelines recommend extended prophylaxis in patients undergoing major cancer surgery.1, 27, 28 Specific recommendations include the following:
-
All patients undergoing major surgical intervention for malignant disease should be considered for VTE prophylaxis with anticoagulants, with or without mechanical prophylaxis;
-
Thromboprophylaxis should be initiated prior to the start of surgery or as early as possible following surgery;
-
Mechanical interventions may supplement pharmacologic prophylaxis, especially in patients who have the highest risk;
-
Prophylaxis with a LMWH should be initiated 12 to 24 hours after the surgical procedure;
-
Continue prophylaxis at least 7 to 10 days postoperatively;
-
Consider prolonged prophylaxis (ie, up to 4 weeks) with a LMWH for high‐risk patients (eg, patients undergoing major abdominal or pelvic surgery, those with residual malignant disease after surgery, obese patients, and patients with a history of VTE).
Routine prophylaxis with anticoagulants is not recommended for most outpatients, except for those with high‐risk factors (eg, thrombogenic chemotherapy or a central venous catheter). The strategy of restricting thromboprophylaxis to cancer outpatients with specific indications, however, may miss an opportunity to reduce VTE in this vulnerable patient population. In the PROTECHT study, 1166 ambulatory cancer patients were randomly assigned to placebo or the LMWH nadroparin for the duration of their chemotherapy. Treatment with nadroparin reduced the rate of clinical thrombosis by 47.2% compared with placebo (3.9% vs. 2.1%; P = 0.033). The risk reduction was consistent across all measured events, including DVT, PE, stroke, and visceral venous thrombosis.31
Pregnancy
Prophylaxis should be considered in pregnant women with known risk factors for VTE such as prior VTE, thrombophilia, and a history of prolonged immobility. In addition, women with a moderate to high risk of VTE associated with a cesarean section should be considered for postpartum thromboprophylaxis. For example, 1 of the following regimens may be appropriate for high‐risk women following a cesarean section:32
-
UFH 5000 units subcutaneously every 12 hours until fully mobile;
-
LMWH subcutaneously once daily for 5 days (such as enoxaparin 20 mg daily).
For pregnant women already receiving anticoagulant prophylaxis (eg, for hypercoagulable state, structural heart disease, or prior DVT/PE), ACCP guidelines recommend discontinuing VKAs before 6 weeks of fetal gestation to minimize the risk of birth defects and miscarriage. In general, a LMWH should be substituted for VKAs as soon as pregnancy is confirmed or prior to conception in preparation for pregnancy, as VKAs cross the placental barrier, but LMWH and UFH do not.1, 33
Renal Insufficiency
The ACCP guidelines recommend that renal function be considered when making decisions about the use and/or dose of LMWHs and fondaparinux. Because these agents are eliminated primarily via renal clearance, changes in renal function can reduce drug clearance, prolong the half‐life, and increase plasma concentrations. Consequently, the risk of treatment‐related bleeding complications is elevated in patients with renal impairment.1 Depending on the circumstances, one of the following options should be considered1:
-
Avoid using an anticoagulant that bioaccumulates in the presence of renal impairment;
-
Use a lower dose of the agent;
-
Monitor the drug level or its anticoagulant effect.
In severe renal impairment (creatinine clearance < 30 mL/minute):710
-
The prophylactic dose of enoxaparin should be adjusted to 30 mg subcutaneously once daily; no specific dosing adjustments have been recommended for dalteparin or tinzaparin;
-
Fondaparinux is contraindicated.
Epidural Anesthesia
Neuraxial blockade has several advantages over systemic opioids, but the risk of spinal or epidural hematoma may be increased with the concomitant use of antithrombotic drugs. Therefore, these agents must be used cautiously in patients with neuraxial blockade.1 Guidelines from the American Society of Regional Anesthesia and Pain Medicine (ASRA) contain the following recommendations:34
-
Subcutaneous UFH: No contraindication, consider delaying heparin until after block if technical difficulty is anticipated;
-
LMWH: Since twice daily dosing may be associated with an increased risk of spinal hematoma, delay initiation of LMWH until at least 24 hours after surgery, regardless of anesthetic technique; for single daily dosing, administer the first dose of LMWH 6 to 8 hours postoperatively and second dose no sooner than 24 hours after the first dose;
-
Warfarin: Document normal INR after discontinuation (prior to neuraxial technique); remove catheter when INR 1.5 (initiation of therapy).
Complications of Thromboprophylaxis
Before initiating thromboprophylaxis, it is important to evaluate the risk of bleeding, and patients should be assessed for contraindications that could increase that risk. HIT should also be considered.
Bleeding Risk
The ACCP and ASCO guidelines emphasize the importance of weighing the potential benefits of thromboprophylaxis against the potential risks of bleeding in individual patients. According to the ACCP, the overall risk of bleeding with intravenous UFH in patients with VTE is less than 3%, and thromboprophylaxis has not been shown to increase the risk of bleeding compared with placebo in major clinical trials.13, 15, 35 However, bleeding risk may increase in older patients and with higher doses of heparin. Warfarin therapy can be monitored with an INR to reduce the risk of bleeding during thromboprophylaxis.1
Anticoagulation therapy may be contraindicated in patients with certain factors and conditions that increase the risk of bleeding. These include:
-
Clinically significant active or chronic bleeding;
-
Recent central nervous system or spinal surgery with increased risk of bleeding;
-
Thrombocytopenia (excluding HIT) or severe platelet dysfunction;
-
Abnormalities associated with clotting factors.
The NCCN provides specific contraindications to anticoagulation therapy for the prevention and treatment of VTE in cancer patients.28 These include:
-
Recent central nervous system bleed; intracranial, or spinal lesions at high risk of bleeding;
-
Active major bleeding (> 2 units transfused in 24 hours);
-
Chronic, clinically significant measurable bleeding for more than 48 hours;
-
Thrombocytopenia (platelets < 50,000/L);
-
Severe platelet dysfunction;
-
Recent major operation with high risk of bleeding;
-
Underlying coagulopathy (eg, clotting factor abnormalities or elevated prothrombin time or activated partial thromboplastin time [aPTT]);
-
Spinal anesthesia or lumbar puncture;
-
High risk of falls.
HIT
HIT is a serious complication that can occur as a result of exposure to heparin. It is an immune response that causes platelet activation and platelet aggregation, among other effects, and is capable of leading to severe thrombosis, amputation, or death.36 The incidence of HIT varies with subpopulations of patients and more commonly develops in patients receiving heparin in therapeutic doses. Early diagnosis (through an interpretation of clinical and laboratory information) is important to improve clinical outcomes, but difficult to achieve.36 The ACCP guidelines note that enzyme‐linked immunosorbent assay (ELISA)‐based tests for HIT are often falsely positive after surgery. As an alternative, serotonin‐release tests are more specific, although they are not as widely available.1
Substantial clinical evidence suggests that LMWH poses less of a risk of HIT than UFH. Martel et al,37 for example, conducted a meta‐analysis of 15 randomized and nonrandomized controlled trials (a total of 7287 patients) that included studies that compared prophylactic doses of UFH and LMWH and assessed postoperative or medical inpatients who received prophylaxis. The analysis revealed that the risk of HIT was 2.6% following UFH use compared with 0.2% following LMWH use.37 Despite the inclusion of UFH in the ASCO guidelines, ASCO acknowledges that a lower risk of HIT is one of the potential advantages of LMWH over UFH in cancer surgery prophylaxis.27 In addition, the recommendation to transition to outpatient therapy as soon as possible is an indirect way of stating a preference for LMWH. For cancer patients with established VTE, the recommendation is more direct: LMWH is clearly preferred over UFH for both initial and continuing antithrombotic therapy.27
Conclusions
Thromboprophylaxis should be considered in all hospitalized patients who have a risk of VTE. Anticoagulants are the mainstays of prophylaxis, and recent clinical trials have clearly demonstrated the efficacy of LMWHs and fondaparinux in preventing VTE. Each class of anticoagulant carries a number of side effects and contraindications, and frequent patient evaluation and monitoring may be required. This is especially true in those with renal impairment, for whom UFH may be a logical choice. A number of organizations have released guidelines for VTE prophylaxis that provide specific recommendations regarding thromboprophylaxis in special patient populations and scenarios.
Thromboprophylaxis with anticoagulants has proven benefits in hospitalized patients. Despite this, venous thromboembolism (VTE) prophylaxis is underused and VTE remains the leading cause of preventable hospital mortality.1 Medical patients have a particularly high risk; those who develop a deep vein thrombosis (DVT) are significantly less likely to have received prophylaxis prior to the diagnosis of DVT than nonmedical patients. Even within the high‐risk setting of the intensive care unit (ICU), medical patients receive thromboprophylaxis only two‐thirds as often as nonmedical patients.2
In this article we summarize the evidence concerning the various prophylaxis options, including current guideline recommendations for VTE prevention in medical and surgical patients. We also discuss strategies for thromboprophylaxis in special populations and potential complications of prophylaxis.
Efficacy of Prophylaxis in Medical Patients
Several meta‐analyses have demonstrated the marked benefits of anticoagulant prophylaxis in medical patients. Dentali et al3 conducted a meta‐analysis of 9 randomized controlled trials enrolling a total of 19,958 at‐risk hospitalized medical patients. The selected trials compared standard anticoagulant regimens with no treatment and only included studies with objectively documented and independently adjudicated outcomes. Compared with patients receiving placebo, those receiving thromboprophylaxis had significant reductions in any PE by 57% (95% CI, 0.26‐0.71; absolute risk reduction, 0.29%) and fatal pulmonary embolism (PE) by 62% (95% CI, 0.21‐0.69; absolute risk reduction, 0.25%), with a nonsignificant reduction in symptomatic DVT (relative risk [RR], 0.47; 95% CI, 0.22‐1.00) and a nonsignificant increase in major bleeding (RR, 1.32; 95% CI, 0.73‐2.37). The researchers concluded that anticoagulant prophylaxis is effective in preventing symptomatic VTE in medical patients, though the optimal duration of therapy is not yet defined.3
Another meta‐analysis focusing on subclinical DVT in acutely ill medical patients examined the therapeutic effects of various prophylaxis regimens. Overall, anticoagulant prophylaxis reduced the risk of any asymptomatic DVT (assessed by venogram or ultrasound) by 49% (95% CI, 0.39‐0.67) and asymptomatic proximal DVT by 55% (95% CI, 0.31‐0.65) compared with placebo (absolute risk reduction, 2.6% and 1.8%, respectively). Although prophylaxis was associated with a 0.5% absolute risk increase in major bleeding, the authors concluded that the benefits of prophylaxis outweighed the risks of bleeding.4
Anticoagulant Agents in the Prevention of VTE
Currently available anticoagulants for the prevention of VTE include unfractionated heparin (UFH), low‐molecular‐weight heparins (LMWHs), fondaparinux, and warfarin. These agents interrupt thrombus formation, either indirectly (through interaction with antithrombin) or directly (by inhibiting the action of thrombin). Each class of therapy has advantages and limitations. Table 1 lists common anticoagulant options for VTE prophylaxis, along with dosing information and other important information.510
| Prophylactic Dose | Warnings/Contraindications/Adverse Reactions | |
|---|---|---|
| ||
| Warfarin | 5 to 10 mg daily initially;* adjust dose based on INR; therapeutic INR goal: 2.5 (2‐3) | Warning: bleeding risk; requires frequent monitoring; contraindicated in patients for whom hazard of hemorrhage outweighs potential benefit (eg, in pregnant women) |
| UFH | 5000 IU every 8‐12 hours subcutaneously | Contraindicated in the presence of active bleeding, uncontrolled hypertension, or severe thrombocytopenia; monitor platelet count every 4‐7 days for HIT |
| Dalteparin | 5000 IU daily subcutaneously | Warning: spinal/epidural hematoma; monitor for signs and symptoms of neurological impairment. LMWHs should be used with caution in renal impairment; anti‐Xa monitoring and dose adjustments may be required. Follow prescribing information for dose adjustments and body weight‐based dosing. Most common adverse reactions: bleeding, anemia, thrombocytopenia, elevation of serum aminotransferase, diarrhea, and nausea |
| Enoxaparin | 40 mg daily subcutaneously; reduce to 30 mg daily in renal impairment | |
| Tinzaparin | 3500 IU daily subcutaneously | |
| Fondaparinux | 2.5 mg daily subcutaneously | Warning: spinal/epidural hematoma; monitor for signs and symptoms of neurological impairment; contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/minute) and in patients < 50 kg |
UFH
UFH, which is typically administered by subcutaneous injection, has the longest history as an anticoagulant in the prevention and treatment of VTE. It is an attractive option in patients with severe renal failure or those who may require a procedure in the near future. Although UFH is partially cleared by the kidney, its short half‐life can be perceived as a safety advantage in patients with severe renal impairment and an increased risk of bleeding. For most other patients, UFH holds several disadvantages compared with newer therapies, including the need for injections to be administered 3 times a day to be optimally effective, its effect on platelets, and its association with heparin‐induced thrombocytopenia (HIT).1 Given the costs of administration and potential complications, it is not less expensive than LMWHs, and it appears to be less cost‐effective.11
LMWHs
LMWHs have a higher bioavailability and longer half‐life than UFH, which translates to reliable anticoagulation levels when given subcutaneously on a weight‐based dosing schedule. Unlike UFH, LMWHs do not require laboratory tests to monitor the intensity of anticoagulation, except in special circumstances.1 The LMWHs dalteparin, enoxaparin, and tinzaparin are widely used for the prevention and treatment of VTE in the United States.
Two landmark clinical trials demonstrated the efficacy of appropriate thromboprophylaxis with LMWHs in reducing the burden of VTE in acutely ill, hospitalized medical patients. The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial and Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT) demonstrated the benefits of enoxaparin and dalteparin, respectively, in reducing the risk of VTE. As shown in Table 2, thromboprophylaxis with these agents was associated with a 45% to 63% relative reduction in the risk of VTE compared with placebo.12, 13
| Trial | Number of Patients | Agent (vs. placebo) | Detection of VTE | Relative Risk Reduction | Number Needed to Treat |
|---|---|---|---|---|---|
| |||||
| MEDENOX | 866 | Enoxaparin 20 mg or 40 mg SC daily for 6‐14 days | Distal and proximal venographic DVT or documented PE | 63% (with 40 mg; 97.6 CI, 0.22‐0.63; P <0.001) | 10 |
| PREVENT | 3706 | Dalteparin 5000 IU SC daily for up to 14 days | CUS DVT, symptomatic VTE, and fatal PE | 45% (95% CI, 0.38‐0.80; P = 0.0015) | 45 |
| ARTEMIS | 849 | Fondaparinux 2.5 mg SC daily for 6‐14 days | Distal and proximal venographic DVT, symptomatic VTE, and fatal PE | 47% (95% CI, 0.077‐0.693) | 20 |
Pentasaccharides
Fondaparinux is a synthetic factor Xa antagonist that shares many features of LMWHs, including a high bioavailability and long half‐life. Fondaparinux does not require monitoring, but it is contraindicated in patients with renal failure (CrCl < 30 mL/minute) and in patients weighing less than 50 kg.1 Although PF4 antibodies have been associated with fondaparinux administration, this drug has not, to date, been associated with HIT.14 The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS) trial demonstrated the advantage of fondaparinux over placebo in reducing the risk of VTE (Table 2).15 The American College of Chest Physicians (ACCP) guidelines state that fondaparinux appears to be as effective and safe as LMWH.1
Vitamin K Antagonists
Vitamin K antagonists (VKAs) such as warfarin inhibit the production of prothrombin, clotting factors VII, IX, and X, and the anticoagulants protein C and protein S. Warfarin is challenging to manage because of its narrow therapeutic window, its tendency to exhibit considerable variability in dose‐response, the time required to reach target international normalized ratio (INR), its potential for interaction with diet and concomitant medications, and its need for ongoing monitoring.5 Warfarin should usually be initiated within the same 24 hours as parenteral anticoagulation, with a goal of achieving INR results between 2.0 and 3.0. An initial dose of 5 to 10 mg for the first 1 or 2 days is appropriate for most patients, and subsequent dosing should be based on INR response.5 Warfarin prophylaxis is primarily used in patients in the US undergoing orthopedic surgery, including total hip replacement and hip and knee arthroplasty.1
Future Anticoagulants
New oral agents have the potential to improve the management of patients who have a moderate to high risk of thromboembolic disease.
Rivaroxaban
This oral factor Xa inhibitor is showing promise in patients undergoing major orthopedic surgery. A prespecified pooled analysis was performed on data from the four Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism (RECORD) trials to evaluate the effect of rivaroxaban on the composite of symptomatic VTE (DVT or PE) and death, and bleeding. In the analysis, patients undergoing hip or knee arthroplasty had a VTE rate of 0.8% with rivaroxaban vs. 1.6% with enoxaparin, the current gold standard for surgical prophylaxis (P < 0.001). Bleeding rates were not significantly different between treatment arms (P = 0.376).16
Apixaban
This oral, direct, reversible factor Xa inhibitor is under evaluation for the prevention and treatment of VTE. In the Apixaban Prophylaxis in Patients Undergoing Total Knee Replacement Surgery (APROPOS) study of patients undergoing knee replacement, apixaban had a lower composite rate of DVT, PE, and all‐cause mortality when compared with enoxaparin or warfarin.17 In the ADVANCE‐1 study of patients undergoing knee surgery, however, apixaban failed to meet criteria for noninferiority when compared with enoxaparin.18 Apixaban is now being evaluated for VTE prophylaxis in acutely ill medical patients.
Dabigatran
This oral direct thrombin inhibitor reversibly binds to free and fibrin‐bound thrombin. In the RE‐NOVATE trial, dabigatran was noninferior to enoxaparin in reducing the events of DVT, PE, and all‐cause mortality following total hip replacement surgery.19 In a Phase II dose‐ranging trial in patients with atrial fibrillation (Prevention of Embolic and Thrombotic Events in Patients with Persistent [AFPETRO]), dabigatran with or without aspirin was as effective as warfarin in reducing embolic events.20 In the RE‐MODEL study, dabigatran was as effective as enoxaparin in preventing VTE and all‐cause mortality following knee replacement surgery, but failed to show equivalence to a higher dose of enoxaparin in the RE‐MOBILIZE trial.21, 22 It should be noted that in the RE‐MODEL study, enoxaparin was not administered at the dosage recommended by the U.S. Food and Drug Administration (FDA) for knee replacement surgery.
Mechanical Prophylaxis
Mechanical methods of thromboprophylaxis include graduated compression stockings (GCS), intermittent pneumatic compression (IPC) devices, and the venous foot pump (VFP). Mechanical approaches to thromboprophylaxis should be used primarily in patients who have a high risk of bleeding or as an adjunct to pharmacotherapeutic prophylaxis.1 The ACCP guidelines summarize the advantages and limitations of mechanical prophylaxis in patients at risk of developing VTE (Table 3).1
| Advantages | Limitations |
|---|---|
| |
| Does not increase the risk of bleeding | Not as intensively studied as pharmacologic thromboprophylaxis (fewer studies and smaller) |
| Can be used in patients who have a high risk of bleeding | No established standards for size, pressure, or physiologic features |
| Efficacy has been demonstrated in a number of patient groups | Many specific mechanical devices have never been assessed in any clinical trial |
| May enhance the effectiveness of anticoagulant thromboprophylaxis | Almost all mechanical thromboprophylaxis trials were unblinded and therefore have a potential for bias |
| May reduce leg swelling | Are less effective in high‐risk groups than anticoagulant thromboprophylaxis |
| Greater effect in reducing calf DVT than proximal DVT | |
| Effect on PE and death unknown | |
| May reduce or delay the use of more effective anticoagulant thromboprophylaxis | |
| Compliance by patients and staff is often poor | |
| Trials may overestimate the protection compared with routine use | |
| Cost associated with purchase, storage, dispensing, and cleaning of the devices, as well as ensuring optimal compliance | |
When properly fitted, GCS increase venous blood return through external pressure, thereby reducing venous stasis. IPC devices or sequential compression devices are usually applied over compression stockings. In addition to improving venous blood flow, these devices stimulate endogenous fibrinolysis. Compliance is often a problem in medical patients, who may not use the devices properly. Furthermore, for patients with severe vascular insufficiency (ankle brachial index <0.05), IPC may worsen vascular insufficiency and digital gangrene.
Inferior vena cava (IVC) filters are barrier devices that may benefit patients with major bleeding risk in the acute VTE setting by preventing PE. These devices, however, do not prevent DVT and may promote further venous stasis and clotting below the device. Importantly, patients with HIT should not have IVC filters placed due to a very high thrombogenic state that could lead to limb ischemia or cerulea phlegmasia dolens.23
Thromboprophylaxis in Medical Patients
Duration
Although major trials support the use of short‐term prophylaxistypically 6 to 14 daysin‐hospital for acutely ill medical patients, the optimal duration of thromboprophylaxis in these patients is unclear.24 The Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial is the first randomized trial to evaluate the potential benefits of extended prophylaxis in acutely ill medical patients. In this study, 5101 hospitalized patients with varying levels of reduced mobility due to cancer, ischemic stroke, heart failure, respiratory failure, infection, and other acute medical conditions received open‐label enoxaparin 40 mg daily for a mean duration of 10 days. Patients were then randomly assigned to additional therapy with enoxaparin or placebo for a mean duration of 28 additional days.25 Preliminary findings from this trial suggest that high‐risk medical patients can benefit from extended thromboprophylaxis following hospital discharge, with significantly reduced VTE events (RR reduction, 44%; P = 0.0011). The benefits of thromboprophylaxis were apparent during the extended treatment period and persistent through 90 days.26
Guideline Recommendations
Incorrect use of thromboprophylaxis does not stem from a lack of evidence‐based recommendations. Within the past year, the ACCP, the American Society of Clinical Oncology (ASCO), and the National Comprehensive Cancer Network (NCCN) have published updated guidelines for thromboprophylaxis in hospitalized patients at risk of VTE.1, 27, 28 The 2008 ACCP guidelines include more than 700 recommendations for VTE risk assessment and management, to be implemented by a variety of physicians, including pulmonologists, cardiologists, cardiothoracic surgeons, and critical care medicine specialists.1
The ACCP guidelines organize prophylaxis recommendations on the basis of patient risk (Table 4).1 Risk assessment remains relatively subjective, however, and validated risk assessment models are not yet widely available. The prudent approach is to consider thromboprophylaxis for all hospitalized medically ill patients who do not have a specific contraindication.
| Levels of Risk | Approximate DVT Risk Without Thromboprophylaxis (%) | Suggested Thromboprophylaxis Options |
|---|---|---|
| ||
| Low risk | ||
| Minor surgery in mobile patients | < 10 | No specific thromboprophylaxis; early and aggressive ambulation |
| Medical patients who are fully mobile | ||
| Moderate risk | ||
| Most general, open gynecologic, or urologic surgery patients | 10‐40 | LMWH (at recommended doses), low‐dose UFH bid or tid, fondaparinux |
| Medical patients, bed rest or sick | ||
| Moderate VTE risk plus high bleeding risk | Mechanical thromboprophylaxis | |
| High risk | ||
| Hip or knee arthroplasty, HFS | 40‐80 | LMWH (at recommended doses), fondaparinux, oral vitamin K antagonist (INR 2‐3) |
| Major trauma, SCI | ||
| High VTE risk plus high bleeding risk | Mechanical thromboprophylaxis | |
Key evidence‐based recommendations regarding thromboprophylaxis for hospitalized, acutely ill patients include the following:1
-
Every hospital should develop a formal strategy to addresses VTE prophylaxis;
-
Aspirin alone is not recommended to prevent VTE for any patient group;
-
Mechanical methods of thromboprophylaxis should be used primarily for patients who have a high bleeding risk or possibly as an adjunct to anticoagulant thromboprophylaxis;
-
Thromboprophylaxis with LMWH, UFH, or fondaparinux is recommended for patients admitted to hospital with an acute medical illness (Note: fondaparinux is recommended, but not FDA‐approved, for this indication in the United States);
-
On admission to the ICU, all patients should be assessed for risk of VTE, and most should receive thromboprophylaxis;
-
All major trauma and all spinal‐cord injury patients should receive thromboprophylaxis.
Thromboprophylaxis in Surgical Patients
For hospitalized surgical patients, the ACCP guidelines indicate the importance of the type of surgery (eg, gynecologic, urologic, or neurologic) in determining the appropriate prophylaxis strategy. In general, routine thromboprophylaxis is recommended for patients undergoing major general, gynecologic, or orthopedic surgery, as well as bariatric and coronary artery bypass surgery.1 Some specific recommendations regarding thromboprophylaxis for surgical patients include the following:
-
Major general surgery: LMWH, low‐dose UFH, or fondaparinux;
-
Major gynecologic surgery and major open urologic procedures: LMWH, low‐dose UFH, fondaparinux, and/or a mechanical device;
-
Elective hip or knee arthroplasty: Anticoagulant therapy (LMWH, fondaparinux, or a VKA);
-
Hip‐fracture surgery: Fondaparinux, LMWH, a VKA, or low‐dose UFH;
-
Patients undergoing hip or knee arthroplasty or hip‐fracture surgery should receive thromboprophylaxis for a minimum of 10 days; for hip arthroplasty and hip‐fracture surgery, thromboprophylaxis should continue for more than 10 days and up to 35 days.
Although the ACCP guidelines recommend against aspirin monotherapy for any patient group, the American Academy of Orthopaedic Surgeons (AAOS) guidelines state that aspirin alone is an effective option in preventing VTE in standard‐risk patients who are undergoing hip or knee replacement surgery.29 However, evidence for aspirin monotherapy is currently limited.1
The 2008 ACCP guidelines include a new chapter on the perioperative management of patients receiving long‐term antithrombotic treatment who must undergo surgery or other invasive procedures. To minimize surgical bleeding, the ACCP recommends the temporary discontinuation of antithrombotic treatment immediately before and during surgery for most patients. Discontinuing antithrombotics can increase the risk of a thromboembolic event, but this risk must be weighed against the risk of bleeding.1 The guidelines also offer specific recommendations for the use of perioperative bridging therapy in patients receiving VKAs based on the risk of VTE and whether the patient has a mechanical heart valve or atrial fibrillation. Guidelines recommend discontinuing bridging anticoagulation 24 hours prior to surgery if therapeutic subcutaneous LMWH is the agent used and approximately 4 hours prior to surgery if intravenous UFH is the agent used.
Thromboprophylaxis in Special Populations
Care must be taken when using thromboprophylaxis in certain high‐risk populations. The following section provides recommendations regarding prophylaxis in the presence of cancer, pregnancy, renal insufficiency, and epidural anesthesia.
Cancer Patients
The ASCO and NCCN guidelines endorse the use of VTE prophylaxis with anticoagulants in all hospitalized patients with active cancer or suspicion of cancer in the absence of contraindications.27, 28 The ACCP guidelines restrict this recommendation to hospitalized cancer patients who are bedridden.1 Thromboprophylaxis should continue at least through the duration of the hospital stay. Acceptable subcutaneous regimens include fondaparinux, dalteparin, or enoxaparin at the doses presented in Table 1; if UFH is chosen, the dose should be 5000 units every 8 hours.
Cancer patients who are scheduled to undergo major surgery require a different prophylaxis strategy. Even with prophylaxis, cancer patients have a 2‐fold higher risk of postoperative VTE compared with noncancer patients and more than a 3‐fold higher risk of fatal PE.30 To manage this risk, the ASCO, NCCN, and ACCP guidelines recommend extended prophylaxis in patients undergoing major cancer surgery.1, 27, 28 Specific recommendations include the following:
-
All patients undergoing major surgical intervention for malignant disease should be considered for VTE prophylaxis with anticoagulants, with or without mechanical prophylaxis;
-
Thromboprophylaxis should be initiated prior to the start of surgery or as early as possible following surgery;
-
Mechanical interventions may supplement pharmacologic prophylaxis, especially in patients who have the highest risk;
-
Prophylaxis with a LMWH should be initiated 12 to 24 hours after the surgical procedure;
-
Continue prophylaxis at least 7 to 10 days postoperatively;
-
Consider prolonged prophylaxis (ie, up to 4 weeks) with a LMWH for high‐risk patients (eg, patients undergoing major abdominal or pelvic surgery, those with residual malignant disease after surgery, obese patients, and patients with a history of VTE).
Routine prophylaxis with anticoagulants is not recommended for most outpatients, except for those with high‐risk factors (eg, thrombogenic chemotherapy or a central venous catheter). The strategy of restricting thromboprophylaxis to cancer outpatients with specific indications, however, may miss an opportunity to reduce VTE in this vulnerable patient population. In the PROTECHT study, 1166 ambulatory cancer patients were randomly assigned to placebo or the LMWH nadroparin for the duration of their chemotherapy. Treatment with nadroparin reduced the rate of clinical thrombosis by 47.2% compared with placebo (3.9% vs. 2.1%; P = 0.033). The risk reduction was consistent across all measured events, including DVT, PE, stroke, and visceral venous thrombosis.31
Pregnancy
Prophylaxis should be considered in pregnant women with known risk factors for VTE such as prior VTE, thrombophilia, and a history of prolonged immobility. In addition, women with a moderate to high risk of VTE associated with a cesarean section should be considered for postpartum thromboprophylaxis. For example, 1 of the following regimens may be appropriate for high‐risk women following a cesarean section:32
-
UFH 5000 units subcutaneously every 12 hours until fully mobile;
-
LMWH subcutaneously once daily for 5 days (such as enoxaparin 20 mg daily).
For pregnant women already receiving anticoagulant prophylaxis (eg, for hypercoagulable state, structural heart disease, or prior DVT/PE), ACCP guidelines recommend discontinuing VKAs before 6 weeks of fetal gestation to minimize the risk of birth defects and miscarriage. In general, a LMWH should be substituted for VKAs as soon as pregnancy is confirmed or prior to conception in preparation for pregnancy, as VKAs cross the placental barrier, but LMWH and UFH do not.1, 33
Renal Insufficiency
The ACCP guidelines recommend that renal function be considered when making decisions about the use and/or dose of LMWHs and fondaparinux. Because these agents are eliminated primarily via renal clearance, changes in renal function can reduce drug clearance, prolong the half‐life, and increase plasma concentrations. Consequently, the risk of treatment‐related bleeding complications is elevated in patients with renal impairment.1 Depending on the circumstances, one of the following options should be considered1:
-
Avoid using an anticoagulant that bioaccumulates in the presence of renal impairment;
-
Use a lower dose of the agent;
-
Monitor the drug level or its anticoagulant effect.
In severe renal impairment (creatinine clearance < 30 mL/minute):710
-
The prophylactic dose of enoxaparin should be adjusted to 30 mg subcutaneously once daily; no specific dosing adjustments have been recommended for dalteparin or tinzaparin;
-
Fondaparinux is contraindicated.
Epidural Anesthesia
Neuraxial blockade has several advantages over systemic opioids, but the risk of spinal or epidural hematoma may be increased with the concomitant use of antithrombotic drugs. Therefore, these agents must be used cautiously in patients with neuraxial blockade.1 Guidelines from the American Society of Regional Anesthesia and Pain Medicine (ASRA) contain the following recommendations:34
-
Subcutaneous UFH: No contraindication, consider delaying heparin until after block if technical difficulty is anticipated;
-
LMWH: Since twice daily dosing may be associated with an increased risk of spinal hematoma, delay initiation of LMWH until at least 24 hours after surgery, regardless of anesthetic technique; for single daily dosing, administer the first dose of LMWH 6 to 8 hours postoperatively and second dose no sooner than 24 hours after the first dose;
-
Warfarin: Document normal INR after discontinuation (prior to neuraxial technique); remove catheter when INR 1.5 (initiation of therapy).
Complications of Thromboprophylaxis
Before initiating thromboprophylaxis, it is important to evaluate the risk of bleeding, and patients should be assessed for contraindications that could increase that risk. HIT should also be considered.
Bleeding Risk
The ACCP and ASCO guidelines emphasize the importance of weighing the potential benefits of thromboprophylaxis against the potential risks of bleeding in individual patients. According to the ACCP, the overall risk of bleeding with intravenous UFH in patients with VTE is less than 3%, and thromboprophylaxis has not been shown to increase the risk of bleeding compared with placebo in major clinical trials.13, 15, 35 However, bleeding risk may increase in older patients and with higher doses of heparin. Warfarin therapy can be monitored with an INR to reduce the risk of bleeding during thromboprophylaxis.1
Anticoagulation therapy may be contraindicated in patients with certain factors and conditions that increase the risk of bleeding. These include:
-
Clinically significant active or chronic bleeding;
-
Recent central nervous system or spinal surgery with increased risk of bleeding;
-
Thrombocytopenia (excluding HIT) or severe platelet dysfunction;
-
Abnormalities associated with clotting factors.
The NCCN provides specific contraindications to anticoagulation therapy for the prevention and treatment of VTE in cancer patients.28 These include:
-
Recent central nervous system bleed; intracranial, or spinal lesions at high risk of bleeding;
-
Active major bleeding (> 2 units transfused in 24 hours);
-
Chronic, clinically significant measurable bleeding for more than 48 hours;
-
Thrombocytopenia (platelets < 50,000/L);
-
Severe platelet dysfunction;
-
Recent major operation with high risk of bleeding;
-
Underlying coagulopathy (eg, clotting factor abnormalities or elevated prothrombin time or activated partial thromboplastin time [aPTT]);
-
Spinal anesthesia or lumbar puncture;
-
High risk of falls.
HIT
HIT is a serious complication that can occur as a result of exposure to heparin. It is an immune response that causes platelet activation and platelet aggregation, among other effects, and is capable of leading to severe thrombosis, amputation, or death.36 The incidence of HIT varies with subpopulations of patients and more commonly develops in patients receiving heparin in therapeutic doses. Early diagnosis (through an interpretation of clinical and laboratory information) is important to improve clinical outcomes, but difficult to achieve.36 The ACCP guidelines note that enzyme‐linked immunosorbent assay (ELISA)‐based tests for HIT are often falsely positive after surgery. As an alternative, serotonin‐release tests are more specific, although they are not as widely available.1
Substantial clinical evidence suggests that LMWH poses less of a risk of HIT than UFH. Martel et al,37 for example, conducted a meta‐analysis of 15 randomized and nonrandomized controlled trials (a total of 7287 patients) that included studies that compared prophylactic doses of UFH and LMWH and assessed postoperative or medical inpatients who received prophylaxis. The analysis revealed that the risk of HIT was 2.6% following UFH use compared with 0.2% following LMWH use.37 Despite the inclusion of UFH in the ASCO guidelines, ASCO acknowledges that a lower risk of HIT is one of the potential advantages of LMWH over UFH in cancer surgery prophylaxis.27 In addition, the recommendation to transition to outpatient therapy as soon as possible is an indirect way of stating a preference for LMWH. For cancer patients with established VTE, the recommendation is more direct: LMWH is clearly preferred over UFH for both initial and continuing antithrombotic therapy.27
Conclusions
Thromboprophylaxis should be considered in all hospitalized patients who have a risk of VTE. Anticoagulants are the mainstays of prophylaxis, and recent clinical trials have clearly demonstrated the efficacy of LMWHs and fondaparinux in preventing VTE. Each class of anticoagulant carries a number of side effects and contraindications, and frequent patient evaluation and monitoring may be required. This is especially true in those with renal impairment, for whom UFH may be a logical choice. A number of organizations have released guidelines for VTE prophylaxis that provide specific recommendations regarding thromboprophylaxis in special patient populations and scenarios.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest.2008;133(6 suppl):381S–453S.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , , , , .Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- , , , , .Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta‐analysis.J Thromb Haemost.2008;6:405–414.
- , , , et al.Pharmacology and Management of the Vitamin K Antagonists. American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. 8th Edition.Chest.2008;133:160S–198S.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
- Fragmin prescribing information.Last updated April 2007.Woodcliff Lake, NJ:Eisai, Inc.;2007.
- Lovenox prescribing information.Last updated 2008.Bridgewater, NJ:sanofi‐aventis U.S.;2008.
- Innohep prescribing information.Last updated July 2000.Wilmington, DE:DuPont Pharma;2000.
- Arixtra prescribing information.Last updated October 2008.Research Triangle Park, NC:GlaxoSmithKline;2008.
- , , , et al.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10(9):632–642.
- , , , et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341(11):793–800.
- , , , et al.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- , , , et al.A pooled analysis of four pivotal studies of rivaroxaban for the prevention of venous thromboembolism after orthopaedic surgery: effect on symptomatic venous thromboembolism, death, and bleeding.Blood.2008;112:19–20.
- , , , , , .The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement.J Thromb Haemost.2007;5:2368–2375.
- Bristol‐Myers Squibb and Pfizer. Bristol‐Myers Squibb and Pfizer provide update on apixaban clinical development program [Press release]. August 26, 2008. Available at: http://www.pfizer.com. Accessed August2009.
- , , , et al.Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double blind, non‐inferiority trial.Lancet.2007;370:949–956.
- , , , et al.Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with non‐valvular atrial fibrillation (PETRO Study).Am J Cardiol.2007;100:1419–1426.
- , , , et al.Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE‐MODEL randomized trial.J Thromb Haemost.2007;5:2178–2185.
- , , , et al.Dabigatran etexilate versus enoxaparin in preventing venous thromboembolism following total knee arthroplasty.J Thromb Haemost.2007;5(suppl 2):Abstract O‐W‐051.
- , , , .Treatment and prevention of heparin‐induced thrombocytopenia: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th edition.Chest.2008;133:340–380.
- .Extended duration of thromboprophylaxis in acutely ill medical patients: optimizing therapy?J Thromb Haemost.2007;5:5–11.
- , , , et al.Extended‐duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study.J Thromb Thrombolysis.2006;22:31–38.
- , , , et al.Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. 2007 Congress of the International Society on Thrombosis and Hemostasis; July 7–13,2007; Geneva, Switzerland. Abstract O‐S‐001.
- , , , et al.American Society of Clinical Oncology Guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer.J Clin Oncol.2007;25:5490–5505.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice guidelines in oncology. V.1. 2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2006.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty. May 18, 2007. Available at http://www.aaos.org/research/guidelines/PE_guideline.pdf. Accessed August2009.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.A randomized double‐blind placebo‐controlled study on nadroparin for prophylaxis of thromboembolic events in cancer patients receiving chemotherapy: The PROTECHT Study.Blood.2008;112(11):Abstract 6.
- , , , et al.Prevention and treatment of venous thromboembolism (VTE) in obstetrics.J SOGC.2000;22(9):736–749.
- , , , et al.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Am Fam Med.2007;5:74–80.
- , , , et al.Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation).Reg Anesth Pain Med.2003;28(3):172–197.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , .The laboratory diagnosis and clinical management of patients with HIT: an update.Semin Thromb Hemost.2008;34(1):86–93.
- , , .Risk for heparin‐induced thrombocytopenia with unfractionated and low‐molecular‐weight heparin thromboprophylaxis: a meta‐analysis.Blood.2005;106(8):2710–2715.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest.2008;133(6 suppl):381S–453S.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , , , , .Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- , , , , .Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta‐analysis.J Thromb Haemost.2008;6:405–414.
- , , , et al.Pharmacology and Management of the Vitamin K Antagonists. American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. 8th Edition.Chest.2008;133:160S–198S.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
- Fragmin prescribing information.Last updated April 2007.Woodcliff Lake, NJ:Eisai, Inc.;2007.
- Lovenox prescribing information.Last updated 2008.Bridgewater, NJ:sanofi‐aventis U.S.;2008.
- Innohep prescribing information.Last updated July 2000.Wilmington, DE:DuPont Pharma;2000.
- Arixtra prescribing information.Last updated October 2008.Research Triangle Park, NC:GlaxoSmithKline;2008.
- , , , et al.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10(9):632–642.
- , , , et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.N Engl J Med.1999;341(11):793–800.
- , , , et al.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110:874–879.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- , , , et al.A pooled analysis of four pivotal studies of rivaroxaban for the prevention of venous thromboembolism after orthopaedic surgery: effect on symptomatic venous thromboembolism, death, and bleeding.Blood.2008;112:19–20.
- , , , , , .The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement.J Thromb Haemost.2007;5:2368–2375.
- Bristol‐Myers Squibb and Pfizer. Bristol‐Myers Squibb and Pfizer provide update on apixaban clinical development program [Press release]. August 26, 2008. Available at: http://www.pfizer.com. Accessed August2009.
- , , , et al.Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double blind, non‐inferiority trial.Lancet.2007;370:949–956.
- , , , et al.Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with non‐valvular atrial fibrillation (PETRO Study).Am J Cardiol.2007;100:1419–1426.
- , , , et al.Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE‐MODEL randomized trial.J Thromb Haemost.2007;5:2178–2185.
- , , , et al.Dabigatran etexilate versus enoxaparin in preventing venous thromboembolism following total knee arthroplasty.J Thromb Haemost.2007;5(suppl 2):Abstract O‐W‐051.
- , , , .Treatment and prevention of heparin‐induced thrombocytopenia: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th edition.Chest.2008;133:340–380.
- .Extended duration of thromboprophylaxis in acutely ill medical patients: optimizing therapy?J Thromb Haemost.2007;5:5–11.
- , , , et al.Extended‐duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study.J Thromb Thrombolysis.2006;22:31–38.
- , , , et al.Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. 2007 Congress of the International Society on Thrombosis and Hemostasis; July 7–13,2007; Geneva, Switzerland. Abstract O‐S‐001.
- , , , et al.American Society of Clinical Oncology Guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer.J Clin Oncol.2007;25:5490–5505.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice guidelines in oncology. V.1. 2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2006.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty. May 18, 2007. Available at http://www.aaos.org/research/guidelines/PE_guideline.pdf. Accessed August2009.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.A randomized double‐blind placebo‐controlled study on nadroparin for prophylaxis of thromboembolic events in cancer patients receiving chemotherapy: The PROTECHT Study.Blood.2008;112(11):Abstract 6.
- , , , et al.Prevention and treatment of venous thromboembolism (VTE) in obstetrics.J SOGC.2000;22(9):736–749.
- , , , et al.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Am Fam Med.2007;5:74–80.
- , , , et al.Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation).Reg Anesth Pain Med.2003;28(3):172–197.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , .The laboratory diagnosis and clinical management of patients with HIT: an update.Semin Thromb Hemost.2008;34(1):86–93.
- , , .Risk for heparin‐induced thrombocytopenia with unfractionated and low‐molecular‐weight heparin thromboprophylaxis: a meta‐analysis.Blood.2005;106(8):2710–2715.