User login
Venous thromboembolism (VTE) is a common and potentially devastating complication of medical illness and surgical intervention. Among patients discharged from acute‐care hospitals in 2003, more than 12 million (31%) had a moderate or high risk of VTE during hospitalization, including 11% at risk due to surgical procedures and 20% at risk due to medical illnesses.1 The incidence of VTEwhich can present as deep vein thrombosis (DVT) or pulmonary embolism (PE)is rising in hospitalized patients.2 Despite the availability of effective prophylaxis, VTE is the third most common cause of hospital‐related death and the most common preventable cause of hospital mortality.3
The clinical impact of VTE is significant. While total incidence, prevalence, and mortality rates of VTE are elusive, the annual incidence of DVT is thought to be as high as 2 million.4 The most serious complication of DVT is acute PE, which occurs in approximately 600,000 patients per year, one‐third of whom die.5 DVT may also be complicated by recurrent episodes of VTE and postthrombotic sequelae such as chronic venous stasis, venous ulceration, debilitating pain, and intractable edema.6 One prospective cohort study found that 30% of patients who had experienced a first episode of DVT developed recurrent VTE within 8 years, and the incidence of postthrombotic syndrome (PTS) was 23% after just 2 years.6
The economic burden of VTE is substantial, due both to the initial event and to the high rate of hospital readmission. The estimated average cost of DVT management (including initial acute care and 6 months of follow‐up care) is $10,072 per patient, and the corresponding cost for PE is $14,649.7 Hospital readmission occurs in 5% to 14% of patients, more than one‐half of whom are readmitted within 90 days.8 For patients with recurrent DVT or PE, the mean total hospitalization costs of readmission are $11,862 and $14,722, respectively.8
The clinical and economic burden of VTE can be significantly mitigated by the use of effective prophylaxis. Because VTE is difficult to diagnose antemortem, it is easier and safer to prevent with appropriate prophylaxis than to diagnose after it has occurred. Unfortunately, VTE prophylaxis is markedly underused, particularly among high‐risk, hospitalized medical patients who would most benefit from it.9
This article summarizes specific risk factors for VTE and provides guidance in identifying patients who may require thromboprophylaxis. Barriers to optimal VTE prophylaxis in the hospital setting will also be explored.
Methodology
For this article and the ones that follow, relevant literature was identified through a Medline search (January 1980 to December 2008) using the following search terms: venous thromboembolism, pulmonary embolism, deep vein thrombosis, epidemiology, risk factors, prophylaxis, mechanical prophylaxis, diagnosis, treatment, anticoagulants, monitoring, secondary prevention, guideline, adherence, treatment protocol, performance measure, and quality improvement. The bibliographies of all key texts were searched for additional relevant articles. The websites of the American College of Chest Physicians (ACCP), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and Society of Hospital Medicine (SHM) were also searched for annual meeting abstracts, position statements, and other key publications.
Evidence‐based clinical guidelines were identified through a search of the National Guideline Clearinghouse (
Pathogenesis of VTE
Venous thrombosis occurs as a result of at least 1 of 3 underlying factors: alterations in blood flow, vascular endothelial injury, and alterations in the constitution of the blood.10 Each potential underlying factor encompasses a wide range of risk factors and clinical scenarios. Alterations in venous blood flow can include several situations, including venous stasis, venous hypertension, and valvular incompetence. Endothelial injury can arise from shear stress, direct trauma, infection, hypertension, or other sources of endothelial damage. Hypercoagulability from alterations in the constitution of the blood may be due to antithrombin deficiency, cancer, surgery, pregnancy, or other risk factors. The presence of any of these factors indicates an elevated risk of VTE, and the presence of multiple factors further increases risk.10
Risk Factors for VTE
VTE can occur in a wide variety of clinical circumstances. Recognized risk factors for VTE include hospitalization for an acute medical illness, cardiovascular disease, pulmonary disease, major surgery, multiple trauma, obesity, and increasing age.10 Additional factors that place patients at increased risk of VTE (independent of age) include a history of prior VTE, known hypercoagulable states, active cancer, and acute infection.11 Hospital‐acquired risk factors such as immobility, acute illness, or medical interventions may lead to the development of VTE in these patients. Severity of illness must be factored into the risk assessment, and all patients need to be assessed for VTE risk at the time of hospital admission and daily thereafter if pharmacologic therapy is not initiated.
In a review of 1231 consecutive patients treated for acute DVT and/or PE, 96.3% had at least 1 risk factor for VTE, and more than one‐third (39%) had 3 or more risk factors (Table 1).10 The incidence of VTE in hospitalized patients is directly related to the number of risk factors present:10
-
1 risk factor: 11%
-
2 risk factors: 24%
-
3 risk factors: 36%
-
4 risk factors: 50%
-
5 risk factors: > 90%
| Risk Factor | Patients (%) |
|---|---|
| |
| Age 40 years | 88.5 |
| Obesity | 37.8 |
| History of VTE | 26.0 |
| Cancer | 22.3 |
| Bed rest 5 days | 12.0 |
| Major surgery | 11.2 |
| Congestive heart failure | 8.2 |
| Varicose veins | 5.8 |
| Fracture (hip or leg) | 3.7 |
| Estrogen treatment | 2.0 |
| Stroke | 1.8 |
| Multiple trauma | 1.1 |
| Childbirth | 1.1 |
| Myocardial infarction | 0.7 |
Current or Recent Prior Hospitalization
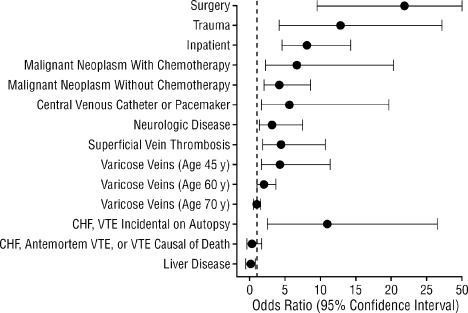
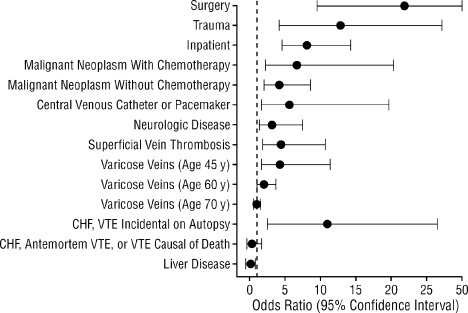
The risk of VTE is elevated among hospitalized patients. The prevalence of DVT varies across hospital specialties, reaching up to 80% in major trauma, spinal cord injury, and critical care.3 The Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study evaluated the prevalence of VTE risk factors in the acute hospital care setting.12 Among the 68,183 patients enrolled, more than one‐half (52%) were judged to be at risk of VTE. In a case‐control study examining 625 patients with a first lifetime VTE, confinement to a hospital (among other risk factors) was found to be an independent and important predictor of VTE (odds ratio [OR], 8.0; 95% confidence interval [CI], 4.514.2) (Figure 1).13 Recent hospitalization is also an important risk factor for VTE, and patients who are readmitted to the hospital should be considered moderate or high risk.13
Age
Patient age must be considered when assessing VTE risk. VTE is predominantly a disease of older age, and age older than 75 years is an important risk factor for the condition.11 In general, patients older than 40 years have a significantly increased risk compared with younger patients, and the risk approximately doubles with each additional decade.10 Given the aging population, the prevalence of VTE and its complications are expected to increase.
Women of childbearing age experience VTE more frequently than men of the same age, due to pregnancy and exposure to contraceptive therapy.14 This risk, however, is modest compared with the risk among older patients. After age 45 years, the incidence of VTE increases markedly for both sexes, becoming more prominent in men.14 Compared with women, men also have an increased risk of recurrent VTE.15
Despite the effect of age on VTE risk, the risk among patients younger than 40 years may be underestimated because this subgroup has not been extensively studied. For reasons that are not well‐understood, the risk of VTE associated with heart failure is higher in patients younger than 40 years, and the relative risk of PE in patients with chronic obstructive pulmonary disease (COPD) is also higher in younger patients.16, 17
Cancer and Its Treatment
Cancer patients, on average, have twice the risk of VTE compared with noncancer patients.18 This risk, however, varies considerably by cancer type. According to an assessment of nearly 41 million hospitalized patients in the National Hospital Discharge Survey (NHDS), the relative risk of VTE varied from 1.02 in patients with bladder cancer to 4.34 in patients with cancer of the pancreas.18
VTE is one of the most common complications of cancer and cancer therapy, and it is the second leading cause of death among hospitalized cancer patients.19 Molecular mechanisms underlying thromboembolic events in cancer patients include tumor cell procoagulants, inflammatory cell cytokines, mediators of platelet adhesion, and tumor‐related stasis and endothelial damage.20 The clinical implications of these processes are severe. Cancer exacerbates the natural course of VTE, increasing the risk of recurrent VTE and major bleeding, and VTE worsens the prognosis of cancer, increasing the risk of death among cancer patients.
Various cancer therapiesincluding surgery, chemotherapy, hematopoietic stem cell transplantation, and even growth factor supportalso increase the risk of VTE, in part because extrinsic factors such as surgery or chemotherapy can intensify the hypercoagulable process.18, 2123 In the NHDS, cancer patients undergoing surgery had at least twice the risk of postoperative DVT and more than 3 times the risk of PE compared with noncancer patients undergoing similar procedures.18
Cancer is an independent predictor of thromboprophylaxis failure following surgery. The @RISTOS Project found that VTE was the most common cause of death among 2373 patients undergoing general, urologic, or gynecologic surgery for cancer.24 A multivariate analysis identified 5 independent risk factors for VTE after cancer surgery:
-
Previous VTE (OR, 5.98; 95% CI, 2.1316.80)
-
Anesthesia 2 hours (OR, 4.50; 95% CI, 1.0619.04)
-
Bed rest 4 days (OR, 4.37; 95% CI, 2.457.78)
-
Age 60 years (OR, 2.63; 95% CI, 1.215.71)
-
Advanced‐stage cancer (OR, 2.68; 95% CI, 1.375.24)
Cardiovascular Disease
The risk of VTE is pronounced among patients with cardiovascular disease. After stroke and coronary disease, VTE is the third most common cardiovascular disorder, and PE causes more deaths each year than myocardial infarction (MI).25 Several cardiovascular diseases, including hypertension, stroke, acute MI, and heart failure, are independently associated with VTE.10, 2627 Related disorders, including diabetes and the metabolic syndrome, also increase the risk of VTE.26, 28
Congestive heart failure (CHF) is a risk factor for VTE, and the severity of illness increases risk. In the DVT‐Free Prospective Registry, 13% of patients with ultrasound‐confirmed DVT had CHF.29 In a subgroup analysis of patients of the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, the incidence of VTE exceeded 20% in patients with New York Heart Association (NYHA) class IV heart failure, compared with 12% in patients with NYHA class III heart failure.30 Another study found that VTE risk increases as left ventricular ejection fraction (LVEF) decreases, with an LVEF of less than 20% associated with a VTE OR of 38.3 (95% CI, 9.6152.5).31
Infectious Disease
Acute infection may increase the relative risk of VTE by as much as 50% and is associated with VTE event rates of up to 26%.11 Acute infections may be associated with acute inflammation, adverse effects on cardiac or pulmonary function, and prolonged immobilization.30, 32, 33 Human immunodeficiency virus (HIV) patients may also have an increased risk of VTE due to a circulating lupus anticoagulant and/or the presence of acute infection.34
Obesity
The 2008 ACCP guideline update recognizes obesity, for the first time, as a risk factor for VTE.3 Obesity was 1 of the 5 most frequent comorbidities found in patients with DVT in the DVT‐Free Prospective Registry.29 It increases the risk of both incident and recurrent VTE, with every 1‐point increase in body mass index (BMI) increasing the risk of recurrent VTE by 4.4% (95% CI, 1.37.6%; P < 0.001).35
Pregnancy and Puerperium
Pregnancy, particularly the postpartum period, is associated with an increased risk of VTE in women, even though the absolute risk is small.36 Still, PE is one of the leading causes of maternal death following childbirth.10 Smoking, prior VTE, and inherited thrombophilias all increase the risk of VTE in pregnant women.10 The risk begins to rise in the first trimester, and when prophylaxis is needed, it should be started early in gestation.37
Pulmonary Disease
COPD is another risk factor for the development of VTE. COPD patients who develop VTE tend to be older, hospitalized in the intensive care unit (ICU), and on mechanical ventilation.38 In the DVT‐Free Prospective Registry, 12% of patients with ultrasound‐confirmed DVT had COPD.29
Trauma and Surgery
Injury to the body tissue, via trauma or surgery, stimulates the body's clotting mechanism and increases the risk of thromboembolic complications. During the perioperative period, the circulatory system must balance a variety of assaults: an immune response to surgical stress, prolonged immobilization during surgery and recovery, vasodilation associated with general or regional anesthesia, and hypercoagulability due to venous stasis and vascular injury.39 Renal transplant recipients have an increased risk of VTE due to a chronic hypercoagulable state.40 In surgery patients, perioperative complications such as dehydration and acute infection increase the risk of VTE beyond the risk associated with the surgical procedure itself.10
VTE risk is increased approximately 13‐fold by recent major trauma or lower‐extremity injury.13 In the absence of prophylaxis, the overall risk of VTE among patients undergoing major surgery is increased nearly 22‐fold.13 After controlling for the type of surgery, additional independent risk factors for VTE within 3 months of major surgery include:41, 42
-
Obesity
-
Central venous catheter placement
-
Malignancy
-
Smoking
-
Heart failure
-
Previous DVT
-
Prolonged immobility
-
Infection
Many surgical and medical inpatients share common risk factors, and without prophylaxis, the incidence of hospital‐acquired DVT ranges from 10% to 40% for both groups.3
Inherited or Acquired Risk Factors
VTE is a multifactorial disease, and recent evidence indicates that some heritable traits may be potent risk factors for VTE.43 Approximately 35% of patients with DVT will have at least 1 of 5 traits related to an inherited blood clotting disorder:43
-
Deficiencies in the anticoagulation factors protein C, protein S, or antithrombin, or
-
Mutations in the factor V and prothrombin genes, resulting in Factor V Leiden and prothrombin G20210A, respectively.
Certain inherited traits and genetic polymorphisms increase the risk of VTE by interacting with clinical risk factors such as contraceptive use, pregnancy, surgery, trauma, and cancer. One recent study found that oral estrogen therapy among women with the CYP3A5*1 allele was associated with a particularly high risk of VTE.44 Although widespread screening for inherited risk factors is not currently practical, future tools may incorporate genetic polymorphisms to more precisely identify patients who would benefit from aggressive prophylaxis.
Lifestyle Factors
Lifestyle factors have a significant effect on VTE risk. Smoking increases the risk of VTE by 20% to 30%, and a sedentary lifestyle also increases the risk of VTE.26, 45 In fact, women who exercise regularly and consume alcohol in moderation have one‐half the risk of VTE as women who have a sedentary lifestyle and drink little or no alcohol.42 For both men and women, a diet high in fruits, vegetables, and fish is associated with a lower lifetime risk of VTE.46
Medications
Medications may also increase the risk of VTE. In cancer patients with anemia, for example, the use of erythropoiesis‐stimulating agents such as recombinant erythropoietin and darbepoetin was recently shown to increase the risk of VTE by 57% (95% CI, 3187%) and increase mortality risk by 10% (95% CI, 120%).23 In addition, combination hormone replacement therapy in women is associated with a higher risk of VTE compared with estrogen monotherapy, and transdermal contraceptive systems more than double the risk of VTE compared with oral contraceptives (95% CI, 1.33.8).47, 48 Recent studies have also reported an increased risk of VTE with some psychiatric drugs, including amitriptyline, clozapine, olanzapine, and risperidone.4952
Thromboprophylaxis in the Hospital Setting
Despite the prevalence of risk factors and compelling evidence regarding the value of prophylaxis, VTE prophylaxis is suboptimal in hospitalized medical and surgical patients. In a study of 123,304 hospitalized patients who were determined to be at risk of VTE, only 13.3% received prophylaxis in accordance with ACCP guidelines.53 Compliance ranged from a high of 52.4% among patients undergoing orthopedic surgery to a low of 2.8% among patients undergoing neurosurgery.53 Results from several other large trials echo these findings (Table 2).12, 5456
| Trial | Patient Type | Total Patients | Patients at Risk of VTE (Based on ACCP Criteria) (%) | At‐Risk Patients Receiving Recommended Prophylaxis | |
|---|---|---|---|---|---|
| Medical Patients (%) | Surgical Patients (%) | ||||
| |||||
| IMPROVE | Medical patients | 15,156 | 52 | 61 | n/a |
| ENDORSE | Medical and surgical patients | 68,183 | 51.8 | 39.5 | 58.5 |
| ADHERE | Hospitalized heart failure patients | 155,073 | 46 | 30.6 | n/a |
| Amin et al.56 (2008) | Medical and surgical patients | 258,556 | 26.4 | 9.8 | 17.9 |
Reasons for Inadequate Prophylaxis
Researchers have identified a range of barriers to adequate VTE prophylaxis (Table 3).57 Some of these barriers are outlined below.
|
| Variability in clinician knowledge of risk assessment and appropriate prophylaxis |
| Lack of agreement with, and inconsistency between, guidelines in certain patient populations |
| Perceived lack of need |
| Concerns about adverse effects |
| Lack of hospital support systems and policies |
| Lack of established responsibilities for prophylaxis |
Underestimation of Risk of Clotting
VTE is often clinically silent, leading some physicians to mistakenly believe that it is rare.58 In hospitalized surgical patients, for example, the incidence of thromboembolic complications during a short postoperative stay may be low. Given that many cases of symptomatic VTE occur after hospital discharge, hospitalists and surgeons may be unaware of the true incidence of DVT.59
Overestimation of the Risk of Bleeding
Physicians may also overestimate the risk of possible side effects of prophylaxis, such as major bleeding or heparin‐induced thrombocytopenia (HIT).58 Fear of excess bleeding has been cited by physicians as a leading reason for their decision to withhold thromboprophylaxis from at‐risk hospitalized patients.60 Physicians are particularly fearful of complications among elderly patients, who are less likely to receive adequate prophylaxis than younger patients with a similar risk of VTE.61 When bleeding does occur, it rarely results in death. On the other hand, PE may account for as many as 10% of hospital deaths.9
Guideline Confusion and Complexity
Discrepancies between guidelines published by different medical societies contribute to confusion in choosing a management approach. The American Academy of Orthopedic Surgeons (AAOS), for example, describes aspirin alone as a reasonable choice for VTE prophylaxis in some patients, but the ACCP guidelines advise against the use of aspirin monotherapy.58 The cumbersome nature of multiple risk‐assessment and treatment algorithms can also be problematic.61 Furthermore, certain patient subgroups, such as those with cirrhosis, severe renal failure, and epidural catheters, have been excluded from randomized controlled trials, and the management of such patients is not straightforward.
Absence of Institutional Protocols and Information Technology Support
The lack of institution‐level guidance and support can have a detrimental effect on patient care. In a 2007 survey of 127 community hospitals, the prevalence of institutional protocols related to VTE was low: only 60% had protocols to encourage prophylaxis in at‐risk patients, 54% had guidelines to assist in appropriate drug selection, and 43% had guidelines for the dosing of prophylaxis regimens.62 A lack of systems for data collection and audit has also been identified as a barrier to the implementation of prophylaxis guidelines.57 Thus, hospitals need to adopt protocols such as:3
-
Written, institution‐wide thromboprophylaxis policies
-
Preprinted order forms and computer decision‐support systems
-
Policies specifying responsibilities for assessing VTE risk and prescribing prophylaxis
Conclusions
VTE is the most common preventable cause of hospital death, and prophylaxis is underused in hospitalized patients. Although VTE risk factors are numerous and complex, deciding whether to use prophylaxis need not be complicated. In general, elderly patients, medically‐ill patients, and patients undergoing surgery will benefit from prophylaxis, as well as those who are hospitalized for more than 1 night. Hospitalized patients with at least 1 risk factor should be considered for pharmacologic prophylaxis. In general, the risk of hospital‐acquired VTE greatly exceeds the risk of bleeding with prophylactic doses of anticoagulation. A patient's risk of VTE may change, and regular assessment of this risk should be mandated if pharmacologic therapy is not initiated at the time of admission.
Numerous barriers to the optimal use of VTE prophylaxis exist, and hospitals must implement systems changes and multidisciplinary approaches to overcome these barriers. The fourth article in this supplement provides detailed strategies for meeting VTE performance measures and overcoming barriers to the optimal use of prophylaxis.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82(9):777–782.
- , , .Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients.Am J Cardiol.2005;95(12):1525–1526.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th ed.Chest.2008;133(6 suppl):381S–453S.
- , .Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association.Circulation.1996;93(12)2212–2245.
- , , , et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- , , , et al.The long‐term clinical course of acute deep venous thrombosis.Ann Intern Med.1996;125(1):1–7.
- , .Direct medical cost of managing deep vein thrombosis according to the occurrence of complications.Pharmacoeconomics.2002;20(9):603–615.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , .Risk factors for venous thromboembolism.Circulation.2003;107(23 suppl 1):I9–I16.
- , , , et al.Risk factors for VTE in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- , , , et al.;ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , et al.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160(6):809–815.
- , .Range of published estimates of venous thromboembolism incidence in young women.Contraception.2007;75(5):328–336.
- , , , , , .The risk of recurrent venous thromboembolism in men and women.N Engl J Med.2004;350(25):2558–2563.
- , , , et al.Risk of venous thromboembolism in patients hospitalized with heart failure.Am J Cardiol.2006;98(6):793–795.
- , , , et al.Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease.J Cardiovasc Med.2007;8(4):253–257.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.Causes of death in cancer patients.J Med.1975;6(1):61–64.
- , .Tissue factor and angiogenesis in cancer.Curr Opin Hematol.2002;9(5):401–406.
- , , , et al.Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel‐containing first‐line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group.J Clin Oncol.2008;26(16):2683–2689.
- , , , , , .The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention.Blood.2008;112(3):504–510.
- , , , et al.Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia.JAMA.2008;299(8):914–924.
- , , , et al.A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project.Ann Surg.2006;243(1):89–95.
- .Pulmonary embolism in thrombolysis: a clarion call for international collaboration.J Am Coll Cardiol.1992;19:246–247.
- , , , et al.Cardiovascular risk factors and venous thromboembolism: a meta‐analysis.Circulation.2008;117(1):93–102.
- , , , , , .Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study.J Thromb Haemost.2008;6(11):1851–1857.
- , , , et al.Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome—the Tromsø study.J Thromb Haemost.2009;7(5):739–745.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , , .Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case‐control study.J Clin Epidemiol.2001;54(8):810–816.
- , , , et al;ARTEMIS Investigators.Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- , , , et al.HIV infection is a risk factor for venous thromboembolism.AIDS Patient Care STDS.2002;16(5):205–209.
- , , , et al.Overweight, obesity, and the risk of recurrent venous thromboembolism.Arch Intern Med.2008;168(15):1678–1683.
- .Venous thromboembolism in women.Vasc Med.2008;13(3):255–266.
- , , .Thrombosis during pregnancy and the postpartum period.Am J Obstet Gynecol.2005;193(1):216–219.
- , , , et al.Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination.J Thromb Thrombolysis.2008;26(1):35–40.
- , , .Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability?J Trauma.2003;54(2):224–231.
- , .Acquired hypercoagulable state in renal transplant recipients.Thromb Haemost.2004;91(4):646–654.
- , , , .Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion.Thromb Haemost.2007;98(6):1220–1225.
- , , , , , .A 10‐year analysis of venous thromboembolism on the surgical service: the effect of practice guidelines for prophylaxis.Surgery.2008;144(1):3–11.
- , , , et al;EDITH Collaborative Study Group.Family history as a risk factor for venous thromboembolism.Thromb Res.2008;122(5):624–629.
- , , , et al;Estrogen and Thromboembolism Risk (ESTHER) Study Group.Synergism between oral estrogen therapy and cytochrome P450 3A5*1 allele on the risk of venous thromboembolism among postmenopausal women.J Clin Endocrinol Metab.2008;93(8):3082–3087.
- , , .The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study.Br J Haematol.2009;144(2):234–240.
- , , , , .Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology.Circulation.2007;115(2):188–195.
- , , .Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta‐analysis.Eur Heart J.2008;29(16):2031–2041.
- , , , .Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users.Obstet Gynecol.2007;109(2 Pt 1):339–346.
- , , , , , .Pulmonary thromboembolism associated with olanzapine and risperidone.J Emerg Med.2008;35(2):159–161.
- , , , .Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions.Drug Saf.2008;31(8):685–694.
- .Evaluating the association between clozapine and venous thromboembolism.Am J Health Syst Pharm.2008;65(19):1825–1829.
- , .Antidepressant drug use and risk of venous thromboembolism.Pharmacotherapy.2008;28(2):144–150.
- , , , .Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64(1):69–76.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients.Chest.2007;132:936–945.
- , , , et al.Adhere Scientific Advisory Committee and Investigators. Venous thromboembolism prophylaxis in hospitalized heart failure patients.J Card Fail.2008;14(2):127–132.
- , , , et al. Are hospitals following guidelines for VTE Prevention? The Venous Thromboembolism Study to Assess the Rate of Thromboprophylaxis. Presented at the 50th Annual Meeting of the American College of Hematology, San Francisco, CA, December 6–9, 2008. Abstract 1286.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- .A need for a simplified approach to venous thromboembolism prophylaxis in acute medical inpatients.Int J Clin Pract.2007;61(2):336–340.
- .Barriers to the optimal use of anticoagulants after orthopaedic surgery.Arch Orthop Trauma Surg.2008 (Published online Oct, 8.). [http://dx.doi.org/DOI 10.1007/s00402‐008‐0765‐9]
- , , , , .Venous thrombosis in cancer patients: Insights from the FRONTLINE survey.Oncologist.2003;8:381–388.
- , , , et al.Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross‐sectional study in Brazil.J Thromb Haemost.2006;4(6):1266–1270.
- , , , , .Survey of hospitals for guidelines, policies, and protocols for anticoagulants.Am J Health Syst Pharm.2007;64(11):1203–1208.
Venous thromboembolism (VTE) is a common and potentially devastating complication of medical illness and surgical intervention. Among patients discharged from acute‐care hospitals in 2003, more than 12 million (31%) had a moderate or high risk of VTE during hospitalization, including 11% at risk due to surgical procedures and 20% at risk due to medical illnesses.1 The incidence of VTEwhich can present as deep vein thrombosis (DVT) or pulmonary embolism (PE)is rising in hospitalized patients.2 Despite the availability of effective prophylaxis, VTE is the third most common cause of hospital‐related death and the most common preventable cause of hospital mortality.3
The clinical impact of VTE is significant. While total incidence, prevalence, and mortality rates of VTE are elusive, the annual incidence of DVT is thought to be as high as 2 million.4 The most serious complication of DVT is acute PE, which occurs in approximately 600,000 patients per year, one‐third of whom die.5 DVT may also be complicated by recurrent episodes of VTE and postthrombotic sequelae such as chronic venous stasis, venous ulceration, debilitating pain, and intractable edema.6 One prospective cohort study found that 30% of patients who had experienced a first episode of DVT developed recurrent VTE within 8 years, and the incidence of postthrombotic syndrome (PTS) was 23% after just 2 years.6
The economic burden of VTE is substantial, due both to the initial event and to the high rate of hospital readmission. The estimated average cost of DVT management (including initial acute care and 6 months of follow‐up care) is $10,072 per patient, and the corresponding cost for PE is $14,649.7 Hospital readmission occurs in 5% to 14% of patients, more than one‐half of whom are readmitted within 90 days.8 For patients with recurrent DVT or PE, the mean total hospitalization costs of readmission are $11,862 and $14,722, respectively.8
The clinical and economic burden of VTE can be significantly mitigated by the use of effective prophylaxis. Because VTE is difficult to diagnose antemortem, it is easier and safer to prevent with appropriate prophylaxis than to diagnose after it has occurred. Unfortunately, VTE prophylaxis is markedly underused, particularly among high‐risk, hospitalized medical patients who would most benefit from it.9
This article summarizes specific risk factors for VTE and provides guidance in identifying patients who may require thromboprophylaxis. Barriers to optimal VTE prophylaxis in the hospital setting will also be explored.
Methodology
For this article and the ones that follow, relevant literature was identified through a Medline search (January 1980 to December 2008) using the following search terms: venous thromboembolism, pulmonary embolism, deep vein thrombosis, epidemiology, risk factors, prophylaxis, mechanical prophylaxis, diagnosis, treatment, anticoagulants, monitoring, secondary prevention, guideline, adherence, treatment protocol, performance measure, and quality improvement. The bibliographies of all key texts were searched for additional relevant articles. The websites of the American College of Chest Physicians (ACCP), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and Society of Hospital Medicine (SHM) were also searched for annual meeting abstracts, position statements, and other key publications.
Evidence‐based clinical guidelines were identified through a search of the National Guideline Clearinghouse (
Pathogenesis of VTE
Venous thrombosis occurs as a result of at least 1 of 3 underlying factors: alterations in blood flow, vascular endothelial injury, and alterations in the constitution of the blood.10 Each potential underlying factor encompasses a wide range of risk factors and clinical scenarios. Alterations in venous blood flow can include several situations, including venous stasis, venous hypertension, and valvular incompetence. Endothelial injury can arise from shear stress, direct trauma, infection, hypertension, or other sources of endothelial damage. Hypercoagulability from alterations in the constitution of the blood may be due to antithrombin deficiency, cancer, surgery, pregnancy, or other risk factors. The presence of any of these factors indicates an elevated risk of VTE, and the presence of multiple factors further increases risk.10
Risk Factors for VTE
VTE can occur in a wide variety of clinical circumstances. Recognized risk factors for VTE include hospitalization for an acute medical illness, cardiovascular disease, pulmonary disease, major surgery, multiple trauma, obesity, and increasing age.10 Additional factors that place patients at increased risk of VTE (independent of age) include a history of prior VTE, known hypercoagulable states, active cancer, and acute infection.11 Hospital‐acquired risk factors such as immobility, acute illness, or medical interventions may lead to the development of VTE in these patients. Severity of illness must be factored into the risk assessment, and all patients need to be assessed for VTE risk at the time of hospital admission and daily thereafter if pharmacologic therapy is not initiated.
In a review of 1231 consecutive patients treated for acute DVT and/or PE, 96.3% had at least 1 risk factor for VTE, and more than one‐third (39%) had 3 or more risk factors (Table 1).10 The incidence of VTE in hospitalized patients is directly related to the number of risk factors present:10
-
1 risk factor: 11%
-
2 risk factors: 24%
-
3 risk factors: 36%
-
4 risk factors: 50%
-
5 risk factors: > 90%
| Risk Factor | Patients (%) |
|---|---|
| |
| Age 40 years | 88.5 |
| Obesity | 37.8 |
| History of VTE | 26.0 |
| Cancer | 22.3 |
| Bed rest 5 days | 12.0 |
| Major surgery | 11.2 |
| Congestive heart failure | 8.2 |
| Varicose veins | 5.8 |
| Fracture (hip or leg) | 3.7 |
| Estrogen treatment | 2.0 |
| Stroke | 1.8 |
| Multiple trauma | 1.1 |
| Childbirth | 1.1 |
| Myocardial infarction | 0.7 |
Current or Recent Prior Hospitalization
The risk of VTE is elevated among hospitalized patients. The prevalence of DVT varies across hospital specialties, reaching up to 80% in major trauma, spinal cord injury, and critical care.3 The Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study evaluated the prevalence of VTE risk factors in the acute hospital care setting.12 Among the 68,183 patients enrolled, more than one‐half (52%) were judged to be at risk of VTE. In a case‐control study examining 625 patients with a first lifetime VTE, confinement to a hospital (among other risk factors) was found to be an independent and important predictor of VTE (odds ratio [OR], 8.0; 95% confidence interval [CI], 4.514.2) (Figure 1).13 Recent hospitalization is also an important risk factor for VTE, and patients who are readmitted to the hospital should be considered moderate or high risk.13

Age
Patient age must be considered when assessing VTE risk. VTE is predominantly a disease of older age, and age older than 75 years is an important risk factor for the condition.11 In general, patients older than 40 years have a significantly increased risk compared with younger patients, and the risk approximately doubles with each additional decade.10 Given the aging population, the prevalence of VTE and its complications are expected to increase.
Women of childbearing age experience VTE more frequently than men of the same age, due to pregnancy and exposure to contraceptive therapy.14 This risk, however, is modest compared with the risk among older patients. After age 45 years, the incidence of VTE increases markedly for both sexes, becoming more prominent in men.14 Compared with women, men also have an increased risk of recurrent VTE.15
Despite the effect of age on VTE risk, the risk among patients younger than 40 years may be underestimated because this subgroup has not been extensively studied. For reasons that are not well‐understood, the risk of VTE associated with heart failure is higher in patients younger than 40 years, and the relative risk of PE in patients with chronic obstructive pulmonary disease (COPD) is also higher in younger patients.16, 17
Cancer and Its Treatment
Cancer patients, on average, have twice the risk of VTE compared with noncancer patients.18 This risk, however, varies considerably by cancer type. According to an assessment of nearly 41 million hospitalized patients in the National Hospital Discharge Survey (NHDS), the relative risk of VTE varied from 1.02 in patients with bladder cancer to 4.34 in patients with cancer of the pancreas.18
VTE is one of the most common complications of cancer and cancer therapy, and it is the second leading cause of death among hospitalized cancer patients.19 Molecular mechanisms underlying thromboembolic events in cancer patients include tumor cell procoagulants, inflammatory cell cytokines, mediators of platelet adhesion, and tumor‐related stasis and endothelial damage.20 The clinical implications of these processes are severe. Cancer exacerbates the natural course of VTE, increasing the risk of recurrent VTE and major bleeding, and VTE worsens the prognosis of cancer, increasing the risk of death among cancer patients.
Various cancer therapiesincluding surgery, chemotherapy, hematopoietic stem cell transplantation, and even growth factor supportalso increase the risk of VTE, in part because extrinsic factors such as surgery or chemotherapy can intensify the hypercoagulable process.18, 2123 In the NHDS, cancer patients undergoing surgery had at least twice the risk of postoperative DVT and more than 3 times the risk of PE compared with noncancer patients undergoing similar procedures.18
Cancer is an independent predictor of thromboprophylaxis failure following surgery. The @RISTOS Project found that VTE was the most common cause of death among 2373 patients undergoing general, urologic, or gynecologic surgery for cancer.24 A multivariate analysis identified 5 independent risk factors for VTE after cancer surgery:
-
Previous VTE (OR, 5.98; 95% CI, 2.1316.80)
-
Anesthesia 2 hours (OR, 4.50; 95% CI, 1.0619.04)
-
Bed rest 4 days (OR, 4.37; 95% CI, 2.457.78)
-
Age 60 years (OR, 2.63; 95% CI, 1.215.71)
-
Advanced‐stage cancer (OR, 2.68; 95% CI, 1.375.24)
Cardiovascular Disease
The risk of VTE is pronounced among patients with cardiovascular disease. After stroke and coronary disease, VTE is the third most common cardiovascular disorder, and PE causes more deaths each year than myocardial infarction (MI).25 Several cardiovascular diseases, including hypertension, stroke, acute MI, and heart failure, are independently associated with VTE.10, 2627 Related disorders, including diabetes and the metabolic syndrome, also increase the risk of VTE.26, 28
Congestive heart failure (CHF) is a risk factor for VTE, and the severity of illness increases risk. In the DVT‐Free Prospective Registry, 13% of patients with ultrasound‐confirmed DVT had CHF.29 In a subgroup analysis of patients of the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, the incidence of VTE exceeded 20% in patients with New York Heart Association (NYHA) class IV heart failure, compared with 12% in patients with NYHA class III heart failure.30 Another study found that VTE risk increases as left ventricular ejection fraction (LVEF) decreases, with an LVEF of less than 20% associated with a VTE OR of 38.3 (95% CI, 9.6152.5).31
Infectious Disease
Acute infection may increase the relative risk of VTE by as much as 50% and is associated with VTE event rates of up to 26%.11 Acute infections may be associated with acute inflammation, adverse effects on cardiac or pulmonary function, and prolonged immobilization.30, 32, 33 Human immunodeficiency virus (HIV) patients may also have an increased risk of VTE due to a circulating lupus anticoagulant and/or the presence of acute infection.34
Obesity
The 2008 ACCP guideline update recognizes obesity, for the first time, as a risk factor for VTE.3 Obesity was 1 of the 5 most frequent comorbidities found in patients with DVT in the DVT‐Free Prospective Registry.29 It increases the risk of both incident and recurrent VTE, with every 1‐point increase in body mass index (BMI) increasing the risk of recurrent VTE by 4.4% (95% CI, 1.37.6%; P < 0.001).35
Pregnancy and Puerperium
Pregnancy, particularly the postpartum period, is associated with an increased risk of VTE in women, even though the absolute risk is small.36 Still, PE is one of the leading causes of maternal death following childbirth.10 Smoking, prior VTE, and inherited thrombophilias all increase the risk of VTE in pregnant women.10 The risk begins to rise in the first trimester, and when prophylaxis is needed, it should be started early in gestation.37
Pulmonary Disease
COPD is another risk factor for the development of VTE. COPD patients who develop VTE tend to be older, hospitalized in the intensive care unit (ICU), and on mechanical ventilation.38 In the DVT‐Free Prospective Registry, 12% of patients with ultrasound‐confirmed DVT had COPD.29
Trauma and Surgery
Injury to the body tissue, via trauma or surgery, stimulates the body's clotting mechanism and increases the risk of thromboembolic complications. During the perioperative period, the circulatory system must balance a variety of assaults: an immune response to surgical stress, prolonged immobilization during surgery and recovery, vasodilation associated with general or regional anesthesia, and hypercoagulability due to venous stasis and vascular injury.39 Renal transplant recipients have an increased risk of VTE due to a chronic hypercoagulable state.40 In surgery patients, perioperative complications such as dehydration and acute infection increase the risk of VTE beyond the risk associated with the surgical procedure itself.10
VTE risk is increased approximately 13‐fold by recent major trauma or lower‐extremity injury.13 In the absence of prophylaxis, the overall risk of VTE among patients undergoing major surgery is increased nearly 22‐fold.13 After controlling for the type of surgery, additional independent risk factors for VTE within 3 months of major surgery include:41, 42
-
Obesity
-
Central venous catheter placement
-
Malignancy
-
Smoking
-
Heart failure
-
Previous DVT
-
Prolonged immobility
-
Infection
Many surgical and medical inpatients share common risk factors, and without prophylaxis, the incidence of hospital‐acquired DVT ranges from 10% to 40% for both groups.3
Inherited or Acquired Risk Factors
VTE is a multifactorial disease, and recent evidence indicates that some heritable traits may be potent risk factors for VTE.43 Approximately 35% of patients with DVT will have at least 1 of 5 traits related to an inherited blood clotting disorder:43
-
Deficiencies in the anticoagulation factors protein C, protein S, or antithrombin, or
-
Mutations in the factor V and prothrombin genes, resulting in Factor V Leiden and prothrombin G20210A, respectively.
Certain inherited traits and genetic polymorphisms increase the risk of VTE by interacting with clinical risk factors such as contraceptive use, pregnancy, surgery, trauma, and cancer. One recent study found that oral estrogen therapy among women with the CYP3A5*1 allele was associated with a particularly high risk of VTE.44 Although widespread screening for inherited risk factors is not currently practical, future tools may incorporate genetic polymorphisms to more precisely identify patients who would benefit from aggressive prophylaxis.
Lifestyle Factors
Lifestyle factors have a significant effect on VTE risk. Smoking increases the risk of VTE by 20% to 30%, and a sedentary lifestyle also increases the risk of VTE.26, 45 In fact, women who exercise regularly and consume alcohol in moderation have one‐half the risk of VTE as women who have a sedentary lifestyle and drink little or no alcohol.42 For both men and women, a diet high in fruits, vegetables, and fish is associated with a lower lifetime risk of VTE.46
Medications
Medications may also increase the risk of VTE. In cancer patients with anemia, for example, the use of erythropoiesis‐stimulating agents such as recombinant erythropoietin and darbepoetin was recently shown to increase the risk of VTE by 57% (95% CI, 3187%) and increase mortality risk by 10% (95% CI, 120%).23 In addition, combination hormone replacement therapy in women is associated with a higher risk of VTE compared with estrogen monotherapy, and transdermal contraceptive systems more than double the risk of VTE compared with oral contraceptives (95% CI, 1.33.8).47, 48 Recent studies have also reported an increased risk of VTE with some psychiatric drugs, including amitriptyline, clozapine, olanzapine, and risperidone.4952
Thromboprophylaxis in the Hospital Setting
Despite the prevalence of risk factors and compelling evidence regarding the value of prophylaxis, VTE prophylaxis is suboptimal in hospitalized medical and surgical patients. In a study of 123,304 hospitalized patients who were determined to be at risk of VTE, only 13.3% received prophylaxis in accordance with ACCP guidelines.53 Compliance ranged from a high of 52.4% among patients undergoing orthopedic surgery to a low of 2.8% among patients undergoing neurosurgery.53 Results from several other large trials echo these findings (Table 2).12, 5456
| Trial | Patient Type | Total Patients | Patients at Risk of VTE (Based on ACCP Criteria) (%) | At‐Risk Patients Receiving Recommended Prophylaxis | |
|---|---|---|---|---|---|
| Medical Patients (%) | Surgical Patients (%) | ||||
| |||||
| IMPROVE | Medical patients | 15,156 | 52 | 61 | n/a |
| ENDORSE | Medical and surgical patients | 68,183 | 51.8 | 39.5 | 58.5 |
| ADHERE | Hospitalized heart failure patients | 155,073 | 46 | 30.6 | n/a |
| Amin et al.56 (2008) | Medical and surgical patients | 258,556 | 26.4 | 9.8 | 17.9 |
Reasons for Inadequate Prophylaxis
Researchers have identified a range of barriers to adequate VTE prophylaxis (Table 3).57 Some of these barriers are outlined below.
|
| Variability in clinician knowledge of risk assessment and appropriate prophylaxis |
| Lack of agreement with, and inconsistency between, guidelines in certain patient populations |
| Perceived lack of need |
| Concerns about adverse effects |
| Lack of hospital support systems and policies |
| Lack of established responsibilities for prophylaxis |
Underestimation of Risk of Clotting
VTE is often clinically silent, leading some physicians to mistakenly believe that it is rare.58 In hospitalized surgical patients, for example, the incidence of thromboembolic complications during a short postoperative stay may be low. Given that many cases of symptomatic VTE occur after hospital discharge, hospitalists and surgeons may be unaware of the true incidence of DVT.59
Overestimation of the Risk of Bleeding
Physicians may also overestimate the risk of possible side effects of prophylaxis, such as major bleeding or heparin‐induced thrombocytopenia (HIT).58 Fear of excess bleeding has been cited by physicians as a leading reason for their decision to withhold thromboprophylaxis from at‐risk hospitalized patients.60 Physicians are particularly fearful of complications among elderly patients, who are less likely to receive adequate prophylaxis than younger patients with a similar risk of VTE.61 When bleeding does occur, it rarely results in death. On the other hand, PE may account for as many as 10% of hospital deaths.9
Guideline Confusion and Complexity
Discrepancies between guidelines published by different medical societies contribute to confusion in choosing a management approach. The American Academy of Orthopedic Surgeons (AAOS), for example, describes aspirin alone as a reasonable choice for VTE prophylaxis in some patients, but the ACCP guidelines advise against the use of aspirin monotherapy.58 The cumbersome nature of multiple risk‐assessment and treatment algorithms can also be problematic.61 Furthermore, certain patient subgroups, such as those with cirrhosis, severe renal failure, and epidural catheters, have been excluded from randomized controlled trials, and the management of such patients is not straightforward.
Absence of Institutional Protocols and Information Technology Support
The lack of institution‐level guidance and support can have a detrimental effect on patient care. In a 2007 survey of 127 community hospitals, the prevalence of institutional protocols related to VTE was low: only 60% had protocols to encourage prophylaxis in at‐risk patients, 54% had guidelines to assist in appropriate drug selection, and 43% had guidelines for the dosing of prophylaxis regimens.62 A lack of systems for data collection and audit has also been identified as a barrier to the implementation of prophylaxis guidelines.57 Thus, hospitals need to adopt protocols such as:3
-
Written, institution‐wide thromboprophylaxis policies
-
Preprinted order forms and computer decision‐support systems
-
Policies specifying responsibilities for assessing VTE risk and prescribing prophylaxis
Conclusions
VTE is the most common preventable cause of hospital death, and prophylaxis is underused in hospitalized patients. Although VTE risk factors are numerous and complex, deciding whether to use prophylaxis need not be complicated. In general, elderly patients, medically‐ill patients, and patients undergoing surgery will benefit from prophylaxis, as well as those who are hospitalized for more than 1 night. Hospitalized patients with at least 1 risk factor should be considered for pharmacologic prophylaxis. In general, the risk of hospital‐acquired VTE greatly exceeds the risk of bleeding with prophylactic doses of anticoagulation. A patient's risk of VTE may change, and regular assessment of this risk should be mandated if pharmacologic therapy is not initiated at the time of admission.
Numerous barriers to the optimal use of VTE prophylaxis exist, and hospitals must implement systems changes and multidisciplinary approaches to overcome these barriers. The fourth article in this supplement provides detailed strategies for meeting VTE performance measures and overcoming barriers to the optimal use of prophylaxis.
Venous thromboembolism (VTE) is a common and potentially devastating complication of medical illness and surgical intervention. Among patients discharged from acute‐care hospitals in 2003, more than 12 million (31%) had a moderate or high risk of VTE during hospitalization, including 11% at risk due to surgical procedures and 20% at risk due to medical illnesses.1 The incidence of VTEwhich can present as deep vein thrombosis (DVT) or pulmonary embolism (PE)is rising in hospitalized patients.2 Despite the availability of effective prophylaxis, VTE is the third most common cause of hospital‐related death and the most common preventable cause of hospital mortality.3
The clinical impact of VTE is significant. While total incidence, prevalence, and mortality rates of VTE are elusive, the annual incidence of DVT is thought to be as high as 2 million.4 The most serious complication of DVT is acute PE, which occurs in approximately 600,000 patients per year, one‐third of whom die.5 DVT may also be complicated by recurrent episodes of VTE and postthrombotic sequelae such as chronic venous stasis, venous ulceration, debilitating pain, and intractable edema.6 One prospective cohort study found that 30% of patients who had experienced a first episode of DVT developed recurrent VTE within 8 years, and the incidence of postthrombotic syndrome (PTS) was 23% after just 2 years.6
The economic burden of VTE is substantial, due both to the initial event and to the high rate of hospital readmission. The estimated average cost of DVT management (including initial acute care and 6 months of follow‐up care) is $10,072 per patient, and the corresponding cost for PE is $14,649.7 Hospital readmission occurs in 5% to 14% of patients, more than one‐half of whom are readmitted within 90 days.8 For patients with recurrent DVT or PE, the mean total hospitalization costs of readmission are $11,862 and $14,722, respectively.8
The clinical and economic burden of VTE can be significantly mitigated by the use of effective prophylaxis. Because VTE is difficult to diagnose antemortem, it is easier and safer to prevent with appropriate prophylaxis than to diagnose after it has occurred. Unfortunately, VTE prophylaxis is markedly underused, particularly among high‐risk, hospitalized medical patients who would most benefit from it.9
This article summarizes specific risk factors for VTE and provides guidance in identifying patients who may require thromboprophylaxis. Barriers to optimal VTE prophylaxis in the hospital setting will also be explored.
Methodology
For this article and the ones that follow, relevant literature was identified through a Medline search (January 1980 to December 2008) using the following search terms: venous thromboembolism, pulmonary embolism, deep vein thrombosis, epidemiology, risk factors, prophylaxis, mechanical prophylaxis, diagnosis, treatment, anticoagulants, monitoring, secondary prevention, guideline, adherence, treatment protocol, performance measure, and quality improvement. The bibliographies of all key texts were searched for additional relevant articles. The websites of the American College of Chest Physicians (ACCP), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and Society of Hospital Medicine (SHM) were also searched for annual meeting abstracts, position statements, and other key publications.
Evidence‐based clinical guidelines were identified through a search of the National Guideline Clearinghouse (
Pathogenesis of VTE
Venous thrombosis occurs as a result of at least 1 of 3 underlying factors: alterations in blood flow, vascular endothelial injury, and alterations in the constitution of the blood.10 Each potential underlying factor encompasses a wide range of risk factors and clinical scenarios. Alterations in venous blood flow can include several situations, including venous stasis, venous hypertension, and valvular incompetence. Endothelial injury can arise from shear stress, direct trauma, infection, hypertension, or other sources of endothelial damage. Hypercoagulability from alterations in the constitution of the blood may be due to antithrombin deficiency, cancer, surgery, pregnancy, or other risk factors. The presence of any of these factors indicates an elevated risk of VTE, and the presence of multiple factors further increases risk.10
Risk Factors for VTE
VTE can occur in a wide variety of clinical circumstances. Recognized risk factors for VTE include hospitalization for an acute medical illness, cardiovascular disease, pulmonary disease, major surgery, multiple trauma, obesity, and increasing age.10 Additional factors that place patients at increased risk of VTE (independent of age) include a history of prior VTE, known hypercoagulable states, active cancer, and acute infection.11 Hospital‐acquired risk factors such as immobility, acute illness, or medical interventions may lead to the development of VTE in these patients. Severity of illness must be factored into the risk assessment, and all patients need to be assessed for VTE risk at the time of hospital admission and daily thereafter if pharmacologic therapy is not initiated.
In a review of 1231 consecutive patients treated for acute DVT and/or PE, 96.3% had at least 1 risk factor for VTE, and more than one‐third (39%) had 3 or more risk factors (Table 1).10 The incidence of VTE in hospitalized patients is directly related to the number of risk factors present:10
-
1 risk factor: 11%
-
2 risk factors: 24%
-
3 risk factors: 36%
-
4 risk factors: 50%
-
5 risk factors: > 90%
| Risk Factor | Patients (%) |
|---|---|
| |
| Age 40 years | 88.5 |
| Obesity | 37.8 |
| History of VTE | 26.0 |
| Cancer | 22.3 |
| Bed rest 5 days | 12.0 |
| Major surgery | 11.2 |
| Congestive heart failure | 8.2 |
| Varicose veins | 5.8 |
| Fracture (hip or leg) | 3.7 |
| Estrogen treatment | 2.0 |
| Stroke | 1.8 |
| Multiple trauma | 1.1 |
| Childbirth | 1.1 |
| Myocardial infarction | 0.7 |
Current or Recent Prior Hospitalization
The risk of VTE is elevated among hospitalized patients. The prevalence of DVT varies across hospital specialties, reaching up to 80% in major trauma, spinal cord injury, and critical care.3 The Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study evaluated the prevalence of VTE risk factors in the acute hospital care setting.12 Among the 68,183 patients enrolled, more than one‐half (52%) were judged to be at risk of VTE. In a case‐control study examining 625 patients with a first lifetime VTE, confinement to a hospital (among other risk factors) was found to be an independent and important predictor of VTE (odds ratio [OR], 8.0; 95% confidence interval [CI], 4.514.2) (Figure 1).13 Recent hospitalization is also an important risk factor for VTE, and patients who are readmitted to the hospital should be considered moderate or high risk.13

Age
Patient age must be considered when assessing VTE risk. VTE is predominantly a disease of older age, and age older than 75 years is an important risk factor for the condition.11 In general, patients older than 40 years have a significantly increased risk compared with younger patients, and the risk approximately doubles with each additional decade.10 Given the aging population, the prevalence of VTE and its complications are expected to increase.
Women of childbearing age experience VTE more frequently than men of the same age, due to pregnancy and exposure to contraceptive therapy.14 This risk, however, is modest compared with the risk among older patients. After age 45 years, the incidence of VTE increases markedly for both sexes, becoming more prominent in men.14 Compared with women, men also have an increased risk of recurrent VTE.15
Despite the effect of age on VTE risk, the risk among patients younger than 40 years may be underestimated because this subgroup has not been extensively studied. For reasons that are not well‐understood, the risk of VTE associated with heart failure is higher in patients younger than 40 years, and the relative risk of PE in patients with chronic obstructive pulmonary disease (COPD) is also higher in younger patients.16, 17
Cancer and Its Treatment
Cancer patients, on average, have twice the risk of VTE compared with noncancer patients.18 This risk, however, varies considerably by cancer type. According to an assessment of nearly 41 million hospitalized patients in the National Hospital Discharge Survey (NHDS), the relative risk of VTE varied from 1.02 in patients with bladder cancer to 4.34 in patients with cancer of the pancreas.18
VTE is one of the most common complications of cancer and cancer therapy, and it is the second leading cause of death among hospitalized cancer patients.19 Molecular mechanisms underlying thromboembolic events in cancer patients include tumor cell procoagulants, inflammatory cell cytokines, mediators of platelet adhesion, and tumor‐related stasis and endothelial damage.20 The clinical implications of these processes are severe. Cancer exacerbates the natural course of VTE, increasing the risk of recurrent VTE and major bleeding, and VTE worsens the prognosis of cancer, increasing the risk of death among cancer patients.
Various cancer therapiesincluding surgery, chemotherapy, hematopoietic stem cell transplantation, and even growth factor supportalso increase the risk of VTE, in part because extrinsic factors such as surgery or chemotherapy can intensify the hypercoagulable process.18, 2123 In the NHDS, cancer patients undergoing surgery had at least twice the risk of postoperative DVT and more than 3 times the risk of PE compared with noncancer patients undergoing similar procedures.18
Cancer is an independent predictor of thromboprophylaxis failure following surgery. The @RISTOS Project found that VTE was the most common cause of death among 2373 patients undergoing general, urologic, or gynecologic surgery for cancer.24 A multivariate analysis identified 5 independent risk factors for VTE after cancer surgery:
-
Previous VTE (OR, 5.98; 95% CI, 2.1316.80)
-
Anesthesia 2 hours (OR, 4.50; 95% CI, 1.0619.04)
-
Bed rest 4 days (OR, 4.37; 95% CI, 2.457.78)
-
Age 60 years (OR, 2.63; 95% CI, 1.215.71)
-
Advanced‐stage cancer (OR, 2.68; 95% CI, 1.375.24)
Cardiovascular Disease
The risk of VTE is pronounced among patients with cardiovascular disease. After stroke and coronary disease, VTE is the third most common cardiovascular disorder, and PE causes more deaths each year than myocardial infarction (MI).25 Several cardiovascular diseases, including hypertension, stroke, acute MI, and heart failure, are independently associated with VTE.10, 2627 Related disorders, including diabetes and the metabolic syndrome, also increase the risk of VTE.26, 28
Congestive heart failure (CHF) is a risk factor for VTE, and the severity of illness increases risk. In the DVT‐Free Prospective Registry, 13% of patients with ultrasound‐confirmed DVT had CHF.29 In a subgroup analysis of patients of the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, the incidence of VTE exceeded 20% in patients with New York Heart Association (NYHA) class IV heart failure, compared with 12% in patients with NYHA class III heart failure.30 Another study found that VTE risk increases as left ventricular ejection fraction (LVEF) decreases, with an LVEF of less than 20% associated with a VTE OR of 38.3 (95% CI, 9.6152.5).31
Infectious Disease
Acute infection may increase the relative risk of VTE by as much as 50% and is associated with VTE event rates of up to 26%.11 Acute infections may be associated with acute inflammation, adverse effects on cardiac or pulmonary function, and prolonged immobilization.30, 32, 33 Human immunodeficiency virus (HIV) patients may also have an increased risk of VTE due to a circulating lupus anticoagulant and/or the presence of acute infection.34
Obesity
The 2008 ACCP guideline update recognizes obesity, for the first time, as a risk factor for VTE.3 Obesity was 1 of the 5 most frequent comorbidities found in patients with DVT in the DVT‐Free Prospective Registry.29 It increases the risk of both incident and recurrent VTE, with every 1‐point increase in body mass index (BMI) increasing the risk of recurrent VTE by 4.4% (95% CI, 1.37.6%; P < 0.001).35
Pregnancy and Puerperium
Pregnancy, particularly the postpartum period, is associated with an increased risk of VTE in women, even though the absolute risk is small.36 Still, PE is one of the leading causes of maternal death following childbirth.10 Smoking, prior VTE, and inherited thrombophilias all increase the risk of VTE in pregnant women.10 The risk begins to rise in the first trimester, and when prophylaxis is needed, it should be started early in gestation.37
Pulmonary Disease
COPD is another risk factor for the development of VTE. COPD patients who develop VTE tend to be older, hospitalized in the intensive care unit (ICU), and on mechanical ventilation.38 In the DVT‐Free Prospective Registry, 12% of patients with ultrasound‐confirmed DVT had COPD.29
Trauma and Surgery
Injury to the body tissue, via trauma or surgery, stimulates the body's clotting mechanism and increases the risk of thromboembolic complications. During the perioperative period, the circulatory system must balance a variety of assaults: an immune response to surgical stress, prolonged immobilization during surgery and recovery, vasodilation associated with general or regional anesthesia, and hypercoagulability due to venous stasis and vascular injury.39 Renal transplant recipients have an increased risk of VTE due to a chronic hypercoagulable state.40 In surgery patients, perioperative complications such as dehydration and acute infection increase the risk of VTE beyond the risk associated with the surgical procedure itself.10
VTE risk is increased approximately 13‐fold by recent major trauma or lower‐extremity injury.13 In the absence of prophylaxis, the overall risk of VTE among patients undergoing major surgery is increased nearly 22‐fold.13 After controlling for the type of surgery, additional independent risk factors for VTE within 3 months of major surgery include:41, 42
-
Obesity
-
Central venous catheter placement
-
Malignancy
-
Smoking
-
Heart failure
-
Previous DVT
-
Prolonged immobility
-
Infection
Many surgical and medical inpatients share common risk factors, and without prophylaxis, the incidence of hospital‐acquired DVT ranges from 10% to 40% for both groups.3
Inherited or Acquired Risk Factors
VTE is a multifactorial disease, and recent evidence indicates that some heritable traits may be potent risk factors for VTE.43 Approximately 35% of patients with DVT will have at least 1 of 5 traits related to an inherited blood clotting disorder:43
-
Deficiencies in the anticoagulation factors protein C, protein S, or antithrombin, or
-
Mutations in the factor V and prothrombin genes, resulting in Factor V Leiden and prothrombin G20210A, respectively.
Certain inherited traits and genetic polymorphisms increase the risk of VTE by interacting with clinical risk factors such as contraceptive use, pregnancy, surgery, trauma, and cancer. One recent study found that oral estrogen therapy among women with the CYP3A5*1 allele was associated with a particularly high risk of VTE.44 Although widespread screening for inherited risk factors is not currently practical, future tools may incorporate genetic polymorphisms to more precisely identify patients who would benefit from aggressive prophylaxis.
Lifestyle Factors
Lifestyle factors have a significant effect on VTE risk. Smoking increases the risk of VTE by 20% to 30%, and a sedentary lifestyle also increases the risk of VTE.26, 45 In fact, women who exercise regularly and consume alcohol in moderation have one‐half the risk of VTE as women who have a sedentary lifestyle and drink little or no alcohol.42 For both men and women, a diet high in fruits, vegetables, and fish is associated with a lower lifetime risk of VTE.46
Medications
Medications may also increase the risk of VTE. In cancer patients with anemia, for example, the use of erythropoiesis‐stimulating agents such as recombinant erythropoietin and darbepoetin was recently shown to increase the risk of VTE by 57% (95% CI, 3187%) and increase mortality risk by 10% (95% CI, 120%).23 In addition, combination hormone replacement therapy in women is associated with a higher risk of VTE compared with estrogen monotherapy, and transdermal contraceptive systems more than double the risk of VTE compared with oral contraceptives (95% CI, 1.33.8).47, 48 Recent studies have also reported an increased risk of VTE with some psychiatric drugs, including amitriptyline, clozapine, olanzapine, and risperidone.4952
Thromboprophylaxis in the Hospital Setting
Despite the prevalence of risk factors and compelling evidence regarding the value of prophylaxis, VTE prophylaxis is suboptimal in hospitalized medical and surgical patients. In a study of 123,304 hospitalized patients who were determined to be at risk of VTE, only 13.3% received prophylaxis in accordance with ACCP guidelines.53 Compliance ranged from a high of 52.4% among patients undergoing orthopedic surgery to a low of 2.8% among patients undergoing neurosurgery.53 Results from several other large trials echo these findings (Table 2).12, 5456
| Trial | Patient Type | Total Patients | Patients at Risk of VTE (Based on ACCP Criteria) (%) | At‐Risk Patients Receiving Recommended Prophylaxis | |
|---|---|---|---|---|---|
| Medical Patients (%) | Surgical Patients (%) | ||||
| |||||
| IMPROVE | Medical patients | 15,156 | 52 | 61 | n/a |
| ENDORSE | Medical and surgical patients | 68,183 | 51.8 | 39.5 | 58.5 |
| ADHERE | Hospitalized heart failure patients | 155,073 | 46 | 30.6 | n/a |
| Amin et al.56 (2008) | Medical and surgical patients | 258,556 | 26.4 | 9.8 | 17.9 |
Reasons for Inadequate Prophylaxis
Researchers have identified a range of barriers to adequate VTE prophylaxis (Table 3).57 Some of these barriers are outlined below.
|
| Variability in clinician knowledge of risk assessment and appropriate prophylaxis |
| Lack of agreement with, and inconsistency between, guidelines in certain patient populations |
| Perceived lack of need |
| Concerns about adverse effects |
| Lack of hospital support systems and policies |
| Lack of established responsibilities for prophylaxis |
Underestimation of Risk of Clotting
VTE is often clinically silent, leading some physicians to mistakenly believe that it is rare.58 In hospitalized surgical patients, for example, the incidence of thromboembolic complications during a short postoperative stay may be low. Given that many cases of symptomatic VTE occur after hospital discharge, hospitalists and surgeons may be unaware of the true incidence of DVT.59
Overestimation of the Risk of Bleeding
Physicians may also overestimate the risk of possible side effects of prophylaxis, such as major bleeding or heparin‐induced thrombocytopenia (HIT).58 Fear of excess bleeding has been cited by physicians as a leading reason for their decision to withhold thromboprophylaxis from at‐risk hospitalized patients.60 Physicians are particularly fearful of complications among elderly patients, who are less likely to receive adequate prophylaxis than younger patients with a similar risk of VTE.61 When bleeding does occur, it rarely results in death. On the other hand, PE may account for as many as 10% of hospital deaths.9
Guideline Confusion and Complexity
Discrepancies between guidelines published by different medical societies contribute to confusion in choosing a management approach. The American Academy of Orthopedic Surgeons (AAOS), for example, describes aspirin alone as a reasonable choice for VTE prophylaxis in some patients, but the ACCP guidelines advise against the use of aspirin monotherapy.58 The cumbersome nature of multiple risk‐assessment and treatment algorithms can also be problematic.61 Furthermore, certain patient subgroups, such as those with cirrhosis, severe renal failure, and epidural catheters, have been excluded from randomized controlled trials, and the management of such patients is not straightforward.
Absence of Institutional Protocols and Information Technology Support
The lack of institution‐level guidance and support can have a detrimental effect on patient care. In a 2007 survey of 127 community hospitals, the prevalence of institutional protocols related to VTE was low: only 60% had protocols to encourage prophylaxis in at‐risk patients, 54% had guidelines to assist in appropriate drug selection, and 43% had guidelines for the dosing of prophylaxis regimens.62 A lack of systems for data collection and audit has also been identified as a barrier to the implementation of prophylaxis guidelines.57 Thus, hospitals need to adopt protocols such as:3
-
Written, institution‐wide thromboprophylaxis policies
-
Preprinted order forms and computer decision‐support systems
-
Policies specifying responsibilities for assessing VTE risk and prescribing prophylaxis
Conclusions
VTE is the most common preventable cause of hospital death, and prophylaxis is underused in hospitalized patients. Although VTE risk factors are numerous and complex, deciding whether to use prophylaxis need not be complicated. In general, elderly patients, medically‐ill patients, and patients undergoing surgery will benefit from prophylaxis, as well as those who are hospitalized for more than 1 night. Hospitalized patients with at least 1 risk factor should be considered for pharmacologic prophylaxis. In general, the risk of hospital‐acquired VTE greatly exceeds the risk of bleeding with prophylactic doses of anticoagulation. A patient's risk of VTE may change, and regular assessment of this risk should be mandated if pharmacologic therapy is not initiated at the time of admission.
Numerous barriers to the optimal use of VTE prophylaxis exist, and hospitals must implement systems changes and multidisciplinary approaches to overcome these barriers. The fourth article in this supplement provides detailed strategies for meeting VTE performance measures and overcoming barriers to the optimal use of prophylaxis.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82(9):777–782.
- , , .Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients.Am J Cardiol.2005;95(12):1525–1526.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th ed.Chest.2008;133(6 suppl):381S–453S.
- , .Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association.Circulation.1996;93(12)2212–2245.
- , , , et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- , , , et al.The long‐term clinical course of acute deep venous thrombosis.Ann Intern Med.1996;125(1):1–7.
- , .Direct medical cost of managing deep vein thrombosis according to the occurrence of complications.Pharmacoeconomics.2002;20(9):603–615.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , .Risk factors for venous thromboembolism.Circulation.2003;107(23 suppl 1):I9–I16.
- , , , et al.Risk factors for VTE in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- , , , et al.;ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , et al.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160(6):809–815.
- , .Range of published estimates of venous thromboembolism incidence in young women.Contraception.2007;75(5):328–336.
- , , , , , .The risk of recurrent venous thromboembolism in men and women.N Engl J Med.2004;350(25):2558–2563.
- , , , et al.Risk of venous thromboembolism in patients hospitalized with heart failure.Am J Cardiol.2006;98(6):793–795.
- , , , et al.Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease.J Cardiovasc Med.2007;8(4):253–257.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.Causes of death in cancer patients.J Med.1975;6(1):61–64.
- , .Tissue factor and angiogenesis in cancer.Curr Opin Hematol.2002;9(5):401–406.
- , , , et al.Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel‐containing first‐line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group.J Clin Oncol.2008;26(16):2683–2689.
- , , , , , .The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention.Blood.2008;112(3):504–510.
- , , , et al.Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia.JAMA.2008;299(8):914–924.
- , , , et al.A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project.Ann Surg.2006;243(1):89–95.
- .Pulmonary embolism in thrombolysis: a clarion call for international collaboration.J Am Coll Cardiol.1992;19:246–247.
- , , , et al.Cardiovascular risk factors and venous thromboembolism: a meta‐analysis.Circulation.2008;117(1):93–102.
- , , , , , .Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study.J Thromb Haemost.2008;6(11):1851–1857.
- , , , et al.Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome—the Tromsø study.J Thromb Haemost.2009;7(5):739–745.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , , .Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case‐control study.J Clin Epidemiol.2001;54(8):810–816.
- , , , et al;ARTEMIS Investigators.Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- , , , et al.HIV infection is a risk factor for venous thromboembolism.AIDS Patient Care STDS.2002;16(5):205–209.
- , , , et al.Overweight, obesity, and the risk of recurrent venous thromboembolism.Arch Intern Med.2008;168(15):1678–1683.
- .Venous thromboembolism in women.Vasc Med.2008;13(3):255–266.
- , , .Thrombosis during pregnancy and the postpartum period.Am J Obstet Gynecol.2005;193(1):216–219.
- , , , et al.Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination.J Thromb Thrombolysis.2008;26(1):35–40.
- , , .Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability?J Trauma.2003;54(2):224–231.
- , .Acquired hypercoagulable state in renal transplant recipients.Thromb Haemost.2004;91(4):646–654.
- , , , .Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion.Thromb Haemost.2007;98(6):1220–1225.
- , , , , , .A 10‐year analysis of venous thromboembolism on the surgical service: the effect of practice guidelines for prophylaxis.Surgery.2008;144(1):3–11.
- , , , et al;EDITH Collaborative Study Group.Family history as a risk factor for venous thromboembolism.Thromb Res.2008;122(5):624–629.
- , , , et al;Estrogen and Thromboembolism Risk (ESTHER) Study Group.Synergism between oral estrogen therapy and cytochrome P450 3A5*1 allele on the risk of venous thromboembolism among postmenopausal women.J Clin Endocrinol Metab.2008;93(8):3082–3087.
- , , .The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study.Br J Haematol.2009;144(2):234–240.
- , , , , .Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology.Circulation.2007;115(2):188–195.
- , , .Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta‐analysis.Eur Heart J.2008;29(16):2031–2041.
- , , , .Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users.Obstet Gynecol.2007;109(2 Pt 1):339–346.
- , , , , , .Pulmonary thromboembolism associated with olanzapine and risperidone.J Emerg Med.2008;35(2):159–161.
- , , , .Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions.Drug Saf.2008;31(8):685–694.
- .Evaluating the association between clozapine and venous thromboembolism.Am J Health Syst Pharm.2008;65(19):1825–1829.
- , .Antidepressant drug use and risk of venous thromboembolism.Pharmacotherapy.2008;28(2):144–150.
- , , , .Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64(1):69–76.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients.Chest.2007;132:936–945.
- , , , et al.Adhere Scientific Advisory Committee and Investigators. Venous thromboembolism prophylaxis in hospitalized heart failure patients.J Card Fail.2008;14(2):127–132.
- , , , et al. Are hospitals following guidelines for VTE Prevention? The Venous Thromboembolism Study to Assess the Rate of Thromboprophylaxis. Presented at the 50th Annual Meeting of the American College of Hematology, San Francisco, CA, December 6–9, 2008. Abstract 1286.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- .A need for a simplified approach to venous thromboembolism prophylaxis in acute medical inpatients.Int J Clin Pract.2007;61(2):336–340.
- .Barriers to the optimal use of anticoagulants after orthopaedic surgery.Arch Orthop Trauma Surg.2008 (Published online Oct, 8.). [http://dx.doi.org/DOI 10.1007/s00402‐008‐0765‐9]
- , , , , .Venous thrombosis in cancer patients: Insights from the FRONTLINE survey.Oncologist.2003;8:381–388.
- , , , et al.Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross‐sectional study in Brazil.J Thromb Haemost.2006;4(6):1266–1270.
- , , , , .Survey of hospitals for guidelines, policies, and protocols for anticoagulants.Am J Health Syst Pharm.2007;64(11):1203–1208.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82(9):777–782.
- , , .Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients.Am J Cardiol.2005;95(12):1525–1526.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th ed.Chest.2008;133(6 suppl):381S–453S.
- , .Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association.Circulation.1996;93(12)2212–2245.
- , , , et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- , , , et al.The long‐term clinical course of acute deep venous thrombosis.Ann Intern Med.1996;125(1):1–7.
- , .Direct medical cost of managing deep vein thrombosis according to the occurrence of complications.Pharmacoeconomics.2002;20(9):603–615.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , , .Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent.Chest.2007;132(2):554–561.
- , .Risk factors for venous thromboembolism.Circulation.2003;107(23 suppl 1):I9–I16.
- , , , et al.Risk factors for VTE in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- , , , et al.;ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , et al.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160(6):809–815.
- , .Range of published estimates of venous thromboembolism incidence in young women.Contraception.2007;75(5):328–336.
- , , , , , .The risk of recurrent venous thromboembolism in men and women.N Engl J Med.2004;350(25):2558–2563.
- , , , et al.Risk of venous thromboembolism in patients hospitalized with heart failure.Am J Cardiol.2006;98(6):793–795.
- , , , et al.Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease.J Cardiovasc Med.2007;8(4):253–257.
- , , , et al.Incidence of venous thromboembolism in patients hospitalized with cancer.Am J Med.2006;119(1):60–68.
- , , , et al.Causes of death in cancer patients.J Med.1975;6(1):61–64.
- , .Tissue factor and angiogenesis in cancer.Curr Opin Hematol.2002;9(5):401–406.
- , , , et al.Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel‐containing first‐line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group.J Clin Oncol.2008;26(16):2683–2689.
- , , , , , .The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention.Blood.2008;112(3):504–510.
- , , , et al.Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia.JAMA.2008;299(8):914–924.
- , , , et al.A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project.Ann Surg.2006;243(1):89–95.
- .Pulmonary embolism in thrombolysis: a clarion call for international collaboration.J Am Coll Cardiol.1992;19:246–247.
- , , , et al.Cardiovascular risk factors and venous thromboembolism: a meta‐analysis.Circulation.2008;117(1):93–102.
- , , , , , .Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromsø study.J Thromb Haemost.2008;6(11):1851–1857.
- , , , et al.Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome—the Tromsø study.J Thromb Haemost.2009;7(5):739–745.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- , , , et al.Prevention of venous thromboembolism in medical patients with enoxaparin; a subgroup analysis of the MEDENOX study.Blood Coagul Fibrinolysis.2003;14:341–348.
- , , .Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case‐control study.J Clin Epidemiol.2001;54(8):810–816.
- , , , et al;ARTEMIS Investigators.Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial.BMJ.2006;332(7537):325–329.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- , , , et al.HIV infection is a risk factor for venous thromboembolism.AIDS Patient Care STDS.2002;16(5):205–209.
- , , , et al.Overweight, obesity, and the risk of recurrent venous thromboembolism.Arch Intern Med.2008;168(15):1678–1683.
- .Venous thromboembolism in women.Vasc Med.2008;13(3):255–266.
- , , .Thrombosis during pregnancy and the postpartum period.Am J Obstet Gynecol.2005;193(1):216–219.
- , , , et al.Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination.J Thromb Thrombolysis.2008;26(1):35–40.
- , , .Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability?J Trauma.2003;54(2):224–231.
- , .Acquired hypercoagulable state in renal transplant recipients.Thromb Haemost.2004;91(4):646–654.
- , , , .Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion.Thromb Haemost.2007;98(6):1220–1225.
- , , , , , .A 10‐year analysis of venous thromboembolism on the surgical service: the effect of practice guidelines for prophylaxis.Surgery.2008;144(1):3–11.
- , , , et al;EDITH Collaborative Study Group.Family history as a risk factor for venous thromboembolism.Thromb Res.2008;122(5):624–629.
- , , , et al;Estrogen and Thromboembolism Risk (ESTHER) Study Group.Synergism between oral estrogen therapy and cytochrome P450 3A5*1 allele on the risk of venous thromboembolism among postmenopausal women.J Clin Endocrinol Metab.2008;93(8):3082–3087.
- , , .The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study.Br J Haematol.2009;144(2):234–240.
- , , , , .Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology.Circulation.2007;115(2):188–195.
- , , .Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta‐analysis.Eur Heart J.2008;29(16):2031–2041.
- , , , .Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users.Obstet Gynecol.2007;109(2 Pt 1):339–346.
- , , , , , .Pulmonary thromboembolism associated with olanzapine and risperidone.J Emerg Med.2008;35(2):159–161.
- , , , .Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions.Drug Saf.2008;31(8):685–694.
- .Evaluating the association between clozapine and venous thromboembolism.Am J Health Syst Pharm.2008;65(19):1825–1829.
- , .Antidepressant drug use and risk of venous thromboembolism.Pharmacotherapy.2008;28(2):144–150.
- , , , .Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64(1):69–76.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients.Chest.2007;132:936–945.
- , , , et al.Adhere Scientific Advisory Committee and Investigators. Venous thromboembolism prophylaxis in hospitalized heart failure patients.J Card Fail.2008;14(2):127–132.
- , , , et al. Are hospitals following guidelines for VTE Prevention? The Venous Thromboembolism Study to Assess the Rate of Thromboprophylaxis. Presented at the 50th Annual Meeting of the American College of Hematology, San Francisco, CA, December 6–9, 2008. Abstract 1286.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- .A need for a simplified approach to venous thromboembolism prophylaxis in acute medical inpatients.Int J Clin Pract.2007;61(2):336–340.
- .Barriers to the optimal use of anticoagulants after orthopaedic surgery.Arch Orthop Trauma Surg.2008 (Published online Oct, 8.). [http://dx.doi.org/DOI 10.1007/s00402‐008‐0765‐9]
- , , , , .Venous thrombosis in cancer patients: Insights from the FRONTLINE survey.Oncologist.2003;8:381–388.
- , , , et al.Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross‐sectional study in Brazil.J Thromb Haemost.2006;4(6):1266–1270.
- , , , , .Survey of hospitals for guidelines, policies, and protocols for anticoagulants.Am J Health Syst Pharm.2007;64(11):1203–1208.