User login
Despite the availability of effective thromboprophylaxis, the prevalence of venous thromboembolism (VTE) is increasing in the hospital setting. In 2008, the Fifth Annual Health Grades Patient Safety in American Hospitals Study reported on key patient safety incidents among nearly 41 million hospitalizations in the Medicare population between 2004 and 2006. Although many areas showed improvementincluding reduced rates of hospital‐related infections, postoperative bleeding, transfusion reactions, and other injuriesthe number of cases of postoperative VTE increased by 11% during this period.1
Even with optimal thromboprophylaxis, VTE will develop in some at‐risk patients. Early diagnosis and treatment of VTE is critical to reduce morbidity and mortality, but no single tool can definitively confirm its presence. Consequently, the detection of deep vein thrombosis (DVT) and pulmonary embolism (PE) requires a stepwise diagnostic strategy that combines clinical, biochemical, and imaging modalities.
In addition to outlining diagnostic strategies for DVT and PE, this article summarizes VTE treatment guidelines from various organizations and societies and discusses long‐term management strategies to prevent recurrent VTE and other complications.
Diagnosis of DVT
The clinical symptoms and signs of DVT are nonspecific and include unilateral calf, leg, or thigh swelling and pain. Despite the limited sensitivity and specificity of individual signs and symptoms of DVT, the combination of these variables can be useful in assessing the probability of VTE. Patients can be risk stratified according to the likelihood of DVT, as determined by implicit clinical judgment or by a validated prediction rule.2
Assessment of Clinical Probability
The Wells prediction rule is used in assessing the probability of DVT.3 It incorporates signs, symptoms, and risk factors of DVT to calculate a clinical probability rating. Specifically, 1 point is assigned to each of the following factors, if present:3
-
Active cancer (treatment ongoing, within 6 months, or palliative)
-
Calf swelling >3 cm asymptomatic side (measured 10 cm below tibial tuberosity)
-
Collateral superficial veins (nonvaricose)
-
Entire leg swelling
-
Localized tenderness along the distribution of the deep venous system
-
Paralysis, paresis, or recent plaster immobilization of the lower extremities
-
Pitting edema confined to the symptomatic leg
-
Recently bedridden more than 3 days or major surgery within 4 weeks
In addition, 2 points are subtracted if an alternative diagnosis is as likely as or more likely than DVT. In patients with symptoms in both legs, the more symptomatic leg is used.
Patients with low (score <1), moderate (score 1‐2), and high (score 3) pretest probability of DVT have been shown to have DVT prevalence rates of 3%, 17%, and 75%, respectively.3
D‐Dimer Testing
D‐dimer testing measures the small protein fragments remaining in the blood after a cross‐linked fibrin clot is degraded by fibrinolysis. A low clinical probability assessment combined with a negative result in a highly sensitive, enzyme‐linked immunosorbent assay (ELISA)‐based D‐dimer test can safely exclude DVT, with a negative predictive value of 99.1% (95% confidence interval [CI]; 96.7‐99.9).4
Due to its poor specificity, D‐dimer testing has limited utility in unselected inpatients, especially older patients and those who have undergone prolonged hospitalization.5 However, it is reasonable to obtain a highly sensitive, ELISA‐based D‐dimer test in carefully selected inpatients with a low pretest probability of DVT.5, 6 In such patients, a negative result indicates that DVT is highly unlikely, while a positive result indicates a need for further testing. D‐dimer testing is likely not helpful in moderate‐risk or high‐risk patients.
Diagnostic Imaging
For patients with a moderate to high pretest probability of DVT, ultrasound is recommended.6 Compression ultrasonography (CUS) is currently the preferred imaging tool in patients with suspected DVT because it is noninvasive, can be repeated serially, and offers high sensitivity (+90%) and high specificity (95%) for detecting proximal vein thrombosis.7, 8 If the clinical suspicion of DVT persists after an initial negative CUS study, imaging can be repeated after 3 to 7 days to detect the propagation of any thrombosis to the proximal veins. Limitations of CUS include poor visualization of deep iliac and pelvic veins and poor sensitivity in isolated or nonocclusive calf vein thrombi.2
Contrast venography was considered the gold standard for the detection of DVT of the lower extremity, but this modality is invasive, painful, and offers poor visualization of the deep femoral vein and the internal iliac vein. In addition, contrast venography is associated with an increased risk of new thrombosis, renal failure, and hypersensitivity reaction to contrast media. Consequently, contrast venography is currently used in symptomatic patients only when noninvasive testing is inconclusive or unavailable.2 Other second‐line diagnostic tools include computed tomography venography (CTV) and magnetic resonance venography (MRV).9
Diagnostic Strategy
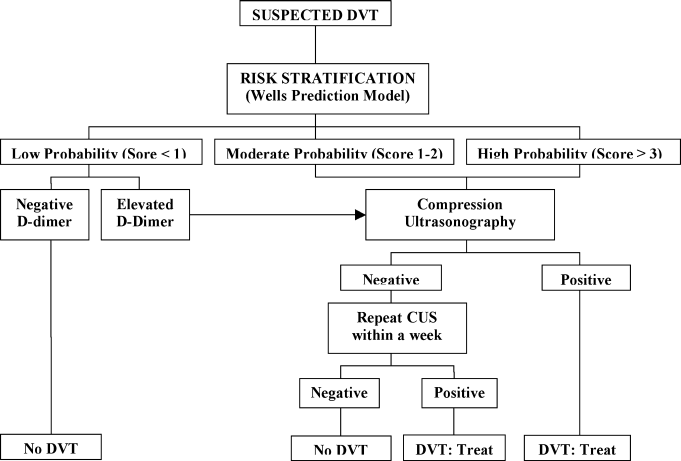
A diagnostic algorithm for DVT is presented in Figure 1. First, a validated clinical prediction scale such as the Wells prediction rule should be used to estimate the pretest probability of DVT, and the result of the clinical assessment should influence the choice and interpretation of subsequent testing.
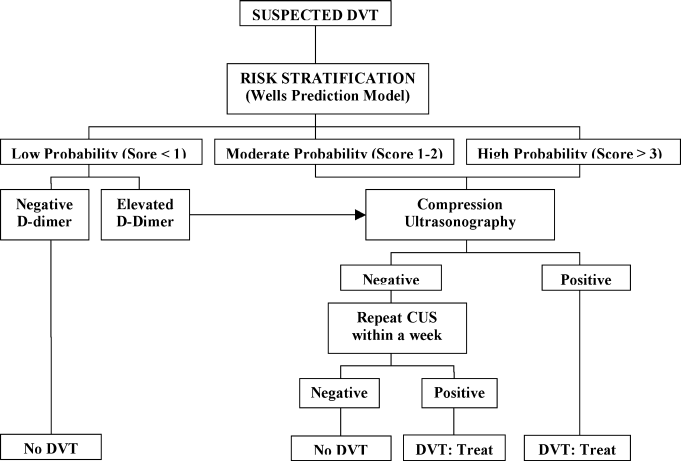
Abbreviations: CUS, compression ultrasonography; DVT, deep vein thrombosis (DVT).
Diagnosis of PE
Clinical symptoms and signs such as dyspnea, chest pain, tachycardia, tachypnea, and syncope raise the suspicion of PE. Individual signs and symptoms, however, cannot confirm or exclude acute PE, as they are neither sensitive nor specific.10 Furthermore, although the likelihood of PE increases with the number of predisposing risk factors, approximately 30% of PE cases are unprovoked or idiopathic, meaning that they occur in the absence of predisposing factors. Diagnosis, therefore, depends on an integrated strategy involving similar tools as those used in diagnosing DVT.
Assessing Clinical Probability
Wells et al.11 also developed a clinical prediction rule for the risk stratification of patients with suspected PE. In this model, 7 specified variables are assigned different scores: clinical signs and symptoms of DVT (3.0); lack of a likely alternative diagnosis (3.0); heart rate greater than 100 beats per minute (1.5); immobilization for more than 3 days or surgery in the previous 4 weeks (1.5); previous DVT/PE (1.5); hemoptysis (1.0); and malignancy (1.0). Although the Wells prediction rule initially categorized 3 levels of probability for PE (low, moderate, or high), a revised model uses a simplified, dichotomized approach to determine whether PE is likely (Wells score >4) or unlikely (4 Wells score).11 An independent, prospective observational study found that the Wells prediction model reliably risk‐stratified pretest probability in patients with suspected PE.12
For patients who are stratified into the low‐risk category, the pulmonary embolism rule‐out criteria (PERC) rule may be helpful in reducing unnecessary diagnostic testing for PE.13 The PERC rule consists of 8 variables designed to offer a pretest probability of PE of less than 1.8%, a probability at which further testing is unnecessary. If the clinical gestalt is that PE is unlikely and all of the following variables are present, further testing can be safely discontinued: (1) pulse <100; (2) age <50; (3) oxygen saturation (SaO2) >94%; (4) no unilateral leg swelling; (5) no hemoptysis; (6) no recent trauma or surgery; (7) no prior DVT or PE; and (8) no hormone use.13 In a large, multicenter study, these criteria combined with a gestalt interpretation of low risk were shown to select a subgroup of patients with a very low probability of PE (<2%).14
D‐Dimer Testing
Evidence suggests that the combination of a low clinical probability assessment and a normal result in a highly sensitive, ELISA‐based D‐dimer test can safely exclude PE in hospitalized patients.15 Due to the large number of comorbidities among hospitalized patients, however, this combination occurs in only approximately 10% of inpatients.15 D‐dimer levels may be elevated in patients with a variety of nonthrombotic conditions, and it is therefore most useful in the diagnosis of otherwise healthy patients who have symptoms of PE. D‐dimer testing is not appropriate in moderate‐risk or high‐risk patients.
Diagnostic Imaging
Computed tomography (CT) is a leading imaging modality for the exclusion or confirmation of PE, as well as for the detection of alternative diagnoses. The diagnostic algorithms endorsed by the European Society of Cardiology (ESC) rely on both single‐detector and multidetector CT. However, multidetector CT scanners are now preferred because, in contrast to single‐detector CT, they can detect pulmonary emboli in smaller pulmonary arteries.10 Because single‐detector CT has a limited sensitivity of approximately 70%, it must be used in conjunction with lower limb venous CUS.16 In contrast, multidetector CT angiography has high sensitivity (83%) and specificity (96%) for the detection of PE and does not require the additional use of lower limb venous CUS.16, 17
Diagnostic Strategy
The Christopher Study demonstrated the utility of a diagnostic algorithm that incorporates a dichotomized decision rule, D‐dimer testing, and CT. In this approach, PE is excluded in patients with an unlikely clinical probability score (Wells score 4) and a normal D‐dimer test result. In all other patients, CT is the sole imaging method used to make management decisions.18 However, in patients with massive pulmonary embolism, if CT angiography is not immediately available, selective pulmonary angiography has been performed to identify and localize the emboli before aggressive therapy is instituted (Figure 2).19 If the patient is critically ill (hypotensive, severely hypoxemic), empiric treatment is appropriate while diagnostic strategy is being formulated.
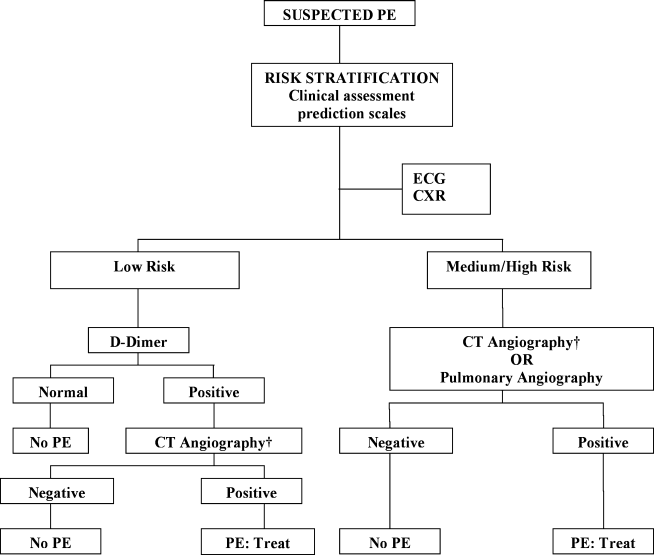
Abbreviations: CT, computed tomography; CXR, plain chest X‐ray; ECG, electrocardiogram; PE, pulmonary embolism. †CT angiography using multidetector instruments.
Treatment Options for VTE
For patients with VTE, the American College of Chest Physicians (ACCP) guidelines recommend initial treatment with low‐molecular‐weight heparin (LMWH), intravenous unfractionated heparin (UFH), or adjusted‐dose subcutaneous UFH, followed by at least 3 months of oral anticoagulation therapy.20
When VTE is diagnosed, anticoagulation should be initiated immediately unless contraindications are present. In addition, patients without contraindications to anticoagulation should receive treatment before diagnostic testing if such testing is delayed or if the clinical suspicion of VTE is high.20
Anticoagulant Treatment
For decades, parenteral administration of UFH for 5 to 7 days followed by long‐term warfarin therapy has been the conventional treatment of patients with VTE. Although UFH can be administered subcutaneously or by intravenous (IV) infusion, continuous IV infusion has been preferred because of superior dosing precision. The anticoagulation effect of intravenous UFH must be monitored to ensure a therapeutic activated partial thromboplastin time (aPTT). Consequently, the use of intravenous UFH requires frequent aPTT assessment and dose adjustment.20
Given their ease of use and improved pharmacokinetic and pharmacodynamic profiles, LMWHs have replaced UFH for the treatment of VTE in many institutions. Fondaparinux is also a safe and effective alternative to both intravenous UFH and LMWH in the treatment of VTE.20 It has a longer half‐life (15‐20 hours) than LMWH, permitting a once‐daily administration, and in patients with submassive PE, its efficacy and safety are comparable to UFH.21 Platelet count monitoring is not necessary with fondaparinux because it is given at weight‐adjusted doses, and only 1 case of heparin‐induced thrombocytopenia (HIT) has been reported.22 It is, however, contraindicated in renal failure with a creatinine clearance of <30 mL/minute.10
Warfarin is very effective in the long‐term management of VTE and should be started concurrently with rapid‐acting injectable anticoagulation therapy. Warfarin requires overlap with injectable anticoagulants for a minimum of 5 days until a therapeutic international normalized ratio (INR) has been achieved.20
Other Treatments
Most patients with VTE can be treated effectively with only anticoagulation therapy. However, in cases of massive PE (with or without systemic arterial hypotension and usually with significant hypoxemia not generally responsive to supplemental oxygen), removal of the occluding thrombus by thrombolytic agents, special clot‐removing catheters, or surgical procedures may be necessary to prevent or ameliorate shock and subsequent death.23 In other cases, such as when anticoagulants are ineffective or contraindicated, an inferior vena cava (IVC) filter may be an appropriate option for VTE treatment. Importantly, guidelines do not recommend filters in patients who can tolerate anticoagulation.
Permanent and retrievable IVC filters are effective at preventing PE and are generally associated with a low complication rate.24 However, nonfatal complications are relatively common with permanent IVC filters. One early complication is insertion‐site thrombosis, which occurs in about 10% of patients. Subsequent complications are more frequent and include recurrent DVT and post‐thrombotic syndrome (PTS), which occur in approximately 20% and 40% of patients, respectively. At 5 and 9 years, about 22% and 33% of the filters are occluded, regardless of the use and duration of anticoagulation.2527 To minimize these complications, retrievable filters have been increasingly used, but most filters are not retrieved and are subject to the same complications as permanent IVC filters.28
Catheter‐directed thrombolysis, with or without IVC filter placement, is safe and effective in treating acute DVT.29 Additional measures, such as the use of graduated compression stockings, can reduce the risk of developing PTS.20
Guideline Recommendations
Guidelines from the ACCP, the American College of Physicians (ACP), and the American Academy of Family Physicians (AAFP) address the treatment of VTE in a broad spectrum of patients. Additional guidelines provide recommendations for specific presentations or patient groups. For example, the ESC guidelines address the treatment of acute PE, and several groupsthe American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the French Working Group (FWG)have published guidelines for the treatment of VTE in patients with cancer. The following sections summarize the most important recommendations from several of these organizations and societies.
ACCP Guidelines
The ACCP guideline recommendations are assigned grades of 1 or 2, denoting a stronger or weaker recommendation, as well as a grade of A, B, or C, indicating high‐quality evidence, moderate‐quality evidence, and low‐quality evidence, respectively. Physicians must supplement the guideline recommendations with informed clinical judgment to ensure proper use of treatment in at‐risk hospitalized patients.20
The 2008 ACCP guidelines suggest several options for the initial treatment of VTE, which are listed, along with acceptable dosing regimens, in Table 1.20, 30, 31 Fixed‐dose, unmonitored, subcutaneous UFH and fondaparinux are new Grade 1A additions to the 2008 update. In general, LMWH is preferred over intravenous UFH, except in patients with severe renal failure.20
| Initial Anticoagulation Therapy | Grade | Acceptable Treatment Regimen* |
|---|---|---|
| ||
| SC LMWH | 1A | Enoxaparin: 1 mg/kg every 12 hours or 1.5 mg/kg once daily; Dalteparin: 200 IU/kg once daily (can be administered out of hospital) |
| Intravenous UFH | 1A | Get baseline aPTT, PT, and platelet count; if no abnormalities, proceed with a weight‐based heparin infusion protocol such as: |
| Bolus of 80 U/kg, followed by an infusion of 18 U/kg per hour (treatment duration 72 days); check aPTT every 4‐6 hours and adjust according to the normogram; monitor platelet count every 3‐4 days for HIT | ||
| Monitored SC UFH; fixed‐dose, unmonitored, SC UFH | 1A; 1A | Initial dose of 333 U/kg, followed by a fixed dose of 250 U/kg every 12 hours (can be administered out of hospital) |
| SC fondaparinux | 1A | 5 mg (body weight <50 kg), 7.5 mg (body weight 50‐100 kg), or 10 mg (body weight >100 kg) once daily (treatment duration 72 days) |
| IVC filter if anticoagulation contraindicated | 1C | |
Warfarin should also be initiated on the same day as UFH or LMWH and adjusted to a target INR of 2.5 (range, 2.0‐3.0). Treatment with UFH or LMWH should be continued concomitantly for a minimum of 5 days and should not be discontinued until the INR has been over 2.0 for 24 hours. The ACCP guidelines also recommend systematic follow‐up of oral anticoagulation therapy.
ACP/AAFP Guidelines
In 2007, the ACP and the AAFP collaborated to develop joint guidelines for the management of VTE.32 Several of their key recommendations are the following:32
-
LMWH, rather than UFH, should be used whenever possible for the initial inpatient treatment of DVT
-
Either UFH or LMWH is appropriate for the initial treatment of PE
-
Anticoagulation should be continued for 3 to 6 months for VTE secondary to transient risk factors, and for more than 12 months for recurrent VTE
-
LMWH is safe and effective for the long‐term treatment of VTE in selected patients (and may be preferable for patients with cancer)
ESC Guidelines for the Treatment of PE
According to the 2008 ESC guidelines, anticoagulation with UFH, LMWH, or fondaparinux should be initiated immediately in patients with confirmed PE, as well as in those with a high or intermediate clinical probability of PE while the diagnostic workup is ongoing. Subcutaneous LMWH or fondaparinux is preferable to intravenous UFH for initial treatment in most patients. UFH, however, should be used in patients with a high risk of bleeding due to its capacity for reversal and short half‐life, as well as in those with severe renal dysfunction.10
According to the ESC, patients with high‐risk PE (presenting with cardiogenic shock or persistent arterial hypotension) should receive thrombolytic therapy as first‐line therapy. Hemodynamic and respiratory support is also necessary for these patients.10 Routine thrombolysis is not recommended in patients with non‐high‐risk PE, but it may be considered in select patients with intermediate‐risk PE (characterized by severe right ventricular dysfunction on echocardiography and/or myocardial injury), depending on the patient's risk of bleeding. Thrombolytic therapy should not be used in patients with low‐risk PE (presenting without shock, hypotension, right ventricular dysfunction, or myocardial injury).10
Like the ESC guidelines, the ACCP guidelines recommend against the use of thrombolytic therapy for the majority of patients with PE (Grade 1B), but they do recommend its use in patients with evidence of hemodynamic compromise and no major contraindications owing to bleeding risk (Grade 1B) and in certain other high‐risk patients (Grade 2B).20
The ESC states that pulmonary embolectomy has recently become a reasonable option for patients with massive, high‐risk PE and an absolute contraindication to thrombolysis, or in whom thrombolysis has failed, when appropriate expertise is available. In the past, it was performed as a last resort in patients with massive PE who were in shock and conferred a high risk of mortality (+50%). Recently, however, the procedure has been revived and performed immediately in patients with confirmed massive PE (with severe right ventricular dysfunction but before shock), with mortality rates of less than 10%.19 It should be noted, however, that the ACCP guidelines consider embolectomy a Grade 2C recommendation.20 Alternatively, catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in these patients.10
NCCN Guidelines: Oncology Patients
The NCCN has provided treatment algorithms for the management of DVT and PE in patients with cancer, which are available online at
FWG Guidelines: Oncology Patients
At the 2008 ASH annual meeting, the FWG presented updated guidelines for the treatment of VTE in cancer patients.34 The FWG guidelines contain the following key recommendations:
-
The treatment of VTE should be based on LMWH at curative doses for at least 3 months
-
During the initial treatment (up to 10 days), any approved drug (including LMWH, UFH, and fondaparinux) may be used
-
Beyond the first 10 days, VTE treatment should be based on LMWH at curative doses for at least 3 months and optimally 6 months, as validated with the following drugs and dosage regimens:
-
Dalteparin 200 IU/kg once daily for 1 month, then 150 IU/kg once daily
-
Enoxaparin 150 IU/kg (1.5 mg/kg) once daily
-
Tinzaparin 175 IU/kg once daily
-
Special treatment considerations include the following:
-
In severe renal impairment, UFH should be used and rapidly followed by a vitamin K agonist (VKA) for at least 3 months
-
In severe PE (representing hemodynamic failure), the indications and recommended uses of thrombolytic drugs in noncancer patients apply
-
In patients with an absolute contraindication to anticoagulation or VTE recurrence despite optimal anticoagulation, vena cava filters should be considered
-
In patients with intracranial malignancies, VTE treatment is the same as in cancer patients with nonintracranial tumors
The treatment of central venous catheter thrombosis requires the long‐term use of LMWH according to the FWG guidelines. In patients with severe renal failure, UFH with early VKA must be used as an alternative treatment. Regardless of the therapy used, treatment should be continued as long as the catheter is maintained.34
Long‐Term Management of VTE
The high rate of recurrent VTE after a first episode of DVT or PEapproximately 8% within 90 daysunderscores the importance of maintaining effective prophylaxis postdischarge.35 Inadequate prophylaxis following discharge from the hospital can have severe consequences. In a recent study of 10,744 patients who were discharged from the hospital following hip or knee replacement surgery, fewer than 1 in 5 received postdischarge thromboprophylaxis. The 3‐month risk of mortality was significantly lower among those who received thromboprophylaxis at discharge (adjusted hazard ratio, 0.34; 95% CI, 0.20‐0.57).36
Detailed patient education at the time of discharge may be one of the most effective ways to prevent or minimize the burden of long‐term complications such as PTS or recurrent VTE. Accordingly, proper discharge planning and postdischarge support, including an appropriate anticoagulant, are critical steps toward reducing mortality, morbidity, and healthcare costs.
PTS
As many as 50% of patients with VTE will develop PTS, a serious but preventable complication that leads to pain, swelling, and skin changes in the affected limb. Female gender, older age, higher body mass index (BMI), and DVT of the common femoral or iliac vein (vs. distal DVT) are associated with an increased risk of PTS.37 To prevent PTS in a patient who has had a symptomatic proximal DVT, current guidelines recommend the use of graduated elastic compression stockings with an ankle pressure of 30 to 40 mm Hg, if feasible. Compression therapy should start as soon as possible after the initiation of anticoagulation therapy and be encouraged for a minimum of 2 years.20
Recurrent VTE
After discontinuing anticoagulation, the risk of recurrent VTE increases steadily over time. In a recent long‐term study of patients with acute proximal DVT or PE, the risk of recurrent VTE was 11% after 1 year, 20% after 3 years, 30% after 5 years, and 40% after 10 years. In this study, risk factors for recurrent VTE included unprovoked initial VTE, thrombophilia, increasing age, and a shorter duration of anticoagulation (6 months or less).38 Another study identified residual venous thrombosis as an important risk factor for recurrent VTE.39 In addition, 1 meta‐analysis found that men had a 50% higher risk of recurrent VTE than women.40 Recurrent DVT events are associated with a 21% greater cost than the initial event, suggesting that recurrent VTE is a preventable drain on healthcare resources.41
Secondary Prevention
The risk of recurrent VTE is determined by the effectiveness of treatment for the acute episode of VTE and by the patient's intrinsic risk of thromboembolism. The ACCP recommends different durations of warfarin or LMWH anticoagulant therapy according to these features (Table 2).20 Attaching a high value to prevention of recurrent VTE and a lower value to the burden of long‐term anticoagulant treatment, the ACCP recommends long‐term treatment for patients with a first unprovoked proximal DVT, no risk factors for bleeding, and the ability to monitor the anticoagulant effectively (Grade 1A).20
| Clinical Features | Duration | Grade |
|---|---|---|
| ||
| First episode and transient risk factors | 3 months | 1A |
| Unprovoked episode | 3 months | 1A |
| Unprovoked proximal DVT with low bleed risk | Long‐term | 1A |
| Cancer | 3 to 6 months with LMWH; then with a VKA or a LMWH indefinitely or until cancer is resolved | 1A; 1C |
| Second unprovoked episode | Indefinite | 2A |
Transition to Outpatient Therapy
The use of outpatient LMWH has changed the course of long‐term anticoagulation therapy and is listed as the preferred option for anticoagulation in the ACCP guidelines.20 With the availability of subcutaneous LMWHs, patients with acute VTE no longer have to be hospitalized for the initiation of oral therapy. In addition, patients undergoing invasive procedures that require temporary discontinuation of warfarin can opt for bridge therapy with LMWH.42
Conclusions
The diagnosis of VTE is challenging and depends on the integration of clinical, biochemical, and imaging modalities. In the absence of contraindications, treatment should be initiated immediately after a diagnosis of VTE is confirmed. Anticoagulant therapy alone is sufficient for most patients, but some patients may require thrombolytics or other strategies. Various societies and organizations have issued recommendations regarding the optimal use of these therapies in specific patient populations. Following these recommendations carefully may reduce the risk of complications in patients with VTE.
- Health Grades, Inc. The Fifth Annual Health Grades Patient Safety in American Hospitals Study. Available at: http://www.healthgrades.com/media/dms/pdf/PatientSafetyInAmericanHospitalsStudy2008.pdf. Accessed August2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest2008;133(6 suppl):381S–453S.
- , , , et al.Value of assessment of pretest probability of deep‐vein thrombosis in clinical management.Lancet.1997;350(9094):1795–1798.
- , , , et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- , , , et al.Limitations of D‐dimer testing in unselected inpatients with suspected venous thromboembolism.Am J Med.2003;114(4):276–282.
- , , , et al.Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians.Ann Fam Med.2007;5(1):57–62.
- , , .The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism.Ann Intern Med.1998;129:1044–1049.
- , .Ultrasonography of leg veins in patients suspected of having pulmonary embolism.Ann Intern Med.1998;128:243–245.
- , .Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism.Circulation.2004;109(12 suppl 1):I15–I21.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology.Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer.Thromb Haemost.2000;83(3):416–420.
- , , , et al.Prospective validation of Wells criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44(5):503–510.
- , , , et al.Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism.J Thromb Haemost.2004;2(8):1247–1255.
- , , , et al.Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria.J Thromb Haemost.2008;6(5):772–780.
- , , , et al.A simple diagnostic strategy in hospitalized patients with clinically suspected pulmonary embolism.J Intern Med.2006;260(5):459–466.
- , .Is computed tomographic venography of lower limbs useful in suspected pulmonary embolism?Rev Med Suisse.2008;4(143):354,356–359.
- , , , et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354(22):2317–2327.
- , , , et al.Christopher Study Investigators.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D‐dimer testing, and computed tomography.JAMA.2006;295(2):172–179.
- , , , .Acute pulmonary embolectomy: a contemporary approach.Circulation.2002;105:1416–1419.
- , , , et al.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 suppl):454S–545S.
- , , , et al.Subcutaneous fondaparinux versus unfractionated heparin in the initial treatment of pulmonary embolism.N Engl J Med.2003;349:1695–1702.
- .Heparin‐induced thrombocytopenia associated with fondaparinux.N Engl J Med.2007;356:2653–2655.
- , , , .Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- , , , et al.Ins and outs of inferior vena cava filters in patients with venous thromboembolism: the experience at Monash Medical Centre and review of the published reports.Intern Med J.2008;38(1):38–43.
- PREPIC Study Group.Eight‐year follow‐up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study.Circulation.2005;112:416–422.
- , , , .Inferior vena cava filters: key considerations.Am J Med Sci.2005;330:82–87.
- , , , .Percutaneous inferior vena cava filters: follow‐up of 7 designs in 320 patients.Radiology.1993;188:851–856.
- , , , et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24.
- , , , , , .Long‐term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement.J Vasc Surg.2007;45(5):992–997.
- , , , et al.Comparison of fixed‐dose weight‐adjusted unfractionated heparin and low‐molecular‐weight heparin for acute treatment of venous thromboembolism.JAMA.2006;296(8):935–942.
- Arixtra prescribing information. Last updated October 2008. Research Triangle Park, NC: GlaxoSmithKline. Available at: http://us.gsk.com/products/assets/us_arixtra.pdf. Accessed August2009.
- , , , et al.American College of Physicians;American Academy of Family Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Ann Intern Med.2007;146(3):204–210.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice Guidelines in Oncology. V.1.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2009.
- , , , et al. Guidelines for the treatment of venous thromboembolism in cancer patients: report from the French Working Group. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1284.
- , , , et al.Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study.Arch Intern Med.2000;160(6):761–768.
- , , , et al.Postdischarge thromboprophylaxis and mortality risk after hip‐or knee‐replacement surgery.CMAJ.2008;178(12):1545–1554.
- , , , et al.Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis.Ann Intern Med.2008;149(10):698–707.
- , , , et al.The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients.Haematologica.2007;92(2):199–205.
- .Risk factors of recurrent venous thromboembolism: the role of residual vein thrombosis.Pathophysiol Haemost Thromb.2003/2004;33(5‐6):351–353.
- , , , et al.Effect of patient's sex on risk of recurrent venous thromboembolism: a meta‐analysis.Lancet.2006;368:371–378.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
Despite the availability of effective thromboprophylaxis, the prevalence of venous thromboembolism (VTE) is increasing in the hospital setting. In 2008, the Fifth Annual Health Grades Patient Safety in American Hospitals Study reported on key patient safety incidents among nearly 41 million hospitalizations in the Medicare population between 2004 and 2006. Although many areas showed improvementincluding reduced rates of hospital‐related infections, postoperative bleeding, transfusion reactions, and other injuriesthe number of cases of postoperative VTE increased by 11% during this period.1
Even with optimal thromboprophylaxis, VTE will develop in some at‐risk patients. Early diagnosis and treatment of VTE is critical to reduce morbidity and mortality, but no single tool can definitively confirm its presence. Consequently, the detection of deep vein thrombosis (DVT) and pulmonary embolism (PE) requires a stepwise diagnostic strategy that combines clinical, biochemical, and imaging modalities.
In addition to outlining diagnostic strategies for DVT and PE, this article summarizes VTE treatment guidelines from various organizations and societies and discusses long‐term management strategies to prevent recurrent VTE and other complications.
Diagnosis of DVT
The clinical symptoms and signs of DVT are nonspecific and include unilateral calf, leg, or thigh swelling and pain. Despite the limited sensitivity and specificity of individual signs and symptoms of DVT, the combination of these variables can be useful in assessing the probability of VTE. Patients can be risk stratified according to the likelihood of DVT, as determined by implicit clinical judgment or by a validated prediction rule.2
Assessment of Clinical Probability
The Wells prediction rule is used in assessing the probability of DVT.3 It incorporates signs, symptoms, and risk factors of DVT to calculate a clinical probability rating. Specifically, 1 point is assigned to each of the following factors, if present:3
-
Active cancer (treatment ongoing, within 6 months, or palliative)
-
Calf swelling >3 cm asymptomatic side (measured 10 cm below tibial tuberosity)
-
Collateral superficial veins (nonvaricose)
-
Entire leg swelling
-
Localized tenderness along the distribution of the deep venous system
-
Paralysis, paresis, or recent plaster immobilization of the lower extremities
-
Pitting edema confined to the symptomatic leg
-
Recently bedridden more than 3 days or major surgery within 4 weeks
In addition, 2 points are subtracted if an alternative diagnosis is as likely as or more likely than DVT. In patients with symptoms in both legs, the more symptomatic leg is used.
Patients with low (score <1), moderate (score 1‐2), and high (score 3) pretest probability of DVT have been shown to have DVT prevalence rates of 3%, 17%, and 75%, respectively.3
D‐Dimer Testing
D‐dimer testing measures the small protein fragments remaining in the blood after a cross‐linked fibrin clot is degraded by fibrinolysis. A low clinical probability assessment combined with a negative result in a highly sensitive, enzyme‐linked immunosorbent assay (ELISA)‐based D‐dimer test can safely exclude DVT, with a negative predictive value of 99.1% (95% confidence interval [CI]; 96.7‐99.9).4
Due to its poor specificity, D‐dimer testing has limited utility in unselected inpatients, especially older patients and those who have undergone prolonged hospitalization.5 However, it is reasonable to obtain a highly sensitive, ELISA‐based D‐dimer test in carefully selected inpatients with a low pretest probability of DVT.5, 6 In such patients, a negative result indicates that DVT is highly unlikely, while a positive result indicates a need for further testing. D‐dimer testing is likely not helpful in moderate‐risk or high‐risk patients.
Diagnostic Imaging
For patients with a moderate to high pretest probability of DVT, ultrasound is recommended.6 Compression ultrasonography (CUS) is currently the preferred imaging tool in patients with suspected DVT because it is noninvasive, can be repeated serially, and offers high sensitivity (+90%) and high specificity (95%) for detecting proximal vein thrombosis.7, 8 If the clinical suspicion of DVT persists after an initial negative CUS study, imaging can be repeated after 3 to 7 days to detect the propagation of any thrombosis to the proximal veins. Limitations of CUS include poor visualization of deep iliac and pelvic veins and poor sensitivity in isolated or nonocclusive calf vein thrombi.2
Contrast venography was considered the gold standard for the detection of DVT of the lower extremity, but this modality is invasive, painful, and offers poor visualization of the deep femoral vein and the internal iliac vein. In addition, contrast venography is associated with an increased risk of new thrombosis, renal failure, and hypersensitivity reaction to contrast media. Consequently, contrast venography is currently used in symptomatic patients only when noninvasive testing is inconclusive or unavailable.2 Other second‐line diagnostic tools include computed tomography venography (CTV) and magnetic resonance venography (MRV).9
Diagnostic Strategy
A diagnostic algorithm for DVT is presented in Figure 1. First, a validated clinical prediction scale such as the Wells prediction rule should be used to estimate the pretest probability of DVT, and the result of the clinical assessment should influence the choice and interpretation of subsequent testing.

Abbreviations: CUS, compression ultrasonography; DVT, deep vein thrombosis (DVT).
Diagnosis of PE
Clinical symptoms and signs such as dyspnea, chest pain, tachycardia, tachypnea, and syncope raise the suspicion of PE. Individual signs and symptoms, however, cannot confirm or exclude acute PE, as they are neither sensitive nor specific.10 Furthermore, although the likelihood of PE increases with the number of predisposing risk factors, approximately 30% of PE cases are unprovoked or idiopathic, meaning that they occur in the absence of predisposing factors. Diagnosis, therefore, depends on an integrated strategy involving similar tools as those used in diagnosing DVT.
Assessing Clinical Probability
Wells et al.11 also developed a clinical prediction rule for the risk stratification of patients with suspected PE. In this model, 7 specified variables are assigned different scores: clinical signs and symptoms of DVT (3.0); lack of a likely alternative diagnosis (3.0); heart rate greater than 100 beats per minute (1.5); immobilization for more than 3 days or surgery in the previous 4 weeks (1.5); previous DVT/PE (1.5); hemoptysis (1.0); and malignancy (1.0). Although the Wells prediction rule initially categorized 3 levels of probability for PE (low, moderate, or high), a revised model uses a simplified, dichotomized approach to determine whether PE is likely (Wells score >4) or unlikely (4 Wells score).11 An independent, prospective observational study found that the Wells prediction model reliably risk‐stratified pretest probability in patients with suspected PE.12
For patients who are stratified into the low‐risk category, the pulmonary embolism rule‐out criteria (PERC) rule may be helpful in reducing unnecessary diagnostic testing for PE.13 The PERC rule consists of 8 variables designed to offer a pretest probability of PE of less than 1.8%, a probability at which further testing is unnecessary. If the clinical gestalt is that PE is unlikely and all of the following variables are present, further testing can be safely discontinued: (1) pulse <100; (2) age <50; (3) oxygen saturation (SaO2) >94%; (4) no unilateral leg swelling; (5) no hemoptysis; (6) no recent trauma or surgery; (7) no prior DVT or PE; and (8) no hormone use.13 In a large, multicenter study, these criteria combined with a gestalt interpretation of low risk were shown to select a subgroup of patients with a very low probability of PE (<2%).14
D‐Dimer Testing
Evidence suggests that the combination of a low clinical probability assessment and a normal result in a highly sensitive, ELISA‐based D‐dimer test can safely exclude PE in hospitalized patients.15 Due to the large number of comorbidities among hospitalized patients, however, this combination occurs in only approximately 10% of inpatients.15 D‐dimer levels may be elevated in patients with a variety of nonthrombotic conditions, and it is therefore most useful in the diagnosis of otherwise healthy patients who have symptoms of PE. D‐dimer testing is not appropriate in moderate‐risk or high‐risk patients.
Diagnostic Imaging
Computed tomography (CT) is a leading imaging modality for the exclusion or confirmation of PE, as well as for the detection of alternative diagnoses. The diagnostic algorithms endorsed by the European Society of Cardiology (ESC) rely on both single‐detector and multidetector CT. However, multidetector CT scanners are now preferred because, in contrast to single‐detector CT, they can detect pulmonary emboli in smaller pulmonary arteries.10 Because single‐detector CT has a limited sensitivity of approximately 70%, it must be used in conjunction with lower limb venous CUS.16 In contrast, multidetector CT angiography has high sensitivity (83%) and specificity (96%) for the detection of PE and does not require the additional use of lower limb venous CUS.16, 17
Diagnostic Strategy
The Christopher Study demonstrated the utility of a diagnostic algorithm that incorporates a dichotomized decision rule, D‐dimer testing, and CT. In this approach, PE is excluded in patients with an unlikely clinical probability score (Wells score 4) and a normal D‐dimer test result. In all other patients, CT is the sole imaging method used to make management decisions.18 However, in patients with massive pulmonary embolism, if CT angiography is not immediately available, selective pulmonary angiography has been performed to identify and localize the emboli before aggressive therapy is instituted (Figure 2).19 If the patient is critically ill (hypotensive, severely hypoxemic), empiric treatment is appropriate while diagnostic strategy is being formulated.

Abbreviations: CT, computed tomography; CXR, plain chest X‐ray; ECG, electrocardiogram; PE, pulmonary embolism. †CT angiography using multidetector instruments.
Treatment Options for VTE
For patients with VTE, the American College of Chest Physicians (ACCP) guidelines recommend initial treatment with low‐molecular‐weight heparin (LMWH), intravenous unfractionated heparin (UFH), or adjusted‐dose subcutaneous UFH, followed by at least 3 months of oral anticoagulation therapy.20
When VTE is diagnosed, anticoagulation should be initiated immediately unless contraindications are present. In addition, patients without contraindications to anticoagulation should receive treatment before diagnostic testing if such testing is delayed or if the clinical suspicion of VTE is high.20
Anticoagulant Treatment
For decades, parenteral administration of UFH for 5 to 7 days followed by long‐term warfarin therapy has been the conventional treatment of patients with VTE. Although UFH can be administered subcutaneously or by intravenous (IV) infusion, continuous IV infusion has been preferred because of superior dosing precision. The anticoagulation effect of intravenous UFH must be monitored to ensure a therapeutic activated partial thromboplastin time (aPTT). Consequently, the use of intravenous UFH requires frequent aPTT assessment and dose adjustment.20
Given their ease of use and improved pharmacokinetic and pharmacodynamic profiles, LMWHs have replaced UFH for the treatment of VTE in many institutions. Fondaparinux is also a safe and effective alternative to both intravenous UFH and LMWH in the treatment of VTE.20 It has a longer half‐life (15‐20 hours) than LMWH, permitting a once‐daily administration, and in patients with submassive PE, its efficacy and safety are comparable to UFH.21 Platelet count monitoring is not necessary with fondaparinux because it is given at weight‐adjusted doses, and only 1 case of heparin‐induced thrombocytopenia (HIT) has been reported.22 It is, however, contraindicated in renal failure with a creatinine clearance of <30 mL/minute.10
Warfarin is very effective in the long‐term management of VTE and should be started concurrently with rapid‐acting injectable anticoagulation therapy. Warfarin requires overlap with injectable anticoagulants for a minimum of 5 days until a therapeutic international normalized ratio (INR) has been achieved.20
Other Treatments
Most patients with VTE can be treated effectively with only anticoagulation therapy. However, in cases of massive PE (with or without systemic arterial hypotension and usually with significant hypoxemia not generally responsive to supplemental oxygen), removal of the occluding thrombus by thrombolytic agents, special clot‐removing catheters, or surgical procedures may be necessary to prevent or ameliorate shock and subsequent death.23 In other cases, such as when anticoagulants are ineffective or contraindicated, an inferior vena cava (IVC) filter may be an appropriate option for VTE treatment. Importantly, guidelines do not recommend filters in patients who can tolerate anticoagulation.
Permanent and retrievable IVC filters are effective at preventing PE and are generally associated with a low complication rate.24 However, nonfatal complications are relatively common with permanent IVC filters. One early complication is insertion‐site thrombosis, which occurs in about 10% of patients. Subsequent complications are more frequent and include recurrent DVT and post‐thrombotic syndrome (PTS), which occur in approximately 20% and 40% of patients, respectively. At 5 and 9 years, about 22% and 33% of the filters are occluded, regardless of the use and duration of anticoagulation.2527 To minimize these complications, retrievable filters have been increasingly used, but most filters are not retrieved and are subject to the same complications as permanent IVC filters.28
Catheter‐directed thrombolysis, with or without IVC filter placement, is safe and effective in treating acute DVT.29 Additional measures, such as the use of graduated compression stockings, can reduce the risk of developing PTS.20
Guideline Recommendations
Guidelines from the ACCP, the American College of Physicians (ACP), and the American Academy of Family Physicians (AAFP) address the treatment of VTE in a broad spectrum of patients. Additional guidelines provide recommendations for specific presentations or patient groups. For example, the ESC guidelines address the treatment of acute PE, and several groupsthe American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the French Working Group (FWG)have published guidelines for the treatment of VTE in patients with cancer. The following sections summarize the most important recommendations from several of these organizations and societies.
ACCP Guidelines
The ACCP guideline recommendations are assigned grades of 1 or 2, denoting a stronger or weaker recommendation, as well as a grade of A, B, or C, indicating high‐quality evidence, moderate‐quality evidence, and low‐quality evidence, respectively. Physicians must supplement the guideline recommendations with informed clinical judgment to ensure proper use of treatment in at‐risk hospitalized patients.20
The 2008 ACCP guidelines suggest several options for the initial treatment of VTE, which are listed, along with acceptable dosing regimens, in Table 1.20, 30, 31 Fixed‐dose, unmonitored, subcutaneous UFH and fondaparinux are new Grade 1A additions to the 2008 update. In general, LMWH is preferred over intravenous UFH, except in patients with severe renal failure.20
| Initial Anticoagulation Therapy | Grade | Acceptable Treatment Regimen* |
|---|---|---|
| ||
| SC LMWH | 1A | Enoxaparin: 1 mg/kg every 12 hours or 1.5 mg/kg once daily; Dalteparin: 200 IU/kg once daily (can be administered out of hospital) |
| Intravenous UFH | 1A | Get baseline aPTT, PT, and platelet count; if no abnormalities, proceed with a weight‐based heparin infusion protocol such as: |
| Bolus of 80 U/kg, followed by an infusion of 18 U/kg per hour (treatment duration 72 days); check aPTT every 4‐6 hours and adjust according to the normogram; monitor platelet count every 3‐4 days for HIT | ||
| Monitored SC UFH; fixed‐dose, unmonitored, SC UFH | 1A; 1A | Initial dose of 333 U/kg, followed by a fixed dose of 250 U/kg every 12 hours (can be administered out of hospital) |
| SC fondaparinux | 1A | 5 mg (body weight <50 kg), 7.5 mg (body weight 50‐100 kg), or 10 mg (body weight >100 kg) once daily (treatment duration 72 days) |
| IVC filter if anticoagulation contraindicated | 1C | |
Warfarin should also be initiated on the same day as UFH or LMWH and adjusted to a target INR of 2.5 (range, 2.0‐3.0). Treatment with UFH or LMWH should be continued concomitantly for a minimum of 5 days and should not be discontinued until the INR has been over 2.0 for 24 hours. The ACCP guidelines also recommend systematic follow‐up of oral anticoagulation therapy.
ACP/AAFP Guidelines
In 2007, the ACP and the AAFP collaborated to develop joint guidelines for the management of VTE.32 Several of their key recommendations are the following:32
-
LMWH, rather than UFH, should be used whenever possible for the initial inpatient treatment of DVT
-
Either UFH or LMWH is appropriate for the initial treatment of PE
-
Anticoagulation should be continued for 3 to 6 months for VTE secondary to transient risk factors, and for more than 12 months for recurrent VTE
-
LMWH is safe and effective for the long‐term treatment of VTE in selected patients (and may be preferable for patients with cancer)
ESC Guidelines for the Treatment of PE
According to the 2008 ESC guidelines, anticoagulation with UFH, LMWH, or fondaparinux should be initiated immediately in patients with confirmed PE, as well as in those with a high or intermediate clinical probability of PE while the diagnostic workup is ongoing. Subcutaneous LMWH or fondaparinux is preferable to intravenous UFH for initial treatment in most patients. UFH, however, should be used in patients with a high risk of bleeding due to its capacity for reversal and short half‐life, as well as in those with severe renal dysfunction.10
According to the ESC, patients with high‐risk PE (presenting with cardiogenic shock or persistent arterial hypotension) should receive thrombolytic therapy as first‐line therapy. Hemodynamic and respiratory support is also necessary for these patients.10 Routine thrombolysis is not recommended in patients with non‐high‐risk PE, but it may be considered in select patients with intermediate‐risk PE (characterized by severe right ventricular dysfunction on echocardiography and/or myocardial injury), depending on the patient's risk of bleeding. Thrombolytic therapy should not be used in patients with low‐risk PE (presenting without shock, hypotension, right ventricular dysfunction, or myocardial injury).10
Like the ESC guidelines, the ACCP guidelines recommend against the use of thrombolytic therapy for the majority of patients with PE (Grade 1B), but they do recommend its use in patients with evidence of hemodynamic compromise and no major contraindications owing to bleeding risk (Grade 1B) and in certain other high‐risk patients (Grade 2B).20
The ESC states that pulmonary embolectomy has recently become a reasonable option for patients with massive, high‐risk PE and an absolute contraindication to thrombolysis, or in whom thrombolysis has failed, when appropriate expertise is available. In the past, it was performed as a last resort in patients with massive PE who were in shock and conferred a high risk of mortality (+50%). Recently, however, the procedure has been revived and performed immediately in patients with confirmed massive PE (with severe right ventricular dysfunction but before shock), with mortality rates of less than 10%.19 It should be noted, however, that the ACCP guidelines consider embolectomy a Grade 2C recommendation.20 Alternatively, catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in these patients.10
NCCN Guidelines: Oncology Patients
The NCCN has provided treatment algorithms for the management of DVT and PE in patients with cancer, which are available online at
FWG Guidelines: Oncology Patients
At the 2008 ASH annual meeting, the FWG presented updated guidelines for the treatment of VTE in cancer patients.34 The FWG guidelines contain the following key recommendations:
-
The treatment of VTE should be based on LMWH at curative doses for at least 3 months
-
During the initial treatment (up to 10 days), any approved drug (including LMWH, UFH, and fondaparinux) may be used
-
Beyond the first 10 days, VTE treatment should be based on LMWH at curative doses for at least 3 months and optimally 6 months, as validated with the following drugs and dosage regimens:
-
Dalteparin 200 IU/kg once daily for 1 month, then 150 IU/kg once daily
-
Enoxaparin 150 IU/kg (1.5 mg/kg) once daily
-
Tinzaparin 175 IU/kg once daily
-
Special treatment considerations include the following:
-
In severe renal impairment, UFH should be used and rapidly followed by a vitamin K agonist (VKA) for at least 3 months
-
In severe PE (representing hemodynamic failure), the indications and recommended uses of thrombolytic drugs in noncancer patients apply
-
In patients with an absolute contraindication to anticoagulation or VTE recurrence despite optimal anticoagulation, vena cava filters should be considered
-
In patients with intracranial malignancies, VTE treatment is the same as in cancer patients with nonintracranial tumors
The treatment of central venous catheter thrombosis requires the long‐term use of LMWH according to the FWG guidelines. In patients with severe renal failure, UFH with early VKA must be used as an alternative treatment. Regardless of the therapy used, treatment should be continued as long as the catheter is maintained.34
Long‐Term Management of VTE
The high rate of recurrent VTE after a first episode of DVT or PEapproximately 8% within 90 daysunderscores the importance of maintaining effective prophylaxis postdischarge.35 Inadequate prophylaxis following discharge from the hospital can have severe consequences. In a recent study of 10,744 patients who were discharged from the hospital following hip or knee replacement surgery, fewer than 1 in 5 received postdischarge thromboprophylaxis. The 3‐month risk of mortality was significantly lower among those who received thromboprophylaxis at discharge (adjusted hazard ratio, 0.34; 95% CI, 0.20‐0.57).36
Detailed patient education at the time of discharge may be one of the most effective ways to prevent or minimize the burden of long‐term complications such as PTS or recurrent VTE. Accordingly, proper discharge planning and postdischarge support, including an appropriate anticoagulant, are critical steps toward reducing mortality, morbidity, and healthcare costs.
PTS
As many as 50% of patients with VTE will develop PTS, a serious but preventable complication that leads to pain, swelling, and skin changes in the affected limb. Female gender, older age, higher body mass index (BMI), and DVT of the common femoral or iliac vein (vs. distal DVT) are associated with an increased risk of PTS.37 To prevent PTS in a patient who has had a symptomatic proximal DVT, current guidelines recommend the use of graduated elastic compression stockings with an ankle pressure of 30 to 40 mm Hg, if feasible. Compression therapy should start as soon as possible after the initiation of anticoagulation therapy and be encouraged for a minimum of 2 years.20
Recurrent VTE
After discontinuing anticoagulation, the risk of recurrent VTE increases steadily over time. In a recent long‐term study of patients with acute proximal DVT or PE, the risk of recurrent VTE was 11% after 1 year, 20% after 3 years, 30% after 5 years, and 40% after 10 years. In this study, risk factors for recurrent VTE included unprovoked initial VTE, thrombophilia, increasing age, and a shorter duration of anticoagulation (6 months or less).38 Another study identified residual venous thrombosis as an important risk factor for recurrent VTE.39 In addition, 1 meta‐analysis found that men had a 50% higher risk of recurrent VTE than women.40 Recurrent DVT events are associated with a 21% greater cost than the initial event, suggesting that recurrent VTE is a preventable drain on healthcare resources.41
Secondary Prevention
The risk of recurrent VTE is determined by the effectiveness of treatment for the acute episode of VTE and by the patient's intrinsic risk of thromboembolism. The ACCP recommends different durations of warfarin or LMWH anticoagulant therapy according to these features (Table 2).20 Attaching a high value to prevention of recurrent VTE and a lower value to the burden of long‐term anticoagulant treatment, the ACCP recommends long‐term treatment for patients with a first unprovoked proximal DVT, no risk factors for bleeding, and the ability to monitor the anticoagulant effectively (Grade 1A).20
| Clinical Features | Duration | Grade |
|---|---|---|
| ||
| First episode and transient risk factors | 3 months | 1A |
| Unprovoked episode | 3 months | 1A |
| Unprovoked proximal DVT with low bleed risk | Long‐term | 1A |
| Cancer | 3 to 6 months with LMWH; then with a VKA or a LMWH indefinitely or until cancer is resolved | 1A; 1C |
| Second unprovoked episode | Indefinite | 2A |
Transition to Outpatient Therapy
The use of outpatient LMWH has changed the course of long‐term anticoagulation therapy and is listed as the preferred option for anticoagulation in the ACCP guidelines.20 With the availability of subcutaneous LMWHs, patients with acute VTE no longer have to be hospitalized for the initiation of oral therapy. In addition, patients undergoing invasive procedures that require temporary discontinuation of warfarin can opt for bridge therapy with LMWH.42
Conclusions
The diagnosis of VTE is challenging and depends on the integration of clinical, biochemical, and imaging modalities. In the absence of contraindications, treatment should be initiated immediately after a diagnosis of VTE is confirmed. Anticoagulant therapy alone is sufficient for most patients, but some patients may require thrombolytics or other strategies. Various societies and organizations have issued recommendations regarding the optimal use of these therapies in specific patient populations. Following these recommendations carefully may reduce the risk of complications in patients with VTE.
Despite the availability of effective thromboprophylaxis, the prevalence of venous thromboembolism (VTE) is increasing in the hospital setting. In 2008, the Fifth Annual Health Grades Patient Safety in American Hospitals Study reported on key patient safety incidents among nearly 41 million hospitalizations in the Medicare population between 2004 and 2006. Although many areas showed improvementincluding reduced rates of hospital‐related infections, postoperative bleeding, transfusion reactions, and other injuriesthe number of cases of postoperative VTE increased by 11% during this period.1
Even with optimal thromboprophylaxis, VTE will develop in some at‐risk patients. Early diagnosis and treatment of VTE is critical to reduce morbidity and mortality, but no single tool can definitively confirm its presence. Consequently, the detection of deep vein thrombosis (DVT) and pulmonary embolism (PE) requires a stepwise diagnostic strategy that combines clinical, biochemical, and imaging modalities.
In addition to outlining diagnostic strategies for DVT and PE, this article summarizes VTE treatment guidelines from various organizations and societies and discusses long‐term management strategies to prevent recurrent VTE and other complications.
Diagnosis of DVT
The clinical symptoms and signs of DVT are nonspecific and include unilateral calf, leg, or thigh swelling and pain. Despite the limited sensitivity and specificity of individual signs and symptoms of DVT, the combination of these variables can be useful in assessing the probability of VTE. Patients can be risk stratified according to the likelihood of DVT, as determined by implicit clinical judgment or by a validated prediction rule.2
Assessment of Clinical Probability
The Wells prediction rule is used in assessing the probability of DVT.3 It incorporates signs, symptoms, and risk factors of DVT to calculate a clinical probability rating. Specifically, 1 point is assigned to each of the following factors, if present:3
-
Active cancer (treatment ongoing, within 6 months, or palliative)
-
Calf swelling >3 cm asymptomatic side (measured 10 cm below tibial tuberosity)
-
Collateral superficial veins (nonvaricose)
-
Entire leg swelling
-
Localized tenderness along the distribution of the deep venous system
-
Paralysis, paresis, or recent plaster immobilization of the lower extremities
-
Pitting edema confined to the symptomatic leg
-
Recently bedridden more than 3 days or major surgery within 4 weeks
In addition, 2 points are subtracted if an alternative diagnosis is as likely as or more likely than DVT. In patients with symptoms in both legs, the more symptomatic leg is used.
Patients with low (score <1), moderate (score 1‐2), and high (score 3) pretest probability of DVT have been shown to have DVT prevalence rates of 3%, 17%, and 75%, respectively.3
D‐Dimer Testing
D‐dimer testing measures the small protein fragments remaining in the blood after a cross‐linked fibrin clot is degraded by fibrinolysis. A low clinical probability assessment combined with a negative result in a highly sensitive, enzyme‐linked immunosorbent assay (ELISA)‐based D‐dimer test can safely exclude DVT, with a negative predictive value of 99.1% (95% confidence interval [CI]; 96.7‐99.9).4
Due to its poor specificity, D‐dimer testing has limited utility in unselected inpatients, especially older patients and those who have undergone prolonged hospitalization.5 However, it is reasonable to obtain a highly sensitive, ELISA‐based D‐dimer test in carefully selected inpatients with a low pretest probability of DVT.5, 6 In such patients, a negative result indicates that DVT is highly unlikely, while a positive result indicates a need for further testing. D‐dimer testing is likely not helpful in moderate‐risk or high‐risk patients.
Diagnostic Imaging
For patients with a moderate to high pretest probability of DVT, ultrasound is recommended.6 Compression ultrasonography (CUS) is currently the preferred imaging tool in patients with suspected DVT because it is noninvasive, can be repeated serially, and offers high sensitivity (+90%) and high specificity (95%) for detecting proximal vein thrombosis.7, 8 If the clinical suspicion of DVT persists after an initial negative CUS study, imaging can be repeated after 3 to 7 days to detect the propagation of any thrombosis to the proximal veins. Limitations of CUS include poor visualization of deep iliac and pelvic veins and poor sensitivity in isolated or nonocclusive calf vein thrombi.2
Contrast venography was considered the gold standard for the detection of DVT of the lower extremity, but this modality is invasive, painful, and offers poor visualization of the deep femoral vein and the internal iliac vein. In addition, contrast venography is associated with an increased risk of new thrombosis, renal failure, and hypersensitivity reaction to contrast media. Consequently, contrast venography is currently used in symptomatic patients only when noninvasive testing is inconclusive or unavailable.2 Other second‐line diagnostic tools include computed tomography venography (CTV) and magnetic resonance venography (MRV).9
Diagnostic Strategy
A diagnostic algorithm for DVT is presented in Figure 1. First, a validated clinical prediction scale such as the Wells prediction rule should be used to estimate the pretest probability of DVT, and the result of the clinical assessment should influence the choice and interpretation of subsequent testing.

Abbreviations: CUS, compression ultrasonography; DVT, deep vein thrombosis (DVT).
Diagnosis of PE
Clinical symptoms and signs such as dyspnea, chest pain, tachycardia, tachypnea, and syncope raise the suspicion of PE. Individual signs and symptoms, however, cannot confirm or exclude acute PE, as they are neither sensitive nor specific.10 Furthermore, although the likelihood of PE increases with the number of predisposing risk factors, approximately 30% of PE cases are unprovoked or idiopathic, meaning that they occur in the absence of predisposing factors. Diagnosis, therefore, depends on an integrated strategy involving similar tools as those used in diagnosing DVT.
Assessing Clinical Probability
Wells et al.11 also developed a clinical prediction rule for the risk stratification of patients with suspected PE. In this model, 7 specified variables are assigned different scores: clinical signs and symptoms of DVT (3.0); lack of a likely alternative diagnosis (3.0); heart rate greater than 100 beats per minute (1.5); immobilization for more than 3 days or surgery in the previous 4 weeks (1.5); previous DVT/PE (1.5); hemoptysis (1.0); and malignancy (1.0). Although the Wells prediction rule initially categorized 3 levels of probability for PE (low, moderate, or high), a revised model uses a simplified, dichotomized approach to determine whether PE is likely (Wells score >4) or unlikely (4 Wells score).11 An independent, prospective observational study found that the Wells prediction model reliably risk‐stratified pretest probability in patients with suspected PE.12
For patients who are stratified into the low‐risk category, the pulmonary embolism rule‐out criteria (PERC) rule may be helpful in reducing unnecessary diagnostic testing for PE.13 The PERC rule consists of 8 variables designed to offer a pretest probability of PE of less than 1.8%, a probability at which further testing is unnecessary. If the clinical gestalt is that PE is unlikely and all of the following variables are present, further testing can be safely discontinued: (1) pulse <100; (2) age <50; (3) oxygen saturation (SaO2) >94%; (4) no unilateral leg swelling; (5) no hemoptysis; (6) no recent trauma or surgery; (7) no prior DVT or PE; and (8) no hormone use.13 In a large, multicenter study, these criteria combined with a gestalt interpretation of low risk were shown to select a subgroup of patients with a very low probability of PE (<2%).14
D‐Dimer Testing
Evidence suggests that the combination of a low clinical probability assessment and a normal result in a highly sensitive, ELISA‐based D‐dimer test can safely exclude PE in hospitalized patients.15 Due to the large number of comorbidities among hospitalized patients, however, this combination occurs in only approximately 10% of inpatients.15 D‐dimer levels may be elevated in patients with a variety of nonthrombotic conditions, and it is therefore most useful in the diagnosis of otherwise healthy patients who have symptoms of PE. D‐dimer testing is not appropriate in moderate‐risk or high‐risk patients.
Diagnostic Imaging
Computed tomography (CT) is a leading imaging modality for the exclusion or confirmation of PE, as well as for the detection of alternative diagnoses. The diagnostic algorithms endorsed by the European Society of Cardiology (ESC) rely on both single‐detector and multidetector CT. However, multidetector CT scanners are now preferred because, in contrast to single‐detector CT, they can detect pulmonary emboli in smaller pulmonary arteries.10 Because single‐detector CT has a limited sensitivity of approximately 70%, it must be used in conjunction with lower limb venous CUS.16 In contrast, multidetector CT angiography has high sensitivity (83%) and specificity (96%) for the detection of PE and does not require the additional use of lower limb venous CUS.16, 17
Diagnostic Strategy
The Christopher Study demonstrated the utility of a diagnostic algorithm that incorporates a dichotomized decision rule, D‐dimer testing, and CT. In this approach, PE is excluded in patients with an unlikely clinical probability score (Wells score 4) and a normal D‐dimer test result. In all other patients, CT is the sole imaging method used to make management decisions.18 However, in patients with massive pulmonary embolism, if CT angiography is not immediately available, selective pulmonary angiography has been performed to identify and localize the emboli before aggressive therapy is instituted (Figure 2).19 If the patient is critically ill (hypotensive, severely hypoxemic), empiric treatment is appropriate while diagnostic strategy is being formulated.

Abbreviations: CT, computed tomography; CXR, plain chest X‐ray; ECG, electrocardiogram; PE, pulmonary embolism. †CT angiography using multidetector instruments.
Treatment Options for VTE
For patients with VTE, the American College of Chest Physicians (ACCP) guidelines recommend initial treatment with low‐molecular‐weight heparin (LMWH), intravenous unfractionated heparin (UFH), or adjusted‐dose subcutaneous UFH, followed by at least 3 months of oral anticoagulation therapy.20
When VTE is diagnosed, anticoagulation should be initiated immediately unless contraindications are present. In addition, patients without contraindications to anticoagulation should receive treatment before diagnostic testing if such testing is delayed or if the clinical suspicion of VTE is high.20
Anticoagulant Treatment
For decades, parenteral administration of UFH for 5 to 7 days followed by long‐term warfarin therapy has been the conventional treatment of patients with VTE. Although UFH can be administered subcutaneously or by intravenous (IV) infusion, continuous IV infusion has been preferred because of superior dosing precision. The anticoagulation effect of intravenous UFH must be monitored to ensure a therapeutic activated partial thromboplastin time (aPTT). Consequently, the use of intravenous UFH requires frequent aPTT assessment and dose adjustment.20
Given their ease of use and improved pharmacokinetic and pharmacodynamic profiles, LMWHs have replaced UFH for the treatment of VTE in many institutions. Fondaparinux is also a safe and effective alternative to both intravenous UFH and LMWH in the treatment of VTE.20 It has a longer half‐life (15‐20 hours) than LMWH, permitting a once‐daily administration, and in patients with submassive PE, its efficacy and safety are comparable to UFH.21 Platelet count monitoring is not necessary with fondaparinux because it is given at weight‐adjusted doses, and only 1 case of heparin‐induced thrombocytopenia (HIT) has been reported.22 It is, however, contraindicated in renal failure with a creatinine clearance of <30 mL/minute.10
Warfarin is very effective in the long‐term management of VTE and should be started concurrently with rapid‐acting injectable anticoagulation therapy. Warfarin requires overlap with injectable anticoagulants for a minimum of 5 days until a therapeutic international normalized ratio (INR) has been achieved.20
Other Treatments
Most patients with VTE can be treated effectively with only anticoagulation therapy. However, in cases of massive PE (with or without systemic arterial hypotension and usually with significant hypoxemia not generally responsive to supplemental oxygen), removal of the occluding thrombus by thrombolytic agents, special clot‐removing catheters, or surgical procedures may be necessary to prevent or ameliorate shock and subsequent death.23 In other cases, such as when anticoagulants are ineffective or contraindicated, an inferior vena cava (IVC) filter may be an appropriate option for VTE treatment. Importantly, guidelines do not recommend filters in patients who can tolerate anticoagulation.
Permanent and retrievable IVC filters are effective at preventing PE and are generally associated with a low complication rate.24 However, nonfatal complications are relatively common with permanent IVC filters. One early complication is insertion‐site thrombosis, which occurs in about 10% of patients. Subsequent complications are more frequent and include recurrent DVT and post‐thrombotic syndrome (PTS), which occur in approximately 20% and 40% of patients, respectively. At 5 and 9 years, about 22% and 33% of the filters are occluded, regardless of the use and duration of anticoagulation.2527 To minimize these complications, retrievable filters have been increasingly used, but most filters are not retrieved and are subject to the same complications as permanent IVC filters.28
Catheter‐directed thrombolysis, with or without IVC filter placement, is safe and effective in treating acute DVT.29 Additional measures, such as the use of graduated compression stockings, can reduce the risk of developing PTS.20
Guideline Recommendations
Guidelines from the ACCP, the American College of Physicians (ACP), and the American Academy of Family Physicians (AAFP) address the treatment of VTE in a broad spectrum of patients. Additional guidelines provide recommendations for specific presentations or patient groups. For example, the ESC guidelines address the treatment of acute PE, and several groupsthe American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the French Working Group (FWG)have published guidelines for the treatment of VTE in patients with cancer. The following sections summarize the most important recommendations from several of these organizations and societies.
ACCP Guidelines
The ACCP guideline recommendations are assigned grades of 1 or 2, denoting a stronger or weaker recommendation, as well as a grade of A, B, or C, indicating high‐quality evidence, moderate‐quality evidence, and low‐quality evidence, respectively. Physicians must supplement the guideline recommendations with informed clinical judgment to ensure proper use of treatment in at‐risk hospitalized patients.20
The 2008 ACCP guidelines suggest several options for the initial treatment of VTE, which are listed, along with acceptable dosing regimens, in Table 1.20, 30, 31 Fixed‐dose, unmonitored, subcutaneous UFH and fondaparinux are new Grade 1A additions to the 2008 update. In general, LMWH is preferred over intravenous UFH, except in patients with severe renal failure.20
| Initial Anticoagulation Therapy | Grade | Acceptable Treatment Regimen* |
|---|---|---|
| ||
| SC LMWH | 1A | Enoxaparin: 1 mg/kg every 12 hours or 1.5 mg/kg once daily; Dalteparin: 200 IU/kg once daily (can be administered out of hospital) |
| Intravenous UFH | 1A | Get baseline aPTT, PT, and platelet count; if no abnormalities, proceed with a weight‐based heparin infusion protocol such as: |
| Bolus of 80 U/kg, followed by an infusion of 18 U/kg per hour (treatment duration 72 days); check aPTT every 4‐6 hours and adjust according to the normogram; monitor platelet count every 3‐4 days for HIT | ||
| Monitored SC UFH; fixed‐dose, unmonitored, SC UFH | 1A; 1A | Initial dose of 333 U/kg, followed by a fixed dose of 250 U/kg every 12 hours (can be administered out of hospital) |
| SC fondaparinux | 1A | 5 mg (body weight <50 kg), 7.5 mg (body weight 50‐100 kg), or 10 mg (body weight >100 kg) once daily (treatment duration 72 days) |
| IVC filter if anticoagulation contraindicated | 1C | |
Warfarin should also be initiated on the same day as UFH or LMWH and adjusted to a target INR of 2.5 (range, 2.0‐3.0). Treatment with UFH or LMWH should be continued concomitantly for a minimum of 5 days and should not be discontinued until the INR has been over 2.0 for 24 hours. The ACCP guidelines also recommend systematic follow‐up of oral anticoagulation therapy.
ACP/AAFP Guidelines
In 2007, the ACP and the AAFP collaborated to develop joint guidelines for the management of VTE.32 Several of their key recommendations are the following:32
-
LMWH, rather than UFH, should be used whenever possible for the initial inpatient treatment of DVT
-
Either UFH or LMWH is appropriate for the initial treatment of PE
-
Anticoagulation should be continued for 3 to 6 months for VTE secondary to transient risk factors, and for more than 12 months for recurrent VTE
-
LMWH is safe and effective for the long‐term treatment of VTE in selected patients (and may be preferable for patients with cancer)
ESC Guidelines for the Treatment of PE
According to the 2008 ESC guidelines, anticoagulation with UFH, LMWH, or fondaparinux should be initiated immediately in patients with confirmed PE, as well as in those with a high or intermediate clinical probability of PE while the diagnostic workup is ongoing. Subcutaneous LMWH or fondaparinux is preferable to intravenous UFH for initial treatment in most patients. UFH, however, should be used in patients with a high risk of bleeding due to its capacity for reversal and short half‐life, as well as in those with severe renal dysfunction.10
According to the ESC, patients with high‐risk PE (presenting with cardiogenic shock or persistent arterial hypotension) should receive thrombolytic therapy as first‐line therapy. Hemodynamic and respiratory support is also necessary for these patients.10 Routine thrombolysis is not recommended in patients with non‐high‐risk PE, but it may be considered in select patients with intermediate‐risk PE (characterized by severe right ventricular dysfunction on echocardiography and/or myocardial injury), depending on the patient's risk of bleeding. Thrombolytic therapy should not be used in patients with low‐risk PE (presenting without shock, hypotension, right ventricular dysfunction, or myocardial injury).10
Like the ESC guidelines, the ACCP guidelines recommend against the use of thrombolytic therapy for the majority of patients with PE (Grade 1B), but they do recommend its use in patients with evidence of hemodynamic compromise and no major contraindications owing to bleeding risk (Grade 1B) and in certain other high‐risk patients (Grade 2B).20
The ESC states that pulmonary embolectomy has recently become a reasonable option for patients with massive, high‐risk PE and an absolute contraindication to thrombolysis, or in whom thrombolysis has failed, when appropriate expertise is available. In the past, it was performed as a last resort in patients with massive PE who were in shock and conferred a high risk of mortality (+50%). Recently, however, the procedure has been revived and performed immediately in patients with confirmed massive PE (with severe right ventricular dysfunction but before shock), with mortality rates of less than 10%.19 It should be noted, however, that the ACCP guidelines consider embolectomy a Grade 2C recommendation.20 Alternatively, catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in these patients.10
NCCN Guidelines: Oncology Patients
The NCCN has provided treatment algorithms for the management of DVT and PE in patients with cancer, which are available online at
FWG Guidelines: Oncology Patients
At the 2008 ASH annual meeting, the FWG presented updated guidelines for the treatment of VTE in cancer patients.34 The FWG guidelines contain the following key recommendations:
-
The treatment of VTE should be based on LMWH at curative doses for at least 3 months
-
During the initial treatment (up to 10 days), any approved drug (including LMWH, UFH, and fondaparinux) may be used
-
Beyond the first 10 days, VTE treatment should be based on LMWH at curative doses for at least 3 months and optimally 6 months, as validated with the following drugs and dosage regimens:
-
Dalteparin 200 IU/kg once daily for 1 month, then 150 IU/kg once daily
-
Enoxaparin 150 IU/kg (1.5 mg/kg) once daily
-
Tinzaparin 175 IU/kg once daily
-
Special treatment considerations include the following:
-
In severe renal impairment, UFH should be used and rapidly followed by a vitamin K agonist (VKA) for at least 3 months
-
In severe PE (representing hemodynamic failure), the indications and recommended uses of thrombolytic drugs in noncancer patients apply
-
In patients with an absolute contraindication to anticoagulation or VTE recurrence despite optimal anticoagulation, vena cava filters should be considered
-
In patients with intracranial malignancies, VTE treatment is the same as in cancer patients with nonintracranial tumors
The treatment of central venous catheter thrombosis requires the long‐term use of LMWH according to the FWG guidelines. In patients with severe renal failure, UFH with early VKA must be used as an alternative treatment. Regardless of the therapy used, treatment should be continued as long as the catheter is maintained.34
Long‐Term Management of VTE
The high rate of recurrent VTE after a first episode of DVT or PEapproximately 8% within 90 daysunderscores the importance of maintaining effective prophylaxis postdischarge.35 Inadequate prophylaxis following discharge from the hospital can have severe consequences. In a recent study of 10,744 patients who were discharged from the hospital following hip or knee replacement surgery, fewer than 1 in 5 received postdischarge thromboprophylaxis. The 3‐month risk of mortality was significantly lower among those who received thromboprophylaxis at discharge (adjusted hazard ratio, 0.34; 95% CI, 0.20‐0.57).36
Detailed patient education at the time of discharge may be one of the most effective ways to prevent or minimize the burden of long‐term complications such as PTS or recurrent VTE. Accordingly, proper discharge planning and postdischarge support, including an appropriate anticoagulant, are critical steps toward reducing mortality, morbidity, and healthcare costs.
PTS
As many as 50% of patients with VTE will develop PTS, a serious but preventable complication that leads to pain, swelling, and skin changes in the affected limb. Female gender, older age, higher body mass index (BMI), and DVT of the common femoral or iliac vein (vs. distal DVT) are associated with an increased risk of PTS.37 To prevent PTS in a patient who has had a symptomatic proximal DVT, current guidelines recommend the use of graduated elastic compression stockings with an ankle pressure of 30 to 40 mm Hg, if feasible. Compression therapy should start as soon as possible after the initiation of anticoagulation therapy and be encouraged for a minimum of 2 years.20
Recurrent VTE
After discontinuing anticoagulation, the risk of recurrent VTE increases steadily over time. In a recent long‐term study of patients with acute proximal DVT or PE, the risk of recurrent VTE was 11% after 1 year, 20% after 3 years, 30% after 5 years, and 40% after 10 years. In this study, risk factors for recurrent VTE included unprovoked initial VTE, thrombophilia, increasing age, and a shorter duration of anticoagulation (6 months or less).38 Another study identified residual venous thrombosis as an important risk factor for recurrent VTE.39 In addition, 1 meta‐analysis found that men had a 50% higher risk of recurrent VTE than women.40 Recurrent DVT events are associated with a 21% greater cost than the initial event, suggesting that recurrent VTE is a preventable drain on healthcare resources.41
Secondary Prevention
The risk of recurrent VTE is determined by the effectiveness of treatment for the acute episode of VTE and by the patient's intrinsic risk of thromboembolism. The ACCP recommends different durations of warfarin or LMWH anticoagulant therapy according to these features (Table 2).20 Attaching a high value to prevention of recurrent VTE and a lower value to the burden of long‐term anticoagulant treatment, the ACCP recommends long‐term treatment for patients with a first unprovoked proximal DVT, no risk factors for bleeding, and the ability to monitor the anticoagulant effectively (Grade 1A).20
| Clinical Features | Duration | Grade |
|---|---|---|
| ||
| First episode and transient risk factors | 3 months | 1A |
| Unprovoked episode | 3 months | 1A |
| Unprovoked proximal DVT with low bleed risk | Long‐term | 1A |
| Cancer | 3 to 6 months with LMWH; then with a VKA or a LMWH indefinitely or until cancer is resolved | 1A; 1C |
| Second unprovoked episode | Indefinite | 2A |
Transition to Outpatient Therapy
The use of outpatient LMWH has changed the course of long‐term anticoagulation therapy and is listed as the preferred option for anticoagulation in the ACCP guidelines.20 With the availability of subcutaneous LMWHs, patients with acute VTE no longer have to be hospitalized for the initiation of oral therapy. In addition, patients undergoing invasive procedures that require temporary discontinuation of warfarin can opt for bridge therapy with LMWH.42
Conclusions
The diagnosis of VTE is challenging and depends on the integration of clinical, biochemical, and imaging modalities. In the absence of contraindications, treatment should be initiated immediately after a diagnosis of VTE is confirmed. Anticoagulant therapy alone is sufficient for most patients, but some patients may require thrombolytics or other strategies. Various societies and organizations have issued recommendations regarding the optimal use of these therapies in specific patient populations. Following these recommendations carefully may reduce the risk of complications in patients with VTE.
- Health Grades, Inc. The Fifth Annual Health Grades Patient Safety in American Hospitals Study. Available at: http://www.healthgrades.com/media/dms/pdf/PatientSafetyInAmericanHospitalsStudy2008.pdf. Accessed August2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest2008;133(6 suppl):381S–453S.
- , , , et al.Value of assessment of pretest probability of deep‐vein thrombosis in clinical management.Lancet.1997;350(9094):1795–1798.
- , , , et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- , , , et al.Limitations of D‐dimer testing in unselected inpatients with suspected venous thromboembolism.Am J Med.2003;114(4):276–282.
- , , , et al.Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians.Ann Fam Med.2007;5(1):57–62.
- , , .The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism.Ann Intern Med.1998;129:1044–1049.
- , .Ultrasonography of leg veins in patients suspected of having pulmonary embolism.Ann Intern Med.1998;128:243–245.
- , .Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism.Circulation.2004;109(12 suppl 1):I15–I21.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology.Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer.Thromb Haemost.2000;83(3):416–420.
- , , , et al.Prospective validation of Wells criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44(5):503–510.
- , , , et al.Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism.J Thromb Haemost.2004;2(8):1247–1255.
- , , , et al.Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria.J Thromb Haemost.2008;6(5):772–780.
- , , , et al.A simple diagnostic strategy in hospitalized patients with clinically suspected pulmonary embolism.J Intern Med.2006;260(5):459–466.
- , .Is computed tomographic venography of lower limbs useful in suspected pulmonary embolism?Rev Med Suisse.2008;4(143):354,356–359.
- , , , et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354(22):2317–2327.
- , , , et al.Christopher Study Investigators.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D‐dimer testing, and computed tomography.JAMA.2006;295(2):172–179.
- , , , .Acute pulmonary embolectomy: a contemporary approach.Circulation.2002;105:1416–1419.
- , , , et al.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 suppl):454S–545S.
- , , , et al.Subcutaneous fondaparinux versus unfractionated heparin in the initial treatment of pulmonary embolism.N Engl J Med.2003;349:1695–1702.
- .Heparin‐induced thrombocytopenia associated with fondaparinux.N Engl J Med.2007;356:2653–2655.
- , , , .Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- , , , et al.Ins and outs of inferior vena cava filters in patients with venous thromboembolism: the experience at Monash Medical Centre and review of the published reports.Intern Med J.2008;38(1):38–43.
- PREPIC Study Group.Eight‐year follow‐up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study.Circulation.2005;112:416–422.
- , , , .Inferior vena cava filters: key considerations.Am J Med Sci.2005;330:82–87.
- , , , .Percutaneous inferior vena cava filters: follow‐up of 7 designs in 320 patients.Radiology.1993;188:851–856.
- , , , et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24.
- , , , , , .Long‐term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement.J Vasc Surg.2007;45(5):992–997.
- , , , et al.Comparison of fixed‐dose weight‐adjusted unfractionated heparin and low‐molecular‐weight heparin for acute treatment of venous thromboembolism.JAMA.2006;296(8):935–942.
- Arixtra prescribing information. Last updated October 2008. Research Triangle Park, NC: GlaxoSmithKline. Available at: http://us.gsk.com/products/assets/us_arixtra.pdf. Accessed August2009.
- , , , et al.American College of Physicians;American Academy of Family Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Ann Intern Med.2007;146(3):204–210.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice Guidelines in Oncology. V.1.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2009.
- , , , et al. Guidelines for the treatment of venous thromboembolism in cancer patients: report from the French Working Group. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1284.
- , , , et al.Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study.Arch Intern Med.2000;160(6):761–768.
- , , , et al.Postdischarge thromboprophylaxis and mortality risk after hip‐or knee‐replacement surgery.CMAJ.2008;178(12):1545–1554.
- , , , et al.Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis.Ann Intern Med.2008;149(10):698–707.
- , , , et al.The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients.Haematologica.2007;92(2):199–205.
- .Risk factors of recurrent venous thromboembolism: the role of residual vein thrombosis.Pathophysiol Haemost Thromb.2003/2004;33(5‐6):351–353.
- , , , et al.Effect of patient's sex on risk of recurrent venous thromboembolism: a meta‐analysis.Lancet.2006;368:371–378.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.
- Health Grades, Inc. The Fifth Annual Health Grades Patient Safety in American Hospitals Study. Available at: http://www.healthgrades.com/media/dms/pdf/PatientSafetyInAmericanHospitalsStudy2008.pdf. Accessed August2009.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th Edition.Chest2008;133(6 suppl):381S–453S.
- , , , et al.Value of assessment of pretest probability of deep‐vein thrombosis in clinical management.Lancet.1997;350(9094):1795–1798.
- , , , et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- , , , et al.Limitations of D‐dimer testing in unselected inpatients with suspected venous thromboembolism.Am J Med.2003;114(4):276–282.
- , , , et al.Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians.Ann Fam Med.2007;5(1):57–62.
- , , .The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism.Ann Intern Med.1998;129:1044–1049.
- , .Ultrasonography of leg veins in patients suspected of having pulmonary embolism.Ann Intern Med.1998;128:243–245.
- , .Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism.Circulation.2004;109(12 suppl 1):I15–I21.
- , , , et al.Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology.Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC).Eur Heart J.2008;29(18):2276–2315.
- , , , et al.Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer.Thromb Haemost.2000;83(3):416–420.
- , , , et al.Prospective validation of Wells criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44(5):503–510.
- , , , et al.Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism.J Thromb Haemost.2004;2(8):1247–1255.
- , , , et al.Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria.J Thromb Haemost.2008;6(5):772–780.
- , , , et al.A simple diagnostic strategy in hospitalized patients with clinically suspected pulmonary embolism.J Intern Med.2006;260(5):459–466.
- , .Is computed tomographic venography of lower limbs useful in suspected pulmonary embolism?Rev Med Suisse.2008;4(143):354,356–359.
- , , , et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354(22):2317–2327.
- , , , et al.Christopher Study Investigators.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D‐dimer testing, and computed tomography.JAMA.2006;295(2):172–179.
- , , , .Acute pulmonary embolectomy: a contemporary approach.Circulation.2002;105:1416–1419.
- , , , et al.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6 suppl):454S–545S.
- , , , et al.Subcutaneous fondaparinux versus unfractionated heparin in the initial treatment of pulmonary embolism.N Engl J Med.2003;349:1695–1702.
- .Heparin‐induced thrombocytopenia associated with fondaparinux.N Engl J Med.2007;356:2653–2655.
- , , , .Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- , , , et al.Ins and outs of inferior vena cava filters in patients with venous thromboembolism: the experience at Monash Medical Centre and review of the published reports.Intern Med J.2008;38(1):38–43.
- PREPIC Study Group.Eight‐year follow‐up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study.Circulation.2005;112:416–422.
- , , , .Inferior vena cava filters: key considerations.Am J Med Sci.2005;330:82–87.
- , , , .Percutaneous inferior vena cava filters: follow‐up of 7 designs in 320 patients.Radiology.1993;188:851–856.
- , , , et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24.
- , , , , , .Long‐term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement.J Vasc Surg.2007;45(5):992–997.
- , , , et al.Comparison of fixed‐dose weight‐adjusted unfractionated heparin and low‐molecular‐weight heparin for acute treatment of venous thromboembolism.JAMA.2006;296(8):935–942.
- Arixtra prescribing information. Last updated October 2008. Research Triangle Park, NC: GlaxoSmithKline. Available at: http://us.gsk.com/products/assets/us_arixtra.pdf. Accessed August2009.
- , , , et al.American College of Physicians;American Academy of Family Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism.Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians.Ann Intern Med.2007;146(3):204–210.
- National Comprehensive Cancer Network (NCCN). Venous thromboembolic disease. Practice Guidelines in Oncology. V.1.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed August2009.
- , , , et al. Guidelines for the treatment of venous thromboembolism in cancer patients: report from the French Working Group. Presented at the 50th Annual Meeting of the American College of Hematology; San Francisco, CA; December 6‐9, 2008. Abstract 1284.
- , , , et al.Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study.Arch Intern Med.2000;160(6):761–768.
- , , , et al.Postdischarge thromboprophylaxis and mortality risk after hip‐or knee‐replacement surgery.CMAJ.2008;178(12):1545–1554.
- , , , et al.Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis.Ann Intern Med.2008;149(10):698–707.
- , , , et al.The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients.Haematologica.2007;92(2):199–205.
- .Risk factors of recurrent venous thromboembolism: the role of residual vein thrombosis.Pathophysiol Haemost Thromb.2003/2004;33(5‐6):351–353.
- , , , et al.Effect of patient's sex on risk of recurrent venous thromboembolism: a meta‐analysis.Lancet.2006;368:371–378.
- , .Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations.J Manag Care Pharm.2007;13(6):475–486.
- , .Outpatient management of anticoagulation therapy.Am Fam Physician.2007;75:1031–1042.