User login
Prevention and treatment of influenza in the primary care office
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
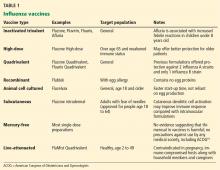
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
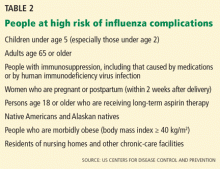
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
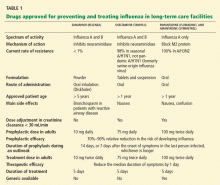
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
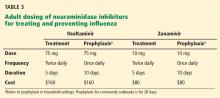
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
KEY POINTS
- Influenza vaccination is effective at preventing influenza-associated disease.
- Influenza vaccine is safe in people with a history of mild egg allergy.
- Many new vaccine formulations exist and may offer benefits to different patient groups.
- Neuraminidase inhibitors are recommended for treatment and postexposure prophylaxis in patients at high risk of influenza-related complications; however, they are not a substitute for vaccination.
Influenza in long-term care facilities: Preventable, detectable, treatable
Influenza vaccination of residents of long-term care facilities (and of health care workers at these facilities) is critical for the prevention of influenza in this frail population. Detection, chemoprophylaxis, and treatment have limitations. Infection control measures should be in place during and between outbreaks. Acute care facilities such as emergency departments and hospitals can assist by testing residents of long-term care facilities who present with influenza-like illness during seasonal epidemics of influenza, and by notifying the receiving facility if a patient with influenza would be arriving.
THE EXTENT OF THE PROBLEM
From 5% to 20% of the US population, including residents and health care workers in long-term care facilities, are infected with influenza every year.1,2 The proportion of those infected who develop clinical illness ranges from 40% to 80%. Each influenza illness is associated with an average of 10 days of respiratory sickness, resulting in approximately 3 days of bed confinement or restricted activity. About 30% to 50% of patients with microbiologically confirmed influenza seek medical care, of whom 16% undergo laboratory tests, 17% undergo radiologic tests, and 75% are recommended an over-the-counter drug or are prescribed a medication. Annual influenza-related hospitalizations range from 200,000 to 400,000, depending on seasonal variations in virulence.2,3 Thus, influenza causes 1.3 hospitalizations per 1,000 people, and 25% of these are in people age 65 and older. In the United States, about 40,000 to 60,000 people die of influenza every year, and 90% of these are age 65 and older.4
POLICIES TO FIGHT ANTIVIRAL RESISTANCE
In January 2006, the US Centers for Disease Control and Prevention (CDC) recommended against the use of the adamantanes—ie, amantadine (Symmetrel) and rimantadine (Flumadine)—for the treatment or prevention of influenza because of a high level of resistance in circulating influenza A/H3N2 in the community. Unfortunately, this resistance trend has not reversed since then, with 96% to 100% of influenza A/H3N2 isolates in the United States showing resistance.5 During the 2007–2008 influenza season, influenza A/H1N1 isolates resistant to oseltamivir (Tamiflu) emerged in Europe, particularly in Norway and France. In the United States, influenza A/H1N1 resistance to oseltamivir increased from 0.7% in the 2006–2007 season, to 10.9% in the 2007–2008 season, and to 98% during the 2008–2009 season.6,7
Fortunately, all oseltamivir-resistant isolates remain susceptible to zanamivir (Relenza). In April 2009, a new influenza A/H1N1 variant (previously referred to as swine-origin influenza virus, or SOIV) emerged in North America and spread to many countries worldwide, and the World Health Organization eventually declared a pandemic. This new variant is susceptible to oseltamivir and zanamivir but resistant to the adamantanes. Resistance patterns for future influenza seasons cannot be predicted, but the current extent of influenza resistance and its development over the past decade8 are alarming.
WHY IS INFLUENZA MORE SERIOUS IN LONG-TERM CARE RESIDENTS?
Influenza is usually introduced to long-term care facilities by workers and visitors. Inside, the closed environment and limited mobility of residents facilitate transmission of infection.
The clinical presentation of influenza in residents of long-term care facilities can be subtle, with a blunted febrile response and a decline in mental and functional status.9
Residents commonly have underlying diseases that can be exacerbated by influenza infection, such as congestive heart failure, chronic obstructive lung disease, chronic kidney disease, and dementia. In addition, residents are at higher risk of serious influenza-related complications than are community-dwelling elderly people.
Impaired oral intake, limited dexterity, and altered consciousness may limit treatment options, thus further adversely affecting outcomes. Bacterial pneumonia secondary to influenza has dire consequences in long-term care residents. Rates of hospitalization for pneumonia and influenza and for exacerbation of chronic lung disease are higher in these patients than in their community-dwelling counterparts.10 Death rates are also higher, exceeding 5% during influenza epidemics.11
PREVENTING INFLUENZA IN LONG-TERM CARE FACILITIES
Immunizing residents is essential
Vaccination is the most important measure in preventing influenza in long-term care facilities, and vaccination programs should include residents and health care workers.
Influenza outbreaks are more common in facilities where the rate of immunization is below 80%, as well as in larger facilities (with > 100 beds), suggesting that herd immunity may play a role.12 Unfortunately, influenza vaccine coverage rates vary widely in this patient population—from 57% to 98% in one report.13
The live-attenuated intranasal vaccine is approved only for healthy people under age 50, so most residents of long-term care facilities should receive only the inactivated trivalent intramuscular vaccine.
The effectiveness of a vaccine in preventing influenza depends in part on the adequacy of the match between vaccine serotypes and circulating strains. Studies of all types—randomized, observational, case-control, and cohort studies, as well as meta-analyses and systematic reviews—have shown preventive efficacy rates of influenza vaccination in elderly residents of long-term care facilities to be 23% to 43% for influenza-like illness, 0% to 58% for influenza, 46% for pneumonia, 45% for hospitalization, 42% for death from influenza or pneumonia, and 60% for death from all causes.14,15 Vaccine performance improved after adjustment for confounders.
Obviously, this protection is variable and incomplete, since influenza outbreaks continue to occur even in long-term care facilities in which most residents are vaccinated.13
Vaccination works, despite the controversy
Whether influenza vaccination prevents deaths in elderly people—or how many deaths it prevents—is a subject of ongoing controversy. 16 Even though influenza vaccination coverage in the elderly increased from 15% to 65% since 1980, the specific influenza-related death rate did not decrease.17
It has been suggested that cohort studies may have overestimated the mortality benefit of influenza vaccination in the elderly because of “frailty selection bias” (ie, extremely frail elderly patients are less likely to be vaccinated and are more likely to die for reasons other than influenza than are less frail, vaccinated elderly people) and because of the use of nonspecific end points such as all-cause mortality. 16 Similarly, observational studies may have overestimated the in-hospital mortality benefit of influenza vaccination in older patients with pneumonia occurring outside of influenza season because of the “healthy user effect” (ie, residual confounding by functional and socioeconomic status).18
One nested case-control study in immunocompetent elderly patients showed that influenza vaccination was not associated with a reduced risk of community-acquired pneumonia after adjusting for the presence and severity of comorbidities.19
Since death is a rare end point, it is hard to show a reduction in the death rate with vaccination in randomized controlled studies. The absolute risk reduction in hospitalization and death with vaccination is two to five times higher in elderly patients at high risk than in the healthy elderly.20
The mortality benefit in elderly patients is increased with annual revaccination, with one death prevented for every 300 vaccinations, and one for every 200 revaccinations.21
The response to influenza vaccination is reduced in elderly people because of immune senescence, and higher doses of vaccine have been shown to be more immunogenic and remain safe.22 This enhanced antibody response may be maintained for subsequent antigenically different influenza variants, even against viruses appearing more than 10 years after vaccination. 23 The 2008–2009 influenza vaccine does not protect against the new, pandemic influenza A/H1N1 variant; efforts to produce such a vaccine are under way.
Influenza vaccination is safe. Recent data showed no association between immunization and Guillain-Barré syndrome.24 In fact, influenza itself may be a triggering agent for Guillain-Barré syndrome during major influenza outbreaks.25
DURATION OF SEROPROTECTION IS MORE THAN 6 MONTHS
Every effort should be made to vaccinate residents of long-term care facilities and their caregivers as early as possible in the influenza season to allow an adequate antibody response to develop before the onset of an influenza outbreak.
In the past, there has been concern that the influenza-vaccine-induced antibody response declines more rapidly in the elderly and may fall below seroprotective levels within 4 months of vaccination. But a recent review of 14 published studies argued against that notion, showing that if seroprotection is achieved in the first month after vaccination, it is then maintained for more than 6 months.26 That review also showed that seroconversion varies inversely with preimmunization titers, but not with age.
Moreover, a prospective study27 in 303 residents of a long-term care facility reported that seroprotection did not correlate with nutritional status. In the study, vaccination was very effective despite a high prevalence of nutritional deficiencies. This study also indicated that although an influenza antibody titer greater than 1:40 is considered protective in the general population, long-term care facility residents may require higher levels for effective immunization.
A recent survey showed that a national shortage of influenza vaccine results in decreased immunization rates in residents and in health care workers in long-term care facilities. 28 In that survey, only 2.3% of facilities expressed concern about emergency preparedness, and this has significant implications for a possible influenza pandemic.
VACCINATION PROGRAMS
Standing-order programs have been shown to significantly increase vaccination rates in ambulatory and hospital settings. However, a recent survey showed that only 9% of long-term care facilities use such programs.29 The greatest use of such programs was in government-owned, nonprofit, dual-certified (ie, by both Medicare and Medicaid), and independent long-term care facilities, and in facilities with a lower index of disease acuity. Use varied substantially by state.
The Healthy People 2010 goal of 90% vaccination may be attained by implementing written protocols for documenting immunization—and refusal of immunization—in a consistent place in the patient’s medical record.30
VACCINATION OF WORKERS
When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die31,32 or develop influenza-like illness, particularly when residents themselves are vaccinated.33 Additional benefits include decreased need for consultations with general practitioners or admissions to the hospital for influenza-like illness during periods of moderate influenza activity.32
The policy of mandatory influenza vaccination for health care workers has its proponents34 and opponents.35 When a large tertiary care center adopted mandatory vaccination, vaccination rates increased significantly over time.36 The Centers for Medicare & Medicaid Services recently mandated public reporting of vaccination rates in health care workers and residents of long-term care facilities, and compliance is expected to increase as a result.
BETWEEN INFLUENZA OUTBREAKS
Studies show that hygienic measures prevent transmission of respiratory viruses.37 Therefore, the cornerstones of any program to prevent transmission of influenza and other microorganisms in long-term care facilities are hand hygiene, cough and sneeze etiquette, maintaining a distance of 3 feet between beds, and education of residents and health care workers.
Wash your hands!
Hands should be washed before and after direct contact with patients or with inanimate objects in their immediate vicinity.38 Contrary to popular belief, hands should also be washed before and after wearing gloves, particularly when handling an invasive device for patient care, and after contact with bodily fluids, excretions, mucous membranes, non-intact skin, or wound dressings. Hands should be washed not only between patients but also during care for the same patient if moving from a contaminated body site to a clean site.
Hands should be washed for 15 to 20 seconds using soap and water or an alcohol-based foam or gel; when hands are visibly soiled, only soap and water should be used. It is not known whether adding virucidals or antiseptics to normal hand-washing further decreases the spread of these viruses.
Continuous education, feedback interventions, and patient-awareness programs can improve hand-washing compliance, but they are not sufficient. Easily accessible dispensers for alcohol-based waterless antiseptic foam or gel can significantly improve hand hygiene rates among health care workers.39
DETECTING INFLUENZA OUTBREAKS
Direct fluorescent antigen detection and nucleic acid detection by polymerase chain reaction (PCR) are the tests recommended for early detection of influenza outbreaks in long-term care facilities. Rapid point-of-care tests to detect influenza antigen are only 60% to 70% sensitive,13,40 viral culture takes several days, and serologic diagnosis requires documentation of seroconversion at least 2 weeks apart, and so none of these is adequate for the early detection of an influenza outbreak.
There is no widely available test to differentiate influenza A/H1N1 from A/H3N2, or to test for drug resistance. In addition, the PCR tests widely used today do not differentiate between seasonal influenza A/H1N1 and the new, pandemic influenza A/H1N1 variant. Efforts are under way to produce and distribute such a test.
Controlling influenza outbreaks
An outbreak should be declared when two or more residents develop an influenza-like illness within 72 hours of each other during the influenza season.41 After influenza infection is confirmed by laboratory testing, testing of all residents who subsequently develop an influenza-like illness may not be feasible, and other respiratory viruses may be responsible for mixed outbreaks during influenza epidemics, particularly respiratory syncytial virus.
Roommates of residents who test positive for influenza have a risk of acquiring influenza three times higher than that of residents living in single rooms.42 Obviously, private rooms for all residents would be optimal, but this is not practical in most facilities. “Cohorting” (ie, housing infected residents together) is reasonable, but in this situation they might infect each other with other viruses or with influenza viruses of different serotypes.
When an outbreak occurs, potential solutions include closing subunits of the facility to new admissions, limiting movement of residents and health care workers from affected to unaffected units, and using curtains or other barriers between roommates. But most important during seasonal outbreaks are hand hygiene, proper cough and sneeze etiquette, and 3-foot separation between roommates. A simulation model showed that during an influenza pandemic, preventing ill residents of long-term care facilities from making contact with other residents may reduce rates of illness and death by about 60%.43 If a long-term care facility resident is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient or when entering the room of a resident with confirmed influenza.
CHEMOPROPHYLAXIS DURING INFLUENZA OUTBREAKS
When influenza outbreaks are recognized early in long-term care facilities, appropriate infection control measures and chemoprophylaxis can be started. Chemoprophylaxis should also be considered in residents in whom influenza vaccination is contraindicated, such as those with severe egg allergy.
Rimantadine is preferred over amantadine in residents of long-term care facilities, since amantadine is associated with a much higher rate of adverse events (18.6% vs 1.9%), especially confusion (10.6% vs 0.6%), resulting in more frequent discontinuation (17.3% vs 1.9%).44 In addition, viral resistance to amantadine develops in about 30% of those who receive it. In long-term care facilities, viral resistance occurs not only when it is used for the management of influenza, but also when it is used to treat Parkinson disease.45
Oseltamivir prophylaxis for 6 weeks in influenza-vaccinated, frail elderly residents of long-term care facilities during influenza epidemics was 91% effective in preventing laboratory-confirmed clinical influenza, and 85% effective in preventing a secondary bacterial complication such as pneumonia or sinusitis.46 The rate of adverse events associated with oseltamivir in that population was similar to that with placebo. Importantly, oseltamivir was not associated with suppression of antibody response to influenza infection or vaccination.
Oseltamivir is very effective in terminating influenza outbreaks in long-term care facilities, even when amantadine fails.4,13 When used in that manner, it is also associated with decreased antibiotic prescriptions, hospitalizations, deaths,47 and substantial cost-savings, even when compared with amantadine, which has a much lower acquisition cost but a higher rate of adverse events, lower efficacy, and individualized dosing requirements.48
Zanamivir prophylaxis for 2 weeks given to unvaccinated residents was 29% effective in preventing all symptomatic influenza confirmed by laboratory testing (by culture, PCR, or seroconversion). It was 65% effective in preventing symptomatic culture-confirmed influenza, 70% effective in preventing febrile, laboratory-confirmed influenza, and 21% effective in preventing complications.49 It was well tolerated in this population and was not associated with the emergence of zanamivir resistance. Zanamivir also provides 61% additional protective efficacy over rimantadine in vaccinated residents of long-term care facilities,50 primarily because of the emergence of viruses resistant to rimantadine. However, up to 50% of elderly people may have difficulty loading and priming the Diskhaler device used to deliver zanamivir.51
While several studies have shown chemoprophylaxis to be effective, it is not possible to ascribe the decrease in cases during an outbreak entirely to antiviral drugs since most studies were not placebo-controlled, and since the natural tendency of outbreaks is to subside.
TREATMENT OF INFLUENZA IN LONG-TERM CARE FACILITIES
A mild illness in someone without a high-risk underlying disease can be managed with symptomatic measures alone, and testing for influenza is at the discretion of the caregiver. Patients who develop a moderate or severe influenza-like illness during an influenza epidemic and who have a high-risk underlying disease or who require hospitalization should be tested for influenza by a sensitive test such as direct fluorescent antigen testing or PCR and should be started on empiric anti-influenza therapy.41
Most elderly residents of long-term care facilities do have comorbid conditions and are thus at high risk of complications of influenza. During influenza outbreaks, clinicians caring for these patients—and for similar patients in outpatient settings, emergency departments, and acute-care hospitals—should consider testing for influenza, even if the patient has only a mild influenza-like illness.
Of note, no randomized treatment study has been done specifically in residents of long-term care facilities.52 Pooled analysis of data from 321 patients at high risk (76 were age 65 or older) in zanamivir treatment studies showed that those who received the drug were sick for 2.5 fewer days than those who received placebo.53 In addition, zanamivir recipients returned to normal activities 3 days sooner, had an 11% reduction in the median total symptom score over 1 to 5 days, and had a rate of complications requiring antibiotics 43% less than placebo recipients. Rates of adverse events were similar in both groups.
A multicenter, randomized, open-label, controlled trial of oseltamivir in the treatment of influenza in high-risk Chinese patients with chronic respiratory diseases (chronic bronchitis, obstructive emphysema, bronchial asthma, or bronchiectasis) or chronic cardiac disease showed that oseltamivir significantly reduced the duration of influenza symptoms by 36.8%, the severity of symptoms by 43.1%, the duration of fever by 45.2%, the time to return to baseline health status by 5 days, and the need for antibiotics, without increasing the total cost of medical care.54
Oseltamivir-resistant isolates of seasonal influenza A/H1N1 do not cause different or more severe symptoms than do oseltamivir-susceptible isolates, and they remain susceptible to zanamivir and the adamantanes. There is no widely available test to differentiate influenza A/H1N1 from A/H3N2 or to test for drug resistance. Influenza B and A/H3N2 remain susceptible to both of the neuraminidase inhibitors, ie, oseltamivir and zanamivir.
All currently circulating influenza A/H3N2 isolates remain resistant to the adamantanes. Influenza B is intrinsically resistant to the adamantanes, and some data show that oseltamivir is less effective against influenza B than against influenza A.
Zanamivir is not available in many pharmacies, is not recommended in patients with underlying reactive airway disease, and requires dexterity for administration.
Amantadine is more likely to cause confusion in the elderly than rimantadine, and it is more susceptible to treatment-emergent antiviral resistance.
No parenteral anti-influenza drug is available, but zanamivir and the experimental drug peramivir are undergoing study for parenteral use. An inhaled, long-acting neuraminidase inhibitor, CS-8958, is currently under study.55 Other agents currently under development include T-705, a polymerase inhibitor, and DAS181, an attachment inhibitor.
Complementary and alternative therapies for influenza are not established. A recent review of 14 randomized, controlled trials56 revealed that the evidence is weak and limited by small sample sizes, poor methodologic quality, or clinically irrelevant effect sizes.
- Cifu A, Levinson W. Influenza. JAMA 2000; 284:2847–2849.
- Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics 1996; 9(suppl 3):26–33.
- Fiore AE, Shay DK, Broder K, et al; US Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:1–60.
- Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol 1993; 22:334–340.
- Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 2007; 196:249–257.
- Centers for Disease Control and Prevention (CDC). Update: Influenza activity—United States, September 28, 2008–January 31, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:115–119.
- CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008–09 influenza season. Distributed via Health Alert Network. 2008. www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279. Accessed 7/14/2009.
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 2009; 301:1066–1069.
- Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998; 158:645–650.
- Menec VH, MacWilliams L, Aoki FY. Hospitalization and deaths due to respiratory illnesses during influenza seasons: a comparison of community residents, senior housing residents, and nursing home residents. J Gerontol A Biol Sci Med Sci 2002; 57:M629–M635.
- Kingston BJ, Wright CV. Influenza in the nursing home. Am Fam Physician 2002; 65:75–78.
- Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis 2006; 194(suppl 2):S133–S138.
- Monto AS, Rotthoff J, Teich E, et al. Detection and control of influenza outbreaks in well-vaccinated nursing home populations. Clin Infect Dis 2004; 39:459–464.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366:1165–1174.
- Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; 3:CD004876.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–666.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Hak E, Nordin J, Wei F, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis 2002; 35:370–377.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis 2008; 198:1016–1018.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Paccalin M, Plouzeau C, Bouche G, et al. Lack of correlation between nutritional status and seroprotection against influenza in a long term care facility. Scand J Infect Dis 2006; 38:894–897.
- Mody L, Langa KM, Malani PN. Impact of the 2004–2005 influenza vaccine shortage on immunization practices in long-term care facilities. Infect Control Hosp Epidemiol 2006; 27:383–387.
- Shefer A, McKibben L, Bardenheier B, Bratzler D, Roberts H. Characteristics of long-term care facilities associated with standing order programs to deliver influenza and pneumococcal vaccinations to residents in 13 states. J Am Med Dir Assoc 2005; 6:97–104.
- Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long-term care facilities: the CMS-CDC standing orders program project. J Am Med Dir Assoc 2005; 6:291–299.
- Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000; 355:93–97.
- Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241.
- Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2006; 3:CD005187.
- Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144–1147.
- Finch M. Point: mandatory influenza vaccination for all health care workers? Seven reasons to say “no”. Clin Infect Dis 2006; 42:1141–1143.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Pittet D, Allegranzi B, Sax H, et al; WHO Global Patient Safety Challenge, World Alliance for Patient Safety. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 2006; 6:641–652.
- Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 2000; 160:1017–1021.
- US Centers for Disease Control and Prevention. Influenza-testing and antiviral-agent prescribing practices—Connecticut, Minnesota, New Mexico, and New York, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:61–65.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Drinka PJ, Krause PF, Nest LJ, Goodman BM, Gravenstein S. Risk of acquiring influenza B in a nursing home from a culture-positive roommate. J Am Geriatr Soc 2005; 53:1437.
- Haber MJ, Shay DK, Davis XM, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis 2007; 13:581–589.
- Keyser LA, Karl M, Nafziger AN, Bertino JS. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med 2000; 160:1485–1488.
- Saito R, Oshitani H, Masuda H, Suzuki H. Detection of amantadineresistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol 2002; 40:84–88.
- Peters PH, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031.
- Bowles SK, Kennie N, Ruston L, Simor A, Louie M, Collins V. Influenza outbreak in a long-term-care facility: considerations for pharmacy. Am J Health Syst Pharm 1999; 56:2303–2307.
- Risebrough NA, Bowles SK, Simor AE, McGeer A, Oh PI. Economic evaluation of oseltamivir phosphate for postexposure prophylaxis of influenza in long-term care facilities. J Am Geriatr Soc 2005; 53:444–451.
- Ambrozaitis A, Gravenstein S, van Essen GA, et al. Inhaled zanamivir versus placebo for the prevention of influenza outbreaks in an unvaccinated long-term care population. J Am Med Dir Assoc 2005; 6:367–374.
- Gravenstein S, Drinka P, Osterweil D, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc 2005; 6:359–366.
- Diggory P, Fernandez C, Humphrey A, Jones V, Murphy M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ 2001; 322:577–579.
- Glezen WP. Clinical practice: prevention and treatment of seasonal influenza. N Engl J Med 2008; 359:2579–2585.
- Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001; 161:212–217.
- Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin 2006; 22:75–82.
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009; 48( suppl 1):S3–S13.
- Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am J Med 2007; 120:923–929.
Influenza vaccination of residents of long-term care facilities (and of health care workers at these facilities) is critical for the prevention of influenza in this frail population. Detection, chemoprophylaxis, and treatment have limitations. Infection control measures should be in place during and between outbreaks. Acute care facilities such as emergency departments and hospitals can assist by testing residents of long-term care facilities who present with influenza-like illness during seasonal epidemics of influenza, and by notifying the receiving facility if a patient with influenza would be arriving.
THE EXTENT OF THE PROBLEM
From 5% to 20% of the US population, including residents and health care workers in long-term care facilities, are infected with influenza every year.1,2 The proportion of those infected who develop clinical illness ranges from 40% to 80%. Each influenza illness is associated with an average of 10 days of respiratory sickness, resulting in approximately 3 days of bed confinement or restricted activity. About 30% to 50% of patients with microbiologically confirmed influenza seek medical care, of whom 16% undergo laboratory tests, 17% undergo radiologic tests, and 75% are recommended an over-the-counter drug or are prescribed a medication. Annual influenza-related hospitalizations range from 200,000 to 400,000, depending on seasonal variations in virulence.2,3 Thus, influenza causes 1.3 hospitalizations per 1,000 people, and 25% of these are in people age 65 and older. In the United States, about 40,000 to 60,000 people die of influenza every year, and 90% of these are age 65 and older.4
POLICIES TO FIGHT ANTIVIRAL RESISTANCE
In January 2006, the US Centers for Disease Control and Prevention (CDC) recommended against the use of the adamantanes—ie, amantadine (Symmetrel) and rimantadine (Flumadine)—for the treatment or prevention of influenza because of a high level of resistance in circulating influenza A/H3N2 in the community. Unfortunately, this resistance trend has not reversed since then, with 96% to 100% of influenza A/H3N2 isolates in the United States showing resistance.5 During the 2007–2008 influenza season, influenza A/H1N1 isolates resistant to oseltamivir (Tamiflu) emerged in Europe, particularly in Norway and France. In the United States, influenza A/H1N1 resistance to oseltamivir increased from 0.7% in the 2006–2007 season, to 10.9% in the 2007–2008 season, and to 98% during the 2008–2009 season.6,7
Fortunately, all oseltamivir-resistant isolates remain susceptible to zanamivir (Relenza). In April 2009, a new influenza A/H1N1 variant (previously referred to as swine-origin influenza virus, or SOIV) emerged in North America and spread to many countries worldwide, and the World Health Organization eventually declared a pandemic. This new variant is susceptible to oseltamivir and zanamivir but resistant to the adamantanes. Resistance patterns for future influenza seasons cannot be predicted, but the current extent of influenza resistance and its development over the past decade8 are alarming.
WHY IS INFLUENZA MORE SERIOUS IN LONG-TERM CARE RESIDENTS?
Influenza is usually introduced to long-term care facilities by workers and visitors. Inside, the closed environment and limited mobility of residents facilitate transmission of infection.
The clinical presentation of influenza in residents of long-term care facilities can be subtle, with a blunted febrile response and a decline in mental and functional status.9
Residents commonly have underlying diseases that can be exacerbated by influenza infection, such as congestive heart failure, chronic obstructive lung disease, chronic kidney disease, and dementia. In addition, residents are at higher risk of serious influenza-related complications than are community-dwelling elderly people.
Impaired oral intake, limited dexterity, and altered consciousness may limit treatment options, thus further adversely affecting outcomes. Bacterial pneumonia secondary to influenza has dire consequences in long-term care residents. Rates of hospitalization for pneumonia and influenza and for exacerbation of chronic lung disease are higher in these patients than in their community-dwelling counterparts.10 Death rates are also higher, exceeding 5% during influenza epidemics.11
PREVENTING INFLUENZA IN LONG-TERM CARE FACILITIES
Immunizing residents is essential
Vaccination is the most important measure in preventing influenza in long-term care facilities, and vaccination programs should include residents and health care workers.
Influenza outbreaks are more common in facilities where the rate of immunization is below 80%, as well as in larger facilities (with > 100 beds), suggesting that herd immunity may play a role.12 Unfortunately, influenza vaccine coverage rates vary widely in this patient population—from 57% to 98% in one report.13
The live-attenuated intranasal vaccine is approved only for healthy people under age 50, so most residents of long-term care facilities should receive only the inactivated trivalent intramuscular vaccine.
The effectiveness of a vaccine in preventing influenza depends in part on the adequacy of the match between vaccine serotypes and circulating strains. Studies of all types—randomized, observational, case-control, and cohort studies, as well as meta-analyses and systematic reviews—have shown preventive efficacy rates of influenza vaccination in elderly residents of long-term care facilities to be 23% to 43% for influenza-like illness, 0% to 58% for influenza, 46% for pneumonia, 45% for hospitalization, 42% for death from influenza or pneumonia, and 60% for death from all causes.14,15 Vaccine performance improved after adjustment for confounders.
Obviously, this protection is variable and incomplete, since influenza outbreaks continue to occur even in long-term care facilities in which most residents are vaccinated.13
Vaccination works, despite the controversy
Whether influenza vaccination prevents deaths in elderly people—or how many deaths it prevents—is a subject of ongoing controversy. 16 Even though influenza vaccination coverage in the elderly increased from 15% to 65% since 1980, the specific influenza-related death rate did not decrease.17
It has been suggested that cohort studies may have overestimated the mortality benefit of influenza vaccination in the elderly because of “frailty selection bias” (ie, extremely frail elderly patients are less likely to be vaccinated and are more likely to die for reasons other than influenza than are less frail, vaccinated elderly people) and because of the use of nonspecific end points such as all-cause mortality. 16 Similarly, observational studies may have overestimated the in-hospital mortality benefit of influenza vaccination in older patients with pneumonia occurring outside of influenza season because of the “healthy user effect” (ie, residual confounding by functional and socioeconomic status).18
One nested case-control study in immunocompetent elderly patients showed that influenza vaccination was not associated with a reduced risk of community-acquired pneumonia after adjusting for the presence and severity of comorbidities.19
Since death is a rare end point, it is hard to show a reduction in the death rate with vaccination in randomized controlled studies. The absolute risk reduction in hospitalization and death with vaccination is two to five times higher in elderly patients at high risk than in the healthy elderly.20
The mortality benefit in elderly patients is increased with annual revaccination, with one death prevented for every 300 vaccinations, and one for every 200 revaccinations.21
The response to influenza vaccination is reduced in elderly people because of immune senescence, and higher doses of vaccine have been shown to be more immunogenic and remain safe.22 This enhanced antibody response may be maintained for subsequent antigenically different influenza variants, even against viruses appearing more than 10 years after vaccination. 23 The 2008–2009 influenza vaccine does not protect against the new, pandemic influenza A/H1N1 variant; efforts to produce such a vaccine are under way.
Influenza vaccination is safe. Recent data showed no association between immunization and Guillain-Barré syndrome.24 In fact, influenza itself may be a triggering agent for Guillain-Barré syndrome during major influenza outbreaks.25
DURATION OF SEROPROTECTION IS MORE THAN 6 MONTHS
Every effort should be made to vaccinate residents of long-term care facilities and their caregivers as early as possible in the influenza season to allow an adequate antibody response to develop before the onset of an influenza outbreak.
In the past, there has been concern that the influenza-vaccine-induced antibody response declines more rapidly in the elderly and may fall below seroprotective levels within 4 months of vaccination. But a recent review of 14 published studies argued against that notion, showing that if seroprotection is achieved in the first month after vaccination, it is then maintained for more than 6 months.26 That review also showed that seroconversion varies inversely with preimmunization titers, but not with age.
Moreover, a prospective study27 in 303 residents of a long-term care facility reported that seroprotection did not correlate with nutritional status. In the study, vaccination was very effective despite a high prevalence of nutritional deficiencies. This study also indicated that although an influenza antibody titer greater than 1:40 is considered protective in the general population, long-term care facility residents may require higher levels for effective immunization.
A recent survey showed that a national shortage of influenza vaccine results in decreased immunization rates in residents and in health care workers in long-term care facilities. 28 In that survey, only 2.3% of facilities expressed concern about emergency preparedness, and this has significant implications for a possible influenza pandemic.
VACCINATION PROGRAMS
Standing-order programs have been shown to significantly increase vaccination rates in ambulatory and hospital settings. However, a recent survey showed that only 9% of long-term care facilities use such programs.29 The greatest use of such programs was in government-owned, nonprofit, dual-certified (ie, by both Medicare and Medicaid), and independent long-term care facilities, and in facilities with a lower index of disease acuity. Use varied substantially by state.
The Healthy People 2010 goal of 90% vaccination may be attained by implementing written protocols for documenting immunization—and refusal of immunization—in a consistent place in the patient’s medical record.30
VACCINATION OF WORKERS
When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die31,32 or develop influenza-like illness, particularly when residents themselves are vaccinated.33 Additional benefits include decreased need for consultations with general practitioners or admissions to the hospital for influenza-like illness during periods of moderate influenza activity.32
The policy of mandatory influenza vaccination for health care workers has its proponents34 and opponents.35 When a large tertiary care center adopted mandatory vaccination, vaccination rates increased significantly over time.36 The Centers for Medicare & Medicaid Services recently mandated public reporting of vaccination rates in health care workers and residents of long-term care facilities, and compliance is expected to increase as a result.
BETWEEN INFLUENZA OUTBREAKS
Studies show that hygienic measures prevent transmission of respiratory viruses.37 Therefore, the cornerstones of any program to prevent transmission of influenza and other microorganisms in long-term care facilities are hand hygiene, cough and sneeze etiquette, maintaining a distance of 3 feet between beds, and education of residents and health care workers.
Wash your hands!
Hands should be washed before and after direct contact with patients or with inanimate objects in their immediate vicinity.38 Contrary to popular belief, hands should also be washed before and after wearing gloves, particularly when handling an invasive device for patient care, and after contact with bodily fluids, excretions, mucous membranes, non-intact skin, or wound dressings. Hands should be washed not only between patients but also during care for the same patient if moving from a contaminated body site to a clean site.
Hands should be washed for 15 to 20 seconds using soap and water or an alcohol-based foam or gel; when hands are visibly soiled, only soap and water should be used. It is not known whether adding virucidals or antiseptics to normal hand-washing further decreases the spread of these viruses.
Continuous education, feedback interventions, and patient-awareness programs can improve hand-washing compliance, but they are not sufficient. Easily accessible dispensers for alcohol-based waterless antiseptic foam or gel can significantly improve hand hygiene rates among health care workers.39
DETECTING INFLUENZA OUTBREAKS
Direct fluorescent antigen detection and nucleic acid detection by polymerase chain reaction (PCR) are the tests recommended for early detection of influenza outbreaks in long-term care facilities. Rapid point-of-care tests to detect influenza antigen are only 60% to 70% sensitive,13,40 viral culture takes several days, and serologic diagnosis requires documentation of seroconversion at least 2 weeks apart, and so none of these is adequate for the early detection of an influenza outbreak.
There is no widely available test to differentiate influenza A/H1N1 from A/H3N2, or to test for drug resistance. In addition, the PCR tests widely used today do not differentiate between seasonal influenza A/H1N1 and the new, pandemic influenza A/H1N1 variant. Efforts are under way to produce and distribute such a test.
Controlling influenza outbreaks
An outbreak should be declared when two or more residents develop an influenza-like illness within 72 hours of each other during the influenza season.41 After influenza infection is confirmed by laboratory testing, testing of all residents who subsequently develop an influenza-like illness may not be feasible, and other respiratory viruses may be responsible for mixed outbreaks during influenza epidemics, particularly respiratory syncytial virus.
Roommates of residents who test positive for influenza have a risk of acquiring influenza three times higher than that of residents living in single rooms.42 Obviously, private rooms for all residents would be optimal, but this is not practical in most facilities. “Cohorting” (ie, housing infected residents together) is reasonable, but in this situation they might infect each other with other viruses or with influenza viruses of different serotypes.
When an outbreak occurs, potential solutions include closing subunits of the facility to new admissions, limiting movement of residents and health care workers from affected to unaffected units, and using curtains or other barriers between roommates. But most important during seasonal outbreaks are hand hygiene, proper cough and sneeze etiquette, and 3-foot separation between roommates. A simulation model showed that during an influenza pandemic, preventing ill residents of long-term care facilities from making contact with other residents may reduce rates of illness and death by about 60%.43 If a long-term care facility resident is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient or when entering the room of a resident with confirmed influenza.
CHEMOPROPHYLAXIS DURING INFLUENZA OUTBREAKS
When influenza outbreaks are recognized early in long-term care facilities, appropriate infection control measures and chemoprophylaxis can be started. Chemoprophylaxis should also be considered in residents in whom influenza vaccination is contraindicated, such as those with severe egg allergy.
Rimantadine is preferred over amantadine in residents of long-term care facilities, since amantadine is associated with a much higher rate of adverse events (18.6% vs 1.9%), especially confusion (10.6% vs 0.6%), resulting in more frequent discontinuation (17.3% vs 1.9%).44 In addition, viral resistance to amantadine develops in about 30% of those who receive it. In long-term care facilities, viral resistance occurs not only when it is used for the management of influenza, but also when it is used to treat Parkinson disease.45
Oseltamivir prophylaxis for 6 weeks in influenza-vaccinated, frail elderly residents of long-term care facilities during influenza epidemics was 91% effective in preventing laboratory-confirmed clinical influenza, and 85% effective in preventing a secondary bacterial complication such as pneumonia or sinusitis.46 The rate of adverse events associated with oseltamivir in that population was similar to that with placebo. Importantly, oseltamivir was not associated with suppression of antibody response to influenza infection or vaccination.
Oseltamivir is very effective in terminating influenza outbreaks in long-term care facilities, even when amantadine fails.4,13 When used in that manner, it is also associated with decreased antibiotic prescriptions, hospitalizations, deaths,47 and substantial cost-savings, even when compared with amantadine, which has a much lower acquisition cost but a higher rate of adverse events, lower efficacy, and individualized dosing requirements.48
Zanamivir prophylaxis for 2 weeks given to unvaccinated residents was 29% effective in preventing all symptomatic influenza confirmed by laboratory testing (by culture, PCR, or seroconversion). It was 65% effective in preventing symptomatic culture-confirmed influenza, 70% effective in preventing febrile, laboratory-confirmed influenza, and 21% effective in preventing complications.49 It was well tolerated in this population and was not associated with the emergence of zanamivir resistance. Zanamivir also provides 61% additional protective efficacy over rimantadine in vaccinated residents of long-term care facilities,50 primarily because of the emergence of viruses resistant to rimantadine. However, up to 50% of elderly people may have difficulty loading and priming the Diskhaler device used to deliver zanamivir.51
While several studies have shown chemoprophylaxis to be effective, it is not possible to ascribe the decrease in cases during an outbreak entirely to antiviral drugs since most studies were not placebo-controlled, and since the natural tendency of outbreaks is to subside.
TREATMENT OF INFLUENZA IN LONG-TERM CARE FACILITIES
A mild illness in someone without a high-risk underlying disease can be managed with symptomatic measures alone, and testing for influenza is at the discretion of the caregiver. Patients who develop a moderate or severe influenza-like illness during an influenza epidemic and who have a high-risk underlying disease or who require hospitalization should be tested for influenza by a sensitive test such as direct fluorescent antigen testing or PCR and should be started on empiric anti-influenza therapy.41
Most elderly residents of long-term care facilities do have comorbid conditions and are thus at high risk of complications of influenza. During influenza outbreaks, clinicians caring for these patients—and for similar patients in outpatient settings, emergency departments, and acute-care hospitals—should consider testing for influenza, even if the patient has only a mild influenza-like illness.
Of note, no randomized treatment study has been done specifically in residents of long-term care facilities.52 Pooled analysis of data from 321 patients at high risk (76 were age 65 or older) in zanamivir treatment studies showed that those who received the drug were sick for 2.5 fewer days than those who received placebo.53 In addition, zanamivir recipients returned to normal activities 3 days sooner, had an 11% reduction in the median total symptom score over 1 to 5 days, and had a rate of complications requiring antibiotics 43% less than placebo recipients. Rates of adverse events were similar in both groups.
A multicenter, randomized, open-label, controlled trial of oseltamivir in the treatment of influenza in high-risk Chinese patients with chronic respiratory diseases (chronic bronchitis, obstructive emphysema, bronchial asthma, or bronchiectasis) or chronic cardiac disease showed that oseltamivir significantly reduced the duration of influenza symptoms by 36.8%, the severity of symptoms by 43.1%, the duration of fever by 45.2%, the time to return to baseline health status by 5 days, and the need for antibiotics, without increasing the total cost of medical care.54
Oseltamivir-resistant isolates of seasonal influenza A/H1N1 do not cause different or more severe symptoms than do oseltamivir-susceptible isolates, and they remain susceptible to zanamivir and the adamantanes. There is no widely available test to differentiate influenza A/H1N1 from A/H3N2 or to test for drug resistance. Influenza B and A/H3N2 remain susceptible to both of the neuraminidase inhibitors, ie, oseltamivir and zanamivir.
All currently circulating influenza A/H3N2 isolates remain resistant to the adamantanes. Influenza B is intrinsically resistant to the adamantanes, and some data show that oseltamivir is less effective against influenza B than against influenza A.
Zanamivir is not available in many pharmacies, is not recommended in patients with underlying reactive airway disease, and requires dexterity for administration.
Amantadine is more likely to cause confusion in the elderly than rimantadine, and it is more susceptible to treatment-emergent antiviral resistance.
No parenteral anti-influenza drug is available, but zanamivir and the experimental drug peramivir are undergoing study for parenteral use. An inhaled, long-acting neuraminidase inhibitor, CS-8958, is currently under study.55 Other agents currently under development include T-705, a polymerase inhibitor, and DAS181, an attachment inhibitor.
Complementary and alternative therapies for influenza are not established. A recent review of 14 randomized, controlled trials56 revealed that the evidence is weak and limited by small sample sizes, poor methodologic quality, or clinically irrelevant effect sizes.
Influenza vaccination of residents of long-term care facilities (and of health care workers at these facilities) is critical for the prevention of influenza in this frail population. Detection, chemoprophylaxis, and treatment have limitations. Infection control measures should be in place during and between outbreaks. Acute care facilities such as emergency departments and hospitals can assist by testing residents of long-term care facilities who present with influenza-like illness during seasonal epidemics of influenza, and by notifying the receiving facility if a patient with influenza would be arriving.
THE EXTENT OF THE PROBLEM
From 5% to 20% of the US population, including residents and health care workers in long-term care facilities, are infected with influenza every year.1,2 The proportion of those infected who develop clinical illness ranges from 40% to 80%. Each influenza illness is associated with an average of 10 days of respiratory sickness, resulting in approximately 3 days of bed confinement or restricted activity. About 30% to 50% of patients with microbiologically confirmed influenza seek medical care, of whom 16% undergo laboratory tests, 17% undergo radiologic tests, and 75% are recommended an over-the-counter drug or are prescribed a medication. Annual influenza-related hospitalizations range from 200,000 to 400,000, depending on seasonal variations in virulence.2,3 Thus, influenza causes 1.3 hospitalizations per 1,000 people, and 25% of these are in people age 65 and older. In the United States, about 40,000 to 60,000 people die of influenza every year, and 90% of these are age 65 and older.4
POLICIES TO FIGHT ANTIVIRAL RESISTANCE
In January 2006, the US Centers for Disease Control and Prevention (CDC) recommended against the use of the adamantanes—ie, amantadine (Symmetrel) and rimantadine (Flumadine)—for the treatment or prevention of influenza because of a high level of resistance in circulating influenza A/H3N2 in the community. Unfortunately, this resistance trend has not reversed since then, with 96% to 100% of influenza A/H3N2 isolates in the United States showing resistance.5 During the 2007–2008 influenza season, influenza A/H1N1 isolates resistant to oseltamivir (Tamiflu) emerged in Europe, particularly in Norway and France. In the United States, influenza A/H1N1 resistance to oseltamivir increased from 0.7% in the 2006–2007 season, to 10.9% in the 2007–2008 season, and to 98% during the 2008–2009 season.6,7
Fortunately, all oseltamivir-resistant isolates remain susceptible to zanamivir (Relenza). In April 2009, a new influenza A/H1N1 variant (previously referred to as swine-origin influenza virus, or SOIV) emerged in North America and spread to many countries worldwide, and the World Health Organization eventually declared a pandemic. This new variant is susceptible to oseltamivir and zanamivir but resistant to the adamantanes. Resistance patterns for future influenza seasons cannot be predicted, but the current extent of influenza resistance and its development over the past decade8 are alarming.
WHY IS INFLUENZA MORE SERIOUS IN LONG-TERM CARE RESIDENTS?
Influenza is usually introduced to long-term care facilities by workers and visitors. Inside, the closed environment and limited mobility of residents facilitate transmission of infection.
The clinical presentation of influenza in residents of long-term care facilities can be subtle, with a blunted febrile response and a decline in mental and functional status.9
Residents commonly have underlying diseases that can be exacerbated by influenza infection, such as congestive heart failure, chronic obstructive lung disease, chronic kidney disease, and dementia. In addition, residents are at higher risk of serious influenza-related complications than are community-dwelling elderly people.
Impaired oral intake, limited dexterity, and altered consciousness may limit treatment options, thus further adversely affecting outcomes. Bacterial pneumonia secondary to influenza has dire consequences in long-term care residents. Rates of hospitalization for pneumonia and influenza and for exacerbation of chronic lung disease are higher in these patients than in their community-dwelling counterparts.10 Death rates are also higher, exceeding 5% during influenza epidemics.11
PREVENTING INFLUENZA IN LONG-TERM CARE FACILITIES
Immunizing residents is essential
Vaccination is the most important measure in preventing influenza in long-term care facilities, and vaccination programs should include residents and health care workers.
Influenza outbreaks are more common in facilities where the rate of immunization is below 80%, as well as in larger facilities (with > 100 beds), suggesting that herd immunity may play a role.12 Unfortunately, influenza vaccine coverage rates vary widely in this patient population—from 57% to 98% in one report.13
The live-attenuated intranasal vaccine is approved only for healthy people under age 50, so most residents of long-term care facilities should receive only the inactivated trivalent intramuscular vaccine.
The effectiveness of a vaccine in preventing influenza depends in part on the adequacy of the match between vaccine serotypes and circulating strains. Studies of all types—randomized, observational, case-control, and cohort studies, as well as meta-analyses and systematic reviews—have shown preventive efficacy rates of influenza vaccination in elderly residents of long-term care facilities to be 23% to 43% for influenza-like illness, 0% to 58% for influenza, 46% for pneumonia, 45% for hospitalization, 42% for death from influenza or pneumonia, and 60% for death from all causes.14,15 Vaccine performance improved after adjustment for confounders.
Obviously, this protection is variable and incomplete, since influenza outbreaks continue to occur even in long-term care facilities in which most residents are vaccinated.13
Vaccination works, despite the controversy
Whether influenza vaccination prevents deaths in elderly people—or how many deaths it prevents—is a subject of ongoing controversy. 16 Even though influenza vaccination coverage in the elderly increased from 15% to 65% since 1980, the specific influenza-related death rate did not decrease.17
It has been suggested that cohort studies may have overestimated the mortality benefit of influenza vaccination in the elderly because of “frailty selection bias” (ie, extremely frail elderly patients are less likely to be vaccinated and are more likely to die for reasons other than influenza than are less frail, vaccinated elderly people) and because of the use of nonspecific end points such as all-cause mortality. 16 Similarly, observational studies may have overestimated the in-hospital mortality benefit of influenza vaccination in older patients with pneumonia occurring outside of influenza season because of the “healthy user effect” (ie, residual confounding by functional and socioeconomic status).18
One nested case-control study in immunocompetent elderly patients showed that influenza vaccination was not associated with a reduced risk of community-acquired pneumonia after adjusting for the presence and severity of comorbidities.19
Since death is a rare end point, it is hard to show a reduction in the death rate with vaccination in randomized controlled studies. The absolute risk reduction in hospitalization and death with vaccination is two to five times higher in elderly patients at high risk than in the healthy elderly.20
The mortality benefit in elderly patients is increased with annual revaccination, with one death prevented for every 300 vaccinations, and one for every 200 revaccinations.21
The response to influenza vaccination is reduced in elderly people because of immune senescence, and higher doses of vaccine have been shown to be more immunogenic and remain safe.22 This enhanced antibody response may be maintained for subsequent antigenically different influenza variants, even against viruses appearing more than 10 years after vaccination. 23 The 2008–2009 influenza vaccine does not protect against the new, pandemic influenza A/H1N1 variant; efforts to produce such a vaccine are under way.
Influenza vaccination is safe. Recent data showed no association between immunization and Guillain-Barré syndrome.24 In fact, influenza itself may be a triggering agent for Guillain-Barré syndrome during major influenza outbreaks.25
DURATION OF SEROPROTECTION IS MORE THAN 6 MONTHS
Every effort should be made to vaccinate residents of long-term care facilities and their caregivers as early as possible in the influenza season to allow an adequate antibody response to develop before the onset of an influenza outbreak.
In the past, there has been concern that the influenza-vaccine-induced antibody response declines more rapidly in the elderly and may fall below seroprotective levels within 4 months of vaccination. But a recent review of 14 published studies argued against that notion, showing that if seroprotection is achieved in the first month after vaccination, it is then maintained for more than 6 months.26 That review also showed that seroconversion varies inversely with preimmunization titers, but not with age.
Moreover, a prospective study27 in 303 residents of a long-term care facility reported that seroprotection did not correlate with nutritional status. In the study, vaccination was very effective despite a high prevalence of nutritional deficiencies. This study also indicated that although an influenza antibody titer greater than 1:40 is considered protective in the general population, long-term care facility residents may require higher levels for effective immunization.
A recent survey showed that a national shortage of influenza vaccine results in decreased immunization rates in residents and in health care workers in long-term care facilities. 28 In that survey, only 2.3% of facilities expressed concern about emergency preparedness, and this has significant implications for a possible influenza pandemic.
VACCINATION PROGRAMS
Standing-order programs have been shown to significantly increase vaccination rates in ambulatory and hospital settings. However, a recent survey showed that only 9% of long-term care facilities use such programs.29 The greatest use of such programs was in government-owned, nonprofit, dual-certified (ie, by both Medicare and Medicaid), and independent long-term care facilities, and in facilities with a lower index of disease acuity. Use varied substantially by state.
The Healthy People 2010 goal of 90% vaccination may be attained by implementing written protocols for documenting immunization—and refusal of immunization—in a consistent place in the patient’s medical record.30
VACCINATION OF WORKERS
When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die31,32 or develop influenza-like illness, particularly when residents themselves are vaccinated.33 Additional benefits include decreased need for consultations with general practitioners or admissions to the hospital for influenza-like illness during periods of moderate influenza activity.32
The policy of mandatory influenza vaccination for health care workers has its proponents34 and opponents.35 When a large tertiary care center adopted mandatory vaccination, vaccination rates increased significantly over time.36 The Centers for Medicare & Medicaid Services recently mandated public reporting of vaccination rates in health care workers and residents of long-term care facilities, and compliance is expected to increase as a result.
BETWEEN INFLUENZA OUTBREAKS
Studies show that hygienic measures prevent transmission of respiratory viruses.37 Therefore, the cornerstones of any program to prevent transmission of influenza and other microorganisms in long-term care facilities are hand hygiene, cough and sneeze etiquette, maintaining a distance of 3 feet between beds, and education of residents and health care workers.
Wash your hands!
Hands should be washed before and after direct contact with patients or with inanimate objects in their immediate vicinity.38 Contrary to popular belief, hands should also be washed before and after wearing gloves, particularly when handling an invasive device for patient care, and after contact with bodily fluids, excretions, mucous membranes, non-intact skin, or wound dressings. Hands should be washed not only between patients but also during care for the same patient if moving from a contaminated body site to a clean site.
Hands should be washed for 15 to 20 seconds using soap and water or an alcohol-based foam or gel; when hands are visibly soiled, only soap and water should be used. It is not known whether adding virucidals or antiseptics to normal hand-washing further decreases the spread of these viruses.
Continuous education, feedback interventions, and patient-awareness programs can improve hand-washing compliance, but they are not sufficient. Easily accessible dispensers for alcohol-based waterless antiseptic foam or gel can significantly improve hand hygiene rates among health care workers.39
DETECTING INFLUENZA OUTBREAKS
Direct fluorescent antigen detection and nucleic acid detection by polymerase chain reaction (PCR) are the tests recommended for early detection of influenza outbreaks in long-term care facilities. Rapid point-of-care tests to detect influenza antigen are only 60% to 70% sensitive,13,40 viral culture takes several days, and serologic diagnosis requires documentation of seroconversion at least 2 weeks apart, and so none of these is adequate for the early detection of an influenza outbreak.
There is no widely available test to differentiate influenza A/H1N1 from A/H3N2, or to test for drug resistance. In addition, the PCR tests widely used today do not differentiate between seasonal influenza A/H1N1 and the new, pandemic influenza A/H1N1 variant. Efforts are under way to produce and distribute such a test.
Controlling influenza outbreaks
An outbreak should be declared when two or more residents develop an influenza-like illness within 72 hours of each other during the influenza season.41 After influenza infection is confirmed by laboratory testing, testing of all residents who subsequently develop an influenza-like illness may not be feasible, and other respiratory viruses may be responsible for mixed outbreaks during influenza epidemics, particularly respiratory syncytial virus.
Roommates of residents who test positive for influenza have a risk of acquiring influenza three times higher than that of residents living in single rooms.42 Obviously, private rooms for all residents would be optimal, but this is not practical in most facilities. “Cohorting” (ie, housing infected residents together) is reasonable, but in this situation they might infect each other with other viruses or with influenza viruses of different serotypes.
When an outbreak occurs, potential solutions include closing subunits of the facility to new admissions, limiting movement of residents and health care workers from affected to unaffected units, and using curtains or other barriers between roommates. But most important during seasonal outbreaks are hand hygiene, proper cough and sneeze etiquette, and 3-foot separation between roommates. A simulation model showed that during an influenza pandemic, preventing ill residents of long-term care facilities from making contact with other residents may reduce rates of illness and death by about 60%.43 If a long-term care facility resident is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient or when entering the room of a resident with confirmed influenza.
CHEMOPROPHYLAXIS DURING INFLUENZA OUTBREAKS
When influenza outbreaks are recognized early in long-term care facilities, appropriate infection control measures and chemoprophylaxis can be started. Chemoprophylaxis should also be considered in residents in whom influenza vaccination is contraindicated, such as those with severe egg allergy.
Rimantadine is preferred over amantadine in residents of long-term care facilities, since amantadine is associated with a much higher rate of adverse events (18.6% vs 1.9%), especially confusion (10.6% vs 0.6%), resulting in more frequent discontinuation (17.3% vs 1.9%).44 In addition, viral resistance to amantadine develops in about 30% of those who receive it. In long-term care facilities, viral resistance occurs not only when it is used for the management of influenza, but also when it is used to treat Parkinson disease.45
Oseltamivir prophylaxis for 6 weeks in influenza-vaccinated, frail elderly residents of long-term care facilities during influenza epidemics was 91% effective in preventing laboratory-confirmed clinical influenza, and 85% effective in preventing a secondary bacterial complication such as pneumonia or sinusitis.46 The rate of adverse events associated with oseltamivir in that population was similar to that with placebo. Importantly, oseltamivir was not associated with suppression of antibody response to influenza infection or vaccination.
Oseltamivir is very effective in terminating influenza outbreaks in long-term care facilities, even when amantadine fails.4,13 When used in that manner, it is also associated with decreased antibiotic prescriptions, hospitalizations, deaths,47 and substantial cost-savings, even when compared with amantadine, which has a much lower acquisition cost but a higher rate of adverse events, lower efficacy, and individualized dosing requirements.48
Zanamivir prophylaxis for 2 weeks given to unvaccinated residents was 29% effective in preventing all symptomatic influenza confirmed by laboratory testing (by culture, PCR, or seroconversion). It was 65% effective in preventing symptomatic culture-confirmed influenza, 70% effective in preventing febrile, laboratory-confirmed influenza, and 21% effective in preventing complications.49 It was well tolerated in this population and was not associated with the emergence of zanamivir resistance. Zanamivir also provides 61% additional protective efficacy over rimantadine in vaccinated residents of long-term care facilities,50 primarily because of the emergence of viruses resistant to rimantadine. However, up to 50% of elderly people may have difficulty loading and priming the Diskhaler device used to deliver zanamivir.51
While several studies have shown chemoprophylaxis to be effective, it is not possible to ascribe the decrease in cases during an outbreak entirely to antiviral drugs since most studies were not placebo-controlled, and since the natural tendency of outbreaks is to subside.
TREATMENT OF INFLUENZA IN LONG-TERM CARE FACILITIES
A mild illness in someone without a high-risk underlying disease can be managed with symptomatic measures alone, and testing for influenza is at the discretion of the caregiver. Patients who develop a moderate or severe influenza-like illness during an influenza epidemic and who have a high-risk underlying disease or who require hospitalization should be tested for influenza by a sensitive test such as direct fluorescent antigen testing or PCR and should be started on empiric anti-influenza therapy.41
Most elderly residents of long-term care facilities do have comorbid conditions and are thus at high risk of complications of influenza. During influenza outbreaks, clinicians caring for these patients—and for similar patients in outpatient settings, emergency departments, and acute-care hospitals—should consider testing for influenza, even if the patient has only a mild influenza-like illness.
Of note, no randomized treatment study has been done specifically in residents of long-term care facilities.52 Pooled analysis of data from 321 patients at high risk (76 were age 65 or older) in zanamivir treatment studies showed that those who received the drug were sick for 2.5 fewer days than those who received placebo.53 In addition, zanamivir recipients returned to normal activities 3 days sooner, had an 11% reduction in the median total symptom score over 1 to 5 days, and had a rate of complications requiring antibiotics 43% less than placebo recipients. Rates of adverse events were similar in both groups.
A multicenter, randomized, open-label, controlled trial of oseltamivir in the treatment of influenza in high-risk Chinese patients with chronic respiratory diseases (chronic bronchitis, obstructive emphysema, bronchial asthma, or bronchiectasis) or chronic cardiac disease showed that oseltamivir significantly reduced the duration of influenza symptoms by 36.8%, the severity of symptoms by 43.1%, the duration of fever by 45.2%, the time to return to baseline health status by 5 days, and the need for antibiotics, without increasing the total cost of medical care.54
Oseltamivir-resistant isolates of seasonal influenza A/H1N1 do not cause different or more severe symptoms than do oseltamivir-susceptible isolates, and they remain susceptible to zanamivir and the adamantanes. There is no widely available test to differentiate influenza A/H1N1 from A/H3N2 or to test for drug resistance. Influenza B and A/H3N2 remain susceptible to both of the neuraminidase inhibitors, ie, oseltamivir and zanamivir.
All currently circulating influenza A/H3N2 isolates remain resistant to the adamantanes. Influenza B is intrinsically resistant to the adamantanes, and some data show that oseltamivir is less effective against influenza B than against influenza A.
Zanamivir is not available in many pharmacies, is not recommended in patients with underlying reactive airway disease, and requires dexterity for administration.
Amantadine is more likely to cause confusion in the elderly than rimantadine, and it is more susceptible to treatment-emergent antiviral resistance.
No parenteral anti-influenza drug is available, but zanamivir and the experimental drug peramivir are undergoing study for parenteral use. An inhaled, long-acting neuraminidase inhibitor, CS-8958, is currently under study.55 Other agents currently under development include T-705, a polymerase inhibitor, and DAS181, an attachment inhibitor.
Complementary and alternative therapies for influenza are not established. A recent review of 14 randomized, controlled trials56 revealed that the evidence is weak and limited by small sample sizes, poor methodologic quality, or clinically irrelevant effect sizes.
- Cifu A, Levinson W. Influenza. JAMA 2000; 284:2847–2849.
- Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics 1996; 9(suppl 3):26–33.
- Fiore AE, Shay DK, Broder K, et al; US Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:1–60.
- Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol 1993; 22:334–340.
- Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 2007; 196:249–257.
- Centers for Disease Control and Prevention (CDC). Update: Influenza activity—United States, September 28, 2008–January 31, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:115–119.
- CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008–09 influenza season. Distributed via Health Alert Network. 2008. www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279. Accessed 7/14/2009.
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 2009; 301:1066–1069.
- Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998; 158:645–650.
- Menec VH, MacWilliams L, Aoki FY. Hospitalization and deaths due to respiratory illnesses during influenza seasons: a comparison of community residents, senior housing residents, and nursing home residents. J Gerontol A Biol Sci Med Sci 2002; 57:M629–M635.
- Kingston BJ, Wright CV. Influenza in the nursing home. Am Fam Physician 2002; 65:75–78.
- Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis 2006; 194(suppl 2):S133–S138.
- Monto AS, Rotthoff J, Teich E, et al. Detection and control of influenza outbreaks in well-vaccinated nursing home populations. Clin Infect Dis 2004; 39:459–464.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366:1165–1174.
- Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; 3:CD004876.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–666.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Hak E, Nordin J, Wei F, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis 2002; 35:370–377.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis 2008; 198:1016–1018.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Paccalin M, Plouzeau C, Bouche G, et al. Lack of correlation between nutritional status and seroprotection against influenza in a long term care facility. Scand J Infect Dis 2006; 38:894–897.
- Mody L, Langa KM, Malani PN. Impact of the 2004–2005 influenza vaccine shortage on immunization practices in long-term care facilities. Infect Control Hosp Epidemiol 2006; 27:383–387.
- Shefer A, McKibben L, Bardenheier B, Bratzler D, Roberts H. Characteristics of long-term care facilities associated with standing order programs to deliver influenza and pneumococcal vaccinations to residents in 13 states. J Am Med Dir Assoc 2005; 6:97–104.
- Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long-term care facilities: the CMS-CDC standing orders program project. J Am Med Dir Assoc 2005; 6:291–299.
- Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000; 355:93–97.
- Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241.
- Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2006; 3:CD005187.
- Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144–1147.
- Finch M. Point: mandatory influenza vaccination for all health care workers? Seven reasons to say “no”. Clin Infect Dis 2006; 42:1141–1143.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Pittet D, Allegranzi B, Sax H, et al; WHO Global Patient Safety Challenge, World Alliance for Patient Safety. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 2006; 6:641–652.
- Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 2000; 160:1017–1021.
- US Centers for Disease Control and Prevention. Influenza-testing and antiviral-agent prescribing practices—Connecticut, Minnesota, New Mexico, and New York, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:61–65.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Drinka PJ, Krause PF, Nest LJ, Goodman BM, Gravenstein S. Risk of acquiring influenza B in a nursing home from a culture-positive roommate. J Am Geriatr Soc 2005; 53:1437.
- Haber MJ, Shay DK, Davis XM, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis 2007; 13:581–589.
- Keyser LA, Karl M, Nafziger AN, Bertino JS. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med 2000; 160:1485–1488.
- Saito R, Oshitani H, Masuda H, Suzuki H. Detection of amantadineresistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol 2002; 40:84–88.
- Peters PH, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031.
- Bowles SK, Kennie N, Ruston L, Simor A, Louie M, Collins V. Influenza outbreak in a long-term-care facility: considerations for pharmacy. Am J Health Syst Pharm 1999; 56:2303–2307.
- Risebrough NA, Bowles SK, Simor AE, McGeer A, Oh PI. Economic evaluation of oseltamivir phosphate for postexposure prophylaxis of influenza in long-term care facilities. J Am Geriatr Soc 2005; 53:444–451.
- Ambrozaitis A, Gravenstein S, van Essen GA, et al. Inhaled zanamivir versus placebo for the prevention of influenza outbreaks in an unvaccinated long-term care population. J Am Med Dir Assoc 2005; 6:367–374.
- Gravenstein S, Drinka P, Osterweil D, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc 2005; 6:359–366.
- Diggory P, Fernandez C, Humphrey A, Jones V, Murphy M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ 2001; 322:577–579.
- Glezen WP. Clinical practice: prevention and treatment of seasonal influenza. N Engl J Med 2008; 359:2579–2585.
- Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001; 161:212–217.
- Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin 2006; 22:75–82.
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009; 48( suppl 1):S3–S13.
- Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am J Med 2007; 120:923–929.
- Cifu A, Levinson W. Influenza. JAMA 2000; 284:2847–2849.
- Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics 1996; 9(suppl 3):26–33.
- Fiore AE, Shay DK, Broder K, et al; US Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:1–60.
- Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol 1993; 22:334–340.
- Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 2007; 196:249–257.
- Centers for Disease Control and Prevention (CDC). Update: Influenza activity—United States, September 28, 2008–January 31, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:115–119.
- CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008–09 influenza season. Distributed via Health Alert Network. 2008. www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279. Accessed 7/14/2009.
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 2009; 301:1066–1069.
- Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998; 158:645–650.
- Menec VH, MacWilliams L, Aoki FY. Hospitalization and deaths due to respiratory illnesses during influenza seasons: a comparison of community residents, senior housing residents, and nursing home residents. J Gerontol A Biol Sci Med Sci 2002; 57:M629–M635.
- Kingston BJ, Wright CV. Influenza in the nursing home. Am Fam Physician 2002; 65:75–78.
- Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis 2006; 194(suppl 2):S133–S138.
- Monto AS, Rotthoff J, Teich E, et al. Detection and control of influenza outbreaks in well-vaccinated nursing home populations. Clin Infect Dis 2004; 39:459–464.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366:1165–1174.
- Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; 3:CD004876.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–666.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Hak E, Nordin J, Wei F, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis 2002; 35:370–377.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis 2008; 198:1016–1018.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Paccalin M, Plouzeau C, Bouche G, et al. Lack of correlation between nutritional status and seroprotection against influenza in a long term care facility. Scand J Infect Dis 2006; 38:894–897.
- Mody L, Langa KM, Malani PN. Impact of the 2004–2005 influenza vaccine shortage on immunization practices in long-term care facilities. Infect Control Hosp Epidemiol 2006; 27:383–387.
- Shefer A, McKibben L, Bardenheier B, Bratzler D, Roberts H. Characteristics of long-term care facilities associated with standing order programs to deliver influenza and pneumococcal vaccinations to residents in 13 states. J Am Med Dir Assoc 2005; 6:97–104.
- Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long-term care facilities: the CMS-CDC standing orders program project. J Am Med Dir Assoc 2005; 6:291–299.
- Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000; 355:93–97.
- Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241.
- Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2006; 3:CD005187.
- Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144–1147.
- Finch M. Point: mandatory influenza vaccination for all health care workers? Seven reasons to say “no”. Clin Infect Dis 2006; 42:1141–1143.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Pittet D, Allegranzi B, Sax H, et al; WHO Global Patient Safety Challenge, World Alliance for Patient Safety. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 2006; 6:641–652.
- Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 2000; 160:1017–1021.
- US Centers for Disease Control and Prevention. Influenza-testing and antiviral-agent prescribing practices—Connecticut, Minnesota, New Mexico, and New York, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:61–65.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Drinka PJ, Krause PF, Nest LJ, Goodman BM, Gravenstein S. Risk of acquiring influenza B in a nursing home from a culture-positive roommate. J Am Geriatr Soc 2005; 53:1437.
- Haber MJ, Shay DK, Davis XM, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis 2007; 13:581–589.
- Keyser LA, Karl M, Nafziger AN, Bertino JS. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med 2000; 160:1485–1488.
- Saito R, Oshitani H, Masuda H, Suzuki H. Detection of amantadineresistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol 2002; 40:84–88.
- Peters PH, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031.
- Bowles SK, Kennie N, Ruston L, Simor A, Louie M, Collins V. Influenza outbreak in a long-term-care facility: considerations for pharmacy. Am J Health Syst Pharm 1999; 56:2303–2307.
- Risebrough NA, Bowles SK, Simor AE, McGeer A, Oh PI. Economic evaluation of oseltamivir phosphate for postexposure prophylaxis of influenza in long-term care facilities. J Am Geriatr Soc 2005; 53:444–451.
- Ambrozaitis A, Gravenstein S, van Essen GA, et al. Inhaled zanamivir versus placebo for the prevention of influenza outbreaks in an unvaccinated long-term care population. J Am Med Dir Assoc 2005; 6:367–374.
- Gravenstein S, Drinka P, Osterweil D, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc 2005; 6:359–366.
- Diggory P, Fernandez C, Humphrey A, Jones V, Murphy M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ 2001; 322:577–579.
- Glezen WP. Clinical practice: prevention and treatment of seasonal influenza. N Engl J Med 2008; 359:2579–2585.
- Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001; 161:212–217.
- Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin 2006; 22:75–82.
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009; 48( suppl 1):S3–S13.
- Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am J Med 2007; 120:923–929.
KEY POINTS
- When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die or develop influenza-like illness, particularly when residents are also vaccinated.
- Easily accessible dispensers for alcohol-based antiseptic foam or gel can significantly improve hand hygiene rates in health care workers.
- If a patient in a long-term care facility is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient.
- All isolates of pandemic influenza A/H1N1 (previously called swine-origin influenza virus) are susceptible to zanamivir (Relenza) and oseltamivir (Tamiflu), but are resistant to amantadine (Symmetrel) and rimantadine (Flumadine).
2008–2009 Influenza update: A better vaccine match
Last year, some people may have lost their faith in flu shots. The three antigens chosen for the vaccine in advance by the US Centers for Disease Control and Prevention (CDC) did not match very well the influenza strains that ultimately circulated in North America, and the overall protective efficacy of the vaccine was estimated at only 40%.
Nevertheless, vaccination remains the primary preventive measure for both epidemic and pandemic influenza, especially in view of a rising rate of resistance to the oral antiviral agent oseltamivir (Tamiflu).
In the 2008–2009 influenza season, we hope to do better. All three antigens contained in the 2008–2009 vaccine are new. Surveillance data from the Southern Hemisphere during the summer of 2008 show that this vaccine is expected to be a good match for the strains circulating in the Northern Hemisphere. And with 146 million doses expected to be manufactured this season by six companies—the largest number of doses ever manufactured in the United States—enough should be available for all.
GREAT STRIDES HAVE BEEN MADE, BUT FLU IS STILL A PROBLEM
We are making great strides against influenza. Over the last 50 years, the rate of influenzarelated deaths in the United States declined by 95%, from an average seasonal rate of 10.2 deaths per 100,000 population in the 1940s to 0.56 per 100,000 by the 1990s.1
However, influenza still accounts for about 10% of patients admitted to intensive care units for acute respiratory failure during epidemics.2
Children and the elderly are still hit the hardest: infants age 0 through 23 months and adults age 65 years and older have the highest peak rates of pneumonia and influenza hospitalization and death.3 School-age children (5–18 years) have an indirect role in anticipating the risk to others and can learn to help avoid spreading the virus by washing their hands more, wearing masks, and adopting other hygienic measures.
In the 1918–1919 pandemic, most deaths were from secondary bacterial pneumonia, a fact that has implications for pandemic preparedness. 4 Currently, Staphylococcus aureus, particularly methicillin-resistant strains (MRSA), is an important cause of secondary bacterial pneumonia, with a high mortality rate.5
UPDATE ON DIAGNOSIS: PCR IS THE BEST TEST
In the hospital, it is important to identify patients who have influenza so that we can give them appropriate antiviral therapy and also protect other patients from getting the flu. Unfortunately, the sensitivity and positive predictive value of fever, cough, and other symptoms for the diagnosis of influenza in hospitalized patients are 40% or less.6
Real-time reverse transcriptase polymerase chain reaction (PCR), compared with direct fluorescent antigen detection or cell culture, has the highest sensitivity (98.7%) and specificity (100%) in both children7 and the elderly.8 Furthermore, cell culture is slow and therefore is not useful in clinical practice. Nasopharyngeal wash sampling appears impractical in nursing home residents, owing to their underlying disabilities, and nasopharyngeal swabs tested by PCR are equally sensitive. 8
However, improvements are needed in molecular detection and subtyping of influenza viruses.9 If a pandemic breaks out, we will need to identify the virus quickly to have enough time for preventive interventions. The US Food and Drug Administration has recently cleared a new test called the Human Virus Real-Time RT-PCR Detection and Characterization Panel to detect and differentiate between seasonal and novel influenza strains.10
UPDATE ON INFLUENZA VACCINE
New recommendations in 2008 by the CDC Advisory Committee on Immunization Practices11 include annual vaccination for all children age 5 through 18 years, and either the trivalent inactivated vaccine (ie, the shot) or the live-attenuated vaccine (ie, the Flu-Mist intranasal spray) for healthy people age 2 through 49 years. The CDC recommendations are summarized at www.cdc.gov/flu/professionals/acip/index.htm.
Has the benefit of vaccination in adults been overestimated?
Jackson et al,12 in an article published in August 2008, suggested that the effect of influenza vaccination on the risk of community-acquired pneumonia in immunocompetent elderly people during influenza season is less than previously estimated. However, some patients in this study who were classified as not having been vaccinated may have actually been vaccinated by other health care providers without notifying their primary care providers. Moreover, influenza infection may cause only a small proportion of cases of pneumonia in this population.
In another study, Eurich et al13 suggested that previous observational studies overestimated the benefit of influenza vaccination on reducing deaths in patients with pneumonia outside the flu season. Although they found the incidence of death to be 51% lower in vaccinated than in unvaccinated adults with community-acquired pneumonia (N = 1,813) admitted to six hospitals, they ascribed it to confounding factors, specifically socioeconomic and functional status. This phenomenon was previously called the “frailty bias” or the “healthy user effect.” However, this study included only patients hospitalized with pneumonia and did not include data on vaccine-induced immunity or the cause of pneumonia, and measures of the healthy user effect were rudimentary. In addition, only outcomes during hospitalization were included.
Most experts still believe that vaccination prevents 50% of influenza-related deaths (with a smaller effect on rates of all-cause mortality14), including deaths in very old people. 15 A recent review found no basis for the historic concern that the antibody response to the influenza vaccine in people age 60 and older declines more rapidly than in younger people and below seroprotective levels within 4 months of immunization.16
Nevertheless, discordance between antibody and T-cell responses to influenza vaccine does exist17 (ie, the vaccine can induce antibodies while not boosting the T-cell-specific response), and we should continue to seek new vaccines that are more effective.
More people are being vaccinated, but we’re still below our goals
Although influenza vaccination rates among adults continue to improve,18 they remain well below the Healthy People 2010 initiative’s target of 90% in adults age 65 and older (the current rate is 72%) and below the target of 60% in people age 18 through 64 who have one or more high-risk conditions, health care workers, and pregnant women19 (currently 35% in people age 18 through 49 and 42% in people age 50 through 64). Thus, we still need to improve vaccination coverage rates.
Health care providers should offer vaccination at every opportunity between October and May.20 Offering vaccination in nontraditional settings such as work sites and pharmacies is likely to be cost-saving for healthy adults due to averted morbidity.21 At many hospitals, health care workers can opt out of being vaccinated, but they must formally state that they are doing so. The use of these declination statements among health care workers is associated with a mean increase of 11.6% in vaccination rates.22
Since influenza is the second most frequent vaccine-preventable infection in travelers, the vaccine should be offered to those crossing to the opposite hemisphere during its peak influenza season (eg, to South America in May through September), as well as to those visiting the tropics at any time of year.23
Vaccination is safe and effective in high-risk groups
Data on vaccination are reassuring in several at-risk groups.
In pregnancy, there is no indication that infants are harmed if their mothers are vaccinated in the first trimester.24 The evidence of excess morbidity during influenza epidemics supports vaccinating healthy pregnant women in the second or third trimester and those with comorbidities any time during pregnancy. Influenza vaccination during pregnancy reduces laboratory-confirmed influenza in infants up to 6 months of age by 63% and prevents 29% of all febrile respiratory illnesses in infants and 36% of those in mothers.25
In patients with chronic obstructive pulmonary disease, vaccination cuts the rate of outpatient visits and hospitalizations due to acute respiratory illness by 67%.26 The antibody response to influenza vaccine in patients with rheumatoid arthritis treated with rituximab (Rituxan), a monoclonal antibody directed against CD20 surface antigen-positive B lymphocytes, was lower than in healthy controls, but was not negligible.27
Dispelling myths about vaccination in children
One recently published study in children younger than 5 years did not find vaccination to be effective in preventing influenza-related hospitalizations and outpatient visits.28 However, in both seasons in which this study was conducted, there was a suboptimal antigenic match between vaccines and circulating strains. Moreover, about 60% of participants were unvaccinated and another 20% were only partially vaccinated, making it difficult to assess vaccine effectiveness. Several other studies have shown that, when there is a good match, vaccine effectiveness in children is 85% to 90%.
Even though the live-attenuated (inhaled) vaccine is more expensive than the inactivated (injected) vaccine, it reduces the number of influenza illness cases and lowers subsequent health care use in children and productivity loss in their parents, with a net total savings of $45.80 relative to the inactivated vaccine.29 The live-attenuated vaccine provides sustained protection against influenza illness for 12 months following vaccination, as well as meaningful efficacy through a second season without revaccination, although at a lower level.30
Several myths about the live-attenuated vaccine should be dispelled.31 It is well tolerated and causes only mild, transient symptoms of upper respiratory infection, even in people with asthma or the early stages of human immunodeficiency virus infection. Genetic reversion of the vaccine strain to a wild-type virus requires independent mutation in four gene segments, an event that has not been observed. Finally, although viral shedding is common for several days after vaccination, transmission to another person has been shown in only one person, who remained asymptomatic.
Unfortunately, rates of influenza vaccination are even worse for children than for adults.32 In children 6 through 23 months old, only 22% are fully vaccinated; in those 24 through 59 months old, only 16.5% are.
One group of immunocompromised children, liver transplant recipients, achieved antibody seroprotection and seroconversion rates similar to those achieved by their healthy siblings, with no vaccine-related serious side effects.33 As in adults, the cell-mediated immune response to the vaccine was diminished, suggesting that other strategies are needed to provide optimal protection.
IF BIRD FLU BREAKS OUT, WE HAVE A VACCINE
In the event of an outbreak of avian influenza in humans, the US government now has a vaccine against H5N1, the causative virus. A two-dose regimen of a whole-virus H5N1 vaccine, which is derived from cell culture, induced neutralizing antibodies against diverse H5N1 virus strains in most subjects in one study.34 Another vaccine, which is egg-independent and adenoviral vector-based and contains conserved nucleoproteins, is broadly protective against globally dispersed H5N1 virus clades.35 The addition of the MF59 adjuvant to a subvirion H5N1 vaccine increased antibody response, but the addition of aluminum hydroxide did not.36
EXERCISE AND HYGIENE PREVENT FLU
Exercise has benefits beyond the usual ones: one study showed that exercising at low to moderate frequency (between once a month and three times a week) is associated with lower rates of influenza-related death.37
A recent meta-analysis38 confirmed that hygienic measures can prevent the spread of respiratory viruses in the community. The investigators calculated that hand-washing at least 10 times daily can prevent a large number of these infections (number needed to treat [NNT] = 4), and wearing surgical masks (NNT = 6), N95 masks (NNT = 3), gloves (NNT = 6), and gowns (NNT = 5) had incremental effects. On the other hand, the value of adding virucidal or antiseptic solutions to normal hand-washing was uncertain. Strict adherence to hand hygiene and masks (including by children) is needed to prevent influenza transmission in the home.39
AMANTADINE, RIMANTADINE ARE OUT; OSELTAMIVIR RESISTANCE IS GROWING
The CDC continues to recommend against using amantadine (Symmetrel) or rimantadine (Flumadine) to treat flu, owing to a high rate (> 90%) of resistance to these drugs.
A nonrandomized study suggests that zanamivir (Relenza) is more effective than oseltamivir (Tamiflu) for treating influenza B.40 A retrospective study in nine lung transplant recipients showed that oseltamivir is well tolerated and may reduce the risk of complications in these patients.41 Large, randomized, multicenter studies are under way to better assess oseltamivir’s preventive and therapeutic efficacy in transplant recipients.
In children, as in adults, oseltamivir is less effective against influenza B than influenza A,42 and both neuraminidase inhibitors, ie, oseltamivir and zanamivir, are equally effective in reducing the febrile period of influenza.43
During the 2007–2008 season, the rate of resistance to oseltamivir increased alarmingly.44 Resistance was restricted to A (H1N1) viruses carrying the H274Y mutation. In March 2008, the frequency of resistance among A (H1N1) viruses in the United States was 8.6%, 10 times higher than during the preceding influenza season. Resistance rates were much higher in several European countries, including Norway and France. During the Southern Hemisphere’s influenza season (May 2008 though September 2008), 46.5% of influenza A (H1N1) viruses received from 14 countries were resistant to oseltamivir. 45 It is worrisome that many of these resistant viruses were isolated from untreated patients. Fortunately, to date, 99% of these isolates remain susceptible to zanamivir.
Microbiologic tests to detect resistance are not currently available for clinical use. During an influenza pandemic, widespread use of neuraminidase inhibitors will likely promote further development of drug resistance. A mathematical model concluded that combined treatment and prophylaxis with antiviral agents will be necessary to control transmission during a pandemic, and that allocating different drugs to cases and contacts would be most effective in curtailing emergence of resistance.46
For now, either oseltamivir or zanamivir is acceptable for patients with flu symptoms and can be started pending results of PCR testing of nasopharyngeal swabs to make sure that the patient really has influenza. The drugs should be taken for 5 days.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Brocas E, Cormier P, Barouk D, Van de Louw A, Tenaillon A. Influenza incidence estimated with a rapid diagnostic test in critically ill patients with acute respiratory failure during the 2005 and 2006 winter flu epidemics. Presse Med 2008; 37:943–947.
- Sebastian R, Skowronski DM, Chong M, Dhaliwal J, Brownstein JS. Age-related trends in the timeliness and prediction of medical visits, hospitalizations and deaths due to pneumonia and influenza, British Columbia, Canada, 1998–2004. Vaccine 2008; 26:1397–1403.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 2008; 121:258–264.
- van den Dool C, Hak E, Wallinga J, van Loon AM, Lammers JW, Bonten MJ. Symptoms of influenza virus infection in hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:314–319.
- Gharabaghi F, Tellier R, Cheung R, et al. Comparison of a commercial qualitative real-time RT-PCR kit with direct immunofluorescence assay (DFA) and cell culture for detection of influenza A and B in children. J Clin Virol 2008; 42:190–193.
- Gooskens J, Swaan CM, Claas EC, Kroes AC. Rapid molecular detection of influenza outbreaks in nursing homes. J Clin Virol 2008; 41:7–12.
- Mackay WG, van Loon AM, Niedrig M, Meijer A, Lina B, Niesters HG. Molecular detection and typing of influenza viruses: are we ready for an influenza pandemic? J Clin Virol 2008; 42:194–197.
- US Department of Health and Human Services. FDA clears new CDC test to detect human influenza. www.pandemicflu.gov. Accessed 11/3/08.
- Fiore AE, Shay DK, Broder K, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57(RR-7):1–60.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Kelly H, Newall AT. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis 2008; 8:462–463.
- Voordouw BC, Sturkenboom MC, Dieleman JP, Stricker BH. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis 2008; 8:461–462.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Co MD, Orphin L, Cruz J, et al. Discordance between antibody and T cell responses in recipients of trivalent inactivated influenza vaccine. Vaccine 2008; 26:1990–1998.
- Centers for Disease Control and Prevention (CDC). Statespecific influenza vaccination coverage among adults— United States, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:1033–1039.
- Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U.S., 1989–2005. Vaccine 2008; 26:1786–1793.
- Poland GA, Johnson DR. Increasing influenza vaccination rates: the need to vaccinate throughout the entire influenza season. Am J Med 2008; 121(suppl 2):S3–S10.
- Prosser LA, O’Brien MA, Molinari NA, et al. Nontraditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics 2008; 26:163–178.
- Polgreen PM, Septimus EJ, Parry MF, et al. Relationship of influenza vaccination declination statements and influenza vaccination rates for healthcare workers in 22 US hospitals. Infect Control Hosp Epidemiol 2008; 29:675–677l.
- Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers’ vaccine? Expert Rev Vaccines 2008; 7:679–687.
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44–52.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- Menon B, Gurnani M, Aggarwal B. Comparison of outpatient visits and hospitalisations, in patients with chronic obstructive pulmonary disease, before and after influenza vaccination. Int J Clin Pract 2008; 62:593–598.
- Oren S, Mandelboim M, Braun-Moscovici Y, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis 2008; 67:937–941.
- Szilagyi PG, Fairbrother G, Griffin MR, et al; New Vaccine Surveillance Network. Inluenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch Pediatr Adolesc Med 2008; 162:943–951.
- Luce BR, Nichol KL, Belshe RB, et al. Cost-effectiveness of live attenuated influenza vaccine versus inactivated influenza vaccine among children aged 24–59 months in the United States. Vaccine 2008; 26:2841–2848.
- Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J 2008; 27:744–748.
- Tosh PK, Boyce TG, Poland GA. Flu myths: dispelling the myths associated with live attenuated influenza vaccine. Mayo Clin Proc 2008; 83:77–84.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among children aged 6–59 months—eight immunization information system sentinel sites, United States, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:1043–1046.
- Madan RP, Tan M, Fernandez-Sesma A, et al. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin Infect Dis 2008; 46:712–718.
- Ehrlich HJ, Muller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- Hoelscher MA, Singh N, Garg S, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis 2008; 197:1185–1188.
- Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008; 197:667–675.
- Wong CM, Lai HK, Ou CQ, et al. Is exercise protective against influenza-associated mortality? PLoS ONE 2008; 3:e2108.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Cowling BJ, Fung RO, Cheng CK, et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS ONE 2008; 3:e2101.
- Kawai N, Ikematsu H, Iwaki N, et al. A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect 2008; 56:51–57.
- Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant 2008; 27:282–288.
- Suzuki E, Ichihara K. The course of fever following influenza virus infection in children treated with oseltamivir. J Med Virol 2008; 80:1065–1071.
- Sugaya N, Tamura D, Yamazaki M, et al. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis 2008; 47:339–345.
- Sheu TG, Deyde VM, Okomo-Adhiambo M, et al. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother 2008; 52:3284–3292.
- Centers for Disease Control and Prevention (CDC). Influenza activity—United States and worldwide, May 18–September 19, 2008. MMWR Morb Mortal Wkly Rep 2008; 57:1046–1049.
- McCaw JM, Wood JG, McCaw CT, McVernon J. Impact of emerging antiviral drug resistance on influenza containment and spread: influence of subclinical infection and strategic use of a stockpile containing one or two drugs. PLoS ONE 2008; 3:e2362.
Last year, some people may have lost their faith in flu shots. The three antigens chosen for the vaccine in advance by the US Centers for Disease Control and Prevention (CDC) did not match very well the influenza strains that ultimately circulated in North America, and the overall protective efficacy of the vaccine was estimated at only 40%.
Nevertheless, vaccination remains the primary preventive measure for both epidemic and pandemic influenza, especially in view of a rising rate of resistance to the oral antiviral agent oseltamivir (Tamiflu).
In the 2008–2009 influenza season, we hope to do better. All three antigens contained in the 2008–2009 vaccine are new. Surveillance data from the Southern Hemisphere during the summer of 2008 show that this vaccine is expected to be a good match for the strains circulating in the Northern Hemisphere. And with 146 million doses expected to be manufactured this season by six companies—the largest number of doses ever manufactured in the United States—enough should be available for all.
GREAT STRIDES HAVE BEEN MADE, BUT FLU IS STILL A PROBLEM
We are making great strides against influenza. Over the last 50 years, the rate of influenzarelated deaths in the United States declined by 95%, from an average seasonal rate of 10.2 deaths per 100,000 population in the 1940s to 0.56 per 100,000 by the 1990s.1
However, influenza still accounts for about 10% of patients admitted to intensive care units for acute respiratory failure during epidemics.2
Children and the elderly are still hit the hardest: infants age 0 through 23 months and adults age 65 years and older have the highest peak rates of pneumonia and influenza hospitalization and death.3 School-age children (5–18 years) have an indirect role in anticipating the risk to others and can learn to help avoid spreading the virus by washing their hands more, wearing masks, and adopting other hygienic measures.
In the 1918–1919 pandemic, most deaths were from secondary bacterial pneumonia, a fact that has implications for pandemic preparedness. 4 Currently, Staphylococcus aureus, particularly methicillin-resistant strains (MRSA), is an important cause of secondary bacterial pneumonia, with a high mortality rate.5
UPDATE ON DIAGNOSIS: PCR IS THE BEST TEST
In the hospital, it is important to identify patients who have influenza so that we can give them appropriate antiviral therapy and also protect other patients from getting the flu. Unfortunately, the sensitivity and positive predictive value of fever, cough, and other symptoms for the diagnosis of influenza in hospitalized patients are 40% or less.6
Real-time reverse transcriptase polymerase chain reaction (PCR), compared with direct fluorescent antigen detection or cell culture, has the highest sensitivity (98.7%) and specificity (100%) in both children7 and the elderly.8 Furthermore, cell culture is slow and therefore is not useful in clinical practice. Nasopharyngeal wash sampling appears impractical in nursing home residents, owing to their underlying disabilities, and nasopharyngeal swabs tested by PCR are equally sensitive. 8
However, improvements are needed in molecular detection and subtyping of influenza viruses.9 If a pandemic breaks out, we will need to identify the virus quickly to have enough time for preventive interventions. The US Food and Drug Administration has recently cleared a new test called the Human Virus Real-Time RT-PCR Detection and Characterization Panel to detect and differentiate between seasonal and novel influenza strains.10
UPDATE ON INFLUENZA VACCINE
New recommendations in 2008 by the CDC Advisory Committee on Immunization Practices11 include annual vaccination for all children age 5 through 18 years, and either the trivalent inactivated vaccine (ie, the shot) or the live-attenuated vaccine (ie, the Flu-Mist intranasal spray) for healthy people age 2 through 49 years. The CDC recommendations are summarized at www.cdc.gov/flu/professionals/acip/index.htm.
Has the benefit of vaccination in adults been overestimated?
Jackson et al,12 in an article published in August 2008, suggested that the effect of influenza vaccination on the risk of community-acquired pneumonia in immunocompetent elderly people during influenza season is less than previously estimated. However, some patients in this study who were classified as not having been vaccinated may have actually been vaccinated by other health care providers without notifying their primary care providers. Moreover, influenza infection may cause only a small proportion of cases of pneumonia in this population.
In another study, Eurich et al13 suggested that previous observational studies overestimated the benefit of influenza vaccination on reducing deaths in patients with pneumonia outside the flu season. Although they found the incidence of death to be 51% lower in vaccinated than in unvaccinated adults with community-acquired pneumonia (N = 1,813) admitted to six hospitals, they ascribed it to confounding factors, specifically socioeconomic and functional status. This phenomenon was previously called the “frailty bias” or the “healthy user effect.” However, this study included only patients hospitalized with pneumonia and did not include data on vaccine-induced immunity or the cause of pneumonia, and measures of the healthy user effect were rudimentary. In addition, only outcomes during hospitalization were included.
Most experts still believe that vaccination prevents 50% of influenza-related deaths (with a smaller effect on rates of all-cause mortality14), including deaths in very old people. 15 A recent review found no basis for the historic concern that the antibody response to the influenza vaccine in people age 60 and older declines more rapidly than in younger people and below seroprotective levels within 4 months of immunization.16
Nevertheless, discordance between antibody and T-cell responses to influenza vaccine does exist17 (ie, the vaccine can induce antibodies while not boosting the T-cell-specific response), and we should continue to seek new vaccines that are more effective.
More people are being vaccinated, but we’re still below our goals
Although influenza vaccination rates among adults continue to improve,18 they remain well below the Healthy People 2010 initiative’s target of 90% in adults age 65 and older (the current rate is 72%) and below the target of 60% in people age 18 through 64 who have one or more high-risk conditions, health care workers, and pregnant women19 (currently 35% in people age 18 through 49 and 42% in people age 50 through 64). Thus, we still need to improve vaccination coverage rates.
Health care providers should offer vaccination at every opportunity between October and May.20 Offering vaccination in nontraditional settings such as work sites and pharmacies is likely to be cost-saving for healthy adults due to averted morbidity.21 At many hospitals, health care workers can opt out of being vaccinated, but they must formally state that they are doing so. The use of these declination statements among health care workers is associated with a mean increase of 11.6% in vaccination rates.22
Since influenza is the second most frequent vaccine-preventable infection in travelers, the vaccine should be offered to those crossing to the opposite hemisphere during its peak influenza season (eg, to South America in May through September), as well as to those visiting the tropics at any time of year.23
Vaccination is safe and effective in high-risk groups
Data on vaccination are reassuring in several at-risk groups.
In pregnancy, there is no indication that infants are harmed if their mothers are vaccinated in the first trimester.24 The evidence of excess morbidity during influenza epidemics supports vaccinating healthy pregnant women in the second or third trimester and those with comorbidities any time during pregnancy. Influenza vaccination during pregnancy reduces laboratory-confirmed influenza in infants up to 6 months of age by 63% and prevents 29% of all febrile respiratory illnesses in infants and 36% of those in mothers.25
In patients with chronic obstructive pulmonary disease, vaccination cuts the rate of outpatient visits and hospitalizations due to acute respiratory illness by 67%.26 The antibody response to influenza vaccine in patients with rheumatoid arthritis treated with rituximab (Rituxan), a monoclonal antibody directed against CD20 surface antigen-positive B lymphocytes, was lower than in healthy controls, but was not negligible.27
Dispelling myths about vaccination in children
One recently published study in children younger than 5 years did not find vaccination to be effective in preventing influenza-related hospitalizations and outpatient visits.28 However, in both seasons in which this study was conducted, there was a suboptimal antigenic match between vaccines and circulating strains. Moreover, about 60% of participants were unvaccinated and another 20% were only partially vaccinated, making it difficult to assess vaccine effectiveness. Several other studies have shown that, when there is a good match, vaccine effectiveness in children is 85% to 90%.
Even though the live-attenuated (inhaled) vaccine is more expensive than the inactivated (injected) vaccine, it reduces the number of influenza illness cases and lowers subsequent health care use in children and productivity loss in their parents, with a net total savings of $45.80 relative to the inactivated vaccine.29 The live-attenuated vaccine provides sustained protection against influenza illness for 12 months following vaccination, as well as meaningful efficacy through a second season without revaccination, although at a lower level.30
Several myths about the live-attenuated vaccine should be dispelled.31 It is well tolerated and causes only mild, transient symptoms of upper respiratory infection, even in people with asthma or the early stages of human immunodeficiency virus infection. Genetic reversion of the vaccine strain to a wild-type virus requires independent mutation in four gene segments, an event that has not been observed. Finally, although viral shedding is common for several days after vaccination, transmission to another person has been shown in only one person, who remained asymptomatic.
Unfortunately, rates of influenza vaccination are even worse for children than for adults.32 In children 6 through 23 months old, only 22% are fully vaccinated; in those 24 through 59 months old, only 16.5% are.
One group of immunocompromised children, liver transplant recipients, achieved antibody seroprotection and seroconversion rates similar to those achieved by their healthy siblings, with no vaccine-related serious side effects.33 As in adults, the cell-mediated immune response to the vaccine was diminished, suggesting that other strategies are needed to provide optimal protection.
IF BIRD FLU BREAKS OUT, WE HAVE A VACCINE
In the event of an outbreak of avian influenza in humans, the US government now has a vaccine against H5N1, the causative virus. A two-dose regimen of a whole-virus H5N1 vaccine, which is derived from cell culture, induced neutralizing antibodies against diverse H5N1 virus strains in most subjects in one study.34 Another vaccine, which is egg-independent and adenoviral vector-based and contains conserved nucleoproteins, is broadly protective against globally dispersed H5N1 virus clades.35 The addition of the MF59 adjuvant to a subvirion H5N1 vaccine increased antibody response, but the addition of aluminum hydroxide did not.36
EXERCISE AND HYGIENE PREVENT FLU
Exercise has benefits beyond the usual ones: one study showed that exercising at low to moderate frequency (between once a month and three times a week) is associated with lower rates of influenza-related death.37
A recent meta-analysis38 confirmed that hygienic measures can prevent the spread of respiratory viruses in the community. The investigators calculated that hand-washing at least 10 times daily can prevent a large number of these infections (number needed to treat [NNT] = 4), and wearing surgical masks (NNT = 6), N95 masks (NNT = 3), gloves (NNT = 6), and gowns (NNT = 5) had incremental effects. On the other hand, the value of adding virucidal or antiseptic solutions to normal hand-washing was uncertain. Strict adherence to hand hygiene and masks (including by children) is needed to prevent influenza transmission in the home.39
AMANTADINE, RIMANTADINE ARE OUT; OSELTAMIVIR RESISTANCE IS GROWING
The CDC continues to recommend against using amantadine (Symmetrel) or rimantadine (Flumadine) to treat flu, owing to a high rate (> 90%) of resistance to these drugs.
A nonrandomized study suggests that zanamivir (Relenza) is more effective than oseltamivir (Tamiflu) for treating influenza B.40 A retrospective study in nine lung transplant recipients showed that oseltamivir is well tolerated and may reduce the risk of complications in these patients.41 Large, randomized, multicenter studies are under way to better assess oseltamivir’s preventive and therapeutic efficacy in transplant recipients.
In children, as in adults, oseltamivir is less effective against influenza B than influenza A,42 and both neuraminidase inhibitors, ie, oseltamivir and zanamivir, are equally effective in reducing the febrile period of influenza.43
During the 2007–2008 season, the rate of resistance to oseltamivir increased alarmingly.44 Resistance was restricted to A (H1N1) viruses carrying the H274Y mutation. In March 2008, the frequency of resistance among A (H1N1) viruses in the United States was 8.6%, 10 times higher than during the preceding influenza season. Resistance rates were much higher in several European countries, including Norway and France. During the Southern Hemisphere’s influenza season (May 2008 though September 2008), 46.5% of influenza A (H1N1) viruses received from 14 countries were resistant to oseltamivir. 45 It is worrisome that many of these resistant viruses were isolated from untreated patients. Fortunately, to date, 99% of these isolates remain susceptible to zanamivir.
Microbiologic tests to detect resistance are not currently available for clinical use. During an influenza pandemic, widespread use of neuraminidase inhibitors will likely promote further development of drug resistance. A mathematical model concluded that combined treatment and prophylaxis with antiviral agents will be necessary to control transmission during a pandemic, and that allocating different drugs to cases and contacts would be most effective in curtailing emergence of resistance.46
For now, either oseltamivir or zanamivir is acceptable for patients with flu symptoms and can be started pending results of PCR testing of nasopharyngeal swabs to make sure that the patient really has influenza. The drugs should be taken for 5 days.
Last year, some people may have lost their faith in flu shots. The three antigens chosen for the vaccine in advance by the US Centers for Disease Control and Prevention (CDC) did not match very well the influenza strains that ultimately circulated in North America, and the overall protective efficacy of the vaccine was estimated at only 40%.
Nevertheless, vaccination remains the primary preventive measure for both epidemic and pandemic influenza, especially in view of a rising rate of resistance to the oral antiviral agent oseltamivir (Tamiflu).
In the 2008–2009 influenza season, we hope to do better. All three antigens contained in the 2008–2009 vaccine are new. Surveillance data from the Southern Hemisphere during the summer of 2008 show that this vaccine is expected to be a good match for the strains circulating in the Northern Hemisphere. And with 146 million doses expected to be manufactured this season by six companies—the largest number of doses ever manufactured in the United States—enough should be available for all.
GREAT STRIDES HAVE BEEN MADE, BUT FLU IS STILL A PROBLEM
We are making great strides against influenza. Over the last 50 years, the rate of influenzarelated deaths in the United States declined by 95%, from an average seasonal rate of 10.2 deaths per 100,000 population in the 1940s to 0.56 per 100,000 by the 1990s.1
However, influenza still accounts for about 10% of patients admitted to intensive care units for acute respiratory failure during epidemics.2
Children and the elderly are still hit the hardest: infants age 0 through 23 months and adults age 65 years and older have the highest peak rates of pneumonia and influenza hospitalization and death.3 School-age children (5–18 years) have an indirect role in anticipating the risk to others and can learn to help avoid spreading the virus by washing their hands more, wearing masks, and adopting other hygienic measures.
In the 1918–1919 pandemic, most deaths were from secondary bacterial pneumonia, a fact that has implications for pandemic preparedness. 4 Currently, Staphylococcus aureus, particularly methicillin-resistant strains (MRSA), is an important cause of secondary bacterial pneumonia, with a high mortality rate.5
UPDATE ON DIAGNOSIS: PCR IS THE BEST TEST
In the hospital, it is important to identify patients who have influenza so that we can give them appropriate antiviral therapy and also protect other patients from getting the flu. Unfortunately, the sensitivity and positive predictive value of fever, cough, and other symptoms for the diagnosis of influenza in hospitalized patients are 40% or less.6
Real-time reverse transcriptase polymerase chain reaction (PCR), compared with direct fluorescent antigen detection or cell culture, has the highest sensitivity (98.7%) and specificity (100%) in both children7 and the elderly.8 Furthermore, cell culture is slow and therefore is not useful in clinical practice. Nasopharyngeal wash sampling appears impractical in nursing home residents, owing to their underlying disabilities, and nasopharyngeal swabs tested by PCR are equally sensitive. 8
However, improvements are needed in molecular detection and subtyping of influenza viruses.9 If a pandemic breaks out, we will need to identify the virus quickly to have enough time for preventive interventions. The US Food and Drug Administration has recently cleared a new test called the Human Virus Real-Time RT-PCR Detection and Characterization Panel to detect and differentiate between seasonal and novel influenza strains.10
UPDATE ON INFLUENZA VACCINE
New recommendations in 2008 by the CDC Advisory Committee on Immunization Practices11 include annual vaccination for all children age 5 through 18 years, and either the trivalent inactivated vaccine (ie, the shot) or the live-attenuated vaccine (ie, the Flu-Mist intranasal spray) for healthy people age 2 through 49 years. The CDC recommendations are summarized at www.cdc.gov/flu/professionals/acip/index.htm.
Has the benefit of vaccination in adults been overestimated?
Jackson et al,12 in an article published in August 2008, suggested that the effect of influenza vaccination on the risk of community-acquired pneumonia in immunocompetent elderly people during influenza season is less than previously estimated. However, some patients in this study who were classified as not having been vaccinated may have actually been vaccinated by other health care providers without notifying their primary care providers. Moreover, influenza infection may cause only a small proportion of cases of pneumonia in this population.
In another study, Eurich et al13 suggested that previous observational studies overestimated the benefit of influenza vaccination on reducing deaths in patients with pneumonia outside the flu season. Although they found the incidence of death to be 51% lower in vaccinated than in unvaccinated adults with community-acquired pneumonia (N = 1,813) admitted to six hospitals, they ascribed it to confounding factors, specifically socioeconomic and functional status. This phenomenon was previously called the “frailty bias” or the “healthy user effect.” However, this study included only patients hospitalized with pneumonia and did not include data on vaccine-induced immunity or the cause of pneumonia, and measures of the healthy user effect were rudimentary. In addition, only outcomes during hospitalization were included.
Most experts still believe that vaccination prevents 50% of influenza-related deaths (with a smaller effect on rates of all-cause mortality14), including deaths in very old people. 15 A recent review found no basis for the historic concern that the antibody response to the influenza vaccine in people age 60 and older declines more rapidly than in younger people and below seroprotective levels within 4 months of immunization.16
Nevertheless, discordance between antibody and T-cell responses to influenza vaccine does exist17 (ie, the vaccine can induce antibodies while not boosting the T-cell-specific response), and we should continue to seek new vaccines that are more effective.
More people are being vaccinated, but we’re still below our goals
Although influenza vaccination rates among adults continue to improve,18 they remain well below the Healthy People 2010 initiative’s target of 90% in adults age 65 and older (the current rate is 72%) and below the target of 60% in people age 18 through 64 who have one or more high-risk conditions, health care workers, and pregnant women19 (currently 35% in people age 18 through 49 and 42% in people age 50 through 64). Thus, we still need to improve vaccination coverage rates.
Health care providers should offer vaccination at every opportunity between October and May.20 Offering vaccination in nontraditional settings such as work sites and pharmacies is likely to be cost-saving for healthy adults due to averted morbidity.21 At many hospitals, health care workers can opt out of being vaccinated, but they must formally state that they are doing so. The use of these declination statements among health care workers is associated with a mean increase of 11.6% in vaccination rates.22
Since influenza is the second most frequent vaccine-preventable infection in travelers, the vaccine should be offered to those crossing to the opposite hemisphere during its peak influenza season (eg, to South America in May through September), as well as to those visiting the tropics at any time of year.23
Vaccination is safe and effective in high-risk groups
Data on vaccination are reassuring in several at-risk groups.
In pregnancy, there is no indication that infants are harmed if their mothers are vaccinated in the first trimester.24 The evidence of excess morbidity during influenza epidemics supports vaccinating healthy pregnant women in the second or third trimester and those with comorbidities any time during pregnancy. Influenza vaccination during pregnancy reduces laboratory-confirmed influenza in infants up to 6 months of age by 63% and prevents 29% of all febrile respiratory illnesses in infants and 36% of those in mothers.25
In patients with chronic obstructive pulmonary disease, vaccination cuts the rate of outpatient visits and hospitalizations due to acute respiratory illness by 67%.26 The antibody response to influenza vaccine in patients with rheumatoid arthritis treated with rituximab (Rituxan), a monoclonal antibody directed against CD20 surface antigen-positive B lymphocytes, was lower than in healthy controls, but was not negligible.27
Dispelling myths about vaccination in children
One recently published study in children younger than 5 years did not find vaccination to be effective in preventing influenza-related hospitalizations and outpatient visits.28 However, in both seasons in which this study was conducted, there was a suboptimal antigenic match between vaccines and circulating strains. Moreover, about 60% of participants were unvaccinated and another 20% were only partially vaccinated, making it difficult to assess vaccine effectiveness. Several other studies have shown that, when there is a good match, vaccine effectiveness in children is 85% to 90%.
Even though the live-attenuated (inhaled) vaccine is more expensive than the inactivated (injected) vaccine, it reduces the number of influenza illness cases and lowers subsequent health care use in children and productivity loss in their parents, with a net total savings of $45.80 relative to the inactivated vaccine.29 The live-attenuated vaccine provides sustained protection against influenza illness for 12 months following vaccination, as well as meaningful efficacy through a second season without revaccination, although at a lower level.30
Several myths about the live-attenuated vaccine should be dispelled.31 It is well tolerated and causes only mild, transient symptoms of upper respiratory infection, even in people with asthma or the early stages of human immunodeficiency virus infection. Genetic reversion of the vaccine strain to a wild-type virus requires independent mutation in four gene segments, an event that has not been observed. Finally, although viral shedding is common for several days after vaccination, transmission to another person has been shown in only one person, who remained asymptomatic.
Unfortunately, rates of influenza vaccination are even worse for children than for adults.32 In children 6 through 23 months old, only 22% are fully vaccinated; in those 24 through 59 months old, only 16.5% are.
One group of immunocompromised children, liver transplant recipients, achieved antibody seroprotection and seroconversion rates similar to those achieved by their healthy siblings, with no vaccine-related serious side effects.33 As in adults, the cell-mediated immune response to the vaccine was diminished, suggesting that other strategies are needed to provide optimal protection.
IF BIRD FLU BREAKS OUT, WE HAVE A VACCINE
In the event of an outbreak of avian influenza in humans, the US government now has a vaccine against H5N1, the causative virus. A two-dose regimen of a whole-virus H5N1 vaccine, which is derived from cell culture, induced neutralizing antibodies against diverse H5N1 virus strains in most subjects in one study.34 Another vaccine, which is egg-independent and adenoviral vector-based and contains conserved nucleoproteins, is broadly protective against globally dispersed H5N1 virus clades.35 The addition of the MF59 adjuvant to a subvirion H5N1 vaccine increased antibody response, but the addition of aluminum hydroxide did not.36
EXERCISE AND HYGIENE PREVENT FLU
Exercise has benefits beyond the usual ones: one study showed that exercising at low to moderate frequency (between once a month and three times a week) is associated with lower rates of influenza-related death.37
A recent meta-analysis38 confirmed that hygienic measures can prevent the spread of respiratory viruses in the community. The investigators calculated that hand-washing at least 10 times daily can prevent a large number of these infections (number needed to treat [NNT] = 4), and wearing surgical masks (NNT = 6), N95 masks (NNT = 3), gloves (NNT = 6), and gowns (NNT = 5) had incremental effects. On the other hand, the value of adding virucidal or antiseptic solutions to normal hand-washing was uncertain. Strict adherence to hand hygiene and masks (including by children) is needed to prevent influenza transmission in the home.39
AMANTADINE, RIMANTADINE ARE OUT; OSELTAMIVIR RESISTANCE IS GROWING
The CDC continues to recommend against using amantadine (Symmetrel) or rimantadine (Flumadine) to treat flu, owing to a high rate (> 90%) of resistance to these drugs.
A nonrandomized study suggests that zanamivir (Relenza) is more effective than oseltamivir (Tamiflu) for treating influenza B.40 A retrospective study in nine lung transplant recipients showed that oseltamivir is well tolerated and may reduce the risk of complications in these patients.41 Large, randomized, multicenter studies are under way to better assess oseltamivir’s preventive and therapeutic efficacy in transplant recipients.
In children, as in adults, oseltamivir is less effective against influenza B than influenza A,42 and both neuraminidase inhibitors, ie, oseltamivir and zanamivir, are equally effective in reducing the febrile period of influenza.43
During the 2007–2008 season, the rate of resistance to oseltamivir increased alarmingly.44 Resistance was restricted to A (H1N1) viruses carrying the H274Y mutation. In March 2008, the frequency of resistance among A (H1N1) viruses in the United States was 8.6%, 10 times higher than during the preceding influenza season. Resistance rates were much higher in several European countries, including Norway and France. During the Southern Hemisphere’s influenza season (May 2008 though September 2008), 46.5% of influenza A (H1N1) viruses received from 14 countries were resistant to oseltamivir. 45 It is worrisome that many of these resistant viruses were isolated from untreated patients. Fortunately, to date, 99% of these isolates remain susceptible to zanamivir.
Microbiologic tests to detect resistance are not currently available for clinical use. During an influenza pandemic, widespread use of neuraminidase inhibitors will likely promote further development of drug resistance. A mathematical model concluded that combined treatment and prophylaxis with antiviral agents will be necessary to control transmission during a pandemic, and that allocating different drugs to cases and contacts would be most effective in curtailing emergence of resistance.46
For now, either oseltamivir or zanamivir is acceptable for patients with flu symptoms and can be started pending results of PCR testing of nasopharyngeal swabs to make sure that the patient really has influenza. The drugs should be taken for 5 days.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Brocas E, Cormier P, Barouk D, Van de Louw A, Tenaillon A. Influenza incidence estimated with a rapid diagnostic test in critically ill patients with acute respiratory failure during the 2005 and 2006 winter flu epidemics. Presse Med 2008; 37:943–947.
- Sebastian R, Skowronski DM, Chong M, Dhaliwal J, Brownstein JS. Age-related trends in the timeliness and prediction of medical visits, hospitalizations and deaths due to pneumonia and influenza, British Columbia, Canada, 1998–2004. Vaccine 2008; 26:1397–1403.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 2008; 121:258–264.
- van den Dool C, Hak E, Wallinga J, van Loon AM, Lammers JW, Bonten MJ. Symptoms of influenza virus infection in hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:314–319.
- Gharabaghi F, Tellier R, Cheung R, et al. Comparison of a commercial qualitative real-time RT-PCR kit with direct immunofluorescence assay (DFA) and cell culture for detection of influenza A and B in children. J Clin Virol 2008; 42:190–193.
- Gooskens J, Swaan CM, Claas EC, Kroes AC. Rapid molecular detection of influenza outbreaks in nursing homes. J Clin Virol 2008; 41:7–12.
- Mackay WG, van Loon AM, Niedrig M, Meijer A, Lina B, Niesters HG. Molecular detection and typing of influenza viruses: are we ready for an influenza pandemic? J Clin Virol 2008; 42:194–197.
- US Department of Health and Human Services. FDA clears new CDC test to detect human influenza. www.pandemicflu.gov. Accessed 11/3/08.
- Fiore AE, Shay DK, Broder K, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57(RR-7):1–60.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Kelly H, Newall AT. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis 2008; 8:462–463.
- Voordouw BC, Sturkenboom MC, Dieleman JP, Stricker BH. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis 2008; 8:461–462.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Co MD, Orphin L, Cruz J, et al. Discordance between antibody and T cell responses in recipients of trivalent inactivated influenza vaccine. Vaccine 2008; 26:1990–1998.
- Centers for Disease Control and Prevention (CDC). Statespecific influenza vaccination coverage among adults— United States, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:1033–1039.
- Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U.S., 1989–2005. Vaccine 2008; 26:1786–1793.
- Poland GA, Johnson DR. Increasing influenza vaccination rates: the need to vaccinate throughout the entire influenza season. Am J Med 2008; 121(suppl 2):S3–S10.
- Prosser LA, O’Brien MA, Molinari NA, et al. Nontraditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics 2008; 26:163–178.
- Polgreen PM, Septimus EJ, Parry MF, et al. Relationship of influenza vaccination declination statements and influenza vaccination rates for healthcare workers in 22 US hospitals. Infect Control Hosp Epidemiol 2008; 29:675–677l.
- Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers’ vaccine? Expert Rev Vaccines 2008; 7:679–687.
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44–52.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- Menon B, Gurnani M, Aggarwal B. Comparison of outpatient visits and hospitalisations, in patients with chronic obstructive pulmonary disease, before and after influenza vaccination. Int J Clin Pract 2008; 62:593–598.
- Oren S, Mandelboim M, Braun-Moscovici Y, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis 2008; 67:937–941.
- Szilagyi PG, Fairbrother G, Griffin MR, et al; New Vaccine Surveillance Network. Inluenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch Pediatr Adolesc Med 2008; 162:943–951.
- Luce BR, Nichol KL, Belshe RB, et al. Cost-effectiveness of live attenuated influenza vaccine versus inactivated influenza vaccine among children aged 24–59 months in the United States. Vaccine 2008; 26:2841–2848.
- Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J 2008; 27:744–748.
- Tosh PK, Boyce TG, Poland GA. Flu myths: dispelling the myths associated with live attenuated influenza vaccine. Mayo Clin Proc 2008; 83:77–84.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among children aged 6–59 months—eight immunization information system sentinel sites, United States, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:1043–1046.
- Madan RP, Tan M, Fernandez-Sesma A, et al. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin Infect Dis 2008; 46:712–718.
- Ehrlich HJ, Muller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- Hoelscher MA, Singh N, Garg S, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis 2008; 197:1185–1188.
- Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008; 197:667–675.
- Wong CM, Lai HK, Ou CQ, et al. Is exercise protective against influenza-associated mortality? PLoS ONE 2008; 3:e2108.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Cowling BJ, Fung RO, Cheng CK, et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS ONE 2008; 3:e2101.
- Kawai N, Ikematsu H, Iwaki N, et al. A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect 2008; 56:51–57.
- Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant 2008; 27:282–288.
- Suzuki E, Ichihara K. The course of fever following influenza virus infection in children treated with oseltamivir. J Med Virol 2008; 80:1065–1071.
- Sugaya N, Tamura D, Yamazaki M, et al. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis 2008; 47:339–345.
- Sheu TG, Deyde VM, Okomo-Adhiambo M, et al. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother 2008; 52:3284–3292.
- Centers for Disease Control and Prevention (CDC). Influenza activity—United States and worldwide, May 18–September 19, 2008. MMWR Morb Mortal Wkly Rep 2008; 57:1046–1049.
- McCaw JM, Wood JG, McCaw CT, McVernon J. Impact of emerging antiviral drug resistance on influenza containment and spread: influence of subclinical infection and strategic use of a stockpile containing one or two drugs. PLoS ONE 2008; 3:e2362.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Brocas E, Cormier P, Barouk D, Van de Louw A, Tenaillon A. Influenza incidence estimated with a rapid diagnostic test in critically ill patients with acute respiratory failure during the 2005 and 2006 winter flu epidemics. Presse Med 2008; 37:943–947.
- Sebastian R, Skowronski DM, Chong M, Dhaliwal J, Brownstein JS. Age-related trends in the timeliness and prediction of medical visits, hospitalizations and deaths due to pneumonia and influenza, British Columbia, Canada, 1998–2004. Vaccine 2008; 26:1397–1403.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 2008; 121:258–264.
- van den Dool C, Hak E, Wallinga J, van Loon AM, Lammers JW, Bonten MJ. Symptoms of influenza virus infection in hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:314–319.
- Gharabaghi F, Tellier R, Cheung R, et al. Comparison of a commercial qualitative real-time RT-PCR kit with direct immunofluorescence assay (DFA) and cell culture for detection of influenza A and B in children. J Clin Virol 2008; 42:190–193.
- Gooskens J, Swaan CM, Claas EC, Kroes AC. Rapid molecular detection of influenza outbreaks in nursing homes. J Clin Virol 2008; 41:7–12.
- Mackay WG, van Loon AM, Niedrig M, Meijer A, Lina B, Niesters HG. Molecular detection and typing of influenza viruses: are we ready for an influenza pandemic? J Clin Virol 2008; 42:194–197.
- US Department of Health and Human Services. FDA clears new CDC test to detect human influenza. www.pandemicflu.gov. Accessed 11/3/08.
- Fiore AE, Shay DK, Broder K, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57(RR-7):1–60.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Kelly H, Newall AT. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis 2008; 8:462–463.
- Voordouw BC, Sturkenboom MC, Dieleman JP, Stricker BH. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis 2008; 8:461–462.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Co MD, Orphin L, Cruz J, et al. Discordance between antibody and T cell responses in recipients of trivalent inactivated influenza vaccine. Vaccine 2008; 26:1990–1998.
- Centers for Disease Control and Prevention (CDC). Statespecific influenza vaccination coverage among adults— United States, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:1033–1039.
- Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U.S., 1989–2005. Vaccine 2008; 26:1786–1793.
- Poland GA, Johnson DR. Increasing influenza vaccination rates: the need to vaccinate throughout the entire influenza season. Am J Med 2008; 121(suppl 2):S3–S10.
- Prosser LA, O’Brien MA, Molinari NA, et al. Nontraditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics 2008; 26:163–178.
- Polgreen PM, Septimus EJ, Parry MF, et al. Relationship of influenza vaccination declination statements and influenza vaccination rates for healthcare workers in 22 US hospitals. Infect Control Hosp Epidemiol 2008; 29:675–677l.
- Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers’ vaccine? Expert Rev Vaccines 2008; 7:679–687.
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44–52.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- Menon B, Gurnani M, Aggarwal B. Comparison of outpatient visits and hospitalisations, in patients with chronic obstructive pulmonary disease, before and after influenza vaccination. Int J Clin Pract 2008; 62:593–598.
- Oren S, Mandelboim M, Braun-Moscovici Y, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis 2008; 67:937–941.
- Szilagyi PG, Fairbrother G, Griffin MR, et al; New Vaccine Surveillance Network. Inluenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch Pediatr Adolesc Med 2008; 162:943–951.
- Luce BR, Nichol KL, Belshe RB, et al. Cost-effectiveness of live attenuated influenza vaccine versus inactivated influenza vaccine among children aged 24–59 months in the United States. Vaccine 2008; 26:2841–2848.
- Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J 2008; 27:744–748.
- Tosh PK, Boyce TG, Poland GA. Flu myths: dispelling the myths associated with live attenuated influenza vaccine. Mayo Clin Proc 2008; 83:77–84.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among children aged 6–59 months—eight immunization information system sentinel sites, United States, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:1043–1046.
- Madan RP, Tan M, Fernandez-Sesma A, et al. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin Infect Dis 2008; 46:712–718.
- Ehrlich HJ, Muller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- Hoelscher MA, Singh N, Garg S, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis 2008; 197:1185–1188.
- Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008; 197:667–675.
- Wong CM, Lai HK, Ou CQ, et al. Is exercise protective against influenza-associated mortality? PLoS ONE 2008; 3:e2108.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Cowling BJ, Fung RO, Cheng CK, et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS ONE 2008; 3:e2101.
- Kawai N, Ikematsu H, Iwaki N, et al. A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect 2008; 56:51–57.
- Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant 2008; 27:282–288.
- Suzuki E, Ichihara K. The course of fever following influenza virus infection in children treated with oseltamivir. J Med Virol 2008; 80:1065–1071.
- Sugaya N, Tamura D, Yamazaki M, et al. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis 2008; 47:339–345.
- Sheu TG, Deyde VM, Okomo-Adhiambo M, et al. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother 2008; 52:3284–3292.
- Centers for Disease Control and Prevention (CDC). Influenza activity—United States and worldwide, May 18–September 19, 2008. MMWR Morb Mortal Wkly Rep 2008; 57:1046–1049.
- McCaw JM, Wood JG, McCaw CT, McVernon J. Impact of emerging antiviral drug resistance on influenza containment and spread: influence of subclinical infection and strategic use of a stockpile containing one or two drugs. PLoS ONE 2008; 3:e2362.
KEY POINTS
- Real-time reverse transcriptase polymerase chain reaction is the most accurate and clinically useful diagnostic test for influenza.
- All children age 6 months to 18 years should be vaccinated, and the live-attenuated vaccine is now approved for use in children 2 years old and older.
- We should continue to pursue traditional and innovative measures to increase influenza vaccination rates.
- Influenza vaccination during pregnancy reduces laboratory-confirmed influenza in infants up to 6 months of age by 63%.
- Hygienic measures (particularly hand-washing) aimed at younger children can prevent the spread of respiratory viruses in the community.
- Primary viral resistance to oseltamivir (Tamiflu) is rising, but almost all isolates remain susceptible to zanamivir (Relenza).