User login
Influenza vaccination of residents of long-term care facilities (and of health care workers at these facilities) is critical for the prevention of influenza in this frail population. Detection, chemoprophylaxis, and treatment have limitations. Infection control measures should be in place during and between outbreaks. Acute care facilities such as emergency departments and hospitals can assist by testing residents of long-term care facilities who present with influenza-like illness during seasonal epidemics of influenza, and by notifying the receiving facility if a patient with influenza would be arriving.
THE EXTENT OF THE PROBLEM
From 5% to 20% of the US population, including residents and health care workers in long-term care facilities, are infected with influenza every year.1,2 The proportion of those infected who develop clinical illness ranges from 40% to 80%. Each influenza illness is associated with an average of 10 days of respiratory sickness, resulting in approximately 3 days of bed confinement or restricted activity. About 30% to 50% of patients with microbiologically confirmed influenza seek medical care, of whom 16% undergo laboratory tests, 17% undergo radiologic tests, and 75% are recommended an over-the-counter drug or are prescribed a medication. Annual influenza-related hospitalizations range from 200,000 to 400,000, depending on seasonal variations in virulence.2,3 Thus, influenza causes 1.3 hospitalizations per 1,000 people, and 25% of these are in people age 65 and older. In the United States, about 40,000 to 60,000 people die of influenza every year, and 90% of these are age 65 and older.4
POLICIES TO FIGHT ANTIVIRAL RESISTANCE
In January 2006, the US Centers for Disease Control and Prevention (CDC) recommended against the use of the adamantanes—ie, amantadine (Symmetrel) and rimantadine (Flumadine)—for the treatment or prevention of influenza because of a high level of resistance in circulating influenza A/H3N2 in the community. Unfortunately, this resistance trend has not reversed since then, with 96% to 100% of influenza A/H3N2 isolates in the United States showing resistance.5 During the 2007–2008 influenza season, influenza A/H1N1 isolates resistant to oseltamivir (Tamiflu) emerged in Europe, particularly in Norway and France. In the United States, influenza A/H1N1 resistance to oseltamivir increased from 0.7% in the 2006–2007 season, to 10.9% in the 2007–2008 season, and to 98% during the 2008–2009 season.6,7
Fortunately, all oseltamivir-resistant isolates remain susceptible to zanamivir (Relenza). In April 2009, a new influenza A/H1N1 variant (previously referred to as swine-origin influenza virus, or SOIV) emerged in North America and spread to many countries worldwide, and the World Health Organization eventually declared a pandemic. This new variant is susceptible to oseltamivir and zanamivir but resistant to the adamantanes. Resistance patterns for future influenza seasons cannot be predicted, but the current extent of influenza resistance and its development over the past decade8 are alarming.
WHY IS INFLUENZA MORE SERIOUS IN LONG-TERM CARE RESIDENTS?
Influenza is usually introduced to long-term care facilities by workers and visitors. Inside, the closed environment and limited mobility of residents facilitate transmission of infection.
The clinical presentation of influenza in residents of long-term care facilities can be subtle, with a blunted febrile response and a decline in mental and functional status.9
Residents commonly have underlying diseases that can be exacerbated by influenza infection, such as congestive heart failure, chronic obstructive lung disease, chronic kidney disease, and dementia. In addition, residents are at higher risk of serious influenza-related complications than are community-dwelling elderly people.
Impaired oral intake, limited dexterity, and altered consciousness may limit treatment options, thus further adversely affecting outcomes. Bacterial pneumonia secondary to influenza has dire consequences in long-term care residents. Rates of hospitalization for pneumonia and influenza and for exacerbation of chronic lung disease are higher in these patients than in their community-dwelling counterparts.10 Death rates are also higher, exceeding 5% during influenza epidemics.11
PREVENTING INFLUENZA IN LONG-TERM CARE FACILITIES
Immunizing residents is essential
Vaccination is the most important measure in preventing influenza in long-term care facilities, and vaccination programs should include residents and health care workers.
Influenza outbreaks are more common in facilities where the rate of immunization is below 80%, as well as in larger facilities (with > 100 beds), suggesting that herd immunity may play a role.12 Unfortunately, influenza vaccine coverage rates vary widely in this patient population—from 57% to 98% in one report.13
The live-attenuated intranasal vaccine is approved only for healthy people under age 50, so most residents of long-term care facilities should receive only the inactivated trivalent intramuscular vaccine.
The effectiveness of a vaccine in preventing influenza depends in part on the adequacy of the match between vaccine serotypes and circulating strains. Studies of all types—randomized, observational, case-control, and cohort studies, as well as meta-analyses and systematic reviews—have shown preventive efficacy rates of influenza vaccination in elderly residents of long-term care facilities to be 23% to 43% for influenza-like illness, 0% to 58% for influenza, 46% for pneumonia, 45% for hospitalization, 42% for death from influenza or pneumonia, and 60% for death from all causes.14,15 Vaccine performance improved after adjustment for confounders.
Obviously, this protection is variable and incomplete, since influenza outbreaks continue to occur even in long-term care facilities in which most residents are vaccinated.13
Vaccination works, despite the controversy
Whether influenza vaccination prevents deaths in elderly people—or how many deaths it prevents—is a subject of ongoing controversy. 16 Even though influenza vaccination coverage in the elderly increased from 15% to 65% since 1980, the specific influenza-related death rate did not decrease.17
It has been suggested that cohort studies may have overestimated the mortality benefit of influenza vaccination in the elderly because of “frailty selection bias” (ie, extremely frail elderly patients are less likely to be vaccinated and are more likely to die for reasons other than influenza than are less frail, vaccinated elderly people) and because of the use of nonspecific end points such as all-cause mortality. 16 Similarly, observational studies may have overestimated the in-hospital mortality benefit of influenza vaccination in older patients with pneumonia occurring outside of influenza season because of the “healthy user effect” (ie, residual confounding by functional and socioeconomic status).18
One nested case-control study in immunocompetent elderly patients showed that influenza vaccination was not associated with a reduced risk of community-acquired pneumonia after adjusting for the presence and severity of comorbidities.19
Since death is a rare end point, it is hard to show a reduction in the death rate with vaccination in randomized controlled studies. The absolute risk reduction in hospitalization and death with vaccination is two to five times higher in elderly patients at high risk than in the healthy elderly.20
The mortality benefit in elderly patients is increased with annual revaccination, with one death prevented for every 300 vaccinations, and one for every 200 revaccinations.21
The response to influenza vaccination is reduced in elderly people because of immune senescence, and higher doses of vaccine have been shown to be more immunogenic and remain safe.22 This enhanced antibody response may be maintained for subsequent antigenically different influenza variants, even against viruses appearing more than 10 years after vaccination. 23 The 2008–2009 influenza vaccine does not protect against the new, pandemic influenza A/H1N1 variant; efforts to produce such a vaccine are under way.
Influenza vaccination is safe. Recent data showed no association between immunization and Guillain-Barré syndrome.24 In fact, influenza itself may be a triggering agent for Guillain-Barré syndrome during major influenza outbreaks.25
DURATION OF SEROPROTECTION IS MORE THAN 6 MONTHS
Every effort should be made to vaccinate residents of long-term care facilities and their caregivers as early as possible in the influenza season to allow an adequate antibody response to develop before the onset of an influenza outbreak.
In the past, there has been concern that the influenza-vaccine-induced antibody response declines more rapidly in the elderly and may fall below seroprotective levels within 4 months of vaccination. But a recent review of 14 published studies argued against that notion, showing that if seroprotection is achieved in the first month after vaccination, it is then maintained for more than 6 months.26 That review also showed that seroconversion varies inversely with preimmunization titers, but not with age.
Moreover, a prospective study27 in 303 residents of a long-term care facility reported that seroprotection did not correlate with nutritional status. In the study, vaccination was very effective despite a high prevalence of nutritional deficiencies. This study also indicated that although an influenza antibody titer greater than 1:40 is considered protective in the general population, long-term care facility residents may require higher levels for effective immunization.
A recent survey showed that a national shortage of influenza vaccine results in decreased immunization rates in residents and in health care workers in long-term care facilities. 28 In that survey, only 2.3% of facilities expressed concern about emergency preparedness, and this has significant implications for a possible influenza pandemic.
VACCINATION PROGRAMS
Standing-order programs have been shown to significantly increase vaccination rates in ambulatory and hospital settings. However, a recent survey showed that only 9% of long-term care facilities use such programs.29 The greatest use of such programs was in government-owned, nonprofit, dual-certified (ie, by both Medicare and Medicaid), and independent long-term care facilities, and in facilities with a lower index of disease acuity. Use varied substantially by state.
The Healthy People 2010 goal of 90% vaccination may be attained by implementing written protocols for documenting immunization—and refusal of immunization—in a consistent place in the patient’s medical record.30
VACCINATION OF WORKERS
When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die31,32 or develop influenza-like illness, particularly when residents themselves are vaccinated.33 Additional benefits include decreased need for consultations with general practitioners or admissions to the hospital for influenza-like illness during periods of moderate influenza activity.32
The policy of mandatory influenza vaccination for health care workers has its proponents34 and opponents.35 When a large tertiary care center adopted mandatory vaccination, vaccination rates increased significantly over time.36 The Centers for Medicare & Medicaid Services recently mandated public reporting of vaccination rates in health care workers and residents of long-term care facilities, and compliance is expected to increase as a result.
BETWEEN INFLUENZA OUTBREAKS
Studies show that hygienic measures prevent transmission of respiratory viruses.37 Therefore, the cornerstones of any program to prevent transmission of influenza and other microorganisms in long-term care facilities are hand hygiene, cough and sneeze etiquette, maintaining a distance of 3 feet between beds, and education of residents and health care workers.
Wash your hands!
Hands should be washed before and after direct contact with patients or with inanimate objects in their immediate vicinity.38 Contrary to popular belief, hands should also be washed before and after wearing gloves, particularly when handling an invasive device for patient care, and after contact with bodily fluids, excretions, mucous membranes, non-intact skin, or wound dressings. Hands should be washed not only between patients but also during care for the same patient if moving from a contaminated body site to a clean site.
Hands should be washed for 15 to 20 seconds using soap and water or an alcohol-based foam or gel; when hands are visibly soiled, only soap and water should be used. It is not known whether adding virucidals or antiseptics to normal hand-washing further decreases the spread of these viruses.
Continuous education, feedback interventions, and patient-awareness programs can improve hand-washing compliance, but they are not sufficient. Easily accessible dispensers for alcohol-based waterless antiseptic foam or gel can significantly improve hand hygiene rates among health care workers.39
DETECTING INFLUENZA OUTBREAKS
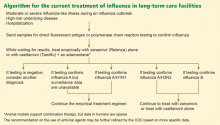
Direct fluorescent antigen detection and nucleic acid detection by polymerase chain reaction (PCR) are the tests recommended for early detection of influenza outbreaks in long-term care facilities. Rapid point-of-care tests to detect influenza antigen are only 60% to 70% sensitive,13,40 viral culture takes several days, and serologic diagnosis requires documentation of seroconversion at least 2 weeks apart, and so none of these is adequate for the early detection of an influenza outbreak.
There is no widely available test to differentiate influenza A/H1N1 from A/H3N2, or to test for drug resistance. In addition, the PCR tests widely used today do not differentiate between seasonal influenza A/H1N1 and the new, pandemic influenza A/H1N1 variant. Efforts are under way to produce and distribute such a test.
Controlling influenza outbreaks
An outbreak should be declared when two or more residents develop an influenza-like illness within 72 hours of each other during the influenza season.41 After influenza infection is confirmed by laboratory testing, testing of all residents who subsequently develop an influenza-like illness may not be feasible, and other respiratory viruses may be responsible for mixed outbreaks during influenza epidemics, particularly respiratory syncytial virus.
Roommates of residents who test positive for influenza have a risk of acquiring influenza three times higher than that of residents living in single rooms.42 Obviously, private rooms for all residents would be optimal, but this is not practical in most facilities. “Cohorting” (ie, housing infected residents together) is reasonable, but in this situation they might infect each other with other viruses or with influenza viruses of different serotypes.
When an outbreak occurs, potential solutions include closing subunits of the facility to new admissions, limiting movement of residents and health care workers from affected to unaffected units, and using curtains or other barriers between roommates. But most important during seasonal outbreaks are hand hygiene, proper cough and sneeze etiquette, and 3-foot separation between roommates. A simulation model showed that during an influenza pandemic, preventing ill residents of long-term care facilities from making contact with other residents may reduce rates of illness and death by about 60%.43 If a long-term care facility resident is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient or when entering the room of a resident with confirmed influenza.
CHEMOPROPHYLAXIS DURING INFLUENZA OUTBREAKS
When influenza outbreaks are recognized early in long-term care facilities, appropriate infection control measures and chemoprophylaxis can be started. Chemoprophylaxis should also be considered in residents in whom influenza vaccination is contraindicated, such as those with severe egg allergy.
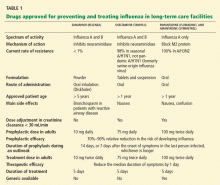
Rimantadine is preferred over amantadine in residents of long-term care facilities, since amantadine is associated with a much higher rate of adverse events (18.6% vs 1.9%), especially confusion (10.6% vs 0.6%), resulting in more frequent discontinuation (17.3% vs 1.9%).44 In addition, viral resistance to amantadine develops in about 30% of those who receive it. In long-term care facilities, viral resistance occurs not only when it is used for the management of influenza, but also when it is used to treat Parkinson disease.45
Oseltamivir prophylaxis for 6 weeks in influenza-vaccinated, frail elderly residents of long-term care facilities during influenza epidemics was 91% effective in preventing laboratory-confirmed clinical influenza, and 85% effective in preventing a secondary bacterial complication such as pneumonia or sinusitis.46 The rate of adverse events associated with oseltamivir in that population was similar to that with placebo. Importantly, oseltamivir was not associated with suppression of antibody response to influenza infection or vaccination.
Oseltamivir is very effective in terminating influenza outbreaks in long-term care facilities, even when amantadine fails.4,13 When used in that manner, it is also associated with decreased antibiotic prescriptions, hospitalizations, deaths,47 and substantial cost-savings, even when compared with amantadine, which has a much lower acquisition cost but a higher rate of adverse events, lower efficacy, and individualized dosing requirements.48
Zanamivir prophylaxis for 2 weeks given to unvaccinated residents was 29% effective in preventing all symptomatic influenza confirmed by laboratory testing (by culture, PCR, or seroconversion). It was 65% effective in preventing symptomatic culture-confirmed influenza, 70% effective in preventing febrile, laboratory-confirmed influenza, and 21% effective in preventing complications.49 It was well tolerated in this population and was not associated with the emergence of zanamivir resistance. Zanamivir also provides 61% additional protective efficacy over rimantadine in vaccinated residents of long-term care facilities,50 primarily because of the emergence of viruses resistant to rimantadine. However, up to 50% of elderly people may have difficulty loading and priming the Diskhaler device used to deliver zanamivir.51
While several studies have shown chemoprophylaxis to be effective, it is not possible to ascribe the decrease in cases during an outbreak entirely to antiviral drugs since most studies were not placebo-controlled, and since the natural tendency of outbreaks is to subside.
TREATMENT OF INFLUENZA IN LONG-TERM CARE FACILITIES
A mild illness in someone without a high-risk underlying disease can be managed with symptomatic measures alone, and testing for influenza is at the discretion of the caregiver. Patients who develop a moderate or severe influenza-like illness during an influenza epidemic and who have a high-risk underlying disease or who require hospitalization should be tested for influenza by a sensitive test such as direct fluorescent antigen testing or PCR and should be started on empiric anti-influenza therapy.41
Most elderly residents of long-term care facilities do have comorbid conditions and are thus at high risk of complications of influenza. During influenza outbreaks, clinicians caring for these patients—and for similar patients in outpatient settings, emergency departments, and acute-care hospitals—should consider testing for influenza, even if the patient has only a mild influenza-like illness.
Of note, no randomized treatment study has been done specifically in residents of long-term care facilities.52 Pooled analysis of data from 321 patients at high risk (76 were age 65 or older) in zanamivir treatment studies showed that those who received the drug were sick for 2.5 fewer days than those who received placebo.53 In addition, zanamivir recipients returned to normal activities 3 days sooner, had an 11% reduction in the median total symptom score over 1 to 5 days, and had a rate of complications requiring antibiotics 43% less than placebo recipients. Rates of adverse events were similar in both groups.
A multicenter, randomized, open-label, controlled trial of oseltamivir in the treatment of influenza in high-risk Chinese patients with chronic respiratory diseases (chronic bronchitis, obstructive emphysema, bronchial asthma, or bronchiectasis) or chronic cardiac disease showed that oseltamivir significantly reduced the duration of influenza symptoms by 36.8%, the severity of symptoms by 43.1%, the duration of fever by 45.2%, the time to return to baseline health status by 5 days, and the need for antibiotics, without increasing the total cost of medical care.54
Oseltamivir-resistant isolates of seasonal influenza A/H1N1 do not cause different or more severe symptoms than do oseltamivir-susceptible isolates, and they remain susceptible to zanamivir and the adamantanes. There is no widely available test to differentiate influenza A/H1N1 from A/H3N2 or to test for drug resistance. Influenza B and A/H3N2 remain susceptible to both of the neuraminidase inhibitors, ie, oseltamivir and zanamivir.
All currently circulating influenza A/H3N2 isolates remain resistant to the adamantanes. Influenza B is intrinsically resistant to the adamantanes, and some data show that oseltamivir is less effective against influenza B than against influenza A.
Zanamivir is not available in many pharmacies, is not recommended in patients with underlying reactive airway disease, and requires dexterity for administration.
Amantadine is more likely to cause confusion in the elderly than rimantadine, and it is more susceptible to treatment-emergent antiviral resistance.
No parenteral anti-influenza drug is available, but zanamivir and the experimental drug peramivir are undergoing study for parenteral use. An inhaled, long-acting neuraminidase inhibitor, CS-8958, is currently under study.55 Other agents currently under development include T-705, a polymerase inhibitor, and DAS181, an attachment inhibitor.
Complementary and alternative therapies for influenza are not established. A recent review of 14 randomized, controlled trials56 revealed that the evidence is weak and limited by small sample sizes, poor methodologic quality, or clinically irrelevant effect sizes.
- Cifu A, Levinson W. Influenza. JAMA 2000; 284:2847–2849.
- Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics 1996; 9(suppl 3):26–33.
- Fiore AE, Shay DK, Broder K, et al; US Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:1–60.
- Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol 1993; 22:334–340.
- Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 2007; 196:249–257.
- Centers for Disease Control and Prevention (CDC). Update: Influenza activity—United States, September 28, 2008–January 31, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:115–119.
- CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008–09 influenza season. Distributed via Health Alert Network. 2008. www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279. Accessed 7/14/2009.
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 2009; 301:1066–1069.
- Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998; 158:645–650.
- Menec VH, MacWilliams L, Aoki FY. Hospitalization and deaths due to respiratory illnesses during influenza seasons: a comparison of community residents, senior housing residents, and nursing home residents. J Gerontol A Biol Sci Med Sci 2002; 57:M629–M635.
- Kingston BJ, Wright CV. Influenza in the nursing home. Am Fam Physician 2002; 65:75–78.
- Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis 2006; 194(suppl 2):S133–S138.
- Monto AS, Rotthoff J, Teich E, et al. Detection and control of influenza outbreaks in well-vaccinated nursing home populations. Clin Infect Dis 2004; 39:459–464.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366:1165–1174.
- Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; 3:CD004876.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–666.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Hak E, Nordin J, Wei F, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis 2002; 35:370–377.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis 2008; 198:1016–1018.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Paccalin M, Plouzeau C, Bouche G, et al. Lack of correlation between nutritional status and seroprotection against influenza in a long term care facility. Scand J Infect Dis 2006; 38:894–897.
- Mody L, Langa KM, Malani PN. Impact of the 2004–2005 influenza vaccine shortage on immunization practices in long-term care facilities. Infect Control Hosp Epidemiol 2006; 27:383–387.
- Shefer A, McKibben L, Bardenheier B, Bratzler D, Roberts H. Characteristics of long-term care facilities associated with standing order programs to deliver influenza and pneumococcal vaccinations to residents in 13 states. J Am Med Dir Assoc 2005; 6:97–104.
- Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long-term care facilities: the CMS-CDC standing orders program project. J Am Med Dir Assoc 2005; 6:291–299.
- Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000; 355:93–97.
- Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241.
- Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2006; 3:CD005187.
- Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144–1147.
- Finch M. Point: mandatory influenza vaccination for all health care workers? Seven reasons to say “no”. Clin Infect Dis 2006; 42:1141–1143.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Pittet D, Allegranzi B, Sax H, et al; WHO Global Patient Safety Challenge, World Alliance for Patient Safety. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 2006; 6:641–652.
- Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 2000; 160:1017–1021.
- US Centers for Disease Control and Prevention. Influenza-testing and antiviral-agent prescribing practices—Connecticut, Minnesota, New Mexico, and New York, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:61–65.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Drinka PJ, Krause PF, Nest LJ, Goodman BM, Gravenstein S. Risk of acquiring influenza B in a nursing home from a culture-positive roommate. J Am Geriatr Soc 2005; 53:1437.
- Haber MJ, Shay DK, Davis XM, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis 2007; 13:581–589.
- Keyser LA, Karl M, Nafziger AN, Bertino JS. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med 2000; 160:1485–1488.
- Saito R, Oshitani H, Masuda H, Suzuki H. Detection of amantadineresistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol 2002; 40:84–88.
- Peters PH, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031.
- Bowles SK, Kennie N, Ruston L, Simor A, Louie M, Collins V. Influenza outbreak in a long-term-care facility: considerations for pharmacy. Am J Health Syst Pharm 1999; 56:2303–2307.
- Risebrough NA, Bowles SK, Simor AE, McGeer A, Oh PI. Economic evaluation of oseltamivir phosphate for postexposure prophylaxis of influenza in long-term care facilities. J Am Geriatr Soc 2005; 53:444–451.
- Ambrozaitis A, Gravenstein S, van Essen GA, et al. Inhaled zanamivir versus placebo for the prevention of influenza outbreaks in an unvaccinated long-term care population. J Am Med Dir Assoc 2005; 6:367–374.
- Gravenstein S, Drinka P, Osterweil D, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc 2005; 6:359–366.
- Diggory P, Fernandez C, Humphrey A, Jones V, Murphy M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ 2001; 322:577–579.
- Glezen WP. Clinical practice: prevention and treatment of seasonal influenza. N Engl J Med 2008; 359:2579–2585.
- Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001; 161:212–217.
- Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin 2006; 22:75–82.
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009; 48( suppl 1):S3–S13.
- Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am J Med 2007; 120:923–929.
Influenza vaccination of residents of long-term care facilities (and of health care workers at these facilities) is critical for the prevention of influenza in this frail population. Detection, chemoprophylaxis, and treatment have limitations. Infection control measures should be in place during and between outbreaks. Acute care facilities such as emergency departments and hospitals can assist by testing residents of long-term care facilities who present with influenza-like illness during seasonal epidemics of influenza, and by notifying the receiving facility if a patient with influenza would be arriving.
THE EXTENT OF THE PROBLEM
From 5% to 20% of the US population, including residents and health care workers in long-term care facilities, are infected with influenza every year.1,2 The proportion of those infected who develop clinical illness ranges from 40% to 80%. Each influenza illness is associated with an average of 10 days of respiratory sickness, resulting in approximately 3 days of bed confinement or restricted activity. About 30% to 50% of patients with microbiologically confirmed influenza seek medical care, of whom 16% undergo laboratory tests, 17% undergo radiologic tests, and 75% are recommended an over-the-counter drug or are prescribed a medication. Annual influenza-related hospitalizations range from 200,000 to 400,000, depending on seasonal variations in virulence.2,3 Thus, influenza causes 1.3 hospitalizations per 1,000 people, and 25% of these are in people age 65 and older. In the United States, about 40,000 to 60,000 people die of influenza every year, and 90% of these are age 65 and older.4
POLICIES TO FIGHT ANTIVIRAL RESISTANCE
In January 2006, the US Centers for Disease Control and Prevention (CDC) recommended against the use of the adamantanes—ie, amantadine (Symmetrel) and rimantadine (Flumadine)—for the treatment or prevention of influenza because of a high level of resistance in circulating influenza A/H3N2 in the community. Unfortunately, this resistance trend has not reversed since then, with 96% to 100% of influenza A/H3N2 isolates in the United States showing resistance.5 During the 2007–2008 influenza season, influenza A/H1N1 isolates resistant to oseltamivir (Tamiflu) emerged in Europe, particularly in Norway and France. In the United States, influenza A/H1N1 resistance to oseltamivir increased from 0.7% in the 2006–2007 season, to 10.9% in the 2007–2008 season, and to 98% during the 2008–2009 season.6,7
Fortunately, all oseltamivir-resistant isolates remain susceptible to zanamivir (Relenza). In April 2009, a new influenza A/H1N1 variant (previously referred to as swine-origin influenza virus, or SOIV) emerged in North America and spread to many countries worldwide, and the World Health Organization eventually declared a pandemic. This new variant is susceptible to oseltamivir and zanamivir but resistant to the adamantanes. Resistance patterns for future influenza seasons cannot be predicted, but the current extent of influenza resistance and its development over the past decade8 are alarming.
WHY IS INFLUENZA MORE SERIOUS IN LONG-TERM CARE RESIDENTS?
Influenza is usually introduced to long-term care facilities by workers and visitors. Inside, the closed environment and limited mobility of residents facilitate transmission of infection.
The clinical presentation of influenza in residents of long-term care facilities can be subtle, with a blunted febrile response and a decline in mental and functional status.9
Residents commonly have underlying diseases that can be exacerbated by influenza infection, such as congestive heart failure, chronic obstructive lung disease, chronic kidney disease, and dementia. In addition, residents are at higher risk of serious influenza-related complications than are community-dwelling elderly people.
Impaired oral intake, limited dexterity, and altered consciousness may limit treatment options, thus further adversely affecting outcomes. Bacterial pneumonia secondary to influenza has dire consequences in long-term care residents. Rates of hospitalization for pneumonia and influenza and for exacerbation of chronic lung disease are higher in these patients than in their community-dwelling counterparts.10 Death rates are also higher, exceeding 5% during influenza epidemics.11
PREVENTING INFLUENZA IN LONG-TERM CARE FACILITIES
Immunizing residents is essential
Vaccination is the most important measure in preventing influenza in long-term care facilities, and vaccination programs should include residents and health care workers.
Influenza outbreaks are more common in facilities where the rate of immunization is below 80%, as well as in larger facilities (with > 100 beds), suggesting that herd immunity may play a role.12 Unfortunately, influenza vaccine coverage rates vary widely in this patient population—from 57% to 98% in one report.13
The live-attenuated intranasal vaccine is approved only for healthy people under age 50, so most residents of long-term care facilities should receive only the inactivated trivalent intramuscular vaccine.
The effectiveness of a vaccine in preventing influenza depends in part on the adequacy of the match between vaccine serotypes and circulating strains. Studies of all types—randomized, observational, case-control, and cohort studies, as well as meta-analyses and systematic reviews—have shown preventive efficacy rates of influenza vaccination in elderly residents of long-term care facilities to be 23% to 43% for influenza-like illness, 0% to 58% for influenza, 46% for pneumonia, 45% for hospitalization, 42% for death from influenza or pneumonia, and 60% for death from all causes.14,15 Vaccine performance improved after adjustment for confounders.
Obviously, this protection is variable and incomplete, since influenza outbreaks continue to occur even in long-term care facilities in which most residents are vaccinated.13
Vaccination works, despite the controversy
Whether influenza vaccination prevents deaths in elderly people—or how many deaths it prevents—is a subject of ongoing controversy. 16 Even though influenza vaccination coverage in the elderly increased from 15% to 65% since 1980, the specific influenza-related death rate did not decrease.17
It has been suggested that cohort studies may have overestimated the mortality benefit of influenza vaccination in the elderly because of “frailty selection bias” (ie, extremely frail elderly patients are less likely to be vaccinated and are more likely to die for reasons other than influenza than are less frail, vaccinated elderly people) and because of the use of nonspecific end points such as all-cause mortality. 16 Similarly, observational studies may have overestimated the in-hospital mortality benefit of influenza vaccination in older patients with pneumonia occurring outside of influenza season because of the “healthy user effect” (ie, residual confounding by functional and socioeconomic status).18
One nested case-control study in immunocompetent elderly patients showed that influenza vaccination was not associated with a reduced risk of community-acquired pneumonia after adjusting for the presence and severity of comorbidities.19
Since death is a rare end point, it is hard to show a reduction in the death rate with vaccination in randomized controlled studies. The absolute risk reduction in hospitalization and death with vaccination is two to five times higher in elderly patients at high risk than in the healthy elderly.20
The mortality benefit in elderly patients is increased with annual revaccination, with one death prevented for every 300 vaccinations, and one for every 200 revaccinations.21
The response to influenza vaccination is reduced in elderly people because of immune senescence, and higher doses of vaccine have been shown to be more immunogenic and remain safe.22 This enhanced antibody response may be maintained for subsequent antigenically different influenza variants, even against viruses appearing more than 10 years after vaccination. 23 The 2008–2009 influenza vaccine does not protect against the new, pandemic influenza A/H1N1 variant; efforts to produce such a vaccine are under way.
Influenza vaccination is safe. Recent data showed no association between immunization and Guillain-Barré syndrome.24 In fact, influenza itself may be a triggering agent for Guillain-Barré syndrome during major influenza outbreaks.25
DURATION OF SEROPROTECTION IS MORE THAN 6 MONTHS
Every effort should be made to vaccinate residents of long-term care facilities and their caregivers as early as possible in the influenza season to allow an adequate antibody response to develop before the onset of an influenza outbreak.
In the past, there has been concern that the influenza-vaccine-induced antibody response declines more rapidly in the elderly and may fall below seroprotective levels within 4 months of vaccination. But a recent review of 14 published studies argued against that notion, showing that if seroprotection is achieved in the first month after vaccination, it is then maintained for more than 6 months.26 That review also showed that seroconversion varies inversely with preimmunization titers, but not with age.
Moreover, a prospective study27 in 303 residents of a long-term care facility reported that seroprotection did not correlate with nutritional status. In the study, vaccination was very effective despite a high prevalence of nutritional deficiencies. This study also indicated that although an influenza antibody titer greater than 1:40 is considered protective in the general population, long-term care facility residents may require higher levels for effective immunization.
A recent survey showed that a national shortage of influenza vaccine results in decreased immunization rates in residents and in health care workers in long-term care facilities. 28 In that survey, only 2.3% of facilities expressed concern about emergency preparedness, and this has significant implications for a possible influenza pandemic.
VACCINATION PROGRAMS
Standing-order programs have been shown to significantly increase vaccination rates in ambulatory and hospital settings. However, a recent survey showed that only 9% of long-term care facilities use such programs.29 The greatest use of such programs was in government-owned, nonprofit, dual-certified (ie, by both Medicare and Medicaid), and independent long-term care facilities, and in facilities with a lower index of disease acuity. Use varied substantially by state.
The Healthy People 2010 goal of 90% vaccination may be attained by implementing written protocols for documenting immunization—and refusal of immunization—in a consistent place in the patient’s medical record.30
VACCINATION OF WORKERS
When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die31,32 or develop influenza-like illness, particularly when residents themselves are vaccinated.33 Additional benefits include decreased need for consultations with general practitioners or admissions to the hospital for influenza-like illness during periods of moderate influenza activity.32
The policy of mandatory influenza vaccination for health care workers has its proponents34 and opponents.35 When a large tertiary care center adopted mandatory vaccination, vaccination rates increased significantly over time.36 The Centers for Medicare & Medicaid Services recently mandated public reporting of vaccination rates in health care workers and residents of long-term care facilities, and compliance is expected to increase as a result.
BETWEEN INFLUENZA OUTBREAKS
Studies show that hygienic measures prevent transmission of respiratory viruses.37 Therefore, the cornerstones of any program to prevent transmission of influenza and other microorganisms in long-term care facilities are hand hygiene, cough and sneeze etiquette, maintaining a distance of 3 feet between beds, and education of residents and health care workers.
Wash your hands!
Hands should be washed before and after direct contact with patients or with inanimate objects in their immediate vicinity.38 Contrary to popular belief, hands should also be washed before and after wearing gloves, particularly when handling an invasive device for patient care, and after contact with bodily fluids, excretions, mucous membranes, non-intact skin, or wound dressings. Hands should be washed not only between patients but also during care for the same patient if moving from a contaminated body site to a clean site.
Hands should be washed for 15 to 20 seconds using soap and water or an alcohol-based foam or gel; when hands are visibly soiled, only soap and water should be used. It is not known whether adding virucidals or antiseptics to normal hand-washing further decreases the spread of these viruses.
Continuous education, feedback interventions, and patient-awareness programs can improve hand-washing compliance, but they are not sufficient. Easily accessible dispensers for alcohol-based waterless antiseptic foam or gel can significantly improve hand hygiene rates among health care workers.39
DETECTING INFLUENZA OUTBREAKS
Direct fluorescent antigen detection and nucleic acid detection by polymerase chain reaction (PCR) are the tests recommended for early detection of influenza outbreaks in long-term care facilities. Rapid point-of-care tests to detect influenza antigen are only 60% to 70% sensitive,13,40 viral culture takes several days, and serologic diagnosis requires documentation of seroconversion at least 2 weeks apart, and so none of these is adequate for the early detection of an influenza outbreak.
There is no widely available test to differentiate influenza A/H1N1 from A/H3N2, or to test for drug resistance. In addition, the PCR tests widely used today do not differentiate between seasonal influenza A/H1N1 and the new, pandemic influenza A/H1N1 variant. Efforts are under way to produce and distribute such a test.
Controlling influenza outbreaks
An outbreak should be declared when two or more residents develop an influenza-like illness within 72 hours of each other during the influenza season.41 After influenza infection is confirmed by laboratory testing, testing of all residents who subsequently develop an influenza-like illness may not be feasible, and other respiratory viruses may be responsible for mixed outbreaks during influenza epidemics, particularly respiratory syncytial virus.
Roommates of residents who test positive for influenza have a risk of acquiring influenza three times higher than that of residents living in single rooms.42 Obviously, private rooms for all residents would be optimal, but this is not practical in most facilities. “Cohorting” (ie, housing infected residents together) is reasonable, but in this situation they might infect each other with other viruses or with influenza viruses of different serotypes.
When an outbreak occurs, potential solutions include closing subunits of the facility to new admissions, limiting movement of residents and health care workers from affected to unaffected units, and using curtains or other barriers between roommates. But most important during seasonal outbreaks are hand hygiene, proper cough and sneeze etiquette, and 3-foot separation between roommates. A simulation model showed that during an influenza pandemic, preventing ill residents of long-term care facilities from making contact with other residents may reduce rates of illness and death by about 60%.43 If a long-term care facility resident is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient or when entering the room of a resident with confirmed influenza.
CHEMOPROPHYLAXIS DURING INFLUENZA OUTBREAKS
When influenza outbreaks are recognized early in long-term care facilities, appropriate infection control measures and chemoprophylaxis can be started. Chemoprophylaxis should also be considered in residents in whom influenza vaccination is contraindicated, such as those with severe egg allergy.
Rimantadine is preferred over amantadine in residents of long-term care facilities, since amantadine is associated with a much higher rate of adverse events (18.6% vs 1.9%), especially confusion (10.6% vs 0.6%), resulting in more frequent discontinuation (17.3% vs 1.9%).44 In addition, viral resistance to amantadine develops in about 30% of those who receive it. In long-term care facilities, viral resistance occurs not only when it is used for the management of influenza, but also when it is used to treat Parkinson disease.45
Oseltamivir prophylaxis for 6 weeks in influenza-vaccinated, frail elderly residents of long-term care facilities during influenza epidemics was 91% effective in preventing laboratory-confirmed clinical influenza, and 85% effective in preventing a secondary bacterial complication such as pneumonia or sinusitis.46 The rate of adverse events associated with oseltamivir in that population was similar to that with placebo. Importantly, oseltamivir was not associated with suppression of antibody response to influenza infection or vaccination.
Oseltamivir is very effective in terminating influenza outbreaks in long-term care facilities, even when amantadine fails.4,13 When used in that manner, it is also associated with decreased antibiotic prescriptions, hospitalizations, deaths,47 and substantial cost-savings, even when compared with amantadine, which has a much lower acquisition cost but a higher rate of adverse events, lower efficacy, and individualized dosing requirements.48
Zanamivir prophylaxis for 2 weeks given to unvaccinated residents was 29% effective in preventing all symptomatic influenza confirmed by laboratory testing (by culture, PCR, or seroconversion). It was 65% effective in preventing symptomatic culture-confirmed influenza, 70% effective in preventing febrile, laboratory-confirmed influenza, and 21% effective in preventing complications.49 It was well tolerated in this population and was not associated with the emergence of zanamivir resistance. Zanamivir also provides 61% additional protective efficacy over rimantadine in vaccinated residents of long-term care facilities,50 primarily because of the emergence of viruses resistant to rimantadine. However, up to 50% of elderly people may have difficulty loading and priming the Diskhaler device used to deliver zanamivir.51
While several studies have shown chemoprophylaxis to be effective, it is not possible to ascribe the decrease in cases during an outbreak entirely to antiviral drugs since most studies were not placebo-controlled, and since the natural tendency of outbreaks is to subside.
TREATMENT OF INFLUENZA IN LONG-TERM CARE FACILITIES
A mild illness in someone without a high-risk underlying disease can be managed with symptomatic measures alone, and testing for influenza is at the discretion of the caregiver. Patients who develop a moderate or severe influenza-like illness during an influenza epidemic and who have a high-risk underlying disease or who require hospitalization should be tested for influenza by a sensitive test such as direct fluorescent antigen testing or PCR and should be started on empiric anti-influenza therapy.41
Most elderly residents of long-term care facilities do have comorbid conditions and are thus at high risk of complications of influenza. During influenza outbreaks, clinicians caring for these patients—and for similar patients in outpatient settings, emergency departments, and acute-care hospitals—should consider testing for influenza, even if the patient has only a mild influenza-like illness.
Of note, no randomized treatment study has been done specifically in residents of long-term care facilities.52 Pooled analysis of data from 321 patients at high risk (76 were age 65 or older) in zanamivir treatment studies showed that those who received the drug were sick for 2.5 fewer days than those who received placebo.53 In addition, zanamivir recipients returned to normal activities 3 days sooner, had an 11% reduction in the median total symptom score over 1 to 5 days, and had a rate of complications requiring antibiotics 43% less than placebo recipients. Rates of adverse events were similar in both groups.
A multicenter, randomized, open-label, controlled trial of oseltamivir in the treatment of influenza in high-risk Chinese patients with chronic respiratory diseases (chronic bronchitis, obstructive emphysema, bronchial asthma, or bronchiectasis) or chronic cardiac disease showed that oseltamivir significantly reduced the duration of influenza symptoms by 36.8%, the severity of symptoms by 43.1%, the duration of fever by 45.2%, the time to return to baseline health status by 5 days, and the need for antibiotics, without increasing the total cost of medical care.54
Oseltamivir-resistant isolates of seasonal influenza A/H1N1 do not cause different or more severe symptoms than do oseltamivir-susceptible isolates, and they remain susceptible to zanamivir and the adamantanes. There is no widely available test to differentiate influenza A/H1N1 from A/H3N2 or to test for drug resistance. Influenza B and A/H3N2 remain susceptible to both of the neuraminidase inhibitors, ie, oseltamivir and zanamivir.
All currently circulating influenza A/H3N2 isolates remain resistant to the adamantanes. Influenza B is intrinsically resistant to the adamantanes, and some data show that oseltamivir is less effective against influenza B than against influenza A.
Zanamivir is not available in many pharmacies, is not recommended in patients with underlying reactive airway disease, and requires dexterity for administration.
Amantadine is more likely to cause confusion in the elderly than rimantadine, and it is more susceptible to treatment-emergent antiviral resistance.
No parenteral anti-influenza drug is available, but zanamivir and the experimental drug peramivir are undergoing study for parenteral use. An inhaled, long-acting neuraminidase inhibitor, CS-8958, is currently under study.55 Other agents currently under development include T-705, a polymerase inhibitor, and DAS181, an attachment inhibitor.
Complementary and alternative therapies for influenza are not established. A recent review of 14 randomized, controlled trials56 revealed that the evidence is weak and limited by small sample sizes, poor methodologic quality, or clinically irrelevant effect sizes.
Influenza vaccination of residents of long-term care facilities (and of health care workers at these facilities) is critical for the prevention of influenza in this frail population. Detection, chemoprophylaxis, and treatment have limitations. Infection control measures should be in place during and between outbreaks. Acute care facilities such as emergency departments and hospitals can assist by testing residents of long-term care facilities who present with influenza-like illness during seasonal epidemics of influenza, and by notifying the receiving facility if a patient with influenza would be arriving.
THE EXTENT OF THE PROBLEM
From 5% to 20% of the US population, including residents and health care workers in long-term care facilities, are infected with influenza every year.1,2 The proportion of those infected who develop clinical illness ranges from 40% to 80%. Each influenza illness is associated with an average of 10 days of respiratory sickness, resulting in approximately 3 days of bed confinement or restricted activity. About 30% to 50% of patients with microbiologically confirmed influenza seek medical care, of whom 16% undergo laboratory tests, 17% undergo radiologic tests, and 75% are recommended an over-the-counter drug or are prescribed a medication. Annual influenza-related hospitalizations range from 200,000 to 400,000, depending on seasonal variations in virulence.2,3 Thus, influenza causes 1.3 hospitalizations per 1,000 people, and 25% of these are in people age 65 and older. In the United States, about 40,000 to 60,000 people die of influenza every year, and 90% of these are age 65 and older.4
POLICIES TO FIGHT ANTIVIRAL RESISTANCE
In January 2006, the US Centers for Disease Control and Prevention (CDC) recommended against the use of the adamantanes—ie, amantadine (Symmetrel) and rimantadine (Flumadine)—for the treatment or prevention of influenza because of a high level of resistance in circulating influenza A/H3N2 in the community. Unfortunately, this resistance trend has not reversed since then, with 96% to 100% of influenza A/H3N2 isolates in the United States showing resistance.5 During the 2007–2008 influenza season, influenza A/H1N1 isolates resistant to oseltamivir (Tamiflu) emerged in Europe, particularly in Norway and France. In the United States, influenza A/H1N1 resistance to oseltamivir increased from 0.7% in the 2006–2007 season, to 10.9% in the 2007–2008 season, and to 98% during the 2008–2009 season.6,7
Fortunately, all oseltamivir-resistant isolates remain susceptible to zanamivir (Relenza). In April 2009, a new influenza A/H1N1 variant (previously referred to as swine-origin influenza virus, or SOIV) emerged in North America and spread to many countries worldwide, and the World Health Organization eventually declared a pandemic. This new variant is susceptible to oseltamivir and zanamivir but resistant to the adamantanes. Resistance patterns for future influenza seasons cannot be predicted, but the current extent of influenza resistance and its development over the past decade8 are alarming.
WHY IS INFLUENZA MORE SERIOUS IN LONG-TERM CARE RESIDENTS?
Influenza is usually introduced to long-term care facilities by workers and visitors. Inside, the closed environment and limited mobility of residents facilitate transmission of infection.
The clinical presentation of influenza in residents of long-term care facilities can be subtle, with a blunted febrile response and a decline in mental and functional status.9
Residents commonly have underlying diseases that can be exacerbated by influenza infection, such as congestive heart failure, chronic obstructive lung disease, chronic kidney disease, and dementia. In addition, residents are at higher risk of serious influenza-related complications than are community-dwelling elderly people.
Impaired oral intake, limited dexterity, and altered consciousness may limit treatment options, thus further adversely affecting outcomes. Bacterial pneumonia secondary to influenza has dire consequences in long-term care residents. Rates of hospitalization for pneumonia and influenza and for exacerbation of chronic lung disease are higher in these patients than in their community-dwelling counterparts.10 Death rates are also higher, exceeding 5% during influenza epidemics.11
PREVENTING INFLUENZA IN LONG-TERM CARE FACILITIES
Immunizing residents is essential
Vaccination is the most important measure in preventing influenza in long-term care facilities, and vaccination programs should include residents and health care workers.
Influenza outbreaks are more common in facilities where the rate of immunization is below 80%, as well as in larger facilities (with > 100 beds), suggesting that herd immunity may play a role.12 Unfortunately, influenza vaccine coverage rates vary widely in this patient population—from 57% to 98% in one report.13
The live-attenuated intranasal vaccine is approved only for healthy people under age 50, so most residents of long-term care facilities should receive only the inactivated trivalent intramuscular vaccine.
The effectiveness of a vaccine in preventing influenza depends in part on the adequacy of the match between vaccine serotypes and circulating strains. Studies of all types—randomized, observational, case-control, and cohort studies, as well as meta-analyses and systematic reviews—have shown preventive efficacy rates of influenza vaccination in elderly residents of long-term care facilities to be 23% to 43% for influenza-like illness, 0% to 58% for influenza, 46% for pneumonia, 45% for hospitalization, 42% for death from influenza or pneumonia, and 60% for death from all causes.14,15 Vaccine performance improved after adjustment for confounders.
Obviously, this protection is variable and incomplete, since influenza outbreaks continue to occur even in long-term care facilities in which most residents are vaccinated.13
Vaccination works, despite the controversy
Whether influenza vaccination prevents deaths in elderly people—or how many deaths it prevents—is a subject of ongoing controversy. 16 Even though influenza vaccination coverage in the elderly increased from 15% to 65% since 1980, the specific influenza-related death rate did not decrease.17
It has been suggested that cohort studies may have overestimated the mortality benefit of influenza vaccination in the elderly because of “frailty selection bias” (ie, extremely frail elderly patients are less likely to be vaccinated and are more likely to die for reasons other than influenza than are less frail, vaccinated elderly people) and because of the use of nonspecific end points such as all-cause mortality. 16 Similarly, observational studies may have overestimated the in-hospital mortality benefit of influenza vaccination in older patients with pneumonia occurring outside of influenza season because of the “healthy user effect” (ie, residual confounding by functional and socioeconomic status).18
One nested case-control study in immunocompetent elderly patients showed that influenza vaccination was not associated with a reduced risk of community-acquired pneumonia after adjusting for the presence and severity of comorbidities.19
Since death is a rare end point, it is hard to show a reduction in the death rate with vaccination in randomized controlled studies. The absolute risk reduction in hospitalization and death with vaccination is two to five times higher in elderly patients at high risk than in the healthy elderly.20
The mortality benefit in elderly patients is increased with annual revaccination, with one death prevented for every 300 vaccinations, and one for every 200 revaccinations.21
The response to influenza vaccination is reduced in elderly people because of immune senescence, and higher doses of vaccine have been shown to be more immunogenic and remain safe.22 This enhanced antibody response may be maintained for subsequent antigenically different influenza variants, even against viruses appearing more than 10 years after vaccination. 23 The 2008–2009 influenza vaccine does not protect against the new, pandemic influenza A/H1N1 variant; efforts to produce such a vaccine are under way.
Influenza vaccination is safe. Recent data showed no association between immunization and Guillain-Barré syndrome.24 In fact, influenza itself may be a triggering agent for Guillain-Barré syndrome during major influenza outbreaks.25
DURATION OF SEROPROTECTION IS MORE THAN 6 MONTHS
Every effort should be made to vaccinate residents of long-term care facilities and their caregivers as early as possible in the influenza season to allow an adequate antibody response to develop before the onset of an influenza outbreak.
In the past, there has been concern that the influenza-vaccine-induced antibody response declines more rapidly in the elderly and may fall below seroprotective levels within 4 months of vaccination. But a recent review of 14 published studies argued against that notion, showing that if seroprotection is achieved in the first month after vaccination, it is then maintained for more than 6 months.26 That review also showed that seroconversion varies inversely with preimmunization titers, but not with age.
Moreover, a prospective study27 in 303 residents of a long-term care facility reported that seroprotection did not correlate with nutritional status. In the study, vaccination was very effective despite a high prevalence of nutritional deficiencies. This study also indicated that although an influenza antibody titer greater than 1:40 is considered protective in the general population, long-term care facility residents may require higher levels for effective immunization.
A recent survey showed that a national shortage of influenza vaccine results in decreased immunization rates in residents and in health care workers in long-term care facilities. 28 In that survey, only 2.3% of facilities expressed concern about emergency preparedness, and this has significant implications for a possible influenza pandemic.
VACCINATION PROGRAMS
Standing-order programs have been shown to significantly increase vaccination rates in ambulatory and hospital settings. However, a recent survey showed that only 9% of long-term care facilities use such programs.29 The greatest use of such programs was in government-owned, nonprofit, dual-certified (ie, by both Medicare and Medicaid), and independent long-term care facilities, and in facilities with a lower index of disease acuity. Use varied substantially by state.
The Healthy People 2010 goal of 90% vaccination may be attained by implementing written protocols for documenting immunization—and refusal of immunization—in a consistent place in the patient’s medical record.30
VACCINATION OF WORKERS
When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die31,32 or develop influenza-like illness, particularly when residents themselves are vaccinated.33 Additional benefits include decreased need for consultations with general practitioners or admissions to the hospital for influenza-like illness during periods of moderate influenza activity.32
The policy of mandatory influenza vaccination for health care workers has its proponents34 and opponents.35 When a large tertiary care center adopted mandatory vaccination, vaccination rates increased significantly over time.36 The Centers for Medicare & Medicaid Services recently mandated public reporting of vaccination rates in health care workers and residents of long-term care facilities, and compliance is expected to increase as a result.
BETWEEN INFLUENZA OUTBREAKS
Studies show that hygienic measures prevent transmission of respiratory viruses.37 Therefore, the cornerstones of any program to prevent transmission of influenza and other microorganisms in long-term care facilities are hand hygiene, cough and sneeze etiquette, maintaining a distance of 3 feet between beds, and education of residents and health care workers.
Wash your hands!
Hands should be washed before and after direct contact with patients or with inanimate objects in their immediate vicinity.38 Contrary to popular belief, hands should also be washed before and after wearing gloves, particularly when handling an invasive device for patient care, and after contact with bodily fluids, excretions, mucous membranes, non-intact skin, or wound dressings. Hands should be washed not only between patients but also during care for the same patient if moving from a contaminated body site to a clean site.
Hands should be washed for 15 to 20 seconds using soap and water or an alcohol-based foam or gel; when hands are visibly soiled, only soap and water should be used. It is not known whether adding virucidals or antiseptics to normal hand-washing further decreases the spread of these viruses.
Continuous education, feedback interventions, and patient-awareness programs can improve hand-washing compliance, but they are not sufficient. Easily accessible dispensers for alcohol-based waterless antiseptic foam or gel can significantly improve hand hygiene rates among health care workers.39
DETECTING INFLUENZA OUTBREAKS
Direct fluorescent antigen detection and nucleic acid detection by polymerase chain reaction (PCR) are the tests recommended for early detection of influenza outbreaks in long-term care facilities. Rapid point-of-care tests to detect influenza antigen are only 60% to 70% sensitive,13,40 viral culture takes several days, and serologic diagnosis requires documentation of seroconversion at least 2 weeks apart, and so none of these is adequate for the early detection of an influenza outbreak.
There is no widely available test to differentiate influenza A/H1N1 from A/H3N2, or to test for drug resistance. In addition, the PCR tests widely used today do not differentiate between seasonal influenza A/H1N1 and the new, pandemic influenza A/H1N1 variant. Efforts are under way to produce and distribute such a test.
Controlling influenza outbreaks
An outbreak should be declared when two or more residents develop an influenza-like illness within 72 hours of each other during the influenza season.41 After influenza infection is confirmed by laboratory testing, testing of all residents who subsequently develop an influenza-like illness may not be feasible, and other respiratory viruses may be responsible for mixed outbreaks during influenza epidemics, particularly respiratory syncytial virus.
Roommates of residents who test positive for influenza have a risk of acquiring influenza three times higher than that of residents living in single rooms.42 Obviously, private rooms for all residents would be optimal, but this is not practical in most facilities. “Cohorting” (ie, housing infected residents together) is reasonable, but in this situation they might infect each other with other viruses or with influenza viruses of different serotypes.
When an outbreak occurs, potential solutions include closing subunits of the facility to new admissions, limiting movement of residents and health care workers from affected to unaffected units, and using curtains or other barriers between roommates. But most important during seasonal outbreaks are hand hygiene, proper cough and sneeze etiquette, and 3-foot separation between roommates. A simulation model showed that during an influenza pandemic, preventing ill residents of long-term care facilities from making contact with other residents may reduce rates of illness and death by about 60%.43 If a long-term care facility resident is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient or when entering the room of a resident with confirmed influenza.
CHEMOPROPHYLAXIS DURING INFLUENZA OUTBREAKS
When influenza outbreaks are recognized early in long-term care facilities, appropriate infection control measures and chemoprophylaxis can be started. Chemoprophylaxis should also be considered in residents in whom influenza vaccination is contraindicated, such as those with severe egg allergy.
Rimantadine is preferred over amantadine in residents of long-term care facilities, since amantadine is associated with a much higher rate of adverse events (18.6% vs 1.9%), especially confusion (10.6% vs 0.6%), resulting in more frequent discontinuation (17.3% vs 1.9%).44 In addition, viral resistance to amantadine develops in about 30% of those who receive it. In long-term care facilities, viral resistance occurs not only when it is used for the management of influenza, but also when it is used to treat Parkinson disease.45
Oseltamivir prophylaxis for 6 weeks in influenza-vaccinated, frail elderly residents of long-term care facilities during influenza epidemics was 91% effective in preventing laboratory-confirmed clinical influenza, and 85% effective in preventing a secondary bacterial complication such as pneumonia or sinusitis.46 The rate of adverse events associated with oseltamivir in that population was similar to that with placebo. Importantly, oseltamivir was not associated with suppression of antibody response to influenza infection or vaccination.
Oseltamivir is very effective in terminating influenza outbreaks in long-term care facilities, even when amantadine fails.4,13 When used in that manner, it is also associated with decreased antibiotic prescriptions, hospitalizations, deaths,47 and substantial cost-savings, even when compared with amantadine, which has a much lower acquisition cost but a higher rate of adverse events, lower efficacy, and individualized dosing requirements.48
Zanamivir prophylaxis for 2 weeks given to unvaccinated residents was 29% effective in preventing all symptomatic influenza confirmed by laboratory testing (by culture, PCR, or seroconversion). It was 65% effective in preventing symptomatic culture-confirmed influenza, 70% effective in preventing febrile, laboratory-confirmed influenza, and 21% effective in preventing complications.49 It was well tolerated in this population and was not associated with the emergence of zanamivir resistance. Zanamivir also provides 61% additional protective efficacy over rimantadine in vaccinated residents of long-term care facilities,50 primarily because of the emergence of viruses resistant to rimantadine. However, up to 50% of elderly people may have difficulty loading and priming the Diskhaler device used to deliver zanamivir.51
While several studies have shown chemoprophylaxis to be effective, it is not possible to ascribe the decrease in cases during an outbreak entirely to antiviral drugs since most studies were not placebo-controlled, and since the natural tendency of outbreaks is to subside.
TREATMENT OF INFLUENZA IN LONG-TERM CARE FACILITIES
A mild illness in someone without a high-risk underlying disease can be managed with symptomatic measures alone, and testing for influenza is at the discretion of the caregiver. Patients who develop a moderate or severe influenza-like illness during an influenza epidemic and who have a high-risk underlying disease or who require hospitalization should be tested for influenza by a sensitive test such as direct fluorescent antigen testing or PCR and should be started on empiric anti-influenza therapy.41
Most elderly residents of long-term care facilities do have comorbid conditions and are thus at high risk of complications of influenza. During influenza outbreaks, clinicians caring for these patients—and for similar patients in outpatient settings, emergency departments, and acute-care hospitals—should consider testing for influenza, even if the patient has only a mild influenza-like illness.
Of note, no randomized treatment study has been done specifically in residents of long-term care facilities.52 Pooled analysis of data from 321 patients at high risk (76 were age 65 or older) in zanamivir treatment studies showed that those who received the drug were sick for 2.5 fewer days than those who received placebo.53 In addition, zanamivir recipients returned to normal activities 3 days sooner, had an 11% reduction in the median total symptom score over 1 to 5 days, and had a rate of complications requiring antibiotics 43% less than placebo recipients. Rates of adverse events were similar in both groups.
A multicenter, randomized, open-label, controlled trial of oseltamivir in the treatment of influenza in high-risk Chinese patients with chronic respiratory diseases (chronic bronchitis, obstructive emphysema, bronchial asthma, or bronchiectasis) or chronic cardiac disease showed that oseltamivir significantly reduced the duration of influenza symptoms by 36.8%, the severity of symptoms by 43.1%, the duration of fever by 45.2%, the time to return to baseline health status by 5 days, and the need for antibiotics, without increasing the total cost of medical care.54
Oseltamivir-resistant isolates of seasonal influenza A/H1N1 do not cause different or more severe symptoms than do oseltamivir-susceptible isolates, and they remain susceptible to zanamivir and the adamantanes. There is no widely available test to differentiate influenza A/H1N1 from A/H3N2 or to test for drug resistance. Influenza B and A/H3N2 remain susceptible to both of the neuraminidase inhibitors, ie, oseltamivir and zanamivir.
All currently circulating influenza A/H3N2 isolates remain resistant to the adamantanes. Influenza B is intrinsically resistant to the adamantanes, and some data show that oseltamivir is less effective against influenza B than against influenza A.
Zanamivir is not available in many pharmacies, is not recommended in patients with underlying reactive airway disease, and requires dexterity for administration.
Amantadine is more likely to cause confusion in the elderly than rimantadine, and it is more susceptible to treatment-emergent antiviral resistance.
No parenteral anti-influenza drug is available, but zanamivir and the experimental drug peramivir are undergoing study for parenteral use. An inhaled, long-acting neuraminidase inhibitor, CS-8958, is currently under study.55 Other agents currently under development include T-705, a polymerase inhibitor, and DAS181, an attachment inhibitor.
Complementary and alternative therapies for influenza are not established. A recent review of 14 randomized, controlled trials56 revealed that the evidence is weak and limited by small sample sizes, poor methodologic quality, or clinically irrelevant effect sizes.
- Cifu A, Levinson W. Influenza. JAMA 2000; 284:2847–2849.
- Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics 1996; 9(suppl 3):26–33.
- Fiore AE, Shay DK, Broder K, et al; US Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:1–60.
- Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol 1993; 22:334–340.
- Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 2007; 196:249–257.
- Centers for Disease Control and Prevention (CDC). Update: Influenza activity—United States, September 28, 2008–January 31, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:115–119.
- CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008–09 influenza season. Distributed via Health Alert Network. 2008. www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279. Accessed 7/14/2009.
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 2009; 301:1066–1069.
- Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998; 158:645–650.
- Menec VH, MacWilliams L, Aoki FY. Hospitalization and deaths due to respiratory illnesses during influenza seasons: a comparison of community residents, senior housing residents, and nursing home residents. J Gerontol A Biol Sci Med Sci 2002; 57:M629–M635.
- Kingston BJ, Wright CV. Influenza in the nursing home. Am Fam Physician 2002; 65:75–78.
- Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis 2006; 194(suppl 2):S133–S138.
- Monto AS, Rotthoff J, Teich E, et al. Detection and control of influenza outbreaks in well-vaccinated nursing home populations. Clin Infect Dis 2004; 39:459–464.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366:1165–1174.
- Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; 3:CD004876.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–666.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Hak E, Nordin J, Wei F, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis 2002; 35:370–377.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis 2008; 198:1016–1018.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Paccalin M, Plouzeau C, Bouche G, et al. Lack of correlation between nutritional status and seroprotection against influenza in a long term care facility. Scand J Infect Dis 2006; 38:894–897.
- Mody L, Langa KM, Malani PN. Impact of the 2004–2005 influenza vaccine shortage on immunization practices in long-term care facilities. Infect Control Hosp Epidemiol 2006; 27:383–387.
- Shefer A, McKibben L, Bardenheier B, Bratzler D, Roberts H. Characteristics of long-term care facilities associated with standing order programs to deliver influenza and pneumococcal vaccinations to residents in 13 states. J Am Med Dir Assoc 2005; 6:97–104.
- Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long-term care facilities: the CMS-CDC standing orders program project. J Am Med Dir Assoc 2005; 6:291–299.
- Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000; 355:93–97.
- Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241.
- Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2006; 3:CD005187.
- Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144–1147.
- Finch M. Point: mandatory influenza vaccination for all health care workers? Seven reasons to say “no”. Clin Infect Dis 2006; 42:1141–1143.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Pittet D, Allegranzi B, Sax H, et al; WHO Global Patient Safety Challenge, World Alliance for Patient Safety. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 2006; 6:641–652.
- Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 2000; 160:1017–1021.
- US Centers for Disease Control and Prevention. Influenza-testing and antiviral-agent prescribing practices—Connecticut, Minnesota, New Mexico, and New York, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:61–65.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Drinka PJ, Krause PF, Nest LJ, Goodman BM, Gravenstein S. Risk of acquiring influenza B in a nursing home from a culture-positive roommate. J Am Geriatr Soc 2005; 53:1437.
- Haber MJ, Shay DK, Davis XM, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis 2007; 13:581–589.
- Keyser LA, Karl M, Nafziger AN, Bertino JS. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med 2000; 160:1485–1488.
- Saito R, Oshitani H, Masuda H, Suzuki H. Detection of amantadineresistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol 2002; 40:84–88.
- Peters PH, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031.
- Bowles SK, Kennie N, Ruston L, Simor A, Louie M, Collins V. Influenza outbreak in a long-term-care facility: considerations for pharmacy. Am J Health Syst Pharm 1999; 56:2303–2307.
- Risebrough NA, Bowles SK, Simor AE, McGeer A, Oh PI. Economic evaluation of oseltamivir phosphate for postexposure prophylaxis of influenza in long-term care facilities. J Am Geriatr Soc 2005; 53:444–451.
- Ambrozaitis A, Gravenstein S, van Essen GA, et al. Inhaled zanamivir versus placebo for the prevention of influenza outbreaks in an unvaccinated long-term care population. J Am Med Dir Assoc 2005; 6:367–374.
- Gravenstein S, Drinka P, Osterweil D, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc 2005; 6:359–366.
- Diggory P, Fernandez C, Humphrey A, Jones V, Murphy M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ 2001; 322:577–579.
- Glezen WP. Clinical practice: prevention and treatment of seasonal influenza. N Engl J Med 2008; 359:2579–2585.
- Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001; 161:212–217.
- Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin 2006; 22:75–82.
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009; 48( suppl 1):S3–S13.
- Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am J Med 2007; 120:923–929.
- Cifu A, Levinson W. Influenza. JAMA 2000; 284:2847–2849.
- Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics 1996; 9(suppl 3):26–33.
- Fiore AE, Shay DK, Broder K, et al; US Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:1–60.
- Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol 1993; 22:334–340.
- Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 2007; 196:249–257.
- Centers for Disease Control and Prevention (CDC). Update: Influenza activity—United States, September 28, 2008–January 31, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:115–119.
- CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008–09 influenza season. Distributed via Health Alert Network. 2008. www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279. Accessed 7/14/2009.
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 2009; 301:1066–1069.
- Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998; 158:645–650.
- Menec VH, MacWilliams L, Aoki FY. Hospitalization and deaths due to respiratory illnesses during influenza seasons: a comparison of community residents, senior housing residents, and nursing home residents. J Gerontol A Biol Sci Med Sci 2002; 57:M629–M635.
- Kingston BJ, Wright CV. Influenza in the nursing home. Am Fam Physician 2002; 65:75–78.
- Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis 2006; 194(suppl 2):S133–S138.
- Monto AS, Rotthoff J, Teich E, et al. Detection and control of influenza outbreaks in well-vaccinated nursing home populations. Clin Infect Dis 2004; 39:459–464.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366:1165–1174.
- Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; 3:CD004876.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–666.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178:527–533.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 2008; 372:398–405.
- Hak E, Nordin J, Wei F, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis 2002; 35:370–377.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis 2008; 198:1016–1018.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502.
- Paccalin M, Plouzeau C, Bouche G, et al. Lack of correlation between nutritional status and seroprotection against influenza in a long term care facility. Scand J Infect Dis 2006; 38:894–897.
- Mody L, Langa KM, Malani PN. Impact of the 2004–2005 influenza vaccine shortage on immunization practices in long-term care facilities. Infect Control Hosp Epidemiol 2006; 27:383–387.
- Shefer A, McKibben L, Bardenheier B, Bratzler D, Roberts H. Characteristics of long-term care facilities associated with standing order programs to deliver influenza and pneumococcal vaccinations to residents in 13 states. J Am Med Dir Assoc 2005; 6:97–104.
- Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long-term care facilities: the CMS-CDC standing orders program project. J Am Med Dir Assoc 2005; 6:291–299.
- Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000; 355:93–97.
- Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241.
- Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2006; 3:CD005187.
- Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144–1147.
- Finch M. Point: mandatory influenza vaccination for all health care workers? Seven reasons to say “no”. Clin Infect Dis 2006; 42:1141–1143.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336:77–80.
- Pittet D, Allegranzi B, Sax H, et al; WHO Global Patient Safety Challenge, World Alliance for Patient Safety. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 2006; 6:641–652.
- Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 2000; 160:1017–1021.
- US Centers for Disease Control and Prevention. Influenza-testing and antiviral-agent prescribing practices—Connecticut, Minnesota, New Mexico, and New York, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2008; 57:61–65.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Drinka PJ, Krause PF, Nest LJ, Goodman BM, Gravenstein S. Risk of acquiring influenza B in a nursing home from a culture-positive roommate. J Am Geriatr Soc 2005; 53:1437.
- Haber MJ, Shay DK, Davis XM, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis 2007; 13:581–589.
- Keyser LA, Karl M, Nafziger AN, Bertino JS. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med 2000; 160:1485–1488.
- Saito R, Oshitani H, Masuda H, Suzuki H. Detection of amantadineresistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol 2002; 40:84–88.
- Peters PH, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031.
- Bowles SK, Kennie N, Ruston L, Simor A, Louie M, Collins V. Influenza outbreak in a long-term-care facility: considerations for pharmacy. Am J Health Syst Pharm 1999; 56:2303–2307.
- Risebrough NA, Bowles SK, Simor AE, McGeer A, Oh PI. Economic evaluation of oseltamivir phosphate for postexposure prophylaxis of influenza in long-term care facilities. J Am Geriatr Soc 2005; 53:444–451.
- Ambrozaitis A, Gravenstein S, van Essen GA, et al. Inhaled zanamivir versus placebo for the prevention of influenza outbreaks in an unvaccinated long-term care population. J Am Med Dir Assoc 2005; 6:367–374.
- Gravenstein S, Drinka P, Osterweil D, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc 2005; 6:359–366.
- Diggory P, Fernandez C, Humphrey A, Jones V, Murphy M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ 2001; 322:577–579.
- Glezen WP. Clinical practice: prevention and treatment of seasonal influenza. N Engl J Med 2008; 359:2579–2585.
- Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001; 161:212–217.
- Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin 2006; 22:75–82.
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009; 48( suppl 1):S3–S13.
- Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am J Med 2007; 120:923–929.
KEY POINTS
- When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die or develop influenza-like illness, particularly when residents are also vaccinated.
- Easily accessible dispensers for alcohol-based antiseptic foam or gel can significantly improve hand hygiene rates in health care workers.
- If a patient in a long-term care facility is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient.
- All isolates of pandemic influenza A/H1N1 (previously called swine-origin influenza virus) are susceptible to zanamivir (Relenza) and oseltamivir (Tamiflu), but are resistant to amantadine (Symmetrel) and rimantadine (Flumadine).