User login
How to better identify and manage women with elevated breast cancer risk
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.
CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
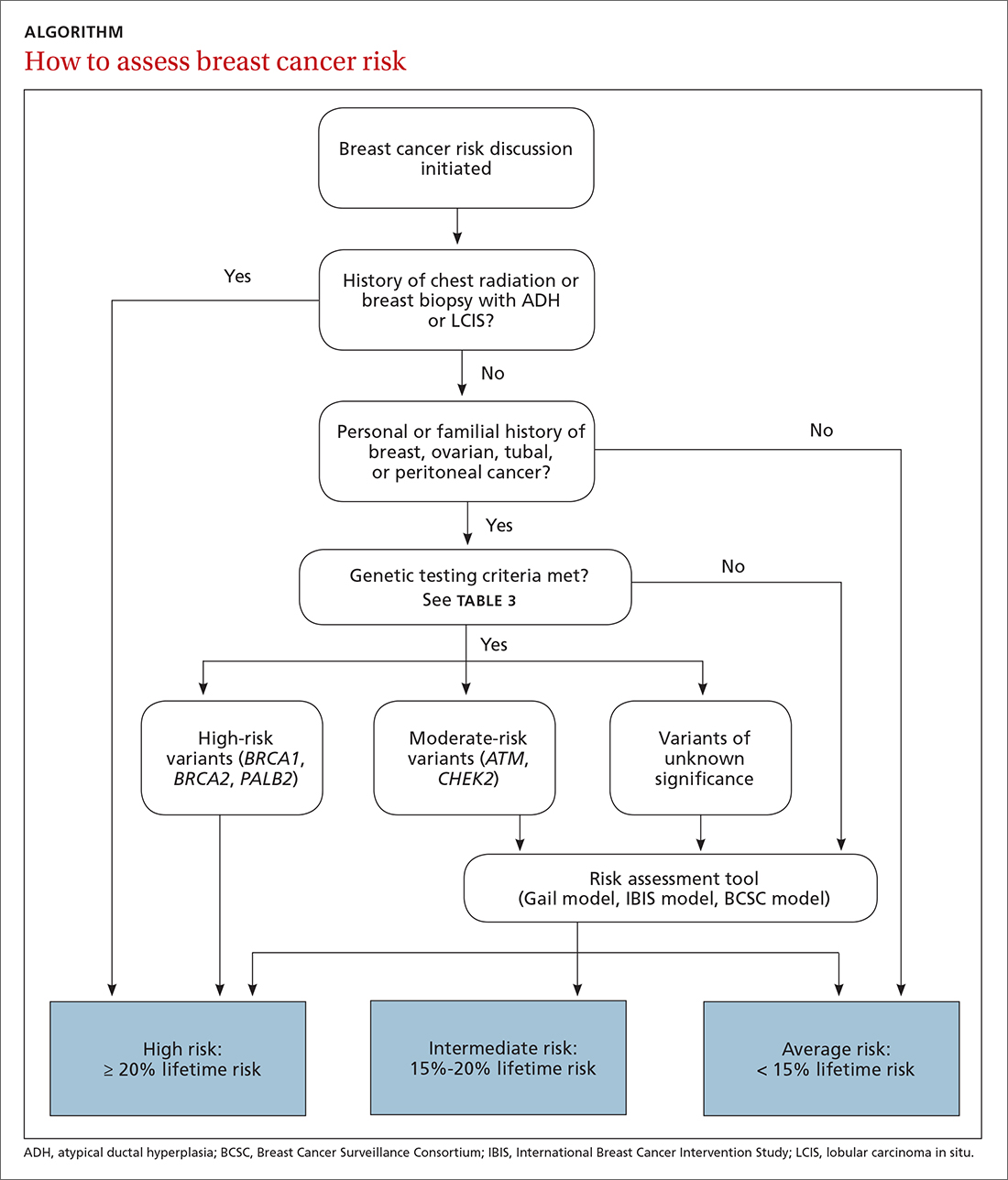
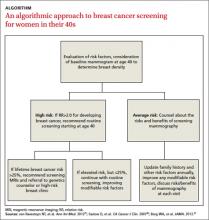
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
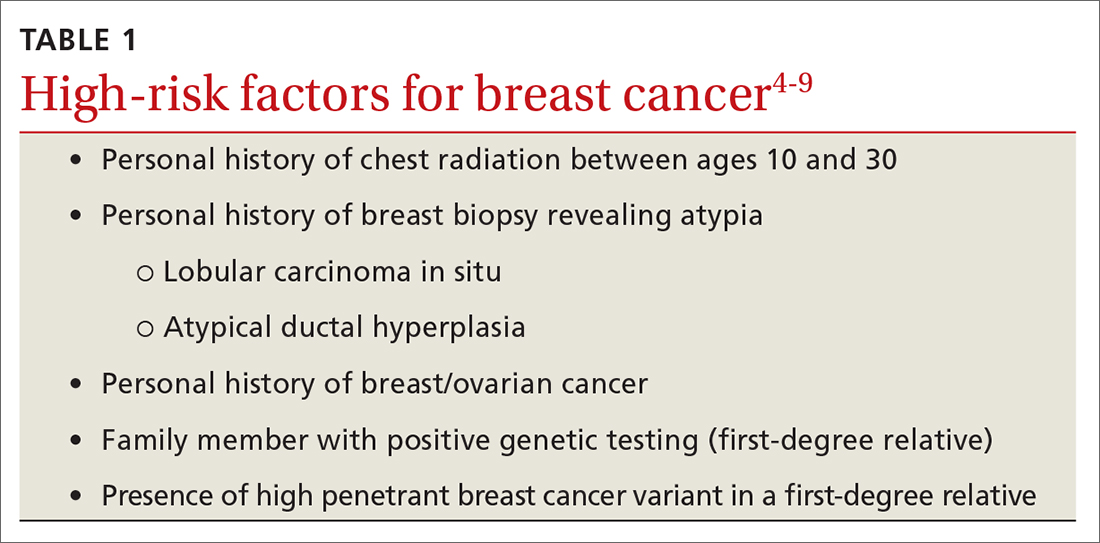
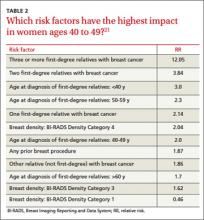
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9
In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
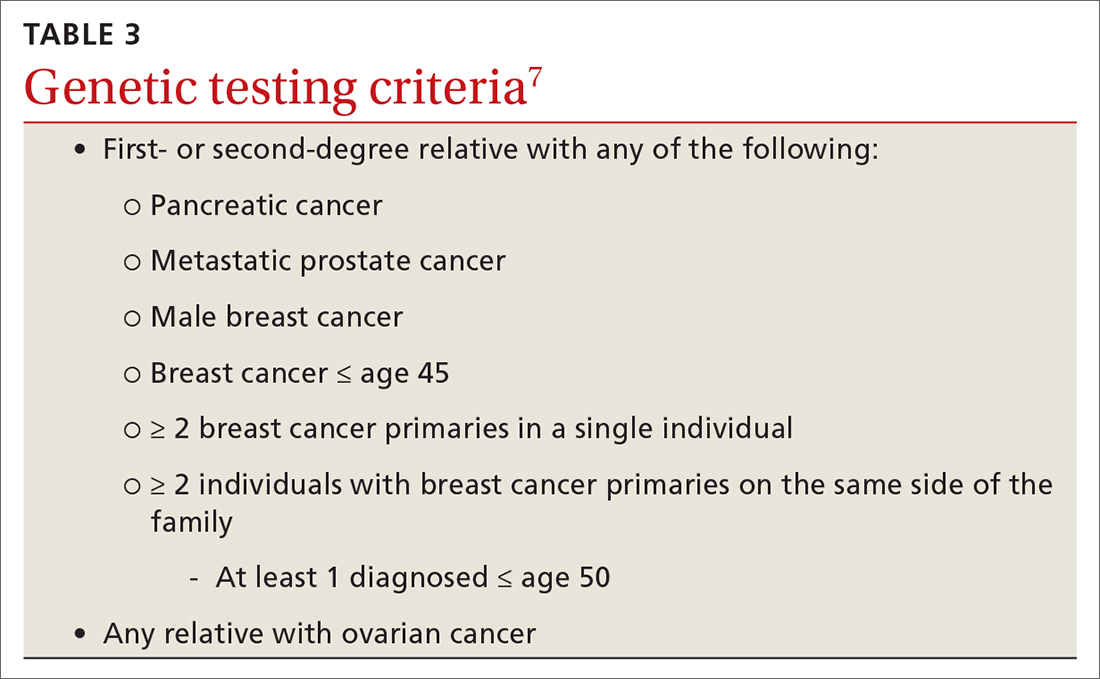
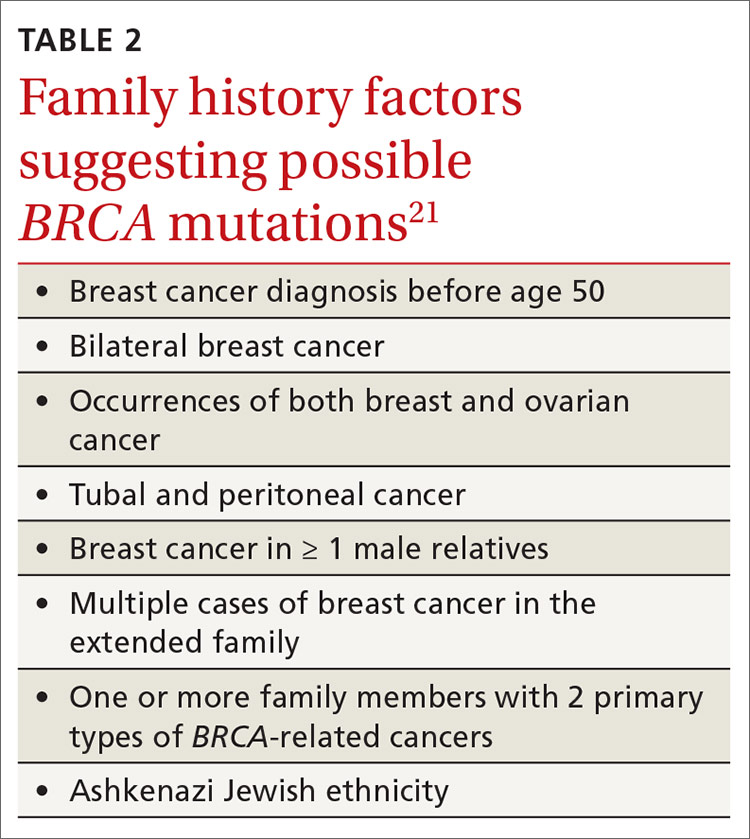
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.
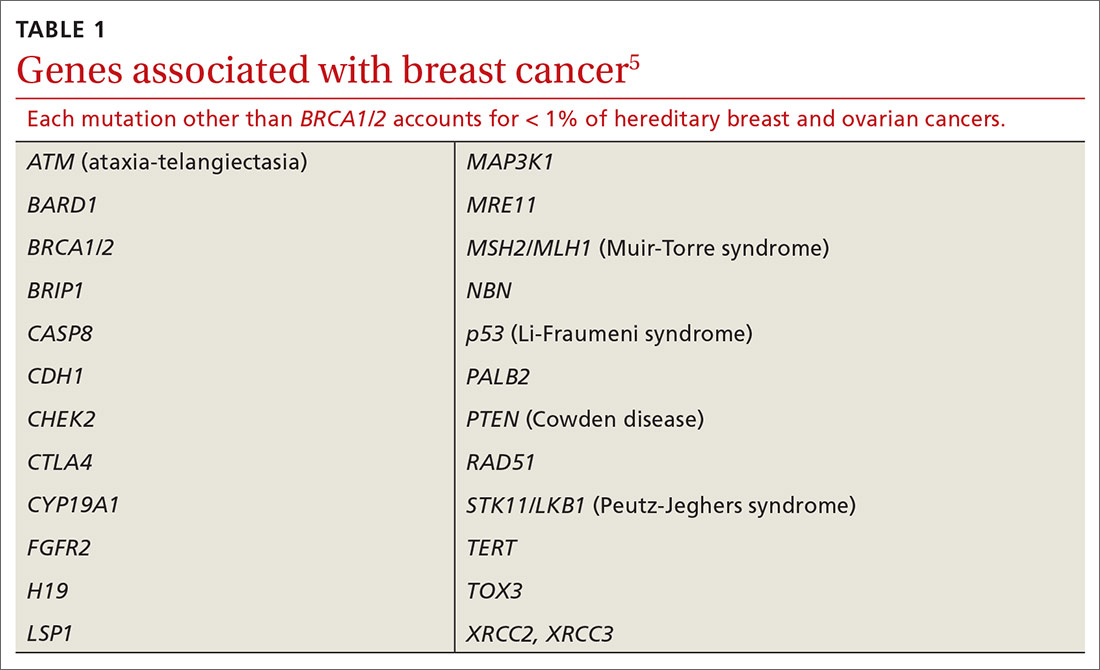
Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
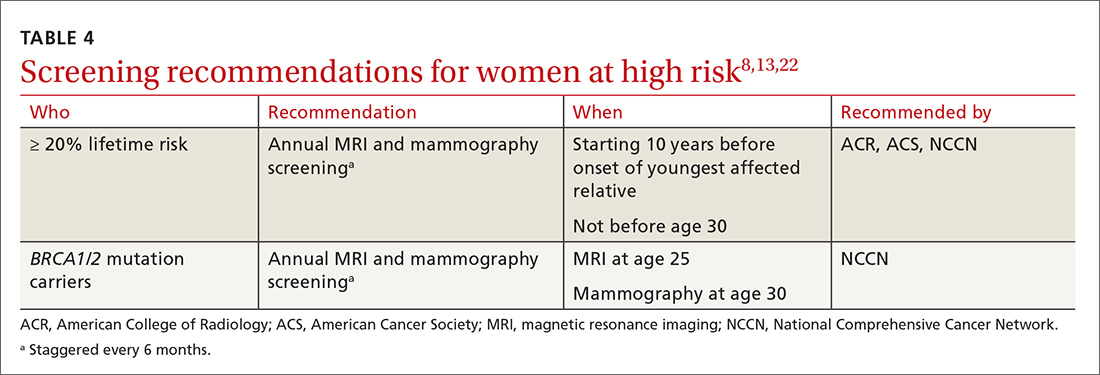
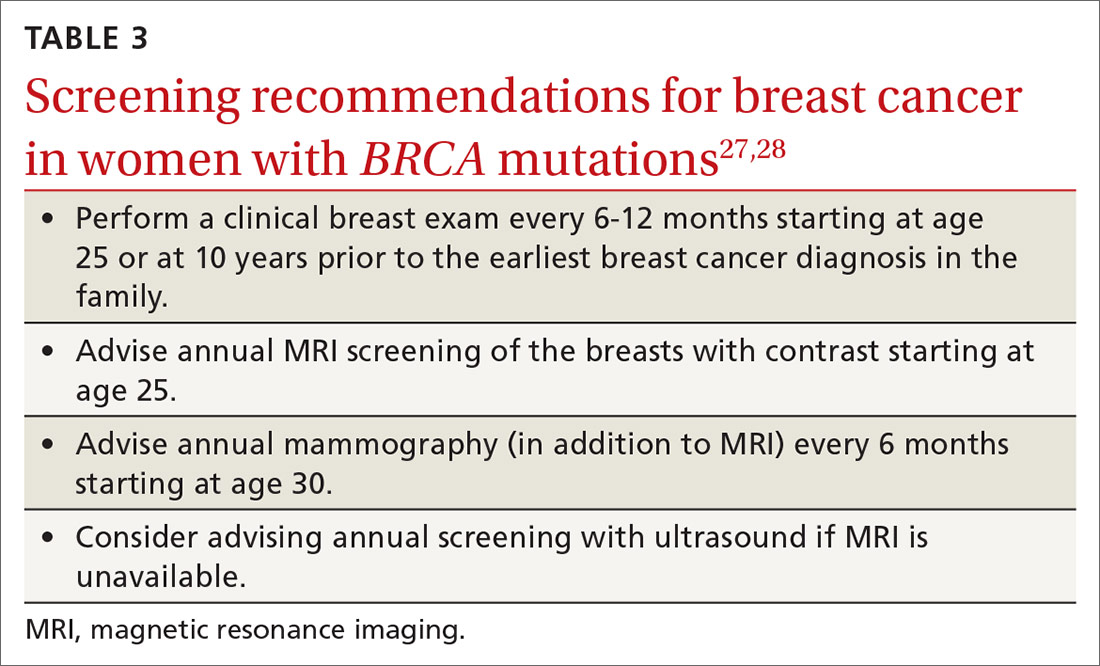
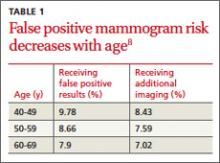
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.
Risk-reduction strategies
Chemoprophylaxis
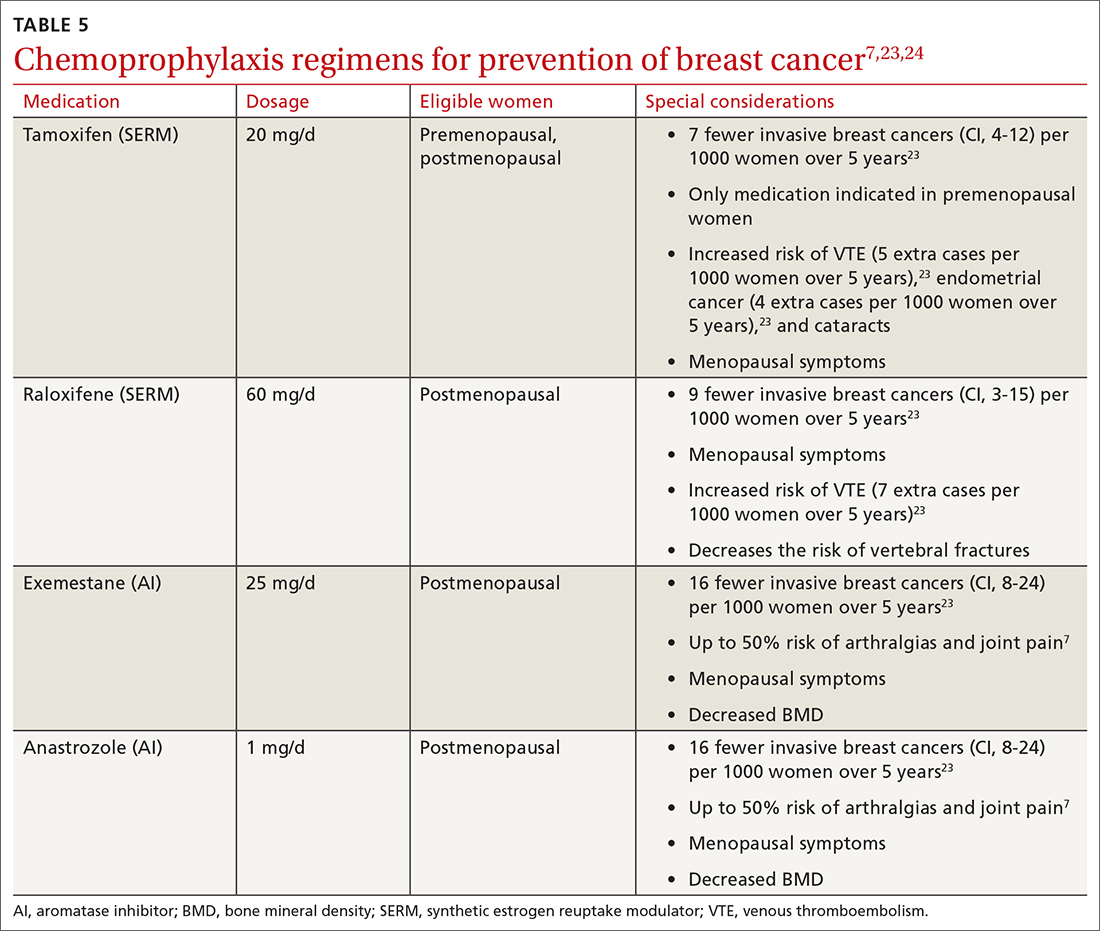
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.
Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.
CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9
In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.
Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.
Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.
Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.
CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9
In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.
Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.
Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.
Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
PRACTICE RECOMMENDATIONS
› Assess breast cancer risk in all women starting at age 35. C
› Perform enhanced screening in all women with a lifetime risk of breast cancer > 20%. A
› Discuss chemoprevention for all women at elevated risk for breast cancer. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Managing a woman with BRCA mutations? Shared decision-making is key
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
PRACTICE RECOMMENDATIONS
› Recommend genetic screening for the BRCA mutation if a patient’s family history includes a breast cancer diagnosis before age 50, occurrences of both breast and ovarian cancers, or other suggestive features. C
› Advise women with the BRCA gene to return for a clinical breast exam every 6 to 12 months starting at age 25, and to start radiologic screening at age 30. C
› Consider recommending bilateral salpingo-oophorectomy to prevent ovarian cancer in women 35 to 40 years of age with a BRCA1 mutation who have completed childbearing. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Menstrual migraines: Which options and when?
› Consider recommending that patients with menstrual migraines try using prophylactic triptans 2 days before the onset of menses. B
› Advise against estrogen-containing contraception for women who have menstrual migraines with aura, who smoke, or are over 35, due to the increased risk of stroke (absolute contraindication). A
› Consider estrogen-containing contraception if the benefits outweigh the risks for women with migraines who are under 35 and do not have aura (relative contraindication). A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mary, a 34-year-old woman, is a new patient to your practice after moving to the area for a job. She has a history of migraine headaches triggered by her menstrual periods. She has been taking combined oral contraceptives (COCs) since she was 17, with a few years off when she had 2 children. Her migraines improved when she was pregnant, but worsened postpartum with each of her daughters to a point where she had to stop breastfeeding at 4 months to go back on the pills.
On the COCs, she gets one or 2 mild-to-moderate headaches a month. She uses sumatriptan for abortive treatment with good relief. She has not missed work in the past 4 years because of her migraines. During the 6 months she was off COCs when trying to get pregnant, she routinely missed 2 to 3 workdays per month due to migraines. She knows when she is going to get a headache because she sees flashing lights in her left visual field. She has no other neurologic symptoms with the headaches, and the character of the headaches has not changed. She is a non-smoker, has normal blood pressure and lipid levels, and no other vascular risk factors.
You review her history and talk to her about the risk of stroke with migraines and with COCs. She is almost 35 years of age and you recommend stopping the COCs due to the risk. She feels strongly that she wants to continue taking the COCs, saying her quality of life is poor when she is off the pills. What should you do?
Migraine headaches are 2 to 3 times more prevalent in women than in men,1 with a lifetime risk of 43% vs 18%, respectively.2 Women account for about 80% of the $1 billion spent each year in the United States in medical expenses and lost work productivity related to migraines.1,2
Clinical patterns suggestive of menstrual migraine. About half of women affected by migraine have menstrually-related migraines (MRM); 3% to 12% have pure menstrual migraines (PMM).3 MRM and PMM are both characterized by the presence of symptoms in at least 2 to 3 consecutive cycles, with symptoms occurring from between 2 days before to 3 days after the onset of menstruation. However, in PMM, symptoms do not occur at any other time of the menstrual cycle; in MRM, symptoms can occur at other times of the cycle. PMM is more likely to respond to hormone therapy than is MRM.
Multiple studies in the United States, Europe, and Asia have noted that migraines related to menses typically last longer, are more severe, less likely to be associated with aura, and more likely to be recurrent and recalcitrant to treatment than non-menstrual migraines.1 TABLE 13 describes diagnostic criteria for migraine without aura.
Possible mechanisms of MRM and PMM. The etiology of migraine is not well understood and is likely multifactorial.4 Incidence of menstrual migraines is related to cyclic changes in female hormones—specifically, the decreasing levels of estrogen that typically happen the week before onset of menses.1 The mechanism is not yet clear, though it is thought that a decline in estrogen levels triggers a decline in serotonin levels, which may lead to cranial vasodilation and sensitization of the trigeminal nerve.5,6 Estrogen decline has also been linked to increased cranial nociception as well as decreased endogenous opioid activity. A study using positron emission tomography found increased activity of serotonergic neurons in migraineurs.7 The evidence that triptans and serotonin receptor agonists are effective in the treatment of migraine also supports the theory that serotonin neurohormonal signaling pathways play a critical role in the pathogenesis of migraines.7
Prevalence patterns point to the role of estrogen. The prevalence of migraines in women increases around puberty, peaks between ages 30 and 40, and decreases after natural menopause.6 Migraine prevalence increases during the first week postpartum, when levels of estrogen and progesterone decrease suddenly and significantly.1 Migraine frequency and intensity decrease in the second and third trimesters of pregnancy and after menopause, when estrogen levels fluctuate significantly less.1 In the Women’s Health Initiative study, women who used hormone replacement therapy (HRT) had a 42% increased risk of migraines compared with women in the study who had never used HRT.8
The association of migraine with female hormones was further supported by a Dutch study of male-to-female transgender patients on estrogen therapy, who had a 26% incidence of migraine, equivalent to the 25% prevalence in natal female controls in this study, compared with just 7.5% in male controls.9 The association between migraine and estrogen withdrawal was investigated in studies performed more than 40 years ago, when women experiencing migraines around the time of menses were given intramuscular estradiol and experienced a delay in symptom onset.10
Abortive and prophylactic treatments: Factors that guide selection
In considering probable menstrual migraine, take a detailed history, review headache diaries if available to determine association of headaches with menses, and perform a thorough neurologic examination. If a diagnosis of menstrual migraine is established, discuss the benefits of different treatment options, both abortive and prophylactic.
For the patient with MRM, take into account frequency of symptoms, predictability of menstruation, medication costs, and comorbidities. Both triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) can be effective treatments for MRM.11 Abortive therapy may be appropriate if a patient prefers to take medication intermittently, if her menses are unpredictable, or if she does not get migraine headaches with every menses. Mefenamic acid, sumatriptan, and rizatriptan have category B recommendations for abortive treatment for menstrual migraines (TABLE 211-16). (For the patient who has regular MRM but unpredictable menses, ovulation predictor kits can be used to help predict the onset of menses, although this would involve additional cost.)
For the patient who has predictable menses and regularly occurring menstrual migraine, some data show that a short-term prophylactic regimen with triptans started 2 to 3 days before the onset of menses and continued for 5 to 7 days total can reduce the incidence of menstrual migraine (TABLE 211-16). At least one high-quality randomized controlled trial (RCT) showed a significant reduction in the incidence of MRM when women were treated prophylactically with frovatriptan, a long-acting triptan with a half-life of approximately 26 hours. Participants received frovatriptan 2.5 mg once a day or twice a day or placebo in the perimenstrual period (day -2 to +3). The incidence of MRM was 52%, 41%, and 67%, respectively (P<.0001).11,17
Another RCT of fair quality examined the effect of naratriptan (half-life 6-8 hours) on the median number of menstrual migraines over 4 menstrual cycles. Women who received 1 mg of naratriptan BID for 2 to 3 days before menses had 2 MRM episodes over the 4 cycles compared with 4 MRM episodes in women who received placebo over the same time period (P<.05).11,18 A third RCT, also of fair quality, compared 2 different regimens of zolmitriptan (half-life 3 hours) with placebo and found that women who received 2.5 mg of zolmitriptan either BID or TID 2 to 3 days prior to menses had a reduction both in frequency of menstrual migraines and in the mean number of breakthrough headaches per menstrual cycle, as well as a reduction in the need for rescue medications.12,19 Triptans are contraindicated in women with a history of cardiac disease or uncontrolled hypertension. Also, triptans can be expensive, precluding their use for some patients.
Evidence is insufficient to recommend for or against the use of NSAIDs as prophylaxis for MRM.11 NSAIDs may be contraindicated in women with a history of peptic ulcer disease or gastrointestinal bleeding. That said, if NSAIDs are not contraindicated, a trial may be reasonable given their low cost.
Data are sparse on the use of vitamins and supplements in treating and preventing PMM or MRM. In one very small double-blind, placebo-controlled study in 1991 (N=24, with efficacy data for 20), participants received a 2-week course of oral magnesium premenstrually. There was a statistically significant reduction in the number of days with headache per month (from 4.7±3.1 days to 2.4±2.2 days; P<.01) and in the total pain index (P<.03).20 A number of studies have demonstrated a correlation between hypomagnesemia and migraine headaches.5,21 The exact mechanism for this relationship is unclear.
Some recent evidence-based reviews have examined the efficacy of nutraceuticals such as magnesium, feverfew, butterbur, coenzyme Q10, and riboflavin on typical migraine, but it is not clear if these results are translatable to the treatment and prophylaxis of menstrual migraine.11,22 A multicenter, single-blind, RCT is underway to examine the efficacy of acupuncture as prophylaxis for MRM.23
Estrogen: Prescribing criteria are strict
Most studies examining the role of exogenous estrogen in reducing menstrual migraines have used topical estrogen (either in patch or gel formulations) in the perimenstrual window (TABLE 211-16). The topical estrogen route has been examined, in particular, as it is presumed to confer less risk of hypercoagulability by avoiding first-pass metabolism. However, there is conflicting evidence on this issue, in particular regarding premenopausal women.13,25 Additionally, many of the studies of estrogen supplementation show a trend toward increased headache once estrogen is discontinued, presumably due to estrogen withdrawal.10,24
That said, one study by MacGregor, et al demonstrates that the use of estradiol gel in the perimenstrual window leads to a 22% reduction in migraine days as well as less severe migrainous symptoms.26 This trend has been demonstrated in other studies examining estrogen supplementation. Of note, the estrogen studies generally are small, older, and of fair to poor quality.11 These studies have used higher doses of estrogen than are commonly used for contraception today because lower doses of estrogen seem not to have the same impact on migraine.5,24
As for COCs, with either normal or extended cycling, data are more mixed than for estrogen supplementation alone; equivalent numbers of women experience improvement, no change, or worsening of their headache pattern. Many women have continuing or worsening migraines in the hormone-free week, and thus most studies have examined the use of extended cycling COCs.5 Sulak, et al demonstrated a statistically significant reduction in headache frequency using extended-cycling COCs, though they did not examine MRM in particular.27 The efficacy of extended-cycling COCs for reduction of MRM was confirmed by Coffee and colleagues with a small but statistically significant decrease in daily headache scores.28
Adverse effects. All estrogen therapies pose the risk of adverse effects (deep vein thrombosis, hypertension, breast tenderness, nausea, etc). Additionally, estrogen supplementation may actually trigger migraines in some women if, when it is discontinued, the blood estrogen level does not remain above a threshold concentration.5,10,24 Estrogen may also trigger migraine in previously headache-free women and may convert migraine without aura into migraine with aura. In either case, therapy should be stopped.5,24
There is promising evidence from 2 small RCTs and one observational trial that progestin-only contraceptive pills (POP) may reduce the frequency and severity of menstrual migraines (TABLE 211-16). More prospective data are needed to confirm this reduction, as there have not been specific studies examining other progesterone-only preparations to prevent menstrual migraines.
Risk of ischemic stroke. Unfortunately, there are population data showing that second-generation and, to a smaller degree, third-generation progestins, which include the desogestrel used in the above studies, may increase the risk of ischemic stroke. This is a particular concern in women who experience migraine.29 Second-generation progestins include levonorgestrel, which is in the levonorgestrel IUD; however, there is no direct evidence for increased ischemic stroke in this particular preparation, and the circulating plasma levels are low. Etonorgestrel, the active ingredient in the contraceptive implant, is a third-generation progestin, though there is no direct evidence of increased ischemic stroke with use of the etonorgestrel implant.
There is a 2- to 4-fold increased risk of ischemic stroke in women who experience migraine.1,5,30 As stated above, this risk may be further increased by some progesterone formulations. But there is also a demonstrable increase in ischemic stroke risk with the use of estrogen, particularly at the higher concentrations that have been shown to prevent MRM.31,32 The overall incidence of ischemic stroke in menstrual-age women is low, which has limited the number of studies with enough power to quantify the absolute increased risk of stroke in conjunction with estrogen use. Nevertheless, exogenous estrogen is thought to increase the risk of ischemic stroke an additional 2- to 4-fold.1,5,29,30,32-34
Women who experience aura. MRM, as it is defined, typically excludes women who experience aura; however, the number of women who experience aura with migraine either in proximity to their menses or throughout the month has not been well documented. The risk of ischemic stroke is higher for women who experience migraine with aura than those with migraine alone, possibly because aura is associated with reduced regional vascular flow leading to hypoperfusion, which sets the stage for a possible ischemic event.4,5,35 The risk of ischemic stroke is amplified further for women who are over 35, who smoke, or who have additional vascular risk factors (eg, uncontrolled hypertension, diabetes, or known vascular or cardiac disease).1,5,34 This array of evidence serves as the basis for the US Medical Eligibility Criteria (USMEC) recommendations36 for hormonal contraceptive use, in particular the absolute contraindication for estrogen use in women who experience migraine with aura (TABLE 336-38).
CASE › Given Mary’s experience of aura with migraine, you talk with her at length about the risk of ischemic stroke and the USMEC recommendation that she absolutely should not be taking COCs. You suggest a progestin-only method of contraception such as depot medroxyprogesterone acetate, a progestin intrauterine device, or a hormonal implant, which may suppress ovulation and decrease her headaches. You discuss that while some women may have headaches with these progestin-only methods, stroke risk is significantly reduced. You also suggest a trial of prophylactic triptans as another possible option.
She says she understands the increased risk of stroke but is still unwilling to try anything else right now due to worries about her quality of life. You decide jointly to refill COCs for 3 months, and you document the shared decision process in the chart. After advising the patient that you will not continue to prescribe COCs for an extended period of time, you also schedule a follow-up appointment to further discuss risks and benefits of migraine treatment and means of reducing other risk factors for stroke.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin, Department of Family Medicine, 1100 Delaplaine Ct, Madison, WI 53715; sbschrag@wisc.edu.
1. MacGregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843-859.
2. Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170-1178.
3. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
4. Garza I, Swanson JW, Cheshire WP Jr, et al. Headache and other craniofacial pain. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1703-1744.
5. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis – part 2. Headache. 2006;46:365-386.
6. Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006; 295:1824-1830.
7. Loder EW. Menstrual migraine: pathophysiology, diagnosis and impact. Headache. 2006;46 (Suppl 2):S55-S60.
8. Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Women’s Health (Larchmt). 2003;12:1027-1036.
9. Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593-594.
10. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355-365.
11. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70:1555-1563.
12. Hu Y, Guan X, Fan L, et al. Triptans in prevention of menstrual migraine: a systematic review with meta-analysis. J Headache Pain. 2013;14:7.
13. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227-1231.
14. Merki-Feld GS, Imthurn B, Langner R, et al. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia. 2013;33:340-346.
15.
16. Morotti M, Remorgida V, Venturini PL, et al. Progestin-only contraception compared with extended combined oral contraceptive in women with migraine without aura: a retrospective pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;183:178-182.
17. Silberstein SD, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261-269.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized double-blind, placebo-controlled study. Headache. 2001;41:248-256.
19. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877-886.
20. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
21. Teigen L, Boes CJ. An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine. Cephalalgia. 2014;35:912-922.
22. Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011;51:484-501.
23. Zhang XZ, Zhang L, Guo J, et al. Acupuncture as prophylaxis for menstrual-related migraine: study protocol for a multicenter randomized controlled trial. Trials. 2013;14:374.
24. MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354-361.
25. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
26. MacGregor EA, Frith A, Ellis J, et al. Prevention of menstrual attacks of migraine: a double blind placebo-controlled crossover study. Neurology. 2006;67:2159-2163.
27. Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47:27-37.
28. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health. 2014;23:310-317.
29. Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65:197-205.
30. Bousser MG. Estrogen, migraine, and stroke. Stroke. 2004;35(Suppl 1):2652-2656.
31. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284:72-78.
32. Donaghy M, Chang CL, Poulter N. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry. 2002;73:747-750.
33. Sacco S, Ricci S, Degan D. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;12:177-189.
34. Merikangas KR, Fenton BT, Cheng SH, et al. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362-368.
35. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
36. Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep
37. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
38. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
› Consider recommending that patients with menstrual migraines try using prophylactic triptans 2 days before the onset of menses. B
› Advise against estrogen-containing contraception for women who have menstrual migraines with aura, who smoke, or are over 35, due to the increased risk of stroke (absolute contraindication). A
› Consider estrogen-containing contraception if the benefits outweigh the risks for women with migraines who are under 35 and do not have aura (relative contraindication). A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mary, a 34-year-old woman, is a new patient to your practice after moving to the area for a job. She has a history of migraine headaches triggered by her menstrual periods. She has been taking combined oral contraceptives (COCs) since she was 17, with a few years off when she had 2 children. Her migraines improved when she was pregnant, but worsened postpartum with each of her daughters to a point where she had to stop breastfeeding at 4 months to go back on the pills.
On the COCs, she gets one or 2 mild-to-moderate headaches a month. She uses sumatriptan for abortive treatment with good relief. She has not missed work in the past 4 years because of her migraines. During the 6 months she was off COCs when trying to get pregnant, she routinely missed 2 to 3 workdays per month due to migraines. She knows when she is going to get a headache because she sees flashing lights in her left visual field. She has no other neurologic symptoms with the headaches, and the character of the headaches has not changed. She is a non-smoker, has normal blood pressure and lipid levels, and no other vascular risk factors.
You review her history and talk to her about the risk of stroke with migraines and with COCs. She is almost 35 years of age and you recommend stopping the COCs due to the risk. She feels strongly that she wants to continue taking the COCs, saying her quality of life is poor when she is off the pills. What should you do?
Migraine headaches are 2 to 3 times more prevalent in women than in men,1 with a lifetime risk of 43% vs 18%, respectively.2 Women account for about 80% of the $1 billion spent each year in the United States in medical expenses and lost work productivity related to migraines.1,2
Clinical patterns suggestive of menstrual migraine. About half of women affected by migraine have menstrually-related migraines (MRM); 3% to 12% have pure menstrual migraines (PMM).3 MRM and PMM are both characterized by the presence of symptoms in at least 2 to 3 consecutive cycles, with symptoms occurring from between 2 days before to 3 days after the onset of menstruation. However, in PMM, symptoms do not occur at any other time of the menstrual cycle; in MRM, symptoms can occur at other times of the cycle. PMM is more likely to respond to hormone therapy than is MRM.
Multiple studies in the United States, Europe, and Asia have noted that migraines related to menses typically last longer, are more severe, less likely to be associated with aura, and more likely to be recurrent and recalcitrant to treatment than non-menstrual migraines.1 TABLE 13 describes diagnostic criteria for migraine without aura.
Possible mechanisms of MRM and PMM. The etiology of migraine is not well understood and is likely multifactorial.4 Incidence of menstrual migraines is related to cyclic changes in female hormones—specifically, the decreasing levels of estrogen that typically happen the week before onset of menses.1 The mechanism is not yet clear, though it is thought that a decline in estrogen levels triggers a decline in serotonin levels, which may lead to cranial vasodilation and sensitization of the trigeminal nerve.5,6 Estrogen decline has also been linked to increased cranial nociception as well as decreased endogenous opioid activity. A study using positron emission tomography found increased activity of serotonergic neurons in migraineurs.7 The evidence that triptans and serotonin receptor agonists are effective in the treatment of migraine also supports the theory that serotonin neurohormonal signaling pathways play a critical role in the pathogenesis of migraines.7
Prevalence patterns point to the role of estrogen. The prevalence of migraines in women increases around puberty, peaks between ages 30 and 40, and decreases after natural menopause.6 Migraine prevalence increases during the first week postpartum, when levels of estrogen and progesterone decrease suddenly and significantly.1 Migraine frequency and intensity decrease in the second and third trimesters of pregnancy and after menopause, when estrogen levels fluctuate significantly less.1 In the Women’s Health Initiative study, women who used hormone replacement therapy (HRT) had a 42% increased risk of migraines compared with women in the study who had never used HRT.8
The association of migraine with female hormones was further supported by a Dutch study of male-to-female transgender patients on estrogen therapy, who had a 26% incidence of migraine, equivalent to the 25% prevalence in natal female controls in this study, compared with just 7.5% in male controls.9 The association between migraine and estrogen withdrawal was investigated in studies performed more than 40 years ago, when women experiencing migraines around the time of menses were given intramuscular estradiol and experienced a delay in symptom onset.10
Abortive and prophylactic treatments: Factors that guide selection
In considering probable menstrual migraine, take a detailed history, review headache diaries if available to determine association of headaches with menses, and perform a thorough neurologic examination. If a diagnosis of menstrual migraine is established, discuss the benefits of different treatment options, both abortive and prophylactic.
For the patient with MRM, take into account frequency of symptoms, predictability of menstruation, medication costs, and comorbidities. Both triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) can be effective treatments for MRM.11 Abortive therapy may be appropriate if a patient prefers to take medication intermittently, if her menses are unpredictable, or if she does not get migraine headaches with every menses. Mefenamic acid, sumatriptan, and rizatriptan have category B recommendations for abortive treatment for menstrual migraines (TABLE 211-16). (For the patient who has regular MRM but unpredictable menses, ovulation predictor kits can be used to help predict the onset of menses, although this would involve additional cost.)
For the patient who has predictable menses and regularly occurring menstrual migraine, some data show that a short-term prophylactic regimen with triptans started 2 to 3 days before the onset of menses and continued for 5 to 7 days total can reduce the incidence of menstrual migraine (TABLE 211-16). At least one high-quality randomized controlled trial (RCT) showed a significant reduction in the incidence of MRM when women were treated prophylactically with frovatriptan, a long-acting triptan with a half-life of approximately 26 hours. Participants received frovatriptan 2.5 mg once a day or twice a day or placebo in the perimenstrual period (day -2 to +3). The incidence of MRM was 52%, 41%, and 67%, respectively (P<.0001).11,17
Another RCT of fair quality examined the effect of naratriptan (half-life 6-8 hours) on the median number of menstrual migraines over 4 menstrual cycles. Women who received 1 mg of naratriptan BID for 2 to 3 days before menses had 2 MRM episodes over the 4 cycles compared with 4 MRM episodes in women who received placebo over the same time period (P<.05).11,18 A third RCT, also of fair quality, compared 2 different regimens of zolmitriptan (half-life 3 hours) with placebo and found that women who received 2.5 mg of zolmitriptan either BID or TID 2 to 3 days prior to menses had a reduction both in frequency of menstrual migraines and in the mean number of breakthrough headaches per menstrual cycle, as well as a reduction in the need for rescue medications.12,19 Triptans are contraindicated in women with a history of cardiac disease or uncontrolled hypertension. Also, triptans can be expensive, precluding their use for some patients.
Evidence is insufficient to recommend for or against the use of NSAIDs as prophylaxis for MRM.11 NSAIDs may be contraindicated in women with a history of peptic ulcer disease or gastrointestinal bleeding. That said, if NSAIDs are not contraindicated, a trial may be reasonable given their low cost.
Data are sparse on the use of vitamins and supplements in treating and preventing PMM or MRM. In one very small double-blind, placebo-controlled study in 1991 (N=24, with efficacy data for 20), participants received a 2-week course of oral magnesium premenstrually. There was a statistically significant reduction in the number of days with headache per month (from 4.7±3.1 days to 2.4±2.2 days; P<.01) and in the total pain index (P<.03).20 A number of studies have demonstrated a correlation between hypomagnesemia and migraine headaches.5,21 The exact mechanism for this relationship is unclear.
Some recent evidence-based reviews have examined the efficacy of nutraceuticals such as magnesium, feverfew, butterbur, coenzyme Q10, and riboflavin on typical migraine, but it is not clear if these results are translatable to the treatment and prophylaxis of menstrual migraine.11,22 A multicenter, single-blind, RCT is underway to examine the efficacy of acupuncture as prophylaxis for MRM.23
Estrogen: Prescribing criteria are strict
Most studies examining the role of exogenous estrogen in reducing menstrual migraines have used topical estrogen (either in patch or gel formulations) in the perimenstrual window (TABLE 211-16). The topical estrogen route has been examined, in particular, as it is presumed to confer less risk of hypercoagulability by avoiding first-pass metabolism. However, there is conflicting evidence on this issue, in particular regarding premenopausal women.13,25 Additionally, many of the studies of estrogen supplementation show a trend toward increased headache once estrogen is discontinued, presumably due to estrogen withdrawal.10,24
That said, one study by MacGregor, et al demonstrates that the use of estradiol gel in the perimenstrual window leads to a 22% reduction in migraine days as well as less severe migrainous symptoms.26 This trend has been demonstrated in other studies examining estrogen supplementation. Of note, the estrogen studies generally are small, older, and of fair to poor quality.11 These studies have used higher doses of estrogen than are commonly used for contraception today because lower doses of estrogen seem not to have the same impact on migraine.5,24
As for COCs, with either normal or extended cycling, data are more mixed than for estrogen supplementation alone; equivalent numbers of women experience improvement, no change, or worsening of their headache pattern. Many women have continuing or worsening migraines in the hormone-free week, and thus most studies have examined the use of extended cycling COCs.5 Sulak, et al demonstrated a statistically significant reduction in headache frequency using extended-cycling COCs, though they did not examine MRM in particular.27 The efficacy of extended-cycling COCs for reduction of MRM was confirmed by Coffee and colleagues with a small but statistically significant decrease in daily headache scores.28
Adverse effects. All estrogen therapies pose the risk of adverse effects (deep vein thrombosis, hypertension, breast tenderness, nausea, etc). Additionally, estrogen supplementation may actually trigger migraines in some women if, when it is discontinued, the blood estrogen level does not remain above a threshold concentration.5,10,24 Estrogen may also trigger migraine in previously headache-free women and may convert migraine without aura into migraine with aura. In either case, therapy should be stopped.5,24
There is promising evidence from 2 small RCTs and one observational trial that progestin-only contraceptive pills (POP) may reduce the frequency and severity of menstrual migraines (TABLE 211-16). More prospective data are needed to confirm this reduction, as there have not been specific studies examining other progesterone-only preparations to prevent menstrual migraines.
Risk of ischemic stroke. Unfortunately, there are population data showing that second-generation and, to a smaller degree, third-generation progestins, which include the desogestrel used in the above studies, may increase the risk of ischemic stroke. This is a particular concern in women who experience migraine.29 Second-generation progestins include levonorgestrel, which is in the levonorgestrel IUD; however, there is no direct evidence for increased ischemic stroke in this particular preparation, and the circulating plasma levels are low. Etonorgestrel, the active ingredient in the contraceptive implant, is a third-generation progestin, though there is no direct evidence of increased ischemic stroke with use of the etonorgestrel implant.
There is a 2- to 4-fold increased risk of ischemic stroke in women who experience migraine.1,5,30 As stated above, this risk may be further increased by some progesterone formulations. But there is also a demonstrable increase in ischemic stroke risk with the use of estrogen, particularly at the higher concentrations that have been shown to prevent MRM.31,32 The overall incidence of ischemic stroke in menstrual-age women is low, which has limited the number of studies with enough power to quantify the absolute increased risk of stroke in conjunction with estrogen use. Nevertheless, exogenous estrogen is thought to increase the risk of ischemic stroke an additional 2- to 4-fold.1,5,29,30,32-34
Women who experience aura. MRM, as it is defined, typically excludes women who experience aura; however, the number of women who experience aura with migraine either in proximity to their menses or throughout the month has not been well documented. The risk of ischemic stroke is higher for women who experience migraine with aura than those with migraine alone, possibly because aura is associated with reduced regional vascular flow leading to hypoperfusion, which sets the stage for a possible ischemic event.4,5,35 The risk of ischemic stroke is amplified further for women who are over 35, who smoke, or who have additional vascular risk factors (eg, uncontrolled hypertension, diabetes, or known vascular or cardiac disease).1,5,34 This array of evidence serves as the basis for the US Medical Eligibility Criteria (USMEC) recommendations36 for hormonal contraceptive use, in particular the absolute contraindication for estrogen use in women who experience migraine with aura (TABLE 336-38).
CASE › Given Mary’s experience of aura with migraine, you talk with her at length about the risk of ischemic stroke and the USMEC recommendation that she absolutely should not be taking COCs. You suggest a progestin-only method of contraception such as depot medroxyprogesterone acetate, a progestin intrauterine device, or a hormonal implant, which may suppress ovulation and decrease her headaches. You discuss that while some women may have headaches with these progestin-only methods, stroke risk is significantly reduced. You also suggest a trial of prophylactic triptans as another possible option.
She says she understands the increased risk of stroke but is still unwilling to try anything else right now due to worries about her quality of life. You decide jointly to refill COCs for 3 months, and you document the shared decision process in the chart. After advising the patient that you will not continue to prescribe COCs for an extended period of time, you also schedule a follow-up appointment to further discuss risks and benefits of migraine treatment and means of reducing other risk factors for stroke.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin, Department of Family Medicine, 1100 Delaplaine Ct, Madison, WI 53715; sbschrag@wisc.edu.
› Consider recommending that patients with menstrual migraines try using prophylactic triptans 2 days before the onset of menses. B
› Advise against estrogen-containing contraception for women who have menstrual migraines with aura, who smoke, or are over 35, due to the increased risk of stroke (absolute contraindication). A
› Consider estrogen-containing contraception if the benefits outweigh the risks for women with migraines who are under 35 and do not have aura (relative contraindication). A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mary, a 34-year-old woman, is a new patient to your practice after moving to the area for a job. She has a history of migraine headaches triggered by her menstrual periods. She has been taking combined oral contraceptives (COCs) since she was 17, with a few years off when she had 2 children. Her migraines improved when she was pregnant, but worsened postpartum with each of her daughters to a point where she had to stop breastfeeding at 4 months to go back on the pills.
On the COCs, she gets one or 2 mild-to-moderate headaches a month. She uses sumatriptan for abortive treatment with good relief. She has not missed work in the past 4 years because of her migraines. During the 6 months she was off COCs when trying to get pregnant, she routinely missed 2 to 3 workdays per month due to migraines. She knows when she is going to get a headache because she sees flashing lights in her left visual field. She has no other neurologic symptoms with the headaches, and the character of the headaches has not changed. She is a non-smoker, has normal blood pressure and lipid levels, and no other vascular risk factors.
You review her history and talk to her about the risk of stroke with migraines and with COCs. She is almost 35 years of age and you recommend stopping the COCs due to the risk. She feels strongly that she wants to continue taking the COCs, saying her quality of life is poor when she is off the pills. What should you do?
Migraine headaches are 2 to 3 times more prevalent in women than in men,1 with a lifetime risk of 43% vs 18%, respectively.2 Women account for about 80% of the $1 billion spent each year in the United States in medical expenses and lost work productivity related to migraines.1,2
Clinical patterns suggestive of menstrual migraine. About half of women affected by migraine have menstrually-related migraines (MRM); 3% to 12% have pure menstrual migraines (PMM).3 MRM and PMM are both characterized by the presence of symptoms in at least 2 to 3 consecutive cycles, with symptoms occurring from between 2 days before to 3 days after the onset of menstruation. However, in PMM, symptoms do not occur at any other time of the menstrual cycle; in MRM, symptoms can occur at other times of the cycle. PMM is more likely to respond to hormone therapy than is MRM.
Multiple studies in the United States, Europe, and Asia have noted that migraines related to menses typically last longer, are more severe, less likely to be associated with aura, and more likely to be recurrent and recalcitrant to treatment than non-menstrual migraines.1 TABLE 13 describes diagnostic criteria for migraine without aura.
Possible mechanisms of MRM and PMM. The etiology of migraine is not well understood and is likely multifactorial.4 Incidence of menstrual migraines is related to cyclic changes in female hormones—specifically, the decreasing levels of estrogen that typically happen the week before onset of menses.1 The mechanism is not yet clear, though it is thought that a decline in estrogen levels triggers a decline in serotonin levels, which may lead to cranial vasodilation and sensitization of the trigeminal nerve.5,6 Estrogen decline has also been linked to increased cranial nociception as well as decreased endogenous opioid activity. A study using positron emission tomography found increased activity of serotonergic neurons in migraineurs.7 The evidence that triptans and serotonin receptor agonists are effective in the treatment of migraine also supports the theory that serotonin neurohormonal signaling pathways play a critical role in the pathogenesis of migraines.7
Prevalence patterns point to the role of estrogen. The prevalence of migraines in women increases around puberty, peaks between ages 30 and 40, and decreases after natural menopause.6 Migraine prevalence increases during the first week postpartum, when levels of estrogen and progesterone decrease suddenly and significantly.1 Migraine frequency and intensity decrease in the second and third trimesters of pregnancy and after menopause, when estrogen levels fluctuate significantly less.1 In the Women’s Health Initiative study, women who used hormone replacement therapy (HRT) had a 42% increased risk of migraines compared with women in the study who had never used HRT.8
The association of migraine with female hormones was further supported by a Dutch study of male-to-female transgender patients on estrogen therapy, who had a 26% incidence of migraine, equivalent to the 25% prevalence in natal female controls in this study, compared with just 7.5% in male controls.9 The association between migraine and estrogen withdrawal was investigated in studies performed more than 40 years ago, when women experiencing migraines around the time of menses were given intramuscular estradiol and experienced a delay in symptom onset.10
Abortive and prophylactic treatments: Factors that guide selection
In considering probable menstrual migraine, take a detailed history, review headache diaries if available to determine association of headaches with menses, and perform a thorough neurologic examination. If a diagnosis of menstrual migraine is established, discuss the benefits of different treatment options, both abortive and prophylactic.
For the patient with MRM, take into account frequency of symptoms, predictability of menstruation, medication costs, and comorbidities. Both triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) can be effective treatments for MRM.11 Abortive therapy may be appropriate if a patient prefers to take medication intermittently, if her menses are unpredictable, or if she does not get migraine headaches with every menses. Mefenamic acid, sumatriptan, and rizatriptan have category B recommendations for abortive treatment for menstrual migraines (TABLE 211-16). (For the patient who has regular MRM but unpredictable menses, ovulation predictor kits can be used to help predict the onset of menses, although this would involve additional cost.)
For the patient who has predictable menses and regularly occurring menstrual migraine, some data show that a short-term prophylactic regimen with triptans started 2 to 3 days before the onset of menses and continued for 5 to 7 days total can reduce the incidence of menstrual migraine (TABLE 211-16). At least one high-quality randomized controlled trial (RCT) showed a significant reduction in the incidence of MRM when women were treated prophylactically with frovatriptan, a long-acting triptan with a half-life of approximately 26 hours. Participants received frovatriptan 2.5 mg once a day or twice a day or placebo in the perimenstrual period (day -2 to +3). The incidence of MRM was 52%, 41%, and 67%, respectively (P<.0001).11,17
Another RCT of fair quality examined the effect of naratriptan (half-life 6-8 hours) on the median number of menstrual migraines over 4 menstrual cycles. Women who received 1 mg of naratriptan BID for 2 to 3 days before menses had 2 MRM episodes over the 4 cycles compared with 4 MRM episodes in women who received placebo over the same time period (P<.05).11,18 A third RCT, also of fair quality, compared 2 different regimens of zolmitriptan (half-life 3 hours) with placebo and found that women who received 2.5 mg of zolmitriptan either BID or TID 2 to 3 days prior to menses had a reduction both in frequency of menstrual migraines and in the mean number of breakthrough headaches per menstrual cycle, as well as a reduction in the need for rescue medications.12,19 Triptans are contraindicated in women with a history of cardiac disease or uncontrolled hypertension. Also, triptans can be expensive, precluding their use for some patients.
Evidence is insufficient to recommend for or against the use of NSAIDs as prophylaxis for MRM.11 NSAIDs may be contraindicated in women with a history of peptic ulcer disease or gastrointestinal bleeding. That said, if NSAIDs are not contraindicated, a trial may be reasonable given their low cost.
Data are sparse on the use of vitamins and supplements in treating and preventing PMM or MRM. In one very small double-blind, placebo-controlled study in 1991 (N=24, with efficacy data for 20), participants received a 2-week course of oral magnesium premenstrually. There was a statistically significant reduction in the number of days with headache per month (from 4.7±3.1 days to 2.4±2.2 days; P<.01) and in the total pain index (P<.03).20 A number of studies have demonstrated a correlation between hypomagnesemia and migraine headaches.5,21 The exact mechanism for this relationship is unclear.
Some recent evidence-based reviews have examined the efficacy of nutraceuticals such as magnesium, feverfew, butterbur, coenzyme Q10, and riboflavin on typical migraine, but it is not clear if these results are translatable to the treatment and prophylaxis of menstrual migraine.11,22 A multicenter, single-blind, RCT is underway to examine the efficacy of acupuncture as prophylaxis for MRM.23
Estrogen: Prescribing criteria are strict
Most studies examining the role of exogenous estrogen in reducing menstrual migraines have used topical estrogen (either in patch or gel formulations) in the perimenstrual window (TABLE 211-16). The topical estrogen route has been examined, in particular, as it is presumed to confer less risk of hypercoagulability by avoiding first-pass metabolism. However, there is conflicting evidence on this issue, in particular regarding premenopausal women.13,25 Additionally, many of the studies of estrogen supplementation show a trend toward increased headache once estrogen is discontinued, presumably due to estrogen withdrawal.10,24
That said, one study by MacGregor, et al demonstrates that the use of estradiol gel in the perimenstrual window leads to a 22% reduction in migraine days as well as less severe migrainous symptoms.26 This trend has been demonstrated in other studies examining estrogen supplementation. Of note, the estrogen studies generally are small, older, and of fair to poor quality.11 These studies have used higher doses of estrogen than are commonly used for contraception today because lower doses of estrogen seem not to have the same impact on migraine.5,24
As for COCs, with either normal or extended cycling, data are more mixed than for estrogen supplementation alone; equivalent numbers of women experience improvement, no change, or worsening of their headache pattern. Many women have continuing or worsening migraines in the hormone-free week, and thus most studies have examined the use of extended cycling COCs.5 Sulak, et al demonstrated a statistically significant reduction in headache frequency using extended-cycling COCs, though they did not examine MRM in particular.27 The efficacy of extended-cycling COCs for reduction of MRM was confirmed by Coffee and colleagues with a small but statistically significant decrease in daily headache scores.28
Adverse effects. All estrogen therapies pose the risk of adverse effects (deep vein thrombosis, hypertension, breast tenderness, nausea, etc). Additionally, estrogen supplementation may actually trigger migraines in some women if, when it is discontinued, the blood estrogen level does not remain above a threshold concentration.5,10,24 Estrogen may also trigger migraine in previously headache-free women and may convert migraine without aura into migraine with aura. In either case, therapy should be stopped.5,24
There is promising evidence from 2 small RCTs and one observational trial that progestin-only contraceptive pills (POP) may reduce the frequency and severity of menstrual migraines (TABLE 211-16). More prospective data are needed to confirm this reduction, as there have not been specific studies examining other progesterone-only preparations to prevent menstrual migraines.
Risk of ischemic stroke. Unfortunately, there are population data showing that second-generation and, to a smaller degree, third-generation progestins, which include the desogestrel used in the above studies, may increase the risk of ischemic stroke. This is a particular concern in women who experience migraine.29 Second-generation progestins include levonorgestrel, which is in the levonorgestrel IUD; however, there is no direct evidence for increased ischemic stroke in this particular preparation, and the circulating plasma levels are low. Etonorgestrel, the active ingredient in the contraceptive implant, is a third-generation progestin, though there is no direct evidence of increased ischemic stroke with use of the etonorgestrel implant.
There is a 2- to 4-fold increased risk of ischemic stroke in women who experience migraine.1,5,30 As stated above, this risk may be further increased by some progesterone formulations. But there is also a demonstrable increase in ischemic stroke risk with the use of estrogen, particularly at the higher concentrations that have been shown to prevent MRM.31,32 The overall incidence of ischemic stroke in menstrual-age women is low, which has limited the number of studies with enough power to quantify the absolute increased risk of stroke in conjunction with estrogen use. Nevertheless, exogenous estrogen is thought to increase the risk of ischemic stroke an additional 2- to 4-fold.1,5,29,30,32-34
Women who experience aura. MRM, as it is defined, typically excludes women who experience aura; however, the number of women who experience aura with migraine either in proximity to their menses or throughout the month has not been well documented. The risk of ischemic stroke is higher for women who experience migraine with aura than those with migraine alone, possibly because aura is associated with reduced regional vascular flow leading to hypoperfusion, which sets the stage for a possible ischemic event.4,5,35 The risk of ischemic stroke is amplified further for women who are over 35, who smoke, or who have additional vascular risk factors (eg, uncontrolled hypertension, diabetes, or known vascular or cardiac disease).1,5,34 This array of evidence serves as the basis for the US Medical Eligibility Criteria (USMEC) recommendations36 for hormonal contraceptive use, in particular the absolute contraindication for estrogen use in women who experience migraine with aura (TABLE 336-38).
CASE › Given Mary’s experience of aura with migraine, you talk with her at length about the risk of ischemic stroke and the USMEC recommendation that she absolutely should not be taking COCs. You suggest a progestin-only method of contraception such as depot medroxyprogesterone acetate, a progestin intrauterine device, or a hormonal implant, which may suppress ovulation and decrease her headaches. You discuss that while some women may have headaches with these progestin-only methods, stroke risk is significantly reduced. You also suggest a trial of prophylactic triptans as another possible option.
She says she understands the increased risk of stroke but is still unwilling to try anything else right now due to worries about her quality of life. You decide jointly to refill COCs for 3 months, and you document the shared decision process in the chart. After advising the patient that you will not continue to prescribe COCs for an extended period of time, you also schedule a follow-up appointment to further discuss risks and benefits of migraine treatment and means of reducing other risk factors for stroke.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin, Department of Family Medicine, 1100 Delaplaine Ct, Madison, WI 53715; sbschrag@wisc.edu.
1. MacGregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843-859.
2. Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170-1178.
3. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
4. Garza I, Swanson JW, Cheshire WP Jr, et al. Headache and other craniofacial pain. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1703-1744.
5. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis – part 2. Headache. 2006;46:365-386.
6. Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006; 295:1824-1830.
7. Loder EW. Menstrual migraine: pathophysiology, diagnosis and impact. Headache. 2006;46 (Suppl 2):S55-S60.
8. Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Women’s Health (Larchmt). 2003;12:1027-1036.
9. Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593-594.
10. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355-365.
11. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70:1555-1563.
12. Hu Y, Guan X, Fan L, et al. Triptans in prevention of menstrual migraine: a systematic review with meta-analysis. J Headache Pain. 2013;14:7.
13. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227-1231.
14. Merki-Feld GS, Imthurn B, Langner R, et al. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia. 2013;33:340-346.
15.
16. Morotti M, Remorgida V, Venturini PL, et al. Progestin-only contraception compared with extended combined oral contraceptive in women with migraine without aura: a retrospective pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;183:178-182.
17. Silberstein SD, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261-269.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized double-blind, placebo-controlled study. Headache. 2001;41:248-256.
19. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877-886.
20. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
21. Teigen L, Boes CJ. An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine. Cephalalgia. 2014;35:912-922.
22. Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011;51:484-501.
23. Zhang XZ, Zhang L, Guo J, et al. Acupuncture as prophylaxis for menstrual-related migraine: study protocol for a multicenter randomized controlled trial. Trials. 2013;14:374.
24. MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354-361.
25. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
26. MacGregor EA, Frith A, Ellis J, et al. Prevention of menstrual attacks of migraine: a double blind placebo-controlled crossover study. Neurology. 2006;67:2159-2163.
27. Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47:27-37.
28. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health. 2014;23:310-317.
29. Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65:197-205.
30. Bousser MG. Estrogen, migraine, and stroke. Stroke. 2004;35(Suppl 1):2652-2656.
31. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284:72-78.
32. Donaghy M, Chang CL, Poulter N. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry. 2002;73:747-750.
33. Sacco S, Ricci S, Degan D. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;12:177-189.
34. Merikangas KR, Fenton BT, Cheng SH, et al. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362-368.
35. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
36. Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep
37. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
38. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
1. MacGregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843-859.
2. Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170-1178.
3. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
4. Garza I, Swanson JW, Cheshire WP Jr, et al. Headache and other craniofacial pain. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1703-1744.
5. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis – part 2. Headache. 2006;46:365-386.
6. Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006; 295:1824-1830.
7. Loder EW. Menstrual migraine: pathophysiology, diagnosis and impact. Headache. 2006;46 (Suppl 2):S55-S60.
8. Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Women’s Health (Larchmt). 2003;12:1027-1036.
9. Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593-594.
10. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355-365.
11. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70:1555-1563.
12. Hu Y, Guan X, Fan L, et al. Triptans in prevention of menstrual migraine: a systematic review with meta-analysis. J Headache Pain. 2013;14:7.
13. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227-1231.
14. Merki-Feld GS, Imthurn B, Langner R, et al. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia. 2013;33:340-346.
15.
16. Morotti M, Remorgida V, Venturini PL, et al. Progestin-only contraception compared with extended combined oral contraceptive in women with migraine without aura: a retrospective pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;183:178-182.
17. Silberstein SD, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261-269.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized double-blind, placebo-controlled study. Headache. 2001;41:248-256.
19. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877-886.
20. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
21. Teigen L, Boes CJ. An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine. Cephalalgia. 2014;35:912-922.
22. Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011;51:484-501.
23. Zhang XZ, Zhang L, Guo J, et al. Acupuncture as prophylaxis for menstrual-related migraine: study protocol for a multicenter randomized controlled trial. Trials. 2013;14:374.
24. MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354-361.
25. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
26. MacGregor EA, Frith A, Ellis J, et al. Prevention of menstrual attacks of migraine: a double blind placebo-controlled crossover study. Neurology. 2006;67:2159-2163.
27. Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47:27-37.
28. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health. 2014;23:310-317.
29. Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65:197-205.
30. Bousser MG. Estrogen, migraine, and stroke. Stroke. 2004;35(Suppl 1):2652-2656.
31. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284:72-78.
32. Donaghy M, Chang CL, Poulter N. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry. 2002;73:747-750.
33. Sacco S, Ricci S, Degan D. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;12:177-189.
34. Merikangas KR, Fenton BT, Cheng SH, et al. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362-368.
35. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
36. Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep
37. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
38. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
Abnormal Uterine Bleeding in Reproductive-Aged Women
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, sbschrag@wisc.edu.
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, sbschrag@wisc.edu.
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, sbschrag@wisc.edu.
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
Mammography at age 40? A risk-based strategy
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
Emergency contraception: An underutilized resource
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
What’s the best treatment for gestational diabetes?
There is no single approach to glycemic control that is better than another for reducing neonatal mortality and morbidity. Glycemic control—regardless of whether it involves diet, glyburide, or insulin—leads to fewer cases of shoulder dystocia, hyperbilirubinemia requiring phototherapy, nerve palsy, bone fracture, being large for gestational age, and fetal macrosomia (strength of recommendation: A).
Customize the intervention
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Achieving solid glucose control for patients with gestational diabetes should be easy—most patients are healthy and motivated to do what is best for their babies. But a new diagnosis and blood sugar monitoring requirements can be daunting. Lifestyle changes and medications can quickly add to the sense of being overwhelmed. Fortunately, whatever brings down the blood sugar will do as therapy, so the patient can negotiate with her doctor to develop an intervention—be it diet, exercise, oral medications, insulin, or a combination—that works for her.
Evidence summary
Findings from 2 studies support the notion that the treatment of gestational diabetes decreases neonatal morbidity and mortality (TABLE).1,2 Both studies found a decrease in neonatal morbidity and mortality for those patients treated either with diet or insulin. One study found a higher rate of NICU admission in the treatment group, but the authors attributed this to physician awareness of the patient having gestational diabetes.1
TABLE
Treatment of gestational diabetes reduces neonatal morbidity and mortality
| TYPE OF STUDY | CONTROL(S) | INTERVENTION | MEONATAL MORBIDITY AND MORTALITY | ADMISSIONS TO NICU | NNT |
|---|---|---|---|---|---|
| RCT1 | GDM routine care (N=510) | GDM treated with diet or insulin (N=490) | Control: 4% Intervention: 1% | 71% diet and insulin vs 61% routine care NNH: 100 | 34 |
| Cohort2 | 1) No GDM (N=1110) | GDM treated diet or insulin (N=1110) | Control 1: 11% Control 2: 59% | Not reported | 2* |
| 2) GDM not treated (due to late entry to care) (N=555) | Intervention: 15% | ||||
| *Compared with patients presenting late. | |||||
| GDM, gestational diabetes mellitus; NNH, number needed to harm; NNT, number needed to treat. | |||||
Glyburide vs insulin
A high-quality randomized controlled trial comparing glyburide with insulin among 404 women found no difference in maternal hypoglycemia, neonatal mortality, or neonatal features and outcomes (including birthweight, NICU admissions, hyperbilirubinemia, and hypoglycemia; P ≥.25).3 Although this was a fairly large trial, it may have been underpowered since it found small differences in such rare outcomes.
Similarly, a retrospective study comparing glyburide with insulin in 584 women found little difference between the 2 approaches. Women in the glyburide group had better glycemic control, but the women in the insulin group started with higher initial blood sugars.4 The glyburide group had fewer NICU admissions than the insulin group (number needed to treat [NNT]=11), but higher rates of jaundice (number needed to harm [NNH]=25), pre-eclampsia (NNH=17), and maternal hypoglycemia (NNH=8). All other neonatal outcomes were similar between groups.
Diet alone vs diet + insulin
A meta-analysis combined 6 RCTs comparing diet alone with diet plus insulin in a total of 1281 women.5 Insulin was moderately superior to diet in preventing fetal macrosomia (NNT=11; 95% confidence interval, 6–36), but not in rates of hypoglycemia, hypocalcemia, hyperbilirubinemia, or congenital malformations.
Recommendations from others
The American Diabetes Association (ADA) recommends that women diagnosed with gestational diabetes by a 3-hour glucose tolerance test receive nutritional counseling from a registered dietician. The ADA also recommends insulin therapy if diet is unsuccessful in achieving fasting glucose <105 mg/dL, 1-hour postprandial <155 mg/dL, or 2-hour postprandial <130 mg/dL.6
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of diet or insulin to achieve 1-hour postprandial blood sugar of 130 mg/dL.7 Both ADA and ACOG indicate that further studies are needed to establish the safety of glyburide before general use can be recommended.
1. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-2486.
2. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol 2005;192:989-997.
3. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134-1138.
4. Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol 2005;193:118-124.
5. Giuffrida FM, Castro AA, Atallah AN, Dib SA. Diet plus insulin compared to diet alone in the treatment of gestational diabetes mellitus: A systematic review. Braz J Med Biol Res 2003;36:1297-1300.
6. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S88-S90.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
There is no single approach to glycemic control that is better than another for reducing neonatal mortality and morbidity. Glycemic control—regardless of whether it involves diet, glyburide, or insulin—leads to fewer cases of shoulder dystocia, hyperbilirubinemia requiring phototherapy, nerve palsy, bone fracture, being large for gestational age, and fetal macrosomia (strength of recommendation: A).
Customize the intervention
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Achieving solid glucose control for patients with gestational diabetes should be easy—most patients are healthy and motivated to do what is best for their babies. But a new diagnosis and blood sugar monitoring requirements can be daunting. Lifestyle changes and medications can quickly add to the sense of being overwhelmed. Fortunately, whatever brings down the blood sugar will do as therapy, so the patient can negotiate with her doctor to develop an intervention—be it diet, exercise, oral medications, insulin, or a combination—that works for her.
Evidence summary
Findings from 2 studies support the notion that the treatment of gestational diabetes decreases neonatal morbidity and mortality (TABLE).1,2 Both studies found a decrease in neonatal morbidity and mortality for those patients treated either with diet or insulin. One study found a higher rate of NICU admission in the treatment group, but the authors attributed this to physician awareness of the patient having gestational diabetes.1
TABLE
Treatment of gestational diabetes reduces neonatal morbidity and mortality
| TYPE OF STUDY | CONTROL(S) | INTERVENTION | MEONATAL MORBIDITY AND MORTALITY | ADMISSIONS TO NICU | NNT |
|---|---|---|---|---|---|
| RCT1 | GDM routine care (N=510) | GDM treated with diet or insulin (N=490) | Control: 4% Intervention: 1% | 71% diet and insulin vs 61% routine care NNH: 100 | 34 |
| Cohort2 | 1) No GDM (N=1110) | GDM treated diet or insulin (N=1110) | Control 1: 11% Control 2: 59% | Not reported | 2* |
| 2) GDM not treated (due to late entry to care) (N=555) | Intervention: 15% | ||||
| *Compared with patients presenting late. | |||||
| GDM, gestational diabetes mellitus; NNH, number needed to harm; NNT, number needed to treat. | |||||
Glyburide vs insulin
A high-quality randomized controlled trial comparing glyburide with insulin among 404 women found no difference in maternal hypoglycemia, neonatal mortality, or neonatal features and outcomes (including birthweight, NICU admissions, hyperbilirubinemia, and hypoglycemia; P ≥.25).3 Although this was a fairly large trial, it may have been underpowered since it found small differences in such rare outcomes.
Similarly, a retrospective study comparing glyburide with insulin in 584 women found little difference between the 2 approaches. Women in the glyburide group had better glycemic control, but the women in the insulin group started with higher initial blood sugars.4 The glyburide group had fewer NICU admissions than the insulin group (number needed to treat [NNT]=11), but higher rates of jaundice (number needed to harm [NNH]=25), pre-eclampsia (NNH=17), and maternal hypoglycemia (NNH=8). All other neonatal outcomes were similar between groups.
Diet alone vs diet + insulin
A meta-analysis combined 6 RCTs comparing diet alone with diet plus insulin in a total of 1281 women.5 Insulin was moderately superior to diet in preventing fetal macrosomia (NNT=11; 95% confidence interval, 6–36), but not in rates of hypoglycemia, hypocalcemia, hyperbilirubinemia, or congenital malformations.
Recommendations from others
The American Diabetes Association (ADA) recommends that women diagnosed with gestational diabetes by a 3-hour glucose tolerance test receive nutritional counseling from a registered dietician. The ADA also recommends insulin therapy if diet is unsuccessful in achieving fasting glucose <105 mg/dL, 1-hour postprandial <155 mg/dL, or 2-hour postprandial <130 mg/dL.6
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of diet or insulin to achieve 1-hour postprandial blood sugar of 130 mg/dL.7 Both ADA and ACOG indicate that further studies are needed to establish the safety of glyburide before general use can be recommended.
There is no single approach to glycemic control that is better than another for reducing neonatal mortality and morbidity. Glycemic control—regardless of whether it involves diet, glyburide, or insulin—leads to fewer cases of shoulder dystocia, hyperbilirubinemia requiring phototherapy, nerve palsy, bone fracture, being large for gestational age, and fetal macrosomia (strength of recommendation: A).
Customize the intervention
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Achieving solid glucose control for patients with gestational diabetes should be easy—most patients are healthy and motivated to do what is best for their babies. But a new diagnosis and blood sugar monitoring requirements can be daunting. Lifestyle changes and medications can quickly add to the sense of being overwhelmed. Fortunately, whatever brings down the blood sugar will do as therapy, so the patient can negotiate with her doctor to develop an intervention—be it diet, exercise, oral medications, insulin, or a combination—that works for her.
Evidence summary
Findings from 2 studies support the notion that the treatment of gestational diabetes decreases neonatal morbidity and mortality (TABLE).1,2 Both studies found a decrease in neonatal morbidity and mortality for those patients treated either with diet or insulin. One study found a higher rate of NICU admission in the treatment group, but the authors attributed this to physician awareness of the patient having gestational diabetes.1
TABLE
Treatment of gestational diabetes reduces neonatal morbidity and mortality
| TYPE OF STUDY | CONTROL(S) | INTERVENTION | MEONATAL MORBIDITY AND MORTALITY | ADMISSIONS TO NICU | NNT |
|---|---|---|---|---|---|
| RCT1 | GDM routine care (N=510) | GDM treated with diet or insulin (N=490) | Control: 4% Intervention: 1% | 71% diet and insulin vs 61% routine care NNH: 100 | 34 |
| Cohort2 | 1) No GDM (N=1110) | GDM treated diet or insulin (N=1110) | Control 1: 11% Control 2: 59% | Not reported | 2* |
| 2) GDM not treated (due to late entry to care) (N=555) | Intervention: 15% | ||||
| *Compared with patients presenting late. | |||||
| GDM, gestational diabetes mellitus; NNH, number needed to harm; NNT, number needed to treat. | |||||
Glyburide vs insulin
A high-quality randomized controlled trial comparing glyburide with insulin among 404 women found no difference in maternal hypoglycemia, neonatal mortality, or neonatal features and outcomes (including birthweight, NICU admissions, hyperbilirubinemia, and hypoglycemia; P ≥.25).3 Although this was a fairly large trial, it may have been underpowered since it found small differences in such rare outcomes.
Similarly, a retrospective study comparing glyburide with insulin in 584 women found little difference between the 2 approaches. Women in the glyburide group had better glycemic control, but the women in the insulin group started with higher initial blood sugars.4 The glyburide group had fewer NICU admissions than the insulin group (number needed to treat [NNT]=11), but higher rates of jaundice (number needed to harm [NNH]=25), pre-eclampsia (NNH=17), and maternal hypoglycemia (NNH=8). All other neonatal outcomes were similar between groups.
Diet alone vs diet + insulin
A meta-analysis combined 6 RCTs comparing diet alone with diet plus insulin in a total of 1281 women.5 Insulin was moderately superior to diet in preventing fetal macrosomia (NNT=11; 95% confidence interval, 6–36), but not in rates of hypoglycemia, hypocalcemia, hyperbilirubinemia, or congenital malformations.
Recommendations from others
The American Diabetes Association (ADA) recommends that women diagnosed with gestational diabetes by a 3-hour glucose tolerance test receive nutritional counseling from a registered dietician. The ADA also recommends insulin therapy if diet is unsuccessful in achieving fasting glucose <105 mg/dL, 1-hour postprandial <155 mg/dL, or 2-hour postprandial <130 mg/dL.6
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of diet or insulin to achieve 1-hour postprandial blood sugar of 130 mg/dL.7 Both ADA and ACOG indicate that further studies are needed to establish the safety of glyburide before general use can be recommended.
1. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-2486.
2. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol 2005;192:989-997.
3. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134-1138.
4. Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol 2005;193:118-124.
5. Giuffrida FM, Castro AA, Atallah AN, Dib SA. Diet plus insulin compared to diet alone in the treatment of gestational diabetes mellitus: A systematic review. Braz J Med Biol Res 2003;36:1297-1300.
6. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S88-S90.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
1. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-2486.
2. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol 2005;192:989-997.
3. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134-1138.
4. Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol 2005;193:118-124.
5. Giuffrida FM, Castro AA, Atallah AN, Dib SA. Diet plus insulin compared to diet alone in the treatment of gestational diabetes mellitus: A systematic review. Braz J Med Biol Res 2003;36:1297-1300.
6. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S88-S90.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best treatment for CIN 2 or 3?
Excision or ablation of the transformation zone are equally effective for treating an initial diagnosis of cervical intraepithelial neoplasia (CIN) grades 2 or 3 in women with a satisfactory colposcopy and no suggestion of microinvasive or invasive disease (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]).
Laser or loop electrosurgical excision procedure (LEEP) are the preferred treatment methods for recurrent CIN 2 and CIN 3 (SOR: B, based on clinical trials without randomization).
For women with an unsatisfactory colposcopy or suspicion of invasive disease, a diagnostic excisional procedure is recommended (SOR: C, based on consensus guidelines).
Observation or deferred treatment may be acceptable for CIN 2 in adolescents with satisfactory colposcopy and negative endocervical sampling (SOR: C, based on consensus guidelines).
Limit diagnostic excisional procedures in pregnancy to cases where suspicion of invasive cancer is high (SOR: C, based on consensus guidelines).
LEEP provides tissue for examination and a short recovery time
Timothy Huber, MD
Oroville, Calif
Close follow-up of observable disease and aggressive intervention continue to drive down the number of cervical cancer deaths each year.
It remains to be seen what the true effect of the HPV vaccine will be, although the presumed result will be a dramatic decline in high-grade lesions (CIN 2 and 3), carcinoma in situ, and invasive disease.
When intervention is necessary, my preferred method is LEEP because it provides tissue for examination and the recovery time is short.
Cryotherapy is an acceptable alternative, but the 4 to 8 weeks of leukorrhea and the lack of a tissue diagnosis often make it a less desirable option for patient and physician.
Evidence summary
Morbidity profile makes LEEP appear best
Similar efficacy. All 7 available surgical techniques were found to have similar efficacy, in a 2005 Cochrane review of 28 randomized trials.1 Resolution of CIN 2 or 3 lesions was 77% to 98%, using knife cone biopsy, laser conization, loop excision, laser ablation, cryotherapy, or 2 techniques not used commonly in the US, cold coagulation and radical diathermy. Surgical techniques were tested in various combinations, but no trial compared all of the techniques with one another. Most studies were underpowered, limiting the results.
Pap test: Good, but underused We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality. Yet, even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. The Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab. 9
Dr. J. Thomas cox, university of California. Member, American Cancer society Cervical guidelines Committee, the 2002 Bethesda Workshop; ACS HPV vaccine Advisory Committee; author, ASCCP guidelines Committee
Each year in the united states approximately 500,000 women are diagnosed with high-grade cervical cancer precursor lesions, CIN 2 and CIN III.4 If left untreated, 22% of CIN 2 lesions progress to carcinoma in situ or invasive cervical cancer, 43% regress, and 35% persist at the same level. Fourteen percent of untreated CIN 3 lesions progress, 32% regress, and 56% persist at the same level.
HPV vaccine: Won’t replace prevention or protection Although an effective vaccine is a major advance in the prevention of genital HPV and cervical cancer, it will not replace other prevention strategies, such as cervical cancer screening for women or protective sexual behaviors. Women should continue to get Pap tests as a safeguard against cervical cancer.
Dr. Anne Schuchat, Director, CDC National Center for Immunization and respiratory Diseases, June 29, 2006 press release (www.cdc.gov/od/oc/media/pressrel/r060629.htm)
The HPV vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18, was approved by the us Food and Drug Administration in June 2006 for use in females 9 to 26 years oof age. Shortly after, the Advisory Committee on Immunization Practices issued guidelines, stating that vaccination is recommended for all women <26 years of age. (www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm).
HPV testing: Adjunct to cytology The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are underway. 10
Dr. Thomas C. Wright, Columbia university. Author, 2001 Consensus guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim guidance for use of HPV DNA testing for Primary screening, and the 2001 Bethesda system
Least morbidity with LEEP. Morbidity was compared with one-on-one trials of different techniques ( TABLE 1 ). The review noted that LEEP has the least morbidity (such as hemorrhage, infection, cervical stenosis, and midtrimester pregnancy loss) while providing the most reliable histology by excising tissue without causing thermal artifact ( FIGURE ).
Higher rate of hemorrhage with cone biopsy. Another systematic review of 21 controlled trials comparing treatments for CIN 2 or 3 found a similar efficacy of all the modalities, including cone biopsy, cryotherapy, laser ablation, and LEEP. However, it also found a trend toward a higher rate of significant hemorrhage among women who received cone biopsies compared with women who received either laser ablation or LEEP.2
FIGURE
CIN 2 and 3 and its treatment by LEEP
TABLE 1
CIN 2 and 3 treatment options: An outcomes comparison
| COMPARISON OF TREATMENTS | OUTCOME/MORBIDITY | ODDS RATIO (95% CI) |
|---|---|---|
| Laser ablation vs cryotherapy | Laser ablation had more perioperative severe bleeding | 7.45 (1.68–33) |
| Laser ablation had higher rates of future adequate colposcopy | 4.64 (2.98–7.27) | |
| Laser conization vs knife conization | Laser conization had higher rates of future adequate colposcopy | 2.73 (1.47–5.08) |
| Laser conization had less cervical stenosis | 0.39 (0.25–0.61) | |
| Laser conization vs LEEP | Laser conization had more severe pain during procedure | 5.36 (1.02–17.2) |
| LEEP had fewer inadequate future colposcopies | 0.27 (0.08–0.89) | |
| Source: Martin-Hirsch et al, Cochrane Database Syst Rev 2000.1 | ||
Surgical treatment raises obstetric risks
There is a concern regarding future obstetric outcomes for women who have undergone surgical treatment of a high-grade cervical lesion. A recent meta-analysis of 27 controlled cohort studies found that cold knife conization and LEEP were associated with increased obstetrical risks, such as delivery at less than 37 weeks’ gestation and a birth weight <2500 g ( TABLE 2 ). Any resection that was more than 10 mm deep increased the risk of prematurity with future pregnancies (pooled relative risk=2.6; 95%] CI, 1.3–5.3).3
TABLE 2
Obstetrical outcomes for CIN 2 and 3 treatment options
| TREATMENT TYPE | OBSTETRICAL OUTCOME | RELATIVE RISK (95% CI) |
|---|---|---|
| Cold knife conization | Preterm delivery | 2.59 (1.80–3.72) |
| Low birth weight | 2.53 (1.19–5.36) | |
| Cesarean delivery | 3.17 (1.07–9.40) | |
| Laser conization | Preterm delivery | 1.71 (0.93–3.14) |
| LEEP | Preterm delivery | 1.70 (1.24–2.35) |
| Low birth weight | 1.82 (1.09–3.06) | |
| Preterm premature rupture of membranes | 2.69 (1.62–4.46) | |
| Source: Kyrgiou et al, Lancet 2006.3 | ||
Recommendations from others
Consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) and a practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) both recommend immediate removal of the entire transformation zone, with either ablative or excisional treatment as initial treatment of CIN 2 and 3 for patients who are not pregnant.4,5
Value of excisional treatment. The ASCCP guidelines note that there is a benefit to excisional treatment, as it allows pathologic assessment of the excised tissue. Some of the ASCCP guideline authors recommend excisional procedures for the management of large CIN 2 and 3 lesions, which are at increased risk of having microinvasive disease.4
For women with unsatisfactory colposcopy and biopsy-proven CIN 2 or 3, there is up to a 7% risk for an occult invasive cervical carcinoma.1,4 Performing a diagnostic excisional procedure is recommended on these patients.4,6
ASCCP and ACOG make special recommendations for both adolescents and pregnant women.
For adolescent patients with biopsy-proven CIN 2, a recent ACOG Committee Opinion recommends close follow-up—with Pap smears or colposcopies every 4 to 6 months—due to the high rates of resolution of CIN 2 in adolescents.7
For pregnant patients, diagnostic excisional procedures are associated with complications such as bleeding and preterm delivery, while there is minimal risk of CIN 2 or 3 progressing to invasive cervical cancer.4,8 In pregnancy, follow CIN 2 and 3 with colposcopy each trimester, and reevaluate at 6 to 12 weeks postpartum. Limit any diagnostic excisional procedures to cases where you cannot rule out invasive cancer.4,5
1. Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;(2)CD001318.-
2. Nuovo J, Melnikow J, Willan AR, Chan BKS. Treatment outcomes for squamous intraepithelial lesions. Int J gynaecol obstet 2000;68:25-33.
3. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-498.
4. Wright TC, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 ASCCP-sponsored Consensus Workshop. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
5. American College of obstetricians and gynecologists. ACOG Practice Bulletin number 66, september 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol 2005;106:645-664.
6. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol 1999;180:276-282.
7. American College of obstetricians and gynecologists. ACOG Committee opinion. Evaluation and management of abnormal cervical cytology and histology in the adolescent. Number 330, April 2006. Obstet Gynecol 2006;107:963-968.
8. Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML. Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol 1993;81:915-918.
9. Cox JT. We’re on the way to ending cervical cancer. OBG Management 2006;18(3):62-72.
10. Wright TC. Cervical disease update. OBG Management 2007;19(3):52-60.
Excision or ablation of the transformation zone are equally effective for treating an initial diagnosis of cervical intraepithelial neoplasia (CIN) grades 2 or 3 in women with a satisfactory colposcopy and no suggestion of microinvasive or invasive disease (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]).
Laser or loop electrosurgical excision procedure (LEEP) are the preferred treatment methods for recurrent CIN 2 and CIN 3 (SOR: B, based on clinical trials without randomization).
For women with an unsatisfactory colposcopy or suspicion of invasive disease, a diagnostic excisional procedure is recommended (SOR: C, based on consensus guidelines).
Observation or deferred treatment may be acceptable for CIN 2 in adolescents with satisfactory colposcopy and negative endocervical sampling (SOR: C, based on consensus guidelines).
Limit diagnostic excisional procedures in pregnancy to cases where suspicion of invasive cancer is high (SOR: C, based on consensus guidelines).
LEEP provides tissue for examination and a short recovery time
Timothy Huber, MD
Oroville, Calif
Close follow-up of observable disease and aggressive intervention continue to drive down the number of cervical cancer deaths each year.
It remains to be seen what the true effect of the HPV vaccine will be, although the presumed result will be a dramatic decline in high-grade lesions (CIN 2 and 3), carcinoma in situ, and invasive disease.
When intervention is necessary, my preferred method is LEEP because it provides tissue for examination and the recovery time is short.
Cryotherapy is an acceptable alternative, but the 4 to 8 weeks of leukorrhea and the lack of a tissue diagnosis often make it a less desirable option for patient and physician.
Evidence summary
Morbidity profile makes LEEP appear best
Similar efficacy. All 7 available surgical techniques were found to have similar efficacy, in a 2005 Cochrane review of 28 randomized trials.1 Resolution of CIN 2 or 3 lesions was 77% to 98%, using knife cone biopsy, laser conization, loop excision, laser ablation, cryotherapy, or 2 techniques not used commonly in the US, cold coagulation and radical diathermy. Surgical techniques were tested in various combinations, but no trial compared all of the techniques with one another. Most studies were underpowered, limiting the results.
Pap test: Good, but underused We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality. Yet, even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. The Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab. 9
Dr. J. Thomas cox, university of California. Member, American Cancer society Cervical guidelines Committee, the 2002 Bethesda Workshop; ACS HPV vaccine Advisory Committee; author, ASCCP guidelines Committee
Each year in the united states approximately 500,000 women are diagnosed with high-grade cervical cancer precursor lesions, CIN 2 and CIN III.4 If left untreated, 22% of CIN 2 lesions progress to carcinoma in situ or invasive cervical cancer, 43% regress, and 35% persist at the same level. Fourteen percent of untreated CIN 3 lesions progress, 32% regress, and 56% persist at the same level.
HPV vaccine: Won’t replace prevention or protection Although an effective vaccine is a major advance in the prevention of genital HPV and cervical cancer, it will not replace other prevention strategies, such as cervical cancer screening for women or protective sexual behaviors. Women should continue to get Pap tests as a safeguard against cervical cancer.
Dr. Anne Schuchat, Director, CDC National Center for Immunization and respiratory Diseases, June 29, 2006 press release (www.cdc.gov/od/oc/media/pressrel/r060629.htm)
The HPV vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18, was approved by the us Food and Drug Administration in June 2006 for use in females 9 to 26 years oof age. Shortly after, the Advisory Committee on Immunization Practices issued guidelines, stating that vaccination is recommended for all women <26 years of age. (www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm).
HPV testing: Adjunct to cytology The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are underway. 10
Dr. Thomas C. Wright, Columbia university. Author, 2001 Consensus guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim guidance for use of HPV DNA testing for Primary screening, and the 2001 Bethesda system
Least morbidity with LEEP. Morbidity was compared with one-on-one trials of different techniques ( TABLE 1 ). The review noted that LEEP has the least morbidity (such as hemorrhage, infection, cervical stenosis, and midtrimester pregnancy loss) while providing the most reliable histology by excising tissue without causing thermal artifact ( FIGURE ).
Higher rate of hemorrhage with cone biopsy. Another systematic review of 21 controlled trials comparing treatments for CIN 2 or 3 found a similar efficacy of all the modalities, including cone biopsy, cryotherapy, laser ablation, and LEEP. However, it also found a trend toward a higher rate of significant hemorrhage among women who received cone biopsies compared with women who received either laser ablation or LEEP.2
FIGURE
CIN 2 and 3 and its treatment by LEEP
TABLE 1
CIN 2 and 3 treatment options: An outcomes comparison
| COMPARISON OF TREATMENTS | OUTCOME/MORBIDITY | ODDS RATIO (95% CI) |
|---|---|---|
| Laser ablation vs cryotherapy | Laser ablation had more perioperative severe bleeding | 7.45 (1.68–33) |
| Laser ablation had higher rates of future adequate colposcopy | 4.64 (2.98–7.27) | |
| Laser conization vs knife conization | Laser conization had higher rates of future adequate colposcopy | 2.73 (1.47–5.08) |
| Laser conization had less cervical stenosis | 0.39 (0.25–0.61) | |
| Laser conization vs LEEP | Laser conization had more severe pain during procedure | 5.36 (1.02–17.2) |
| LEEP had fewer inadequate future colposcopies | 0.27 (0.08–0.89) | |
| Source: Martin-Hirsch et al, Cochrane Database Syst Rev 2000.1 | ||
Surgical treatment raises obstetric risks
There is a concern regarding future obstetric outcomes for women who have undergone surgical treatment of a high-grade cervical lesion. A recent meta-analysis of 27 controlled cohort studies found that cold knife conization and LEEP were associated with increased obstetrical risks, such as delivery at less than 37 weeks’ gestation and a birth weight <2500 g ( TABLE 2 ). Any resection that was more than 10 mm deep increased the risk of prematurity with future pregnancies (pooled relative risk=2.6; 95%] CI, 1.3–5.3).3
TABLE 2
Obstetrical outcomes for CIN 2 and 3 treatment options
| TREATMENT TYPE | OBSTETRICAL OUTCOME | RELATIVE RISK (95% CI) |
|---|---|---|
| Cold knife conization | Preterm delivery | 2.59 (1.80–3.72) |
| Low birth weight | 2.53 (1.19–5.36) | |
| Cesarean delivery | 3.17 (1.07–9.40) | |
| Laser conization | Preterm delivery | 1.71 (0.93–3.14) |
| LEEP | Preterm delivery | 1.70 (1.24–2.35) |
| Low birth weight | 1.82 (1.09–3.06) | |
| Preterm premature rupture of membranes | 2.69 (1.62–4.46) | |
| Source: Kyrgiou et al, Lancet 2006.3 | ||
Recommendations from others
Consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) and a practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) both recommend immediate removal of the entire transformation zone, with either ablative or excisional treatment as initial treatment of CIN 2 and 3 for patients who are not pregnant.4,5
Value of excisional treatment. The ASCCP guidelines note that there is a benefit to excisional treatment, as it allows pathologic assessment of the excised tissue. Some of the ASCCP guideline authors recommend excisional procedures for the management of large CIN 2 and 3 lesions, which are at increased risk of having microinvasive disease.4
For women with unsatisfactory colposcopy and biopsy-proven CIN 2 or 3, there is up to a 7% risk for an occult invasive cervical carcinoma.1,4 Performing a diagnostic excisional procedure is recommended on these patients.4,6
ASCCP and ACOG make special recommendations for both adolescents and pregnant women.
For adolescent patients with biopsy-proven CIN 2, a recent ACOG Committee Opinion recommends close follow-up—with Pap smears or colposcopies every 4 to 6 months—due to the high rates of resolution of CIN 2 in adolescents.7
For pregnant patients, diagnostic excisional procedures are associated with complications such as bleeding and preterm delivery, while there is minimal risk of CIN 2 or 3 progressing to invasive cervical cancer.4,8 In pregnancy, follow CIN 2 and 3 with colposcopy each trimester, and reevaluate at 6 to 12 weeks postpartum. Limit any diagnostic excisional procedures to cases where you cannot rule out invasive cancer.4,5
Excision or ablation of the transformation zone are equally effective for treating an initial diagnosis of cervical intraepithelial neoplasia (CIN) grades 2 or 3 in women with a satisfactory colposcopy and no suggestion of microinvasive or invasive disease (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]).
Laser or loop electrosurgical excision procedure (LEEP) are the preferred treatment methods for recurrent CIN 2 and CIN 3 (SOR: B, based on clinical trials without randomization).
For women with an unsatisfactory colposcopy or suspicion of invasive disease, a diagnostic excisional procedure is recommended (SOR: C, based on consensus guidelines).
Observation or deferred treatment may be acceptable for CIN 2 in adolescents with satisfactory colposcopy and negative endocervical sampling (SOR: C, based on consensus guidelines).
Limit diagnostic excisional procedures in pregnancy to cases where suspicion of invasive cancer is high (SOR: C, based on consensus guidelines).
LEEP provides tissue for examination and a short recovery time
Timothy Huber, MD
Oroville, Calif
Close follow-up of observable disease and aggressive intervention continue to drive down the number of cervical cancer deaths each year.
It remains to be seen what the true effect of the HPV vaccine will be, although the presumed result will be a dramatic decline in high-grade lesions (CIN 2 and 3), carcinoma in situ, and invasive disease.
When intervention is necessary, my preferred method is LEEP because it provides tissue for examination and the recovery time is short.
Cryotherapy is an acceptable alternative, but the 4 to 8 weeks of leukorrhea and the lack of a tissue diagnosis often make it a less desirable option for patient and physician.
Evidence summary
Morbidity profile makes LEEP appear best
Similar efficacy. All 7 available surgical techniques were found to have similar efficacy, in a 2005 Cochrane review of 28 randomized trials.1 Resolution of CIN 2 or 3 lesions was 77% to 98%, using knife cone biopsy, laser conization, loop excision, laser ablation, cryotherapy, or 2 techniques not used commonly in the US, cold coagulation and radical diathermy. Surgical techniques were tested in various combinations, but no trial compared all of the techniques with one another. Most studies were underpowered, limiting the results.
Pap test: Good, but underused We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality. Yet, even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. The Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab. 9
Dr. J. Thomas cox, university of California. Member, American Cancer society Cervical guidelines Committee, the 2002 Bethesda Workshop; ACS HPV vaccine Advisory Committee; author, ASCCP guidelines Committee
Each year in the united states approximately 500,000 women are diagnosed with high-grade cervical cancer precursor lesions, CIN 2 and CIN III.4 If left untreated, 22% of CIN 2 lesions progress to carcinoma in situ or invasive cervical cancer, 43% regress, and 35% persist at the same level. Fourteen percent of untreated CIN 3 lesions progress, 32% regress, and 56% persist at the same level.
HPV vaccine: Won’t replace prevention or protection Although an effective vaccine is a major advance in the prevention of genital HPV and cervical cancer, it will not replace other prevention strategies, such as cervical cancer screening for women or protective sexual behaviors. Women should continue to get Pap tests as a safeguard against cervical cancer.
Dr. Anne Schuchat, Director, CDC National Center for Immunization and respiratory Diseases, June 29, 2006 press release (www.cdc.gov/od/oc/media/pressrel/r060629.htm)
The HPV vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18, was approved by the us Food and Drug Administration in June 2006 for use in females 9 to 26 years oof age. Shortly after, the Advisory Committee on Immunization Practices issued guidelines, stating that vaccination is recommended for all women <26 years of age. (www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm).
HPV testing: Adjunct to cytology The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are underway. 10
Dr. Thomas C. Wright, Columbia university. Author, 2001 Consensus guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim guidance for use of HPV DNA testing for Primary screening, and the 2001 Bethesda system
Least morbidity with LEEP. Morbidity was compared with one-on-one trials of different techniques ( TABLE 1 ). The review noted that LEEP has the least morbidity (such as hemorrhage, infection, cervical stenosis, and midtrimester pregnancy loss) while providing the most reliable histology by excising tissue without causing thermal artifact ( FIGURE ).
Higher rate of hemorrhage with cone biopsy. Another systematic review of 21 controlled trials comparing treatments for CIN 2 or 3 found a similar efficacy of all the modalities, including cone biopsy, cryotherapy, laser ablation, and LEEP. However, it also found a trend toward a higher rate of significant hemorrhage among women who received cone biopsies compared with women who received either laser ablation or LEEP.2
FIGURE
CIN 2 and 3 and its treatment by LEEP
TABLE 1
CIN 2 and 3 treatment options: An outcomes comparison
| COMPARISON OF TREATMENTS | OUTCOME/MORBIDITY | ODDS RATIO (95% CI) |
|---|---|---|
| Laser ablation vs cryotherapy | Laser ablation had more perioperative severe bleeding | 7.45 (1.68–33) |
| Laser ablation had higher rates of future adequate colposcopy | 4.64 (2.98–7.27) | |
| Laser conization vs knife conization | Laser conization had higher rates of future adequate colposcopy | 2.73 (1.47–5.08) |
| Laser conization had less cervical stenosis | 0.39 (0.25–0.61) | |
| Laser conization vs LEEP | Laser conization had more severe pain during procedure | 5.36 (1.02–17.2) |
| LEEP had fewer inadequate future colposcopies | 0.27 (0.08–0.89) | |
| Source: Martin-Hirsch et al, Cochrane Database Syst Rev 2000.1 | ||
Surgical treatment raises obstetric risks
There is a concern regarding future obstetric outcomes for women who have undergone surgical treatment of a high-grade cervical lesion. A recent meta-analysis of 27 controlled cohort studies found that cold knife conization and LEEP were associated with increased obstetrical risks, such as delivery at less than 37 weeks’ gestation and a birth weight <2500 g ( TABLE 2 ). Any resection that was more than 10 mm deep increased the risk of prematurity with future pregnancies (pooled relative risk=2.6; 95%] CI, 1.3–5.3).3
TABLE 2
Obstetrical outcomes for CIN 2 and 3 treatment options
| TREATMENT TYPE | OBSTETRICAL OUTCOME | RELATIVE RISK (95% CI) |
|---|---|---|
| Cold knife conization | Preterm delivery | 2.59 (1.80–3.72) |
| Low birth weight | 2.53 (1.19–5.36) | |
| Cesarean delivery | 3.17 (1.07–9.40) | |
| Laser conization | Preterm delivery | 1.71 (0.93–3.14) |
| LEEP | Preterm delivery | 1.70 (1.24–2.35) |
| Low birth weight | 1.82 (1.09–3.06) | |
| Preterm premature rupture of membranes | 2.69 (1.62–4.46) | |
| Source: Kyrgiou et al, Lancet 2006.3 | ||
Recommendations from others
Consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) and a practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) both recommend immediate removal of the entire transformation zone, with either ablative or excisional treatment as initial treatment of CIN 2 and 3 for patients who are not pregnant.4,5
Value of excisional treatment. The ASCCP guidelines note that there is a benefit to excisional treatment, as it allows pathologic assessment of the excised tissue. Some of the ASCCP guideline authors recommend excisional procedures for the management of large CIN 2 and 3 lesions, which are at increased risk of having microinvasive disease.4
For women with unsatisfactory colposcopy and biopsy-proven CIN 2 or 3, there is up to a 7% risk for an occult invasive cervical carcinoma.1,4 Performing a diagnostic excisional procedure is recommended on these patients.4,6
ASCCP and ACOG make special recommendations for both adolescents and pregnant women.
For adolescent patients with biopsy-proven CIN 2, a recent ACOG Committee Opinion recommends close follow-up—with Pap smears or colposcopies every 4 to 6 months—due to the high rates of resolution of CIN 2 in adolescents.7
For pregnant patients, diagnostic excisional procedures are associated with complications such as bleeding and preterm delivery, while there is minimal risk of CIN 2 or 3 progressing to invasive cervical cancer.4,8 In pregnancy, follow CIN 2 and 3 with colposcopy each trimester, and reevaluate at 6 to 12 weeks postpartum. Limit any diagnostic excisional procedures to cases where you cannot rule out invasive cancer.4,5
1. Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;(2)CD001318.-
2. Nuovo J, Melnikow J, Willan AR, Chan BKS. Treatment outcomes for squamous intraepithelial lesions. Int J gynaecol obstet 2000;68:25-33.
3. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-498.
4. Wright TC, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 ASCCP-sponsored Consensus Workshop. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
5. American College of obstetricians and gynecologists. ACOG Practice Bulletin number 66, september 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol 2005;106:645-664.
6. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol 1999;180:276-282.
7. American College of obstetricians and gynecologists. ACOG Committee opinion. Evaluation and management of abnormal cervical cytology and histology in the adolescent. Number 330, April 2006. Obstet Gynecol 2006;107:963-968.
8. Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML. Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol 1993;81:915-918.
9. Cox JT. We’re on the way to ending cervical cancer. OBG Management 2006;18(3):62-72.
10. Wright TC. Cervical disease update. OBG Management 2007;19(3):52-60.
1. Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;(2)CD001318.-
2. Nuovo J, Melnikow J, Willan AR, Chan BKS. Treatment outcomes for squamous intraepithelial lesions. Int J gynaecol obstet 2000;68:25-33.
3. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-498.
4. Wright TC, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 ASCCP-sponsored Consensus Workshop. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
5. American College of obstetricians and gynecologists. ACOG Practice Bulletin number 66, september 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol 2005;106:645-664.
6. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol 1999;180:276-282.
7. American College of obstetricians and gynecologists. ACOG Committee opinion. Evaluation and management of abnormal cervical cytology and histology in the adolescent. Number 330, April 2006. Obstet Gynecol 2006;107:963-968.
8. Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML. Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol 1993;81:915-918.
9. Cox JT. We’re on the way to ending cervical cancer. OBG Management 2006;18(3):62-72.
10. Wright TC. Cervical disease update. OBG Management 2007;19(3):52-60.
Evidence-based answers from the Family Physicians Inquiries Network