User login
Emergency contraception: An underutilized resource
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
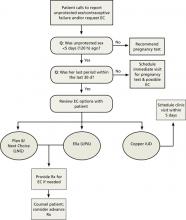
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.