User login
Abnormal Uterine Bleeding in Reproductive-Aged Women
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
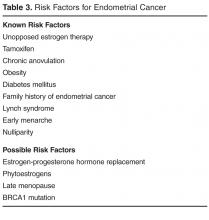
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, sbschrag@wisc.edu.
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, sbschrag@wisc.edu.
From the University of Wisconsin School of Medicine and Public Health, Madison, WI.
Abstract
- Objective: To describe the contributing etiologies, common presentations, diagnosis, evaluation, and management of abnormal uterine bleeding (AUB).
- Methods: Review of the literature in the context of 3 cases.
- Results: AUB is one of the most common reasons that reproductive-aged women seek health care. The causes are varied, depending in large part on the age and life stage of the woman. Diagnosis requires a systematic approach that is driven by a thorough health history and review of presenting symptoms. Determining whether the bleeding is ovulatory or anovulatory is a central part of the evaluation. A methodical history, physical examination, and laboratory evaluation may enable the physician to rule out causes such as pregnancy and pregnancy-related disorders, medications, iatrogenic causes, systemic conditions, and obvious genital tract pathology.
- Conclusion: Clinicians must be knowledgeable about AUB and partner with women to develop appropriate, individualized treatment plans.
Abnormal vaginal bleeding is a common complaint in primary care. The prevalence of some type of abnormal bleeding is up to 30% among women of reproductive age [1].Over 18% of all gynecology outpatient visits in the United States are for menorrhagia alone [2].A retrospective analysis of medical expenditures data compared 1.4 million women with abnormal uterine bleeding to over 50 million women without abnormal bleeding. This study found that women with abnormal bleeding were more likely to be younger, Caucasian, and obese and had poorer physical and mental health quality of life scores [3].
The estimated direct and indirect costs of abnormal bleeding are $1 billion and $12 billion annually, respectively [4]. Indirect costs of abnormal bleeding include time off from work and cost of products to protect clothing from bleeding (eg, tampons and pads). Abnormal bleeding is also a common reason for women to be referred to gynecologists and is an indication for up to 25% of all gynecologic surgeries [5].
History Taking
Taking a menstrual history is an important step in determining whether the current bleeding pattern is normal or abnormal. Regularity of menstrual bleeding is clarified by asking about the frequency of the menses and their duration. Other important questions include age at menarche, presence of premenstrual syndrome symptoms, breast tenderness, cervical mucus changes, and amount of bleeding. An ovulatory cycle will usually include premenstrual symptoms whereas an anovulatory cycle will be random in its symptomatology. Women’s estimates of the amount of menstrual bleeding are notoriously inaccurate. Traditionally, more than 80 cc of menstrual blood loss per cycle is considered menorrhagia. However, women and their health care providers do not measure menstrual blood volume outside of study settings, and one study found that only half of women who presented with menorrhagia actually had more than 80 cc of blood loss [6]. There is movement toward use of more patient-centered measures to diagnose men-orrhagia, such as bleeding interfering with a woman’s daily activities, needing to wake up at night to change tampons or pads, or inability to exercise during menses. Anemia in the setting of menorrhagia by history is a less subjective way to diagnose menorrhagia.
Nomenclature and Differential Diagnosis
Differential diagnosis will vary based on symptomatology as well as age. Pregnancy is a possible cause of any type of abnormal bleeding in any woman of reproductive age (ie, after menarche and before menopause). Many systemic illnesses and medications can affect menstrual bleeding and should be included in a broad differential diagnosis of a presenting woman.
Case 1—Heavy Menstrual Bleeding
Initial Presentation
A 42-year-old woman presents reporting increasingly heavy, somewhat painful periods over the last 6 to 8 months. She experienced menarche at age 12 and has had regular, moderately heavy periods throughout her adult life. She denies any inter-menstrual bleeding.
What additional history should be obtained?
Heavy menstrual bleeding refers to abnormally heavy bleeding that occurs in an ovulatory, cyclical pattern. Women with anovulatory cycles can also have heavy bleeding as well, and distinguishing ovulatory vs anovulatory cycles is often the first step in the evaluation.
The initial evaluation of a woman presenting with heavy menstrual bleeding includes a detailed history and physical examination. The first goal of the history is to establish the severity of bleeding, including any symptoms of hemodynamically significant anemia such as dizziness or exertional dyspnea. Next, the clinician should determine whether the bleeding pattern is ovulatory or anovulatory. Ovulatory heavy menstrual bleeding is most often caused by structural lesions (leiomyomas, endometriosis, adenomyosis, cervical polyps, and endometrial polyps) or a coagulopathy (von Willebrand disease, anticoagulant use, etc). Less commonly, ovulatory heavy menstrual bleeding may be due to systemic illness (including thyroid disease, renal disease, and liver disease) or endometrial hyperplasia or carcinoma.
Once an ovulatory pattern is confirmed, a history of dysmenorrhea, pelvic pain, lower urinary tract symptoms, constipation, dyspareunia, or infertility should be elicited.
Further history taking should seek to identify any symptoms suggestive of thyroid, kidney, or liver disease,
What are key elements of the physical examination?
The physical examination should include visual inspection and palpation of the thyroid gland as well as an abdominal exam to evaluate for hepatosplenomegaly or lower abdominal tenderness or masses. Signs of anemia such as pallor should also be noted. The gynecologic exam should include visual inspection of the external genitalia, a bimanual exam, and a speculum exam. Cervical and endometrial polyps may be visible as masses at the cervical os or extending into the vaginal canal. An enlarged mobile uterus with irregular contours is consistent with leiomyomas [8].Endometriosis may manifest as tenderness, thickening, or nodularity of the uterine corpus, the vaginal canal, the uterosacral ligaments, or the adnexa. Endometriosis may also cause an asymmetric, fixed position of the uterus, the cervix, or the adnexa [9].Adenomyosis may cause diffuse moderate uterine enlargement with or without tenderness [10].Endometrial carcinoma may also cause uterine enlargement and/or immobility.
What laboratory testing should be performed?
What additional testing would be useful in narrowing the differential diagnosis?
If the physical examination and initial laboratory testing is nondiagnostic, the decision to initiate a trial of symptom management or proceed with further testing (imaging and/or tissue sampling) is based on risk of endometrial cancer, severity of symptoms, and patient preference. In many women, body habitus makes a confirmatory pelvic examination difficult, which may lower the threshold for obtaining a pelvic ultrasound.
Women with risk factors for endometrial cancer should undergo office-based endometrial biopsy as the first step in evaluation of heavy menstrual bleeding [7].Risk factors include older age (45 years and older), obesity (BMI > 30), diabetes mellitus, nulliparity, and history of chronic anovulation (eg, polycystic ovary syndrome). Pelvic ultrasound is the first step in the evaluation of women with an abnormal physical exam suggesting a structural lesion [7].If the physical exam is abnormal and the pelvic ultrasound is nondiagnostic, a hysteroscopy or saline-infusion sonohysterogram should be performed, as these tests are more sensitive for the detection of intracavitary lesions and submucosal fibroids [13].Most endometrial polyps will appear as a thickened or irregular endometrium on pelvic ultrasound, but be clearly delineated on sonohysterogram. Women who have a negative initial evaluation but then go on to have persistent bleeding despite a trial of therapy also require further evaluation.
Case Continued
The patient reports that her periods are regular, with a cycle length of 30 to 31 days. She usually notes some bloating and breast tenderness in the days leading up to onset of menses. She experiences lower abdominal cramping during days 1–3 of her period. This has worsened somewhat over the last year, and sometimes radiates to her low back. Her reproductive history is significant for 3 uncomplicated vaginal deliveries and 1 first trimester spontaneous abortion. She did not experience postpartum hemorrhage, and has no history of significant oropharyngeal bleeding or unexplained bruising. Her BMI is 23.3. Her physical exam is unremarkable, including a normal thyroid, abdominal, bimanual and speculum exam. Laboratory evaluation demonstrates a low-normal hemoglobin, hematocrit, and MCV. The TSH is normal and a urine pregnancy test is negative. She had a normal pap smear and HPV assay 2 years ago.
What is the most likely diagnosis?
What treatment is recommended?
Oral tranexamic acid is an anti-fibrinolytic that was recently approved by the FDA for treatment of menorrhagia or heavy menstrual bleeding. It has been used for many years to prevent bleeding during surgery and to treat bleeding disorders. It has been used for over 30 years to treat menorrhagia in Europe. It has a different mechanism of action than NSAIDs and hormonal contraceptives, and is therefore an appropriate alternative for women who cannot tolerate other medication options [16,17].Tranexamic acid is contraindicated in women with an elevated risk of thromboembolic disease.
For women who have insufficient response to medical management or for women who present with more severe symptoms, anemia, or prominent bulk-related symptoms due to fibroids, gynecologic referral should be made for consideration of surgical intervention. The preferred interventional approach to the treatment of uterine fibroid tumors depends upon the type of fibroid (eg submucosal, intramural, subserosal), the number of fibroids, desire for future childbearing, risk for surgical complications, and patient preference. Effective options include myomectomy, uterine artery embolization, endometrial ablation, and hysterectomy [18].
By contrast, good evidence supports the use of medication as first-line therapy for heavy menstrual bleeding when it occurs in the setting of endometriosis. Estrogen-progestin oral contraceptive pills, oral progestins, and depot medroxyprogesterone have all been demonstrated to be effective in decreasing pain [19,20].The levonorgestrel-releasing intrauterine system is also effective in decreasing pain due to endometriosis [21].
Women who do not respond to first-line therapy should be referred to a gynecologist for consideration of other treatment options. Effective second-line treatment options include oral danazol, intramuscular GnRH agonists, and surgical approaches such as laparoscopic ablation and/or excision of endometriosis implants [22].
A similar range of treatment options appears to be effective in the management of heavy menstrual bleeding due to adenomyosis. First-line therapies include oral NSAIDs, oral tranexamic acid, estrogen-progestin oral contraceptive pills, and the levonorgestrel-releasing intrauterine system [23,24].Women with an inadequate response to first-line treatment should be referred to a gynecologist for consideration definitive treatment with hysterectomy versus uterine artery embolization or a trial of a GnRH agonist [24].
For some women with heavy menstrual bleeding, no specific underlying cause is identified. Current evidence suggests that such patients may have disorders of local endometrial hemostasis leading to increased blood loss during otherwise normal menstrual cycles [25].The levonorgestrel-releasing intrauterine system may be the most effective medical therapy for heavy menstrual bleeding in the absence of a specific target lesion [26].For women wishing to avoid hormonal treatment, scheduled oral NSAIDs or oral tranexamic acid are inexpensive and effective options for reducing blood loss [27–29].Other medical treatment options include estrogen-progestin contraceptive pills, cyclic oral progestin, and depot medroxy-progesterone.
For patients who experience treatment failure with pharmaceutical therapy or who desire definitive treatment, both endometrial ablation and hysterectomy have been shown to be effective and associated with high rates of patient satisfaction [30].
Follow-up
The patient reports that she would like to avoid invasive testing if possible. Given her relatively low risk for endometrial cancer, she elects a trial of scheduled NSAIDs. Unfortunately, after a couple of cycles she reports that her heavy bleeding has not been well-controlled. A pelvic ultrasound demonstrates an anterior submucosal fibroid measuring 2.4 cm and a posterior intramural fibroid measuring 1.5 cm. She agrees to insertion of a levonorgestrel IUD and calls 6 months later to report a significant decrease in her bleeding.
Case 2—Anovulation
Initial Presentation
A 27-year-old female presents for pregnancy testing. She is 2 weeks late for her period. She and her husband are attempting pregnancy and she seems disappointed that the pregnancy test is negative. She is having trouble tracking her periods. Her cycles range from 24 to 45 days apart and often she skips cycles altogether. Her flow is scant at times but some months are heavy with soaking tampons/pads.
What are diagnostic considerations in evaluating this bleeding pattern?
Menstrual history can help differentiate between of ovulatory and anovulatory abnormal bleeding. Typically, anovulatory bleeding is marked by irregular or infrequent periods. Flow can be scant to excessive. Women experiencing anovulatory cycles may fail to notice common ovulation symptoms (thin watery cervical mucus) or pre-menstrual symptoms (breast tenderness) [31].
The International Federation of Gynecology and Obstetrics (FIGO) designates AUD-O as “abnormal uterine bleeding due to ovulatory dysfunction” or “anovulatory abnormal uterine bleeding” [7,31].In general, if women are having menses at regular cycles their bleeding is likely to be ovulatory.
Differential Diagnosis
Anovulatory bleeding may be physiologic. After menarche, the hypothalamic-pituitary-ovarian axis is immature. This may result in anovulatory cycles for 2 to 3 years. Women entering perimenopausal transition may also experience intermittent anovulation and subsequent abnormal uterine bleeding. Other physiologic examples include lactation and pregnancy [31].
Physical Examination
A thorough history will help to narrow the differential diagnosis. The physical exam can evaluate for other findings that indicate endocrine dysfunction such as low body weight, hirsutism, balding, acne, high blood pressure, obesity (especially centripetal fat distribution). Acanthosis nigricans is a sign of insulin resistance which is part of the pathophysiology of PCOS. The gynecologic exam is often unremarkable in AUB-O although a bimanual exam can reveal adnexal enlargement indicative of cystic ovaries. Of note, clitoromegally is not common in PCOS. This finding would increase the likelihood of other causes of hyperandrogenism [32].
What is the pathophysiologic basis for this patient’s bleeding pattern?
Pathophysiology of Anovulatory Bleeding
Anovulatory bleeding presumes that there is a normal anatomic and genetic makeup. For example, a woman without ovaries will be, by definition, anovulatory. Using current terminology anovulatory bleeding implies a disruption in the hypothalamic-pituitary-ovarian axis and is therefore primarily an endocrine disorder [31,33].
At the level of the ovary and uterus, anovulation results in prolonged estrogen effect on the endometrium. After ovulation, the corpus luteum produces progesterone which stops endometrial thickening and stabilizes the endometrium. Without ovulation, estrogen continues endometrial stimulation and excess proliferation of endometrial lining. The endometrium becomes unstable, undifferentiated, and sheds unpredictably. The blood vessels become larger, more tortuous and have increased fragility. The result is light or heavy menstrual bleeding, decreased frequency of periods but overall unpredictable menstrual bleeding [33].
Effects of Chronic Anovulation
Irregular cycles can be more than a mere inconvenience. Women who have anovulatory cycles associated with heavy menstrual bleeding are at risk for anemia. Anovulation that is a result of hyperandrogen state or other endocrine disorder has other health ramifications. Infertility and its treatment are common sequelae. Finally, over time, unopposed estrogen in anovulation increases the risk of endometrial hyperplasia, or cancer [7,34].
Case Continued
The patient reports menarche at age 12. Her periods were irregular for the first 1–2 years but became more regular after that. She has been taking combination oral contraceptives since the age of 20 to prevent pregnancy. She stopped this 1 year ago and she and her husband began actively trying to conceive 6 months ago. Her family history is notable for diabetes and hypertension in her father. Her mother had heavy periods leading up to menopause and had a hysterectomy with no malignancy at the age of 47. She has a BMI of 33; blood pressure is mildly elevated at 134/84 mm Hg. She has oily skin and acne along her chin and neck. She has mild hirsutism of her face. Otherwise her skin is normal appearing. She has an elevated waist circumference of 35 inches. The remainder of her exam is normal.
What is the likely diagnosis?
Women with PCOS are at increased risk for metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes and cardiovascular disease, endometrial cancer, and infertility. Women with PCOS who become pregnant have increased risk of pregnancy complications such as hypertensive disorders and gestational diabetes.
What tests are indicated in this patient?
Appropriate laboratory testing is often determined based on findings in the history and physical as well as the patient’s age. Anovulation in the first 18 months to 3 years after menarche is common and testing for pregnancy, infection, and anemia are often sufficient. Menorrhagia in adolescents warrants testing for bleeding disorders as well [7].Within 3 years of menarche, menstrual cycles should become more regular. Persistent anovulatory cycles increase the likelihood of pathologic causes and warrant additional evaluation. Pregnancy testing, thyroid stimulation hormone and prolactin levels are recommended first line evaluation [7,32,34].If PCOS is suspected an ultrasound can be performed but as noted above, polycystic ovaries are not required to make the diagnosis after adolescence.
Additional testing includes testosterone levels to look for androgen secreting tumors. Late onset congenital adrenal hyperplasia is an uncommon cause of hyperandrogenism but is more common in women of Ashkenazi Jewish descent and those with a family history [34].Morning hydroxyprogesterone can be performed to evaluate for this. If women exhibit abrupt change in menstrual pattern and other signs of cortisol excess (hypertension, abdominal striae) 24-hour urine cortisol can detect Cushing’s syndrome [34].
In patients with PCOS, additional testing to evaluate for medical comorbidities is recommended. This includes screening for diabetes, dyslipidemia, and liver dysfunction.
Case Continued
The patient’s prolactin and TSH are normal. Tests for diabetes are normal. Her LDL is elevated to 162, triglycerides are 200, and her HDL is 38. The physician informs her that she meets criteria for PCOS and also that she has obesity and metabolic syndrome.
What factors should be considered when making treatment recommendations for this patient?
Treatment for anovulation is guided by the goals of therapy. Since anovulation is an endocrine abnormality, medical treatment is first line [31].If secondary causes are diagnosed, these should be treated first. Other goals of treatment can include reducing amount and irregularity of menstruation, provide contraception, increasing ovulation in women with desired fertility, and reducing androgenic sequelae such as acne and hirsutism.
When treating the irregular or heavy bleeding associated with anovulation, first-line treatment is exogenous hormone. This can be in the form of combined estrogen/progesterone formulations (pill, patch, and ring). Medroxyprogesterone (medroxyprogesterone acetate 5–10 mg daily) taken 10 to 14 days per month is another option. Standard consideration for medical eligibility in prescribing these agents should be considered (see U.S. medical eligibility criteria for contraceptive use available at www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm). Combined formulations offer contraception, while cyclic progesterone does not. Both offer cyclic withdrawal bleeding. A Cochrane review did not find any RCTs comparing one to the other and therefore either are reasonable options [36].The levonorgestrel IUD is effective at treating AUB as well [31].Women may still experience intermittent vaginal bleeding or amenorrhea so it is less likely to result in cyclic withdrawal bleeding.
All of the above treatments provide the additional benefit of thinning the endometrium and preventing unopposed estrogen effect. This provides further protection the endometrial hyperplasia with chronic anovulatory cycles and unopposed estrogen [31].
In women with PCOS and associated metabolic conditions, first-line treatment is weight loss and other lifestyle interventions to improve or prevent other sequelae of the condition. Weight loss has been shown to reduce circulating androgen levels and increase ovulation. It has been shown to reduce glucose and lipid levels and hirsutism. Pregnancy rates increase as well. Weight loss achieved through medications and gastric bypass has similar effects. There is no special diet that has been shown to be more effective than another [32]. As little as a 5% weight reduction from baseline can improve PCOS symptoms [34,35].
Metformin is also commonly added to lifestyle modifications in women with PCOS to reduce risks for developing diabetes. There is little high quality evidence of added benefit above lifestyle modifications [34].Statin therapy can be considered in women with hyperlipidemia and PCOS [32].
For women with PCOS who desire to conceive, treatment should target increased ovulation. Pre-conception counseling and lifestyle modifications are again first line [32].Ovulation induction interventions carry increased risk of multiple gestation. For ovulation induction, clomiphene citrate is first line therapy. Metformin is commonly used as noted above to improve comorbidities associated with PCOS and can increase ovulation compared to placebo [37].However, RCTs do not support its use as first-line treatment of infertility treatment in PCOS. Clomiphene is 3 times more effective than metformin alone [32].
Medications can improve but often do not resolve hirsutism in women with PCOS. Combined hormonal contraceptives are commonly used off-label and no one type of pill has been shown to be superior. Anti-androgens are also off-label but empirically used. They can also improve lipid and other metabolic variables. They are all teratogenic and therefore should not be used in women who desire conception, and be used with effective contraceptives. Spironolactone is an androgen receptor antagonist. It takes months for effect. Some women will have improved menstrual frequency with this medication as well. Often adjunctive therapy such as eflornithine facial cream or laser therapy or a combination is needed to further treat hirsutism [32,35,38].
Follow-up
After discussion, the patient decides to adopt therapeutic lifestyle changes. She desires to get pregnant and does not opt for hormonal contraceptives at this time. She sees a nutritionist and begins calorie restriction and exercise. Three months later she has lost 20 pounds and feels “healthier.” Her lipid panel shows LDL of 125 and HDL of 43. Her triglycerides are now 160. Her blood pressure in the office is 118/78 mm Hg. She has lost “inches” around her middle. She has had more regular periods as well. She is still not pregnant so the physician asks her to begin tracking ovulation with cervical mucus evaluation and basal body temperature prior to considering further infertility evaluations. Three months after that she misses a period but is pleased to report a positive home pregnancy test.
Case 3—Breakthough Bleeding On Combined Hormonal Contraceptives
A 28-year-old G0P0 in a monogamous relationship presents to her physician. She has been on oral contraceptive pills for 8 years. For the last 3 years she has been taking the pills on an extended cycle schedule. She normally takes an active pill daily for 3 cycles of pills (9 weeks), and then takes a 7-day pill-free week when she gets a menstrual period. This had been working fine until the last 6 months. She has noticed breakthrough spotting up to 2 weeks at a time during the 2nd and 3rd pack of pills.
What is the approach to evaluation and treatment in this patient?
Bleeding in Women on Combined Hormonal Contraception
Many women are now using combined hormonal contraceptives on different schedules. Extended-cycle contraception has been shown to be as effective as the traditional 21/7 schedule of active pills/pill-free week. The FDA has approved several packaged extended-cycle contraceptives. Extended-cycle contraception decreases overall number of bleeding days and improves many menstrual-related symptoms [39].Breakthrough bleeding is the most common side effect of extended cycle contraception. It is classified as AUB-I (abnormal uterine bleeding—iatrogenic). It is most common in the first few months of use, and decreases as use continues. Up to 86% of women will have unscheduled bleeding during the first 3 months of use of extended cycle contraception, but this bleedingdecreases as use continues [40].
There is no consensus as to the underlying mechanism causing this abnormal bleeding. Most clinicians believe that it is related to the balance of estrogen/progestin in each combined hormonal contraceptive. Each woman reacts differently to this combination, making it difficult to predict who will have abnormal bleeding. In women who are beginning an extended-cycle regimen, reassurance is sufficient. Most abnormal bleeding will normalize within the first 2 to 3 months. Missed pills and smoking are consistently related to breakthrough bleeding in women who take combined oral contraceptive pills [41].In women who have previously had stable bleeding patterns and who present with new breakthrough bleeding, evaluation for secondary causes of bleeding may be considered (ie, urine hCG, TSH, STI cultures, evaluation for cervical cancer screening). A pelvic examination may help determine a possible secondary cause of bleeding, but is not necessary.
Treatment of unscheduled bleeding in women on extended-cycle contraception includes shortening the hormone-free interval and adding medications for prevention/treatment of bleeding episodes. The 7-day hormone-free interval in the context of low-dose hormonal contraception may be too long. One study demonstrated that a 7-day hormone-free interval was associated with a lack of pituitary-ovarian suppression, follicular development, and possible ovulation [42].A systematic review found that shortened hormone-free intervals decreased the amount of unscheduled bleeding [39].A small RCT (65 women) of continuous contraceptive ring users found that the group that removed the ring for 4 days during an episode of unscheduled bleeding, and then reinserted it had overall reduction in unscheduled bleeding [43].Some clinicians will also recommend trying a different pill formlation or a different schedule. There is no evidence to support this recommendation, but it can be helpful in some women.
Low-dose doxycycline (40 mg daily) for prevention of unscheduled bleeding shows promise [44].This low- dose doxycycline is also helpful to prevent more unscheduled bleeding in extended-cycle oral contraceptive users [44].However, an RCT found that traditional-dose doxycline (100 mg BID) taken for 5 days at the onset of a bleeding episode, did not decrease the amount or length of unscheduled bleeding [40].Neither estrogen dose [45]nor progestin dose [45]affected bleeding patterns. There is some suggestion based on a small study that women on pills with norethindrone may have less unscheduled bleeding than those who are on pills with levonorgestrel, but more research needs to be done before clinicians change practice [46].A Cochrane review looked at one small study that suggested third-generation progestins had more favorable bleeding profiles than second-generation progestins [47].
Follow-up
The physician investigates for secondary causes of the bleeding. The patient’s urine hCG, TSH, and prolactin levels are all normal. No fibroids or polyps are seen on ultrasound. The physician and patient discuss treatment options, including a low-dose doxycycline pill to help minimize bleeding, trying a different pill formulation, or use of naproxen during the bleeding episodes, but the patient does not want to take 2 pills every day. After further discussion, the patient decides she would like to change to the contraceptive ring with the plan of removing the ring for 4 days at the onset of any unscheduled bleeding. In a phone call 6 months later, the patient states that her unscheduled bleeding has been controlled.
Corresponding author: Sarina Schrager, MD, MS, Dept. of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Ct., Madison, WI 53715, sbschrag@wisc.edu.
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.
1. Singh S, Best C, Dunn S, et al; Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2013 May;35:473-9.
2. Nicholson WR, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Ob Gyn 2001;184:523-30.
3. Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Wom Health 2013;22:959-65.
4. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94.
5. Goodman A. Abnormal genital tract bleeding. Clin Cornerstone 2000;3:25-35.
6. Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6.
7. ACOG Practice Bulletin No. 128. Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197-206.
8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007; 87:725-736.
9. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382-91.
10. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98(3):572-9.
11. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol 1999;50:655-9.
12. Knol HM, Mulder AB, Bogchelman DH, et al. The prevalence of underlying bleeding disorders in patients with heavy menstrual bleeding with and without gynecologic abnormalities. Am J Obstet Gynecol 2013;209:202.e1-7.
13. Kelekci S, Kaya E, Alan M, et al. Comparison of transvaginal sonography, saline infusion sonography, and office hysteroscopy in reproductive-aged women with or without abnormal uterine bleeding. Fertil Steril 2005;84:682–6.
14. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010;82:41-55.
15. Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BWJ. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Systematic Rev 2013, Issue 2.
16. Phillip CS. Antifibrinolytics in women with menorrhagia. Thrombosis Research 2011;127(Sup 3):S113-S115.
17. Hrometz SL. Oral modified release tranexamic acid for heavy menstrual bleeding. Ann Pharmacother 2012;46:1047-53.
18. NICE clinical guidelines. Heavy menstrual bleeding. London: National Institute for Health and Care Excellence.
19. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD001019.
20. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012 Mar 14;3:CD002122.
21. Management of endometriosis. Practice Bulletin No. 114. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36.
22. B. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3.
23. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006 Aug;20:603-16.
24. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93.
25. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:3–13.
26. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37.
27. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database System Rev 2013;1: CD000400.
28. Naoulou BB, MC Ming C Tsai. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand 2012;91:529-37.
29. Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:865-75.
30. Fergusson RJ, Lethaby A, Shepperd, S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding Cochrane Database System Rev 2013;11:CD000329.
31. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin 136. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85.
32. ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Polycystic ovary syndrome. Obstet Gynecol 2009;114 :936-49.
33. Livinstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human Reproduction Update 2002;8:60-67.
34. Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120: 128-132.
35. Cahill D. PCOS. Clinical Evidence. BMJ Publishing Group. 2009;01:1-45.
36. Hickey M, Higam JM, Fraser I. Progestogens with our without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Reviews 2012;9:CD001895.
37. Nothinagle M, Scott-Taylor J. Does metformin improve clinical features of polycystic ovary syndrome? Cochrane for clinicians: putting evidence into practice. Am Fam Physician 2003;68:2163-4.
38. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Systematic Rev 2009;(4): CD002249.
39. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled bleeding in women using extended- or continuous-use combined hormonal contraception: a systematic review. Contraception 2013;87:567-75.
40. Kaneshiro B, Edelman A, Carlson N, et al. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet &Gynecol 2010;115 :1141-9.
41. Grossman MP, Nakajima SP. Menstrual cycle bleeding patterns in smokers. Contraception 2006;73:562-5.
42. Schlaff WD, Lynch Am, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effects on follicular suppression. Am J Obstet Gynecol 2004;190:943-51.
43. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol 2008;112:563-71.
44. Kaneshiro B, Edelman A, Carlson NE, et al. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception 2012;85:351-8.
45. Kaneshiro B, Edelman A, Carlson NE, et al. Unscheduled bleeding with continuous oral contraceptive pills: a comparison of progestin dose. Contraception 2012;86:22-
46. Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol 2006;107:657-65.
47. Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev 2011; (5):CD0004681.