User login
Formaldehyde-Induced Contact Dermatitis From an N95 Respirator Mask
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19
Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19
Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19
Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
Practice Points
- Prolonged wearing of N95 respirator masks has been associated with causing or complicating a number of facial inflammatory dermatoses.
- Consider the possibility of contact dermatitis secondary to formaldehyde exposure in individuals wearing N95 masks for prolonged periods.
- Information on the chemical components of N95 masks would be useful for clinicians tasked with evaluating patients with facial inflammatory dermatoses.
Verrucous Kaposi Sarcoma in an HIV-Positive Man
To the Editor:
Verrucous Kaposi sarcoma (VKS) is an uncommon variant of Kaposi sarcoma (KS) that rarely is seen in clinical practice or reported in the literature. It is strongly associated with lymphedema in patients with AIDS.1 We present a case of VKS in a human immunodeficiency virus (HIV)–positive man with cutaneous lesions that demonstrated minimal response to treatment with efavirenz-emtricitabine-tenofovir, doxorubicin, paclitaxel, and alitretinoin.
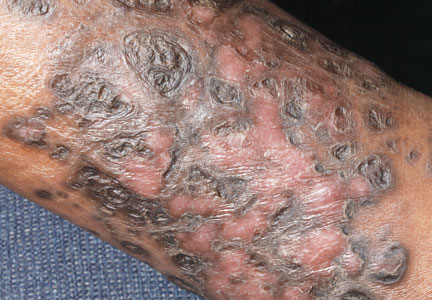
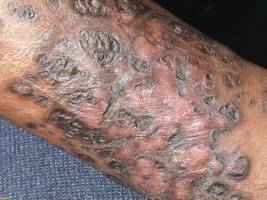
A 48-year-old man with a history of untreated HIV presented with a persistent eruption of heavily scaled, hyperpigmented, nonindurated, thin plaques in an ichthyosiform pattern on the bilateral lower legs and ankles of 4 years’ duration (Figure 1). He also had a number of soft, compressible, cystlike plaques without much overlying epidermal change on the lower extremities. He denied any prior episodes of skin breakdown, drainage, or secondary infection. Findings from the physical examination were otherwise unremarkable.
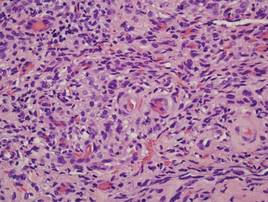
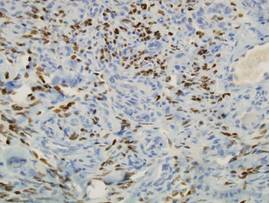
Two punch biopsies were performed on the lower legs, one from a scaly plaque and the other from a cystic area. The epidermis was hyperkeratotic and mildly hyperplastic with slitlike vascular spaces. A dense cellular proliferation of spindle-shaped cells was present in the dermis (Figure 2). Minimal cytologic atypia was noted. Immunohistochemical staining for human herpesvirus 8 (HHV-8) was strongly positive (Figure 3). Histologically, the cutaneous lesions were consistent with VKS.
At the current presentation, the CD4 count was 355 cells/mm3 and the viral load was 919,223 copies/mL. The CD4 count and viral load initially had been responsive to efavirenz-emtricitabine-tenofovir therapy; 17 months prior to the current presentation, the CD4 count was 692 cells/mm3 and the viral load was less than 50 copies/mL. However, the cutaneous lesions persisted despite therapy with efavirenz-emtricitabine-tenofovir, alitretinoin gel, and intralesional chemotherapeutic agents such as doxorubicin and paclitaxel.
Kaposi sarcoma, first described by Moritz Kaposi in 1872, represents a group of vascular neoplasms. Multiple subtypes have been described including classic, African endemic, transplant/AIDS associated, anaplastic, lymphedematous, hyperkeratotic/verrucous, keloidal, micronodular, pyogenic granulomalike, ecchymotic, and intravascular.1-3 Human herpesvirus 8 is associated with all clinical subtypes of KS.3 Immunohistochemical staining for HHV-8 latent nuclear antigen-1 has been shown in the literature to be highly sensitive and specific for KS and can potentially facilitate the diagnosis of KS among patients with similarly appearing dermatologic conditions, such as angiosarcoma, kaposiform hemangioendothelioma, or verrucous hemangioma.1,4 Human herpesvirus 8 infects endothelial cells and induces the proliferation of vascular spindle cells via the secretion of basic fibroblast growth factor and vascular endothelial growth factor.5 Human herpesvirus 8 also can lead to lymph vessel obstruction and lymph node enlargement by infecting cells within the lymphatic system. In addition, chronic lymphedema can itself lead to verruciform epidermal hyperplasia and hyperkeratosis, which has a clinical presentation similar to VKS.1
AIDS-associated KS typically starts as 1 or more purple-red macules that rapidly progress into papules, nodules, and plaques.1 These lesions have a predilection for the head, neck, trunk, and mucous membranes. Albeit a rare presentation, VKS is strongly associated with lymphedema in patients with AIDS.1,3,5 Previously, KS was often the presenting clinical manifestation of HIV infection, but since the use of highly active antiretroviral therapy (HAART) has become the standard of care, the incidence as well as the morbidity and mortality associated with KS has substantially decreased.1,5-7 Notably, in HIV patients who initially do not have signs or symptoms of KS, HHV-8 positivity is predictive of the development of KS within 2 to 4 years.6
In the literature, good prognostic indicators for KS include CD4 count greater than 150 cells/mm3, only cutaneous involvement, and negative B symptoms (eg, temperature >38°C, night sweats, unintentional weight loss >10% of normal body weight within 6 months).7 Kaposi sarcoma cannot be completely cured but can be appropriately managed with medical intervention. All KS subtypes are sensitive to radiation therapy; recalcitrant localized lesions can be treated with excision, cryotherapy, alitretinoin gel, laser ablation, or locally injected interferon or chemotherapeutic agents (eg, vincristine, vinblastine, actinomycin D).5,6 Liposomal anthracyclines (doxorubicin) and paclitaxel are first- and second-line agents for advanced KS, respectively.6
In HIV-associated KS, lesions frequently involute with the initiation of HAART; however, the cutaneous lesions in our patient persisted despite initiation of efavirenz-emtricitabine-tenofovir. He also was given intralesional doxorubicin andpaclitaxel as well as topical alitretinoin but did not experience complete resolution of the cutaneous lesions. It is possible that patients with VKS are recalcitrant to typical treatment modalities and therefore may require unconventional therapies to achieve maximal clearance of cutaneous lesions.
Verrucous Kaposi sarcoma is a rare presentation of KS that is infrequently seen in clinical practice or reported in the literature.3 A PubMed search of articles indexed for MEDLINE using the search term verrucous Kaposi sarcoma yielded 13 articles, one of which included a case series of 5 patients with AIDS and hyperkeratotic KS in Germany in the 1990s.5 Four of the articles were written in French, German, or Portuguese.8-11 The remainder of the articles discussed variants of KS other than VKS.
Although most patients with HIV and KS effectively respond to HAART, it may be possible that VKS is more difficult to treat. In addition, immunohistochemical staining for HHV-8, in particular HHV-8 latent nuclear antigen-1, may be useful to diagnose KS in HIV patients with uncharacteristic or indeterminate cutaneous lesions. Further research is needed to identify and delineate various efficacious therapeutic options for recalcitrant KS, particularly VKS.
Acknowledgment
We are indebted to Antoinette F. Hood, MD, Norfolk, Virginia, who digitized our patient’s histopathology slides.
1. Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
2. Amodio E, Goedert JJ, Barozzi P, et al. Differences in Kaposi sarcoma-associated herpesvirus-specific and herpesvirus-non-specific immune responses in classic Kaposi sarcoma cases and matched controls in Sicily. Cancer Sci. 2011;102:1769-1773.
3. Fagone S, Cavaleri A, Camuto M, et al. Hyperkeratotic Kaposi sarcoma with leg lymphedema after prolonged corticosteroid therapy for SLE. case report and review of the literature. Minerva Med. 2001;92:177-202.
4. Cheuk W, Wong KO, Wong CS, et al. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335-342.
5. Hengge UR, Stocks K, Goos M. Acquired immune deficiency syndrome-related hyperkeratotic Kaposi’s sarcoma with severe lymphedema: report of 5 cases. Br J Dermatol. 2000;142:501-505.
6. James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
7. Thomas S, Sindhu CB, Sreekumar S, et al. AIDS associated Kaposi’s Sarcoma. J Assoc Physicians India. 2011;59:387-389.
8. Mukai MM, Chaves T, Caldas L, et al. Primary Kaposi’s sarcoma of the penis [in Portuguese]. An Bras Dermatol. 2009;84:524-526.
9. Weidauer H, Tilgen W, Adler D. Kaposi’s sarcoma of the larynx [in German]. Laryngol Rhinol Otol (Stuttg). 1986;65:389-391.
10. Basset A. Clinical aspects of Kaposi’s disease [in French]. Bull Soc Pathol Exot Filiales. 1984;77(4, pt 2):529-532.
11. Wlotzke U, Hohenleutner U, Landthaler M. Dermatoses in leg amputees [in German]. Hautarzt. 1996;47:493-501.
To the Editor:
Verrucous Kaposi sarcoma (VKS) is an uncommon variant of Kaposi sarcoma (KS) that rarely is seen in clinical practice or reported in the literature. It is strongly associated with lymphedema in patients with AIDS.1 We present a case of VKS in a human immunodeficiency virus (HIV)–positive man with cutaneous lesions that demonstrated minimal response to treatment with efavirenz-emtricitabine-tenofovir, doxorubicin, paclitaxel, and alitretinoin.
A 48-year-old man with a history of untreated HIV presented with a persistent eruption of heavily scaled, hyperpigmented, nonindurated, thin plaques in an ichthyosiform pattern on the bilateral lower legs and ankles of 4 years’ duration (Figure 1). He also had a number of soft, compressible, cystlike plaques without much overlying epidermal change on the lower extremities. He denied any prior episodes of skin breakdown, drainage, or secondary infection. Findings from the physical examination were otherwise unremarkable.

Two punch biopsies were performed on the lower legs, one from a scaly plaque and the other from a cystic area. The epidermis was hyperkeratotic and mildly hyperplastic with slitlike vascular spaces. A dense cellular proliferation of spindle-shaped cells was present in the dermis (Figure 2). Minimal cytologic atypia was noted. Immunohistochemical staining for human herpesvirus 8 (HHV-8) was strongly positive (Figure 3). Histologically, the cutaneous lesions were consistent with VKS.


At the current presentation, the CD4 count was 355 cells/mm3 and the viral load was 919,223 copies/mL. The CD4 count and viral load initially had been responsive to efavirenz-emtricitabine-tenofovir therapy; 17 months prior to the current presentation, the CD4 count was 692 cells/mm3 and the viral load was less than 50 copies/mL. However, the cutaneous lesions persisted despite therapy with efavirenz-emtricitabine-tenofovir, alitretinoin gel, and intralesional chemotherapeutic agents such as doxorubicin and paclitaxel.
Kaposi sarcoma, first described by Moritz Kaposi in 1872, represents a group of vascular neoplasms. Multiple subtypes have been described including classic, African endemic, transplant/AIDS associated, anaplastic, lymphedematous, hyperkeratotic/verrucous, keloidal, micronodular, pyogenic granulomalike, ecchymotic, and intravascular.1-3 Human herpesvirus 8 is associated with all clinical subtypes of KS.3 Immunohistochemical staining for HHV-8 latent nuclear antigen-1 has been shown in the literature to be highly sensitive and specific for KS and can potentially facilitate the diagnosis of KS among patients with similarly appearing dermatologic conditions, such as angiosarcoma, kaposiform hemangioendothelioma, or verrucous hemangioma.1,4 Human herpesvirus 8 infects endothelial cells and induces the proliferation of vascular spindle cells via the secretion of basic fibroblast growth factor and vascular endothelial growth factor.5 Human herpesvirus 8 also can lead to lymph vessel obstruction and lymph node enlargement by infecting cells within the lymphatic system. In addition, chronic lymphedema can itself lead to verruciform epidermal hyperplasia and hyperkeratosis, which has a clinical presentation similar to VKS.1
AIDS-associated KS typically starts as 1 or more purple-red macules that rapidly progress into papules, nodules, and plaques.1 These lesions have a predilection for the head, neck, trunk, and mucous membranes. Albeit a rare presentation, VKS is strongly associated with lymphedema in patients with AIDS.1,3,5 Previously, KS was often the presenting clinical manifestation of HIV infection, but since the use of highly active antiretroviral therapy (HAART) has become the standard of care, the incidence as well as the morbidity and mortality associated with KS has substantially decreased.1,5-7 Notably, in HIV patients who initially do not have signs or symptoms of KS, HHV-8 positivity is predictive of the development of KS within 2 to 4 years.6
In the literature, good prognostic indicators for KS include CD4 count greater than 150 cells/mm3, only cutaneous involvement, and negative B symptoms (eg, temperature >38°C, night sweats, unintentional weight loss >10% of normal body weight within 6 months).7 Kaposi sarcoma cannot be completely cured but can be appropriately managed with medical intervention. All KS subtypes are sensitive to radiation therapy; recalcitrant localized lesions can be treated with excision, cryotherapy, alitretinoin gel, laser ablation, or locally injected interferon or chemotherapeutic agents (eg, vincristine, vinblastine, actinomycin D).5,6 Liposomal anthracyclines (doxorubicin) and paclitaxel are first- and second-line agents for advanced KS, respectively.6
In HIV-associated KS, lesions frequently involute with the initiation of HAART; however, the cutaneous lesions in our patient persisted despite initiation of efavirenz-emtricitabine-tenofovir. He also was given intralesional doxorubicin andpaclitaxel as well as topical alitretinoin but did not experience complete resolution of the cutaneous lesions. It is possible that patients with VKS are recalcitrant to typical treatment modalities and therefore may require unconventional therapies to achieve maximal clearance of cutaneous lesions.
Verrucous Kaposi sarcoma is a rare presentation of KS that is infrequently seen in clinical practice or reported in the literature.3 A PubMed search of articles indexed for MEDLINE using the search term verrucous Kaposi sarcoma yielded 13 articles, one of which included a case series of 5 patients with AIDS and hyperkeratotic KS in Germany in the 1990s.5 Four of the articles were written in French, German, or Portuguese.8-11 The remainder of the articles discussed variants of KS other than VKS.
Although most patients with HIV and KS effectively respond to HAART, it may be possible that VKS is more difficult to treat. In addition, immunohistochemical staining for HHV-8, in particular HHV-8 latent nuclear antigen-1, may be useful to diagnose KS in HIV patients with uncharacteristic or indeterminate cutaneous lesions. Further research is needed to identify and delineate various efficacious therapeutic options for recalcitrant KS, particularly VKS.
Acknowledgment
We are indebted to Antoinette F. Hood, MD, Norfolk, Virginia, who digitized our patient’s histopathology slides.
To the Editor:
Verrucous Kaposi sarcoma (VKS) is an uncommon variant of Kaposi sarcoma (KS) that rarely is seen in clinical practice or reported in the literature. It is strongly associated with lymphedema in patients with AIDS.1 We present a case of VKS in a human immunodeficiency virus (HIV)–positive man with cutaneous lesions that demonstrated minimal response to treatment with efavirenz-emtricitabine-tenofovir, doxorubicin, paclitaxel, and alitretinoin.
A 48-year-old man with a history of untreated HIV presented with a persistent eruption of heavily scaled, hyperpigmented, nonindurated, thin plaques in an ichthyosiform pattern on the bilateral lower legs and ankles of 4 years’ duration (Figure 1). He also had a number of soft, compressible, cystlike plaques without much overlying epidermal change on the lower extremities. He denied any prior episodes of skin breakdown, drainage, or secondary infection. Findings from the physical examination were otherwise unremarkable.

Two punch biopsies were performed on the lower legs, one from a scaly plaque and the other from a cystic area. The epidermis was hyperkeratotic and mildly hyperplastic with slitlike vascular spaces. A dense cellular proliferation of spindle-shaped cells was present in the dermis (Figure 2). Minimal cytologic atypia was noted. Immunohistochemical staining for human herpesvirus 8 (HHV-8) was strongly positive (Figure 3). Histologically, the cutaneous lesions were consistent with VKS.


At the current presentation, the CD4 count was 355 cells/mm3 and the viral load was 919,223 copies/mL. The CD4 count and viral load initially had been responsive to efavirenz-emtricitabine-tenofovir therapy; 17 months prior to the current presentation, the CD4 count was 692 cells/mm3 and the viral load was less than 50 copies/mL. However, the cutaneous lesions persisted despite therapy with efavirenz-emtricitabine-tenofovir, alitretinoin gel, and intralesional chemotherapeutic agents such as doxorubicin and paclitaxel.
Kaposi sarcoma, first described by Moritz Kaposi in 1872, represents a group of vascular neoplasms. Multiple subtypes have been described including classic, African endemic, transplant/AIDS associated, anaplastic, lymphedematous, hyperkeratotic/verrucous, keloidal, micronodular, pyogenic granulomalike, ecchymotic, and intravascular.1-3 Human herpesvirus 8 is associated with all clinical subtypes of KS.3 Immunohistochemical staining for HHV-8 latent nuclear antigen-1 has been shown in the literature to be highly sensitive and specific for KS and can potentially facilitate the diagnosis of KS among patients with similarly appearing dermatologic conditions, such as angiosarcoma, kaposiform hemangioendothelioma, or verrucous hemangioma.1,4 Human herpesvirus 8 infects endothelial cells and induces the proliferation of vascular spindle cells via the secretion of basic fibroblast growth factor and vascular endothelial growth factor.5 Human herpesvirus 8 also can lead to lymph vessel obstruction and lymph node enlargement by infecting cells within the lymphatic system. In addition, chronic lymphedema can itself lead to verruciform epidermal hyperplasia and hyperkeratosis, which has a clinical presentation similar to VKS.1
AIDS-associated KS typically starts as 1 or more purple-red macules that rapidly progress into papules, nodules, and plaques.1 These lesions have a predilection for the head, neck, trunk, and mucous membranes. Albeit a rare presentation, VKS is strongly associated with lymphedema in patients with AIDS.1,3,5 Previously, KS was often the presenting clinical manifestation of HIV infection, but since the use of highly active antiretroviral therapy (HAART) has become the standard of care, the incidence as well as the morbidity and mortality associated with KS has substantially decreased.1,5-7 Notably, in HIV patients who initially do not have signs or symptoms of KS, HHV-8 positivity is predictive of the development of KS within 2 to 4 years.6
In the literature, good prognostic indicators for KS include CD4 count greater than 150 cells/mm3, only cutaneous involvement, and negative B symptoms (eg, temperature >38°C, night sweats, unintentional weight loss >10% of normal body weight within 6 months).7 Kaposi sarcoma cannot be completely cured but can be appropriately managed with medical intervention. All KS subtypes are sensitive to radiation therapy; recalcitrant localized lesions can be treated with excision, cryotherapy, alitretinoin gel, laser ablation, or locally injected interferon or chemotherapeutic agents (eg, vincristine, vinblastine, actinomycin D).5,6 Liposomal anthracyclines (doxorubicin) and paclitaxel are first- and second-line agents for advanced KS, respectively.6
In HIV-associated KS, lesions frequently involute with the initiation of HAART; however, the cutaneous lesions in our patient persisted despite initiation of efavirenz-emtricitabine-tenofovir. He also was given intralesional doxorubicin andpaclitaxel as well as topical alitretinoin but did not experience complete resolution of the cutaneous lesions. It is possible that patients with VKS are recalcitrant to typical treatment modalities and therefore may require unconventional therapies to achieve maximal clearance of cutaneous lesions.
Verrucous Kaposi sarcoma is a rare presentation of KS that is infrequently seen in clinical practice or reported in the literature.3 A PubMed search of articles indexed for MEDLINE using the search term verrucous Kaposi sarcoma yielded 13 articles, one of which included a case series of 5 patients with AIDS and hyperkeratotic KS in Germany in the 1990s.5 Four of the articles were written in French, German, or Portuguese.8-11 The remainder of the articles discussed variants of KS other than VKS.
Although most patients with HIV and KS effectively respond to HAART, it may be possible that VKS is more difficult to treat. In addition, immunohistochemical staining for HHV-8, in particular HHV-8 latent nuclear antigen-1, may be useful to diagnose KS in HIV patients with uncharacteristic or indeterminate cutaneous lesions. Further research is needed to identify and delineate various efficacious therapeutic options for recalcitrant KS, particularly VKS.
Acknowledgment
We are indebted to Antoinette F. Hood, MD, Norfolk, Virginia, who digitized our patient’s histopathology slides.
1. Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
2. Amodio E, Goedert JJ, Barozzi P, et al. Differences in Kaposi sarcoma-associated herpesvirus-specific and herpesvirus-non-specific immune responses in classic Kaposi sarcoma cases and matched controls in Sicily. Cancer Sci. 2011;102:1769-1773.
3. Fagone S, Cavaleri A, Camuto M, et al. Hyperkeratotic Kaposi sarcoma with leg lymphedema after prolonged corticosteroid therapy for SLE. case report and review of the literature. Minerva Med. 2001;92:177-202.
4. Cheuk W, Wong KO, Wong CS, et al. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335-342.
5. Hengge UR, Stocks K, Goos M. Acquired immune deficiency syndrome-related hyperkeratotic Kaposi’s sarcoma with severe lymphedema: report of 5 cases. Br J Dermatol. 2000;142:501-505.
6. James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
7. Thomas S, Sindhu CB, Sreekumar S, et al. AIDS associated Kaposi’s Sarcoma. J Assoc Physicians India. 2011;59:387-389.
8. Mukai MM, Chaves T, Caldas L, et al. Primary Kaposi’s sarcoma of the penis [in Portuguese]. An Bras Dermatol. 2009;84:524-526.
9. Weidauer H, Tilgen W, Adler D. Kaposi’s sarcoma of the larynx [in German]. Laryngol Rhinol Otol (Stuttg). 1986;65:389-391.
10. Basset A. Clinical aspects of Kaposi’s disease [in French]. Bull Soc Pathol Exot Filiales. 1984;77(4, pt 2):529-532.
11. Wlotzke U, Hohenleutner U, Landthaler M. Dermatoses in leg amputees [in German]. Hautarzt. 1996;47:493-501.
1. Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
2. Amodio E, Goedert JJ, Barozzi P, et al. Differences in Kaposi sarcoma-associated herpesvirus-specific and herpesvirus-non-specific immune responses in classic Kaposi sarcoma cases and matched controls in Sicily. Cancer Sci. 2011;102:1769-1773.
3. Fagone S, Cavaleri A, Camuto M, et al. Hyperkeratotic Kaposi sarcoma with leg lymphedema after prolonged corticosteroid therapy for SLE. case report and review of the literature. Minerva Med. 2001;92:177-202.
4. Cheuk W, Wong KO, Wong CS, et al. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335-342.
5. Hengge UR, Stocks K, Goos M. Acquired immune deficiency syndrome-related hyperkeratotic Kaposi’s sarcoma with severe lymphedema: report of 5 cases. Br J Dermatol. 2000;142:501-505.
6. James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
7. Thomas S, Sindhu CB, Sreekumar S, et al. AIDS associated Kaposi’s Sarcoma. J Assoc Physicians India. 2011;59:387-389.
8. Mukai MM, Chaves T, Caldas L, et al. Primary Kaposi’s sarcoma of the penis [in Portuguese]. An Bras Dermatol. 2009;84:524-526.
9. Weidauer H, Tilgen W, Adler D. Kaposi’s sarcoma of the larynx [in German]. Laryngol Rhinol Otol (Stuttg). 1986;65:389-391.
10. Basset A. Clinical aspects of Kaposi’s disease [in French]. Bull Soc Pathol Exot Filiales. 1984;77(4, pt 2):529-532.
11. Wlotzke U, Hohenleutner U, Landthaler M. Dermatoses in leg amputees [in German]. Hautarzt. 1996;47:493-501.