User login
Teledermatology During the COVID-19 Pandemic: Lessons Learned and Future Directions
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
What Is Your Diagnosis? Verrucous Carcinoma
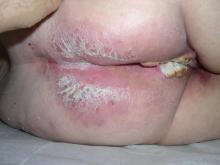
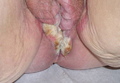
An 81-year-old woman presented for evaluation of a nodule on the right labia majora that had been present for 1 year. She had a history of intertriginous psoriasis, and several biopsies were performed at an outside facility over the last 5 years that revealed psoriasis but were otherwise noncontributory. Physical examination revealed erythema and scaling on the buttocks with maceration in the intertriginous area (top) and the perineum associated with a verrucous nodule (bottom).
The Diagnosis: Verrucous Carcinoma
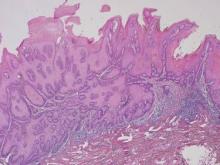
Biopsies of early lesions often may be difficult to interpret without clinicopathological correlation. Our patient’s tumor was associated with intertriginous psoriasis, which was the only abnormality previously noted on superficial biopsies performed at an outside facility. The patient was scheduled for an excisional biopsy due to the large tumor size and clinical suspicion that the prior biopsies were inadequate and failed to demonstrate the primary underlying pathology. Excisional biopsy of the verrucous tumor revealed epithelium composed of keratinocytes with glassy cytoplasm. Papillomatosis was noted along with an endophytic component of well-differentiated epithelial cells extending into the dermis in a bulbous pattern consistent with the verrucous carcinoma variant of squamous cell carcinoma (SCC)(Figure). Verrucous carcinoma often requires correlation with both the clinical and histopathologic findings for definitive diagnosis, as keratinocytes often appear to be well differentiated.1
Verrucous carcinoma may begin as an innocuous papule that slowly grows into a large fungating tumor. Verrucous carcinomas typically are slow growing, exophytic, and low grade. The etiology of verrucous carcinoma is not clear, and the role of human papillomavirus (HPV) infection is controversial.2 Best classified as a well-differentiated SCC, verrucous carcinoma rarely metastasizes but may invade adjacent tissues.
Differential diagnoses include a giant inflamed seborrheic keratosis, condyloma acuminatum, rupioid psoriasis, and inflammatory linear verrucous epidermal nevus (ILVEN). Although large and inflamed seborrheic keratoses may have squamous eddies that mimic SCC, seborrheic keratoses do not invade the dermis and typically have a well-circumscribed stuck-on appearance. Abnormal mitotic figures are not identified. Condylomas are genital warts caused by HPV infection that often are clustered, well circumscribed, and exophytic. Large lesions can be difficult to distinguish from verrucous carcinomas, and biopsy generally reveals koilocytes identified by perinuclear clearing and raisinlike nuclei. Immunohistochemical staining and in situ hybridization studies can be of value in diagnosis and in identifying those lesions that are at high risk for malignant transformation. High-risk condylomas are associated with HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, and HPV-39, as well as other types, whereas low-risk condylomas are associated with HPV-6, HPV-11, HPV-42, and others.2 Differentiating squamous cell hyperplasia from squamous cell carcinoma in situ also can be aided by immunohistochemistry. Squamous cell hyperplasia is usually negative for INK4 p16Ink4A and p53 and exhibits variable Ki-67 staining. Differentiated squamous cell carcinoma in situ exhibits a profile that is p16Ink4A negative, Ki-67 positive, and exhibits variable p53 staining.3 Basaloid and warty intraepithelial neoplasia is consistently p16Ink4A positive, Ki-67 positive, and variably positive for p53.3 Therefore, p16 staining of high-grade areas is a useful biomarker that can help establish diagnosis of associated squamous cell carcinoma.4 The role of papillomaviruses in the development of nonmelanoma skin cancer is an area of active study, and research suggests that papillomaviruses may have a much greater role than previously suspected.5
At times, psoriasis may be markedly hyperkeratotic, clinically mimicking a verrucous neoplasm. This hyperkeratotic type of psoriasis is known as rupioid psoriasis. However, these psoriatic lesions are exophytic, are associated with spongiform pustules, and lack the atypia and endophytic pattern typically seen with verrucous carcinoma. An ILVEN also lacks atypia and an endophytic pattern and usually presents in childhood as a persistent linear plaque, rather than the verrucous plaque noted in our patient. Squamous cell carcinoma has been reported to arise in the setting of verrucoid ILVEN but is exceptionally uncommon.6
Successful treatment of verrucous carcinoma is best achieved by complete excision. Oral retinoids and immunomodulators such as imiquimod also may be of value.7 Our patient’s tumor qualifies as T2N0M0 because it was greater than 2 cm in size.8 A Breslow thickness of 2 mm or greater and Clark level IV are high-risk features associated with a worse prognosis, but clinical evaluation of our patient’s lymph nodes was unremarkable and no distant metastases were identified. Our patient continues to do well with no evidence of recurrence.
1. Bambao C, Nofech-Mozes S, Shier M. Giant condyloma versus verrucous carcinoma: a case report. J Low Genit Tract Dis. 2010;14:230-233.
2. Asiaf A, Ahmad ST, Mohannad SO, et al. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23:206-224.
3. Chaux A, Pfannl R, Rodríguez IM, et al. Distinctive immunohistochemical profile of penile intraepithelial lesions: a study of 74 cases. Am J Surg Pathol. 2011;35:553-562.
4. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297.
5. Aldabagh B, Angeles J, Cardones AR, et al. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1-23.
6. Turk BG, Ertam I, Urkmez A, et al. Development of squamous cell carcinoma on an inflammatory linear verrucous epidermal nevus in the genital area. Cutis. 2012;89:273-275.
7. Erkek E, Basar H, Bozdogan O, et al. Giant condyloma acuminata of Buschke-Löwenstein: successful treatment with a combination of surgical excision, oral acitretin and topical imiquimod. Clin Exp Dermatol. 2009;34:366-368.
8. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301-314.
An 81-year-old woman presented for evaluation of a nodule on the right labia majora that had been present for 1 year. She had a history of intertriginous psoriasis, and several biopsies were performed at an outside facility over the last 5 years that revealed psoriasis but were otherwise noncontributory. Physical examination revealed erythema and scaling on the buttocks with maceration in the intertriginous area (top) and the perineum associated with a verrucous nodule (bottom).
The Diagnosis: Verrucous Carcinoma
Biopsies of early lesions often may be difficult to interpret without clinicopathological correlation. Our patient’s tumor was associated with intertriginous psoriasis, which was the only abnormality previously noted on superficial biopsies performed at an outside facility. The patient was scheduled for an excisional biopsy due to the large tumor size and clinical suspicion that the prior biopsies were inadequate and failed to demonstrate the primary underlying pathology. Excisional biopsy of the verrucous tumor revealed epithelium composed of keratinocytes with glassy cytoplasm. Papillomatosis was noted along with an endophytic component of well-differentiated epithelial cells extending into the dermis in a bulbous pattern consistent with the verrucous carcinoma variant of squamous cell carcinoma (SCC)(Figure). Verrucous carcinoma often requires correlation with both the clinical and histopathologic findings for definitive diagnosis, as keratinocytes often appear to be well differentiated.1
Verrucous carcinoma may begin as an innocuous papule that slowly grows into a large fungating tumor. Verrucous carcinomas typically are slow growing, exophytic, and low grade. The etiology of verrucous carcinoma is not clear, and the role of human papillomavirus (HPV) infection is controversial.2 Best classified as a well-differentiated SCC, verrucous carcinoma rarely metastasizes but may invade adjacent tissues.
Differential diagnoses include a giant inflamed seborrheic keratosis, condyloma acuminatum, rupioid psoriasis, and inflammatory linear verrucous epidermal nevus (ILVEN). Although large and inflamed seborrheic keratoses may have squamous eddies that mimic SCC, seborrheic keratoses do not invade the dermis and typically have a well-circumscribed stuck-on appearance. Abnormal mitotic figures are not identified. Condylomas are genital warts caused by HPV infection that often are clustered, well circumscribed, and exophytic. Large lesions can be difficult to distinguish from verrucous carcinomas, and biopsy generally reveals koilocytes identified by perinuclear clearing and raisinlike nuclei. Immunohistochemical staining and in situ hybridization studies can be of value in diagnosis and in identifying those lesions that are at high risk for malignant transformation. High-risk condylomas are associated with HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, and HPV-39, as well as other types, whereas low-risk condylomas are associated with HPV-6, HPV-11, HPV-42, and others.2 Differentiating squamous cell hyperplasia from squamous cell carcinoma in situ also can be aided by immunohistochemistry. Squamous cell hyperplasia is usually negative for INK4 p16Ink4A and p53 and exhibits variable Ki-67 staining. Differentiated squamous cell carcinoma in situ exhibits a profile that is p16Ink4A negative, Ki-67 positive, and exhibits variable p53 staining.3 Basaloid and warty intraepithelial neoplasia is consistently p16Ink4A positive, Ki-67 positive, and variably positive for p53.3 Therefore, p16 staining of high-grade areas is a useful biomarker that can help establish diagnosis of associated squamous cell carcinoma.4 The role of papillomaviruses in the development of nonmelanoma skin cancer is an area of active study, and research suggests that papillomaviruses may have a much greater role than previously suspected.5
At times, psoriasis may be markedly hyperkeratotic, clinically mimicking a verrucous neoplasm. This hyperkeratotic type of psoriasis is known as rupioid psoriasis. However, these psoriatic lesions are exophytic, are associated with spongiform pustules, and lack the atypia and endophytic pattern typically seen with verrucous carcinoma. An ILVEN also lacks atypia and an endophytic pattern and usually presents in childhood as a persistent linear plaque, rather than the verrucous plaque noted in our patient. Squamous cell carcinoma has been reported to arise in the setting of verrucoid ILVEN but is exceptionally uncommon.6
Successful treatment of verrucous carcinoma is best achieved by complete excision. Oral retinoids and immunomodulators such as imiquimod also may be of value.7 Our patient’s tumor qualifies as T2N0M0 because it was greater than 2 cm in size.8 A Breslow thickness of 2 mm or greater and Clark level IV are high-risk features associated with a worse prognosis, but clinical evaluation of our patient’s lymph nodes was unremarkable and no distant metastases were identified. Our patient continues to do well with no evidence of recurrence.
An 81-year-old woman presented for evaluation of a nodule on the right labia majora that had been present for 1 year. She had a history of intertriginous psoriasis, and several biopsies were performed at an outside facility over the last 5 years that revealed psoriasis but were otherwise noncontributory. Physical examination revealed erythema and scaling on the buttocks with maceration in the intertriginous area (top) and the perineum associated with a verrucous nodule (bottom).
The Diagnosis: Verrucous Carcinoma
Biopsies of early lesions often may be difficult to interpret without clinicopathological correlation. Our patient’s tumor was associated with intertriginous psoriasis, which was the only abnormality previously noted on superficial biopsies performed at an outside facility. The patient was scheduled for an excisional biopsy due to the large tumor size and clinical suspicion that the prior biopsies were inadequate and failed to demonstrate the primary underlying pathology. Excisional biopsy of the verrucous tumor revealed epithelium composed of keratinocytes with glassy cytoplasm. Papillomatosis was noted along with an endophytic component of well-differentiated epithelial cells extending into the dermis in a bulbous pattern consistent with the verrucous carcinoma variant of squamous cell carcinoma (SCC)(Figure). Verrucous carcinoma often requires correlation with both the clinical and histopathologic findings for definitive diagnosis, as keratinocytes often appear to be well differentiated.1
Verrucous carcinoma may begin as an innocuous papule that slowly grows into a large fungating tumor. Verrucous carcinomas typically are slow growing, exophytic, and low grade. The etiology of verrucous carcinoma is not clear, and the role of human papillomavirus (HPV) infection is controversial.2 Best classified as a well-differentiated SCC, verrucous carcinoma rarely metastasizes but may invade adjacent tissues.
Differential diagnoses include a giant inflamed seborrheic keratosis, condyloma acuminatum, rupioid psoriasis, and inflammatory linear verrucous epidermal nevus (ILVEN). Although large and inflamed seborrheic keratoses may have squamous eddies that mimic SCC, seborrheic keratoses do not invade the dermis and typically have a well-circumscribed stuck-on appearance. Abnormal mitotic figures are not identified. Condylomas are genital warts caused by HPV infection that often are clustered, well circumscribed, and exophytic. Large lesions can be difficult to distinguish from verrucous carcinomas, and biopsy generally reveals koilocytes identified by perinuclear clearing and raisinlike nuclei. Immunohistochemical staining and in situ hybridization studies can be of value in diagnosis and in identifying those lesions that are at high risk for malignant transformation. High-risk condylomas are associated with HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, and HPV-39, as well as other types, whereas low-risk condylomas are associated with HPV-6, HPV-11, HPV-42, and others.2 Differentiating squamous cell hyperplasia from squamous cell carcinoma in situ also can be aided by immunohistochemistry. Squamous cell hyperplasia is usually negative for INK4 p16Ink4A and p53 and exhibits variable Ki-67 staining. Differentiated squamous cell carcinoma in situ exhibits a profile that is p16Ink4A negative, Ki-67 positive, and exhibits variable p53 staining.3 Basaloid and warty intraepithelial neoplasia is consistently p16Ink4A positive, Ki-67 positive, and variably positive for p53.3 Therefore, p16 staining of high-grade areas is a useful biomarker that can help establish diagnosis of associated squamous cell carcinoma.4 The role of papillomaviruses in the development of nonmelanoma skin cancer is an area of active study, and research suggests that papillomaviruses may have a much greater role than previously suspected.5
At times, psoriasis may be markedly hyperkeratotic, clinically mimicking a verrucous neoplasm. This hyperkeratotic type of psoriasis is known as rupioid psoriasis. However, these psoriatic lesions are exophytic, are associated with spongiform pustules, and lack the atypia and endophytic pattern typically seen with verrucous carcinoma. An ILVEN also lacks atypia and an endophytic pattern and usually presents in childhood as a persistent linear plaque, rather than the verrucous plaque noted in our patient. Squamous cell carcinoma has been reported to arise in the setting of verrucoid ILVEN but is exceptionally uncommon.6
Successful treatment of verrucous carcinoma is best achieved by complete excision. Oral retinoids and immunomodulators such as imiquimod also may be of value.7 Our patient’s tumor qualifies as T2N0M0 because it was greater than 2 cm in size.8 A Breslow thickness of 2 mm or greater and Clark level IV are high-risk features associated with a worse prognosis, but clinical evaluation of our patient’s lymph nodes was unremarkable and no distant metastases were identified. Our patient continues to do well with no evidence of recurrence.
1. Bambao C, Nofech-Mozes S, Shier M. Giant condyloma versus verrucous carcinoma: a case report. J Low Genit Tract Dis. 2010;14:230-233.
2. Asiaf A, Ahmad ST, Mohannad SO, et al. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23:206-224.
3. Chaux A, Pfannl R, Rodríguez IM, et al. Distinctive immunohistochemical profile of penile intraepithelial lesions: a study of 74 cases. Am J Surg Pathol. 2011;35:553-562.
4. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297.
5. Aldabagh B, Angeles J, Cardones AR, et al. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1-23.
6. Turk BG, Ertam I, Urkmez A, et al. Development of squamous cell carcinoma on an inflammatory linear verrucous epidermal nevus in the genital area. Cutis. 2012;89:273-275.
7. Erkek E, Basar H, Bozdogan O, et al. Giant condyloma acuminata of Buschke-Löwenstein: successful treatment with a combination of surgical excision, oral acitretin and topical imiquimod. Clin Exp Dermatol. 2009;34:366-368.
8. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301-314.
1. Bambao C, Nofech-Mozes S, Shier M. Giant condyloma versus verrucous carcinoma: a case report. J Low Genit Tract Dis. 2010;14:230-233.
2. Asiaf A, Ahmad ST, Mohannad SO, et al. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23:206-224.
3. Chaux A, Pfannl R, Rodríguez IM, et al. Distinctive immunohistochemical profile of penile intraepithelial lesions: a study of 74 cases. Am J Surg Pathol. 2011;35:553-562.
4. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297.
5. Aldabagh B, Angeles J, Cardones AR, et al. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1-23.
6. Turk BG, Ertam I, Urkmez A, et al. Development of squamous cell carcinoma on an inflammatory linear verrucous epidermal nevus in the genital area. Cutis. 2012;89:273-275.
7. Erkek E, Basar H, Bozdogan O, et al. Giant condyloma acuminata of Buschke-Löwenstein: successful treatment with a combination of surgical excision, oral acitretin and topical imiquimod. Clin Exp Dermatol. 2009;34:366-368.
8. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301-314.