User login
Are Text Pages an Effective Nudge to Increase Attendance at Internal Medicine Morning Report Conferences? A Cluster Randomized Controlled Trial
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
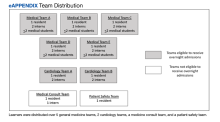
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
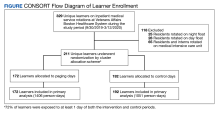
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
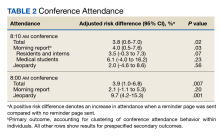
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
Alcohol-Related Hospitalizations During the Initial COVID-19 Lockdown in Massachusetts: An Interrupted Time-Series Analysis
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
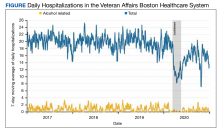
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
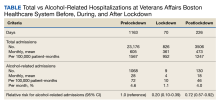
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
A Mission for Graduate Medical Education at VA
More than 65% of all physicians who train in the U.S. rotate through a VA hospital at some point during their training. In 2015 alone, more than 43,000 residents received some or all of their clinical training through VA.1 Of the approximately 120 VAMCs that hold academic affiliations
with medical schools and residency training programs, several hold affiliations with multiple institutions, including VA Boston Healthcare System (VABHS) in Massachusetts. The West Roxbury campus is the home of VA Boston’s acute care hospital, where residents and fellows from Boston Medical Center (BMC), Beth Israel Deaconess Medical Center (BIDMC), and Brigham and Women’s Hospital (BWH) train together. These are 3 of the largest medical training programs in Boston, though each provides a unique training experience for residents due to differences in patient population, faculty expertise, and hospital network affiliations (Table 1).
This diversity brings differences in cultural norms, institutional preferences, and educational expectations. Furthermore, residents from different programs who work together at VA Boston are often meeting one another for the first time, as opportunities for interinstitutional collaboration among these 3 training programs do not exist outside of VA. This training environment presents both an opportunity
and a challenge for medical educators: offering the best possible learning experience for physiciansin-training from multiple programs while providing the best possible care for U.S. veterans.
To guide educators charged with meeting this challenge, the VA Office of Academic Affiliations put forth a mission statement describing its overarching teaching mission (Table 2).2
To address this gap, chief medical residents from the 3 affiliate residency training programs came together to develop a shared mission statement for what we envision as the educational experience for all medical trainees rotating through VABHS (Table 2). In this article, we describe the development of a mission statement for graduate medical education in internal medicine at VABHS and provides examples of how our mission statement guided educational programming.
Methods
Whereas the affiliated institutions assign generic competency-based learning objectives to rotations at VABHS, no specific overarching educational objectives for residents have been defined previously. The directors of the internal medicine residency programs at each of the VABHS affiliate institutions grant their respective VA-based chief medical residents the autonomy to deliver graduate medical education at VA as they see fit, in collaboration with their colleagues from the other affiliated institutions and the VA director of medical resident education. This autonomy and flexibility allowed each of the chief medical residents to articulate an individual vision for VA graduate medical education based on their affiliate program’s goals, values, and mission.
At the beginning of the 2016/2017 academic year, in partnership with the director of medical resident education at VABHS, the chief medical residents met to reconcile these into a single shared mission statement. Special attention was paid to educational gaps at each affiliate institution that could be filled while residents were rotating at VABHS. Once all educational goals and priorities of the shared mission statement were identified, the chief medical residents and director of medical resident education adopted the mission statement as the blueprint for all educational programming for the academic year. Progress toward enacting the various components of the mission statement was reviewed monthly and changes in educational programming to ensure adequate emphasis of all components were made accordingly.
Results
Our first goal was to promote the personal and professional development of residents who rotate through VABHS, particularly interns, in a setting that fosters cross-institutional collaboration, respect, and friendship. The West Roxbury campus of VABHS is the only hospital in the city where internal medicine residents from 3 large training programs work together on teams that have been intentionally built to place residents from different institutions with one another. In educational conferences, we encouraged residents from different training programs to share their experiences with patient populations that others may not see at their home institutions, based on the specialized care that each institution provides. The conferences also give residents the opportunity to provide and receive near-peer teaching in a collegial environment.
Our second goal was to maintain an environment of educational excellence. We produced thought-provoking conferences that prioritized inspiring curiosity and teaching systems of thought over the dissemination of facts. We regularly focused on the broader context of medicine in case conferences and journal club, including topics such as public health, health policy, advocacy, health economics, quality improvement (QI), and high-value care. Our morning reports were interactive and participatory, emphasizing both technical skill practice and sophisticated clinical reasoning.
We embraced the principles of cognitive learning theory by priming learners with preconference “teasers” that previewed conference topics to be discussed. Every Friday, we played a medical version of Jeopardy!, which used spaced learning to consolidate the week’s teaching points in a fun, collaborative, and collegial atmosphere. Our dedicated patient safety conference gave residents the chance to use QI tools to dissect and tackle real problems in the hospital, and our monthly Morbidity and Mortality conference served as inspiration for many of the resident-driven QI projects.
Our third goal was to challenge physicians to provide the best possible care to veterans, including learning about issues unique to this often-marginalized population. We emphasized that training at a VA hospital is a privilege and that the best way to honor our veterans is to take advantage of the unique learning opportunities available at VA. To that end, we exposed residents to veteran-specific educational content, ranging from the structure and payment model of VHA to service-related medical conditions, such as posttraumatic stress disorder, other mental health issues, traumatic brain injury, Agent Orange exposure, and Gulf War Syndrome.
Discussion
Findings from the recently published Accreditation Council for Graduate Medical Education’s (ACGME) 2016 Clinical Learning Environment Review (CLER) Report support the need for mission statements like ours to guide the delivery of graduate medical education.3 A major finding of this report was that the development and implementation of graduate medical education largely occurs separately from other areas of organizational and strategic focus within clinical learning environments. Our mission statement has served as a road map for aligning the delivery of graduate medical education at VABHS with the specific strengths of the clinical learning environment that VA affords.
Additionally, the 2016 CLER report identified a lack of specificity in training on health care disparities and cultural competency for the specific populations served by the surveyed residency programs. The emphasis we placed on learning about issues specific to the care of the veteran population highlights the potential for other mission statements like ours to bridge the gap between articulation and execution of educational priorities. Finally, through the academic partnerships it holds with more than 90% of medical schools in the U.S., VA already has an integral role in both undergraduate and graduate medical education that positions its hospitals as ideal training environments in which to address shortcomings in medical training like those identified by the ACGME.4
Conclusion
We propose this mission statement as a model for the delivery of graduate medical education throughout all VA hospitals with academic affiliations and especially those where trainees from multiple institutions work together. As embodied in our mission statement, our goal was to provide a clinical training experience at VA that complements that of our residents’ home institutions and fosters a respect for and interest in the special care provided at VA. The development of a shared mission statement provides an invaluable tool in accomplishing that goal. We encourage chief medical residents and other leaders in medical education in all specialties at VAMCs to develop their own mission statements that reflect and embody the values of each affiliated training program. For our residents, rotating at VA is an opportunity to learn the practice of medicine for veterans, rather than practicing medicine on veterans. It is our sincere hope that shaping our residents’ educational experience in this fashion will foster a greater appreciation for the care of our nation’s veterans.
1. VA Office of Academic Affiliations. 2015 statistics: health professions trainees. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Published 2016. Accessed September 18, 2017.
2. VA Office of Academic Affiliations. Mission of the Office of Academic Affiliations. http://www.va.gov/oaa/oaa_mission.asp. Updated June 23, 2017. Accessed September 18, 2017.
3. Accreditation Council for Graduate Medical Education. Clinical learning environment review – national report of findings 2016 – executive summary. https://www.acgme.org/Portals/0/PDFs/CLER/ACGME-CLER-ExecutiveSummary.pdf. Published 2016. Accessed September 18, 2017.
4. Association of American Medical Colleges. The VA and academic medicine: partners in health care, training, and research. https://www.aamc.org/download/385612/data/07182014.pdf. Accessed September 14, 2017.
More than 65% of all physicians who train in the U.S. rotate through a VA hospital at some point during their training. In 2015 alone, more than 43,000 residents received some or all of their clinical training through VA.1 Of the approximately 120 VAMCs that hold academic affiliations
with medical schools and residency training programs, several hold affiliations with multiple institutions, including VA Boston Healthcare System (VABHS) in Massachusetts. The West Roxbury campus is the home of VA Boston’s acute care hospital, where residents and fellows from Boston Medical Center (BMC), Beth Israel Deaconess Medical Center (BIDMC), and Brigham and Women’s Hospital (BWH) train together. These are 3 of the largest medical training programs in Boston, though each provides a unique training experience for residents due to differences in patient population, faculty expertise, and hospital network affiliations (Table 1).
This diversity brings differences in cultural norms, institutional preferences, and educational expectations. Furthermore, residents from different programs who work together at VA Boston are often meeting one another for the first time, as opportunities for interinstitutional collaboration among these 3 training programs do not exist outside of VA. This training environment presents both an opportunity
and a challenge for medical educators: offering the best possible learning experience for physiciansin-training from multiple programs while providing the best possible care for U.S. veterans.
To guide educators charged with meeting this challenge, the VA Office of Academic Affiliations put forth a mission statement describing its overarching teaching mission (Table 2).2
To address this gap, chief medical residents from the 3 affiliate residency training programs came together to develop a shared mission statement for what we envision as the educational experience for all medical trainees rotating through VABHS (Table 2). In this article, we describe the development of a mission statement for graduate medical education in internal medicine at VABHS and provides examples of how our mission statement guided educational programming.
Methods
Whereas the affiliated institutions assign generic competency-based learning objectives to rotations at VABHS, no specific overarching educational objectives for residents have been defined previously. The directors of the internal medicine residency programs at each of the VABHS affiliate institutions grant their respective VA-based chief medical residents the autonomy to deliver graduate medical education at VA as they see fit, in collaboration with their colleagues from the other affiliated institutions and the VA director of medical resident education. This autonomy and flexibility allowed each of the chief medical residents to articulate an individual vision for VA graduate medical education based on their affiliate program’s goals, values, and mission.
At the beginning of the 2016/2017 academic year, in partnership with the director of medical resident education at VABHS, the chief medical residents met to reconcile these into a single shared mission statement. Special attention was paid to educational gaps at each affiliate institution that could be filled while residents were rotating at VABHS. Once all educational goals and priorities of the shared mission statement were identified, the chief medical residents and director of medical resident education adopted the mission statement as the blueprint for all educational programming for the academic year. Progress toward enacting the various components of the mission statement was reviewed monthly and changes in educational programming to ensure adequate emphasis of all components were made accordingly.
Results
Our first goal was to promote the personal and professional development of residents who rotate through VABHS, particularly interns, in a setting that fosters cross-institutional collaboration, respect, and friendship. The West Roxbury campus of VABHS is the only hospital in the city where internal medicine residents from 3 large training programs work together on teams that have been intentionally built to place residents from different institutions with one another. In educational conferences, we encouraged residents from different training programs to share their experiences with patient populations that others may not see at their home institutions, based on the specialized care that each institution provides. The conferences also give residents the opportunity to provide and receive near-peer teaching in a collegial environment.
Our second goal was to maintain an environment of educational excellence. We produced thought-provoking conferences that prioritized inspiring curiosity and teaching systems of thought over the dissemination of facts. We regularly focused on the broader context of medicine in case conferences and journal club, including topics such as public health, health policy, advocacy, health economics, quality improvement (QI), and high-value care. Our morning reports were interactive and participatory, emphasizing both technical skill practice and sophisticated clinical reasoning.
We embraced the principles of cognitive learning theory by priming learners with preconference “teasers” that previewed conference topics to be discussed. Every Friday, we played a medical version of Jeopardy!, which used spaced learning to consolidate the week’s teaching points in a fun, collaborative, and collegial atmosphere. Our dedicated patient safety conference gave residents the chance to use QI tools to dissect and tackle real problems in the hospital, and our monthly Morbidity and Mortality conference served as inspiration for many of the resident-driven QI projects.
Our third goal was to challenge physicians to provide the best possible care to veterans, including learning about issues unique to this often-marginalized population. We emphasized that training at a VA hospital is a privilege and that the best way to honor our veterans is to take advantage of the unique learning opportunities available at VA. To that end, we exposed residents to veteran-specific educational content, ranging from the structure and payment model of VHA to service-related medical conditions, such as posttraumatic stress disorder, other mental health issues, traumatic brain injury, Agent Orange exposure, and Gulf War Syndrome.
Discussion
Findings from the recently published Accreditation Council for Graduate Medical Education’s (ACGME) 2016 Clinical Learning Environment Review (CLER) Report support the need for mission statements like ours to guide the delivery of graduate medical education.3 A major finding of this report was that the development and implementation of graduate medical education largely occurs separately from other areas of organizational and strategic focus within clinical learning environments. Our mission statement has served as a road map for aligning the delivery of graduate medical education at VABHS with the specific strengths of the clinical learning environment that VA affords.
Additionally, the 2016 CLER report identified a lack of specificity in training on health care disparities and cultural competency for the specific populations served by the surveyed residency programs. The emphasis we placed on learning about issues specific to the care of the veteran population highlights the potential for other mission statements like ours to bridge the gap between articulation and execution of educational priorities. Finally, through the academic partnerships it holds with more than 90% of medical schools in the U.S., VA already has an integral role in both undergraduate and graduate medical education that positions its hospitals as ideal training environments in which to address shortcomings in medical training like those identified by the ACGME.4
Conclusion
We propose this mission statement as a model for the delivery of graduate medical education throughout all VA hospitals with academic affiliations and especially those where trainees from multiple institutions work together. As embodied in our mission statement, our goal was to provide a clinical training experience at VA that complements that of our residents’ home institutions and fosters a respect for and interest in the special care provided at VA. The development of a shared mission statement provides an invaluable tool in accomplishing that goal. We encourage chief medical residents and other leaders in medical education in all specialties at VAMCs to develop their own mission statements that reflect and embody the values of each affiliated training program. For our residents, rotating at VA is an opportunity to learn the practice of medicine for veterans, rather than practicing medicine on veterans. It is our sincere hope that shaping our residents’ educational experience in this fashion will foster a greater appreciation for the care of our nation’s veterans.
More than 65% of all physicians who train in the U.S. rotate through a VA hospital at some point during their training. In 2015 alone, more than 43,000 residents received some or all of their clinical training through VA.1 Of the approximately 120 VAMCs that hold academic affiliations
with medical schools and residency training programs, several hold affiliations with multiple institutions, including VA Boston Healthcare System (VABHS) in Massachusetts. The West Roxbury campus is the home of VA Boston’s acute care hospital, where residents and fellows from Boston Medical Center (BMC), Beth Israel Deaconess Medical Center (BIDMC), and Brigham and Women’s Hospital (BWH) train together. These are 3 of the largest medical training programs in Boston, though each provides a unique training experience for residents due to differences in patient population, faculty expertise, and hospital network affiliations (Table 1).
This diversity brings differences in cultural norms, institutional preferences, and educational expectations. Furthermore, residents from different programs who work together at VA Boston are often meeting one another for the first time, as opportunities for interinstitutional collaboration among these 3 training programs do not exist outside of VA. This training environment presents both an opportunity
and a challenge for medical educators: offering the best possible learning experience for physiciansin-training from multiple programs while providing the best possible care for U.S. veterans.
To guide educators charged with meeting this challenge, the VA Office of Academic Affiliations put forth a mission statement describing its overarching teaching mission (Table 2).2
To address this gap, chief medical residents from the 3 affiliate residency training programs came together to develop a shared mission statement for what we envision as the educational experience for all medical trainees rotating through VABHS (Table 2). In this article, we describe the development of a mission statement for graduate medical education in internal medicine at VABHS and provides examples of how our mission statement guided educational programming.
Methods
Whereas the affiliated institutions assign generic competency-based learning objectives to rotations at VABHS, no specific overarching educational objectives for residents have been defined previously. The directors of the internal medicine residency programs at each of the VABHS affiliate institutions grant their respective VA-based chief medical residents the autonomy to deliver graduate medical education at VA as they see fit, in collaboration with their colleagues from the other affiliated institutions and the VA director of medical resident education. This autonomy and flexibility allowed each of the chief medical residents to articulate an individual vision for VA graduate medical education based on their affiliate program’s goals, values, and mission.
At the beginning of the 2016/2017 academic year, in partnership with the director of medical resident education at VABHS, the chief medical residents met to reconcile these into a single shared mission statement. Special attention was paid to educational gaps at each affiliate institution that could be filled while residents were rotating at VABHS. Once all educational goals and priorities of the shared mission statement were identified, the chief medical residents and director of medical resident education adopted the mission statement as the blueprint for all educational programming for the academic year. Progress toward enacting the various components of the mission statement was reviewed monthly and changes in educational programming to ensure adequate emphasis of all components were made accordingly.
Results
Our first goal was to promote the personal and professional development of residents who rotate through VABHS, particularly interns, in a setting that fosters cross-institutional collaboration, respect, and friendship. The West Roxbury campus of VABHS is the only hospital in the city where internal medicine residents from 3 large training programs work together on teams that have been intentionally built to place residents from different institutions with one another. In educational conferences, we encouraged residents from different training programs to share their experiences with patient populations that others may not see at their home institutions, based on the specialized care that each institution provides. The conferences also give residents the opportunity to provide and receive near-peer teaching in a collegial environment.
Our second goal was to maintain an environment of educational excellence. We produced thought-provoking conferences that prioritized inspiring curiosity and teaching systems of thought over the dissemination of facts. We regularly focused on the broader context of medicine in case conferences and journal club, including topics such as public health, health policy, advocacy, health economics, quality improvement (QI), and high-value care. Our morning reports were interactive and participatory, emphasizing both technical skill practice and sophisticated clinical reasoning.
We embraced the principles of cognitive learning theory by priming learners with preconference “teasers” that previewed conference topics to be discussed. Every Friday, we played a medical version of Jeopardy!, which used spaced learning to consolidate the week’s teaching points in a fun, collaborative, and collegial atmosphere. Our dedicated patient safety conference gave residents the chance to use QI tools to dissect and tackle real problems in the hospital, and our monthly Morbidity and Mortality conference served as inspiration for many of the resident-driven QI projects.
Our third goal was to challenge physicians to provide the best possible care to veterans, including learning about issues unique to this often-marginalized population. We emphasized that training at a VA hospital is a privilege and that the best way to honor our veterans is to take advantage of the unique learning opportunities available at VA. To that end, we exposed residents to veteran-specific educational content, ranging from the structure and payment model of VHA to service-related medical conditions, such as posttraumatic stress disorder, other mental health issues, traumatic brain injury, Agent Orange exposure, and Gulf War Syndrome.
Discussion
Findings from the recently published Accreditation Council for Graduate Medical Education’s (ACGME) 2016 Clinical Learning Environment Review (CLER) Report support the need for mission statements like ours to guide the delivery of graduate medical education.3 A major finding of this report was that the development and implementation of graduate medical education largely occurs separately from other areas of organizational and strategic focus within clinical learning environments. Our mission statement has served as a road map for aligning the delivery of graduate medical education at VABHS with the specific strengths of the clinical learning environment that VA affords.
Additionally, the 2016 CLER report identified a lack of specificity in training on health care disparities and cultural competency for the specific populations served by the surveyed residency programs. The emphasis we placed on learning about issues specific to the care of the veteran population highlights the potential for other mission statements like ours to bridge the gap between articulation and execution of educational priorities. Finally, through the academic partnerships it holds with more than 90% of medical schools in the U.S., VA already has an integral role in both undergraduate and graduate medical education that positions its hospitals as ideal training environments in which to address shortcomings in medical training like those identified by the ACGME.4
Conclusion
We propose this mission statement as a model for the delivery of graduate medical education throughout all VA hospitals with academic affiliations and especially those where trainees from multiple institutions work together. As embodied in our mission statement, our goal was to provide a clinical training experience at VA that complements that of our residents’ home institutions and fosters a respect for and interest in the special care provided at VA. The development of a shared mission statement provides an invaluable tool in accomplishing that goal. We encourage chief medical residents and other leaders in medical education in all specialties at VAMCs to develop their own mission statements that reflect and embody the values of each affiliated training program. For our residents, rotating at VA is an opportunity to learn the practice of medicine for veterans, rather than practicing medicine on veterans. It is our sincere hope that shaping our residents’ educational experience in this fashion will foster a greater appreciation for the care of our nation’s veterans.
1. VA Office of Academic Affiliations. 2015 statistics: health professions trainees. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Published 2016. Accessed September 18, 2017.
2. VA Office of Academic Affiliations. Mission of the Office of Academic Affiliations. http://www.va.gov/oaa/oaa_mission.asp. Updated June 23, 2017. Accessed September 18, 2017.
3. Accreditation Council for Graduate Medical Education. Clinical learning environment review – national report of findings 2016 – executive summary. https://www.acgme.org/Portals/0/PDFs/CLER/ACGME-CLER-ExecutiveSummary.pdf. Published 2016. Accessed September 18, 2017.
4. Association of American Medical Colleges. The VA and academic medicine: partners in health care, training, and research. https://www.aamc.org/download/385612/data/07182014.pdf. Accessed September 14, 2017.
1. VA Office of Academic Affiliations. 2015 statistics: health professions trainees. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Published 2016. Accessed September 18, 2017.
2. VA Office of Academic Affiliations. Mission of the Office of Academic Affiliations. http://www.va.gov/oaa/oaa_mission.asp. Updated June 23, 2017. Accessed September 18, 2017.
3. Accreditation Council for Graduate Medical Education. Clinical learning environment review – national report of findings 2016 – executive summary. https://www.acgme.org/Portals/0/PDFs/CLER/ACGME-CLER-ExecutiveSummary.pdf. Published 2016. Accessed September 18, 2017.
4. Association of American Medical Colleges. The VA and academic medicine: partners in health care, training, and research. https://www.aamc.org/download/385612/data/07182014.pdf. Accessed September 14, 2017.
A Veteran With Alcohol Use Disorder and Acute Pancreatitis
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.