User login
Alcohol-Related Hospitalizations During the Initial COVID-19 Lockdown in Massachusetts: An Interrupted Time-Series Analysis
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
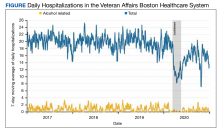
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
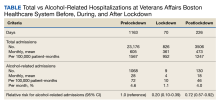
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
Safe Opioid Prescribing for Acute Noncancer Pain in Hospitalized Adults: A Systematic Review of Existing Guidelines
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
© 2018 Society of Hospital Medicine
Inpatient management of opioid use disorder: A review for hospitalists
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
© 2017 Society of Hospital Medicine