User login
A Patient With Recurrent Immune Stromal Keratitis and Adherence Challenges
Herpes simplex keratitis (HSK) is a common yet potentially blinding condition caused by a primary or reactivated herpetic infection of the cornea.1 The Herpetic Eye Disease Study established the standard of care in HSK management.2 Treatments range from oral antivirals and artificial tears to topical antibiotics, amniotic membranes, and corneal transplantation.3 Patients with immune stromal keratitis (ISK) may experience low-grade chronic keratitis for years.4 ISK is classified by a cellular and neovascularization infiltration of the cornea.5 We present a case of a patient with recurrent ISK and review its presentation, diagnosis, and management.
Case Presentation
A 52-year-old man presented to the eye clinic with a watery and itchy right eye with mildly blurred vision. His ocular history was unremarkable. His medical history was notable for hepatitis C, hypertension, alcohol and drug dependence, homelessness, and a COVID-19–induced coma. His medications included trazodone, nifedipine, clonidine HCl, and buprenorphine/naloxone.
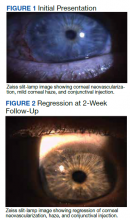
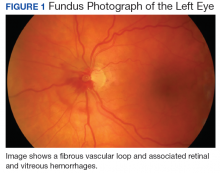
On clinical examination, the patient’s best-corrected visual acuity was 20/40 in the right eye and 20/20 in the left. Corneal sensitivity was absent in the right eye and intact in the left. Anterior segment findings in the right eye included 360-degree superficial corneal neovascularization, deep neovascularization temporally, scattered patches of corneal haze, epithelial irregularity, and 2+ diffuse bulbar conjunctival injection (Figure 1). The anterior segment of the left eye and the posterior segments of both eyes were unremarkable. The differential diagnosis included HSK, syphilis, Cogan syndrome, varicella-zoster virus keratitis, Epstein-Barr virus keratitis, and Lyme disease. With consultation from a corneal specialist, the patient was given the presumptive diagnosis of ISK in the right eye based on unilateral corneal presentation and lack of corneal sensitivity. He was treated with
The patient returned a week later having only used the prednisolone drops for 2 days before discontinuing. Examination showed no change in his corneal appearance from the previous week. The patient was counseled on the importance of adherence to the regimen of topical prednisolone and oral valacyclovir.
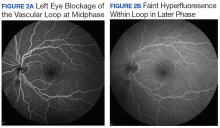
The patient followed up 2 weeks later. He reported good adherence to the ISK medication regimen. His symptoms had resolved, and his visual acuity returned to 20/20 in the right eye. Slit-lamp examination showed improvement in injection, and the superficial corneal neovascularization had cleared. A trace ghost vessel was seen temporally at a site of deep neovascularization (Figure 2). He was instructed to continue valacyclovir once daily and prednisolone drops once daily in the right eye and to follow up in 1 month.
At the 1-month follow-up, the patient’s signs and symptoms had reverted to his original presentation. The patient reported poor adherence to the medication regimen, having missed multiple doses of prednisolone drops as well as valacyclovir. The patient was counseled again on the ISK regimen, and the prednisolone drops and 1-g oral valacyclovir were refilled. A follow-up visit was scheduled for 2 weeks. Additional follow-up revealed a resolved corneal appearance and bimonthly follow-ups were scheduled thereafter.
Discussion
HSK is the most common infectious cause of unilateral blindness and vision impairment in the world.2 This case highlights the diagnosis and management of a patient with ISK, a type of HSK characterized by decreased corneal sensitivity and unilateral stromal opacification or neovascularization.6
ISK is caused by the herpes simplex virus (HSV), a double-stranded enveloped DNA virus that occurs worldwide with little variation, replicates in many types of cells, has rapid growth, and is cytolytic, causing necrosis of nearby cells. Transmission is via direct contact and there is a lifelong latency period in the trigeminal ganglia. Both primary and reactivation infections of HSK can affect a broad array of ocular structures, from the lids to the retina. Infectious epithelial keratitis, also known as dendritic keratitis, is the reactivation of the live virus and is the most common presentation of HSK. ISK is responsible for 20% to 48% of recurrent HSV disease and is the leading cause of vision loss. ISK is the result of an immune-mediated inflammatory response due to a retained viral antigen within the stromal tissue.7 Inflammation in the corneal stroma leads to corneal haze and eventually focal or diffuse scarring, reducing the visual potential.7 This presentation may occur days to years after the initial epithelial episode and may persist for years. Although this patient did not present with infectious epithelial keratitis, it is possible he had a previous episode not mentioned as a history was difficult to obtain, and it can be subtle or innocuous, like pink eye.
Symptoms of ISK include unilateral redness, photophobia, tearing, eye pain, and blurred vision, as described by this patient. On examination, initial manifestations of ISK include corneal haze, edema, scarring, and neovascularization.7 Again, this patient presented with edema and neovascularization. These signs may improve with prompt diagnosis and treatment. More frequent reactivated disease leads to a higher propensity of corneal scarring and irregular astigmatism, reducing the visual outcome.
The standard of care established by the Herpetic Eye Disease Study recommends that a patient with presumed ISK should be started on oral antiviral therapy and, in the absence of epithelial disease, topical steroids. Oral antivirals, such as acyclovir and valacyclovir, have good ocular penetration, a good safety profile, a low susceptibility of resistance, and are well tolerated with long-term treatment.2,8 There were no known interactions between any of the patient’s medications and valacyclovir. Oral antivirals should be used in the initial presentation and for maintenance therapy to help reduce the chance of recurrent disease. Initial treatment for ISK is 1-g valacyclovir 3 times daily. When the eye becomes quiet, that dosage can be tapered to 1 g twice daily, to 1 g once daily, and eventually to a maintenance dose of 500 mg daily. Topical steroids block the inflammatory cascade, therefore reducing the corneal inflammation and potential scarring, further reducing the risk of visual impairment.9 Initial treatment is 1 drop 3 times daily, then can be tapered at the same schedule as the oral acyclovir to help simplify adherence for the patient. After 1 drop once daily, steroids may be discontinued while the oral antiviral maintenance dosage continues. Follow-ups should be performed on a monthly to bimonthly basis to evaluate intraocular pressure, ensuring there is no steroid response.
As seen in this patient, adherence with a treatment regimen and awareness of factors, such as a complex psychosocial history that may impact this adherence, are of utmost importance.7
Conclusions
ISK presents unilaterally with decreased or absent corneal sensitivity and nonspecific symptoms. It should be at the top of the list in the differential diagnosis in any patient with unilateral corneal edema, opacification, or neovascularization, and the patient should be started on oral antiviral therapy.
1. Sibley D, Larkin DFP. Update on Herpes simplex keratitis management. Eye (Lond). 2020;34(12):2219-2226. doi:10.1038/s41433-020-01153-x
2. Chodosh J, Ung L. Adoption of innovation in herpes simplex virus keratitis. Cornea. 2020;39(1)(suppl 1):S7-S18. doi:10.1097/ICO.0000000000002425
3. Pérez-Bartolomé F, Botín DM, de Dompablo P, de Arriba P, Arnalich Montiel F, Muñoz Negrete FJ. Post-herpes neurotrophic keratopathy: pathogenesis, clinical signs and current therapies. Arch Soc Esp Oftalmol. 2019;94(4):171-183. doi:10.1016/j.oftal.2019.01.002
4. Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144-154.
5. Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42(6):e229-e237. doi:10.1016/j.jfo.2019.04.001
6. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;5(57):448-462. doi:10.1016/jsurvophthal.2012.01.005
7. Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020;11:766. Published 2020 May 13. doi:10.3389/fimmu.2020.00766
8. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186(suppl 1):S40-S46. doi:10.1086/342966
9. Dawson CR. The herpetic eye disease study. Arch Ophthalmol. 1990;108(2):191-192. doi:10.1001/archopht.1990.01070040043027
Herpes simplex keratitis (HSK) is a common yet potentially blinding condition caused by a primary or reactivated herpetic infection of the cornea.1 The Herpetic Eye Disease Study established the standard of care in HSK management.2 Treatments range from oral antivirals and artificial tears to topical antibiotics, amniotic membranes, and corneal transplantation.3 Patients with immune stromal keratitis (ISK) may experience low-grade chronic keratitis for years.4 ISK is classified by a cellular and neovascularization infiltration of the cornea.5 We present a case of a patient with recurrent ISK and review its presentation, diagnosis, and management.
Case Presentation
A 52-year-old man presented to the eye clinic with a watery and itchy right eye with mildly blurred vision. His ocular history was unremarkable. His medical history was notable for hepatitis C, hypertension, alcohol and drug dependence, homelessness, and a COVID-19–induced coma. His medications included trazodone, nifedipine, clonidine HCl, and buprenorphine/naloxone.
On clinical examination, the patient’s best-corrected visual acuity was 20/40 in the right eye and 20/20 in the left. Corneal sensitivity was absent in the right eye and intact in the left. Anterior segment findings in the right eye included 360-degree superficial corneal neovascularization, deep neovascularization temporally, scattered patches of corneal haze, epithelial irregularity, and 2+ diffuse bulbar conjunctival injection (Figure 1). The anterior segment of the left eye and the posterior segments of both eyes were unremarkable. The differential diagnosis included HSK, syphilis, Cogan syndrome, varicella-zoster virus keratitis, Epstein-Barr virus keratitis, and Lyme disease. With consultation from a corneal specialist, the patient was given the presumptive diagnosis of ISK in the right eye based on unilateral corneal presentation and lack of corneal sensitivity. He was treated with
The patient returned a week later having only used the prednisolone drops for 2 days before discontinuing. Examination showed no change in his corneal appearance from the previous week. The patient was counseled on the importance of adherence to the regimen of topical prednisolone and oral valacyclovir.
The patient followed up 2 weeks later. He reported good adherence to the ISK medication regimen. His symptoms had resolved, and his visual acuity returned to 20/20 in the right eye. Slit-lamp examination showed improvement in injection, and the superficial corneal neovascularization had cleared. A trace ghost vessel was seen temporally at a site of deep neovascularization (Figure 2). He was instructed to continue valacyclovir once daily and prednisolone drops once daily in the right eye and to follow up in 1 month.
At the 1-month follow-up, the patient’s signs and symptoms had reverted to his original presentation. The patient reported poor adherence to the medication regimen, having missed multiple doses of prednisolone drops as well as valacyclovir. The patient was counseled again on the ISK regimen, and the prednisolone drops and 1-g oral valacyclovir were refilled. A follow-up visit was scheduled for 2 weeks. Additional follow-up revealed a resolved corneal appearance and bimonthly follow-ups were scheduled thereafter.
Discussion
HSK is the most common infectious cause of unilateral blindness and vision impairment in the world.2 This case highlights the diagnosis and management of a patient with ISK, a type of HSK characterized by decreased corneal sensitivity and unilateral stromal opacification or neovascularization.6
ISK is caused by the herpes simplex virus (HSV), a double-stranded enveloped DNA virus that occurs worldwide with little variation, replicates in many types of cells, has rapid growth, and is cytolytic, causing necrosis of nearby cells. Transmission is via direct contact and there is a lifelong latency period in the trigeminal ganglia. Both primary and reactivation infections of HSK can affect a broad array of ocular structures, from the lids to the retina. Infectious epithelial keratitis, also known as dendritic keratitis, is the reactivation of the live virus and is the most common presentation of HSK. ISK is responsible for 20% to 48% of recurrent HSV disease and is the leading cause of vision loss. ISK is the result of an immune-mediated inflammatory response due to a retained viral antigen within the stromal tissue.7 Inflammation in the corneal stroma leads to corneal haze and eventually focal or diffuse scarring, reducing the visual potential.7 This presentation may occur days to years after the initial epithelial episode and may persist for years. Although this patient did not present with infectious epithelial keratitis, it is possible he had a previous episode not mentioned as a history was difficult to obtain, and it can be subtle or innocuous, like pink eye.
Symptoms of ISK include unilateral redness, photophobia, tearing, eye pain, and blurred vision, as described by this patient. On examination, initial manifestations of ISK include corneal haze, edema, scarring, and neovascularization.7 Again, this patient presented with edema and neovascularization. These signs may improve with prompt diagnosis and treatment. More frequent reactivated disease leads to a higher propensity of corneal scarring and irregular astigmatism, reducing the visual outcome.
The standard of care established by the Herpetic Eye Disease Study recommends that a patient with presumed ISK should be started on oral antiviral therapy and, in the absence of epithelial disease, topical steroids. Oral antivirals, such as acyclovir and valacyclovir, have good ocular penetration, a good safety profile, a low susceptibility of resistance, and are well tolerated with long-term treatment.2,8 There were no known interactions between any of the patient’s medications and valacyclovir. Oral antivirals should be used in the initial presentation and for maintenance therapy to help reduce the chance of recurrent disease. Initial treatment for ISK is 1-g valacyclovir 3 times daily. When the eye becomes quiet, that dosage can be tapered to 1 g twice daily, to 1 g once daily, and eventually to a maintenance dose of 500 mg daily. Topical steroids block the inflammatory cascade, therefore reducing the corneal inflammation and potential scarring, further reducing the risk of visual impairment.9 Initial treatment is 1 drop 3 times daily, then can be tapered at the same schedule as the oral acyclovir to help simplify adherence for the patient. After 1 drop once daily, steroids may be discontinued while the oral antiviral maintenance dosage continues. Follow-ups should be performed on a monthly to bimonthly basis to evaluate intraocular pressure, ensuring there is no steroid response.
As seen in this patient, adherence with a treatment regimen and awareness of factors, such as a complex psychosocial history that may impact this adherence, are of utmost importance.7
Conclusions
ISK presents unilaterally with decreased or absent corneal sensitivity and nonspecific symptoms. It should be at the top of the list in the differential diagnosis in any patient with unilateral corneal edema, opacification, or neovascularization, and the patient should be started on oral antiviral therapy.
Herpes simplex keratitis (HSK) is a common yet potentially blinding condition caused by a primary or reactivated herpetic infection of the cornea.1 The Herpetic Eye Disease Study established the standard of care in HSK management.2 Treatments range from oral antivirals and artificial tears to topical antibiotics, amniotic membranes, and corneal transplantation.3 Patients with immune stromal keratitis (ISK) may experience low-grade chronic keratitis for years.4 ISK is classified by a cellular and neovascularization infiltration of the cornea.5 We present a case of a patient with recurrent ISK and review its presentation, diagnosis, and management.
Case Presentation
A 52-year-old man presented to the eye clinic with a watery and itchy right eye with mildly blurred vision. His ocular history was unremarkable. His medical history was notable for hepatitis C, hypertension, alcohol and drug dependence, homelessness, and a COVID-19–induced coma. His medications included trazodone, nifedipine, clonidine HCl, and buprenorphine/naloxone.
On clinical examination, the patient’s best-corrected visual acuity was 20/40 in the right eye and 20/20 in the left. Corneal sensitivity was absent in the right eye and intact in the left. Anterior segment findings in the right eye included 360-degree superficial corneal neovascularization, deep neovascularization temporally, scattered patches of corneal haze, epithelial irregularity, and 2+ diffuse bulbar conjunctival injection (Figure 1). The anterior segment of the left eye and the posterior segments of both eyes were unremarkable. The differential diagnosis included HSK, syphilis, Cogan syndrome, varicella-zoster virus keratitis, Epstein-Barr virus keratitis, and Lyme disease. With consultation from a corneal specialist, the patient was given the presumptive diagnosis of ISK in the right eye based on unilateral corneal presentation and lack of corneal sensitivity. He was treated with
The patient returned a week later having only used the prednisolone drops for 2 days before discontinuing. Examination showed no change in his corneal appearance from the previous week. The patient was counseled on the importance of adherence to the regimen of topical prednisolone and oral valacyclovir.
The patient followed up 2 weeks later. He reported good adherence to the ISK medication regimen. His symptoms had resolved, and his visual acuity returned to 20/20 in the right eye. Slit-lamp examination showed improvement in injection, and the superficial corneal neovascularization had cleared. A trace ghost vessel was seen temporally at a site of deep neovascularization (Figure 2). He was instructed to continue valacyclovir once daily and prednisolone drops once daily in the right eye and to follow up in 1 month.
At the 1-month follow-up, the patient’s signs and symptoms had reverted to his original presentation. The patient reported poor adherence to the medication regimen, having missed multiple doses of prednisolone drops as well as valacyclovir. The patient was counseled again on the ISK regimen, and the prednisolone drops and 1-g oral valacyclovir were refilled. A follow-up visit was scheduled for 2 weeks. Additional follow-up revealed a resolved corneal appearance and bimonthly follow-ups were scheduled thereafter.
Discussion
HSK is the most common infectious cause of unilateral blindness and vision impairment in the world.2 This case highlights the diagnosis and management of a patient with ISK, a type of HSK characterized by decreased corneal sensitivity and unilateral stromal opacification or neovascularization.6
ISK is caused by the herpes simplex virus (HSV), a double-stranded enveloped DNA virus that occurs worldwide with little variation, replicates in many types of cells, has rapid growth, and is cytolytic, causing necrosis of nearby cells. Transmission is via direct contact and there is a lifelong latency period in the trigeminal ganglia. Both primary and reactivation infections of HSK can affect a broad array of ocular structures, from the lids to the retina. Infectious epithelial keratitis, also known as dendritic keratitis, is the reactivation of the live virus and is the most common presentation of HSK. ISK is responsible for 20% to 48% of recurrent HSV disease and is the leading cause of vision loss. ISK is the result of an immune-mediated inflammatory response due to a retained viral antigen within the stromal tissue.7 Inflammation in the corneal stroma leads to corneal haze and eventually focal or diffuse scarring, reducing the visual potential.7 This presentation may occur days to years after the initial epithelial episode and may persist for years. Although this patient did not present with infectious epithelial keratitis, it is possible he had a previous episode not mentioned as a history was difficult to obtain, and it can be subtle or innocuous, like pink eye.
Symptoms of ISK include unilateral redness, photophobia, tearing, eye pain, and blurred vision, as described by this patient. On examination, initial manifestations of ISK include corneal haze, edema, scarring, and neovascularization.7 Again, this patient presented with edema and neovascularization. These signs may improve with prompt diagnosis and treatment. More frequent reactivated disease leads to a higher propensity of corneal scarring and irregular astigmatism, reducing the visual outcome.
The standard of care established by the Herpetic Eye Disease Study recommends that a patient with presumed ISK should be started on oral antiviral therapy and, in the absence of epithelial disease, topical steroids. Oral antivirals, such as acyclovir and valacyclovir, have good ocular penetration, a good safety profile, a low susceptibility of resistance, and are well tolerated with long-term treatment.2,8 There were no known interactions between any of the patient’s medications and valacyclovir. Oral antivirals should be used in the initial presentation and for maintenance therapy to help reduce the chance of recurrent disease. Initial treatment for ISK is 1-g valacyclovir 3 times daily. When the eye becomes quiet, that dosage can be tapered to 1 g twice daily, to 1 g once daily, and eventually to a maintenance dose of 500 mg daily. Topical steroids block the inflammatory cascade, therefore reducing the corneal inflammation and potential scarring, further reducing the risk of visual impairment.9 Initial treatment is 1 drop 3 times daily, then can be tapered at the same schedule as the oral acyclovir to help simplify adherence for the patient. After 1 drop once daily, steroids may be discontinued while the oral antiviral maintenance dosage continues. Follow-ups should be performed on a monthly to bimonthly basis to evaluate intraocular pressure, ensuring there is no steroid response.
As seen in this patient, adherence with a treatment regimen and awareness of factors, such as a complex psychosocial history that may impact this adherence, are of utmost importance.7
Conclusions
ISK presents unilaterally with decreased or absent corneal sensitivity and nonspecific symptoms. It should be at the top of the list in the differential diagnosis in any patient with unilateral corneal edema, opacification, or neovascularization, and the patient should be started on oral antiviral therapy.
1. Sibley D, Larkin DFP. Update on Herpes simplex keratitis management. Eye (Lond). 2020;34(12):2219-2226. doi:10.1038/s41433-020-01153-x
2. Chodosh J, Ung L. Adoption of innovation in herpes simplex virus keratitis. Cornea. 2020;39(1)(suppl 1):S7-S18. doi:10.1097/ICO.0000000000002425
3. Pérez-Bartolomé F, Botín DM, de Dompablo P, de Arriba P, Arnalich Montiel F, Muñoz Negrete FJ. Post-herpes neurotrophic keratopathy: pathogenesis, clinical signs and current therapies. Arch Soc Esp Oftalmol. 2019;94(4):171-183. doi:10.1016/j.oftal.2019.01.002
4. Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144-154.
5. Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42(6):e229-e237. doi:10.1016/j.jfo.2019.04.001
6. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;5(57):448-462. doi:10.1016/jsurvophthal.2012.01.005
7. Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020;11:766. Published 2020 May 13. doi:10.3389/fimmu.2020.00766
8. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186(suppl 1):S40-S46. doi:10.1086/342966
9. Dawson CR. The herpetic eye disease study. Arch Ophthalmol. 1990;108(2):191-192. doi:10.1001/archopht.1990.01070040043027
1. Sibley D, Larkin DFP. Update on Herpes simplex keratitis management. Eye (Lond). 2020;34(12):2219-2226. doi:10.1038/s41433-020-01153-x
2. Chodosh J, Ung L. Adoption of innovation in herpes simplex virus keratitis. Cornea. 2020;39(1)(suppl 1):S7-S18. doi:10.1097/ICO.0000000000002425
3. Pérez-Bartolomé F, Botín DM, de Dompablo P, de Arriba P, Arnalich Montiel F, Muñoz Negrete FJ. Post-herpes neurotrophic keratopathy: pathogenesis, clinical signs and current therapies. Arch Soc Esp Oftalmol. 2019;94(4):171-183. doi:10.1016/j.oftal.2019.01.002
4. Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144-154.
5. Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42(6):e229-e237. doi:10.1016/j.jfo.2019.04.001
6. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;5(57):448-462. doi:10.1016/jsurvophthal.2012.01.005
7. Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020;11:766. Published 2020 May 13. doi:10.3389/fimmu.2020.00766
8. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186(suppl 1):S40-S46. doi:10.1086/342966
9. Dawson CR. The herpetic eye disease study. Arch Ophthalmol. 1990;108(2):191-192. doi:10.1001/archopht.1990.01070040043027
75 Years of the Historic Partnership Between the VA and Academic Medical Centers
The US government has a legacy of providing support for veterans. Pensions were offered to disabled veterans as early as 1776, and benefits were expanded to cover medical needs as the country grew and modernized.1,2 Enacted during the Civil War, the General Pension Act increased benefits for widows and dependents.2 Rehabilitation and vocational training assistance benefits were added after World War I, and the US Department of Veterans Affairs (VA) was created in 1930 to consolidate all benefits under one umbrella organization.2,3
Prior to World War II, the VA lacked the bed capacity for the 4 million veterans who were eligible for care. This shortage became more acute by the end of the war, when the number of eligible veterans increased by 15 million.4 Although the VA successfully built bed capacity through acquisition of military hospitals, VA hospitals struggled to recruit clinical staff.2 Physicians were hesitant to join the VA because civil service salaries were lower than comparable positions in the community, and the VA offered limited opportunities for research or continuing education. These limitations negatively impacted the overall reputation of the VA. The American Medical Association (AMA) was reluctant to directly admit VA physicians for membership because of a “lower” standard of care at VA hospitals.2 This review will describe how passage of 2 legislative actions, the Servicemen’s Readjustment Act and Public Law (PL)79-293, and a key policy memorandum set the foundation for the partnership between the VA and academic medical centers. This led to improved medical care for veterans and expansion of health professions education for VA and the nation.5,6
GI Bill of Rights
The passage of the Servicemen’s Readjustment Act of 1944, better known as the GI Bill of Rights, provided education assistance, guaranteed home loans, and unemployment payments to veterans.5 All medical officers serving during the war were eligible for this benefit, which effectively increased the number of potential physician trainees at the end of World War II by almost 60,000.7 Medical education at the time was simultaneously undergoing a transformation with more rigorous training and a push to standardize medical education across state lines. While prerequisite training was not required for admission to many medical schools and curricula varied in length based on state licensing requirements, more programs were adding premedical education requirements and transitioning to the 4-year curricula seen today. At this time, only 23 states required postgraduate internships for licensure, but this number was growing.8 The American Board of Medical Specialties was established several years prior to World War II in 1934 to elevate the quality of care; the desire for residency training and board certification continued to gain traction during the 1940s.9
Medical Training
In anticipation of an influx of medical trainees, the Committee on Postwar Medical Service conducted a comprehensive survey to understand the training needs of physician veterans returning from World War II.7 The survey collected data from medical officers on their desired length of training, interest in specialty board certification, time served, and type of medical practice prior to enlisting. Length of desired training was categorized as short (up to 6 months), which would serve as a refresher course and provide updates on recent advances in medicine and surgery, and long (> 6 months), which resembled a modern internship or residency. Nineteen percent did not want additional training, 22% wished to pursue short courses, and 51% were interested in longer courses. Most respondents also wished to obtain board certification.7 The AMA played a significant role in supporting the expansion of training opportunities, encouraging all accredited hospitals to assess their capacity to determine the number of additional residents they could accommodate. The AMA also awarded hospitals with existing internship programs temporary accreditation to allow them to add extended training through residency programs.7
Medical schools devised creative solutions to meet the needs of returning physician veterans and capitalize on the available educational benefits. Postgraduate refresher courses that varied in length from hours to months were developed focusing on an array of topics. In addition to basic medical principles, courses covered general topics, such as advances in medicine, to specialty topics, such as nutrition or ophthalmology.7 Although the courses could not be counted toward board certification, participation increased by almost 300% in the 1945/1946 academic year relative to the previous year.7 Increasing access to the longer training courses, including internships and residencies, was often achieved through experiences outside the clinical setting. Yale University modified its curriculum to reduce time devoted to lectures on published materials and encourage active learning and community outreach.10 Northwestern University assigned residents to spend 1 of their 3 years “out of residence” in basic science and clinical instruction provided by the medical school. Tuition assistance from the GI Bill supported the additional expenses incurred by the medical school to fund laboratory space, equipment, and the salaries of the basic science instructors and administrative staff.11
Public Law 79-293
Public Law 79-293 was passed on January 3, 1946, establishing the Department of Medicine and Surgery within the VA. The law, which became the basis for Title 38 chapters 73 and 74, allowed VA hospitals flexibility to hire doctors, dentists, and nurses without regard to the civil service regulations and salary restrictions associated with other federal positions.6
Concerns about quality of care had been mounting for years, and the release of several sensationalized and critical articles motivated VA leadership to make sweeping changes. One article described neglect at VA hospitals.12 Excessive paperwork and low economic benefits were identified as barriers to the recruitment of qualified clinicians at the VA.2 The VA Special Medical Advisory Group investigating the claims recommended that the VA encourage their hospitals to affiliate with medical schools to improve the quality of care. This group also recommended that new VA hospitals be constructed near academic medical centers to allow access to consultants.2 Three large veterans service organizations (American Legion, Veterans of Foreign Wars, and Disabled American Veterans) conducted their own investigations in response to the media reports. The organizations reported that the quality of care in most VA hospitals was already on par with the community but indicated that the VA would benefit from expansion of medical research and training, increased bed capacity, reduction in the administrative burden on clinicians, and increased salaries for clinical staff.2
Policy Memorandum No. 2
The relationship between VA and academic medical centers was solidified on January 30, 1946, with adoption of Policy Memorandum No. 2.13 This memorandum allowed for the establishment of relationships with academic medical centers to provide “the veteran a much higher standard of medical care than could be given him with a wholly full-time medical staff.” Shortly after this memorandum was signed, residents from Northwestern University and the University of Illinois at Chicago began clinical rotations at the Hines VA facility in Chicago, Illinois.2 By 1947, 62 medical schools had committed to an affiliation with local VA hospitals and 21 deans’ committees were in operation, which were responsible for the appointment of physician residents and consultants. The AMA extended direct membership privileges to VA physicians, and by 1947 the number of residency positions doubled nationally.14,15 The almost universal support of the relationship between VA and academic affiliates provided educational opportunities for returning veterans and raised standards for medical education nationally.
Current State
Since the passage of PL 79-293 and PM No. 2, the VA-academic health professions education partnership has grown to include 113,000 trainees rotating through 150 VA medical centers annually from more than 1400 colleges and universities.16 Most VA podiatrists, psychologists, optometrists, and physicians working in VA medical centers also trained at VA, and trainees are 37% more likely to consider a job at VA after completing their clinical rotations. This unique partnership began 76 years ago and continues to provide clinicians “for VA and the nation.”
1. Glasson WH. History of military pension legislation in the United States. Columbia University Press; 1900.
2. Lewis BJ. Veterans Administration medical program relationship with medical schools in the United States. Dissertation. The American University; 1969.
3. Kracke RR. The role of the medical college in the medical care of the veteran. J Med Assoc State Ala. 1950;19(8):225-230.
4. US Department of Veterans Affairs, Office of Public Affairs. VA History in Brief. VA Pamphlet 80-97-2. Washington, DC: United States Department of Veterans Affairs; 1997.
5. Servicesmen’s Readjustment Act of 1944. 38 USC § 370 (1944).
6. To establish a Department of Medicine and Surgery in the Veterans’ Administration. 38 USC § 73-74 (1946). Accessed August 2, 2022.
7. Lueth HC. Postgraduate wishes of medical officers: final report on 21,029 questionnaires. J Am Med Assoc. 1945; 127(13):759-770.
8. Johnson V, Arestad FH, Tipner A. Medical education in the United States and Canada: forty-sixth annual report on medical education in the United States and Canada by the Council on Medical Education and Hospitals of the American Medical Association. J Am Med Assoc. 1946;131(16):1277-1310.
9. Chesney AM. Some impacts of the specialty board movement on medical education. J Assoc Am Med Coll. 1948;23(2):83-89.
10. Hiscock IV. New frontiers in health education. Can J Public Health. 1946;37(11):452-457.
11. Colwell AR. Principles of graduate medical instruction: with a specific plan of application in a medical school. J Am Med Assoc. 1945;127(13):741-746.
12. Maisel, AQ. The veteran betrayed. How long will the Veterans’ Administration continue to give third-rate medical care to first-rate men? Cosmopolitan. 1945(3):45.
13. US Veterans Administration. Policy Memorandum No. 2: Policy in association of veterans’ hospitals with medical schools. January 30, 1946.
14. American Medical Association. Digest of Official Actions: 1846-1958. JAMA. 1946;132:1094.
15. Wentz DK, Ford CV. A brief history of the internship. JAMA. 1984;252(24):3390-3394. doi:10.1001/jama.1984.03350240036035
16. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education academic year 2022-2021. Accessed August 8, 2022. https://www.va.gov/OAA/docs/OAA_Stats_AY_2020_2021_FINAL.pdf
The US government has a legacy of providing support for veterans. Pensions were offered to disabled veterans as early as 1776, and benefits were expanded to cover medical needs as the country grew and modernized.1,2 Enacted during the Civil War, the General Pension Act increased benefits for widows and dependents.2 Rehabilitation and vocational training assistance benefits were added after World War I, and the US Department of Veterans Affairs (VA) was created in 1930 to consolidate all benefits under one umbrella organization.2,3
Prior to World War II, the VA lacked the bed capacity for the 4 million veterans who were eligible for care. This shortage became more acute by the end of the war, when the number of eligible veterans increased by 15 million.4 Although the VA successfully built bed capacity through acquisition of military hospitals, VA hospitals struggled to recruit clinical staff.2 Physicians were hesitant to join the VA because civil service salaries were lower than comparable positions in the community, and the VA offered limited opportunities for research or continuing education. These limitations negatively impacted the overall reputation of the VA. The American Medical Association (AMA) was reluctant to directly admit VA physicians for membership because of a “lower” standard of care at VA hospitals.2 This review will describe how passage of 2 legislative actions, the Servicemen’s Readjustment Act and Public Law (PL)79-293, and a key policy memorandum set the foundation for the partnership between the VA and academic medical centers. This led to improved medical care for veterans and expansion of health professions education for VA and the nation.5,6
GI Bill of Rights
The passage of the Servicemen’s Readjustment Act of 1944, better known as the GI Bill of Rights, provided education assistance, guaranteed home loans, and unemployment payments to veterans.5 All medical officers serving during the war were eligible for this benefit, which effectively increased the number of potential physician trainees at the end of World War II by almost 60,000.7 Medical education at the time was simultaneously undergoing a transformation with more rigorous training and a push to standardize medical education across state lines. While prerequisite training was not required for admission to many medical schools and curricula varied in length based on state licensing requirements, more programs were adding premedical education requirements and transitioning to the 4-year curricula seen today. At this time, only 23 states required postgraduate internships for licensure, but this number was growing.8 The American Board of Medical Specialties was established several years prior to World War II in 1934 to elevate the quality of care; the desire for residency training and board certification continued to gain traction during the 1940s.9
Medical Training
In anticipation of an influx of medical trainees, the Committee on Postwar Medical Service conducted a comprehensive survey to understand the training needs of physician veterans returning from World War II.7 The survey collected data from medical officers on their desired length of training, interest in specialty board certification, time served, and type of medical practice prior to enlisting. Length of desired training was categorized as short (up to 6 months), which would serve as a refresher course and provide updates on recent advances in medicine and surgery, and long (> 6 months), which resembled a modern internship or residency. Nineteen percent did not want additional training, 22% wished to pursue short courses, and 51% were interested in longer courses. Most respondents also wished to obtain board certification.7 The AMA played a significant role in supporting the expansion of training opportunities, encouraging all accredited hospitals to assess their capacity to determine the number of additional residents they could accommodate. The AMA also awarded hospitals with existing internship programs temporary accreditation to allow them to add extended training through residency programs.7
Medical schools devised creative solutions to meet the needs of returning physician veterans and capitalize on the available educational benefits. Postgraduate refresher courses that varied in length from hours to months were developed focusing on an array of topics. In addition to basic medical principles, courses covered general topics, such as advances in medicine, to specialty topics, such as nutrition or ophthalmology.7 Although the courses could not be counted toward board certification, participation increased by almost 300% in the 1945/1946 academic year relative to the previous year.7 Increasing access to the longer training courses, including internships and residencies, was often achieved through experiences outside the clinical setting. Yale University modified its curriculum to reduce time devoted to lectures on published materials and encourage active learning and community outreach.10 Northwestern University assigned residents to spend 1 of their 3 years “out of residence” in basic science and clinical instruction provided by the medical school. Tuition assistance from the GI Bill supported the additional expenses incurred by the medical school to fund laboratory space, equipment, and the salaries of the basic science instructors and administrative staff.11
Public Law 79-293
Public Law 79-293 was passed on January 3, 1946, establishing the Department of Medicine and Surgery within the VA. The law, which became the basis for Title 38 chapters 73 and 74, allowed VA hospitals flexibility to hire doctors, dentists, and nurses without regard to the civil service regulations and salary restrictions associated with other federal positions.6
Concerns about quality of care had been mounting for years, and the release of several sensationalized and critical articles motivated VA leadership to make sweeping changes. One article described neglect at VA hospitals.12 Excessive paperwork and low economic benefits were identified as barriers to the recruitment of qualified clinicians at the VA.2 The VA Special Medical Advisory Group investigating the claims recommended that the VA encourage their hospitals to affiliate with medical schools to improve the quality of care. This group also recommended that new VA hospitals be constructed near academic medical centers to allow access to consultants.2 Three large veterans service organizations (American Legion, Veterans of Foreign Wars, and Disabled American Veterans) conducted their own investigations in response to the media reports. The organizations reported that the quality of care in most VA hospitals was already on par with the community but indicated that the VA would benefit from expansion of medical research and training, increased bed capacity, reduction in the administrative burden on clinicians, and increased salaries for clinical staff.2
Policy Memorandum No. 2
The relationship between VA and academic medical centers was solidified on January 30, 1946, with adoption of Policy Memorandum No. 2.13 This memorandum allowed for the establishment of relationships with academic medical centers to provide “the veteran a much higher standard of medical care than could be given him with a wholly full-time medical staff.” Shortly after this memorandum was signed, residents from Northwestern University and the University of Illinois at Chicago began clinical rotations at the Hines VA facility in Chicago, Illinois.2 By 1947, 62 medical schools had committed to an affiliation with local VA hospitals and 21 deans’ committees were in operation, which were responsible for the appointment of physician residents and consultants. The AMA extended direct membership privileges to VA physicians, and by 1947 the number of residency positions doubled nationally.14,15 The almost universal support of the relationship between VA and academic affiliates provided educational opportunities for returning veterans and raised standards for medical education nationally.
Current State
Since the passage of PL 79-293 and PM No. 2, the VA-academic health professions education partnership has grown to include 113,000 trainees rotating through 150 VA medical centers annually from more than 1400 colleges and universities.16 Most VA podiatrists, psychologists, optometrists, and physicians working in VA medical centers also trained at VA, and trainees are 37% more likely to consider a job at VA after completing their clinical rotations. This unique partnership began 76 years ago and continues to provide clinicians “for VA and the nation.”
The US government has a legacy of providing support for veterans. Pensions were offered to disabled veterans as early as 1776, and benefits were expanded to cover medical needs as the country grew and modernized.1,2 Enacted during the Civil War, the General Pension Act increased benefits for widows and dependents.2 Rehabilitation and vocational training assistance benefits were added after World War I, and the US Department of Veterans Affairs (VA) was created in 1930 to consolidate all benefits under one umbrella organization.2,3
Prior to World War II, the VA lacked the bed capacity for the 4 million veterans who were eligible for care. This shortage became more acute by the end of the war, when the number of eligible veterans increased by 15 million.4 Although the VA successfully built bed capacity through acquisition of military hospitals, VA hospitals struggled to recruit clinical staff.2 Physicians were hesitant to join the VA because civil service salaries were lower than comparable positions in the community, and the VA offered limited opportunities for research or continuing education. These limitations negatively impacted the overall reputation of the VA. The American Medical Association (AMA) was reluctant to directly admit VA physicians for membership because of a “lower” standard of care at VA hospitals.2 This review will describe how passage of 2 legislative actions, the Servicemen’s Readjustment Act and Public Law (PL)79-293, and a key policy memorandum set the foundation for the partnership between the VA and academic medical centers. This led to improved medical care for veterans and expansion of health professions education for VA and the nation.5,6
GI Bill of Rights
The passage of the Servicemen’s Readjustment Act of 1944, better known as the GI Bill of Rights, provided education assistance, guaranteed home loans, and unemployment payments to veterans.5 All medical officers serving during the war were eligible for this benefit, which effectively increased the number of potential physician trainees at the end of World War II by almost 60,000.7 Medical education at the time was simultaneously undergoing a transformation with more rigorous training and a push to standardize medical education across state lines. While prerequisite training was not required for admission to many medical schools and curricula varied in length based on state licensing requirements, more programs were adding premedical education requirements and transitioning to the 4-year curricula seen today. At this time, only 23 states required postgraduate internships for licensure, but this number was growing.8 The American Board of Medical Specialties was established several years prior to World War II in 1934 to elevate the quality of care; the desire for residency training and board certification continued to gain traction during the 1940s.9
Medical Training
In anticipation of an influx of medical trainees, the Committee on Postwar Medical Service conducted a comprehensive survey to understand the training needs of physician veterans returning from World War II.7 The survey collected data from medical officers on their desired length of training, interest in specialty board certification, time served, and type of medical practice prior to enlisting. Length of desired training was categorized as short (up to 6 months), which would serve as a refresher course and provide updates on recent advances in medicine and surgery, and long (> 6 months), which resembled a modern internship or residency. Nineteen percent did not want additional training, 22% wished to pursue short courses, and 51% were interested in longer courses. Most respondents also wished to obtain board certification.7 The AMA played a significant role in supporting the expansion of training opportunities, encouraging all accredited hospitals to assess their capacity to determine the number of additional residents they could accommodate. The AMA also awarded hospitals with existing internship programs temporary accreditation to allow them to add extended training through residency programs.7
Medical schools devised creative solutions to meet the needs of returning physician veterans and capitalize on the available educational benefits. Postgraduate refresher courses that varied in length from hours to months were developed focusing on an array of topics. In addition to basic medical principles, courses covered general topics, such as advances in medicine, to specialty topics, such as nutrition or ophthalmology.7 Although the courses could not be counted toward board certification, participation increased by almost 300% in the 1945/1946 academic year relative to the previous year.7 Increasing access to the longer training courses, including internships and residencies, was often achieved through experiences outside the clinical setting. Yale University modified its curriculum to reduce time devoted to lectures on published materials and encourage active learning and community outreach.10 Northwestern University assigned residents to spend 1 of their 3 years “out of residence” in basic science and clinical instruction provided by the medical school. Tuition assistance from the GI Bill supported the additional expenses incurred by the medical school to fund laboratory space, equipment, and the salaries of the basic science instructors and administrative staff.11
Public Law 79-293
Public Law 79-293 was passed on January 3, 1946, establishing the Department of Medicine and Surgery within the VA. The law, which became the basis for Title 38 chapters 73 and 74, allowed VA hospitals flexibility to hire doctors, dentists, and nurses without regard to the civil service regulations and salary restrictions associated with other federal positions.6
Concerns about quality of care had been mounting for years, and the release of several sensationalized and critical articles motivated VA leadership to make sweeping changes. One article described neglect at VA hospitals.12 Excessive paperwork and low economic benefits were identified as barriers to the recruitment of qualified clinicians at the VA.2 The VA Special Medical Advisory Group investigating the claims recommended that the VA encourage their hospitals to affiliate with medical schools to improve the quality of care. This group also recommended that new VA hospitals be constructed near academic medical centers to allow access to consultants.2 Three large veterans service organizations (American Legion, Veterans of Foreign Wars, and Disabled American Veterans) conducted their own investigations in response to the media reports. The organizations reported that the quality of care in most VA hospitals was already on par with the community but indicated that the VA would benefit from expansion of medical research and training, increased bed capacity, reduction in the administrative burden on clinicians, and increased salaries for clinical staff.2
Policy Memorandum No. 2
The relationship between VA and academic medical centers was solidified on January 30, 1946, with adoption of Policy Memorandum No. 2.13 This memorandum allowed for the establishment of relationships with academic medical centers to provide “the veteran a much higher standard of medical care than could be given him with a wholly full-time medical staff.” Shortly after this memorandum was signed, residents from Northwestern University and the University of Illinois at Chicago began clinical rotations at the Hines VA facility in Chicago, Illinois.2 By 1947, 62 medical schools had committed to an affiliation with local VA hospitals and 21 deans’ committees were in operation, which were responsible for the appointment of physician residents and consultants. The AMA extended direct membership privileges to VA physicians, and by 1947 the number of residency positions doubled nationally.14,15 The almost universal support of the relationship between VA and academic affiliates provided educational opportunities for returning veterans and raised standards for medical education nationally.
Current State
Since the passage of PL 79-293 and PM No. 2, the VA-academic health professions education partnership has grown to include 113,000 trainees rotating through 150 VA medical centers annually from more than 1400 colleges and universities.16 Most VA podiatrists, psychologists, optometrists, and physicians working in VA medical centers also trained at VA, and trainees are 37% more likely to consider a job at VA after completing their clinical rotations. This unique partnership began 76 years ago and continues to provide clinicians “for VA and the nation.”
1. Glasson WH. History of military pension legislation in the United States. Columbia University Press; 1900.
2. Lewis BJ. Veterans Administration medical program relationship with medical schools in the United States. Dissertation. The American University; 1969.
3. Kracke RR. The role of the medical college in the medical care of the veteran. J Med Assoc State Ala. 1950;19(8):225-230.
4. US Department of Veterans Affairs, Office of Public Affairs. VA History in Brief. VA Pamphlet 80-97-2. Washington, DC: United States Department of Veterans Affairs; 1997.
5. Servicesmen’s Readjustment Act of 1944. 38 USC § 370 (1944).
6. To establish a Department of Medicine and Surgery in the Veterans’ Administration. 38 USC § 73-74 (1946). Accessed August 2, 2022.
7. Lueth HC. Postgraduate wishes of medical officers: final report on 21,029 questionnaires. J Am Med Assoc. 1945; 127(13):759-770.
8. Johnson V, Arestad FH, Tipner A. Medical education in the United States and Canada: forty-sixth annual report on medical education in the United States and Canada by the Council on Medical Education and Hospitals of the American Medical Association. J Am Med Assoc. 1946;131(16):1277-1310.
9. Chesney AM. Some impacts of the specialty board movement on medical education. J Assoc Am Med Coll. 1948;23(2):83-89.
10. Hiscock IV. New frontiers in health education. Can J Public Health. 1946;37(11):452-457.
11. Colwell AR. Principles of graduate medical instruction: with a specific plan of application in a medical school. J Am Med Assoc. 1945;127(13):741-746.
12. Maisel, AQ. The veteran betrayed. How long will the Veterans’ Administration continue to give third-rate medical care to first-rate men? Cosmopolitan. 1945(3):45.
13. US Veterans Administration. Policy Memorandum No. 2: Policy in association of veterans’ hospitals with medical schools. January 30, 1946.
14. American Medical Association. Digest of Official Actions: 1846-1958. JAMA. 1946;132:1094.
15. Wentz DK, Ford CV. A brief history of the internship. JAMA. 1984;252(24):3390-3394. doi:10.1001/jama.1984.03350240036035
16. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education academic year 2022-2021. Accessed August 8, 2022. https://www.va.gov/OAA/docs/OAA_Stats_AY_2020_2021_FINAL.pdf
1. Glasson WH. History of military pension legislation in the United States. Columbia University Press; 1900.
2. Lewis BJ. Veterans Administration medical program relationship with medical schools in the United States. Dissertation. The American University; 1969.
3. Kracke RR. The role of the medical college in the medical care of the veteran. J Med Assoc State Ala. 1950;19(8):225-230.
4. US Department of Veterans Affairs, Office of Public Affairs. VA History in Brief. VA Pamphlet 80-97-2. Washington, DC: United States Department of Veterans Affairs; 1997.
5. Servicesmen’s Readjustment Act of 1944. 38 USC § 370 (1944).
6. To establish a Department of Medicine and Surgery in the Veterans’ Administration. 38 USC § 73-74 (1946). Accessed August 2, 2022.
7. Lueth HC. Postgraduate wishes of medical officers: final report on 21,029 questionnaires. J Am Med Assoc. 1945; 127(13):759-770.
8. Johnson V, Arestad FH, Tipner A. Medical education in the United States and Canada: forty-sixth annual report on medical education in the United States and Canada by the Council on Medical Education and Hospitals of the American Medical Association. J Am Med Assoc. 1946;131(16):1277-1310.
9. Chesney AM. Some impacts of the specialty board movement on medical education. J Assoc Am Med Coll. 1948;23(2):83-89.
10. Hiscock IV. New frontiers in health education. Can J Public Health. 1946;37(11):452-457.
11. Colwell AR. Principles of graduate medical instruction: with a specific plan of application in a medical school. J Am Med Assoc. 1945;127(13):741-746.
12. Maisel, AQ. The veteran betrayed. How long will the Veterans’ Administration continue to give third-rate medical care to first-rate men? Cosmopolitan. 1945(3):45.
13. US Veterans Administration. Policy Memorandum No. 2: Policy in association of veterans’ hospitals with medical schools. January 30, 1946.
14. American Medical Association. Digest of Official Actions: 1846-1958. JAMA. 1946;132:1094.
15. Wentz DK, Ford CV. A brief history of the internship. JAMA. 1984;252(24):3390-3394. doi:10.1001/jama.1984.03350240036035
16. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education academic year 2022-2021. Accessed August 8, 2022. https://www.va.gov/OAA/docs/OAA_Stats_AY_2020_2021_FINAL.pdf
A First Look at the VA MISSION Act Veteran Health Administration Medical School Scholarship and Loan Repayment Programs
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
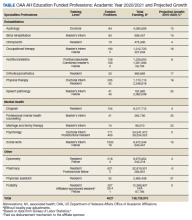
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
The Role of Diagnostic Imaging in Macular Telangiectasia Type 2
While uncommon with subtle findings, macular telangiectasia type 2 can be diagnosed with careful retinal examination and selective use of diagnostic imaging.
Macular telangiectasia type 2 (MacTel2) is an uncommon, bilateral, and asymmetric condition that typically presents between the ages of 40 and 60 years without sex predilection.1-9 Its estimated prevalence ranges from 0.02 to 0.10%.2,8 The disease can manifest in either a nonproliferative or proliferative phase; the latter is far less common. The etiology of MacTel2 is poorly understood, but it is believed to have neurodegenerative as well as vascular components.1-6,8-10 We present a case of MacTel2 and highlight the role of diagnostic imaging in early diagnosis prior to development of classic funduscopic features.
Case Presentation
A 66-year-old White male with a 10-year history of type 2 diabetes mellitus (T2DM) presented to the eye clinic for an annual eye examination. The patient was taking metformin, and 6 months prior to presentation, his hemoglobin A1c was 7.4%. He had a history of mild nonproliferative diabetic retinopathy in the left eye without diabetic macular edema. He reported no ocular concerns.
On examination, best-corrected visual acuity (VA) was 20/20 in each eye. Slit-lamp examination was notable only for bilateral mild nuclear sclerosis. Dilated fundus examination showed a blunted foveal reflex consistent with the appearance of a macular pseudo-hole in the right eye and was unremarkable in the left eye (Figure 1).
Macular optical coherence tomography (OCT) revealed an intraretinal cyst without thickening in the temporal fovea of both eyes with mild disruption of the underlying ellipsoid zone in the right eye (Figure 2). A presumptive diagnosis of MacTel2 vs diabetic macular edema was made, and the patient was referred to the retina clinic for further evaluation.
At the 1-month follow-up in the retina clinic, VA, macula OCT, and fundus examination were stable. Fundus autofluorescence (FAF), optical coherence tomography angiography (OCT-A), and fluorescein angiography (FA) were performed. The FAF revealed a hyperreflective crescent in the temporal aspect of the fovea of both eyes, greater in the right eye than the left (Figure 3). The OCT-A showed abnormal dilation of the vessels in the deep capillary plexus of the temporal fovea of both eyes (Figure 4). This area of abnormality correlated to the area of hyperreflectivity seen on FAF. The early- phase FA revealed telangiectatic vessels in the temporal fovea in both eyes; in the late phase, there was leakage of telangiectatic vessels, which remained localized to the temporal perifovea and spared the central fovea of both eyes (Figure 5). The patient was diagnosed with MacTel2.
Discussion
This case highlights several important management considerations in MacTel2. These include symptoms, disease stage, and diagnostic imaging, which can allow more precise staging of the disease.
The etiology of MacTel2 is unknown.6 It is believed to be primarily a neurodegenerative condition that damages Müller cells and photoreceptors, leading to vascular changes.1-6,8-10 Müller cells may play a role in creating and maintaining the integrity of the blood-retinal barrier, particularly in the deep capillary plexus where the vascular abnormalities begin.6,10 These early changes in the deep capillary plexus may evolve to include the superficial capillary plexus in intermediate stages with anastomoses forming between the 2 layers.2,6-10 Late proliferative stages show significant alterations of the juxtafoveal capillary network, subretinal neovascularization and retinochoroidal anastomoses.6,7,9,11 In one cohort study, 81% of patients with MacTel2 were White, and a genetic link is still under investigation.2,4-9
Presentation
The most common symptoms of MacTel2 include blurred vision, microscotoma, metamorphopsia, and difficulty reading, with missing or distorted letters a common concern.1,2,4-8 Best-corrected VA at presentation is usually better than 20/30, and disease progression tends to be slow.2,6 Microscotomata are best mapped with microperimetry.1-3,5-7
There are several classic fundus findings (Table). In the early stages, these findings are subtle or entirely absent funduscopically.1,2,4-10 In intermediate stages, fundus findings become apparent and include a loss of retinal clarity (grayish perifoveal sheen), telangiectatic macular vessels, retinal pigment epithelium hypertrophy, blunted right-angled vessels, and superficial retinal crystalline deposits.2,4-11 Right-angled vessels may have a greater association with choroidal neovascularization, with growth into the outer retina in particular being a marker of disease progression.9 The crystalline deposits have been hypothesized to be the footplates of degenerated Müller cells.6
An important vision-threatening complication of MacTel2 is progression to proliferative disease.1,2,5-10 Choroidal neovascularization is present in a minority of cases and is associated with rapid vision loss.2,6 It is often accompanied by subretinal hemorrhage and lipid exudation.6,7,9 If untreated, the result can be disciform scarring and fibrosis.2,5,6 Additional complications of MacTel2 are foveal atrophy and full thickness macular holes.1,2,4-8, Macular holes secondary to MacTel2 respond poorly to pars plana vitrectomy with inner limiting membrane (ILM) peel.2,6
Diagnostic Testing
Diagnostic retinal imaging is invaluable in the diagnosis of MacTel2. The OCT can detect hyporeflectivity within the ellipsoid zone in early disease corresponding to ellipsoid zone loss, which increases as the disease progresses.1-8,10 This loss most often begins in the temporal parafoveal region and correlates with the progression of both relative and absolute scotomas perceived by affected individuals.2,3,5,8
Intraretinal foveal hyporeflective spaces on the OCT represent cavity formation after Müller cell and photoreceptor loss and do not correlate with increased thickness.1,2,4,6,7 This is important in differentiating from diabetic macular edema, which will often show thickening.6 In most cases of MacTel2, foveal thickness is decreased.4-6 The ILM remains intact overlying this space and is referred to as ILM drape.6,7 This can cause blunting or absence of the foveal light reflex and mimic the appearance of a macular pseudohole.4
The OCT-A allows visualization of capillary changes through every layer of the retina, which could not otherwise be appreciated, allowing early detection as well as precise staging of the disease.2,6-10 Anastomoses present in late-stage disease also can be imaged using OCT-A.7,9 These anastomoses can be seen as hyperreflective vasculature present between the retinal layers where there is little to no vasculature visible in normal eyes.7
A lesser-known occurrence in MacTel2 is the depletion of macular luteal pigment, with many eyes possessing an abnormal distribution.2,4,6-8,10 This depletion and abnormal distribution can be visualized with FAF. In particular, short wavelength fundus autofluorescence (SW-FAF) is the most effective at highlighting these changes.10 The characteristic finding is a hyperreflective halo surrounding the fovea.2,6 Fluorescence life imaging ophthalmoscopy (FLIO) is a recent development in FAF that measures FAF lifetime, which is the duration of time a structure autofluoresces.8 A cross-sectional study published in 2018 showed prolonged FAF lifetime in the temporal fovea of patients with early and moderate stage MacTel2 when compared with normal patients.8 More advanced stages showed a ring encircling the entire fovea.8
Classic FA findings in MacTel2 include early hyperfluorescence of temporal foveal telangiectatic capillaries and late-stage leakage with sparing of the central fovea.1,2,4,6,7,11
Management and Prognosis
Management of MacTel2 depends on the stage of the disease. In the absence of proven treatment, management in nonproliferative stages is conservative.2,6 Intravitreal anti-VEGF does not offer any benefit in nonproliferative disease.2,5,6 Indeed, as VEGF may have a neuroprotective effect on the retina, anti-VEGF may result in more harm than benefit in earlier disease stages.5 In proliferative stages, intravitreal anti-VEGF can help limit scarring and prevent vision loss.2,5
Long-term prognosis of MacTel2 is variable with VA typically better than 20/100.2 Vision loss in MacTel2 most often begins paracentrally; it can then progress centrally, leading to significant reduction in VA.12 The progression of this functional vision loss and corresponding structural damage is typically slow.3 VA worse than 20/100 is usually a result of proliferative disease; in such cases, there is potential for severe central vision loss and legal blindness.1
Conclusions
This case of MacTel2 underscores the subtle retinal findings in the earliest stages of the disease and the importance of a complete retinal examination and diagnostic imaging with macula OCT, OCT-A, and FAF to establish the correct diagnosis.
1. Chew EY, Clemons TE, Jaffe GJ, et al. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology. 2019;126(4):540-549. doi:10.1016/j.ophtha.2018.09.041
2. Christakis PG, Fine HF, Wiley HE. The diagnosis and management of macular telangiectasia. Ophthalmic Surg Lasers Imaging Retina. 2019;50(3):139-144. doi:10.3928/23258160-20190301-02
3. Heeren TFC, Kitka D, Florea D, et al. Longitudinal correlation of ellipsoid zone loss and functional loss in macular telangiectasia type 2. Retina. 2018;38 Suppl 1(suppl 1):S20-S26. doi:10.1097/IAE.0000000000001715
4. Charbel Issa P, Heeren TF, Kupitz EH, Holz FG, Berendschot TT. Very early disease manifestations of macular telangiectasia type 2. Retina. 2016;36(3):524-534. doi:10.1097/IAE.0000000000000863
5. Khodabande A, Roohipoor R, Zamani J, et al. Management of idiopathic macular telangiectasia type 2. Ophthalmol Ther. 2019;8(2):155-175. doi:10.1007/s40123-019-0170-1
6. Wu L. When is macular edema not macular edema? An update on macular telangiectasia type 2. Taiwan J Ophthalmol. 2015;5(4):149-155. doi:10.1016/j.tjo.2015.09.001
7. Roisman L, Rosenfeld PJ. Optical Coherence Tomography Angiography of Macular Telangiectasia Type 2. Dev Ophthalmol. 2016;56:146-158. doi:10.1159/000442807
8. Sauer L, Gensure RH, Hammer M, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy: a novel way to assess macular telangiectasia type 2. Ophthalmol Retina. 2018;2(6):587-598. doi:10.1016/j.oret.2017.10.008
9. Tzaridis S, Heeren T, Mai C, et al. Right-angled vessels in macular telangiectasia type 2. Br J Ophthalmol. 2021;105(9):1289-1296. doi:10.1136/bjophthalmol-2018-313364
10. Micevych PS, Lee HE, Fawzi AA. Overlap between telangiectasia and photoreceptor loss increases with progression of macular telangiectasia type 2. PLoS One. 2019;14(10):e0224393. Published 2019 Oct 28. doi:10.1371/journal.pone.0224393
11. Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100(5):769-780. doi:10.1001/archopht.1982.01030030773010
12. Heeren TF, Clemons T, Scholl HP, Bird AC, Holz FG, Charbel Issa P. Progression of vision loss in macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2015;56(6):3905-3912. doi:10.1167/iovs.15-16915
While uncommon with subtle findings, macular telangiectasia type 2 can be diagnosed with careful retinal examination and selective use of diagnostic imaging.
While uncommon with subtle findings, macular telangiectasia type 2 can be diagnosed with careful retinal examination and selective use of diagnostic imaging.
Macular telangiectasia type 2 (MacTel2) is an uncommon, bilateral, and asymmetric condition that typically presents between the ages of 40 and 60 years without sex predilection.1-9 Its estimated prevalence ranges from 0.02 to 0.10%.2,8 The disease can manifest in either a nonproliferative or proliferative phase; the latter is far less common. The etiology of MacTel2 is poorly understood, but it is believed to have neurodegenerative as well as vascular components.1-6,8-10 We present a case of MacTel2 and highlight the role of diagnostic imaging in early diagnosis prior to development of classic funduscopic features.
Case Presentation
A 66-year-old White male with a 10-year history of type 2 diabetes mellitus (T2DM) presented to the eye clinic for an annual eye examination. The patient was taking metformin, and 6 months prior to presentation, his hemoglobin A1c was 7.4%. He had a history of mild nonproliferative diabetic retinopathy in the left eye without diabetic macular edema. He reported no ocular concerns.
On examination, best-corrected visual acuity (VA) was 20/20 in each eye. Slit-lamp examination was notable only for bilateral mild nuclear sclerosis. Dilated fundus examination showed a blunted foveal reflex consistent with the appearance of a macular pseudo-hole in the right eye and was unremarkable in the left eye (Figure 1).
Macular optical coherence tomography (OCT) revealed an intraretinal cyst without thickening in the temporal fovea of both eyes with mild disruption of the underlying ellipsoid zone in the right eye (Figure 2). A presumptive diagnosis of MacTel2 vs diabetic macular edema was made, and the patient was referred to the retina clinic for further evaluation.
At the 1-month follow-up in the retina clinic, VA, macula OCT, and fundus examination were stable. Fundus autofluorescence (FAF), optical coherence tomography angiography (OCT-A), and fluorescein angiography (FA) were performed. The FAF revealed a hyperreflective crescent in the temporal aspect of the fovea of both eyes, greater in the right eye than the left (Figure 3). The OCT-A showed abnormal dilation of the vessels in the deep capillary plexus of the temporal fovea of both eyes (Figure 4). This area of abnormality correlated to the area of hyperreflectivity seen on FAF. The early- phase FA revealed telangiectatic vessels in the temporal fovea in both eyes; in the late phase, there was leakage of telangiectatic vessels, which remained localized to the temporal perifovea and spared the central fovea of both eyes (Figure 5). The patient was diagnosed with MacTel2.
Discussion
This case highlights several important management considerations in MacTel2. These include symptoms, disease stage, and diagnostic imaging, which can allow more precise staging of the disease.
The etiology of MacTel2 is unknown.6 It is believed to be primarily a neurodegenerative condition that damages Müller cells and photoreceptors, leading to vascular changes.1-6,8-10 Müller cells may play a role in creating and maintaining the integrity of the blood-retinal barrier, particularly in the deep capillary plexus where the vascular abnormalities begin.6,10 These early changes in the deep capillary plexus may evolve to include the superficial capillary plexus in intermediate stages with anastomoses forming between the 2 layers.2,6-10 Late proliferative stages show significant alterations of the juxtafoveal capillary network, subretinal neovascularization and retinochoroidal anastomoses.6,7,9,11 In one cohort study, 81% of patients with MacTel2 were White, and a genetic link is still under investigation.2,4-9
Presentation
The most common symptoms of MacTel2 include blurred vision, microscotoma, metamorphopsia, and difficulty reading, with missing or distorted letters a common concern.1,2,4-8 Best-corrected VA at presentation is usually better than 20/30, and disease progression tends to be slow.2,6 Microscotomata are best mapped with microperimetry.1-3,5-7
There are several classic fundus findings (Table). In the early stages, these findings are subtle or entirely absent funduscopically.1,2,4-10 In intermediate stages, fundus findings become apparent and include a loss of retinal clarity (grayish perifoveal sheen), telangiectatic macular vessels, retinal pigment epithelium hypertrophy, blunted right-angled vessels, and superficial retinal crystalline deposits.2,4-11 Right-angled vessels may have a greater association with choroidal neovascularization, with growth into the outer retina in particular being a marker of disease progression.9 The crystalline deposits have been hypothesized to be the footplates of degenerated Müller cells.6
An important vision-threatening complication of MacTel2 is progression to proliferative disease.1,2,5-10 Choroidal neovascularization is present in a minority of cases and is associated with rapid vision loss.2,6 It is often accompanied by subretinal hemorrhage and lipid exudation.6,7,9 If untreated, the result can be disciform scarring and fibrosis.2,5,6 Additional complications of MacTel2 are foveal atrophy and full thickness macular holes.1,2,4-8, Macular holes secondary to MacTel2 respond poorly to pars plana vitrectomy with inner limiting membrane (ILM) peel.2,6
Diagnostic Testing
Diagnostic retinal imaging is invaluable in the diagnosis of MacTel2. The OCT can detect hyporeflectivity within the ellipsoid zone in early disease corresponding to ellipsoid zone loss, which increases as the disease progresses.1-8,10 This loss most often begins in the temporal parafoveal region and correlates with the progression of both relative and absolute scotomas perceived by affected individuals.2,3,5,8
Intraretinal foveal hyporeflective spaces on the OCT represent cavity formation after Müller cell and photoreceptor loss and do not correlate with increased thickness.1,2,4,6,7 This is important in differentiating from diabetic macular edema, which will often show thickening.6 In most cases of MacTel2, foveal thickness is decreased.4-6 The ILM remains intact overlying this space and is referred to as ILM drape.6,7 This can cause blunting or absence of the foveal light reflex and mimic the appearance of a macular pseudohole.4
The OCT-A allows visualization of capillary changes through every layer of the retina, which could not otherwise be appreciated, allowing early detection as well as precise staging of the disease.2,6-10 Anastomoses present in late-stage disease also can be imaged using OCT-A.7,9 These anastomoses can be seen as hyperreflective vasculature present between the retinal layers where there is little to no vasculature visible in normal eyes.7
A lesser-known occurrence in MacTel2 is the depletion of macular luteal pigment, with many eyes possessing an abnormal distribution.2,4,6-8,10 This depletion and abnormal distribution can be visualized with FAF. In particular, short wavelength fundus autofluorescence (SW-FAF) is the most effective at highlighting these changes.10 The characteristic finding is a hyperreflective halo surrounding the fovea.2,6 Fluorescence life imaging ophthalmoscopy (FLIO) is a recent development in FAF that measures FAF lifetime, which is the duration of time a structure autofluoresces.8 A cross-sectional study published in 2018 showed prolonged FAF lifetime in the temporal fovea of patients with early and moderate stage MacTel2 when compared with normal patients.8 More advanced stages showed a ring encircling the entire fovea.8
Classic FA findings in MacTel2 include early hyperfluorescence of temporal foveal telangiectatic capillaries and late-stage leakage with sparing of the central fovea.1,2,4,6,7,11
Management and Prognosis
Management of MacTel2 depends on the stage of the disease. In the absence of proven treatment, management in nonproliferative stages is conservative.2,6 Intravitreal anti-VEGF does not offer any benefit in nonproliferative disease.2,5,6 Indeed, as VEGF may have a neuroprotective effect on the retina, anti-VEGF may result in more harm than benefit in earlier disease stages.5 In proliferative stages, intravitreal anti-VEGF can help limit scarring and prevent vision loss.2,5
Long-term prognosis of MacTel2 is variable with VA typically better than 20/100.2 Vision loss in MacTel2 most often begins paracentrally; it can then progress centrally, leading to significant reduction in VA.12 The progression of this functional vision loss and corresponding structural damage is typically slow.3 VA worse than 20/100 is usually a result of proliferative disease; in such cases, there is potential for severe central vision loss and legal blindness.1
Conclusions
This case of MacTel2 underscores the subtle retinal findings in the earliest stages of the disease and the importance of a complete retinal examination and diagnostic imaging with macula OCT, OCT-A, and FAF to establish the correct diagnosis.
Macular telangiectasia type 2 (MacTel2) is an uncommon, bilateral, and asymmetric condition that typically presents between the ages of 40 and 60 years without sex predilection.1-9 Its estimated prevalence ranges from 0.02 to 0.10%.2,8 The disease can manifest in either a nonproliferative or proliferative phase; the latter is far less common. The etiology of MacTel2 is poorly understood, but it is believed to have neurodegenerative as well as vascular components.1-6,8-10 We present a case of MacTel2 and highlight the role of diagnostic imaging in early diagnosis prior to development of classic funduscopic features.
Case Presentation
A 66-year-old White male with a 10-year history of type 2 diabetes mellitus (T2DM) presented to the eye clinic for an annual eye examination. The patient was taking metformin, and 6 months prior to presentation, his hemoglobin A1c was 7.4%. He had a history of mild nonproliferative diabetic retinopathy in the left eye without diabetic macular edema. He reported no ocular concerns.
On examination, best-corrected visual acuity (VA) was 20/20 in each eye. Slit-lamp examination was notable only for bilateral mild nuclear sclerosis. Dilated fundus examination showed a blunted foveal reflex consistent with the appearance of a macular pseudo-hole in the right eye and was unremarkable in the left eye (Figure 1).
Macular optical coherence tomography (OCT) revealed an intraretinal cyst without thickening in the temporal fovea of both eyes with mild disruption of the underlying ellipsoid zone in the right eye (Figure 2). A presumptive diagnosis of MacTel2 vs diabetic macular edema was made, and the patient was referred to the retina clinic for further evaluation.
At the 1-month follow-up in the retina clinic, VA, macula OCT, and fundus examination were stable. Fundus autofluorescence (FAF), optical coherence tomography angiography (OCT-A), and fluorescein angiography (FA) were performed. The FAF revealed a hyperreflective crescent in the temporal aspect of the fovea of both eyes, greater in the right eye than the left (Figure 3). The OCT-A showed abnormal dilation of the vessels in the deep capillary plexus of the temporal fovea of both eyes (Figure 4). This area of abnormality correlated to the area of hyperreflectivity seen on FAF. The early- phase FA revealed telangiectatic vessels in the temporal fovea in both eyes; in the late phase, there was leakage of telangiectatic vessels, which remained localized to the temporal perifovea and spared the central fovea of both eyes (Figure 5). The patient was diagnosed with MacTel2.
Discussion
This case highlights several important management considerations in MacTel2. These include symptoms, disease stage, and diagnostic imaging, which can allow more precise staging of the disease.
The etiology of MacTel2 is unknown.6 It is believed to be primarily a neurodegenerative condition that damages Müller cells and photoreceptors, leading to vascular changes.1-6,8-10 Müller cells may play a role in creating and maintaining the integrity of the blood-retinal barrier, particularly in the deep capillary plexus where the vascular abnormalities begin.6,10 These early changes in the deep capillary plexus may evolve to include the superficial capillary plexus in intermediate stages with anastomoses forming between the 2 layers.2,6-10 Late proliferative stages show significant alterations of the juxtafoveal capillary network, subretinal neovascularization and retinochoroidal anastomoses.6,7,9,11 In one cohort study, 81% of patients with MacTel2 were White, and a genetic link is still under investigation.2,4-9
Presentation
The most common symptoms of MacTel2 include blurred vision, microscotoma, metamorphopsia, and difficulty reading, with missing or distorted letters a common concern.1,2,4-8 Best-corrected VA at presentation is usually better than 20/30, and disease progression tends to be slow.2,6 Microscotomata are best mapped with microperimetry.1-3,5-7
There are several classic fundus findings (Table). In the early stages, these findings are subtle or entirely absent funduscopically.1,2,4-10 In intermediate stages, fundus findings become apparent and include a loss of retinal clarity (grayish perifoveal sheen), telangiectatic macular vessels, retinal pigment epithelium hypertrophy, blunted right-angled vessels, and superficial retinal crystalline deposits.2,4-11 Right-angled vessels may have a greater association with choroidal neovascularization, with growth into the outer retina in particular being a marker of disease progression.9 The crystalline deposits have been hypothesized to be the footplates of degenerated Müller cells.6
An important vision-threatening complication of MacTel2 is progression to proliferative disease.1,2,5-10 Choroidal neovascularization is present in a minority of cases and is associated with rapid vision loss.2,6 It is often accompanied by subretinal hemorrhage and lipid exudation.6,7,9 If untreated, the result can be disciform scarring and fibrosis.2,5,6 Additional complications of MacTel2 are foveal atrophy and full thickness macular holes.1,2,4-8, Macular holes secondary to MacTel2 respond poorly to pars plana vitrectomy with inner limiting membrane (ILM) peel.2,6
Diagnostic Testing
Diagnostic retinal imaging is invaluable in the diagnosis of MacTel2. The OCT can detect hyporeflectivity within the ellipsoid zone in early disease corresponding to ellipsoid zone loss, which increases as the disease progresses.1-8,10 This loss most often begins in the temporal parafoveal region and correlates with the progression of both relative and absolute scotomas perceived by affected individuals.2,3,5,8
Intraretinal foveal hyporeflective spaces on the OCT represent cavity formation after Müller cell and photoreceptor loss and do not correlate with increased thickness.1,2,4,6,7 This is important in differentiating from diabetic macular edema, which will often show thickening.6 In most cases of MacTel2, foveal thickness is decreased.4-6 The ILM remains intact overlying this space and is referred to as ILM drape.6,7 This can cause blunting or absence of the foveal light reflex and mimic the appearance of a macular pseudohole.4
The OCT-A allows visualization of capillary changes through every layer of the retina, which could not otherwise be appreciated, allowing early detection as well as precise staging of the disease.2,6-10 Anastomoses present in late-stage disease also can be imaged using OCT-A.7,9 These anastomoses can be seen as hyperreflective vasculature present between the retinal layers where there is little to no vasculature visible in normal eyes.7
A lesser-known occurrence in MacTel2 is the depletion of macular luteal pigment, with many eyes possessing an abnormal distribution.2,4,6-8,10 This depletion and abnormal distribution can be visualized with FAF. In particular, short wavelength fundus autofluorescence (SW-FAF) is the most effective at highlighting these changes.10 The characteristic finding is a hyperreflective halo surrounding the fovea.2,6 Fluorescence life imaging ophthalmoscopy (FLIO) is a recent development in FAF that measures FAF lifetime, which is the duration of time a structure autofluoresces.8 A cross-sectional study published in 2018 showed prolonged FAF lifetime in the temporal fovea of patients with early and moderate stage MacTel2 when compared with normal patients.8 More advanced stages showed a ring encircling the entire fovea.8
Classic FA findings in MacTel2 include early hyperfluorescence of temporal foveal telangiectatic capillaries and late-stage leakage with sparing of the central fovea.1,2,4,6,7,11
Management and Prognosis
Management of MacTel2 depends on the stage of the disease. In the absence of proven treatment, management in nonproliferative stages is conservative.2,6 Intravitreal anti-VEGF does not offer any benefit in nonproliferative disease.2,5,6 Indeed, as VEGF may have a neuroprotective effect on the retina, anti-VEGF may result in more harm than benefit in earlier disease stages.5 In proliferative stages, intravitreal anti-VEGF can help limit scarring and prevent vision loss.2,5
Long-term prognosis of MacTel2 is variable with VA typically better than 20/100.2 Vision loss in MacTel2 most often begins paracentrally; it can then progress centrally, leading to significant reduction in VA.12 The progression of this functional vision loss and corresponding structural damage is typically slow.3 VA worse than 20/100 is usually a result of proliferative disease; in such cases, there is potential for severe central vision loss and legal blindness.1
Conclusions
This case of MacTel2 underscores the subtle retinal findings in the earliest stages of the disease and the importance of a complete retinal examination and diagnostic imaging with macula OCT, OCT-A, and FAF to establish the correct diagnosis.
1. Chew EY, Clemons TE, Jaffe GJ, et al. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology. 2019;126(4):540-549. doi:10.1016/j.ophtha.2018.09.041
2. Christakis PG, Fine HF, Wiley HE. The diagnosis and management of macular telangiectasia. Ophthalmic Surg Lasers Imaging Retina. 2019;50(3):139-144. doi:10.3928/23258160-20190301-02
3. Heeren TFC, Kitka D, Florea D, et al. Longitudinal correlation of ellipsoid zone loss and functional loss in macular telangiectasia type 2. Retina. 2018;38 Suppl 1(suppl 1):S20-S26. doi:10.1097/IAE.0000000000001715
4. Charbel Issa P, Heeren TF, Kupitz EH, Holz FG, Berendschot TT. Very early disease manifestations of macular telangiectasia type 2. Retina. 2016;36(3):524-534. doi:10.1097/IAE.0000000000000863
5. Khodabande A, Roohipoor R, Zamani J, et al. Management of idiopathic macular telangiectasia type 2. Ophthalmol Ther. 2019;8(2):155-175. doi:10.1007/s40123-019-0170-1
6. Wu L. When is macular edema not macular edema? An update on macular telangiectasia type 2. Taiwan J Ophthalmol. 2015;5(4):149-155. doi:10.1016/j.tjo.2015.09.001
7. Roisman L, Rosenfeld PJ. Optical Coherence Tomography Angiography of Macular Telangiectasia Type 2. Dev Ophthalmol. 2016;56:146-158. doi:10.1159/000442807
8. Sauer L, Gensure RH, Hammer M, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy: a novel way to assess macular telangiectasia type 2. Ophthalmol Retina. 2018;2(6):587-598. doi:10.1016/j.oret.2017.10.008
9. Tzaridis S, Heeren T, Mai C, et al. Right-angled vessels in macular telangiectasia type 2. Br J Ophthalmol. 2021;105(9):1289-1296. doi:10.1136/bjophthalmol-2018-313364
10. Micevych PS, Lee HE, Fawzi AA. Overlap between telangiectasia and photoreceptor loss increases with progression of macular telangiectasia type 2. PLoS One. 2019;14(10):e0224393. Published 2019 Oct 28. doi:10.1371/journal.pone.0224393
11. Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100(5):769-780. doi:10.1001/archopht.1982.01030030773010
12. Heeren TF, Clemons T, Scholl HP, Bird AC, Holz FG, Charbel Issa P. Progression of vision loss in macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2015;56(6):3905-3912. doi:10.1167/iovs.15-16915
1. Chew EY, Clemons TE, Jaffe GJ, et al. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology. 2019;126(4):540-549. doi:10.1016/j.ophtha.2018.09.041
2. Christakis PG, Fine HF, Wiley HE. The diagnosis and management of macular telangiectasia. Ophthalmic Surg Lasers Imaging Retina. 2019;50(3):139-144. doi:10.3928/23258160-20190301-02
3. Heeren TFC, Kitka D, Florea D, et al. Longitudinal correlation of ellipsoid zone loss and functional loss in macular telangiectasia type 2. Retina. 2018;38 Suppl 1(suppl 1):S20-S26. doi:10.1097/IAE.0000000000001715
4. Charbel Issa P, Heeren TF, Kupitz EH, Holz FG, Berendschot TT. Very early disease manifestations of macular telangiectasia type 2. Retina. 2016;36(3):524-534. doi:10.1097/IAE.0000000000000863
5. Khodabande A, Roohipoor R, Zamani J, et al. Management of idiopathic macular telangiectasia type 2. Ophthalmol Ther. 2019;8(2):155-175. doi:10.1007/s40123-019-0170-1
6. Wu L. When is macular edema not macular edema? An update on macular telangiectasia type 2. Taiwan J Ophthalmol. 2015;5(4):149-155. doi:10.1016/j.tjo.2015.09.001
7. Roisman L, Rosenfeld PJ. Optical Coherence Tomography Angiography of Macular Telangiectasia Type 2. Dev Ophthalmol. 2016;56:146-158. doi:10.1159/000442807
8. Sauer L, Gensure RH, Hammer M, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy: a novel way to assess macular telangiectasia type 2. Ophthalmol Retina. 2018;2(6):587-598. doi:10.1016/j.oret.2017.10.008
9. Tzaridis S, Heeren T, Mai C, et al. Right-angled vessels in macular telangiectasia type 2. Br J Ophthalmol. 2021;105(9):1289-1296. doi:10.1136/bjophthalmol-2018-313364
10. Micevych PS, Lee HE, Fawzi AA. Overlap between telangiectasia and photoreceptor loss increases with progression of macular telangiectasia type 2. PLoS One. 2019;14(10):e0224393. Published 2019 Oct 28. doi:10.1371/journal.pone.0224393
11. Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100(5):769-780. doi:10.1001/archopht.1982.01030030773010
12. Heeren TF, Clemons T, Scholl HP, Bird AC, Holz FG, Charbel Issa P. Progression of vision loss in macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2015;56(6):3905-3912. doi:10.1167/iovs.15-16915
The Expansion of Associated Health Training in the VA
The US Department of Veterans Affairs (VA) is the largest health care delivery system in the United States, comprising 1293 sites of care, including 171 medical centers.1 One of the 4 statutory missions of the VA is to train health care professionals (HCPs) to meet the needs of the VA and the nation.2 Through partnerships with more than 1800 accredited colleges, universities, and training programs, the VA provides training annually to nearly 118,000 health professions trainees (HPTs) across a variety of health care professions, and all of whom provide direct clinical care to veterans.3
In the VA, the Office of Academic Affiliations (OAA) is charged with overseeing health professions training and the VA’s partnership with medical and associated health (AH) professions schools, which was first codified in Policy Memorandum No. 2 in 1946.4,5 Given the scope and breadth of health professions education offered through the VA, OAA is in a unique position to address health care shortage areas as well as influence the educational standards for certain professions.
Many of these health care professions fall under the rubric of AH, which include mental health (MH) specialties, rehabilitative specialties, and others. These professions are critical to address in the expanding world of health care in the United States with its increased specialization and emphasis on coordination of care with interprofessional teams. During the 2019/2020 academic year, the VA provided clinical training to approximately 21,000 AH HPTs from > 40 professions with just over 20% receiving financial support through the OAA. Of the HPTs who train at VA without compensation, most spend shorter amounts of time in clinical rotations in the VA, are in pregraduate-degree education programs where payment for clinical rotations is not expected and may not be eligible for hire immediately on completion of their clinical training experience. The 17 funded professions have been strategically selected by the OAA to ensure a robust pipeline of HCPs to meet the needs of veterans and the nation.
To meet the demands of AH professionals (AHPs), the OAA implemented targeted expansion over the past 10 years. While not exhaustive, this paper describes several expansion efforts based on VA special initiatives, including enhancing clinical access in rural settings and shifting toward postgraduate-degree training and specialization. By aligning expansion with VA priorities as well as trends in health care more broadly, the OAA can ensure that there is a supply of well-trained AHPs who have developed the requisite competencies to contribute to our nation’s health care needs. Further, expansion can help train and recruit health professionals who can be hired into VA positions ready to care for the complex needs of veterans.
Associated Health Professionals
Overseen by the OAA, AH expansion is designed to address the specific needs of the VA and the US health care system. Data from the VA Workforce Management and Consulting (WMC) shows that the VA employment of AHPs has grown from 87,351 AHPs hired in fiscal year (FY) 2010 to 119,120 as of April 2020. This represents an average yearly growth rate of 3.4% and a total growth rate of 36%. The Bureau of Labor Statistics predictions for 2019/2029 suggest that certain AHPs are expected to have a 10-year growth rates of 20% or more to meet the changing health care needs of patients especially as the population ages; the growth rates for many AHPs far surpasses that of physicians, which is anticipated to be 4% (Table).6,7 The VA WMC expects an additional 52,283 AHPs will be hired by the VA by FY 2030 based on the 10-year average growth rate (Kali Clark, Veterans Health Administration Workforce Management and Consulting Office, email communication, May 28, 2020).
One of the driving forces behind the growth rate is the move toward using AHPs to supplement health care for a variety of health conditions.8,9 Examples include the integration of rehabilitation professionals, alternative care professionals (eg, massage therapists, practitioners who offer training in yoga and meditation), chiropractors, MH professionals, and pharmacists in the treatment of chronic pain, the use of a wider range of professionals in the treatment of MH conditions, and the integration of MH professionals into traditional medical settings, such as primary care. This intentional move to a more well-integrated model of interprofessional care is apparent in many other health care systems throughout the United States. Within the VA, this shift may be most evident through the introduction of the Whole Health model of care. The Whole Health model of care uses an interprofessional team to assess and care for veterans, using a personalized health plan addressing medical and MH conditions as well as behavioral, social, or spiritual concerns.10 The Whole Health model of care provides veterans with access to a variety of health care services, including but not limited to MH services, spiritual interventions, exercise-based programs, yoga, meditation, and nutrition counseling.
The OAA and AH education division have focused expansion to meet the increased need for MH and rehabilitation providers, to enhance interprofessional education, and to emphasize postgraduate-degree clinical training. This focus reflects the trends seen in health care training broadly throughout the nation and the intentional pivot is a model of these trends and a model for how to intentionally address these trends. Specific to the VA, focused expansion plans have allowed OAA to address VA strategic initiatives such as pain management and caring for rural veterans.
Funded Training Positions
As a result of recent AH expansion efforts, there has been a 33% increase in stipend-funded positions during the past 10 years, a rate that directly corresponds with the growth of AHPs in the VA. Recent AH expansion efforts can contribute to a particularly positive impact in highly rural and underserved areas where recruiting providers remains challenging.
The OAA launched the Mental Health Education Expansion (MHEE) initiative in 2012, which has now added 782 funded training slots across 10 health professions, 8 of which are psychology, pharmacy, chaplaincy, professional MH counseling, marriage and family therapy (MFT), social work (SW), occupational therapy (OT), and physician assistant (PA). Through the MHEE initiative, the VA has established funded internships for licensed professional mental health counselors and marriage and family therapists, as these professions are targeted for expanding the overall MH workforce in the VA. The OAA currently funds more than 50 total HPT positions for these 2 professions with an aim of increasing their recruitment to the VA MH workforce over the next decade. The MHEE is aligned with specified VA priorities to train a future VA workforce prepared for interprofessional collaboration and clinical care in an increasingly integrated and complex environment. This expansion effort also aligns with an increasing understanding of the importance of addressing the MH needs of our nation by ensuring there is an adequate supply of competent, well-trained clinicians entering the workforce.
The OAA has created and expanded residencies and fellowships in multiple rehabilitation professions, including chiropractic, physical therapy (PT), and OT. With the increased focus on the management of chronic pain in the nation combined with a specific emphasis on this clinical need in the VA, chiropractors have been deemed essential HCPs. In 2014, the VA established 5 chiropractic residency programs while partnering with the Council on Chiropractic Education to develop accreditation standards for residency training. OAA’s efforts have yielded 5 accredited residency programs, the first in the United States. In 2020, the VA doubled the number of available chiropractic residency programs, and future expansion is anticipated. Since 2010, PT residencies have expanded from 1 to 28 programs (42 funded positions) across 4 board certification specialties: cardiovascular-pulmonary, geriatric, neurologic, and orthopedic. Similarly, the VA was one of the first organizations to achieve accreditation for OT fellowships; there are currently 5 accredited OT fellowship programs across 3 areas of practice: assistive technology, MH, and physical rehabilitation. The VA OT fellowship program focused on assistive technology is the only program in the United States at this time.
Interprofessional Education
As one of the primary focus areas for AH expansion, interprofessional education (IPE) has been recognized as increasingly important for the provision of health care and the development of HPT programs. IPE can develop professionals who appreciate the roles of diverse professions and can use teamwork to enhance clinical outcomes for patients.11 There also are a growing number of professional organizations supporting the Interprofessional Education Collaborative with many representing AHPs.12 Collaboration across HCPs is an important way of reducing health care costs by enhancing clinical outcomes, communication, and teamwork.13-16 The VA and the nation’s health care system benefit from the by-products of interprofessional collaboration through investment in targeted training programs. In each phase of the AH expansion, special consideration was given to applicant programs offering unique and innovative clinical and educational experiences consistent with the promotion of interprofessional care. In doing so, increased numbers of AH HPTs have engaged in team-based clinical care.
Pain Management Pharmacy
The efforts of AH to align expansion with high-priority agency-wide efforts has resulted in the growth of pharmacy residency positions focused on pain management. Pharmacy postgraduate year (PGY) 2 residencies focusing on opioid reduction are an example of VA efforts to improve response to managing chronic pain and the long-term risks from opioid use during this national public health crisis.17 These residency programs focus on strategies to reduce the use of opioid medications in the clinical setting and teaching effective clinical interventions for reducing the rates of opioid addiction in veterans while still recognizing the need to identify and treat chronic pain. Before expansion efforts in 2018, there were 6 pharmacy residency programs focused on opioid use reduction in the VA, 8 pharmacy PGY2 residency positions were added in academic year 2019/2020, an additional 5 positions are being added in academic year 2021/2022 with the explicit goal of managing patients with high-risk chronic pain.
Rural Health
The lack of MH providers in rural areas has received much attention and is particularly important in the VA because veterans are more likely to live in less populated areas.18 The VA mandate to address this population was codified by the creation of the Office of Rural Health in 2006 via 38 USC § 7308.19Creating health professions training programs in rural settings provides HPTs the opportunity to learn professional competencies and train with faculty knowledgeable about this population—all of which provide a comprehensive training experience and serve as a recruitment pathway to hire HPTs into staff positions at these sites.19
When MHEE was initiated, not all regions of the country had funded VA psychology training programs, and this geographic gap in psychology training was a contributing factor to recruitment difficulties for psychologists in rural areas. As a result, the request for proposal process in the OAA highlighted and incentivized rurality when considering funding for new training programs. The OAA defined rurality as the number of patients served by the proposed health care facility who lived in a rural or highly rural zip code according to VA Support Service Center Capital Assets data.20 As a result, VA psychology doctoral internships expanded to be available in all states, the District of Columbia, and Puerto Rico. MH training programs were started in the highly rural states of Montana and Wyoming. These expansion efforts promise to be an essential component to addressing the gaps in coverage in rural settings as noted in recent research.21
Pregraduate to Postgraduate Programs
The OAA AH education division supports a significant number of pregraduate-degree and postgraduate-degree training. Some professions, such as psychology, pharmacy, SW, PT, speech pathology, OT, and nutrition/dietetics receive funding at both levels of training. More recent, the OAA has started to move funding from pregraduate to postgraduate-degree positions, specifically within professions where pregraduate funding is uncommon for both federal and nonfederal training positions. The effort is designed to better align stipend-paid training programs with the VA Professional Qualification Standards and the final level of training required for employment in the VA.22This means that HPTs receive stipend support during the highest level of their clinical training before degree conferral, eligibility for VA employment, or while participating in a postgraduate-degree residency or fellowship.
Additionally, this shift in focus and the resulting internal assessment of professions has allowed the OAA to fund more specialized training opportunities, which sometimes go beyond what is required by accrediting bodies or for recruitment into VA positions. For example, the OAA is supporting SW fellowship programs and PA residency positions to allow for greater specialization within these professions; the accrediting agencies for both professions have recently finalized their accreditation standards, and the OAA played a role in moving these standards forward.
While postgraduate residencies and fellowships are not required for all AH HPTs or for employment in the VA, there is a shift in some professions to encourage postgraduate training in advanced competencies in specialized areas. Participation in a residency or fellowship training program affords HPTs additional time and diverse clinical experiences to acquire clinical skills, all while under the supervision of a highly trained practitioner. This additional training also allows for a longitudinal assessment of the HPT to ensure an alignment of the HPTs’ knowledge, abilities, and skills with the expectation should they pursue VA employment.
In academic year 2019/2020, the OAA AH education division in conjunction with the PA national program office transitioned the entirety of the PA pregraduate-degree student positions (415 funded positions) to residency positions, increasing residency positions from 19 to 32 funded positions. This shift in emphasis for funding did not negatively impact the total number of pregraduate PA students receiving training in the VA and has created a pipeline of residency graduates who are ready to enter VA staff positions. To date, the VA has 14 PA residency programs across 3 specialties: emergency medicine (EM), MH, and primary care/geriatrics. Of these tracks, the VA offers 5 EM and 4 MH residencies that position graduates to be eligible for specialty certification. The National Commission on Certification of Physician Assistants established Certificates of Added Qualifications (CAQ) to recognize and document specialty knowledge, skills, and experience. The VA MH residency programs have been established to align with the CAQ expectations, and residents immediately qualify to take the CAQ examination after the completion of training.
Currently, the same process to move pregraduate to postgraduate funding is being implemented for PT and OT. Within the PT profession, there is increased momentum toward residency and fellowship training programs to respond to the changing complexity of the health care systemand reduce the need of complex care to be provided by non-VA providers in the community.23 Both PT and OT have entered the initial phases of transitioning to residency or fellowship-funded positions. The OAA is partnering with these professions to move positions to postgraduate degree within the next 3 years with a commensurate increase in funding. The initial data indicate that 80% of graduated VA PT residents are board-certification eligible, and 89% of those who are eligible passed the examination on their first attempt.
Since 2013, the VA psychology training also has realized a growth in postgraduate-degree residencies. Psychology residency positions have increased 99% to 453 funded positions. This growth represents increased specialization in neuropsychology, geropsychology, rehabilitation psychology, and health psychology. Additionally, postgraduate residencies meet most jurisdictional requirements for postdoctoral supervised experience and better prepare HPTs to enter specialty staff positions that are necessary to care for aging veterans.
Additional professions are being targeted for postgraduate-degree training programs, including dietetics and speech pathology, to align with upcoming changes in the qualification standards for employment. While the process to transition positions to postgraduate-degree training programs can take 3 to 5 years, the outcomes are expected to result in better prepared HPTs who can fill staff vacancies in the VA.
Conclusions
Through its funding and oversight of numerous professions, the OAA is uniquely situated to adapt its portfolio to meet the needs of the VA and the nation. Over the past 10 years, the OAA has expanded its total number of HPT positions to enhance interprofessional care, respond to the VA’s strategic initiatives, address the care needs of rural veterans, and shift positions to postgraduate training programs. The OAA’s investment in high-quality training programs builds a strong health care workforce ready to meet the needs of an increasingly complex and integrated health care environment.
The OAA anticipates future expansion, especially related to promoting rural training opportunities and shifting to postgraduate training programs as a means of promoting advanced health care and health system competencies while continuing to align with workforce projections. Furthermore, while there are data on the percentage of VA staff who participated in OAA training program through the VA All Employee Survey (AES), the range for AH professions is wide. For example, about 37% of rehabilitative staff reported participating in an OAA training program, and 72% of VA psychologists reported having an OAA training experience. To maximize the hiring of HPTs, OAA will continue its partnership with WMC to enact programs aimed at streamlining the hiring process so that veterans have access to HCPs who are specifically trained to work with them.
1. US Department of Veterans Affairs. Providing health care for veterans. Updated April 23, 2021. Accessed July 15, 2021. https://www.va.gov/health
2. Veterans’ Benefits. 38 USC §7301 and §7302 (1991). Accessed May 18, 2020. https://www.govinfo.gov/content/pkg/USCODE-2018-title38/pdf/USCODE-2018-title38-partV-chap73-subchapI-sec7302.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2021. Accessed July 15, 2021. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
4. US Department of Veterans Affairs, VHA Office of Academic Affiliations. VA Policy Memorandum # 2. Policy in association of veterans’ hospitals with medical schools. Published January 30, 1946. Accessed October 13, 2020. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Mission of the office of academic affiliations. Updated September 24, 2019. Accessed July 15, 2021. https://www.va.gov/oaa/oaa_mission.asp
6. US Bureau of Labor Statistics, Office of Occupational Statistics and Employment Projections Occupational Outlook Handbook. Healthcare occupations. Updated May 14, 2021. Accessed July 15, 2021. https://www.bls.gov/ooh/healthcare/home.htm
7. Windmill IM, Freeman BA. Demand for audiology services: 30-yr projections and impact on academic programs. J Am Acad Audiol. 2013;24(5):407-416. doi:10.3766/jaaa.24.5.7
8. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. HRSA health workforce: behavioral health workforce projections, 2017-2030. Accessed July 15, 2021. https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/bh-workforce-projections-fact-sheet.pdf
9. Centers for Disease Control and Prevention, National Center for Health Statistics. NCHS data brief, No. 325. Use of yoga, meditation, and chiropractors among US adults aged 18 and over. Published November 2018. Accessed September 24, 2020. https://www.cdc.gov/nchs/data/databriefs/db325-h.pdf
10. US Department of Veterans Affairs, Veterans Health Administration Whole Health. Updated July 6, 2021. Accessed July 15, 2021. https://www.va.gov/wholehealth
11. Clark KM. Interprofessional education: making our way out of the silos. Respir Care. 2018;63(5): 637-639. doi:10.4187/respcare.06234
12. Interprofessional Education Collaborative. What is interprofessional education (IPE)? Accessed July 15, 2021. https://www.ipecollaborative.org/about-us
13. Nester J. The importance of interprofessional practice and education in the era of accountable care. N C Med J. 2016;77(2):128-132. doi.10.18043/ncm.77.2.128
14.. Hardin L, Kilian A, Murphy E. Bundled payments for care improvement: preparing for the medical diagnosis-related groups. J Nurs Adm. 2017;47(6): 313-319. doi:10.1097/NNA.0000000000000492
15. Guraya SY, Barr H. The effectiveness of interprofessional education in healthcare: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2018;34(2):125-184. doi:10.1016/j.kjms.2017.12.009
16. Ateah CA, Snow W, Wenter P, et al. Stereotyping as a barrier to collaboration: does interprofessional education make a difference? Nurse Educ Today. 2011;31(2):208-213. doi:10.1016/j.nedt.2010.06.004
17. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for Managing Opioid Therapy for Chronic Pain. Published May 7, 1991. Updated February 2017. Accessed July 15, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
18. US Department of Veterans Affairs, Office of Rural Health. VHA office of rural health. Updated March 17, 2021. Accessed July 15, 2021. https://www.ruralhealth.va.gov19. Curran V, Rourke J. The role of medical education in the recruitment and retention of rural physicians. Med Teach. 2004;26(3):265-272. doi:10.1080/0142159042000192055
20. US Department of Veterans Affairs. VHA Support Service Center Capital Assets. Updated December 1, 2020. Accessed July 15, 2021. https://www.data.va.gov/dataset/VHA-Support-Service-Center-Capital-Assets-VSSC-/2fr5-sktm
21. Domino ME, Lin CC, Morrisey JP, et al. Training psychologists for rural practice: exploring opportunities and constraints. J Rural Health. 2019;35(1):35-41. doi:10.1111/jrh.12299
22. US Department of Veterans Affairs. VA Directive 5005: Staffing. Published March 4, 2020. Accessed July 15, 2021. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=1140&FType=2
23. Furze JA, Freeman BA. Physical therapy and fellowship education: reflections on the past, present, and future. Phys Ther. 2016;96(7):949-960. doi:10.2522/ptj.20150473
The US Department of Veterans Affairs (VA) is the largest health care delivery system in the United States, comprising 1293 sites of care, including 171 medical centers.1 One of the 4 statutory missions of the VA is to train health care professionals (HCPs) to meet the needs of the VA and the nation.2 Through partnerships with more than 1800 accredited colleges, universities, and training programs, the VA provides training annually to nearly 118,000 health professions trainees (HPTs) across a variety of health care professions, and all of whom provide direct clinical care to veterans.3
In the VA, the Office of Academic Affiliations (OAA) is charged with overseeing health professions training and the VA’s partnership with medical and associated health (AH) professions schools, which was first codified in Policy Memorandum No. 2 in 1946.4,5 Given the scope and breadth of health professions education offered through the VA, OAA is in a unique position to address health care shortage areas as well as influence the educational standards for certain professions.
Many of these health care professions fall under the rubric of AH, which include mental health (MH) specialties, rehabilitative specialties, and others. These professions are critical to address in the expanding world of health care in the United States with its increased specialization and emphasis on coordination of care with interprofessional teams. During the 2019/2020 academic year, the VA provided clinical training to approximately 21,000 AH HPTs from > 40 professions with just over 20% receiving financial support through the OAA. Of the HPTs who train at VA without compensation, most spend shorter amounts of time in clinical rotations in the VA, are in pregraduate-degree education programs where payment for clinical rotations is not expected and may not be eligible for hire immediately on completion of their clinical training experience. The 17 funded professions have been strategically selected by the OAA to ensure a robust pipeline of HCPs to meet the needs of veterans and the nation.
To meet the demands of AH professionals (AHPs), the OAA implemented targeted expansion over the past 10 years. While not exhaustive, this paper describes several expansion efforts based on VA special initiatives, including enhancing clinical access in rural settings and shifting toward postgraduate-degree training and specialization. By aligning expansion with VA priorities as well as trends in health care more broadly, the OAA can ensure that there is a supply of well-trained AHPs who have developed the requisite competencies to contribute to our nation’s health care needs. Further, expansion can help train and recruit health professionals who can be hired into VA positions ready to care for the complex needs of veterans.
Associated Health Professionals
Overseen by the OAA, AH expansion is designed to address the specific needs of the VA and the US health care system. Data from the VA Workforce Management and Consulting (WMC) shows that the VA employment of AHPs has grown from 87,351 AHPs hired in fiscal year (FY) 2010 to 119,120 as of April 2020. This represents an average yearly growth rate of 3.4% and a total growth rate of 36%. The Bureau of Labor Statistics predictions for 2019/2029 suggest that certain AHPs are expected to have a 10-year growth rates of 20% or more to meet the changing health care needs of patients especially as the population ages; the growth rates for many AHPs far surpasses that of physicians, which is anticipated to be 4% (Table).6,7 The VA WMC expects an additional 52,283 AHPs will be hired by the VA by FY 2030 based on the 10-year average growth rate (Kali Clark, Veterans Health Administration Workforce Management and Consulting Office, email communication, May 28, 2020).
One of the driving forces behind the growth rate is the move toward using AHPs to supplement health care for a variety of health conditions.8,9 Examples include the integration of rehabilitation professionals, alternative care professionals (eg, massage therapists, practitioners who offer training in yoga and meditation), chiropractors, MH professionals, and pharmacists in the treatment of chronic pain, the use of a wider range of professionals in the treatment of MH conditions, and the integration of MH professionals into traditional medical settings, such as primary care. This intentional move to a more well-integrated model of interprofessional care is apparent in many other health care systems throughout the United States. Within the VA, this shift may be most evident through the introduction of the Whole Health model of care. The Whole Health model of care uses an interprofessional team to assess and care for veterans, using a personalized health plan addressing medical and MH conditions as well as behavioral, social, or spiritual concerns.10 The Whole Health model of care provides veterans with access to a variety of health care services, including but not limited to MH services, spiritual interventions, exercise-based programs, yoga, meditation, and nutrition counseling.
The OAA and AH education division have focused expansion to meet the increased need for MH and rehabilitation providers, to enhance interprofessional education, and to emphasize postgraduate-degree clinical training. This focus reflects the trends seen in health care training broadly throughout the nation and the intentional pivot is a model of these trends and a model for how to intentionally address these trends. Specific to the VA, focused expansion plans have allowed OAA to address VA strategic initiatives such as pain management and caring for rural veterans.
Funded Training Positions
As a result of recent AH expansion efforts, there has been a 33% increase in stipend-funded positions during the past 10 years, a rate that directly corresponds with the growth of AHPs in the VA. Recent AH expansion efforts can contribute to a particularly positive impact in highly rural and underserved areas where recruiting providers remains challenging.
The OAA launched the Mental Health Education Expansion (MHEE) initiative in 2012, which has now added 782 funded training slots across 10 health professions, 8 of which are psychology, pharmacy, chaplaincy, professional MH counseling, marriage and family therapy (MFT), social work (SW), occupational therapy (OT), and physician assistant (PA). Through the MHEE initiative, the VA has established funded internships for licensed professional mental health counselors and marriage and family therapists, as these professions are targeted for expanding the overall MH workforce in the VA. The OAA currently funds more than 50 total HPT positions for these 2 professions with an aim of increasing their recruitment to the VA MH workforce over the next decade. The MHEE is aligned with specified VA priorities to train a future VA workforce prepared for interprofessional collaboration and clinical care in an increasingly integrated and complex environment. This expansion effort also aligns with an increasing understanding of the importance of addressing the MH needs of our nation by ensuring there is an adequate supply of competent, well-trained clinicians entering the workforce.
The OAA has created and expanded residencies and fellowships in multiple rehabilitation professions, including chiropractic, physical therapy (PT), and OT. With the increased focus on the management of chronic pain in the nation combined with a specific emphasis on this clinical need in the VA, chiropractors have been deemed essential HCPs. In 2014, the VA established 5 chiropractic residency programs while partnering with the Council on Chiropractic Education to develop accreditation standards for residency training. OAA’s efforts have yielded 5 accredited residency programs, the first in the United States. In 2020, the VA doubled the number of available chiropractic residency programs, and future expansion is anticipated. Since 2010, PT residencies have expanded from 1 to 28 programs (42 funded positions) across 4 board certification specialties: cardiovascular-pulmonary, geriatric, neurologic, and orthopedic. Similarly, the VA was one of the first organizations to achieve accreditation for OT fellowships; there are currently 5 accredited OT fellowship programs across 3 areas of practice: assistive technology, MH, and physical rehabilitation. The VA OT fellowship program focused on assistive technology is the only program in the United States at this time.
Interprofessional Education
As one of the primary focus areas for AH expansion, interprofessional education (IPE) has been recognized as increasingly important for the provision of health care and the development of HPT programs. IPE can develop professionals who appreciate the roles of diverse professions and can use teamwork to enhance clinical outcomes for patients.11 There also are a growing number of professional organizations supporting the Interprofessional Education Collaborative with many representing AHPs.12 Collaboration across HCPs is an important way of reducing health care costs by enhancing clinical outcomes, communication, and teamwork.13-16 The VA and the nation’s health care system benefit from the by-products of interprofessional collaboration through investment in targeted training programs. In each phase of the AH expansion, special consideration was given to applicant programs offering unique and innovative clinical and educational experiences consistent with the promotion of interprofessional care. In doing so, increased numbers of AH HPTs have engaged in team-based clinical care.
Pain Management Pharmacy
The efforts of AH to align expansion with high-priority agency-wide efforts has resulted in the growth of pharmacy residency positions focused on pain management. Pharmacy postgraduate year (PGY) 2 residencies focusing on opioid reduction are an example of VA efforts to improve response to managing chronic pain and the long-term risks from opioid use during this national public health crisis.17 These residency programs focus on strategies to reduce the use of opioid medications in the clinical setting and teaching effective clinical interventions for reducing the rates of opioid addiction in veterans while still recognizing the need to identify and treat chronic pain. Before expansion efforts in 2018, there were 6 pharmacy residency programs focused on opioid use reduction in the VA, 8 pharmacy PGY2 residency positions were added in academic year 2019/2020, an additional 5 positions are being added in academic year 2021/2022 with the explicit goal of managing patients with high-risk chronic pain.
Rural Health
The lack of MH providers in rural areas has received much attention and is particularly important in the VA because veterans are more likely to live in less populated areas.18 The VA mandate to address this population was codified by the creation of the Office of Rural Health in 2006 via 38 USC § 7308.19Creating health professions training programs in rural settings provides HPTs the opportunity to learn professional competencies and train with faculty knowledgeable about this population—all of which provide a comprehensive training experience and serve as a recruitment pathway to hire HPTs into staff positions at these sites.19
When MHEE was initiated, not all regions of the country had funded VA psychology training programs, and this geographic gap in psychology training was a contributing factor to recruitment difficulties for psychologists in rural areas. As a result, the request for proposal process in the OAA highlighted and incentivized rurality when considering funding for new training programs. The OAA defined rurality as the number of patients served by the proposed health care facility who lived in a rural or highly rural zip code according to VA Support Service Center Capital Assets data.20 As a result, VA psychology doctoral internships expanded to be available in all states, the District of Columbia, and Puerto Rico. MH training programs were started in the highly rural states of Montana and Wyoming. These expansion efforts promise to be an essential component to addressing the gaps in coverage in rural settings as noted in recent research.21
Pregraduate to Postgraduate Programs
The OAA AH education division supports a significant number of pregraduate-degree and postgraduate-degree training. Some professions, such as psychology, pharmacy, SW, PT, speech pathology, OT, and nutrition/dietetics receive funding at both levels of training. More recent, the OAA has started to move funding from pregraduate to postgraduate-degree positions, specifically within professions where pregraduate funding is uncommon for both federal and nonfederal training positions. The effort is designed to better align stipend-paid training programs with the VA Professional Qualification Standards and the final level of training required for employment in the VA.22This means that HPTs receive stipend support during the highest level of their clinical training before degree conferral, eligibility for VA employment, or while participating in a postgraduate-degree residency or fellowship.
Additionally, this shift in focus and the resulting internal assessment of professions has allowed the OAA to fund more specialized training opportunities, which sometimes go beyond what is required by accrediting bodies or for recruitment into VA positions. For example, the OAA is supporting SW fellowship programs and PA residency positions to allow for greater specialization within these professions; the accrediting agencies for both professions have recently finalized their accreditation standards, and the OAA played a role in moving these standards forward.
While postgraduate residencies and fellowships are not required for all AH HPTs or for employment in the VA, there is a shift in some professions to encourage postgraduate training in advanced competencies in specialized areas. Participation in a residency or fellowship training program affords HPTs additional time and diverse clinical experiences to acquire clinical skills, all while under the supervision of a highly trained practitioner. This additional training also allows for a longitudinal assessment of the HPT to ensure an alignment of the HPTs’ knowledge, abilities, and skills with the expectation should they pursue VA employment.
In academic year 2019/2020, the OAA AH education division in conjunction with the PA national program office transitioned the entirety of the PA pregraduate-degree student positions (415 funded positions) to residency positions, increasing residency positions from 19 to 32 funded positions. This shift in emphasis for funding did not negatively impact the total number of pregraduate PA students receiving training in the VA and has created a pipeline of residency graduates who are ready to enter VA staff positions. To date, the VA has 14 PA residency programs across 3 specialties: emergency medicine (EM), MH, and primary care/geriatrics. Of these tracks, the VA offers 5 EM and 4 MH residencies that position graduates to be eligible for specialty certification. The National Commission on Certification of Physician Assistants established Certificates of Added Qualifications (CAQ) to recognize and document specialty knowledge, skills, and experience. The VA MH residency programs have been established to align with the CAQ expectations, and residents immediately qualify to take the CAQ examination after the completion of training.
Currently, the same process to move pregraduate to postgraduate funding is being implemented for PT and OT. Within the PT profession, there is increased momentum toward residency and fellowship training programs to respond to the changing complexity of the health care systemand reduce the need of complex care to be provided by non-VA providers in the community.23 Both PT and OT have entered the initial phases of transitioning to residency or fellowship-funded positions. The OAA is partnering with these professions to move positions to postgraduate degree within the next 3 years with a commensurate increase in funding. The initial data indicate that 80% of graduated VA PT residents are board-certification eligible, and 89% of those who are eligible passed the examination on their first attempt.
Since 2013, the VA psychology training also has realized a growth in postgraduate-degree residencies. Psychology residency positions have increased 99% to 453 funded positions. This growth represents increased specialization in neuropsychology, geropsychology, rehabilitation psychology, and health psychology. Additionally, postgraduate residencies meet most jurisdictional requirements for postdoctoral supervised experience and better prepare HPTs to enter specialty staff positions that are necessary to care for aging veterans.
Additional professions are being targeted for postgraduate-degree training programs, including dietetics and speech pathology, to align with upcoming changes in the qualification standards for employment. While the process to transition positions to postgraduate-degree training programs can take 3 to 5 years, the outcomes are expected to result in better prepared HPTs who can fill staff vacancies in the VA.
Conclusions
Through its funding and oversight of numerous professions, the OAA is uniquely situated to adapt its portfolio to meet the needs of the VA and the nation. Over the past 10 years, the OAA has expanded its total number of HPT positions to enhance interprofessional care, respond to the VA’s strategic initiatives, address the care needs of rural veterans, and shift positions to postgraduate training programs. The OAA’s investment in high-quality training programs builds a strong health care workforce ready to meet the needs of an increasingly complex and integrated health care environment.
The OAA anticipates future expansion, especially related to promoting rural training opportunities and shifting to postgraduate training programs as a means of promoting advanced health care and health system competencies while continuing to align with workforce projections. Furthermore, while there are data on the percentage of VA staff who participated in OAA training program through the VA All Employee Survey (AES), the range for AH professions is wide. For example, about 37% of rehabilitative staff reported participating in an OAA training program, and 72% of VA psychologists reported having an OAA training experience. To maximize the hiring of HPTs, OAA will continue its partnership with WMC to enact programs aimed at streamlining the hiring process so that veterans have access to HCPs who are specifically trained to work with them.
The US Department of Veterans Affairs (VA) is the largest health care delivery system in the United States, comprising 1293 sites of care, including 171 medical centers.1 One of the 4 statutory missions of the VA is to train health care professionals (HCPs) to meet the needs of the VA and the nation.2 Through partnerships with more than 1800 accredited colleges, universities, and training programs, the VA provides training annually to nearly 118,000 health professions trainees (HPTs) across a variety of health care professions, and all of whom provide direct clinical care to veterans.3
In the VA, the Office of Academic Affiliations (OAA) is charged with overseeing health professions training and the VA’s partnership with medical and associated health (AH) professions schools, which was first codified in Policy Memorandum No. 2 in 1946.4,5 Given the scope and breadth of health professions education offered through the VA, OAA is in a unique position to address health care shortage areas as well as influence the educational standards for certain professions.
Many of these health care professions fall under the rubric of AH, which include mental health (MH) specialties, rehabilitative specialties, and others. These professions are critical to address in the expanding world of health care in the United States with its increased specialization and emphasis on coordination of care with interprofessional teams. During the 2019/2020 academic year, the VA provided clinical training to approximately 21,000 AH HPTs from > 40 professions with just over 20% receiving financial support through the OAA. Of the HPTs who train at VA without compensation, most spend shorter amounts of time in clinical rotations in the VA, are in pregraduate-degree education programs where payment for clinical rotations is not expected and may not be eligible for hire immediately on completion of their clinical training experience. The 17 funded professions have been strategically selected by the OAA to ensure a robust pipeline of HCPs to meet the needs of veterans and the nation.
To meet the demands of AH professionals (AHPs), the OAA implemented targeted expansion over the past 10 years. While not exhaustive, this paper describes several expansion efforts based on VA special initiatives, including enhancing clinical access in rural settings and shifting toward postgraduate-degree training and specialization. By aligning expansion with VA priorities as well as trends in health care more broadly, the OAA can ensure that there is a supply of well-trained AHPs who have developed the requisite competencies to contribute to our nation’s health care needs. Further, expansion can help train and recruit health professionals who can be hired into VA positions ready to care for the complex needs of veterans.
Associated Health Professionals
Overseen by the OAA, AH expansion is designed to address the specific needs of the VA and the US health care system. Data from the VA Workforce Management and Consulting (WMC) shows that the VA employment of AHPs has grown from 87,351 AHPs hired in fiscal year (FY) 2010 to 119,120 as of April 2020. This represents an average yearly growth rate of 3.4% and a total growth rate of 36%. The Bureau of Labor Statistics predictions for 2019/2029 suggest that certain AHPs are expected to have a 10-year growth rates of 20% or more to meet the changing health care needs of patients especially as the population ages; the growth rates for many AHPs far surpasses that of physicians, which is anticipated to be 4% (Table).6,7 The VA WMC expects an additional 52,283 AHPs will be hired by the VA by FY 2030 based on the 10-year average growth rate (Kali Clark, Veterans Health Administration Workforce Management and Consulting Office, email communication, May 28, 2020).
One of the driving forces behind the growth rate is the move toward using AHPs to supplement health care for a variety of health conditions.8,9 Examples include the integration of rehabilitation professionals, alternative care professionals (eg, massage therapists, practitioners who offer training in yoga and meditation), chiropractors, MH professionals, and pharmacists in the treatment of chronic pain, the use of a wider range of professionals in the treatment of MH conditions, and the integration of MH professionals into traditional medical settings, such as primary care. This intentional move to a more well-integrated model of interprofessional care is apparent in many other health care systems throughout the United States. Within the VA, this shift may be most evident through the introduction of the Whole Health model of care. The Whole Health model of care uses an interprofessional team to assess and care for veterans, using a personalized health plan addressing medical and MH conditions as well as behavioral, social, or spiritual concerns.10 The Whole Health model of care provides veterans with access to a variety of health care services, including but not limited to MH services, spiritual interventions, exercise-based programs, yoga, meditation, and nutrition counseling.
The OAA and AH education division have focused expansion to meet the increased need for MH and rehabilitation providers, to enhance interprofessional education, and to emphasize postgraduate-degree clinical training. This focus reflects the trends seen in health care training broadly throughout the nation and the intentional pivot is a model of these trends and a model for how to intentionally address these trends. Specific to the VA, focused expansion plans have allowed OAA to address VA strategic initiatives such as pain management and caring for rural veterans.
Funded Training Positions
As a result of recent AH expansion efforts, there has been a 33% increase in stipend-funded positions during the past 10 years, a rate that directly corresponds with the growth of AHPs in the VA. Recent AH expansion efforts can contribute to a particularly positive impact in highly rural and underserved areas where recruiting providers remains challenging.
The OAA launched the Mental Health Education Expansion (MHEE) initiative in 2012, which has now added 782 funded training slots across 10 health professions, 8 of which are psychology, pharmacy, chaplaincy, professional MH counseling, marriage and family therapy (MFT), social work (SW), occupational therapy (OT), and physician assistant (PA). Through the MHEE initiative, the VA has established funded internships for licensed professional mental health counselors and marriage and family therapists, as these professions are targeted for expanding the overall MH workforce in the VA. The OAA currently funds more than 50 total HPT positions for these 2 professions with an aim of increasing their recruitment to the VA MH workforce over the next decade. The MHEE is aligned with specified VA priorities to train a future VA workforce prepared for interprofessional collaboration and clinical care in an increasingly integrated and complex environment. This expansion effort also aligns with an increasing understanding of the importance of addressing the MH needs of our nation by ensuring there is an adequate supply of competent, well-trained clinicians entering the workforce.
The OAA has created and expanded residencies and fellowships in multiple rehabilitation professions, including chiropractic, physical therapy (PT), and OT. With the increased focus on the management of chronic pain in the nation combined with a specific emphasis on this clinical need in the VA, chiropractors have been deemed essential HCPs. In 2014, the VA established 5 chiropractic residency programs while partnering with the Council on Chiropractic Education to develop accreditation standards for residency training. OAA’s efforts have yielded 5 accredited residency programs, the first in the United States. In 2020, the VA doubled the number of available chiropractic residency programs, and future expansion is anticipated. Since 2010, PT residencies have expanded from 1 to 28 programs (42 funded positions) across 4 board certification specialties: cardiovascular-pulmonary, geriatric, neurologic, and orthopedic. Similarly, the VA was one of the first organizations to achieve accreditation for OT fellowships; there are currently 5 accredited OT fellowship programs across 3 areas of practice: assistive technology, MH, and physical rehabilitation. The VA OT fellowship program focused on assistive technology is the only program in the United States at this time.
Interprofessional Education
As one of the primary focus areas for AH expansion, interprofessional education (IPE) has been recognized as increasingly important for the provision of health care and the development of HPT programs. IPE can develop professionals who appreciate the roles of diverse professions and can use teamwork to enhance clinical outcomes for patients.11 There also are a growing number of professional organizations supporting the Interprofessional Education Collaborative with many representing AHPs.12 Collaboration across HCPs is an important way of reducing health care costs by enhancing clinical outcomes, communication, and teamwork.13-16 The VA and the nation’s health care system benefit from the by-products of interprofessional collaboration through investment in targeted training programs. In each phase of the AH expansion, special consideration was given to applicant programs offering unique and innovative clinical and educational experiences consistent with the promotion of interprofessional care. In doing so, increased numbers of AH HPTs have engaged in team-based clinical care.
Pain Management Pharmacy
The efforts of AH to align expansion with high-priority agency-wide efforts has resulted in the growth of pharmacy residency positions focused on pain management. Pharmacy postgraduate year (PGY) 2 residencies focusing on opioid reduction are an example of VA efforts to improve response to managing chronic pain and the long-term risks from opioid use during this national public health crisis.17 These residency programs focus on strategies to reduce the use of opioid medications in the clinical setting and teaching effective clinical interventions for reducing the rates of opioid addiction in veterans while still recognizing the need to identify and treat chronic pain. Before expansion efforts in 2018, there were 6 pharmacy residency programs focused on opioid use reduction in the VA, 8 pharmacy PGY2 residency positions were added in academic year 2019/2020, an additional 5 positions are being added in academic year 2021/2022 with the explicit goal of managing patients with high-risk chronic pain.
Rural Health
The lack of MH providers in rural areas has received much attention and is particularly important in the VA because veterans are more likely to live in less populated areas.18 The VA mandate to address this population was codified by the creation of the Office of Rural Health in 2006 via 38 USC § 7308.19Creating health professions training programs in rural settings provides HPTs the opportunity to learn professional competencies and train with faculty knowledgeable about this population—all of which provide a comprehensive training experience and serve as a recruitment pathway to hire HPTs into staff positions at these sites.19
When MHEE was initiated, not all regions of the country had funded VA psychology training programs, and this geographic gap in psychology training was a contributing factor to recruitment difficulties for psychologists in rural areas. As a result, the request for proposal process in the OAA highlighted and incentivized rurality when considering funding for new training programs. The OAA defined rurality as the number of patients served by the proposed health care facility who lived in a rural or highly rural zip code according to VA Support Service Center Capital Assets data.20 As a result, VA psychology doctoral internships expanded to be available in all states, the District of Columbia, and Puerto Rico. MH training programs were started in the highly rural states of Montana and Wyoming. These expansion efforts promise to be an essential component to addressing the gaps in coverage in rural settings as noted in recent research.21
Pregraduate to Postgraduate Programs
The OAA AH education division supports a significant number of pregraduate-degree and postgraduate-degree training. Some professions, such as psychology, pharmacy, SW, PT, speech pathology, OT, and nutrition/dietetics receive funding at both levels of training. More recent, the OAA has started to move funding from pregraduate to postgraduate-degree positions, specifically within professions where pregraduate funding is uncommon for both federal and nonfederal training positions. The effort is designed to better align stipend-paid training programs with the VA Professional Qualification Standards and the final level of training required for employment in the VA.22This means that HPTs receive stipend support during the highest level of their clinical training before degree conferral, eligibility for VA employment, or while participating in a postgraduate-degree residency or fellowship.
Additionally, this shift in focus and the resulting internal assessment of professions has allowed the OAA to fund more specialized training opportunities, which sometimes go beyond what is required by accrediting bodies or for recruitment into VA positions. For example, the OAA is supporting SW fellowship programs and PA residency positions to allow for greater specialization within these professions; the accrediting agencies for both professions have recently finalized their accreditation standards, and the OAA played a role in moving these standards forward.
While postgraduate residencies and fellowships are not required for all AH HPTs or for employment in the VA, there is a shift in some professions to encourage postgraduate training in advanced competencies in specialized areas. Participation in a residency or fellowship training program affords HPTs additional time and diverse clinical experiences to acquire clinical skills, all while under the supervision of a highly trained practitioner. This additional training also allows for a longitudinal assessment of the HPT to ensure an alignment of the HPTs’ knowledge, abilities, and skills with the expectation should they pursue VA employment.
In academic year 2019/2020, the OAA AH education division in conjunction with the PA national program office transitioned the entirety of the PA pregraduate-degree student positions (415 funded positions) to residency positions, increasing residency positions from 19 to 32 funded positions. This shift in emphasis for funding did not negatively impact the total number of pregraduate PA students receiving training in the VA and has created a pipeline of residency graduates who are ready to enter VA staff positions. To date, the VA has 14 PA residency programs across 3 specialties: emergency medicine (EM), MH, and primary care/geriatrics. Of these tracks, the VA offers 5 EM and 4 MH residencies that position graduates to be eligible for specialty certification. The National Commission on Certification of Physician Assistants established Certificates of Added Qualifications (CAQ) to recognize and document specialty knowledge, skills, and experience. The VA MH residency programs have been established to align with the CAQ expectations, and residents immediately qualify to take the CAQ examination after the completion of training.
Currently, the same process to move pregraduate to postgraduate funding is being implemented for PT and OT. Within the PT profession, there is increased momentum toward residency and fellowship training programs to respond to the changing complexity of the health care systemand reduce the need of complex care to be provided by non-VA providers in the community.23 Both PT and OT have entered the initial phases of transitioning to residency or fellowship-funded positions. The OAA is partnering with these professions to move positions to postgraduate degree within the next 3 years with a commensurate increase in funding. The initial data indicate that 80% of graduated VA PT residents are board-certification eligible, and 89% of those who are eligible passed the examination on their first attempt.
Since 2013, the VA psychology training also has realized a growth in postgraduate-degree residencies. Psychology residency positions have increased 99% to 453 funded positions. This growth represents increased specialization in neuropsychology, geropsychology, rehabilitation psychology, and health psychology. Additionally, postgraduate residencies meet most jurisdictional requirements for postdoctoral supervised experience and better prepare HPTs to enter specialty staff positions that are necessary to care for aging veterans.
Additional professions are being targeted for postgraduate-degree training programs, including dietetics and speech pathology, to align with upcoming changes in the qualification standards for employment. While the process to transition positions to postgraduate-degree training programs can take 3 to 5 years, the outcomes are expected to result in better prepared HPTs who can fill staff vacancies in the VA.
Conclusions
Through its funding and oversight of numerous professions, the OAA is uniquely situated to adapt its portfolio to meet the needs of the VA and the nation. Over the past 10 years, the OAA has expanded its total number of HPT positions to enhance interprofessional care, respond to the VA’s strategic initiatives, address the care needs of rural veterans, and shift positions to postgraduate training programs. The OAA’s investment in high-quality training programs builds a strong health care workforce ready to meet the needs of an increasingly complex and integrated health care environment.
The OAA anticipates future expansion, especially related to promoting rural training opportunities and shifting to postgraduate training programs as a means of promoting advanced health care and health system competencies while continuing to align with workforce projections. Furthermore, while there are data on the percentage of VA staff who participated in OAA training program through the VA All Employee Survey (AES), the range for AH professions is wide. For example, about 37% of rehabilitative staff reported participating in an OAA training program, and 72% of VA psychologists reported having an OAA training experience. To maximize the hiring of HPTs, OAA will continue its partnership with WMC to enact programs aimed at streamlining the hiring process so that veterans have access to HCPs who are specifically trained to work with them.
1. US Department of Veterans Affairs. Providing health care for veterans. Updated April 23, 2021. Accessed July 15, 2021. https://www.va.gov/health
2. Veterans’ Benefits. 38 USC §7301 and §7302 (1991). Accessed May 18, 2020. https://www.govinfo.gov/content/pkg/USCODE-2018-title38/pdf/USCODE-2018-title38-partV-chap73-subchapI-sec7302.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2021. Accessed July 15, 2021. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
4. US Department of Veterans Affairs, VHA Office of Academic Affiliations. VA Policy Memorandum # 2. Policy in association of veterans’ hospitals with medical schools. Published January 30, 1946. Accessed October 13, 2020. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Mission of the office of academic affiliations. Updated September 24, 2019. Accessed July 15, 2021. https://www.va.gov/oaa/oaa_mission.asp
6. US Bureau of Labor Statistics, Office of Occupational Statistics and Employment Projections Occupational Outlook Handbook. Healthcare occupations. Updated May 14, 2021. Accessed July 15, 2021. https://www.bls.gov/ooh/healthcare/home.htm
7. Windmill IM, Freeman BA. Demand for audiology services: 30-yr projections and impact on academic programs. J Am Acad Audiol. 2013;24(5):407-416. doi:10.3766/jaaa.24.5.7
8. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. HRSA health workforce: behavioral health workforce projections, 2017-2030. Accessed July 15, 2021. https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/bh-workforce-projections-fact-sheet.pdf
9. Centers for Disease Control and Prevention, National Center for Health Statistics. NCHS data brief, No. 325. Use of yoga, meditation, and chiropractors among US adults aged 18 and over. Published November 2018. Accessed September 24, 2020. https://www.cdc.gov/nchs/data/databriefs/db325-h.pdf
10. US Department of Veterans Affairs, Veterans Health Administration Whole Health. Updated July 6, 2021. Accessed July 15, 2021. https://www.va.gov/wholehealth
11. Clark KM. Interprofessional education: making our way out of the silos. Respir Care. 2018;63(5): 637-639. doi:10.4187/respcare.06234
12. Interprofessional Education Collaborative. What is interprofessional education (IPE)? Accessed July 15, 2021. https://www.ipecollaborative.org/about-us
13. Nester J. The importance of interprofessional practice and education in the era of accountable care. N C Med J. 2016;77(2):128-132. doi.10.18043/ncm.77.2.128
14.. Hardin L, Kilian A, Murphy E. Bundled payments for care improvement: preparing for the medical diagnosis-related groups. J Nurs Adm. 2017;47(6): 313-319. doi:10.1097/NNA.0000000000000492
15. Guraya SY, Barr H. The effectiveness of interprofessional education in healthcare: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2018;34(2):125-184. doi:10.1016/j.kjms.2017.12.009
16. Ateah CA, Snow W, Wenter P, et al. Stereotyping as a barrier to collaboration: does interprofessional education make a difference? Nurse Educ Today. 2011;31(2):208-213. doi:10.1016/j.nedt.2010.06.004
17. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for Managing Opioid Therapy for Chronic Pain. Published May 7, 1991. Updated February 2017. Accessed July 15, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
18. US Department of Veterans Affairs, Office of Rural Health. VHA office of rural health. Updated March 17, 2021. Accessed July 15, 2021. https://www.ruralhealth.va.gov19. Curran V, Rourke J. The role of medical education in the recruitment and retention of rural physicians. Med Teach. 2004;26(3):265-272. doi:10.1080/0142159042000192055
20. US Department of Veterans Affairs. VHA Support Service Center Capital Assets. Updated December 1, 2020. Accessed July 15, 2021. https://www.data.va.gov/dataset/VHA-Support-Service-Center-Capital-Assets-VSSC-/2fr5-sktm
21. Domino ME, Lin CC, Morrisey JP, et al. Training psychologists for rural practice: exploring opportunities and constraints. J Rural Health. 2019;35(1):35-41. doi:10.1111/jrh.12299
22. US Department of Veterans Affairs. VA Directive 5005: Staffing. Published March 4, 2020. Accessed July 15, 2021. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=1140&FType=2
23. Furze JA, Freeman BA. Physical therapy and fellowship education: reflections on the past, present, and future. Phys Ther. 2016;96(7):949-960. doi:10.2522/ptj.20150473
1. US Department of Veterans Affairs. Providing health care for veterans. Updated April 23, 2021. Accessed July 15, 2021. https://www.va.gov/health
2. Veterans’ Benefits. 38 USC §7301 and §7302 (1991). Accessed May 18, 2020. https://www.govinfo.gov/content/pkg/USCODE-2018-title38/pdf/USCODE-2018-title38-partV-chap73-subchapI-sec7302.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2021. Accessed July 15, 2021. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
4. US Department of Veterans Affairs, VHA Office of Academic Affiliations. VA Policy Memorandum # 2. Policy in association of veterans’ hospitals with medical schools. Published January 30, 1946. Accessed October 13, 2020. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Mission of the office of academic affiliations. Updated September 24, 2019. Accessed July 15, 2021. https://www.va.gov/oaa/oaa_mission.asp
6. US Bureau of Labor Statistics, Office of Occupational Statistics and Employment Projections Occupational Outlook Handbook. Healthcare occupations. Updated May 14, 2021. Accessed July 15, 2021. https://www.bls.gov/ooh/healthcare/home.htm
7. Windmill IM, Freeman BA. Demand for audiology services: 30-yr projections and impact on academic programs. J Am Acad Audiol. 2013;24(5):407-416. doi:10.3766/jaaa.24.5.7
8. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. HRSA health workforce: behavioral health workforce projections, 2017-2030. Accessed July 15, 2021. https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/bh-workforce-projections-fact-sheet.pdf
9. Centers for Disease Control and Prevention, National Center for Health Statistics. NCHS data brief, No. 325. Use of yoga, meditation, and chiropractors among US adults aged 18 and over. Published November 2018. Accessed September 24, 2020. https://www.cdc.gov/nchs/data/databriefs/db325-h.pdf
10. US Department of Veterans Affairs, Veterans Health Administration Whole Health. Updated July 6, 2021. Accessed July 15, 2021. https://www.va.gov/wholehealth
11. Clark KM. Interprofessional education: making our way out of the silos. Respir Care. 2018;63(5): 637-639. doi:10.4187/respcare.06234
12. Interprofessional Education Collaborative. What is interprofessional education (IPE)? Accessed July 15, 2021. https://www.ipecollaborative.org/about-us
13. Nester J. The importance of interprofessional practice and education in the era of accountable care. N C Med J. 2016;77(2):128-132. doi.10.18043/ncm.77.2.128
14.. Hardin L, Kilian A, Murphy E. Bundled payments for care improvement: preparing for the medical diagnosis-related groups. J Nurs Adm. 2017;47(6): 313-319. doi:10.1097/NNA.0000000000000492
15. Guraya SY, Barr H. The effectiveness of interprofessional education in healthcare: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2018;34(2):125-184. doi:10.1016/j.kjms.2017.12.009
16. Ateah CA, Snow W, Wenter P, et al. Stereotyping as a barrier to collaboration: does interprofessional education make a difference? Nurse Educ Today. 2011;31(2):208-213. doi:10.1016/j.nedt.2010.06.004
17. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for Managing Opioid Therapy for Chronic Pain. Published May 7, 1991. Updated February 2017. Accessed July 15, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
18. US Department of Veterans Affairs, Office of Rural Health. VHA office of rural health. Updated March 17, 2021. Accessed July 15, 2021. https://www.ruralhealth.va.gov19. Curran V, Rourke J. The role of medical education in the recruitment and retention of rural physicians. Med Teach. 2004;26(3):265-272. doi:10.1080/0142159042000192055
20. US Department of Veterans Affairs. VHA Support Service Center Capital Assets. Updated December 1, 2020. Accessed July 15, 2021. https://www.data.va.gov/dataset/VHA-Support-Service-Center-Capital-Assets-VSSC-/2fr5-sktm
21. Domino ME, Lin CC, Morrisey JP, et al. Training psychologists for rural practice: exploring opportunities and constraints. J Rural Health. 2019;35(1):35-41. doi:10.1111/jrh.12299
22. US Department of Veterans Affairs. VA Directive 5005: Staffing. Published March 4, 2020. Accessed July 15, 2021. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=1140&FType=2
23. Furze JA, Freeman BA. Physical therapy and fellowship education: reflections on the past, present, and future. Phys Ther. 2016;96(7):949-960. doi:10.2522/ptj.20150473
Refractive Outcomes for Cataract Surgery With Toric Intraocular Lenses at a Veterans Affairs Medical Center
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
A Primary Care Provider’s Guide to Cataract Surgery in the Very Elderly
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
Vitreous Hemorrhage in the Setting of a Vascular Loop
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.