User login
The Burden of Skin Cancer in the Military Health System, 2017-2022
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.
Results
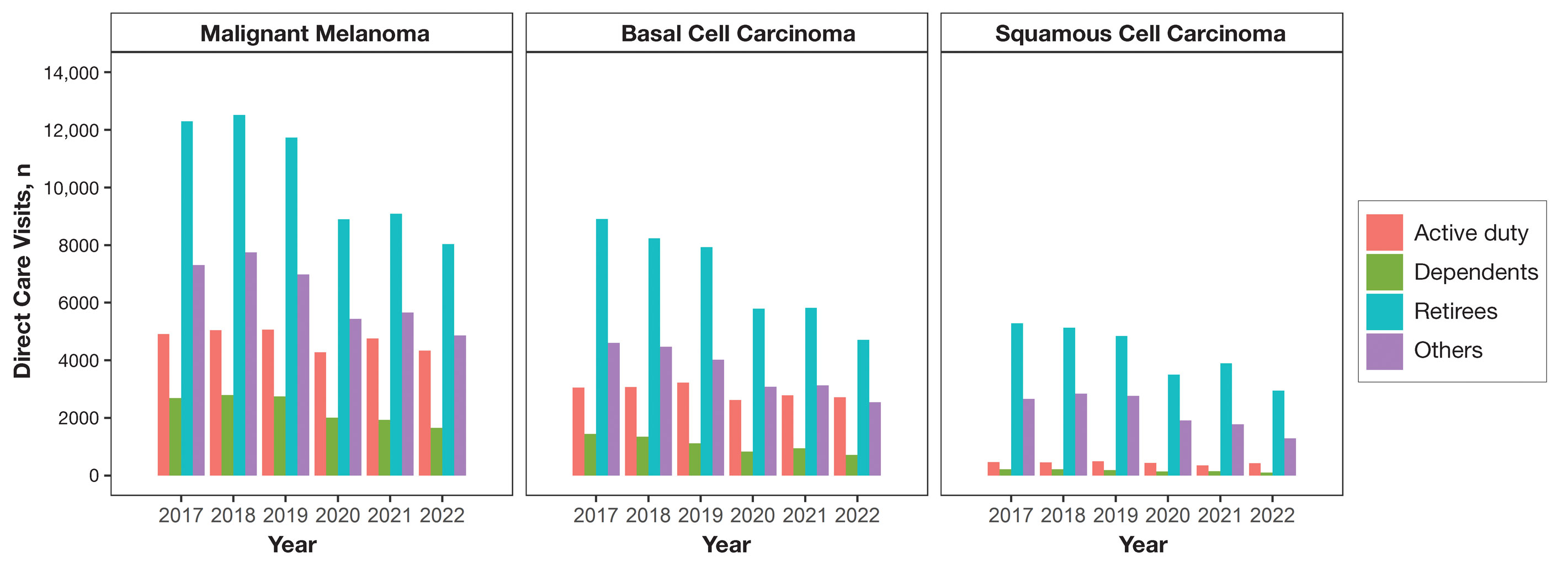
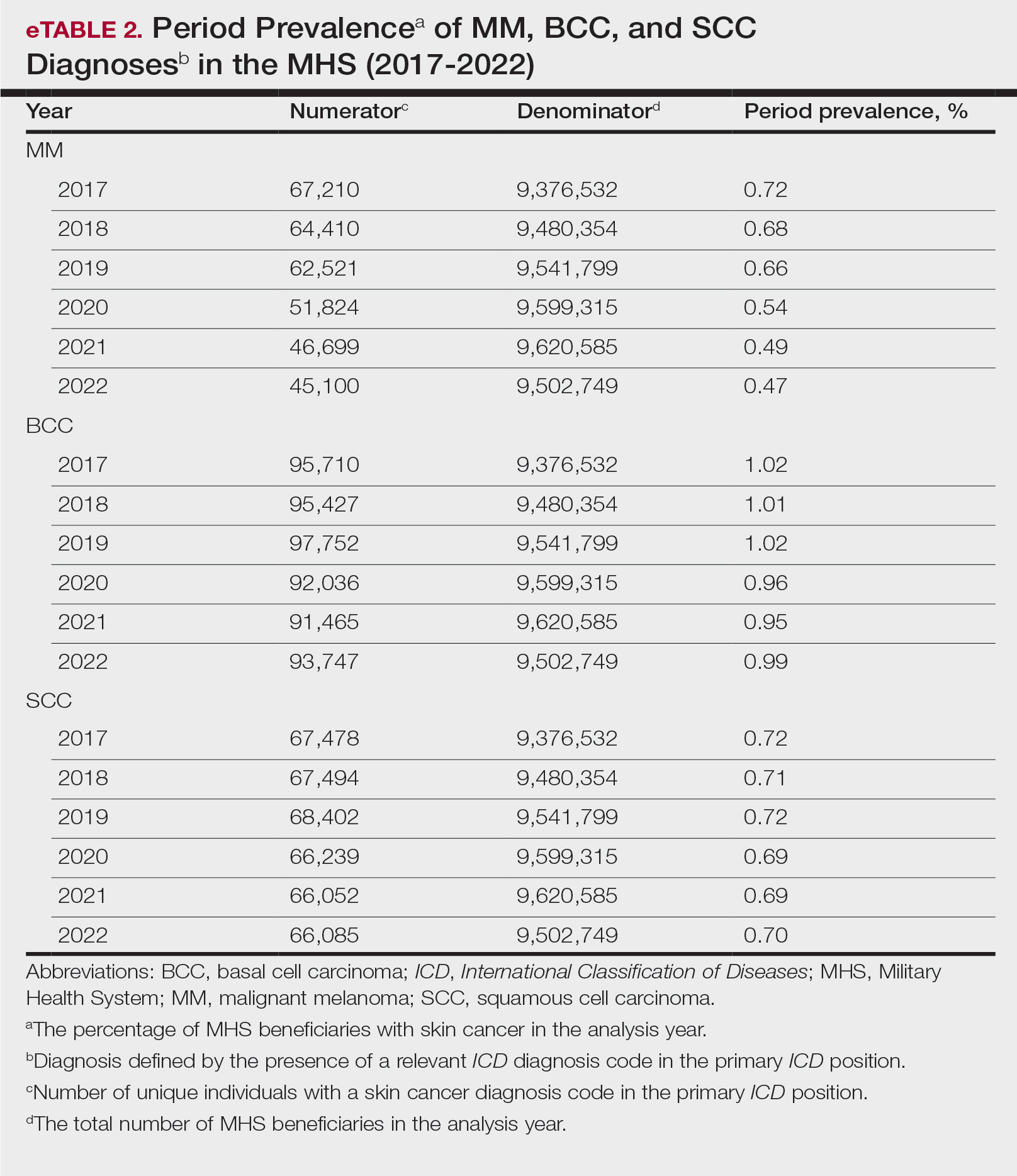
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.
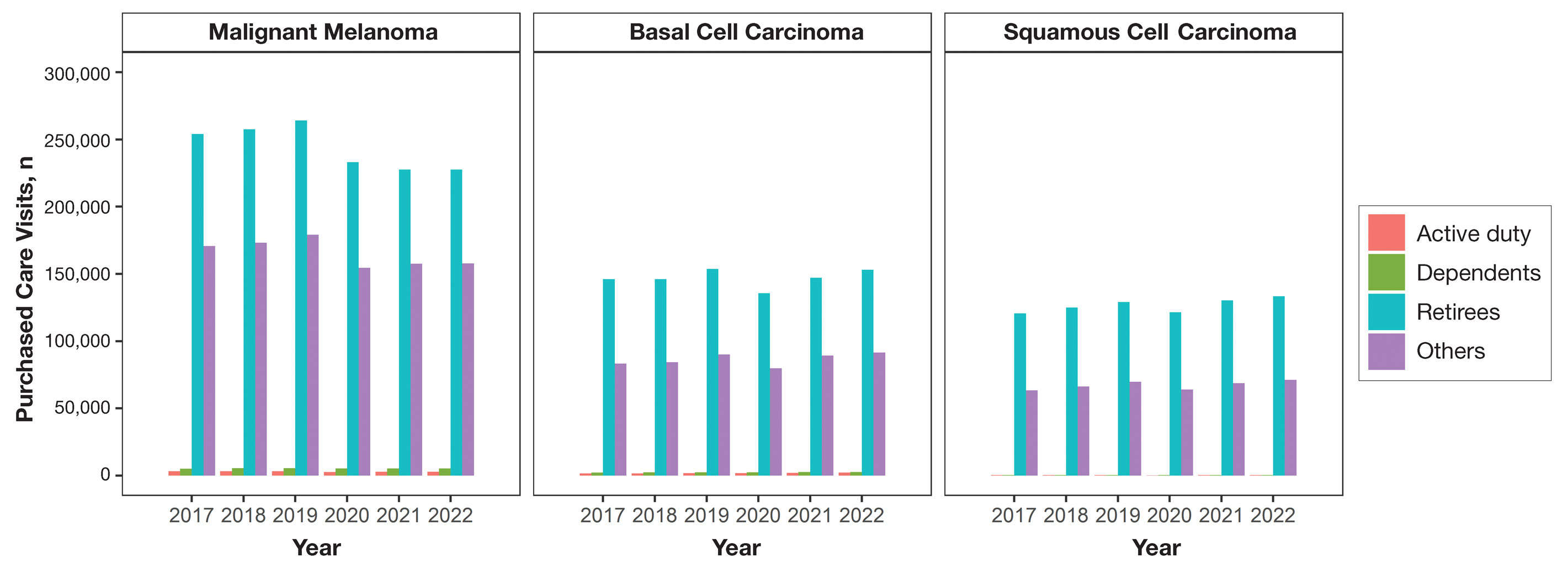
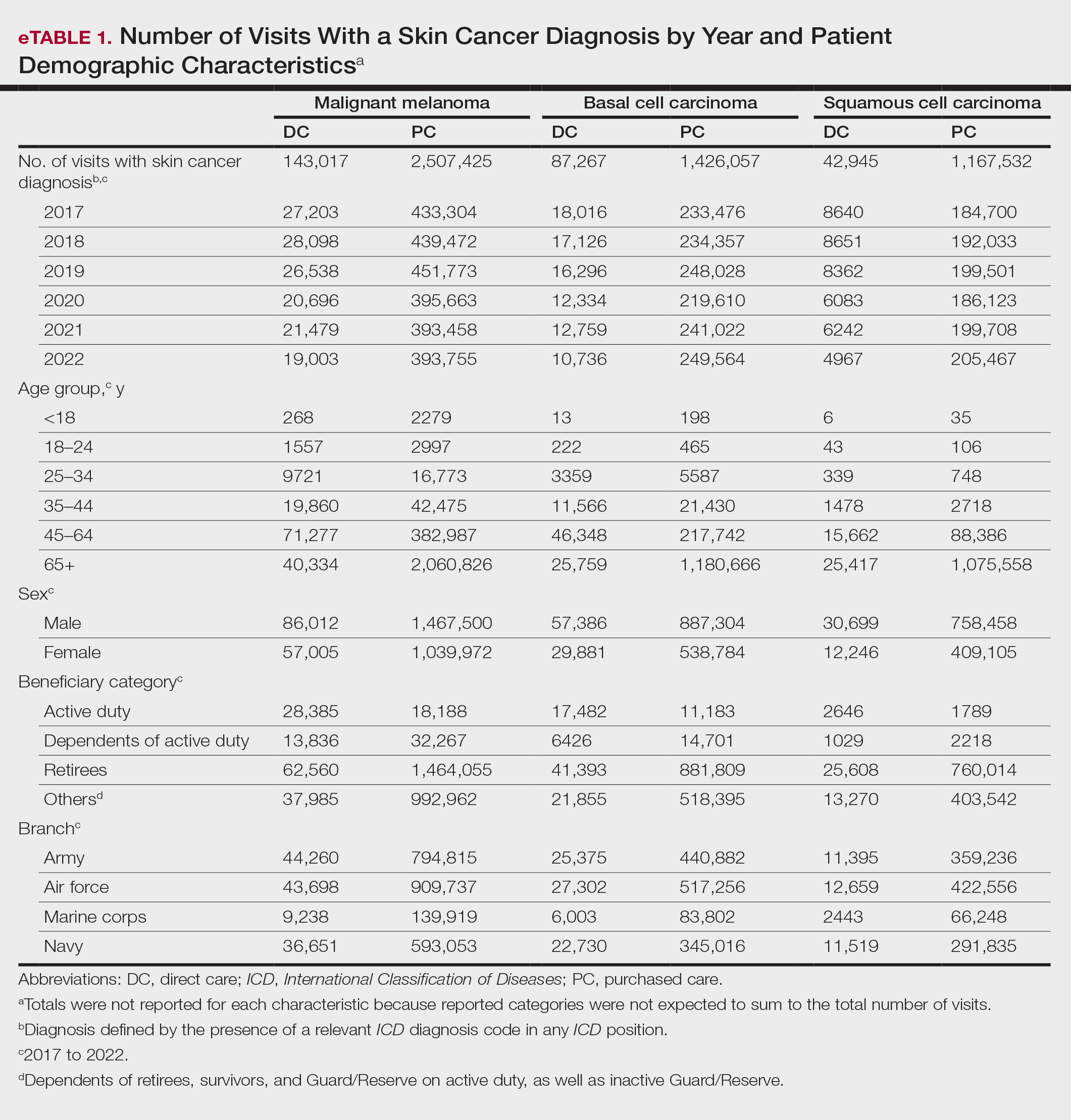
When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.
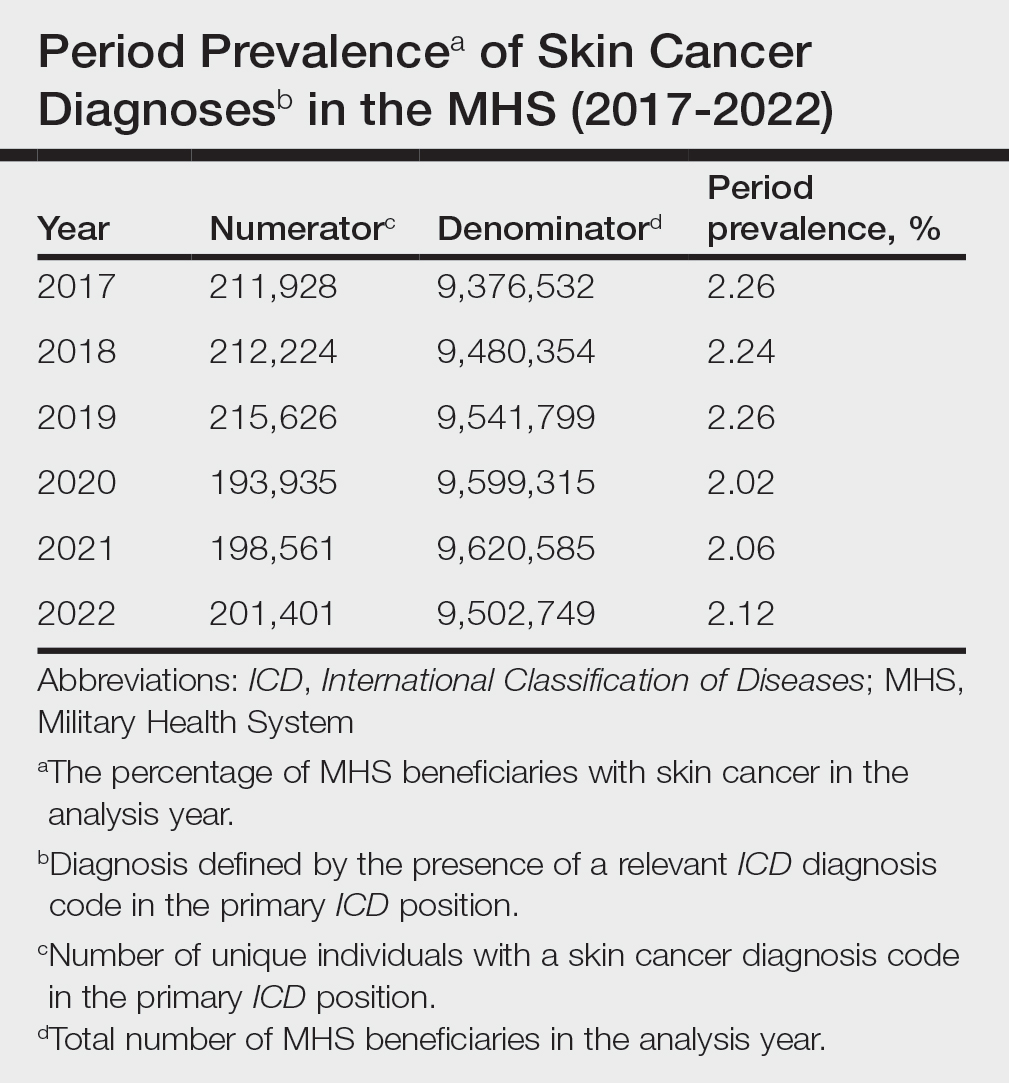
The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.
Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
PRACTICE POINTS
- Study data showed an overall decreasing prevalence of skin cancer in the Military Health System (MHS) from 2019 to 2021, possibly attributable to underdiagnosis resulting from the COVID-19 pandemic. Providers should be mindful of this trend when screening patients who have experienced interruptions in care.
- An overall increased prevalence of skin cancer was noted in the military beneficiary population compared with publicly available civilian data—and thus this diagnosis should be given special consideration within this population.
Dissociating Fibroepithelioma of Pinkus From Internal Malignancy: A Single-Center Retrospective Study
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
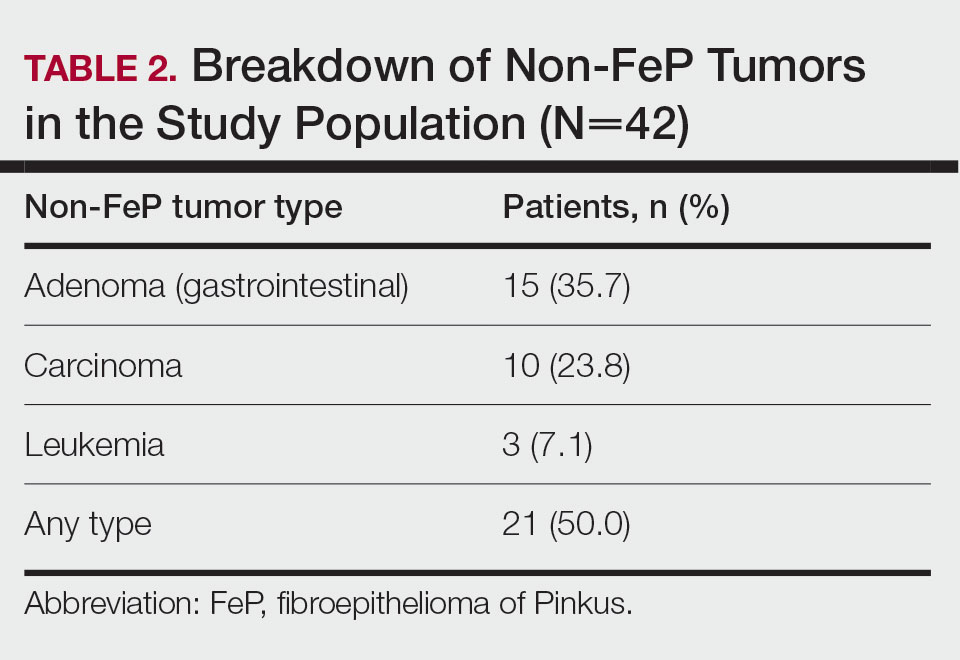
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).
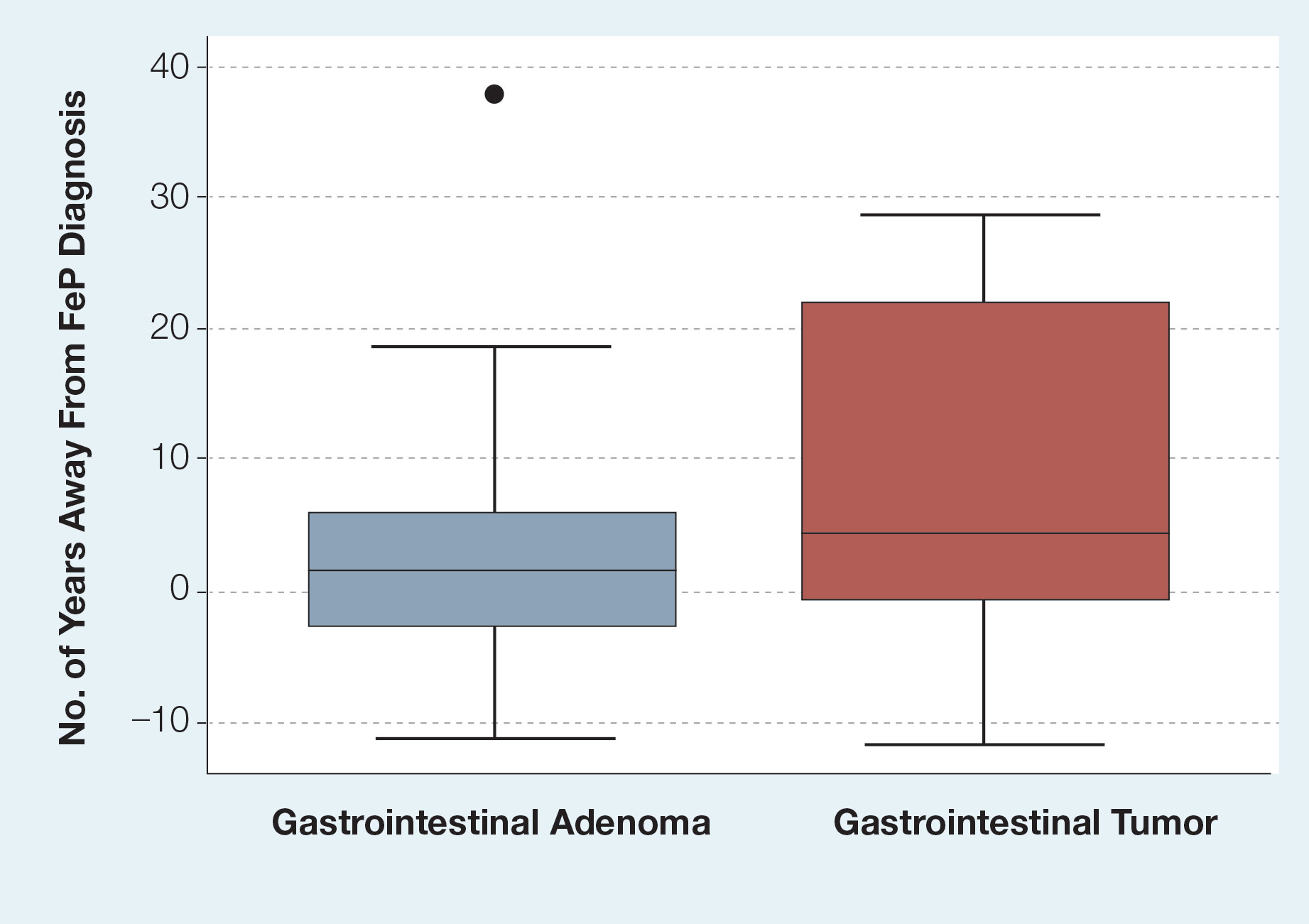
Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.
Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.
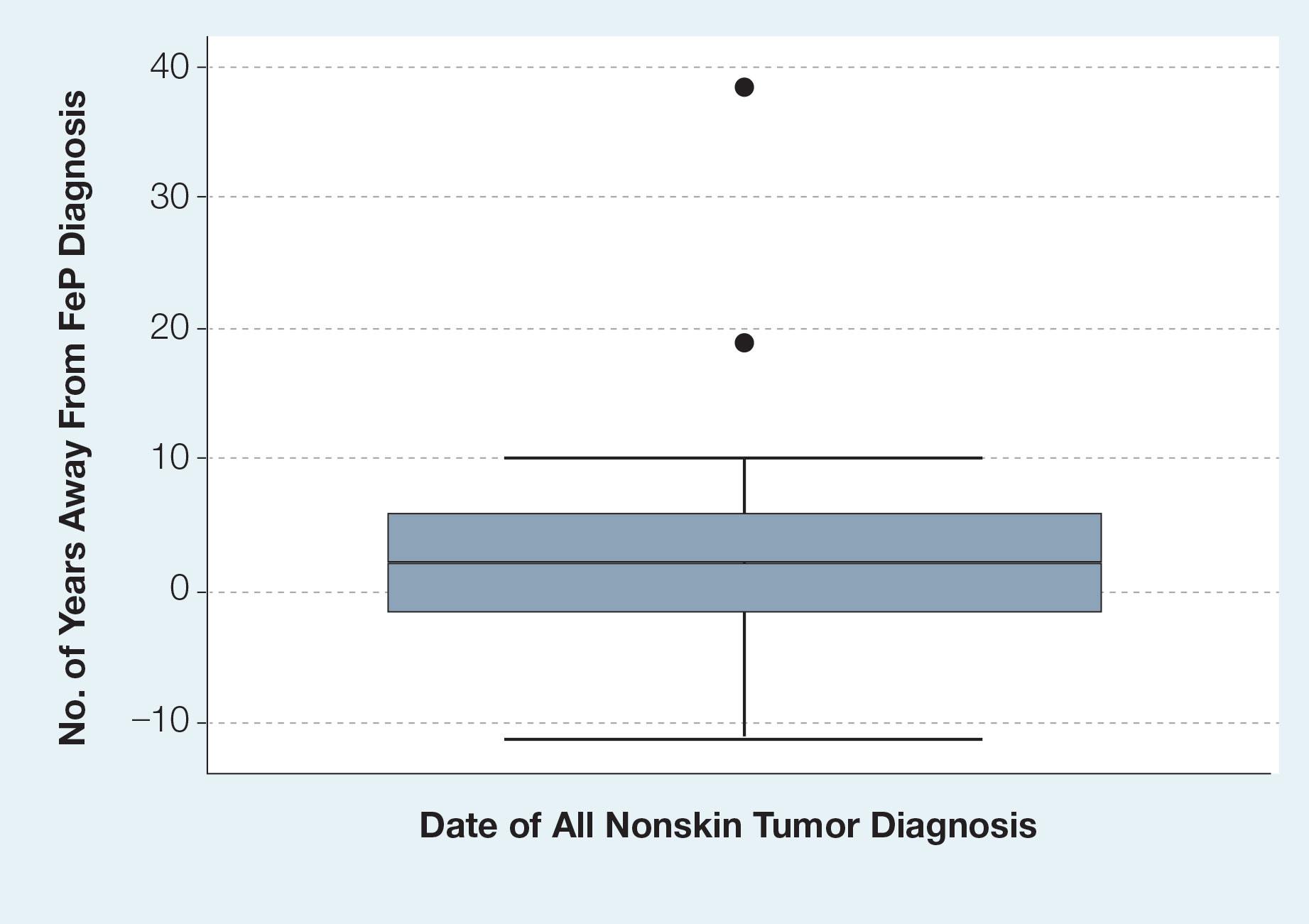
Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
PRACTICE POINTS
- Dermatologic reactions may be the initial presentation of an internal malignancy.
- Fibroepithelioma of Pinkus is considered on the spectrum between adnexal neoplasms and a nonaggressive variant of basal cell carcinoma (BCC).
- Fibroepithelioma of Pinkus should be managed similar to nonaggressive variants of BCC such as nodular BCC.
- Fibroepithelioma of Pinkus is not associated with internal malignancy.
Photoprotection Prevents Skin Cancer: Let’s Make It Fashionable to Wear Sun-Protective Clothing
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Practice Points
- Routine wear of wide-brimmed hats is the simplest, most inexpensive, and only reusable form of photoprotection for the head and neck and should be an everyday practice for reducing the risk for preventable skin cancers.
- The regular wear of clothing and head cover with adequate UV protection factor is equally as important to utilize in the prevention of UV-induced skin cancers as the application of topical sunscreens and sunblocks.
- The medical community should make a concerted effort to dispel any public policy or fashion trend that does not promote personal protection from sun-induced skin cancers. Policies that restrict wearing photoprotective garments, such as in schools and in the military, need to be changed.
